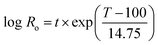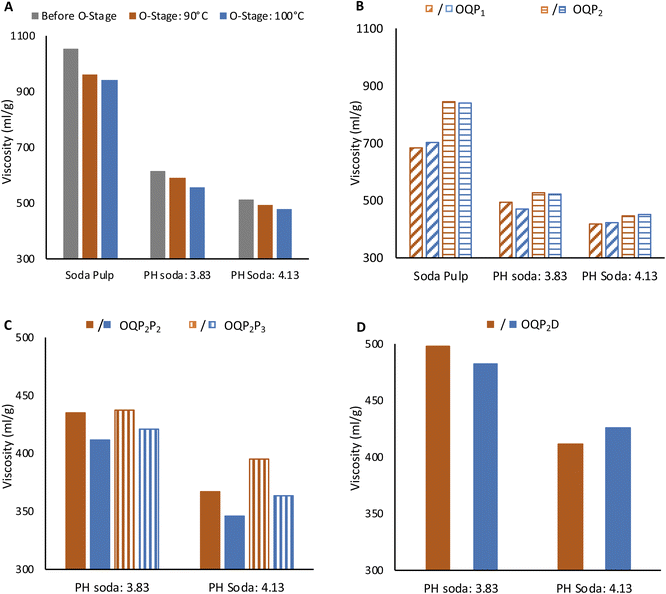Open Access Article
Open Access ArticlePrehydrolysis soda pulping of Enset fiber for production of dissolving grade pulp and biogas
Hanna Berhanu Lemma *a,
Christian Freund
*a,
Christian Freund a,
Abubeker Yimamb,
Friedrich Steffena and
Bodo Saake
a,
Abubeker Yimamb,
Friedrich Steffena and
Bodo Saake a
a
aUniversity of Hamburg, Chemial Wood Technology, Hamburg, Germany. E-mail: berhanu.hanna@gmail.com
bAddis Ababa Institute of Technology, Addis Ababa University, Chemical, and Bio Engineering, Addis Ababa, Ethiopia
First published on 1st February 2023
Abstract
Massive tonnes of fibrous residues are produced during the harvesting of the Enset plant for food preparation. The fibers are characterized by high cellulose and hemicellulose content and low lignin and extractive content. These make the fiber a good candidate for its concurrent valorization aimed at dissolving grade pulp and biogas. Prehydrolysis soda pulping was performed using steam pretreatment as a prehydrolysis step at a severity ranging from 2.95 to 4.13. The steamed fiber (PH fiber) was subjected to subsequent soda pulping under mild (160 °C and 16% alkali concentration) and severe (180 °C and 24% alkali concentration) pulping conditions. At higher steaming severity, a pulp with a xylose content of <4% and glucose content of 96% was obtained. A simple bleaching stage was envisaged to develop oxygen-peroxide (OQP1), oxygen-double peroxide (OQP2P2, and OQP2P3) and oxygen-peroxide-chlorine dioxide (OQP2D) sequences. Brightnesses up to ∼85% ISO could be reached for all sequences with CUEN viscosities of ∼350–500 ml g−1. Higher viscosities with higher brightness were achieved mainly by OQP2D sequence. However, even with OQP1 and OQP2P3 sequences the pulps met the requirements for lyocell production. An intense steam treatment reduces the biochemical methane potential (BMP) of prehydrolysis liquid (PHL) from 462 ml g−1 vs to 315 ml g−1 vs. The reduction might be due to the inhibition effect of furan concentration increase in the corresponding PHL from 2 ppm to 24 ppm. However, due to the higher yield and carbohydrate concentration of the prehydrolysis liquid, the biogas production volumes per initial raw material were still higher at higher steaming severity.
1. Introduction
Enset fibers are obtained as a residual agricultural byproduct of the Enset plant during harvesting for food in southern and southwestern Ethiopia. The fiber bundles mainly originated from scraping of the leaf sheath's lower part to obtain starchy food. A small proportion of the fiber is traditionally used for making sacks, bags, ropes, mats, and sieves. However, large quantities of the fiber have been accumulated and misused in the Enset growing areas. Various research has been done in the past few years regarding the fiber characteristics and scheming of different valorization options. Previous studies have reported that Enset fiber bundles consist of 5–10.5% lignin, 77.7–87.5% holocellulose, and 54.3–69.6% cellulose. The hemicellulose in the fibers is mostly highly acetylated xylanes ranging from 15.6–27.6%.1–4 The high cellulose and low lignin content of Enset fiber promotes its utilization for the production of dissolving pulp.Dissolving pulp is a starting material for producing viscose and lyocell fibers or cellulose derivatives such as cellulose esters and ethers. It is high-purity cellulose pulp (90–99%), with low contents of hemicellulose (<4%) and lignin (<0.05%) and a small amount of inorganic compounds. Besides, it has unique properties, such as high brightness, uniform molecular weight distribution, and high cellulose reactivity.5–7 The quality of dissolving pulp mainly depends on the raw material used and the processing technique.8 Dissolving-grade pulp is commercially produced from 85% hardwood or softwood and 10% cotton linters.9 Due to increased demand, much research has been devoted to producing dissolving-grade pulp from non-wood materials such as bamboo, straw and bagasse.9–12
Moreover, extensive research on innovative techniques for dissolving grade pulp production directly from lignocellulose biomass or paper-grade pulp has also been investigated in response to environmental and cost considerations. But, the typical processes for commercial uses of wood are still the prehydrolysis kraft (PHK) and acid sulfite (AS) processes.13,14 Soda pulping is solely used for processing monocotyledons and especially cotton linter. The AS process is better suited than the regular kraft process due to the spontaneous cleavage of glycosidic bonds in the hemicellulose chain under the acidic condition.13 An acidic prehydrolysis is added to the kraft process to achieve a comparable result by removing the hemicelluloses before the alkaline cooking process.9
Removal of hemicellulose is one of the primary intents of dissolving pulp production processes. A high amount of short-chain hemicellulose in the dissolving pulp has an adverse effect on the further processing of the end product. Prehydrolysis (PH) dissolves a portion of the hemicellulose into the PHL. PHL comprises short-chain carbohydrates, polysaccharides, and other chemical compounds such as acetic acid, phenolic, and furfural. These compounds can be converted into valuable products.6,14
Water in the liquid or vapor phase is a low-cost and effective medium to pre-hydrolyze lignocellulosic materials.15,16 The process provides several potential advantages, including no requirement for catalysts due to its simplicity and a significant decrease in chemical and material construction costs compared to other pretreatment techniques.17 In steam treatment, the biomass is treated with high-pressure saturated steam at high temperatures for a certain period. During the treatment, hemicellulose is partially degraded by autohydrolysis, whereas the lignin structure is altered.5 The more severe treatment causes lignin condensation and a viscosity drop after the subsequent alkaline pulping process.18
On the other hand, Harsono et al. reported that pre-hydrolyzed biomass could be delignified with fewer chemicals and shorter time than the non-prehydrolyzed material because the treatment disrupts the lignocellulose matrix and increases the accessibility of lignin during the subsequent pulping and bleaching.8,19 The mechanism of steam PH is similar to weak acid hydrolysis, which degrades polysaccharides to monosaccharides.20 Acetic acids are cleaved off from hemicellulose, assisting the further breakdown of the cellulose–hemicellulose–lignin matrics by creating an acidic medium.20,21 This theory is anticipated by Garrote et al. during the autohydrolysis of wood. The prehydrolysis step does not cause delignification and complete dissolution of hemicellulose.22,23 The alkaline pulping step is necessary for delignification and further hemicellulose removal. Sulfur-free soda pulping in the subsequent delignification stage is preferable and suitable for non-wood material by considering the environmental concerns.19 Both prehydrolysis (PH) and pulping are essential to achieve dissolving pulp with the required purity.6,14
Soda pulping is the chemical process of using sodium hydroxide as the active chemical to produce pulp from lignocellulosic materials. Soda pulping only makes up a minor share of the commercially used pulping techniques for wood pulping but is the preferred process for pulping annual plants.24 Soda pulping utilizes sodium hydroxide (NaOH) as an active chemical under high pressure and temperatures well above the boiling point of the cooking liquor, commonly between 150–170 °C.25 This achieves swelling of the fibers to increase the accessibility of the cooking chemicals and for better penetration into the raw material.25
The degradation of carbohydrates other than lignin could be of considerable concern when using soda pulping. It results in significant yield losses as mainly hemicelluloses are degraded by peeling reactions and dissolved into the cooking liquor.24 Some random alkaline hydrolysis of glucosidic bonds also occurs during soda pulping.24 This is, however, to some extent desired for the production of dissolving-grade pulp, as the carbohydrates that are especially prone to degradation are the hemicelluloses due to their lower degree of polymerization compared to cellulose.24,26
Dissolving grade pulp must meet specific properties and conditions such as high α-cellulose content and DP, low ash content and metal ion and remarkable reactivity or solubility to produce cellulose derivatives or regenerated cellulose fiber (RCF).27 Modern RCF products are lyocell fibers used in clothing, non-wovens, industrial fiber material and even in the medical field. The α-cellulose and hemicellulose content of the lyocell-grade dissolving pulps should be ≥92% and <4%, respectively.28 The target intrinsic CUEN viscosity for lyocell fiber should be between 400 ml g−1 and 500 ml g−1.28,29 Asaadi et al. reported the difficulty of spinning cellulose pulp with intrinsic viscosity higher or lower than 450–550 ml g−1.28
A multi-stage bleaching step is usually added to achieve the desired goal.9 The most environmentally friendly method of bleaching pulp is the TCF method, where no chlorine is used. This method has been proven to reduce emissions while offering excellent bleaching properties. TCF bleaching is often performed by using oxygen delignification before the further bleaching steps, where hydrogen peroxide or ozone bleaching is used. An additional extraction of hemicelluloses might be required after the cooking process in the form of cold caustic extraction.30
This study aimed to produce dissolving pulp and biogas using prehydrolysis soda pulping of Enset fiber in a biorefinery approach. It employed the steam treatment as a prehydrolysis step followed by a soda pulping process and a short ECF or TCF bleaching sequence. The effect of steaming severity on the composition and biomethane potential of the prehydrolysis liquor (PHL) was also investigated.
2. Materials and method
2.1. Raw material characterization
The Enset fiber was obtained from the southwestern part of Ethiopia, Woliata and Wolkite. The samples were cleaned, air-dried, and stored in a polyethylene bag until further processing.2.2. Steaming of Enset fiber
Enset fiber was steamed in a cylindrical 10 L reactor (Martin Busch und Sohn, Schermbeck, Germany). 200 g (O.D.) of Enset fiber with 2–5 cm length was used for each steaming experiment. The temperature ranges of the experiments varied between 150–190 °C for 30 min and 60 min. The effects of time and temperature during steam treatment were interpreted based on the severity factor, Ro.31where T (°C) is the temperature, t (min) is time, and 14.75 is an empirical parameter related to activation energy and temperature. The steaming condition and the corresponding severity are given below in Table 1.
| Steaming temperature (°C) | Time (min) | Severity factor log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R0 R0 |
|---|---|---|
| 150 | 30 | 2.95 |
| 160 | 30 | 3.24 |
| 170 | 30 | 3.54 |
| 180 | 30 | 3.83 |
| 190 | 30 | 4.13 |
| 150 | 60 | 3.25 |
| 160 | 60 | 3.54 |
| 170 | 60 | 3.84 |
| 180 | 60 | 4.13 |
After the steaming, the extract fraction was subjected to freeze drying, and the yield of both fractions was calculated based on the initial oven-dry raw material weight. The PH fiber fractions were air-dried and milled using T-1000 disc mill (Siebtechnick GmbH, Mülheim and der Ruhr, Germany). The carbohydrate and lignin composition of raw fiber, PH fiber, and lyophilisate were analyzed as described in the characterization section.
2.3. Subsequent soda pulping
Soda pulping of raw Enset fiber and PH fiber was performed using a 15 L rotary electric digester. 300 g (O.D.) of raw fiber and PH fiber were cooked at mild and severe pulping conditions pre-selected based on the preliminary experiment. The pulping conditions were 160 °C and 16% NaOH concentration and 180 °C and 24% NaOH concentration for 60 + 60 min preheating. The NaOH concentrations were calculated based on the oven dry weight, and the solid-to-liquid ratio was kept at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 for all experiments.
4 for all experiments.
The yield was calculated based on the oven dry weight of the resulting pulp and reported as the percentage of the initial weight of raw material. The carbohydrate and lignin content of soda pulp was determined by acid hydrolysis and AEC, as described in the section below.
2.4. Characterization of the PH fiber and PH soda pulp
The extractive content of the raw fiber bundle was analyzed using Accelerated Solvent Extraction ASE 350 (Thermo scientific T. M. Dionex TM, Waltham, Ma, USA) using petrol ether, acetone water (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and water consecutively.
1), and water consecutively.
Monomeric carbohydrate and lignin content of raw fiber, PH fiber, and PH soda were determined by two step sulphuric acid hydrolysis of the milled samples as described in Lorenz et al., 2016.32 The solid residue was weighed and calculated as the Klason lignin content. The carbohydrate content was determined from the acid-hydrolyzed filtrates by Borate-AEC using a Dionex Ultimate 3000 (Dionex, Sunnyvale, CA, USA).32 The acid-soluble lignin content was determined according to Maekawa et al. with a UV-spectrophotometer LAMBDA 650 (PerkinElmer, Waltham, MA, USA) at a wavelength of 205 nm.33
Furan composition was analyzed directly from the extracted fraction by reverse-phase high-performance liquid chromatography (Jasco, Tokyo, Japan) according to Krafft et al.34 The carbohydrate composition and acid-soluble lignin in the extract fractions were analyzed after freeze drying and acid hydrolysis of lyophilisate. All hydrolysis was done in triplicate.
The acetic acid content in the fiber and extracted fraction was determined after deacetylation by 1.5 ml of 0.6 M NaOH solution for 70 hours at room temperature. The suspension and the extracted liquor were centrifuged for 15 min before determining the acetic acid using the K-ACETRM microplate assay procedure (BioTECH, EPOCH/2 Microplate reader).
Kappa number was determined according to TAPPI T236 cm 35. Pulp viscosity was measured by cupri-ethylenediamine (CED) according to the ISO 5351 standard. The brightness of pulp was determined by TAPPI T525 with an ELREPHO 450X from Data color (Rotkreuz, Switzerland).
2.5. Bleaching of PH soda pulp
PH soda pulps produced at higher steaming severity and mild soda pulping condition was selected for the subsequent bleaching step. Non-prehydrolysis soda pulp was selected as a reference. Total chlorine-free (TCF) bleaching (OQP1 and OQP2P3) and elemental chlorine-free (ECF) bleaching with chlorine dioxide (OQP2D) was performed, and the resulting pulp was analyzed to select the appropriate condition for lyocell grade pulp. The detailed bleaching conditions are shown in Table 2.| Parameter | O | Q | P1 | P2 | P3 | D |
|---|---|---|---|---|---|---|
| Consistency (%) | 10 | 3 | 10 | 10 | 10 | 7 |
| Temperature (°C) | 90 | 65 | 70 | 70 | 70 | 70 |
| 100 | ||||||
| Time (min) | 60 | 60 | 120 | 120 | 120 | 120 |
| MgSO4 (%) | 0.2 | — | — | — | — | |
| NaOH (%) | 3 | — | 2 | 1 | 1.5 | — |
| O2 (MPa) | 0.6 | — | — | — | — | — |
| DTPA (%) | — | 0.3 | — | — | — | — |
| H2SO4 (mol l−1) | — | 1 | — | — | — | — |
| H2O2 (%) | — | — | 4 | 2 | 3 | — |
| ClO2 (%) | — | — | — | — | — | 0.4 |
All peroxide bleaching stage was made at 70 °C for 2 hours. The peroxide/NaOH concentrations were 4%/2% (P1), 2%/1% (P2) and 3%/1.5% (P3). At the end of the stage, the pulp was washed and air-dried for analysis based on Section 2.4.
Chlorine dioxide was also used as the last bleaching stage for (OQP2D) bleaching sequence. The pulp consistency of 7% and chlorine dioxide concentration of 0.4% was used for the chlorine dioxide stage.
The final pulp brightness, viscosity and kappa number were analyzed after each bleaching sequence.
2.6. Evaluation of biochemical methane potential of PHL
The biochemical methane potential (BMP) test was performed in a multi-batch reactor system (AMPTS II, bioprocess control) for 21 days. The inoculum for the BMP test was obtained from Seevetal municipal sewage plant. The blank inoculum and microcrystalline cellulose (Avicel @ PH-101, Sigma Aldrich) were used as a negative and positive control to evaluate the activity of the inoculum. The detailed BMP test is stated by Steffen et al.373. Results
3.1. Chemical characterization of Enset fiber
The composition of Enset fiber used in this research work is presented in Table 3. The lignin content was 13.3%, of which 10.7% was Klason lignin. It has a low content of extractives (4.4%), of which about 20% were soluble in acetone: water and more than 65% were soluble in water. The monomeric composition of carbohydrates consists mainly of glucose (59%), and hemicellulose sugar is dominated by xylose (10.7%).| Extractives (%) | Petrol ether | 0.1 |
| Acetone | 0.9 | |
| Water | 3.4 | |
| Σ | 4.4 | |
| Lignin (%) | Klason lignin | 10.8 |
| Acid soluble lignin | 2.5 | |
| Σ | 13.3 | |
| Monosaccharide (%) | Other sugar (Rha, Man, Ara, Gal) | 2.4 |
| Xylose | 10.7 | |
| Glucose | 58.9 | |
| Σ | 72.1 | |
| Ash (%) | Silica | 2.6 |
| Σ | 5.4 |
3.2. Steam treatment of Enset fiber
Steam pretreatment of Enset fiber was performed at severity varied from 2.95 to 4.13, corresponding to steaming temperature and time. The main objective of the pretreatment was to remove hemicellulose sugar and enhances the accessibility of fiber components by removing resistant barriers from the fiber. As shown in Fig. 1A, the PH fiber fraction yield gradually decreased, and the PHL fraction increased with steaming severity. The dissolution of the fiber components, especially hemicellulose sugar and water extractives, which are more than 3% of the material, are responsible for the mass loss in PH Enset fiber. The acidic nature of the PHL further facilitates the hemicellulose dissolution due to releasing acetic acid from acetylated xylan of the hemicellulose.12,16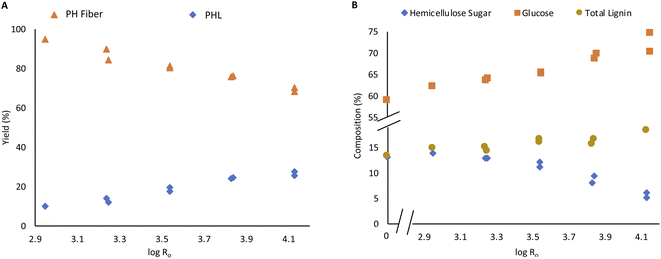 | ||
| Fig. 1 PH fiber and PHL yield based on raw material (A), carbohydrate composition and total lignin content of PH fiber (B). | ||
The amount of lignin increased from 11.6% to 16.6% with steaming intensity, as shown in Fig. 1B. The increase of lignin content in steamed fibers is mainly due to a shift of composition due to the removal of hemicelluloses. This underlines that only minor lignin solubilization occurs during steaming. On the other hand, some generation of pseudo-lignin might occur under high-severity conditions contributing to the high lignin content. It results from the re-polymerization of carbohydrate degradation products and condensation of lignin components.12,17,38
The xylose content in PH fiber decreased to 4.2% with steaming intensity. On the other hand, the glucose content increased from 62.1% to 74.5%, as shown in Fig. 1B. It has been observed that hemicellulose is indeed dissolved during the treatment, while cellulose is not degraded to soluble products leading to a significant increase in the proportion of cellulose in PH fiber. A similar phenomenon is reported by Berhanu et al. during hot water pretreatment of Enset fiber.16 Various authors also report the same occurrences in the steam treatment of different biomass source.12
3.3. Soda pulping of steam treated Enset fiber
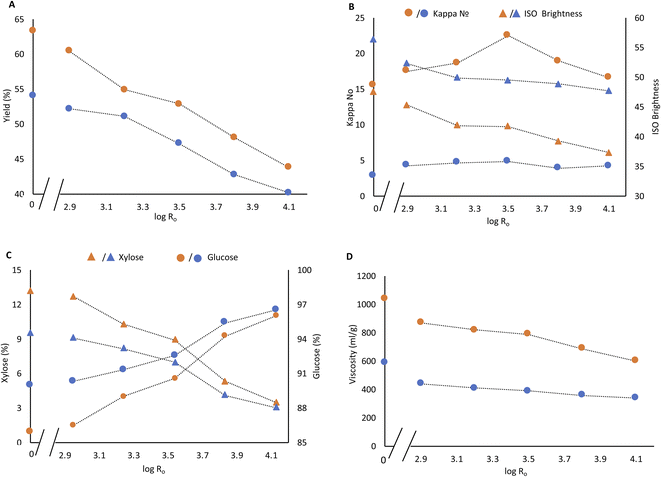
Soda pulping is an effective process for the delignification of non-wood plants and agricultural residues. Soda pulping of raw Enset fiber and PH fiber was performed at mild and severe pulping conditions. The yield from starting Enset fiber during mild and severe pulping was 63% and 54%, respectively. The yield drops continuously with steaming severity (Fig. 2A). The kappa numbers range from 17.5 to 22.5 at mild pulping conditions, while under intense pulping conditions, values between 3.9 to 4.8 are obtained (Fig. 2B). The kappa numbers in PH soda pulp are slightly higher than in soda pulp of Enset fiber, even for steaming at low severity (Fig. 2B).It was not possible to obtain a high purity cellulose pulp during soda pulping solely or from low-severity PH fiber, as it is also reported by Jahan et al.7 The xylose content of soda pulp without prehydrolysis was 13.2% and 9.5% for mild and severe soda pulping, respectively. Xylose content dropped only slightly after low severity steaming (2.95) and subsequent soda pulping, which were 12.7% and 9.1%. However, the values decreased continuously with steaming severity for both pulping conditions. At higher steaming severity, the difference in xylose content between mild and intense soda pulping conditions becomes smaller and smaller (Fig. 2C). At the severity of 4.13, the xylose content of PH soda pulps are 3.5% and 3%. The corresponding cellulose content of pulp reached 96% and 96.5% for both mild and severe pulping conditions, as shown in Fig. 2C. The pulp yield was 44% and 40% at this severity level based on the starting material, and the kappa number was 16 and 4, respectively. The same phenomenon is also reported by Jahan et al. that increasing prehydrolysis temperature improved the cellulose content in the resulting pulp.7
The viscosity of dissolving pulp is one essential parameter that reflects the average degree of polymerization. It indicates the degradation of cellulose molecular weight during steaming and soda pulping.14 The viscosity of PH soda and soda pulps was determined to evaluate the effect of process severity on cellulose degradation.
A huge viscosity increment was already observed for mild and intense soda pulping without prehydrolysis (Fig. 2D). There is a gradual decrease in viscosity from 870 ml g−1 to 600 ml g−1 and from 440 ml g−1 to 340 ml g−1 with steaming severity for mild and severe pulping. Accordingly, the reduction of xylose content in the PH soda, down to less than 4%, was obtained at the expense of reducing pulp viscosity.
The pulp brightness decreased with steaming intensity and increased with pulping intensity. It decreased from 48% to 40% brightness and from 58% to 50% brightness for mild and severe pulping, respectively (Fig. 2B). The condensation reaction of lignin at high temperatures might explain the brightness drops due to streaming severity. Lourenço et al. also report this phenomenon in the steam explosion pretreatment of cardoon.5,8,15 An increment in alkali dosage results in a decrease in kappa number, viscosity and hemicellulose content but an increase in brightness and cellulose content, as reported by Salaghi et al.39 An appropriate bleaching sequence is still necessary to improve the brightness and lower the kappa number in the resulting pulp.
A viscosity target of 400–500 ml g−1 is defined for the envisaged lyocell application.28,29 Furthermore, additional viscosity reduction can not be avoided in subsequent bleaching. Therefore the PH soda pulps produced under severe pulping conditions can not meet the target. For further bleaching, two PH soda pulps were selected and produced under mild pulping conditions with a previous PH severity of 3.83 (180 °C, 30 min) and 4.13 (190 °C, 30 min).
3.4. Bleaching of PH soda pulp
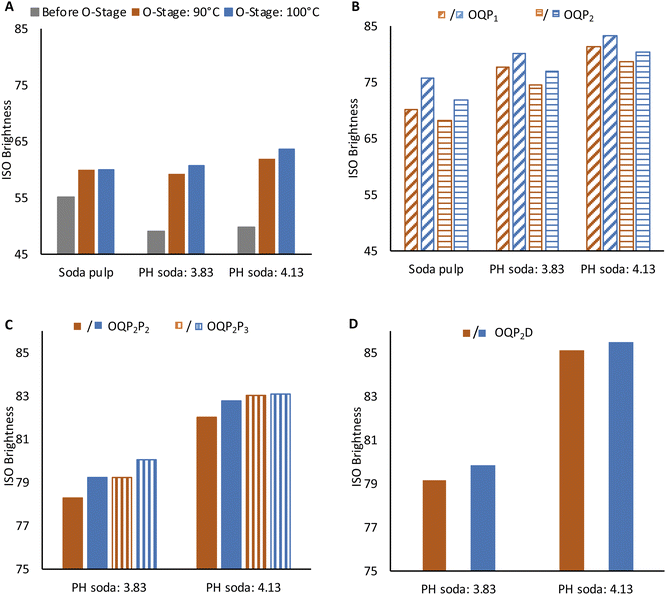
High brightness at moderately acceptable viscosity and degree of polymerization is crucial for high-grade dissolving grade pulp. Production of lyocell grade pulp from PH Enset fiber was achieved using simple elemental chlorine-free bleaching sequences OQP1, OQP2P2, OQP2P3, and OQP2D. The effect of each bleaching stage on viscosity and pulp brightness is shown in Fig. 3 and 4.The O stage was performed at 90 and 100 °C. Pulp viscosity after 90 °C O-stage was 590 ml g−1 and 494 ml g−1 for steaming severity of 3.83 and 4.13, respectively. The pulp viscosities were lower for 100 °C oxygen bleaching (555 ml g−1 and 478 ml g−1), as shown in Fig. 3A. The viscosity of PH soda pulps after the O-stage were lower than the soda pulp without prehydrolysis. For the latter 962 ml g−1 and 943 ml g−1 for 90 °C and 100 °C oxygen bleaching were obtained, respectively. The effect of oxygen bleaching on the PH soda pulp brightness was more pronounced with prehydrolysis severity (Fig. 4A). The soda pulp along 90 °C O-stage gained 4.8%. For PH soda pulps, the brightness gain were 10.1% and 12.1% for 3.83 and 4.13 steaming severity, respectively. During O-bleaching, the kappa number dropped to 6 and 3.3. This sound effect of the O-stage on the PH soda pulp is remarkable since, after pulping, a slightly negative effect of PH on kappa number and brightness indicated condensation reactions. Fortunately, this could not be confirmed after the O-stage.
The oxygen-bleached pulp was then subjected to a complexing step using 0.3% DTPA before the peroxide bleaching stages.
The first P-bleaching stage with high (P1: 4% H2O2, 2% NaOH) and low peroxide charge (P2: 2% H2O2, 1% NaOH) were compared.
The pulps from 100 °C O-stage showed slightly higher bleachability in the subsequent peroxide bleaching, as shown in Fig. 4B. The positive effect of the higher temperature in the O-stage on brightness remains effective after both P stages (Fig. 4B). The brightness of PH soda pulp after the P1 stage was better than P2 stage by 3% and 2.8% for steaming severity of 3.83 and 4.13, respectively. On the other hand, the pulp viscosity was lower by 37 ml g−1 and 53 ml g−1 (Fig. 3B). However, the negative effect of O-stage on viscosity is equalized in the P-stage, at least for pulps with high PH severity. This indicates that with a high temperature in the O-stage and a high peroxide charge (P1), good bleaching efficiency can be obtained by simple OQP1 sequence reaching a brightness of 83.2% and a viscosity of 424 ml g−1 for the PH soda pulp with the severity of 4.13.
For PH soda pulps after O-bleaching followed by the mild P2 stage, a second peroxide bleaching stage P2 (2% H2O2, 1% NaOH) and P3 (3% H2O2, 1.5% NaOH) was performed (Fig. 3C and 4C). The second P-stage gives an additional brightness increment, reaching 83% ISO for pulp with high PH severity and high peroxide charge in P3. This holds for both samples from O-stage at 90 °C and 100 °C. However, the viscosity of these samples has been reduced to 400 ml g−1 (O: 90 °C) and 364 ml g−1 (O: 100 °C). Accordingly, samples are at the lower limit of the targeted viscosity range.
In order to better preserve the viscosity, chlorine dioxide was investigated as a final step with a low dosage in an OQP2D sequence as an alternative to the second P stage.
Chlorine dioxide bleaching shows slightly higher brightness reaching 85% for 4.13 severity pulp. The pulp viscosity after the full bleaching sequence was higher for the OQP2D sequence in both prehydrolysis severity levels. The final viscosity of 3.83 and 4.13 PH soda pulp was 412 ml g−1 and 426 ml g−1, respectively. At these bleaching conditions, the kappa number was 0.8.18 The PH soda pulp followed by bleaching fulfills the requirement of dissolving grade pulp for lyocell fiber regarding brightness, viscosity, and α-cellulose content.27
3.5. Composition and biomethane production potential of PHL
The PHL was characterized directly regarding its pH and acetic acid content. Carbohydrates and furans were analyzed after freeze drying. The PHL yield and concentration of recovered carbohydrates increase with steaming severity intensification. The yield was increased from 9.4% to 27.5% with severity mainly due to the accumulation of dissolved carbohydrates in the PHL (Fig. 1A).The acetic acid concentration in PHL increased due to xylan deacetylation, so the pH dropped with severity, as shown in Fig. 3A.17 This is in agreement with Joanna Wojtasz-Mucha et al. report.23
The glucose concentration in PHL decreased with steaming severity intensification. On the other hand, xylose concentration increased from 2.1% to 21% of lyophilisate, as shown in the Fig. 5B. At high steaming severity levels, the rise in xylose content became insignificant.
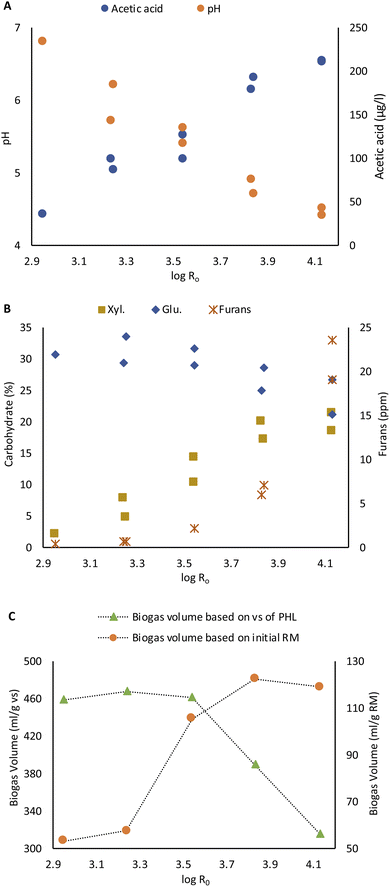 | ||
| Fig. 5 Effect of steaming severity on acetic acid content and pH of PHL (A) on carbohydrate and furan content of PHL (B) and on BMP of PHL based on volatile solid (vs.) and raw material (RM) (C). | ||
Besides carbohydrates and organic acids, furan compounds, such as furfural and HMF, are also found in PHL. These compounds are generated from the degradation of pentoses such as xylose and arabinose and hexoses such as glucose. The concentration of furans also increased with steaming severity. A rise in steaming severity beyond 3.83 caused a dramatic increment in furan concentration (Fig. 5B).
The biochemical methane potential test was determined from PHL obtained from 30 min steaming. The total accumulated biomethane yield was measured daily. The accumulated biomethane for all PHL samples became constant after the 10th day. The highest biogas volume was obtained as 468 ml g−1 of volatile solid (vs.) at the severity level of 3.14 (Fig. 5C). The BMP yield dropped to 390 ml g−1 vs. at severity of 3.83 and to 316 ml g−1 vs. at the severity of 4.14. The decrease in biogas formation may be related to the high concentration of furan compounds in PHL at higher steaming severity of 3.83 and 4.14. High concentrations of furan compounds may hinder biogas production. Janzon et al. reported that high concentrations of furan compound in PHL have only a small effect on biogas formation,40 while other authors report the potential of high-concentration furan compounds to reduce biogas production.41,42 Besides furans, other factors such as pH and acetic acid concentration of PHL could as well contribute to inhibition processes. However, the volume of biogas produced per initial raw material was still higher due to the high yield and carbohydrate content of PHL at higher steaming severity (Fig. 5C).
4. Conclusion
Prehydrolysis soda pulping of Enset fiber using steaming pretreatment followed by soda pulping effectively fractionates hemicellulose sugar and cellulose-rich fiber. An intense prehydrolysis and severe soda pulping condition enable high-purity cellulose fiber of 96.5%. However, the viscosity was much lower under severe soda pulping conditions. Accordingly, the severe prehydrolysis and mild soda pulping conditions were selected for further OQP1, OQP2P2, OQP2P3 and OQP2D bleaching to obtain high-grade dissolving grade pulp for lyocell application. All the pulping sequences give lyocell grade pulp with acceptable quality. Pulp with high brightness (>83%) and high viscosity (>400 ml g−1) was obtained by OQP1 sequence at high temperature O-stage and by the OQP2D bleaching sequence. A high BMP between 390–468 ml g−1 vs. was obtained for all PHL. However, an intensification of prehydrolysis severity increased the formation of furan compounds. This might be the reason for the reduction of biogas formation per volatile solid beyond steaming severity of 3.54. However, the volume of accumulated biogas per initial raw material was still increased until steaming severity of 3.83 as a result of the high PHL yield and increased carbohydrate concentration. The volume slightly decreased from 122 ml g−1 RM to 119 ml g−1 RM for steaming severity of 3.83 to 4.14, respectively.All in all, the study shows that the residues of the Enset plant are highly suitable for a dissolving pulp biorefinery, producing lyocell grade dissolving pulp and biogas. The pulp bleaching can be achieved in a relatively simple 3 or 4 step bleaching sequence. Further optimization potential exists by fine tuning the PH and soda pulping conditions. This will certainly further facilitate bleaching and improve the final product brightness and viscosity.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The Schlumberger Foundation, faculty for the future program, funds the first author for two years post-doctoral research stay at the University of Hamburg. The authors gratefully acknowledge Andreas Schreiber for his valuable assistance during laboratory work.References
- H. Lemma, Z. Kiflie, S. Feleke and A. Yimam, Chemical and Morphological Analysis of Enset (Ensete Ventricosum) Fiber, Leaf, and Pseudo stem, Lignocellulose, 2016, 5(2), 139–151 Search PubMed.
- H. Berhanu, Z. Kiflie, I. Miranda, A. Louerenco, J. Ferreira, S. Feleke, A. Yimam and H. Pereira, Characterization of crop residues from false banana/ensete ventricosum/in Ethiopia in view of a full-resource valorization, PLoS One, 2018, 13(7), 1–21, DOI:10.1371/journal.pone.0199422.
- A. M. Dube, Isolation and characterization of cellulose nanocrystals from Ensete ventricosum pseudo – stem fiber using acid hydrolysis, Biomass Convers. Biorefin., 2022 DOI:10.1007/s13399-022-02987-z.
- N. Seid, P. Griesheimer and A. Neumann, Investigating the Processing Potential of Ethiopian Agricultural Residue Enset/Ensete ventricosum for Biobutanol Production, Bioengineering, 2022, 9(4), 1–14, DOI:10.3390/bioengineering9040133.
- M. Borrega, L. K. Tolonen, F. Bardot, L. Testova and H. Sixta, Potential of hot water extraction of birch wood to produce high-purity dissolving pulp after alkaline pulping, Bioresour. Technol., 2013, 135, 665–671, DOI:10.1016/j.biortech.2012.11.107.
- H. Kumar and L. P. Christopher, Recent trends and developments in dissolving pulp production and application, Cellulose, 2017, 24(6), 2347–2365, DOI:10.1007/s10570-017-1285-y.
- M. S. Jahan, L. Ahsan, A. Noori and M. A. Quaiyyum, Process for the production of dissolving pulp, BioResources, 2008, 3(3), 816–828 Search PubMed.
- M. Chem, K. Tanifuji, S. P. Utami, A. S. Putra, H. Ohi and A. Nakagawa-izumi, Development of dissolving pulp from Phyllostachys pubescens stem by prehydrolysis soda cooking with 2-methylanthraquinone, Ind. Crops Prod., 2022, 178, 114570, DOI:10.1016/j.indcrop.2022.114570.
- C. Chen, C. Duan, J. Li and et al, ., Cellulose (Dissolving Pulp) Manufacturing Processes and Properties: A Mini-Review, BioResources, 2016, 11(2), 5553–5564, DOI:10.15376/biores.11.4.5553-5564.
- M. F. Andrade and J. L. Colodette, Dissolving pulp production from sugar cane bagasse, Ind. Crops Prod., 2014, 52, 58–64, DOI:10.1016/j.indcrop.2013.09.041.
- Z. Yuan, Y. Wen, N. S. Kapu, R. Beatson and D. Mark Martinez, A biorefinery scheme to fractionate bamboo into high-grade dissolving pulp and ethanol, Biotechnol. Biofuels, 2017, 10(1), 1–16, DOI:10.1186/s13068-017-0723-2.
- Y. Dong, H. Ji, C. Dong, W. Zhu, Z. Long and Z. Pang, Preparation of high-grade dissolving pulp from radiata pine, Ind. Crops Prod., 2020, 143, 111880, DOI:10.1016/j.indcrop.2019.111880.
- C. Duan, J. Li, X. Ma and et al, ., Comparison of acid sulfite (AS)- and prehydrolysis kraft (PHK)-based dissolving pulps, Cellulose, 2015, 22(6), 4017–4026, DOI:10.1007/s10570-015-0781-1.
- H. Sixta, Handbook of Pulp, 2006 Search PubMed.
- A. Lourenço, J. Gominho, M. D. Curt, E. Revilla, J. C. Villar and H. Pereira, Steam explosion as a pretreatment of Cynara cardunculus prior to delignification, Ind. Eng. Chem. Res., 2017, 56(1), 424–433, DOI:10.1021/acs.iecr.6b03854.
- H. Berhanu, D. Neiva, J. Gominho and et al, ., Bio-refinery potential of Enset/Ensete ventricosum/fiber bundle using Non-catalyzed and alkali catalyzed hydrothermal pretreatment, Waste Biomass Valorization, 2021, 12(2), 663–672, DOI:10.1007/s12649-020-01015-3.
- F. Hu and A. Ragauskas, Pretreatment and lignocellulosic chemistry, BioEnergy Res., 2012, 5(4), 1043–1066, DOI:10.1007/s12155-012-9208-0.
- X. Luo, J. Liu, H. Wang, L. Huang and L. Chen, Comparison of hot-water extraction and steam treatment for production of high purity-grade dissolving pulp from green bamboo, Cellulose, 2014, 21(3), 1445–1457, DOI:10.1007/s10570-014-0234-2.
- H. Harsono, A. S. Putra, R. Maryana and et al, ., Preparation of dissolving pulp from oil palm empty fruit bunch by prehydrolysis soda-anthraquinone cooking method, J. Wood Sci., 2016, 62(1), 65–73, DOI:10.1007/s10086-015-1526-3.
- Y. Lu, Q. He, G. Fan, Q. Cheng and G. Song, Extraction and modification of hemicellulose from lignocellulosic biomass: A review, Green Process. Synth., 2021, 10(1), 779–804, DOI:10.1515/gps-2021-0065.
- T. R. Sarker, F. Pattnaik, S. Nanda, A. K. Dalai, V. Meda and S. Naik, Hydrothermal pretreatment technologies for lignocellulosic biomass: A review of steam explosion and subcritical water hydrolysis, Chemosphere, 2021, 284, 131372, DOI:10.1016/j.chemosphere.2021.131372.
- G. Garrote, H. Domínguez and J. C. Parajó, Study on the deacetylation of hemicelluloses during the hydrothermal processing of eucalyptus wood, Holz Roh- Werkst., 2001, 59(1–2), 53–59, DOI:10.1007/s001070050473.
- J. Wojtasz-Mucha, M. Hasani and H. Theliander, Dissolution of wood components during hot water extraction of birch, Wood Sci. Technol., 2021, 55(3), 811–835, DOI:10.1007/s00226-021-01283-9.
- M. Ek, G. Gellerstedt and G. Henriksson, Pulping Chemistry and Technology, vol. 2, 2009, DOI:10.1515/9783110213423.
- C. Heitner; D. Dimmel and J. Schmidt, Lignin and Lignans: Advances in Chemistry, Taylor and Francis, 2016 Search PubMed.
- R. C. Francis, R. B. Hanna, S. J. Shin, A. F. Brown and D. Riemenschneider, Papermaking characteristics of three Populus clones grown in the north-central United States, Biomass Bioenergy, 2006, 30(8–9), 803–808 CrossRef CAS.
- X. Jiang, Y. Bai, X. Chen and W. Liu, A review on raw materials, commercial production and properties of lyocell fiber, J. Bioresour. Bioprod., 2020, 5(1), 16–25, DOI:10.1016/j.jobab.2020.03.002.
- S. Asaadi, M. Hummel, S. Hellsten and et al, ., Renewable high-performance fibers from the chemical recycling of cotton waste utilizing an ionic Liquid, ChemSusChem, 2016, 9(22), 3250–3258, DOI:10.1002/cssc.201600680.
- A. Michud, M. Hummel and H. Sixta, Influence of molar mass distribution on the final properties of fibers regenerated from cellulose dissolved in ionic liquid by dry-jet wet spinning, Polymer, 2015, 75, 1–9, DOI:10.1016/j.polymer.2015.08.017.
- E. Kärkkäinen, Industrial Production of Different Pre-hydrolysis Kraft dissolving pulp grades, Aalto University, 2021 Search PubMed.
- R. P. Overend, E. Chornet and J. A. Gascoigne, Fractionation of lignocellulosic by steam-aqueous pretreatments, Philos. Trans. R. Soc. London, Ser. A, 1987, 321(1561), 523–536, DOI:10.1098/rsta.1987.0029.
- D. Lorenz, N. Erasmy, Y. Akil and B. Saake, A new method for the quantification of monosaccharides, uronic acids and oligosaccharides in partially hydrolyzed xylans by HPAEC-UV/VIS, Carbohydr. Polym., 2016, 140, 181–187, DOI:10.1016/j.carbpol.2015.12.027.
- E. Maekawa, T. Ichizawa and T. Koshijima, An evaluation of the acid-soluble lignin determination in analyses of lignin by the sulfuric acid method, J. Wood Chem. Technol., 1989, 9(4), 549–567, DOI:10.1080/02773818908050315.
- M. J. Krafft, J. Berger and B. Saake, Analytical Characterization and inhibitor detection in liquid phases obtained after steam refining of corn stover and maize silage, Front. Chem., 2021, 9, 1–13, DOI:10.3389/fchem.2021.760657.
- A. Wuorimaa, R. Jokela and R. Aksela, Recent developments in the stabilization of hydrogen peroxide bleaching of pulps: An overview, Nord. Pulp Pap. Res. J., 2006, 21(4), 435–443, DOI:10.3183/npprj-2006-21-04-p435-443.
- R. Janzon, B. Saake and J. Puls, Upgrading of paper-grade pulps to dissolving pulps by nitren extraction: Properties of nitren extracted xylans in comparison to NaOH and KOH extracted xylans, Cellulose, 2008, 15(1), 161–175, DOI:10.1007/s10570-007-9154-8.
- F. Steffen, A. Requejo, C. Ewald, R. Janzon and B. Saake, Anaerobic digestion of fines from recovered paper processing - Influence of fiber source, lignin and ash content on biogas potential, Bioresour. Technol., 2016, 200, 506–513, DOI:10.1016/j.biortech.2015.10.014.
- X. Guo, Y. Fu, F. Zhang, X. Li and N. Liu, Change of structural features and relocalization of chemical components in the autohydrolyzed poplar wood chips enhance the accessibility of remaining components, Ind. Crops Prod., 2021, 167, 113508, DOI:10.1016/j.indcrop.2021.113508.
- A. Salaghi, A. S. Putra, A. T. Rizaluddin, M. Kajiyama and H. Ohi, Totally chlorine-free bleaching of prehydrolysis soda pulp from plantation hardwoods consisting of various lignin structures, J. Wood Sci., 2019, 65(1), 1–12, DOI:10.1186/s10086-019-1785-5.
- R. Janzon, F. Schütt, S. Oldenburg, E. Fischer, I. Körner and B. Saake, Steam pretreatment of spruce forest residues: Optimal conditions for biogas production and enzymatic hydrolysis, Carbohydr. Polym., 2014, 100, 202–210, DOI:10.1016/j.carbpol.2013.04.093.
- J. R. Kim and K. G. Karthikeyan, Effects of severe pretreatment conditions and lignocellulose-derived furan byproducts on anaerobic digestion of dairy manure, Bioresour. Technol., 2021, 340, 125632, DOI:10.1016/j.biortech.2021.125632.
- C. Huang, L. Xiong, H. J. Guo and et al, ., Anaerobic digestion of elephant grass hydrolysate: Biogas production, substrate metabolism and outlet effluent treatment, Bioresour. Technol., 2019, 283(2), 191–197, DOI:10.1016/j.biortech.2019.03.079.
| This journal is © The Royal Society of Chemistry 2023 |