Effects of feeding mode on synthesis of polyalkylsilsesquioxane emulsion and latex properties
Bo Liu,
Zhengguang Sun,
Jun Wu and
Shiqiang Huang*
Faculty of Materials Science & Engineering, Ministry of Education Key Laboratory for the Green Preparation and Application of Functional Materials, Hubei University, Wuhan 430062, China. E-mail: Huangsq@hubu.edu.cn
First published on 18th September 2014
Abstract
A stable polyalkylsilsesquioxane emulsion was prepared by batch and semi-continuous emulsion polymerization of methyl triethoxysilane (MTES) and n-propyltrimethoxysilane (PTMS). The influence of different feeding modes on the emulsion particle size and morphology structure was investigated by dynamic laser scattering (DLS), transmission electron microscopy (TEM), scanning electron microscopy (SEM). The results indicated that the particle sizes of the polyalkylsilsesquioxane emulsion increased and finally tended to be constant with the extension of reaction time. The latex particle size distribution index (PDI) decreased with the extension of reaction time. The particle size prepared by the batch method was larger than that by the semi-continuous method, while the PDI was smaller. The actual solid content of the emulsion was 15.6% (by batch) and 15.5% (by semi-continuous) when the monomer mass fraction was 30% in the total system. The polymerization stability and storage stability of the emulsions prepared by the two methods are good. The morphologies of latex particles prepared by the two methods were spherical. There was a core–shell structure. There were some similar crystal structures in the molecular chain of polyalkylsilsesquioxane prepared with alkyltrialkoxysilane. The thermal degradation weightlessness peak of the polymer in a nitrogen atmosphere was at 450–600 °C, while in air atmosphere it was at 250–300 °C. The final residues were 68% in nitrogen, 65.1% in air atmosphere.
1. Introduction
Polysiloxane is widely used in textile finishing agents, waterproofing agents, adhesives and so on because of its specific physical and chemical properties.1,2 The methods for preparation of polysiloxane emulsions are generally classified in two categories: mechanical means and emulsion polymerization. Mechanical means usually entail taking the polysiloxane and using mechanical means such as homogenizers or vigorous agitation to emulsify the siloxanes in water.3–5 Emulsion polymerization typically entails combining a reactive silicone monomers or oligomers, emulsifiers, polymerization catalyst and water. (e.g.: octamethylcyclotetrasiloxane (D4) emulsion).6–8 Emulsion polymerization method is much more flexible in raw material selection and much easier to control the polymer structure during preparing polysiloxane emulsion. For example, for this polysiloxane emulsion prepared by ring-opening polymerization of D4, the molecular structure of polysiloxane is mainly linear structure. In order to increase the diversity of polysiloxane structure, a small amount of alkoxysilane monomer could be added into the D4 ring-opening emulsion polymerization system. Silicone resin dispersions were synthesized by emulsion polymerization of three silicone monomers: D4, methyltrimethoxysilane (METMS) and methacryloyltrimethoxysilane (MATMS) in the presence of dodecylbenzenesulphonic acid playing the role of both surfactant and polymerization catalyst.9The hydrolytic condensation mechanism of alkylalkoxysilane (RnSiX4−n, n = 1, 2, 3; R is alkyl, X for alkoxy) shows that the siloxy is easy hydrolysis and condensation in the acidic or alkaline aqueous medium.10–12 Pühringer13 synthesized polyalkylsilsesquioxane emulsion using alkylalkoxysilane monomer. During the emulsion polymerization, the reaction rate of alkylalkoxysilane was controlled using just the right amount of water and organic solvent. It has a negative impact to the natural environment to use organic solvent. However, a few reports are focused on the synthesis of polyalkylsilsesquioxane emulsion via the hydrolysis/condensation of sole alkyltrialkoxysilanes.
In this paper, the stable polyalkylsilsesquioxane emulsion was prepared by batch method and semi-continuous method emulsion polymerization of methyl triethoxysilane (MTES) and n-propyltrimethoxysilane (PTMS). The influence of different feeding modes on emulsion particle size was discussed. The thermal properties and X-ray powder diffraction (XRD) of polyalkylsilsesquioxane was also analyzed.
2. Experimental
2.1 Materials
Methyltriethoxysilane (MTES) (99.2%), and n-propyltrimethoxysilane (PTMS) (99.2%) were purchased from Jingzhou Jianghan Fine Chemical Co., Ltd. (China). Sodium dodecylsulfate (SDS) (CP) was purchased from Shanghai Maxam Co., Ltd. (China). Sodium dodecylbenzesulfonate (SDBS) (CP) was purchased from Nanjing Tianxi Fine Chemical Co., Ltd. (China). Polyoxyethylene octylphenol ether (OP-10) (CP) was purchased from Sinopharm Chemical Reagent Co., Ltd. (China). Deionized water was used throughout experiments.2.2 Synthesis polyalkylsilsesquioxane emulsion by batch
All the monomers (mole ratio of PTMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MTES = 1
MTES = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, mass fraction of total system w = 30%) and emulsifiers (mass ratio of SDS
1, mass fraction of total system w = 30%) and emulsifiers (mass ratio of SDS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OP-10
OP-10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SDBS = 2
SDBS = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were mixed with 50 mL water at room temperature for 30 minutes, and then the temperature was increased to a certain temperature and kept there for 3–5 h.
1) were mixed with 50 mL water at room temperature for 30 minutes, and then the temperature was increased to a certain temperature and kept there for 3–5 h.
2.3 Synthesis polyalkylsilsesquioxane emulsion by semi-continuous
All the emulsifiers (mass ratio of SDS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OP-10
OP-10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SDBS = 2
SDBS = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were mixed with 50 mL water at room temperature for 30 minutes, and then the temperature was increased to a certain temperature. After that, the monomers (mole ratio of PTMS
1) were mixed with 50 mL water at room temperature for 30 minutes, and then the temperature was increased to a certain temperature. After that, the monomers (mole ratio of PTMS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MTES = 1
MTES = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, mass fraction of total system w = 30%) were added into the system using semi-continuous process (feed rate = 2–3 seconds per drop). The polymerization was continued for about 2–5 h after the monomer dripping off.
1, mass fraction of total system w = 30%) were added into the system using semi-continuous process (feed rate = 2–3 seconds per drop). The polymerization was continued for about 2–5 h after the monomer dripping off.
2.4 Characterization
| ω = (M2 − M1)/M0 × 100% |
| σ = W1/W0 × 100% |
The morphology and structure of hybrid core–shell particles were observed by transmission electron microscopy (TEM, Tecnai G20) and scanning electron microscopy (SEM, JSM6510LV), field emission scanning electron microscopy (FESEM, JSM7100F). SEM samples were gold-coated before analysis. TEM 200 mesh-sized Cu grids were supplied by Beijing Xinxing Braim Technology Co., Ltd (China).
3. Results and discussion
3.1 Preparation of polyalkylsilsesquioxane emulsion
The stable polyalkylsilsesquioxane emulsion was prepared by batch method and semi-continuous method emulsion polymerization of methyl triethoxysilane (MTES) and n-propyltrimethoxysilane (PTMS). For these two methods, the initial reaction time was marked by starting adding monomers. The emulsion polymerization was alkaline because SDBS aqueous solution is alkaline.The Fig. 2A is the particle size change curves of polyalkylsilsesquioxane emulsion prepared by batch. It shows that under the condition of different reaction temperature, particle size increased gradually, while PDI decreased as the extension of reaction time. After 120 min, the particle size and PDI did not change significantly. The hydrolysis and condensation reactions could be happened between alkylalkoxysilane and water, alkylalkoxysilane and oligomers, even between alkylalkoxysilane themselves (Fig. 1)10–12 and there was no specific reaction activity centre. As result, micellar nucleation, droplet nucleation and homogeneous nucleation were likely to exist. The Fig. 3A shows that the curve had two peaks when reaction time was 5 min at 45 °C, which one peak existed on the left side of the abscissa point 100 nm, the other one existed on the right side of the abscissa point 1000 nm. As the extension of reaction time, the peak existed on the right side of 1000 nm was disappeared (reaction time 15 min), the peak on the left side of the 100 nm translated to the right. For the emulsion prepared by batch, a lot of oligomers were generated during the hydrolysis and condensation of alkylalkoxysilane in the initial reaction because of enough monomers in the system. Meanwhile, the latex particles were formed, and part of monomers were existed in the oil drops. The particle PDI was polydispersion in this stage. With the extension of reaction time, the polycondensation among oligomers in the system continued. Thus, the particle size increased and PDI gradually narrowed.
 | ||
| Fig. 2 Relationship between Z-average particle size and time under different reaction temperature: (A) batch method, and (B) semi-continuous method. | ||
 | ||
| Fig. 3 Curves of particle size distribution with different reaction time: (A) batch method, and (B) semi-continuous method. | ||
The Fig. 2B is the particle size change curves of polyalkylsilsesquioxane emulsion synthesized by semi-continuous. Compared with polyalkylsilsesquioxane emulsion synthesized by batch, particle size also increased gradually, and PDI decreased as the extension of reaction time, after 120 min, the particle size and PDI did not change significantly. The difference between them is that the particle size was smaller and the PDI was larger. The Fig. 3B shows that the curve also had two peaks in the initial reaction at 45 °C. Similar to the emulsion prepared by batch, in the semi-continuous emulsion polymerization, the peak on the right side of point 1000 nm was gradually weak, the peak on the left side of point 100 nm moved right, and the peak on the right side of point 1000 nm was disappeared after 30 min. Because the monomer was added using semi-continuous process (feed rate = 2–3 seconds per drop), the coexistence time of the two peak was longer than the former by batch. The monomer concentration in the initial stage of emulsion polymerization was low, and the latex particle size would be small. During the monomer dropping process, the particle size would increase. However, after all monomers added to the system, the change of latex particle size would be not apparent with the decrease of monomer concentration.
3.2 Stabilities of polyalkylsilsesquioxane emulsion
In this paper, the mass fraction ratio of alkylsilsequioxane is 30%. However, the actual solid content of polyalkylsilsesquioxane emulsion is lower because of volatile small molecule (H2O, CH3OH, CH3CH2OH) generated with alkylsilsequioxane hydrolysis condensation reaction. Table 1 shows that after the emulsion polymerization process was finished, the actual solid content of emulsion ω1 was 15.6% (by batch) and 15.5% (by semi-continuous). After stored 12 months, the ω2 of polyalkylsilsesquioxane emulsion (by batch) was 14.4%, while the ω2 of polyalkylsilsesquioxane emulsion (by semi-continuous) was 15.5%. Because latex particle size by batch was larger than that by semi-continuous, coagulation was more likely to happen. Thus, the actual solid content of emulsion by batch was smaller.The gel ratio was 0.3% (by batch) and 0.2% (by semi-continuous). It suggests that the polymerization stabilities of polyalkylsilsesquioxane emulsion prepared by batch and semi-continuous were very good. On the other hand, the storage stability of emulsion was very good. After stored 6 months, the emulsion appearance had no obvious change, and there were a few physical crosslinking floccules at the bottom of the bottle. Nevertheless, the physical crosslinking floccules would be disappear after shaking.
3.3 Morphology of the polyalkylsilsesquioxane emulsion latex particles
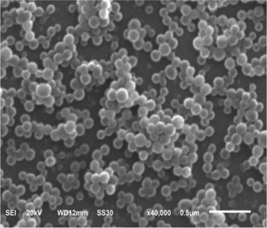
Fig. 4 shows the TEM photographs of polyalkylsilsesquioxane emulsion particles with different feeding modes. It could be found that all latex particles were sphere shape, and there were core–shell structure particles.16 It was consistent with particle size measured with DLS that the latex particles sizes by batch were larger. Besides, the polydispersibility of particle size by batch was more obvious. The SEM photographs of polyalkylsilsesquioxane emulsion particles by semi-continuous provided further evidence that the latex particle morphology was spherical (Fig. 5). | ||
| Fig. 4 TEM images of polyalkylsilsesquioxane emulsion particles: (A) batch method, and (B) semi-continuous method. | ||
3.4 XRD analysis
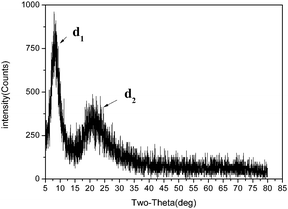
Based on the literature,17–20 Fig. 6 shows the XRD pattern of polyalkylsilsesquioxane, showing two distinct diffraction halos. The first halo (d1), appearing at 8.123°, indicates an intramolecular chain-to-chain distance of approximately 1.088 nm. The second diffuse halo (d2), which covers a wider range of diffraction angles, appears at approximately 21.691° and indicates that average intermolecules was approximately 0.409 nm. These peaks represent the ladder width and the ladder thickness.FTIR spectra of polyalkylsilsesquioxane are shown in Fig. 7. Strong absorption bands attributed to asymmetric Si–O–Si stretching were observed at 1130 and 1034 cm−1. Hiroyasu Seki et al.21 also reported two sharp Si–O–Si stretching absorption bands at 1130 and 1030 cm−1 for ladder polymethylsilsesquioxane (PMSQ).
These results indicate that the ordered and unordered polymer regions in the molecular chain of polyalkylsilsesquioxane. Therefore, we deduced that there was the existence of some similar crystal structures in the molecular chain of polyalkylsilsesquioxane prepared with alkyltrialkoxysilane.
3.5 TG analysis
In polysiloxane polymer, the bond energy of four kinds of main covalent bonds Si–O, Si–C and C–C is 460.5 KJ mol−1, 304 KJ mol−1, 326 KJ mol−1, respectively.22,23 The degradation of the sample should start from initial cleavage of Si–C and C–C bonds in the polymer. Fig. 8 shows that there was one step loss mass process in the nitrogen atmosphere. Fig. 9 shows that the weight loss rate was max at 515 °C. It could be found that the loss mass step at 274 °C was rapid in the air atmosphere (Fig. 9). The residual mass in the nitrogen atmosphere (68%) was higher than the residual mass in the air atmosphere (65.1%). There was still carbon residue after thermal degradation of organic groups in the nitrogen atmosphere, while there was nothing in the air atmosphere. These results indicate that thermal properties of polysiloxane are good.4. Conclusions
The stable polyalkylsilsesquioxane emulsion was prepared using batch method and semi-continuous method emulsion polymerization respectively. Under the condition of different reaction temperature, particle size of polyalkylsilsesquioxane emulsion of prepared by batch method and semi-continuous method increased gradually, and PDI decreased as the extension of reaction time. Finally, the particle size and PDI do not change significantly. The latex particle size by batch was larger than that by semi-continuous, while the PDI smaller. The actual solid content of emulsion ω1 was 15.6% (by batch) and 15.5% (by semi-continuous). The polymerization stability and storage stability of emulsion prepared by both batch method and semi-continuous method are good. The morphologies of latex particle prepared by both methods were spherical and core–shell structure. The polyalkylsilsesquioxane possessed an ordered structure. Thermal degradation weightlessness peaks of polymer in nitrogen atmosphere was at 450–600 °C, while in air atmosphere was at 250–300 °C and the final residues were 68% in nitrogen, 65.1% in air atmosphere.References
- P. Bajaj, J. Appl. Polym. Sci., 2002, 83(3), 631–659 CrossRef CAS.
- B. Liu, X. W. Xu, F. Gao and S. Q. Huang, Silicone material (China), 2013, 27(4), 313–317 CAS.
- J. H. Merrifield, R. J. Thimineur and F. J. Traver, US Pat., 5244598[P], 14 September 1993.
- F. Wu, Z. P. Cao, X. Zhong and Y. Xu, US Pat., 8258192[P], 4 September 2012.
- A. S. McAuliffe, M. J. Sarrazin, D. B. Selley and A. Stammer, US Pat., 8354480[P], 15 January 2013.
- J. F. Hyde and J. R. Wehrly, US Pat., 2891920[P], 23 June 1959.
- Y. Q. Zhuang, X. Ke, X. L. Zhan and Z. H. Luo, Powder Technol., 2010, 201(2), 146–152 CrossRef CAS PubMed.
- I. Mohorič and U. Šebenik, Polymer, 2011, 52(20), 4423–4428 CrossRef PubMed.
- J. Kozakiewicz, I. Ofat, I. Legocka and J. Trzaskowska, Prog. Org. Coat., 2014, 77(3), 568–578 CrossRef CAS PubMed.
- F. D. Osterholtz and E. R. Pohl, J. Adhes. Sci. Technol., 1992, 6, 127–149 CrossRef CAS PubMed.
- Z. C. Liang, X. G. Zang, G. G. Shan, Y. G. Shao and Z. X. Weng, Polymer Bull., 2006, 11, 31–35 Search PubMed.
- H. M. Jiang, Z. Zheng, Z. M. Li and X. L. Wang, Ind. Eng. Chem. Res., 2006, 45(25), 8617–8622 CrossRef CAS.
- J. A. Pühringer, US Pat., 4937104[P], 26 June 1990.
- S. Q. Huang, D. Q. Fan, Y. Q. Lei and H. Huang, J. Appl. Polym. Sci., 2004, 94(3), 954–960 CrossRef CAS.
- S. Q. Huang, H. Peng and J. Zhu, Acta Polym. Sin., 1998, 6, 692–697 Search PubMed.
- B. Liu, S. Q. Huang, Z. S. Xu, F. Gao and J. Zhu, New J. Chem., 2014, 38, 4996–5002 RSC.
- G. Kumaraswamy, Y. Deshmukh, V. V. Agrawal and P. Rajmohanan, J. Phys. Chem. B, 2005, 109(33), 16034–16039 CrossRef CAS PubMed.
- Z. Z. Chen, Z. J. Ren, J. T. Zhang, W. X. Fu and R. B. Zhang, React. Funct. Polym., 2012, 72(8), 503–508 CrossRef CAS PubMed.
- W. P. Chuang, Y. C. Sheen, S. M. Wei, M. Y. Yen and C. C. M. Ma, Eur. Polym. J., 2013, 49(3), 646–651 CrossRef CAS PubMed.
- Z. J. Ren, P. Xie, S. D. Jiang, S. K. Yan and R. B. Zhang, Macromolecules, 2010, 43(5), 2130–2136 CrossRef CAS.
- H. S. T. Kajiwara, Y. Abe and T. Cunji, J. Organomet. Chem., 2010, 695, 1363–1369 CrossRef PubMed.
- Z. Z. Yang, S. Han, R. Zhang, S. Y. Feng, C. Q. Zhang and S. Y. Zhang, Polym. Degrad. Stab., 2011, 96(12), 2145–2151 CrossRef CAS PubMed.
- Z. Z. Yang, L. Feng, S. Diao, S. Y. Feng and C. Q. Zhang, Thermochim. Acta, 2011, 521(1–2), 170–175 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |