Facile synthesis of carbon-decorated single-crystalline Fe3O4nanowires and their application as high performance anode in lithium ion batteries†
Theivanayagam
Muraliganth
,
Arumugam
Vadivel Murugan
and
Arumugam
Manthiram
*
Electrochemical Energy Laboratory, Materials Science and Engineering Program, The University of Texas at Austin, Texas, 78712, USA. E-mail: rmanth@mail.utexas.edu; Fax: (+1) 512-471-768; Tel: (+1) 512-471-1791
First published on 22nd October 2009
Abstract
A facile microwave-hydrothermal approach has been used to synthesize single-crystalline Fe3O4nanowires within 15 min at 150 °C. The Fe3O4nanowires, after decorating with carbon, exhibit excellent cyclability and rate performance when employed as an anode in lithium ion batteries.
In the past decade, considerable attention has been drawn towards one-dimensional (1-D) nanostructures, such as nanowires, nanorods, and nanotubes, because of their high surface to volume ratio, interesting physical properties, and potential applications in a wide range of fields like optoelectronic devices, field effect transistors, chemical/biological sensors, fuel cells, solar cells, and energy storage.1 Particularly, there is a growing interest in utilizing the 1-D nanostructures as electrodes in lithium-ion batteries.2,3Magnetite (Fe3O4) is an attractive anode material for next generation lithium-ion batteries because of its high capacity, eco-friendliness, natural abundance, and high electronic conductivity.3d,4 However, its application in practical lithium-ion batteries is hindered due to its low rate performance arising from kinetic limitations and poor cycling stability resulting from large volume expansion occurring during cycling.
In this context, use of nanowire architectures will offer high capacity and improved cyclic stability because of their high interfacial contact area with the electrolyte and better accommodation of strain and volume change without any structural change or fracture.2,3 Moreover, the 1-D nanowire morphology is beneficial for achieving high rate performance since it facilitates better electron and lithium ion transport than electrodes comprising small nanoparticles in which the electrons and lithium-ions have to move through particles and are limited by the inter-particle contacts. In this regard, development of 1-D Fe3O4nanowires can enhance the viability of Fe3O4 as a high performance anode in practical lithium ion cells. Moreover, as Fe3O4 is magnetic, the synthesized 1-D nanowires can have potential applications in a wide variety of fields such as data storage, magnetic resonance, gas sensors, spintronic devices, and biomedical applications.5
Various solution-based approaches, such as co-precipitation, reverse micelle, thermal decomposition, and hydrothermal methods, have been pursued in the literature for the synthesis of Fe3O4nanostructures.6Magnetitenanocrystals with irregular morphology have also been synthesized recently by a microwave assisted sol –gel method.7 However, synthesis of Fe3O4nanowires is quite complex because of its cubic crystal structure, so it requires the use of specialized templates or external applied magnetic field to orient the growth of the nanocrystals and the procedures are mostly time and energy consuming.6f–g Recently, microwave-assisted hydrothermal (MW-HT) and solvothermal (MW-ST) methods are gaining increasing popularity for nanomaterials synthesis as they offer a clean, low-cost approach to synthesize nanocrystals within a very short reaction time (in minutes) with high yields.8 Herein, we report a facile MW-HT approach to synthesize Fe3O4nanowires within 15 min using polyethylene glycol (PEG-400) as a soft template in water at temperatures as low as 150 °C. The as-synthesized Fe3O4nanowires after decorating with carbon exhibit a high reversible capacity of ∼830 mA h g−1 with excellent cyclability and high rate performance when employed as an anode in lithium cells.
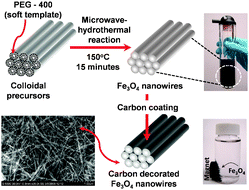 | ||
| Scheme 1 Schematic illustration of the microwave-hydrothermal synthesis of single-crystalline Fe3O4nanowires, employing PEG-400 as a soft template and subsequent carbon decoration for application as high performance anode in lithium ion batteries. | ||
Our synthetic strategy involves the use of PEG-400 as a soft template for the synthesis of Fe3O4nanowires by the MW-HT method (see ESI† for experimental details).9 The short chain polymer (PEG-400) preferentially adsorbs on the surface of a growing colloid during the synthesis, confining the colloid and facilitating anisotropic growth of the nanocrystals. The use of PEG as a soft template enables synthesis of bulk quantities of nanowires without requiring complex procedures or specialized hard templates.
Carbon coating has often been shown in recent years to improve the electrochemical performances of nanostructured anode and cathode materials due to its high electronic conductivity, good lithium permeability, and electrochemical stability.10 Accordingly, the Fe3O4nanowires were decorated with carbon by a two step procedure. In the first step, the as-synthesized Fe3O4nanowires were coated with glucose-derived carbon-rich polysaccharide (GCP) by sonicating with glucose solution, followed by subjecting them to the MW-HT process at 180 °C for 15 min. Typically, hydrothermal carbonization involves an initial dehydration of the carbohydrate followed by subsequent polymerization and carbonization. It is well known that GCP can be easily carbonized and structurally ordered by heating in an inert atmosphere at lower temperatures.11 Accordingly, the carbon-decorated Fe3O4nanowires were obtained after heating the GCP coated Fe3O4 at 400 °C for 3 h in Ar atmosphere. The carbon content in the composite thus obtained was determined to be ∼10 wt% from thermogravimetric analysis (TGA).
We examined the structural morphology of the as-synthesized Fe3O4 with ultra high resolution field emission scanning electron microscopy (FE-SEM) and high resolution transmission electron microscopy (HRTEM). The representative SEM images of the as-synthesized Fe3O4 shown in Fig. 1a and b reveal uniform nanowire-like morphology with a diameter ranging from 20 to 50 nm and a length of several micrometres. As shown in Fig. 1c, the nanowire morphology is maintained even after the MW-HT carbonization and subsequent heat treatment at 400 °C. Fig. 1d and e show the overall view and high resolution TEM imagesof the carbon-decorated Fe3O4nanowires. The HRTEM image and fast Fourier transform (FFT) shown in Fig. 1e and f reveal that the Fe3O4nanowires are single crystalline and they preferentially grow along the [110] direction, which is one of the easy magnetic axes of Fe3O4.12Fig. 1d clearly shows the amorphous carbon nano-coating on the surface of the highly crystalline Fe3O4nanowire and the thickness of the carbon layer is found to be ∼4 nm.
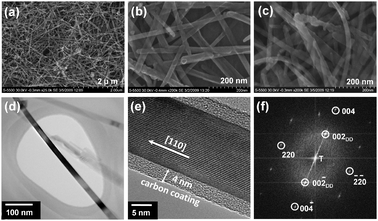 | ||
| Fig. 1 Morphological characterization of Fe3O4nanowires: (a) low and (b) high magnification FE-SEM images of as-synthesized Fe3O4nanowires, (c) FE-SEM image of carbon-decorated Fe3O4nanowires, (d) TEM and (e) HRTEM images showing crystalline Fe3O4nanowires surrounded by amorphous carbon, and (f) FFT image of single-crystalline Fe3O4nanowire. The subscript DD refers to double diffraction. | ||
Fig. 2a shows the XRD patterns of the as-synthesized and carbon-coated Fe3O4nanowires. All the reflections could be indexed on the basis of the cubic magnetite spinel phase (space group: Fd3m) with a lattice parameter of 8.382 Å. The carbon-decorated Fe3O4 did not show any impurity or peaks corresponding to carbon due to its amorphous nature or low content. Fig. 2b compares the Raman spectra of the as-synthesized and carbon-coated Fe3O4nanowire samples. Raman spectrum of the pristine Fe3O4nanowire shows the characteristic peaks of magnetite at 331, 524 and 670 cm−1 that are attributed, respectively, to the Eg, T2g and A1g vibrational modes,13 confirming that the as-synthesized nanowires are composed of the magnetiteFe3O4 phase. On the other hand, the Raman spectrum of the carbon-decorated Fe3O4nanowire shows the fundamental D and G bands for carbon at around, respectively, 1365 and 1590 cm−1 in addition to the characteristic bands for Fe3O4. However, the carbon spectrum could be deconvoluted into four Gaussian bands at 1590, 1487, 1365, and 1223 cm−1, which are referred to, respectively, as G, D′′, D, and I, following the convention of Bonhomme et al.14 used for peak fitting. The peak intensity ratio between D and G bands (ID/IG) generally provides a useful index for comparing the degree of crystallinity of various carbon materials, i.e., smaller the ratio of ID/IG, higher the degree of ordering in the carbon material. The ID/IG ratio was found to be 1.03, demonstrating that the carbon formed is fairly ordered, which will be beneficial for achieving better electronic conduction between adjacent nanowires.
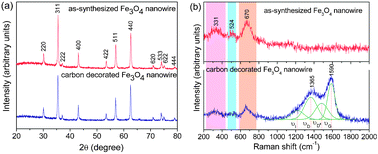 | ||
| Fig. 2 (a) X-ray diffraction and (b) Raman spectra of Fe3O4nanowires before and after carbon decoration. | ||
Fig. 3a shows the first charge–discharge profiles of Fe3O4 and carbon-decorated Fe3O4nanowires charged at various current rates from C/10 to 5C but discharged at a constant rate of C/10. For the as-synthesized Fe3O4nanowires, the first discharge capacity was found to be around 1240 mA h g−1, corresponding to the insertion of almost 11 Li per Fe3O4 formula. Although a small amount of capacity coming from the alloying of lithium with the copper current collector cannot be ruled out, it is usually too small to cause any significant change in the overall capacity. In the following charge, only about 8 Li could be removed reversibly resulting in a charge capacity of about 922 mA h g−1. The reversible reaction occurring with lithium can be summarized as,
| Fe3O4 + 8e− + 8Li+ ↔ 3Fe0 + 4Li2O |
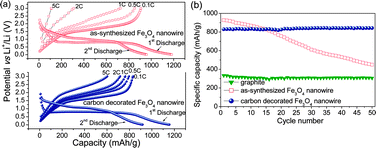 | ||
| Fig. 3 (a) Galvanostatic charge–discharge curves of as-synthesized and carbon-decorated Fe3O4nanowires in lithium cells (charged at various C-rates from 0.1C to 5C but discharged at a constant rate of 0.1C) and (b) cycling performances of the Fe3O4nanowires before and after carbon decoration at 0.1C rate. For a comparison, data for a natural graphiteelectrode is also shown. | ||
The enhanced cycling performance of the carbon-coated samples is due to the presence of the carbon buffer layer around the Fe3O4nanowires, preventing direct contact among the adjacent nanowires and thereby minimizing aggregation of the nanowires during electrochemical cycling. The carbon nano-coating also provides an elastic inactive matrix that can absorb the massive volume expansion and contraction occurring during charge–discharge cycling. More importantly, the carbon-decorated Fe3O4nanowires exhibit improved rate performance compared to the as-synthesized Fe3O4nanowires. For example, as seen in Fig. 3a, the carbon-decorated Fe3O4nanowires can still deliver a high capacity of 600 mA h g−1 at a high charge rate of 5C. In other words, 73% of the initial capacity could be retained at a high rate of 5C. In contrast, only 12% of the initial capacity could be retained with the as-synthesized Fe3O4nanowires at such at a high charge rate of 5C. Herein, the improvement in rate capability is mainly attributed to the conductive carbon coating layer formed on the magnetitenanowire. Moreover, with a capacity of 830 mA h g−1 and with the magnetite (5.22 g cm−3) having two times higher density than carbon (2.22 g cm−3), the carbon decorated Fe3O4nanowires provide almost 5 times higher volumetric capacity than the currently used graphite anodes.
In summary, we have described a facile, rapid microwave-assisted hydrothermal synthesis approach to prepare single-crystalline Fe3O4nanowires and carbon-coated Fe3O4nanowires. The MW-HT method significantly reduces the reaction time, cost, and energy required compared to the conventional solution-based methods, and it can be employed for large scale synthesis of these technologically important magnetitenanowires that are of interest for energy storage, data storage, spintronics devices, and biomedical applications. The Fe3O4nanowires after decorating with carbon provide excellent capacity retention and high rate capability when used as an anode in lithium-ion batteries. Thus, the carbon-decorated Fe3O4nanowires offer an attractive possibility to be used as a high capacity anode material in next generation lithium-ion batteries with high energy and power densities.
This work was supported by the Office of Vehicle Technologies of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231.
Notes and references
- (a) Y. Li, F. Qian, J. Xiang and C. M. Lieber, Mater. Today, 2006, 9, 18 CrossRef CAS; (b) Y. Kim, H. J. Joyce, Q. Gao, H. H. Tan, C. Jagadish, M. Paladugu, J. Zou and A. Suvorova, Nano Lett., 2006, 6, 599 CrossRef CAS; (c) M. Muccini, Nat. Mater., 2006, 5, 605 CrossRef CAS; (d) J. Shui and J. C. M. Li, Nano Lett., 2009, 9, 1307 CrossRef CAS.
- C. K. Chan, H. Peng, G. Liu, K. Mcilwrath, X. F. Zhang, R. A. Huggins and Y. Cui, Nat. Nanotechnol., 2008, 3, 31 CrossRef CAS.
- (a) A. S. Aricò, P. Bruce, B. Scrosati, W. V. Shalkwijk and J.-M. Tarascon, Nat. Mater., 2005, 4, 366 CrossRef CAS; (b) Y. Wang and G. Cao, Adv. Mater., 2008, 20, 2251 CrossRef CAS; (c) A. Manthiram, A. Vadivel Murugan, A. Sarkar and T. Muraliganth, Energy Environ. Sci., 2008, 1, 621 RSC; (d) P. L. Tarascon, S. Mitra, P. Poizot, P. Simon and J.-M. Tarascon, Nat. Mater., 2006, 5, 567 CrossRef CAS; (e) K. T. Nam, D.-W. Kim, P. J. Yoo, C.-Y. Chiang, N. Meethong, P. T. Hammond, Y.-M. Chiang and A. M. Belcher, Science, 2006, 312, 885 CrossRef CAS; (f) Y. Li, B. Tan and Y. Wu, Nano Lett., 2008, 8, 265 CrossRef CAS.
- (a) H. Liu, G. Wang, J. Wang and D. Wexler, Electrochem. Commun., 2008, 10, 1879 CrossRef CAS; (b) S. Ito, K. Nakaoko, M. Kawamura, K. Ui, K. Fujimoto and N. Koura, J. Power Sources, 2005, 146, 319 CrossRef CAS.
- L. Zhang and Y. Zhang, J. Magn. Magn. Mater., 2009, 321, L15 CrossRef CAS.
- (a) X. Wang, J. Zhuang, Q. Peng and Y. Li, Nature, 2005, 437, 121 CrossRef CAS; (b) J. Wang, Q. Chen, C. Zeng and B. Hou, Adv. Mater., 2004, 16, 137 CrossRef CAS; (c) R. Fan, X. H. Chen, Z. Gui, L. Liu and Z. Y. Chen, Mater. Res. Bull., 2001, 36, 497 CrossRef; (d) S. Lian, Z. Kang, E. Wang, M. Jiang, C. Hu and L. Xu, Solid State Commun., 2003, 127; (e) J. B. Yang, H. Xu, S. X. You, X. D. Zhou, C. S. Wang, W. B. Yelon and W. J. James, J. Appl. Phys., 2006, 99, 08Q507 CrossRef; (f) K. He, C. Y. Xu, L. Zhen and W.-Z. Shao, Mater. Lett., 2007, 61, 3159 CrossRef CAS; (g) Z. Liu, S. Han, C. Li, B. Lei, M. P. Stewart, J. M. Tour and C. Zhou, Nano Lett., 2004, 4, 2151 CrossRef CAS.
- I. Bilecka, I. Djerdj and M. Niederberger, Chem. Commun., 2008, 886 RSC.
- (a) A. B. Panda, G. Glaspell and M. S. M. El-Shall, J. Am. Chem. Soc., 2006, 128, 2790 CrossRef CAS; (b) J. A. Gerbec, D. Magana, A. Washington and G. F. Strouse, J. Am. Chem. Soc., 2005, 127, 15791 CrossRef CAS; (c) A. Vadivel Murugan, T. Muraliganth, P. J. Ferreira and A. Manthiram, Inorg. Chem., 2009, 48, 946 CrossRef CAS; (d) T. Muraliganth, A. Vadivel Murugan and A. Manthiram, J. Mater. Chem., 2008, 18, 5661 RSC.
- Z. Li, Y. Xiong and Y. Xie, Inorg. Chem., 2003, 42, 8105 CrossRef CAS.
- (a) X. W. Lou, C. M. Li and L. A. Archer, Adv. Mater., 2009, 21, 2536 CrossRef CAS; (b) Y.-S. Hu, R. D. Cakan, M.-M. Titirici, J.-O. Müller, R. Schlöge, M. Antonietti and J. Maier, Angew. Chem., Int. Ed., 2008, 47, 1645 CrossRef CAS.
- X. M. Sun, J. F. Liu and Y. D. Li, Chem. Mater., 2006, 18, 3486 CrossRef CAS.
- Y. L. Li and G. D. Li, in Physics of Ferrites, Science Publishing Corporation, Beijing, 1978, p. 381 Search PubMed.
- O. N. Shebanova and P. Lazor, J. Solid State Chem., 2003, 174, 424 CrossRef CAS.
- F. Bonhomme, J. C. Lassegues and L. Servant, J. Electrochem. Soc., 2001, 148(11), E450 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details. See DOI: 10.1039/b916376j |
| This journal is © The Royal Society of Chemistry 2009 |
