Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recyclable thermosets based on modified epoxy-amine network polymers†
Lynn
Anderson
 a,
Edward W.
Sanders
a,
Edward W.
Sanders
 ab and
Matthew G.
Unthank
ab and
Matthew G.
Unthank
 *a
*a
aDepartment of Applied Sciences, Northumbria University, Newcastle upon Tyne, NE1 8ST, UK. E-mail: matthew.unthank@northumbria.ac.uk
bDepartment of Chemistry, University of Cambridge, Cambridge, CB2 1EW, UK
First published on 16th December 2022
Abstract
The development of high performance, recyclable thermoset materials for applications in plastics, composites, coatings and adhesives requires a synthetic approach where recyclability is designed into the molecular structure of the material. This paper describes a single stage process for the creation of materials from simple, low-cost molecular building blocks, where the polymerisation of liquid epoxy resins and aliphatic amines in the presence of an n-butyl diboronic ester, delivers epoxy-amine-dioxazaborocane materials with tunable physical properties including glass transition temperature (Tg). Mechanical (thermal) recycling and reprocessing of the epoxy-amine-dioxazaborocane thermoset is demonstrated, with retention of Young's modulus and ultimate tensile strength. Most notably, an efficient and low-cost process for the chemical recycling, disassembly and dissolution of the thermoset is demonstrated via two complementary processes using either pinacol (diol) or mono-functional phenylboronic ester.
New conceptsThis paper describes a simple, single stage process for the creation of a recyclable, high performance thermoset material from readily available epoxy resins and aliphatic amines, crosslinked with diboronic esters. Our approach to recyclable thermoset (network) polymers is distinguished from others in this field in creating an opportunity to form high performance, recyclable materials from established molecular building blocks, common to the plastics, composites, coatings and adhesive industries. The epoxy-amine-dioxazaborocane thermoset materials can be synthesised via a single stage mixing and casting process, though exploitation of a scarcely reported latent cross-linking reaction. Most notably, an efficient and low-cost process for the chemical recycling, disassembly and dissolution of the thermoset material is demonstrated via two complementary synthetic processes, using either pinacol (diol) or mono-functional phenylboronic esters. We anticipate that this approach to designing high performance thermoset materials from simple, accessible building blocks, with recyclability built into the molecular architecture, will inspire other researchers working across the field of material science. |
Introduction
High performance thermoset plastics and polymers deliver lightweight, durable and mechanically robust materials for a wide range of industrial applications.1 These include high durability coatings,2,3 structural composites4,5 (some of which are used in the renewable energy sector), lightweight components for aerospace and automotive application, and structural adhesives.6,7 Whilst focus is dominated on the challenge of plastic recycling of commoditised (low-cost) thermoplastic materials used in bottles, packaging and films,8 the significant environmental and scientific challenge of recyclable thermoset materials has received proportionately less attention. Thermoset polymers, which make up ∼20% of all polymeric materials,9 are based on high density crosslinked polymer networks traditionally assembled using non-reversible covalent bonding. However, their covalent network structure renders them single-use, and non-recyclable in their current form. Epoxy-resins are one of the leading thermoset technologies for industrial applications, due to their excellent mechanical strength, versatility and robustness with over 323![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes of epoxy resin produced in Europe per annum.10
000 tonnes of epoxy resin produced in Europe per annum.10
The increasing focus on sustainable development goals11 and Net Zero Strategy,12 (including sustainable and recyclable materials), highlights a major limitation of epoxy-resin technology, in the limited opportunity for chemical or mechanical recycling.13,14 This issue is highlighted in applications such as wind turbine blades, where it is estimated that ∼250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes of epoxy thermoset polymers reside, that are destined for landfill or incineration, once the wind turbines have reached the end of their useful life.10 Without lightweight, structural epoxy-thermosets renewable wind-energy would not be possible, yet their inherent ‘almost indestructible’ molecular structure renders them unsuitable for conventional recycling processes.4
000 tonnes of epoxy thermoset polymers reside, that are destined for landfill or incineration, once the wind turbines have reached the end of their useful life.10 Without lightweight, structural epoxy-thermosets renewable wind-energy would not be possible, yet their inherent ‘almost indestructible’ molecular structure renders them unsuitable for conventional recycling processes.4
The development of new materials, that have mechanical or chemical recyclability designed into their molecular structure, offer an opportunity to replace existing single-use materials with more sustainable alternatives. Dynamic materials, such as covalent adaptive networks (CANs), offer a potential solution to this issue,15–20 but the creation of such materials would need to be based on readily available high-performance building blocks, if a rapid pathway to impact and market acceptance is to be realised.13 Further to this, such materials would need to be designed in a way that offered versatile material performance, with tuneable properties spanning hard, high Tg glassy materials to more flexible adhesives.21–23 If the new class of materials could be both mechanically recycled (thermally reprocessed) and chemically recycled (i.e. molecule recovery and separation from mixed material), then this would represent a significant contribution to this important area of polymer chemistry.
The research in this report describes a scalable and versatile approach to tackle this challenge. The approach exploits widely used and structurally diverse epoxy resins, polymerised with equally diverse aliphatic amines.22 When crosslinked with diboronic acid esters (to form thermoset materials), a range of tailored material types are accessible, with tuneable physical properties. Unlike ‘traditional’ epoxy-amine thermosets, this approach allows the polymer backbone to be based on epoxy-amine polymerisation, generating β-amino diol groups, which are subsequently complexed with boronic esters24–26 to form dioxazaborocane crosslinks. Whilst epoxy resins have been chemically modified previously to create dynamic or reversible thermoset materials27–32 such materials rarely benefit from the high Tg or rapid dynamic exchange kinetics described in our approach, both desirable properties for recyclable materials.33–36 Other research to exploit β-amino diol functionalities (from epoxy-amine polymerisation) have been based on a modification of a pre-prepared polymeric material, and not directly from a single stage synthetic process.37–39 Further to this, an efficient process for the chemical or mechanical recycling of these materials has not been demonstrated.
Materials and methods
All materials were purchased from commercial vendors listed in the supplementary information (Table S1, ESI†) and used as received. Methods for the preparation of all thermoset materials used in this study can be found in the ESI.†Epoxy-amine-dioxazaborocane preparation
1,4-Butanediol glycidyl ether (BGE, 4.00 g, 0.020 moles), phenyl glycidyl ether (PGE, 1.49 g, 0.010 moles) and n-hexylamine (HA, 2.50 g, 0.025 moles) were combined in a round bottom flask under nitrogen with stirring. To the reaction mixture the appropriate mass of 1,4-phenylenediboronic acid tetrabutyl ester (2d) was added according to Table S2 (see ESI† for details). The reaction mixture was stirred at 55 °C for 1 hour and 45 minutes under nitrogen whilst viscosity slowly increased, before drawing down onto a non-stick PTFE sheet using a 400 μm draw-down bar to control film thickness. The resulting films were allowed to cure at room temperature for 16 hours under nitrogen, followed by a post cure schedule ramp at 80 (1 hour), 120 (1 hour) then 170 °C (1 hour, total 3 hours thermal cure). After cooling to room temperature, the resulting polymeric films were removed from the PTFE sheet to give dioxazaborocane crosslinked epoxy-amine polymers (D0–D100) or viscous liquid films (D0–D40).Epoxy-amine (control) preparation
1,4-Butanediol glycidyl ether (4.00 g, 0.020 moles) and phenyl glycidyl ether (1.49 g, 0.010 moles) were combined in a round bottom flask under nitrogen with stirring. To the reaction mixture, the appropriate mass of n-hexylamine (HA), cyclo-hexylamine, 1,6-hexanediamine and 4,4′-MDH was added according to Tables S3–S5 (see ESI† for details). The reaction mixture was stirred at 55 °C for 1 hour and 45 minutes under nitrogen whilst viscosity slowly increased, before drawing down onto a non-stick PTFE sheet using a 400 μm draw-down bar to control film thickness. The resulting film was allowed to cure at room temperature for 16 hours under nitrogen, followed by a post cure schedule ramp at 80 (1 hour), 120 (1 hour) then 170 °C (1 hour, total 3 hours thermal cure). After cooling to room temperature, the resulting polymeric films were removed from the PTFE sheet to give solid sheets of epoxy-amine polymer (A60–A100) or viscous liquid films (A0–A40).Nuclear magnetic resonance (NMR)
NMR analyses were acquired at 25 °C using a JEOL ECS400 Delta spectrometer at frequencies of 399.78 MHz for 1H-NMR, 100.53 MHz for 13C-NMR and 128.28 MHz for 11B-NMR. All chemical shifts are quoted as parts per million (ppm) relative to tetramethylsilane (TMS, δ = 0 ppm) as an internal standard in either deuterated chloroform (CDCl3) or deuterated dimethyl sulfoxide (DMSO-d6). OH group analyses were confirmed by doping samples with a drop of deuterated water (D2O). 13C-NMR assignment was confirmed by DEPT analysis. The spectral data is recorded as chemical shift (δ), relative integral, multiplicity (s = singlet, br = broad, d = doublet, t = triplet, q = quartet, quin = quintet, sext = sextet, dd = doublet of doublets, m = multiplet) and coupling constant (J = Hz).Fourier transformed infrared spectroscopy (FTIR)
Infrared spectroscopy was performed on a Bruker alpha Platinum-ATR and the output data analysed in OPUS software. Absorption maxima are expressed in wavenumbers (cm−1).Gel permeation chromatography (GPC)
Samples were dissolved in THF (2 mg mL−1) and filtered through 0.2 μm nylon filters. Samples were analysed using an Agilent 1260 infinity II system equipped with a RI and viscometry detector, fitted with PLgel MiniMIX-E and PLgel MiniMIX-D columns in sequence, using a THF mobile phase and a flow rate of 0.6 mL min−1. Analysis was performed against a calibration curve of polystyrene standards (EasiVial PS-M supplied by Agilent).Dynamic mechanical thermal analysis (DMTA)
DMTA analyses were conducted for network polymers on a PerkinElmer DMA 8000 and analysed in Pyris software (version 11.1.1.0492). Temperature scans were performed on 30 mm × 5 mm × 0.3 mm samples and were analysed in tension geometry with a fixed length of 11.52 mm, a frequency of 1 Hz and a constant temperature ramp of 3 °C per minute from −80 °C to 200 °C. Storage modulus (E′), loss modulus (E′′) and tan delta (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) signals were recorded.
δ) signals were recorded.
Tensile testing
Thin film polymer samples were laser cut to a modified ASTM standard 638-14-type V40 and experiments were conducted on either an Instron 5969 or Instron 3343 with a displacement ramp rate of 10 mm per minute.Hot press recycling
Mechanical recycling was conducted using a 2′′ × 3′′ Mini Manual Hot Press Machine (TTLIFE) with LCD Controller at 700–1000 lbs pressure. Ground polymer samples were placed between PTFE sheets before pressing at 170 °C for 30 minutes, then allowed to cool to room temperature (∼25 °C) overnight. Samples were then removed from the press and prepared for testing.Results and discussion
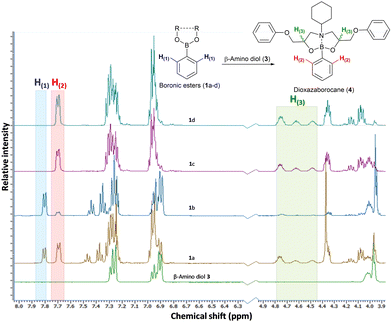
The preparation of epoxy-amine-dioxazaborocane thermosets requires controlled sequential epoxy-amine polymerisation, followed by dioxazaborocane cross-linking. To achieve a single stage synthesis process suitable for film casting or resin infusion processes, multifunctional boronic esters must be designed and synthesised for optimal miscibility with epoxy resins, whilst maintaining a balance of storage stability and chemical reactivity to allow latent cross-linking of the developing epoxy-amine polymeric network. Previous work in our group24–26 reported latent reactivity in epoxy-amine-borate (EAB) complexation which could potentially be exploited in the formation of a thermoset/network polymer. In the previous work, epoxy-amine polymerisation resulted in the formation of β-amino diol functional groups, which underwent latent complexation with borate esters. It was hypothesised that this chemo-selective and latent reactive sequence could be applied to the ‘one-pot’ synthesis of epoxy-amine polymers, with latent crosslinking through diboronic esters, to form epoxy-amine-dioxazaborocane thermosets. Since dioxazaborocane functional groups are known to undergo reversable bond exchange with adjacent β-amino diol functionalities,41 this presented an attractive potential route for the preparation of high-performance dynamic thermosets, from low-cost commodity starting materials, such as epoxy resins and aliphatic amines. To explore this concept, the preparation of suitable boronic esters that combined miscibility (in general purpose epoxy resins), stability and chemical reactivity (with a β-amino diol) was required. A range of mono-boronic esters (1a–d) were prepared from readily available starting materials (Fig. 2)42 and their physical and chemical reactivity properties were studied.To explore the relative reactivity of the selected boronic esters with a β-amino diol functional group, the model β-amino diol 3 was prepared24 and reacted (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) with the series of mono-boronic esters (Fig. 2, 1a–d) in CDCl3 (5 minutes at 25 °C). Direct analysis of the equilibrium reaction mixtures was conducted using 1H-NMR. The reaction of β-amino diol 3 with cyclic boronic ester 1a and 1b did not deliver a high reaction yield of the desired dioxazaborocane 4, even after extended reaction times. The non-substituted cyclic ethane-1,2-diol derived boronic ester (1a) achieved partial conversion to dioxazaborocane 4 (1a
1 molar ratio) with the series of mono-boronic esters (Fig. 2, 1a–d) in CDCl3 (5 minutes at 25 °C). Direct analysis of the equilibrium reaction mixtures was conducted using 1H-NMR. The reaction of β-amino diol 3 with cyclic boronic ester 1a and 1b did not deliver a high reaction yield of the desired dioxazaborocane 4, even after extended reaction times. The non-substituted cyclic ethane-1,2-diol derived boronic ester (1a) achieved partial conversion to dioxazaborocane 4 (1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio, 27
4 ratio, 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 73, Fig. 1, entry 1a) whilst the more hindered 2,3-dimethyl-2,3-butanediol (pinacol) based ester (1b)43 achieved a poor conversion of 78
73, Fig. 1, entry 1a) whilst the more hindered 2,3-dimethyl-2,3-butanediol (pinacol) based ester (1b)43 achieved a poor conversion of 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 (1b
12 (1b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio) after 96 hours (Fig. 1, entry 1b, comparison of electron-rich aromatic signal at 7.7 ppm (4) vs. 7.81 ppm (1a/b)). Equally, cyclic boronic ester 1a and 1b were found to be crystalline solids with relatively poor solubility in general purpose epoxy-resins such as diglycidylether of bisphenol-A (DGEBA, DER-331) and butane-1,4-diglycidyl ether. The linear (non-cyclic) esters 1c (iso-propyl) and 1d (n-butyl) in-contrast, showed quantitative conversion to the desired dioxazaborocane 4, indicating the required reactivity for the proposed ‘one-pot’ latent network assembly. They were also found to be liquids and miscible with general purpose epoxy-resins. Based on this study, the n-butyl diboronic ester 2d was selected for further studies, and it was found to deliver the ideal combination of solubility, chemical reactivity and scalability (synthesised from n-butanol and benzene-1,4-diboronic acid via azeotropic distillation).
4 ratio) after 96 hours (Fig. 1, entry 1b, comparison of electron-rich aromatic signal at 7.7 ppm (4) vs. 7.81 ppm (1a/b)). Equally, cyclic boronic ester 1a and 1b were found to be crystalline solids with relatively poor solubility in general purpose epoxy-resins such as diglycidylether of bisphenol-A (DGEBA, DER-331) and butane-1,4-diglycidyl ether. The linear (non-cyclic) esters 1c (iso-propyl) and 1d (n-butyl) in-contrast, showed quantitative conversion to the desired dioxazaborocane 4, indicating the required reactivity for the proposed ‘one-pot’ latent network assembly. They were also found to be liquids and miscible with general purpose epoxy-resins. Based on this study, the n-butyl diboronic ester 2d was selected for further studies, and it was found to deliver the ideal combination of solubility, chemical reactivity and scalability (synthesised from n-butanol and benzene-1,4-diboronic acid via azeotropic distillation).
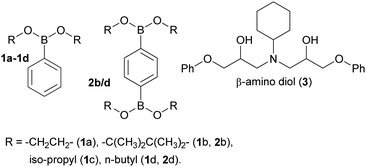 | ||
| Fig. 2 The mono- and di-boronic esters prepared for reactivity and miscibility studies, as well as β-amino diol model ligand 3. | ||
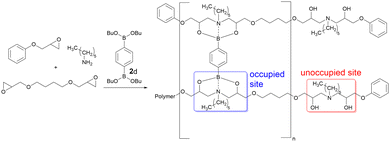
A series of epoxy-amine-dioxazaborocane thermoset materials were prepared via a single-pot film casting process using butane-1,4-diglycidyl ether (BDGE, two-functional epoxy resin), phenylglycidyl ether (PGE, mono-functional epoxy resin) and n-hexylamine (Fig. 3). As the epoxy-amine reaction progresses, β-amino diol functional groups are created which subsequently undergo cross-linking with n-butyl diboronic ester 2d (to a range of degrees based on predetermined stoichiometry, evidenced by the FTIR). PGE controls the degree of polymerisation of the epoxy-amine polymer and therefore by definition the average functionality of β-amino diol groups per polymer chain. Since the critical gelation point (pgel) is proportional to average functionality, this approach also controls the extent of reaction required for gelation.44 This strategy allows for molecular-level material design, controlling factors critical to material performance including (i) polymer molecular weight (ii) average polymer functionality and (iii) cross-link density.
 | ||
| Fig. 3 The synthesis of epoxy-amine-dioxazaborocane (D series) materials via a controlled molecular assembly approach. | ||
Epoxy-amine-dioxazaborocane materials, spanning 20, 40, 60, 80 and 100% occupied crosslinking positions were prepared (Table 1, D20–D100), with the remaining positions unoccupied.45 It was anticipated that varying the percentage of occupied sites would impact both the resulting material properties and recycling behaviour, which could then be correlated to molecular structures via this controlled assembly approach. A sequential study of the epoxy-amine polymerisation reaction in the presence of n-butyl boronic ester 1d, confirmed that partially polymerised secondary β-amino alcohols (i.e., from hexylamine reacting with one epoxy group) still underwent facile reaction with a second equivalent of epoxide (to form tertiary β-amino diols). This exemplifies the latent and chemoselectivity of dioxazaborocane cross-linking, where stable (dioxazaborocane) bond formation only occurs in the presence of tertiary β-amino diols.24
| Sample | % occupied | T g (°C) | Residual mass (wt%) |
|---|---|---|---|
| D20 | 20 | −13.9 | 5.6 |
| D40 | 40 | −7.7 | 7.3 |
| D60 | 60 | 11.7 | 10.3 |
| D80 | 80 | 48.3 | 12.2 |
| D100 | 100 | 49.7 | 14.0 |
| A20 | 20 | −22.5 | 2.2 |
| A40 | 40 | −14.1 | 2.7 |
| A60 | 60 | −8.1 | 3.5 |
| A80 | 80 | 1.4 | 4.5 |
| A100 | 100 | 9.1 | 5.4 |
| A220 | 20 | −17.2 | 1.7 |
| A240 | 40 | −4.5 | 2.5 |
| A260 | 60 | 16.4 | 3.3 |
| A280 | 80 | 32.9 | 4.3 |
| A2100 | 100 | 41.5 | 4.3 |
| A360 | 60 | 87.8 | 8.0 |
| A380 | 80 | 88.0 | 8.0 |
| A3100 | 100 | 97.1 | 6.7 |
To understand the impact of dioxazaborocane cross-linking on material properties, a series of complementary diamine crosslinked control materials were prepared (Table 1, A20–A100). Crosslink density (‘occupied sites’) was controlled using 1,6-diaminohexane (DAH), whilst corresponding ‘unoccupied sites’ retained n-hexylamine functional groups. This approach ensures maximum molecular synergy between the dioxazaborocane and diamine crosslinked materials, for performance and recyclability evaluation (Fig. 4). All materials were characterised using infrared spectroscopy, DSC, TGA and DMTA to gain insight into the structure and properties of the two complementary material types.
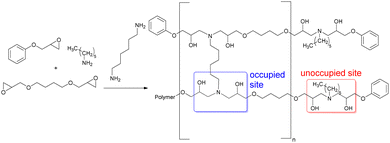 | ||
| Fig. 4 The synthesis of epoxy-amine (A series) ‘non-dynamic’ materials with structural synergy to epoxy-amine-dioxazaborocane (D series) materials. | ||
DSC analysis delivered insight into the effect of the rigid, bicyclic dioxazaborocane structure on the material's Tg. Across the epoxy-amine-dioxazaborocane (D20–D100) and the control epoxy-amine (A20–A100) materials, both series exhibited the ability to tune Tg by increasing the degree of cross-linking (and associated cross-link density). The epoxy-amine-dioxazaborocane materials exhibited significantly higher Tg's across the series, with ΔTg of ∼40–46 °C in the more crosslinked D80/D100 examples. This increase in Tg is likely a direct result of the reduced molecular mobility of the rigid bicyclic (5,5-fused) dioxazaborocane functionality (Fig. 3).41 Residual mass analysis by TGA confirmed increasing inorganic (boron) content across the D20–D100 series (5.6–14.0 wt%). This is presumably due to the formation of high thermal stability boron-oxide structures, the mass of which increase proportionally to the mass of diboronic ester used in epoxy-amine polymer crosslinking (i.e., increasing from D20 to D100). This contrasts with the organic epoxy-amine (A20–A100 series) polymeric materials, which show significantly lower residual mass percentages of 2.2–5.4 wt%.
DMTA analysis supported the Tg trends observed by DSC, with apparent higher Tg's for the D60–D100 vs. the A60–A100 series, as characterised by either Tgonset (sharp decline in E′, Fig. 5a) or peak of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ (Fig. 5c).46 Both the storage and loss modulus curves for the epoxy-amine-dioxazaborocane (D60–D100) materials show additional transitions after Tgonset, in comparison to the A60–A100 epoxy-amine materials (Fig. 5a and b, non-smooth decline in E′ and E′′). Given the well-established exchange reaction of boronic esters with diols and amino-diols18,38,41 it is proposed that these transitions are related to rapid bond exchange reactions and related rapid topological rearrangements, between ‘occupied’ (dioxazaborocane) and ‘unoccupied’ (β-amino diol) groups, enabled by the transition of the materials from the glassy to rubbery material state. It has been suggested that, when the topological freezing temperatures (Tv) is significantly lower than Tg, facile topological rearrangements would be expected at T > Tg.33 In the case of the dioxazaborocane materials described here, as temperatures progressed beyond Tgonset the D-series materials softened and at Tg + 30 °C demonstrated flow behaviour, indicative of a material with potential for mechanical recycling (vide infra). This behaviour also resulted in a significant broadening of the tan
δ (Fig. 5c).46 Both the storage and loss modulus curves for the epoxy-amine-dioxazaborocane (D60–D100) materials show additional transitions after Tgonset, in comparison to the A60–A100 epoxy-amine materials (Fig. 5a and b, non-smooth decline in E′ and E′′). Given the well-established exchange reaction of boronic esters with diols and amino-diols18,38,41 it is proposed that these transitions are related to rapid bond exchange reactions and related rapid topological rearrangements, between ‘occupied’ (dioxazaborocane) and ‘unoccupied’ (β-amino diol) groups, enabled by the transition of the materials from the glassy to rubbery material state. It has been suggested that, when the topological freezing temperatures (Tv) is significantly lower than Tg, facile topological rearrangements would be expected at T > Tg.33 In the case of the dioxazaborocane materials described here, as temperatures progressed beyond Tgonset the D-series materials softened and at Tg + 30 °C demonstrated flow behaviour, indicative of a material with potential for mechanical recycling (vide infra). This behaviour also resulted in a significant broadening of the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ in the case of D60–D100 materials, accounting for the apparent large increase in Tg value when compared to the corresponding DSC result (Table 1). Equivalent post-Tg transitions are not present in the control epoxy-amine materials (A60–A100), presumably due to the absence of dynamic bond exchange reactions in these ‘non-reversible’ covalently cross-linked materials.
δ in the case of D60–D100 materials, accounting for the apparent large increase in Tg value when compared to the corresponding DSC result (Table 1). Equivalent post-Tg transitions are not present in the control epoxy-amine materials (A60–A100), presumably due to the absence of dynamic bond exchange reactions in these ‘non-reversible’ covalently cross-linked materials.
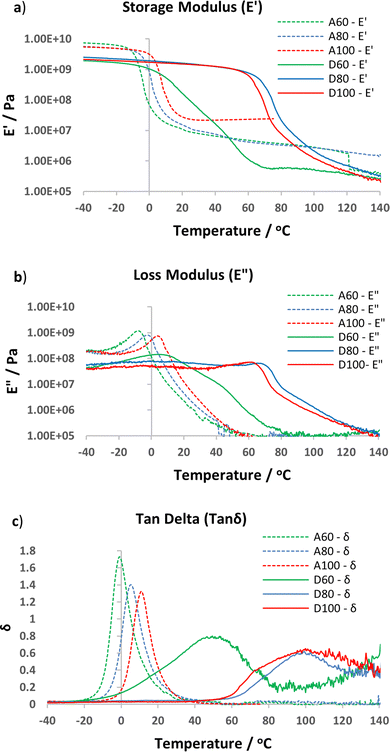 | ||
Fig. 5 DMTA analysis of D60-D100 and A60-A100 materials. (a) overlay of storage modulus curves (E′), (b) overlay of loss modulus curves (E′′) and (c) overlay of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ curves (E′′/E′). δ curves (E′′/E′). | ||
To address the significant difference in Tg between the D-series and A-series materials (which was considered important for comparison of material properties), higher Tg epoxy-amine thermoset materials were prepared as control materials. The A2-series were based on 4,4′-methylenedicyclohexanamine (4,4′-MDH) as the crosslinking agent, replacing the more flexible 1,6-diaminohexane (DAH). The bicycloaliphatic structure of 4,4′-MDH (see ESI†) resulted in an increase in material Tg (Table 1, A220–A2100), reducing the ΔTg between D100 and A2100 to ∼8 °C. The A3 series utilised 4,4′-MDH (crosslinking) and DER-332 (bisphenol-A epoxide from Olin Epoxy) with cyclohexylamine for the epoxy-amine polymerisation to deliver materials with Tg's in the region of 87.8–97.1 °C (Table 1, A360–A3100).
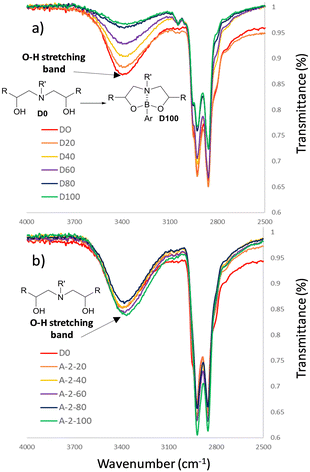
Characterisation of the D20–D100 series and A220–A2100 by infrared spectroscopy delivered insight into the structural difference between the dynamic and non-dynamic materials (Fig. 6). Increasing the dioxazaborocane content (i.e., increasing %-occupied vs. %-unoccupied in D0–D100) results in a proportional decrease in OH concentration (Fig. 6a, indicated region). This can be assigned to conversion of β-amino diol (OH) groups into dioxazaborocane complexes (see Fig. 3 for illustration), consuming the OH functionality proportional to the degree of crosslinking. This trend is absent in the infrared spectra of the epoxy-amine series (A20–A2100), where cross-linking does not consume hydroxyl groups, thus OH concentration should be approximately equal in all examples (Fig. 6b, indicated region).
Chemical resistance and recycling
A chemical recycling process that allows selective disassembly and dissolution of epoxy-amine-dioxazaborocane could allow separation and purification of the molecular building blocks, ready for reuse. However, it is essential that high-performance epoxy-thermosets exhibit a broad spectrum chemical resistance, remaining undamaged and insoluble upon exposure to chemically aggressive liquids and solutions.47–49 Immersion of the epoxy-amine-dioxazaborocane D100, in a range of solvents including ethanol, DMSO, toluene, acetone, THF, distilled water and 1 M NaOH showed no material integrity loss with the expected excellent chemical resistance and insoluble properties of a ‘traditional/epoxy-amine’ thermoset, network polymer. The hydrolytic stability under aqueous conditions, can be attributed to the enhanced stability of boronic esters containing tetra-coordinate bonding via N-atom coordination,50,51 such as that seen in the bicyclic dioxazaborocane structures described here. The solvent resistance can be clearly seen in Fig. 9a (THF control), where both D100 and A100 remain unchanged and undissolved after 24 hours. Polymer-network disassembly was demonstrated with the dioxazaborocane D-series, using (mono-functional) n-butyl phenylboronic ester 1d in THF. Using an excess of 1d, the D100 polymer-network can be disassembled to form a soluble (non-crosslinked) epoxy-amine polymer as illustrated in Fig. 7. This is demonstrated visually in Fig. 9b (THF + 1d) where, in contrast to THF only (Fig. 9a), the D100 sample has fully dissolved after 24 hours. In all cases, the control epoxy-amine A100 remains undissolved, as expected for a ‘traditional’ epoxy thermoset. The same result was achieved using pinacol (2,3-dimethyl-2,3-butanediol) to disassemble the dioxazaborocane D100 polymer network, liberating the epoxy-amine polymer, with concomitant formation of di-pinacol boronic ester 2b (Fig. 7, method b). 1H-NMR analyses support this, with characteristic dioxazaborocane signals (Fig. 8, boronic ester model complex, 4.2–4.8 ppm) absent from the product 1H-NMR spectra (Fig. 8, D100 + pinacol) indicating release of the non-complexed, linear epoxy-amine polymer (equivalent to polymer D0). The dipinacol ester of phenyl diboronic acid (2b) has a peak also clearly present in the product 1H-NMR spectra (Ar-H at 7.8 ppm), further supporting our understanding of the chemical disassembly process.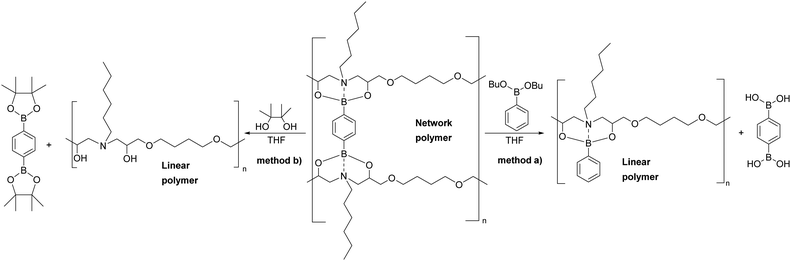 | ||
| Fig. 7 Chemical recycling of epoxy-amine-dioxazaborocane D100 via either (a) phenylboronic ester or (b) 2,3-dimethyl-2,3-butanediol (pinacol) methodologies. | ||
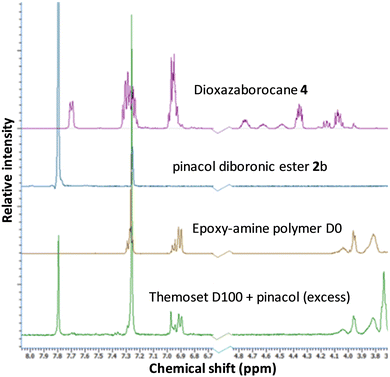 | ||
| Fig. 8 1H-NMR spectra of epoxy-amine-dioxazaborocane thermoset D100 after chemical recycling via pinacol methodology (according to Fig. 7b). | ||
GPC analysis of the chemical recycling solutions supported the results of the 1H-NMR analysis, through comparison of molecular weight distributions from both disassembly methods (Fig. 7, method (a) and (b)) vs. authentic prepared samples of linear epoxy-amine polymer (i.e., D100 without dioxazaborocane crosslinking = D0). The polymer molecular weight distributions of the two chemical disassembly methods (Fig. 10, ‘D100 post THF/1d immersion’ and D100 post THF/pinacol immersion’) show excellent correlation with the parent control polymers (Fig. 10, ‘D0 control’ and ‘D0 control with 1d). In comparison, immersion of the epoxy-amine-dioxazaborocane in THF only (Fig. 10, D100 post THF immersion) did not result in dissolution of high molecular weight polymeric material, further evidencing the chemoselectivity of the disassembly process. These experiments not only demonstrate a viable mechanism for recovery of molecular building blocks from epoxy-amine-dioxazaborocane crosslinked polymer networks, but also provided evidence for the molecular structure of the epoxy-amine polymeric component within the epoxy-amine-dioxazaborocane crosslinked network.
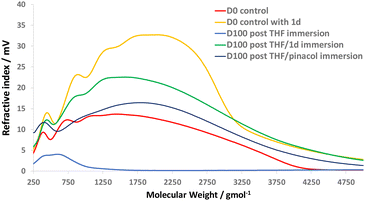 | ||
| Fig. 10 GPC analysis of the solutions from 1d and pinacol chemical recycling methodologies (according to Fig. 7) vs. D0 controls (with and without 1d). | ||
Mechanical recycling
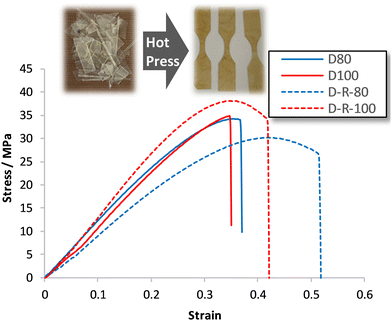
A covalent adaptable network, such as proposed with materials D60–D100, that undergoes rapid bond and topological rearrangement at T > Tgonset would be an attractive material for mechanical recycling. Epoxy-amine-dioxazaborocane materials D60–D100 were ground into small particles, then heated under compression at 170 °C for 30 minutes, using a simple bench-top hot press, before slowly cooling overnight (see ESI† for details). In all cases this resulted in the formation of a cohesive sheet of polymeric material, where tensile testing of the mechanically recycled materials (DR80–DR100) vs. the parent materials (D80–D100), showed similar stress-strain curves (Fig. 12), across both D80 and D100 materials.Key physical parameters such as Young's modulus and ultimate tensile strength were statistically unchanged in the higher crosslink density D80 and D100 materials. Both series of materials measured Young's moduli of 96–119 MPa for the D80/DR80 examples, increasing to 128–136 MPa for the higher crosslinked D100/DR100 example (Fig. 11b, entries 2 and 3), demonstrating the ability to both control material properties and maintain such properties post-recycling. For comparison to a ‘traditional’ epoxy-amine control, the high Tg A3 series was selected for analysis. Whilst the D-series materials were lower in Young's modulus than the corresponding A3-series (309–321 MPa), these results demonstrate significant promise for this new material class. Similar trends were observed for the ultimate tensile strength data of DR100 (Fig. 11a) with the pre- and post-recycling results for D80/DR80 and D100/DR100 statistically indistinguishable from each other (30–38 MPa). Again, whilst the A3 control demonstrated higher strength (68–69 MPa), the results for the epoxy-amine-dioxazaborocane were very promising. These results support the hypothesis that the dioxazaborocane bond exchange results in sufficient rheological flow for material fragments to reform into a single cohesive polymer sheet, and that bond interchange restores the polymer network and associated mechanical properties.
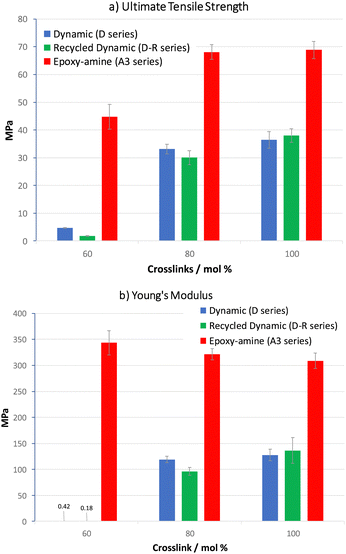 | ||
| Fig. 11 (a) Ultimate tensile strength and (b) Young's modulus, data for D60–D100 and A360–A3100 materials. | ||
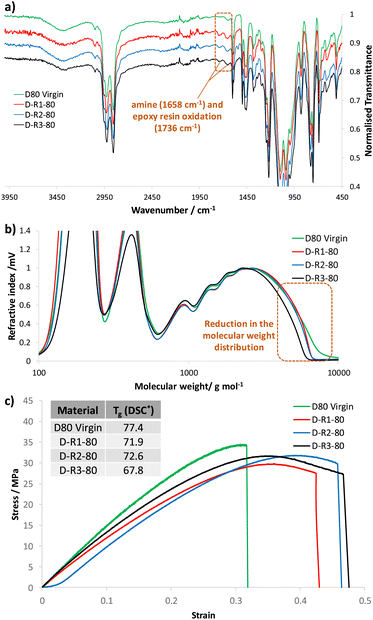
Extended evaluation to study the impact of repeated mechanical recycling on the thermal, chemical and mechanical properties of dioxazaborocane material D80 was conducted. A virgin sample of D80 was prepared and subjected to three cycles of mechanical recycling, with characterization by infra-red spectroscopy, GPC (after chemical disassembly in THF/pinacol), tensile testing and thermal analysis (Tg by DSC) at each stage of recycling (plus characterisation of the virgin D80). Analysis by infra-red spectroscopy revealed low levels of apparent oxidation upon subjecting the D80 material to repeated mechanical recycling at elevated temperatures (170 °C). This is characterised by the presence of weak absorption bands at 1658 cm−1, associated with amine oxidation and at 1738 cm−1, characteristic of epoxy resin oxidation (Fig. 13a, highlighted region).52 To examine the impact of mechanical recycling on the molecular weight distribution of the dioxazaborocane D80 material, the same samples were subjected to chemical disassembly in THF/pinacol according to our experimental procedure (see previously described method) and analyzed by GPC. A slight reduction in the molecular weight distribution profile was observed upon mechanical recycling, proportional to the number of recycling processes to which the material was subjected. Since oxidation of polymers is a well-recognized route to degradation (including molecular weight reduction),52,53 it seems reasonable to assume that these properties are connected, and that the apparent oxidation is responsible for the modest reduction in the molecular weight distribution by GPC (Fig. 13b). The tensile properties of the dioxazaborocane D80 materials were generally maintained on repeated mechanical recycling (Fig. 13c). The recycled samples (R1, R2 ad R3) all demonstrated similar stress-strain curves, Young's moduli and ultimate tensile strength compared to the D80 virgin sample (Fig. 13c). In addition to this, the recycled materials (R1–R3) all showed improved ductile behavior in comparison to the virgin material (D80), likely linked to the slow cooling of the recycled materials in the mechanical hot-press, allowing annealing of the polymeric networks. Finally, thermal analysis of the 4 samples by DSC, revealed a slight depression in Tg after repeated mechanical recycling (Fig. 13c, inset), again likely linked to the reduction in molecular weight distribution observed by GPC54,55 and associated polymer oxidation.56
Conclusions
A new methodology for the synthesis of recyclable thermoset polymers has been described, based on epoxy-amine building blocks which are common to the coatings, adhesives, and composites industries. Practical limitations associated with miscibility and reactivity of boronic esters (used in crosslinking) has been overcome to allow the preparation of epoxy-amine-dioxazaborocane, covalent adaptive networks, via a single stage film casting process, which also demonstrated the inherent latent reactivity of dioxazaborocane crosslinking. Comparative ‘traditional’ epoxy-amine thermoset materials were also prepared and the physical, mechanical and recyclable properties studied, alongside the epoxy-amine-dioxazaborocane materials.Two complementary synthetic methodologies for the subsequent chemical disassembly and dissolution of epoxy-amine-dioxazaborocane thermosets has been demonstrated. This process uses simple and readily available reagents (pinacol or phenylboronic ester) and highlights a potential route to recycling, separation and recovery of molecular building blocks. The dynamic nature of epoxy-amine-dioxazaborocane thermosets allows for facile topological rearrangement and flow when heated above Tg, delivering an effective mechanism for mechanical reprocessing or recycling of the materials. This recycling process has been shown to retain important material properties including Young's modulus and ultimate tensile strength. Overall, this work highlights the opportunity to create high performance thermoset materials for industrial applications, with mechanical and chemical recyclability designed into the molecular architecture.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The author acknowledges Northumbria University for the financial support of this research, Olin Epoxy for supporting the research through the provision of epoxy resins.References
- H. Dodiuk and S. H. Goodman, Handbook of Thermoset Plastics, Elsevier Inc, 3rd Edn, 2014, ISBN 978-1-4557-3107-7 Search PubMed.
- A. R. Marrion, The Chemistry and Physics of Coatings, RSC, 2nd Edn, 2004, ISBN-13: 9780854046041 Search PubMed.
- F. Dawson, W. C. Yew, B. Orme, C. Markwell, R. Ledesma-Aguilar, J. J. Perry, I. M. Shortman, D. Smith, H. Torun, G. Wells and M. G. Unthank, Langmuir, 2022, 38, 10632 CrossRef CAS.
- S. Rana, R. Alagirusamy and M. Joshi, J. Reinf. Plast. Compos., 2009, 28(4), 461 CrossRef CAS.
- T. W. Clyne and D. Hull, An Introduction to Composite Materials, Cambridge University Press, 3rd edn, 2019, ISBN-13: 978-0521860956 Search PubMed.
- F. Stoeckel, J. Konnerth and W. Gindl-Altmutter, Int. J. Adhes. Adhes., 2013, 45, 32 CrossRef CAS.
- D. Ratna, Recent Advances and Applications of Thermoset Resins, Elsevier Inc, 2nd Edn, 2022, ISBN 9780323856645 Search PubMed.
- https://www.ukri.org/what-we-offer/our-main-funds/industrial-strategy-challenge-fund/clean-growth/smart-sustainable-plastic-packaging-challenge/, accessed 7th July 2022.
- P. Shieh, W. Zhang, K. E. L. Husted, S. L. Kristufek, B. Xiong, D. J. Lundberg, J. Lem, D. Veysset, Y. Sun, K. A. Nelson, D. L. Plata and J. A. Johnson, Nature, 2020, 583, 542–547 CrossRef CAS.
- Epoxy resin commission report, 2019.
- United Nations, The 2030 Agenda and the Sustainable Development Goals: An opportunity for Latin America and the Caribbean (LC/G. 2681-P/Rev. 3), Santiago, 2018.
- HM Government, Net Zero Strategy: Build Back Greener, October 2021, ISBN 978-1-5286-2938-6.
- Y. Yang, R. Boom, B. Irion, D. van Heerden, P. Kuiper and H. de Wit, Chem. Eng. Process., 2012, 51, 53 CrossRef CAS.
- W. Post, A. Susa, R. Blaauw, K. Molenveld and R. J. I. Knoop, Polym. Rev., 2020, 60, 359 CrossRef CAS.
- S. Ma and D. C. Webster, Prog. Polym. Sci., 2018, 76, 65 CrossRef CAS.
- C. J. Kloxin, T. F. Scott, B. J. Adzima and C. N. Bowman, Macromolecules, 2010, 43, 2643 CrossRef CAS PubMed.
- J. M. Winne, L. Leibler and F. E. Du Prez, Polym. Chem., 2019, 10, 6091 RSC.
- M. Röttger, T. Domenech, R. van der Weegen, A. Breuillac, R. Nicolaÿ and L. Leibler, Science, 2017, 356, 62 CrossRef PubMed.
- W. Li, H.-Q. Wang, W.-T. Gao, Z. Wang, P. Xu, H. Ma and C.-H. Li, CCS Chem., 2022, 1–17 Search PubMed.
- J. Zhu, S. Zhao, J. Luo, W. Niu, J. T. Damron, Z. Zhang, M. Anisur Rahman, M. A. Arnould, T. Saito, R. Advincula, A. P. Sokolov, B. G. Sumpter and P.-F. Cao, CCS Chem., 2022, 1–13 Search PubMed.
- J. C. Capricho, B. Fox and N. Hameed, Polym. Rev., 2020, 60(1), 1 CrossRef CAS.
- B. Ellis, Chemistry and Technology of Epoxy Resins, Blackie Academic and Professional, 1993, ISBN 0-7514-0095-5 Search PubMed.
- C. May, Epoxy resins: chemistry and technology, Marcel Dekker, New York, 2nd edn, 1988 Search PubMed.
- M. G. Unthank, C. Cameron, A. Wright, D. Hughes, M. A. Alam and M. R. Probert, Polym. Chem., 2019, 10(36), 4920 RSC.
- C. Cameron, A. Wright and M. G. Unthank, Coating method for surfaces in chemical installations, WO 2015165808, 2015 Search PubMed.
- C. Cameron, A. Wright, M. G. Unthank and J. Wood, Coating method for surfaces in chemical installations, WO 2017068015, 2017 Search PubMed.
- Q. Shi, K. Yu, X. Kuang, X. Mu, C. K. Dunn, M. L. Dunn, T. Wang and H. Q. Jerry, Mater. Horiz., 2017, 4(4), 598 RSC.
- F. I. Altuna, C. E. Hoppe and R. J. J. Williams, Polymers, 2018, 10(1), 43 CrossRef.
- F. I. Altuna, C. E. Hoppe and R. J. J. Williams, RSC Adv., 2016, 6(91), 88647 RSC.
- X. Yang, L. Guo, X. Xu, S. Shang and H. Liu, Mater. Des., 2020, 186, 1 Search PubMed.
- Q. A. Poutrel, J. J. Blaker, C. Soutis, F. Tournilhac and M. Gresil, Polym. Chem., 2020, 11(33), 5327 RSC.
- K. Tangthana-Umrung, Q. A. Poutrel and M. Gresil, Macromolecules, 2021, 54(18), 8393 CrossRef CAS.
- M. L. Henriksen, J. B. Ravnsbæk, M. Bjerring, T. Vosegaard, K. Daasbjerg and M. Hinge, ChemSusChem, 2017, 10(14), 2936 CrossRef CAS.
- W. Denissen, G. Rivero, R. Nicolaÿ, L. Leibler, J. M. Winne and F. E. du Prez, Adv. Funct. Mater., 2015, 25(16), 2451 CrossRef CAS.
- S. Julia and A. A. Kalow, Polym. Chem., 2020, 11, 5339 RSC.
- B. Krishnakumar, R. V. S. Prasanna Sanka, W. H. Binder, V. Parthasarthy, S. Rana and N. Karak, Chem. Eng. J., 2020, 385, 123820 CrossRef CAS.
- Y. Ito, J. Kida, D. Aoki and H. Otsuka, Chem. Commun., 2018, 54(92), 12930 RSC.
- Y. Ito, D. Aoki and H. Otsuka, Polym. Chem., 2020, 11(33), 5356 RSC.
- H. Xu, J. Nishida, W. Ma, H. Wu, M. Kobayashi, H. Otsuka and A. Takahara, ACS Macro Lett., 2012, 1(4), 457 CrossRef CAS PubMed.
- See supporting information for full details and dog-bone dimensions. All samples were prepared and tested under identical conditions to allow direct comparison of material types.
- H. Bonin, T. Delacroix and E. Gras, Org. Biomol. Chem., 2011, 9(13), 4714 RSC.
- G. Wesela-Bauman, M. Urban, S. Luliński, J. Serwatowski and K. Woźniak, Org. Biomol. Chem., 2015, 13, 3268 RSC.
- J. Clayden, N. Greeves and S. G. Warren, Organic Chemistry, Oxford University Press, Oxford, 2012, 808–810 Search PubMed.
- G. Odian, Principles of Polymerization, John Wiley and Sons inc., 2004, 4th edn, pp. 108–110 Search PubMed.
- The occupied vs. unoccupied sites are controlled by the stoichiometric ratio of theoretical boronic ester functional groups to amine functional groups.
- D20 and D40 could not be tested due to inadequate cohesive strength for sample preparation, presumably due to failure to achieve the critical gelation point, pgel.
- M. Jackson, M. Kaushik, S. Nazarenko, S. Ward, R. Maskell and J. Wiggins, Polymer, 2011, 52(20), 4528 CrossRef CAS.
- S. T. Knox, A. Wright, C. Cameron and J. P. A. Fairclough, Macromolecules, 2019, 52(18), 6861 CrossRef CAS.
- S. T. Knox, A. Wright, C. Cameron and J. P. A. Fairclough, ACS Appl. Polym. Mater., 2021, 3(7), 3438 CrossRef CAS PubMed.
- H. Steinberg and D. L. Hunter, Ind. Eng. Chem. Res., 1957, 49, 174 CrossRef CAS.
- S. Gulyuz, Y. Yagci and B. Kiskan, Polym. Chem., 2022, 13, 3631 RSC.
- S. Morsch, Y. Liu, S. B. Lyon, S. R. Gibbon, B. Gabriele, M. Malanin and K. J. Eichhorn, Polym. Degrad. Stab., 2020, 176, 109147 CrossRef CAS.
- B. Gewert, M. M. Plassmann and M. Macleod, Environ. Sci.: Processes Impacts, 2015, 17, 1513 RSC.
- T. G. Fox and P. J. Flory, J. Polym. Sci., 1954, 14, 315 CrossRef CAS.
- J. Hintermeyer, A. Herrmann, R. Kahlau, C. Goiceanu and E. A. Rössler, Macromolecules, 2008, 41, 9335 CrossRef CAS.
- G. Z. Xiao and M. E. R. Shanahan, J. Appl. Polym. Sci., 1998, 69, 363 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2mh01211a |
| This journal is © The Royal Society of Chemistry 2023 |