Open Access Article
Open Access ArticleAdvances in chromone-based copper(II) Schiff base complexes: synthesis, characterization, and versatile applications in pharmacology and biomimetic catalysis†
Sumit Kumar‡
ab,
Aditi Arora‡
a,
Vipin K. Maikhuri‡
 a,
Ankita Chaudhary
c,
Rajesh Kumar
ad,
Virinder S. Parmar
abe,
Brajendra K. Singh
a,
Ankita Chaudhary
c,
Rajesh Kumar
ad,
Virinder S. Parmar
abe,
Brajendra K. Singh
 *a and
Divya Mathur
*a and
Divya Mathur
 *af
*af
aDepartment of Chemistry, Bioorganic Research Laboratory, University of Delhi, Delhi, India. E-mail: singhbk@chemistry.du.ac.in
bDepartment of Chemistry and Environmental Science, Medgar Evers College, 1638 Bedford Avenue, Brooklyn, New York 11225, USA
cDepartment of Chemistry, Maitreyi College, University of Delhi, Delhi, India
dDepartment of Chemistry, R. D. S College, B. R. A. Bihar University, Muzaffarpur, India
eAmity Institute of Click Chemistry and Research Studies, Amity University, Sector 125, Noida 201313, Uttar Pradesh, India
fDepartment of Chemistry, Daulat Ram College, University of Delhi, Delhi, India. E-mail: dmchem05@gmail.com; divyamathur@dr.du.ac.in
First published on 28th May 2024
Abstract
Chromones are well known as fundamental structural elements found in numerous natural compounds and medicinal substances. The Schiff bases of chromones have a much wider range of pharmacological applications such as antitumor, antioxidant, anti-HIV, antifungal, anti-inflammatory, and antimicrobial properties. A lot of research has been carried out on chromone-based copper(II) Schiff-base complexes owing to their role in the organometallic domain and promise as potential bioactive cores. This review article is centered on copper(II) Schiff-base complexes derived from chromones, highlighting their diverse range of pharmacological applications documented in the past decade, as well as the future research opportunities they offer.
1. Introduction
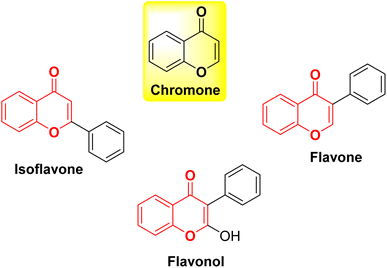
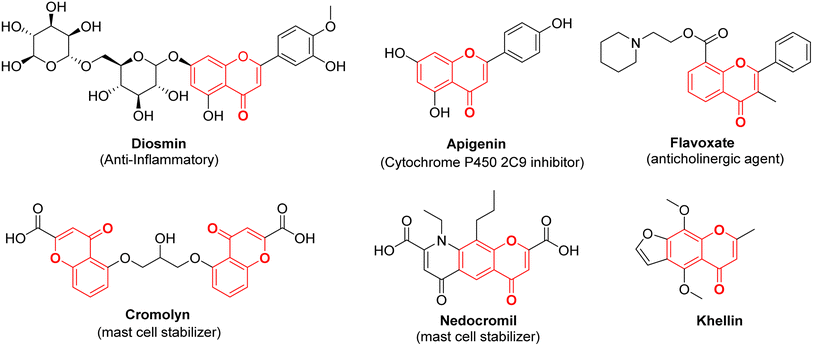
Chromone, also known as 4H-chromen-4-one or 4H-1-benzopyran-4-one, is an O-heterocyclic compound characterized by its benzo-γ-pyrone structure. It is part of a category of naturally occurring molecules that are prevalent in the plant kingdom, ranging from algae to coniferous plants.1 It forms the central backbone in flavonoids such as flavone, isoflavone, and flavonol (Fig. 1).2,3 Chromones have become a compelling presence in the field of medicinal chemistry and drug development initiatives due to their potential to offer a wide range of chemical variations along with a diverse array of pharmacological effects.4–6 Both naturally existing and artificially synthesized compounds containing a chromone component have exhibited a diverse range of biological characteristics including anti-inflammatory7,8 anticancer9,10 antitumor,11 antimicrobial12,13 anti-HIV,14 antioxidant,15 and many other activities.16,17 Khellin, a furanochromone obtained from the seeds of the Ammi visnaga plant, serves as an illustrative instance of a natural chromone employed as a bronchodilator for asthma. Presently, it is also utilized in the management of vitiligo and psoriasis (Fig. 2).18 Exploration rooted in khellin has given rise to novel chromone derivatives like cromolyn and nedocromil, which are employed as mast cell stabilizers for the treatment of allergic asthma and allergic conjunctivitis.19 Diosmin, a flavone glycoside is known for its antioxidative, anti-inflammatory, antihyperglycemic, and anti-ulcer properties.20 Another bioflavonoid, apigenin has been reported to modulate histamine release, is a bronchodilator, and also possesses anti-cancer properties.21 Flavoxate is known as an anticholinergic agent because of its antimuscarinic effects (Fig. 2).22Apart from the development of new chromone derivatives with promising properties, combining chromones with other pharmacophores to achieve hybrid structures with enhanced and multitarget therapeutic applications is an interesting proposition. One such pharmacophore is Schiff bases with varied applications across various domains, including pharmaceutical chemistry,23 catalysis,24 dye industry,25 corrosion inhibitors26,27 and chemo-sensors.28,29 The conventional method for creating Schiff bases, which bears the name of Hugo Schiff, encompasses the combination of a carbonyl compound with an appropriate amine, employing suitable solvents and catalysts.30 Schiff bases, a subclass of imines, feature a carbon–nitrogen double bond bound exclusively to alkyl or aryl groups, without any attached hydrogen atoms. Imines, organic compounds characterized by a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond, are pivotal entities in organic chemistry. They play essential roles in various fields such as synthesis, medicinal chemistry, coordination chemistry, and materials science. With their distinct reactivity and diverse structures, the imine functional group serves as a versatile and crucial component, facilitating the construction of intricate molecular architectures and functional materials.31,32 Owing to the electron-rich nature of the azomethine nitrogen atom through which they coordinate with metal ions, Schiff bases are known as one of the strongest chelators. Schiff bases possess remarkable pharmacological activities such as antibacterial, antifungal, and anticancer activities due to the presence of C
N double bond, are pivotal entities in organic chemistry. They play essential roles in various fields such as synthesis, medicinal chemistry, coordination chemistry, and materials science. With their distinct reactivity and diverse structures, the imine functional group serves as a versatile and crucial component, facilitating the construction of intricate molecular architectures and functional materials.31,32 Owing to the electron-rich nature of the azomethine nitrogen atom through which they coordinate with metal ions, Schiff bases are known as one of the strongest chelators. Schiff bases possess remarkable pharmacological activities such as antibacterial, antifungal, and anticancer activities due to the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N moiety.33–35 A noteworthy number of Schiff base complexes are analogs of naturally occurring molecules and thus are of critical importance.36 In this regard, Schiff bases can also be combined with chromones as they are ideal organic fluorescent probes possessing structural flexibility and pharmacological activity. 3-Formyl chromone serves as a commonly employed starting molecule in the construction of chromone Schiff bases, and it is typically generated through the Vilsmeier–Haack reaction.37
N moiety.33–35 A noteworthy number of Schiff base complexes are analogs of naturally occurring molecules and thus are of critical importance.36 In this regard, Schiff bases can also be combined with chromones as they are ideal organic fluorescent probes possessing structural flexibility and pharmacological activity. 3-Formyl chromone serves as a commonly employed starting molecule in the construction of chromone Schiff bases, and it is typically generated through the Vilsmeier–Haack reaction.37
Chromone Schiff bases are organic compounds resulting from the condensation reaction between chromone derivatives and primary amines. Chromone-based Schiff bases have been the focal point of organic, medicinal, and organometallic chemistry. Known for their pharmacological potential, these compounds exhibit diverse activities such as anti-inflammatory, antioxidant, anticancer, antibacterial, and antifungal properties.38 Owing to their established reputation as chelating ligands, these compounds are recognized for their enhanced bioavailability, decreased toxicity, ability to form stable metal complexes, and robust binding affinity for metal ions.39–41 The synthesis of chromone Schiff bases typically involves mild conditions and allows for structural modifications to enhance their biological activities. Research has shown that these compounds possess promising therapeutic effects, making them valuable candidates for drug discovery and development.42 A tremendous increase in the biological property of Schiff bases is observed when they are chelated with transition metal ions. Such metal complexes can be efficiently prepared through the addition of a suitable Schiff-base ligand to a metal precursor in an appropriate ratio and experimental conditions (Fig. 3). This is also true for chromone-based Schiff bases as exemplified by many examples. Chromone Schiff-base nano-complexes (I) such as those of Cu2+, Ni2+, Co2+, Fe3+, Zn2+, Cd2+, and UO26+, and Zn2+ have been successfully prepared and evaluated as antimicrobial and antitumor agents, exhibiting lower toxicity compared to cis-platin.43 Chromone Schiff base (II) complexes with Pd2+ have also been successfully prepared, characterized, and investigated for circulating tumor DNA (ctDNA) binding, radical scavenging, and antimicrobial potency.44 In a recent study, a fluorescent molecule appended with rhodamine and chromone (III) was synthesized as an efficient sensor for Fe3+, Al3+, and Cr3+ at trace levels. Further, they were assessed for anticancer activity and were found to be effective against MCF-7 cells, glucocorticoid, and progesterone receptors.45 Barve et al.46 synthesized copper ion conjugated chromone Schiff base complexes (IV) these compounds demonstrated their effectiveness as inhibitors against BT20, PC-3, as well as both COLO 357 and BxPC-3 cancer cell lines. Thus, it can be affirmatively suggested that the metal complexes exhibit better biological activity. The increase in activity can be ascribed to structural modifications resulting from coordination with the metal, and thus chelation makes metal complexes more potent compared to Schiff bases.47 The report by El-Saghier et al.48 established that Cu2+ complexes exhibit superior inhibition in comparison to the other complexes. This was ascribed to the presence of free mobilized electrons of Cu2+ complexes that permit strong oxidative inhibitory activity against microorganisms. This re-emphasizes that chromone-based Schiff bases are capable of forming d-block metal complexes amongst which copper complexes are predominantly effective as they have exhibited potent radical scavenging, anti-cancer, antimicrobial, and chemosensing properties.
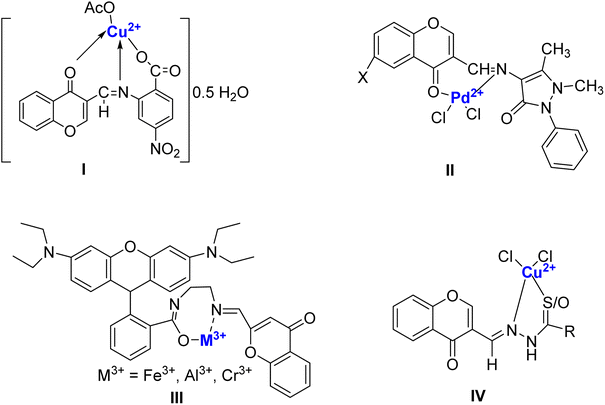 | ||
| Fig. 3 Pharmacologically significant chromone-based Schiff base complexes reported in the literature. | ||
The exploration of copper coordination chemistry is influenced by the crucial role copper assumes in numerous physiological processes, where it serves as an intrinsic component within metalloproteins like tyrosinase, cytochrome C oxidase, Cu/Zn superoxide dismutase, and blue copper proteins.49,50 Equally significant is the fact that there are numerous copper(II) complexes with four, five or six-coordinate and different geometries such as square planar, flattened tetrahedral, trigonal bipyramidal, square-pyramidal, distorted octahedral, and penta-coordinated structures.51–53 Owing to the pharmacological importance of chromones and Schiff bases, together with the multifaceted role of copper, there is a substantial interest in the development of chromone Schiff base copper complexes.
The primary goal of the current study is to perform an extensive review of published data from the past decade (2013–2023) pertaining to the synthesis and diverse applications of copper complexes derived from chromone-based Schiff bases. The impetus for undertaking this research stemmed from the observation that no comprehensive review had been available until now on Cu2+ complexes involving chromone Schiff bases. In this work, we initially delve into the use of chromone-based Schiff base ligands as a selective colorimetric tool for detecting Cu2+ ions. Subsequently, we provide a comprehensive examination of ongoing research on chromone Schiff base copper complexes, highlighting their wide-ranging medicinal and pharmaceutical applications, such as antimicrobial, antioxidant, and antitumor properties. Further, we have explained the biomimetic catalytic activity of chromone-based Schiff bases together with some miscellaneous applications. Finally, the challenges and future developments have been deliberated upon to promote the research on copper chromone Schiff base agents.
2. Copper detection by chromone-based Schiff bases
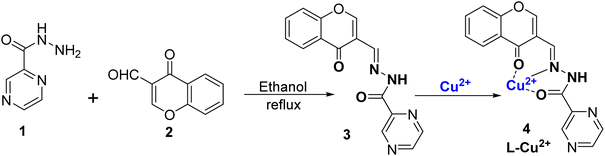
Copper is regarded as an abundant and important metal which play a very useful role in various biological, medicinal, and environmental fields.54 On one hand, excessive use of copper metal can cause serious problems in humans such as retardation of growth, Wilson's disease, and liver destruction while on the other, less intake of copper is also harmful, causing anemia, leukopenia, and myelopathy.55 Excess Cu2+ ions in drinking water sources can cause damage to animals, humans, and plants. According to the U. S. EPA, the highest range of Cu2+ ions in water should not be more than 20 μM.56,57 According to WHO, the maximum limit of Cu2+ ions is 31.4 μM.58 Thus, preparing an efficient sensor for tracing Cu2+ ions in environmental bodies and organic species becomes all the more important.59–61 Many traditional methods are available, which are otherwise costly or difficult to handle such as electrochemical sensing and atomic absorption spectroscopy (AAS).62–64 Therefore, there is a need for a simple, cost-effective, accurate method for monitoring Cu2+ ions in samples. Colorimetric sensors are the solution for the detection of copper because they not only reduce the cost but also make the process of detection easy and fast. Due to this, the design and use of colorimetric probes is a flourishing area of research.65,66 Nowadays, different variety of organic receptors and nanomaterials are used as colorimetric sensors.67–69 In recent times, Schiff bases and chromone-derived colorimetric and optical sensors have also been used for metal ion detection.70–72 This is due to their specific structure, and large binding affinity with metal ions.73,74 Herein, we have discussed chromone Schiff base sensors which bind to Cu2+ ions making exclusive coloured copper complexes and thus can be used as colorimetric sensors.In 2021, Tomer et al.75 successfully synthesized a Schiff base ligand denoted as ligand 3, which was derived from chromone. The synthesis of this ligand involved a condensation reaction between pyrazine-2-carbohydrazide 1 and 3-formylchromone 2, as illustrated in Scheme 1. The synthesized ligand 3 underwent characterization such as 1H-NMR, HRMS, FT-IR, and UV-Vis spectroscopy. Notably, ligand 3 was employed as a highly effective colorimetric probe for the detection of Cu2+ ions in different water sources, including canal water, groundwater, and tap water. The addition of Cu2+ ions to ligand 3 resulted in an immediate change in color from colorless to yellow, and the color change was because of the complex formation between the ligand and Cu2+ ions, referred to as complex 4. Importantly, there was no color change with other tested metal ions. The absorption spectra of ligand 3 displayed two prominent absorption peaks at 258 nm and 311 nm. Nonetheless, with the introduction of Cu2+ ions, a fresh absorption peak became apparent at 428 nm, while the 311 nm band diminished. This shift in absorption, known as a bathochromic shift, was attributed to the complexation of Cu2+ ions and ligands through an intramolecular charge transfer process. When Cu2+ ions were incrementally introduced into the solution, another band at 428 nm continued to appear, while the 311 nm band gradually decreased. The researchers observed that ligand 3 displayed remarkable sensitivity across a wide pH range, particularly in neutral and basic conditions within the pH range of 6–11, although it was not effective at very low pH values, such as 2–3. The limit of detection for Cu2+ ions was determined to be 3.9 × 10−7 M, and the association constant was calculated to be 2.3 × 105 M−1 using the Benesi–Hildebrand equation. The stoichiometry between ligand 3 and Cu2+ ions as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was confirmed through Job's plot, which was also substantiated by HRMS and DFT. Moreover, the practical applicability of this developed ligand was assessed by testing its performance in actual water samples for the identification and quantification of Cu2+ ions.
1 was confirmed through Job's plot, which was also substantiated by HRMS and DFT. Moreover, the practical applicability of this developed ligand was assessed by testing its performance in actual water samples for the identification and quantification of Cu2+ ions.
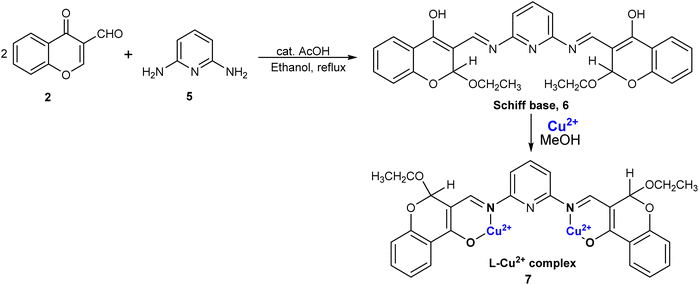
A chromone-functionalized pyridine-based chemosensor was also prepared by the same group for the sensing of Cu2+ ions.76 In their study, the researchers reported the synthesis of a novel Schiff base labeled as Schiff base 6. This compound was prepared by condensing 2 with 2,6-aminopyridine 5 in ethanol as the solvent, and glacial acetic acid as catalyst (Scheme 2). A significant finding was that the binding stoichiometry between Schiff base 6 and Cu2+ ions was established as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, a conclusion supported by both Job's plot and HRMS spectra data. Schiff base 6 demonstrated exceptional sensitivity and selectivity for cupric ions in comparison to other metal ions, as revealed by absorbance and emission titration studies. The absorption spectrum of Schiff base 6 exhibited three distinct absorption bands at 306 nm, 357 nm, and 401 nm. Upon the introduction of cupric ions, a new absorption band emerged at 347 nm, attributable to ligand-to-metal charge transfer, while a reduction in intensity was observed at 306 nm and 401 nm. Regarding fluorescence emission spectra, Schiff base 6 displayed a peak at 465 nm. The addition of Cu2+ ions resulted in a decrease in fluorescence intensity because of the paramagnetic nature of Cu2+ ions, accompanied by the appearance of a new peak at 458 nm. This fluorescence quenching was specific to the binding of Schiff base 6 to Cu2+ ions, with no similar response observed with other ions. pH studies indicated that the optimal pH range for detecting cupric ions was between 6 and 11. At lower pH values, protonation of the azomethine linkage occurred, leading to reduced sensing capability. Schiff base 6 exhibited an association constant of 3.26 × 104 M−1 and a regression coefficient of 98.7% for Cu2+ ions. It displayed a detection value of 1.2 μM for the Schiff base–Cu2+ complex 7, which was below the permissible limit for Cu2+ ions, thus confirming its effectiveness as a probe. Schiff base 6 was successfully employed in actual water samples, including canal water, distilled water, groundwater, and tap water for the detection of Cu2+ ions.
2, a conclusion supported by both Job's plot and HRMS spectra data. Schiff base 6 demonstrated exceptional sensitivity and selectivity for cupric ions in comparison to other metal ions, as revealed by absorbance and emission titration studies. The absorption spectrum of Schiff base 6 exhibited three distinct absorption bands at 306 nm, 357 nm, and 401 nm. Upon the introduction of cupric ions, a new absorption band emerged at 347 nm, attributable to ligand-to-metal charge transfer, while a reduction in intensity was observed at 306 nm and 401 nm. Regarding fluorescence emission spectra, Schiff base 6 displayed a peak at 465 nm. The addition of Cu2+ ions resulted in a decrease in fluorescence intensity because of the paramagnetic nature of Cu2+ ions, accompanied by the appearance of a new peak at 458 nm. This fluorescence quenching was specific to the binding of Schiff base 6 to Cu2+ ions, with no similar response observed with other ions. pH studies indicated that the optimal pH range for detecting cupric ions was between 6 and 11. At lower pH values, protonation of the azomethine linkage occurred, leading to reduced sensing capability. Schiff base 6 exhibited an association constant of 3.26 × 104 M−1 and a regression coefficient of 98.7% for Cu2+ ions. It displayed a detection value of 1.2 μM for the Schiff base–Cu2+ complex 7, which was below the permissible limit for Cu2+ ions, thus confirming its effectiveness as a probe. Schiff base 6 was successfully employed in actual water samples, including canal water, distilled water, groundwater, and tap water for the detection of Cu2+ ions.
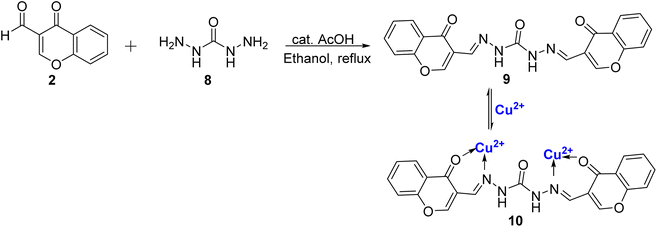
Tomer et al.77 presented another Schiff base probe based on chromone 9, in their study. This probe exhibited remarkable selectivity for Cu2+ ions and possessed the added capability of detecting para-nitrotoluene (pNT) with impressively low detection limits. The synthesis of this probe 9, was accomplished through a straightforward, one-step condensation reaction involving 2 and 8, (Scheme 3). Notably, Schiff base probe 9 displayed noticeable naked-eye colorimetric changes, shifting from colorless to yellow upon the introduction of Cu2+ ions, setting it apart from the response to other ions tested. In terms of its spectroscopic features, the Schiff base probe 9 exhibited two distinct absorption bands at 315 nm and 278 nm. With the gradual addition of Cu2+ ions, a new absorption band emerged at 421 nm, accompanied by a reduction in the intensity of the bands at 315 nm and 278 nm. The binding stoichiometry of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 between the complex and Cu2+ ions was confirmed through computational studies, Job's plot, and HRMS. The sensor exhibited reliable performance within a pH range of 5–9, and the sensing mechanism was found to be reversible, enhancing its practical utility. The binding constant was determined to be 4.5 × 105 M−1 for complex 10, with a low detection limit of 11.4 × 10−7 M. For practical applications, it was demonstrated that the most noticeable color change can occur at a Cu2+ concentration of 10−4 M, using a paper strip test which underscores the efficacy of the probe 9 in real-world scenarios.
2 between the complex and Cu2+ ions was confirmed through computational studies, Job's plot, and HRMS. The sensor exhibited reliable performance within a pH range of 5–9, and the sensing mechanism was found to be reversible, enhancing its practical utility. The binding constant was determined to be 4.5 × 105 M−1 for complex 10, with a low detection limit of 11.4 × 10−7 M. For practical applications, it was demonstrated that the most noticeable color change can occur at a Cu2+ concentration of 10−4 M, using a paper strip test which underscores the efficacy of the probe 9 in real-world scenarios.
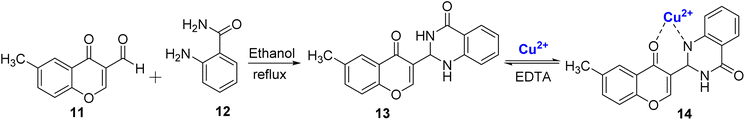
In another study, Mohammadi et al.78 reported a novel optical sensor 13 for selective sensing of Cu2+ ions in acetonitrile–water solution. The sensor was synthesized by condensation reaction between 11 and 2-amino benzamide 12 followed by cyclization resulting in quinazolinone ring formation (Scheme 4). The chromone-based sensor 13 exhibited exceptional selectivity by undergoing a visible color change, transitioning from colorless to yellow when just 1 μM of Cu2+ ions were added. Notably, it retained its colorless appearance when tested individually with all twelve other metal ions at the same concentration. Upon the subsequent introduction of Cu2+ ions to the synthesized complex, a reduction in the intensity of the strong absorption band of the free ligand at 379 nm was observed, accompanied by the appearance of new absorption bands at approximately 306 nm and 456 nm. The stoichiometric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 between the colorimetric sensor 13 and Cu2+ ions was corroborated through Job's plot and DFT studies. The binding constant for the 13–Cu2+ complex was determined to be 3.27 × 104 M−1, emphasizing the strength of the interaction of the sensor and Cu2+ ions. In anticipation of potential commercial applications, detection test strips were developed by coating filter paper with this chemosensor. These test strips allowed for the immediate naked-eye detection of copper ions in a wide concentration range, spanning from 10−3 M to 10−7 M, which enhances the practical utility and versatility of this sensor. The near nano detection limit (4.6 × 10−7 M), wide concentration range, short response time, and reversible properties of 14 were some of the salient features of the developed sensor.
1 between the colorimetric sensor 13 and Cu2+ ions was corroborated through Job's plot and DFT studies. The binding constant for the 13–Cu2+ complex was determined to be 3.27 × 104 M−1, emphasizing the strength of the interaction of the sensor and Cu2+ ions. In anticipation of potential commercial applications, detection test strips were developed by coating filter paper with this chemosensor. These test strips allowed for the immediate naked-eye detection of copper ions in a wide concentration range, spanning from 10−3 M to 10−7 M, which enhances the practical utility and versatility of this sensor. The near nano detection limit (4.6 × 10−7 M), wide concentration range, short response time, and reversible properties of 14 were some of the salient features of the developed sensor.
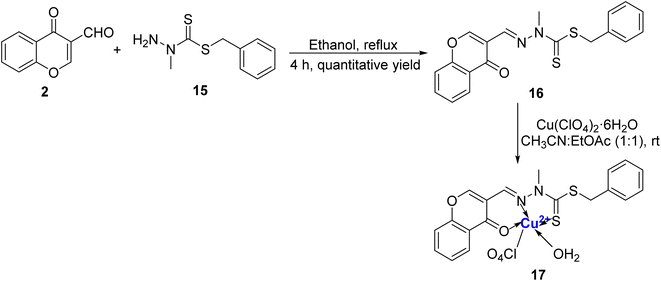
Rahman et al.79 prepared a sensitive and selective chromone and benzyldithiocarbazate based ligand 16 for colorimetric detection of Cu2+ ion in HEPES buffer media in a mixture of H2O/CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The ligand 16 was synthesized as a colourless cotton-like solid by taking N-methyl-S-benzyldithiocarbazate 15 with 3-formylchromone 2 in ethanol under reflux (Scheme 5). 100% selectivity was achieved in the case of Cu2+ with colourless to yellow colour change and there was no change in the presence of other metal ions and anions. Single crystal analysis of the green complex 17 formed with Cu2+ and 16 was found to be penta-coordinated with square pyramidal orientation. Complex 17 showed an absorption peak around 420 nm and also displayed a 1
1). The ligand 16 was synthesized as a colourless cotton-like solid by taking N-methyl-S-benzyldithiocarbazate 15 with 3-formylchromone 2 in ethanol under reflux (Scheme 5). 100% selectivity was achieved in the case of Cu2+ with colourless to yellow colour change and there was no change in the presence of other metal ions and anions. Single crystal analysis of the green complex 17 formed with Cu2+ and 16 was found to be penta-coordinated with square pyramidal orientation. Complex 17 showed an absorption peak around 420 nm and also displayed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio for Cu2+: 16. The detection limit was 0.12 nM and the association constant for 16 with Cu2+ was 5.24 × 106 M−1. A colorimetric detection kit for Cu2+ ions was developed using the sensor and it was found that it could easily detect even 2 μM concentration of Cu2+ that is far beneath the WHO acceptable level.
1 stoichiometric ratio for Cu2+: 16. The detection limit was 0.12 nM and the association constant for 16 with Cu2+ was 5.24 × 106 M−1. A colorimetric detection kit for Cu2+ ions was developed using the sensor and it was found that it could easily detect even 2 μM concentration of Cu2+ that is far beneath the WHO acceptable level.
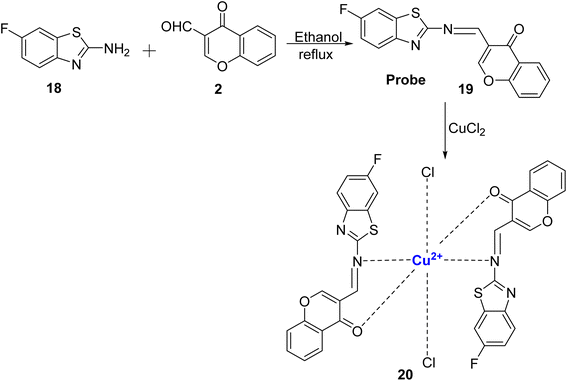
Kouser et al.80 reported the development of a chemosensor probe denoted as 19, based on the chromone molecule. This probe, 19, was synthesized by reacting 2 with 2-amino-6-fluorobenzothiazole 18 under reflux conditions, (Scheme 6). Notably, probe 19 exhibited exceptional selectivity and sensitivity towards Cu2+ ions as opposed to a range of other metal ions. Two distinct absorption bands at 371 nm and 260 nm were assigned to n–π* and π–π* transitions. Upon the introduction of Cu2+ ions into the solution of 19, there was a noticeable change from daffodil yellow to green, and new bands emerged at 355 and 452 nm. These changes were attributed to the formation of charge transfer complexes, d–d transitions, and π–π* transitions. The sensor demonstrated rapid recognition of Cu2+ ions and a low detection limit of 0.273 × 10−6 mol L−1. Furthermore, it displayed an increase in fluorescence intensity and a high association constant of 8.48 × 108 M−2 with the addition of Cu2+ ions. By utilizing Job's plot analysis, it was determined that the stoichiometric ratio between probe 19 and Cu2+ in the complex was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The sensor's effectiveness in detecting copper-containing biomolecules was also assessed in various biological samples, including healthy liver tissues, F. gigantica-infected liver tissues, and adult F. gigantica worms.
1. The sensor's effectiveness in detecting copper-containing biomolecules was also assessed in various biological samples, including healthy liver tissues, F. gigantica-infected liver tissues, and adult F. gigantica worms.
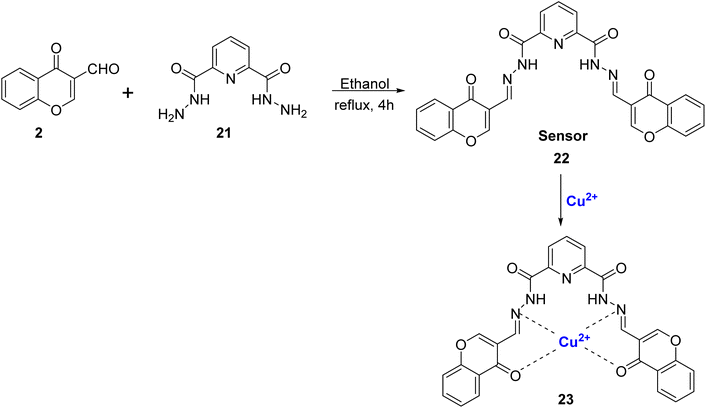
Rezaeian et al. reported a chemo-sensor 22 obtained by the condensation reaction of 2 and 2,6-pyridinedicarbohydrazide 21 in high yields (Scheme 7).81 The synthesized ligand 22 was used to sense and spot Cu2+ and Zn2+ ions both visually and spectrophotometrically among the different cations tested. With the increase in Cu2+ ions absorbance at 310 nm decreased with the concomitant formation of complex 23 and a new band at 425 nm. Cu2+ ion and ligand 22 had 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry and a binding constant of 3.41 × 104 M−1. From the experimental data, the detection limit for Cu2+ obtained was 5.5 × 10−7 mol L−1 which was lower as compared to the limit set for safe drinking water by WHO. Further, multiple and complex Boolean operations such as OR, NOR, AND, INH, and half adders such as AND and XOR were also conducted by varying anions and cations as chemical inputs and the absorption intensity as the logic output.
1 stoichiometry and a binding constant of 3.41 × 104 M−1. From the experimental data, the detection limit for Cu2+ obtained was 5.5 × 10−7 mol L−1 which was lower as compared to the limit set for safe drinking water by WHO. Further, multiple and complex Boolean operations such as OR, NOR, AND, INH, and half adders such as AND and XOR were also conducted by varying anions and cations as chemical inputs and the absorption intensity as the logic output.
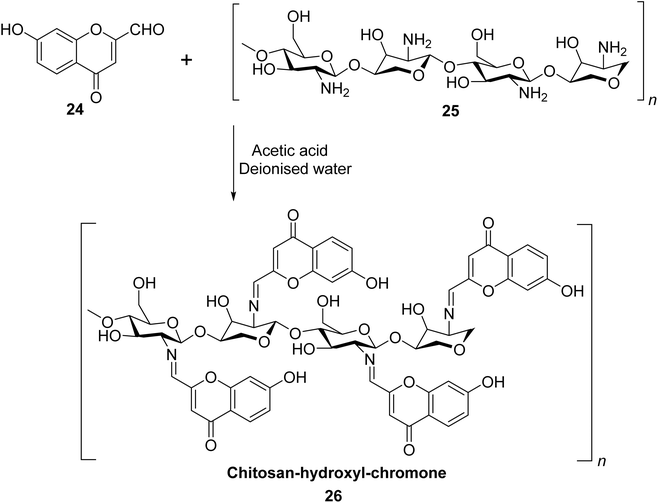
Gaidhane et al. used chitosan, which is a naturally occurring, environment-friendly bio-polysaccharide derived from chitin to synthesize a new chromone-based polymer 26.82 Polymer 26 was prepared by the treatment of 24 with chitosan 25 in deionized water (Scheme 8). It displayed specific, selective, and pH-influenced chelating efficiency with Cu2+ ions in comparison to Ni2+, Co2+, and Cd2+ heavy metal ions. At pH 4–6, polymer 26 showed selectivity in the order of Cu2+ > Ni2+ > Cd2+ > Co2+. The adsorption capacity was also highest for Cu2+ with a concentration of 3 mmol g−1. Chromone-based chitosan polymer 26 significantly inhibited lipid peroxidation emulsion system. It also demonstrated excellent results against Gram-negative bacteria Pseudomonas aeruginosa and decent activities with Escherichia coli, Staphylococcus aureus, and Candida albicans.
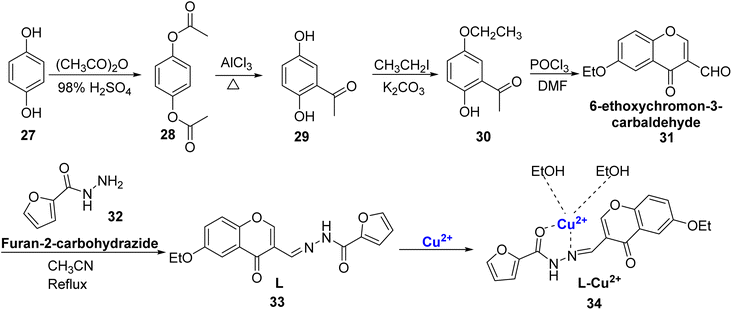
Yang et al. reported a new probe i.e., 6-ethoxychromone-3 carbaldehyde-(furanyl)hydrazone 33 with turn-on fluorescence and colorimetric properties.83 The dual functional probe 33 was designed by refluxing a mixture of furan-2-carbohydrazide 32 and 6-ethoxychromon-3-carbaldehyde 31 in acetonitrile for 24 hours (Scheme 9). It showed colorimetric changes and high selectivity for Al3+ and Cu2+ as compared to other metal ions. When exposed to Cu2+ ions, it underwent a visible color change from colorless to yellow. When Cu2+ was introduced, the absorption peak at 319 nm steadily diminished, and a novel absorption band appeared at 422 nm. Analyzing the data through Job's plot and HRMS confirmed that the 33–Cu2+ complex involved a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 coordination ratio. The probe 33 demonstrated an impressive detection limit of 2.857 × 10−7 M for Cu2+ ions, which met the standards set by the WHO. Furthermore, the practical applicability of 33 as a solid-state probe was assessed by utilizing silica-coated slides and test papers immersed in the chromone-based probe 33. Based on the findings, it is evident that 33 could serve as a prototype for a wide range of practical applications in environmental and biological systems.
1 coordination ratio. The probe 33 demonstrated an impressive detection limit of 2.857 × 10−7 M for Cu2+ ions, which met the standards set by the WHO. Furthermore, the practical applicability of 33 as a solid-state probe was assessed by utilizing silica-coated slides and test papers immersed in the chromone-based probe 33. Based on the findings, it is evident that 33 could serve as a prototype for a wide range of practical applications in environmental and biological systems.
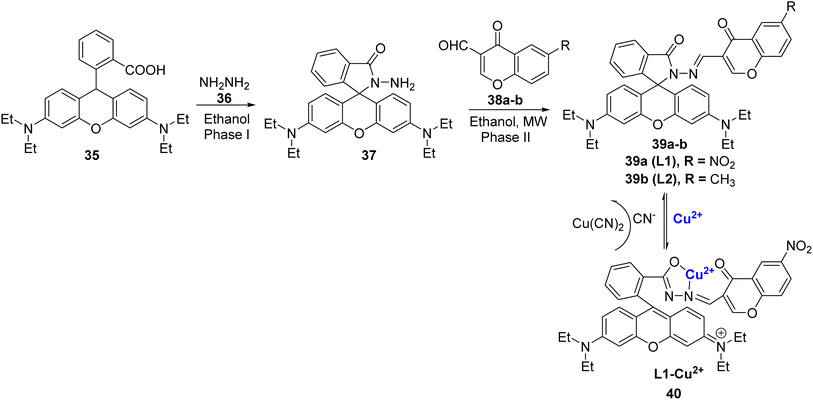
Abebe et al. synthesized two rhodamine B-based chemo-sensors, 39a and 39b using microwave irradiation protocol (Scheme 10).84 They were prepared in a two-step process, firstly by reacting rhodamine B 35 and hydrazine hydrate 36 in ethanol as green solvent to afford compound 37, which further underwent condensation reaction with chromone aldehydes 38a–b to furnish the final products 39a–b (Scheme 10). The ligands 39a–b were found to be selective for Cu2+ ions in the aqueous media. The ability of both the ligands 39a–b was measured by using fluorescence titration, Job's plot, 1H NMR, and DFT studies. Out of the two synthesized sensors, 39a with electron-withdrawing substituent i.e., the nitro group exhibited better response, good selectivity, and high sensitivity to Cu2+ ions in the aqueous solution. The fluorescence emission wavelengths for 39a with Cu2+ ions appeared at 580 nm. The fluorescence intensity was observed mainly due to the formation of ring-open spirolactam 40 with Cu2+ ions whereas the other metal ions produced no substantial effect. Job's plot confirmed that both sensors bind to copper ions in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. The association constant of 39a with Cu2+ was estimated to be 3.12 × 104 M−1 and the limit of detection was found to be 2.11 μM. The in situ synthesis of the L1–Cu2+ complex 40 displayed excellent selectivity for cyanide ions through the metal-displacement method. It is noteworthy that the addition of cyanide ions to complex 40 led to a change in colour from pink to colourless. However, anions, such as Cl−, I−, F−, ClO4−, CH3COO−, HSO4−, H2SO42−, PO43−, SCN2−, HS−, and OH−, did not give any results.
1 stoichiometry. The association constant of 39a with Cu2+ was estimated to be 3.12 × 104 M−1 and the limit of detection was found to be 2.11 μM. The in situ synthesis of the L1–Cu2+ complex 40 displayed excellent selectivity for cyanide ions through the metal-displacement method. It is noteworthy that the addition of cyanide ions to complex 40 led to a change in colour from pink to colourless. However, anions, such as Cl−, I−, F−, ClO4−, CH3COO−, HSO4−, H2SO42−, PO43−, SCN2−, HS−, and OH−, did not give any results.
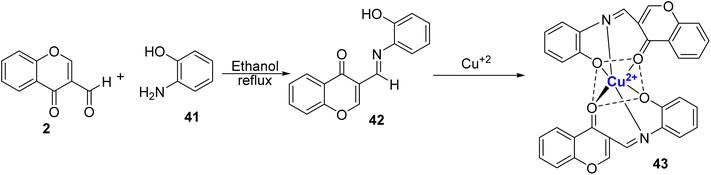
Recently, Alorabi et al. designed a multidentate Schiff base ligand, 42 through condensation of 2 and 2-aminophenol 41 (Scheme 11).85 They examined its role as a colorimetric chemosensor. There was no significant colour change for metal ions other than Cu2+, Fe3+, and V5+. It was proposed that the synthesized Schiff base ligand coordinates with metal ions through nitrogen atom from the azomethine group and with the oxygen atom from the phenolic group, and the ketonic group. Utilizing Job's plot, it was determined that the binding stoichiometry between the ligand and Cu2+ ions was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The detection limit for copper ions was 7.03 μM, and the binding constant was calculated to be 1.37 × 104 M−1. To evaluate the real-world practicality of the chemosensor, various water samples were gathered, encompassing distilled water, household water sources, and a sample from the Al-Aqiq water reservoir dam.
1. The detection limit for copper ions was 7.03 μM, and the binding constant was calculated to be 1.37 × 104 M−1. To evaluate the real-world practicality of the chemosensor, various water samples were gathered, encompassing distilled water, household water sources, and a sample from the Al-Aqiq water reservoir dam.
3. Pharmacological activities of chromone-based Schiff bases
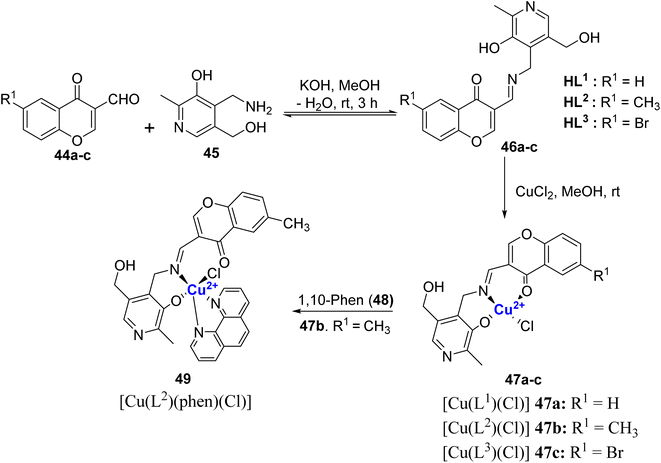
Transition metal ion such as copper that is biocompatible combined with a ligand framework of Schiff base induces a unique multi-modal mechanism of biological action. Incorporation of copper ions into the chromone moiety enhances their chemical and biological diversity. Thus, Schiff base-based metallo-drugs has great potential to combat disease like cancer, and microbial infections and circumvent multi-drug resistance problem. Copper complexes of chromone-based Schiff bases can cause oxidative DNA damage, base modification, and strand breaks.86 The inhibition potential of metal complexes is far better than their ligands because of chelation which causes the delocalization of electrons over the chelate ring and the sharing of positive charge between metals and ligands enhances the lipophilic nature of the complex.87 These factors boost the penetration of the metal complexes across the lipid membrane of the microbial cell wall thereby disrupting the membranes, blocking the enzyme's metal binding sites, and stopping the microbial growth.88 Moreover, metal complexes can generate the bioactive compound in situ which could be another mode of action for metallodrugs. In this regard, chromone-based Cu2+ complexes Schiff bases with invigorating structural and electronic properties have received substantial attention.In 2022, Nunes et al.89 reported chromone Schiff bases 46a–c synthesized via the condensation of 6-substituted-3-formyl-chromones 44a–c with pyridoxamine 45 (Scheme 12). The Schiff bases 46a–c were then treated with copper(II) chloride in MeOH at room temperature to furnish Cu(II)-Schiff base complexes [Cu(Ln)Cl] 47a–c. Another ternary complex 49 [Cu(L2)(phen)Cl] was also synthesized by the addition of bidentate 1,10-phenanthroline ligand 48 to complex 47b. The synthesized complexes were subjected to elemental analysis such as FT-IR, 1H- and 13C-NMR spectroscopy, UV-Vis, EPR, and mass spectrometry. The investigations indicated that the chloride ion is bonded to the metal ions, which are coordinated to Schiff bases through donor atoms such as O-phenolate, N-imine, and O-carbonyl in compounds 47a–c and 49.
Amongst all the complexes, [Cu(L2)(phen)Cl] 49 was the most stable and displayed higher stability in aqueous media. The presence of BSA protein further increased its stability. For other metal complexes it was predicted that ligand substitution, metal hydroxide formation, precipitation and partial oxidation in aqueous solution could be the reason for their instability. Compounds 46b, 46c as well as 47c and 49 were tested for their cytotoxic characteristics on human cancer cells (MC7, HeLa, A2780, LN-229, and MCF7) and normal cells (RPE-1) with concentration 1.6–100 μM. The incubation periods ranged from 24–72 h with an interval of 24 h each. The metal complexes showed cytotoxicity in comparison to their ligand precursors but were not selective toward cancer cells. Complex 49 induced DNA cleavage, nicking supercoiled DNA to completion, activated higher levels of ROS and genotoxic damage. It also triggered cell death through apoptosis, as determined by apoptotic body formation, condensation, and fragmentation of DNA and TUNEL assay. The major challenge in this work was low aqueous solubility and less selectivity for cancer vs. normal cells.
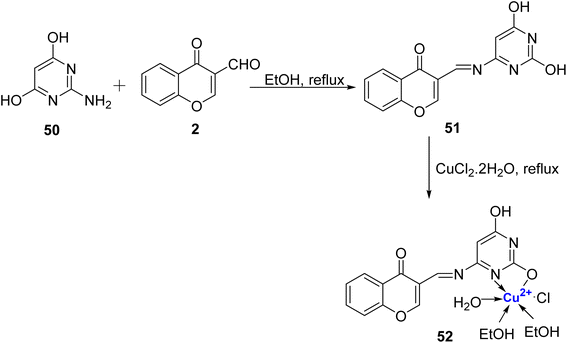
In 2019, Gaber et al.90 developed a Schiff base ligand, (E)-3-((2,6-dihydroxypyrimidin-4-ylimino) methyl)-4H-chromen-4-one 51 that was prepared by condensation of 2 with 4-amino-2,6-dihydroxypyrimidine 50 in absolute ethanol in good yields. Further, Cu2+ metal complexes were made using CuCl2·2H2O. The spectral and magnetic studies revealed that the binding affinity of Cu(II) complex 52 was greater than the ligand 51 and the complexe have octahedral structure (Scheme 13). The ligand and Cu(II), complexes were assessed against E. coli and S. aureus for their antibacterial activity and C. albicans and Aspergillus niger for antifungal activity. However, complex 52 as well as ligand 51 did not exhibit promising antibacterial and antifungal activity, and inhibition results against human cancer cell line HepG2 was also not very encouraging. The IC50 values for ligand 51 and its corresponding complex 52 against HepG2 were found to be 39.56 and 62.73 μg mL−1. The intrinsic DNA-binding constant for Cu(II) complex 52 was found to be 4.3 × 105 M−1 indicating better intercalative-binding interaction with DNA.
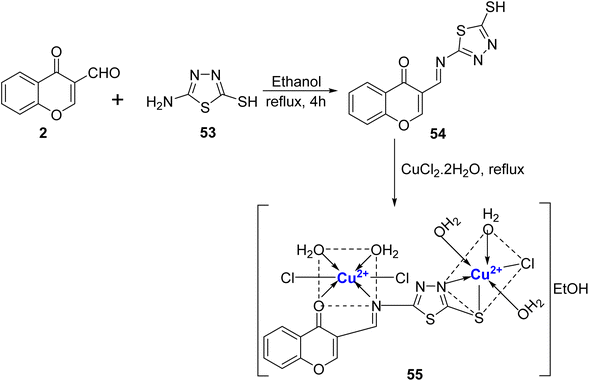
The same research group91 also designed novel Schiff base, 54 and its Cu(II)-complexes (Scheme 14). The Schiff base 54 was synthesized from chromon-3-carbaldehyde 2 and 53 in ethanol and its Cu(II) complex 55 was prepared by refluxing with hydrated metal chloride, CuCl2·2H2O. Characterization of the Cu(II) complex 55 was done by elemental analysis, inductive coupled plasma, X-ray, IR, EI mass, UV-Vis, ESR, and 1H- and 13C-NMR which revealed octahedral shape for the complex. The complexes showed weak antimicrobial and anticancer activity. The IC50 values for the ligand 54 as well as its complex with Cu2+, 55, were quite high i.e. 23.00 μg mL−1 and 18.40 μg mL−1, respectively against HepG2 in comparison to that of doxorubicin, the standard drug (IC50 = 4.73 μg mL−1).
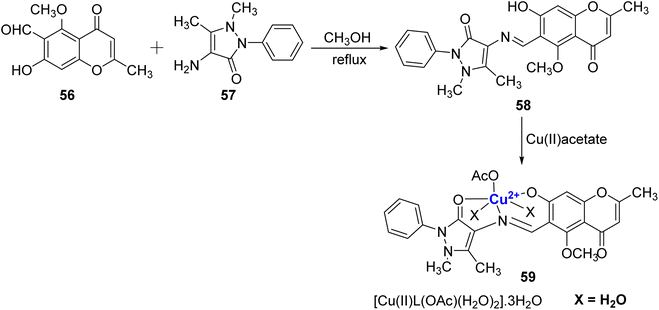
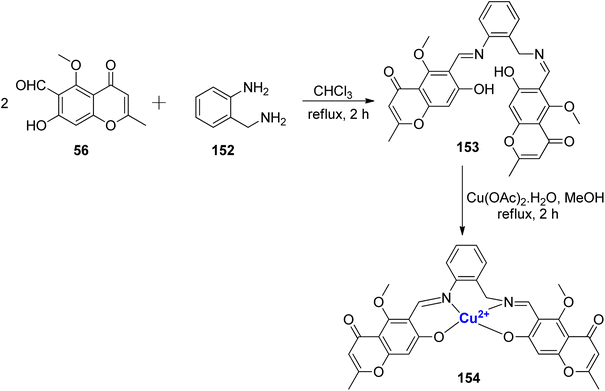
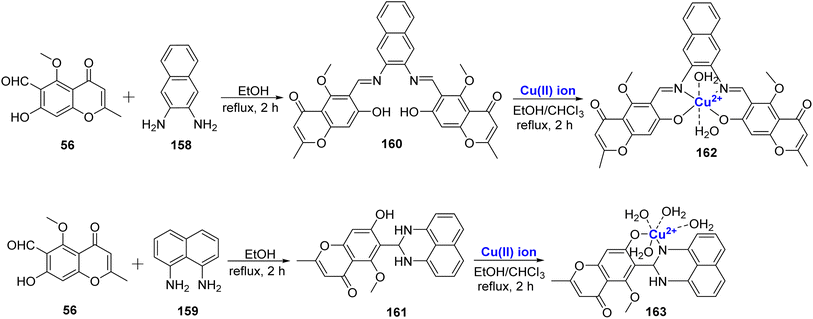
A Cu2+ complexes of a new chromone Schiff base was prepared by Shakdofa et al.92 The ligand 58 was synthesized by refluxing 56 and 57 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 M ratio (Scheme 15). Copper(II)-based chromone Schiff base complex 59 displayed a tetragonal distorted octahedral geometry. It was synthesized by refluxing a methanolic solution of copper(II)-acetate and the ligand 58 for three hours. The designed molecules were analyzed for inhibition of tumor suppressor protein p53 ubiquitination. Ligand 58 exhibited an in vivo IC50 value of 12.13 μM. It is worth noting that inhibiting the interaction between p53 and the oncoprotein, MDM2, resulted in MDM2 blocking the ability of p53 to activate transcription. Copper complex 59 was found to disrupt the p53-MDM2 binding with IC50 values of 0.21 μM in vitro. Moreover, complex 59 with IC50 = 1.79 μM also activated the tumor suppressor p53 present in cancer cells and showed promising results for p53 ubiquitination in vivo when compared to the reference drug i.e., diphenylimidazole (IC50 = 0.26 μM).
1 M ratio (Scheme 15). Copper(II)-based chromone Schiff base complex 59 displayed a tetragonal distorted octahedral geometry. It was synthesized by refluxing a methanolic solution of copper(II)-acetate and the ligand 58 for three hours. The designed molecules were analyzed for inhibition of tumor suppressor protein p53 ubiquitination. Ligand 58 exhibited an in vivo IC50 value of 12.13 μM. It is worth noting that inhibiting the interaction between p53 and the oncoprotein, MDM2, resulted in MDM2 blocking the ability of p53 to activate transcription. Copper complex 59 was found to disrupt the p53-MDM2 binding with IC50 values of 0.21 μM in vitro. Moreover, complex 59 with IC50 = 1.79 μM also activated the tumor suppressor p53 present in cancer cells and showed promising results for p53 ubiquitination in vivo when compared to the reference drug i.e., diphenylimidazole (IC50 = 0.26 μM).
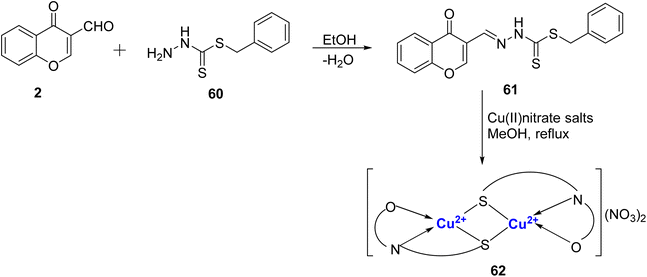
A new monobasic tridentate ligand 61 with sulfur, nitrogen and oxygen of γ-pyrone chelating centers was prepared by the condensation reaction of chromone-3-carboxaldehyde 2 and 5 benzyldithiocarbazate 60 by Adly et al. (Scheme 16).93 Further, dimeric complex with Cu2+, was made. The energy gap of the prepared complexes was lower than the free ligand and because of that these complexes were more reactive. The HOMO → LUMO electron transition and energy gap (ΔE) of the Cu(II) complex 62 was found to be 0.434 eV. The smaller energy gap of validates its high reactivity and low kinetic stability. The dimeric complex 62 was found to exist in square planar geometry. The ligand 61 (IC50 value = 14.90 mg mL−1) and Cu(II)-complex 62 (IC50 value = 7.37 mg mL−1) were active against HepG2 cancer cell lines when compared to the standard cis-platin (IC50 value = 3.67 mg mL−1) and it was the electrophilicity and electronegativity that played a crucial role in their antitumor activities. Complex 62 also showed antimicrobial activity towards the Gram-positive/-negative bacteria as well as with fungus strains.
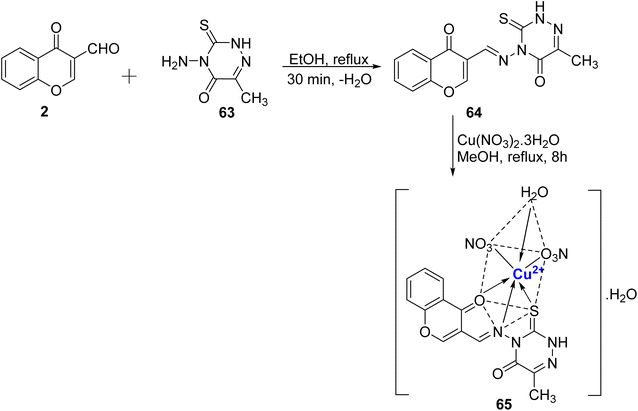
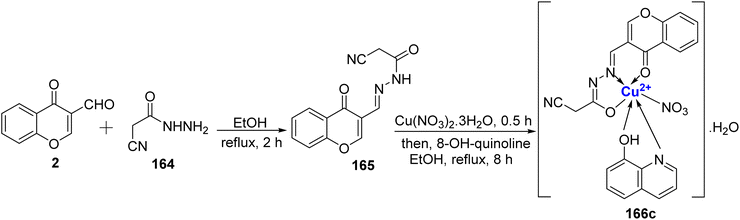
Recently, novel Cu2+ nano complex 65 based on hydrazone ligand 64 containing chromone and triazine moieties were synthesized.94 The ligand 64 was synthesized by refluxing a mixture of 63 and 2 in ethanol (Scheme 17). The Cu2+ nano complex 65 was prepared by refluxing the methanolic solution of the neutral tridentate ligand 64 and Cu(NO3)2·3H2O and was found to possess nano-rode morphology. The nanocomplex 65 exhibited excellent inhibition towards the cancer cell line HepG2 with IC50 value of 0.027 μM. The effectiveness of this sensor can be attributed to two key mechanisms. Firstly, it operates through reactive oxygen species generated by copper ions. These reactive species are known to inflict damage on the DNA within tumor cells, making it a promising tool in cancer treatment. Secondly, the chelation theory comes into play, where the positive charge on the metal ion boosts the ligand's acidity, enabling it to accept protons. This, in turn, strengthens the hydrogen bonds that pivotally enhance the sensor's biological activity. Docking results revealed Cu2+ nano complex 65 as a probable inhibitor of the CDK2 enzyme. Cu2+ nano-complex 65 showed better orientation with amino acids Phe82, Leu83, Asp86, and Lys89. It was also noticed that nano-formulations result in better cytotoxicity and proliferation.
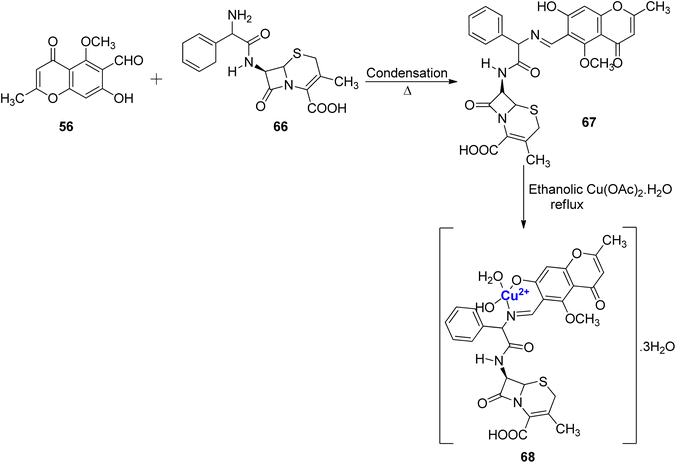
A new Schiff base ligand 67 via the condensation between 56 and cephradine drug 66 was developed (Scheme 18).95 The ligand was monobasic bidentate, and its Cu(II), complex was also prepared. From experimental data, it was found that Cu(II)–ligand complex 68 was in the stoichiometric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (M
1 (M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L). The spectrum of Cu(II)–L complex showed emission band at 443 and 726 nm. The intense fluorescence band emission was assigned to the intra-ligand fluorescence and intramolecular charge transfer between ligand and ion. As corroborated by the photostability studies, the complex 68 was less photostable than the Schiff base ligand 67 as absorption of photonic energy caused irreversible photochemical decomposition–bleaching. The photobiological larvicidal activity was conducted using mosquito larvae to examine the efficiency of complexes in controlling insects using direct sunlight. However, Cu(II) complexes did not show appreciable results. Cytotoxicity of the ligand 67 and complex 68 was also studied on the HepG2 cell line. The Schiff base ligand 67 displayed a better inhibitory effect of 55% towards the HepG2 cancer cell lines when compared to its Cu(II) complex 68 with 35% inhibition.
L). The spectrum of Cu(II)–L complex showed emission band at 443 and 726 nm. The intense fluorescence band emission was assigned to the intra-ligand fluorescence and intramolecular charge transfer between ligand and ion. As corroborated by the photostability studies, the complex 68 was less photostable than the Schiff base ligand 67 as absorption of photonic energy caused irreversible photochemical decomposition–bleaching. The photobiological larvicidal activity was conducted using mosquito larvae to examine the efficiency of complexes in controlling insects using direct sunlight. However, Cu(II) complexes did not show appreciable results. Cytotoxicity of the ligand 67 and complex 68 was also studied on the HepG2 cell line. The Schiff base ligand 67 displayed a better inhibitory effect of 55% towards the HepG2 cancer cell lines when compared to its Cu(II) complex 68 with 35% inhibition.
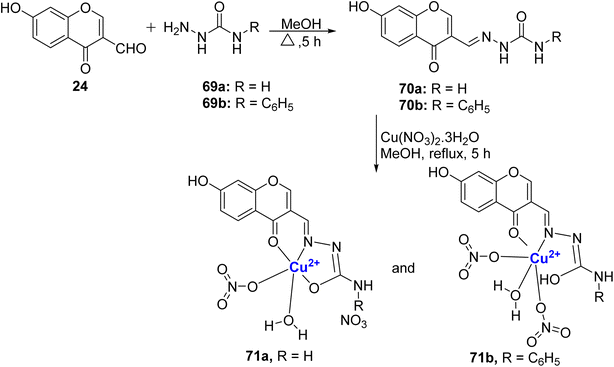
In 2018, Kalaiarasi et al.96 prepared new Schiff base ligands 70a–b by the reaction of 24 with 69a and phenyl semicarbazone 69b (Scheme 19). The Cu(II)-complexes 71a–b were synthesized by the reaction of ligand 70a–b with copper(II)-nitrate salts in methanol under reflux. Single crystal XRD studies established the structure of cationic complex 71a as distorted square pyramidal geometry and the neutral complex 71b as octahedral geometry. The intercalative mode of binding compounds with calf thymus DNA was confirmed by ethidium bromide displacement and viscosity measurement studies. Most pronounced EB-DNA fluorescence emission suppression was demonstrated by complex 71a. The same complex was observed to induce an increase in the separation of base pairs at the intercalation site within DNA, subsequently leading to an extension in the molecular length of the DNA. Furthermore, in a protein binding study, it was demonstrated that the ligands and complexes exhibited a binding capacity with both BSA and HSA through a static quenching mechanism. To assess their potential medical relevance, the compounds were tested against MCF-7 and A549 cancer cell lines (Table 1). Notably, complexes 71a and 71b displayed superior cytotoxicity results when compared to the drug cis-platin. The enhanced activity of 71a may be attributed to its cationic nature, which sets it apart from the neutral octahedral complex 71b. These results were further checked by lactate dehydrogenase release assay and nitric oxide assay. The complexes 71a–b also displayed antimicrobial properties. The antibacterial activity of the compounds had the order: 71b > 71a > 70b > 70a for S. aureus, A. baumannii as well as S. Pneumonie, while for P. aeruginosa, the compounds 71a and 71b had similar MIC values. For Aspergillus niger, Candida tropicalis, and Aspergillus fumigatus, compound 71b had more potency as an antifungal agent over 71a.
| Compounds | IC50 (μM) | |
|---|---|---|
| MCF-7 | A549 | |
| Cu(NO3)2·3H2O | >50 | >50 |
| 70b | 18.44 ± 0.16 | 19.15 ± 0.14 |
| 70a | 15.96 ± 0.14 | 17.19 ± 0.29 |
| 71b | 3.52 ± 0.09 | 3.69 ± 0.06 |
| 71a | 2.49 ± 0.10 | 3.33 ± 0.09 |
| Cisplatin | 15.10 ± 0.05 | 16.79 ± 0.08 |
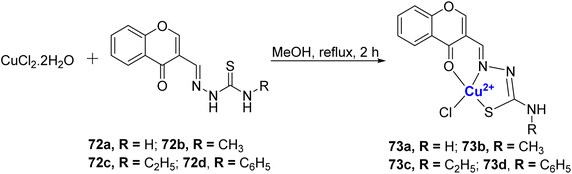
The same research group also reported four water-soluble Cu(II)–chromone complexes 73a–d.97 The complexes 73a–d were synthesized from CuCl2·2H2O and 3-formyl chromone-4(N)-substituted thiosemicarbazones 72a–d ligands and it coordinated with the metal in a tridentate monobasic ONS donor fashion (Scheme 20). The binding ability of synthesized copper thiosemicarbazone to calf thymus DNA was analyzed and the results were in the order 73c > 73b > 73a > 73d. Notably, among the four complexes, the one with an ethyl group, 73c, demonstrated a stronger affinity for DNA as evidenced by its performance in the ethidium bromide (EB) displacement assay and viscosity measurements. The interaction of complexes 73a–d with plasmid pBR322 DNA showed that they cleave the supercoiled DNA. A static quenching mechanism was noted for BSA and HSA serum albumins by the complexes. These Cu(II) complexes 73a–d displayed considerable antibacterial as well as antifungal activity, where 73c was the most potent. Anti-proliferation studies on MCF-7, HeLa, and HaCaT revealed that complexes 73a–d could overcome cis-platin resistance in both cell lines. Significant cytotoxicity was observed with IC50 values of 5.05 ± 0.09 to 7.64 ± 0.13 μM for the MCF-7 cell line and for A549, it was 7.57 ± 0.10 to 8.67 ± 0.13 μM whereas for cisplatin the values are 16.79 ± 0.08 μM for MCF-7 and 15.10 ± 0.05 μM for A549. The synthesized compounds 72a–d and 73a–d were non-toxic toward the HaCaT. Complex 73c was the most effective of all.
The same group further reported four Schiff bases 75a–d and their resultant water-soluble Cu(II)-complexes 76a–d.98 The Cu(II)-complexes 76a–d were prepared from different thiosemicarbazones 75a–d, which were initially synthesized by refluxing the 24 with thiosemicarbazide 74a–d in glacial acetic acid for 5 hours (Scheme 21). The compounds showed good antibacterial and antifungal properties towards the different tested microbial species. For fungi T. rubrum and C. albicans, the compounds displayed the activity in the following order: 76c > 76b > 76a > 76d; for C. tropicalis: 76c > 76d > 76b > 76a; for A. fumigatus: 76c > 76a > 76b > 76d; for A. niger: 76c > 76b > 76d > 76a. Complex 76c was the most effective against S. aureus and P. aeruginosa and activity order was as follows, 76c > 76d > 76b > 76a. In the case of S. pneumoniae, the activities of the complexes followed the order 76c > 76b > 76a > 76d, and against A. baumannii, complex 76d stood out with 76d > 76a > 76c > 76b. The cytotoxic assessment was also conducted against MCF-7 and A549 cells. Cu(II)-complexes followed the order with IC50 values for MCF-7 as 76c (2.94 ± 0.09 μM) > 76b (3.71 ± 0.06 μM) > 76d (3.87 ± 0.08 μM) > 76a (4.21 ± 0.09 μM); for A549 the inhibitory activity was as follows: 76c (2.31 ± 0.07 μM) > 76b (3.20 ± 0.05 μM) > 76d (4.00 ± 0.09 μM) > 76a (4.30 ± 0.09 μM). The results were better than the standard drug cis-platin for which IC50 for MCF-7 is 15.10 ± 0.05 μM and for A549 it is 16.79 ± 0.08 μM. It is noteworthy that complex 76c which contains an electron-rich ethyl group displayed the highest activity that further authenticated by LDH and NO release assays. The binding affinity of the complexes with calf thymus DNA confirmed intercalative binding mode, which was also supported by EB displacement and viscosity measurements. Also, the copper complexes quenched the fluorescence of serum albumins through a static mechanism. It is motivating to note that N-terminal ethyl substituted thiosemicarbazone exhibited higher cytotoxicity and thus it will be interesting to assess compounds with longer alkyl chain lengths.
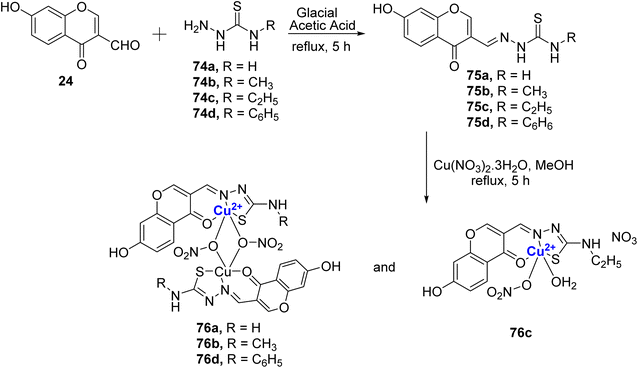 | ||
| Scheme 21 Schiff base ligands 75a–d, were synthesized, and subsequently, Cu(II)-complexes 76a–d were formed. | ||
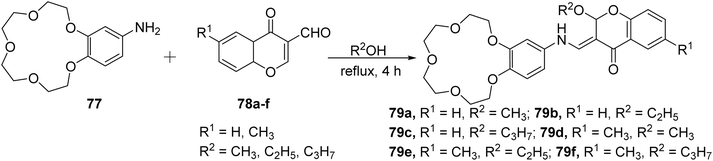
Sahin Gul et al. synthesized a series of chromone-crown ether-based Schiff base ligands 79a–f.99 The chromone-crown ethers 79a–f were prepared by the reaction of 77 with 6-substituted-3-formylchromones 78a–f (Scheme 22). The synthesized Schiff base ligands 79a–f were tested as chemosensors using UV-visible and fluorescence spectroscopy and were found to be selective for Cu2+ and Fe3+ ions in the presence of various other competing ions. A sharp blue shift in the absorption spectrum and fluorescence quenching was detected for chromone compounds 79a–f, on increasing the metal concentration. The synthesized ligands were also found active against both Gram-positive, Gram-negative bacteria and displayed good antifungal activity.
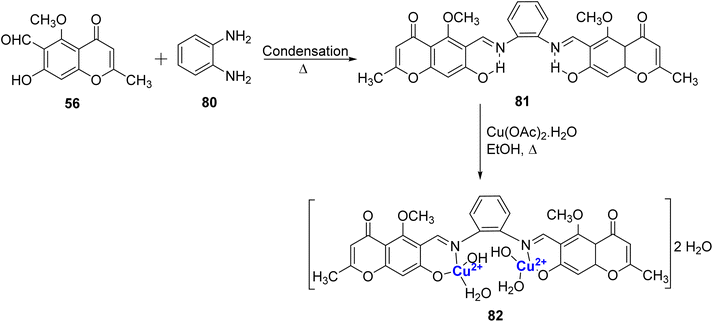
Synthesis of a Cu(II)-complex 82 from Schiff base ligand 81 has been attempted.100 The ligand 81 was prepared by the reaction amid 80 and 56 (Scheme 23). Characterization of both the ligand 81 and the complex 82 was done by elemental analysis, Mass, FT-IR, thermal analysis, electronic spectra, magnetic susceptibility measurements, and conductivity. The analytical data confirmed 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry for the Cu2–81 complex with square planar geometry. However, the copper complex showed minimal biological activity towards S. aureus, B. Subtilis, P. aeruginosa and E. coli bacteria. This may be due to their lower permeability across the bacterial cell membrane. The studies show that penetration of the complexes through the lipid membrane is crucial for the inhibition of microbial growth.
1 stoichiometry for the Cu2–81 complex with square planar geometry. However, the copper complex showed minimal biological activity towards S. aureus, B. Subtilis, P. aeruginosa and E. coli bacteria. This may be due to their lower permeability across the bacterial cell membrane. The studies show that penetration of the complexes through the lipid membrane is crucial for the inhibition of microbial growth.
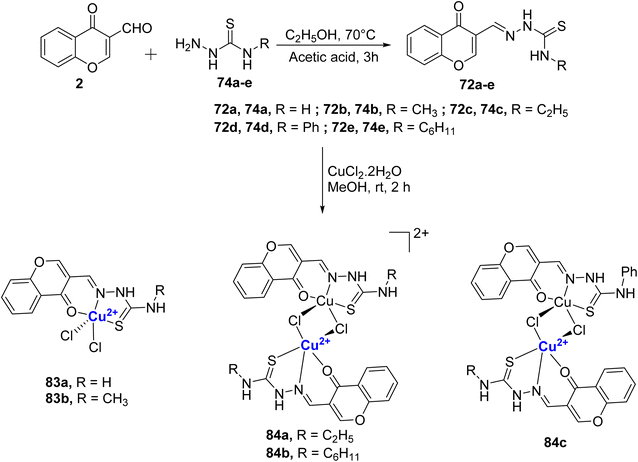
Balakrishnan et al. synthesized different chromone appended thiosemicarbazone ligands 72a–e by refluxing 2 with thiosemicarbazides 74a–e and treated them with copper salt to procure copper complexes 83a–b and 84a–c (Scheme 24).101 It was inferred that the variation in the terminal N-substitution in the ligands influenced the complexes' stoichiometry and due to the bulkiness of –C2H5, –C6H11 and –C6H5 in the ligands, dicationic bimetallic complexes 84a–b and neutral bimetallic 84c were formed. Monometallic complexes of Cu(II), 83a and 84b were obtained from 72a and 72b, respectively. The complexes were found to be stable under physiological conditions. The designed complexes 83a–b and 84a–c displayed catecholase-mimicking activity, and the result showed that except 84c with the bulky phenyl group, all other complexes 83a–b, 84a–b could oxidize 3,5-di-tert-butylcatechol into 3,5-di-tert-butylquinone molecule in the presence of air or aerobic conditions. It was seen that the bimetallic complexes 84a and 84b underwent dissociation into monomers as a necessary step to participate in the catalytic cycle. The catalytic activity followed the order 84a > 84b > 83b > 83a. Phosphatase like activity of the complexes were also studied with the help of 4-nitrophenylphosphate (4-NPP). The catalytic ability of the Cu(II) complexes to hydrolyse the phosphomonoester followed the order 84a > 84b > 83a > 83b > 83d. Complexes 84a–b showed superior radical scavenging activity due to their cationic nature and electron-releasing group. On the other hand, mononuclear 83b with electron-donating methyl group showed better activity in comparison to the binuclear complex 84c with an electron-withdrawing group. Each complex exhibited the capacity to prevent hemolysis and, importantly, did not display any toxicity towards red blood cells. Three complexes 84a–c also showed better cytotoxicity towards the HeLa-cancer cells exhibiting IC50 values of 2.24 μM (84a), 2.25 μM (84b), and 3.77 μM (84c) that is two times higher activity when compared to the standard drug cis-platin. The complex 84a–b displayed full inhibition of the colony formation at 10 μM. The results are promising and further development could lead to a potential anticancer metallodrug.
Slomiak et al.102 prepared a library of hydrazine 87a–h and hydrazide derivatives 88 of 3-formylchromone 2. The ligands 87a–h and 88 were synthesized by reacting 2 with different hydrazines 85a–h or hydrazide 86 (Scheme 25). The neutral mononuclear copper(II)-complex 89 was synthesized by the treatment of the ligand 88 with copper(II) chloride. On studying their antimicrobial and antiproliferative properties, it was observed that the compounds 87a–h, 88, and 89 were capable of inhibiting the growth of microorganisms. Complex 89 was found to have improved antiproliferative properties than ligand 88, with its IC50 value 35.01 μmol L−1 for the L929 and 0.04 μmol L−1 for EA.hy926.
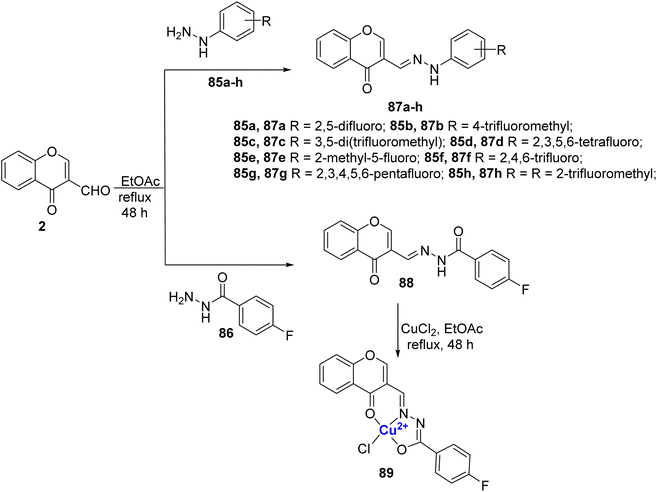 | ||
| Scheme 25 Hydrazine derivatives 87a–h and hydrazide derivatives 88, originating from 3-formylchromone, were synthesized, and Cu(II)-complex denoted as 89 was also prepared. | ||
Copper complexes 91a–f were synthesized from the ligand, 3-formyl-6-methylchromone-4-phenylthiosemicarbazone 8 using copper(II) salt solutions in molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (M
2 (M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
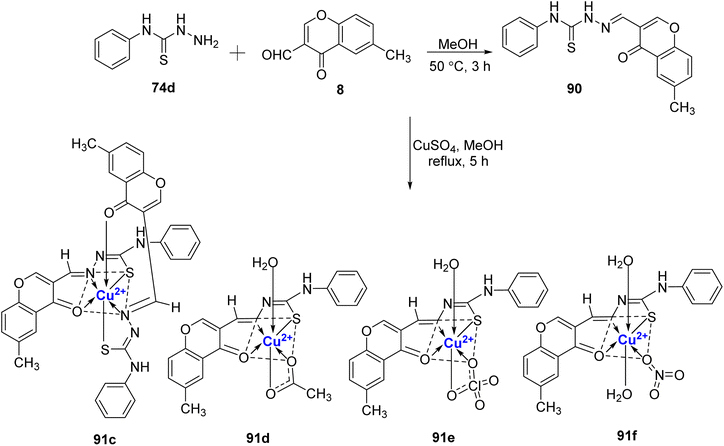
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L) by Ilies et al.103 The ligand was synthesized by treating 4-phenylthiosemicarbazide 74d with 3-formyl-6-methylchromone 8 in a methanolic solution (Scheme 26).104 Complex 91a was found to have a distorted square-planar shape and complex 91b was found to be square-pyramidal. All of the Cu(II)-complexes 91a–f exhibited antimicrobial activity against S. aureus, E. faecalis, E. coli, S. enteritidis and C. albicans. Complex 91e displayed the most promising activity towards all the strains with MIC values between 16 μg mL−1 to 64 μg mL−1 and it was attributed to the bulky ClO4− anion. The antifungal and antibacterial data showed that the metal complexes 91a–f have higher activity in comparison to the free ligand 90.
L) by Ilies et al.103 The ligand was synthesized by treating 4-phenylthiosemicarbazide 74d with 3-formyl-6-methylchromone 8 in a methanolic solution (Scheme 26).104 Complex 91a was found to have a distorted square-planar shape and complex 91b was found to be square-pyramidal. All of the Cu(II)-complexes 91a–f exhibited antimicrobial activity against S. aureus, E. faecalis, E. coli, S. enteritidis and C. albicans. Complex 91e displayed the most promising activity towards all the strains with MIC values between 16 μg mL−1 to 64 μg mL−1 and it was attributed to the bulky ClO4− anion. The antifungal and antibacterial data showed that the metal complexes 91a–f have higher activity in comparison to the free ligand 90.
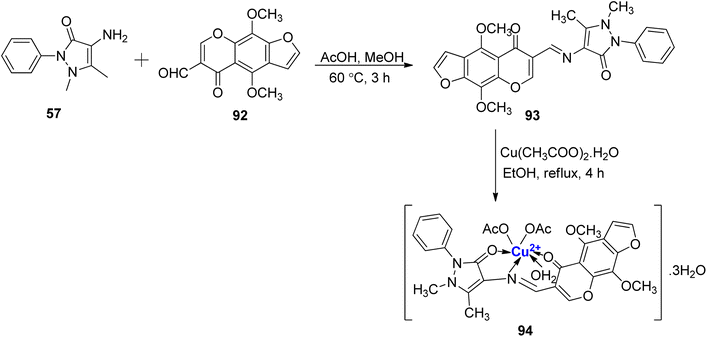
Furochromone-based Cu(II)-complexe of Schiff base, resulting from the reaction of 57 and 92, has been reported.105 Initially, the furochromone based ligand 93 was synthesized from 57 and 92 (Scheme 27). Copper complex 94 was prepared by the treatment of ligand 93 with the metal salt Cu(CH3COO)2·H2O. The structure of the complex 94 was reported to be of distorted octahedral geometry that was confirmed via spectral techniques and elemental analysis. All substances were tested for their in vitro antimicrobial activity. The findings demonstrated that copper complex 94 to be effective against C. albicans and A. niger. It showed moderate activity against E. coli and S. aureus whereas with A. faecalis and B. subtilis, very little activity was reported.
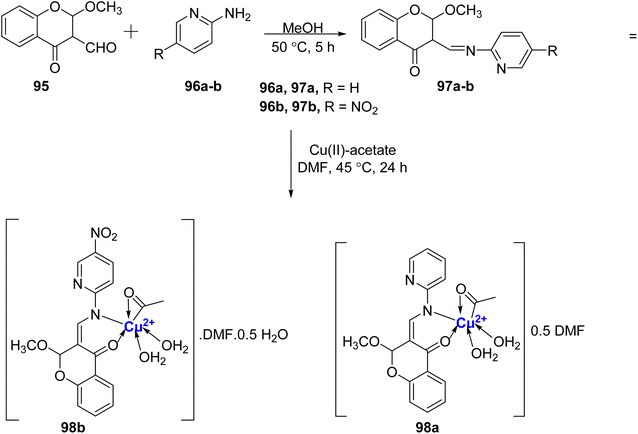
In the same year, Cu(II)-chromanone complexes 98a–b was reported by Jose et al.106 Firstly, the methoxy-substituted ligands 97a–b were synthesized by the reaction of 95 with 2-aminopyridine 96a/2-amino-5-nitropyridine 98b in the presence of methanol (Scheme 28). The complexes 98a–b were then prepared by treating the ligands 97a–b with the metal salt at 45 °C. The Cu(II)-complexes 98a–b thus synthesized were found to show exceptional stability due to tetragonal distortion and Jahn–Teller effect. Among the prepared compounds, The complex CuL 98b exhibited the most potent α-amylase inhibitory activity, yielding an IC50 value of 0.251 ± 0.2 mM. Conversely, the complex CuL 98a demonstrated the highest α-glucosidase activity, with an IC50 value of 0.060 ± 0.3 mM. Furthermore, these compounds were screened for their antimicrobial effectiveness. Moreover, the complexes 98a–b also displayed excellent antibacterial activity with MIC of 15.3 μg mL−1 against S. aureus which was found equivalent to that of the standard drug.
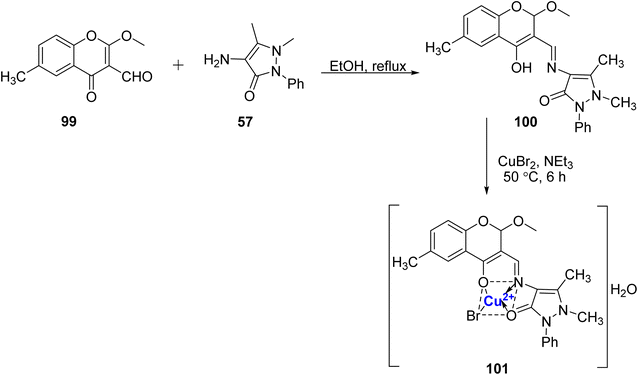
A Cu(II), complex was designed and prepared using Schiff base 100 was synthesized by Pahontu et al.107 The ligand 100, was prepared from 57 and 99 in ethanol (Scheme 29), and it was further treated with methanolic CuBr2 solution to yield the desired complex 101 in 73% yield. The structure of complex 101 was confirmed as tetrahedral with the aid of analytical techniques. Copper complex 101 showed both bacteriostatic and bactericidal properties against Gram-positive (concentration: 0.007–0.25 mg mL−1) as well as Gram-negative bacteria (concentration: 0.0312–0.5 mg mL−1). Additionally, ten cancer cell lines, namely MSC, A375, B16 4A5, HT-29, MCF-7, HEp-2, BxPC-3, RD, MDCK, and L20B, were employed to evaluate the in vitro antiproliferative properties of the ligand and complex. It was found that ligand 100 also has promising antimicrobial properties. The ligand 100 showed promising antifungal results, but the copper complex 101 was found to be inactive in comparison to the standard drugs nystatin and miconazole.
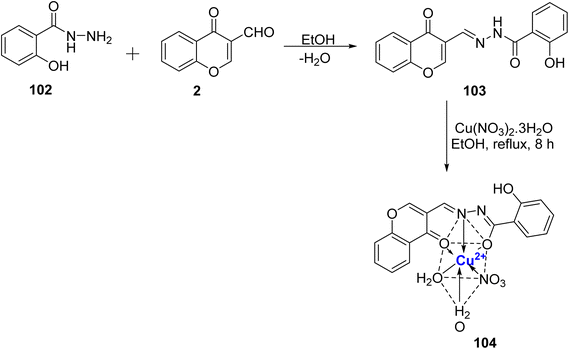
A new class of octahedral nano-complex of Cu2+ 104 with chromone Schiff base 103 was prepared by Saif et al.108 (Scheme 30). The complex 104 was synthesized by refluxing 103 with copper–nitrate salt in ethanol as a reaction medium. The synthesized complex 104 exhibited excellent antioxidant activity (IC50 = 0.93 μM) as compared to the standard used (ascorbic acid). The Cu(II) nano-complex 104 demonstrated significant effectiveness in inhibiting the growth of EAC cells, with an IC50 value of 47 μM. This efficacy surpassed that of its parent compound and the other complexes that were synthesized. Moreover, Cu(II) 104 nano-complex was also found to show cytotoxic effects and was less toxic than cis-platin. The chemical structure of complex 104 was confirmed via elemental analysis.
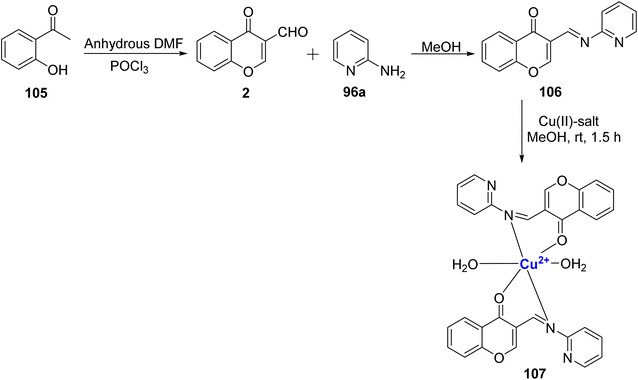
Kavitha et al.109 used 3-formylchromone 2 and 2-aminopyridine 96a as the reactants to synthesize the ligand 106, and its complex 107 with Cu2+ ion (Scheme 31). Characterization of the ligand 106 and complex 107 was done by analytical methods including IR, ESR, XRD, and SEM. Based on magnetic and electronic spectrum data, the complexes had octahedral geometry. The nematicidal and antibacterial effects of the metal complex were stronger than those of the parent ligand. In the presence of H2O2, the ligand and its metal complex DNA cleaving activity was detected.
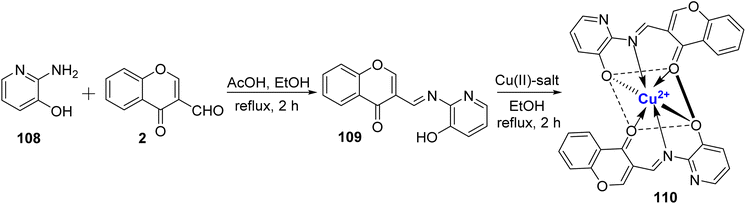
Ammar et al.110 conducted a study in which they synthesized a metal Cu(II) complex 110. These complexe were derived from a tridentate ligand 109, which was prepared using readily available starting materials, 108 and 2, with the assistance of a catalytic amount of acetic acid (Scheme 32). Both the ligand 109 and its corresponding complex 110 were thoroughly characterized using spectral data and elemental analysis. The octahedral geometry of complex 110 was verified through various methods, including DFT calculations, UV-Vis spectroscopy, and ligand field parameters. Furthermore, the researchers assessed the antibacterial properties of both the ligand and the metal complex in vitro against a range of bacterial and fungal strains. The collected findings support the investigated compounds potential as bactericides and fungicides. The most effective cytotoxic compound against malignant cells is the Cu(II) complex. Against B. subtilis, every substance exhibited antibacterial action. The data demonstrates that the Cu(II) complex had substantial activity against S. aureus and E. coli, respectively. Cu(II) complex also displayed the antioxidant activity.
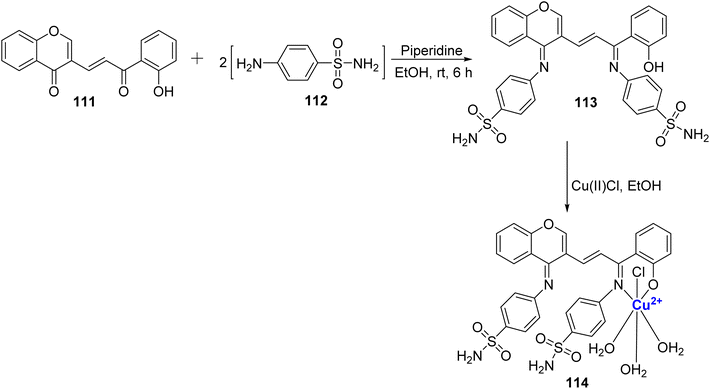
A fluorescent, octahedral, and non-electrolytic Cu(II)-complex 114 was reported by Sumathi et al.111 (Scheme 33). The ligand 113 was synthesized using 111 and sulphanilamide 112 in the presence of piperidine. The structure of ligand 113 and complex 114 was further evaluated via various analytical techniques such as IR, NMR, etc. Generally, mostly metal chelates show higher biological activity because of the chelation theory.112 In this context as well, it's worth noting that most metal chelates exhibit superior antimicrobial activity when compared to ligand 113. The developed complex 114 may also assist as a photoactive compound as shown by its fluorescence studies.
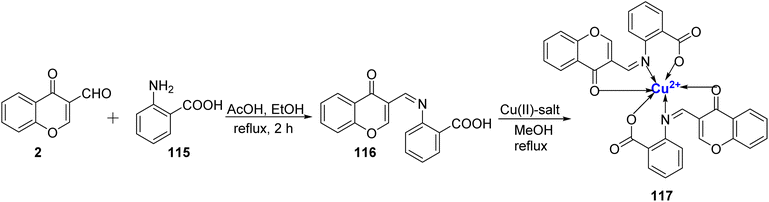
Padmaja et al.113 synthesized a Cu(II) complexes with ligand 116 and further characterized both the ligand 116 as well as the metal complex 117 via elemental/thermal analysis, ESR studies, magnetic susceptibility, and spectroscopic techniques. All six complexes share a common octahedral coordination geometry surrounding the metal ion. Ligand 116 was synthesized through a condensation reaction between 2 and 115 (Scheme 34). In these complexes, the ligand 116 interacts with the metal ion in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometric ratio. It's worth noting that the metal complexes 117 displayed superior antimicrobial properties compared to the unbound ligand 116.
2 stoichiometric ratio. It's worth noting that the metal complexes 117 displayed superior antimicrobial properties compared to the unbound ligand 116.
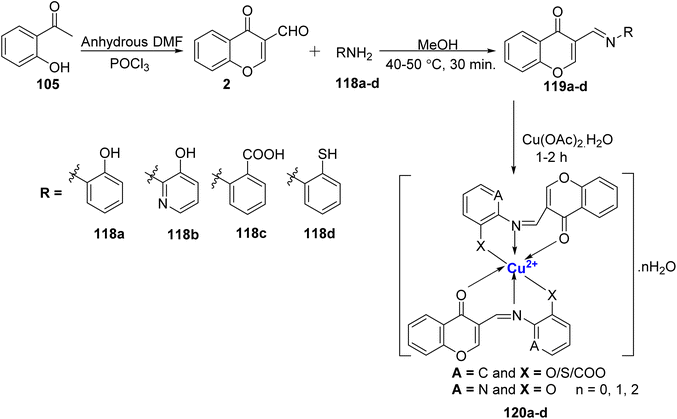
In yet another research by Kavitha et al.,114 fluorescent Cu(II)-complexes 120a–d were synthesized with four Schiff bases, 119a, 119b, 119c, and 119d (Scheme 35). The ligands, 119a–d, along with the corresponding complexes 120a–d, were subjected to comprehensive characterization using techniques such as mass spectrometry, as well as 1H and 13C nuclear magnetic resonance (NMR) spectroscopy. The complexes 120a–d adopted a tetragonally distorted octahedral geometry, while the ligands 119a–d coordinated with the Cu(II) metal ion in a tridentate manner. Significantly, the complexes 120a–d displayed superior antimicrobial properties when compared to the ligands 119a–d. Furthermore, the radical scavenging activities of the synthesized compounds were assessed based on their IC50 values. Notably, complex 120a exhibited a particularly low IC50 value of 0.16 μg mL−1, indicating strong radical scavenging activity. Further, the scavenging properties based on the IC50 values was in the following order: 120a > 120b > 120c > 120d.
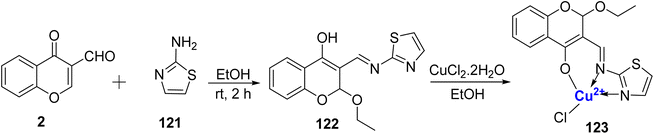
In 2011, Kalanithi et al.115 synthesized coordination compound involving Cu(II), with Schiff base 122. This Schiff base was obtained through the condensation of 3-formyl chromone 2 and 2-aminothiazole 121, as outlined in Scheme 36. The structural confirmation of these complex was established through various spectroscopic techniques, including EPR, NMR, mass spectrometry, and magnetic susceptibility measurements. The ligand 122 coordinated to the metal ion from three sites namely, enolic oxygen, the nitrogen of the thiazole ring as well as from nitrogen of the azomethine group, thereby behaving as a tridentate entity. Due to chelation effects, the copper complex 123 was potent against pathogens. Therefore, chelation affected the biological outcome of the synthesized copper complex. The inhibition ability of the complex 123 against bacteria C. albicans also showed promising outcomes.
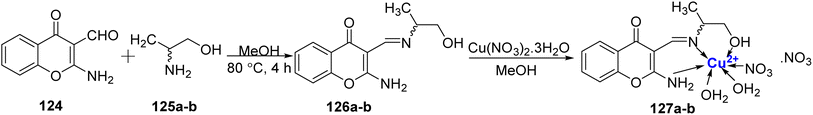
DNA cleavage ability of Cu(II)-metal complex 127a–b was studied by Arjmand et al.116 in 2012. (R)- and (S)-2-amino-3-(((1-hydroxypropan-2-yl)imino)methyl)-4H-chromen-4-one 126a–b were prepared from 124 and 125a–b, which on complexation with Cu(II) ion afford complexes 127a–b (Scheme 37). They were then characterized by NMR, mass, IR, elemental analysis, and molar conductance calculations. It was observed that the complexes 127a–b preferred to attach themselves to the guanine–cytosine region of the DNA molecule and the (R) enantiomer 127a was found to be more active as compared to the (S) enantiomer 127b. Topoisomerase II inhibition property as well as the cytotoxic effects of the complexes 127a–b against human carcinoma lines were also examined. Complex 127a was found to be selective for two cancer cell lines: A2780 (GI50 value = 17.6 μg mL−1) as well as MCF-7 (GI50 value = 18.4 μg mL−1), however complex 127b displayed moderate activity with GI50 value of 20 μg mL−1 for A2780 and 26.6 μg mL−1 for MCF-7 cell lines. For cell lines Zr-75-1, SiHa as well as A549, the activity was observed to be very marginal.
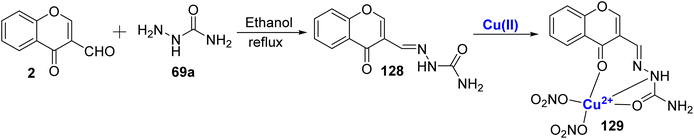
In 2010, Li et al.117 also utilized Cu(II)-complex 129 in studying DNA binding properties. The characterization of 3-carbaldehyde-chromone semicarbazone 128 and its complex with Cu(II) was carried out using a variety of methods, including crystallography. Complexes showed better binding interactions as compared to the free ligand 128 as studied by spectroscopic measurements (Scheme 38). The developed complex 129 interacted with DNA through intercalation binding mode. The antioxidant activity of these compounds is quantified by their IC50 in μM against hydroxyl radicals (HO˙). The IC50 values for ligand 128 and its Cu(II) against HO˙ are 10.170, and 1.195 μM, respectively. Notably, the metal complex exhibit significantly higher scavenging activity against hydroxyl radicals when compared to standard antioxidants like mannitol (IC50: 10.19 μM). For scavenging superoxide anions, the IC50 values for the ligand, and Cu(II) complex are 32.810, and 0.943 μM, respectively. The metal complex 129 exhibit superior antioxidant properties compared to the ligand. Thus, the study showed that the metal ions can act as selective scavenging agents in biological systems and paved a pathway for further advancements in this field.
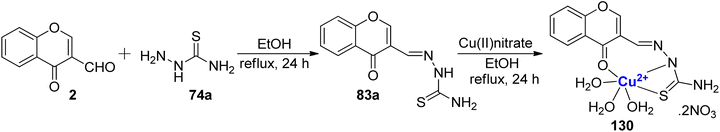
Yang et al.118 developed the transition metal complexes of 83a to study its fluorescence as well as DNA binding by spectral and viscosity studies (Scheme 39). The ligand 83a was synthesized from ethanolic solutions of 3-carbaldehyde chromone 2 and thiosemicarbazide 74a. Ligand 83a was reacted with copper(II)nitrate in ethanol at reflux to afford the complex 130. Moreover, the antioxidant properties (superoxide dismutase activity) for ligand 83a (IC50 = 263.028 μM) and its corresponding Cu2+ complex 130 (0.799 μM) were also tested and found to be significant and higher than that of the standards used (IC50 for vitamin C = 852 μM). The ligand 83a and complex 130 were characterized by various structural methodologies. The Cu(II) complex demonstrates superior antioxidant activity against superoxide and hydroxyl radicals and exhibits stronger scavenging effects.
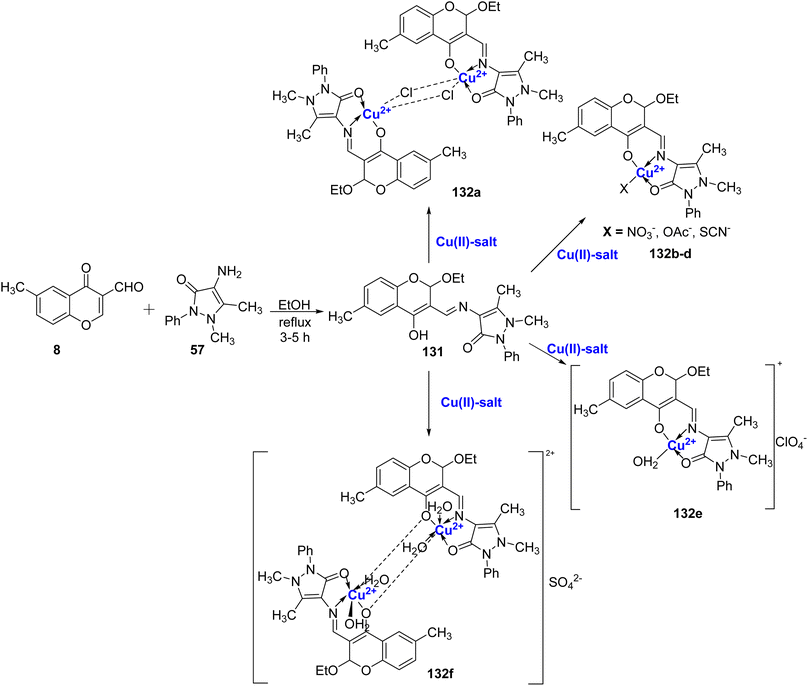
Rosu et al.119 conducted a study in which they synthesized coordination compounds 132a–f with Cu(II) ions, using the Schiff base ligand 131. The Schiff base, 131, was obtained through the reaction between 57 and 8 as outlined in Scheme 40. To characterize these compounds, various techniques such as NMR, FT-IR, UV-Vis, ESR spectroscopy, X-ray diffraction, molar electric conductibility, and elemental analysis were employed. Furthermore, the antibacterial activity of the synthesized compounds was investigated in vitro. The results of the antibacterial study clearly indicated that the antibacterial properties of the Schiff base compounds were significantly enhanced when coordinated with metal ions.
Anitha et al.120 designed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
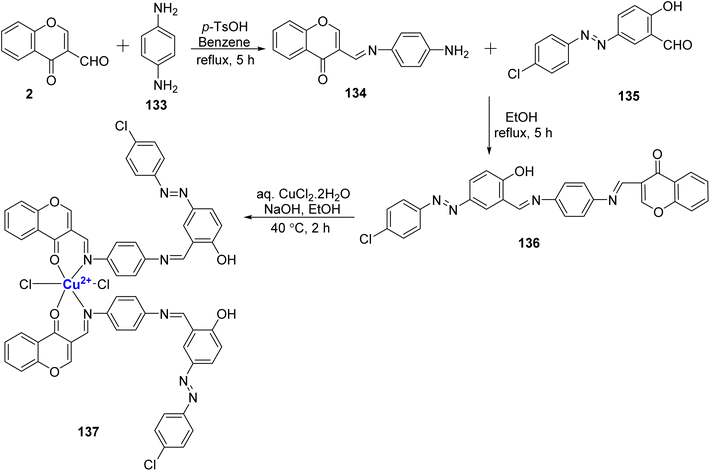
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex of Cu(II) ions in conjunction with azo Schiff base 136. Conductance data indicate that the complex are generally non-electrolytic in nature. The synthesis of Schiff base 136 involved the condensation of p-phenylenediamine 133, 135, and 2. Subsequently, this Schiff base was treated with copper(II) chloride, resulting in the desired metal complex 137 (Scheme 41). The Schiff bases and their corresponding metal complex were also subjected to antibacterial and antifungal studies. The Cu(II) complexes showed promising antibacterial activity towards bacteria, S. aureus, E. coli, S. enterica typhi, and B. subtilis.
2 complex of Cu(II) ions in conjunction with azo Schiff base 136. Conductance data indicate that the complex are generally non-electrolytic in nature. The synthesis of Schiff base 136 involved the condensation of p-phenylenediamine 133, 135, and 2. Subsequently, this Schiff base was treated with copper(II) chloride, resulting in the desired metal complex 137 (Scheme 41). The Schiff bases and their corresponding metal complex were also subjected to antibacterial and antifungal studies. The Cu(II) complexes showed promising antibacterial activity towards bacteria, S. aureus, E. coli, S. enterica typhi, and B. subtilis.
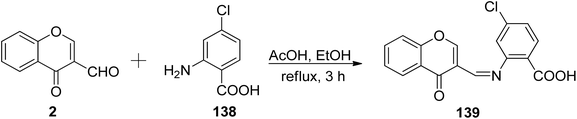
Mendu et al.121 explored the synthesis of the Schiff base known as 4-chloro-2-((4-oxo-4H-chromen-3-yl)methyleneamino)benzoic acid 139, along with a Cu(II) complex (Scheme 42). The Schiff base ligand 139 obtained by the reaction of 2 and 4-chloroanthranilic acid 138. The synthesized ligand 139 and its respective complex were also subjected to antimicrobial assessments against different bacteria using well disc and fusion methods. The metal complex exhibited higher potency against microorganisms when compared to the free Schiff base ligand. The Cu(II)-complex function through redox chemistry in cleaving DNA. The structure (octahedral geometry) and characterization were done via analytical and spectroscopic measurements. The complexation of the ligand 139 was found to occur from three sites: the nitrogen atom of azomethine, the oxygen atom of ketonic functionality as well as the hydroxyl of the carbonyl functional group.
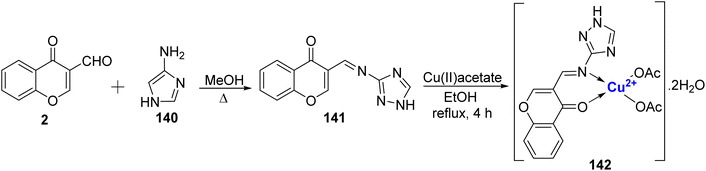
Bheemarasetti et al.122 developed a novel Schiff base ligand known as 3-(((1H-1,2,4-triazol-3-yl)imino)methyl)-4H-chromen-4-one (L) 141 through the reaction of 2 and 140 in methanol (Scheme 43). Additionally, they synthesized Cu(II), complex. Comprehensive characterization of both the ligand 141 and the complex 142 was carried out using techniques such as FT-IR, ESR, UV-Vis, SEM, NMR, mass spectrometry, TGA, and X-ray analysis. The shape of complex 142 was found to be square planar. The complex 142 also demonstrated good antiproliferative and anticancer results compared to the remaining compounds. They could be used as a promising antitumor agent. Additionally, DNA binding studies revealed that these compounds interact with CT-DNA via an intercalative mode.
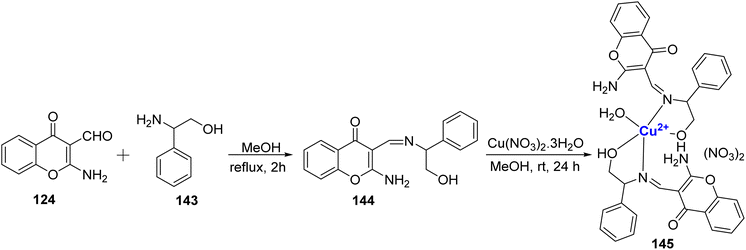
In 2011, Arjmand et al.123 conducted a study in which they synthesized a Cu(II) complex based on chromone and investigated its interactions with DNA molecules, as well as its DNA cleavage properties. The ligand 144 was prepared by combining 124 and 143, (Scheme 44). The Cu(II)-complex 145 was then synthesized with a metal-to-ligand ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Significant absorption enhancements were observed, confirming the binding of complex 145 with the DNA backbone, particularly in the 5′-GMP region. Consistent findings supporting this interaction were obtained through other experimental measurements. Gel electrophoretic mobility assay was employed to assess the complex's artificial nuclease activity, revealing that complex 145 effectively cleaves plasmid pBR322 DNA, transforming it from form I to form II and ultimately generating linearized form III as the complex concentration increases.
2. Significant absorption enhancements were observed, confirming the binding of complex 145 with the DNA backbone, particularly in the 5′-GMP region. Consistent findings supporting this interaction were obtained through other experimental measurements. Gel electrophoretic mobility assay was employed to assess the complex's artificial nuclease activity, revealing that complex 145 effectively cleaves plasmid pBR322 DNA, transforming it from form I to form II and ultimately generating linearized form III as the complex concentration increases.
In a study conducted by Alturiqi et al.,124 they synthesized new complex of Cu(II), 148 by combining 146 with 2, as outlined in Scheme 45. The complex was then evaluated for their potential as agents against microbial infections and tumors. The researchers assessed the antimicrobial properties of complex against a range of bacteria, as well as fungi. The hexacoordinated octahedral copper complex 148 demonstrated the most robust antibacterial activity, with a zone of inhibition ranging from 13 to 20 mm. This enhanced activity was attributed to the larger atomic radius and electronegativity of the Cu(II) ion, which reduced the effective positive charge on the complex and facilitated its interaction with cellular membranes. Additionally, the complex exhibited outstanding scavenging activity against the DPPH radical, with the copper complex showing an IC50 value of 83.28. However, when it came to their impact on human tumor cell lines, including A427, LCLC-103H, and SISO, none of the tested compounds displayed toxicity. Remarkably, the Cu(II) complex displayed moderate activity, with IC50 values of 18.365 μM.
Singh et al.125 conducted a study where they synthesized a metal complex involving Cu(II), using Schiff base ligand 150 (Scheme 46). Through UV absorption studies, they identified characteristic peaks for the Cu(II) complexes, designated as 151, in combination with the chromone ligand. These peaks appeared at 274 nm, 278 nm, and 279 nm, respectively, representing π–π* transitions, and also showed charge transfer transitions in the range of 465–481 nm. The geometric structure of the copper complexes was determined through DFT studies, revealing a distorted octahedral geometry. To investigate how these metal complexes interacted with DNA, the researchers employed various techniques, including UV-vis absorption spectroscopy, fluorescence titration, and viscosity measurements. Changes in spectral position and intensity were observed to assess the interaction between the metal complexes and DNA. Notably, when the metal complexes bound to DNA, a hypochromic effect was observed, accompanied by a bathochromic shift. The copper complexes associated with chromone displayed absorption peaks at 299, 310, and 356 nm due to π–π* transitions. These complexes were found to bind to DNA through non-covalent interactions or by causing the uncoiling of the DNA double helix. As a result, the complexes adopted an intercalation mode of binding, effectively stacking among the aromatic chromophores and DNA base pairs. Fluorescence quenching studies revealed a decrease in emission intensity, signifying the interaction of the complexes with DNA. Further confirmation of the intercalation mode of interaction was provided by replacing EB with the complex molecules in EB-bound DNA. Hydrodynamic viscosity measurements furnished additional evidence by showing that the complexes inserted themselves between the DNA base pairs. This action caused the separation of the DNA double helix, resulting in an increase in DNA length and viscosity.
4. Biomimetic catalytic activity of chromone-based Schiff bases
Copper has a vital role in different enzymatic activities.126 The reason for the significant interest in Schiff base metal complexes is not only because of their high stability and biological activity127,128 but also because of their unique features of functioning as catalysts.129 Within the array of bio-inspired structures capable of emulating catecholase activity, copper complexes of Schiff bases emerge as a particularly promising candidate. While the active site of the catecholase enzyme typically features a hydroxo-bridged dicopper(II) center, it is widely acknowledged that numerous monometallic copper(II) complexes exhibit catecholase activity. In this context, we have presented a study on the synthesis, characterization, and catalytic attributes of certain chromone-based metal complexes of Schiff bases.In 2016, Beyazit et al.130 reported a novel tetradentate, unsymmetrical Schiff base ligand 153 and its Cu2+ ion complex 154. The ligand 153 was obtained by reacting a mixture of 56 (prepared by oxidation of visnagin 131) and 2-aminobenzylamine 152 in CHCl3 for 2 hours under reflux condition (Scheme 47). The complex 154 was obtained as a dark green precipitate by refluxing the ligand 153 and Cu(CH3COO)2·H2O in methanol for 5 hours. The ligand 153 and the complex 154 were confirmed by using characterization techniques such as NMR, mass, elemental analysis, and electronic spectra. The complex 154 complex exhibited catecholase-like biocatalytic activity against the oxidation of 3,5-di-tert-butylcatechol to quinone form. This revealed that complex 154 complex had moderate catalytic activity.
In continuation of their previous work (Scheme 47), they synthesized two chromone-based ligands 156a–b and their corresponding transition metal complexes (Scheme 48).132 The complexes 157a–b thus obtained were screened for their catechol oxidase activity. The study revealed that the presence of substituents on ligands 156a–b significantly influenced the catalytic activity of the resulting metal complexes 157a–b. Notably, electron-donating substituents led to an enhancement in catalytic performance. The oxidation of catechol was found to adhere to first-order kinetics, according to the results of the kinetic measurements. All the synthesized compounds 156a–b and 157a–b were well characterized via thermal/elemental analysis, FT-IR, UV-vis spectroscopy, and NMR.
Beyazit et al.133 also utilized visnagin derivative 56 to synthesize Schiff base ligands 160 and 161 by reacting it with the 2,3-diaminonaphthalene 158 and 1,8-diaminonaphthalene 159 (Scheme 49). The synthesized ligands 160 and 161 were treated with copper salts in a mixture of EtOH/CHCl3 to afford Cu2+–L complexes 162 and 163. The structure of the ligands 160 and 161 as well as the complex 162 and 163 were confirmed via various spectroscopic techniques and elemental analysis. The complexes Cu(II) thus prepared were investigated for its catecholase potential and found to display moderate activity against 3,5-DTBC oxidation.
In 2021, Shebl et al.134 synthesized novel mononuclear Cu(II)–hydrazone complexes 166a–c with the aid of ligand 165 (Scheme 50). Characterization was done by elemental analysis, IR, TEM, powder XRD, thermal analysis, conductivity, mass, and ESR. Three different complexes of Cu(II) were reported: [Cu(L)(NO3)]EtOH 166a, [Cu(L2)]·5H2O 166b, and [Cu(L)(8-HQ)NO3]·H2O 166c. These complexes 166a–c also showed phenoxazinone synthase property by oxidizing 2-aminophenol into the 2-aminophenoxazine-3-one. The complexes 166a–c were found to be active against Candida albicans. The ligand 165 also displayed activity against Candida albicans but was found to be less than that of the complexes 166a–c.
5. Miscellaneous
A tridentate chromone-based ligand 169 was developed by Alaghaz et al.135 When an equimolar mixture of Girard T (2-ethyl-5-methoxy-6-formyl-7-hydroxy chromone) 167 and trimethylammoniumacetyl hydrazine chloride 168 was refluxed, it gave chromone-based Schiff base ligand 169 (Scheme 51). This tridentate ligand which was confirmed via an elemental analysis as well as spectral data, was further utilized by the authors to synthesize its Cu(II)-complex 170 which was found to be non-electrolytic in nature. DFT calculations affirmed the octahedral geometry of complex 170.6. Conclusion and future perspectives
Schiff bases hold a significant position within the realm of organic compounds, owing to their pharmacological attributes and their capacity to establish stable bonds with transition metal ions, notably copper. Copper complexes featuring Schiff bases have garnered notable attention in recent times because of their diverse applications in biological processes and their role in the advancement of novel therapeutic drugs. Consequently, chromone-based Schiff base complexes with copper constitute a noteworthy category of compounds. It has been revealed that Cu(II) complexes formed with chromone Schiff bases exhibit potent in vivo antitumor properties by impeding DNA replication in tumor cells, thereby inhibiting tumor growth. These compounds have also gained prominence for their utility as not only anticancer agents but also as antimicrobial agents, antioxidants, and more.Nevertheless, there remain certain challenges, particularly concerning water solubility and selectivity issues between cancer and normal cells. These challenges can potentially be addressed by incorporating them into nano-level formulations. Furthermore, Schiff base ligands have demonstrated their potential as excellent probes for detecting cupric ions. Chromone-based Schiff base ligands, in particular, exhibit high specificity and selectivity in detecting Cu2+ ions, with potential applications in environmental and biological monitoring, as well as various pharmaceutical applications.
Abbreviations
| AAS | Atomic absorption spectroscopy |
| HIV | Human immunodeficiency virus |
| NMR | Nuclear magnetic resonance |
| CS | Colorimetric sensor |
| U. S. | United States |
| FTIR | Fourier transform infra-red |
| UV-Vis | Ultraviolet-visible |
| EPA | Environmental Protection Agency |
| TGA | Thermal Gravimetric Analysis |
| EPR | Electron paramagnetic resonance |
| IR | Infra-red |
| EI Mass | Electron ionization mass |
| HRMS | High resolution mass spectrometry |
| TGA | Thermal gravimetric analysis |
| ICP | Inductively coupled plasma |
| 5-BDTC | 5-Benzyldithiocarbazate |
| DFT | Density functional theory |
| PNT | Para-Nitrotoluene |
| ESR | Electron spin resonance |
| FESEM | Field emission scanning electron microscopy |
| DTCB | N-methyl-5-benzyldithiocarbazate |
| TEM | Transmission electron microscopy |
| WHO | World Health Organization |
| SEM | Scanning electron microscopy |
| DTBC | Di-tert-butylcatechol |
| CTAB | Cetrimonium bromide |
| TG–DSC | Thermogravimetry and differential scanning colorimetry |
| TD-DFT | Time dependent density-functional theory |
| EPDM | Ethylene propylenediene monomer |
| XRD | X-ray diffraction |
| DAN | Diaminonaphthalene |
| IC50 | Half-maximal inhibitory concentration |
| DNA | Deoxyribonucleic acid |
| GMP | Guanosine monophosphate |
| GI50 | Growth inhibition by 50% |
| CT-DNA | Circulating-tumor DNA |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We express our gratitude to the University of Delhi's Institute of Eminence for their generous financial support, which has greatly contributed to bolstering our research and development efforts. Additionally, Divya Mathur extends her appreciation for the Navdhara research grant awarded by Daulat Ram College, University of Delhi. Furthermore, Aditi Arora and Sumit Kumar convey their thanks to CSIR, New Delhi, India, for granting them the Senior Research Fellowship award.References
- J. Reis, A. Gaspar, N. Milhazes and F. Borges, Chromone as a Privileged Scaffold in Drug Discovery: Recent Advances, J. Med. Chem., 2017, 60, 7941–7957 CrossRef CAS PubMed.
- G. W. Kabalka and A. R. Mereddy, Microwave-assisted synthesis of functionalized flavones and chromones, Tetrahedron Lett., 2005, 46, 6315–6317 CrossRef CAS.
- S. Tsanova-Savova and F. Ribarova, Flavonols and Flavones in some Bulgarian Plant Foods, Pol. J. Food Nutr. Sci., 2013, 63, 173–177 CrossRef CAS.
- S. Kumar, B. K. Singh, A. K. Pandey, A. Kumar, S. K. Sharma, H. G. Raj, A. K. Prasad, E. V. der Eycken, V. S. Parmar and B. Ghosh, A chromone analog inhibits TNF-α induced expression of cell adhesion molecules on human endothelial cells via blocking NF-κB activation, Bioorg. Med. Chem., 2007, 15, 2952–2962 CrossRef CAS PubMed.
- S. K. Sharma, S. Kumar, K. Chand, A. Kathuria, A. Gupta and R. Jain, An Update on Natural Occurrence and Biological Activity of Chromones, Curr. Med. Chem., 2011, 18, 3825–3852 CrossRef CAS PubMed.
- A. Kumar, B. K. Singh, N. K. Sharma, K. Gyanda, S. K. Jaim, Y. K. Tyagi, A. S. Baghel, M. Pandey, S. K. Sharma, A. K. Prasad, S. C. Jain, R. C. Rastogi, H. G. Raj, A. C. Watterson, E. V. der Eycken and V. S. Parmar, Specificities of acetoxy derivatives of coumarins, biscoumarins, chromones, flavones, isoflavones and xanthones for acetoxy drug: Protein transacetylase, Eur. J. Med. Chem., 2007, 42, 447–455 CrossRef CAS PubMed.
- S. Amaral, L. Mira, J. M. F. Nogueira, A. P. da Silva and M. H. Florêncio, Plant extracts with anti-inflammatory properties-a new approach for characterization of their bioactive compounds and establishment of structure-antioxidant activity relationships, Bioorg. Med. Chem., 2009, 17, 1876–1883 CrossRef CAS PubMed.
- K. M. Khan, N. Ambreen, U. R. Mughal, S. Jalil, S. Perveen and M. I. Choudhary, 3-Formylchromones: Potential anti-inflammatory agents, Eur. J. Med. Chem., 2010, 45, 4058–4064 CrossRef CAS PubMed.
- E. Venkateswararao, V. K. Sharma, M. Manickam, J. Yun and S.-H. Jung, Synthesis and SAR studies of bis-chromenone derivatives for anti-proliferative activity against human cancer cells, Bioorg. Med. Chem. Lett., 2014, 24, 5256–5259 CrossRef CAS.
- B.-D. Wang, Z.-Y. Yang, M.-H. Lü, J. Hai, Q. Wang and Z.-N. Chen, Synthesis, characterization, cytotoxic activity and DNA binding Ni(II) complex with the 6-hydroxy chromone-3-carbaldehyde thiosemicarbazone, J. Organomet. Chem., 2009, 694, 4069–4075 CrossRef CAS.
- G. Atassi, P. Briet, J.-J. Berthelon and F. Collonges, Synthesis and antitumor activity of some 8-substituted-4-oxo-4H-1-benzopyrans, Eur. J. Med. Chem., 1985, 20, 393–402 CAS.
- A. A. el-Gammal and R. M. Mansour, Antimicrobial activities of some flavonoid compounds, Zentralbl. Microbiol., 1986, 141, 561–565 CrossRef CAS.
- J. Nawrot-Modranka, E. Nawrot and J. Graczyk, In-vivo antitumor, in vitro antibacterial activity and alkylating properties of phosphorohydrazine derivatives of coumarin and chromone, Eur. J. Med. Chem., 2006, 41, 1301–1309 CrossRef CAS PubMed.
- T. Zhou, Q. ShIIi, C.-H. Chen, H. Zhu, L. Huang, P. Ho and K.-H. Lee, Anti-AIDS agents 79.Design, synthesis, molecular modelling and structure-activity relationships of novel dicamphanoyl-2’,2’-dimethyldihydropyranochromone (DCP) analogs as potent anti-HIV agents, Bioorg. Med. Chem., 2010, 18, 6678–6689 CrossRef CAS PubMed.
- P. B. Kaufman, J. A. Duke, H. Brielmann, J. Boik and J. E. Hoyt, A comparative survey of leguminous plants as sources of the isoflavones, genistein and daidzein:implications for human nutrition and health, J. Altern. Complementary Med., 1997, 3, 7–12 CrossRef CAS PubMed.
- A. Kandil, W. Gobran, H. A. Samaan and H. Abu-Shady, The spasmolytic potential of a new khellin derivative, J. Drug Res., 1977, 9, 35 CAS.
- R. S. Keri, S. Budagumpi, R. K. Pai and R. G. Balakrishna, Chromones as a privileged scaffold in drug discovery: A review, Eur. J. Med. Chem., 2014, 78, 340–374 CrossRef CAS.
- D. Vedaldi, S. Caffieri, F. Dall'Acqua, L. Andreassi, L. Bovalini, P. Martelli and P. Khellin, a naturally occurring furochromone, used for the photochemotherapy of skin diseases: mechanism of action, Farm. Sci., 1988, 43, 333 CAS.
- C. F. M. Silva, V. F. Batista, D. C. G. A. Pinto and M. S. Artur, Challenges with chromone as a privileged scaffold in drug discovery, Expert Opin. Drug Discovery, 2018, 13, 795 CrossRef CAS.
- E. Huwait and M. Mobashir, Potential and Therapeutic Roles of Diosmin in Human Diseases, Biomedicines, 2022, 10, 1076 CrossRef CAS.
- E. F. DeRango-Adem and J. Blay, Does Oral Apigenin Have Real Potential for a Therapeutic Effect in the Context of Human Gastrointestinal and Other Cancers?, Front. Pharmacol, 2021, 8, 681477 CrossRef PubMed.
- R. Ruffmann, A Review of Flavoxate Hydrochloride in the Treatment of Urge Incontinence, J. Int. Med. Res., 1988, 16, 317–330 CrossRef CAS.
- M. Pervaiz, S. Sadiq, A. Sadiq, U. Younas, A. Ashraf, Z. Saeed and A. Adnan, Azo-Schiff base derivatives of transition metal complexes as antimicrobial agents, Coord. Chem. Rev., 2021, 447, 214128 CrossRef CAS.
- D. Djamel, T. Douadi, S. Issaadi and S. Chafaa, Adsorption and corrosion inhibition of new synthesized thiophene Schiff base on mild steel X52 in HCl and H2SO4 solutions, Corros. Sci., 2014, 79, 50–58 CrossRef.
- M. S. Refat, H. A. Saad, A. A. Gobouri, M. Alsawat, A. M. Adam and S. M. El-Megharbel, Charge transfer complexation between some transition metal ions with azo Schiff base donor as a smart precursor for synthesis of nano oxides: An adsorption efficiency for treatment of Congo red dye in wastewater, J. Mol. Liq., 2022, 345, 117140 CrossRef CAS.
- S. K. Saha, M. Murmu, N. C. Murmu and P. Banerjee, Synthesis, characterization and theoretical exploration of pyrene-based Schiff base molecules as corrosion inhibitor, J. Mol. Struct., 2021, 1245, 131098 CrossRef CAS.
- C. Verma and M. A. Quraishi, Recent progresses in Schiff bases as aqueous phase corrosion inhibitors: Design and applications, Coord. Chem. Rev., 2021, 446, 214105 CrossRef CAS.
- C. J. Liu, Z. Y. Yang, L. Fan, X. L. Jin, J. M. An, X. Y. Cheng and B. D. Wang, Novel optical selective chromone Schiff base chemosensor for Al3+ ion, J. Lumin., 2015, 158, 172–175 CrossRef CAS.
- D. Udhayakumari and V. Inbaraj, A Review on Schiff Base Fluorescent Chemosensors for Cell Imaging Applications, J. Fluoresc., 2020, 30, 1203–1223 CrossRef CAS PubMed.
- W. Qin, S. Long, M. Panunzio and S. Biondi, Schiff Bases: A Short Survey on an Evergreen Chemistry Tool, Molecules, 2013, 18, 12264–12289 CrossRef CAS.
- M. E. Belowich and J. F. Stoddart, Dynamic imine chemistry, Chem. Soc. Rev., 2012, 41, 2003–2024 RSC.
- H. Vardhan, A. Mehta, I. Nath and F. Verpoort, Dynamic imine chemistry in metal–organic polyhedra, RSC Adv., 2015, 5, 67011–67030 RSC.
- M. S. More, P. G. Joshi, Y. K. Mishra and P. K. Khanna, Metal complexes driven from Schiff bases and semicarbazones for biomedical and allied applications: a review, Mater. Today Chem., 2019, 14, 100195 CrossRef CAS.
- C. Boulechfar, H. Ferkous, A. Delimi, A. Djedouani, A. Kahlouche, A. Boublia, A. S. Darwish, T. Lemaoui, R. Verma and Y. Benguerba, Schiff bases and their metal Complexes: A review on the history, synthesis, and applications, Inorg. Chem. Commun., 2023, 15, 110451 CrossRef.
- X. Liu and J. R. Hamon, Recent developments in penta-, hexa- and heptadentate Schiff base ligands and their metal complexes, Coord. Chem. Rev., 2019, 389, 94–118 CrossRef CAS.
- C. M. Da Silva, D. L. da Silva, L. V. Modolo, R. B. Alves, M. A. de Resende, C. V. B. Martins and A. de Fátima, Schiff bases: A short review of their antimicrobial activities, J. Adv. Res., 2011, 2, 1–8 CrossRef.
- G. P. Ellis, in Chromenes, Chromanones, and Chromones: the Chemistry of Heterocyclic Compounds, ed. Ellis, G. P., John Wiley and Sons, Inc., New York, 1977, vol. 31, pp. 1–10 Search PubMed.
- A. T. Benny, S. D. Arikkatt, C. G. Vazhappilly, S. Kannadasan, R. Thomas, M. S. Leelabaiamma, E. K. Radhakrishnan and P. Shanmugam, Chromone, a privileged scaffold in drug discovery: Developments in the synthesis and bioactivity, Mini-Rev. Med. Chem., 2022, 22, 1030–1063 CrossRef CAS.
- X.-J. Yan, Z. Li, H.-B. Liu, Z.-G. Wang, J. Fan, C.-Z. Xie, Q.-Z. Li and J.-Y. Xu, A chromone hydrazide Schiff base fluorescence probe with high selectivity and sensitivity for the detection and discrimination of human serum albumin (HSA) and bovine serum albumin (BSA), J. Photochem. Photobiol., A, 2022, 422, 113576 CrossRef CAS.
- K. M. Khan, N. Ambreen, S. Hussain, S. Perveen and M. I. Choudhary, Schiff bases of 3-formylchromone as thymidine phosphorylase inhibitors, Bioorg. Med. Chem., 2009, 17, 2983–2988 CrossRef CAS.
- P. Bhalla, K. Malhotra, N. Tomer and R. Malhotra, Binding interactions and Sensing applications of chromone derived Schiff base chemosensors via absorption and emission studies: A comprehensive review, Inorg. Chem. Commun., 2022, 146, 110026 CrossRef CAS.
- M. Grazul and E. Budzisz, Biological activity of metal ions complexes of chromones, coumarins and flavones, Coord. Chem. Rev., 2009, 253, 2588–2598 CrossRef CAS.
- M. Shebl, M. Saif, A. I. Nabeel and R. Shokry, New non-toxic transition metal nanocomplexes and Zn complex-silica xerogel nanohybrid: Synthesis, spectral studies, antibacterial, and antitumor activities, J. Mol. Struct., 2016, 1118, 335–343 CrossRef CAS.
- A. Husain, P. ACh and B. Anupama, DNA binding affinities, anti-oxidant, antimicrobial and molecular docking activities of Pd (II) complexes of chromone Schiff bases, J. Mol. Struct., 2022, 1254, 132341 CrossRef CAS.
- M. B. Maity, D. Talukdar, B. Dutta, G. Bairy, N. Murmu, G. Das and C. Sinha, Application of a Rhodamine-chromone Schiff base probe for the sensing of Fe3+, Al3+, Cr3+ at low concentration and exploration of the anticancer activity and bio-imaging, Inorg. Chim. Acta, 2023, 545, 121276 CrossRef.
- V. Barve, F. Ahmed, S. Adsule, S. Banerjee, S. Kulkarni, P. Katiyar, C. E. Anson, A. K. Powell, S. Padhye and F. H. Sarkar, Synthesis, Molecular Characterization, and Biological Activity of Novel Synthetic Derivatives of Chromen-4-one in Human Cancer Cells, J. Med. Chem., 2006, 49, 3800–3808 CrossRef CAS.
- D. Karati, S. Mukherjee and S. Roy, An Explicative Review on the Current Advancement in Schiff Base-Metal Complexes as Anticancer Agents Evolved in the Past Decade: Medicinal Chemistry Aspects, Med. Chem., 2023, 19, 960–985 CrossRef CAS.
- A. M. El-Saghier, H. F. Abd El-Halim, L. H. Abdel-Rahman and A. Kadry, Green synthesis of new triazole based heterocyclic amino acids ligands and their transition metal complexes. Characterization, kinetics, antimicrobial and docking studies, Appl. Organomet. Chem., 2019, 33, e4641 CrossRef.
- A. Chakraborty, P. Kumar, K. Ghosh and P. Roy, Evaluation of a Schiff base copper complex compound as potent anticancer molecule with multiple targets of action, Eur. J. Pharmacol., 2010, 647, 1–12 CrossRef CAS.
- J. Costamagna, J. Vargas, R. Latorre, A. Alvarado and G. Mena, Coordination compounds of copper, nickel and iron with Schiff bases derived from hydroxynaphthaldehydes and salicylaldehydes, Coord. Chem. Rev., 1992, 119, 67–88 CrossRef CAS.
- B. Evertsson, The crystal structure of bis-L-histidinecopper(II) dinitrate dihydrate, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1969, 25, 30–41 CrossRef CAS.
- F. S. Stephens, R. S. Vagg and P. A. Williams, The crystal and molecular structure of bis (l-asparaginato) copper (II),[Cu(OOCCHNH2CH2CONH2)2]n, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1975, 31, 841–845 CrossRef.
- C. M. Weeks, A. Cooper and D. A. Norton, The crystal structure of the copper (II) complex of L-isoleucine, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1969, 25, 443–450 CrossRef CAS.
- W. Kaim and J. Rall, Copper—A“Modern” Bioelement, Angew Chem. Int. Ed. Engl., 1996, 35, 43–60 CrossRef CAS.
- D. B. Milne, Copper intake and assessment of copper status, Am. J. Clin. Nutr., 1998, 67, 1041S–1045S CrossRef CAS.
- M. Sahu, A. K. Manna and G. K. Patra, A dihydrazone based conjugated bis Schiff base chromogenic chemosensor for selectively detecting copper ion, Inorg. Chim. Acta, 2021, 517, 120199 CrossRef CAS.
- D. E. Kang, C. S. Lim, J. Y. Kim, E. S. Kim, H. J. Chun and B. R. Cho, Two-Photon Probe for Cu2+ with an Internal Reference: Quantitative Estimation of Cu2+ in Human Tissues by Two-Photon Microscopy, Anal. Chem., 2014, 86, 5353–5359 CrossRef CAS PubMed.
- D. Zhu, A. Ren, X. He, Y. Luo, Z. Duan, X. Yan, Y. Xiong and X. Zhong, A novel ratiometric fluorescent probe for selective and sensitive detection of Cu2+ in complete aqueous solution, Sens. Actuators, B, 2017, 252, 134–141 CrossRef CAS.
- A. Verma, P. Gahlyan, R. Bawa, S. R. Dash, A. K. Prasad and R. Kumar, Glycerol-Triazole Conjugated Rhodamine as Colorimetric and Fluorimetric Sensor for Cu2+, Chemistryselect, 2021, 6, 9288–9292 CrossRef CAS.
- D. Vashisht, S. Sharma, R. Kumar, V. Saini, V. Saini, A. Ibhadon, S. C. Sahoo, S. Sharma, S. K. Mehta and R. Kataria, Dehydroacetic acid derived Schiff base as selective and sensitive colorimetric chemosensor for the detection of Cu (II) ions in aqueous medium, Microchem. J., 2020, 155, 104705 CrossRef CAS.
- J. Yao, K. Zhang, H. Zhu, F. Ma, M. Sun, H. Yu, J. Sun and S. Wang, Efficient Ratiometric Fluorescence Probe Based on Dual-Emission Quantum Dots Hybrid for On-Site Determination of Copper Ions, Anal. Chem., 2013, 85, 6461–6468 CrossRef CAS PubMed.
- S. L. Ting, S. J. Ee, A. Ananthanarayanan, K. C. Leong and P. Chen, Graphene quantum dots functionalized gold nanoparticles for sensitive electrochemical detection of heavy metal ions, Electrochim. Acta, 2015, 172, 7–11 CrossRef CAS.
- H. Bagheri, A. Afkhami, M. Saber-Tehrani and H. Khoshsafar, Preparation and characterization of magnetic nanocomposite of Schiff base/silica/magnetite as a preconcentration phase for the trace determination of heavy metal ions in water, food and biological samples using atomic absorption spectrometry, Talanta, 2012, 97, 87–95 CrossRef CAS PubMed.
- Z. Zhou, Q. Shang, Y. Shen, L. Zhang, Y. Zhang, Y. Lv, Y. Li, S. Liu and Y. Zhang, Chemically modulated carbon nitride nanosheets for highly selective electrochemiluminescent detection of multiple metal-ions, Anal. Chem., 2016, 88, 6004–6010 CrossRef CAS PubMed.
- S. Mukherjee, S. Talukder, S. Chowdhury, P. Mal and H. Stoeckli-Evans, Synthesis, structure and sensing behavior of hydrazone based chromogenic chemosensors for Cu2+ in aqueous environment, Inorg. Chim. Acta, 2016, 450, 216–224 CrossRef CAS.
- H. Kim, Y. J. Na, E. J. Song, K. B. Kim, J. M. Bae and C. Kim, A single colorimetric sensor for multiple target ions: the simultaneous detection of Fe2+ and Cu2+ in aqueous media, RSC Adv., 2014, 4, 22463 RSC.
- P. Kumar, K. H. Kim, V. Bansal, T. Lazarides and N. Kumar, Progress in the sensing techniques for heavy metal ions using nanomaterials, J. Ind. Eng. Chem., 2017, 54, 30–43 CrossRef CAS.
- D. Vashisht, K. Kaur, R. Jukaria, A. Vashisht, S. Sharma and S. K. Mehta, Colorimetric chemosensor based on coumarin skeleton for selective naked eye detection of cobalt (II) ion in near aqueous medium, Sens. Actuators, B, 2019, 280, 219–226 CrossRef CAS.
- A. Mohammadi and M. Kianfar, A simple colorimetric chemosensor with highly performance for detection of cyanide and copper ions and its practical application in real samples, J. Photochem. Photobiol., A, 2018, 367, 22–31 CrossRef CAS.
- A. Goel and R. Malhotra, Efficient detection of Picric acid by pyranone based Schiff base as a chemosensor, J. Mol. Struct., 2021, 1249, 131619 CrossRef.
- G. P. Rao, K. Seshaiah, Y. K. Rao and M. C. Wang, Solid phase extraction of Cd, Cu, and Ni from leafy vegetables and plant leaves using amberlite XAD-2 functionalized with 2-hydroxy-acetophenone-thiosemicarbazone (HAPTSC) and determination by inductively coupled plasma atomic emission spectroscopy, J. Agric. Food Chem., 2006, 54, 2868–2872 CrossRef CAS.
- X. Tang, J. Han, Y. Wang, L. Ni, X. Bao, L. Wang and W. Zhang, A multifunctional Schiff base as a fluorescence sensor for Fe3+ and Zn2+ ions and a colorimetric sensor for Cu2+ and applications, Spectrochim. Acta, Part A, 2017, 173, 721–726 CrossRef CAS.
- R. Malhotra, A. Ravesh and V. Singh, Synthesis, characterization, antimicrobial activities, and QSAR studies of organotin (IV) complexes, Phosphorus, Sulfur, Silicon Relat. Elem., 2017, 192, 73–80 CrossRef.
- A. Prakash and R. Malhotra, Co (II), Ni (II), Cu (II) and Zn (II) complexes of aminothiazole-derived Schiff base ligands: Synthesis, characterization, antibacterial and cytotoxicity evaluation, bovine serum albumin binding and density functional theory studies, Appl. Organomet. Chem., 2018, 32, 4098 CrossRef.
- N. Tomer, A. Goel, V. D. Ghule and R. Malhotra, A chromone based Schiff base: An efficient colorimetric sensor for specific detection of Cu (II) ion in real water samples, J. Mol. Struct., 2020, 1227, 129549 CrossRef.
- P. Bhalla, N. Tomer, P. Bhagat and R. Malhotra, Chromone functionalized pyridine chemosensor for cupric ions detection, Spectrochim. Acta, Part A, 2022, 264, 120279 CrossRef CAS.
- N. Tomer, A. Goel, P. Bhalla, P. Bhagat and R. Malhotra, Chromone derived effective probe for the detection of metal ion (Cu2+) and chemical explosive (p-nitrotoluene), J. Photochem. Photobiol., A, 2022, 427, 113823 CrossRef CAS.
- A. Mohammadi, B. Khalili and A. S. Haghayegh, A novel chromone based colorimetric sensor for highly selective detection of copper ions: Synthesis, optical properties and DFT calculations, Spectrochim. Acta, Part A, 2019, 222, 117193 CrossRef CAS.
- F.-U. Rahman, S.-B. Yu, S. K. Khalil, Y. P. Wu, S. Koppireddi, Z.-T. Li, H. Wang and D.-W. Zhang, Chromone and benzyldithiocarbazate based probe: A highly selective and sensitive platform for colorimetric sensing of Cu2+, single crystal of the complex and DFT calculations, Sens. Actuators, B, 2018, 263, 594–604 CrossRef CAS.
- R. Kouser, A. Rehman, S. M. A. Abidi, F. Arjmand and S. Tabassum, A chromone-based colorimetric fluorescence sensor for selective detection of Cu2+ ions, and its application for in-situ imaging, J. Mol. Struct., 2022, 1256, 132533 CrossRef CAS.
- K. Rezaeian, H. Khanmohammadi and A. Talebbaigy, Detection of CN−, Cu2+ and Zn2+ ions using a new chromone-based colorimetric chemosensor: half-adder and integrated circuits, Anal. Methods, 2020, 12, 1759–1766 RSC.
- M. K. Gaidhane, A. M. Ghatole and K. R. Lanjewar, Synthesis of Chromone Functionalized Chitosan Polymer: Application/Screening of Its Physical Parameters, Polym. Sci., Ser. B, 2020, 62, 206–217 CrossRef CAS.
- L. Tian, J. Xue, S. Li and Z. Yang, A novel chromone derivative as dual probe for selective sensing of Al(III) by fluorescent and Cu(II) by colorimetric methods in aqueous solution, J. Photochem. Photobiol., A, 2019, 382, 111955 CrossRef CAS.
- F. Abebe, P. Perkins, R. Shaw and S. Tadesse, A rhodamine-based fluorescent sensor for selective detection of Cu2+ in aqueous media: Synthesis and spectroscopic properties, J. Mol. Struct., 2020, 1205, 127594 CrossRef CAS PubMed.
- A. Q. Alorabi, S. A. Zabin, M. M. Alam and M. Abdelbaset, Schiff Base Ligand 3-(-(2-Hydroxyphenylimino) Methyl)-4H-Chromen-4-One as Colorimetric Sensor for Detection of Cu2+, Fe3+, and V5+ in Aqueous Solutions, Int. J. Anal. Chem., 2022, 2022, 4899145 Search PubMed.
- A. Erxleben, Interactions of copper complexes with nucleic acids, Coord. Chem. Rev., 2018, 360, 92–121 CrossRef CAS.
- M. A. Malik, O. A. Dar, P. Gull, M. Y. Wani and A. A. Hashmi, Heterocyclic Schiff base transition metal complexes in antimicrobial and anticancer chemotherapy, Med. Chem. Commun., 2018, 9, 409–436 RSC.
- M. Claudel, J. V. Schwarte and K. M. Fromm, New Antimicrobial Strategies Based on Metal Complexes, Chemistry, 2020, 2, 849–899 CrossRef CAS.
- P. Nunes, Y. Yildizhan, Z. Adiguzel, F. Marques, J. C. Pessoa, C. Acilan and I. Correia, Copper(II) and oxidovanadium(IV) complexes of chromone Schiff bases as potential anticancer agents, J. Biol. Inorg. Chem., 2022, 27, 89–109 CrossRef CAS PubMed.
- M. Gaber, K. El-Baradie, N. El-Wakiel and S. Hafez, Synthesis and characterization studies of 3-formyl chromone Schiff base complexes and their application as antitumor, antioxidant and antimicrobial, Appl. Organomet. Chem., 2020, 34, e5348 CrossRef CAS.
- M. Gaber, N. El-Wakiel, K. El-Baradie and S. Hafez, Chromone Schiff base complexes: synthesis, structural elucidation, molecular modeling, antitumor, antimicrobial, and DNA studies of Co(II), Ni(II), and Cu(II) complexes, J. Iran. Chem. Soc., 2019, 16, 169–182 CrossRef CAS.
- M. M. E. Shakdofa, H. A. Mousa, A. A. Labib, A. S. Abd-El-All, A. A. El-Beih and M. M. Abdalla, Synthesis and characterization of novel chromone Schiff base complexes as p53 activators, Appl. Organomet. Chem., 2018, 32, e4345 CrossRef.
- O. M. I. Adly and H. F. El-Shafiy, New metal complexes derived from S-benzyldithiocarbazate (SBDTC) and chromone-3-carboxaldehyde: synthesis, characterization, antimicrobial, antitumor activity and DFT calculations, J. Coord. Chem., 2019, 72, 218–238 CrossRef CAS.
- R. Fouad and O. M. I. Adly, Novel Cu(II) and Zn(II) Nanocomplexes Drug Based on Hydrazone Ligand Bearings Chromone and Triazine moieties: Structural, Spectral, DFT, Molecular Docking and Cytotoxic Studies, J. Mol. Struct., 2020, 1225, 129158 CrossRef.
- N. S. Abdel-Kader, A. L. El-Ansary, T. A. El-Tayeb and M. M. F. Elnagdi, Synthesis and characterization of Schiff base complexes derived from cephradine: Fluorescence, photostability and photobiological applications, J. Photochem. Photobiol., A, 2016, 321, 223–237 CrossRef CAS.
- G. Kalaiarasi, S. Rex Jeya Rajkumar, S. Dharani, N. P. Rath and R. Prabhakaran, New cationic and neutral copper(II) complexes containing 7-hydroxy-4-oxo-4[H]-chromene derived ONO pincer ligands: Synthesis, characterization and in vitro biological evaluations, J. Photochem. Photobiol., B, 2018, 180, 77–88 CrossRef CAS PubMed.
- G. Kalaiarasi, S. R. J. Rajkumar, S. Dharani, V. M. Lynch and R. Prabhakaran, Synthesis, spectral characterization and biological evaluation of some copper(II) complexes containing 4-oxo-4H-chromene-3-carbaldehyde-4-(N)-substituted thiosemicarbazones, Inorg. Chim. Acta, 2018, 471, 759–776 CrossRef CAS.
- G. Kalaiarasi, S. Rex Jeya Rajkumar, S. Dharani, N. P. Rath and R. Prabhakaran, In vitro cytotoxicity of new water-soluble copper(II) metallates containing 7-hydroxy-4-oxo-4H-chromene thiosemicarbazones, Polyhedron, 2019, 173, 114120 CrossRef CAS.
- D. Şahin Gül, H. Ogutcu and Z. Hayvalı, Investigation of photophysical behaviours and antimicrobial activity of novel benzo-15-crown-5 substituted coumarin and chromone derivatives, J. Mol. Struct., 2020, 1204, 127569 CrossRef.
- O. E. Sherif and N. S. Abdel-Kader, DFT calculations, spectroscopic studies, thermal analysis and biological activity of supramolecular Schiff base complexes, Arabian J. Chem., 2018, 11, 700–713 CrossRef CAS.
- N. Balakrishnan, J. Haribabu, A. K. Dhanabalan, S. Swaminathan, S. Sun, D. F. Dibwe, N. Bhuvanesh, S. Awale and R. Karvembu, Thiosemicarbazone(s)-anchored water-soluble mono- and bimetallic Cu(II) complexes: Enzymes-like activities, biomolecular interactions, anticancer property and real-time live cytotoxicity, Dalton Trans., 2020, 49, 9411–9424 RSC.
- K. Słomiak, A. Łazarenkow, L. Chęcińska, J. Kusz, J. Ochocki and J. Nawrot-Modranka, Synthesis, Spectroscopic Analysis and Assessment of the Biological Activity of New Hydrazine and Hydrazide Derivatives of 3-Formylchromone, Molecules, 2018, 23, 2067 CrossRef PubMed.
- D.-C. Ilies, E. Pahontu, S. Shova, R. Georgescu, N. Stanica, R. Olar, A. Gulea and T. Rosu, Synthesis, characterization, crystal structure and antimicrobial activity of copper(II) with a thiosemicarbazone derived from 3-formyl-6-methylchromone, Polyhedron, 2014, 81, 123–131 CrossRef CAS.
- K. M. Ibrahim and M. M. Bekheit, Synthesis and characterization of new metal complexes of thiosemicarbazone derived from 4-phenyl-3-thiosemicarbazide and chromone-3-carboxaldehyde, Transition Met. Chem., 1988, 13, 230–232 CrossRef CAS.
- M. M. Shakdofa, H. A. H. Mousa, A. A. Iabib, A. Abd El-All and F. Bassyouni, In vitro Antimicrobial Activity Evaluation of Newly Synthesized Furochromone Schiff Base Complexes, Egypt. J. Chem., 2018, 61, 295–304 Search PubMed.
- E. S. Jose, J. E. Philip, A. A. Shanty, M. R. P. Kurup and P. V. Mohanan, Novel class of mononuclear 2-methoxy-4-chromanones ligated Cu(II), Zn(II), Ni(II) complexes: synthesis, characterization and biological studies, Inorg. Chim. Acta, 2018, 478, 155–165 CrossRef.
- E. Pahontu, M. Proks, S. Shova, G. Lupaşcu, D.-C. Ilieş, S.-F. Bărbauceanu, L.-I. Socea, M. Badea, V. Păunescu, D. Istrati, A. Gulea, D. Drăgănescu and C. E. D. Pîrvu, Synthesis, characterization, molecular docking studies and in vitro screening of new metal complexes with Schiff base as antimicrobial and antiproliferative agents, Appl. Organomet. Chem., 2019, 33, e5185 CrossRef CAS.
- M. Saif, H. F. El-Shafiy, M. M. Mashaly, M. F. Eid, A. I. Nabeel and R. Fouad, Synthesis, characterization, and antioxidant/cytotoxicity activity of new chromone Schiff base, J. Mol. Struct., 2016, 1118, 75–82 CrossRef CAS.
- P. Kavitha and K. L. Reddy, Synthesis, spectral characterization, morphology, biological activity and DNA cleavage studies of metal complexes with chromone Schiff base, Arabian J. Chem., 2016, 9, 596–605 CrossRef CAS.
- R. A. Ammar, A. N. M. Alaghaz, M. E. Zayed and L. A. Al-Bedair, Synthesis, spectroscopic, molecular structure, antioxidant, antimicrobial and antitumor behavior of Mn(II), Co(II), Ni(II), Cu(II) and Zn(II) complexes of O2N type tridentate chromone-2-carboxaldehyde Schiff's base ligand, J. Mol. Struct., 2017, 1141, 368–381 CrossRef CAS.
- S. Sumathi, P. Tharmaraj, C. D. Sheela, R. Ebenezer and P. Saravana Bhava, Synthesis, characterization, NLO study, and antimicrobial activities of metal complexes derived from 3-(3-(2-hydroxyphenyl)-3-oxoprop-1-enyl)-4H-chromen-4-one and sulfanilamide, J. Coord. Chem., 2011, 64, 1673–1682 CrossRef CAS.
- N. Dharamaraj, P. Viswanathamurthi and K. Natarajan, Ruthenium(II) complexes containing bidentate Schiff bases and their antifungal activity, Transition Met. Chem., 2001, 26, 105–109 CrossRef.
- M. Padmaja, J. Pragathi and C. G. Kumari, Synthesis, spectral characterization, molecular modeling and biological activity of first row transition metal complexes with Schiff base ligand derived from chromone-3-carbaldehyde and o-amino benzoic acid, J. Chem. Pharm. Res., 2011, 3, 602–613 CAS.
- P. Kavitha, M. Saritha and K. Laxma Reddy, Synthesis, structural characterization, fluorescence, antimicrobial, antioxidant and DNA cleavage studies of Cu(II) complexes of formyl chromone Schiff bases, Spectrochim. Acta, Part A, 2013, 102, 159–168 CrossRef CAS PubMed.
- M. Kalanithi, D. Kodimunthiri, M. Rajarajan and P. Tharmaraj, Synthesis, characterization and biological activity of some new VO(IV), Co(II), Ni(II), Cu(II) and Zn(II) complexes of chromone based NNO Schiff base derived from 2-aminothiazole, Spectrochim. Acta, Part A, 2011, 82, 290–298 CrossRef CAS PubMed.
- F. Arjmand, A. Jamsheera, M. Afzal and S. Tabassum, Enantiomeric Specificity of Biologically Significant Cu(II) and Zn(II) Chromone Complexes Towards DNA, Chirality, 2012, 24, 977–986 CrossRef CAS PubMed.
- Y. Li, Z.-Y. Yang, Z.-C. Liao, Z.-C. Han and Z.-C. Liu, Synthesis, crystal structure, DNA binding properties and antioxidant activities of transition metal complexes with 3-carbaldehyde-chromone semicarbazone, Inorg. Chem. Commun., 2010, 13, 1213–1216 CrossRef CAS.
- Y. Li, Z.-Y. Yang and J.-C. Wu, Synthesis, crystal structures, biological activities and fluorescence studies of transition metal complexes with 3-carbaldehyde chromone thiosemicarbazone, Eur. J. Med. Chem., 2010, 45, 5692–5701 CrossRef CAS PubMed.
- T. Rosu, E. Pahontu, C. Maxim, R. Georgescu, N. Stanica, G. L. Almajan and A. Gulea, Synthesis, characterization and antibacterial activity of some new complexes of Cu(II), Ni(II), VO(II), Mn(II) with Schiff base derived from 4-amino-2,3-dimethyl-1-phenyl-3- pyrazolin-5-one, Polyhedron, 2010, 29, 757–766 CrossRef CAS.
- C. Anitha, C. D. Sheela, P. Tharmaraj and S. Johnson Raja, Synthesis and characterization of VO(II), Co(II), Ni(II), Cu(II) and Zn(II) complexes of chromone based azo-linked Schiff base ligand, Spectrochim. Acta, Part A, 2012, 98, 35–42 CrossRef CAS PubMed.
- P. Mendu, C. G. Kumari and R. Ragi, Synthesis, Characterization, DNA Binding, DNA Cleavage and Antimicrobial Studies of Schiff Base Ligand and its Metal Complexes, J. Fluoresc., 2015, 25, 369–378 CrossRef CAS PubMed.
- M. Bheemarasetti, K. Palakuri, S. Raj, P. Saudagar, D. Gandamalla, N. R. Yellu and L. R. Kotha, Novel Schiff base metal complexes: synthesis, characterization, DNA binding, DNA cleavage and molecular docking studies, J. Iran. Chem. Soc., 2018, 15, 1377–1389 CrossRef CAS.
- F. Arjmand, F. Sayeed and M. Muddassir, Synthesis of new chiral heterocyclic Schiff base modulated Cu(II)/Zn(II) complexes: Their comparative binding studies with CT-DNA, mononucleotides and cleavage activity, J. Photochem. Photobiol., B, 2011, 103, 166–179 CrossRef CAS PubMed.
- A. S. Alturiqi, A.-N. M. A. Alaghaz and R. A. Ammar, Synthesis, Spectral Characterization, Antitumor, Antioxidant, and Antimicrobial Studies of New Potential ONS Schiff Base Complexes, J. Chin. Chem. Soc., 2017, 64, 1270–1285 CrossRef CAS.
- A. Singh, S. K. Maiti, H. P. Gogoi and P. Barman, Purine-based Schiff base Co(II), Cu(II), and Zn(II) complexes: Synthesis, characterization, DFT calculations, DNA binding study, and molecular docking, Polyhedron, 2023, 230, 116244 CrossRef CAS.
- G. Tamasi, L. Chiasserini, L. Savini, A. Sega and R. Cini, Structural Study of Ribonucleotide Reductase Inhibitor Hydrazones. Synthesis and X-Ray Diffraction Analysis of a Copper(II)-Benzoylpyridine-2-Quinolinyl Hydrazone Complex, J. Inorg. Biochem., 2005, 99, 1347–1359 CrossRef CAS PubMed.
- W. Al Zoubi, Biological activities of Schiff bases and their complexes: a review of recent works, Int. J. Org. Chem., 2013, 3, 73–95 CrossRef.
- S. Kumar, D. N. Dhar and P. N. Saxena, Applications of metal complexes of Schiff bases-A review, J. Sci. Ind. Res., 2009, 68, 181–187 CAS.
- K. C. Gupta and A. K. Sutar, Catalytic activities of Schiff base transition metal complexes, Coord. Chem. Rev., 2008, 252, 1420–1450 CrossRef CAS.
- N. Beyazit, B. Çatıkkaş, Ş. Bayraktar and C. Demetgül, Synthesis, characterization and catecholase-like activity of new Schiff base metal complexes derived from visnagin: Theoretical and experimental study, J. Mol. Struct., 2016, 1119, 124–132 CrossRef CAS.
- A. Schonberg, N. Badran and N. A. Starkowsky, Furo-chromones and -Coumarins. VII. Degradation of visnagin, khellin and related substances; experiments with chromic acid and hydrogen peroxide; and a synthesis of eugenitin, J. Am. Chem. Soc., 1953, 75, 4992–4995 CrossRef CAS.
- N. Beyazit, D. Çakmak and C. Demetgül, Chromone-based Schiff base metal complexes as catalysts for catechol oxidation: Synthesis, kinetics and electrochemical studies, Tetrahedron, 2017, 73, 2774–2779 CrossRef CAS.
- N. Beyazit, S. Çobanoğlu and C. Demetgül, Metal complexes of perimidine and Schiff base ligands bearing both naphthalene and chromone, Bulg. Chem. Commun., 2017, 49, 115–121 Search PubMed.
- M. Shebl, A. A. Saleh, S. M. E. Khalil, M. Dawy and A. A. M. Ali, Synthesis, spectral, magnetic, DFT calculations, antimicrobial studies and phenoxazinone synthase biomimetic catalytic activity of new binary and ternary Cu(II), Ni(II) and Co(II) complexes of a tridentate ONO hydrazone ligand, Inorg. Nano-Met. Chem., 2021, 51, 195–209 CrossRef CAS.
- S. I. Al-Saeedi, A.-N. M. A. Alaghaz and R. A. Ammar, Synthesis, spectroscopic characterization and electrochemical studies of Girard's T chromone complexes, J. Mol. Struct., 2016, 1111, 201–213 CrossRef CAS.
Footnotes |
| † This article is a tribute to the cherished memory of our esteemed (late) Professor Ashok K. Prasad, who served as our enduring friend, collaborator, colleague, and mentor for many years. His enduring influence and guidance throughout the past several decades have profoundly impacted the careers of numerous aspiring scientists. |
| ‡ All three authors have equal contribution. |
| This journal is © The Royal Society of Chemistry 2024 |