Nisin anchored cellulose nanofibers for long term antimicrobial active food packaging
Seema Sainiab,
Cecile Sillardab,
Mohamed Naceur Belgacemab and
Julien Bras*ab
aUniv. Grenoble Alpes, LGP2, F-38000 Grenoble, France
bCNRS, LGP2, F-38000 Grenoble, France. E-mail: Julien.Bras@grenoble-inp.fr
First published on 13th January 2016
Abstract
Increasing consumer demand for high performance bio-based materials in order to develop microbiologically safer foods has forced the food industry to revise their packaging strategies. One of the emerging fields within this industry is active packaging which prevents an excess of antimicrobials in food formulation and, meanwhile, inhibits or reduces bacterial growth. In this study, a green process of immobilizing a peptide on bio-based carboxylated cellulose nanofibers (CNF) using a coupling agent has been proposed. Validity of the grafting reaction between nanocellulose, the coupling agent and peptide was first analyzed by Quartz crystal microbalance-dissipation (QCM-D). Efficiency of the grafting reaction was characterized with Fourier Transform Infrared spectroscopy and thermogravimetric analysis which suggest the grafting of nisin on CNF. Finally, the amount of grafted nisin was quantified on the surface of CNF by conductometric titration and nitrogen content analysis. The antimicrobial activity and release experiment demonstrated that the nisin grafted CNF displayed excellent antimicrobial activity against different Gram +ve bacteria (Bacillus subtilis and Staphylococcus aureus) with significant 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction. The obtained results are promising and confirm the interest in such strategy.
log reduction. The obtained results are promising and confirm the interest in such strategy.
Introduction
Infectious diseases frequently spread through food quality deterioration. According to the World health organization, globally, 351![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people die every year due to food poisoning.1 Therefore, to assure the safety of food products, it is necessary to control the contamination by limiting the growth of pathogenic bacteria during processing and storage.
000 people die every year due to food poisoning.1 Therefore, to assure the safety of food products, it is necessary to control the contamination by limiting the growth of pathogenic bacteria during processing and storage.
In traditional packaging, antimicrobial active compounds are mixed with the food formulation for preservation. But, the presence of antimicrobials in the bulk is unable to target the food surface where a large portion of spoilage and contamination occurs. Hence, bioactive antimicrobial packaging was introduced as an innovative approach to diminish microbial growth predominantly at the surface of food.2,3 Various researchers have demonstrated the use of antimicrobial compounds, such as organic acids (sorbic acid, acetic acid), essential oils (thymol, rosemary oil), enzymes (lysozyme), bacteriocins (nisin and pediocin), and metal particles (silver, zinc oxide)4–7 incorporated or coated over polymer matrices.
Indeed, this study primarily focuses on the use of a cationic, amphiphilic antimicrobial peptide nisin composed of 34 amino acids produced by strains of the bacterium Lactococcus lactis lactis.8 Nisin has already been exploited for decades as a food preservative and is also approved by the European food safety authority with acceptable uptake of 0.13 mg per kg per day per person.9
Nisin intervenes with cell wall synthesis by binding to lipid II, a core precursor of cell wall synthesis, and efficiently permeabilizing the cell membrane via the formation of pores.10 Converse to antibiotics, their mode of action does not encourage pathogenic resistance, hence, suppresses the evolution of new infectious diseases.11
In last decade, nisin has been either incorporated into various polymer matrices, for instance polyethylene brushes,12 LDPE,13 polypropylene,14 soy protein,15 or starch,16 or in coatings over them17–19_ENREF_17. However, these direct applications have been limited in their beneficial effects and exhibit a loss in long-term activity.
Still, only very few investigations have taken place with chemical covalent immobilization of nisin for example some studies tried to graft nisin on compounds such as multiwalled carbon nanotubes,20 or calcium alginates.21–23
Due to bio-based material requirements and needs from society, intensive research is being carried out to develop environmentally-friendly packaging materials to replace petroleum-based synthetic polymers. Nevertheless, by virtue of its properties (renewable, biodegradable, biocompatible and cost effective), cellulose carry on gaining attention for bioactive packaging.24,25
Cellulose nanofibers (also called microfibrillated cellulose or nanofibrillated cellulose) is a novel cellulosic nanomaterial developed by a researcher team at ITT Rayonier Inc. in 1983.26 These slender materials possess a diameter between 10–50 nm and length >1 μm. CNF is a very promising material with unique properties such as being light-weight, low density, high barrier, low coefficient of thermal expansion, extremely high surface area, flexible, and good strength properties.27–29 It is prepared by high shearing mechanical disintegration26 which is major concern in its preparation as fibrillation consume energy as high as 78.8 MJ kg−1.30
Pretreatment of the cellulose fibers before mechanical disintegration, not only decreases the energy consumption, but can also add novel surface properties to fibers. Thus, pretreatment with 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) selectively converts the C6 groups of primary hydroxyl into carboxyl functions, which attracts attention for diverse applications.31,32
Very recently, conventional CNF have been grafted with silanes, isocyanate, anhydrides, isothiocyanate and antibiotics33–39 to impart antimicrobial properties. The novelty of this work lies in the selection of the type of CNF and active molecule (nisin), which was never attempted in previous investigations. It also proposes to use a new green strategy for grafting active molecule. Indeed even if such grafting has been recently performed onto nanocellulose for various molecules, none of them targeted antimicrobial properties using rather large molecule weight molecule like nisin.
Consequently, in this study, we have developed a novel antimicrobial film with covalently linked nisin on the surface of TEMPO oxidized CNF especially for food packaging. In addition to reduction of energy consumption during mechanical disintegration of fibers, TEMPO pretreatment imparts additional properties to nanofibers which could be exploited for different application. Our novel TEMPO oxidized CNF film has enhanced the antimicrobial activity of the film against Bacillus subtilis and Staphylococcus aureus.
Materials and methods
Materials
For the preparation of nanocellullose, high quality bleached softwood cellulose (domsjo, Sweden) produced from a controlled mixture of spruce and pine (respectively 60% and 40%) was used. Nisin Z was procured by Handary S.A. Various chemicals were purchased such as 2,2,6,6-tetramethylpiperidine-1-oxyl radical (Sigma Aldrich), 2-(N-morpholino)ethanesulfonic acid (sigma Aldrich), N-hydroxysuccinimide (Sigma Aldrich), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (Sigma Aldrich), L-α-lecithin (Merck), sodium thiosulphate (Roth), L-histidine (Merck), Tween 80 (Merck), potassium dihydrogen phosphate (KH2PO4) (Roth). A suspension of Bacillus subtilis (107 spores per mL) was also purchased from Humeau (France). Spores were revived by growing in nutrient broth for 16 hours. A nutrient agar adapted to the development of the spores was also purchased from Humeau (France). Staphylococcus aureus ATCC 6538 was bought from Thermo scientific, USA. Petri dishes with a diameter of 90 mm and 60 mm were purchased from Roth (France) and used for the antibacterial tests.Preparation carboxylated CNF
The dried cellulose fibers (15 g) were soaked in water (1 L) overnight aiming for a better dispersion of fibers during reaction. After redispersion, the cellulose fibers were transferred to a reactor with heating mantle, after TEMPO (0.24 g, 0.1 mmol) and sodium bromide (1.5 g, 1 mmol) were added. The TEMPO-mediated oxidation was started by pouring the 4.9 mL of the sodium hypochlorite solution (5.0 mmol NaClO per gram of cellulose at pH 10) slowly into the reactor and then consistency was filled with deionised water till 1.5 L. Then, the reaction was carried out at room temperature by stirring at 200 rpm. The pH was maintained at 10 by adding 0.5 M sodium hydroxide (NaOH) using a pH regulator until no further decrease of pH was observed.The reaction was quenched by lowering the pH till 7 with 0.1 M HCl. TEMPO-oxidized cellulose was thoroughly washed with deionized water by vacuum filtration using a nylon sieve with a pore diameter of 1 μm until the filtrate conductivity value reached less than 5 μS cm−1. After that, the TEMPO cellulose was stored at 4 °C for further treatment. Recovery ratios of the TEMPO oxidized celluloses after washing were over 90%. The carboxylate content of the TEMPO-oxidized cellulose was determined using conductivity titration method. For the preparation of nanofibers, the pretreated fibers were passed through Masuko® grinder at 2500 rpm for 45 min under continuous recirculation.40
Chemical grafting of nisin on carboxylated CNF
1 g of dry TEMPO CNF was diluted with 160 mL 2-(N-morpholino)ethanesulfonic acid (MES) buffer (pH 4.5; MES 10 mM). The CNF suspension was stirred for 30 minutes. 0.84 g of EDC (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide) was solubilized in 10 mL of MES buffer; 0.5 g of NHS (N-hydroxysuccinimide) was solubilized in 10 mL of MES buffer. The required quantity of nisin was added to the TEMPO CNF suspension and then, EDC–NHS was added to the mixture. Finally the mixture was stirred during 24 hours at room temperature.The quantity of nisin was calculated on the basis carboxyl group on the surface of CNF. Two quantities were used to analyze the efficiency of grafting i.e. 1 and 4 equivalents with respect to the carboxyl group.
Validation of grafting reaction between CNF and nisin (QCM-D)
Adsorption measurements were performed with a Quartz Crystal Microbalance with Dissipation (QCM-D). The instrument (Q-Sense® E1, Biolin Scientific, Sweden) measures the resonance frequency of the oscillation of a quartz crystal based on the fundamental frequency (5 MHz) and selected overtones (15, 25, 35, 45, 55, and 75 MHz). However, only the seventh overtone is used in the data evaluation. Sorption curves were acquired using 100 nm gold-coated quartz crystals (QSX301, Biolin Scientific, Sweden). All measurements were performed under constant flow rate (100 μL min−1) and temperature (23 °C). The adsorbed mass (ng nm−2) was estimated from the frequency change (Δf) using Sauerbrey's equation.41
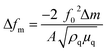 | (1) |
Grafting characterization
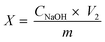 | (2) |
Finally, percentage of grafting was determined using the total carboxylate content before and after the reaction on each sample:
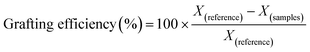 | (3) |
Antibacterial activity
| log reduction = log CFU T24 untreated sample − log CFU T24 treated sample | (4) |
All experiments were at least duplicated.
Results and discussion
Influence of the coupling agent on the interaction between CNF and nisin
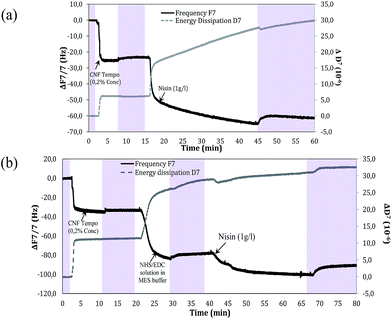
First, tempo oxidised cellulose was prepared and characterized. Gel like suspension and semi-transparent dried film was obtained as expected. Morphological nanostructure was confirmed by the AFM as detailed later in Fig. 5. Dimension of CNF calculated with the image J are 18 ± 4 nm which was in the range of classic Tempo CNF.Since, this was the first investigation for such strategy, it was extremely important to confirm the efficacy of nisin immobilization on the surface of nanofibers with the coupling agent. Therefore, Quartz Crystal Microbalance with Dissipation Monitoring (QCM-D) was first used to monitor the validity of the grafting reaction. QCM-D is a highly sensitive technique which is used to study the reaction binding kinetics of a molecule by analyzing its sorption and desorption from the quartz surface. Two different deposition cycles were preformed using CNF and nisin.
A detailed description of the QCM-D, which represents the normalized change in frequency and dissipation of the 7th overtone in each deposition cycle, is shown in Fig. 1. In first experiment, nisin solution (1 g L−1) was directly in the flow contact on the carboxylated nanofibers previously coated on the functionalized gold plates.
The introduction of carboxylated CNF on PEI treated gold quartz results in the decrease of the Δf (−30 Hz) and an increase in the corresponding ΔD. This change is attributed to the strong absorption of cellulose on the PEI. After each deposition, the quartz was rinsed with deionized water to remove the excess of unbound compound and to negotiate the frequency response generated by them.
Next, when peptide was passed into QCM-D module, there is instantaneous decrease in Δf and increase in ΔD in less than 2 minutes corresponding to the strong adsorption of nisin on the nanofibers. After 3 minutes of deposition, there was slow depression which was contributed by swelling of the Tempo CNF owning to the absorption of water. The adsorption of nisin on CNF is mainly related to the weak forces such as hydrogen bonding and van der Waals interactions between the two.
In another system of deposition, EDC/NHS was first absorbed on the surface of CNF and then nisin was allowed to interact with EDC/NHS. The EDC/NHS treatment of oxidized nanofibers film decreased the frequency (change over −44 Hz after rinsing) which indicates the formation of semi-stable NHS-esters. It is worthy to notice that no significant desorption of NHS-ester took place even after washing for 20 minutes. Nonetheless, these systems were found to differ with respect to the absorption of nisin. There is very low change in dissipation energy due to the formation of NHS ester, which reduces the swelling of the TEMPO CNF, thus resulting in a rigid layer of nisin which indeed confirms the reaction of CNF with nisin.42,43
The mass/thickness of the layers can be determined by the Sauerbrey equation. However, this equation is only applicable to rigid layers. Another indication of a non-rigid viscoelastic film, which leads to a deviation from the Sauerbrey relation, is the frequency dependence of the overtones (Δf/n). The mass for the coupled TEMPO CNF after water rinsing was found to be 6 mg m−2 which corresponds the thickness of 4 nm on the gold surface considering a cellulose nanofibril density of 1500 kg m−3. The mass of CNF was quite sufficient for uniform distribution on the gold plate reducing the non-specific adsorption of nisin on gold plates.44
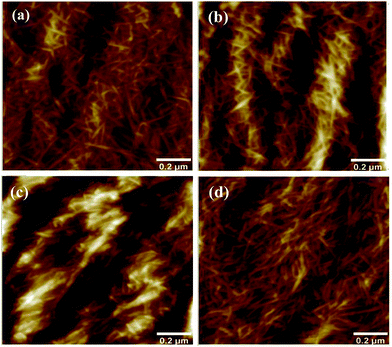
AFM images are recorded to control the morphology of the cellulose after each injection (Fig. 2). No large voids or pores were observed indicating the uniform distribution of TEMPO oxidized cellulose nanofibrils on the surface of the PEI treated gold surface. When EDC/NHS was deposited on the surface of cellulose, a gel like structure was formed with water and NHS/EDC, however, after rinsing and addition of nisin, fibrillar structure similar to the reference CNF was observed.45 EDC/NHS removes the charge on the nanofibrils by forming NHS esters that reduce the water binding capacity of fibrils. Therefore, rigid absorption of peptide nisin took place mainly on the CNF film surface, when EDC–NHS coupling agent is used.
Grafting of CNF with nisin
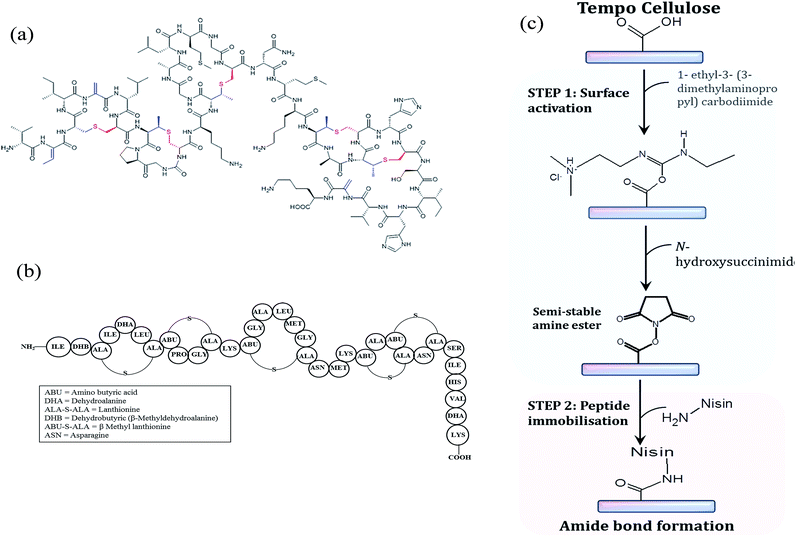
Actually, the pathway for covalent immobilization of nisin peptides on the surface of CNF consists of two prime steps. In a first step, COOH on nanofibers were activated by using an EDC–NHS mixture. Then, the nisin peptide was covalently immobilized onto the surface through the nucleophilic reaction occurring between the terminal NH2 group of the peptide and the activated carboxylic functional group of the surface. Fig. 3 illustrates the structure of nisin and the reaction pathway demonstrating the main steps involved in the grafting of nisin peptide.This EDC–NHS intermediate grafting reaction is well known by peptidic reaction but is still barely used with nanocellulose, only very recent papers deals with such strategy and up our knowledge; nobody used it for grafting the peptide on Tempo CNF.46–49 In this case the risk of “homopolymerisation” (condensation) of nisin is present and can be limited by favoring the adsorption of EDC–NHA as shown with QCM-D. The main advantage of this grafting strategy is the use of aqueous system at room temperature.
Prior to all the characterization, modified nanofibers were thoroughly washed with water using centrifugation to remove all physically adsorbed peptide. ATR-FTIR analysis was performed to characterize and determine changes in the infrared bands related to the nisin immobilization on the CNF. Normalized FTIR spectra of the neat and grafted CNF are presented in Fig. 4. Unmodified CNF display several bands characteristics of cellulose at 3350 cm−1 (OH), 1110 cm−1 (C–O of secondary alcohol and used for the normalization of all spectra), and 2868 and 2970 cm−1 (C–H from –CH2–).
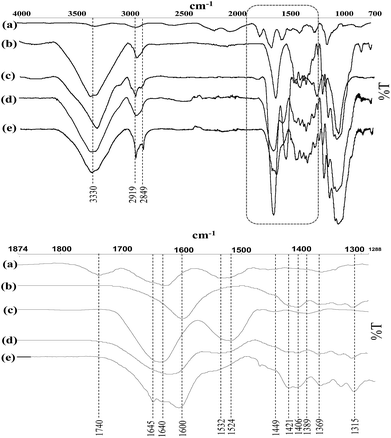 | ||
| Fig. 4 FT-IR spectra of the (a) nisin, (b) CNF Tempo, (c) CNF Tempo-m-nisin (d) CNF Tempo-g-1-eq. and (e) CNF Tempo-g-4eq. | ||
In addition, TEMPO oxidized fibers also show a new peak 1550–1610 cm−1 corresponding to the asymmetric –COO− stretching from TEMPO CNF carboxylates. The spectra reported characteristic changes in the region of 1750–850 cm−1 due to the grafting of nisin on nanocellulose. The spectra of nisin-grafted CNF demonstrated distinct new characteristic bands at 1640 cm−1, 1560 cm−1 and 1402 cm−1 associated with the presence of amide I group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch combined with N–H deformation), amide II group (N–H deformation in amides combined with –NH3+ deformation), and amide III group (C–N stretch in primary amides combined with COO− symmetric stretch in carboxylic acid salts), respectively.50 The band around 2919 and 2849 has increased significantly after the addition or grafting of nisin. Moreover, direct mixing of the nisin into CNF demonstrated the band characteristic to both CNF and nisin.
O stretch combined with N–H deformation), amide II group (N–H deformation in amides combined with –NH3+ deformation), and amide III group (C–N stretch in primary amides combined with COO− symmetric stretch in carboxylic acid salts), respectively.50 The band around 2919 and 2849 has increased significantly after the addition or grafting of nisin. Moreover, direct mixing of the nisin into CNF demonstrated the band characteristic to both CNF and nisin.
Atomic force microscopy (Fig. 5) of unmodified CNF suspension showed the morphology of nanofibers in dimension of 18 ± 4 nm. Generally, the estimated diameter of TEMPO nanofibers should be around 3–4 nm 40. It is important to mention here that the mechanical treatment used in this investigation was ultra-friction grinding system, which provides nanofibers of higher dimension compared to nanofibers treated with homogenizer.51 The average diameter of grafted CNF was 20 ± 7 nm and 21 ± 6 nm for CNF TEMPO-g-1eq. and CNF-TEMPO-g-4eq., respectively, which is not significantly different from unmodified CNF. In conclusion, no change in the morphology was observed after grafting owning to the non-swelling grafting solvent (MES buffer) used for the reaction process.
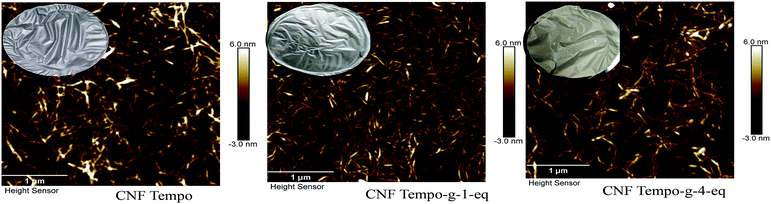 | ||
| Fig. 5 AFM pictures of CNF Tempo, CNF Tempo-g-1-eq. and CNF Tempo-g-4eq. with films after grafting on the surface of cellulose (conc. 0.1%, tapping mode in air, area 3.3 × 3.3 μm2). | ||
Apparent change in colour (from white to brown) was noticed after the grafting with the higher concentration of peptide of the film. In contrast, no change in colour was observed when the concentration used for grafting is 1 equivalent. This observation is in agreement with the FTIR results i.e. more pronounced bands were obtained with CNF Tempo-g-4eq.
Influence of peptide grafting on thermal degradation
Thermogravimetric analyses (TGA) were accomplished to determine the influence of nisin on the thermal stability of cellulose. Weight loss and derivative weight loss thermographs for reference and modified samples are represented in Fig. 6. The formation of sodium carboxylate groups from the C6 primary hydroxyls of cellulose microfibrils surfaces by the TEMPO oxidation affects the thermal degradation.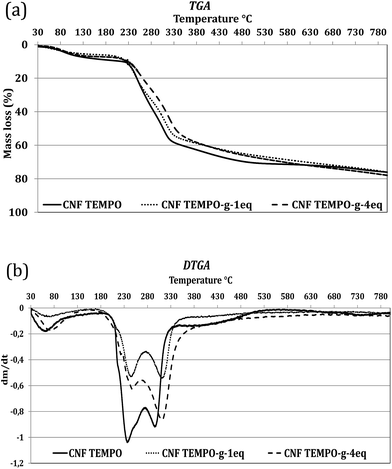 | ||
| Fig. 6 Thermographs for the control (CNF and nisin) and grafted CNF with different initial concentrations. | ||
Degradation of TEMPO cellulose fibers starts at 200 °C under nitrogen while with the conventional cellulose, its decomposition start at 300 °C. The initial decreases in weight observed at about 60–100 °C in the reference TEMPO CNF is a consequence of the loss of the residual moisture. But, this depression in weight for residual moisture is slightly lower in the grafted samples stating the presence of less moisture comparatively after grafting which is classical for all grafting procedures.52 As already shown in previous literature, small modification might prove undirectly the grafting. Indeed it is well-known that nanocellulose grafting with large molecule like nisin can protect the nanocellulose from thermal degradation.46,53,54
As a result, grafting (or adsorption) of nisin on the CNF slightly improves the thermal stability of the cellulose (Table 1). 70% weight loss was obtained at 490 °C, 619 °C and 570 °C for CNF Tempo, CNF Tempo-g-1eq. and CNF Tempo-g-4eq., respectively. However, at higher temperatures, disintegration of nisin-grafted cellulose occurs, comparatively at lower temperature with the reference nanofibers due to the decomposition of grafting agent. Moreover, presence of higher quantity of nisin in CNF Tempo-g-4eq. makes it more sensitive to higher temperatures than the CNF Tempo-g-1eq.
| Weight loss (%) | CNF Tempo | CNF Tempo-g-1eq. | CNF Tempo-g-4eq. |
|---|---|---|---|
| 10 | 214 | 223 | 226 |
| 30 | 265 | 271 | 289 |
| 50 | 306 | 318 | 332 |
| 70 | 490 | 619 | 570 |
| 80 | 900 | 866 | 850 |
The DTGA curves of TEMPO CNF demonstrate two evident peaks at 239 °C and 290 °C. The first peak in the DTGA curves come from the degradation of sodium anhydroglucuronate units, and the other peak at 290 °C indicates the thermally unstable anhydroglucuronate groups present at the crystal's surface. Grafting with nisin slightly enhances the stability of anhydroglucuronate groups present on the crystal surfaces. Thus, the second peak increases to the 309 °C and 305 °C for CNF Tempo-g-1eq. and CNF Tempo-g-4eq., respectively.
Quantification of peptide grafting on CNF
Nitrogen content for neat and grafted CNF are shown in Table 2 using bulk elemental analysis. With the grafting of nisin on CNF, there is high content of nitrogen in grafted CNF compared to the neat CNF which verifies the grafting of CNF. Moreover, when grafted with 4 equivalent of nisin, nitrogen content on grafted fibers increased to 2.9% compared to 1.8% of 1 equivalent CNF. Therefore, higher nitrogen content in grafted CNF is in accordance with the immobilization of nisin on the surface of nanocellulose.| Nitrogen content (%) | Carboxyl content (mmol g−1) | Grafting (%) | |
|---|---|---|---|
| CNF Tempo | 0 | 1087.5 | |
| CNF Tempo-g-1eq. | 1.8 | 787 | 27.5 |
| CNF Tempo-g-4eq. | 2.9 | 590 | 45.7 |
The presence of carboxyl content on the surface of TEMPO CNF was used to calculate the grafting efficiency. Carboxyl content on the surface was analysed by conductomertic titration and found to be 1088, 787 and 590 mmol g−1 for CNF Tempo, CNF Tempo-g-1eq. and CNF Tempo-g-4eq. respectively. From these values, it was concluded that 27.5% of carboxyl group in CNF Tempo-g-1eq. and 45.7% in CNF Tempo-g-4eq. were modified with nisin.
Antibacterial activity of grafted CNF films
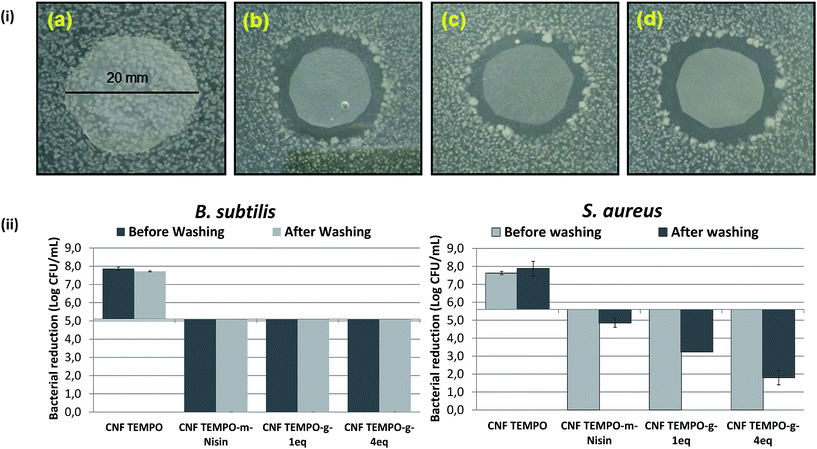
Zone of inhibition was also formed by CNF Tempo-g-1-eq. and CNF Tempo-g-4eq. which can be attributed to the presence of non-covalent bonded nisin present in the CNF.
In a previous study, it was stated that uncontrolled and non-covalent immobilization of peptide forms an unstable layer but on the other hand activation of surface with EDC/NHS leads to the stable immobilization of peptide.50 Furthermore, this hypothesis was also proved with Quartz crystal microbalance with dissipation (QCM-D).
It was observed that regardless of the concentration or method of nisin incorporation, complete killing effect was seen against B. subtilis (Fig. 7(ii)). After that, the authors tried to remove the non-immobilised or free nisin from inside the nanoporous structure of CNF by washing for 48 hours. Washing with water efficiently removes 94% (w/w) of the nisin which was recovered from CNF Tempo-m-nisin when the nisin physically mixed with CNF fibers, but from the grafted CNF, less than 1.6% (w/w) of the nisin was released.
Subsequently, antimicrobial activity was carried with these washed samples. Nevertheless, again complete killing was examined with B. subtilis. Despite, when treated with stronger and more resistant bacteria, S. aureus, physically mixed nisin after washing exhibited very low bactericidal activity but still showed a bacteriostatic activity with reduction of 0.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log of the bacterial population over 24 hours. Moreover, this slight inhibition with CNF Tempo-m-nisin could be attributed to the nisin strongly crosslinked with CNF which is difficult to remove after washing.
log of the bacterial population over 24 hours. Moreover, this slight inhibition with CNF Tempo-m-nisin could be attributed to the nisin strongly crosslinked with CNF which is difficult to remove after washing.
Although, grafting with higher quantity (CNF Tempo-g-4eq.) illustrates a significant reduction of bacterial population after 24 hours i.e. 3.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log CFU reduction. The different bacterial efficiency against S. aureus was obtained because of its high level of resistance abiding the effect of antimicrobials56_ENREF_53. Even more, grafted CNF at lower concentration (1 eq.) was successful, which is evident of bactericidal activity against these highly resistant bacteria.
log CFU reduction. The different bacterial efficiency against S. aureus was obtained because of its high level of resistance abiding the effect of antimicrobials56_ENREF_53. Even more, grafted CNF at lower concentration (1 eq.) was successful, which is evident of bactericidal activity against these highly resistant bacteria.
Nisin classically used to preserve cheese or canned fruits and vegetable. These products retains significant amount of water which can wash off nisin from the packaging matrix. After grafting with nisin, peptide molecule stays on the surface of the matrix for longer period of time and provides longer shelf life to food products. In addition, the activity of nisin was not affected after grafting since only one amino group was changed among the large amount available.
Conclusions
In conclusion, nisin can be implemented with CNF either by direct incorporation or grafting on its surface. QCM-D confirmed the interface reaction between nisin and CNF with EDC/NHS but also gave evidence of the strong interaction between nisin and CNF without any coupling agent.In this study, a two-step procedure was proposed and developed for fabrication of antimicrobial film. Surface modification of CNF was performed with an active molecule, nisin, was activated by using the coupling agents EDC/NHS. Surface functionalization was confirmed by the emergence of new band for amide group in FTIR and the presence of nitrogen after grafting. The presence of nisin improves thermal stability of CNF and washing of mixed nisin onto CNF is strongly more efficient than the grafted one.
Conversely, qualitative antimicrobial test against B. subtilis revealed the leaching of nisin from the grafted nanofibers film thanks to the strong interaction between the nisin and CNF. Direct mixing of nisin with CNF demonstrated complete killing against B. subtilis even after washing, but the activity decreases when applied to more resistant bacteria, S. aureus. Even after washing for 48 hours, high bacterial killing (2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log CNF Tempo-g-1-eq. and 3.8
log CNF Tempo-g-1-eq. and 3.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log CNF Tempo-g-4eq.) was reported.
log CNF Tempo-g-4eq.) was reported.
It is noteworthy that direct mixing loses its activity after the release of active molecule within the time period or in continuous washing by the liquid present in food formulation. The grafting ensures food preservation for a long period of time as molecules are situated at the surface of the packaging system.
Nevertheless, it can be inferred from all the characteristics that the peptide grafted CNF have outstanding potential in antimicrobial active food packaging for products like cheese or canned vegetables.
Acknowledgements
This research was supported by new generation packaging (NEWGENPAK) project of the seven framework program of European research under grant agreement no. 290098. LGP2 is part of the LabEx Tec 21 (Investissements d'Avenir – grant agreement no. ANR-11-LABX-0030) and of the Énergies du Futur and PolyNat Carnot Institutes (Investissements d'Avenir – grant agreements no. ANR-11-CARN-007-01 and ANR-11-CARN-030-01). This research was made possible thanks to the facilities of the TekLiCell platform funded by the Région Rhône-Alpes (ERDF: European regional development fund).References
- 351,000 People Die of Food Poisoning Globally Every Year, ed. A. Sifferlin, World Health Organisation, 2015, TIME Search PubMed.
- P. Suppakul, J. Miltz, K. Sonneveld and S. W. Bigger, J. Food Sci., 2003, 68, 408–420 CrossRef CAS.
- L. Vermeiren, F. Devilghere, M. Ran beest, N. Kruijef and J. Debevere, Trends Food Sci. Technol., 1999, 10(3), 77–86 CrossRef CAS.
- A. Watthanaphanit, P. Supaphol, H. Tamura, S. Tokura and R. Rujiravanit, Carbohydr. Polym., 2010, 79, 738–746 CrossRef CAS.
- S. W. Ali, S. Rajendran and M. Joshi, Carbohydr. Polym., 2011, 83, 438–446 CrossRef CAS.
- D. S. Cha, J. H. Choi, M. S. Chinnan and H. J. Park, Lebensm.-Wiss. Technol., 2002, 35, 715–719 CrossRef CAS.
- D. Ding, F. Wang, X. Liu and C. Han, Appl. Mech. Mater., 2015, 731, 385–388 CrossRef CAS.
- R. D. Joerger, Poult. Sci., 2003, 82, 640–647 CrossRef CAS PubMed.
- European Food Safety Authority (EFSA), The use of nisin (E 234) as a food additive, EFSA, 2006, vol. 314, pp. 1–16 Search PubMed.
- J. Lubelski, R. Rink, R. Khusainov, G. N. Moll and O. P. Kuipers, Cell. Mol. Life Sci., 2008, 65, 455–476 CrossRef CAS PubMed.
- N. E. Kramer, H. E. Hasper, P. T. C. v. d. Bogaard, S. Morath, B. d. Kruijff, T. Hartung, E. J. Smid, E. Breukink, J. Kok and O. P. Kuipers, Microbiology, 2008, 154, 1755–1762 CrossRef CAS PubMed.
- J. A. Auxier, K. F. Schilke and J. McGuire, J. Food Prot., 2014, 6, 1624–1629 CrossRef PubMed.
- M. K. Heshmati, N. Hamdami, M. Shahedi, M. A. Hejazi, A. A. Motalebi and A. Nasirpour, Food Sci. Technol. Res., 2013, 19, 749–758 CrossRef CAS.
- A. Cagri, Z. Ustunol and E. T. Ryser, J. Food Prot., 2004, 67, 833–848 CAS.
- S. Eswaranandam, N. Hettiarachchy and M. Johnson, J. Food Sci., 2006, 69, 79–84 CrossRef.
- C. P. O. Resa, L. N. Gerschenson and R. J. Jagus, Food Control, 2014, 44, 146–151 CrossRef.
- A. La Storia, G. Mauriello, F. Villani and D. Ercolini, Food Bioprocess Technol., 2013, 6, 2770–2779 CrossRef.
- M. E. Janes, S. Kooshesh and M. G. Johnson, J. Food Sci., 2002, 67, 2754–2757 CrossRef.
- M. Guo, T. Z. Jin, L. Wang, O. J. Scullen and C. H. Sommers, Food Control, 2014, 40, 64–70 CrossRef CAS.
- X. Qi, G. Poernomo, K. Wang, Y. Chen, M. B. Chan-Park, R. Xu and M. W. Chang, Nanoscale, 2011, 3, 1874–1880 RSC.
- J. Wan, J. B. Gordon, K. Muirhead, M. W. Hickey and M. J. Coventry, Lett. Appl. Microbiol., 1997, 24, 153–158 CAS.
- K. Ku and B. S. Kyung, J. Microbiol. Biotechnol., 2007, 17, 520–523 CAS.
- M. Millette, C. Le Tien, W. Smoragiewicz and M. Lacroix, Food Control, 2007, 18, 878–884 CrossRef CAS.
- M. Imran, S. El-Fahmy, A. Revol-Junelles and S. Desobry, Carbohydr. Polym., 2010, 81, 219–225 CrossRef CAS.
- B. Matthews, S. Mangalasary, D. Darby and K. Cooksey, Packag. Technol. Sci., 2010, 23, 267–273 CAS.
- A. F. Turbak, F. W. Snyder and K. R. Sandberg, J. Appl. Polym. Sci.: Appl. Polym. Symp., 1983, 37, 815–827 CAS.
- K. Syverud and P. Stenius, Cellulose, 2009, 16, 75–85 CrossRef CAS.
- I. Siro and D. Plackett, Cellulose, 2010, 17, 459–494 CrossRef CAS.
- N. Lavoine, I. Desloges, A. Dufresne and J. Bras, Carbohydr. Polym., 2012, 90, 735–764 CrossRef CAS PubMed.
- K. L. Spence, R. A. Venditti, Y. Habibi, O. J. Rojas and J. J. Pawlak, Bioresour. Technol., 2010, 101, 5961–5968 CrossRef CAS PubMed.
- T. Saito, S. Kimura, Y. Nishiyama and A. Isogai, Biomacromolecules, 2007, 8, 2485–2491 CrossRef CAS PubMed.
- Y. Habibi, Chem. Soc. Rev., 2014, 43, 1519–1542 RSC.
- M. Andresen, P. Stenstad, T. Møretrø, S. Langsrud, K. Syverud, L. Johansson and P. Stenius, Biomacromolecules, 2007, 8, 2149–2155 CrossRef CAS PubMed.
- S. Saini, M. N. Belgacem, J. Mendes, G. Elegir and J. Bras, ACS Appl. Mater. Interfaces, 2015, 7, 18076–18085 CAS.
- S. C. M. Fernandes, P. Sadocco, P. Teodoro, A. Alonso-Varona, A. Eceiza, A. J. D. Silvestre, I. Mondragon and C. S. R. Freire, ACS Appl. Mater. Interfaces, 2013, 5, 3290–3297 CAS.
- K. Missoum, P. Sadocco, J. Causio, M. N. Belgacem and J. Bras, Mater. Sci. Eng., C, 2014, 45, 477–483 CrossRef CAS PubMed.
- S. Saini, M. N. Belgacem, K. Missoum and J. Bras, Ind. Crops Prod., 2015, 78, 82–90 CrossRef CAS.
- S. Saini, Ç. Yücel-Falco, M. N. Belgacem and B. Julien, Carbohydr. Polym., 2016, 135, 239–247 CrossRef CAS PubMed.
- S. Saini, M. N. Belgacem, M. C. Brochier Salon and J. Bras, Cellulose, 2016, 23(1), 795–810 CrossRef CAS.
- A. Isogai, T. Saito and H. Fukuzumi, Nanoscale, 2011, 3, 71–85 RSC.
- G. Z. Sauerbrey, Z. Phys., 1959, 155, 206 CrossRef CAS.
- X. Turon, O. J. Rojas and R. S. Deinhammer, Langmuir, 2008, 24, 3880–3887 CrossRef CAS PubMed.
- C. Aulin, E. Johansson, L. Wågberg and T. Lindstrom, Biomacromolecules, 2010, 11, 872–882 CrossRef CAS PubMed.
- R. Bardet, V. Perrin, N. Belgacem and J. Bras, ACS Appl. Mater. Interfaces, 2015, 7, 7 Search PubMed.
- H. Orelma, I. Filpponen, L.-S. Johansson, M. Osterberg, O. J. Rojas and J. Laine, Biointerphases, 2012, 7, 61 CrossRef CAS PubMed.
- A. Benkaddour, K. Jradi, S. Robert and C. Daneault, Nanomaterials, 2013, 3, 141–157 CrossRef CAS.
- I. Filpponen and D. S. Argyropoulos, Biomacromolecules, 2010, 11, 1060–1066 CrossRef CAS PubMed.
- H. Orelma, I. Filpponen, L.-S. Johansson, M. Osterberg, O. J. Rojas and J. Laine, Biointerphases, 2012, 7, 61 CrossRef CAS PubMed.
- H. Sadeghifar, I. Filpponen, S. P. Clarke, D. F. Brougham and D. S. Argyropoulos, J. Mater. Sci., 2011, 46, 7344–7355 CrossRef CAS.
- R. Mauchauffe, M. Moreno-Couranjou, N. D. Boscher, C. e. V. D. Weerdt, A.-S. Duwez and P. Choquet, J. Mater. Chem. B, 2014, 2, 5168–5177 RSC.
- O. Nechyporchuk, F. Pignon and N. Belgacem, J. Mater. Sci., 2015, 50, 531–541 CrossRef CAS.
- H. Fukuzumi, T. Saito, T. Iwata, Y. Kumamoto and A. Isogai, Biomacromolecules, 2009, 10, 162–165 CrossRef CAS PubMed.
- K. Missoum, M. N. Belgacem and J. Bras, Materials, 2013, 6, 1745–1766 CrossRef CAS.
- H. Lönnberg, L. Fogelström, S. Samir, L. A. Berglund, E. Malmström and A. Hult, Eur. Polym. J., 2008, 44, 2991–2997 CrossRef.
- T. Huq, B. Riedl, J. Bouchard, S. Salmieri and M. Lacroix, Cellulose, 2014, 21, 4309–4321 CrossRef CAS.
- I. T. Paulsen, T. G. Littlejohn, P. Rådström, L. Sundström, O. Sköld, G. Swedberg and R. A. Skurray, Antimicrob. Agents Chemother., 1993, 37, 761–768 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |