Flower-like TiO2 nanostructures with exposed {001} facets: Facile synthesis and enhanced photocatalysis†
Min
Liu
a,
Lingyu
Piao
*b,
Weiming
Lu
ac,
Siting
Ju
b,
Lei
Zhao
a,
Chunlan
Zhou
a,
Hailing
Li
a and
Wenjing
Wang
*a
aKey Laboratory of Solar Thermal Energy and Photovoltaic System, Institute of Electrical Engineering, Chinese Academy of Sciences, No. 6 Beiertiao, Zhongguancun, 100190 Beijing, China. E-mail: wjwangwj@126.com; Fax: (+86) 10-82547041; Tel: (+86) 10-82547042
bNational Center for Nanoscience and Technology, No.11 Beiyitiao, Zhongguancun, 100190 Beijing, China. E-mail: piaoly@nanoctr.cn; Fax: (+86) 10-82545505; Tel: (+86) 10-82545505
cInstitute of Metal Research, Chinese Academy of Sciences, Shenyang, 110016, China
First published on 17th March 2010
Abstract
Flower-like TiO2 nanostructures with exposed {001} facets were synthesized by a low-temperature hydrothermal process from Ti powders for the first time, and they exhibited enhanced photocatalytic degradation of methylene blue dye under ultraviolet light irradiation.
Titanium oxide (TiO2), as one of the most important oxides, has been widely investigated due to its numerous applications in photovoltaic cells,1 photocatalysis,2,3 Li-ion battery materials,4 sensors5 and so on. These applications originate from the unique physical and chemical properties of TiO2 which depend not only on the crystal phase and particle size but also on the exposed facet.6–10 For anatase TiO2, both theoretical and experimental studies have found that the minority {001} facets in the equilibrium state are especially reactive.8–11 However, it has been demonstrated that the average surface energies are γ{001} (0.90 J m−2) > γ{100} (0.53 J m−2) > γ{101} (0.44 J m−2).7,9,10 Although the higher-surface-energy {001} facets have much higher chemical activities, almost all of anatase TiO2 micro- and nanostructures reported to date are usually dominated by less-reactive {101} facets, which are thermodynamically stable due to their lower surface energy. Therefore, anatase TiO2 nanostructures with exposed {001} facets are rarely observed.
An important step forward in the preparation of anatase TiO2 single crystals with exposed {001} facets were achieved by Lu and co-workers.11 They reported the synthesis of anatase TiO2 microcrystals with exposed {001} facets by using TiF4 as the raw material. Since then, several researchers have prepared anatase TiO2 single crystals with exposed {001} facets from different raw materials, such as titanium tetrafluorine, titanium chloride, tetrabutyl titanate, titanium tetraisopropoxide, and so on.12–16 However, these materials have high hydrolytic reaction rates which make it difficult to control their reaction processes. Very recently, anatase TiO2 sheets with a high percentage of {001} facets were prepared from titanium nitride by Lu and co-workers.17 However, the price of titanium nitride is still very high. Moreover, all of the previously mentioned experimental processes require high temperatures,11–18 even as high as 1300 °C,15 which not only increases the production cost but also hinders scaled-up production.
Furthermore, nanomaterials with hierarchical architectures have attracted much attention, since they can possess a number of attractive properties and have a wide range of applications in catalysis, solar cells, Li-ion batteries, and so on.19–24 For example, rose-like ZnO nanostructures, which consist of many nanosheets, exhibit enhanced light conversion efficiency in dye-sensitized solar cells (DSSC).23 Flower-like ZnMn2O4 nanostructures, which are composed of numerous nanorods, showed enhanced electrochemical lithium storage.24 However, it remains a great challenge to develop feasible methods for the synthesis of well-defined hierarchical TiO2 nanostructures.
Herein, we report a simple and facile route for the one-pot synthesis of flower-like TiO2 nanostructures with exposed {001} facets by a low-temperature hydrothermal process from Ti powders. These flower-like TiO2 nanostructures with exposed {001} facets have never been reported until now. In agreement with calculations, it can be confirmed that these flower-like TiO2 nanostructures contain about 10–30% exposed highly reactive {001} facets. Moreover, these flower-like TiO2 nanostructures exhibited enhanced photocatalytic degradation of methylene blue (MB) dye under ultraviolet (UV) light irradiation, and they also have potential applications in photonic and optoelectronic devices, solar cells, sensors, and so on.
In a typical synthesis, 0.1 g Ti powder (200 mesh, 99.9% purity, see Fig. S1 of the ESI†), 40 ml H2O and 0.25 ml hydrofluoric acid (40 wt %) were added into a Teflon-lined autoclave. Then, the mixture was kept at 120 °C for 10 h. After completion of the reaction, the products were collected by centrifugation and thoroughly washed with high-purity water (18 MΩ) until pH 7.0 was reached. Finally, the products were dried at 80 °C.
Fig. 1 a and b show typical field-emission scanning electron microscope (FE-SEM) images of the flower-like TiO2 nanostructures. It can be seen that the TiO2 product contains numerous flower-like aggregates, and almost all of them show the same morphology. These flower-like TiO2 nanostructures have a size around 300–700 nm, and they are composed of large numbers of truncated tetragonal pyramidal TiO2 nanocrystals. The width of their top surface is ca. 50–150 nm. The length of the truncated tetragonal pyramidal TiO2 nanocrystals is ca. 100–200 nm. Fig. 1c shows an X-ray diffraction (XRD) pattern of the flower-like TiO2 nanostructures. All XRD peaks of the synthesized flower-like TiO2 nanostructures can be indexed to the anatase TiO2 phase (JCPDS No. 21-1272) and no residual Ti phase can be detected. Remarkably enhanced (101) and (004) peaks indicate that the TiO2 nanostructures are dominant in {101} and {001} facets.
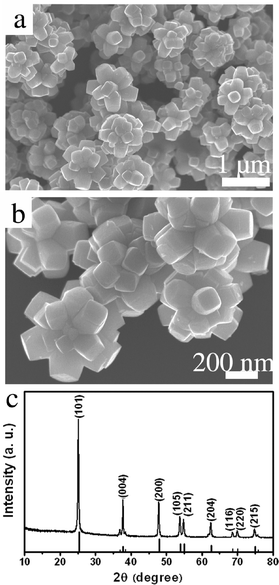 | ||
| Fig. 1 (a, b) FE-SEM images of the flower-like TiO2 nanostructures at low and high magnifications, respectively. (c) XRD pattern of the flower-like TiO2 nanostructures. Vertical bars indicate peak position and intensity of anatase TiO2 (JCPDS No. 21-1272). | ||
Fig. 2a shows a transmission electron microscopy (TEM, FEI F-20) image of the flower-like TiO2 nanostructures. It confirms that the flower-like TiO2 nanostructures were composed of many truncated tetragonal pyramidal TiO2 nanocrystals. An angle of 68.3° ± 0.3°, which is consistent with the interfacial angle between (001) and (101),11,14,15 was observed on the truncated tetragonal pyramidal TiO2 nanocrystals. It suggests that the truncated tetragonal pyramidal TiO2 nanocrystals expose facets of {001} and {101}. Fig. 2b gives a schematic illustration of the flower-like TiO2 nanostructures. Fig. 2c shows a high-resolution TEM (HRTEM) image of a truncated tetragonal pyramidal TiO2 nanocrystal with clear crystalline lattice fringes. The lattice spacing of 0.352 nm corresponds to the {101} planes, while the lattice spacing of 0.237 nm corresponds to the {004} planes. The angle labeled in the corresponding fast-Fourier transform (FFT) image (Fig. 2d) is 68.3° ± 0.3°, which is identical to the theoretical value for the angle between the {101} and {001} facets. On the basis of the above characterizations, it can be confirm that the flower-like TiO2 nanostructures have exposed {001} facets.
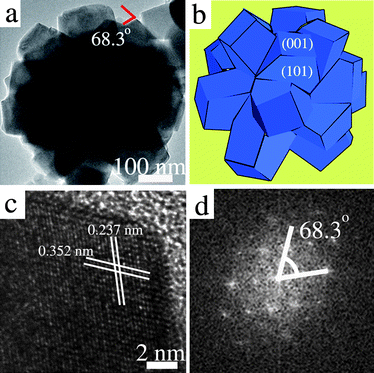 | ||
| Fig. 2 (a) TEM image of the flower-like TiO2 nanostructures. (b) Schematic diagram of the flower-like TiO2 nanostructures. (c) HRTEM image of a truncated tetragonal pyramidal TiO2 nanocrystal. (d) The corresponding fast-Fourier transform (FFT) pattern. | ||
It has been demonstrated that metal Ti could react with HF forming TiO2, under hydrothermal conditions.25 Due to their low surface energy, TiO2 crystals grow with exposed {101} facets mainly during the early stages of synthesis. However, fluorine ions can markedly reduce the surface energy of {001} facets to a level lower than that of {101} facets.11 As a result, {001} facets are exposed during growth, and flower-like TiO2 nanostructures with exposed {001} facets are formed. Therefore, hydrofluoric acid is believed to have a triple role here: to dissolve the Ti powders, to retard the hydrolysis of the titanium precursor, and to reduce the surface energy.18
The photocatalytic activity of the flower-like TiO2 nanostructures was evaluated in terms of the decolorization of MB dye under UV light irradiation. For photocatalytic reaction, the surface fluorine was removed using a heat treatment process at 600 °C in O2 for 2 h, without altering the morphology (see Fig. S2 of the ESI†). After that, 30 mg flower-like TiO2 nanostructures was dispersed in 100 ml of aqueous solution containing 0.01 M NaOH and 25 ppm MB.12 Before exposure to UV light irradiation, the suspension was stirred in the dark for 1 h to allow it reach a complete adsorption–desorption equilibrium. Then the solution was irradiated with ∼0.5 mW cm−2 UV light (with a wavelength peak at 365 nm) under continuous stirring. The concentration of MB was determined from the absorbance at the wavelength of 665 nm. For comparison, the same procedure was also done for P25 TiO2 powders.
As shown in Fig. 3, the flower-like TiO2 nanostructures exhibited higher activity than that of the commercial P25 TiO2 powders. The linear relationship of ln C0/Cvs. time (inset of Fig. 3) shows that the photocatalytic degradation of MB follows pseudo-first-order kinetics, ln(C0/C) = kt, where C/C0 is the normalized MB concentration, t is the reaction time, and k is the pseudo-first-rate constant. The apparent photochemical degradation rate constant for the flower-like TiO2 nanostructures is 1.23 × 10−2 min−1, which is almost two times of that for the P25 TiO2 powders, 6.73 × 10−3 min−1. This further confirms that the flower-like TiO2 nanostructures exhibit high photocatalytic efficiency. The enhanced photocatalytic activity of the flower-like TiO2 nanostructures can be attributed to their three-dimensional (3D) hierarchical nanostructures and exposed {001} facets. 3D hierarchical nanostructures are regarded to have a greater number of active sites than either 1D or 0D architectures.26,27 Furthermore, it has been demonstrated that the {001} facets are more reactive toward dissociative adsorption of reactant molecules compared with {101} facets.28–32 Therefore, high photocatalytic efficiency is expected for the flower-like TiO2 nanostructures with {001} facets.
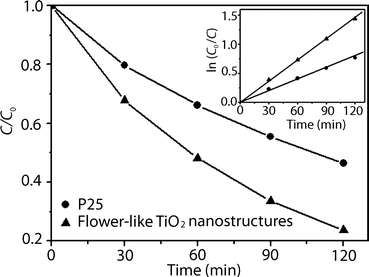 | ||
| Fig. 3 The variation of MB concentration by photoelectrocatalytic reaction with P25 TiO2 powders and flower-like TiO2 nanostructures. The inset shows the pseudo-first-order kinetic rate plots for the photochemical degradation of MB. | ||
In conclusion, flower-like TiO2 nanostructures with exposed {001} facets have been successfully synthesized by a low-temperature hydrothermal process from Ti powders for the first time. Owing to their chemically active {001} facets, these TiO2 nanostructures exhibited enhanced photocatalytic efficiency, and they could possibly be further used in photovoltaic cells, photonic and optoelectronic devices, sensors and so on.
Acknowledgements
This work was supported by the National Natural Science Foundation of China under Grant Nos 60576065 and 50602008, the Knowledge Innovation Program of the Chinese Academy of Sciences under Grant No. KGCXZ-YW-351, and the National High-Technology Research and Development Programme of China under Grant Nos 2006AA05Z405 and 2007AA05Z437.References
- B. O'Regan and M. Gratzel, Nature, 1991, 353, 737 CrossRef CAS.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37 CAS.
- A. L. Linsebigler, G. Q. Lu and J. T. Yates, Chem. Rev., 1995, 95, 735 CrossRef CAS.
- V. Subramanian, A. Karki, K. I. Gnanasekar, F. P. Eddy and B. Rambabu, J. Power Sources, 2006, 159, 186 CrossRef CAS.
- N. Wu, S. Wang and I. A. Rusakova, Science, 1999, 285, 1375 CrossRef CAS.
- X. B. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891 CrossRef CAS.
- U. Diebold, Surf. Sci. Rep., 2003, 48, 53 CrossRef CAS.
- X. Gong and A. Selloni, J. Phys. Chem. B, 2005, 109, 19560 CrossRef CAS.
- M. Lazzeri, A. Vittadini and A. Selloni, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 63, 155409 CrossRef.
- M. Lazzeri, A. Vittadini and A. Selloni, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 65, 119901 CrossRef.
- H. G. Yang, C. H. Sun, S. Z. Qiao, J. Zou, G. Liu, S. C. Smith, H. M. Cheng and G. Q. Lu, Nature, 2008, 453, 638 CrossRef CAS.
- H. G. Yang, G. Liu, S. Z. Qiao, C. H. Sun, Y. G. Jin, S. C. Smith, J. Zou, H. M. Cheng and G. Q. Lu, J. Am. Chem. Soc., 2009, 131, 4078 CrossRef CAS.
- X. Han, Q. Kuang, M. Jin, Z. Xie and L. Zheng, J. Am. Chem. Soc., 2009, 131, 3152 CrossRef CAS.
- Y. Dai, C. M. Cobley, J. Zeng, Y. M. Sun and Y. Xia, Nano Lett., 2009, 9, 2455 CrossRef CAS.
- F. Amano, O. Prieto-Mahaney, Y. Terada, T. Yasumoto, T. Shibayama and B. Ohtani, Chem. Mater., 2009, 21, 2601 CrossRef CAS.
- D. Zhang, G. Li, X. Yang and J. C. Yu, Chem. Commun., 2009, 4381 RSC.
- G. Liu, H. G. Yang, X. W. Wang, L. Cheng, J. Pan, G. Q. Lu and H. M. Cheng, J. Am. Chem. Soc., 2009, 131, 12868 CrossRef CAS.
- M. Liu, L. Pao, L. Zhao, S. Ju, Z. Yan, T. He, C. Zhou and W. Wang, Chem. Commun., 2010, 46, 1664 RSC.
- F. Lu, W. Cai and Y. Zhang, Adv. Funct. Mater., 2008, 18, 1047 CrossRef CAS.
- B. Li, G. Rong, Y. Xie, L. Huang and C. Feng, Inorg. Chem., 2006, 45, 6404 CrossRef CAS.
- Y. Ma, S. Weiner and L. Addadi, Adv. Funct. Mater., 2007, 17, 2693 CrossRef CAS.
- Z. Liu, X. Zhang, S. Nishimoto, M. Jin, D. A. Tryk, T. Murakami and A. Fujishima, Langmuir, 2007, 23, 10916 CrossRef CAS.
- E. Hosono, S. Fujihara and T. Kimura, Electrochim. Acta, 2004, 49, 2287 CrossRef CAS.
- L. Xiao, Y. Yang, J. Yin, Q. Li and L. Zhang, J. Power Sources, 2009, 194, 1089 CrossRef CAS.
- G. Wu, J. Wang, D. F. Thomas and A. Chen, Langmuir, 2008, 24, 3503 CrossRef CAS.
- C. Z. Wu, Y. Xie, L. Y. Lei, S. Q. Hu and C. Z. OuYang, Adv. Mater., 2006, 18, 1727 CrossRef CAS.
- X. L. Hu, J. C. Yu and J. M. Gong, J. Phys. Chem. C, 2007, 111, 11180 CrossRef CAS.
- A. Vittadini, A. Selloni, F. P. Rotzinger and M. Gratzel, Phys. Rev. Lett., 1998, 81, 2954 CrossRef CAS.
- G. S. Herman, Z. Dohnalek, N. Ruzycki and U. Diebold, J. Phys. Chem. B, 2003, 107, 2788 CrossRef CAS.
- A. Tilocca and A. Selloni, J. Phys. Chem. B, 2004, 108, 19314 CrossRef CAS.
- C. Arrouvel, M. Digne, M. Breysse, H. Toulhoat and P. Raybaud, J. Catal., 2004, 222, 152 CrossRef CAS.
- A. Selloni, Nat. Mater., 2008, 7, 613 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional FE-SEM images. See DOI: 10.1039/c0nr00050g |
| This journal is © The Royal Society of Chemistry 2010 |
