Discovery of anti-inflammatory components from Guge Fengtong tablet based on inflammatory markers and exploration of its molecular mechanism†
Qun Liu‡
,
Zhen Wang‡,
Le-Le Liu,
Ping Li* and
E-Hu Liu*
State Key Laboratory of Natural Medicines, China Pharmaceutical University, No. 24 Tongjia Lane, Nanjing 210009, China. E-mail: liuehu2011@163.com; liping2004@126.com; Fax: +86 25 83271379; Tel: +86 25 83271379
First published on 30th September 2016
Abstract
Guge Fengtong tablet (GGFTT) is a Chinese herbal preparation which is widely applied to treat rheumatoid arthritis. In this work, we aim to discover the main anti-inflammatory components from GGFTT based on inflammatory markers and to explore its molecular mechanism. The components in GGFTT were screened by lipopolysaccharide induced macrophage RAW264.7 cells and TNF-α induced 293 cells which co-transfected with NF-κB. Based on the results of anti-inflammatory effects, we established and validated a reliable liquid chromatography-mass spectrometry method for the simultaneous determination of the 10 active compounds (10C) screened in GGFTT. The results of bioactive equivalence indicated that the combination of 10 active compounds could be considered as the bioactive combinatorial components in GGFTT. Further investigations suggested that GGFTT and 10C could significantly decrease the production of TNF-α, IL-1β, IL-6 and iNOS. 10C exerted comparable anti-inflammatory effect against NF-κB and MAPKs signaling pathways as GGFTT. Both of them could inhibit the phosphorylation of p65 and IκB-α, block the nuclear translocation of p65 as well as decrease the phosphorylation of ERK1/2 and JNK. The results of the present study has a great potential in promoting standardization of GGFTT and could be extended to improve quality control of herbal medicines.
1. Introduction
Rheumatoid arthritis (RA) is an autoimmune joint disease characterized by inflammation and destruction of articular surfaces and bone.1 The pathogenesis of RA is complex and marked by synovial hyperplasia, leukocyte infiltration and pro-inflammatory cytokine production, however, stimulated macrophages play a pivotal role in inflammatory diseases via excess production of these cytokines,2,3 such as tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6).4 Lipopolysaccharide (LPS) could activate several intracellular signaling transduction pathways, such as nuclear factor kappa B (NF-κB) pathway and the mitogen-activated protein kinase (MAPK) pathway.5,6 NF-κB, a heterodimeric transcription factor composed of p65 and p50 subunits, plays a significant role in the expression of inflammatory genes.7 When the cells are stimulated by LPS, IκB-α can be phosphorylated and degraded. The disassociation of IκB-α allows p65/p50 to translocate into the nucleus to bind the promoter regions of immune and inflammatory genes.8,9 These signaling pathways are also involved in inducible nitric oxide synthase (iNOS) expression in macrophages, which catalyze the production of NO.10 The use of herbal medicines to inhibit the expression of these inflammatory mediators has recently become a topic of interest in the regulation of inflammatory diseases.Traditional Chinese medicine has been used to treat RA for thousands years in China.11 Guge Fengtong tablet (GGFTT), a popular traditional Chinese herbal preparation designed for the treatment of RA, is made from three medicinal herbs including Dioscorea nipponica Rhizoma, Spatholobi Caulis and Zingiberis Rhizoma. Our previous studies have illustrated the chemical profiling and the pharmacological effects of GGFTT in vivo.12,13 The major components identified in GGFTT includes saponins, gingerols and flavonoids.13 However, little is known about its active components responsible for the efficacy as well as the mechanism of action of GGFTT.
It is widely accepted that the therapeutic efficacy of herbal medicines is achieved by combinatorial components rather than single compound. Nevertheless, previous works have been focused on isolating and screening single effective component from herbal medicines. The combinatorial role and integrative therapeutic effects of multiple active compounds are rarely investigated. Hence, it is incumbent to elucidate the exact effective component combination in herbal preparation.
In the present study, the anti-inflammatory effects of 20 major compounds in GGFTT were firstly investigated by NO and NF-κB inhibition in LPS-induced RAW264.7 cells. Based on the anti-inflammatory results, the contents of the bioactive components were determined by liquid chromatography-mass spectrometry (LC-MS) method, and the anti-inflammatory contribution ratio of the effective compound combination was calculated. Also, the anti-inflammatory mechanisms of GGFTT and the active compound combination were investigated in intracellular level. This work offers evidence-based data to answer the key question of which are real bioactive components for GGFTT and its molecular mechanism.
2. Experimental
2.1. Materials
Guge Fengtong tablet (GGFTT) was obtained from GMP-approved Xiuzheng Pharmaceutical Co., Ltd. Lipopolysaccharide (Escherichia coli 055:B5) and dimethyl sulphoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Mouse TNF-α and IL-1β ELISA (enzyme-linked immunosorbent assay) kits were obtained from CUSABIO BIOTECH CO., LTD. Primary antibodies for NF-κB p65, phospho-NF-κB p65, IκB-α, phospho-IκB-α, ERK1/2, phospho-ERK1/2, JNK, phospho-JNK, p38, phospho-p38 were purchased from Cell Signaling Technology (Danvers, MA, USA). β-Actin antibody was from Santa Cruz biotechnology (Santa Cruz, CA, USA) and HRP-conjugated goat anti-rabbit and goat anti-mouse second bodies were provided by Zhongshan Golden Bridge Bio-technology Co., Ltd (Beijing, China).The reference compounds of daidzein, genistein, liquiritigenin, ononin, hesperetin, calycosin, formononetin, naringenin, epigallocatechi, epicatechin, protocatechuic acid, catechin, biochanin A, 6-gingerol, 8-gengerol, 6-shogaol, protodioscin, protogarcillin, pseudoprotodioscin, dioscin and internal standard amentoflavone, ginsenoside Rg3, capsaicin were purchased from Chengdu Must Bio-technology Co., Ltd. (Chengdu, China). The purities of these compounds were determined to be higher than 95% by high performance liquid chromatography-diode array detection analysis.
2.2. The preparation of GGFTT
The GGFTT samples were pulverized into powder after removed the coating. The powder (0.5 g) was extracted with ultrasonic in 100% methanol (25 mL) for 45 min at room temperature. After cooling to room temperature, the extracted solution was centrifuged at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm min−1 for 10 min for LC-MS experiment. Another resultant extract solution was condensed with rotary vacuum evaporator, then dissolved with water. The extract was lyophilized and stored at 4 °C before used. The lyophilized powder was re-suspended in DMSO to prepare GGFTT for cell experiments.
000 rpm min−1 for 10 min for LC-MS experiment. Another resultant extract solution was condensed with rotary vacuum evaporator, then dissolved with water. The extract was lyophilized and stored at 4 °C before used. The lyophilized powder was re-suspended in DMSO to prepare GGFTT for cell experiments.
2.3. Cell culture
The RAW264.7 and 293 cell lines derived from murine macrophages were purchased from the Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China. Cells were cultured in RPMI 1640 medium (RAW264.7 cells) or Dulbecco's modified Eagle's medium (293 cells) supplemented with 10% fetal bovine serum (FBS, Gibco, Grand Island, New York, USA) and antibiotics (100 U mL−1 of penicillin and 100 U mL−1 of streptomycin) at 37 °C in an atmosphere of 5% (v/v) CO2.2.4. Nitrite analysis
RAW264.7 cells were seeded into 96-well plates 5 × 105 cells per mL for 24 h, the cells were treated with various concentrations of compounds (ESI Table S5†) for 1 h before LPS (1 μg mL−1) stimulation and then incubated at 37 °C for an additional 24 h. NO synthesis was then determined by assaying the culture supernatants for nitrite using the Griess reagent. Briefly, the conditioned medium (100 μL) was mixed with the same volume of Griess reagent and incubated for 10 min. The absorbance of the mixture was measured at 540 nm with a plate reader.2.5. Transient transfection and luciferase assay
For the luciferase reporter assay, 293 cells were seeded into 96-well plates 2 × 105 cells per mL for 24 h. Cells were transfected with pNF-κB-TA-luc and SV40 plasmids using LipofectamineTM 2000 according to the manufacturer's protocol (Invitrogen). After transfection for 24 h, 293 cells were treated with TNF-α for 4 h and then incubated with different concentrations of compounds (ESI Table S6†) for 3 h. The transcriptional activity was determined by a dual-luciferase reporter assay system (Promega, Madison, WI) according to the manufacturer's instructions with a luminometer.2.6. LC-MS conditions
Chromatographic separations performed by an Agilent 1100 Series LC system (Agilent Technologies, Germany) was used for quantitative analyses. Chromatographic separation was conducted on an Agilent Extend-C18 column (5 μm, 4.6 × 250 mm). The separation and determination were carried out in a linear gradient programme, in which the mobile phase consisted of 0.1% (v/v) formic acid in water (A) and acetonitrile (B). The mobile phase was programmed as follows: 0–5 min, 30–35% B; 5–8 min, 35% B; 8–15 min, 35–42% B; 15–24 min, 42% B; 24–42 min, 42–50% B; 42–57 min, 50% B; 57–67 min, 50–65% B; 67–71 min, 65–100% B; 71–76 min, 100% B. The flow rate was 1 mL min−1 and the column temperature was set at 30 °C.The MS conditions were as follows: drying gas temperature, 300 °C; drying gas flow, 10 L min−1; nebulizer pressure, 35 psi; capillary voltage, 3000 V; fragmentor voltage, 120 V. The MS spectra were acquired in selective ion monitoring (SIM) mode for detecting the compounds. SIM for each compound was restricted to specific retention time windows: 0–12.4 min at m/z 271.0 (genistein), 12.4–14.5 min at m/z 303.0 (hesperetin), 14.5–19 min at m/z 539.0 (amentoflavone), 19–76 min at m/z 285.0 (biochanin A) by channel 1; and 0–7 min at m/z 1047.0 (protogracillin), 7–10 min at m/z 1031.0 (pseudoprotodioscin), 10–13 min at m/z 273.0 (naringenin), 13–21 min at m/z 539.0 (amentoflavone), 21–26 min at m/z 277.0 (6-gingerol), 26–36 min, at m/z 407.0 (ginsenoside Rg3) and 294.0 (capsaicin), 36–44 min at m/z 869.0 (dioscin), 44–49 min at m/z 305.0 (8-gingerol), 49–76 min at m/z 277.0 (6-shogaol) by channel 2.
2.7. Preparation of standard and sample solutions
Each standard solution containing ten mixed reference compounds (genistein, hesperetin, biochanin A, naringenin, protogracillin, pseudoprotodioscin, dioscin, 6-gingerol, 8-gingerol and 6-shogaol) was accurately weighed, and then dissolved in methanol to give the stock solutions. The working solutions were prepared by appropriate dilution of the stock solutions with methanol to yield six concentrations, an aliquot of stock solution of internal standard amentoflavone, ginsenoside Rg3 and capsaicin were added to the final concentration of 0.8 μg mL−1, 10 μg mL−1 and 0.7 μg mL−1. The solutions were all stored in 4 °C.2.8. Measurement production of IL-1β, TNF-α and IL-6
RAW264.7 cells were seeded in 6-well plates at 7.5 × 105 cells per mL for 24 h, then in the presence of either 1 μg mL−1 LPS alone, or LPS plus 200 μg mL−1, 150 μg mL−1 and 100 μg mL−1 GGFTT and 10C for 24 h. Cell-free supernatants were subsequently employed for the cytokine assays using a mouse ELISA kit according to the manufacturer's instructions.2.9. Preparation of total cellular extract, cytosolic and nuclear extracts
RAW264.7 cells were seeded in 6-well plates pretreated with GGFTT and 10C for 1 h before treatment with or without LPS (1 μg mL−1) for 30 min. After washed twice with PBS, cells were lysed in RIPA lyse solution (Beyotime Institute of Biotechnology) containing protease inhibitors cocktail (F. Hoffmann-La Roche, Ltd) and phosSTOP (F. Hoffmann-La Roche, Ltd) on the ice for 1 h. The homogenates were centrifuged at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min at 4 °C, supernatant were used for total cellular extract. Cytosolic and nuclear fractions were isolated using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific, Rockford, IL, USA) according to the procedure described by the manufacturer.
000 rpm for 10 min at 4 °C, supernatant were used for total cellular extract. Cytosolic and nuclear fractions were isolated using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific, Rockford, IL, USA) according to the procedure described by the manufacturer.
2.10. Western blot
The protein concentrations were determined using BCA Protein Assay Kit (Beyotime Institute of Biotechnology). The protein of each sample was resolved using 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins were transferred to a nitrocellulose membrane (Millipore, Billerica, MA, USA), followed by blocking with 5% nonfat dried-milk proteins (w/v) in TBST for 2 h at room temperature, incubated with one of the following antibodies (overnight at 4 °C): IκB-α, p-IκB-α, NF-κB p65, p-NF-κB p65, p38, p-p38, ERK1/2, p-ERK1/2, JNK, p-JNK, or β-actin antibodies (all diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000). After washing with TBST 10 min for 3 times, proteins were detected by incubating with rabbit or mouse secondary antibodies for 2 h at room temperature. Bands were visualized using an enhanced chemiluminescence reagent (Millipore Cooperation, Billerica, MA).
1000). After washing with TBST 10 min for 3 times, proteins were detected by incubating with rabbit or mouse secondary antibodies for 2 h at room temperature. Bands were visualized using an enhanced chemiluminescence reagent (Millipore Cooperation, Billerica, MA).
2.11. Statistical analysis
All data were analyzed as means ± standard deviation (SD). Differences between means are determined by one-way ANOVA. A value of p < 0.05 was considered statistically significant.3. Results
3.1. Screening of anti-inflammatory compounds in GGFTT in LPS-induced RAW264.7 cells
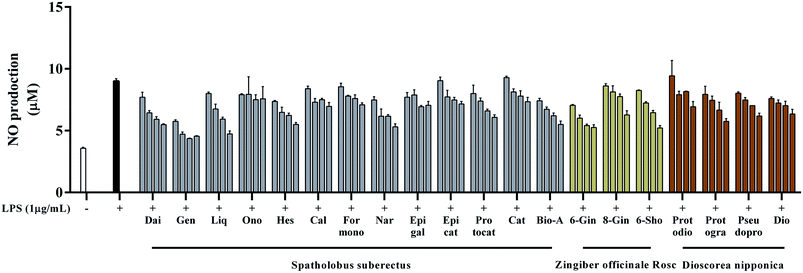
In our previous study, we had identified 47 compounds in GGFTT including 18 phenolic acids, 8 saponins, 14 gingerol-related compounds, and 7 diarylhepatonoids based on their MS fragmentation and literature information.13 To identify the active ingredients of GGFTT responsible for the anti-inflammatory activities, 20 major compounds (ESI Fig. S1†) from GGFTT were selected to investigate their effects on LPS-induced NO production in RAW264.7 cells. As shown in Fig. 1, 13 compounds (daidzein, genistein, liquiritigenin, hesperetin, naringenin, protocatechuic acid, biochanin A, 6-gingerol, 6-shogaol, 8-gingerol, protogarcillin, pseudoprotodioscin and dioscin) at their high concentrations, could significantly reduce an amount of 50% NO production, respectively. According to the MTT assay (ESI Fig. S2†), the inhibitory activity of these compounds on NO production in RAW264.7 cells was not caused by their cytotoxic effects. The main text of the article should appear here with headings as appropriate.3.2. Screening of active compounds that inhibit NF-κB gene expression in TNF-α induced 293 cells
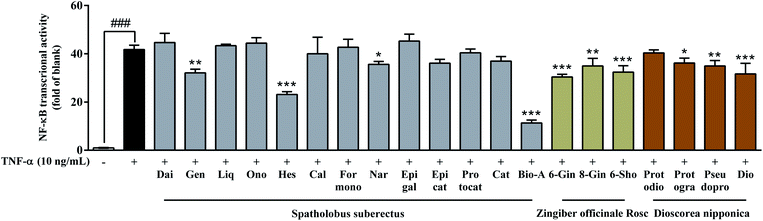
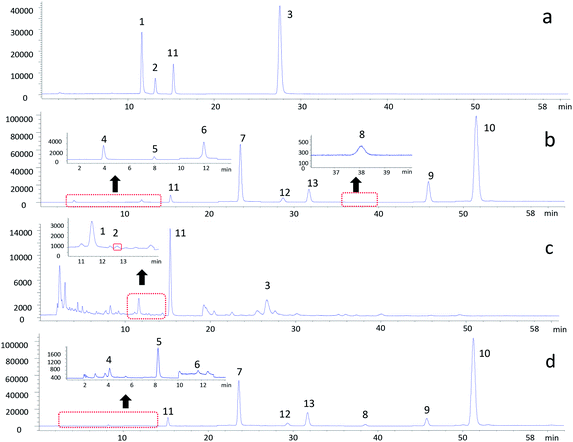
To investigate whether these 20 compounds could inhibit NF-κB pathway, TNF-α induced 293 cells were co-transfected with NF-κB-driven luciferase reporter gene (p-NF-κB Luc) and SV40. As shown in Fig. 2, 10 compounds including genistein, dioscin, hesperetin, biochanin A, 6-gingerol, 8-gengerol, 6-shogaol, protogarcillin, pseudoprotodioscin and naringenin could reduce the levels of NF-κB, respectively. Based on the results of cell viability (ESI Fig. S3†), the inhibitory activity of these compounds on NF-κB expression in 293 cells was not caused by their cytotoxic effects. Taking together the results of NO inhibition and NF-κB gene expression, we considered these 10 active compounds including 4 flavonoids, 3 gingerols and 3 saponins had made a majority contribution to the anti-inflammatory effect of the herbal preparation. The 10 anti-inflammatory compounds in GGFTT were quantified by LC-MS.To verify the above hypothesis, we next determined the content of these 10 compounds in GGFTT and validated the anti-inflammatory activities of the compound combination. The 10 anti-inflammatory compounds screened in GGFTT were quantified by LC-MS method. Chromatograms for the GGFTT and standard mixture obtained using LC-MS are shown in Fig. 3. After optimization of chromatographic conditions, the method validation results demonstrated that the LC-MS method had desirable specificity, linearity, precision and accuracy (ESI Tables S1–S4†). As the result shown in Table 1, the contents of these 10 compounds in 5 batches of GGFTT were relatively stable. The most abundant compound in GGFTT was dioscin, which was derived from Dioscorea nipponica Rhizoma. Our data indicated that the contents of saponins (e.g., dioscin, pseudoprotodioscin and protogarcillin) were higher than other types of compounds in GGFTT. It should be noted that the contents of the 4 flavonoids (genistein, naringenin, hesperetin and biochanin A) were remarkably low in GGFTT. In all, the total amounts of these 10 active compounds ranged from 7.01 to 8.97 mg g−1 in GGFTT.
| Peak | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Compounds | Genistein | Hesperetin | Biochanin A | Protogarcillin | Pseudoprotodioscin | Naringenin | 6-Gingerol | Dioscin | 8-Gingerol | 6-Shogaol | |
| a Three replicates of the 5 batches samples were extracted and analyzed the content. Trace means under the limit of quantification. | |||||||||||
| Sample 1 | Content mean ± SD | 0.00135 ± 0.00001 | 0.00008 ± 0.00002 | 0.00197 ± 0.00007 | 0.62751 ± 0.01101 | 1.85652 ± 0.03981 | 0.00077 ± 0.00002 | 0.28487 ± 0.00335 | 4.92373 ± 0.20904 | 0.09078 ± 0.00073 | 0.89364 ± 0.01552 |
| Sample 2 | Content mean ± SD | 0.00154 ± 0.00002 | 0.00011 ± 0.00003 | 0.00223 ± 0.00007 | 0.47681 ± 0.0184 | 1.24577 ± 0.03807 | 0.00102 ± 0.00002 | 0.17062 ± 0.00145 | 4.27917 ± 0.18532 | 0.0523 ± 0.0001 | 0.78287 ± 0.00164 |
| Sample 3 | Content mean ± SD | 0.00101 ± 0.00005 | 0.00009 ± 0.00001 | 0.00366 ± 0.00002 | 1.94262 ± 0.01872 | 1.70151 ± 0.02108 | Trace | 0.2483 ± 0.00153 | 2.8816 ± 0.049 | 0.07161 ± 0.00028 | 0.96791 ± 0.00781 |
| Sample 4 | Content mean ± SD | 0.00113 ± 0.00002 | 0.00007 ± 0.00001 | 0.00244 ± 0.00004 | 0.4626 ± 0.00663 | 2.47871 ± 0.0263 | 0.00089 ± 0.00005 | 0.15191 ± 0.00107 | 4.88063 ± 0.12105 | 0.05275 ± 0.00062 | 0.94183 ± 0.01176 |
| Sample 5 | Content mean ± SD | 0.00062 ± 0.00002 | 0.00008 ± 0.000001 | 0.00165 ± 0.00004 | 0.29794 ± 0.0042 | 1.44043 ± 0.04284 | 0.00076 ± 0.000001 | 0.34941 ± 0.01429 | 5.37194 ± 0.254 | 0.08956 ± 0.00306 | 0.92534 ± 0.01651 |
3.3. Evaluation of bioactive equivalence between compound combination and GGFTT
To obtain the combination of the 10 anti-inflammatory candidates (10C), we prepared it by mixing 10 reference compounds in proportion to the corresponding average contents of the 10 compounds in GGFTT. To test the contribution of these three types of compounds, flavonoids, saponins or gingerols were removed from 10C respectively and their anti-inflammatory activities were subsequently evaluated. As shown in Table 2, the combination of any two groups could not show equivalent bioactivity to original GGFTT. The results also indicated that each type of ingredients was indispensable. In the bioactive equivalence assessment, 90% CI showed that ratios of efficacies between GGFTT and 10C lie in average within the range of 66.70–112.30% (Table 3). Although 10C could not fully achieve bioactive equivalence as compared to the original GGFTT, it could be regarded as the major bioactive combinatorial components in GGFTT.| Group | Concentration μg mL−1 | Inhibition% |
|---|---|---|
| a “-” represented the removal of this type of compounds in 10C.b p 0.05, comparing with LPS group. | ||
| GGFTT | 200 | 77.73 ± 5.97b |
| 150 | 59.48 ± 0.5b | |
| 100 | 37.49 ± 3.38b | |
| 10C | 200 | 59.78 ± 0.53b |
| 150 | 37.49 ± 3.38b | |
| 100 | 27.97 ± 2.68b | |
| -Saponins | 200 | 53.24 ± 2.36b |
| 150 | 33.33 ± 3.57b | |
| 100 | 8.92 ± 3.61 | |
| -Gingerols | 200 | 6.54 ± 0.89 |
| 150 | 8.62 ± 4.12 | |
| 100 | 7.73 ± 2.87 | |
| -Flavones | 200 | 58.62 ± 5.74b |
| 150 | 35.11 ± 3.57b | |
| 100 | 28.56 ± 7.8b | |
| Concentration of GGFTT and 10C | ||||
|---|---|---|---|---|
| 90% CI of relative efficacy | 200 μg mL−1 | 150 μg mL−1 | 100 μg mL−1 | Average |
| 67.20–111.23% | 52.19–123.53% | 80.70–102.15% | 66.70–112.30% | |
3.4. GGFTT and 10C inhibited LPS-induced activation of pro-inflammatory cytokines in RAW264.7 cells
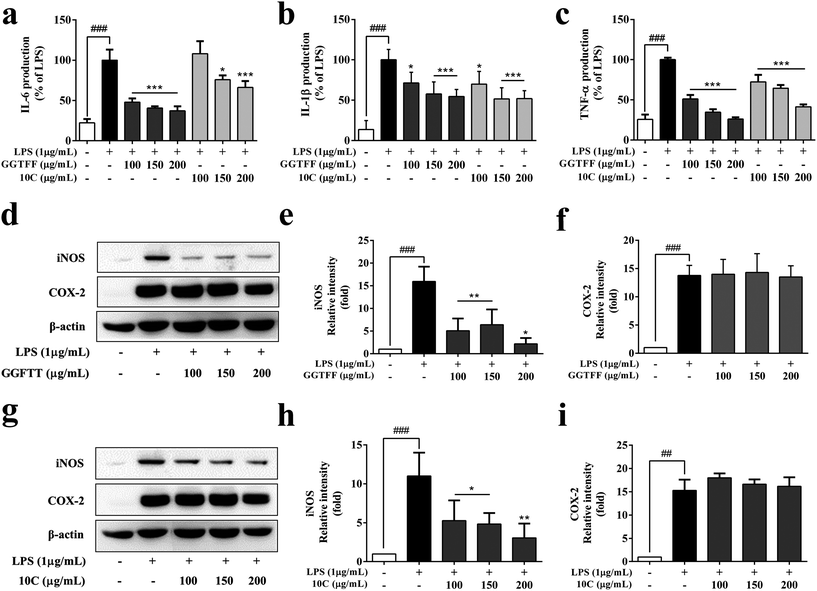
To investigate the anti-inflammatory molecular mechanism of GGFTT and verify whether 10C had comparable effect on pro-inflammatory cytokines and related signaling pathway, we firstly determined the effect of GGFTT and 10C on expression of IL-1β, TNF-α and IL-6 in the LPS-induced RAW264.7 cells. The results showed that both GGFTT and 10C could statistically inhibit the release of IL-1β, TNF-α and IL-6 in a dose-dependent manner (Fig. 4a–c). Subsequently, we evaluated the protein expression levels of iNOS and COX-2 to determine the inhibitory effects of GGFTT and 10C in RAW264.7 macrophages. In contrast to the dramatic reduction of protein expression of iNOS, GGFTT had no significant suppressive effects on the expression of COX-2 by Western blot analysis (Fig. 4d–f). Similarly, the results demonstrated that the effect of candidate 10C against iNOS and COX-2 is almost comparable to that of original GGFTT (Fig. 4g–i). These results suggested that the anti-inflammatory action of GGFTT and 10C was involved in the suppression of NO production by regulating the expression of IL-1β, TNF-α, IL-6 and iNOS in LPS-stimulated RAW264.7 macrophages.3.5. GGFTT and 10C inhibited LPS-induced activation of NF-κB in RAW264.7 cells
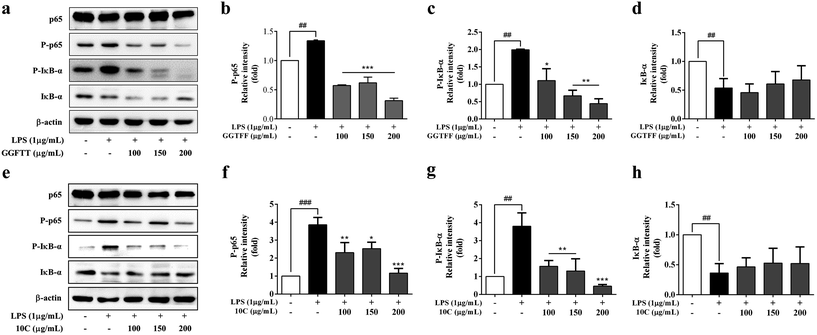
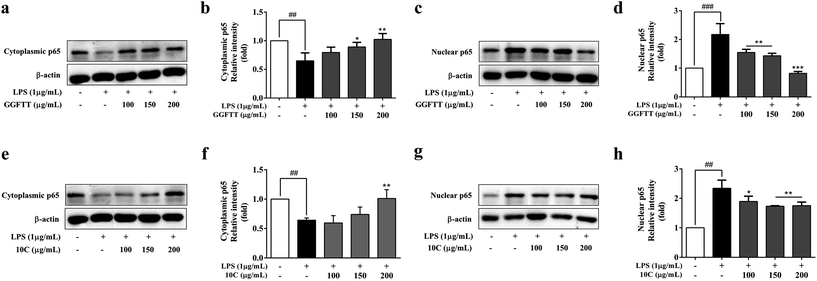
In order to elucidate the inhibitory effects of GGFTT and 10C on intracellular signaling transduction pathways, the major LPS-regulated proteins involved in the NF-κB signaling pathways were next detected. As shown in Fig. 5a–d, GGFTT could suppress the phosphorylation of NF-κB factor p65 and IκB-α compared with LPS-induced RAW264.7 cells, and 10C also showed similar inhibitory effect on NF-κB factor p65 and IκB-α to GGFTT (Fig. 5e–h). Moreover, we found that the translocation of p65, which is normally translocated from the cytoplasm to the nucleus after exposure to LPS, was strongly inhibited by GGFTT in a dose-dependent manner (Fig. 6a–d). Also, the effect of candidate 10C against NF-κB activation was almost comparable to that of original GGFTT (Fig. 6e–h). These results suggested that the effects of GGFTT and 10C on the production of inflammatory cytokines and mediators were at least partially mediated by the suppression of the NF-κB signaling pathway.3.6. GGFTT and 10C inhibited LPS-induced activation of MAPKs in RAW264.7 cells
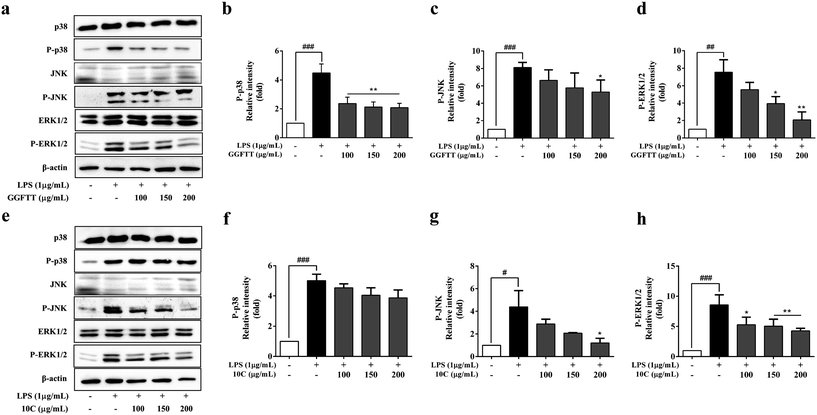
MAPKs activation also plays a key role for the expression of pro-inflammatory genes. We next investigated whether the MAPKs signaling pathway was involved in the anti-inflammatory activity of GGFTT and 10C. The expression of MAPKs proteins including ERK1/2, JNK and p38 were examined by Western blot in the present study. As shown in Fig. 7a–d, LPS induced phosphorylation of ERK1/2, JNK and p38 in non-treated cells, whereas pretreatment with GGFTT suppressed LPS-induced MAPK phosphorylation in a dose-dependent manner. Likewise, 10C showed similar inhibitory effect on these MAPKs proteins, such as p-ERK1/2 and p-JNK (Fig. 7e–h). These results indicated that GGFTT and 10C may exert anti-inflammatory effect via suppression of NF-κB activation and nuclear translocation as well as blocking of MAPKs signaling in LPS-stimulated RAW264.7 cells.4. Discussion
Rheumatoid arthritis is a chronic, immune-mediated, systematic inflammatory disease affecting approximately 1% of the population.14 Recent therapeutic interventions, including TNF-α, IL-6 and IL-1 inhibitors, strongly support the importance of cytokines in RA.15,16 Herbal medicines have played an important role in treatment of RA for thousands of years. It is wildly reported that the suppression of cytokines, such as IL-1β and TNF-α expression by natural product could ameliorate RA.17 GGFTT, which is composed of three medicinal herbs including Dioscorea nipponica Rhizoma, Spatholobi Caulis and Zingiberis Rhizoma, is a popular traditional Chinese herbal preparation designed for the treatment of RA. Our recent study has demonstrated that saponins, gingerols and flavonoids are the major components in GGFTT. In particular, 6-shogaol, 6-gingerol and dioscin, which are mostly explained by the inhibition of cyclooxygenase and tumor necrosis factor,18–20 are considered to be effective in ameliorating rheumatism and immunosuppressive conditions. However, to our knowledge, the active components responsible for the efficacy as well as the mechanism of action of GGFTT have not been reported.It is believed that not all the components contribute to the efficacy of herbal formulae, also, any single compound cannot account for the whole therapeutic efficacies of herbal formulae. In order to uncover the bioactive combinatorial components of GGFTT, we screened the main anti-inflammatory components in GGFTT by determination of different inflammatory markers. Simulation of LPS causes inflammation in RAW264.7 macrophages by releasing a variety of inflammatory cytokines such as TNF-α, IL-1β, IL-6, iNOS and COX-2.21 Since excessive NO induced by iNOS could significantly correlate with the degree of inflammation,22–24 and targeting NF-κB is an effective therapeutic strategy in many models of arthritis, we detected the release of NO in RAW264.7 and the inhibition of NF-κB gene in 293 cells. Our data revealed that 10 active compounds including the above reported anti-inflammatory ones were screened as the candidate bioactive components. In a previous study, our group developed a bioactive equivalence oriented feedback screening method and discovered the bioactive equivalent combinatorial components from Cardiotonic Pill.25 To verify whether these 10 compounds had made a majority contribution to the anti-inflammatory effect of GGFTT, we assessed the bioactive equivalence between 10C and GGFTT in vitro. Before the bioactive assay, the contents of these 10 components in GGFTT were quantified by LC-MS. The LC-MS method has been widely accepted to be the predominant tool for quantitative analysis of chemical components in complex samples for it provides favorable specificity and sensitivity. Our data indicated that the LC-MS method established had desirable specificity, linearity, precision and accuracy for quantification of these 10 active components in GGFTT, and their contents were relatively stable in 5 batches of GGFTT.
It is believed that candidate compound combinations are considered to be bioactive equivalents with original herbal medicines if the ratio of their efficacies falls within an acceptable range for a given assay. Our group once engineered a real-time components trapping and combining system to obtain the combination of candidate active compounds, which ensure the feasibility and reliability of the preparation system.25 In this work, to obtain 10C, we prepared it by mixing 10 reference compounds in proportion to the corresponding average contents of the 10 compounds in GGFTT, and then we evaluated the bioactivities of candidate 10C and the original GGFTT simultaneously. The results indicated that although 10C could not fully achieve bioactive equivalence as compared to the original GGFTT, it could be regarded as the major bioactive combinatorial components in GGFTT.
Knowledge of molecular mechanisms of these effects is essential for the safe and effective application of GGFTT. In the present study, our results demonstrated that pretreatment with GGFTT significantly decreased the production of IL-1β, TNF-α, IL-6 and iNOS in LPS-induced RAW264.7 cells. Understanding the intracellular targets that regulate cytokines in RA can potentially lead to new therapeutic interventions.26 It is widely accepted various intracellular signal pathways such as the activation of the transcription factor NF-κB pathway, is involved in the regulation of the inflammatory reaction in stimulated macrophages.27 When treated with LPS, RAW264.7 cells are activated due to phosphorylation and subsequent degradation of IκB-α, which leads to the activation of NF-κB and its translocation to the nucleus.28 Our data revealed that GGFTT potently inhibited LPS-induced the phosphorylation of NF-κB p65 subunit into the nucleus. The results also demonstrated that 10C made a majority contribution to the anti-inflammatory effect through the same mechanism.
The MAPKs are also key regulators of cytokine production and could also be targeted in RA.26 MAPKs are a group of serine/threonine protein kinases comprising three sub families: ERK1/2, JNK, and p38,29 which were all expressed in rheumatoid synovial tissue and subjected to pro-inflammatory cytokines induces phosphorylation.30 To obtain more details regarding the mechanism of the inhibitory effect of GGFTT and 10C on cytokine production, we investigated their effects on MAPK signaling pathways. The results demonstrated that GGFTT and 10C could significantly inhibit MAPKs signaling pathways by suppress the levels of phosphorylation of ERK1/2 and JNK. Currently, the MAPKs have attracted considerable attractions as potential therapeutic targets in RA. SP600125, a selective JNK inhibitor, provides predominant protection effects against bone and cartilage destruction.31 The suppression of JNK by GGFTT and 10C could be a potential therapeutic target for the treatment of RA.
5. Conclusions
GGFTT is a popular traditional Chinese herbal preparation designed for the treatment of RA, but its activity components are not clear. Hence, it is urgent to elucidate the effective component combination in GGFTT. Based on inflammatory markers and combined with molecular mechanism exploration. We have demonstrated that the 10C were as effective as GGFTT in cell models of rheumatoid arthritis diseases. The discovering of 10C could be extended to improve quality control of GGFTT and answer the key question of which are real bioactive components for GGFTT. We believe that the anti-inflammatory mechanism underlying GGFTT and its bioactive combinatorial components will contribute to the further application of other Chinese herbal preparations.Acknowledgements
This work was supported by Project in the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period (2012BAI29B07), National Natural Science Foundation of China (81202898) and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).References
- T. Pap, W. H. V. D. Laan, K. R. Aupperle, R. E. Gay, J. H. Verheijen, G. S. Firestein, S. Gay and M. Neidhart, Arthritis Rheumatol., 2000, 43, 2531 CrossRef CAS.
- J. R. Vane and D. A. Willoughby, Proc. Natl. Acad. Sci. U. S. A., 1994, 91, 2046 CrossRef CAS.
- J. Marks-Konczalik, S. C. Chu and J. Moss, J. Biol. Chem., 1998, 273, 22201 CrossRef CAS PubMed.
- P. Li, I. Sanz, R. J. O'Keefe and E. M. Schwarz, J. Immunol., 2000, 164, 5990 CrossRef CAS.
- E. S. Shin, H. J. Hwang, I. H. Kim and T. J. Nam, Int. J. Mol. Med., 2011, 28, 809 CAS.
- S. H. Huang, C. H. Lee, H. M. Wang, Y. W. Chang, C. Y. Lin, C. Y. Chen and Y. H. Chen, J. Agric. Food Chem., 2014, 62, 9171 CrossRef CAS PubMed.
- M. S. Hayden and S. Ghosh, Cell, 2008, 132, 344 CrossRef CAS PubMed.
- X. Fan, Y. Zhang, H. Dong, B. Wang, H. Ji and X. Liu, Food Chem., 2015, 166, 609 CrossRef CAS PubMed.
- R. Velagapudi, M. Aderogba and O. A. Olajide, Biochim. Biophys. Acta, 2014, 1840, 3311 CrossRef CAS PubMed.
- K. K. Jung, H. S. Lee, J. Y. Cho, W. C. Shin, M. H. Rhee, T. G. Kim, J. H. Kang, S. H. Kim, S. Hong and S. Y. Kang, Life Sci., 2006, 79, 2022 CrossRef CAS PubMed.
- C. Lu, Q. Zha, A. Chang, Y. He and A. Lu, J. Altern. Complement. Med., 2009, 15, 1021 CrossRef PubMed.
- X. L. Cheng, L. W. Qi, Q. Wang, X. G. Liu, B. Boubertakh, J. Y. Wan, E. H. Liu and P. Li, Analyst, 2013, 138, 2279 RSC.
- S. L. Zeng, X. G. Liu, C. J. S. Lai, E. H. Liu and P. Li, Chin. J. Nat. Med., 2015, 13, 390 Search PubMed.
- S. E. Gabriel, Rheum. Dis. Clin. North Am., 2001, 27, 269 CrossRef CAS PubMed.
- P. Charles, M. J. Elliott, D. Davis, A. Potter, J. R. Kalden, C. Antoni, F. C. Breedveld, J. S. Smolen, G. Eberl, K. deWoody, M. Feldmann and R. N. Maini, J. Immunol., 1999, 163, 1521 CAS.
- P. Emery, E. Keystone, H. P. Tony, A. Cantagrel, R. van Vollenhoven, A. Sanchez, E. Alecock, J. Lee and J. Kremer, Ann. Rheum. Dis., 2008, 67, 1516 CrossRef CAS PubMed.
- T. Pap, U. Muller-Ladner, R. E. Gay and S. Gay, Arthritis Res., 2000, 2, 1 CrossRef PubMed.
- M. H. Pan, M. C. Hsieh, P. C. Hsu, S. Y. Ho, C. S. Lai, H. Wu, S. Sang and C. T. Ho, Mol. Nutr. Food Res., 2008, 52, 1467 CAS.
- T. Y. Lee, K. C. Lee, S. Y. Chen and H. H. Chang, Biochem. Biophys. Res. Commun., 2009, 382, 134 CrossRef CAS PubMed.
- X. X. Zhang, Y. Ito, J. R. Liang, J. L. Liu, J. He and W. J. Sun, Int. Immunopharmacol., 2014, 23, 407 CrossRef CAS PubMed.
- J. Y. Tao, G. H. Zheng, L. Zhao, J. G. Wu, X. Y. Zhang, S. L. Zhang, Z. J. Huang, F. L. Xiong and C. M. Li, J. Ethnopharmacol., 2009, 123, 97 CrossRef CAS PubMed.
- E. Y. Kim and K. D. Moudgil, Immunol. Lett., 2008, 120, 1 CrossRef CAS PubMed.
- F. Feihl, B. Waeber and L. Liaudet, Pharmacol. Ther., 2001, 91, 179 CrossRef CAS PubMed.
- N. McCartney-Francis, J. B. Allen, D. E. Mizel, J. E. Albina, Q. W. Xie, C. F. Nathan and S. M. Wahl, J. Exp. Med., 1993, 178, 749 CrossRef CAS PubMed.
- P. Liu, H. Yang, F. Long, H. P. Hao, X. Xu, Y. Liu, X. W. Shi, D. D. Zhang, H. C. Zheng, Q. Y. Wen, W. W. Li, H. Ji, X. J. Jiang, B. L. Zhang, L. W. Qi and P. Li, Pharm. Res., 2014, 31, 1788 CrossRef CAS PubMed.
- G. S. Firestein, Evolving concepts of rheumatoid arthritis, Nature, 2003, 423, 356 CrossRef CAS PubMed.
- J. Shao, Y. Li, Z. Wang, M. Xiao, P. Yin, Y. Lu, X. Qian, Y. Xu and J. Liu, Int. Immunopharmacol., 2013, 17, 216 CrossRef CAS PubMed.
- S. Ghosh and M. Karin, Missing pieces in the NF-kappaB puzzle, Cell, 2002, 109, S81 CrossRef CAS PubMed.
- M. C. Medeiros, S. C. Frasnelli, A. S. Bastos, S. R. Orrico and C. Rossa Jr, J. Appl. Oral Sci., 2014, 22, 185 CrossRef PubMed.
- G. Schett, M. Tohidast-Akrad, J. S. Smolen, B. J. Schmid, C. W. Steiner, P. Bitzan, P. Zenz, K. Redlich, Q. Xu and G. Steiner, Arthritis Rheumatol., 2000, 43, 2501 CrossRef CAS.
- Z. Han, D. L. Boyle, L. Chang, B. Bennett, M. Karin, L. Yang, A. M. Manning and G. S. Firestein, J. Clin. Invest., 2001, 108, 73 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra17737a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |