Filled and peptide-modified single-walled carbon nanotubes: synthesis, characterization, and in vitro test for cancer cell targeting†
Abstract
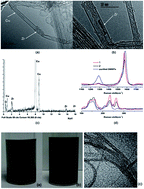
Multi-functional single-walled carbon nanotubes (SWNTs) with metal endohedral filling and a high degree of polycarboxylation on the sidewalls were synthesized without affecting the SWNT σ-framework. The encapsulation of Zr4+ ions within SWNTs from aqueous solutions by the wet chemistry method was achieved using tubes “corked” with C70 at both ends to prevent the loss of the loaded metal. Carboxylic acid functionalities were introduced on the sidewalls of the tubes without introducing holes in the framework through a modified Birch-type reaction. Moreover, these metal-filled, surface polycarboxylated SWNTs can serve as versatile anchor points for coupling to specific peptides, which can target integrins for potential applications in diagnostic imaging and therapy. This directly leads to the construction of multi-functional SWNT conjugates, equipped with peptides on the surface for the targeted delivery of radionuclides to specific sites in vivo. The different SWNT adducts were characterized by Raman spectroscopy, ultraviolet-visible/near-infrared (UV-vis/NIR) spectroscopy, thermogravimetric analysis combined with mass spectrometry and also transmission electron microscopy (TEM). The aqueous solubility, cytotoxicity and cancer cell binding assay were studied for investigating the potential of these multi-functional SWNT conjugates in biomedical applications.

 Please wait while we load your content...
Please wait while we load your content...