Copper-catalyzed and iodide-promoted aerobic C–C bond cleavage/C–N bond formation toward the synthesis of amides†
Kun
Wu
a,
Zhiliang
Huang
a,
Yiyang
Ma
a and
Aiwen
Lei
*ab
aCollege of Chemistry and Molecular Sciences, The Institute for Advanced Studies (IAS), Wuhan University, Wuhan, Hubei 430072, P. R. China. E-mail: aiwenlei@whu.edu.cn; Fax: +86-27-68754067; Tel: +86-27-68754672
bState Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou, 730000, P. R. China
First published on 26th February 2016
Abstract
A copper-catalyzed and iodide-promoted aerobic C–C bond cleavage/C–N bond formation reaction between ketone and simple amine was developed toward the synthesis of amides, which are useful synthetic intermediates in organic synthesis and important skeletons of biologically active molecules. Notably, the reaction conditions are very mild, and preliminary mechanistic investigations indicate that molecular oxygen might be involved in the C–C bond cleavage process.
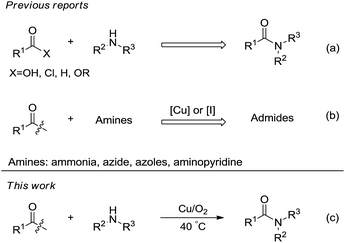
Amides are one of the most well-known and important classes of compounds for life, which exhibit extraordinary properties in the fields of chemistry and biology.1 In particular, in recent years, with the development of the pharmaceutical industry, more and more researchers project amidation to be one of the most preferred reactions that require universal and sustainable alternatives.2 To date, various amidation reactions have been intensively investigated (Scheme 1(a)).3 However, most of these methods involve corrosive reagents, toxic gas, sensitivity toward moisture and highly harsh conditions. Practical approaches to realize amidation under simple and mild conditions are still desirable.
During the past years, C–C bond activation and functionalization have emerged as an attractive and powerful strategy for the generation of carbon–carbon and carbon–heteroatom bonds.4 Of which, the selective oxidative cleavage of C–C single bonds still remains a great challenge in chemistry and biology.5 In very recent times, several achievements about the oxidative C(CO)–C(alkyl) bond cleavage of ketones have been reported.6 With this strategy, some kinds of amides could be synthesized from ketones and amines via copper or iodine catalyzed (Scheme 1(b)).7 However, in these reactions, ammonia,7a,7b,7d azide,7f azoles7c or aminopyridine7e are usually chosen as reagents, more universal amine rarely has been reported up to now.8 Herein, we present a copper-catalyzed and iodide promoted aerobic C–C bond cleavage/C–N bond formation reaction between ketone and simple amine toward the synthesis of amides (Scheme 1(c)).
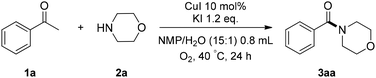
The combination of CuI and KI in NMP and H2O (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 40 °C gave the best result for the reaction between 1a and 2a under 1 atm O2 (Table 1, entry 1). With these conditions, a 60% yield of morpholino(phenyl)methanone (3aa) was obtained. CuI played a critical role in the reaction, since no 3aa was formed without CuI. Other cuprous salts, such as CuCl, CuBr were also effective (Table 1, entries 2–4). As to additives, KI gave the highest yield for this reaction (Table 1, entry 5), nBu4NI was also effective and afforded 3aa in 55% yield (Table 1, entry 7), but I2 and NIS showed less efficiency in terms of chemical yields (Table 1, entries 6 and 8). In addition, H2O was beneficial for the reaction, the yield of desired product was decreased to 38% without H2O, MeOH and EtOH could also promote the reaction (Table 1, entries 9–11). Notably, this reaction did not proceed in the absence of molecular oxygen (Table 1, entry 12).
1) at 40 °C gave the best result for the reaction between 1a and 2a under 1 atm O2 (Table 1, entry 1). With these conditions, a 60% yield of morpholino(phenyl)methanone (3aa) was obtained. CuI played a critical role in the reaction, since no 3aa was formed without CuI. Other cuprous salts, such as CuCl, CuBr were also effective (Table 1, entries 2–4). As to additives, KI gave the highest yield for this reaction (Table 1, entry 5), nBu4NI was also effective and afforded 3aa in 55% yield (Table 1, entry 7), but I2 and NIS showed less efficiency in terms of chemical yields (Table 1, entries 6 and 8). In addition, H2O was beneficial for the reaction, the yield of desired product was decreased to 38% without H2O, MeOH and EtOH could also promote the reaction (Table 1, entries 9–11). Notably, this reaction did not proceed in the absence of molecular oxygen (Table 1, entry 12).
| Entry | Variation from “standard conditions” | Yield of 3aab [%] |
|---|---|---|
| a Reaction conditions: 1a (0.5 mmol), 2a (1.0 mmol), KI (0.6 mmol), NMP (0.75 mL), H2O (0.05 mL), 40 °C, 24 h, under O2 (1 atm). b The yield was determined by GC analysis, calibrated using biphenyl as the internal standard, the yield in parenthesis was isolated yield. | ||
| 1 | None | 62(60) |
| 2 | No CuI | 0 |
| 3 | CuCl, instead of CuI | 50 |
| 4 | CuBr, instead of CuI | 55 |
| 5 | No KI | 37 |
| 6 | I2, instead of KI | 15 |
| 7 | n BuN4I, instead of KI | 55 |
| 8 | NIS, instead of KI | 12 |
| 9 | No H2O | 38 |
| 10 | MeOH, instead of H2O | 54 |
| 11 | EtOH, instead of H2O | 55 |
| 12 | N2, instead of O2 | 0 |
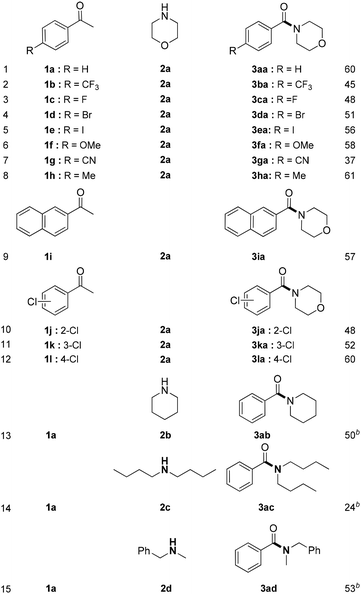
With the above optimized reaction conditions in hand, we subsequently tested the aerobic amidation of aryl ketones and 2a, and the results are summarized in Table 2. All substrates proceeded well and afforded corresponding products in moderate yields. Aryl methyl ketones with electron-donating group such as methyl, methoxy (Table 2, 3fa, 3ha) and electron-withdrawing group such as trifluoromethyl, cyano (Table 2, 3ba, 3ga) could react with 2a smoothly to afford the desired products in moderate yields. It is noteworthy that F, Cl, Br and I substituents on the phenyl rings were well tolerated, which enabled a potential application in further functionalization (Table 2, 3ca, 3da, 3ea, 3ja, 3ka, 3la). In addition, 1-(naphthalen-2-yl)ethanone was also suitable substrate for this transformation (Table 2, 3ia).
Furthermore, we explored the reactions between acetophenone with other secondary amines. Preliminary results showed that piperidine 2b and N-methyl benzylamine 2d were good substrates, generating the target amides in 50% and 53% yields, respectively (Table 2, 3ab, 3ad). Di-n-butylamine 2c could also obtain desired product smoothly (Table 2, 3ac). Besides, primary amines such as n-butyl amine and aniline were also tested, but they were not compatible with this reaction.
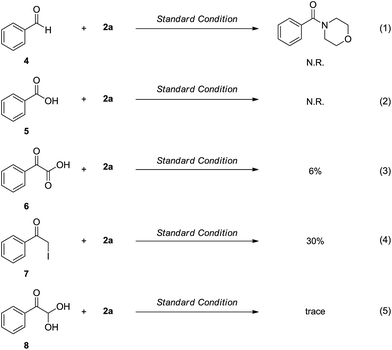
To explore the mechanism of this transformation, some potential intermediates were subjected to the reaction system (Scheme 2). When benzaldehyde (4) and benzoic acid (5) were employed to react with 2a under the standard conditions, no 3aa was detected, which suggested that 4 and 5 were not the active intermediates for this reaction (eqn (1) and (2)). In addition, the reactions of the intermediates 6 and 8 with morpholine (2a) afforded amides in a very low yields (eqn (3) and (5)). These results indicated that they might not be involved in the transformation. Furthermore, 2-iodo-1-phenylethanone (7) was also investigated and the desired product was afforded in 30% yield, demonstrating that 7 might be an alternative intermediate (eqn (4)).
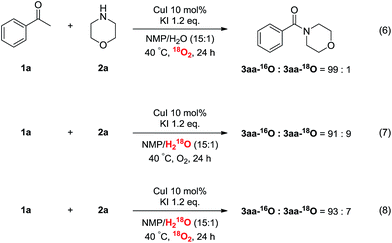
To further elucidate the mechanism, 18O isotope labeling experiments were performed. As shown in Scheme 3, only 1% of 18O-labeled 3aa was obtained when the reaction was performed under 1 atm 18O2 (eqn (6)). 9% of 18O-labeled 3aa was observed when H218O instead of H2O was employed for the transformation (eqn (7)). In addition, when the reaction was performed under both 18O2 and H218O, also only 7% of 18O-labeled 3aa was detected (eqn (8)). These results indicated that the carbonyl oxygen atom of products should be from the ketones, rather than O2 or H2O.
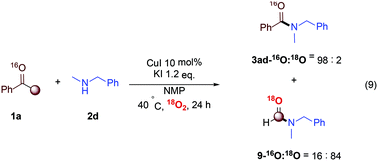
Besides, when amine 2d was subjected to the standard conditions under 1 atm 18O2 in the absence of H2O, some interesting results were observed (eqn (9)). Firstly, except for the desired product 3ad, compound 9 was also detected by GC-MS, which was believed to be a cleavage byproduct in this transformation. Then, 2% of 18O-labeled 3ad and 84% of 18O-labeled 9 were detected, indicating that molecular oxygen might be involved in C–C bond cleavage process, but not be a part of the desired amides 3ad.
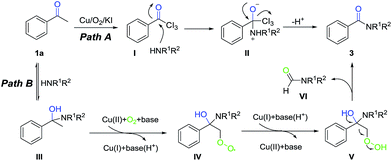
Based on the results of mechanistic investigations and literature reports,9 two possible pathways were proposed in Scheme 4. In the path A, initially, three sequential α-halogenations took place to afford the triiodoacetophenone I in the presence of Cu/O2/KI. Then the compound I was attacked by the amines to form the intermediate II. Finally, the product 3 was obtained via the C–C bond cleavage and electron transfer of intermediate II. Alternatively, in path B, the intermediate III was formed by the nucleophilic addition of ketone with amine, which could further transform into the radical intermediate IV in the presence of Cu(II) and O2via a single electron transfer (SET) process. Subsequently, the SET reduction and protonation of intermediate IV by Cu(I) and base(H+) generated hydroperoxide intermediate V. Then a series of rearrangement, and electron transfer happened, 3 and VI10 was afforded finally.
In conclusion, we have demonstrated a novel copper-catalyzed and iodide promoted aerobic C–C bond cleavage/C–N bond formation reaction between ketone and amine under simple and mild conditions. Preliminary mechanistic investigations indicated that molecular oxygen might be involved in C–C bond cleavage process, but not be a part of the desired amides. Detailed mechanism research and the synthetic applications of this methodology are currently ongoing in our laboratory.
Acknowledgements
This work was supported by the 973 Program (2012CB725302), the National Natural Science Foundation of China (21390400, 21520102003, 21272180, 21302148), the Hubei Province Natural Science Foundation of China (2013CFA081), the Research Fund for the Doctoral Program of Higher Education of China (20120141130002), and the Ministry of Science and Technology of China (2012YQ120060). The Program of Introducing Talents of Discipline to Universities of China (111 Program) is also appreciated.Notes and references
- (a) C. L. Allen and J. M. J. Williams, Chem. Soc. Rev., 2011, 40, 3405–3415 RSC; (b) J. M. Humphrey and A. R. Chamberlin, Chem. Rev., 1997, 97, 2243–2266 CrossRef CAS; (c) C. A. G. N. Montalbetti and V. Falque, Tetrahedron, 2005, 61, 10827–10852 CrossRef CAS.
- D. J. C. Constable, P. J. Dunn, J. D. Hayler, G. R. Humphrey, J. J. L. Leazer, R. J. Linderman, K. Lorenz, J. Manley, B. A. Pearlman, A. Wells, A. Zaks and T. Y. Zhang, Green Chem., 2007, 9, 411–420 RSC.
- (a) A. Brennführer, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2009, 48, 4114–4133 CrossRef; (b) H. Huang, G. Yuan, X. Li and H. Jiang, Tetrahedron Lett., 2013, 54, 7156–7159 CrossRef CAS; (c) N. D. Kokare, R. R. Nagawade, V. P. Rane and D. B. Shinde, Synthesis, 2007, 766–772 CAS; (d) H. Nakatsuji, M. Morimoto, T. Misaki and Y. Tanabe, Tetrahedron, 2007, 63, 12071–12080 CrossRef CAS; (e) V. R. Pattabiraman and J. W. Bode, Nature, 2011, 480, 471–479 CrossRef CAS; (f) M. Pilo, A. Porcheddu and L. De Luca, Org. Biomol. Chem., 2013, 11, 8241–8246 RSC; (g) A. Schoenberg and R. F. Heck, J. Org. Chem., 1974, 39, 3327–3331 CrossRef CAS; (h) P. Subramanian, G. C. Rudolf and K. P. Kaliappan, Chem.–Asian J., 2016, 11, 168–192 CrossRef CAS; (i) E. Valeur and M. Bradley, Chem. Soc. Rev., 2009, 38, 606–631 RSC.
- (a) F. Chen, T. Wang and N. Jiao, Chem. Rev., 2014, 114, 8613–8661 CrossRef CAS; (b) C.-H. Jun, Chem. Soc. Rev., 2004, 33, 610–618 RSC; (c) C.-H. Jun and J.-W. Park, in C–C Bond Activation, ed. G. Dong, Springer Berlin Heidelberg, Berlin, Heidelberg, 2014 Search PubMed; (d) M. Murakami and N. Ishida, in Cleavage of Carbon–Carbon Single Bonds by Transition Metals, Wiley-VCH Verlag GmbH & Co. KGaA, 2015 CrossRef; (e) B. Rybtchinski and D. Milstein, Angew. Chem., Int. Ed., 1999, 38, 870–883 CrossRef.
- (a) C. Aïssa, D. Crépin, D. J. Tetlow and K. Y. T. Ho, Org. Lett., 2013, 15, 1322–1325 CrossRef; (b) S. C. Bart and P. J. Chirik, J. Am. Chem. Soc., 2003, 125, 886–887 CrossRef CAS; (c) M. Murakami, T. Itahashi and Y. Ito, J. Am. Chem. Soc., 2002, 124, 13976–13977 CrossRef CAS; (d) T. Seiser and N. Cramer, J. Am. Chem. Soc., 2010, 132, 5340–5341 CrossRef CAS; (e) C. Q. Sun, H. L. Bai, B. K. Tay, S. Li and E. Y. Jiang, J. Phys. Chem. B, 2003, 107, 7544–7546 CrossRef CAS.
- (a) L. Huang, K. Cheng, B. Yao, Y. Xie and Y. Zhang, J. Org. Chem., 2011, 76, 5732–5737 CrossRef CAS; (b) X. Huang, X. Li, M. Zou, S. Song, C. Tang, Y. Yuan and N. Jiao, J. Am. Chem. Soc., 2014, 136, 14858–14865 CrossRef CAS; (c) F. Minisci, F. Recupero, F. Fontana, H.-R. Bjørsvik and L. Liguori, Synlett, 2002, 0610–0612 CrossRef CAS; (d) P. Sathyanarayana, O. Ravi, P. R. Muktapuram and S. R. Bathula, Org. Biomol. Chem., 2015, 13, 9681–9685 RSC; (e) J. Wang, W. Chen, S. Zuo, L. Liu, X. Zhang and J. Wang, Angew. Chem., Int. Ed., 2012, 51, 12334–12338 CrossRef CAS; (f) C. Zhang, P. Feng and N. Jiao, J. Am. Chem. Soc., 2013, 135, 15257–15262 CrossRef CAS; (g) L. Zhang, X. Bi, X. Guan, X. Li, Q. Liu, B.-D. Barry and P. Liao, Angew. Chem., Int. Ed., 2013, 52, 11303–11307 CrossRef CAS; (h) W. Zhou, Y. Yang, Y. Liu and G.-J. Deng, Green Chem., 2013, 15, 76–80 RSC.
- (a) N. A. Angeles, F. Villavicencio, C. Guadarrama, D. Corona and E. Cuevas-Yañez, J. Braz. Chem. Soc., 2010, 21, 905–908 CrossRef CAS; (b) L. Cao, J. Ding, M. Gao, Z. Wang, J. Li and A. Wu, Org. Lett., 2009, 11, 3810–3813 CrossRef CAS; (c) W. Ding and Q. Song, Org. Chem. Front., 2015, 2, 765–770 RSC; (d) M. Sharif, J. Chen, P. Langer, M. Beller and X.-F. Wu, Org. Biomol. Chem., 2014, 12, 6359–6362 RSC; (e) P. Subramanian, S. Indu and K. P. Kaliappan, Org. Lett., 2014, 16, 6212–6215 CrossRef CAS; (f) C. Tang and N. Jiao, Angew. Chem., Int. Ed., 2014, 53, 6528–6532 CrossRef CAS.
- J. Ding, L. Cao, J. Wang, W. Xue, Y. Zhu and A. Wu, J. Chem. Res., 2011, 35, 298–301 CrossRef CAS.
- (a) S. E. Allen, R. R. Walvoord, R. Padilla-Salinas and M. C. Kozlowski, Chem. Rev., 2013, 113, 6234–6458 CrossRef CAS; (b) A. L. Gemal and J. L. Luche, J. Org. Chem., 1979, 44, 4187–4189 CrossRef CAS; (c) Q. Lu, J. Zhang, F. Wei, Y. Qi, H. Wang, Z. Liu and A. Lei, Angew. Chem., Int. Ed., 2013, 52, 7156–7159 CrossRef CAS; (d) Q. Lu, J. Zhang, G. Zhao, Y. Qi, H. Wang and A. Lei, J. Am. Chem. Soc., 2013, 135, 11481–11484 CrossRef CAS; (e) T. Taniguchi, Y. Sugiura, H. Zaimoku and H. Ishibashi, Angew. Chem., Int. Ed., 2010, 49, 10154–10157 CrossRef CAS; (f) C. Zhang, Z. Xu, L. Zhang and N. Jiao, Angew. Chem., Int. Ed., 2011, 50, 11088–11092 CrossRef CAS.
- (a) S. C. Ghosh, J. S. Y. Ngiam, A. M. Seayad, D. T. Tuan, C. L. L. Chai and A. Chen, J. Org. Chem., 2012, 77, 8007–8015 CrossRef CAS; (b) M. Zhu, K.-i. Fujita and R. Yamaguchi, J. Org. Chem., 2012, 77, 9102–9109 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra02153k |
| This journal is © The Royal Society of Chemistry 2016 |