 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Emerging frontiers in chiral metal–organic framework membranes: diverse synthesis techniques and applications
Yun
Fan
a and
Mengyun
Chen
 *b
*b
aKey Laboratory of Flexible Electronics (KLOFE), Institute of Advanced Materials (IAM) & School of Flexible Electronics (Future Technologies), Nanjing Tech University, 30 South Puzhu Road, Nanjing 211816, China
bDepartment of Physics, Chemistry and Biology (IFM), Linköping University, Linköping, 58183 Sweden. E-mail: mengyun.chen@liu.se
First published on 28th April 2025
Abstract
Chirality is a basic and universal property in nature, refering to the asymmetry of molecules, where they do not coincide with their mirror images. Chiral materials, in multiple forms, usually exhibit unique physical phenomena such as chiral luminescence and distinctive chemical properties. Metal–organic framework (MOF) membranes have high porosity and abundant active sites; thus, they are an excellent candidate for functionalization. With the involvement of chiral units, chiral MOF membranes demonstrate great potential in applications such as chiral sensing, separation and luminescence. In this review, we first introduce the up-to-date preparation methods for chiral MOF membranes, including direct and indirect methods, and then discuss their applications in enantiomer recognition, chiral separation, and circularly polarized luminescence. Finally, we summarize the challenges in developing chiral MOF membranes and provide a perspective on future developments.
1. Introduction
Metal–organic frameworks are a type of porous compound formed via the self-assembly of metal ions or clusters with organic ligands.1–3 Owing to their unique properties such as high porosity, large surface area, adjustable pore structure, and accessible active sites, MOFs have broad applications in separation, catalysis, sensing, and energy storage.4,5 Research on MOF materials is gradually shifting from bulk materials to membrane materials, as membranes offer advantages such as low cost, low energy consumption and reusability, which are important for their practical applications.6–8Chiral molecules are interesting materials and exhibit unique physical phenomena, such as chiral luminescence and distinctive chemical properties. Chiral materials share similar structures but can possibly present completely different physicochemical or biological properties.9,10 For example, one enantiomer performs the desired function, while the other shows an inactive state or, in some cases, even causes unwanted side effects, which are significant in drug and food safety as well as chemical synthesis.11,12
Owing to the abundant active sites of MOFs, chiral MOFs have been successfully fabricated by incorporating chiral units via various methods (e.g. addition of chiral ligands or chiral secondary structural units, post modification, or chiral induction).13–16 Based on chiral MOF materials, researchers have developed chiral MOF membranes for practical applications.17 With an ordered pore structure resulting from the arrangement of crystal growth, chiral MOF membranes provide better recognition and separation of enantiomers, e.g. chiral drugs and chiral gas molecules.18–20 By integrating fluorescent units, chiral MOF membranes present circularly polarized luminescence (CPL), which has potential for applications in information storage, anti-counterfeiting, and encryption.21 In summary, chiral MOF membranes, as emerging functional materials, have seen great progress in preparation and applications, which inspired us to provide a summary.
In this review, we first introduce the preparation of chiral MOF membranes, including direct methods (solvothermal synthesis, layer-by-layer (lbl) synthesis, and template-assisted synthesis) and indirect methods (solution casting and thermally induced phase separation-hot pressing (TIPS-HoP)) (Fig. 1).22–24 Then, we summarize the applications of chiral MOF membranes in chiral sensing, chiral separation, and CPL. Finally, we provide insights into the preparation of high-quality chiral MOF membranes for enhanced applications.
2. Preparation of chiral MOF membranes
There are two main forms of chiral MOF membranes—pure membranes and mixed matrix membranes (MMMs)—based on the presence of a matrix. Direct methods are typically used for the synthesis of pure chiral MOF membranes, while indirect methods are used for the synthesis of MMMs. The synthesis of pure chiral MOF membranes is simple and usually involves a one-step process on the substrate. Self-supporting MMMs are prepared by synthesizing chiral MOF materials followed by membrane fabrication. Due to their combination with various matrices, chiral MOF MMMs offer the advantages of enhanced mechanical stability and processability, which ensures a simultaneous increase in permeability and selectivity.25 The methods for preparing pure chiral MOF membranes and MMMs are discussed in the following section.2.1. Pure chiral MOF membranes
Pure chiral MOF membranes are synthesized from chiral MOF precursors via in situ synthesis on substrates, including one-pot solvothermal, lbl, and template-assisted synthesis.In addition, metal source substrates are usually used to prepare MOF membranes, which not only serve as substrates but also participate in the synthesis.26 Qiu et al. prepared a Ni2(L-asp)2(bipy) membrane on a Ni net substrate using a solvothermal method with a thickness of 10–20 μm (Fig. 2a).27 The Ni net was horizontally placed in the autoclave and reacted with a solution of 4,4′-bipyridine (bipy) and L-aspartic acid (L-asp) ligands. The Ni ions in the Ni net acted as anchoring active sites, coordinating with organic ligands to promote the nucleation and growth of MOFs. Ni2(L-asp)2(bipy) grew around the wires of the Ni net and then alternately grew to form a continuous Ni2(L-asp)2(bipy) membrane, creating strong adhesion between the membrane and the Ni net substrate. A thin and defect-free Ni2(L-asp)2(bipy) membrane was prepared using this single metal source method because the formation of a layer of the membrane led to the cessation of the growth process due to the absence of a metal source. X-ray diffraction and scanning electron microscopy (SEM) characterization indicated the high crystallinity and continuity of the Ni2(L-asp)2(bipy) membrane.
 | ||
| Fig. 2 (a) Preparation of thin chiral Ni2(L-asp)2(bipy) membrane with Ni net asboth substrate and metal source. Reproduced with permission from ref. 27. Copyright 2013, The Royal Society of Chemistry. (b) Preparation of chiral MOF-808 membranes by introduction of chiral molecules to MOF-808 membranes. Reproduced with permission from ref. 23. Copyright 2024, American Chemical Society. | ||
In situ secondary growth of MOFs on substrates was used to obtain continuous and well-intergrown MOF membranes. Recently, Zhao et al. synthesized chiral MOF-808 membranes on α-aluminum oxide (α-Al2O3) substrate through secondary growth and post-synthetic modification (Fig. 2b).23 First, the α-Al2O3 substrate was vertically placed in the precursor solution of MOF-808 for a solvothermal reaction to prepare a seeded MOF-808 membrane. The membrane was then placed in a precursor solution of MOF-808 for secondary growth to obtain a continuous MOF-808 membrane. Subsequently, the chiral MOF-808 membranes MOF-808-Ala, MOF-808-Thr, and MOF-808-His were grown after reacting in solutions containing the chiral molecules L-alanine (L-Ala), L-threonine (L-Thr), and L-His, respectively. The introduced amino acid molecules partially replaced the trifluoroacetic acid regulator on the Zr cluster to coordinate with the unoccupied Zr cluster, thus being integrated into the MOF-808 structure. The chiral MOF-808 membranes obtained by post-synthetic modification retained the crystallinity of MOF-808, and their thicknesses could be controlled by regulating the concentration of metal ions.
 | ||
| Fig. 3 (a) Preparation of γCD-SURMOF membrane by assembly of metal ions and ligands layer by layer onto γCD(SH)8 modified Au substrates. Reproduced with permission from ref. 29. Copyright 2022, Chinese Chemical Society. (b) Preparation of M-CMOF membranes with hierarchical porous structures by using PS arrays as templates. Reproduced with permission from ref. 30. Copyright 2024, American Chemical Society. | ||
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch of the chiral molecule L-His and the disappearance of N–H vibration indicated that the chiral molecule was incorporated into the MOF framework.
O stretch of the chiral molecule L-His and the disappearance of N–H vibration indicated that the chiral molecule was incorporated into the MOF framework.
2.2. Chiral MOF MMMs
Chiral MOF MMMs are hybrid membranes in which chiral MOF fillers are embedded in a continuous matrix.31 The chiral MOF MMMs exhibit higher mechanical strength, fewer defects, and better continuity due to the combination of the matrix. The synthesis of chiral MOF materials and the preparation of MMMs are described in the following sections.In situ synthesis. The in situ synthesis is the most widely used method for synthesizing chiral MOFs, and the synthesized chiral MOFs exhibit bulk uniformity and high enantiomeric purity.34–36 The method involves enantiomeric chiral ligands, which are expensive, causing complexity and high cost in the synthesis.
Tanaka et al. synthesized chiral ligands (R)-2,2′-dihydroxy-1,1′-binaphthyl-5,5′-dibenzoic acid (H2L1) and (R)-2,2′-dimethoxy-1,1′-binaphthyl-5,5′-dibenzoic acid (H2L2) for chiral interpenetrating (R)-MOF-1 and non-interpenetrating (R)-MOF-2, respectively (Fig. 5a).37 In (R)-MOF-1, Cu ions coordinated with the four oxygen atoms in the carboxylic acid group of H2L1 to form layers in the topological structure. Then, each two-dimensional (2D) network was interpenetrated in a parallel or parallel tilted manner to form (R)-MOF-1. The 2D network structure in (R)-MOF-2 was like that in (R)-MOF-1, but the 2D network did not interpenetrate but overlapped to form (R)-MOF-2. In addition, Wang et al. synthesized homochiral L-His-ZIF-8 using mixed ligands.19 The chiral molecule L-His was introduced into the precursor solution of ZIF-8, and then L-His-ZIF-8 was formed by the solvothermal self-assembly of L-His with Hmim and Zn ions.
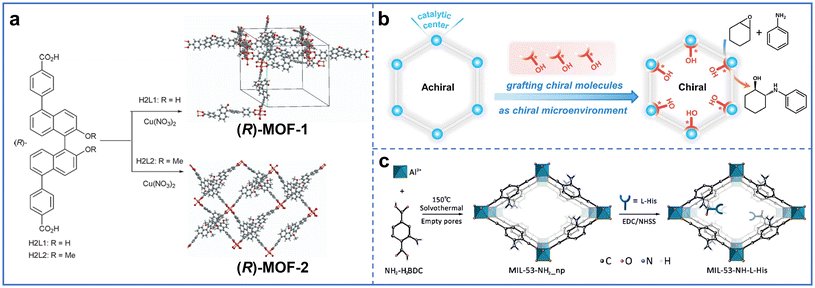 | ||
| Fig. 5 (a) Preparation of chiral (R)-MOF-1 and (R)-MOF-2 with chiral molecules H2L1 and H2L2 as ligands through in situ synthesis. Reproduced with permission from ref. 37. Copyright 2015, The Royal Society of Chemistry. (b) Preparation of chiral (R)-Cn@PCN-222(Cu) by anchoring chiral molecules onto Zr clusters in PCN-222(Cu). Reproduced with permission from ref. 43. Copyright 2023, Wiley-VCH. (c) Preparation of chiral MIL-53-NH-L-His by grafting L-His onto the ligands of MIL-53-NH2(Al). Reproduced with permission from ref. 44. Copyright 2019, Wiley-VCH. | ||
The in situ synthesis method has also been used to synthesize chiral MOF nanosheets with highly ordered in-plane nanopores, which exhibit excellent performance in catalysis, sensing, and separation. For example, Cui et al. synthesized chiral layered Eu-MOFs using dicarboxylic acids derived from 1,1′-biphenyl phosphoric acid as chiral ligands.38 The Eu ions were coordinated with four bidentate bridges and four chelating carboxylate groups in the six ligands. Two adjacent Eu ions were connected by four carboxylate groups to form a planar framework, which was then stacked vertically to form the layered structure. The layered Eu-MOF was exfoliated into chiral MOF nanosheets (1-MONs) using solvent-assisted liquid sonication due to weak interlayer interactions between the layers.
Post-synthetic modification. This method involves introducing chiral molecules into the as-synthesized achiral MOFs to endow them with chiral characteristics without altering their topological structure.39–42 When there are active sites in MOFs, chiral molecules can be grafted onto the metal clusters or organic ligands of MOFs through coordination or covalent interactions. This method has been successfully applied to a wide range of chiral MOFs, as it starts from achiral materials, with the chirality introduced in a separate process. However, compatibility and interaction between the chiral molecules and the MOF framework must be carefully considered during the selection of chiral molecules.
PCN-222(Cu) is an achiral MOF formed by the coordination of Zr ions, [5,10,15,20-tetrakis(4-carboxyphenyl) porphyrinato]-Cu(II) (Cu-TCPP), and benzoic acid (Fig. 5b).43 By anchoring chiral hydroxylated molecules with different carbon chain lengths onto unsaturated coordination sites of Zr clusters, a series of (R)-Cn@PCN-222(Cu) (n = 1, 2, 3) was obtained. There were approximately 3.8–3.9 chiral molecules grafted onto each Zr cluster on average, indicating that chiral molecules completely replaced the –OH groups on the Zr clusters and coordinated with the Zr clusters.
Wang et al. introduced the chiral molecule L-His, which coordinates with the functional groups of ligands, to convert achiral MIL-53-NH2(Al) into chiral MIL-53-NH-L-His (Fig. 5c).44 The achiral MIL-53-NH2(Al) was formed by the coordination assembly of Al clusters with 2-aminoterephthalic acid (NH2BDC) with a flexible framework structure, which was conducive to immobilizing guest molecules. The carboxylic group in L-His underwent an amidation reaction with the amino group in NH2BDC to graft L-His into the MOF framework, endowing MIL-53-NH2(Al) with chirality.
In addition to grafting chiral molecules onto ligands, chiral MOFs can be formed by replacing achiral ligands in MOFs. Cui et al. used a solvent-assisted ligand exchange strategy to incorporate chiral ligand linear dicarboxylate linkers (H2LM) into UiO-68-Me.45 The H2LM molecule had a similar length and connectivity with the ligand (2-methyl-terphenyl dicarboxylate ligand) H2TPDC-Me in UiO-68-Me.
Incorporating chiral guests into the pores of MOFs is also a post-synthetic modification method. The chiral molecules [Rh(Me-BPE)(cod)]OTf (Me-BPE = 1,2-bis(2,5-dimethylphospholano)ethane, cod = 1,5-cyclooctadiene, OTf = triflate) were encapsulated into the pores of UMCM-1-NH2, endowing the MOF with chiral properties, which exhibited excellent enantioselectivity in the asymmetric hydrogenation reactions of unsaturated olefins.46 During the post-synthetic modification process, it is necessary to strictly control the reaction conditions to avoid damaging the MOF framework structure.
Chiral induction. Chiral induction is based on achiral precursors, where the symmetry breaking and induction of chiral arrangement in building units are caused by physical stimulation such as CPL irradiation or adding inducing agents.47–49 The chiral induction method is relatively simpler and less expensive but gives less pure products.
Wu et al. synthesized the coordination polymer [{P/M-Cu(succinate)(4,4′-bipyridine)}n]·(4H2O)n using CPL irradiation.50 According to the analysis of CPL chirality and the corresponding obtained products, the product after left-handed CPL irradiation showed a left-handed helical structure, whereas right-handed CPL irradiation was beneficial for the production of right-handed helical structure crystals. The Cu ions coordinated with succinic acid to form an intermediate fragment [Cu(succinate)]x that exhibited helicity. CPL irradiation stimulated the conversion of fragment [Cu(succinate)]x into the preferential configuration, which then assembled into a 3D structure with chirality by coordination with 4,4′-bipyridine.
In addition to physical stimulation, the introduction of chemical reagents can induce chirality by affecting the self-assembly of MOFs. Enantiomeric pure organic acids D-(+)-camphoric acid (D-(+)-H2cam) and L-(−)-camphoric acid (L-(−)-H2cam) were used to induce the asymmetric crystallization of achiral manganese damantane-1,3-dicarboxylic ([Mn(adc)]) (Fig. 6a).51 When using D-(+)-H2cam as a chiral inducer, the main product was (+)-[Mn3(HCOO)4(adc)]; when L-(−)-H2cam was used as a chiral inducer, the main product was (−)-[Mn3(HCOO)4(adc)]. The synergistic effect of chiral H2cam and achiral H2adc led to the asymmetric crystallization of the product. H2cam coordinated with Mn clusters to control the absolute helicity of the [Mn3(HCOO)4]n2n+ framework. The H2cam in the crystal was then replaced by H2adc ligands to form chiral MOFs, and H2cam was not incorporated into the MOF structure. Hong et al. introduced chiral intermediates I(M) or I(P) to the assembly with achiral pyridine, ligand benzene-1,3,5-tris(4-benzoic acid) (H3BTB) and Zn ions to prepare chiral FJI-H27(M) and FJI-H27(P) (Fig. 6b).52 Pyridine, as a template, participated in the coordination-driven assembly, the amount of which provided the precise regulation of the chirality.
 | ||
| Fig. 6 (a) Preparation of chiral [Mn3(HCOO)4(adc)] by chiral induction with H2cam. Reproduced with permission from ref. 51. Copyright 2010, Wiley-VCH. (b) Preparation of chiral FJI-H27(M) and FJI-H27(P) chiral induction with pyridine. Reproduced with permission from ref. 52. Copyright 2021, Wiley-VCH. | ||
In conclusion, in situ synthesis offers high enantiomer purity and stability, even though it is complex and expensive and usually requires high temperatures and pressures. Post-synthetic modification is a general method in which the compatibility of the introduced chiral molecules and MOFs is required. The chiral induction method is simple and cost-effective, even though the chiral purity achieved is modest. Depending on the requirements of chiral MOFs, various preparation methods are available.
Solution casting. The solution casting method involves mixing chiral MOFs with polymers and casting them into a membrane, followed by appropriate post-treatment to obtain the final MMMs, which is simple and suitable for large-scale production. Wang et al. prepared MIL-53-NH-L-His-based MMMs by depositing the membrane onto a substrate using a casting blade to apply solution or paste (Fig. 7a).44 First, a small amount of polymer polyethersulfone (PES) was added to chiral MOF particle dispersions to reduce the aggregation of MOF particles in the polymer. Then, the remaining PES was added to the dispersion, and the mixture was stirred evenly. Subsequently, the mixture was dropped onto a glass substrate and spread onto a membrane on the substrate using a casting blade. As the solvent evaporated, MIL-53-NH-L-His-based MMM was formed, which could be peeled off from the glass substrate after cooling to obtain a self-supporting membrane. MIL-53-NH-L-His particles were uniformly distributed in the membrane without cracks and defects (Fig. 7b). The loading of MOFs in the membrane could be regulated by changing the ratio of added MOFs. MMMs with different loading ratios of MOFs retained the crystal structure of the MOF particles, indicating that the manufacturing process of the membrane did not affect the crystal structure of the MOFs.
 | ||
| Fig. 7 (a) Preparation of self-supporting MMMs by solution casting method with chiral MIL-53-NH-L-His dispersed in PES. (b) SEM image of the cross-section of MMMs with 20 wt% MIL-53-NH-L-His loading. Reproduced with permission from ref. 44. Copyright 2019, Wiley-VCH. (c) Prrparation of MMMs with high MOF loading by chiral Zn-BLD dispersed inmixed HDPE and UHMWPE polymers through TIPS-HoP method. Reproduced with permission from ref. 55. Copyright 2019, Springer Nature. | ||
Subsequently, the group used the same preparation method to mix chiral MOF γ-CD-MOF with PES to prepare CD-MOF/PES MMMs.53 In addition to PES as the polymer substrate, poly(vinylidene fluoride) (PVDF) was also employed as the dispersed matrix for yolk-shell L-His&R6G@ZIF-8 particles in the preparation of MMMs.54 The casting blade technology is simple and effective for preparing MMMs with controlled thickness and surface roughness.
TIPS-HoP. This method involves roll-to-roll hot pressing of a mixture of ultra-high molecular weight polymer interwoven MOF particles onto a belt to form MMMs, which usually help achieve membranes with good flexibility and ultrahigh MOF loading. MOFs act as molecular sieving channels in MMMs, in which the separation performance can be effectively improved by increasing the loading of MOFs.
Wang et al. prepared MMMs using the TIPS-HoP approach, which overcame the challenge of loss of mechanical strength in high MOF-loading membranes (Fig. 7c).55 First, chiral Zn-BLD was added to the paraffin, high-density polyethylene (HDPE), and ultrahigh-molecular-weight polyethylene (UHMWPE) mixed melt, and it was continuously stirred to disperse evenly. UHMWPE was introduced into the membrane to connect MOF particles, ensuring the flexibility of the membrane under ultrahigh MOF loading. Paraffin was used as the flowable agent to reduce the melt viscosity of HDPE and UHMWPE, thereby avoiding the formation of membranes with brittleness and impermeability. Then, the mixture was dropped onto a belt and transferred to the middle of two rollers for roll-to-roll hot pressing to form the MMM. Finally, the Zn-BLD PE MMM was formed by soaking the membrane in dichloromethane to remove the paraffin from the membrane. In addition to chiral Zn-BLD, achiral MOFs such as NH2-UiO-66, MIL-100(Cr), MOF-5, and ZIF-8 could be prepared as MMMs using this approach, demonstrating the universality of this strategy. Typically, the mechanical properties of MMMs decrease with the increase in MOF loading.56,57 However, the MMM with 86% MOF (Zn-BLD PE MMM-86%) loading maintained flexibility without forming cracks after bending. MOF particle aggregates and long-chain polymers interwove in Zn-BLD PE MMM-86%, forming hierarchical porous structured MMM with the inherent micropores of MOFs and the macropores originating from the interweaving of MOF particles.
Recently, some reports have investigated new matrices for chiral MOF MMMs. A chiral liquid crystal (CLC) was introduced to combine with MOFs to prepare a CLC MOF (CLCMOF) membrane. Gu et al. prepared a CLCMOF membrane by combining CLC with Zn2(sdc)2 (Fig. 8a).58 The CLC solution was a mixture of R-obob, 2-methyl-1,4-phenylene bis(4-(3-(acryloyloxy)propoxy)benzoate), and 4-[[6-[(1-oxo-2-propen-1-yl)oxy]hexyl]oxy]-,4-methoxyphenyl ester in a certain proportion, which encapsulated into the pores of Zn2(sdc)2 due to cross-linking reaction between alkenyl groups in CLC and stilbene groups in MOF ligands under ultraviolet light (UV) irradiation. The CLCMOF membrane exhibits LC thermotropic properties and excellent transparency. The crystal structure of Zn2(sdc)2 was not destroyed by the cross-linking reaction with CLC, allowing the CLCMOF membrane to retain the crystallinity of the MOFs.
 | ||
| Fig. 8 (a) Preparation of CLCMOF membrane by encapsulateion of CLC into the pores of achiral MOFs. Reproduced with permission from ref. 58. Copyright 2024, American Chemical Society. (b) Preparation of chiral MOF membrane (MPN-DMOF) by reaction of chiral ligands with MPN matrix. Reproduced with permission from ref. 59. Copyright 2024, Wiley-VCH. | ||
Xu et al. used an amorphous metal-polyphenol network (MPN) as the matrix for an amorphous/crystalline heterogeneous structure chiral MOF membrane (MPN-DMOF) (Fig. 8b).59 MPN was prepared by a mixed solution of Cu2+, polyethylenimine, and tannic acid, and provided abundant nucleation sites, where the D-methionine chelated with Cu ions to form MOFs. The MPN-DMOF coating retained the crystal structure and phase purity of D-Met@MOF, which was constructed by Cu clusters with D-methionine ligands.
Pure chiral MOF membranes provide high porosity and more accessible active sites despite their high cost and lack of self-supporting possibility. Chiral MOF MMMs have excellent mechanical strength and more convenient applications as self-supporting membranes. However, the interface compatibility between MOFs and matrices, as well as the aggregation of MOF particles, still requires improvement for high-quality MMMs.
3. Applications of chiral MOF membranes
The high porosity and high density of reactive sites ensure that chiral MOF membranes have great potential for chiral recognition and enantiomer separation.60–62 By the involvement of luminescent units in various ways, chiral MOF membranes also present excellent CPL properties, which make them potential for applications in optical display and information storage fields.63 We discuss the applications of chiral MOF membranes in sensing, separation, and CPL in the following section.3.1. Sensing
Due to the abundant active sites and decoration of chiral units, chiral MOF membranes interact differently with chiral enantiomers, and they have been successfully applied as sensors to distinguish chiral isomers.64–67Cui et al. dispersed chiral 1-MONs into PES to prepare self-supporting MMM (1-MONs-MMM) for detecting terpenes and terpenoids, which was a challenging enantiomer detection issue.38 1-MONs-MMM exhibited an emission peak at 355 nm, which was blue shifted compared to 386 nm of 1-MONs in acetonitrile solvent. This may be due to the influence of the PES matrix in the MMM on the fluorescence emission. The phosphoric acid active sites in 1-MONs interacted with the double bonds or carbonyl groups in terpenes and terpenoids, which induced changes in the structure of the nanosheets, such as conformational and exciton binding changes or hardening, leading to fluorescence quenching and selective recognition of enantiomers (Fig. 9a). When MMMs were exposed to (−)-linalool vapor, the fluorescence of (S)-1-MONs-MMM and (R)-1-MONs-MMM was quenched (Fig. 9b). After 420 s, the quenching percentage of (S)-1-MONs-MMM reached 38.5%, whereas the quenching percentage of (R)-1-MONs-MMM could only reach 6.5%, corresponding to the enantioselectivity factor of 9.03 (Fig. 9c). MMMs selectively recognized not only chiral vapors with single functional groups such as α-pinene, limonene, and fenchone, but also terpenoids with chiral bifunctional groups, including terpinen-4-ol and β-citronellol. In addition to chiral MOF nanosheets, L-His&R6G@ZIF-8 with a unique yolk-shell structure, MMMs have been prepared as optical sensors for highly sensitive and selective detection of L-proline.54
 | ||
| Fig. 9 (a) Structures of monofunctional and difunctional terpenes and terpenoids. (b) Stern–Völmer plots of (−)-linalool added to 1-MONs. (c) Quenching percentage of fluorescence by (−)-linalool with exposure time in (S)-1-MONs-MMM and (R)-1-MONs-MMM. Reproduced with permission from ref. 38. Copyright 2021, American Chemical Society. (d) Radar plots of the responses of sensors exposed to five enantiomers. Reproduced with permission from ref. 69. Copyright 2021, Wiley-VCH. | ||
To simultaneously identify multiple molecules and their enantiomers, a sensor array comprising different sensors for identifying enantiomers was proposed.68 Heinke et al. prepared an enantioselective electronic nose (e-nose) based on a chiral MOF sensor array and used for the detection of chiral odor molecules through machine learning.69 Three chiral MOFs and three achiral MOFs were prepared as quartz-crystal microbalance (QCM) sensors, which were integrated into the sensor array. In the e-nose, achiral MOFs were only used to distinguish different molecules that exhibited the same response as chiral isomers, while chiral MOFs were used to recognize enantiomers. When the sensor array was exposed to odor molecules, the contact between the MOF membranes and the odor molecules caused a frequency shift in resonance frequency to achieve a response to the odor molecules, and the sensor response was linearly correlated with the concentration of the odor molecules. The sensor array exhibited selective recognition of ten isomers with an average accuracy of 96.1%, achieving high selectivity recognition (Fig. 9d).
Subsequently, the same group used MOF membrane sensor arrays to detect mixtures of ternary xylene isomers.70 The accuracy of e-nose in distinguishing isomers of xylene mixtures with concentrations of 10 and 100 ppm was evaluated. Using machine learning algorithms to analyze the sensor array data, the composition of the mixture was determined. The accuracy of the mixture with a 10 ppm concentration was 86%, and the accuracy of the mixture with a 100 ppm concentration was 96%. When the concentration of the mixture was 100 ppm, the sensor array could recognize sixteen different xylene mixtures.
Recently, Gu et al. prepared ZnCar SURMOFs membranes with dynamic structural changes for selective recognition of six fragrance enantiomers ((+)/(−)-carvone, (+)/(−)-menthol, and (+)/(−)-limonene), mimicking the conformational changes of olfactory receptor proteins.71 ZnCar SURMOFs were prepared using ligands of L-carnosine (Car) and Zn ions via the lbl method. A chiral sensor was constructed by integrating ZnCar SURMOFs into a QCM, achieving high recognition accuracy (98.58%) for fragrance enantiomers.
Electrochemical devices are widely used for chiral sensing due to their advantages of high sensitivity, high selectivity, low cost, and good stability.72,73 Chiral MOFs can be deposited on electrodes for electrochemical chiral recognition.74 For example, Zheng et al. immobilized chiral MOF nanofibers in chitosan (CS) to prepare a MOF/CS electrochemical sensor for selective detection of tyrosine (Tyr) enantiomers.75 MOF and MOF/CS-modified glassy carbon electrodes (GCE) exhibited higher interfacial charge-transfer resistances than CS-modified GCEs, indicating that the introduction of CS improved electron transfer. Due to the synergistic effect of chiral MOF nanofibers and chiral CS, MOF/CS displayed the maximum peak current ratio, which was more sensitive for the detection of Tyr enantiomers. The concentrations of D/L-Tyr in the range of 0.007–0.055 mM were linearly correlated with the peak current of L/L-Tyr oxidation, demonstrating the quantitative detection of Tyr isomers. Compared with the initial current, the current loss after 10 days was only 8.9%, indicating that the MOF/CS electrochemical sensor exhibits good repeatability and stability.
The chiral environment, such as nanoconfined spaces, has a significant impact on chiral recognition. Xia et al. revealed the influence of the chiral environment on chiral recognition by comparing the detection of three amino acids of enantiomers (tryptophan (Trp), Ala, and leucine (Leu)) with different sizes.76 The dense chiral c-ZIF-8 layer was deposited on a gold-coated poly(ethylene terephtalate) (PET) nanochannel array using the cathodic deposition method, forming a c-ZIF-8-PET chiral membrane for electrochemical recognition. The selectivity is only found in enantiomers of Trp, among the three amino acids, as the chiral cavity size of c-ZIF-8 (8.1 Å) matched the molecular size of Trp but not Ala and Leu (Fig. 10a). After adding L-Trp, the zeta potential of c-ZIF-8 increased, while the zeta potential remained almost unchanged after adding D-Trp (Fig. 10b). This is attributed to the selective adsorption of negatively charged L-Trp molecules by the c-ZIF-8 layer, which increased the surface-charge density and led to an increase in the zeta potential. The continuous expansion of the D-Trp downward peak at 234 nm further indicated the sustained adsorption of L-Trp by c-ZIF-8 (Fig. 10c). The imidazole ring in D-Trp exhibits a steric hindrance effect, making it difficult to enter the chiral cavity of c-ZIF-8. The high adsorption capacity of the c-ZIF-8 layer for L-Trp was beneficial for the interaction between L-Trp and chiral sites, thereby facilitating chiral recognition. The study indicated the important role of the size-matching effect in chiral recognition.
 | ||
| Fig. 10 (a) Schematic of the interaction and adsorption differences between D-Trp and L-Trp with c-ZIF-8. (b) Zeta potential of the c-ZIF-8 membrane before and after the addition of D-Trp or L-Trp. (c) CD spectra of c-ZIF-8 after interaction with the Trp racemic mixture. Reproduced with permission from ref. 76. Copyright 2023, Wiley-VCH. (d) Different interactions of proteins with L-His-ZIF-8 membrane and M-L-His-ZIF-8 membrane. (e) Quenching ratio of M-His-ZIF-8 and His-ZIF-8 membranes after contact with INS, HSA, and BSA. Reproduced with permission from ref. 30. Copyright 2024, American Chemical Society. | ||
Typically, chiral MOF membranes have microporous structures in which biomolecules cannot easily enter, which makes the recognition of biomolecules challenging. To address this challenge, Cui et al. prepared a chiral MOF M-L-His-ZIF-8 membrane with hierarchical pores for detecting both small molecules and large proteins.30 The chiral MOF M-L-His-ZIF-8 membrane enables highly selective detection of phenylethanol enantiomers. In detail, the fluorescence intensity of the membrane was significantly enhanced by (R)-phenylethanol but did not change with (S)-phenylethanol. The L-His part in MOFs interacted with the hydroxyl group in phenylethanol through hydrogen bonding, and the interaction between L-His-ZIF-8 and (R)-phenylethanol was stronger than that between (S)-phenylethanol.
Furthermore, the M-CMOF membrane with macropore and micropore structures exhibited excellent chiral recognition for proteins such as human serum albumin (HSA), bovine serum albumin (BSA), and insulin (INS) with high enantioselectivity (Fig. 10d and e). The M-L-His-ZIF-8 membrane retained the integrity of the macropore structure after 3 months and could be reused at least 5 times, indicating its excellent stability and recyclability.
Wearable smart biosensors for human health monitoring were also prepared using chiral MOF membranes. Xie et al. constructed flexible MMMs with RT@CDMOF and PES for wearable sensors to detect lactic acid in sweat and monitor fatigue levels during high-intensity exercise.25 In RT@CDMOF, a dual response was achieved by encapsulation of both rhodamine 6G hydrazide (RGH) with fluorescence response and tetracyanovinylindane (TCN) with colorimetric response in γ-CDMOF. With the distinguishable color change and different affinity of L-lactic and D-lactic to MOF materials, RT@CDMOF MMMs exhibited rapid dual color sensing of lactic acid enantiomers with biocompatibility, flexibility, and mechanical stability, which was beneficial for health monitoring.
Chiral MOF membranes have been used for the recognition of various chemical enantiomers. The selective sensitivity was achieved by different interactions between the target molecules and chiral sites or structures. The various signal outputs, such as fluorescence and colorimetry, provide a diverse visual view. However, some improvements are still needed for chiral MOF membranes, e.g., recognition ability of target molecules in complex matrices and the stability of chiral MOF membranes in specific chemical or physical environments.
3.2. Separation
To avoid ineffective or even harmful effects from the enantiomer of a designed chiral material in drugs or food additives, it is significant to separate the enantiomers for a pure designed single configuration.77,78 By designing pore structures and surface chemistry, chiral MOF membranes have also been successfully used for chiral enantiomer separation.79,80Chiral alcohols are important precursors for the synthesis of chiral drugs.81 Among them, 1-phenylethanol is widely used. Specifically, S-1-phenylethanol is used for drugs to treat depression and asthma, while R-1-phenylethanol is a useful ophthalmic preservative and cholesterol adsorption inhibitor. Therefore, the separation of chiral enantiomers in 1-phenylethanol is of great significance. Wang et al. prepared an L-His-ZIF-8 membrane for the separation of racemic 1-phenylethanol (Fig. 11a).19L-His introduced a chiral environment into the MOF channel, thereby endowing the MOF with enantioselectivity. The peak shift of the membrane after permeation with 1-phenylethanol at 14 ppm and 124 ppm in solid-state nuclear magnetic resonance (NMR) indicated that the interaction between chiral molecules and MOF was very close at the molecular level to the two sites in space (Fig. 11b). Due to the different interaction of S-1-phenylethanol and R-1-phenylethanol with L-His-ZIF-8, they permeated through the membrane at a difference rate, fulfilling the enantiomeric separation with an enantiometric excess (ee) value of 76% (Fig. 11c). The membrane separated the enantiomers three times without losing selectivity, and the crystal structure of L-His-ZIF-8 remained unchanged. To further improve the separation performance, they used chiral MIL-53-NH-L-His MMMs for separating racemic 1-phenylethanol at 100% ee.44
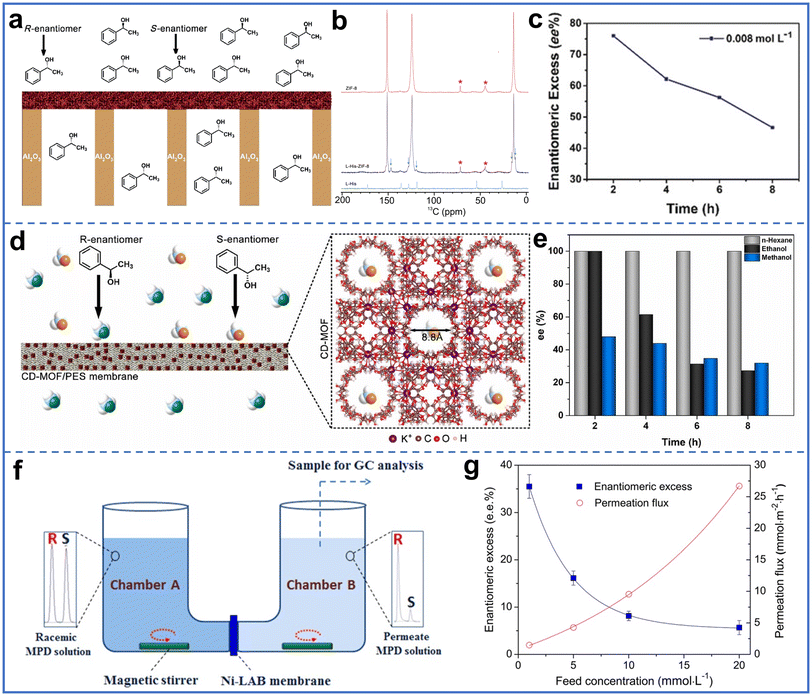 | ||
| Fig. 11 (a) Schematic of the L-His-ZIF-8 membrane separating racemic 1-phenylethanol. (b) 13C CPMAS NMR spectra of ZIF-8, L-His-ZIF-8, and L-His. (c) Enantiometric excess with time in separation of racemic 1-phenylethanol by L-His-ZIF-8 membrane. Reproduced with permission from ref. 19. Copyright 2018, Wiley-VCH. (d) Schematic of the separation of the 1-phenylethanol racemic mixture by CD-MOF/PES MMM. (e) Effect of solvents on separation of 1-phenylethanol racemates by MMMs. Reproduced with permission from ref. 53. Copyright 2021, Elsevier B.V. (f) Schematic of the separation of racemic MPD at low temperature through a Ni2(L-asp)2(bipy) membrane. (g) Enantiometric excess and permeation flux with feed concentrations at 30 °C in the separation of racemic MPD by Ni2(L-asp)2(bipy) membrane . Reproduced with permission from ref. 83. Copyright 2013, Wiley-VCH. | ||
The same group also prepared CD-MOF/PES MMMs for the separation of racemic 1-phenylethanol with 100% ee (Fig. 11d).53 In this study, the influence of solvent polarity on the separation performance was studied. The increase in solvent polarity led to a decrease in the enantioselectivity of the membrane, as polar solvents preferentially occupied chiral sites in MOFs, leading to reduced 1-phenylethanol adsorption in MOFs (Fig. 11e). In addition, chiral MOF membranes were assembled into a capillary column for the separation of racemic 1-phenylethanol. The chiral pillar-layered Cu2(D-cam)2P (D-cam was (1R,3S)-(+)-camphorate, P was 1,4-diazobicyclo[2.2.2] octane (dabco) and bipy) was grown layer by layer in a capillary column to form the membrane, which exhibited excellent separation efficiency for 1-phenylethanol.82
In addition to racemic 1-phenylethanol, 2-methyl-2,4-pentanediol (MPD), a type of dihydric alcohol, is an important intermediate in the synthesis of fine chemicals and chiral drugs. Qiu et al. prepared chiral Ni2(L-asp)2(bipy) membranes on nickel net via an in situ growth method for the separation of MPD isomer mixtures.27 Due to the interaction between S-MPD and chiral channels in MOFs, (S)-MPD was adsorbed on the active sites, whereas (R)-MPD had a higher permeability. The chiral Ni2(L-asp)2(bipy) membrane also exhibited excellent thermal stability in addition to the separation effect. Raising the separation temperature of the membrane from room temperature to 200 °C resulted in a higher permeability of R-MPD, leading to an ee value of 32.5% for racemic separation.
In the same year, Jin et al. reported the separation of MPD racemates at low temperatures using a Ni2(L-asp)2(bipy) membrane (Fig. 11f).83 Unlike the preparation method reported by Qiu et al., they decreased the crystal size of the as-synthesized Ni2(L-asp)2(bipy) crystals by ball milling and then followed by secondary growth to form the membrane. R-MPD was more easily passed through a Ni2(L-asp)2(bipy) membrane with a higher permeability than S-MPD, achieving an ee value of approximately 35.5% at 30 °C (Fig. 11g).
Chiral 2,5-hexandiol (HDO) is another important precursor of chiral drugs and agricultural chemicals. It was successfully separated by chiral MOF membranes ((±)-[{Zn2(cam)2(dabco)}n]).84 The compatible size of HDO with the pores of MOFs and the hydrogen bond with the Zn-carboxylate groups in MOFs ensured the adsorption of HDO by MOFs. The separation of R-HDO and S-HDO was achieved by their different adsorption rates on the ((±)-[{Zn2(cam)2(dabco)}n]) membranes.
Most drugs are racemate with enantiomers, and generally, only one enantiomer possesses therapeutic effects, whereas the other may be ineffective or cause side effects.85 Yan et al. reported CD-MOF MMMs for the separation of phenylalanine (Phe) enantiomers with 100% separation efficiency (Fig. 12a).86 When the load of CD-MOF in MMMs was 5%, the ee value reached the maximum (Fig. 12b). The ee was lower when the MOF loading was 3% because the CD-MOF content was too low to provide sufficient chiral recognition sites. The separation performance at CD-MOF loading levels of 7% and 9% was also lower than that at a loading level of 5% due to the aggregation of CD-MOF in the membrane, which led to the generation of defects in the membrane and decreased binding sites. The binding affinity between L-Phe and the chiral sites in CD-MOF was stronger, allowing it to adsorb in the membrane. D-Phe did not interact with the CD-MOF and preferentially passed through the membrane to achieve the chiral separation of Phe enantiomers.
 | ||
Fig. 12 (a) Schematic of the separation of Phe enantiomers by CD-MOF MMMs. (b) Enantiometric excess with time in the separation of Phe enantiomers by CD-MOF MMMs. Reproduced with permission from ref. 86. Copyright 2023, Elsevier B.V. (c) Adsorption isotherms of C3H6 (blue), C3H8 (red), CO2 (purple), CH4 (black), and N2 (yellow) at 298 K for activated CuII2(S,S)-hismox·5H2O. Experimental column breakthrough curves of (d) N2/CO2 (volume ratio was 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25), (e) CH4/CO2 (volume ratio was 75 25), (e) CH4/CO2 (volume ratio was 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25), and (f) C3H6/C3H8 (volume ratio was 50 25), and (f) C3H6/C3H8 (volume ratio was 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) gas mixtures measured at 298 K and 1 bar. Reproduced with permission from ref. 89. Copyright 2019, American Chemical Society. 50) gas mixtures measured at 298 K and 1 bar. Reproduced with permission from ref. 89. Copyright 2019, American Chemical Society. | ||
In addition, chiral MOF membranes can also be used to separate naproxen to obtain S-naproxen with pain relief and anti-inflammatory effects.23 Compared with S-naproxen, R-naproxen exhibited a stronger affinity with chiral MOF-808. When enantiomers passed through the membrane, R-naproxen underwent noncovalent interaction with chiral MOFs within the membrane, whereas S-naproxen preferentially permeated through the membrane. The molecular dynamics simulation showed almost no significant difference in the diffusion rates of the two enantiomers in MOF-808, whereas the diffusivity of S-naproxen in chiral MOF-808 was higher than that of R-naproxen. The chiral MOF-808-Ala obtained from L-Ala post-modification showed an ee value of approximately 95.0%. Due to the excellent structural stability of chiral MOF-808, the membrane exhibited good integrity after separation.
The separation of ibuprofen enantiomers is significant because S-(+)-ibuprofen is 160 times more pharmacologically active than R-(−)-ibuprofen and is more easily to be metabolized. Ben et al. prepared chiral (P)-CoMOF membranes by symmetrical breaking in ligand dimethyl pyridine-2,5-dicarboxylate and cobalt ions.87 Efficient separation of ibuprofen enantiomers (100% ee) was achieved according to the different adsorption and desorption rates of S-(+)-ibuprofen and R-(−)-ibuprofen on (P)-CoMOF membrane.
Chiral MOF membranes are also used for the separation of gas mixtures due to their differences in interactions with different gas molecules. Jin et al. prepared chiral [Ni2(mal)2(bpy)]·2H2O membrane for the selective separation of H2/CO2.88 The ideal selectivity of H2/CO2 was calculated to be 89 based on the permeability of the membrane to H2 and CO2. Due to the significant quadrupole moment and polarizability, the strong interaction between CO2 and the chiral MOF frameworks resulted in powerful adsorption onto the membrane. The lower permeability of CO2 compared to H2 led to excellent H2/CO2 separation of the chiral MOF membrane.
Pardo et al. achieved the separation of gas mixtures including natural gas, flue gas, and petrochemical industrial gas using a chiral MOF membrane.89 The chiral CuII2(S,S)-hismox·5H2O (hismox was bis[(S)-histidine]oxalyl diamide) was dispersed into poly(ether-co-amide) multiblock copolymer to prepare the MMMs as the stationary phase for the chromatographic column, achieving the separation of CO2/N2, CO2/CH4, and C3H8/C3H6 gas mixtures. The chiral MOFs possessed significant adsorption capacity for CO2, C3H8, and C3H6, but almost no adsorption capacity for CH4 and N2, indicating their potential for separating gas mixtures (Fig. 12c–f). The noninteracting channels were formed with MOFs, which preferentially passed through the membrane, consistent with the poor adsorption of CH4 and N2 by MOFs. This chiral MOF membrane enabled the separation of gas mixtures for economical or environmental values.
The separation of chiral drug precursors, drug molecules, and gas molecules was achieved by chiral MOF membranes through distinguishable absorption or desorption rates of enantiomers from the membranes. Compared to traditional chromatographic separation, chiral MOF membrane separation has the advantages of low energy consumption, easy adjustment of pore size and functional groups, and large-scale separation. However, more types of high-quality chiral MOF membranes still need to be developed for higher separation efficiency, adaptability for a broader range of enantiomers and mechanical research.
3.3. Circularly polarized luminescence
CPL refers to left-handed or right-handed circularly polarized luminescence, and it has broad application prospects in fields such as 3D displays, chiral recognition, information storage, and optoelectronic devices.90 MOF materials are ideal chiral luminophores because their specific porous structures provide multiple routes for chiral emission, including chiral units on the MOF blocks and in the pores.91Gu et al. encapsulated the lanthanide complexes Ln(acac)3 (acac was acetylacetone) into the pores of chiral MOF [Zn2(cam)2dabco]n to obtain Ln(acac)3@SURchirMOF membrane with excellent CPL property (Fig. 13a).92 The lanthanide complexes Eu(acac)3, Tb(acac)3, and Gd(acac)3 emitted primary red, green, and blue light under ultraviolet irradiation, respectively. The chiral MOFs with weak photoluminescent emission in the membrane hardly affected the photoluminescent emission of the lanthanide complexes. The chirality of the MOFs was maintained after the encapsulation of Ln(acac)3, as indicated by the same circular dichroism (CD) signals (Fig. 13c). The Ln(acac)3@SURchirMOF membrane exhibited a photoluminescent emission similar to that of Ln(acac)3, thereby achieving the tunable CPL of the membrane (Fig. 13d). The increase in the fluorescent lifetime of chiral MOFs in the membrane and the decrease in fluorescent lifetime of lanthanide complexes indicated energy transfer from Ln(acac)3 to chiral MOFs, where Ln(acac)3 acted as the donor and chiral MOFs acted as the acceptor. The encapsulation of lanthanide complexes in chiral MOF pores was crucial for achieving CPL property, as the coordination or intermolecular interactions between acac in the complex and Zn nodes in the MOF resulted in efficient energy transfer (Fig. 13b).
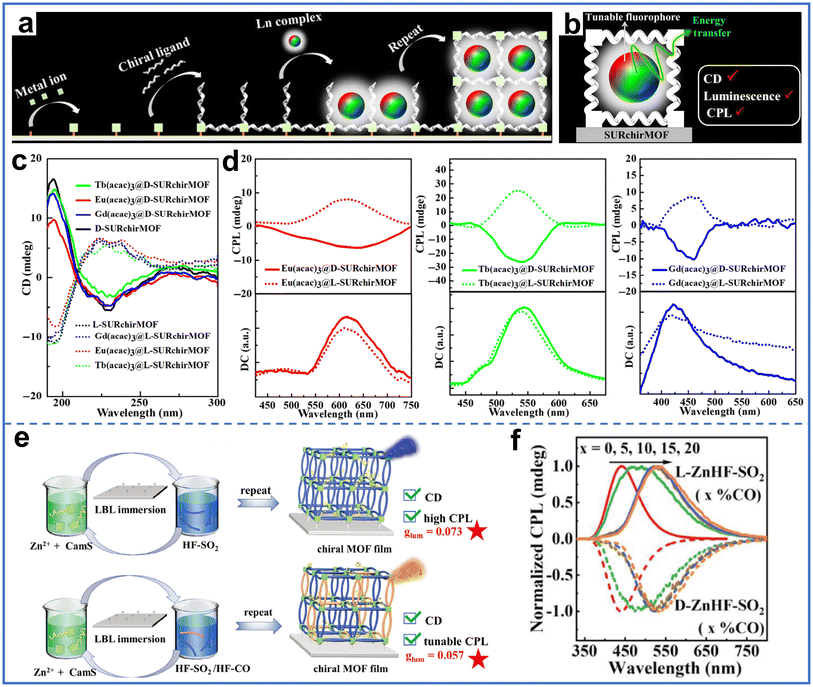 | ||
| Fig. 13 (a) Schematic of the preparation of Ln(acac)3@SURchirMOF membrane by encapsulating the Ln complex into chiral MOF pores. (b) Schematic of energy transfer in the host–guest membrane of Ln(acac)3@SURchirMOFs. (c) CD spectra of Ln(acac)3@SURchirMOFs and chiral MOFs. (d) CPL spectra of Ln(acac)3@SURchirMOFs. Reproduced with permission from ref. 92. Copyright 2022, Springer Nature. (e) Preparation of D/L-ZnHF-SO2 membranes and D/L-ZnHF-SO2/COmembranes by lbl chiral induction method. (f) Normalized CPL spectra of D/L-ZnHF-SO2 (x% CO represented the proportion of HF-CO ligands) membranes. Reproduced with permission from ref. 94. Copyright 2024, Wiley-VCH. | ||
Another method to endow MOF membranes with CPL properties is to encapsulate chiral molecules into luminescent MOF membranes. Zhang's group prepared luminescent MOF membranes using the lbl spraying method and then loaded chiral molecules into the pores of MOFs to endow the membrane with CPL properties.93 The molecule 4,4′-(1,2-diphenylethene-1,2-diyl) dibenzoic acid (TPE) with aggregation-induced emission was used as the ligand to self-assemble with Zn ions via the lbl method, resulting in a ZnTPE membrane with photoluminescence. Then, chiral dibenzoyl-tartaric acid was loaded into the pores of ZnTPE via a post modification method. The fluorescence emission of the MOF membrane loaded with chiral molecules maintained strong fluorescence with a shift from 495 to 478 nm. The membrane exhibited a uniform CPL signal due to the π–π conjugation effect between the benzene ring of the loaded chiral molecule and ZnTPE.
In addition, the same group also prepared chiral D/L-ZnHF-SO2 (HF-SO2 was 3,7-dibenzothiophenedicarboxylate, 5,5-dioxide) membranes using camphorsulfonic acid as a chiral inductor via the lbl chiral induction method (Fig. 13e).94 The ZnHF-SO2 membranes exhibited strong CPL properties, with the CPL signal appearing at 440 nm, consistent with the fluorescent emission of the membranes. The 9-fluororenone-2,7-dicarboxylate (HF-CO) molecule has a similar structure to the HF-SO2 ligand in MOFs and can therefore be introduced as a mixed ligand to prepare photoluminescent MOFs. By adjusting the proportion of ligands, CPL colors in the MOF membranes could be modulated (Fig. 13f). The membranes with different ligand ratios exhibited CPL signals at different positions due to energy transfer between HF-SO2 ligands and Zn ions and between Zn ions and HF-CO ligands.
Furthermore, Zhang's group crosslinked CLC with MOFs to prepare a CLCMOF membrane for achieving CPL performance in photo-thermal switching, which was used for information anticounterfeiting and encryption.58 CLC was a thermally induced liquid crystal that gradually changed from an amorphous solid-like state to a transparent liquid state with increasing temperature, and the transmittance of the membrane also increased with increasing temperature. The UV–vis spectra of the CLCMOF membrane are consistent with the MOF membrane loaded with CLC without cycloaddition reaction, indicating that the CLCMOF membrane retained photochromic properties. The S-LCMOF membrane selectively reflected right-handed circularly polarized light and transmitted left-handed circularly polarized light, while the R-LCMOF membrane was the opposite. When the membrane was heated at 140 °C and irradiated with UV, the CPL changed from yellow to blue fluorescence. Since the photoluminescence and CPL signals of the CLCMOF membrane were regulated through irradiation and heating, molecular logic gates were designed for Morse code information encryption.
Chiral MOF membranes have been proven an ideal platform for CPL development due to their diverse structures and helical channels. Long-range periodic stacking in chiral MOF membranes is advantageous for the improvement of photoluminescence quantum yield and asymmetric factor. In addition, guest luminescent materials with different wavelengths are easy to be encapsulated into chiral MOFs for colorful CPLs. In the future, organic ligands or metal ions with specific chiral centers and coordination geometries are expected to be introduced for more efficient chiral transfer and CPL.
Table 1 summarizes MOF materials and matrices used for chiral MOF membranes, as well as their applications.
| MOF materials | Matrices | Applications | |
|---|---|---|---|
| Pure chiral MOF membranes | Ni2(L-asp)2(bipy) | Separation of racemic MPD27 | |
| L-His-ZIF-8 | Separation of racemic 1-phenylethanol19 | ||
| MOF-808-Ala, MOF-808-Thr, MOF-808-His | Separation of racemic naproxen enantiomers23 | ||
| Cu2(DCam)2(AzoBiPyB) | Enantiopure uptakes of racemic phenylethanol28 | ||
| γCD-SURMOF | Recognition and separation of enantiomeric tripeptide pair29 | ||
| His-ZIF-8, [Cd(LTP)2]n, [Cu(mal)bpy]·H2O | Recognition of racemic phenylethanol, HAS, BSA, or INS30 | ||
| Meso-L-CuMOF | Recognition of monosaccharide enantiomers64 | ||
| Cu2(DCam)2(dabco), Cu2(DCam)2(BiPy), Cu2(DCam)2(BiPyB), HKUST-1, Cu(BDC), Cu(BPDC) | Recognition of limonene, 2-octanol, 1-phenylethanol, 1-phenylethylamine, or methyl lactate enantiomers69 | ||
| c-ZIF-8 | Detection of Trp, Ala, and Leu enantiomers76 | ||
| Cu2(D-cam)2P | Separation of racemic 1-phenylethanol82 | ||
| Ni2(L-asp)2(bipy) | Separation of racemic MPD at low temperatures83 | ||
| [Ni2(mal)2(bpy)]·2H2O | Separation of H2/CO288 | ||
| Ln(acac)3@SURchirMOF | CPL performance92 | ||
| ZnTPE | CPL luminescence performance93 | ||
| D/L-ZnHF-SO2 | CPL properties94 | ||
| MMMs | RT@CDMOF | PES | Recognition of lactic acid enantiomers25 |
| MIL-53-NH-L-His | PES | Separation of racemic 1-phenylethanol44 | |
| γ-CD-MOF | PES | Separation of racemic 1-phenylethanol53 | |
| Zn-BLD | HDPE and UHMWPE | Separation of racemic methyl phenyl sulfoxide55 | |
| Zn2(sdc)2 | CLC | Information encryption of Morse code58 | |
| D-Met@MOF | MPN | Anti-biofouling efficacy59 | |
| [Cu2((+)-Cam)2Dabco]n, [Cu2((+)-Cam)2Bipy]n | Poly(dopamine) and polycarbonate trail etched membrane | Separation of racemic 1-phenylethanol62 | |
| 1-MONs | PES | Sensing of terpenes and terpenoids38 | |
| L-His&R6G@ZIF-8 | PVDF | Sensing of proline enantiomers54 | |
| MOF nanofibers | CS | Electrochemical sensing of Tyr isomers75 | |
| Eu(BTC)(H2O)·DMF | PIM-1 | Separation of 2-amino-1-butanol enantiomers79 | |
| CD-MOF | PVDF | Separation of Phe enantiomers86 | |
| CuII2(S,S)-hismox·5H2O | Poly(ether-co-amide) multiblock copolymer | Separation of CO2/N2, CO2/CH4, and C3H8/C3H689 |
4. Conclusions and outlook
Chiral MOF membranes show great potential for chiral recognition or separation and chiral emission due to their porous structure and simplicity of functionalization. In this review, we first summarized the various methods for synthesizing chiral MOF membranes, including direct methods for preparing pure chiral MOF membranes (e.g. solvothermal synthesis, lbl synthesis, and template-assisted synthesis) and indirect methods for fabricating chiral MOF MMMs (e.g. solution casting and TIPS-HoP). Pure chiral MOF membranes contain a high density of functional sites, whereas MMMs exhibit excellent flexibility and stability.Second, we summarized the applications of chiral MOF membranes in chiral sensing, separation, and CPL. Chiral MOF membranes have been used in chiral sensing and separation of various molecules with high detectivity and selectivity. Membranes that combine photoluminescent units with chiral MOFs, or chiral molecules with luminescent MOFs, exhibit tunable CPL, indicating potential applications in imaging, optoelectronic devices, information anticounterfeiting and encryption.
However, the successful applications of chiral MOF membranes are still limited, which can be attributed to the challenges associated with the fabrication of high-quality membranes despite the abundance of chiral MOF materials. To improve the quality of chiral MOF membranes, research efforts can be directed towards the following areas.
From the perspective of MOF materials, the exploration of functional groups, such as groups with chirality or catalytic sites, is important to broaden their applications. In addition, stable ligands and metal ions should be selected to improve the stability of chiral MOFs. Furthermore, synthesis methods and conditions, such as the metal-ion-to-organic-ligand ratio, should be creatively modified to optimize the size of the MOF particles.
From the perspective of membranes, the interface between MOF particles and the matrix in MMMs is a key concern. To produce high-quality membranes (defect-free, uniform, and dense), the MOF materials must be compatible with the polymer matrix. Thus, the functional groups on organic ligands in chiral MOFs and matrices should be carefully selected to promote covalent or coordination interactions. In addition, the introduction of specific functional groups, such as hydrophobic groups and corrosion-resistant groups, should be considered to enhance water resistance and chemical stability. Furthermore, membrane preparation techniques, such as electrochemical deposition and microwave-assisted methods, should be broadened to develop better and multifunctional membranes.
Chiral MOF membranes, as emerging functional materials, have demonstrated excellent performance in chiral recognition and luminescence. With further improvements in quality and functional investigations, chiral MOF membranes are expected to play a more important role in these fields.
Author contributions
Yun Fan conceived and wrote the manuscript. Mengyun Chen conceptualized and revised the review.Data availability
No primary research results, software or code has been included, and no new data were generated or analyzed as part of this review.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China 22205100.References
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- G. Lu, S. Li, Z. Guo, O. K. Farha, B. G. Hauser, X. Qi, Y. Wang, X. Wang, S. Han, X. Liu, J. S. DuChene, H. Zhang, Q. Zhang, X. Chen, J. Ma, S. C. J. Loo, W. D. Wei, Y. Yang, J. T. Hupp and F. Huo, Nat. Chem., 2012, 4, 310–316 CrossRef CAS PubMed.
- J. Guo, Y. Qin, Y. Zhu, X. Zhang, C. Long, M. Zhao and Z. Tang, Chem. Soc. Rev., 2021, 50, 5366–5396 RSC.
- Q. Zhou, Q. Ding, Z. Geng, C. Hu, L. Yang, Z. Kan, B. Dong, M. Won, H. Song, L. Xu and J. S. Kim, Nano-Micro Lett., 2025, 17, 50 CrossRef CAS PubMed.
- J. He, X. Wen, L. Wu, H. Chen, J. Hu and X. Hou, TrAC, Trends Anal. Chem., 2022, 156, 116715 CrossRef CAS.
- Z. Huang, X. Shen, Y. Wei, J. W. Chew, E. H. Ang and M. Pan, Mater. Horiz., 2024, 11, 6098–6106 RSC.
- Y. Wang, N. Wu, Y. Wang, H. Ma, J. Zhang, L. Xu, M. K. Albolkany and B. Liu, Nat. Commun., 2019, 10, 2500 CrossRef PubMed.
- Z. Liu, J. Ai, T. Bai, Y. Fang, K. Ding, Y. Duan, L. Han and S. Che, Chem, 2022, 8, 186–196 CAS.
- J. E. Rekoske, AIChE J., 2001, 47, 2–5 CrossRef CAS.
- D. S. Bradshaw, J. M. Leeder, M. M. Coles and D. L. Andrews, Chem. Phys. Lett., 2015, 626, 106–110 CrossRef CAS.
- X. Su, J. Sun, J. Liu, Y. Wang, J. Wang, W. Tang and J. Gong, Angew. Chem., Int. Ed., 2024, 63, e202402886 CrossRef CAS PubMed.
- N. Chhabra, M. L. Aseri and D. Padmanabhan, Int. J. Appl. Basic Med. Res., 2013, 3, 16–18 CrossRef PubMed.
- C. D. Wu, A. Hu, L. Zhang and W. B. Lin, J. Am. Chem. Soc., 2005, 127, 8940–8941 CrossRef CAS PubMed.
- Z. Sharifzadeh, S. A. A. Razavi and A. Morsali, Green Chem., 2023, 25, 8661–8678 RSC.
- M. Zhang, Z.-J. Pu, X.-L. Chen, X.-L. Gong, A.-X. Zhu and L.-M. Yuan, Chem. Commun., 2013, 49, 5201–5203 RSC.
- Z. Gu, Y. Luo, X. Zhang, Z. Zhu, Y. Wang, T. Tang, S. Zhang and W. Zhang, TrAC, Trends Anal. Chem., 2024, 179, 117864 CrossRef CAS.
- H.-J. Choi and D.-Y. Koh, Membranes, 2022, 12, 357 CrossRef CAS PubMed.
- T. Duerinck and J. F. M. Denayer, Chem. Eng. Sci., 2015, 124, 179–187 CrossRef CAS.
- J. Y. Chan, H. Zhang, Y. Nolvachai, Y. Hu, H. Zhu, M. Forsyth, Q. Gu, D. E. Hoke, X. Zhang, P. J. Marriot and H. Wang, Angew. Chem., Int. Ed., 2018, 57, 17130–17134 CrossRef CAS PubMed.
- J. Liang, Y. Song, H. Xing, L. Ma, F. Wang, M. Zhang, H. Zhang, G. Zou and G. Yang, Nanoscale, 2024, 16, 22011–22020 RSC.
- Y.-H. Xiao, Z.-Z. Ma, X.-X. Yang, D.-S. Li, Z.-G. Gu and J. Zhang, ACS Nano, 2023, 17, 19136–19143 CrossRef CAS PubMed.
- W. Wang, X. Dong, J. Nan, W. Jin, Z. Hu, Y. Chen and J. Jiang, Chem. Commun., 2012, 48, 7022–7024 RSC.
- T. Chen, H. Li, X. Shi, J. Imbrogno and D. Zhao, J. Am. Chem. Soc., 2024, 146, 14433–14438 CrossRef CAS PubMed.
- H.-J. Choi, Y.-H. Ahn and D.-Y. Koh, Membranes, 2021, 11, 279 CrossRef CAS PubMed.
- X.-L. Yang, Z.-Y. Yang, R. Shao, R.-F. Guan, S.-L. Dong and M.-H. Xie, Adv. Mater., 2023, 35, 2304046 CrossRef CAS PubMed.
- J. He, J. He, L. Tang, Y. Xia, J. Zhou, X. Jiang and X. Hou, TrAC, Trends Anal. Chem., 2024, 175, 117709 CrossRef CAS.
- Z. Kang, M. Xue, L. Fan, J. Ding, L. Guo, L. Gao and S. Qiu, Chem. Commun., 2013, 49, 10569–10571 RSC.
- A. B. Kanj, J. Buerck, S. Grosjean, S. Braese and L. Heinke, Chem. Commun., 2019, 55, 8776–8779 RSC.
- L.-M. Chang, Q.-h. Li, P. Weidler, Z.-G. Gu, C. Wöll and J. Zhang, CCS Chem., 2022, 4, 3472–3481 CrossRef CAS.
- J. Yang, Q. Song, T. Zhang, Y. Yan, C. Yuan, Y. Cui and X. Hou, Anal. Chem., 2024, 96, 17280–17289 CrossRef CAS PubMed.
- X. Liang, F. Zhang, W. Feng, X. Zou, C. Zhao, H. Na, C. Liu, F. Sun and G. Zhu, Chem. Sci., 2013, 4, 983–992 RSC.
- L. A. Hall, D. M. D'Alessandro and G. Lakhwani, Chem. Soc. Rev., 2023, 52, 3567–3590 RSC.
- M. Ma, J. Chen, H. Liu, Z. Huang, F. Huang, Q. Li and Y. Xu, Nanoscale, 2022, 14, 13405–13427 RSC.
- Z. F. Chen, J. Zhang, R. G. Xiong and X. Z. You, Inorg. Chem. Commun., 2000, 3, 493–496 CrossRef CAS.
- N. Corella-Ochoa, J. B. Tapia, H. N. Rubin, V. Lillo, J. González-Cobos, J. Luis Núñez-Rico, S. R. G. Balestra, N. Almora-Barrios, M. Lledós, A. Güell-Bara, J. Cabezas-Giménez, E. C. Escudero-Adán, A. Vidal-Ferran, S. Calero, M. Reynolds, C. Martí-Gastaldo and J. Ramón Galán-Mascarós, J. Am. Chem. Soc., 2019, 141, 14306–14316 CrossRef PubMed.
- M. P. Yutkin, M. S. Zavakhina, D. G. Samsonenko, D. N. Dybtsev and V. P. Fedin, Inorg. Chim. Acta, 2013, 394, 367–372 CrossRef CAS.
- K. Tanaka, D. Yanamoto, K. Yoshimura, T. Anami and Z. Urbanczyk-Lipkowska, CrystEngComm, 2015, 17, 1291–1295 RSC.
- Y. Liu, L. Liu, X. Chen, Y. Liu, Y. Han and Y. Cui, J. Am. Chem. Soc., 2021, 143, 3509–3518 CrossRef CAS PubMed.
- K. Berijani and A. Morsali, J. Catal., 2019, 378, 28–35 CrossRef CAS.
- P. Cui, P. Wang, Y. Zhao and W.-Y. Sun, Cryst. Growth Des., 2019, 19, 1454–1470 CrossRef CAS.
- Z. Han, K. Wang, Y. Guo, W. Chen, J. Zhang, X. Zhang, G. Siligardi, S. Yang, Z. Zhou, P. Sun, W. Shi and P. Cheng, Nat. Commun., 2019, 10, 5117 CrossRef PubMed.
- W.-T. Kou, C.-X. Yang and X.-P. Yan, J. Mater. Chem. A, 2018, 6, 17861–17866 RSC.
- G. Yang, W. Shi, Y. Qian, X. Zheng, Z. Meng and H.-L. Jiang, Angew. Chem., Int. Ed., 2023, 62, e202308089 CrossRef CAS PubMed.
- Y. Lu, H. Zhang, J. Y. Chan, R. Ou, H. Zhu, M. Forsyth, E. M. Marijanovic, C. M. Doherty, P. J. Marriott, M. M. B. Holl and H. Wang, Angew. Chem., Int. Ed., 2019, 58, 16928–16935 CrossRef CAS PubMed.
- C. Tan, X. Han, Z. Li, Y. Liu and Y. Cui, J. Am. Chem. Soc., 2018, 140, 16229–16236 CrossRef CAS PubMed.
- B. Vilhanová, M. Ranocchiari and J. A. van Bokhoven, ChemCatChem, 2016, 8, 308–312 CrossRef.
- A. B. Kanj, J. Buerck, N. Vankova, C. Li, D. Mutruc, A. Chandresh, S. Hecht, T. Heine and L. Heinke, J. Am. Chem. Soc., 2021, 143, 7059–7068 CrossRef CAS PubMed.
- J.-S. Feng, M. Ren, Z.-S. Cai, K. Fan, S.-S. Bao and L.-M. Zheng, Chem. Commun., 2016, 52, 6877–6880 RSC.
- T. Yamada, T. Eguchi, T. Wakiyama, T. Narushima, H. Okamoto and N. Kimizuka, Chem. – Eur. J., 2019, 25, 6698–6702 CrossRef PubMed.
- S.-T. Wu, Z.-W. Cai, Q.-Y. Ye, C.-H. Weng, X.-H. Huang, X.-L. Hu, C.-C. Huang and N.-F. Zhuang, Angew. Chem., Int. Ed., 2014, 53, 12860–12864 CrossRef CAS PubMed.
- J. Zhang, S. Chen, R. A. Nieto, T. Wu, P. Feng and X. Bu, Angew. Chem., Int. Ed., 2010, 49, 1267–1270 CrossRef CAS PubMed.
- D. Wu, K. Zhou, J. Tian, C. Liu, J. Tian, F. Jiang, D. Yuan, J. Zhang, Q. Chen and M. Hong, Angew. Chem., Int. Ed., 2021, 60, 3087–3094 CrossRef CAS PubMed.
- Y. Lu, J. Y. Chan, H. Zhang, X. Li, Y. Nolvachai, P. J. Marriott, X. Zhang, G. P. Simon, M. M. B. Holl and H. Wang, J. Membr. Sci., 2021, 620, 118956 CrossRef CAS.
- Y.-H. Geng, Y. Xin, J. Du, M.-Y. Cui, Y.-Y. Liu, L.-X. Zhang and B. Ding, Spectrochim. Acta, Part A, 2024, 305, 123468 CrossRef CAS PubMed.
- H. Wang, S. Zhao, Y. Liu, R. Yao, X. Wang, Y. Cao, D. Ma, M. Zou, A. Cao, X. Feng and B. Wang, Nat. Commun., 2019, 10, 4204 CrossRef PubMed.
- B. D. Freeman, Macromolecules, 1999, 32, 375–380 CrossRef CAS.
- L. M. Robeson, J. Membr. Sci., 2008, 320, 390–400 CrossRef CAS.
- X.-X. Yang, N. Li, C. Li, Z.-B. Jin, Z.-Z. Ma, Z.-G. Gu and J. Zhang, J. Am. Chem. Soc., 2024, 146, 16213–16221 CrossRef CAS PubMed.
- Z. Yu, X. Li, Z. Wang, Y. Fan, W. Zhao, D. Li, D. Xu, T. Gu and F. Wang, Adv. Mater., 2024, 36, 2407409 CrossRef CAS PubMed.
- C. Li and L. Heinke, Symmetry, 2020, 12, 686 CrossRef CAS.
- X. Niu, M. Yuan, X. Yang, H. Li, R. Zhao, Y. Liu, H. Zhao and K. Wang, ACS Appl. Nano Mater., 2024, 7, 19175–19183 CrossRef CAS.
- M. Chen, X. Li, J. Li, C. Liu, A. Yu and S. Zhang, Sep. Purif. Technol., 2024, 331, 125704 CrossRef CAS.
- L. Geng, Y. Qiao, R. Sun, L. Guo, Z.-Q. Li, Y. Ma, M.-H. Yu, Z. Chang and X.-H. Bu, Adv. Mater., 2025, 37, 2415511 CrossRef CAS PubMed.
- J. Guo, X. Liu, J. Zhao, H. Xu, Z. Gao, Z.-Q. Wu and Y.-Y. Song, Chem. Sci., 2023, 14, 1742–1751 RSC.
- X. Niu, R. Zhao, Y. Liu, M. Yuan, H. Zhao, H. Li, X. Yang, H. Xu and K. Wang, J. Mater. Chem. A, 2023, 11, 23376–23386 RSC.
- J.-Y. Liu, Y.-H. Geng, T.-T. Wang, B. Ding, Y.-H. Qiao, J.-Z. Huo and B. Ding, ACS Appl. Nano Mater., 2023, 6, 398–409 CrossRef CAS.
- Y.-W. Zhao, L.-E. Guo, F.-Q. Zhang, J. Yao and X.-M. Zhang, ACS Appl. Mater. Interfaces, 2021, 13, 20821–20829 CrossRef CAS PubMed.
- Y. Zhang, Y. Wang, Y. Dai, X. Bai, X. Hu, L. Du, H. Hu, X. Yang, D. Li, Q. Dai, T. Hasan and Z. Sun, Sci. Adv., 2022, 8, eabq8246 CrossRef CAS PubMed.
- S. Okur, P. Qin, A. Chandresh, C. Li, Z. Zhang, U. Lemmer and L. Heinke, Angew. Chem., Int. Ed., 2021, 60, 3566–3571 CrossRef CAS PubMed.
- P. Qin, B. A. Day, S. Okur, C. Li, A. Chandresh, C. E. Wilmer and L. Heinke, ACS Sens., 2022, 7, 1666–1675 CrossRef CAS PubMed.
- N. Li, J.-B. Zhang, C. Woell, Z.-G. Gu and J. Zhang, Adv. Funct. Mater., 2025, 2422860 CrossRef.
- L. Lan, X. Kuang, X. Sun, Q. Wei and R. Kuang, Anal. Chem., 2023, 95, 18295–18302 CrossRef CAS PubMed.
- X. Niu, S. Yan, R. Zhao, H. Li, X. Liu and K. Wang, ACS Appl. Mater. Interfaces, 2023, 15, 22435–22444 CrossRef CAS PubMed.
- X. Niu, Y. Liu, R. Zhao, M. Yuan, H. Zhao, H. Li and K. Wang, ACS Appl. Mater. Interfaces, 2024, 16, 17361–17370 CrossRef CAS PubMed.
- L. Tong, X. Kuang, Q. Duan and X. Zheng, Starke, 2021, 73, 2100112 CrossRef CAS.
- M.-Y. Wu, R.-J. Mo, X.-L. Ding, L.-Q. Huang, Z.-Q. Li and X.-H. Xia, Small, 2023, 19, 2301460 CrossRef CAS PubMed.
- F. Wang, K. He, R. Wang, H. Ma, P. J. Marriott, M. R. Hill, G. P. Simon, M. M. B. Holl and H. Wang, Adv. Mater., 2024, 36, 2400709 CrossRef CAS PubMed.
- G. Sun, Y. Luo, Z. Yan, H. Qiu and W. Tang, Chin. Chem. Lett., 2024, 35, 109787 CrossRef CAS.
- S. Das, S. Xu, T. Ben and S. Qiu, Angew. Chem., Int. Ed., 2018, 57, 8629–8633 CrossRef CAS PubMed.
- Y. Duan, L. Li, Z. Shen, J. Cheng and K. He, Membranes, 2023, 13, 480 CrossRef CAS PubMed.
- Z.-J. Li, J. Yao, Q. Tao, L. Jiang and T.-B. Lu, Inorg. Chem., 2013, 52, 11694–11696 CrossRef CAS PubMed.
- Z.-G. Gu, W.-Q. Fu, X. Wu and J. Zhang, Chem. Commun., 2016, 52, 772–775 RSC.
- K. Huang, X. Dong, R. Ren and W. Jin, AIChE J., 2013, 59, 4364–4372 CrossRef CAS.
- B. Liu, O. Shekhah, H. K. Arslan, J. Liu, C. Woell and R. A. Fischer, Angew. Chem., Int. Ed., 2012, 51, 807–810 CrossRef CAS PubMed.
- F. Zhang, L. He, W. Sun, Y. Cheng, J. Liu and Z. Ren, RSC Adv., 2015, 5, 41729–41735 RSC.
- Q. Ye, J. Li, Y. Huang, H. Wu, Y. Li and B. Yan, J. Environ. Chem. Eng., 2023, 11, 109250 CrossRef CAS.
- B. Li, Y. Feng, D. Zhou, M. Yang, D. Li, S. Zhang, J. Fu and T. Ben, J. Mater. Chem. A, 2025, 13, 8375–8384 RSC.
- Q. Li, G. Liu, K. Huang, J. Duan and W. Jin, Asia-Pac. J. Chem. Eng., 2016, 11, 60–69 CrossRef CAS.
- M. Mon, R. Bruno, E. Tiburcio, A. Grau-Atienza, A. Sepúlveda-Escribano, E. V. Ramos-Fernandez, A. Fuoco, E. Esposito, M. Monteleone, J. C. Jansen, J. Cano, J. Ferrando-Soria, D. Armentano and E. Pardo, Chem. Mater., 2019, 31, 5856–5866 CrossRef CAS.
- Y. Zhou, Y. Wang, Y. Song, S. Zhao, M. Zhang, G. Li, Q. Guo, Z. Tong, Z. Li, S. Jin, H.-B. Yao, M. Zhu and T. Zhuang, Nat. Commun., 2024, 15, 251 CrossRef CAS PubMed.
- C. Li, H. Schopmans, L. Langer, S. Marschner, A. Chandresh, J. Buerck, Y. Tsuchiya, A. Chihaya, W. Wenzel, S. Braese, M. Kozlowska and L. Heinke, Angew. Chem., Int. Ed., 2023, 62, e202217377 CrossRef CAS PubMed.
- R. Zhai, Y. Xiao, Z. Gu and J. Zhang, Nano Res., 2022, 15, 1102–1108 CrossRef CAS.
- X.-X. Yang, C. Li, S.-M. Chen, Z.-G. Gu and J. Zhang, Chem. – Eur. J., 2024, 30, e202400350 CrossRef CAS PubMed.
- C. Li, X.-X. Yang, M.-Y. Zheng, Z.-G. Gu and J. Zhang, Adv. Funct. Mater., 2024, 34, 2401102 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |


