DOI:
10.1039/C4RA12086H
(Paper)
RSC Adv., 2015,
5, 16785-16791
Copolymers based on telechelic benzoxazine with a reactive main-chain and anhydride: monomer and polymer synthesis, and thermal and mechanical properties of carbon fiber composites
Received
15th October 2014
, Accepted 20th January 2015
First published on 22nd January 2015
Abstract
Enhanced thermomechanical properties of polybenzoxazine based on allylamine-terminated oligomeric benzoxazine (Allyl-oligomer) are obtained by copolymerizing the oligomer with maleic anhydride (MA) in the presence of a free radical initiator. MA is introduced to react with the oxazine ring and allyl groups via different mechanisms. From 10% to 30% of MA are added into benzoxazine systems to explore the optimum copolymerization ratio. The polymerization behavior, glass transition temperature (Tg) and thermal stability of the corresponding polymers are investigated by differential scanning calorimetry (DSC), in situ Fourier transform infrared spectroscopy (FTIR), dynamic mechanical analysis (DMA) and thermogravimetric analysis (TGA). Addition of 10% MA is found to give the highest thermal stability, while maintaining the highest Tg of the polymer matrix. Using the resulting high performance polymer, carbon fiber composites are prepared and studied. The mechanical properties are evaluated by ASTM tensile and flexural tests. The tensile strength, tensile modulus, and flexural modulus of the composites exceed those of benchmark products with epoxy matrices.
1. Introduction
Polybenzoxazines have been used as attractive polymer matrices to prepare fiber reinforced high performance composites. This is due to many unique advantages, such as no release of volatiles, no catalyst required and near zero shrinkage during polymerization,1,2 high thermal stability,3–5 self-extinguishing ability,6 and great molecular design flexibility. Recently, the composites using glass fibers,7–9 cellulose fibers,10–13 silica fibers,14 and carbon fibers15–18 have been successfully prepared. However, improving the thermal and mechanical properties and processability of the composite by changing the polymer matrix is of continuing interest. The goal of this study is to develop a new type of benzoxazine resin as a composite matrix based on a telechelic architecture having a reactive group in the main chain. Such molecules are a departure from the traditional telechelic molecules where the main-chain usually consists of inert structures.
Copolymerization of monomeric benzoxazine resins with anhydrides to enhance thermal and mechanical properties have been first reported by Rimdusit et al.,19–21 they observed enhanced thermomechanical properties by reacting bisphenol A-aniline type benzoxazine (abbreviated as BA-a) with different carboxylic dianhydrides. The property enhancement was attributed to the additional ester linkage formation between the hydroxyl group of poly (BA-a) and the carbonyl group of anhydride during thermal curing. However, addition of anhydride to main-chain type polybenzoxazines has not been reported. Main-chain type benzoxazine was developed by Liu et al.22–24 to form a more ductile cross-linked network upon polymerization thus leading to enhanced thermal properties comparing to the monomeric type. However, the liquefication temperature of main-chain type benzoxazine is usually rather high. In order to achieve good processability without sacrificing excellent thermal and mechanical properties, oligomeric main chain type benzoxazines have been developed.25,26 The stoichiometry is manipulated in such a way to yield short, main-chain type precursors.
In this study, allyl amine-terminated main-chain type oligomeric benzoxazine is chosen mainly for three reasons. First of all, the addition of allyl group is reported to ease processibility, reduce cost and improve thermal and mechanical properties of polybenzoxazines due to the extra crosslinking sites.27 Secondly, with the existence of free radical initiator, maleate double bonds in anhydride are expected to copolymerize with allyl group to form an alternating co-polymers. Finally, allyl group termination eliminates the reactive end groups and thus improves the shelf life. This co-polymerizability of maleate double bonds and vinyl double bounds has been extensively used in hardening of unsaturated polyesters.28–32 Furthermore, copolymerization between the anhydride group and phenolic group of polybenzoxazine can also occur.19–21 However, the optimum amount of anhydride must be determined to obtain the best thermal and mechanical properties of the polymer. Very high elastic modulus; high tensile strength; high chemical inertness; high thermal conductivity, assisting good fatigue properties; excellent creep resistance; and low coefficient of thermal expansion33–36 compliments the excellent properties of polybenzoxazine as a matrix.
2. Experimental
2.1. Materials
Mixed isomers of bisphenol-F were kindly supplied from Hexion Specialty Chemicals. Para-formaldehyde (96%), 4,4′-diamino-diphenylmethane (DDM) (>99%) and allylamine (98%) were purchased from Aldrich Chemical Company. N,N′-Dimethylformamide (DMF), toluene, hexanes (a mixture of isomers) tetrahydrofuran, methanol, ethanol and amine terminated butadiene–acrylonitrile rubber were obtained from Fisher Scientific Company. All the other chemicals were used as received. Toray T300 Plain Weave Carbon Fiber Fabric was used as reinforcement for making composites.
2.2. Synthesis of allylamine terminated, main-chain type oligomeric benzoxazine (Allyl-oligomer)
In a 250 mL round bottom flask were added 10 g (0.0499 mol) bisphenol F isomer mixtures, 4.95 g (0.025 mol) 4,4′-methylenedianiline, 5.99 g (0.200 mol) paraformaldehyde. To this mixture, 100 mL 1,4-dioxane was added to assist better mixing. After the temperature was gradually increased to 101 °C, which is the reflux temperature of this solvent, the temperature was maintained for 15 min, and 2.85 g (0.05 mol) allylamine was carefully added into the system through the top of the condenser. A small amount of dioxane was used to rinse the tube to make sure all the allylamine has been added to the mixture. Since allylamine is highly volatile, it is crucial to make sure that the condenser functions effectively. The reaction mixture was kept at 101 °C and stirred vigorously for another 5 h, after which a clear yellowish solution was obtained. Thin layer chromatography was used to monitor the completion of the reaction by tracing the consumption of the starting material. 1H NMR was used to confirm the formation of benzoxazine structure. The molecular weight of oligomer (Mn) is around 1500–2000 Da.
2.3. Copolymerization of allyl group and maleic anhydride
The weight ratios of binary mixtures at 90/10, 80/20, 70/30 benzoxazine–MA were evaluated as potential matrices for carbon fiber-reinforced composites. The exact amount of maleic anhydride and benzoxazine oligomer was measured and dissolved in chloroform, and 3 wt% dicumyl peroxide was added into each system as a free radical initiator. The solution was magnetically stirred for about half an hour to ensure homogeneous mixing, resulting in a clear yellowish solution. For DSC samples, chloroform was removed by a rotary evaporator at room temperature. Sample size of about 3–5 mg was used. For TGA and DMA samples, the polymerized samples were obtained by stepwise polymerization of the mixtures with a heating cycle of 75 °C, 125 °C, 150 °C, 175 °C, 200 °C for 2 h at each temperature and 30 min at 225 °C to achieve full polymerization. Sample size of about 10 mg was used for TGA measurement and the sample dimension of 40 mm × 5 mm × 1 mm was used for DMA measurement (Table 1).
Table 1 Benzoxazine–maleic anhydride systems investigated in this study
| Sample name |
Benzoxazine |
Maleic anhydride |
Initiator |
| Neat Allyl-oligomer |
100% |
0 |
0 |
| Allyl-oligomer–10% MA |
90% |
10% |
3% |
| Allyl-oligomer–20% MA |
80% |
20% |
3% |
| Allyl-oligomer–30% MA |
70% |
30% |
3% |
2.4. Production of benzoxazine–carbon fiber prepregs
Benzoxazine–carbon fiber prepregs were prepared as follows. The carbon fabrics were first immersed in a solution of 0.005% by fiber weight of amine terminated butadiene–acrylonitrile (ATBN) rubber in a THF bath first. They were then mechanically pulled out from the bath at a constant speed.29,30 The fabric was dried in air overnight, forming a very thin elastomer coating on the carbon fiber surface. Subsequently, the surface treated carbon cloth was immersed in a THF solution of the resin at 40 wt% and again the fabric was taken out of the solution at a constant speed. The coated carbon fiber cloths were dried in air at 50 °C overnight and subsequently in a vacuum oven at 100 °C for 6 h. The resin concentration was calculated and varied with respect to achieve target resin content of 26% by fiber volume. Property comparison with composites of other resins reported in the literature were also made by normalization to a fiber volume of 60%.
2.5. Fabrication of polybenzoxazine–carbon fiber test specimens
Test specimens for physical and mechanical testing were fabricated from carbon fiber/polybenzoxazine prepregs through two main steps: (1) compression molding of the prepreg plies to produce plaques approximately 152.4 mm × 152.4 mm (6.0 × 6.0 inch) in size and (2) machining the plaques to produce test specimens of desired size and shape. A Tetrahedron Associates compression molding machine was used to fabricate carbon-fiber reinforced benzoxazine composite samples. Six plies of coated carbon fiber cloths were laminated and then subjected to a step polymerization procedure as follows: 140 °C, 1.38 MPa (2 h), 160 °C, 1.38 MPa (2 h), 180 °C, 1.38 MPa (2 h), and 200 °C, 1.38 MPa (2 h). The samples were then slowly cooled to room temperature.
2.6. Measurement
1H NMR spectra were acquired in chloroform with tetramethylsilane as an internal standard on a Varian Oxford AS300 at a proton frequency of 300 MHz. The average number of transients for 1H NMR was 64. A relaxation time of 10 s was used for the integrated intensity determination of 1H NMR spectra. 13C NMR spectra were also obtained at the corresponding carbon frequency of 75.43 MHz with the number of transients of 2000. TA Instruments DSC model 2920 was used with a heating rate of 10 °C min−1 and a nitrogen flow rate of 60 mL min−1 for all tests of the differential scanning calorimetric (DSC) study. All samples were crimped in hermetic aluminum pans with lids. Thermogravimetric analyses (TGA) were performed on a TA Instruments Q500 TGA with a heating rate of 10 °C min−1 in a nitrogen atmosphere at a flow rate of 40 mL min−1. Fourier transform infrared spectra (FT-IR) were obtained using a Bomem Michelson MB100 FT-IR spectrometer which was equipped with a deuterated triglycine sulfate (DTGS) detector. Co-addition of 16 scans was recorded at a resolution of 4 cm−1 after a 20 minute purge with dry air. Dynamic mechanical analyses (DMA) were performed on a TA Instruments Q800 DMA applying controlled strain tension mode with a constant frequency of 1.0 Hz over a temperature sweep range of 0 °C to 300 °C with a ramp rate of 3 °C min−1.
To fabricate individual test specimens for flexural and tensile testing, plaques were first roughly cut using a diamond blade saw. The specimens were then deburred and machined to final dimensions using a polishing wheel with progressively finer grades of silicon-carbide paper. Flexural testing of carbon fiber reinforced composites was performed using a three point bending test according to ASTM 790-92. The dimensions of the specimens were 76.2 mm × 12.7 mm × 1.6 mm. All tests were conducted on an Instron model 5567 Universal Testing Apparatus at room temperature in air and six samples were used to average for each test.
Tensile testing of T300/Allyl-oligomer/MA composite specimens was conducted in accordance with ASTM D3039 to determine the tensile strength and modulus of the composite material. Targeted nominal dimensions of the finished specimens were 152.4 mm × 12.7 mm × 1.6 mm (6.0 × 0.5 × 0.063 inch). To prevent failure at the grips, fiberglass tabs were added to both ends of the specimens. The tabs were affixed with an epoxy resin and were approximately 25.6 mm in length with a 30 degree bevel on the medial end of the tab. To accurately measure strain, a strain gauge was affixed to the center of each specimen. The leads of the strain gauge were connected to a high resolution Ohm-meter. Strain was measured during testing by recording the change in strain gauge resistance at a rate of four measurements per second using data-logging software.
3. Results and discussion
3.1. Preparation of allylamine terminated oligomeric benzoxazine
As shown in Scheme 1, allylamine terminated oligomeric benzoxazine was prepared by end capping the main-chain type benzoxazine by allylamine monomers. The chemical structure of oligomeric benzoxazine was confirmed by both 1H and 13C NMR. Fig. 1 shows the 1H NMR spectrum of the allylamine-terminated oligomeric benzoxazine. The two multiplets at 5.25 and 5.95 ppm are characteristic protons of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 and
CH2 and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– in the allyl group, respectively. The protons of –CH2– of ally group showed a doublet at 3.39 ppm.27 Typically, benzoxazine monomers have two equal intensity singlet peaks in 1H NMR due to the two CH2's in the oxazine ring.31 The characteristic protons of oxazine ring appeared at 5.87 and 4.83 ppm which are assigned to –O–CH2–N– and –Ar–CH2–N–, respectively. The aromatic protons appeared as multiple resonances at 6.77–7.16 ppm. The chemical structure was further confirmed by 13C NMR, as shown in Fig. 2, characteristic resonances for oxazine ring located at 81.91 ppm and 51.47 ppm, respectively. The resonances located at 118.4 ppm, 135.0 ppm come from the carbon double bond in allyl group.
CH– in the allyl group, respectively. The protons of –CH2– of ally group showed a doublet at 3.39 ppm.27 Typically, benzoxazine monomers have two equal intensity singlet peaks in 1H NMR due to the two CH2's in the oxazine ring.31 The characteristic protons of oxazine ring appeared at 5.87 and 4.83 ppm which are assigned to –O–CH2–N– and –Ar–CH2–N–, respectively. The aromatic protons appeared as multiple resonances at 6.77–7.16 ppm. The chemical structure was further confirmed by 13C NMR, as shown in Fig. 2, characteristic resonances for oxazine ring located at 81.91 ppm and 51.47 ppm, respectively. The resonances located at 118.4 ppm, 135.0 ppm come from the carbon double bond in allyl group.
 |
| | Scheme 1 Synthesis of allylamine terminated oligomeric benzoxazine (the main-chain polybenzoxazine structure is shown using the para-isomer bisphenol as an example). | |
 |
| | Fig. 1 1H NMR spectrum of allylamine-terminated oligomeric benzoxazine. | |
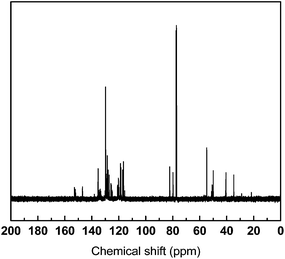 |
| | Fig. 2 13C NMR spectrum of allylamine-terminated oligomeric benzoxazine. | |
3.2. Copolymerization of benzoxazine and maleic anhydride
3.2.1 Benzoxazine–maleic anhydride polymerization behavior by DSC. As shown in Fig. 3, the polymerization behavior of oligomeric benzoxazine–maleic anhydride mixture is quite complex. For the neat oligomer, a broad exothermic peak at 234 °C with onset temperature around 172 °C was observed, which corresponds to the ring opening polymerization of benzoxazine. However, for the oligomer mixed with 10%, 20%, 30% MA, another peak around 175–180 °C appeared, indicating multiple polymerization reactions. In all three cases, the peak onset shifts to around 130 °C; this could be due to the presence of dicumyl peroxide, which works as a free radical initiator to induce the copolymerization between the anhydride group and allyl group. For 10% and 20% mixture, a broad peak with a shoulder around 225 °C was observed, corresponding to the allyl-anhydride reaction and oxazine ring opening polymerization respectively. The peak onset and maximum peak temperature is almost independent of the amount of maleic anhydride added, however, for 30% mixture, the shoulder of the peak decreased drastically, while the maximum peak temperature shifts from 234 °C to 179 °C (Table 2).
 |
| | Fig. 3 Polymerization behavior of benzoxazine–maleic anhydride mixture. | |
Table 2 Thermal properties of benzoxazine–maleic anhydride oligomers
| Monomer |
Exothermal heat |
| Onset (°C) |
Max (°C) |
(J g−1) |
| Neat Allyl-oligomer |
172 |
234 |
209 |
| Allyl-oligomer–10% MA |
134 |
185 |
318 |
| Allyl-oligomer–20% MA |
132 |
188 |
361 |
| Allyl-oligomer–30% MA |
128 |
179 |
305 |
3.2.2 FT-IR study of benzoxazine–maleic anhydride polymerization behavior. In situ FT-IR measurement was used to further understand the polymerization behavior of the material. Spectra of neat Allyl-oligomer benzoxazine along with that of the benzoxazine–MA copolymer were measured for comparison. The addition of 10% MA was used as an example to discuss here. In Fig. 4, the characteristic absorptions are observed for the benzoxazine structure at 1500–1491 cm−1 (trisubstituted of benzene ring), 1340–1327 cm−1 (–CH2–wagging), 1236–1230 cm−1 (asymmetric stretching of C–O–C), 1036–1028 cm−1 (symmetric stretching of C–O–C), and at 950–920 cm−1 out-of-the-plane mode of benzene to which oxazine group is attached.32 Characteristic absorption bands assigned to allyl group appeared at 3084 cm−1 (stretching of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H), 1644 cm−1 (stretching of C
C–H), 1644 cm−1 (stretching of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond). In addition, the out-of-the-plane CH bending at 864–860 cm−1 and 997–991 cm−1 also indicates the presence of allyl group. Upon heating, the peak intensity of 952 cm−1 is gradually decreased and eventually disappeared by the end of the polymerization, suggesting the completion of ring-opening of benzoxazine to form polybenzoxazine. Besides, the intensity of allyl group out-of-plane CH bending (864–860 cm−1) decreased upon temperature increase, indicating the occurrence of allyl polymerization. For example with 10% addition of maleic anhydride, in addition to the characteristic peaks from benzoxazine and allyl groups, one can observe two strong bands located at 1830–1800 cm−1 and 1755–1740 cm−1 which correspond to the C
C bond). In addition, the out-of-the-plane CH bending at 864–860 cm−1 and 997–991 cm−1 also indicates the presence of allyl group. Upon heating, the peak intensity of 952 cm−1 is gradually decreased and eventually disappeared by the end of the polymerization, suggesting the completion of ring-opening of benzoxazine to form polybenzoxazine. Besides, the intensity of allyl group out-of-plane CH bending (864–860 cm−1) decreased upon temperature increase, indicating the occurrence of allyl polymerization. For example with 10% addition of maleic anhydride, in addition to the characteristic peaks from benzoxazine and allyl groups, one can observe two strong bands located at 1830–1800 cm−1 and 1755–1740 cm−1 which correspond to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch in anhydride group as shown in Fig. 5. Upon temperature increase, similar to neat Allyl-oligomer benzoxazine, the peak intensity of 952 cm−1 gradually decreased and eventually completely disappeared due to the oxazine ring polymerization. The peak intensity of the allyl group at 1644 cm−1 and 864–860 cm−1 decreased much faster compared to those in the neat benzoxazine system, which could be due to the initiation by the free radical initiator. Meanwhile, the anhydride group is involved in two reactions: (1) reaction with allyl group to form alternating copolymers; (2) reaction with hydroxyl group from ring opened benzoxazines to form ester linkage.19,20 The characteristic band of C–O at 1250–1310 cm−1 was observed.
O stretch in anhydride group as shown in Fig. 5. Upon temperature increase, similar to neat Allyl-oligomer benzoxazine, the peak intensity of 952 cm−1 gradually decreased and eventually completely disappeared due to the oxazine ring polymerization. The peak intensity of the allyl group at 1644 cm−1 and 864–860 cm−1 decreased much faster compared to those in the neat benzoxazine system, which could be due to the initiation by the free radical initiator. Meanwhile, the anhydride group is involved in two reactions: (1) reaction with allyl group to form alternating copolymers; (2) reaction with hydroxyl group from ring opened benzoxazines to form ester linkage.19,20 The characteristic band of C–O at 1250–1310 cm−1 was observed.
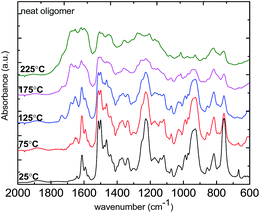 |
| | Fig. 4 In situ FT-IR spectrum of neat oligomeric allyl-benzoxazine upon heating. | |
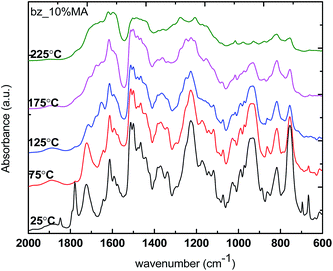 |
| | Fig. 5 In situ FT-IR spectrum of benzoxazine–10% maleic anhydride upon heating. | |
3.3. Thermal stability of polymerized benzoxazine–maleic anhydride
The thermal stability of polymerized resin is studied by heating sample in a nitrogen atmosphere to 900 °C in a TGA furnace. As shown in Fig. 6, a similar weight loss pattern, while showing different char yield, was observed. With the addition of MA, the thermal stability of fully polymerized resin increased compared to the neat resin. This could be attributed to the copolymerization between anhydride group and allyl group increasing the crosslink density of the network. However, 10% MA addition gives the highest thermal stability, whereas the thermal stability decrease as the amount of MA increases thereafter. This indicates the relative content of Mannich bridge is the driving factor of thermal stability. With the increased amount of anhydride group, the thermal stability decreased, which could be due to the dissolution of maleic anhydride in the thermosetting materials.
 |
| | Fig. 6 Thermal stability of polymerized benzoxazine–maleic anhydride. | |
3.4. Dynamic mechanical analysis of polymer matrix
The glass transition temperature (Tg) of the polymer matrix was evaluated by dynamic mechanical analysis (DMA). As shown in Fig. 7 and Table 3, the Tg is increased from 220 °C to 249 °C and 234 °C for the resins polymerized with 10% and 20% MA. However, for the resin polymerized with 30% MA, the Tg appears to be around 155 °C. It is postulated that two phenomena are overlapped. The first event is the copolymerization of the allyl group and anhydride. This increases the Tg. However, as the anhydride concentration increases, some of the anhydride may not be able to copolymerize with the allyl group and remain as unreacted or small oligomers. The dissolution of maleic anhydride causes Tg decrease as shown in 30% MA sample. The very weak peaks in the E′′ spectra in the range of 125–150 °C may be due to the β transition of these polymers. Based on both TGA and DMA results, we decided to use the 10% anhydride system for composite preparation so as not to dilute the positive effect of benzoxazines (Table 4).
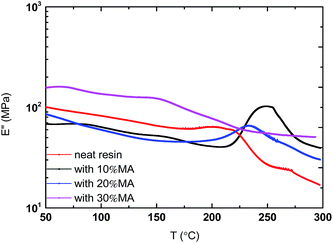 |
| | Fig. 7 DMA study of polymerized resins. | |
Table 3 Thermal properties of poly(benzoxazine–maleic anhydride)
| Type |
Weight-loss temperature (°C) |
Char yield at 800 °C (%) |
| 5% |
10% |
| Neat Allyl-oligomer |
370 |
414 |
52 |
| Allyl-oligomer–10% MA |
365 |
404 |
62 |
| Allyl-oligomer–20% MA |
350 |
409 |
58 |
| Allyl oligomer-30% MA |
338 |
395 |
56 |
Table 4 Glass transition temperature of poly(benzoxazine–maleic anhydride)
| BZ/MA polymers |
Tg (°C) |
| Neat Allyl-oligomer |
220 |
| Allyl-oligomer–10% MA |
249 |
| Allyl-oligomer–20% MA |
234 |
| Allyl-oligomer–30% MA |
155 |
3.5. Evaluation of mechanical properties of carbon fiber reinforced polymer composites
The T300/Allyl-oligomer/MA composite's physical and mechanical properties were compared to the properties of commercially available polymer matrix composites containing similar types of fiber reinforcement (i.e. standard modulus, 228 GPa/33 Msi class, plain weave carbon fiber cloth). The reported mechanical properties of commercially available composites are regularly normalized to values representative of a composite with a fiber volume fraction of 60%. To make as direct a comparison as possible, the mechanical properties of the T300/Allyl-oligomer/MA composite were also normalized for a fiber volume fraction of 60%. In order to perform the normalization, the actual fiber volume fraction for the T300/Allyl-oligomer/MA composite produced during this project needed to be estimated first. This estimate was made using the rule of mixtures as shown below in eqn (1) and the definition of the composite volume fraction as shown in eqn (1) and (2):where, ρc = composite density, g cm−3, ρm = matrix density, g cm−3, Vm = matrix volume fraction, ρf = fiber density, g cm−3, Vf = fiber volume fraction.
To solve for the fiber volume fraction, Vf, the following values were used:
ρc = 1.31 g cm−3 (the average density measurements of the benzoxazine composite), ρm = 1.15 g cm−3 (estimated density of the crosslinked Allyl-oligomer resin), ρf = 1.76 g cm−3 (density reported on the Toray T300 technical datasheet).
The resulting estimate of the actual fiber volume fraction was calculated to be 26 vol%. Using this value, the mechanical properties of the composite were normalized to a fiber volume fraction of 60 vol% using the preferred method described in Section 2.5.7 of the Department of Defense Composites Materials Handbook, vol. 1 (MIL-HDBK-17-1F). This method makes the assumption that fiber-dominated strength and stiffness properties vary linearly with fiber volume fraction and therefore material properties such as strength and modulus can be normalized using the following equation:
| |
 | (3) |
where,
Xn = normalized test value,
Xa = actual test value,
Vn = fiber volume fraction that test values with be normalized to,
Vf = fiber volume fraction of the actual tested composite (
Tables 5 and
6).
Table 5 Summary of T300/Allyl-oligomer/MA composite flexural testing results (actual values for composites with 26 vol% fiber)
| |
Average |
| Maximum load (N) |
142 |
| Deflection at maximum load (mm) |
5.56 |
| Percent strain at maximum load |
1.28% |
| Flexural strength (ksi) |
63.9 |
| Flexural strength (MPa) |
440.4 |
| Flexural modulus (Msi) |
6.4 |
| Flexural modulus (GPa) |
43.9 |
Table 6 Comparison of benzoxazine carbon fiber composite with commercial composite products (normalized to 60 vol% fiber contents)
| Composite ID |
T300/Allyl-oligomer/MA |
Commercial composite products |
| Matrix resin |
Allyl-oligomer/MA polybenzoxazine |
Cycom® 934 epoxy1 |
Tencate RS-12 toughened polycyanate2 |
Cycom® 997 epoxy3 |
| Reinforcement fiber |
Toray T300 plain weave carbon fiber fabric |
Cytec HMF 322/34 plain weave carbon fiber fabric |
T300 plain weave carbon fiber fabric |
Standard modulus plain weave carbon fiber fabric |
| Fiber volume fraction |
60% |
60% |
60% |
60% |
| Density, g cm−3 |
1.52 |
1.59 |
1.54 |
N/A |
| Tensile strength, MPa (ksi) |
1037.2 (150.5) |
637.5 (92.5) |
N/A |
782.5 (113.5) |
| Tensile modulus, GPa (Msi) |
135.3 (19.6) |
69.0 (10.0) |
N/A |
69.0 (10.0) |
| Flexural strength, MPa (ksi) |
1016.4 (147.4) |
827.0 (120.0) |
1055.0 (153.0) |
879.0 (127.5) |
| Flexural modulus, GPa (Msi) |
101.4 (14.7) |
57.0 (10.0) |
66.2 (9.6) |
62.0 (9.0) |
4. Conclusion
Allyl terminated oligomeric benzoxazine has been prepared and characterized in this study. Maleic anhydride was introduced as a comonomer with allyl group; free radical initiator was used to improve the conversion of anhydride and allyl group during the polymerization. The polymerized product has shown excellent thermal and mechanical properties and good processibility. By utilizing an initiator, rubber interlayer and copolymerization with maleic anhydride, carbon fiber composites were fabricated with improved properties without decreasing the thermal properties. A Tg of 249 °C was observed from the optimized composition of the polymerized benzoxazine–maleic anhydride resin. The highest flexural strength of the composite exceeded 1 GPa.
Acknowledgements
The authors gratefully acknowledge partial financial support through NASA SBIR Project contract #NNX12CF55P. One of the authors (J.L.) is also grateful for the partial financial support of China Scholarship Council.
Reference and notes
- M. Alcoutlabi, G. B. Mckenna and S. L. Simon, J. Appl. Polym. Sci., 2003, 88, 227 CrossRef CAS.
- J. D. Russell, Cure shrinkage of thermoset composites, SAMPE Q., 1993, 24, 28 CAS.
- H. Y. Low and H. Ishida, Polymer, 1999, 40, 4365 CrossRef CAS.
- H. Y. Low and H. Ishida, J. Polym. Sci., Part B: Polym. Phys., 1998, 36, 1935 CrossRef CAS.
- A. Laobuthee, S. Chirachanchai, H. Ishida and K. Tashiro, J. Am. Chem. Soc., 2001, 123, 9947 CrossRef CAS PubMed.
- W. R. L. Lyon, J. Appl. Polym. Sci., 2003, 87, 548 CrossRef.
- H. Ishida and H. Y. Low, J. Appl. Polym. Sci., 1998, 69, 2559 CrossRef CAS.
- H. Ishida and T. Chaisuwan, Polym. Mater.: Sci. Eng., 2001, 85, 534 CAS.
- H. Kimura, A. Matsumoto and K. Ohtsuka, J. Appl. Polym. Sci., 2009, 114, 1256 CrossRef CAS.
- N. Dansiri, N. Yanumet, J. W. Ellis and H. Ishida, Polym. Compos., 2002, 23, 352 CrossRef CAS.
- S. Tragoonwichian, N. Yanumet and H. Ishida, J. Appl. Polym. Sci., 2007, 106, 2925 CrossRef CAS.
- S. Tragoonwichian, N. Yanumet and H. Ishida, Compos. Interfaces, 2008, 15, 321 CrossRef CAS PubMed.
- C. Jubsilp, T. Takeichi, S. Hiziroglu and S. Rimdusit, Bioresour. Technol., 2008, 99, 8880 CrossRef CAS PubMed.
- K. S. Santhosh Kumar, C. P. R. Nair and K. N. Ninan, J. Appl. Polym. Sci., 2008, 107, 1091 CrossRef.
- S. B. Shen and H. Ishida, Polym. Compos., 1996, 17, 710 CrossRef CAS.
- T. Chaisuwan and H. Ishida, J. Appl. Polym. Sci., 2006, 101, 548 CrossRef CAS.
- J. S. Jang and H. Yang, Compos. Sci. Technol., 2000, 60, 457 CrossRef CAS.
- H. Ishida and T. Chaisuwan, Polym. Compos., 2003, 24, 597 CrossRef CAS.
- C. Jubsilp, T. Takeichi and S. Rimdusit, Polym. Degrad. Stab., 2011, 96, 1047 CrossRef CAS PubMed.
- T. Takeichi, Y. Guo and S. Rimdusit, Polymer, 2005, 46, 4909 CrossRef CAS PubMed.
- A. Tuzun, B. Kiskan, N. Alemdar, A. T. Erciyes and Y. Yagci, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 4279 CrossRef CAS.
- J. Liu, Ph.D. thesis, Case Western Reserve University, Cleveland, Ohio, May 1995.
- T. Takeichi, T. Kano and T. Agag, Polymer, 2005, 46, 12172 CrossRef CAS PubMed.
- A. Chernykh, J. P. Liu and H. Ishida, Polymer, 2006, 47, 7664 CrossRef CAS PubMed.
- J. Liu, T. Agag and H. Ishida, Polymer, 2010, 54, 5688 CrossRef PubMed.
- J. Liu, T. Agag and H. Ishida, in Handbook of Benzoxazine Resins, ed. Elsevier, Amsterdam, 2011, ch. 18, pp. 355–362 Search PubMed.
- T. Agag and T. Takeichi, Macromolecules, 2003, 36, 6010 CrossRef CAS.
- C. P. Hsu and L. J. Lee, Polymer, 1991, 32, 2263 CrossRef CAS.
- G. Negrell-Guriao, G. David, B. Boutevin and K. Chougrani, J. Polym. Sci., Part A: Polym. Chem., 2011, 18, 3905 CrossRef.
- H. W. Liu and C. E. Wilen, Macromolecules, 2001, 14, 5067 CrossRef.
- A. Boztug and S. Basan, Des. Monomers Polym., 2006, 6, 617 CrossRef PubMed.
- H. W. Liu and C. E. Wilen, J. Appl. Polym. Sci., 2005, 5, 1941 CrossRef.
- J. Jang and H. Yang, J. Mater. Sci., 2000, 35, 2297 CrossRef CAS.
- J. Jang and H. Yang, Compos. Sci. Technol., 2000, 60, 457 CrossRef CAS.
- H. Ishida and D. Allen, Polymer, 1996, 37, 4487 CrossRef CAS.
- J. Dunkers and H. Ishida, Spectrochim. Acta, Part A, 1995, 51, 1061 CrossRef.
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 and
CH2 and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– in the allyl group, respectively. The protons of –CH2– of ally group showed a doublet at 3.39 ppm.27 Typically, benzoxazine monomers have two equal intensity singlet peaks in 1H NMR due to the two CH2's in the oxazine ring.31 The characteristic protons of oxazine ring appeared at 5.87 and 4.83 ppm which are assigned to –O–CH2–N– and –Ar–CH2–N–, respectively. The aromatic protons appeared as multiple resonances at 6.77–7.16 ppm. The chemical structure was further confirmed by 13C NMR, as shown in Fig. 2, characteristic resonances for oxazine ring located at 81.91 ppm and 51.47 ppm, respectively. The resonances located at 118.4 ppm, 135.0 ppm come from the carbon double bond in allyl group.
CH– in the allyl group, respectively. The protons of –CH2– of ally group showed a doublet at 3.39 ppm.27 Typically, benzoxazine monomers have two equal intensity singlet peaks in 1H NMR due to the two CH2's in the oxazine ring.31 The characteristic protons of oxazine ring appeared at 5.87 and 4.83 ppm which are assigned to –O–CH2–N– and –Ar–CH2–N–, respectively. The aromatic protons appeared as multiple resonances at 6.77–7.16 ppm. The chemical structure was further confirmed by 13C NMR, as shown in Fig. 2, characteristic resonances for oxazine ring located at 81.91 ppm and 51.47 ppm, respectively. The resonances located at 118.4 ppm, 135.0 ppm come from the carbon double bond in allyl group.

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H), 1644 cm−1 (stretching of C
C–H), 1644 cm−1 (stretching of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond). In addition, the out-of-the-plane CH bending at 864–860 cm−1 and 997–991 cm−1 also indicates the presence of allyl group. Upon heating, the peak intensity of 952 cm−1 is gradually decreased and eventually disappeared by the end of the polymerization, suggesting the completion of ring-opening of benzoxazine to form polybenzoxazine. Besides, the intensity of allyl group out-of-plane CH bending (864–860 cm−1) decreased upon temperature increase, indicating the occurrence of allyl polymerization. For example with 10% addition of maleic anhydride, in addition to the characteristic peaks from benzoxazine and allyl groups, one can observe two strong bands located at 1830–1800 cm−1 and 1755–1740 cm−1 which correspond to the C
C bond). In addition, the out-of-the-plane CH bending at 864–860 cm−1 and 997–991 cm−1 also indicates the presence of allyl group. Upon heating, the peak intensity of 952 cm−1 is gradually decreased and eventually disappeared by the end of the polymerization, suggesting the completion of ring-opening of benzoxazine to form polybenzoxazine. Besides, the intensity of allyl group out-of-plane CH bending (864–860 cm−1) decreased upon temperature increase, indicating the occurrence of allyl polymerization. For example with 10% addition of maleic anhydride, in addition to the characteristic peaks from benzoxazine and allyl groups, one can observe two strong bands located at 1830–1800 cm−1 and 1755–1740 cm−1 which correspond to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch in anhydride group as shown in Fig. 5. Upon temperature increase, similar to neat Allyl-oligomer benzoxazine, the peak intensity of 952 cm−1 gradually decreased and eventually completely disappeared due to the oxazine ring polymerization. The peak intensity of the allyl group at 1644 cm−1 and 864–860 cm−1 decreased much faster compared to those in the neat benzoxazine system, which could be due to the initiation by the free radical initiator. Meanwhile, the anhydride group is involved in two reactions: (1) reaction with allyl group to form alternating copolymers; (2) reaction with hydroxyl group from ring opened benzoxazines to form ester linkage.19,20 The characteristic band of C–O at 1250–1310 cm−1 was observed.
O stretch in anhydride group as shown in Fig. 5. Upon temperature increase, similar to neat Allyl-oligomer benzoxazine, the peak intensity of 952 cm−1 gradually decreased and eventually completely disappeared due to the oxazine ring polymerization. The peak intensity of the allyl group at 1644 cm−1 and 864–860 cm−1 decreased much faster compared to those in the neat benzoxazine system, which could be due to the initiation by the free radical initiator. Meanwhile, the anhydride group is involved in two reactions: (1) reaction with allyl group to form alternating copolymers; (2) reaction with hydroxyl group from ring opened benzoxazines to form ester linkage.19,20 The characteristic band of C–O at 1250–1310 cm−1 was observed.








