DOI:
10.1039/C6RA21144E
(Paper)
RSC Adv., 2016,
6, 96049-96056
Synthesis of 3-arylindole derivatives from nitroalkane precursors†
Received
23rd August 2016
, Accepted 2nd October 2016
First published on 3rd October 2016
Abstract
3-Arylindole derivatives were synthesized by Cu(I) catalysed intramolecular Ullmann coupling of 2-bromoarylaminoalkanes. 2-Bromoarylaminoalkanes were synthesized from 2-bromoarylnitroalkanes, which in turn prepared through AlCl3-mediated Friedel–Crafts alkylation of bromo-substituted β-nitrostyrenes and arenes.
Introduction
The indole skeleton is a key structural component in various natural products, pharmaceutical compounds, dyes, agricultural compounds, cosmetics, nutraceuticals, and flavourings.1 3-Aryl indole, a derivative of indole also exhibit various interesting activities as shown in Fig. 1. For example, 4-fluoro-3-phenylindole can inhibit brassinin glucosyltransferase, a kind of phytoalexin detoxification enzyme which comes from fungus Sclerotinia sclerotiorum.2 Another example is murrapanine, it was isolated from the root bark of Murraya paniculata var. omphalocarpa and used for the treatments for lymphocytic leukaemia, lung adenocarcinoma and colon adenocarcinoma.3
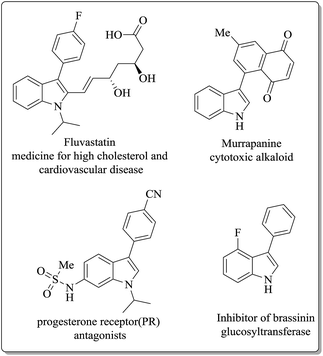 |
| | Fig. 1 Pharmaceutically active 3-arylindole derivatives. | |
Fluvastatin, a multi-purpose medicine for high cholesterol and cardiovascular disease prevention, also found to have antiviral activity against hepatitis C.4 Also, 3-arylindoles acts as a potent and efficacious progesterone receptor (PR) antagonists and can be used for the treatment of uterine fibroids.5
In the light of the importance of these bioactive molecules, several synthetic protocols have been reported.6 Various methods known for the exclusive synthesis of 3-arylindoles are conventional Fischer indole synthesis,7 transition-metal-catalyzed coupling of indoles with coupling partners such as aryl halides,8 diaryl-iodine(III) reagent,9 carboxylic acids,10 arylhydrazine/amine,11 other miscellaneous methods.12 In 2010, Siva Murru et al. reported Au and Cu versions of annulation of nitrosoarenes and alkynes for the synthesis of 3-arylindoles.13 Recently, Kumar et al. reported the t-BuOK-mediated transition metal free synthesis of 3-(2/4-nitroaryl)-indoles through intermolecular oxidative coupling of indole with nitroarenes.14 Most of the developed methodologies requires N-protection or C-2 substitution of indoles or requires costly additives/ligands and harsh reaction conditions to obtain exclusively 3-arylindoles. Hence there is a need to develop an alternative and direct method to access the valuable 3-arylindoles.
Results and discussion
In 1991, Russell and Yao et al. reported the synthesis of 3-arylderivatives from α-phenyl-β-nitrostyrenes in the presence of triethylphosphite15 (Scheme 1). Since the starting material α-phenyl-β-nitrostyrenes are very tedious to prepare, the same protocol is applied on easily available starting material β-nitrostyrenes. But didn't find any desired 3-arylindole product and end up with a mixture of products.16 As a part of our interest in developing fascinating methodologies for the synthesis of biologically active heterocycles17 using easily available starting materials, we envisioned that 3-arylindoles can be synthesized from Cu catalyzed intramolecular Ullmann coupling of 2-bromoarylamino alkanes. The required aminoalkanes prepared from racemic 2-bromoarylnitroalkanes, which in turn prepared from our reported method18 using AlCl3-mediated Friedel–Crafts alkylation of bromo-substituted β-nitrostyrenes and arenes as shown in Scheme 2. 2-Bromoarylnitroalkanes may undergo reduction to form 2-bromoarylaminoalkanes followed by Cu catalyzed intramolecular coupling may afford the desired 3-arylindole product.
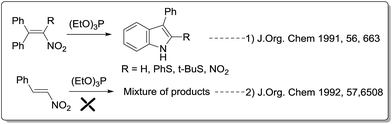 |
| | Scheme 1 Earlier synthetic approaches from nitrostyrenes. | |
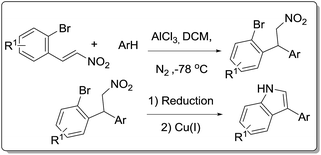 |
| | Scheme 2 Synthetic strategy for 3-aryl indoles. | |
In order to check the validity of our hypothesis we initially synthesized various 2-bromo-β-nitrostyrene derivatives (1) from nitromethane and corresponding 2-bromobenzaldehydes through Knoevenagel condensation followed by Friedel–Crafts alkylation with AlCl3 to obtain the corresponding nitroalkane derivatives (3a–3i) (Table 1). Most of the substrates produced nitroalkanes in moderate to good yields. Starting from simple 2-bromo-β-nitrostyrene various substituents such as OMe, OCH2O, OPr, naphthyl afford the corresponding nitroalkane derivatives 3a–3f in good yield with its coupling partner anisole. Its noteworthy that the current protocol not only works well with strong donating groups like OMe on aryl ring but also works well with Me, i-Pr, H substituents (3g, 3h and 3i) with simple 2-bromo-β-nitrostyrene. In all these entries, the insertion of bromo group on β-nitrostyrene was needed which confined the versatility of our method. For this reason, we attempted to develop another synthetic route, using 3-bromoanisole as substrate in the Friedel–Crafts alkylation. We synthesized another series of nitroalkanes (3j–3r) Table 2 as precursors from the same Friedel–Crafts alkylation procedure. The reactions took longer times compared to its former entries due to both steric hindrance and electron-withdrawing effect of bromo group. Most of the substrates produced nitroalkanes in moderate to good yields, while few substrates like 2-methyl, 1-naphthyl and 3-chloro substituted β-nitrostyrene produced poor yields of corresponding nitroalkanes (3k, 3o, 3p) as shown in Table 2.
Table 1 Synthesis of 2-bromoarylnitroalkane derivativesa,b
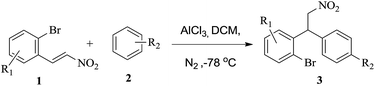
|
| Entry |
R1 |
R2 |
3 |
Time |
Yield% |
| Reaction conditions: 1 (5 mmol), 2 arene (10 mmol), AlCl3 (10 mmol), DCM (20 mL), −78 °C, under N2. Isolated yields. |
| 1 |
H |
OMe |
3a |
3 h |
98% |
| 2 |
5-OMe |
OMe |
3b |
3 h |
70% |
| 3 |
4,5-DiOMe |
OMe |
3c |
3 h |
71% |
| 4 |
4,5-OCH2O– |
OMe |
3d |
5 h |
64% |
| 5 |
5-OPr |
OMe |
3e |
5 h |
64% |
| 6 |
3,4-(CH4) |
OMe |
3f |
1 h |
76% |
| 7 |
H |
H |
3g |
5 h |
89% |
| 8 |
H |
Me |
3h |
4 h |
72% |
| 9 |
H |
iPr |
3i |
2 h |
67% |
Table 2 Synthesis of 4-methoxy-2-bromoarylnitroalkane derivativesa,b
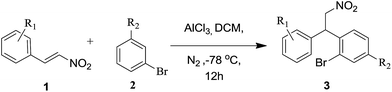
|
| Entry |
R1 |
R2 |
3 |
Yield% |
| Reaction conditions: 1 (5 mmol), 2 arene (10 mmol), AlCl3 (10 mmol), DCM (20 mL), −78 °C, under N2. Isolated yields. |
| 1 |
H |
OMe |
3j |
97% |
| 2 |
2-Me |
OMe |
3k |
27% |
| 3 |
4-Me |
OMe |
3l |
80% |
| 4 |
4-t-Bu |
OMe |
3m |
90% |
| 5 |
3-OMe |
OMe |
3n |
61% |
| 6 |
2,3-(CH)4 |
OMe |
3o |
36% |
| 7 |
3-Cl |
OMe |
3p |
34% |
| 8 |
4-Cl |
OMe |
3q |
70% |
| 9 |
2-NO2 |
OMe |
3r |
94% |
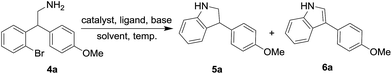
After the preparation of various nitroalkane derivatives, 1-bromo-2-(1-(4-methoxyphenyl)-2-nitroethyl)benzene (3a) was taken as the model substrate and reduced with reagents Fe/AcOH and NaBH4/BF3·THF. A mixture of 2-(2-bromophenyl)-2-(4-methoxyphenyl)ethan-1-amine (4a) and debromonated amine was observed. If we use LAH as reducing reagent, the reaction produced the desired 2-(2-bromophenyl)-2-(4-methoxyphenyl)ethan-1-amine as sole product in quantitative yield, as a result, we utilized the 2-bromoarylamine 4a for the coupling reactions without further purification. Initially we conducted the reaction of 2-bromoarylamine 4a with 10 mol% of CuI, 20 mol% of L-proline and 2 eq. of K2CO3 in DMSO at 80 °C as the model reaction (Entry 1, Table 3). The reaction produced 49% of indoline (5a) and 16% of desired indole product (6a). We then focused on variation of other reaction parameters in order to increase the yield of indole product as presented in Table 3. In the ligand tuning, except L-phenylalanine other ligands resulted in lower yields (Entries 2–6). Using L-phenylalanine as ligand, showed better result as the temperature raised from 80 °C to 100 °C (Entries 4, 5). In the solvent tuning, DMSO prevailed other solvents. In 1986, Speier et al. reported a kinetics and mechanism research about Cu-catalyzed oxidation of indolines to indoles, the experiments showed that there is a positive correlation between the oxidant's concentration and the oxidation rate in the presence of Cu(I) catalysts.19 Based on this, we ran a reaction under O2 atmosphere, and the yield of indole (6a) improved to 72%, and the reaction time was shortened from 1 h to 30 min (Entry 9), showing that O2 indeed assisted the oxidation process of indoline in the reaction. As a result, the following optimizations were conducted under O2 atmosphere. We further tested different copper catalysts including Cu(I), Cu(II) and Cu(I)/Cu(II) as co-catalyst, but did not receive good result (Entries 10–14). Interestingly, if the reaction was conducted under microwave irradiation, bromoamine was consumed within 5 min and produced 60% of indoline 5a and 13% of indole 6a (Entry 15). Whereas, as we prolonged the reaction time, indoline experienced decomposition without any yield increase of desired indole. Finally, after a series of tunings, we found the coupling of bromoamine 4a had best performance when treated with 10 mol% of CuI, 20 mol% of L-phenylalanine and 2 eq. of K2CO3 in DMSO at 100 °C under O2 atmosphere. The other bromoamine derivatives were also directly subjected to the coupling reactions without further purification under optimized reaction conditions as shown in Table 4. In the case of 5-methoxy-substituted bromoamine (Table 4, Entry 2), the reaction yielded desired indole derivative in 72% yield. The current protocol works with other strong electron donating groups in obtaining the desired product in low to moderate yields (Table 4, Entries 3, 4 and 5). In case of naphthyl derivative also the desired indole product was obtained in low yield (Table 3, Entry 6). There is a significant enhancement in the yield of the desired indole product in case of substituents like Me, i-Pr and unsubstituted derivatives (Table 4, Entries 7, 8 and 9).
Table 3 Optimization of indolization of amine
Table 4 Synthesis of 3-arylindole derivatives from 2-bromonitroalkane precursorsa,b
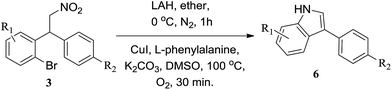
|
| Entry |
R1 |
R2 |
6 |
Yield% |
| Reaction conditions: (i) 3 (1 mmol), LAH (4 mmol), ether (10 mL), 0 °C, N2, 1 h. (ii) CuI (0.1 mmol), L-phenylalanine (0.2 mmol), K2CO3 (2 mmol), DMSO (1 mL), 100 °C, under O2, 30 min. Isoated yields. |
| 1 |
H |
OMe |
6a |
70% |
| 2 |
5-OMe |
OMe |
6b |
72% |
| 3 |
4,5-DiOMe |
OMe |
6c |
57% |
| 4 |
4,5-OCH2O– |
OMe |
6d |
23% |
| 5 |
5-OPr |
OMe |
6e |
28% |
| 6 |
3,4-(CH4) |
OMe |
6f |
24% |
| 7 |
H |
H |
6g |
84% |
| 8 |
H |
Me |
6h |
63% |
| 9 |
H |
iPr |
6i |
67% |
Latter the scope of the current protocol was examined on other nitroalkane derivatives derived from 3-bromoanisole and subjected to optimized reaction conditions. But we found that under optimized reaction conditions along with the formation of desired 3-arylindole derivatives, 10–20% of indolines were observed which was difficult to separate by column chromatography. Subsequently we treated the crude mixtures with MnO2 as oxidant and it oxidized the indolines to the corresponding indole products without decomposition. Therefore, for the synthesis of these 3-arylindoles (6j–6q), we conducted additional oxidation process of crude products after Ullmann reaction, and achieved 3-arylindole derivatives with moderate to good yields as shown in Table 5. The present method works well with various substituents such as Me, OMe, Cl and naphthyl in delivering the desired indole derivatives in good yield. But in the presence of t-butyl substituent desired indole product (6m) obtained in low yield. Interestingly, as we trying to reduce nitroalkane (3r) with LAH, the reaction did not produce the expected bromoamine, but the mixture of indoline and indole (6r) was observed in poor yield. Latter nitroalkane 3r treated with SiO2-supported with Fe in refluxing AcOH/EtOH for 10 minutes and got excellent yield of 3-arylindole (6r) as sole product (Scheme 3).
Table 5 Synthesis of 3-arylindole derivatives from 4-methoxy-2-bromonitroalkane precursorsa,b,c
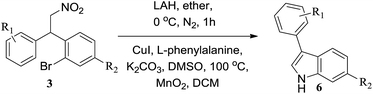
|
| Entry |
R1 |
R2 |
6 |
Yield% |
| Reaction conditions: (i) 3 (1 mmol), LAH (4 mmol), ether (10 mL), 0 °C, N2, 1 h. (ii) CuI (0.1 mmol), L-phenylalanine (0.2 mmol), K2CO3 (2 mmol), DMSO (1 mL), 100 °C, under O2, 30 min. Isoated yields. MnO2 (10 eq.), DCM (5 mL), silica gel (0.25 g), reflux, 12 h. |
| 1 |
H |
OMe |
6j |
62% |
| 2 |
2-Me |
OMe |
6k |
65% |
| 3 |
4-Me |
OMe |
6l |
74% |
| 4 |
4-t-Bu |
OMe |
6m |
21% |
| 5 |
3-OMe |
OMe |
6n |
62% |
| 6 |
2,3-(CH)4 |
OMe |
6o |
80% |
| 7 |
3-Cl |
OMe |
6p |
56% |
| 8 |
4-Cl |
OMe |
6q |
60% |
| 9 |
2-NO2 |
OMe |
6r |
00% |
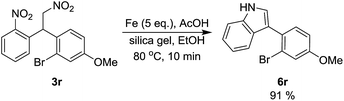 |
| | Scheme 3 Synthesis of 3-arylindole 6r. | |
Experimental section
General information
Reagents and solvents were purchased from various commercial sources and were used directly without any further purification, unless otherwise stated. Column chromatography was performed with 63–200 mesh silica gel. 1H and 13C NMR spectra were recorded at 400 and 100 MHz, or 500 and 125 MHz, respectively. Chemical shifts are reported in parts per million (δ) using TMS and chloroform as internal standards and coupling constants are expressed in Hertz. Melting points were recorded using an electro thermal capillary melting point apparatus and are uncorrected. HRMS spectra were recorded using ESI-TOF or EI+ mode. The starting material β-nitrostyrene derivatives 1 were synthesized from functionalized benzaldehydes and nitromethane followed by the reported literatures.
General procedure for nitroalkanes 3. β-Nitrostyrene derivative 1 (5 mmol) and granular AlCl3 (1.334 g, 10 mmol) were placed in an oven-dried round bottom flask and thoroughly filled it with N2. Dry DCM (20 mL) was then added to the flask, and the mixture was stirred at −78 °C for 10 min. The arene (6 mmol) was then added dropwise to the flask via a syringe, and the reaction was monitored by TLC. After the reaction was finished, the reaction was quenched with ice-cold brine. The reaction mixture was extracted with DCM and water, and the DCM layer was separated, dried over anhydrous MgSO4 and concentrated under vacuum to give the crude product. The crude product was furthered purified by column chromatography using EA/hexane as eluent to yield the desired product 3.
General procedure for indole 6a–6i. An oven-dried 50 mL round bottom flask with dropping funnel was added LAH (0.1518 g, 4 mmol) and filled with N2. The flask was cooled to 0 °C and added dry ether/THF (4 mL), and the nitroalkane derivative 3 (1 mmol) in ether/THF (6 mL) was slowly added to the flask, and stirred for 1 h. After that, the reaction was carefully quenched with H2O (1.5 mL) and the precipitate was filtered. The filtrate was dried over anhydrous MgSO4, concentrated at low temperature and dried under vacuum to give the amine 4.The amine 4 was then dissolved in DMSO (1 mL) and added CuI (0.019 g, 0.1 mmol) and L-phenylalanine (0.033 g, 0.2 mmol), which was then heated to 100 °C for 2–5 min before K2CO3 (0.2764 g, 2 mmol) was added. After that, the reaction was stirred with an O2 balloon for 30 min, and then quenched with water. The reaction mixture was extracted with EA and water, the EA layer was washed with water, dried over anhydrous MgSO4 and concentrated under vacuum to give the crude product. The crude product was furthered purified by column chromatography using EA/hexane as eluent to yield the desired product 6a–6j. For indole 6b, the crude product was dissolved in DCM and treated with DDQ (0.227 g, 1 mmol) for 1 h before purification.
General procedure for indole 6k–6r. The general procedure was as same as the procedure for 6a–6i. The crude product 6k–6r was dissolved in DCM (5 mL) and added MnO2 (0.87 g, 10 mmol), SiO2 (0.25 g), and heated to reflux for 12 h. After the indoline was consumed, the solvent was dried and the cruRepublic of Chinade was purified by column chromatography using EA/hexane as eluent to yield the desired product 6k–6r.
General procedure for indole 6r. A mixture of nitroalkane 3r (0.381 g, 1 mmol), iron powder (0.28 g, 5 mmol) and SiO2 (0.25 g) in EtOH (1.25 mL) was added AcOH (1.25 mL) and heated to 80 °C for 10 min. The reaction was filtered through celite and washed with EA, the filtrate was then concentrated under vacuum to give the crude product. The crude product was furthered purified by column chromatography using EA/hexane as eluent to yield the desired product 3r.
Spectral data of compounds
1-Bromo-2-(1-(4-methoxyphenyl)-2-nitroethyl)benzene (3a). Yield: 329 mg, 98%. Colorless solid. Mp: 78–80 °C. 1H-NMR (400 MHz, CDCl3) δ 7.59 (d, J = 7.9 Hz, 1H), 7.30 (td, J = 8.0, 1.0 Hz, 1H), 7.23–7.16 (m, 3H), 7.16–7.10 (td, J = 7.1, 1.6 Hz, 1H), 6.86 (d, J = 4.4 Hz, 2H), 5.38 (t, J = 8.1 Hz, 1H), 4.97 (dd, J = 13.2, 7.5 Hz, 1H), 4.89 (dd, J = 13.2, 8.8 Hz, 1H), 3.78 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 159.3, 138.7, 134.0, 129.8, 129.3, 129.3, 128.4, 128.1, 125.1, 114.6, 78.3, 55.5, 47.2; HRMS (EI) m/z calcd for C15H14NO3Br (M+) 335.0157, found 335.0158.
1-Bromo-4-methoxy-2-(1-(4-methoxyphenyl)-2-nitroethyl)benzene (3b). Yield: 256 mg, 70%. Yellow oil. 1H-NMR (400 MHz, CDCl3) δ 7.47 (d, J = 7.8 Hz, 1H), 7.20 (d, J = 8.4 Hz, 2H), 6.86 (d, J = 8.4 Hz, 2H), 6.75 (d, J = 2.5 Hz, 1H), 6.68 (dd, J = 8.8, 2.6 Hz, 1H), 5.32 (t, J = 8.0 Hz, 1H), 5.00–4.83 (m, 2H), 3.77 (s, 3H), 3.74 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 159.3, 159.2, 139.6, 134.4, 129.6, 129.3, 115.3, 115.2, 114.6, 113.9, 78.2, 55.6, 55.4, 47.2. HRMS (EI) m/z calcd for C16H16NO4Br (M+) 365.0263, found 365.0255.
1-Bromo-4,5-dimethoxy-2-(1-(4-methoxyphenyl)-2-nitroethyl)benzene (3c). Yield: 281 mg, 71%. Yellow solid. Mp: 71–72 °C. 1H-NMR (400 MHz, CDCl3) δ 7.17 (d, J = 8.6 Hz, 2H), 7.03 (s, 1H), 6.85 (d, J = 8.6 Hz, 2H), 6.66 (s, 1H), 5.29 (t, J = 8.3 Hz, 1H), 4.97–4.84 (m, 2H), 3.81 (s, 3H), 3.77 (s, 3H), 3.75 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 159.1, 149.0, 148.8, 130.3, 130.0, 128.9, 116.3, 114.8, 114.5, 111.3, 78.2, 56.2, 55.3, 46.8; HRMS (ESI) m/z calcd for C17H18NO5BrNa (M+ + Na) 418.0260, found 418.0252.
5-Bromo-6-(1-(4-methoxyphenyl)-2-nitroethyl)benzo[d][1,3]dioxole (3d). Yield: 243 mg, 64%. Yellow solid. Mp: 106–107 °C. 1H-NMR (400 MHz, CDCl3) δ 7.18 (d, J = 8.7 Hz, 2H), 7.03 (s, 1H), 6.87 (d, J = 8.7 Hz, 2H), 6.67 (s, 1H), 5.93 (dd, J = 6.6, 1.1 Hz, 2H), 5.32 (t, J = 8.2 Hz, 1H), 4.84 (d, J = 8.2 Hz, 2H), 3.77 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 159.1, 148.0, 147.8, 131.7, 129.9, 128.9, 115.1, 114.6, 113.5, 108.2, 102.2, 78.1, 55.4, 46.9; HRMS (EI) m/z calcd for C16H14NO5Br (M+) 379.0048, found 379.0055.
1-Bromo-2-(1-(4-methoxyphenyl)-2-nitroethyl)-4-propoxybenzene (3e). Yield: 315 mg, 64%. Yellow oil. 1H-NMR (400 MHz, CDCl3) δ 7.45 (d, J = 8.7 Hz, 1H), 7.20 (d, J = 8.7 Hz, 2H), 6.86 (d, J = 8.7 Hz, 2H), 6.75 (d, J = 2.9 Hz, 1H), 6.67 (dd, J = 8.7, 2.9 Hz, 1H), 5.31 (t, J = 8.2 Hz, 1H), 4.98–4.82 (m, 2H), 3.84 (t, J = 6.6 Hz, 2H) 3.77 (s, 3H), 1.83–1.73 (m, 2H), 1.02 (t, J = 7.4 Hz, 3H); 13C-NMR (100 MHz, CDCl3) δ 159.2, 158.9, 139.5, 134.3, 129.7, 129.3, 115.7, 114.9, 114.5, 78.2, 70.0, 55.4, 47.2, 22.6, 10.6; HRMS (EI) m/z calcd for C19H22NO4Br (M+) 393.0576, found 393.0585.
1-Bromo-2-(1-(4-methoxyphenyl)-2-nitroethyl)naphthalene (3f). Yield: 294 mg, 76%. Red oil. 1H-NMR (400 MHz, CDCl3) δ 8.39 (d, J = 8.5 Hz, 1H), 7.81 (d, J = 6.6 Hz, 2H), 7.64 (t, J = 7.2 Hz, 1H), 7.55 (t, J = 7.4 Hz, 1H), 7.35–7.28 (m, 3H), 6.94 (d, J = 8.6 Hz, 2H), 5.85 (t, J = 8.1 Hz, 1H), 5.07 (d, J = 8.1 Hz, 2H) 3.80 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 159.1, 136.5, 133.8, 132.8, 130.1, 129.1, 128.6, 128.3, 128.1, 128.0, 127.1, 125.1, 124.9, 114.6, 78.1, 55.4, 47.9; HRMS (EI) m/z calcd for C19H16NO3Br (M+) 385.0314, found 385.0314.
1-Bromo-2-(2-nitro-1-phenylethyl)benzene (3g). Yield: 272 mg, 89%. Yellow oil. 1H-NMR (400 MHz, CDCl3) δ 7.63 (d, J = 7.9 Hz, 1H), 7.40–7.34 (m, 2H), 7.34–7.28 (m, 4H), 7.28–7.23 (m, 1H), 7.20–7.14 (m, 1H), 5.49 (t, J = 8.1 Hz, 1H), 5.06–4.93 (m, 2H); 13C-NMR (100 MHz, CDCl3) δ 138.3, 137.9, 134.0, 129.3, 129.2, 128.5, 128.2, 128.1, 127.9, 125.1, 78.1, 47.8; HRMS (EI) m/z calcd for C14H12NO2Br (M+) 305.0051, found 305.0053.
1-Bromo-2-(2-nitro-1-(p-tolyl)ethyl)benzene (3h). Yield: 231 mg, 72%. Yellow oil. 1H-NMR (400 MHz, CDCl3) δ 7.60 (d, J = 8.0 Hz, 1H), 7.32.7.25 (m, 1H), 7.24–7.20 (m, 1H), 7.19–7.11 (m, 5H), 5.42 (t, J = 8.1 Hz, 1H), 5.01–4.88 (m, 2H); 13C-NMR (100 MHz, CDCl3) δ 138.6, 137.7, 134.8, 134.0, 129.9, 129.2, 128.5, 128.1, 125.1, 78.2, 47.5, 21.2; HRMS (EI) m/z calcd for C15H14NO2Br (M+) 319.0208, found 319.0208.
1-Bromo-2-(1-(4-isopropylphenyl)-2-nitroethyl)benzene (3i). Yield: 233 mg, 67%. Yellow oil. 1H-NMR (400 MHz, CDCl3) δ 7.59 (d, J = 8.0 Hz, 1H), 7.33–7.25 (m, 1H), 7.25–7.20 (m, 1H), 7.18 (s, 4H), 7.16–7.10 (m, 1H), 5.42 (t, J = 8.1 Hz, 1H), 5.01–4.88 (m, 2H), 1.23 (s, 3H), 1.21 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 148.6, 138.6, 135.1, 134.0, 129.2, 128.6, 128.1, 127.3, 125.1, 78.2, 47.5, 33.9, 24.1; HRMS (EI) m/z calcd for C17H18NO2Br (M+) 347.0521, found 347.0519.
2-Bromo-4-methox-1-(2-nitro-1-phenylethyl)benzene (3j). Yield: 326 mg, 97%. Yellow oil. 1H-NMR (400 MHz, CDCl3) δ 7.39–7.31 (m, 2H), 7.31–7.24 (m, 3H), 7.16 (d, J = 2.5 Hz, 1H), 7.12 (d, J = 8.7 Hz, 1H), 6.85 (dd, J = 8.6, 2.5 Hz, 1H), 5.39 (t, J = 8.2 Hz, 1H), 4.96 (dd, J = 13.4, 7.7 Hz, 1H), 4.92 (dd, J = 13.2, 8.6 Hz, 1H), 3.77 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 159.4, 138.3, 130.2, 129.1, 128.9, 128.0, 127.8, 125.2, 119.1, 114.1, 78.2, 55.7, 47.1; HRMS (EI) m/z calcd for C15H14NO3Br (M+) 335.0157, found 335.0151.
2-Bromo-4-methoxy-1-(2-nitro-1-(o-tolyl)ethyl)benzene (3k). Yield: 95 mg, 27%. Yellow oil. 1H-NMR (400 MHz, CDCl3) δ 7.29–7.20 (m, 4H), 7.21 (d, J = 2.4 Hz, 1H), 6.98 (d, J = 8.7 Hz, 1H), 6.80 (dd, J = 8.7, 2.6 Hz, 1H), 5.51 (dd, J = 9.1, 7.1 Hz, 1H), 4.95–4.82 (m, 2H), 3.79 (s, 3H), 2.31 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 159.5, 137.1, 136.3, 131.5, 129.8, 129.4, 127.8, 126.4, 125.9, 125.2, 118.8, 114.0, 76.6, 55.7, 44.0, 19.6; HRMS (EI) m/z calcd for C16H16NO3Br (M+) 349.0314, found 349.0308.
2-Bromo-4-methoxy-1-(2-nitro-1-(p-tolyl)ethyl)benzene (3l). Yield: 280 mg, 80%. Yellow oil. 1H-NMR (400 MHz, CDCl3) δ 7.16–7.13 (m, 5H), 7.11 (d, J = 8.7 Hz, 1H), 6.84 (dd, J = 8.7, 2.6 Hz, 1H), 5.34 (t, J = 8.2 Hz, 1H), 4.96–4.86 (m, 2H), 3.77 (s, 3H), 2.32 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 159.2, 137.2, 135.2, 130.3, 129.6, 128.7, 127.7, 125.0, 118.8, 113.8, 78.1, 55.4, 46.6, 20.9; HRMS (EI) m/z calcd for C16H16NO3Br (M+) 349.0315, found 349.0308.
2-Bromo-1-(1-(4-isobutylphenyl)-2-nitroethyl)-4-methoxybenzene (3m). Yield: 353 mg, 90%. Yellow oil. 1H-NMR (400 MHz, CDCl3) δ 7.17–7.09 (m, 6H), 6.84 (dd, J = 8.6, 2.6 Hz, 1H), 5.35 (t, J = 8.2 Hz, 1H), 4.97–4.87 (m, 2H), 3.77 (s, 3H), 2.44 (d, J = 7.2 Hz, 2H), 1.88–1.78 (m, 1H), 0.89 (d, J = 6.6 Hz, 6H); 13C-NMR (100 MHz, CDCl3) δ 159.4, 141.3, 135.6, 130.5, 129.9, 129.0, 127.7, 125.3, 119.0, 114.1, 78.4, 55.7, 46.8, 45.2, 30.3, 22.6; HRMS (ESI) m/z calcd for C19H22NO3Br (M+ + Na) 414.0675, found 414.0683.
2-Bromo-4-methoxy-1-(1-(3-methoxyphenyl)-2-nitroethyl)benzene (3n). Yield: 223 mg, 61%. Yellow oil. 1H-NMR (400 MHz, CDCl3) δ 7.27–7.23 (m, 1H), 7.15 (d, J = 2.7 Hz, 1H), 7.12 (d, J = 8.7 Hz, 1H), 6.86–6.80 (m, 4H), 5.36 (t, J = 8.2 Hz, 1H), 4.96–4.87 (m, 2H), 3.77 (s, 3H), 3.76 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 160.1, 159.4, 139.9, 130.1, 128.9, 125.2, 120.1, 119.0, 114.3, 114.0, 112.7, 78.1, 55.6, 55.3, 47.0, 29.8; HRMS (EI) m/z calcd for C16H16NO4Br (M+) 365.0263, found 365.0268.
1-(1-(2-Bromo-4-methoxyphenyl)-2-nitroethyl)naphthalene (3o). Yield: 139 mg, 36%. Yellow oil. 1H-NMR (400 MHz, CDCl3) δ 8.08 (d, J = 8.3 Hz, 1H), 7.89 (d, J = 7.8 Hz, 1H), 7.84 (d, J = 7.9 Hz, 1H), 7.58–7.43 (m, 4H), 7.26 (d, J = 2.8, 1H), 7.00 (d, J = 8.7 Hz, 1H), 6.71 (dd, J = 8.7, 2.6 Hz, 1H), 6.20 (dd, J = 8.8, 7.2 Hz, 1H), 5.09 (dd, J = 13.7, 9.2 Hz, 1H), 4.97 (dd, J = 13.7, 9.2 Hz, 1H), 3.71 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 159.5, 134.3, 134.0, 131.4, 129.7, 129.7, 129.1, 128.7, 127.0, 126.1, 125.2, 124.9, 124.0, 123.2, 119.0, 114.0, 76.6, 55.5, 43.5; HRMS (ESI) m/z calcd for C19H16NO3Br (M + Na+) 408.0205, found 408.0206.
2-Bromo-1-(1-(3-chlorophenyl)-2-nitroethyl)-4-methoxybenzene (3p). Yield: 126 mg, 34%. Yellow oil. 1H-NMR (400 MHz, CDCl3) δ 7.30–7.26 (m, 3H), 7.21–7.19 (m, 2H), 7.12 (d, J = 8.6 Hz, 1H), 6.89 (dd, J = 8.6, 2.6 Hz, 1H), 5.4 (t, J = 8.1 Hz, 1H), 4.97–4.91 (m, 2H), 3.81 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 159.6, 140.4, 135.0, 130.4, 129.4, 128.8, 128.3, 128.1, 126.2, 125.2, 119.2, 114.2, 77.9, 55.7, 46.8; HRMS (EI) m/z calcd for C15H13NO3ClBr (M+) 368.9767, found 368.9775.
2-Bromo-1-(1-(4-chlorophenyl)-2-nitroethyl)-4-methoxybenzene (3q). Yield: 259 mg, 70%. Yellow oil. 1H-NMR (400 MHz, CDCl3) δ 7.30 (d, J = 8.4 Hz, 2H), 7.19 (d, J = 8.4 Hz, 2H), 7.15 (d, J = 2.6 Hz, 1H), 7.07 (d, J = 8.7 Hz, 1H), 6.85 (dd, J = 8.7, 2.6 Hz, 1H), 5.34 (t, J = 16.3 Hz, 1H), 4.96–4.85 (m, 2H), 3.78 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 159.6, 136.9, 133.8, 129.7, 129.5, 129.3, 128.8, 125.2, 119.2, 114.2, 78.1, 55.8, 46.6; HRMS (EI) m/z calcd for C15H13NO3ClBr (M+) 368.9767, found 368.9775.
2-Bromo-4-methoxy-1-(2-nitro-1-(2-nitrophenyl)ethyl)benzene (3r). Yield: 358 mg, 94%. Yellow solid. Mp: 115–116 °C 1H-NMR (400 MHz, CDCl3) δ 7.94 (dd, J = 8.1, 1.2 Hz, 1H), 7.57–7.53 (m, 1H), 7.47–7.42 (m, 1H), 7.26–7.24 (m, 1H), 7.16 (d, J = 8.6 Hz, 1H), 7.12 (d, J = 2.6 Hz, 1H), 6.85 (dd, J = 8.6, 2.6 Hz, 1H), 5.86 (dd, J = 8.6, 7.3 Hz, 1H), 5.09–4.97 (m, 2H), 3.76 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 159.9, 150.0, 133.5, 132.8, 130.0, 128.9, 128.6, 128.3, 125.9, 125.5, 119.7, 113.8, 76.2, 55.8, 43.1; HRMS (ESI) m/z calcd for C15H13N2O5Br (M + Na+) 402.9900, found 402.9915.
2-(2-Bromophenyl)-2-(4-methoxyphenyl)ethanamine (4a). Yield: 306 mg, 99%. Colorless oil. 1H-NMR (400 MHz, CDCl3) δ 7.51 (d, J = 7.8 Hz, 1H), 7.35–7.25 (m, 2H), 7.21 (d, J = 8.6 Hz, 2H), 7.08 (ddd, J = 7.8, 6.6, 2.2 Hz, 1H), 6.88 (d, J = 8.6 Hz, 2H), 4.48 (t, J = 7.5 Hz, 1H), 3.80 (s, 3H), 3.30 (dd, J = 12.9, 7.2 Hz, 1H), 3.27 (dd, J = 13.2, 7.9 Hz, 1H), 1.28 (s, 2H); 13C-NMR (100 MHz, CDCl3) δ 158.5, 142.4, 133.6, 133.5, 129.6, 128.7, 128.1, 127.9, 125.9, 114.2, 55.4, 53.0, 47.0; HRMS (EI) m/z calcd for C15H16NOBr (M+) 305.0415, found 305.0421.
3-(4-Methoxyphenyl)indoline (5a). Colorless solid. Mp: 67–69 °C. 1H-NMR (400 MHz, CDCl3) δ 7.20 (d, J = 8.5 Hz, 2H), 7.11–7.04 (m, 1H), 6.91 (d, J = 7.0 Hz, 1H), 6.86 (d, J = 8.7 Hz, 2H), 6.68–6.75 (m, 2H), 4.45 (t, J = 9.2 Hz, 1H), 3.91 (t, J = 9.0 Hz, 1H), 3.80 (s, 3H), 3.46 (t, J = 9.0 Hz, 1H); 13C-NMR (100 MHz, CDCl3) δ 158.7, 151.7, 135.9, 132.9, 139.3, 127.9, 125.2, 119.3, 114.2, 110.0, 57.0, 55.5, 48.2. HRMS (EI) m/z calcd for C15H15NO (M+) 225.1154, found 225.1156.
3-(4-Methoxyphenyl)-1H-indole (6a). Yield: 156 mg, 70%. Colorless solid. Mp: 133–134 °C. 1H-NMR (400 MHz, CDCl3) δ 8.17 (s, 1H), 7.96 (d, J = 7.9 Hz, 1H), 7.64 (d, J = 8.7 Hz, 2H), 7.44 (d, J = 8.0 Hz, 1H), 7.34–7.21 (m, 3H), 7.06 (d, J = 8.7 Hz, 2H), 3.91 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 158.3, 136.8, 128.8, 128.3, 126.1, 122.5, 121.4, 120.3, 119.9, 118.2, 114.5, 111.6, 55.6. HRMS (EI) m/z calcd for C15H13NO (M+) 223.0997, found 223.1002.
5-Methoxy-3-(4-methoxyphenyl)-1H-indole (6b). Yield: 182 mg, 72%. Colorless solid. Mp: 193–194 °C. 1H-NMR (400 MHz, CDCl3) δ 7.50 (d, J = 8.7 Hz, 2H), 7.39 (d, J = 8.9 Hz, 1H), 7.30 (d, J = 2.3 Hz, 1H), 7.24 (s, 1H), 6.98 (d, J = 8.7 Hz, 2H), 6.94 (dd, J = 8.9, 2.3 Hz, 1H), 6.31 (s, 1H), 3.85 (s, 3H), 3.84 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 158.5, 155.3, 132.0, 128.9, 127.9, 127.7, 124.8, 118.6, 114.6, 113.1, 110.4, 102.6, 56.2, 55.6. HRMS (EI) m/z calcd for C16H15NO2 (M+) 253.1103, found 253.1106.
5,6-Dimethoxy-3-(4-methoxyphenyl)-1H-indole (6c). Yield: 161 mg, 57%. Colorless solid. Mp: 207–208 °C. 1H-NMR (400 MHz, CDCl3) δ 8.04 (s, 1H), 7.56 (d, J = 8.7 Hz, 2H), 7.30 (s, 1H), 7.16 (d, J = 2.3 Hz, 1H), 7.01 (d, J = 8.7 Hz, 2H), 6.92 (s, 1H), 3.93 (s, 6H), 3.87 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 158.3, 147.6, 145.7, 131.2, 128.7, 128.6, 119.9, 119.0, 118.2, 114.5, 101.8, 94.9, 56.7, 56.4, 55.6. HRMS (EI) m/z calcd for C17H17NO3 (M+) 283.1208, found 283.1213.
7-(4-Methoxyphenyl)-5H-[1,3]dioxolo[4,5-f]indole (6d). Yield: 61 mg, 23%. Colorless solid. Mp: 169–170 °C. 1H-NMR (400 MHz, CDCl3) δ 8.04 (s, 1H), 7.54–7.50 (m, 2H), 7.25 (s, 1H), 7.16 (d, J = 2.5 Hz, 1H), 7.01–6.97 (m, 2H), 6.87 (s, 1H), 5.95 (s, 2H), 3.86 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 158.3, 145.3, 143.7, 131.7, 128.7, 128.3, 120.1, 120.0, 118.6, 114.5, 100.9, 98.6, 92.3, 55.6. HRMS (EI) m/z calcd for C16H13NO3 (M+) 267.0895, found 267.0898.
3-(4-Methoxyphenyl)-5-propoxy-1H-indole (6e). Yield: 79 mg, 28%. Colorless oil. 1H-NMR (400 MHz, CDCl3) δ 8.08 (s, 1H), 7.56 (d, J = 7.9 Hz, 2H), 7.34–7.26 (m, 3H), 7.01 (d, J = 7.9 Hz, 2H), 6.91 (d, J = 8.8 Hz, 1H), 3.97 (t, J = 6.6 Hz, 2H), 3.87 (s, 3H), 1.88–1.81 (m, 2H), 1.06 (t, J = 4.8 Hz, 3H); 13C-NMR (100 MHz, CDCl3) δ 158.3, 154.3, 132.0, 128.7, 128.5, 126.6, 122.1, 118.1, 114.5, 113.3, 112.1, 103.1, 70.8, 55.6, 23.0, 10.8. HRMS (EI) m/z calcd for C18H19NO2 (M+) 281.1416, found 281.1412.
3-(4-Methoxyphenyl)-1H-benzo[g]indole (6f). Yield: 66 mg, 24%. Gray solid. Mp: 251–252 °C. 1H-NMR (400 MHz, CDCl3) δ 8.91 (s, 1H), 8.03 (d, J = 8.6 Hz, 1H), 7.95 (d, J = 8.9 Hz, 2H), 7.64 (d, J = 8.6 Hz, 2H), 7.59–7.53 (m, 2H), 7.47–7.44 (m, 1H), 7.36 (d, J = 2.4 Hz, 1H), 7.03 (d, J = 8.6 Hz, 2H), 3.88 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 158.5, 131.4, 130.8, 129.1, 128.3, 125.8, 124.3, 122.0, 122.0, 121.2, 120.2, 119.9, 119.5, 119.5, 114.5, 55.6. HRMS (EI) m/z calcd for C19H15NO (M+) 273.1154, found 273.1149.
3-Phenyl-1H-indole (6g). Yield: 162 mg, 84%. Colorless solid. Mp: 85–87 °C. 1H-NMR (400 MHz, CDCl3) δ 8.23 (s, 1H), 7.96 (d, J = 7.9 Hz, 1H), 7.69 (d, J = 7.8 Hz, 2H), 7.49–7.42 (m, 3H), 7.38 (d, J = 2.6 Hz, 1H), 7.33–7.18 (m, 3H); 13C-NMR (100 MHz, CDCl3) δ 136.9, 135.8, 129.0, 127.7, 126.2, 126.0, 122.6, 121.9, 120.5, 120.0, 118.6, 111.6. HRMS (EI) m/z calcd for C14H11N (M+) 193.0891, found 193.0891.
3-(p-Tolyl)-1H-indole (6h). Yield: 130 mg, 63%. Colorless solid. Mp: 88–90 °C. 1H-NMR (400 MHz, CDCl3) δ 8.21 (s, 1H), 7.98 (d, J = 7.8 Hz, 1H), 7.62 (d, J = 8.0 Hz, 2H), 7.45 (d, J = 8.0 Hz, 1H), 7.36 (d, J = 1.2 Hz, 1H), 7.33–7.20 (m, 4H), 2.45 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 136.8, 135.8, 132.8, 129.7, 127.6, 126.0, 122.5, 121.7, 120.4, 120.1, 118.5, 111.5, 21.4. HRMS (EI) m/z calcd for C15H13N (M+) 207.1048, found 207.1049.
3-(4-Isopropylphenyl)-1H-indole (6i). Yield: 157 mg, 63%. Colorless solid. Mp: 163–165 °C. 1H-NMR (400 MHz, CDCl3) δ 8.20 (s, 1H), 7.95 (d, J = 7.88 Hz, 1H), 7.61 (d, J = 8.2 Hz, 2H), 7.43 (d, J = 8.0 Hz, 1H), 7.35 (d, J = 2.5 Hz, 1H), 7.32 (d, J = 8.2 Hz, 2H), 7.28–7.16 (m, 2H), 3.00–2.94 (m, 1H), 1.33 (s, 3H), 1.31 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 146.8, 136.9, 133.2, 127.7, 127.0, 126.1, 122.5, 121.7, 120.4, 120.1, 118.6, 111.5, 34.1, 24.3. HRMS (EI) m/z calcd for C17H17N (M+) 235.1361, found 235.1361.
6-Methoxy-3-phenyl-1H-indole (6j). Yield: 138 mg, 62%. Colorless solid. Mp: 147–148 °C. 1H-NMR (500 MHz, CDCl3) δ 8.10 (s, 1H), 7.81 (d, J = 8.8 Hz, 1H), 7.65 (d, J = 7.3 Hz, 2H), 7.43 (t, J = 7.6 Hz, 2H), 7.30–7.24 (m, 2H), 6.90 (d, J = 2.1 Hz, 1H), 6.87 (dd, J = 8.8, 2.1 Hz, 1H), 3.87 (s, 3H); 13C-NMR (125 MHz, CDCl3) δ 156.9, 137.7, 135.8, 128.9, 127.5, 126.1, 120.7, 120.7, 120.4, 118.6, 110.5, 95.0, 55.9. HRMS (EI) m/z calcd for C15H13NO (M+) 223.0997, found 223.1001.
6-Methoxy-3-(o-tolyl)-1H-indole (6k). Yield: 154 mg, 65%. Colorless oil. 1H-NMR (400 MHz, CDCl3) δ 8.09 (s, 1H), 7.43–7.38 (m, 2H), 7.33–7.31 (m, 1H), 7.27–7.24 (m, 2H), 7.08 (s, 1H), 6.92 (s, 1H), 6.82 (d, J = 8.7 Hz, 1H), 3.87 (s, 3H), 2.33 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 156.8, 137.0, 136.9, 134.8, 131.0, 130.5, 126.9, 125.8, 121.9, 121.7, 121.0, 117.7, 110.1, 94.9, 55.9, 20.9. HRMS (EI) m/z calcd for C16H15NO (M+) 237.1154, found 237.1148.
6-Methoxy-3-(p-tolyl)-1H-indole (6l). Yield: 176 mg, 74%. Colorless solid. Mp: 110–111 °C. 1H-NMR (400 MHz, CDCl3) δ 8.05 (s, 1H), 7.79 (d, J = 8.8 Hz, 1H), 7.55 (d, J = 8.0 Hz, 2H), 7.24–7.23 (m, 2H), 6.91 (d, J = 2.2 Hz, 1H), 6.86 (dd, J = 8.8, 2.2 Hz, 1H), 3.87 (s, 3H), 2.40 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 156.9, 137.7, 135.7, 132.9, 129.6, 127.4, 120.7, 120.5, 120.4, 118.5, 110.3, 95.0, 55.9, 21.3. HRMS (EI) m/z calcd for C16H15NO (M+) 237.1154, found 237.1151.
3-(4-Isobutylphenyl)-6-methoxy-1H-indole (6m). Yield: 59 mg, 21%. Colorless solid. Mp: 77–78 °C. 1H-NMR (400 MHz, CDCl3) δ 8.06 (s, 1H), 7.81 (d, J = 8.7 Hz, 1H), 7.56 (d, J = 8.1 Hz, 2H), 7.24 (d, J = 2.3 Hz, 1H), 7.21 (d, J = 8.1 Hz, 2H), 6.90 (d, J = 2.2 Hz, 1H), 6.85 (dd, J = 8.8, 2.3 Hz, 1H), 3.87 (s, 3H), 2.51 (d, J = 7.1 Hz, 2H), 1.96–1.86 (m, 1H), 0.95 (d, J = 6.6 Hz, 6H); 13C-NMR (100 MHz, CDCl3) δ 156.9, 139.6, 137.7, 133.1, 129.7, 127.2, 120.8, 120.6, 120.4, 118.6, 110.3, 95.0, 55.9, 45.4, 30.5, 22.6. HRMS (EI) m/z calcd for C19H21NO (M+) 279.1623, found 279.1629.
6-Methoxy-3-(3-methoxyphenyl)-1H-indole (6n). Yield: 157 mg, 62%. Colorless oil. 1H-NMR (400 MHz, CDCl3) δ 8.09 (s, 1H), 7.81 (d, J = 8.8 Hz, 1H), 7.37–7.33 (m, 1H), 7.27 (d, J = 2.1 Hz, 1H), 7.23–7.20 (m, 1H), 6.91–6.83 (m, 3H), 3.87 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 160.2, 157.0, 137.7, 137.2, 129.9, 120.8, 120.8, 120.4, 120.1, 118.5, 113.2, 111.6, 110.5, 95.0, 55.9, 55.5. HRMS (EI) m/z calcd for C16H15NO2 (M+) 253.1103, found 253.1105.
6-Methoxy-3-(naphthalen-1-yl)-1H-indole (6o). Yield: 219 mg, 80%. Red oil. 1H-NMR (400 MHz, CDCl3) δ 8.21 (s, 1H), 8.10 (d, J = 8.5 Hz, 1H), 7.92 (d, J = 8.1 Hz, 1H), 7.86 (d, J = 8.1 Hz, 1H), 7.60–7.48 (m, 3H), 7.43–7.36 (m, 2H), 7.27–7.26 (m, 1H), 6.97 (d, J = 2.1 Hz, 1H), 6.80 (dd, J = 8.8, 2.1 Hz, 1H), 3.89 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 157.0, 137.0, 134.2, 133.3, 132.8, 128.5, 127.8, 127.3, 126.8, 125.9, 125.9, 125.8, 122.5, 122.4, 121.2, 116.8, 110.3, 94.9, 56.0. HRMS (EI) m/z calcd for C19H15NO (M+) 273.1154, found 273.1151.
3-(3-Chlorophenyl)-6-methoxy-1H-indole (6p). Yield: 144 mg, 56%. Colorless solid. Mp: 195–196 °C. 1H-NMR (400 MHz, CDCl3) δ 7.79 (d, J = 8.7 Hz, 1H), 7.60–7.59 (m, 1H), 7.51–7.48 (m, 1H), 7.38–7.34 (m, 1H), 7.28–7.25 (m, 2H), 6.98 (d, J = 2.1 Hz, 2H), 6.92 (dd, J = 8.8, 2.2 Hz, 1H), 6.31 (s, 1H), 3.86 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 157.5, 137.7, 136.9, 134.8, 130.2, 127.5, 126.5, 125.7, 123.6, 121.2, 121.1, 118.2, 110.8, 93.8, 55.9. HRMS (EI) m/z calcd for C15H12NOCl (M+) 257.0607, found 257.0601.
3-(4-Chlorophenyl)-6-methoxy-1H-indene (6q). Yield: 155 mg, 60%. Yellow solid. Mp: 154–155 °C. 1H-NMR (400 MHz, CDCl3) δ 8.10 (s, 1H), 7.75 (d, J = 8.7 Hz, 1H), 7.59–7.55 (m, 2H), 7.41–7.38 (m, 2H), 7.24 (d, J = 2.5 Hz, 1H), 6.91 (d, J = 2.2 Hz, 1H), 6.87 (dd, J = 8.7, 2.2 Hz, 1H), 3.87 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 157.0, 137.7, 134.3, 131.7, 129.1, 128.6, 120.8, 120.5, 120.1, 117.3, 110.7, 95.1, 55.9. HRMS (EI) m/z calcd for C15H12NOCl (M+) 257.0607, found 257.0602.
3-(2-Bromo-4-methoxyphenyl)-1H-indole (6r). Yield: 275 mg, 91%. Yellow solid. Mp: 122–123 °C. 1H-NMR (400 MHz, CDCl3) δ 8.26 (s, 1H), 7.58 (d, J = 7.9 Hz, 1H), 7.43 (d, J = 4.2 Hz, 2H), 7.36 (d, J = 2.5 Hz, 1H), 7.29 (d, J = 2.5 Hz, 1H), 7.26–7.22 (m, 1H), 7.18–7.14 (m, 1H), 6.95 (dd, J = 8.5, 2.6 Hz, 1H), 3.86 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 159.0, 135.9, 132.7, 128.3, 127.1, 124.5, 123.9, 122.5, 120.3, 120.2, 118.6, 116.6, 113.7, 111.4, 55.8. HRMS (EI) m/z calcd for C15H12NOBr (M+) 301.0102, found 301.0109.
Conclusions
In summary, we successfully synthesized unprotected NH 3-arylindole derivatives via Cu catalysed intramolecular Ullmann reaction of bromoamines starting from easily available nitrostyrene starting materials. The substructures on both indole and 3-aryl groups can be flexible based on the choices of β-nitrostyrenes and arenes.
Acknowledgements
Financial support by the Ministry of Science and Technology of the Republic of China (MOST 103-2113-M-003-008-MY3), National Taiwan Normal University (103-07-C) and Instrumentation Centre at National Taiwan Normal University is gratefully acknowledged. The authors are grateful to Ms Hsiu-Ni Huan, Ms Chiu-Hui He and Ting-Shen Kuo for providing HRMS, NMR spectral and crystallographic data respectively.
Notes and references
- T. Barden, in Heterocyclic Scaffolds II, ed. G. W. Gribble, Springer, Berlin, Heidelberg, 2011, vol. 26, p. 31 Search PubMed.
-
(a) M. S. Pedras and M. Hossain, Bioorg. Med. Chem., 2007, 15, 5981 CrossRef CAS PubMed;
(b) T. C. Leboho, J. P. Michael, W. A. L. van Otterlo, S. F. van Vuuren and C. B. de Koning, Bioorg. Med. Chem., 2009, 19, 4948 CrossRef CAS PubMed.
- T.-S. Wu, M.-J. Liou, C.-J. Lee, T.-T. Jong, A. T. McPhail, D. R. McPhail and K.-H. Lee, Tetrahedron Lett., 1989, 30, 6649 CrossRef CAS.
- S. Murru, A. A. Gallo and R. S. Srivastava, Eur. J. Org. Chem., 2011, 2035 CrossRef CAS.
- T. I. Richardson, C. A. Clarke, K.-L. Yu, Y. K. Yee, T. J. Bleisch, J. E. Lopez, S. A. Jones, N. E. Hughes, B. S. Muehl, C. W. Lugar, T. L. Moore, P. K. Shetler, R. W. Zink, J. J. Osborne, C. Montrose-Rafizadeh, N. Patel, A. G. Geiser, R. J. Sells Galvin and J. A. Dodge, ACS Med. Chem. Lett., 2011, 2, 148 CrossRef CAS PubMed.
- L. Jouclaa and L. Djakovitch, Adv. Synth. Catal., 2009, 351, 673 CrossRef.
-
(a) E. Siddalingamurthy, K. M. Mahadevan, J. N. Masagalli and H. N. Harishkumar, Tetrahedron Lett., 2013, 54, 5591 CrossRef CAS;
(b) S. Gore, S. Baskaran and B. König, Org. Lett., 2012, 14, 4568 CrossRef CAS PubMed.
-
(a) Z. Zhang, Z. Hu, Z. Yu, P. Lei, H. Chi, Y. Wang and R. He, Tetrahedron Lett., 2007, 48, 2415 CrossRef CAS;
(b) G. Cusati and L. Djakovitch, Tetrahedron Lett., 2008, 49, 2499 CrossRef CAS;
(c) F. Bellina, F. Benelli and R. Rossi, J. Org. Chem., 2008, 73, 5529 CrossRef CAS PubMed;
(d) L. Joucla, N. Batail and L. Djakovitch, Adv. Synth. Catal., 2010, 352, 2929 CrossRef CAS.
- R. J. Phipps, N. P. Grimster and M. J. Gaunt, J. Am. Chem. Soc., 2008, 130, 8172 CrossRef CAS PubMed.
- J. Cornella, P. Lu and I. Larrosa, Org. Lett., 2009, 11, 5506 CrossRef CAS PubMed.
-
(a) Y. Chen, S. Guo, K. Li, J. Qu, H. Yuan, Q. Hua and B. Chen, Adv. Synth. Catal., 2013, 355, 711 CrossRef CAS;
(b) Y.-P. Zhang, X.-L. Feng, Y.-S. Yang and B.-X. Cao, Tetrahedron Lett., 2016, 57, 2298 CrossRef CAS.
-
(a) T. H. H. Hsieh and V. M. Dong, Tetrahedron, 2009, 65, 3062 CrossRef CAS;
(b) M. Shen, B. E. Leslie and T. G. Driver, Angew. Chem., Int. Ed., 2008, 47, 5056 CrossRef CAS PubMed;
(c) F. Zhou, D.-S. Wang and T. G. Driver, Adv. Synth. Catal., 2015, 357, 3463 CrossRef CAS;
(d) S. Tong, Z. Xu, M. Mamboury, Q. Wang and J. Zhu, Angew. Chem., Int. Ed., 2015, 54, 11809 CrossRef CAS PubMed.
-
(a) S. Murru, A. A. Gallo and R. S. Srivastava, ACS Catal., 2011, 1, 29 CrossRef CAS;
(b) S. Murru, A. A. Gallo and R. S. Srivastava, Eur. J. Org. Chem., 2011, 2035 CrossRef CAS.
- S. Kumar, V. Rathore, A. Verma, C. D. Prasad, A. Kumar, A. Yadav, S. Jana, M. Sattar, Meenakshi and S. Kumar, Org. Lett., 2015, 17, 82 CrossRef CAS PubMed.
- G. A. Russell, C.-F. Yao, H. I. Tashtoush, J. E. Russell and D. F. Dedolph, J. Org. Chem., 1991, 56, 663 CrossRef CAS.
- G. A. Russell and C.-F. Yao, J. Org. Chem., 1992, 57, 6508 CrossRef CAS.
- Some selected publications
(a) C.-W. Kuo, A. Konala, L. Lin, T.-T. Chiang, C.-Y. Huang, T.-H. Yang, V. Kavala and C.-F. Yao, Chem. Commun., 2016, 52, 7870 RSC;
(b) V. Kavala, D. Janreddy, M. J. Raihan, C.-W. Kuo, C. Ramesh and C.-F. Yao, Adv. Synth. Catal., 2012, 354, 2229 CrossRef CAS;
(c) T. Kotipalli, V. Kavala, D. Janreddy, C.-W. Kuo, T.-S. Kuo, H.-N. Huang, C.-H. He and C.-F. Yao, RSC Adv., 2014, 4, 2274 RSC;
(d) V. Kavala, C.-C. Wang, Y.-H. Wang, C.-W. Kuo, D. Janreddy, W.-C. Huang, T.-S. Kuo, C.-H. He, M.-L. Chen and C.-F. Yao, Adv. Synth. Catal., 2014, 356, 2609 CrossRef CAS;
(e) S. D. Gawande, V. Kavala, M. R. Zanwar, C.-W. Kuo, W.-C. Huang, T.-S. Kuo, H.-N. Huang, C.-H. He and C.-F. Yao, Adv. Synth. Catal., 2014, 356, 2599 CrossRef CAS;
(f) S. D Gawande, M. R Zanwar, V. Kavala, C.-W. Kuo, R. R. Rajawinslin and C.-F. Yao, Adv. Synth. Catal., 2015, 357, 168 CrossRef.
- Z. Tu, B. R. Raju, T.-R. Liou, V. Kavala, C.-W. Kuo, Y. Jang, Y.-H. Shih, C.-C. Wang and C.-F. Yao, Tetrahedron, 2009, 65, 2436 CrossRef CAS.
- É. Balogh-Hergovich and G. Speier, J. Mol. Catal., 1986, 37, 309 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra21144e |
| ‡ Equal contribution. |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu(OAc)2 = 5 mol%
Cu(OAc)2 = 5 mol%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 mol%.d Microwave 100 W.e NMR yields.
5 mol%.d Microwave 100 W.e NMR yields.