Open Access Article
Open Access ArticleDesign and synthesis of a deep-cavity aluminium-organic macrocycle to trap dyes and generate enhanced non-linear optical performance†
Zhuang-Hua
Liu
a,
Si-Hao
Shen
a,
Cheng-Yang
Zhang
a,
Jingyang
Niu
 b,
Qiao-Hong
Li
b,
Qiao-Hong
Li
 *a,
Jian
Zhang
*a,
Jian
Zhang
 a and
Wei-Hui
Fang
a and
Wei-Hui
Fang
 *a
*a
aState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, 350002 Fuzhou, P.R. China. E-mail: lqh2382@fjirsm.ac.cn; fwh@fjirsm.ac.cn
bHenan Key Laboratory of Polyoxometalate, College of Chemistry and Molecular Science, Henan University, 475004 Kaifeng, P.R. China
First published on 14th May 2024
Abstract
The development of a “two birds with one stone” strategy for capturing pollutant molecules and incorporating new functions provides a promising solution for sustainability. In this work, we designed an unprecedented deep-cavity aluminum–organic macrocycle to trap dye molecules and enhance non-linear optical performance. Using long building blocks and inorganic aluminum ions at the midriff, we successfully isolated a deep-cavity (1.8 nm) macrocycle, with a deeper cavity than classic pure organic macrocycles, such as crown ether and calixarenes. We report the accurate locking of the HAO7 dye molecule in the deep-cavity macrocycle and reveal its trapping mechanism at the molecular level for the first time. The combined host–guest compound AlOC-136-HAO7 displays altered physical properties, such as a decreased optical band gap and increased proton conductivity but also exhibits enhanced third-order non-linear optical (NLO) properties. Combined with theoretical calculations, we confirmed that the enhancement was attributed to abundant host–guest interactions and the guest-to-guest charge transfer. Our findings provide a strategy for isolating deep-cavity macrocycles and further demonstrate their enormous potential for capturing contaminants and forming valuable materials.
Introduction
In recent years, water contamination with dyes from industries has become a global concern.1 This is not only due to vast amounts discharged (global emissions are nearly 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons per year) but also because of the toxicity2 and resistance to biodegradation (i.e., the half-life of hydrolyzed reactive blue 19 is about 46 years) of dyes.3 Particular attention should be given to azo dyes4,5 because they are the most commonly used chromophore and account for up to 70% of all produced textiles. Compared with chemical methods,6–9 physical adsorption is generally considered a more efficient, low-cost, and environmentally friendly method for removing dyes from wastewater.10–12 Ideally, adsorbents should be designed to utilize these trapped or adsorbed dyes to realize “Waste to Wealth”. According to some recent research, such an idea is not a castle in the sky. It has been reported that confining conjugated photonic active dye molecules in metal–organic frameworks with regular internal cavities could help amplify their function and minimize aggregation-caused quenching effects.13,14
000 tons per year) but also because of the toxicity2 and resistance to biodegradation (i.e., the half-life of hydrolyzed reactive blue 19 is about 46 years) of dyes.3 Particular attention should be given to azo dyes4,5 because they are the most commonly used chromophore and account for up to 70% of all produced textiles. Compared with chemical methods,6–9 physical adsorption is generally considered a more efficient, low-cost, and environmentally friendly method for removing dyes from wastewater.10–12 Ideally, adsorbents should be designed to utilize these trapped or adsorbed dyes to realize “Waste to Wealth”. According to some recent research, such an idea is not a castle in the sky. It has been reported that confining conjugated photonic active dye molecules in metal–organic frameworks with regular internal cavities could help amplify their function and minimize aggregation-caused quenching effects.13,14
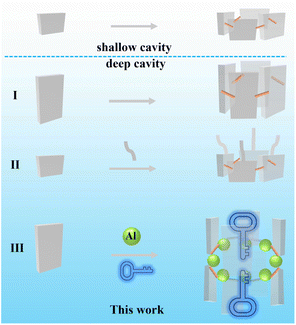
The first step in turning “Waste to Wealth” is to design periodic shape-matched cavities that can simultaneously be beneficial for investigating the adsorption mechanism. In terms of crystalline materials, macrocycles, famous for their central cavities for guest inclusion, are promising candidates. Recent research on macrocycle-dye complexes has been carried out in aqueous solution,15 while their unambiguous host–guest interaction in the solid state is relatively challenging because the specific structures of shallow cavity macrocycles interact very weakly with the dyes. Therefore, one solution is constructing a cavity that is deep enough to bury the dyes. The next step that we should consider is how to transform host–guest compounds into functional materials. Despite extensive study and advances in large-cavity macrocycles,16–19 those with deep and stable cavities are far from abundant.
Based on above presented considerations and our recent extensive research regarding aluminium molecular rings,20–24 we are particularly interested in designing deep-cavity macrocycles to realize “Waste to Wealth”. Although we have developed efficient synthetic strategies for the isolation of aluminum molecular rings, most of these are shallow molecular rings lacking a sufficient buried surface area to encapsulate large-sized guest molecules. Inspired by the two methods that are currently commonly used, self-assembly cyclization by long building blocks25,26 and postmodification27–29 (extending the cavity depth of an existing macrocycle), we herein aim to create a deep-cavity inorganic–organic macrocycle by employing long building blocks and inorganic aluminum ions at the midriff (Scheme 1). Considering the linear and aromatic characteristics of dye molecules, we chose the long linear ligand indole-2-carboxylic acid (HL 7.3 Å) to simultaneously act as pillars for constructing deep cavities and bring potential photo-related applications. The choice of azo dyes as the target is due to their large proportion in production applications and their enormous potential as non-linear optical (NLO) materials due to red-shifted absorption maxima, large dipole moments, and good thermal stability.30,31
Thus, we designed and synthesized a macrocycle with a deep cavity depth of approximately 2 nm to lock Acid Orange 7 (NaAO7) molecules in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio and simultaneously enhance optical performance. It is worth noting that the host–guest interactions are unambiguously revealed by single-crystal X-ray diffraction. Such a “lock and key” host–guest complex possesses a low band gap and notably better third-order non-linear optical (NLO) effect than those of separate pristine component materials and physically doped materials.
2 ratio and simultaneously enhance optical performance. It is worth noting that the host–guest interactions are unambiguously revealed by single-crystal X-ray diffraction. Such a “lock and key” host–guest complex possesses a low band gap and notably better third-order non-linear optical (NLO) effect than those of separate pristine component materials and physically doped materials.
Results and discussion
Assembly of deep-cavity aluminum–organic macrocycles and structural characterization
First, we reviewed the aluminium–organic molecular rings synthesized in recent years. We found a clear trend, whereby the macrocycles with alcohol ligands are shallow and those containing only one type of aromatic carboxylic acid ligand have a large dihedral angle (between the ligand and aluminum inorganic ring plane) (Fig. 1a) and deeper cavity. Our literature investigation of all metal–organic ring molecules showed that although some also have large dihedral angles,32–36 most of them are fatty carboxylic acid ligands37–39 and have not formed regular deep cavities.40–42 First, under the regulation of alcohol solvents, despite employing long ligands, we consistently obtained similar shallow rings, concordant with the pattern mentioned above. Therefore, when designing and synthesizing deep-cavity macrocycles, selecting the right solvent and using long aromatic ligands are crucial. This review and discussion provide a foundation for this work's design and synthesis of metallocycles with deep cavities.Colorless decahedral crystals of [Al8(OH)8L16] (AlOC-136) were readily obtained in considerable yield by reacting aluminum isopropoxide and indole-2-carboxylic acid (HL) in a stoichiometric ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) in the presence of acetonitrile. Acetonitrile effectively prevents the generation of products that are similar to previous shallow bowl-shaped metallocycles.20,23 Single-crystal X-ray diffraction (SCXRD) analysis revealed that AlOC-136 is an eight-membered macro-metallocycle with eight bridging –OH pointing into the center (Fig. 1b and c) and crystallizes in the P4nc space group (Table S1†). The asymmetric unit of AlOC-136 includes two Al(III) centers, four L ligands, and two μ2-OH groups (Fig. S1a†).
2) in the presence of acetonitrile. Acetonitrile effectively prevents the generation of products that are similar to previous shallow bowl-shaped metallocycles.20,23 Single-crystal X-ray diffraction (SCXRD) analysis revealed that AlOC-136 is an eight-membered macro-metallocycle with eight bridging –OH pointing into the center (Fig. 1b and c) and crystallizes in the P4nc space group (Table S1†). The asymmetric unit of AlOC-136 includes two Al(III) centers, four L ligands, and two μ2-OH groups (Fig. S1a†).
Its most prominent structural feature is the presence of a cavity with a depth of around 1.8 nm, which is significantly larger than that observed in many classic pure organic macrocycles (Fig. 1b and d, and Table S2†).17,25,43–48AlOC-136 possesses the greatest depth among all the cyclic compounds in the system (Fig. S2 and S3†). The octahedral coordination geometry of aluminum is completed by a deprotonated L ligand, resulting in 16 L ligands surrounding each metallocycle (Fig. 1c and Fig. S1†). Intuitively, these ligands can be divided into axial position (labeled as α, β) and equatorial position (labeled as γ, η) (Fig. 1b). From the top view, the outer diameter of the ring is 2.5 nm, defined as the distance between two equatorial positions (γ or η) ligands, while the inner diameter is 0.5 nm, defined as the distance between two opposite –OH (Fig. 1c). The inner diameter of the aluminum molecular macrocycles is similar to that of α-cyclodextrin,49 larger than that of crown50 and smaller than that of non-porous pillar[n]arene (Table S2†).17,51 From the side view, four ligands in the α position form a calixarene-like cavity (defined as the α-cavity), while four ligands in the β position form the β-cavity (Fig. 1b). Thus, an hourglass-shaped macrocycle is formed with two large hydrophobic ends and a small middle hydrophilic cavity. Its dimensions are 0.8 nm and 1.1 nm for upper-rim lengths, 0.5 nm and 0.8 nm for lower-rim sizes (that is, distances between the centers of the benzene rings of indole unit) (Fig. S4†), which stems from the difference in the dihedral angle (75° vs. 85°) (Fig. 1a and Fig. S5†). AlOC-136 not only has deep intramolecular cavities (404 Å3, the sum of two α-cavities) (Fig. 1b and Fig. S6†) but also intermolecular pores (27.1% according to PLATON calculations) to provide space for guest inclusion (Fig. S6c†). Gas adsorption further demonstrated that AlOC-136 has a permanent microporous structure. Nitrogen adsorption–desorption isotherms collected at 77 K on activated AlOC-136 exhibited type I behavior (Brunauer–Emmett–Teller (BET) surface area is 371 m2 g−1) (Fig. S6d†).
| 8Al(OiPr)3 + 16HL + 8H2O → [Al8(L)16(OH)8] + 24HOiPr |
Adsorption performance for dye molecules by deep-cavity aluminium–organic macrocycle
To verify the structural stability of AlOC-136, we immersed the crystal sample of AlOC-136 in an aqueous solution with pH values ranging from 2–7 and common organic solvents. The powder X-ray diffraction (PXRD) results confirm its air and water structural stability (Fig. S17 and S18†). The single-crystal X-ray diffraction images prove that AlOC-136 maintains good diffraction points after soaking in aqueous acidic solution and organic solvents such as toluene, ethanol, cyclohexane, DMSO, DCM, and petroleum ether for 48 h (Fig. S20 and S21†). This water stability is attributed to the abundance of hydrophobic groups on its structural surface, as demonstrated by its large contact angle observed in the wettability investigation (Fig. S22†). Considering the inherent deep-cavity tubular structure of the compound, water stability, and facile synthesis (Fig. 3a), we performed adsorption experiments with Acid Orange 7 (NaAO7), Alizarin Yellow (AY), and Acid Red 27 (AR27) azo dye molecules at 60 °C in water (Fig. S23†). The hybrid deep-cavity macrocycle exhibited superior adsorption of NaAO7 molecules (Fig. S24†), which may stem from the linear-shaped geometry and different hydrophilic and hydrophobic features of the two ends. This result encouraged us to study its capture mechanism. However, attempts to determine its microscopic capture principle through immersion experiments were unsuccessful because crystallization remained while no suitable peak could be defined as NaAO7 molecules after immersion (defined as AlOC-136′, Table S1†).To clarify the NaAO7 capture mechanism, we tried the cocrystallization method. Fortunately, the red flaky single crystals of AlOC-136-HAO7 were successfully isolated. The one-pot approach closely relates to the proposed mechanism whereby anionic dye (AO7) tends to be removed using a highly acidic solution.52 We speculate that excess HL acid ligands during synthesis create an acidic reaction environment (pH ≈ 4) to form protonated HAO7, which drives the host–guest combination process. Such speculation is confirmed by isolating the same host–guest product using other acidic substances, such as aluminum chloride and methylacrylic acid. A more intuitive analysis comes from the electrostatic potential map (ESP) calculation (Fig. 2c).53 We discovered that the hydrophilic center of the cavity of AlOC-136 is highly electropositive (0.06), and should thus be inclined to interact with the protonated HAO7 species. For the first time, we made a clear elucidation at the molecular level, as the mechanism of anionic AO7 capture has been speculative for a long time.54
Single-crystal analysis showed that AlOC-136-HAO7 crystallized in the C2/c space group (ESI video S1†). Its asymmetric unit included five Al(III) centers, eight L ligands, a protonated AO7 guest molecule (HAO7), and four –OH, as well as two acetonitrile molecules and two water molecules as guests (Fig. S7†). Notably, AlOC-136-HAO7 has significant host–guest interactions and apparent adaptive behavior. On the one hand, different kinds of guest molecules are comfortably located in various positions owing to their different size and structural characteristics. The small molecule guests of acetonitrile and water interact adhesively in the hydrophobic voids between macrocyclic molecules (Fig. S8†) through hydrogen bonding (Fig. S9 and Table S3†). The linear-shaped HAO7 molecules are partially wrapped in deep-cavity rings with the hydrophilic sulfonic group facing inward and hydrophobic naphthalene facing outward, consistent with the hydrophilic characteristics of the central cavity. As can be seen from Fig. 2a, each large ring captures two HAO7 molecules (the naphthol ring on HAO7 has a statistical phenomenon) that exhibit a torsion angle of 90° (Fig. S10†), which is caused by a 45° misalignment of aromatic hydrophobic walls in the upper and lower parts of the cavity (Fig. S11†). This combination of host and guest is attributed to shape matching and four different host–guest forces (summarized in Table S4†), namely: (I) strong hydrogen bonding (O–H⋯O, 2.6–2.7 Å) between the sulfonic group and hydrophilic –OH site in the center of the ring; (II) sandwich-configuration π–π (3.9–4.0 Å) and T-shape configuration π–π interactions (4.7–4.9 Å) (Fig. S12†) between the benzoate ring of HAO7 and hydrophilic aromatic walls of the ring; (III) hydrogen bond interactions between the azo moiety and ing body (C–H⋯N, 3.3–3.8 Å); and (IV) the naphthol ring on HAO7 extends out of the central cavity involving abundant C–H⋯π interactions (3.3–3.8 Å) with the ligand aromatic ring in the perpendicular and equatorial directions of the adjacent aluminum ring (Fig. 2b and Fig. S13†). Furthermore, the ring host adapts to the most comfortable state with the entry of the HAO7 molecule. The shape of the annular cavity changed significantly from different sizes at both the ends to the same length (Fig. S14†), which is also related to the host–guest adaptation described above. To determine the quantitative distribution of these weak interaction forces, we used Hirshfeld surface analysis55 (Fig. S25†) to calculate the bond energy relationship within or between molecules (see the 2D fingerprint plot in Fig. S26†). Compared with the pristine -AB- alternating supramolecular stacking in AlOC-136, the compound AlOC-136-HAO7 exhibits A-type stacking after the trapping of the HAO7 guest (retaining a porosity of 21%) (Fig. S15 and S16†).
In addition to the single-crystal analysis method described above to provide visual evidence for capturing HAO7 molecules, we obtained additional evidence from the bulk sample. The first manifestation is its significant color change from colorless to red (Fig. 3b). In addition, two other direct pieces of evidence of the presence of HAO7 come from energy dispersive spectroscopy (EDS) and Fourier transform infrared (FT-IR) spectroscopy. EDS proved the presence of S, a characteristic element of HAO7 (Fig. 3c and d). The absorption peak at 1031 cm−1 is attributed to the symmetric O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of –SO3H groups56 in AlOC-136-HAO7 (Fig. S27 and S28†). The physical properties of the macrocycle changed significantly after the capture of the guest molecule, mainly reflected in the decreased optical band gap and wettability, improved thermal stability, and increased proton conductivity. The solid-state UV-vis diffuse reflection data indicates that the band gap energy reduces from 3.4 eV (AlOC-136) to 2.1 eV (AlOC-136-HAO7), which is consistent with the color change (Fig. 3b). The thermogravimetric (TGA) curve shows that AlOC-136-HAO7 exhibits better thermal stability than that of AlOC-136 (Fig. S29 and S30†). Since hydrophilic –SO3H groups are confined within the ring, the contact angle of AlOC-136-HAO7 is lower than that of AlOC-136 (Fig. S22†). Considering that the –SO3H group has been recognized as a superior proton donor and an enabler for high proton conductivity,56,57 we investigated the proton conductivity (Fig. S31†). The results showed that the bulk conductivity of AlOC-136-HAO7 is six orders of magnitude higher than that of vacant AlOC-136, increasing from 6.7 × 10−9 S cm−1 to 7.78 × 10−3 S cm−1 at 98 °C and 98% RH. Activation energies (Ea) of the materials were derived from Arrhenius plots to be 0.23 eV for AlOC-136-HAO7 (Fig. S32†), indicating that the proton transfer follows the conventional Grotthuss mechanism.58
O stretching vibration of –SO3H groups56 in AlOC-136-HAO7 (Fig. S27 and S28†). The physical properties of the macrocycle changed significantly after the capture of the guest molecule, mainly reflected in the decreased optical band gap and wettability, improved thermal stability, and increased proton conductivity. The solid-state UV-vis diffuse reflection data indicates that the band gap energy reduces from 3.4 eV (AlOC-136) to 2.1 eV (AlOC-136-HAO7), which is consistent with the color change (Fig. 3b). The thermogravimetric (TGA) curve shows that AlOC-136-HAO7 exhibits better thermal stability than that of AlOC-136 (Fig. S29 and S30†). Since hydrophilic –SO3H groups are confined within the ring, the contact angle of AlOC-136-HAO7 is lower than that of AlOC-136 (Fig. S22†). Considering that the –SO3H group has been recognized as a superior proton donor and an enabler for high proton conductivity,56,57 we investigated the proton conductivity (Fig. S31†). The results showed that the bulk conductivity of AlOC-136-HAO7 is six orders of magnitude higher than that of vacant AlOC-136, increasing from 6.7 × 10−9 S cm−1 to 7.78 × 10−3 S cm−1 at 98 °C and 98% RH. Activation energies (Ea) of the materials were derived from Arrhenius plots to be 0.23 eV for AlOC-136-HAO7 (Fig. S32†), indicating that the proton transfer follows the conventional Grotthuss mechanism.58
Improved third-order non-linear optical properties
The introduction of HAO7 decreases the optical band gap and wettability, increases the thermal stability and proton conductivity, and brings new functions. Taking into account weak interactions, especially π⋯π interactions that might contribute to optical properties, we further investigated the third-order NLO properties. As control experiments, we performed same tests on the pristine constitute component, including the blank AlOC-136 macrocycle and NaAO7 powder, as well as physically doped materials (that is, a mixture of ground powder of AlOC-136 and NaAO7 with the same chemical molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2).
2).
The third-order NLO properties and optical limiting (OL) activities were investigated through a facile Z-scan technique, which is widely applied for analyzing the NLO response due to its simplicity, suitable operation, and high sensitivity (Fig. 4a). By dispersing uniformly in N,N-dimethylformamide (DMF) solution (Fig. 4b), AlOC-136 showed an unobvious NLO response even though there are conjugated ligands on the molecular ring, which is possibly due to the lack of intramolecular interactions and relatively weak intermolecular π conjugation interactions. As shown in Fig. 4c, the OA Z-scan curves of AlOC-136-HAO7, NaAO7 powder, and physically doped material exhibited an apparent saturation absorption (SA) response, which results from the surface defect effect, the quantum confinement effect, and complex interactions between molecules.59,60 OL curves confirmed that the normalized transmittance of samples depends on the input laser pulse energy according to eqn (1):
 | (1) |
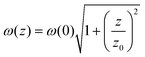 | (2) |
Among them, AlOC-136-HAO7 gave the most prominent SA response and had the largest normalized transmittance of ≈1.28 at the laser focus, where the input fluence is at its maximum. Moreover, the output fluence of AlOC-136-HAO7 linearly increased at low-incident fluence. However, it deviates from the linearity at high-incident fluence, which indicates the typical OL effect (Fig. S33b†). Furthermore, to quantitatively evaluate the NLO effects of the samples, NLO absorption coefficients were obtained by fitting open aperture Z-scan curves using eqn (3):61
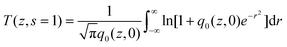 | (3) |
 . The non-linear absorption coefficient β value for AlOC-136-HAO7 is −15.98 cm per GW, as listed in Table S5.† It is superior to the majority of azo dyes,62 although it is not comparable with transition metal-based materials.63 Besides, the imaginary part of the third-order non-linear optical susceptibility Im
. The non-linear absorption coefficient β value for AlOC-136-HAO7 is −15.98 cm per GW, as listed in Table S5.† It is superior to the majority of azo dyes,62 although it is not comparable with transition metal-based materials.63 Besides, the imaginary part of the third-order non-linear optical susceptibility Im![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) χ(3) was calculated according to eqn (4):
χ(3) was calculated according to eqn (4):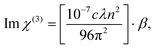 | (4) |
 (Table S5†). The expected results were consistent with the analysis of the host–guest interactions in the previous structural description. Such performance originates from HAO7 molecules because blank AlOC-136 only exhibits a weak signal. However, we can see that the physically doped material displays close the NLO effects to those of the NaAO7 powder.
(Table S5†). The expected results were consistent with the analysis of the host–guest interactions in the previous structural description. Such performance originates from HAO7 molecules because blank AlOC-136 only exhibits a weak signal. However, we can see that the physically doped material displays close the NLO effects to those of the NaAO7 powder.
To achieve devices for practical OL application, we prepared transparent and flexible films by evenly dispersing the samples into a polydimethylsiloxane (PDMS) matrix (Fig. 4b). Compared with the solution method, it is worth noting that AlOC-136-HAO7 exhibited an opposite reverse saturable absorption (RSA) response (Fig. 4d and Fig. S34, Table S6†) but with the same trend. This phenomenon was also observed in Zhan's system research of nanostructured noble metals64 and is related to the hindered electronic relaxation process in solid-state environments.65 Considering that AlOC-136-HAO7 reveals an SA response (applied in fast optical switching and passive mode-locking technique) and RSA response (applied in protecting human eyes and sensitive optical devices) in different matrices, we could prepare devices according to requirements.
Considering why such chemical doping with precise host–guest structure behaves better than physical doping, we suspect it is related to periodic structures with confined effects. To further clarify this, we performed density-functional theory (DFT) calculations. In the AlOC-136 macrocycle, the highest occupied molecular orbital (HOMO) is composed of equatorial position ligands, while the lowest unoccupied frontier (LUMO) is composed of the axial position ligand (Fig. 4e). However, in AlOC-136-HAO7, the guest-to-guest charge transfer is between the two linear HAO7 molecules (Fig. 4e). Therefore, combining structural analysis and theoretical calculations, the enhancement of NLO behaviour could be attributed to the periodic abundant host–guest interactions and guest-to-guest charge transfer.66
Conclusions
In summary, we have demonstrated the designed synthesis of a deep-cavity aluminum–organic macrocycle (AlOC-136) with a hydrophobic inner wall and hydrophilic center. The crucial factors for the generation of deep-cavity aluminum–organic macrocycles lie in introducing more extended ligands and selecting aprotic solvents. Combined with the feature of a deep cavity, we explored an in situ self-assembly strategy to capture linear dye Acid Orange 7 (AlOC-136-HAO7) and revealed the HAO7 capture mechanism at the molecular level for the first time. NLO experiment results illustrated that AlOC-136-HAO7 exhibited significantly enhanced NLO behavior compared with pristine constituent components and physically doped components. Theoretical calculations demonstrated that the enhancement of NLO behavior could be attributed to the guest-to-guest charge transfer. In addition, AlOC-136-HAO7 realized SA and RSA response conversion in different matrices, which could play a corresponding role in diverse environments. This work brings a bright future for the promising inorganic–organic hybrid deep-cavity macrocycle system, reveals their particular application in guest capture, and incorporates valuable functions.Data availability
The datasets supporting this article have been uploaded as part of the ESI.† X-ray crystallographic data for the structures reported in the article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2304752 (AlOC-136) and 2304753 (AlOC-136-HAO7).† The dataset is also provided as ESI† with this paper. All other data supporting the findings of this study are available within the paper and its ESI† and from the corresponding author.Author contributions
All authors contributed extensively to the work presented in this paper. W.-H. F., J. N. and J. Z. conceived and designed this project. Z.-H. L. carried out the synthesis, characterization and non-linear optical study. S.-H. S. assisted with proton conductivity measurements. Q.-H. Li carried out the theoretical calculations. C.-Y. Z. gave support on the writing. W.-H. F. and Z.-H. L. wrote the manuscript. All the authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Natural Science Foundation of China (22371278, U23A2095, and 92061104), Natural Science Foundation of Fujian Province (2021J06035) and Youth Innovation Promotion Association Chinese Academy of Sciences (Y2021081).References
- M. A. Hassaan, A. El Nemr and A. Hassaan, Health and Environmental Impacts of Dyes: Mini Review, Am. J. Environ. Sci. Eng., 2017, 1, 64–67 Search PubMed.
- J. Liang, X.-a. Ning, J. Sun, J. Song, J. Lu, H. Cai and Y. Hong, Toxicity evaluation of textile dyeing effluent and its possible relationship with chemical oxygen demand, Ecotoxicol. Environ. Saf., 2018, 166, 56–62 CrossRef CAS PubMed.
- O. J. Hao, H. Kim and P.-C. Chiang, Decolorization of Wastewater, Crit. Rev. Environ. Sci. Technol., 2000, 30, 449–505 CrossRef CAS.
- A. M. M. Vargas, A. L. Cazetta, A. C. Martins, J. C. G. Moraes, E. E. Garcia, G. F. Gauze, W. F. Costa and V. C. Almeida, Kinetic and equilibrium studies: Adsorption of food dyes Acid Yellow 6, Acid Yellow 23, and Acid Red 18 on activated carbon from flamboyant pods, Chem. Eng. J., 2012, 181–182, 243–250 CrossRef CAS.
- A. M. M. Vargas, A. C. Martins and V. C. Almeida, Ternary adsorption of acid dyes onto activated carbon from flamboyant pods (Delonix regia): Analysis by derivative spectrophotometry and response surface methodology, Chem. Eng. J., 2012, 195–196, 173–179 CrossRef CAS.
- S.-F. Kang, C.-H. Liao and S.-T. Po, Decolorization of textile wastewater by photo-fenton oxidation technology, Chemosphere, 2000, 41, 1287–1294 CrossRef CAS PubMed.
- E. J. Ruiz, C. Arias, E. Brillas, A. Hernández-Ramírez and J. M. Peralta-Hernández, Mineralization of Acid Yellow 36azo dye by electro-Fenton and solar photoelectro-Fenton processes with a boron-doped diamond anode, Chemosphere, 2011, 82, 495–501 CrossRef CAS PubMed.
- Z. Xia, L. Wang, Q. Zhang, F. Li and L. Xu, Fast degradation of phenol over porphyrin-polyoxometalate composite photocatalysts under visible light, Polyoxometalates, 2022, 1, 9140001 CrossRef.
- Z. Ni, N. Liu, C. Zhao and L. Mi, Hexanuclear nickel-added silicotungstates as high-efficiency electrocatalysts for nitrate reduction to ammonia, Polyoxometalates, 2024, 3, 9140044 CrossRef.
- M. Sultan, Polyurethane for removal of organic dyes from textile wastewater, Environ. Chem. Lett., 2017, 15, 347–366 CrossRef CAS.
- S. Rojas and P. Horcajada, Metal–Organic Frameworks for the Removal of Emerging Organic Contaminants in Water, Chem. Rev., 2020, 120, 8378–8415 CrossRef CAS PubMed.
- A. Kausar, S. T. Zohra, S. Ijaz, M. Iqbal, J. Iqbal, I. Bibi, S. Nouren, N. El Messaoudi and A. Nazir, Cellulose-based materials and their adsorptive removal efficiency for dyes: A review, Int. J. Biol. Macromol., 2023, 224, 1337–1355 CrossRef CAS PubMed.
- H. He, E. Ma, Y. Cui, J. Yu, Y. Yang, T. Song, C.-D. Wu, X. Chen, B. Chen and G. Qian, Polarized three-photon-pumped laser in a single MOF microcrystal, Nat. Commun., 2016, 7, 11087 CrossRef CAS PubMed.
- H. Li, L. Zhang, H. He, Y. Yang, Y. Cui and G. Qian, Tunable nonlinear optical responses based on host–guest MOF hybrid materia, Sci. China Mater., 2021, 64, 698–705 CrossRef CAS.
- R. N. Dsouza, U. Pischel and W. M. Nau, Fluorescent Dyes and Their Supramolecular Host/Guest Complexes with Macrocycles in Aqueous Solution, Chem. Rev., 2011, 111, 7941–7980 CrossRef CAS PubMed.
- S. M. Biros and J. Rebek, Structure and binding properties of water-soluble cavitands and capsules, Chem. Soc. Rev., 2007, 36, 93–104 RSC.
- K. Jie, Y. Zhou, Y. Yao and F. Huang, Macrocyclic amphiphiles, Chem. Soc. Rev., 2015, 44, 3568–3587 RSC.
- Z. Liu, S. K. M. Nalluri and J. F. Stoddart, Surveying macrocyclic chemistry: from flexible crown ethers to rigid cyclophanes, Chem. Soc. Rev., 2017, 46, 2459–2478 RSC.
- G. Zhang, W. Lin, F. Huang, J. Sessler and N. M. Khashab, Industrial Separation Challenges: How Does Supramolecular Chemistry Help?, J. Am. Chem. Soc., 2023, 145, 19143–19163 CrossRef CAS PubMed.
- L. Geng, C.-H. Liu, S.-T. Wang, W.-H. Fang and J. Zhang, Designable Aluminum Molecular Rings: Ring Expansion and Ligand Functionalization, Angew. Chem., Int. Ed., 2020, 59, 16735–16740 CrossRef CAS PubMed.
- S. Yao, W.-H. Fang, Y. Sun, S.-T. Wang and J. Zhang, Mesoporous Assembly of Aluminum Molecular Rings for Iodine Capture, J. Am. Chem. Soc., 2021, 143, 2325–2330 CrossRef CAS PubMed.
- C.-H. Liu, W.-H. Fang, Y. Sun, S. Yao, S.-T. Wang, D. Lu and J. Zhang, Designable Assembly of Aluminum Molecular Rings for Sequential Confinement of Iodine Molecules, Angew. Chem., Int. Ed., 2021, 60, 21426–21433 CrossRef CAS PubMed.
- Y. Li, C. Zheng, S.-T. Wang, Y.-J. Liu, W.-H. Fang and J. Zhang, Record Aluminum Molecular Rings for Optical Limiting and Nonlinear Optics, Angew. Chem., Int. Ed., 2022, 61, e202116563 CrossRef CAS PubMed.
- D. Luo, H. Xiao, M.-Y. Zhang, S.-D. Li, L. He, H. Lv, C.-S. Li, Q.-P. Lin, W.-H. Fang and J. Zhang, Accurate binding of porous aluminum molecular ring catalysts with the substrate, Chem. Sci., 2023, 14, 5396–5404 RSC.
- H. Han, R. Fu, R. Wang, C. Tang, M.-M. He, J.-Y. Deng, D.-S. Guo, J. F. Stoddart and K. Cai, Corralarenes: A Family of Conjugated Tubular Hosts, J. Am. Chem. Soc., 2022, 144, 20351–20362 CrossRef CAS PubMed.
- X. Wan, S. Li, Y. Tian, J. Xu, L.-C. Shen, H. Zuilhof, M. Zhang and A. C.-H. Sue, Twisted pentagonal prisms: AgnL2 metal-organic pillars, Chem, 2022, 8, 2136–2147 CAS.
- R. K. Juneja, K. D. Robinson, C. P. Johnson and J. L. Atwood, Synthesis and characterization of rigid, deep-cavity calix[4]arenes, J. Am. Chem. Soc., 1993, 115, 3818–3819 CrossRef CAS.
- S. M. Biros and J. J. Rebek, Structure and binding properties of water-soluble cavitands and capsules, Chem. Soc. Rev., 2007, 36, 93–104 RSC.
- G. Peñuelas-Haro and P. Ballester, Efficient hydrogen bonding recognition in water using aryl-extended calix[4]pyrrole receptors, Chem. Sci., 2019, 10, 2413–2423 RSC.
- C. R. Moylan, R. J. Twieg, V. Y. Lee, S. A. Swanson, K. M. Betterton and R. D. Miller, Nonlinear optical chromophores with large hyperpolarizabilities and enhanced thermal stabilities, J. Am. Chem. Soc., 1993, 115, 12599–12600 CrossRef CAS.
- C. W. Ghanavatkar, V. R. Mishra and N. Sekar, Review of NLOphoric azo dyes – Developments in hyperpolarizabilities in last two decades, Dyes Pigm., 2021, 191, 109367 CrossRef CAS.
- G. A. Timco, T. B. Faust, F. Tuna and R. E. P. Winpenny, Linking heterometallic rings for quantum information processing and amusement, Chem. Soc. Rev., 2011, 40, 3067–3075 RSC.
- P. King, T. C. Stamatatos, K. A. Abboud and G. Christou, Reversible Size Modification of Iron and Gallium Molecular Wheels: A Ga10 “Gallic Wheel” and Large Ga18 and Fe18 Wheels, Angew. Chem., Int. Ed., 2006, 45, 7379–7383 CrossRef CAS PubMed.
- T. C. Stamatatos, A. G. Christou, C. M. Jones, B. J. O'Callaghan, K. A. Abboud, T. A. O'Brien and G. Christou, “Squaring the Circle”: Molecular Squares and Rectangles from Chelate-Induced Structural Transformations of Known Fe10 and New Fe12 Ferric Wheels, J. Am. Chem. Soc., 2007, 129, 9840–9841 CrossRef CAS PubMed.
- D. Schray, G. Abbas, Y. Lan, V. Mereacre, A. Sundt, J. Dreiser, O. Waldmann, G. E. Kostakis, C. E. Anson and A. K. Powell, Combined Magnetic Susceptibility Measurements and 57Fe Mössbauer Spectroscopy on a Ferromagnetic {FeIII4Dy4} Ring, Angew. Chem., Int. Ed., 2010, 49, 5185–5188 CrossRef CAS PubMed.
- L. Qin, J. Singleton, W.-P. Chen, H. Nojiri, L. Engelhardt, R. E. P. Winpenny and Y.-Z. Zheng, Quantum Monte Carlo Simulations and High-Field Magnetization Studies of Antiferromagnetic Interactions in a Giant Hetero-Spin Ring, Angew. Chem., Int. Ed., 2017, 56, 16571–16574 CrossRef CAS PubMed.
- G. A. Timco, A. Fernandez, A. K. Kostopoulos, C. A. Muryn, R. G. Pritchard, I. Strashnov, I. J. Vitorica-Yrezebal, G. F. S. Whitehead and R. E. P. Winpenny, An Extensive Family of Heterometallic Titanium(IV)–Metal(III) Rings with Structure Control through Templates, Angew. Chem., Int. Ed., 2017, 56, 13629–13632 CrossRef CAS PubMed.
- W.-P. Chen, P.-Q. Liao, P.-B. Jin, L. Zhang, B.-K. Ling, S.-C. Wang, Y.-T. Chan, X.-M. Chen and Y.-Z. Zheng, The Gigantic {Ni36Gd102} Hexagon: A Sulfate-Templated “Star-of-David” for Photocatalytic CO2 Reduction and Magnetic Cooling, J. Am. Chem. Soc., 2020, 142, 4663–4670 CrossRef CAS PubMed.
- H.-L. Zhang, Y.-Q. Zhai, H. Nojiri, C. Schröder, H.-K. Hsu, Y.-T. Chan, Z. Fu and Y.-Z. Zheng, {ScnGdn} Heterometallic Rings: Tunable Ring Topology for Spin-Wave Excitations, J. Am. Chem. Soc., 2022, 144, 15193–15202 CrossRef CAS PubMed.
- J. Ferrando-Soria, A. Fernandez, E. Moreno Pineda, S. A. Varey, R. W. Adams, I. J. Vitorica-Yrezabal, F. Tuna, G. A. Timco, C. A. Muryn and R. E. P. Winpenny, Controlled Synthesis of Nanoscopic Metal Cages, J. Am. Chem. Soc., 2015, 137, 7644–7647 CrossRef CAS PubMed.
- J. Ferrando-Soria, A. Fernandez, D. Asthana, S. Nawaz, I. J. Vitorica-Yrezabal, G. F. S. Whitehead, C. A. Muryn, F. Tuna, G. A. Timco, N. D. Burton and R. E. P. Winpenny, A [13]rotaxane assembled via a palladium molecular capsule, Nat. Commun., 2019, 10, 3720 CrossRef PubMed.
- M.-H. Du, S.-H. Xu, G.-J. Li, H. Xu, Y. Lin, W.-D. Liu, L.-S. Long, L.-S. Zheng and X.-J. Kong, Hierarchical Assembly of Coordination Macromolecules with Atypical Geometries: Gd44Co28 Crown and Gd95Co60 Cage, Angew. Chem., Int. Ed., 2022, 61, e202200537 CrossRef CAS PubMed.
- C. J. Pedersen, Cyclic polyethers and their complexes with metal salts, J. Am. Chem. Soc., 1967, 89, 2495–2496 CrossRef CAS.
- C. J. Pedersen, The Discovery of Crown Ethers, Science, 1988, 241, 536–540 CrossRef CAS PubMed.
- A. Ikeda and S. Shinkai, Novel Cavity Design Using Calix[n]arene Skeletons: Toward Molecular Recognition and Metal Binding, Chem. Rev., 1997, 97, 1713–1734 CrossRef CAS PubMed.
- T. Hishiya, H. Asanuma and M. Komiyama, Spectroscopic Anatomy of Molecular-Imprinting of Cyclodextrin. Evidence for Preferential Formation of Ordered Cyclodextrin Assemblies, J. Am. Chem. Soc., 2002, 124, 570–575 CrossRef CAS PubMed.
- K. I. Assaf and W. M. Nau, Cucurbiturils: from synthesis to high-affinity binding and catalysis, Chem. Soc. Rev., 2015, 44, 394–418 RSC.
- T. Ogoshi, T.-a. Yamagishi and Y. Nakamoto, Pillar-Shaped Macrocyclic Hosts Pillar[n]arenes: New Key Players for Supramolecular Chemistry, Chem. Rev., 2016, 116, 7937–8002 CrossRef CAS PubMed.
- M. V. Rekharsky and Y. Inoue, Complexation Thermodynamics of Cyclodextrins, Chem. Rev., 1998, 98, 1875–1918 CrossRef CAS PubMed.
- G. W. Gokel, W. M. Leevy and M. E. Weber, Crown Ethers: Sensors for Ions and Molecular Scaffolds for Materials and Biological Models, Chem. Rev., 2004, 104, 2723–2750 CrossRef CAS PubMed.
- K. Jie, Y. Zhou, E. Li, Z. Li, R. Zhao and F. Huang, Reversible Iodine Capture by Nonporous Pillar[6]arene Crystals, J. Am. Chem. Soc., 2017, 139, 15320–15323 CrossRef CAS PubMed.
- S. Yoon, J. Calvo and M. So, Removal of Acid Orange 7 from Aqueous Solution by Metal-Organic Frameworks, Crystals, 2018, 9, 17 CrossRef.
- P. J. Stephens, F. J. Devlin, C. F. Chabalowski and M. J. Frisch, Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields, J. Phys. Chem., 1994, 98, 11623–11627 CrossRef CAS.
- M. Zhou, Z. Ju and D. Yuan, A new metal–organic framework constructed from cationic nodes and cationic linkers for highly efficient anion exchange, Chem. Commun., 2018, 54, 2998–3001 RSC.
- C. B. Pinto, L. H. R. Dos Santos and B. L. Rodrigues, Response of Hirshfeld Surface to Structural Modifications in Transition-Metal Coordination Compounds, Cryst. Growth Des., 2020, 20, 4827–4838 CrossRef CAS.
- B. Shi, X. Pang, S. Li, H. Wu, J. Shen, X. Wang, C. Fan, L. Cao, T. Zhu, M. Qiu, Z. Yin, Y. Kong, Y. Liu, M. Zhang, Y. Liu, F. Pan and Z. Jiang, Short hydrogen-bond network confined on COF surfaces enables ultrahigh proton conductivity, Nat. Commun., 2022, 13, 6666 CrossRef CAS PubMed.
- X. Li, H. Zhang, J. Hou, R. Ou, Y. Zhu, C. Zhao, T. Qian, C. D. Easton, C. Selomulya, M. R. Hill and H. Wang, Sulfonated Sub-1 nm Metal–Organic Framework Channels with Ultrahigh Proton Selectivity, J. Am. Chem. Soc., 2020, 142, 9827–9833 CAS.
- X. Meng, H.-N. Wang, S.-Y. Song and H.-J. Zhang, Proton-conducting crystalline porous materials, Chem. Soc. Rev., 2017, 46, 464–480 RSC.
- H. Pan, H. Chu, X. Wang, Y. Li, S. Zhao, G. Li and D. Li, Optical nonlinearity of zeolitic imidazolate framework-67 in the near-infrared region, Mater. Chem. Front., 2020, 4, 2081–2088 RSC.
- G.-H. Chen, Y.-P. He, Z.-R. Wang, Q.-H. Li, Z.-Z. Ma and J. Zhang, Tunable third-order nonlinear optical effect via modifying Ti4(embonate)6 cage-based ionic pairs, Inorg. Chem. Front., 2022, 9, 1984–1991 RSC.
- M. Sheik-Bahae, A. A. Said, T. H. Wei, D. J. Hagan and E. W. V. Stryland, Sensitive measurement of optical nonlinearities using a single beam, IEEE J. Quantum Electron., 1990, 26, 760–769 CrossRef CAS.
- C. W. Ghanavatkar, V. R. Mishra and N. Sekar, Review of NLOphoric azo dyes – Developments in hyperpolarizabilities in last two decades, Dyes Pigm., 2021, 191, 109367 CrossRef CAS.
- D.-J. Li, Z.-G. Gu and J. Zhang, Auto-controlled fabrication of a metal-porphyrin framework thin film with tunable optical limiting effects, Chem. Sci., 2020, 11, 1935–1942 RSC.
- C. Zheng, Y. Du, M. Feng and H. Zhan, Shape dependence of nonlinear optical behaviors of nanostructured silver and their silica gel glass composites, Appl. Phys. Lett., 2008, 93, 143108 CrossRef.
- C. Zheng, X. Ye, S. Cai, M. Wang and X. Xiao, Observation of nonlinear saturable and reverse-saturable absorption in silver nanowires and their silica gel glass composite, Appl. Phys. B, 2010, 101, 835–840 CrossRef CAS.
- D.-J. Li, Q.-h. Li, Z.-R. Wang, Z.-Z. Ma, Z.-G. Gu and J. Zhang, Interpenetrated Metal-Porphyrinic Framework for Enhanced Nonlinear Optical Limiting, J. Am. Chem. Soc., 2021, 143, 17162–17169 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, detail structure charts, stability analysis, characterization, tables and supplement videos. CCDC 2304752 (AlOC-136) and 2304753 (AlOC-136-HAO7). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qi00976b |
| This journal is © the Partner Organisations 2024 |