Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ion-induced bias in Ag2S luminescent nanothermometers†
Marina
París Ogáyar
 a,
Diego
Mendez-Gonzalez
a,
Diego
Mendez-Gonzalez
 bc,
Irene
Zabala Gutierrez
c,
Álvaro
Artiga
bc,
Irene
Zabala Gutierrez
c,
Álvaro
Artiga
 a,
Jorge
Rubio-Retama
bc,
Oscar G.
Calderón
a,
Jorge
Rubio-Retama
bc,
Oscar G.
Calderón
 d,
Sonia
Melle
d,
Sonia
Melle
 d,
Aida
Serrano
d,
Aida
Serrano
 e,
Ana
Espinosa
f,
Daniel
Jaque
e,
Ana
Espinosa
f,
Daniel
Jaque
 *abgh and
Riccardo
Marin
*abgh and
Riccardo
Marin
 *agh
*agh
aNanoBIG, Departamento de Física de Materiales, Facultad de Ciencias, Universidad Autónoma de Madrid, C/Francisco Tomás y Valiente 7, Madrid, Spain. E-mail: daniel.jaque@uam.es; riccardo.marin@uam.es
bNanobiology Group, Instituto Ramón y Cajal de Investigación Sanitaria (IRYCIS), Ctra. De Colmenar Viejo, Km. 9100, Madrid, Spain
cDepartment of Chemistry in Pharmaceutical Sciences, Faculty of Pharmacy, Complutense University of Madrid, Plaza Ramon y Cajal 2, Madrid 28040, Spain
dDepartment of Optics, Faculty of Optics and Optometry, Complutense University of Madrid, Arcos de Jalón 118, Madrid E-28037, Spain
eInstituto de Cerámica y Vidrio | CSIC, Campus de Cantoblanco, C. Kelsen, 5, 28049 Madrid, Spain
fInstituto de Ciencia de Materiales de Madrid | CSIC, Campus de Cantoblanco, C. Sor Juana Inés de la Cruz, 3, 28049 Madrid, Spain
gInstitute for Advanced Research in Chemical Sciences (IAdChem), Universidad Autónoma de Madrid, Madrid 28049, Spain
hInstituto Nicolás Cabrera, Universidad Autónoma de Madrid, 28049 Madrid, Spain
First published on 19th October 2023
Abstract
Luminescence nanothermometry allows measuring temperature remotely and in a minimally invasive way by using the luminescence signal provided by nanosized materials. This technology has allowed, for example, the determination of intracellular temperature and in vivo monitoring of thermal processes in animal models. However, in the biomedical context, this sensing technology is crippled by the presence of bias (cross-sensitivity) that reduces the reliability of the thermal readout. Bias occurs when the impact of environmental conditions different from temperature also modifies the luminescence of the nanothermometers. Several sources that cause loss of reliability have been identified, mostly related to spectral distortions due to interaction between photons and biological tissues. In this work, we unveil an unexpected source of bias induced by metal ions. Specifically, we demonstrate that the reliability of Ag2S nanothermometers is compromised during the monitoring of photothermal processes produced by iron oxide nanoparticles. The observed bias occurs due to the heat-induced release of iron ions, which interact with the surface of the Ag2S nanothermometers, enhancing their emission. The results herein reported raise a warning to the community working on luminescence nanothermometry, since they reveal that the possible sources of bias in complex biological environments, rich in molecules and ions, are more numerous than previously expected.
1. Introduction
Luminescence nanothermometry makes use of luminescent nanomaterials with temperature-dependent luminescence, which are referred to as luminescent nanothermometers (LNThs).1 Although LNThs have been successfully used to obtain remote thermal readings in, e.g., miniaturized electronics, it is in the field of biomedicine where the most impressive advances have been made.2–6 LNThs have been used for diagnosis of tumors,7,8 diagnosis of affections of the circulatory and nervous systems,9,10 monitoring of inflammation events and even in studies of single cells.11–13 Despite the great results and the variety of LNThs reported, luminescence thermometry is being questioned.14,15 This is because of biases that cast doubts on the reliability of the thermal readouts provided by LNThs.16Biases appear when the luminescence of LNThs – used as signal for achieving a thermal readout – also depends on parameters other than temperature (cross-sensitivity).17–19 In this situation, changes in the emission spectrum or luminescence decay curves can be erroneously attributed to temperature changes, thus leading to inaccurate thermal readouts. The most frequent source of bias is the induction of spectral distortions caused by changes in the photon extinction by the surrounding medium.20 There is no “one-size-fits-all” solution to bias, because each combination of LNTh and environment is unique. Thus, to achieve reliable luminescence nanothermometry, effort should be put into understanding the different mechanisms giving rise to biases.
In a recent work, it was demonstrated how bias reduces the reliability of Ag2S nanoparticles as LNThs during real-time monitoring of a photothermal treatment enabled by magnetic nanoparticles.21 Therein, bias could not be attributed to environment-induced spectral distortions and a definitive explanation to the observed lack of reliability in the thermal readout was not given. What is therefore the source of this bias?
In this work, we provide an answer to this question, systematically investigating the performance of Ag2S LNThs when mixed with magnetic nanoparticles during photothermal processes. The bias mechanism has been identified by combining a series of measurements including (i) study of the impact of the irradiation conditions on the thermal readouts provided by the Ag2S LNThs, alongside (ii) spectroscopic characterization of the Ag2S LNThs and (iii) chemical analysis of the dispersant before and after the photothermal procedure. Through these analyses we have uncovered a new bias mechanism involving interaction between Ag2S LNThs and Fe3+ ions. Lastly, we include a critical discussion on the potential impact of this new bias mechanism in luminescence nanothermometry broadly speaking.
2. Results and discussion
2.1. Evidence of bias
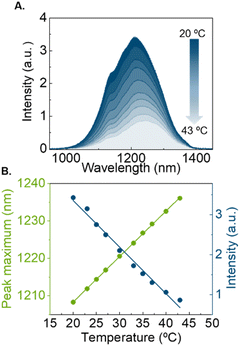
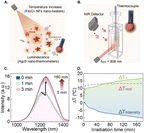
The prepared near-infrared (NIR)-emitting Ag2S LNThs (mean diameter 9 ± 1 nm, HS-PEG-OMe surface coating Fig. S1A†) were first calibrated recording their NIR emission spectrum luminescence in the 20–43 °C temperature range using 0.5 mg mL−1 of Ag2S LNThs in 100 μL of deionized water (Fig. 1A). The behavior is the one expected from the literature:22,23 a linear decrease in intensity (quenching) is accompanied by a linear red-shift of the peak maximum when increasing the temperature (Fig. 1B).20,24 The possibility of using multiple thermometric parameters (intensity and peak position) is key in the context of this study, since the thermal readouts obtained with these two parameters are in agreement only if no bias is present.25The Ag2S LNThs were subsequently mixed in an aqueous dispersion with commercial γ-Fe2O3 nanoflowers (NFs; 23 ± 4 nm nominal diameter, dextran-NH2 surface coating Fig. S1B†) featuring photothermal (PT) properties – meaning that they produce heat under photoexcitation (Fig. 2A), as generally reported for magnetic nanoparticles.26,27 This mixed dispersion is henceforth referred to as nanofluid (Fig. 1SC†). We initially attempted to measure the temperature increase during irradiation of the nanofluid in a 3 × 3 mm2 cuvette, using a concentration of 0.5 mg mL−1 of Ag2S LNThs and 9.75 mg mL−1 of NFs in 100 μL of deionized water. Both Ag2S LNThs and NFs are known to behave as efficient photothermal transducers. Nevertheless, heating measurements revealed that, under the above concentrations, the heating is mainly generated by NFs (Fig. S2†). The nanofluid was continuously irradiated for 15 min (as a standard for PT treatment) with an 808 nm laser diode (200 mW), while monitoring the temperature with a thermocouple and recording the emission spectrum of the Ag2S LNThs (Fig. 2B). Two regimes were observed for the Ag2S LNThs emission during irradiation (Fig. 2C). For the first 3 min, an expected intensity reduction accompanied by a red-shift of the peak maximum was observed due to the heat generated by the NFs. This initial behavior is followed by an increase of intensity to 153% of the initial value after 3 h of irradiation. Note that while the irradiation time was extended up to 3 h to properly study the observed phenomenon, after a few minutes into the PT treatment the intensity enhancement was already noticeable. This intensity increment goes hand in hand with a counterintuitive red-shift of the peak position. These two observations do not seem to be reconcilable, since they would correspond to diametrically opposite thermal readouts: a temperature decrease according to the intensity variation and a temperature increase according to the shift in peak position (Fig. 2D). The thermocouple reading is in fairly good agreement – although with significant discrepancies in slopes and measured temperature increase – with the temperature increase obtained from the analysis of the peak shift (Fig. 2D). A difference in temperature is partially expected between the thermocouple readout and the one given by Ag2S LNThs due to heat dissipation through water and the macroscopic average readout achieved with the thermocouple.28 The opposite behavior shown by the readouts obtained through the two luminescence thermometry parameters points to a clear bias in the Ag2S LNThs thermometric performance upon mixing them with NFs (compare Fig. 1A and 2C). The origin of this bias is explored in the next section.
2.2. Identification of the mechanism inducing bias
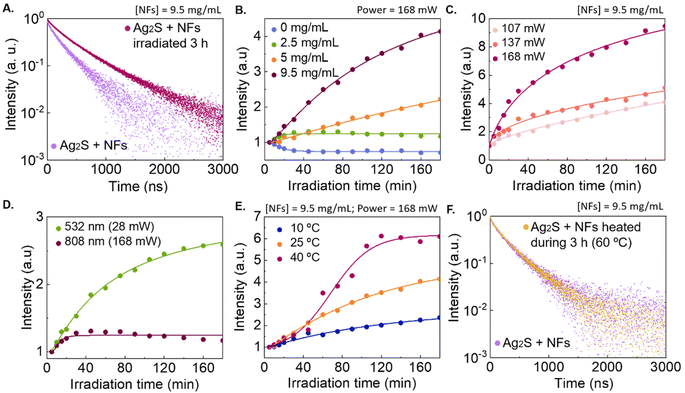
Aside from spectral information, we decided to also explore the photoluminescence dynamics of the system, since it brings additional insight into the changes occurring in the emitting species. Specifically, we recorded photoluminescence decay curves for the Ag2S LNThs in the nanofluid before and after irradiation, using a concentration of 0.5 mg mL−1 of Ag2S LNThs and 9.75 mg mL−1 of NFs in 100 μL of deionized water (Fig. 3A). Upon irradiation, the average lifetime increased by 100% (from 216 to 428 ns). These results suggest a structural or compositional change at the surface or within the volume of the Ag2S LNThs, which are mirrored by changes in the electronic structure of the emitters. Indeed, it is widely reported that the nonradiative decay of Ag2S nanoparticles can be reduced simply altering the chemical composition of their surface.29–32 To better understand the processes involved, we systematically investigated the impact that different parameters have on the observed intensity enhancement:- Effect of NF concentration. We monitored the enhancement in the Ag2S LNThs emitted intensity during PT processes for different NF concentrations (Fig. 3B). In the absence of NFs, a 30% intensity decrease was observed, revealing a local thermal increment of approximately 10 °C. Once the NFs are incorporated, the Ag2S LNThs start failing to provide indications of laser induced heating: instead of an intensity quenching (indicating heating) an intensity enhancement was observed. The magnitude of the intensity enhancement is proportional to the NFs concentration, with up to a 4-fold increase at the highest studied NF concentration (9.5 mg mL−1; corresponding to 3 Ag2S LNThs per NF). These results highlight the presence of an interaction between Ag2S LNThs and NFs that is more relevant as the concentration of the NFs increases.
- Effect of laser power and wavelength. Upon fixing the NF concentration to 9.5 mg mL−1, we studied the effect of the 808 nm laser power (Fig. 3C), observing that higher powers correspond to a greater emission intensity enhancement and a faster rate of change. For example, a power of 107 and 168 mW leads to a 260 and 860% enhancement, respectively. Furthermore, we decided to investigate the effect of using an irradiation wavelength more efficiently absorbed by the NFs (Fig. S3†).33,34 Under 532 nm irradiation with laser power of 28 mW (Fig. 3D), a greater enhancement is observed compared to the one obtained using a 808 nm laser and a power of 168 mW (20 vs. 160%). The higher photon absorption at 532 nm also forced us to bring down the NF concentration to 2.5 mg mL−1, so that the emission of the Ag2S LNThs could be properly monitored (i.e., more excitation photons could reach the Ag2S LNThs). This difference in NF concentration between the experiments conducted with the 532 and 808 nm lasers makes the results even more staggering. Both the excitation with a higher laser power and the use of a wavelength more efficiently absorbed by the NFs lead to higher local temperatures (due to the PT effect described previously). We thus decided to specifically investigate the effect of this parameter.
- Effect of the nanofluid temperature. For this set of experiments, the temperature of the nanofluid was externally set to 10, 25, and 45 °C, observing that the degree of emission intensity enhancement is higher for higher temperatures (Fig. 3E). These results are well in agreement with the above observations that highlight how temperature plays a role in the process.
Altogether, these results point towards an enhancement of the Ag2S LNTh emission intensity (and lifetime lengthening) driven by a photo-induced interaction with the NFs, which is accelerated at higher temperatures. Additionally, we confirmed that the effect does not occurr by simply heating the nanofluid for 3 h (Fig. 3F), suggesting that localized heating at the nanoparticle level is required for the effect to be noticeable.
These observations allowed us to narrow down our search to two possible sources of changes in spectroscopic properties of the Ag2S LNThs and hence, the presence of bias: a photo-induced release of Fe2+/3+ ions or a photocatalytic effect. The following experiments were thus conducted to identify the source of the bias.
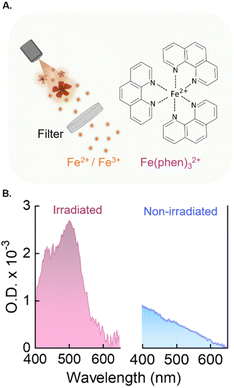
- Iron ion release. Iron oxide nanoparticles in aqueous media are known to release a small amount of Fe2+/3+ ions over time.35 We thus postulated that the NFs used in this study would be induced to release Fe2+/3+ ions at faster rates during laser irradiation due to the local heating. To verify this, we used a colorimetric assay (Fig. 4A and Fig. S4A†) to identify the presence of free iron ions in the medium before and after irradiation of a 9.5 mg mL−1 dispersion of NFs (no Ag2S LNThs were added at this stage). The assay relies on the use of 1,10-Phenanthroline as chelating agent. This molecule forms a strongly colored orange-red metal complex in the presence of Fe2+, which can be detected via absorption spectrophotometry. To account for all free iron ions, after removing the NFs via centrifugation, the medium was treated with an hydroxylamine solution to reduce possible Fe3+ ions to Fe2+.36 The results of these tests prove that a measurable amount of iron is released only when the NFs are irradiated (Fig. 4B). Upon extrapolating the values of the calibration curve, we estimated that the irradiation led to a release of iron amounting to a final concentration of 2 μM (see ESI Fig. S4B and 4SC†).
- Photocatalytic effects. We also evaluated the possible production of reactive oxygen species (ROS) by NFs during 808 nm irradiation by monitoring the degradation of Rhodamine B (RhB) under irradiation.37 Comparing the results obtained irradiating with a 808-nm laser an RhB solution (12.25 μM) in the presence or absence of NFs, we could not observe significant differences in the decomposition of RhB (Fig. S5A and S5B†). Therefore, we concluded that if ROS were generated during the irradiation, these were not in high enough quantity to substantially contribute to the observed changes in the spectroscopic properties of the Ag2S LNThs.
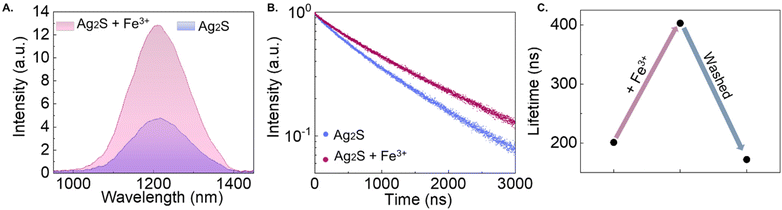
To unequivocally verify that the released iron ions are responsible for the change in the spectroscopic features of Ag2S LNThs (note that no change in the pH value was observed during the experiments), we compared the emission spectrum and photoluminescence decay curves of an Ag2S LNThs dispersion before and after addition of Fe3+ ions.
Upon adding 2 μL of 1 mM FeCl3 to 98 μL 0.5 mg mL−1 Ag2S LNThs dispersion (approximately 20 μM of Fe3+ in the final solution), an enhancement in the emission intensity (280%, Fig. 5A) and a lengthening of the lifetime (30%, Fig. 5B) was observed. We should also point out that a different Ag2S LNThs batch was used for these experiments. As such, the discrepancies in the absolute values of lifetime and relative changes observed compared to the results showed in Fig. 3 are to be ascribed to interbatch variabilities. Although there is also the possibility that other parameters are contributing to the changes in the optical properties of Ag2S LNThs, such as local temperature increases. The confirmation that these changes are induced by surface modifications was obtained from observation of the reversibility of the effect. Indeed, after washing the Ag2S LNThs via centrifugation after the addition of the FeCl3 solution, the lifetime values were recovered (Fig. 5C). This result suggests that iron ions did not diffuse into the crystal structure of the Ag2S LNThs but rather remained weakly tethered to their surface, possibly due to the lack of ligands in the solution supporting incorporation by cation exchange.38
It should be noted that the concentration of Fe3+ ions added in this test is 10 times higher than the one estimated during the irradiation (Fig. 4 and Fig. S4†). This is because no significant changes in the spectral properties were detectable using a concentration of 2 μM. This discrepancy could be explained considering that the concentration obtained using the colorimetric assay is an underestimation, since part of the Fe2+/3+ ions released under irradiation could be retained at the surface of the NFs by NH2 (present in the amino-dextran coating of the NFs). Moreover, a concentration of iron ions of approximately 20 μM is on par with the serum free iron concentration found in human body.39
The involvement of the surface rather than the bulk of Ag2S LNThs in this process is also supported by comparison of our results with the ones reported in the literature. Previous studies show that intentional incorporation of ions like Au3+ and Pb2+ in the lattice leads to an increased emission efficiency of silver chalcogenide nanocrystals, which is also accompanied by a shift of the emission peak.29,40–42 The absence of relevant incorporation of iron ions into the volume of Ag2S LNThs is supported by the absence of such shift in the emission peak position (Fig. 5A).
We also attempted to get direct evidence of the presence of iron attached to the Ag2S LNThs, performing elemental mapping of the nanofluid after irradiation (Fig. S6†). Yet, this analysis was not conclusive probably due to the low concentration of the released ions during the irradiation process. Lastly, the possible presence of contribution of partial oxidation and incorporation of iron into the Ag2S LNThs was also studied with X-ray absorption spectroscopy (XAS) (Fig. S7A†) measurements at the Ag K-edge (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 514 eV) performed at the BM30 beamline (ESRF synchrotron, Grenoble, France). A change in valence (to be expected in the case of oxidation) was discarded by extended X-ray absorption fine structure (EXAFS) (Fig. S7B and S7C†). At larger interatomic distances, a variation in the position and the intensity is detected in the irradiated sample. The observed changes could be related to the incorporation of Fe in the structure. Therefore, changes in the surface chemistry of the Ag2S LNThs are to be considered the main responsible for the observed variations in their spectroscopic properties.
514 eV) performed at the BM30 beamline (ESRF synchrotron, Grenoble, France). A change in valence (to be expected in the case of oxidation) was discarded by extended X-ray absorption fine structure (EXAFS) (Fig. S7B and S7C†). At larger interatomic distances, a variation in the position and the intensity is detected in the irradiated sample. The observed changes could be related to the incorporation of Fe in the structure. Therefore, changes in the surface chemistry of the Ag2S LNThs are to be considered the main responsible for the observed variations in their spectroscopic properties.
2.3. Ion-induced bias: a broader issue in luminescence nanothermometry?
We have shown how mixing Ag2S and γ-Fe2O3 nanoparticles leads to changes in the optical properties of the luminescent species used as LNThs under irradiation, and hence to a faulty thermal readout. The effect reported herein indicates that the thermal readout capability of the LNThs is somehow retained using one thermometric parameter (spectral shift), while the other parameter (absolute intensity) becomes completely unreliable. The case study herein reported is admittedly a specific one, but the processes involved are general in nature.In fact, LNThs are supposed to work in complex biological environments, where several ions are naturally present: some in higher, other in lower concentrations. The most abundant metal ions constituting the human body include alkali and alkaline earth ions like Na+, K+, Ca2+ and Mg2+. These metal ions are crucial for many functions in our body such as neurotransmission or blood circulation,43 and are present at concentrations up to 102 mM.44 Other oligo-element metals (i.e. trace elements that are found in smaller quantities in the body but are necessary for humans)45 include Fe2+/3+, Cu2+, Zn2+, and Mn2+.46 Among the different transition metal, iron is the most prevalent one in the body, in both oxidation states Fe2+/3+,43 with serum free iron concentration in concentrations between 10–30 μM.39 When LNThs (but any nanoparticle, really) are introduced in the human body, they are therefore bound to interact with these ions. In addition, local variations in the ionic strength and in temperature in living organisms are to be expected and external irradiation might lead to further localized heating. These temperature changes might promote mechanisms like cation exchange between ions already present in solution and the LNThs. Heat-promoted incorporation of ions has been reported in semiconductor nanocrystals as well as in lanthanide-doped nanoparticles (the two classes of luminescent nanoparticles used the most in luminescence nanothermometry), and the associated changes in optical properties are sizeable.38,47–49 Therefore, we claim that, in the evaluation of the reliability of a luminescence nanothermometry approach, care should be taken in considering possible ion-induced effects beyond the ones merely induced by the ionic strength of the medium. As a last remark, we would like to highlight that strategies like coating with suitable polymeric and inorganic shells may prove useful to avoid detrimental effects such as the one discussed previously. Efforts in this direction are expected to contribute solving the issue of frequent lack of reliability of luminescence nanothermometry in complex biological media.
3. Conclusions
In this study, we showed that the performance of Ag2S luminescent nanothermometers (LNThs) can be affected by a previously overseen source of bias: the interaction of the LNThs with metal ions in solution. We discovered this effect by investigating the possibility of measuring the magnitude of temperature increase during photothermal (PT) processes in a nanofluid composed of Ag2S LNThs and iron oxide nanoflowers (NFs). Specifically, during the irradiation with a near infrared laser (808 nm), the Ag2S LNThs in the nanofluid exhibited an unexpected enhancement of emission intensity instead of the expected heating-induced luminescence quenching. We verified that the enhancement in emission intensity depends on the NF concentration, the temperature of the nanofluid, as well as the power and wavelength of the laser. With the help of a colorimetric assay, a release of iron ions from the NFs under irradiation was observed. These ions appear to be weakly bound to the surface of the Ag2S LNThs, modifying their luminescent properties. As such, the Ag2S LNThs cease to be reliable luminescent nanothermometers.This work therefore raises an important caveat: the interaction between LNThs and free ions in the medium could affect the reliability of the thermometric approach. These interactions could be especially significant when working in biological media since different ions are naturally present. Such observations reinforce the need for a thorough understanding of how the LNThs can interact with complex media (particularly in the biological context) to achieve a reliable thermal readout.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financed by the Spanish Ministerio de Ciencia e Innovación under project NANONERV PID2019-106211RB-I00. R. M. is grateful to the Spanish Ministerio de Ciencia e Innovación for support to research through a Ramón y Cajal Fellowship (RYC2021-032913-I). M. P. acknowledges the financial support from the Spanish Ministerio de Ciencia e Innovación for a FPU (FPU20/03166). A. S. and A. E. acknowledge the MICIN for the financial support from Ramón y Cajal program (RYC2021-031236-I funded by the Recovery, Transformation and Resilience plan and RYC2020-029282-I, respectively). A. A. is grateful to the Spanish Ministerio de Ciencia e Innovación for financial support through a Juan de la Cierva Fellowship (FJC2021-047006-I). We also thank the BM30 beamline staff at ESRF synchrotron for their technical support.References
- D. Jaque and F. J. N. Vetrone, Nanoscale, 2012, 4, 4301–4326 RSC.
- M. Runowski, P. Woźny, N. Stopikowska, I. R. Martín, V. Lavín and S. Lis, ACS Appl. Mater. Interfaces, 2020, 12, 43933–43941 CrossRef CAS PubMed.
- R. G. Geitenbeek, A.-E. Nieuwelink, T. S. Jacobs, B. B. Salzmann, J. Goetze, A. Meijerink and B. M. Weckhuysen, ACS Catal., 2018, 8, 2397–2401 CrossRef CAS PubMed.
- J. Drabik, R. Kowalski and L. Marciniak, Sci. Rep., 2020, 10, 11190 CrossRef CAS PubMed.
- A. Bednarkiewicz, L. Marciniak, L. D. Carlos and D. Jaque, Nanoscale, 2020, 12, 14405–14421 RSC.
- I. V. Barbosa, L. J. Maia, A. Ibanez and G. Dantelle, Opt. Mater.: X, 2023, 100236 Search PubMed.
- H. D. Santos, E. C. Ximendes, M. d. C. Iglesias-de la Cruz, I. Chaves-Coira, B. del Rosal, C. Jacinto, L. Monge, I. Rubia-Rodríguez, D. Ortega and S. Mateos, Adv. Funct. Mater., 2018, 28, 1803924 CrossRef.
- Q. Fan, C. Sun, B. Hu and Q. Wang, Mater. Today Bio, 2023, 100646 CrossRef CAS PubMed.
- E. C. Ximendes, U. Rocha, B. Del Rosal, A. Vaquero, F. Sanz-Rodríguez, L. Monge, F. Ren, F. Vetrone, D. Ma and J. García-Solé, Adv. Healthcare Mater., 2017, 6, 1601195 CrossRef PubMed.
- P. Rodríguez-Sevilla, R. Marin, E. Ximendes, B. Del Rosal, A. Benayas and D. Jaque, Front. Chem., 2022, 10, 941861 CrossRef PubMed.
- Y. Shen, J. Lifante, I. Zabala-Gutierrez, M. de la Fuente-Fernández, M. Granado, N. Fernández, J. Rubio-Retama, D. Jaque, R. Marin and E. Ximendes, Adv. Mater., 2022, 34, 2107764 CrossRef CAS PubMed.
- M. Garvas, S. Acosta, I. Urbančič, T. Koklič, J. Štrancar, L. A. Nunes, P. Guttmann, P. Umek and C. Bittencourt, Nano Sel., 2021, 2, 1208–1217 CrossRef CAS.
- H. Liu, Y. Fan, J. Wang, Z. Song, H. Shi, R. Han, Y. Sha and Y. Jiang, Sci. Rep., 2015, 5, 14879 CrossRef CAS PubMed.
- L. Labrador-Paez, M. Pedroni, A. Speghini, J. Garcia-Sole, P. Haro-Gonzalez and D. Jaque, Nanoscale, 2018, 10, 22319–22328 RSC.
- J. C. Martins, A. R. Bastos, R. A. Ferreira, X. Wang, G. Chen and L. D. Carlos, Adv. Photonics Res., 2021, 2, 2000169 CrossRef CAS.
- Y. Shen, J. Lifante, N. Fernandez, D. Jaque and E. Ximendes, ACS Nano, 2020, 14, 4122–4133 CrossRef CAS PubMed.
- P. Rodríguez-Sevilla, G. Spicer, A. Sagrera, A. P. Adam, A. Efeyan, D. Jaque and S. A. Thompson, Adv. Opt. Mater., 2023, 2201664 CrossRef.
- A. D. Pickel, A. Teitelboim, E. M. Chan, N. J. Borys, P. J. Schuck and C. Dames, Nat. Commun., 2018, 9, 4907 CrossRef PubMed.
- M. Suta, Ž. Antić, V. Đorđević, S. Kuzman, M. D. Dramićanin and A. Meijerink, Nanomaterials, 2020, 10, 543 CrossRef CAS PubMed.
- J. Zhou, B. Del Rosal, D. Jaque, S. Uchiyama and D. Jin, Nat. Methods, 2020, 17, 967–980 CrossRef CAS PubMed.
- D. Mendez-Gonzalez, J. Lifante, I. Z. Gutierrez, R. Marin, E. Ximendes, E. Sanz-de Diego, M. C. Iglesias-de la Cruz, F. J. Teran, J. Rubio-Retama and D. Jaque, Nanoscale, 2022, 14, 16208–16219 RSC.
- R. Marin, A. Benayas, N. García-Carillo, J. Lifante, J. Yao, D. Mendez-Gonzalez, F. Sanz-Rodríguez, J. Rubio-Retama, L. V. Besteiro and D. Jaque, Adv. Photonics Res., 2022, 3, 2100260 CrossRef CAS.
- Y. Shen, J. Lifante, E. Ximendes, H. D. Santos, D. Ruiz, B. H. Juárez, I. Z. Gutiérrez, V. T. Vera, J. R. Retama and E. M. Rodríguez, Nanoscale, 2019, 11, 19251–19264 RSC.
- Y. Shen, H. D. Santos, E. C. Ximendes, J. Lifante, A. Sanz-Portilla, L. Monge, N. Fernandez, I. Chaves-Coira, C. Jacinto and C. D. Brites, Adv. Funct. Mater., 2020, 30(49), 2002730 CrossRef CAS.
- T. A. Alrebdi, A. N. Alodhayb, Z. Ristić and M. D. Dramićanin, Sensors, 2023, 23, 3839 CrossRef CAS PubMed.
- S. Shaw, J. Kailashiya, A. Gangwar, S. Alla, S. K. Gupta, C. Prajapat, S. S. Meena, D. Dash, P. h. Maiti and N. Prasad, Appl. Surf. Sci., 2021, 560, 150025 CrossRef CAS.
- A. Espinosa, R. Di Corato, J. Kolosnjaj-Tabi, P. Flaud, T. Pellegrino and C. Wilhelm, ACS Nano, 2016, 10, 2436–2446 CrossRef CAS PubMed.
- E. Glais, A. Maître, B. Viana and C. Chanéac, Nanoscale Adv., 2021, 3, 2862–2869 RSC.
- H. Yang, R. Li, Y. Zhang, M. Yu, Z. Wang, X. Liu, W. You, D. Tu, Z. Sun and R. Zhang, J. Am. Chem. Soc., 2021, 143, 2601–2607 CrossRef CAS PubMed.
- Z. Wang, T. Gu, T. Kadohira, T. Tada and S. Watanabe, J. Chem. Phys., 2008, 128(1), 014704 CrossRef PubMed.
- M. Hamilton, A. Barnes, W. Howells and H. Fischer, J. Phys.: Condens. Matter, 2001, 13, 2425 CrossRef CAS.
- H. D. Santos, I. Zabala Gutiérrez, Y. Shen, J. Lifante, E. Ximendes, M. Laurenti, D. Méndez-González, S. Melle, O. G. Calderón and E. López Cabarcos, Nat. Commun., 2020, 11, 2933 CrossRef CAS PubMed.
- J. R. Albani, Chapter 2-Fluorescence: Principles and Observables, in Structure and Dynamics of Macromolecules: Absorption and Fluorescence Studies, Elsevier Science, 2004, pp. 55–98 Search PubMed.
- M. A. Omary and H. H. Patterson, in Encyclopedia of Spectroscopy and Spectrometry, ed. J. C. Lindon, G. E. Tranter and D. W. Koppenaal, Academic Press, Oxford, 3rd edn, 2017, pp. 636–653 Search PubMed.
- L. Voss, E. Hoché, V. Stock, L. Böhmert, A. Braeuning, A. F. Thünemann and H. Sieg, Arch. Toxicol., 2021, 95, 895–905 CrossRef CAS PubMed.
- E. Agustina, J. Goak, S. Lee, Y. Seo, J. Y. Park and N. Lee, ChemistryOpen, 2015, 4, 613–619 CrossRef CAS PubMed.
- P. Van Viet, D. Van Chuyen, N. Q. Hien, N. N. Duy and C. M. Thi, J. Sci.: Adv. Mater. Devices, 2020, 5, 308–315 Search PubMed.
- L. De Trizio and L. Manna, Chem. Rev., 2016, 116, 10852–10887 CrossRef CAS PubMed.
- P. Bennett, C. Williamson, L. Sykes, D. A. MacIntyre and P. H. Dixon, Basic Science in Obstetrics and Gynaecology E-Book: A Textbook for MRCOG Part 1, Elsevier Health Sciences, 2022 Search PubMed.
- Y. Shu, J. Yan, Q. Lu, Z. Ji, D. Jin, Q. Xu and X. Hu, Sens. Actuators, B, 2020, 307, 127593 CrossRef.
- M. Yu, X. Yang, Y. Zhang, H. Yang, H. Huang, Z. Wang, J. Dong, R. Zhang, Z. Sun and C. Li, Small, 2021, 17, 2006111 CrossRef CAS PubMed.
- H. Yang, H. Huang, X. Ma, Y. Zhang, X. Yang, M. Yu, Z. Sun, C. Li, F. Wu and Q. Wang, Adv. Mater., 2021, 33, 2103953 CrossRef CAS PubMed.
- X. Zheng, W. Cheng, C. Ji, J. Zhang and M. Yin, Rev. Anal. Chem., 2020, 39, 231–246 CrossRef CAS.
- A. Melkikh and M. Sutormina, J. Theor. Biol., 2008, 252, 247–254 CrossRef CAS PubMed.
- D. C. Carvalho, L. M. Coelho, M. S. M. Acevedo and N. M. Coelho, Handbook of mineral elements in food, 2015, pp. 109–122 Search PubMed.
- N. P. Zaksas, S. E. Soboleva and G. A. Nevinsky, Sci. World J., 2019, 1, 1–9 Search PubMed.
- L. Jin, J. Liu, X. Liu, D. Benetti, G. S. Selopal, X. Tong, E. Hamzehpoor, F. Li, D. F. Perepichka and Z. M. Wang, Small Methods, 2023, 2300133 CrossRef PubMed.
- C. Dong and F. C. van Veggel, ACS Nano, 2009, 3, 123–130 CrossRef CAS PubMed.
- L. Labrador-Páez, M. Pedroni, K. Smits, A. Speghini, F. Jaque, J. García-Solé, D. Jaque and P. Haro-González, Part. Part. Syst. Charact., 2017, 34, 1700276 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3nr03728b |
| This journal is © The Royal Society of Chemistry 2023 |