Separation of palladium from high level liquid waste – A review
R. Ruhela*a,
A. K. Singha,
B. S. Tomarb and
R. C. Hublia
aMaterials Processing Division, Bhabha Atomic Research Centre, Mumbai, India. E-mail: riteshr@barc.gov.in; Tel: +91 22 25592605
bRadioanalytical Chemistry Division, Bhabha Atomic Research Centre, Mumbai, India
First published on 16th May 2014
Abstract
The present paper provides a review of the various processes/schemes developed for the separation and recovery of palladium from high level liquid waste (HLLW) generated during reprocessing of spent nuclear fuel. This separation is necessary in view of various problems posed by the presence of palladium during vitrification of HLLW. Further, to meet the ever increasing demand for palladium in various applications, HLLW can be considered as one of the possible secondary sources of this valuable metal. In this regard several processes are proposed involving liquid–liquid extraction, solid phase extraction, precipitation electro-deposition, for recovering palladium from HLLW. The focus of the present review is to evaluate various liquid–liquid extraction processes proposed for palladium separation from HLLW.
Introduction
Palladium is one of the platinum group metals, which occur in very small concentration in the earth's crust (∼10−6%).1–3 However, some of its unique physicochemical properties have made this element indispensable in numerous fields, namely, electronics, catalysis, medicine, chemical technology, etc.3–5 In recent years, some significant applications of palladium, such as, hydrogen storage materials,6–8 photovoltaic materials,9,10 gas sensors,11,12 metallic glass formations,13–15 catalyst for degradation of toxic organic compounds in aqueous streams,16,17 etc., have made this even more sought after element. The numerous applications of palladium have increased the demand for palladium thereby putting the pressure on the scarce natural resources.To circumvent this problem, there is a need to look out for alternative sources of palladium. One such source can be the spent nuclear fuel (arising from power reactors) which contains significant amount of palladium along with other fission products and actinides.4,5 Theoretical calculations of Pd in high level liquid waste (HLLW) obtained from reprocessing of spent nuclear fuel of a PHWR having a burn up of 6700 MWD per te show 165 mg L−1 Pd for a HLLW solution of 800 L per te.18 On the other hand, the corresponding value for HLLW from FBTR spent fuel reprocessing is 30 mg L−1 for 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L per te & burn up of 150 GWD per te.18 The fission palladium comprises of stable isotopes 104Pd (17 wt%), 105Pd (29 wt%), 106Pd (21 wt%), 108Pd (12 wt%), 110Pd (4 wt%) and a radioactive isotope 107Pd (17 wt%) with half life of 6.5 × 106 years. The radioactivity of 107Pd (soft β-emittor with Emax of 35 keV) is a matter of concern and will have to be taken care of by isotope enrichment techniques for industrial usage.19 However, there are various applications in nuclear field where the associated radioactivity of 107Pd may be insignificant compared to the inherent radioactivity of the major components. Some of the applications of Pd in nuclear technology include improvement in the properties of structural elements of nuclear fuel and various other components of reactor,20–23 hydrogen isotope separation and storage,24–27 storage of long lived fission products and actinides28 and waste management.29 Further, the separation of Pd from HLLW will provide an additional advantage of mitigating various problems associated with the presence of palladium during vitrification where it is known to form separate phases within glass matrix thereby destabilizing the latter.5,30
000 L per te & burn up of 150 GWD per te.18 The fission palladium comprises of stable isotopes 104Pd (17 wt%), 105Pd (29 wt%), 106Pd (21 wt%), 108Pd (12 wt%), 110Pd (4 wt%) and a radioactive isotope 107Pd (17 wt%) with half life of 6.5 × 106 years. The radioactivity of 107Pd (soft β-emittor with Emax of 35 keV) is a matter of concern and will have to be taken care of by isotope enrichment techniques for industrial usage.19 However, there are various applications in nuclear field where the associated radioactivity of 107Pd may be insignificant compared to the inherent radioactivity of the major components. Some of the applications of Pd in nuclear technology include improvement in the properties of structural elements of nuclear fuel and various other components of reactor,20–23 hydrogen isotope separation and storage,24–27 storage of long lived fission products and actinides28 and waste management.29 Further, the separation of Pd from HLLW will provide an additional advantage of mitigating various problems associated with the presence of palladium during vitrification where it is known to form separate phases within glass matrix thereby destabilizing the latter.5,30
The separation and recovery of palladium from various waste solutions has been reviewed in great details by various groups.19,31–33 These reviews have not only broadened the understanding of various problems associated with palladium recovery from spent nuclear fuel but also elaborated the various applications where the recovered palladium may find its use. Subsequently, several reports have been appeared from various laboratories suggesting effective solutions for Pd recovery from HLLW. It is therefore worthwhile to evaluate the current status of this subject and to discuss various upcoming solutions. The present paper is aimed at reviewing the recent work in the area of Pd separation from HLLW and finally presenting a scheme for large scale operation for the same.
Designing the scheme for palladium separation from spent nuclear fuel necessitates the knowledge of the states in which palladium can exist during fuel irradiation as well as various stages of spent fuel reprocessing. Though, it is difficult to perform the chemical analysis of the irradiated fuel, various reports containing the detailed examination of irradiated fuel from different types of power reactors at the micro level have shown that platinum group metals generally form part of intermetallic inclusions, forming an independent phase in irradiated fuel.34,35
During reprocessing, the irradiated (spent) fuel undergoes decladding, chopping and dissolution in nitric acid medium, wherein, palladium along with other fission products was found to be distributed between two streams, namely, the dissolver solution and the undissolved residue, known as high level solid waste (HLSW). The exact proportion of palladium in the above two streams, of course, is dictated by its physico-chemical state in the fuel (metal, alloy, or oxide) and the various conditions followed during the dissolution. The dissolver solution, after acidity adjustment, is reprocessed to obtain pure uranium and plutonium (PUREX process), wherein, palladium along with other fission products and actinides goes into the raffinate, known as high level liquid waste (HLLW). Various methods have been proposed to recover palladium from above two streams. However, we shall restrict our discussions on HLLW. Some of the most prominent ones are discussed below.
Recovery of palladium from high level liquid waste (HLLW) arising from PUREX process raffinate
It has been established that during spent fuel dissolution, majority of fission palladium goes into the nitric acid solution along with other fission products and actinides. Generally, palladium occurs in divalent state in PUREX process raffinate. However, the exact composition of various palladium species that may occur will depend upon the redox conditions as well as the acidity of the PUREX process raffinate. Speciation data of Pd in nitric acid medium36 show that it exists as Pd2+ and Pd(NO3)+ at lower acidities (0.5 M to 3.0 M). However, at higher nitric acid/nitrate ion concentrations (≥3.0 M), palladium generally exists as Pd(NO3)3− or Pd(NO3)42−. Therefore, the selection of extractant for effective complexation of palladium will mainly depend up on the nature of palladium species to be extracted and, the chosen ligand should comply with the following criteria:(i) The extractant should be highly selective for palladium; otherwise additional steps will be required to further separate it from co-extracted metal ions which would increase additional waste volumes.
(ii) The extractant should show appreciable uptake of palladium so that minimum possible extraction steps will suffice to recover palladium quantitatively.
(iii) Back extraction of palladium from loaded organic solvent should be easily achievable so that the extractant can be recycled.
(iv) The extractant should have sufficient solubility in normal paraffinic diluents like n-dodecane.
(v) It should be sufficiently stable in nitric acid as well as in radiation environment.
(vi) It should have high flash and boiling point.
(vii) It should be easily available on large scale and at low cost.
For liquid–liquid extraction system, the uptake of metal ion is conventionally expressed in terms of distribution ratio (DM) and percentage extraction (%E) while the selectivity for a metal ion is presented in terms of separation factor (SF). These terms are defined as
| DM = [M]org./[M]raff. |
{for equal vol of org. and aq. phase}
| SF = DPd/DM |
Various extractants have been proposed for palladium separation from HLLW, which can be further categorized as follows:
Hard donor extractants
where R = butyl, isoamyl or octyl.
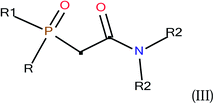
where R = phenyl, R1 = alkyl or phenyl and R2-isobutyl.
where R1 = methyl, R2 = butyl and R = tetradecyl.
where R = octyl.
On the other hand, tetradentate ligand, namely, N,N,N′,N′-tetraoctyl-3,6-dioxo-1,8-diamide (DOODA), (VII) has shown lower DPd for 0.1 M DOODA/n-dodecane from nitric acid medium.44
where R = phenyl.
From the above discussion, it is evident that extractants with hard donor atoms possess very low extraction capacity for Pd at nitric acid concentrations (∼3.0 M) that generally prevail during reprocessing of HLW. Also, when these extractants are used in higher concentrations in order to get appreciable extraction for palladium, co-extraction of other fission products is generally observed. It is to be noted that since most of the above extractants have been initially designed for other purposes in nuclear fuel reprocessing, therefore, they do not meet the essential criteria for Pd extraction.
Soft donor extractants
Palladium being a soft acid will form stronger complexes with ligands having soft donor atoms like ‘N’ and ‘S’. Based upon this presumption, several extractants have been developed. Among them, the most studied ones are as follows:(i) Amines and quaternary ammonium compounds. Palladium extraction with amines and quaternary ammonium compounds has been studied in detail at various laboratories. Both the type of extractants usually works effectively at lower nitric acid concentrations. However, in both cases, DPd was found to decrease with increase of acidity. For instance, 0.5 M trioctyl amine (TOA) dissolved in benzene showed DPd of 2.3 and 0.11 at 3.0 M and 8.0 M nitric acid respectively.47 Similar concentrations of tricaprylmethyl ammonium nitrate (Aliquat 336 nitrate) showed DPd of 18 & 0.3 at 3.0 M and 8.0 M nitric acid respectively. It was proposed that effective extraction at 3.0 M nitric acid and stripping at 6–8 M nitric acid would suffice to recover palladium from HLLW.47 However, co-extraction of some fission products has also been reported.48 Furthermore, the stability of these extractants in radiation environment has been found to be reasonable,49 thus showing promise for their use in processing of HLLW. However, very low extraction capacity for palladium was observed when normal paraffinic diluents like n-dodecane were used in place of aromatic diluents like benzene, toluene, etc. Since commercial scale use of aromatic diluents in processing of HLLW is still being debated, the use of these extractants for the said purpose becomes uncertain. However, improvement in the existing schemes can be made by the use of suitable phase modifiers (alcohol, mono-amides, etc.) to improve the solubility and hence the extractability of Pd species in to the organic phase (n-dodecane).
where R = octyl.
(ii) Benzoin oxime. Oximes have been known for their preferential ligation with soft metals. However, very few studies have been reported involving these extractants for extraction of palladium from nitric acid medium. Recently, benzoin oxime (XII) has been extensively studied for palladium extraction from HLLW.50 With 0.0001 M α-benzoin oxime dissolved in solvesso-100 (aromatic diluent), DPd of 1.7 was obtained from 2.0 M nitric acid medium. Thus, appreciable extraction of palladium can be achieved with higher concentration of α-benzoin oxime for application in actual HLLW. However, the co-extraction of other fission products and use of aromatic diluents will have to be justified before their commercial application.
(iii) (Methylimino) bis (N,N-dioctylacetamide) MIDOA. Tridentate extractant having appropriately placed tertiary amine and amide moieties have recently been reported to give high extraction capacity for palladium even at higher nitric acid concentrations. With 0.1 M MIDOA (XIII) dissolved in n-dodecane, DPd = 10 was observed from 3.0 M nitric acid medium.51 However, the extractant also shows significant uptake of various other metal ions like Tc, Zr, Re, etc.51 Thus, appreciable decontamination factor cannot be achieved for palladium vis a vis other metal ions present in HLLW necessitating further separation schemes to separate palladium from co-extracted fission products.51
where R = octyl.
(iv) Dicarboxypyridine diamide (DCPDA). These ligands were initially studied for extraction of trivalent lanthanides and actinides from HLLW. However along with these metal ions, palladium was found to be efficiently extracted. Thus, 0.1 M solution of DCPDA (XIV) in FS-13 diluent gave DPd greater than 100 in 3.0 M nitric acid solution.52 However, the co-extraction of other metal ions remains a problem with these extractants which may require further separation of Pd from co-extracted metal ions.
where R = methyl, phenyl.
(i) Dialkyl sulphide. These extractants have been widely studied for palladium extraction from different acidic media particularly HCl. They exhibit very high extraction efficiency for palladium over other metal ions. For instance, 0.005 M dioctyl sulphide (XV) in solvesso-100 gave DPd of 13.7 from 3.0 M nitric acid medium.53 Another homologue namely, dihexyl sulphide (10%(v/v)) in n-dodecane, gave a distribution ratio of palladium of order of 103 from 0.1 to 6.0 M nitric acid medium.54 Further, dialkyl disulphides (XVI) in aromatic diluents have been reported to show even higher uptake of palladium. For example, dilute solution of dihexyl dithioether (0.001 M) in chloroform gave DPd of 39 from 1.0 M nitric acid solutions.55
Though these extractants appear to be very attractive in view of high distribution ratios for palladium, their use is limited owing to very slow kinetics of extraction and instability in nitric acid medium. Further, these extractants were found to decompose readily in radiation environment.53
(ii) Dialkyl sulphoxides. With 0.05 M dioctyl sulphoxides (DOSO) in solvesso-100, DPd of 16.5 was achieved from 3.1 M nitric acid solution.53 However, with branched homologue of the extractant the distribution ratios were found to decrease. DPd of 3.55 was observed for 0.05 M bis-(2-ethylhexyl) sulphoxide (BESO) (XVII) in toluene. Both the extractants provide appreciable DPd in simulated HLLW conditions. However, the notable co-extraction of other fission products along with actinides may pose difficulties in selective separation of palladium from HLLW.56
where R = octyl, 2-ethylhexyl, etc.
(iii) Diesel fuel. It is known to be the cheapest source of sulphide extractant and thus can be used for palladium extraction. Diesel fuel after appropriate treatment with HNO3/K2Cr2O7 and NaOH/NaNO3 solution can be taken as ready to use solvent. When dissolved in mixed paraffin and aromatic diluent, it can extract Pd(II) from 2–3 M HNO3 with a DPd of 10–100.57 However, their use in palladium recovery from HLLW is questionable in view of the low flash point of diesel fuel. Further, the behavior of sulphur species present in the diesel when exposed to radiation environment has not been studied.
(iv) Triisobutyl phosphine sulphide (TIPS). The extractant has been found to be even more effective than dialkyl sulphides. For instance, a dilute solution of 0.0001 M TIPS (XVIII) in solvesso-100 extracts palladium with DPd of 1.4 from 3.0 M nitric acid and equilibrium was attained within 45 minutes.53 An aromatic homologue, namely, triphenylphosphine sulphide (0.005 M) in benzene shows DPd of 5.0 at 2.5 M nitric acid.58 However, these extractants were found to be insoluble in normal paraffinic diluents. Also, their instability towards oxidation in nitric acid medium will remain problematic.53
where R = isobutyl, phenyl.
(v) Thiamacrocycles. Thiamacrocycles, particularly, 2-octyl-1,4,7-trithiacyclononane (2-octyl-9S3) and 1,4,7-trithiacyclodecane (10S3) (XX) form extractable palladium complexes when used along with dinonyl naphthalene sulfonic acid (HDNNS) (XIX). Thus, with 0.005 M 10S3 and 0.01 M HDNNS, more than 90% extraction of palladium is achieved from 1.0 M nitric acid.59 However, the extraction decreases drastically on further increasing the nitric acid concentration. Hence, these extractants can be used in separation of palladium from weakly acidic solutions (≤1.0 M HNO3). However, their stability in nitric acid and radiation environment has to be evaluated before their further applications.
where R = nonyl.
(vi) Thiodiglycolamides. Thiodiglycolamides have been reported as one of the most promising class of extractants for palladium removal from HLLW. Thus, using 0.01 M N,N,N′,N′-tetra-(2-ethylhexyl)thiodiglycolamide ((T(2 EH)TDGA)) (XXI) dissolved in n-dodecane, almost complete extraction was achieved in a single contact from 3.0 M nitric acid solutions.60 The kinetics of extraction was very fast, equilibrium was achieved within five minutes. Complete recovery of palladium from organic phase was obtained with 0.01 M thiourea in 0.1 M nitric acid. Studies with both simulated as well actual HLLW have shown very high separation factors (≥105) for palladium over other metal ions.61 Hydrolytic and radiolytic stability studies have shown that the extractant is fairly stable to be used in actual conditions of HLLW.62
Recently, another new extractant, namely, N,N,N′,N′-tetra-(2-ethylhexyl) dithiodiglycolamide (DTDGA) (XXII) has been reported to show the highest DPd as well as separation factor for palladium over other metal ions present in HLLW.63 Studies with simulated HLLW solutions have shown very encouraging results. Detailed hydrolysis and radiolysis studies have shown fair stability of the extractant to be used in actual conditions of HLLW.
Conclusions
Separation and recovery of palladium from high level liquid waste (HLLW) can be accomplished using various methods. Amongst them the most suited one will be the liquid–liquid extraction processes using palladium selective extractants. If hard donor extractants are used for the said purpose, further separation will be required to recover palladium, since these extractants will also co-extract other fission products along with palladium. However, using soft donor extractants, single stage separation will be sufficient to recover palladium. Among these soft donor extractants the possible candidate can be tertiary/quaternary amines and the extractants containing thioetheric linkage. While tertiary/quaternary amines can only be used for palladium recovery from weakly acidic solutions, thioetheric based extractants provides better extractions from strongly acidic medium, however, the later one generally shows less stability in nitric acid medium. Thiodiglycolamides and dithiodiglycolamides, having thioetheric moiety along with suitably spaced amidic moieties, have been found to be promising extractants for recovery of palladium from HLLW. Very high extractability for palladium and excellent separation factor with respect to most actinides and other fission products present in HLLW as well as their fair hydrolytic and radiolytic stability make them one of the most promising extractant for palladium.References
- C. C. Allen, The Platinum Metals; Mineral Resources Division, Ottawa, 1960, Mineral Report No. 3, p. 7 Search PubMed.
- J. B. Mertre, Jr, The good news platinum deposits, U.S. Geol. Surv. Bull., Alaska, 1940, vol. 918, p. 97 Search PubMed.
- R. E. Kirk and D. F. Othmer, Encyclopedia of chemical technology, Wiley Publishers, New York, 3rd edn, 1973, vol. 18, p. 228 Search PubMed.
- G. J. K. Acres, Platinum Met. Rev., 1984, 28(4), 150–157 CAS.
- H. J. Ache, L. H. Baetsle, R. P. Bush, A. F. Nechaev, U. P. Popik and Yu. Ying, IAEA technical report series, 1989, p. 308.
- R. Schulz, J. Strom-Olsen, L. Zaluski and A. Zaluska, US Pat. 5,964,965, 1999.
- C. T. Fogg, J. L. Cornellisson, Platinum Met. Rev., 1993, 37, 2, 77–101 Search PubMed.
- A. Omoto, K. Moriya and H. Karasawa, Nucl. Eng. Des., 2000, 197(3), 281–299 CrossRef CAS.
- L. I. Hernandez and A. Perez-Quintana, Martel Pages, 1996, 44(4), 367–373 CAS.
- I. J. Ferrer, P. Diaz-Chao, A. Pascual and C. Sanchez, Thin Solid Films, 2007, 515, 5783–5786 CrossRef CAS PubMed.
- B. Orel, N. Grošelj, U. Opara Krašovec, M. Gabršček, P. Bukovec and R. Reisfeld, Sens. Actuators, B, 1998, 50(3), 234–245 CrossRef CAS.
- D. Bilenko, O. Belobrovaya, E. Jarkova, O. Coldobanova, I. Mysenko and E. Khasina, Sensors on low-dimensional silicon structures, 1997, vol. 62, pp. 1–3, 621–623 Search PubMed.
- R. B. Schwarz and Y. He, Mater. Sci. Forum, 1997, 231–240 CrossRef CAS.
- L. F. Chua and H. W. Kui, J. Appl. Phys., 1998, 84, 5993–5996 CrossRef CAS PubMed.
- C. Park, M. Saito, Y. Waseda, N. Nishiyama and A. Inoue, Mater. Trans. JIM, 1999, 40, 491–497 CrossRef CAS.
- A. Sen, US DOE Office of Environmental Management, Annual Progress Report, September 15, 1996–September 14, 1997.
- A. Pifer, T. Hogan, B. Snedeker, R. Simpson, M. Lin, C. Shen and A. Sen, J. Am. Chem. Soc., 1999, 121, 7485–7492 CrossRef CAS.
- (a) B. S. Tomar, Internal Report, BARC, 2011; (b) R. Ruhela, PhD thesis, Homi Bhabha National Institute, 2013.
- Z. Kolarik and E. V. Renard, Platinum Met. Rev., 2003, 47, 74–87 CAS.
- A. Sawatzky and G. A. Ledoux, Proc. 2nd Int. Congr. Hydrogen in metals, Pregamon Press, Paris, France, New York, June 1977, vol. 1, pp. 6–10, Paper 1C8 Search PubMed.
- C. L. Stokes and R. E. Buxbaum, Nucl. Technol., 1992, 98(2), 207–216 CAS.
- K. K. Nippon Engeruharudo, Power Reactor & Nuclear Fuel Dev. Corp., Jpn. Util. Pat. Appl., 1980, 502, 55–126 Search PubMed.
- W. T. Grubb, US Pat. 4,097,402, General Electric Co., 1978.
- F. Collins Martin and M. Romuald, US Pat. 4,178,350, Engelhard Minerals & Chemical Corp., 1979.
- Yu. A. Sakharovski, M. B. Rozenkevich, B. M. Andreev, E. P. Magomedbekov, Yu. S. Pak, D. M. Nikitin, I. Z. Khairullin and V. V. Uborskii, At. Energy, 1998, 85(1), 462–467 CrossRef.
- K. K. Mitsubishi, Genshiryoku Japanese Patent Appl., 49–002,000, 1974.
- K. K. Hitachi Seisakusho Japanese Patent Appl., 57–046,198, 1982.
- Y. A. Pokhitonov, The outlook for some fission product utilization with the aim to immobilize long lived radionuclides, WM'08 Conference, Phoenix, AZ, Feb. 24–28, 2008.
- A. Michaelis, S. Kudelka and J. W. Schultze, Electochim. Acta, 1998, 43(1–2), 119–130 CrossRef CAS.
- S. K. Sundaram and J. M. Perez Jr, Noble metals and spinel settlings in high level waste melters, PNNL-13347, 2, Pacific Northwest National Laboratory, Richland, WA, 2000 Search PubMed.
- Z. Kolarik and E. V. Renard, Platinum Met. Rev., 2003, 47, 123–131 CAS.
- Y. A. Pokhitonov and V. N. Romanovskii, Radiochemistry, 2005, 47(1), 1–13 CrossRef CAS PubMed.
- L. V. Arsenkov, B. S. Zakharkin, K. P. Lunichkina, E. V. Renard, V. Yu. Rogozkhin and N. A. Shorokhov, At. Energ., 1992, 72(5), 462–473 CrossRef.
- H. Kleykamp, J. O. Paschoal, R. Pejsa and F. Thummler, J. Nucl. Mater., 1985, 130, 426–433 CrossRef CAS.
- H. Kleykamp, Nucl. Mater., 1985, 131, 221–246 CrossRef CAS.
- (a) E. C. Frias, H. K. Pitsch, J. Ly and C. Poitrenaud, Talanta, 1995, 42, 1675–1683 CrossRef CAS; (b) J. Purans, B. Fourest, C. Cannes, V. Sladkov, F. David, L. Venault and M. Lecomte, J. Phys. Chem. B, 2005, 109, 11074–11082 CrossRef CAS PubMed; (c) T. Fujii, S. Egusa, A. Uehara, A. Kirishima, I. Yamagishi, Y. Morita and H. Yamana, J. Radioanal. Nucl. Chem., 2011, 290, 475–478 CrossRef CAS PubMed.
- K. P. Lunichkina, E. V. Renard and V. B. Shevchenko, Zh. Neorg. Khim., 1974, 19(1), 205–209 CAS ; J. Inorg. Chem., 19(1), 205–209.
- (a) B. N. Zaitsev and E. N. Invoka, Radiochemistry, 1981, 23, 817 CAS; (b) V. S. Shmidt and N. A. Shorokhov, At. Energ., 1988, 64(2), 103–110 CAS.
- V. S. Shmidt, N. A. Shorokov, S. S. Novikova, E. G. Teterin and A. N. Penteleeva, Soviet Radiochemistry, 1983, 252, 189–194 Search PubMed.
- B. N. Zaitsev, I. B. Kvasnitskii, V. A. Korolev, V. A. Babain and Yu. A. Pokhitonov, Radiochemistry, 2005, 47(4), 374–377 CrossRef CAS PubMed.
- (a) I. V. Smirnov, M. D. Karavan, T. I. Efremora, V. A. Babain, S. I. Miroshnichenko, S. A. Cherenok and V. I. Kalchenko, Radiochemistry, 2007, 49, 482–492 CrossRef CAS; (b) I. V. Smirnov, M. D. Karavan, V. A. Babain, I. Kvasnitskii, E. Stoyanov and S. I. Miroshnichenko, Radiochim. Acta, 2007, 95, 97–102 CrossRef CAS.
- O. Courson, M. Lebrun, R. Malmbeck, G. Pagliosa, K. Romer, B. Satmark and J. P. Glatz, Radiochim. Acta, 2000, 88, 857–863 CrossRef CAS.
- Z. Zhu, Y. Sasaki, H. Suzuki, S. Suzuki and T. Kimura, Anal. Chim. Acta, 2004, 527, 163–168 CrossRef CAS PubMed.
- Y. Sasaki, Y. Morita, Y. Kitatsuji and T. Kimura, Solvent Extr. Ion Exch., 2010, 28, 335–349 CrossRef CAS.
- N. T. Hung, M. Watanabe and T. Kimura, Solvent Extr. Ion Exch., 2007, 25, 407–416 CrossRef CAS.
- M. Mohan Raj, A. Dharmaraja, Y. Panchanatheswaran, K. A. Venkatesan, T. G. Srinivasan and V. P. R. Rao, Hydrometallurgy, 2006, 84, 118–124 CrossRef PubMed.
- E. A. Mezhov, V. A. Kuchmunov and V. V. Druzhenkov, Radiochemistry, 2002, 44(2), 135–140 CrossRef CAS.
- E. A. Mezhov, V. V. Druzhenkov and A. N. Sirotinn, Radiochemistry, 2002, 44(2), 146–150 CrossRef CAS.
- E. A. Mezhov, I. A. Kulikov and E. G. Teterin, Radiochemistry, 2002, 44(2), 141–145 CrossRef CAS.
- A. Dakshinamoorthy, P. S. Dhami, P. W. Naik, S. K. Dudwadkar, S. K. Munshi, P. K. Dey and V. Venugopal, Desalination, 2008, 232, 26–36 CrossRef CAS PubMed.
- Y. Sasaki, M. Ozawa, T. Kimura and K. Ohashi, Solvent Extr. Ion Exch., 2009, 27, 378–394 CrossRef CAS.
- M. Yu. Alyapyshev, V. A. Babain, L. I. Tkachenko, I. I. Didenko, A. V. Eliseev and M. L. Petrov, Solvent Extr. Ion Exch., 2011, 29, 619–636 CrossRef CAS.
- G. H. Rizvi, J. N. Mathur, M. S. Murali and R. H. Iyer, Sep. Sci. Technol., 1996, 31, 1805–1813 CrossRef CAS.
- (a) K. Kirishima, H. Shibayama, H. Nakahira, H. Shimauchi, M. Myochin, Y. Wada, K. Kawase and K. Kishimoto, Proc. Int. Conf. Nucl. Waste Management Environ. Remed., 1993, 5–11, Sept., Prague, Czech Rep, ASME, New York, NY, U.S.A., vol. 1, p. 667 Search PubMed; (b) S. J. Al-Bazi and H. Freiser, Solvent Extr. Ion Exch., 1987, 5, 265–271 CrossRef CAS.
- V. Guyon, A. Guy, J. Foos, R. Chomel, T. Moutarde, G. Lebuzyte and M. J. Lemaire, Radioanal. Nucl. Lett., 1994, 187(1), 19–24 CrossRef CAS.
- J. P. Shukla, R. K. Singh, S. R. Sawant and N. Varadarajan, Anal. Chim. Acta, 1993, 276, 181–187 CrossRef CAS.
- (a) V. G. Torogov, V. V. Tatarchuk, I. A. Druzhinina, T. M. Korda, A. N. Tatarchuk and E. V. Renard, At. Energ., 1994, 76(6), 478 Search PubMed ; Sov. At. Energy, 76(6) 442–447; (b) L. V. Arseenkov, B. S. Zakharkin, K. P. Lunichkina, E. V. Renard, V. Yu. Rogozhkin and N. A. Shorokhov, At. Energ., 1992, 72(5), 462 CrossRef CAS ; Sov. At. Energy, 72(5) 411.
- J. A. Daoud, S. A. El-Reefy and H. F. J. Aly, Radioanal. Nucl. Chem. Lett., 1992, 166(5), 441–449 CrossRef CAS.
- M. Draye, A. Reguillon Favre, G. Le. Buzit, J. Foos, M. Lemaire and A. J. Guy, Radioanal. Nucl. Chem., 1997, 220(1), 105–107 CrossRef CAS.
- R. Ruhela, J. N. Sharma, B. S. Tomar, S. Panja, S. C. Tripathi, R. C. Hubli and A. K. Suri, Radiochim. Acta, 2010, 98, 209–214 CrossRef CAS.
- R. Ruhela, J. N. Sharma, B. S. Tomar, V. C. Adya, T. K. Seshagiri, R. C. Hubli and A. K. Suri, Sep. Sci. Technol., 2011, 46, 1–7 CrossRef.
- R. Ruhela, J. N. Sharma, B. S. Tomar, K. K. Singh, M. Kumar, P. N. Bajaj, R. C. Hubli and A. K. Suri, Radiochim. Acta, 2012, 100, 37–44 CrossRef CAS.
- R. Ruhela, J. N. Sharma, B. S. Tomar, M. S. Murali, R. C. Hubli and A. K. Suri, Tetrahedron Lett., 2011, 52, 3929–3932 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |