Wettability of graphene: from influencing factors and reversible conversions to potential applications
Jing
Feng
ab and
Zhiguang
Guo
 *ab
*ab
aHubei Collaborative Innovation Centre for Advanced Organic Chemical Materials and Ministry of Education Key Laboratory for the Green Preparation and Application of Functional Materials, Hubei University, Wuhan 430062, People's Republic of China
bState Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, People's Republic of China. E-mail: zguo@licp.cas.cn; Fax: +86-931-8277088; Tel: +86-931-4968105
First published on 30th November 2018
Abstract
As a member of the carbon material family, graphene has long been the focus of research on account of its abundant excellent properties. Nevertheless, many previous research works have attached much importance to its mechanical capacity and electrical properties, and not to its surface wetting properties with respect to water. In this review, a series of methods are put forward for characterization of the water contact angle of graphene, such as experimental measurements, classic molecular dynamics simulations, and formula calculations. A series of factors that affect the wettability of graphene, including defects, controllable atmosphere, doping, and electric field, are also discussed in detail, and have rarely have been covered in other review articles before. Finally, with the developments of smart surfaces, a reversible wettability variation of graphene from hydrophobic to hydrophilic is important in the presence of external stimulation and is discussed in detail herein. It is anticipated that graphene could serve as a tunable wettability coating for further developments in electronic devices and brings a new perspective to the construction of smart material surfaces.
1. Introduction
Graphene, a single-atom-thick sheet comprised of sp2-hybridized carbon atoms, is a fascinating two-dimensional (2D) structured nanomaterial packed in a honeycomb lattice.1 Single-layer graphene is successfully obtained from graphite with the aid of mechanical exfoliation2 and has been intensively studied on account of its ultrahigh specific surface area,3 high electron mobility,4,5 superior thermal conductivity,6,7 exceptional optical transmittance,8 and extraordinary mechanical strength.9 Over the past ten years, graphene, a member of the carbon material family, has manifested a cornucopia of both basic science insights and practical applications thanks to these excellent properties.10 Graphene has exhibited enormous potential in a wide range of applications; for example, transparent flexible electrodes,11 multifunctional coating materials,12,13 high-performance strain sensors,14 and optoelectronic devices.15–17 Moreover, graphene plays a critical role in all the carbon material family. Many carbon nanomaterials, such as three-dimensional (3D) graphite, one-dimensional (1D) carbon nanotubes, and zero-dimensional (0D) fullerenes, can be prepared from graphene by virtue of various methods, specifically including the stacking, rolling, and wrapping of graphene.18 Nevertheless, much previous research work has given much importance to its mechanical capacity and electrical properties, and not to its surface wetting properties with respect to water.19–26The wettability, which is one of the most fundamental properties of a material surface, has always played an important role in addressing certain issues, such as those related to fog harvesting,27–30 oil/water emulsion separation,31–33 and self-cleaning.34,35 In order to achieve an efficient water-harvesting ability, a hydrophilic–superhydrophobic patterned hybrid surface that imitates the structure of a beetle's back has been widely fabricated by virtue of various ingenious approaches.36,37 A membrane with hydrophobicity and underwater superoleophilicity can be used to separate water-in-oil emulsions. Analogously, a membrane with hydrophilicity and underwater superoleophobicity is capable of separating oil-in-water emulsions.31 It is possible to design a superhydrophilic surface upon which a continuous thin water film can be generated to remove adsorbed particles.38 The wettability of a material surface, i.e., the hydrophobicity (superhydrophobicity) or hydrophilicity (superhydrophilicity), is usually strongly dependent on the surface chemical modification and surface topography.39–41 For example, Liu et al. reported organosilane-polymerized carbon dots inverse opals with a closed-cell structure, which showed unique lyophilic but non-wettable characteristics.42 The closed-cell structure made this material non-wettable toward many organic solvents, while the introduction of silane's chemical composition imparted lyophilic properties to the material, with its unique wettability attributed to the combination of closed-cell structures and silane's chemical composition. Therefore, the surface wettability of graphene can be regulated by means of introducing a microstructure or by the surface chemical composition. For instance, Nguyen et al. fabricated a sort of graphene sheet deposited on the pore-wall surface of melamine foams by a facile dip-coating method.43 These graphene-based foams showed enhanced hydrophobicity, which was primarily ascribed to the synergistic effect of the micro/nanotextured structure of the graphene sheets and the microporous structure of the melamine foams.44 Jiang et al. prepared periodically structured superhydrophobic graphene films by means of a two-beam laser holography interference technique. Micro-scale grating-like structures with nanometer roughness could be easily generated on graphene films by a laser-induced erosion effect. Simultaneously, the surface of graphene films was chemically modified through removing the abundant hydrophilic oxygen-containing groups. The graphene films presented unique superhydrophobicity owing to the combination of the micro/nanotextured structure and the removal of the hydrophilic oxygen-containing functional groups.45 Lin et al. prepared a neoteric superhydrophobic graphene aerogel via chemical surface modification.46
The wetting properties of a specific material surface is defined by the interactions between water molecules and the material surface.47–49 When the binding energies between water molecules are less than the associated adsorption energies on the material surface, the water molecules tend to spread out on the material surface to form a thin liquid film, and the material surface exhibits hydrophilic properties and yields a contact angle less than 90°. On the contrary, when the associated adsorption energies between water molecules and the material surface are less than the binding energies between water molecules, the water molecules are inclined to agglomerate on the material surface to form spherical droplets, and the material surface shows a hydrophobic nature and yields a contact angle more than 90°.50 It can be said that up to now, in all types of liquid–solid interactions, none is more interesting than water–carbon interactions because many carbon-based electronic devices are generally submerged in water solution.51 Graphene, a neoteric surface material in which almost all the carbon atoms are exposed to the environment, still remains the central material of choice in all carbon interfacial materials and is favorable to help establishing a thorough comprehension of water–carbon interactions. Therefore, it is particularly vital to research the wettability of graphene with respect to water.52–54 Graphene with hydrophobic properties can reduce liquid deposition and prevent contamination during the fabrication of electronic devices. The hydrophilic surface of graphene has important applications in biomaterials and microfluids. However, several recent studies have proclaimed that there is no definitive conclusion as to whether graphene is hydrophobic or hydrophilic.55,56 The wettability of graphene has been shown to have a significant impact on the performance of graphene-based electronic devices, such as on the capacitive energy storage and heat-transfer coefficient.57–60 In addition, monolayer graphene possesses wettability transparency, which makes it an ideal coating material for metal substrates.60–63 Gaining an extensive understanding of the wetting properties of graphene in contact with water would not only provide a reference for the microstructural design of graphene-based electronic devices, but would also lead to improvements in the performances of these devices, which is essential for the reliable and repeatable production of such devices.64,65
In this review, first, a series of methods are put forward for the characterization of the water contact angle (WCA) of graphene, such as experimental measurements, classic molecular dynamics simulations, and formula calculations. In addition, one of the most important and controversial properties of graphene, namely the wettability transparency, is discussed in detail in this article. Then, a series of factors that affect the wettability of graphene, including defects, controllable atmosphere, doping, and electric field, are also discussed in detail, and these have rarely been covered in other review articles before. Finally, with the developments of smart surfaces, the reversible wettability variation of graphene from hydrophobic to hydrophilic is important in the presence of external stimulation. It is anticipated that graphene could serve as a tunable wettability coating for further development in electronic devices and will bring a new perspective to the construction of smart material surfaces.
2. Intrinsic wettability of graphene
Graphene can be widely used in many fields of nanotechnology, such as optoelectronics and water desalination.17,66 The dispersion of a series of graphene sheets in polymers or the interactions between lots of graphene flakes and brine lixiviant are both strongly determined by the wettability of graphene.67 It is generally recognized that the wetting properties of a specific material (i.e., whether it is hydrophobic or hydrophilic) can be identified by measuring its contact angle with respect to water. Hence the most intuitive method for characterizing the wetting properties of graphene is to place droplets on the graphene surface and then to measure the WCA. A series of contact angle values of graphene with respect to water have been determined in the literature utilizing various methods, including experimental measurements (static contact angle measurements and dynamic contact angle measurements), classic molecular dynamics simulations, linear fitting, and formula calculations. Due to the extremely thin atomic thickness of graphene, classic molecular dynamics simulation is more convenient than actual experimental measurements. A series of formulas can also be derived to calculate the contact angle value of a water droplet on graphene surface, as discussed in the third part of this review. Table 1 lists the contact angles of single-layer graphene measured by various methods in the presence or absence of a substrate. It is worth noting that the graphene listed in the table refers to single-layered graphene, which was deposited on different types of substrates or not deposited on any substrate.| Year | Method | WCA | Substrate | Ref. |
|---|---|---|---|---|
| 2009 | Static contact angle measurement | 127° | Without | Wang et al.68 |
| 2010 | Contact angle measurement | 92° | With SiC | Shin et al.69 |
| 2012 | Quantum molecular dynamics simulations | 87° | Without | Li et al.70 |
| 2012 | Contact angle measurement | 86.2° | With Cu | Rafiee et al. and Mugele60,61 |
| 2012 | Formula calculation | 96° | Without | Shih et al.62 |
| 2013 | Well-founded estimation | 95°–100° | Without | Taherian et al.56 |
| 2013 | Molecular dynamics simulations | 40° | With/SiO2 | Raj et al.71 |
| 0° | With/superhydrophilic Cu | |||
| 2014 | Static water contact angle measurement | 125° | With/superhydrophobic Cu | Kim et al.63 |
| 2016 | Linear fitting | 85° ± 5° | Without | Thomas et al.72 |
The contact angle of a water droplet deposited on a separated graphene surface has long been a point of controversy. The contact angle value of graphene with respect to water has been investigated ever since the first successful exfoliation of graphene flake, yet it still does not have a definite value. Measurement of the contact angle of water droplets on graphene surface is very sophisticated due to the fact that graphene peeled from graphite can be affected by various factors. Graphene sheets are produced first by the chemical stripping of natural graphite sheets, followed by hydrazine conversion. Subsequently, the graphene flakes stemming from a series of suspensions, such as graphene–ethanol suspensions and graphene–isopropanol suspensions, are assembled into a thin graphene film. Micron-sized drops are placed onto the graphene film surface to measure its wettability and contact angle. For instance, Wang et al. demonstrated through experimental measurement that the static contact angle of graphene surface is 127°, which is larger than 90° and thus shows it has a hydrophobic property.68 The contact angle of water droplets on monolayer epitaxial graphene-coated SiC substrates is 92°. Shin et al. also found that increasing the number of graphene layers deposited on SiC substrates had no significant impact on the contact angle.69 Quantum molecular dynamics simulations represented that the graphene flakes yield a contact angle of 87°, while a monolayer boron nitride sheet gave rise to a contact angle of 86°.70 Rafiee et al. reported that a water droplet on a graphene-coated copper substrate gives rise to a contact angle of 86.2°, which is almost the same as that on pure copper (85.9°).60,61 With the aid of a series of formula calculations, Shih et al. described the process of deriving the contact angle of water droplets on graphene surface from the van der Waals (vdW) force between the water droplets and graphene.62 The series of formulas showed that a water droplet on an isolated graphene surface yields a contact angle of 96°. By virtue of classical molecular dynamics simulations and empirical force-field theory, Taherian et al. proved that the value of 127° is an unrealistic estimate and the expected value would be 95°–100°.56 Graphene coatings have been reportedly used for dynamic contact angle measurements on substrates with widely varying intrinsic wettability (∼0° contact angle on superhydrophilic copper to ∼40° contact angle on SiO2).71 Similarly, after graphene growth on an electroplated and thermally reduced copper surface, the water contact angle (∼125°) remained close to the value before graphene deposition.63 Note, these two similar experiments were actually concerned with the following referred graphene wetting transparency instead of its intrinsic wettability. The wetting transparency of graphene is discussed in further detail in the following section. Through a linear fitting, Thomas et al.72 demonstrated that completely isolated graphene gives rise to a water contact angle of 85° ± ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5°, which is higher than that of partially suspended or supported graphene in the absence of a substrate.
5°, which is higher than that of partially suspended or supported graphene in the absence of a substrate.
In summary, graphene possesses excellent hydrophobic properties on account of its slightly higher contact angle, which is favorable for greatly reducing the deposition of a liquid and thus can help prevent liquid contamination of advanced electronic devices.73
3. Theoretical analysis of graphene wettability
This part mainly introduces determination of the contact angle of water droplets on graphene surface utilizing formula calculation. Shih et al.62 described the process of deriving the contact angle of water droplets on graphene surface from the vdW force between water droplets and graphene. Then Kim et al. corrected some errors in Shih's paper. Briefly, the attractive vdW interaction potential between one carbon atom of graphene and one liquid molecule, VCL, is given by,74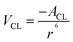 | (1) |
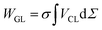 | (2) |
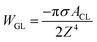 | (3) |
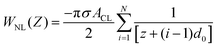 | (4) |
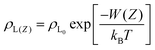 | (5) |
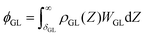 | (6) |
The vdW interaction potential per unit area between the whole liquid molecule and mutual N-layer graphene, ϕNL, is then given by,
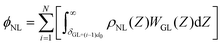 | (7) |
Nevertheless, mathematical flaws were found in Shin's work, which Kim et al.76 corrected. The mistake in Shih's work involved the calculation of the total vdW interaction potential per unit area between the liquid and the mutual N-layer graphene. The integral in the square brackets underestimated the vdW interaction from all layers except i = 1, because the liquid density at the bottom was underestimated. Thus, ϕNL must be corrected as:
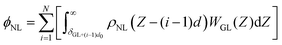 | (8) |
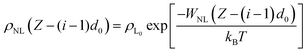 | (9) |
On the basis of Young's equation, the contact angle can be given as follows:77
γS = γSL + γL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ | (10) |
On the other hand, the work of adhesion between a solid surface and liquid can be described by:78,79
| WSL = γS + γL − γSL | (11) |
Combining eqn (10) and (11) results in eqn (12):
WSL = γL(1 + cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) θ) | (12) |
By convention, stronger vdW interactions correspond to a more negative value of ϕ, that is:
γL(1 + cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) = −ϕ θ) = −ϕ | (13) |
4. Wetting transparency of graphene
When a thin coating is added to the top of a substrate, the wettability of the original substrate is different from the wettability of the coated substrate. This is because the added coating is likely to affect the vdW forces between the original substrate and water molecules, further destroying the wettability behavior of the substrate. However, writing in Nature Materials, for the first time Rafiee et al. found that monolayer graphene as a functional coating on a particular substrate did not affect the substrate wetting behavior, and they defined this peculiar phenomenon as the wettability transparency of graphene.60 Specifically, the property means that a single-layer graphene coating has no significant impact on the wetting behavior of a substrate and remains transparent and non-aggressive to the substrate and water interface. It was found that, using graphene monolayer as a special coating, when coated on copper, gold, or silicon metal substrates, within the experimental error allowable range, the contact angle values for these virgin metal substrates were in good agreement with the contact angle values for the graphene-coated metal substrates. Peterson et al. also studied the wettability transparency of graphene-coated silicon surfaces.80 This experimental phenomenon is depicted in Fig. 1(a–d), where molecular dynamics simulations and theoretical calculations on a graphene-coated copper surface validated this phenomenon. It is shown in Fig. 1(a–d) that the contact angle of bare copper is almost the same as the contact angle of copper coated with monolayer graphene when monolayer graphene is coated on a copper substrate. With the number of graphene layers increasing, the contact angle of water on the supported graphene increases, eventually converging to a fixed value, close to the contact angle of water on graphite. With the increase in graphene layers, the interactions between the water molecules and copper substrate move away from the substrate, and the water molecules then interact with more and more carbon atoms.61 By utilizing the chemical vapor deposition growth method and changing the deposition time of graphene on the substrate, the number of graphene layers can be controlled.81 The additional graphene layer thickness inhibits the apparent wetting transparency of the original graphene sheet.82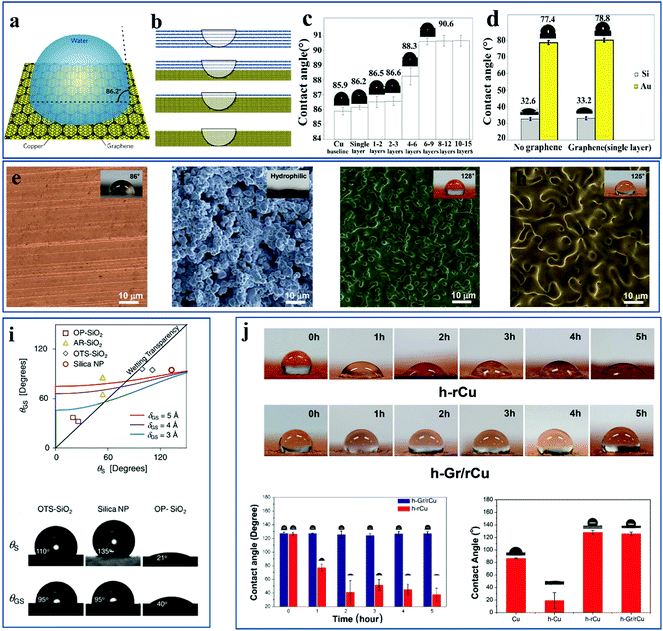 | ||
| Fig. 1 (a) A water droplet on a copper substrate coated with a single layer of graphene giving rise to a contact angle of 86.2°.61 (b) Increasing the number of graphene layers on the copper substrate.61 (c) After gradually covering a series of graphene layers on a copper substrate, the contact angle values of water droplets on the graphene layer were measured in order.60 (d) Contact angle measurements for Si substrate and Au substrate with and without a single layer graphene, where the contact angle values for the Si substrate and Au substrate were in good agreement with that for the graphene-coated Si and Au substrate.60 (e–h) Scanning electron microscopy (SEM) images of: (e) pure copper substrate (Cu), (f) electroplated copper substrate (h-Cu), (g) electroplated and thermal annealed copper substrate (h-rCu), (h) electroplated and thermal annealed copper substrate coated with a monolayer graphene coating (h-Gr/rCu) by an in situ growth method. The illustrations show the contact angle value of water on each substrate.63 (i) The contact angle of a substrate coated with a single layer of graphene as a function of contact angle of the bare substrate. These substrates include OP-SiO2, AR-SiO2, OTS-SiO2, silica NP.62 (j) When both h-rCu and h-Gr/rCu were immersed in the brine solution, the contact angle of h-Gr/rCu remained almost unchanged, which indicated that graphene could serve as a corrosion-resistant coating for the protective substrate.63 Reproduced from ref. 60 with permission from Nature, copyright [2012]. Reproduced from ref. 61 with permission from Nature, copyright [2012]. Reproduced from ref. 62 with permission from Physical Review Letters, copyright [2012]. Reproduced from ref. 63 with permission from Wiley-VCH, copyright [2014]. | ||
Why does single-layer graphene have such an excellent feature? Why does double or multi-layer graphene not have this characteristic? For these questions, Rafiee also analyzed the causes of this phenomenon after he first proposed this feature. Taking a copper substrate as an example, the wettability transparency of graphene on a copper substrate is related to the adsorption energy of water on a copper substrate. The number of graphene layers affects the size of the adsorption energy.60 When a single layer of graphene is inserted between a water molecule and a copper substrate, only the topmost layer of copper is replaced with a carbon atom. Since the geometrical shape of graphene is in the form of 2D laminates, monolayer graphene hardly influences the adsorption energy between water molecules and the copper substrate. With and without monolayer graphene coating, the adsorption of water on the copper substrate nearly remains unchanged. With the number of graphene layers increasing, the adsorption energy of water on copper can be reduced monotonically, which is contrary to the variation of the contact angle with the number of graphene layers. These observations, where the thickness of the graphene layer affects the graphene wettability transparency, were shown to be determined by the adsorption energy of water on the substrate.
Later, Shih et al. put forward a viewpoint contradicting the previous argument reported by Rafieee et al.62 They demonstrated that monolayer graphene is not completely transparent to wetting. More specifically, if the substrate coated with monolayer graphene is initially superhydrophilic or superhydrophobic, the wettability transparency of monolayer graphene breaks down significantly on these substrates. Therefore, they performed an experiment to prove their point of view. As is qualitatively illustrated in Fig. 1i, for those substrates with superhydrophobicity or superhydrophilicity, there clearly exists a deviation from the wettability transparency.
Nevertheless, Kim et al.63 demonstrated that a single-layer graphene coating is totally wetting-transparent for rough copper substrate with superhydrophobicity, which is inconsistent with the opinion reported by Shin et al. The original copper surface gives rise to a contact angle of 86° (Fig. 1e), while after electroplating and thermal annealing, the copper surface becomes rough and the contact angle is then 128° (Fig. 1g). When the copper surface is coated with single-layer graphene, the contact angle values for the rough copper substrate (128°) hardly differ from the contact angle values for monolayer graphene-coated copper substrate (125°) (Fig. 1h). The reason for this incompatible phenomenon is closely related to the deposition method of graphene on the copper substrate. When graphene is grown in situ on a copper surface by chemical vapor deposition, graphene can definitely maintain the hydrophobicity of the rough copper surface. However, when graphene is deposited on a hydrophobic copper surface by a wet chemical method,83 the wettability of the original hydrophobic copper substrate is certain to be altered. In addition, when both h-rCu and h-Gr/rCu were immersed in brine solution, the contact angle of h-Gr/rCu remained almost unchanged, which indicated that graphene can served as a corrosion-resistant coating for a protective substrate (Fig. 1j).
Based on research interest in the transparency of graphene wetting, the views of different researchers on the wettability of graphene have been comprehensively summarized. Discovering whether the wettability transparency of graphene on the substrate is effective depends on the following points: (1) the range of a substrate's attraction to water; (2) the thickness of the graphene layer; (3) the specific substrate type on which the graphene is located; (4) the degree of wettability of the original substrate, namely the contact angle of the pure substrate; (5) the growth method of graphene on the substrate.
Taking into account the first key factor, Shih et al. used molecular dynamics simulations and theoretical calculations to perform quantitative comparisons.84 The continuum model of the effective interface potential method is an important way to understand the transparency of graphene wettability.85,86 The vdW interaction potentials per unit area between water molecule and a substrate, ϕW(G+S) is a combination of two parts: ϕW(G+S) = ϕWG + ϕWS, where W, G, and S correspond to water, graphene, and the substrate, respectively.74 If the value of ϕWG is much smaller than ϕWS, the entire vdW forces are controlled by the substrate, which means that graphene is transparent to the interaction between the substrate and water. In contrast, if the value of ϕWG is much larger than ϕWS, the total vdW interactions are controlled by graphene, namely that graphene is opaque to the interaction between the substrate and water. Kim studied the wetting transparency of multilayer graphene and found an implication on the transparency of atomically thin coatings.76 The total graphene layers will contribute the dominant portion of the vdW forces if more graphene layers are stacked on substrates.80 Kim et al. further studied the transparency of graphene wettability and discovered that the liquid bulk modulus and superficial roughness are the keys to accurately calculating the vdW interactions between a liquid and the surface.76
Considering the number of graphene layers and the specific substrate type of these two factors, the influence of the number of graphene layers on the transparency of graphene wettability was mentioned in the previous article, while the effect of the substrate type on this property will be highlighted in the following. Rafiee60 reported that if the substrate is glass, the graphene coating on the glass surface will change the wetting properties of the glass. The transparency of graphene wetting plays a role in copper, gold, and silicon; conversely, this property disappears on glass. The wettability of glass is governed by a shorter range of interactions (hydrogen bonds between water and glass), but although graphene is extremely thin, the presence of graphene in the water and the glass interface will destroy the short-range chemical interactions.60,61 In contrast, the wettability of copper, gold, and silicon substrates is controlled by the relatively long-range vdW interactions. This indicates that a single-layer graphene coating does not provide wetting transparency to the glass surface. Shin et al. studied the variation of the contact angle of epitaxial graphene deposited on SiC.69 When a single layer of graphene was coated on the top of the SiC substrate, there existed a sharp variation in the contact angle of a water droplet with the graphene-coated SiC substrate (92°) compared to that of the original SiC (69.3°). This showed that, like the glass substrate, the wettability transparency of single-layer graphene disappears on the SiC substrate.
5. Factors affecting graphene wettability
The contact angle of water on graphene is not fixed, and constantly varies with the external conditions. Many researchers have measured the contact angle of water droplets on a graphene surface under various conditions, further obtaining a series of factors that affect the wettability of the graphene surface. For example, the wrinkled morphology of graphene has a certain effect on the wettability of its surface, and is capable of improving the hydrophobicity of the graphene surface. In addition, tension and vibration can also remarkably improve the hydrophobicity of the graphene surface. When the tensile strain is less than 10%, the contact angle of water droplets on graphene increases linearly with increasing strain, and when the strain is larger than 10%, the contact angle of water droplets is a constant value, about 110°. With an increase in vibration amplitude and decrease in vibration period, the contact angle of the graphene with respect to water increases, both of which make graphene more hydrophobic.87 Graphene with a hydrophobic property can reduce liquid deposition and prevent contamination during the fabrication of electronic devices. The hydrophilic surface of graphene has important applications in biomaterials and microfluids. Hence, it is particularly vital to study the factors affecting graphene wettability. In the next section, we summarize several classic factors that affect the wettability of graphene, such as low-density and high-density defects, controlled atmosphere, doping, and electric field.5.1 Defects
At present, chemical vapor deposition (CVD), especially plasma-enhanced chemical vapor deposition (PECVD), has become feasible technology for fabricating freestanding few-layer graphene such as vertical graphene nanosheets (VGNs).88 VGNs have been proven to be able to serve as an ideal electrode material for high-performance supercapacitors due to their large specific surface area and excellent electrical conductivity.89,90 Since the electrode material is inevitably in contact with the electrolyte solution, it is particularly vital to study the factors affecting the wettability of VGN-based electrode materials.91 Zhang et al.92 reported four formation steps for VGNs grown vertically on a silicon wafer substrate in detail, and these are depicted in Fig. 2(a–d). First, a series of carbon buffer layers germinate in an orientation parallel to the silicon crystal plane substrate. Then, via argon ion sputtering, a series of defects are inevitably generated in the carbon buffer layers. These defects provide nucleation sites for VGNs growth and are favorable for the subsequent formation of VGNs. Deng et al. proposed a mechanism termed as defect-induced formation, which was suitable for fewer-layer graphene, such as VGNs.93 After that, VGNs were generated in a series of defects sites and grown vertically to the silicon crystal plane substrate and carbon buffer layers. Finally, a series of new graphene layers were generated parallel to the graphene layer that was previously formed, which will thicken the VGNs.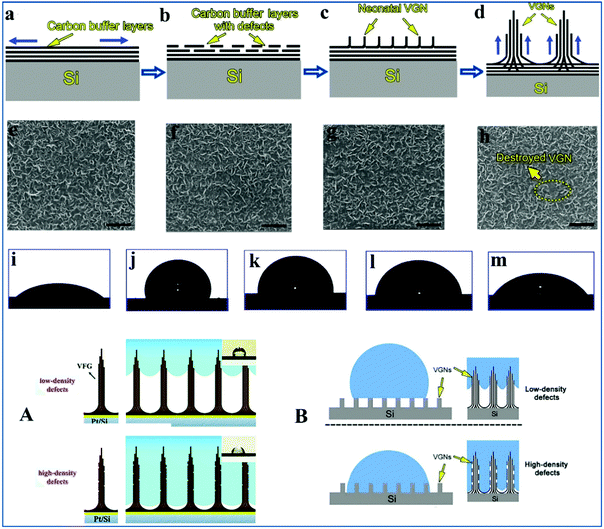 | ||
| Fig. 2 (a–d) Schematic illustration of the formation steps of the VGNs. (a) Formation of carbon buffer layers on silicon wafer substrate. (b) By virtue of argon ion sputtering, a series of defects were formed on carbon buffer layers. (c) The defects were used for the nucleation and growth of VGNs on the carbon buffer layers. (d) Generation of a series of new graphene layers. (e–h) SEM images of VGNs sprayed for (e) 0 s (initial VGNs without argon ion sputtering), (f) 30 s, (g) 60 s, (h) 120 s. (i–m) Schematic illustration of WCA for: (i) silicon crystal plane substrate, (j) initial VGNs deposited on silicon crystal plane substrate, (k) VGNs deposited on silicon crystal plane substrate sputtered for 30 s, (l) VGNs deposited on silicon crystal plane substrate sputtered for 60 s, (m) VGNs deposited on silicon crystal plane substrate sputtered for 120 s.92 (A and B) Graphic of the wetting of VFG with high-density defects and low-density defects with respect to water. Schematic illustration of the wetting of VGNs with high-density defects and low-density defects. Illustrations inside show the appearance of water droplets on VFG surface and VGNs surface, respectively.64,92 Reproduced from ref. 64 with permission from the Royal Society of Chemistry, copyright [2015]. Reproduced from ref. 92 with permission from Elsevier, copyright [2016]. | ||
By regulating the sputtering time of argon plasma to VGNs, different defects densities will be generated on VGNs, which eventually yield WCA values. As is qualitatively illustrated in Fig. 2(i–m), with the plasma sputtering time increasing, the VGNs become more and more hydrophilic. It is worth noting that argon plasma sputtering does not destroy the surface morphology of VGNs. Nevertheless, when the spraying period is extended to 120 s, the surface morphology of VGNs are slightly damaged Fig. 2(e–h). In brief, the wettability of VGNs can be manipulated by producing various degrees of defects via argon plasma treatment. VGNs with high-density defects show much better wettability, while VGNs with low-density defects show a hydrophobic nature (Fig. 2B). The reason for this phenomenon is that defects are favorable for the generation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds when VGNs are in contact with water. The C
O bonds when VGNs are in contact with water. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds impart hydrophilic properties to the VGNs, allowing water to easily permeate into the interval between the VGNs.
O bonds impart hydrophilic properties to the VGNs, allowing water to easily permeate into the interval between the VGNs.
Another representative example where defects have an impact on the wettability of vertically-oriented few-layer graphene (VFG) was reported by Fei et al.64 VFG grown on a nickel substrate by PECVD technology could also serve as an electrode material for high-performance supercapacitors.64,65 A hydrophilic electrode surface is more favorable for contact with the electrolyte solution for better electrocatalysis. A wettability improvement between the electrode and electrolyte solution is crucial for VFG-based supercapacitors. VFG with high-density defects shows a much better wettability, while VFG with low-density defects shows a hydrophobic nature (Fig. 2A). In summary, the wettability of graphene (such as VGNs and VFG) can be regulated by producing various degrees of defects via argon plasma treatment. VGN samples and VFG samples with a high density of defects thus overcome the drawbacks of difficulties in the contact with electrolyte solution and open up a wide horizon for ultrathin high-performance supercapacitor electrodes.
5.2 Controlled atmosphere
Over the past decade, various routes for fabricating different kinds of graphene have been widely researched, including the thermal exfoliation of graphite oxide,94 epitaxial growth on silicon carbide,95 thermal CVD techniques,96 PECVD techniques,97 and epitaxial growth on single crystal metal substrates.98 Different from these conventional methods, recently Lin et al. fabricated a special graphene material termed as laser-induced graphene (LIG) by using a facile laser induction process.99 This method specifically refers to using a carbon dioxide infrared laser to irradiate a polyimide (PI) film under environmental conditions. The laser is capable of burning away all the other components on the top layer of PI plastic film except carbon. The remaining material to be treated by laser is a form of graphene, which is a porous film with a flexible surface. In general, high-temperature treatment100–102 and multi-step chemical synthesis routes103–105 are two traditional methods to fabricate porous graphene films, but these approaches are cumbersome and economically inefficient.99 In contrast, a one-step laser engraving technology from PI films is a relatively simple method.99,106 Although this manufacturing technology has some similarities with the previously mentioned methods for producing graphene, it has a paramount advantage that laser irradiation is substituted for prolonged high-temperature heating.Via vertical irradiation of the pulsed laser, the PI film can be transformed into a graphene pattern, which exhibits the shape of an owl. The right side of Fig. 3a is the corresponding SEM image.99 It is worth noting that not only can LIG be produced from PI films, but also it can be made from virgin wood (Fig. 3b).107 Peng et al. reported that boron-doped LIG (B-LIG) can be synthesized by the laser irradiation of PI sheet surfaces mixed with boric acid, leading to the incorporation of heteroatoms into graphene (Fig. 3c).108 Another representative example is that of porous graphene films doped with crystal nanoparticles, which can be fabricated by the laser irradiation of PI films containing metal complexes (Fig. 3d).109 After describing the manufacturing method of LIG in detail, the impact of the controllable atmosphere on the LIG surface wettability will be carefully illustrated below.
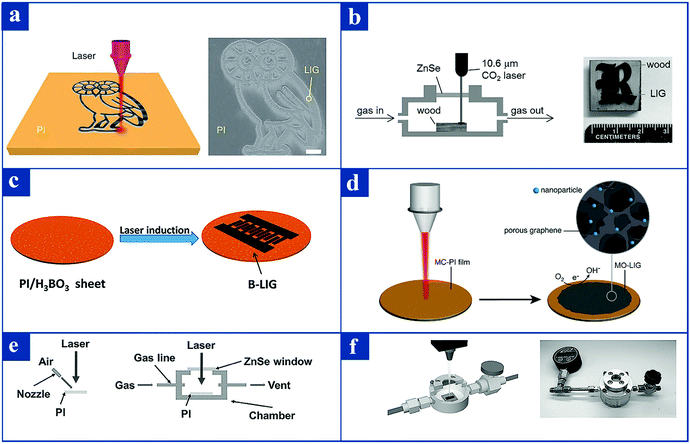 | ||
| Fig. 3 Schematic illustration of the formation of LIG formed from PI film or virgin timber via laser irradiation. (a) Schematic of the fabrication procedure of LIG patterned into an owl shape from commercial PI films; the right side of Fig. 1(a) is the corresponding SEM image.99 (b) Schematic of the fabrication procedure of LIG derived from virgin wood via laser irradiation; the right side of Fig. 1(b) is the corresponding photograph of LIG patterned into the letter R on virgin timber.107 (c) B-LIG synthesized by laser irradiation of a PI sheet surface mixed with boric acid.108 (d) Porous graphene films doped with crystal nanoparticles fabricated by the laser irradiation of PI films containing metal complexes.109 (e) The fabrication of LIG with gas assistance or in a controlled atmosphere room.106 (f) Physical photograph of the homemade controllable atmosphere chamber.106 Reproduced from ref. 99 with permission from Nature, copyright [2014]. Reproduced from ref. 106 with permission from Wiley-VCH, copyright [2017]. Reproduced from ref. 107 with permission from Wiley-VCH, copyright [2017]. Reproduced from ref. 108 with permission from the American Chemical Society, copyright [2015]. Reproduced from ref. 109 with permission from the American Chemical Society, copyright [2015]. | ||
By regulating the selected gas growth environment of LIG, the wettability of the as-prepared LIG surface can be altered in a tunable method.106 LIG can not only be fabricated via a gas-assisted method, but also it can be prepared in a controllable atmosphere chamber (Fig. 3(e and f)). Taking into account the gas-assisted condition, the LIG surface exhibits a superhydrophilic nature with an ≈0° contact angle regardless of air assistance or 3% H2/Ar assistance. Considering the case of the controllable atmosphere chamber, when LIG is prepared in O2 chamber or air chamber, it shows superhydrophilicity with an ≈0° contact angle; whereas, for LIG made in a H2 chamber or Ar chamber, it demonstrates superhydrophobicity with an ≈152° contact angle. This peculiar phenomenon was reported by Li et Al. (Fig. 4(a and b)).106 It is worth mentioning that LIG surface wettability can also be changed by altering the laser grating orientation relative to the gas-assisted orientation during LIG preparation. If the laser raster direction is identical with the gas-assistance orientation, the as-prepared LIG surface is hydrophilic. In contrast, the manufactured LIG surface demonstrates hydrophobicity under the condition that the laser raster direction is opposite to the gas-assistance orientation.106
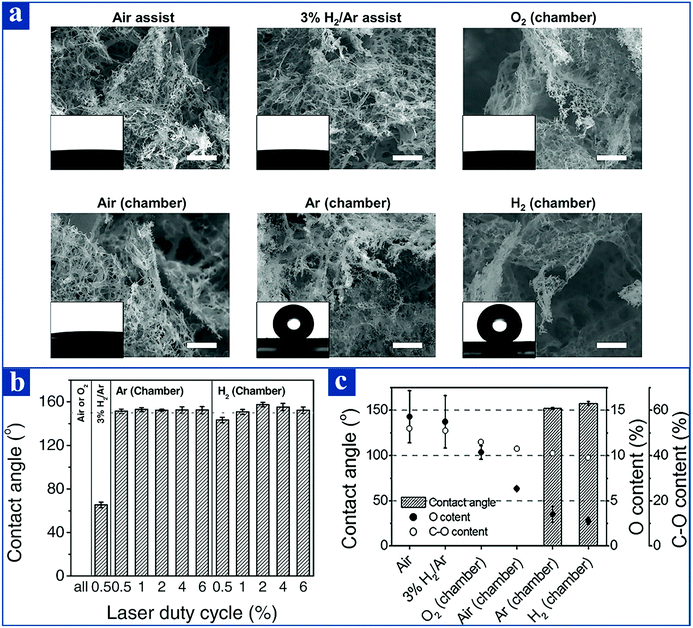 | ||
| Fig. 4 (a) SEM images of LIG samples fabricated with the aid of a gas-assisted method or under a controllable atmosphere chamber. Illustrations inside each SEM image show the appearance of a water droplet on the as-prepared LIG surfaces. (b) Image of the relationship between the contact angles of the LIG samples and the laser duty cycles. (c) The relationship between C–O bond content, O content, and WCA for LIG prepared with the aid of a gas-assisted method or under various atmosphere chambers.106 Reproduced from ref. 106 with permission from Wiley-VCH, copyright [2017]. | ||
The reason why LIG samples fabricated under various different atmosphere chambers have different contact angle values with respect to water is due to the distinct surface chemical composition of LIG. As is quantitatively illustrated in Fig. 4c, LIG samples made in a H2 chamber or Ar chamber have a relatively lower O content and C–O content. Therefore, a lower O content indicates that the LIG samples are more unfavorable to interact with water and thus become more hydrophobic. This great discovery not only shows the great potential for LIG with different wettability properties under various atmosphere chambers, but also amplifies the scope of applications for LIG samples.
5.3 Doping
Graphene is a kind of zero-band-gap semiconductor material with a special band structure.110 In the absence of doping, the conduction and valence bands of graphene meet at the Dirac point, which is also termed as the Fermi level. The p-doping of graphene means that its Fermi level moves downward with respect to the Dirac point, allowing a large number of holes to enter the valence bands. Whereas the n-doping of graphene represents that its Fermi level moves upward with respect to the Dirac point, causing a mass of electrons to enter the conduction bands (Fig. 5a).111 The p-type doping of graphene leads to a lower π charge density distribution than the undoped graphene, but the n-type doping of graphene is just the opposite.112 More specifically, graphene generates a partial charge transfer (electron donation) when interacting with electron-withdrawing groups, such as fluoropolymers, water, nitrogen dioxide,113 Br2, and I2,114 all of which result in p-type doping of graphene. Accordingly, graphene can form n-type doping when molecules with strong electron-donating ability are adsorbed on the graphene surface.115,116 Generally speaking, the doping of graphene is briefly classified as positive and negative electrical voltage doping (Fig. 5b), subsurface polyelectrolytes doping (Fig. 5c), doping with a subsurface metal (Fig. 5d), or chemical doping (fluorination) (Fig. 5e), all of which lead to p-type doping or n-type doping of graphene.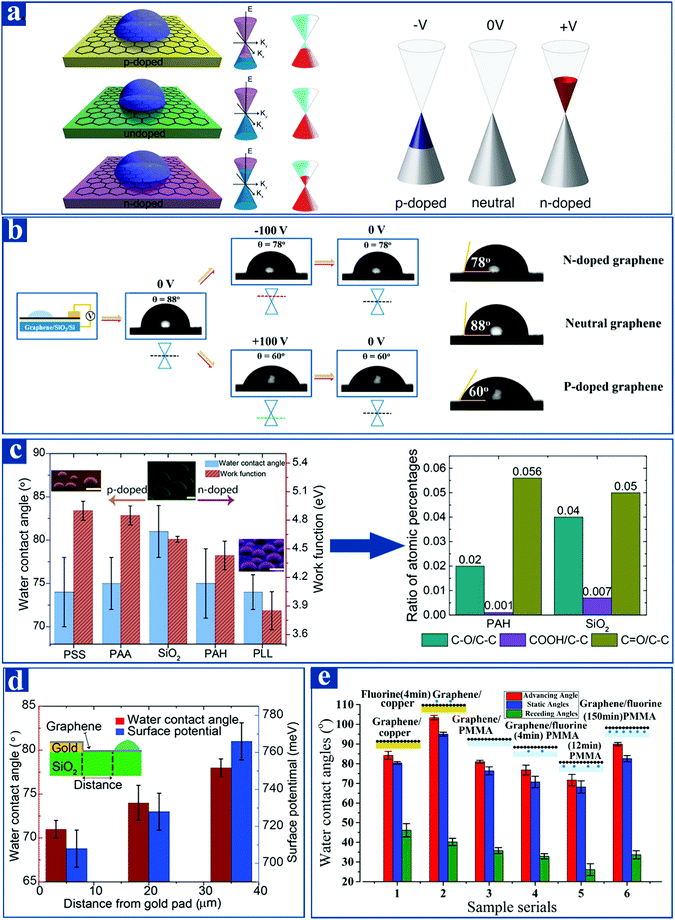 | ||
| Fig. 5 Tunable wetting of graphene surface caused by various doping methods. (a) Schematic representations of graphene wetting manipulation by either p-type doping or n-type doping.118–120 (b) The wettability of graphene deposited on SiO2/Si substrate can be affected by positive and negative electrical voltage doping, which is equivalent to the p-doping of graphene and the n-doping of graphene, respectively.118 (c) Upon either p-type doping or n-type doping with subsurface polyelectrolytes, the wetting of graphene exhibits increased hydrophilicity compared to nominally undoped graphene. The chart on the right side of Fig. 1(c) refers to the XPS contrast of hydrophilic functional groups of undoped graphene samples and a polyelectrolyte-doped graphene sample (PAH).120 (d and e) The interaction between graphene and water can be influenced by doping with a subsurface metal or by chemical doping (fluorination). Reprinted with permission.118,120 Reproduced from ref. 118 with permission from the American Chemical Society, copyright [2016]. Reproduced from ref. 119 with permission from the American Chemical Society, copyright [2014]. Reproduced from ref. 120 with permission from the American Chemical Society, copyright [2016]. | ||
The wettability of graphene can be altered via various doping methods. Park et al.117 reported that the graphene surface wetting properties can be transformed by AuCl3 doping, such that the performance of devices using graphene as an electrode material is further improved. As quantitatively illustrated in Fig. 5b, the Fermi level deviates from the Dirac point significantly when subjecting graphene to the applied positive and negative electrical voltage doping.118,119 When applying a negative voltage of 100 V to graphene deposited on a SiO2/Si substrate, which has the equivalent impact as the n-type doping of graphene, the Fermi level moves above the Dirac point and the static WCA of graphene drops to 78°. Similarly, when applying a positive voltage of 100 V to graphene deposited on a SiO2/Si substrate, which is identical to the p-type doping of graphene, the WCA decreases to 60°. As qualitatively illustrated in Fig. 4c, subsurface polyelectrolytes doping contributes to strong interactions between graphene and water and an enhanced wettability.120 Poly(sodium 4-styrenesulfonate) [PSS] and poly(acrylic acid) [PAA] have strong electron-withdrawing abilities, but poly(allylamine hydrochloride) [PAH] and poly-L-lysine [PLL] have strong electron-donating abilities, both of which lead to the p-type doping and n-type doping of graphene, respectively, thus further influencing the WCA of graphene. To further underpin the validity of graphene wettability alternation induced by subsurface polyelectrolytes doping, the chart on the right side of Fig. 5c exhibits the X-ray photoelectron spectroscopy (XPS) contrast of hydrophilic functional groups of undoped graphene samples and polyelectrolyte-doped graphene sample (PAH). Compared to the undoped graphene sample, polyelectrolyte PAH-doped graphene has a similar (carbonyl) or lower (hydroxyl and carboxyl) amount of hydrophilic oxygen-containing functional groups. This phenomenon fully demonstrates that the enhanced wetting property of graphene results from doping with subsurface polyelectrolytes rather than by hydrophilic oxygen-containing functional groups. In addition, with the aid of doping with a subsurface metal or chemical doping (fluorination), the wettability of graphene surface can be altered in a tunable manner (Fig. 5(d and e)).118,120
The phenomenon that doping leads to tunable wettability is typical of graphene and some similar two-dimensional materials. The wettability of the graphene surface can be affected via doping because doping can regulate the electron density of the graphene surface. Doping-induced electron–hole in graphene affect the vdW interaction of graphene with external molecules,112 further altering the way that graphene interacts with external molecules. These findings explain the link between the quantum level charge transfer and the macroscopic wetting properties of the graphene surface. This great discovery not only provides a feasible path for the fabrication of graphene surface coating with tunable wettability, but also brings new opportunities for the development of electrowetting without an applied current, which will make a significant contribution in energy conservation.
5.4 Electric field
Electrowetting refers to a process where the wettability of a material surface with respect to water can be altered in a tunable manner by manipulating the electric field.121,122 The quantitative relationship between the voltage and contact angle can be obtained by the Young–Lippmann equation, that is,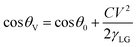 ,123 where θ0 is the contact angle when no electric field is applied, θV is the contact angle when an electric field is applied, V represents the electric voltage, C stands for the electric capacitance per unit area in the contact area between the material surface and droplet, and γLG is the surface tension of the droplet. It can be seen from the above equation that the contact angle of a material surface decreases as the electric voltage increases. Electrowetting is characterized by enhancing the wettability of the original material without changing its geometric component and surface morphology. Ren et al.124 investigated the wetting behavior of water droplets on graphene under a static electric field using classical molecular dynamics simulations. They concluded that when the applied electric field is parallel to the graphene plate, the external electric field reduces the contact angle of graphene with respect to water droplets, which makes graphene more hydrophilic. Pu et al.125 reported the effect of an electric field on the wettability of graphene/carbon nanotube conductive film mixed in various proportions. They reported how, as the applied electric voltage increased, the contact angle of a pure carbon nanotube conductive film with respect to water hardly changed significantly, whereas the contact angle of the water droplet on pure graphene conductive film varied greatly. Specifically, as the applied electrical voltage was increased up to 9 V, the contact angle of pure graphene versus water decreased rapidly. When the applied electrical voltage was further increased, the contact angle of water droplets on the graphene surface decreased slowly and then reached a fixed value. Tan et al.126 reported that flexible, conducting, and transparent single-layer graphene can served as a neoteric electrode material in a dielectric wetting apparatus. By exerting an electric potential difference between a graphene electrode and water droplets, when the applied electrical voltage was varied from 0 V to 40 V, the contact angle of the graphene electrode with respect to the water droplets changed from 117° to 86°. In short, an electrical field can be used as a powerful and effective means to regulate the wettability of graphene surface.
,123 where θ0 is the contact angle when no electric field is applied, θV is the contact angle when an electric field is applied, V represents the electric voltage, C stands for the electric capacitance per unit area in the contact area between the material surface and droplet, and γLG is the surface tension of the droplet. It can be seen from the above equation that the contact angle of a material surface decreases as the electric voltage increases. Electrowetting is characterized by enhancing the wettability of the original material without changing its geometric component and surface morphology. Ren et al.124 investigated the wetting behavior of water droplets on graphene under a static electric field using classical molecular dynamics simulations. They concluded that when the applied electric field is parallel to the graphene plate, the external electric field reduces the contact angle of graphene with respect to water droplets, which makes graphene more hydrophilic. Pu et al.125 reported the effect of an electric field on the wettability of graphene/carbon nanotube conductive film mixed in various proportions. They reported how, as the applied electric voltage increased, the contact angle of a pure carbon nanotube conductive film with respect to water hardly changed significantly, whereas the contact angle of the water droplet on pure graphene conductive film varied greatly. Specifically, as the applied electrical voltage was increased up to 9 V, the contact angle of pure graphene versus water decreased rapidly. When the applied electrical voltage was further increased, the contact angle of water droplets on the graphene surface decreased slowly and then reached a fixed value. Tan et al.126 reported that flexible, conducting, and transparent single-layer graphene can served as a neoteric electrode material in a dielectric wetting apparatus. By exerting an electric potential difference between a graphene electrode and water droplets, when the applied electrical voltage was varied from 0 V to 40 V, the contact angle of the graphene electrode with respect to the water droplets changed from 117° to 86°. In short, an electrical field can be used as a powerful and effective means to regulate the wettability of graphene surface.
6. Reversible transition of graphene surface wettability
The reversible conversion of a material surface wettability between hydrophilicity and hydrophobicity has always been a consequential subject in surface chemistry and can be manipulated in a tunable manner via a series of external stimuli.127,128 It is well-known that surface energy and surface morphology are two key parameters regulating a material surface wettability.129 External stimuli are an effective approach to induce an alternation in the surface energy or surface morphology, further resulting in transformations of the material surface wettability from hydrophobic/hydrophilic to hydrophilic/hydrophobic. The wettability switching of various material surfaces with the aid of different external stimuli has been reported. For instance, Sun et al. reported a poly(N-isopropylacrylamide)-modified surface, which exhibited thermally responsive wettability switching between hydrophilicity and hydrophobicity.130 Mertens et al. fabricated a single-layer atomic structure similar to graphene, namely boron nitride, also known as white graphene, which was mainly composed of alternating nitrogen and boron atoms. By changing the atomic angle of a single layer of boron nitride, the boron nitride could be converted from hydrophilic to hydrophobic with or without power applied.131 The wettability of graphene films can be transformed from superhydrophobic to superhydrophilic by changing the volume ratio of acetone to water in solvent.132 However, in these classic examples, the surface wettability switching of some materials is reversible, while some are irreversible.133 This section primarily outlines the current state-of-the-art method for reversibly switching the surface wettability of graphene materials (Fig. 6).6.1. Air plasma treatment and Joule heating
The reversible conversion between superhydrophobicity and superhydrophilicity of graphene materials has become a research hotspot,134 although lengthy transformation periods and sophisticated conversion processes severely restrict the practical application in many aspects of such materials. It has been found that nearly all previously reported reversible wettability transition processes of graphene require a prolonged period to complete.135 For instance, Zhang et al.136 demonstrated that graphene is capable of undergoing a reversible wettability transition between superhydrophilic and superhydrophobic with the aid of a cyclical transformation of ultraviolet (UV) irradiation and air storage. Although the alternation procedure is invertible and controllable, the graphene wettability can change from superhydrophilic to superhydrophobic or from superhydrophobic to superhydrophilic, but both take a long time. Specifically, the graphene can turn into the superhydrophilic form via UV irradiation treatment for 1 h, and then recover superhydrophobicity after being reserved in a vacuum environment for 4 days.137 Based on the long-term transformation processes, more and more researchers are beginning to explore superior methods to shorten the cycle time of transition for graphene wettability. Recently, Ding et al.138 fabricated a superhydrophobic graphene material, which had an open-cell microsphere structure with an interconnected network by the simple method of Pickering emulsion technology and vapor ejection. The superhydrophobic open-cell graphene network (OCGN) could accomplish an ultrafast transition from superhydrophobicity to superhydrophilicity in a very short time (1 s) by exposure to air plasma, which significantly shortened the conversion time. In addition, how to recover the superhydrophobicity of OCGN quickly through a feasible route is still a severe challenge. Based on the same report, Ding et al. also reported the ultrafast recovery of superhydrophobic OCGN within only 6 min by virtue of the simple method of Joule heating. This kind of graphene material with ultrafast reversible wettability transition has broad application prospects in the field of constructing intelligent superwetting surfaces.The fabrication processes of superhydrophobic OCGN via Pickering emulsion technology and vapor ejection is shown in detail in Fig. 7a. Due to its open-cell microstructure, the OCGN shows superhydrophobicity, which also can be quantitatively explained by the Cassie–Baxter equation:139
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ* = f θ* = f![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ − (1 − f) θ − (1 − f) |
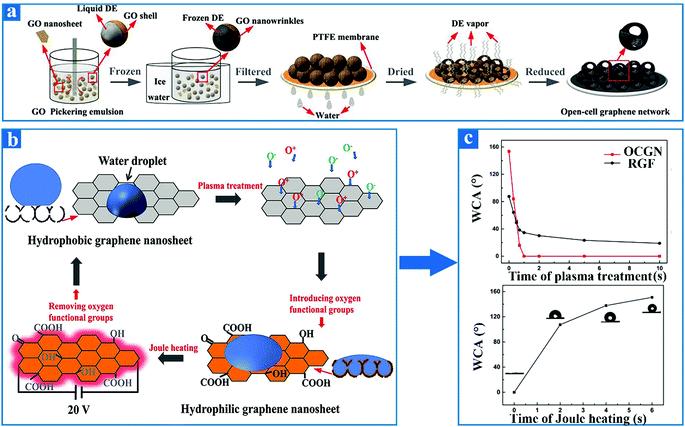 | ||
| Fig. 7 (a) Schematic illustration of the fabrication procedure for the superhydrophobic OCGN by Pickering emulsion technology and vapor ejection. (b) Diagrams of the surface composition and surface morphology of OCGN during the reversible wettability transformation procedure. (c) The upper graph in Fig. 1(a) shows the OCGN contact angle values as a function of plasma processing time, which presents a transition from superhydrophobicity to superhydrophilicity in a very short time (1 s) by exposure in air plasma. Similarly, the picture below shows the OCGN contact angle as a function of Joule heating time, which recovers superhydrophobicity within only 6 min by Joule heating.138 Reproduced from ref. 138 with permission from Wiley-VCH, copyright [2018]. | ||
In this equation, θ* represents the apparent contact angle of the OCGN; θ is the balance contact angle or the intrinsic contact angle of the OCGN, which is determined by Young's equation;140 and f is the fraction of water droplets in contact with OCGN. The actual contact area between OCGNs and water droplets is very small on account of the open-cell mesh microstructures of OCGN. As can be seen from the above equation, the contact angle of OCGN is inversely proportional to the value of f. Therefore, the OCGN exhibits superhydrophobicity, attributed to its large apparent contact angle value.
The mechanism of ultrafast, reversible transition between superhydrophobicity and superhydrophilicity of OCGN by means of air plasma treatment and Joule heating is described in detail in this paragraph. The superhydrophobic OCGN has a unique open microcell hierarchical structure that is capable of being converted into superhydrophilic OCGN in a very short time (within only 1 s) by exposure in air plasma. It is well-known that air plasma contains a mass of ions, especially oxygen ions. It is shown in Fig. 7b that both oxygen positive ions and oxygen negative ions will attack the superhydrophobic OCGN surface when they are exposed to an air plasma atmosphere. These oxygen ions naturally react with the carbon atoms on the highly active graphene and introduce to the OCGN a variety of hydrophilic oxygen-containing functional groups, such as carboxyl, hydroxyl, and carbonyl. By comparing the SEM images of OCGN before and after plasma treatment, it can be clearly seen that the overall surface morphologies and physical structures show almost no variations. Both of the SEM images have bowl-shaped hierarchical microstructures with graphene wrinkles and open-cell microcells. The wettability of a material surface is mainly attributed to the surface chemical composition and surface topography. The surface morphology of OCGN is barely affected by plasma treatment, but the surface composition is altered to some extent. Hence, it is the variation of the chemical composition that dominates the transition process. After plasma treatment for a short time, the content of oxygen atoms shows a dramatic rise, as demonstrated by the X-ray photoelectron spectroscopy (XPS) spectra and Raman spectra. Moreover, the XPS spectra manifest that the variation in the chemical components merely exists to a very shallow depth (a few molecular layers), which promotes the rapid recovery from superhydrophilicity to superhydrophobicity later. It is evident that these oxygen-containing functional groups have an excellent affinity toward water droplets. At the same time, on account of its typical configuration, the OCGN represents a minor apparent contact angle and ultimately achieves reversible wettability transition from superhydrophobicity to superhydrophilicity. In addition, the unique physical microstructure of OCGN plays a key role in the wettability transition process. For the sake of studying the effect of the physical microstructure, as a comparison, reduced GO films (RGF) with smooth surfaces were prepared by percolating graphene oxide (GO) liquor on a PTFE membrane. As illustrated in the upper graphs of Fig. 7c, air plasma treatment can only convert graphene into a hydrophilic material, but not to a superhydrophilic material. This phenomenon demonstrates that the open-cell physical microstructure is favorable for accelerating the transition process from superhydrohobicity to superhydrophilicity. Then, in order to achieve a reversible alternation from superhydrophilicity to superhydrophobicity, voltage treatment was applied to the superhydrophilic OCGN, which generated Joule heating. The OCGN exhibited a superfast Joule heating velocity of up to 20 °C s−1 at 20 V voltage on account of the good conductivity of the pure graphene network. This Joule heating rate is very fast, which causes the OCGN to be heated up rapidly. High temperatures can destroy the chemical bond between OCGN and oxygen, further resulting in the loss of hydrophilic oxygen-containing functional groups, which is similar to the thermal reduction of graphene oxide. The rapid removal of a series of oxygen-containing functional groups leads to the recovery of superhydrophobic OCGN, as demonstrated by the XPS spectra. The superhydrophilic OCGN is able to be converted into superhydrophobic OCGN rapidly in a very short time (within only 1 min) by Joule heating. The two graphs in Fig. 7c quantitatively describe curves of the air plasma treatment time and Joule heating time versus OCGN contact angle values. One of the significant applications of this research is that superhydrophilic OCGN can serve as a rapid drying agent because the shortest propagation time of water droplets on this material is no more than 20 ms. In addition, this excellent graphene material overcomes the shortcomings of a long wettability conversion time and complicated wettability conversion processes and opens up a wide horizon for constructing intelligent graphene materials.
6.2. Acid and alkali regulation
At present, pH-responsive surfaces with adjustable wetting property have been intensively investigated, and can be used in large quantities as controlled separating systems141 and microfluidic systems.142 For instance, Cheng et al.143 reported a neoteric pH-controllable copper mesh film as an oil and water separation film, which was synthesized by first growing nanoparticles on a copper grid and then modifying this with responsive thiol molecules. Specifically, the novel modified copper mesh membrane turned superhydrophobic when the water pH was acidic, then recovering superhydrophilicity after being submerged in an alkaline water environment. In addition, the neoteric pH-controllable copper mesh film meanwhile maintained superoleophobicity when immersed in an alkaline water environment. This copper mesh film thus was able to overcome the difficulties in separation on demand, thus opening up a wide horizon for oil and water separation films. Wang et al.144 reported a reversible pH-responsive wettability surface, which was prepared by the chemisorption of HS(CH2)9CH3 (60%, molar fraction) and HS(CH2)10COOH (40%, molar fraction) on a gold substrate. The pH-responsive wettability surface showed superhydrophobicity when exposed to acidic conditions, and superhydrophilicity after being submerged in an alkaline water environment.Recently, it has been found that the wettability of the graphene surface can also be reversibly switched between hydrophobicity and hydrophilicity by acid and alkali regulation.145 As shown in Fig. 8a, the graphene membrane from a graphene–ethanol suspension exhibited a hydrophobic nature when exposed to an acidic environment, then recovered its hydrophilicity after being submerged in an alkaline environment. Furthermore, the adjustable wetting pH-responsive sensitivity of graphene surface is repetitious. On the one hand, when the pH value is less than 6, the contact angle of graphene surface increases with the increase in pH, and when the pH value is equal to 6, the contact angle of the graphene surface reaches the maximum value. On the other hand, when the pH is more than 6, the contact angle of the graphene surface decreases with the increase in pH (Fig. 8b). This discovery reveals that the graphene surface demonstrates excellent stimuli-responsive wettability with the aid of altering the pH value.
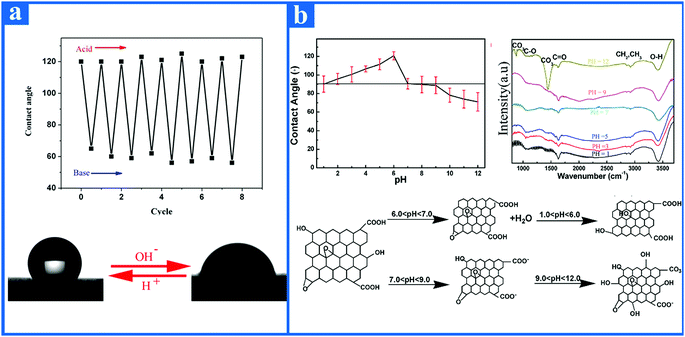 | ||
| Fig. 8 (a) Contact angle of graphene film from graphene–ethanol suspension as a function of the number of repetitions with the aid of alternating regulation of acid and alkali. When the pH is varied from acid to alkali, the graphene film can convert from hydrophobicity to hydrophilicity. Similarly, when the pH is varied from alkali to acid, the graphene film can convert from hydrophilicity to hydrophobicity. (b) The contact angle value of graphene film from graphene–ethanol suspension is different when exposed to different pH conditions. The graph on the right represents the Fourier infrared spectrum of the graphene film from graphene–ethanol suspension under various pH conditions. In order to explain the reason why reversible wettability of graphene film can be tuned by acid and alkali, the alternation mechanism of reversible wettability of graphene films from graphene–ethanol suspension is listed.145 Reproduced from ref. 145 with permission from Applied Physics Letters, copyright [2013]. | ||
The mechanism for the reversible wettability transition of graphene surface in response to acid and alkali is described in detail in this paragraph. As an external stimulus, the pH regulates the wettability of the graphene surface mainly through the acidic functional groups, such as carboxylic acid on the graphene surface. These carboxylic acid groups exhibit two different states of protonation (COOH) and deprotonation (COOH−) as the external pH changes. For acidic aqueous solution, the protonated carboxylic acid on the graphene surface is a relatively hydrophobic group. As the pH of the solution increases, deprotonated carboxylic acid groups with better hydrophilicity are formed on the graphene surface. To further explore the reason why the wettability of the graphene surface can be reversibly regulated by an acid and alkali, an alternation mechanism graph of the reversible wettability of graphene film from a graphene–ethanol suspension is presented in Fig. 8b. It is worth noting that on the graphene surface fabricated by chemical redox treatment inevitably there exist some oxygen-containing functional groups, such as carbonyl groups, carboxylic acid groups, and hydroxyl groups, as can be seen by Fourier transform infrared spectroscopy.146–149 The carboxylic acid groups in these oxygen-containing functional groups spontaneously offer the opportunity for the high pH-responsive behavior of the graphene surface. Therefore, the reason why the wettability of the graphene surface can be reversible switched from hydrophobicity to hydrophilicity by acid and alkali regulation is mainly due to the carbonyl and hydroxyl groups of graphene surface undergoing protonation and deprotonation under both external conditions of an acid and alkali. A graphene surface with an excellent high pH-responsive wettability property opens up broad prospects for the design of many engineered products.
6.3. O3 and annealing treatment
Graphene, a 2D monolayer sheet composed of sp2-hybridized conjugated carbon atoms, can be assembled into multiform 3D carbon macrostructures with very low density, such as 3D foam-like graphene macrostructures,100 3D graphene aerogels,150 3D porous graphene sponges,151 or 3D high-performance self-assembled graphene hydrogels.152 The reason why scattered graphene flakes are capable of forming 3D structures by interstratified arbitrary folding is mainly due to them possessing extraordinary flexibility and strength.153,154 Recently, A 3D cross-linked reduced graphene oxide material (3DG) was fabricated by a modified conventional hydrothermal method that included three specific synthesis steps.134,155 First, GO ethanol solution was solvothermally treated in a polytetrafluoroethylene-lined autoclave at 180 °C for 12 h. Subsequently, the product obtained in the first step was carefully removed from the autoclave and then a solvent exchange reaction with water was performed. Ultimately, the 3DG is obtained by annealing at 800 °C in an argon atmosphere for 1 h.Reduced graphene oxide (RGO) is generally regarded as a sort of chemically derived graphene material.156,157 It also has some other names, such as chemically converted graphene,158 chemically modified graphene,159 functionalized graphene,160 or reduced graphene.161 GO is a chemically modified graphene with a high specific surface area and plentiful oxygen functional groups, such as carboxyl, hydroxy, and epoxy groups.162 These ample oxygen-containing functional groups impart some excellent properties to GO, such as better dispersibility, hydrophilicity, and compatibility. By means of the chemical or thermal reduction of GO, RGO can be prepared without any complicated process. There are significant wettability differences between GO and RGO.163 For example, GO has long been regarded as a hydrophilic material due to the presence of plentiful oxygen-containing functional groups. However, RGO exhibits hydrophobic properties, attributed to the elimination of oxygen-containing functional groups.164 In addition to the wettability distinction, there are other significant differences between GO and RGO. The electrical conductivity of RGO is superior to that of GO. The reason for the poor electrical conductivity of GO is that the introduction of oxygen-containing functional groups destroys the π bonds in the graphene oxide sheet and makes it lose the ability to conduct electrons. Nevertheless, RGO has good electrical conductivity due to elimination of the oxygen-containing functional groups and restoration of the conjugate structure.165 With the help of an optical microscope, it can be seen that the colors of GO and RGO are distinctly different. The RGO film is silver with a metallic luster, while GO film is dark brown.166 A significant microscopic scale difference between GO and RGO deposited on SiO2/Si substrate can also be observed. GO sheets are almost transparent with a very subtle optical contrast with the SiO2/Si substrate, while after reduction, RGO sheets present an enhanced contrast with the substrate.167 With the help of reduction of GO, graphene with a relatively perfect structure and excellent performance can be prepared in large quantities. Different reduction processes lead to diverse properties and ultimately affect the performance of materials or devices consisting of RGO. RGO and RGO are still hot topics in the development and applications of graphene, especially in the large-scale production of graphene. Compared with RGO, GO is more suitable as a component of capacitors due to its good hydrophilicity.
The 3DG material is a kind of unique graphene-based material that is capable of achieving a reversible transition between hydrophobic and hydrophilic by using a periodical alternation of O3 and through annealing treatment.134 As depicted in Fig. 9a, the original hydrophobic 3DG is able to turn into the hydrophilic form with the aid of O3 treatment, and then can recover its hydrophobicity by a simple annealing treatment under an inert atmosphere. The mechanism for the reversible transition between hydrophobic and hydrophilic 3DG by means of air plasma treatment and Joule heating is described in detail. Specifically, when the original hydrophobic 3DG is exposed to an O3 atmosphere environment, O3 molecules are favorably diffused into the interior of the material on account of the high specific surface area and large porosity of 3DG and then they further react with carbon atoms to produce a higher content of oxygen-containing groups, such as ketone groups, hydroxy groups, and esters in the basal and edges of this material. Simultaneously, Gao et al. demonstrated that the reaction of graphene-based materials with O3 molecules results in a high level of oxidation.168 The capillary effect of 3DG's porous topography is also capable of promoting the wetting property of graphene with respect to water after being treated with O3 in an inert atmosphere. On the one hand, once the 3DG material is in contact with water molecules, the abundant oxygen-containing groups of this material quickly form plenty of hydrogen bonds with the water molecules, which increases its hydrophilicity to some extent (Fig. 9b). On the other hand, when the hydrophilic 3DG material is annealed in argon atmosphere, the hydrophilic 3DG quickly restores its hydrophobicity. The fundamental reason for this phenomenon is that annealing treatment removes the enhancive oxygen-containing groups without destroying its intrinsic basic structure, hence recovering the original hydrophobicity of 3DG. The value of ID/IG in the Raman spectrum also demonstrates the recovery from the hydrophilic state to the hydrophobic state of 3DG. In brief, the reversible wettability transformation of this material between hydrophobicity and hydrophilicity is mainly attributed to its change in surface chemical composition.
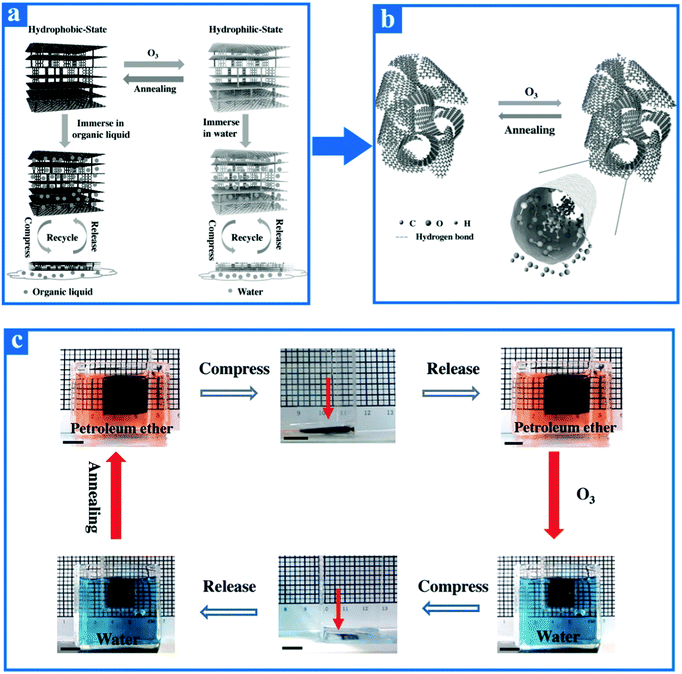 | ||
| Fig. 9 (a) Schematic illustration of reversible wettability transformation and the reversible absorption of 3DG. The original hydrophobic 3DG can turn into hydrophilic via O3 treatment, then recover its hydrophobicity by virtue of a simple annealing treatment under an inert atmosphere. Whether 3DG is in a hydrophobic state or hydrophilic state, it shows highly reproducible and reversible assimilation during constant compression and release processes. (b) Changes in surface chemical composition of 3DG during the reversible transition process. (c) Schematic illustration of the reversible and highly recyclable assimilation process between water and petroleum ether. In order to more clearly observe the experimental phenomenon, the petroleum ether is shown as orange because it is colored by Sudan III, while water is represented as a blue due to it being stained with bromocresol green.134 Reproduced from ref. 134 with permission from Wiley-VCH, copyright [2016]. | ||
To date, the wettability reversible changes of graphene-based materials under hydrophilic and hydrophobic conditions have been extensively reported, but the recyclable, reversible, and large-scale absorption of graphene-based materials under both conditions have rarely been reported. The 3DG material not only exhibits reversible conversion between hydrophilic and hydrophobic conditions by O3 and annealing treatment, but also satisfies the conditions foe a continuous reversible compression-releasing process on account of its Poisson's ratio being close to zero.155,169 As vividly depicted in Fig. 9c, when the petroleum ether-saturated hydrophobic-state 3DG material is compressed, almost of the absorbed petroleum ether is extruded, and then when the material is submerged in petroleum ether once again, it can be totally restored to its initial bulk and will have the same absorption capacity as before. Simultaneously, hydrophilic-state 3DG material also has a reversible and reproducible ability with respect to water during a continuous compression-releasing process. The extremely low density, extremely high porosity, and structural integrity maintained by the 3D cross-linking bonds between graphene sheets result in excellent reproducibility of the 3DG material.155 The 3DG material thus overcomes the shortcoming that in general, a film coated on a substrate cannot reversibly absorb liquid and thus it opens up a broad horizon for large-scale and reversible absorption under both hydrophobic and hydrophilic environments.
6.4. Positive and negative electric field regulation
Beyond that, the graphene surface can also be reversibly switched between hydrophobicity and hydrophilicity under the regulation of positive and negative electric fields.170 The main reason for this phenomenon is that positive and negative electric fields can affect the absorption energy of water molecules on graphene, further influencing the wettability of graphene. The electric field can be used as a powerful and effective means to change the chemical potential energy of the material, and thereby to change the reactivity of the material. For instance, Ao et al.171 reported that exerting an electric field perpendicular to the graphene surface can reduce the absorption energy of hydrogen on the graphene surface. Ao et al.172 further explained this phenomenon in another article in that a negative perpendicular electric field can serve as a catalyst and decreases the absorption energy of hydrogen on the graphene surface. By virtue of density functional theory, Medlin et al.173 found that a negative electric field increased the absorption energy of oxygen molecule on Pt(111), whereas a positive electric field reduced the adsorption energy.In order to better understand how the positive and negative electric field affect the absorption energy of water on graphene, Jiang et al.170 first discussed the optimal adsorption position of water molecules on graphene and then used a formula to calculate the adsorption energy of water molecules on graphene. It is shown in Fig. 10a that top, bridge, and hollow are three possible absorption positions of water molecules on the graphene surface.174 The adsorption energy of water molecules on graphene can be calculated by the following equation:
| Ead = EH2O/graphene − (Egraphene − EH2O) |
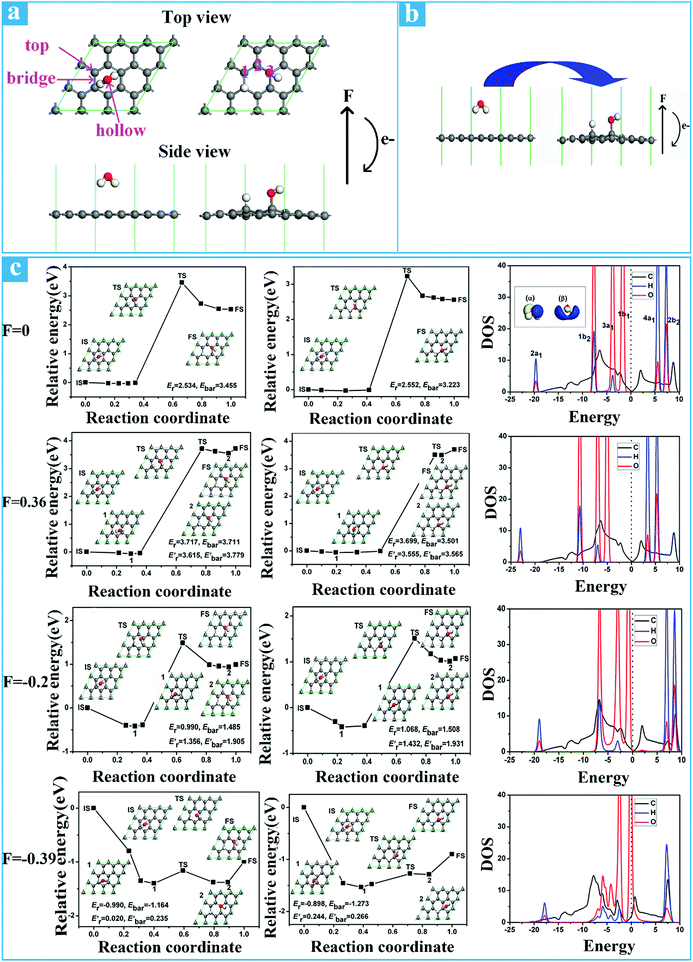 | ||
| Fig. 10 (a) The initial state and final state of water molecules absorbed on the graphene surface. Top, bridge, and hollow are three possible absorption positions of water molecules on graphene surface. (b) By manipulating the electric field, which is opposite to the direction of electron flow, a water molecule is easily absorbed on the graphene surface. (c) Two interaction paths of water molecule on graphene under different electric fields. The four pictures on the right are the PDOS of hydrogen atoms, oxygen atoms, and carbon atoms at different electric field intensities.170 Reproduced from ref. 170 with permission from the American Chemical Society, copyright [2012]. | ||
The results indicate that the hollow sites with two OH bonds pointing down to the graphene surface is the optimum absorption position of a water molecule on graphene (Fig. 10b).175Fig. 10c exhibits two interaction paths of a water molecule on graphene and the partial density of states (PDOS) of hydrogen atoms, oxygen atoms, and carbon atoms under different electric fields. Water molecules first need to overcome energy barriers and to be decomposed into hydrogen atoms and hydroxyl groups, and then the hydrogen atoms and hydroxyl groups are respectively absorbed on carbon atoms of graphene. By virtue of analyzing the PDOS, the effect of F on the adsorption energy barrier can be obtained by considering the interactions between H2O and graphene bands. The interactions between graphene and water molecules are strongly dependent on the forces of the H-s and C-p bands. When no electric field is applied, the interaction energy between carbon and hydrogen is exceedingly weak, resulting in a large energy barrier for the decomposition of water molecules on the graphene surface. When a negative electric field is applied, the peak of hydroxyl band shifts to the right, which indicates that the influence of the hydroxyl bond is weakened and the decomposition of water molecules becomes easier than that without the negative electric field. In addition, the negative electric field can convert the endothermic reaction into an exothermic reaction. When the applied voltage is low enough, water molecules can be decomposed into hydrogen atoms and hydroxyl groups automatically. Therefore, graphene can be transformed from hydrophobic to hydrophilic by the effect of the hydroxyl groups. In brief, the negative electric field can act as a catalyst to promote the decomposition adsorption of water molecules on the graphene, which makes the graphene become more hydrophilic. On the contrary, when a positive electric field is applied, the peak of hydroxyl band shifts to the left, which indicates that the influence of hydroxyl bond is strengthened and the decomposition of water molecules becomes more difficult than that without the negative electric field. The positive electric field is favorable to promote the desorption of hydrogen atoms and hydroxyl groups on the graphene surface, which makes the graphene become more hydrophobic. In other words, the wettability of the graphene film surface can be controllably switched from hydrophobic to hydrophilic by positive and negative electric field regulation.
Another representative example of how the wettability property of graphene can be reversibly transformed with the aid of positive and negative electric bias voltage regulation was demonstrated by Jeon et al.176 It is shown in Fig. 11a that oxygen atoms can be evenly distributed on single-layer graphene by thermal oxidation. When a positive electric bias voltage is exerted, a large number of oxygen atoms migrate near the positive voltage, which makes graphene more hydrophilic. On the contrary, when a negative electric bias voltage is exerted, abundant oxygen atoms deviate from the position where the negative electric bias voltage is applied, which results in hydrophobic graphene (Fig. 11b). The reason why the wettability of single-layer graphene can be reversibly converted under positive and negative electric bias voltage regulation is mainly attributed to the migration of oxygen atoms and is not ascribed to any kind of electrochemical redox reaction (Fig. 11c).175,177 The location and coverage of oxygen atoms are controlled by the positive and negative electric bias voltage, which determines the degree of graphene wettability. The reversible variation of oxygen atoms coverage under positive and negative electric bias voltage regulation demonstrates that oxygen atoms move from the negative potential location to the positive potential location. The fundamental cause for this is primarily that oxygen atoms obtain the drift speed from the kinetic momentum and move toward the orientation of the electron motion according to the electromigration theory.178,179 These findings provide the opportunity to reversibly change the wettability of the graphene surface by regulation under a positive and negative electric field, which is of importance for the movement of droplets on the graphene surface.
 | ||
| Fig. 11 (a) Single-layer graphene deposited on a SiO2/p-type silicon substrate becomes graphene oxide by virtue of thermal oxidization under a mixture of argon and air. Oxygen atoms are evenly distributed on single-layer graphene. (b) When a positive or negative electric bias voltage is applied, a large number of absorbed oxygen atoms migrate to the position where a positive electric bias voltage is applied. (c) Graphene undergoes a reversible transition from hydrophobic to hydrophilic or from hydrophilic to hydrophobic by positive and negative electric bias voltage regulation.176 Reproduced from ref. 176 with permission from Wiley-VCH, copyright [2016]. | ||
6.5. UV irradiation and dark storage
UV irradiation and dark storage can serve as two external conditions to reversibly change the wettability of some material surface. For instance, the wettability of many transition metal oxides, such as Fe2O3, V2O5, and WO3, can be reversibly transformed under UV irradiation and dark storage.147,148,180 Carbon nanotubes, a member of the carbon nanomaterial family, also have tunable surface wettability in response to alternative UV irradiation and dark storage.181 Similarly, graphene as a carbon nanomaterial also exhibits the same wettability transformation behavior. The reversible switching between superhydrophobicity and superhydrophilicity of graphene films under alternative UV illumination and dark storage has been carefully explored.135 Although the reversible transformation of graphene wettability has been previously reported, the control conditions are alternative UV irradiation and air storage.136,137 For example, graphene film from graphene–ethanol suspensions exhibit excellent reversible wettability transformation between hydrophobicity and hydrophilicity by virtue of recurrent variations of UV irradiation and air storage.136 Zhang et al. further explained the reason why the wettability of graphene can be reversibly converted in response to alternative UV irradiation and air storage.136 Graphene is favorable to assimilate substantial oxygen molecules on account of its high specific area. With the aid of UV irradiation, the oxygen molecules on graphene are motivated from the ground spin triplet state to a higher energy spin singlet, which leads to a series of hydrophilic groups being created on the graphene surface. When graphene is exposed to an air environment, the oxygen molecules will gradually replace water molecules absorbed on graphene, which make the graphene surface more hydrophilic. Later, Huang et al. reported that the reversible wettability variation of graphene between hydrophobic and hydrophilic can be ascribed to the dissociative adsorption of water molecules on graphene rather than a dissociative process involving the chemical dissociation adsorption of oxygen molecules.135Graphene films derived from THF, water, and DMF suspensions exhibit reversible wettability transformation between hydrophobicity and hydrophilicity by virtue of recurrent variations of UV irradiation and air storage (Fig. 12a).135 In addition, the reversible wettability change process of graphene is repeatable (Fig. 12b). After several periodic hydrophobic and hydrophilic transitions, the contact angle of water on graphene remains almost constant. Fig. 12c shows the contact angle of the graphene surface as a function of dark storage time and UV exposure time. It is clear that graphene can quickly become hydrophilic within the first 30 min of UV irradiation. Compared with the wettability alternation from hydrophobic to hydrophilic, the recovery procedure of graphene hydrophobicity takes more time.
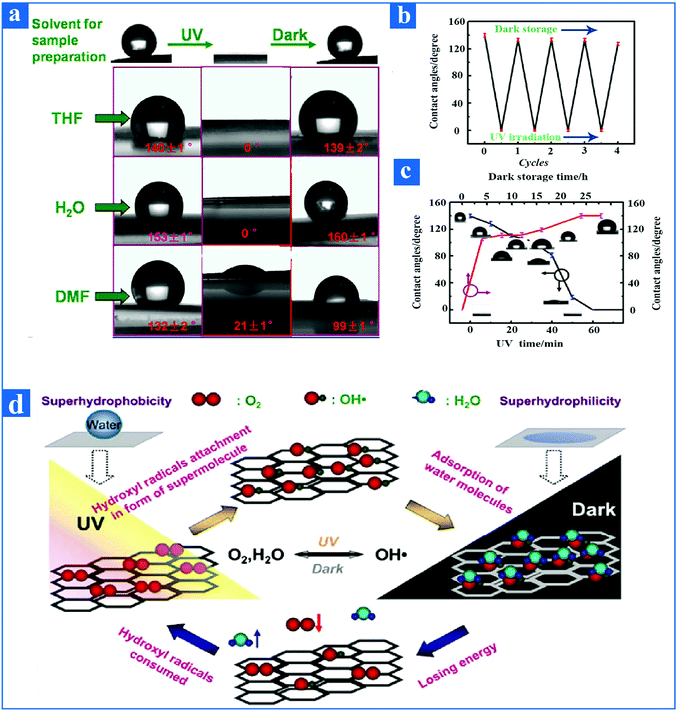 | ||
| Fig. 12 (a) Graphene film exhibiting reversible wettability transformation between hydrophobicity and hydrophilicity by recurrent variations of UV irradiation and air storage. Graphene films derived from THF, water, and DMF suspensions respectively. (b) The reversible wettability change process of graphene from THF solution is repeatable. (c) The contact angle of the graphene surface as a function of dark storage time and UV exposure time. (d) The reaction mechanism graphics of the reversible wettability variation of graphene surface.135 Reproduced from ref. 135 with permission from Springer Link, copyright [2014]. | ||
The reaction mechanism graphics of the reversible wettability variation of graphene surface are schematically drawn in Fig. 12d. With the aid of UV irradiation, the oxygen molecules on graphene are motivated from the ground spin triplet state to a higher energy spin singlet, resulting in electron (e−)–hole (h+) pairs.182,183 Superoxide radicals are formed through the reaction of oxygen molecules and electrons. The depletion of a large number of oxygen molecules leads to a continuous diffusion of oxygen vacancies on the graphene surface. Water molecules in the air capture vacancies to generate hydroxyl free radicals. More specifically, hydroxyl free radicals are generated through two methods: one is through the hydroxyl bond to capture vacancies, the other is through the reaction of superoxide radicals with water molecules. The hydroxyl free radicals are attached to the carbon atom of the graphene in a supramolecular form rather than in the form of a covalent bond, which converts the original superhydrophobic graphene into hydrophilicity.184,185 The work of Duong et al.186 confirmed the opinion that hydroxyl free radicals are attached to sp2-carbon in a supramolecular form in the case of UV irradiation. The adsorption of water molecules on graphene defect surface further imparts it with superhydrophilicity. Under dark storage, these supramolecules gradually disappear on account of a lack of adequate energy supply.186 Oxygen molecules substitute for water molecules deposited on the graphene defect surface, which induces the return of superhydrophobic graphene. It is worth noting that if the hydroxyl free radicals are attached to the carbon atom of graphene in the form of a covalent bond, the hydroxyl free radicals will not disappear in the absence of energy. For example, the hydroxyl groups on GO are covalently bonded to carbon atoms. After a long period of dark storage, the GO surface is not capable of converting into the superhydrophobic form on account of the presence of hydroxyl groups. The following equations can be used to describe the reaction mechanisms:
| graphene + hν → graphene (e− + h+) |
| O2 + e− ↔ O2−˙ |
| h+ + OH− ↔ OH˙ |
| 2O2−˙ + 2H2O → 2OH˙ + 2OH− + O2 |
In short, graphene is capable of undergoing reversible wettability transition between superhydrophilic and superhydrophobic by using a cyclical transformation of UV irradiation and dark storage. From the above formulas and mechanism diagram, it can be seen that the switching manner of wettability is closely related to the attachment and detachment of hydroxyl radicals on the graphene surface.
7. Conclusions
As a member of carbon material family, graphene has long been the focus of research on account of its abundant excellent properties. Nevertheless, a variety of previous research works attached much importance to its mechanical capacity and electrical properties rather than to its surface wetting properties with respect to water. However, it is particularly vital to study the wettability of graphene as graphene with a hydrophobic property can reduce liquid deposition and prevent contamination during the fabrication of electronic devices, while the hydrophilic surface of graphene has important applications in biomaterials and microfluids.In this review, we comprehensively discussed the wetting behavior of water droplets on the graphene surface. First, we summarized a series of contact angle values of single-layer graphene with respect to water as determined by various methods, such as experimental measurements, classic molecular dynamics simulations, and formula calculations. As a result, the contact angle of water droplets on single-layer graphene does not have a definite value, albeit in general we can say that graphene possesses outstanding hydrophobic properties on account of its slightly higher contact angle. However, the challenge that graphene is intrinsically hydrophilic with a water contact angle of ∼45° has recently been put forward.118,187,188 There exist two reasons for this unique phenomenon. One reason is that the adsorption of hydrocarbons in the air impart hydrophobicity to graphene, which covers the original hydrophilicity of graphene. Another reason is that single-layer graphene is more prone to hydrophobicity but multilayer graphene is more inclined to represent hydrophilicity. The higher contact angle value of water droplets on monolayer graphene is ascribed to its lower surface tensions, whereas the addition of one or two graphitic layers to single-layer graphene increases the total vdW interaction potential and thus reduces the contact angle. The above report on the hydrophilicity of graphene is attributed to the fact that the measured substrate is multilayer graphene. Subsequently, the wetting transparency of graphene, involving the transmission of the substrate wetting property over a graphene coating, has aroused great interest on account of its versatility for many potential applications. A graphene coating can prevent copper oxidation without destroying the original wetting properties of the surface based on this specific property. This excellent capacity to regulate the surface properties without disrupting their wetting properties has enormous potential in the design of conductive and conformal surface coatings. Next, several classic factors that affect the wettability of graphene were presented in detail, such as low-density defects and high-density defects, controlled atmosphere, doping and electric field, all of which rarely have been put covered in other review articles before. Because graphene is inevitably in contact with the electrolyte solution, it is crucial to learn more about the factors that affect the wettability of graphene. Finally, the wettability reversible switching of the graphene surface under various external stimuli was summarized, which is important for building a smart graphene material surface.
The special superhydrophobic property of the lotus leaf surface is mainly ascribed to the combination of a micro/nanoscale layered structure and waxy nanocrystalline formation on top of the papillae. Enlightened by this natural phenomenon, the wettability concept with regard to nanoscale control and nanoscale fabrication has been widely used to fabricate graphene material surfaces with special wettability.57,189 Nanoscale manufacturing processes are generally categorized into two procedures: first, a series of micro/nanostructures are generated on the material surface with the aid of using some particles or polymers. Regulating the nanoscale structure of material is capable of altering the wettability of material surface effectively.190 Hence, a superhydrophobic material surface can be prepared by means of creating a rough surface with architectures at the micro/nanoscale. Second, surface chemistry is also essential in adjusting the range and size of wettability interactions on a material surface. The introduction of nanoscale chemical heterogeneity can effectively reduce the hydrophobicity of the material surface, while the addition of low surface energy substances can increase the hydrophobicity of the material surface.191,192
In brief, it is particularly vital to study the wettability of graphene. A graphene sample with a high density of defects is capable of overcoming the difficulties involved in the contact with electrolyte solution and opens up a wide horizon for ultrathin high-performance supercapacitor electrodes. In addition, the wettability of a graphene surface can be changed by doping, which not only provides a feasible path for the fabrication of a graphene surface coating with tunable wettability, but also brings new opportunities for the development of electrowetting displays without an applied current, which will make a significant contribution in the energy conservation field.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Nature Science Foundation of China (No. 51522510, 51675513, and 51735013).Notes and references
- L.-Y. Meng and S.-J. Park, Carbon Lett., 2014, 15, 89–104 CrossRef.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306(5696), 666–669 CrossRef CAS PubMed.
- C. Wang, L. Zhang, Z. Guo, J. Xu, H. Wang, K. Zhai and X. Zhuo, Microchim. Acta, 2010, 169, 1–6 CrossRef CAS.
- Y. Zhang, Y.-W. Tan, H. L. Stormer and P. Kim, Nature, 2005, 438, 201 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, M. I. Katsnelson, I. V. Grigorieva, S. V. Dubonos and A. A. Firsov, Nature, 2005, 438, 197 CrossRef CAS PubMed.
- H. Malekpour, K. H. Chang, J. C. Chen, C. Y. Lu, D. L. Nika, K. S. Novoselov and A. A. Balandin, Nano Lett., 2014, 14, 5155–5161 CrossRef CAS PubMed.
- A. A. Balandin, S. Ghosh, W. Bao, I. Calizo, D. Teweldebrhan, F. Miao and C. N. Lau, Nano Lett., 2008, 8, 902–907 CrossRef CAS PubMed.
- J. Wu, H. A. Becerril, Z. Bao, Z. Liu, Y. Chen and P. Peumans, Appl. Phys. Lett., 2008, 92, 263302 CrossRef.
- Y. Zhu, S. Murali, W. Cai, X. Li, J. W. Suk, J. R. Potts and R. S. Ruoff, Adv. Mater., 2010, 22, 3906–3924 CrossRef CAS PubMed.
- J. N. Wang, Y. L. Zhang, Y. Liu, W. Zheng, L. P. Lee and H. B. Sun, Nanoscale, 2015, 7, 7101–7114 RSC.
- H. Chang, G. Wang, A. Yang, X. Tao, X. Liu, Y. Shen and Z. Zheng, Adv. Funct. Mater., 2010, 20, 2893–2902 CrossRef CAS.
- S. C. Sahu, A. K. Samantara, M. Seth, S. Parwaiz, B. P. Singh, P. C. Rath and B. K. Jena, Electrochem. Commun., 2013, 32, 22–26 CrossRef CAS.
- X. Xu, D. Yi, Z. Wang, J. Yu, Z. Zhang, R. Qiao, Z. Sun, Z. Hu, P. Gao, H. Peng, Z. Liu, D. Yu, E. Wang, Y. Jiang, F. Ding and K. Liu, Adv. Mater., 2018, 30, 1702944 CrossRef PubMed.
- S.-H. Bae, Y. Lee, B. K. Sharma, H.-J. Lee, J.-H. Kim and J.-H. Ahn, Carbon, 2013, 51, 236–242 CrossRef CAS.
- Q. Bao and K. P. Loh, ACS Nano, 2012, 6, 3677–3694 CrossRef CAS PubMed.
- S. Jang, E. Hwang, Y. Lee, S. Lee and J. H. Cho, Nano Lett., 2015, 15, 2542–2547 CrossRef CAS PubMed.
- F. Bonaccorso, Z. Sun, T. Hasan and A. C. Ferrari, Nat. Photonics, 2010, 4, 611 CrossRef CAS.
- M. J. Allen, V. C. Tung and R. B. Kaner, Chem. Rev., 2010, 110, 132–145 CrossRef CAS PubMed.
- X. Li, L. Li, Y. Wang, H. Li and X. Bian, J. Phys. Chem. C, 2013, 117, 14106–14112 CrossRef CAS.
- C. Melios, C. E. Giusca, V. Panchal and O. Kazakova, 2D Mater., 2018, 5, 022001 CrossRef.
- Y. Wang, S. Sinha, L. Hu and S. Das, Phys. Chem. Chem. Phys., 2017, 19, 27421–27434 RSC.
- F. Du, J. Huang, H. Duan, C. Xiong and J. Wang, Appl. Surf. Sci., 2018, 454, 249–255 CrossRef CAS.
- Y. Y. Song, Y. Liu, H. B. Jiang, S. Y. Li, C. Kaya, T. Stegmaier, Z. W. Han and L. Q. Ren, Nanoscale, 2018, 10, 3813–3822 RSC.
- A. V. Prydatko, L. A. Belyaeva, L. Jiang, L. M. C. Lima and G. F. Schneider, Nat. Commun., 2018, 9, 4185 CrossRef PubMed.
- A. Khan, M. R. Habib, R. R. Kumar, S. M. Islam, V. Arivazhagan, M. Salman, D. Yang and X. Yu, J. Mater. Chem. A, 2018, 6, 22437–22464 RSC.
- M. Wang, Y. Zhao, L.-D. Wang, Y.-P. Zhu, X.-J. Wang, J. Sheng, Z.-Y. Yang, H.-L. Shi, Z.-D. Shi and W.-D. Fei, Carbon, 2018, 139, 954–963 CrossRef CAS.
- L. Zhong, H. Zhu, Y. Wu and Z. Guo, J. Colloid Interface Sci., 2018, 525, 234–242 CrossRef CAS PubMed.
- J. Li and Z. Guo, Nanoscale, 2018, 10, 13814–13831 RSC.
- X. Jing and Z. Guo, J. Mater. Chem. A, 2018, 6, 16731–16768 RSC.
- C. Huang and Z. Guo, Nanoscale, 2018, 10, 19659–19672 RSC.
- W. Zhang, N. Liu, Q. Zhang, R. Qu, Y. Liu, X. Li, Y. Wei, L. Feng and L. Jiang, Angew. Chem., Int. Ed., 2018, 57, 5740–5745 CrossRef CAS PubMed.
- Y. Peng and Z. Guo, J. Mater. Chem. A, 2016, 4, 15749–15770 RSC.
- Y. Peng, F. Guo, Q. Wen, F. Yang and Z. Guo, Sep. Purif. Technol., 2017, 184, 72–78 CrossRef CAS.
- B. B. Rich and B. Pokroy, Soft Matter, 2018, 14, 7782–7792 RSC.
- S. S. Latthe, P. Sudhagar, A. Devadoss, A. M. Kumar, S. Liu, C. Terashima, K. Nakata and A. Fujishima, J. Mater. Chem. A, 2015, 3, 14263–14271 RSC.
- Y. Gao, J. Wang, W. Xia, X. Mou and Z. Cai, ACS Sustainable Chem. Eng., 2018, 6, 7216–7220 CrossRef CAS.
- Z. Yu, F. F. Yun, Y. Wang, L. Yao, S. Dou, K. Liu, L. Jiang and X. Wang, Small, 2017, 13, 1701403 CrossRef PubMed.
- T. Adachi, S. S. Latthe, S. W. Gosavi, N. Roy, N. Suzuki, H. Ikari, K. Kato, K.-I. Katsumata, K. Nakata, M. Furudate, T. Inoue, T. Kondo, M. Yuasa, A. Fujishima and C. Terashima, Appl. Surf. Sci., 2018, 458, 917–923 CrossRef CAS.
- J. Wang, Y. Zhang, S. Wang, Y. Song and L. Jiang, Acc. Chem. Res., 2011, 44, 405–415 CrossRef CAS PubMed.
- J. Wang, Y. Wen, J. Hu, Y. Song and L. Jiang, Adv. Funct. Mater., 2007, 17, 219–225 CrossRef CAS.
- Q. Li and Z. Guo, J. Colloid Interface Sci., 2019, 536, 507–515 CrossRef CAS PubMed.
- J. Liu, Z. Xie, Y. Shang, J. Ren, R. Hu, B. Guan, J. Wang, T. Ikeda and L. Jiang, ACS Appl. Mater. Interfaces, 2018, 10, 6701–6710 CrossRef CAS PubMed.
- D. D. Nguyen, N.-H. Tai, S.-B. Lee and W.-S. Kuo, Energy Environ. Sci., 2012, 5, 7908 RSC.
- Z. Chen, L. Dong, D. Yang and H. Lu, Adv. Mater., 2013, 25, 5352–5359 CrossRef CAS PubMed.
- H.-B. Jiang, Y.-L. Zhang, D.-D. Han, H. Xia, J. Feng, Q.-D. Chen, Z.-R. Hong and H.-B. Sun, Adv. Funct. Mater., 2014, 24, 4595–4602 CrossRef CAS.
- Y. Lin, G. J. Ehlert, C. Bukowsky and H. A. Sodano, ACS Appl. Mater. Interfaces, 2011, 3, 2200–2203 CrossRef CAS PubMed.
- I. Mata-Cruz, A. Vargas-Caamal, B. Yañez-Soto, A. López-Valdivieso, G. Merino and M. Quintana, Carbon, 2017, 121, 472–478 CrossRef CAS.
- L. Tie, J. Li, M. Liu, Z. Guo, Y. Liang and W. Liu, J. Mater. Chem. A, 2018, 6, 11682–11687 RSC.
- Q. Li and Z. Guo, J. Mater. Chem. A, 2018, 6, 13549–13581 RSC.
- S. Tian, L. Li, W. Sun, X. Xia, D. Han, J. Li and C. Gu, Sci. Rep., 2012, 2, 511 CrossRef PubMed.
- L. L. Zhang and X. S. Zhao, Chem. Soc. Rev., 2009, 38, 2520–2531 RSC.
- B. Bouali, F. Ganachaud, J.-P. Chapel, C. Pichot and P. Lanteri, J. Colloid Interface Sci., 1998, 208, 81–89 CrossRef CAS PubMed.
- Y. Zhao, G. Wang, W. Huang, X. Fan, Y. Deng, J. Zhang, T. Wei, R. Duan, J. Wang and L. Sun, RSC Adv., 2016, 6, 1999–2003 RSC.
- H. Lin, A. Schilo, A. R. Kamoka, N. Severin, I. M. Sokolov and J. P. Rabe, Phys. Rev. B, 2017, 95, 195414 CrossRef.
- Z. Li, Y. Wang, A. Kozbial, G. Shenoy, F. Zhou, R. McGinley, P. Ireland, B. Morganstein, A. Kunkel, S. P. Surwade, L. Li and H. Liu, Nat. Mater., 2013, 12, 925–931 CrossRef CAS PubMed.
- F. Taherian, V. Marcon, N. F. A. van der Vegt and F. Leroy, Langmuir, 2013, 29, 1457–1465 CrossRef CAS PubMed.
- Z. Bo, Y. Tian, Z. J. Han, S. Wu, S. Zhang, J. Yan, K. Cen and K. Ostrikov, Nanoscale Horiz., 2017, 2, 89–98 RSC.
- S. Ghosh, X. An, R. Shah, D. Rawat, B. Dave, S. Kar and S. Talapatra, J. Phys. Chem. C, 2012, 116, 20688–20693 CrossRef CAS.
- D. J. Preston, D. L. Mafra, N. Miljkovic, J. Kong and E. N. Wang, Nano Lett., 2015, 15, 2902–2909 CrossRef CAS PubMed.
- J. Rafiee, X. Mi, H. Gullapalli, A. V. Thomas, F. Yavari, Y. Shi, P. M. Ajayan and N. A. Koratkar, Nat. Mater., 2012, 11, 217 CrossRef CAS PubMed.
- F. Mugele, Nat. Mater., 2012, 11, 182 CrossRef CAS PubMed.
- C.-J. Shih, Q. H. Wang, S. Lin, K.-C. Park, Z. Jin, M. S. Strano and D. Blankschtein, Phys. Rev. Lett., 2012, 109, 176101 CrossRef PubMed.
- G.-T. Kim, S.-J. Gim, S.-M. Cho, N. Koratkar and I.-K. Oh, Adv. Mater., 2014, 26, 5166–5172 CrossRef CAS PubMed.
- J. L. Qi, X. Wang, J. H. Lin, F. Zhang, J. C. Feng and W.-D. Fei, Nanoscale, 2015, 7, 3675–3682 RSC.
- M. D. Stoller, S. Park, Y. Zhu, J. An and R. S. Ruoff, Nano Lett., 2008, 8, 3498–3502 CrossRef CAS PubMed.
- D. Cohen-Tanugi and J. C. Grossman, Nano Lett., 2012, 12, 3602–3608 CrossRef CAS PubMed.
- H. Kim, A. A. Abdala and C. W. Macosko, Macromolecules, 2010, 43, 6515–6530 CrossRef CAS.
- S. Wang, Y. Zhang, N. Abidi and L. Cabrales, Langmuir, 2009, 25, 11078–11081 CrossRef CAS PubMed.
- Y. J. Shin, Y. Wang, H. Huang, G. Kalon, A. T. S. Wee, Z. Shen, C. S. Bhatia and H. Yang, Langmuir, 2010, 26, 3798–3802 CrossRef CAS PubMed.
- H. Li and X. C. Zeng, ACS Nano, 2012, 6, 2401–2409 CrossRef CAS PubMed.
- R. Raj, S. C. Maroo and E. N. Wang, Nano Lett., 2013, 13, 1509–1515 CrossRef CAS PubMed.
- T. Ondarçuhu, V. Thomas, M. Nuñez, E. Dujardin, A. Rahman, C. T. Black and A. Checco, Sci. Rep., 2016, 6, 24237 CrossRef PubMed.
- Z. Zhang and T. Li, J. Appl. Phys., 2011, 110, 083526 CrossRef.
- H. C. Hamaker, Physica, 1937, 4, 1058–1072 CrossRef CAS.
- D. D. L. Chung, Asian J. Mater. Sci., 2002, 37, 1475–1489 CrossRef CAS.
- D. Kim, N. M. Pugno, M. J. Buehler and S. Ryu, Sci. Rep., 2015, 5, 15526 CrossRef CAS PubMed.
- B. Bouali, F. Ganachaud, J.-P. Chapel, C. Pichot and P. Lanteri, J. Colloid Interface Sci., 1998, 208, 81–89 CrossRef CAS PubMed.
- D. B. Hough and L. R. White, Adv. Colloid Interface Sci., 1980, 14, 3–41 CrossRef CAS.
- C. J. Drummond and D. Y. C. Chan, Langmuir, 1997, 13, 3890–3895 CrossRef CAS.
- B. Ramos-Alvarado, S. Kumar and G. P. Peterson, J. Chem. Phys., 2016, 144, 014701 CrossRef PubMed.
- A. Srivastava, C. Galande, L. Ci, L. Song, C. Rai, D. Jariwala, K. F. Kelly and P. M. Ajayan, Chem. Mater., 2010, 22, 3457–3461 CrossRef CAS.
- J. Driskill, D. Vanzo, D. Bratko and A. Luzar, J. Chem. Phys., 2014, 141, 18C517 CrossRef PubMed.
- E. Singh, A. V. Thomas, R. Mukherjee, X. Mi, F. Houshmand, Y. Peles, Y. Shi and N. Koratkar, ACS Nano, 2013, 7, 3512–3521 CrossRef CAS PubMed.
- C. J. Shih, M. S. Strano and D. Blankschtein, Nat. Mater., 2013, 12, 866–869 CrossRef CAS PubMed.
- J. Y. Wang, S. Betelu and B. M. Law, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2001, 63, 031601 CrossRef CAS PubMed.
- R. Seemann, S. Herminghaus and K. Jacobs, Phys. Rev. Lett., 2001, 86, 5534–5537 CrossRef CAS PubMed.
- C. Huang, F. Xu and Y. Sun, Comput. Mater. Sci., 2017, 139, 216–224 CrossRef CAS.
- A. Malesevic, R. Vitchev, K. Schouteden, A. Volodin, L. Zhang, G. V. Tendeloo, A. Vanhulsel and C. V. Haesendonck, Nanotechnol., 2008, 19, 305604 CrossRef PubMed.
- D. H. Seo, S. Yick, Z. J. Han, J. H. Fang and K. K. Ostrikov, ChemSusChem, 2014, 7, 2317–2324 CrossRef CAS PubMed.
- R. A. Quinlan, M. Cai, R. A. Outlaw, S. M. Butler, J. R. Miller and A. N. Mansour, Carbon, 2013, 64, 92–100 CrossRef CAS.
- B. Jia and L. Zou, Chem. Phys. Lett., 2012, 548, 23–28 CrossRef CAS.
- L. X. Zhang, Z. Sun, J. L. Qi, J. M. Shi, T. D. Hao and J. C. Feng, Carbon, 2016, 103, 339–345 CrossRef CAS.
- J.-H. Deng, R.-T. Zheng, Y. Zhao and G.-A. Cheng, ACS Nano, 2012, 6, 3727–3733 CrossRef CAS PubMed.
- H. C. Schniepp, J.-L. Li, M. J. McAllister, H. Sai, M. Herrera-Alonso, D. H. Adamson, R. K. Prud'homme, R. Car, D. A. Saville and I. A. Aksay, J. Phys. Chem. B, 2006, 110, 8535–8539 CrossRef CAS PubMed.
- C. Berger, Z. Song, T. Li, X. Li, A. Y. Ogbazghi, R. Feng, Z. Dai, A. N. Marchenkov, E. H. Conrad, P. N. First and W. A. de Heer, J. Phys. Chem. B, 2004, 108, 19912–19916 CrossRef CAS.
- P. R. Somani, S. P. Somani and M. Umeno, Chem. Phys. Lett., 2006, 430, 56–59 CrossRef CAS.
- A. N. Obraztsov, A. A. Zolotukhin, A. O. Ustinov, A. P. Volkov, Y. Svirko and K. Jefimovs, Diamond Relat. Mater., 2003, 12, 917–920 CrossRef CAS.
- Y. Pan, H. Zhang, D. Shi, J. Sun, S. Du, F. Liu and H.-J. Gao, Adv. Mater., 2009, 21, 2777–2780 CrossRef CAS.
- J. Lin, Z. Peng, Y. Liu, F. Ruiz-Zepeda, R. Ye, E. L. G. Samuel, M. J. Yacaman, B. I. Yakobson and J. M. Tour, Nat. Commun., 2014, 5, 5714 CrossRef CAS PubMed.
- Z. Chen, W. Ren, L. Gao, B. Liu, S. Pei and H.-M. Cheng, Nat. Mater., 2011, 10, 424 CrossRef CAS PubMed.
- X. Wang, Y. Zhang, C. Zhi, X. Wang, D. Tang, Y. Xu, Q. Weng, X. Jiang, M. Mitome, D. Golberg and Y. Bando, Nat. Commun., 2013, 4, 2905 CrossRef PubMed.
- J. R. Miller, R. A. Outlaw and B. C. Holloway, Science, 2010, 329, 1637–1639 CrossRef CAS PubMed.
- Z. S. Wu, A. Winter, L. Chen, Y. Sun, A. Turchanin, X. Feng and K. Mullen, Adv. Mater., 2012, 24, 5130–5135 CrossRef CAS PubMed.
- M. F. El-Kady, V. Strong, S. Dubin and R. B. Kaner, Science, 2012, 335, 1326–1330 CrossRef CAS PubMed.
- X. Yang, C. Cheng, Y. Wang, L. Qiu and D. Li, Science, 2013, 341, 534–537 CrossRef CAS PubMed.
- Y. Li, D. X. Luong, J. Zhang, Y. R. Tarkunde, C. Kittrell, F. Sargunaraj, Y. Ji, C. J. Arnusch and J. M. Tour, Adv. Mater., 2017, 29, 1700496 CrossRef PubMed.
- R. Ye, Y. Chyan, J. Zhang, Y. Li, X. Han, C. Kittrell and J. M. Tour, Adv. Mater., 2017, 29, 1702211 CrossRef PubMed.
- Z. Peng, R. Ye, J. A. Mann, D. Zakhidov, Y. Li, P. R. Smalley, J. Lin and J. M. Tour, ACS Nano, 2015, 9, 5868–5875 CrossRef CAS PubMed.
- R. Ye, Z. Peng, T. Wang, Y. Xu, J. Zhang, Y. Li, L. G. Nilewski, J. Lin and J. M. Tour, ACS Nano, 2015, 9, 9244–9251 CrossRef CAS PubMed.
- C. Berger, Z. Song, X. Li, X. Wu, N. Brown, C. Naud, D. Mayou, T. Li, J. Hass, A. N. Marchenkov, E. H. Conrad, P. N. First and W. A. de Heer, Science, 2006, 312, 1191–1196 CrossRef CAS PubMed.
- H. Liu, Y. Liu and D. Zhu, J. Mater. Chem., 2011, 21, 3335–3345 RSC.
- F. Huttmann, A. J. Martínez-Galera, V. Caciuc, N. Atodiresei, S. Schumacher, S. Standop, I. Hamada, T. O. Wehling, S. Blügel and T. Michely, Phys. Rev. Lett., 2015, 115, 236101 CrossRef PubMed.
- T. O. Wehling, K. S. Novoselov, S. V. Morozov, E. E. Vdovin, M. I. Katsnelson, A. K. Geim and A. I. Lichtenstein, Nano Lett., 2008, 8, 173–177 CrossRef CAS PubMed.
- N. Jung, N. Kim, S. Jockusch, N. J. Turro, P. Kim and L. Brus, Nano Lett., 2009, 9, 4133–4137 CrossRef CAS PubMed.
- J. Choi, H. Lee, K.-J. Kim, B. Kim and S. Kim, J. Phys. Chem. Lett., 2010, 1, 505–509 CrossRef CAS.
- X. Dong, D. Fu, W. Fang, Y. Shi, P. Chen and L. J. Li, Small, 2009, 5, 1422–1426 CrossRef CAS PubMed.
- P. Hyesung, A. R. Jill, K. Ki Kang, B. Vladimir and K. Jing, Nanotechnol., 2010, 21, 505204 CrossRef PubMed.
- G. Hong, Y. Han, T. M. Schutzius, Y. Wang, Y. Pan, M. Hu, J. Jie, C. S. Sharma, U. Müller and D. Poulikakos, Nano Lett., 2016, 16, 4447–4453 CrossRef CAS PubMed.
- J. H. J. Ostrowski and J. D. Eaves, J. Phys. Chem. B, 2014, 118, 530–536 CrossRef CAS PubMed.
- A. Ashraf, Y. Wu, M. C. Wang, K. Yong, T. Sun, Y. Jing, R. T. Haasch, N. R. Aluru and S. Nam, Nano Lett., 2016, 16, 4708–4712 CrossRef CAS PubMed.
- A. Quinn, R. Sedev and J. Ralston, J. Phys. Chem. B, 2005, 109, 6268–6275 CrossRef CAS PubMed.
- D. Klarman, D. Andelman and M. Urbakh, Langmuir, 2011, 27, 6031–6041 CrossRef CAS PubMed.
- R. Shamai, D. Andelman, B. Berge and R. Hayes, Soft Matter, 2008, 4, 38–45 RSC.
- H. Ren, L. Zhang, X. Li, Y. Li, W. Wu and H. Li, Phys. Chem. Chem. Phys., 2015, 17, 23460–23467 RSC.
- J. Pu, S. Wan, Z. Lu, G.-A. Zhang, L. Wang, X. Zhang and Q. Xue, J. Mater. Chem. A, 2013, 1, 1254–1260 RSC.
- X. B. Tan, et al., Electrowetting on flexible, transparent and conducting single-layer graphene, Micro Electro Mechanical Systems (MEMS), 2012 IEEE 25th International Conference on IEEE, 2012.
- B. Xin and J. Hao, Chem. Soc. Rev., 2010, 39, 769–782 RSC.
- S. Naghdi, B. Jaleh and N. Shahbazi, Appl. Surf. Sci., 2016, 368, 409–416 CrossRef CAS.
- X. Yao, Y. Song and L. Jiang, Adv. Mater., 2011, 23, 719–734 CrossRef CAS PubMed.
- T. Sun, G. Wang, L. Feng, B. Liu, Y. Ma, L. Jiang and D. Zhu, Angew. Chem., Int. Ed., 2004, 43, 357–360 CrossRef CAS PubMed.
- S. F. L. Mertens, A. Hemmi, S. Muff, O. Gröning, S. De Feyter, J. Osterwalder and T. Greber, Nature, 2016, 534, 676 CrossRef CAS PubMed.
- J. Rafiee, M. A. Rafiee, Z. Z. Yu and N. Koratkar, Adv. Mater., 2010, 22, 2151–2154 CrossRef CAS PubMed.
- M. Yi, W. Zhang, Z. Shen, X. Zhang, X. Zhao, Y. Zheng and S. Ma, J. Nanopart. Res., 2013, 15, 1811 CrossRef PubMed.
- H. Chang, J. Qin, P. Xiao, Y. Yang, T. Zhang, Y. Ma, Y. Huang and Y. Chen, Adv. Mater., 2016, 28, 3504–3509 CrossRef CAS PubMed.
- Y. Huang, X. Chen and M. Q. Zhang, J. Mater. Sci., 2014, 49, 3025–3033 CrossRef CAS.
- X. Zhang, S. Wan, J. Pu, L. Wang and X. Liu, J. Mater. Chem., 2011, 21, 12251 RSC.
- Z. Xu, Z. Ao, D. Chu, A. Younis, C. M. Li and S. Li, Sci. Rep., 2014, 4, 6450 CrossRef CAS PubMed.
- G. Ding, W. Jiao, R. Wang, Y. Niu, L. Chen and L. Hao, Adv. Funct. Mater., 2018, 28, 1706686 CrossRef.
- S. Wang, K. Liu, X. Yao and L. Jiang, Chem. Rev., 2015, 115, 8230–8293 CrossRef CAS PubMed.
- N. K. Adam, Nature, 1957, 180, 809 CrossRef.
- N. Nidhi and A. Chilkoti, Adv. Mater., 2002, 14(17), 1243–1247 CrossRef.
- A. Kikuchi and T. Okano, Prog. Polym. Sci., 2002, 27, 1165–1193 CrossRef CAS.
- Z. Cheng, J. Wang, H. Lai, Y. Du, R. Hou, C. Li, N. Zhang and K. Sun, Langmuir, 2015, 31, 1393–1399 CrossRef CAS PubMed.
- X. Yu, Z. Wang, Y. Jiang, F. Shi and X. Zhang, Adv. Mater., 2005, 17, 1289–1293 CrossRef CAS.
- S. Wan, J. Pu, X. Zhang, L. Wang and Q. Xue, Appl. Phys. Lett., 2013, 102, 011603 CrossRef.
- C. M. Weber, D. M. Eisele, J. P. Rabe, Y. Liang, X. Feng, L. Zhi, K. Mullen, J. L. Lyon, R. Williams, D. A. Vanden Bout and K. J. Stevenson, Small, 2010, 6, 184–189 CrossRef CAS PubMed.
- H. S. Lim, D. Kwak, D. Y. Lee, S. G. Lee and K. Cho, J. Am. Chem. Soc., 2007, 129, 4128–4129 CrossRef CAS PubMed.
- B. Yan, J. Tao, C. Pang, Z. Zheng, Z. Shen, C. H. A. Huan and T. Yu, Langmuir, 2008, 24, 10569–10571 CrossRef CAS PubMed.
- C. K. Chua and M. Pumera, Chem. Soc. Rev., 2014, 43, 291–312 RSC.
- H. Hu, Z. Zhao, W. Wan, Y. Gogotsi and J. Qiu, Adv. Mater., 2013, 25, 2219–2223 CrossRef CAS PubMed.
- H. Bi, X. Xie, K. Yin, Y. Zhou, S. Wan, R. S. Ruoff and L. Sun, J. Mater. Chem. A, 2014, 2, 1652–1656 RSC.
- Y. Xu, K. Sheng, C. Li and G. Shi, ACS Nano, 2010, 4, 4324–4330 CrossRef CAS PubMed.
- Y. Zhao, C. Hu, Y. Hu, H. Cheng, G. Shi and L. Qu, Angew. Chem., Int. Ed., 2012, 51, 11371–11375 CrossRef CAS PubMed.
- L. Qiu, J. Z. Liu, S. L. Y. Chang, Y. Wu and D. Li, Nat. Commun., 2012, 3, 1241 CrossRef PubMed.
- Y. Wu, N. Yi, L. Huang, T. Zhang, S. Fang, H. Chang, N. Li, J. Oh, J. A. Lee, M. Kozlov, A. C. Chipara, H. Terrones, P. Xiao, G. Long, Y. Huang, F. Zhang, L. Zhang, X. Lepró, C. Haines, M. D. Lima, N. P. Lopez, L. P. Rajukumar, A. L. Elias, S. Feng, S. J. Kim, N. T. Narayanan, P. M. Ajayan, M. Terrones, A. Aliev, P. Chu, Z. Zhang, R. H. Baughman and Y. Chen, Nat. Commun., 2015, 6, 6141 CrossRef CAS PubMed.
- A. Bagri, C. Mattevi, M. Acik, Y. J. Chabal, M. Chhowalla and V. B. Shenoy, Nat. Chem., 2010, 2, 581 CrossRef CAS PubMed.
- S. V. Tkachev, E. Y. Buslaeva, A. V. Naumkin, S. L. Kotova, I. V. Laure and S. P. Gubin, Inorg. Mater., 2012, 48, 796–802 CrossRef CAS.
- H. Bai, C. Li and G. Shi, Adv. Mater., 2011, 23, 1089–1115 CrossRef CAS PubMed.
- W. Yuan, A. Liu, L. Huang, C. Li and G. Shi, Adv. Mater., 2013, 25, 766–771 CrossRef CAS PubMed.
- K. N. Kudin, B. Ozbas, H. C. Schniepp, R. K. Prud'homme, I. A. Aksay and R. Car, Nano Lett., 2008, 8, 36–41 CrossRef CAS PubMed.
- G. Eda, G. Fanchini and M. Chhowalla, Nat. Nanotechnol., 2008, 3, 270 CrossRef CAS PubMed.
- Y. Qiu, M. Wang, W. Zhang, Y. Liu, Y. V. Li and K. Pan, Nanoscale, 2018, 10, 14060–14066 RSC.
- S. Pei and H.-M. Cheng, Carbon, 2012, 50, 3210–3228 CrossRef CAS.
- D. Wan, C. Yang, T. Lin, Y. Tang, M. Zhou, Y. Zhong, F. Huang and J. Lin, ACS Nano, 2012, 6, 9068–9078 CrossRef CAS PubMed.
- S. Pei, J. Zhao, J. Du, W. Ren and H.-M. Cheng, Carbon, 2010, 48, 4466–4474 CrossRef CAS.
- L. Gao, W. Ren, F. Li and H.-M. Cheng, ACS Nano, 2008, 2, 1625–1633 CrossRef CAS PubMed.
- J. Zhao, S. Pei, W. Ren, L. Gao and H.-M. Cheng, ACS Nano, 2010, 4, 5245–5252 CrossRef CAS PubMed.
- W. Gao, G. Wu, M. T. Janicke, D. A. Cullen, R. Mukundan, J. K. Baldwin, E. L. Brosha, C. Galande, P. M. Ajayan, K. L. More, A. M. Dattelbaum and P. Zelenay, Angew. Chem., Int. Ed., 2014, 53, 3588–3593 CrossRef CAS PubMed.
- J. Zhang and Q. Xiong, Phys. Chem. Chem. Phys., 2018, 20, 4597–4605 RSC.
- Q. G. Jiang, Z. M. Ao, D. W. Chu and Q. Jiang, J. Phys. Chem. C, 2012, 116, 19321–19326 CrossRef CAS.
- Z. M. Ao and F. M. Peeters, J. Phys. Chem. C, 2010, 114, 14503–14509 CrossRef CAS.
- Z. M. Ao and F. M. Peeters, Appl. Phys. Lett., 2010, 96, 253106 CrossRef.
- M. P. Hyman and J. W. Medlin, J. Phys. Chem. Lett., 2005, 109, 6304–6310 CrossRef CAS PubMed.
- M. Topsakal, H. H. Gürel and S. Ciraci, J. Phys. Chem. C, 2013, 117, 5943–5952 CrossRef CAS.
- J. Ito, J. Nakamura and A. Natori, J. Appl. Phys., 2008, 103, 113712 CrossRef.
- K. Vijayarangamuthu, S. Ahn, H. Seo, S. H. Yoon, C. M. Park and K. J. Jeon, Adv. Mater., 2016, 28, 661–667 CrossRef CAS PubMed.
- O. Ö. Ekiz, M. Ürel, H. Güner, A. K. Mizrak and A. Dâna, ACS Nano, 2011, 5, 2475–2482 CrossRef CAS PubMed.
- K. A. Velizhanin, N. Dandu and D. Solenov, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 89, 155414 CrossRef.
- D. Solenov and K. A. Velizhanin, Phys. Rev. Lett., 2012, 109, 095504 CrossRef PubMed.
- S. Wang, X. Feng, J. Yao and L. Jiang, Angew. Chem., Int. Ed., 2006, 118, 1286–1289 CrossRef.
- J. Yang, Z. Zhang, X. Men, X. Xu and X. Zhu, Langmuir, 2010, 26, 10198–10202 CrossRef CAS PubMed.
- X. Feng, J. Zhai and L. Jiang, Angew. Chem., Int. Ed., 2005, 117, 5245–5248 CrossRef.
- S. B. Kim, H. T. Hwang and S. C. Hong, Chemosphere, 2002, 48, 437–444 CrossRef CAS PubMed.
- H. Zhou, P. Ganesh, V. Presser, M. C. F. Wander, P. Fenter, P. R. C. Kent, D.-E. Jiang, A. A. Chialvo, J. McDonough, K. L. Shuford and Y. Gogotsi, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 035406 CrossRef.
- Z. Luo, N. J. Pinto, Y. Davila and A. T. Charlie Johnson, Appl. Phys. Lett., 2012, 100, 253108 CrossRef.
- D. L. Duong, G. H. Han, S. M. Lee, F. Gunes, E. S. Kim, S. T. Kim, H. Kim, Q. H. Ta, K. P. So, S. J. Yoon, S. J. Chae, Y. W. Jo, M. H. Park, S. H. Chae, S. C. Lim, J. Y. Choi and Y. H. Lee, Nature, 2012, 490, 235 CrossRef CAS PubMed.
- A. Ashraf, Y. Wu, M. C. Wang, N. R. Aluru, S. A. Dastgheib and S. Nam, Langmuir, 2014, 30, 12827–12836 CrossRef CAS PubMed.
- Y. Wu and N. R. Aluru, J. Phys. Chem. B, 2013, 117, 8802–8813 CrossRef CAS PubMed.
- M. Ge, C. Cao, J. Huang, X. Zhang, Y. Tang, X. Zhou, K. Zhang, Z. Chen and Y. Lai, Nanoscale Horiz., 2018, 3, 235–260 RSC.
- Y. Sun and Z. Guo, Nanoscale Horiz., 2019 10.1039/c8nh00223a.
- Y. Zhang, Z. Cai, Y. Zhao, X. Wen, W. Xu, Y. Zhong, L. Bai, W. Liu, Y. Zhang, Y. Zhang, Y. Kuang and X. Sun, Nanoscale Horiz., 2018 10.1039/c8nh00259b.
- X. Cui, J. Liu, L. Xie, J. Huang, Q. Liu, J. N. Israelachvili and H. Zeng, Angew. Chem., Int. Ed., 2018, 57, 11903–11908 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2019 |