Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Selective hydrogenation of guaiacol to 2-methoxycyclohexanone over supported Pd catalysts†
Yota
Taniwaki
a,
Yoshinao
Nakagawa
 *ab,
Mizuho
Yabushita
*ab,
Mizuho
Yabushita
 ab and
Keiichi
Tomishige
ab and
Keiichi
Tomishige
 *abc
*abc
aDepartment of Applied Chemistry, School of Engineering, Tohoku University, 6-6-07 Aoba, Aramaki, Aoba-ku, Sendai, Miyagi 980-8579, Japan. E-mail: yoshinao@erec.che.tohoku.ac.jp; tomishige@tohoku.ac.jp
bResearch Center for Rare Metal and Green Innovation, Tohoku University, 468-1 Aoba, Aramaki, Aoba-ku, Sendai, Miyagi 980-0845, Japan
cAdvanced Institute for Materials Research (WPI-AIMR), Tohoku University, 2-1-1 Katahira, Aoba-ku, Sendai, Miyagi 980-8577, Japan
First published on 7th September 2024
Abstract
Selective hydrogenation of guaiacol to 2-methoxycyclohexanone was investigated with various Pd catalysts. This reaction is much more difficult than the hydrogenation of phenol to cyclohexanone, namely in terms of the low reactivity of guaiacol and the reduced selectivity of 2-methoxycyclohexanone due to the demethoxylation reaction. Pd/TiO2 catalysts were found to be superior to other supported Pd catalysts in terms of activity and selectivity to 2-methoxycyclohexanone. The Pd dispersion did not affect the selectivity of Pd/TiO2 catalysts. Meanwhile, the increase of Pd dispersion decreased the turnover frequency, and the optimum Pd dispersion was about 25%. The presence of residual chloride ions had a negative effect on the selectivity to 2-methoxycyclohexanone. The optimal Pd/TiO2 catalyst gave 65% yield of 2-methoxycyclohexanone. The catalyst was reusable after washing with toluene solvent to extract residual organic species from the catalyst surface. The catalyst was capable of hydrogenating various phenolic compounds, namely methoxyphenols, into the corresponding cyclohexanone derivatives.
1. Introduction
Amid concerns over the depletion of petroleum, there is growing interest in producing chemicals from biomass resources.1–4 Lignocellulosic biomass mainly consisting of cellulose, hemicellulose, and lignin is a promising renewable resource due to its abundance. Fast pyrolysis of lignocellulosic biomass or lignin yields liquid compounds (bio-oil) that can be used as both fuel and chemical feedstock.5–11 However, bio-oil contains a larger number of oxygen atoms and/or double bonds than petroleum. To this end, it is necessary to decrease the O/C ratio and increase the H/C ratio. Moreover, bio-oil is rich in lignin-derived phenolic derivatives and has great potential as a raw material for aromatic compounds and their derivatives. Guaiacol, a simple aromatic compound with a C6 carbon backbone possessing both hydroxy and methoxy groups, shares structural similarities with lignin and many lignin-derived compounds. In addition, the pyrolysis of lignin under appropriate conditions offers bio-oil in which guaiacol is a major component.12 In this respect, the conversion of guaiacol into valuable chemical products such as benzene, phenol, and cyclohexanol has garnered considerable attention.13–25Adipic acid is one of the most important C6 monomers that serves as a raw material for synthetic polymers and has been a target product of guaiacol conversion. Adipic acid is currently synthesized from petroleum-derived benzene by hydrogenation to cyclohexane, oxidation to K/A-oil, which is a mixture of cyclohexanone and cyclohexanol, and further oxidation with nitric acid.26,27 Although the environmentally-benign oxidant O2 is an attractive oxidant, the oxidation of K/A-oil with O2 is less selective than that with nitric acid and is not used in industrial processes. The production of adipic acid from guaiacol is thus a promising approach for synthesizing renewable adipic acid.26,28,29
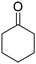
One approach is the synthesis of K/A-oil by hydrodeoxygenation of guaiacol. However, this route requires a large amount of hydrogen (min 3 eq.) (eqn (1)), and nitric acid oxidation is still necessary. Furthermore, there have been no reports on the direct synthesis of cyclohexanone and methanol expressed as eqn (1). The primary mono-oxygenate product is phenol or cyclohexanol. Hydrogenation of phenol to cyclohexanone is possible, but the process becomes more complex. Cyclohexanol can be used as a substrate for the nitric acid oxidation to adipic acid, but the synthesis of cyclohexanol from guaiacol consumes a larger amount of hydrogen than eqn (1). In addition, most reported systems decompose the methoxy group in guaiacol to methane, which further increases hydrogen consumption.
 | (1) |
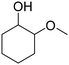
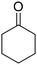
Recently, our group reported that adipic acid can be synthesized by the oxidative cleavage of 2-methoxycyclohexanone (2-MCO), a partial hydrogenation product of guaiacol, with a heteropoly acid catalyst and O2 (eqn (2)).30 The methoxy group can be recovered by methanol. Ideally, the route of adipic acid synthesis via 2-MCO consumes less hydrogen (2 eq., consumed in guaiacol to 2-MCO) than the route via mono-oxygenates (3 eq. or more) and can also avoid the nitric acid oxidation step.
 | (2) |
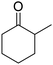
To establish the production route of adipic acid from guaiacol via 2-MCO, selective partial hydrogenation of guaiacol to 2-MCO is necessary (eqn (3)). However, few studies have examined this reaction, and the maximum reported yield with H2 as a reductant was only 37%.31 On the other hand, the selective partial hydrogenation of phenol to cyclohexanone, which is a closely related reaction to the current target reaction, has been intensively investigated, and excellent yields have been reported in many studies.32,33 For the hydrogenation of phenol to cyclohexanone, catalysts with platinum group metals, especially Pd, have been mainly used (Table S1†).34–46 Pd is known to have excellent hydrogen activation ability and high activity in the hydrogenation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, while the activity of Pd in C
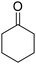
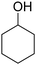
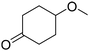
C bonds, while the activity of Pd in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O hydrogenation is low; these features make Pd a good catalyst for the hydrogenation of phenol to cyclohexanone. The effect of supports has been intensively investigated, and base sites and/or Lewis acid sites have been proposed to have a positive effect on the selectivity to cyclohexanone.47–51 Some studies investigated the selective hydrogenation of phenolic compounds to the corresponding cyclohexanone derivatives. Alkylphenols such as o-cresol (2-methylphenol) are typical substrates used in such studies, and excellent yields have also been reported.31,36,41,44,52–56 Meanwhile, reports on the selective hydrogenation of methoxyphenols to target ketones have been limited, and the performance of reported catalysts was lower than those of other phenolic compounds. Most of the studies tested only 4-methoxyphenol as the substrate among methoxyphenols, despite the fact that guaiacol is a more abundant phenolic compound than methoxyphenol. Even in the studies that investigated guaiacol hydrogenation, the performance of catalysts in the guaiacol hydrogenation was always much lower than those in the hydrogenation of 4-methoxyphenol, o-cresol, and catechol. The most typical case is the Pd/HAP (HAP = hydroxyapatite) catalyst which gave the highest yield of 2-MCO. Cyclohexanone, 4-methoxycyclohexanone, and 2-hydroxycyclohexanone were obtained in quantitative yields from phenol, 4-methoxyphenol, and catechol at 3 h, 3.5 h, and 8 h, respectively, while 2-MCO was obtained from guaiacol in only 37% yield (98% selectivity) at 24 h.31
O hydrogenation is low; these features make Pd a good catalyst for the hydrogenation of phenol to cyclohexanone. The effect of supports has been intensively investigated, and base sites and/or Lewis acid sites have been proposed to have a positive effect on the selectivity to cyclohexanone.47–51 Some studies investigated the selective hydrogenation of phenolic compounds to the corresponding cyclohexanone derivatives. Alkylphenols such as o-cresol (2-methylphenol) are typical substrates used in such studies, and excellent yields have also been reported.31,36,41,44,52–56 Meanwhile, reports on the selective hydrogenation of methoxyphenols to target ketones have been limited, and the performance of reported catalysts was lower than those of other phenolic compounds. Most of the studies tested only 4-methoxyphenol as the substrate among methoxyphenols, despite the fact that guaiacol is a more abundant phenolic compound than methoxyphenol. Even in the studies that investigated guaiacol hydrogenation, the performance of catalysts in the guaiacol hydrogenation was always much lower than those in the hydrogenation of 4-methoxyphenol, o-cresol, and catechol. The most typical case is the Pd/HAP (HAP = hydroxyapatite) catalyst which gave the highest yield of 2-MCO. Cyclohexanone, 4-methoxycyclohexanone, and 2-hydroxycyclohexanone were obtained in quantitative yields from phenol, 4-methoxyphenol, and catechol at 3 h, 3.5 h, and 8 h, respectively, while 2-MCO was obtained from guaiacol in only 37% yield (98% selectivity) at 24 h.31
 | (3) |
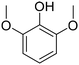
Syringol (2,6-dimethoxyphenol; possessing two methoxy groups at both o-positions of phenol) is another model compound having typical substructures of lignin. The reports on the hydrogenation of syringol to 2,6-dimethoxycyclohexanone are few, and there have been no reports with considerable yield. Partial hydrogenation of these o-methoxyphenols to o-methoxycyclohexanones is quite challenging, and there have been no reports focusing on this reaction. In this study, therefore, we investigated the partial hydrogenation of guaiacol to 2-MCO over various Pd catalysts because of their good ability for hydrogen activation and low activity for the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O hydrogenation of Pd species, as alluded to above. The relationship between Pd dispersion and catalytic performance was confirmed, and good yield (65%) was obtained with the optimized Pd/TiO2 catalyst.
O hydrogenation of Pd species, as alluded to above. The relationship between Pd dispersion and catalytic performance was confirmed, and good yield (65%) was obtained with the optimized Pd/TiO2 catalyst.
2. Experimental
2.1. Catalyst preparation
All the reagents were commercially available (Table S2†). Pd catalysts were prepared by the typical impregnation method. The Pd precursor was Pd(OAc)2 unless otherwise noted, and the typical Pd loading amount was 3 wt%. The properties of supports are summarized in Table S3.† First, an acetone solution of the Pd precursor (Pd(OAc)2, Pd(acac)2) or an aqueous solution (PdCl2, Pd(NO3)2, Pd(NH3)4Cl2) was added to the support in a beaker until the surface of the support was saturated, and the mixture was stirred well with a glass bar. The beaker containing the wet sample was then placed on a heating plate, and the mixture was dried at 343 K (in the case using either acetone solution or 373 K (aqueous solution)). This process was repeated until the entire precursor solution was consumed. The solids were further dried in a drying oven at 383 K overnight. The dried catalysts except those using carbon-based supports (AC (activated carbon) and CB (carbon black)) were calcined in air at 573 K for 3 h at a heating rate of 10 K min−1. Pd/AC and Pd/CB were directly reduced at 573 K for 1 h under a H2 flow (30 mL min−1) at a heating rate of 10 K min−1 instead of calcination.2.2. Activity test and product analysis
The activity tests were carried out in a stainless steel autoclave (190 mL) equipped with a glass inner vessel. The substrate, solvent (typically water), and catalyst were put into the autoclave together with a spinner. After the purge process of the autoclave with H2 three times, the autoclave was filled with 1 MPa H2 (at r.t.) and then heated to the designated temperature. The reaction time was counted from the time when the inner temperature monitored with a thermocouple reached 5 K below the target one. After an appropriate reaction time, the reactor was rapidly cooled in a cold-water bath. The gas phase was collected in a gas bag. The liquid phase was collected with 1,4-dioxane, and the catalyst powder was removed by filtration with a syringe filter.The quantification of products in the gas phase was carried out with a gas chromatograph (GC; Shimadzu GC-2014) equipped with a Porapak N packed column, a methanator, and a flame-ionization detector (FID). The products in the liquid phase were analyzed with a GC (Shimadzu GC-2025) equipped with a TC-WAX column (diameter 0.25 mm, length 30 m) and a FID. The conversion, yield, selectivity, carbon balance, and conversion rate were calculated using the following equations:
 | (4) |
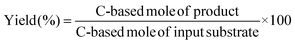 | (5) |
 | (6) |
 | (7) |
 | (8) |
 | (9) |
The carbon balance values were typically 100 ± 5% as shown in any tables in the ESI.† The selectivity calculated using eqn (6) might involve some overestimation because of the formation of unidentified products (within a few percent considering the carbon balance values).
Catalyst reuse tests were conducted as follows: the reaction was carried out in multiple autoclaves under the same conditions. The used catalysts were collected from each autoclave by filtration and mixed together. The mixed sample was washed with water (30 g) and dried at 383 K overnight. The dried catalyst was calcined at 573 K, if necessary. The total loss of catalyst during the recovery and regeneration processes was less than the amount necessary for one batch, and the next cycle of reaction was carried out with fewer autoclaves by one under the same conditions. For the washing process with toluene, the collected catalyst samples were first washed with water (30 g), followed by washing with toluene (30 g); afterward, the resulting samples were dried at 383 K overnight.
2.3. Characterization
Thermogravimetry-differential thermal analysis (TG-DTA) was carried out with a Rigaku Thermo Plus EVO-2 in an air flow (30 mL min−1). Wavelength dispersive X-ray fluorescence spectroscopy (XRF) was carried out with Bruker S8 Tiger apparatus, and Si in SiO2 was used as an internal standard. A Rigaku MiniFlex600 diffractometer (Cu Kα radiation) was used to acquire the X-ray diffraction (XRD) patterns of each sample. A catalyst analyzer BELCAT II (MicrotracMRB; equipped with a thermal conductivity detector) was used for CO chemisorption measurement. Prior to each run, the catalyst was reduced under a H2 flow (50 mL min−1) at 473 K for 30 min; afterward, CO pulses were introduced to the catalyst bed at 323 K. Pd dispersion was calculated as the ratio of the adsorbed CO amount to the total Pd amount. Scanning transmission electron microscopy (STEM) images were taken with a HITACHI HD-2700. For STEM measurement, the catalyst was reduced in water with 1 MPa H2 at 373 K (i.e., liquid phase reduction), dispersed in ethanol, and dropped on a Cu microgrid. 1H nuclear magnetic resonance (NMR) spectroscopy was carried out with a Bruker AV400 spectrometer (1H 400 MHz), and toluene-d8 was employed as a deuterated solvent. The methyl group (2.08 ppm) of toluene-d8 was used to calibrate the chemical shift.3. Results and discussion
3.1. Optimization of the catalyst
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 6
methanol = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Pd/CeO2, Pd/SiO2, Pd/TiO2, and Pd/AC gave relatively high conversion and 2-MCO yield. The high activity can be mostly explained by the higher dispersion of Pd, although there were some exceptions such as Pd/Nb2O5 with low activity and high dispersion. Among the three TiO2 supports, the activity of Pd/TiO2 catalysts decreased in the order of anatase > P-25 (anatase-rich anatase-rutile mixture) > rutile. Even with consideration of Pd dispersion, anatase provided higher activity than rutile. Furthermore, Pd/TiO2 (anatase) showed the highest selectivity to 2-MCO when compared at a similar conversion level (Table S5†). The Pd/HAP catalyst, which was reported to exhibit high selectivity to 2-MCO,31 showed low activity regardless of its comparable Pd dispersion to Pd/TiO2 (anatase). The selectivity of Pd/HAP to 2-MCO was similarly high to Pd/TiO2 (anatase), agreeing with the literature. Pd/CeO2 showed the highest conversion of guaiacol; however, the formation of byproducts such as 2-MCol and cyclohexanone was significantly large. This result is consistent with previous reports where Pd/CeO2 was reported to be an effective catalyst for the hydrogenolysis of guaiacol to cyclohexanol and cyclohexanone.57
1). Pd/CeO2, Pd/SiO2, Pd/TiO2, and Pd/AC gave relatively high conversion and 2-MCO yield. The high activity can be mostly explained by the higher dispersion of Pd, although there were some exceptions such as Pd/Nb2O5 with low activity and high dispersion. Among the three TiO2 supports, the activity of Pd/TiO2 catalysts decreased in the order of anatase > P-25 (anatase-rich anatase-rutile mixture) > rutile. Even with consideration of Pd dispersion, anatase provided higher activity than rutile. Furthermore, Pd/TiO2 (anatase) showed the highest selectivity to 2-MCO when compared at a similar conversion level (Table S5†). The Pd/HAP catalyst, which was reported to exhibit high selectivity to 2-MCO,31 showed low activity regardless of its comparable Pd dispersion to Pd/TiO2 (anatase). The selectivity of Pd/HAP to 2-MCO was similarly high to Pd/TiO2 (anatase), agreeing with the literature. Pd/CeO2 showed the highest conversion of guaiacol; however, the formation of byproducts such as 2-MCol and cyclohexanone was significantly large. This result is consistent with previous reports where Pd/CeO2 was reported to be an effective catalyst for the hydrogenolysis of guaiacol to cyclohexanol and cyclohexanone.57
| Entry | Catalyst | D (%) | Conv. (%) | Yield [C based] (%) | ||||
|---|---|---|---|---|---|---|---|---|
| 2-MCO | 2-MCol | Others | Methanol | |||||
Reaction conditions: guaiacol (0.62 g), catalyst (Pd = 3 wt%, 100 mg), water (10 g), 373 K, H2 (1 MPa at r.t.), 4 h. HAP: hydroxyapatite, AC: activated carbon, CB: carbon black.a  . . |
||||||||
| 1 | Pd/α-Al2O3 | 13.5 | 16.2 | 9.6 | 0.4 | 1.3 | 0.5 | 0.2 |
| 2 | Pd/BN | 6.5 | 8.1 | 3.2 | 0.8 | 0.1 | 0.3 | 0.0 |
| 3 | Pd/H-ZSM-5 | 19.2 | 23.5 | 0.3 | 14.9 | 3.4 | 1.3 | 2.0 |
| 4 | Pd/CeO2 | 57.6 | 56.4 | 20.8 | 13.8 | 9.0 | 6.7 | 2.3 |
| 5 | Pd/SiO2 | 29.4 | 45.0 | 33.0 | 6.7 | 4.6 | 2.9 | 1.0 |
| 6 | Pd/Nb2O5 | 43.6 | 13.6 | 6.0 | 1.9 | 0.6 | 1.0 | 0.2 |
| 7 | Pd/TiO2 (anatase) | 24.4 | 40.6 | 29.6 | 5.7 | 2.0 | 0.6 | 0.4 |
| 8 | Pd/TiO2 (rutile) | 15.3 | 15.3 | 7.2 | 1.6 | 1.0 | 1.6 | 0.2 |
| 9 | Pd/TiO2 (P25) | 17.4 | 33.3 | 24.0 | 3.3 | 2.0 | 1.1 | 0.4 |
| 10 | Pd/HAP | 21.4 | 10.4 | 7.3 | 0.7 | 0.4 | 0.3 | 0.1 |
| 11 | Pd/AC | 18.2 | 29.8 | 18.7 | 6.1 | 0.3 | 0.5 | 0.1 |
| 12 | Pd/CB | 6.9 | 4.6 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 |
Next, the time courses of the hydrogenation of guaiacol were compared among Pd/TiO2 (anatase), Pd/SiO2, and Pd/AC (Fig. 1; detailed data: Table S6†). The conversion reached 100% for all the three catalysts with enough reaction time. The yield of 2-MCO increased at the initial stage and then decreased because of the over-hydrogenation of 2-MCO to 2-MCol. The highest yield of 2-MCO (65%-C) was obtained over Pd/TiO2 (anatase). This yield value significantly surpasses previously reported yields of up to 37%.31 The lower selectivity of Pd/SiO2 and Pd/AC than that of Pd/TiO2 (anatase) was also confirmed by the conversion–selectivity plots (Fig. 1D): the selectivity of Pd/TiO2 (anatase) was higher than those of Pd/SiO2 and Pd/AC in the entire conversion range. These results indicate that TiO2 (anatase) is the best among the supports tested for the Pd-catalyzed guaiacol hydrogenation to 2-MCO. Pd/TiO2 (anatase) was thus employed in the following study and hereafter denoted as Pd/TiO2.
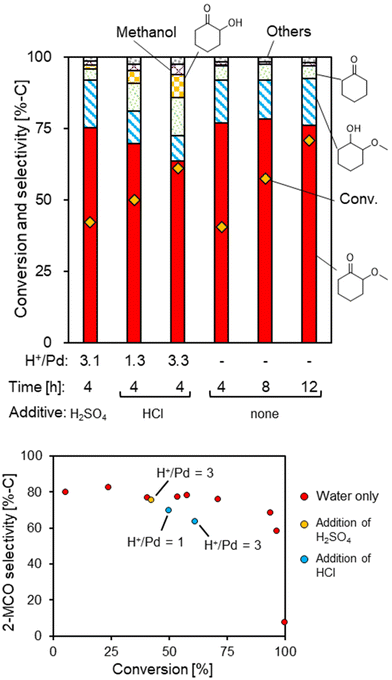
For the selectivity to 2-MCO at about 20% level of conversion (Fig. 2C), all the Pd/TiO2 catalysts showed similar selectivity to 2-MCO regardless of the Pd dispersion. Nevertheless, some Pd/TiO2 catalysts showed slightly lower selectivity, and the common feature was the use of a Cl−-containing precursor. To this end, the effect of residual Cl− in the reaction system was investigated through control reactions by adding HCl with the Cl−-free catalyst (Fig. 3; detailed data: Table S8†). The addition of HCl to the system with the Pd/TiO2 catalyst prepared from Pd(OAc)2 slightly increased the activity while decreasing the selectivity to 2-MCO. The increased byproducts were cyclohexanone and 2-hydroxycyclohexanone (hydrolysis product of 2-MCO). The effect of H+, which was inevitably added to the system due to the use of HCl, was checked by further control experiments using H2SO4 as an additive; however, the addition of H2SO4 did not affect the activity or selectivity. These results indicated that the presence of Cl− has negative effects on the selective hydrogenation to 2-MCO. In view of both activity and selectivity, the Pd/TiO2 catalyst prepared from the Cl−-free precursor with about 25% dispersion was the optimum catalyst; such a catalyst was obtained successfully with 3 wt% loading, Pd(OAc)2 as the precursor, and calcination at 573 K after Pd loading, i.e., the Pd/TiO2 (anatase) catalyst displayed in Table 1 and Fig. 1.
Here we discuss the possible active structure in the guaiacol hydrogenation to 2-MCO. The strong negative correlation between the TOF and Pd dispersion of the Pd/TiO2 catalysts (Fig. 2 and Fig. S1†) suggests that the simple flat surface (terrace sites) of the Pd metal phase is active for this reaction, and the corner and/or edge sites are rather inactive. This structure sensitivity can be explained by the reaction model drawn in Fig. 5 where the aromatic ring of guaiacol is adsorbed in a planar mode onto the terrace site of the Pd metal and the hydrogenation proceeds by the reaction of the adsorbed aromatic ring and hydrogen atoms also adsorbed on the Pd surface.60,61 In addition, the effect of the TiO2 support is not positive because the support effect becomes stronger with the decrease of particle size (increase of dispersion). The reaction mechanism will be further discussed in the later section after discussing the solvent effect, kinetics, and substrate scope. For the catalysts with other supports, the activity and selectivity of the Pd-support interface sites can be considered. Some catalysts (Pd/BN, Pd/TiO2 (rutile), and Pd/HAP) show lower activity than the Pd/TiO2 catalysts (Fig. 2). The possible explanation is that the Pd-support interface sites have lower activity than the Pd-TiO2 ones. Moreover, Pd/HAP and Pd/α-Al2O3 exhibited comparable selectivity toward 2-MCO to the Pd/TiO2 catalysts. The contribution of Pd-support interface sites in these two catalysts to side reactions was small: the interface sites had negligible activity (Pd/HAP: much lower activity than Pd/TiO2) or similar selectivity (Pd/α-Al2O3: only slightly lower activity than Pd/TiO2) to non-interface Pd sites. The Pd/CeO2 catalyst showed much higher activity while much lower selectivity compared to the Pd/TiO2 catalysts. This behavior can be explained by the high activity but low selectivity of Pd–CeO2 interface sites. The good performance of the optimized Pd/TiO2 catalyst would be due to the small effect of the TiO2 support on the catalytic performance of Pd-support interfacial sites and appropriate Pd particle size to expose the terrace sites.
3.2. Effect of the reaction conditions
| Entry | Solvent | Amount of catalyst (g) | Conv. (%) | Yield [C based] (%) | ||||
|---|---|---|---|---|---|---|---|---|
| 2-MCO | 2-MCol | Others | Methanol | |||||
| Conditions: guaiacol (0.62 g), Pd/TiO2 (Pd = 3 wt%), solvent (10 g), 373 K, H2 (1 MPa at r.t.), 4 h. | ||||||||
| 1 | Water | 0.1 | 40.6 | 29.6 | 5.7 | 2.0 | 0.7 | 0.4 |
| 2 | Methanol | 0.1 | 1.5 | 0.0 | 0.1 | 0.0 | 0.1 | 0.0 |
| 3 | Ethanol | 0.1 | 8.4 | 4.1 | 1.2 | 0.3 | 0.3 | 0.1 |
| 4 | 2-Propanol | 0.1 | 1.5 | 0.4 | 0.1 | 0.0 | 0.2 | 0.0 |
| 5 | Tetrahydrofuran | 0.1 | 7.2 | 3.2 | 1.8 | 0.5 | 1.1 | 0.2 |
| 6 | Cyclohexane | 0.1 | 12.7 | 7.2 | 3.3 | 0.8 | 0.4 | 0.1 |
| 7 | Dodecane | 0.1 | 14.9 | 6.1 | 2.9 | 0.4 | 0.3 | 0.0 |
| 8 | Dodecane | 0.3 | 38.0 | 17.1 | 15.2 | 1.4 | 1.1 | 0.2 |
| 9 | No solvent | 0.1 | 13.9 | 6.9 | 3.0 | 0.4 | 0.4 | 0.0 |
To examine the adsorption behavior of the substrate on the catalyst surface in each solvent, the effect of guaiacol concentration on the selective hydrogenation of guaiacol over the Pd/TiO2 catalyst was investigated at a low conversion level in each solvent (Fig. 6; detailed data: Table S10† (raw data) and Fig. S2† (conversion–time plot)). The detailed data including the guaiacol conversion rate are shown in Table S10 and Fig. S2.† The reaction order with respect to the guaiacol concentration was ca. 0.5 in ethanol and 0 in water and dodecane. These results suggest that the low activity of Pd/TiO2 in ethanol was due to the competitive adsorption of alcohol and substrate molecules onto the active sites. Meanwhile, the adsorption sites of the Pd/TiO2 catalyst were fully occupied with guaiacol in the water solvent. For the selective hydrogenation of guaiacol to 2-MCO, the adsorption with the hydrophobic aromatic ring of guaiacol is necessary, and that with the hydrophilic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of 2-MCO decreases the selectivity by promoting over-hydrogenation to 2-MCol.
O group of 2-MCO decreases the selectivity by promoting over-hydrogenation to 2-MCol.
The effect of solvent was also investigated in the hydrogenation of 2-MCO, which is the most impactful side reaction, over Pd/TiO2 (entries 10–13, Table S9†). The difference in activity among solvents was smaller in 2-MCO hydrogenation than that in guaiacol hydrogenation. The water solvent accelerates the hydrogenation of guaiacol to 2-MCO but does not accelerate the hydrogenation of 2-MCO to 2-MCol. This behavior can explain the higher selectivity to 2-MCO in water solvent than that in inert solvents, and therefore, the promotion mechanism by water solvent is important for both activity and selectivity in guaiacol hydrogenation to 2-MCO. The promotion ability of water solvent in the guaiacol hydrogenation will be discussed later together with the reaction mechanism.
| Entry | H2 pressure (MPa at r.t.) | Time (h) | Conv. (%) | Yield [C based] (%) | ||||
|---|---|---|---|---|---|---|---|---|
| 2-MCO | 2-MCol | Others | Methanol | |||||
| Conditions: guaiacol (0.62 g), Pd/TiO2 (Pd = 3 wt%, 100 mg), water (10 g), 373 K. | ||||||||
| 1 | 0.1 | 4 | 21.9 | 13.9 | 1.2 | 1.2 | 0.3 | 0.2 |
| 2 | 0.1 | 6 | 41.5 | 27.0 | 5.1 | 2.0 | 0.8 | 0.4 |
| 3 | 1 | 4 | 40.6 | 29.6 | 5.7 | 2.0 | 0.7 | 0.4 |
| 4 | 3 | 4 | 45.2 | 28.3 | 7.9 | 1.8 | 0.8 | 0.3 |
| 5 | 5 | 4 | 43.1 | 26.9 | 9.8 | 1.6 | 0.8 | 0.3 |
3.3. Reusability of the Pd/TiO2 catalyst
The reusability of the Pd/TiO2 catalyst was evaluated in the selective hydrogenation of guaiacol (Fig. 8; detailed data: Table S12†). The activity of the Pd/TiO2 catalyst decreased upon its reuse when the recovered catalyst was reused after filtration, washing with water, and drying. The TG-DTA profile for the spent catalyst showed an exothermic weight loss at around 523 K (Fig. S3B†), suggesting that the deposition of organic species on the catalyst surface led to the loss of activity. Thus, the calcination at 573 K as regeneration was then applied to the recovered catalyst after washing with water. Although the activity was partially recovered, the activity of the Pd/TiO2 catalyst still decreased. The Pd dispersion of the calcined catalysts measured by CO adsorption gradually decreased in the reuses (Table S13†), suggesting that the decrease in the number of active sites due to the agglomeration of Pd particles during the calcination caused the decrease in activity. The increase of Pd particle size was also confirmed in the XRD pattern (Fig. S4†). On the other hand, the washing treatment for the used Pd/TiO2 catalyst with toluene succeeded in the preservation of catalytic activity. The TG-DTA profile for the catalyst washed with toluene indicated the absence of organic species on the catalyst surface. The organic species dissolved in toluene was analyzed by 1H NMR spectroscopy (Fig. S5†). Various signals were observed, such as those for alkyl (0.8–1.7 ppm), methoxy (3.0–3.3 ppm; singlet signals), and olefinic (5.5 ppm) protons. Although the possibility of overlapping with toluene signals cannot be ruled out completely, the peaks attributable to aromatic compounds were not detected. These NMR data indicate that polymeric hydrogenated compounds can be the organic species covering the used catalyst. | ||
| Fig. 8 Reusability of the Pd/TiO2 catalyst in guaiacol hydrogenation. Conditions: guaiacol (0.62 g), Pd/TiO2 (Pd = 3 wt%, 200 mg), water (10 g), 373 K, H2 (1 MPa at r.t.), 4 h. | ||
3.4. Substrate scope of Pd/TiO2
The Pd/TiO2 catalyst was applied to the hydrogenation of various phenolic compounds (Table 4). The detailed data including the time course for each substrate are shown in Table S14.† The hydrogenation of phenol was very fast, and a high yield (92%) of cyclohexanone was obtained. The hydrogenation of 4-methoxyphenol (entry 3) was faster than that of guaiacol (2-methoxyphenol), and demethoxylation from 4-methoxyphenol proceeded more slowly than that from guaiacol. The significantly higher reactivity of 4-methoxyphenol than that of guaiacol has also been reported with other Pd catalysts.31,55,62 In addition, the effect of substituents at the ortho position on the reactivity in the aromatic ring hydrogenation followed the order of –H ∼ –OH > –CH3 ≥ –CF3 ≫ –OCH3 (entries 1, 2, and 5–7). This tendency can be mostly explained by steric hindrance due to the size of substituents, while guaiacol is an exception with very low reactivity. The reactivity of 2,6-dimethoxyphenol (syringol) was further lower than guaiacol, and the selectivity to 2,6-dimethoxycyclohexanone was also very low (entry 8). The presence of 2-methoxy groups thus drastically decreased the reactivity. On the other hand, 2-ethoxyphenol showed slightly higher reactivity than guaiacol, although the ethoxy group is bulkier than the methoxy group. The presence of an intramolecular hydrogen bond between the adjacent alkoxy and hydroxy groups is a possible reason for the decrease in reactivity.We discuss the effect of the methoxy group at the ortho position and the reaction mechanism. In the hydrogenation of phenol to cyclohexanone, which is closely related to the selective hydrogenation of guaiacol, two reaction routes have been proposed thus far based on density functional theory (DFT) studies;60,61,63 (i) direct hydrogenation of adsorbed phenol and (ii) dissociation of adsorbed phenol to adsorbed phenoxide and H followed by hydrogenation. For route (i), the hydrogenation of adsorbed phenol first produces adsorbed dihydrophenol. The dihydrophenol is then transformed into cyclohexanone by keto–enol tautomerization, and the cyclohexanone is hydrogenated to cyclohexanone. Lercher et al. investigated the effect of water solvent over Pt and Ni catalysts using DFT-based molecular dynamics calculations; the calculated activation energy for keto–enol tautomerization was greatly reduced by the proton transfer via proton jumping in solvent molecules compared to the direct proton transfer from oxygen to carbon.63 The thermodynamic stability of the adsorbed keto form was also found to be enhanced. The positive effect of water solvent observed for guaiacol hydrogenation, but not for 2-MCO hydrogenation, in this study agrees with the calculation report of route (i). Fig. 9 depicts the possible reaction routes for the selective hydrogenation of guaiacol over the Pd/TiO2 catalyst in a water solvent, and route (i) is the more likely one. The presence of an intramolecular hydrogen bond between the adjacent methoxy and hydroxy groups in guaiacol may suppress the dissociation of the O–H bond and the reaction route (ii). The reaction route (i) is also slowed down by the presence of the 2-methoxy group because of the stabilization of the enol form. Still, the water solvent promotes the keto–enol formation, and 2-MCO is produced in this route.
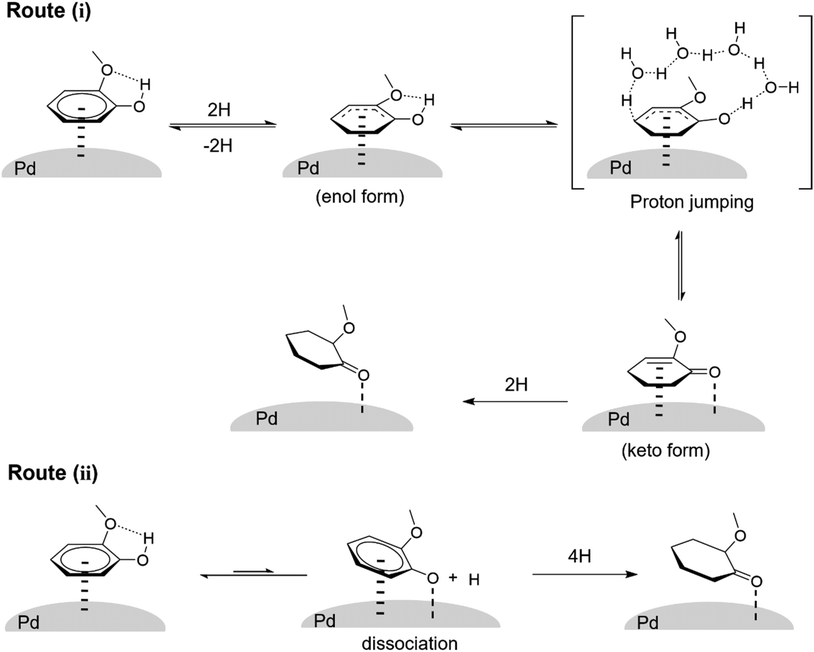 | ||
| Fig. 9 Possible reaction routes for the selective hydrogenation of guaiacol over the Pd/TiO2 catalyst in water solvent. | ||
The formation ratios of cis/trans isomers of 2-MCol from guaiacol and 2-MCO as substrates were compared (Table S15†). From 2-MCO, the cis![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trans ratio of 2-MCol was almost 1
trans ratio of 2-MCol was almost 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 regardless of the reaction time. On the other hand, the cis
1 regardless of the reaction time. On the other hand, the cis![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trans ratio was about 2
trans ratio was about 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 from guaiacol when the overreaction of 2-MCO was minor. When the produced 2-MCO was almost completely converted to 2-MCol (24 h), the cis
1 from guaiacol when the overreaction of 2-MCO was minor. When the produced 2-MCO was almost completely converted to 2-MCol (24 h), the cis![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trans ratio became about 1.5
trans ratio became about 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. These data indicated that part of 2-MCol was directly produced from guaiacol by the syn addition of hydrogen species to the unsaturated ring adsorbed on the metal surfaces. The large contribution of the direct total hydrogenation of guaiacol can be more reasonably explained by route (i) in Fig. 9. The enol form of adsorbed dihydroguaiacol is more stabilized by the hydrogen bond than the cases of other phenolic compounds, and the further hydrogenation of the adsorbed enol form, which can be regarded as the direct hydrogenation of guaiacol, becomes easier. These results and considerations further support the aforementioned possibility of route (i) as the more plausible route than route (ii).
1. These data indicated that part of 2-MCol was directly produced from guaiacol by the syn addition of hydrogen species to the unsaturated ring adsorbed on the metal surfaces. The large contribution of the direct total hydrogenation of guaiacol can be more reasonably explained by route (i) in Fig. 9. The enol form of adsorbed dihydroguaiacol is more stabilized by the hydrogen bond than the cases of other phenolic compounds, and the further hydrogenation of the adsorbed enol form, which can be regarded as the direct hydrogenation of guaiacol, becomes easier. These results and considerations further support the aforementioned possibility of route (i) as the more plausible route than route (ii).
The performance of the Pd/TiO2 catalyst is compared with that of other catalysts in previous reports (Table S1†). For the hydrogenation of phenolic compounds without methoxy groups such as o-cresol (2-methylphenol) and catechol (2-hydroxyphenol), the Pd/TiO2 catalyst exhibited slightly lower selectivity towards the target ketones than the reported best catalysts with sophisticated supports because of the over-hydrogenation. In contrast, in the selective hydrogenation of phenol derivatives containing methoxy groups, the Pd/TiO2 catalyst performed comparable to or even better than other catalysts. A difference from reported catalysts is the lower activity of the Pd/TiO2 catalyst for demethoxylation. Many reported catalysts used basic supports to offer adsorption sites for the weakly acidic phenolic group to promote phenol hydrogenation over cyclohexanone hydrogenation; however, basic sites also promote demethoxylation during the hydrogenation of methoxyphenols to decrease the selectivity to methoxycyclohexanones.47,48,51
4. Conclusions
We have reported the hydrogenation of guaiacol to 2-methoxycyclohexanone in good yields (up to 65%) using the Pd/TiO2 catalyst in an aqueous solution. The chloride ion promotes the demethoxylation of guaiacol to decrease the selectivity, and thus the use of a Cl-free precursor is preferred. In reusable tests, the recovered catalyst after washing with water or calcination exhibited lower activity than the fresh catalyst; in stark contrast, the catalyst could be reused by washing with toluene. Furthermore, the catalyst was applicable to the selective hydrogenation of various phenolic compounds and produced the corresponding ketones in high yields. In particular, the Pd/TiO2 catalyst is good at the selective hydrogenation of methoxyphenols in comparison with reported catalysts using a Pd + basic support. Through the characterization of Pd dispersion by CO adsorption, we determined the optimal Pd particle size for the formation of 2-MCO from guaiacol to be approximately 3 nm because of the balance of good dispersion and exposure to flat metal surfaces. Considering the solvent effect and cis/trans formation ratio of 2-methoxycyclohexanol, the reaction route involving keto–enol tautomerization of intermediates is more plausible.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by JSPS KAKENHI grants 23H05404, 23K20034, and 23K26451. The authors also appreciate the assistance provided by the Technical Division, School of Engineering, Tohoku University, in STEM measurement.References
- A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef CAS PubMed
.
- M. Besson, P. Gallezot and C. Pinel, Chem. Rev., 2014, 114, 1827–1870 CrossRef CAS PubMed
.
- B. M. Stadler, C. Wulf, T. Werner, S. Tin and J. G. Vries, ACS Catal., 2019, 9, 8012–8067 CrossRef CAS
.
- D. M. Alonso, J. Q. Bond and J. A. Dumesic, Green Chem., 2010, 12, 1493–1513 RSC
.
- G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106, 4044–4098 CrossRef CAS PubMed
.
- H. Wang, J. Male and Y. Wang, ACS Catal., 2013, 3, 1047–1070 CrossRef CAS
.
- Q. Lu, X. Jiang, Z. Zhang, X. Zhang, G. Song, H. Wang, Y. Yuan and Y. Chang, Green Chem., 2021, 23, 9348–9376 RSC
.
- J. Yang, S. Luan, M. Dong, Y. Wu, X. Xing, X. Liu, J. Xiang, Z. Zhao, S. Li, B. Zhang, H. Liu and B. Han, CCS Chem., 2024, 6, 709–718 CrossRef CAS
.
- B. Zhang, Q. Meng, H. Liu and B. Han, Acc. Chem. Res., 2023, 56, 3558–3571 CrossRef CAS PubMed
.
- K. Yamazaki, A. Segawa, H. Mazaki, N. Hiyoshi, N. Mimura, O. Sato and A. Yamaguchi, RSC Adv., 2023, 13, 13472–13476 RSC
.
- B. Liu, M. Sanchez, J. Truong, P. C. Ford and M. M. Abu-Omar, Green Chem., 2022, 24, 4958–4968 RSC
.
- D. J. Nowakowski, A. V. Bridgwater, D. C. Elliott, D. Meier and P. Wild, J. Anal. Appl. Pyrolysis, 2010, 88, 53–72 CrossRef CAS
.
- T. S. Nguyen, D. Laurenti, P. Afanasiev, Z. Konuspayeva and L. Piccolo, J. Catal., 2016, 344, 136–140 CrossRef CAS
.
- J. Mao, J. Zhou, Z. Xia, Z. Wang, Z. Xu, W. Xu, P. Yan, K. Liu, X. Guo and Z. C. Zhang, ACS Catal., 2017, 7, 695–705 CrossRef CAS
.
- X. Jin, B. Yin, Q. Xia, T. Fang, J. Shen, L. Kuang and C. Yang, ChemSusChem, 2018, 12, 71–92 CrossRef PubMed
.
- A. Gutierrez, R. K. Kaika, M. L. Honkela, R. Slioor and A. O. I. Krause, Catal. Today, 2009, 147, 239–246 CrossRef CAS
.
- M. V. Bykova, D. Y. Ermakov, V. V. Kaichev, O. A. Bulavchenko, A. A. Saraev, M. Y. Lebedev and V. A. Yakovlev, Appl. Catal., B, 2012, 113, 296–307 CrossRef
.
- R. N. Olcese, M. Bettahar, D. Petitjean, B. Malaman, F. Giovanella and A. Dufour, Appl. Catal., B, 2012, 115, 63–73 CrossRef
.
- Y. C. Lin, C. L. Li, H. P. Wan, H. T. Lee and C. F. Liu, Energy Fuels, 2011, 25, 890–896 CrossRef CAS
.
- K. Zhang, Q. Meng, H. Wu, J. Yan, X. Mei, P. An, L. Zheng, J. Zhang, M. He and B. Han, J. Am. Chem. Soc., 2022, 144, 20834–20846 CrossRef CAS PubMed
.
- M. M. Ambursa, J. C. Juan, Y. Yahaya, Y. H. T. Yap, Y. C. Lin and H. V. Lee, Renewable Sustainable Energy Rev., 2021, 138, 110667 CrossRef CAS
.
- V. S. Prabhudesai, L. Gurrala and R. Vinu, Energy Fuels, 2022, 36, 1155–1188 CrossRef CAS
.
- X. Wang, M. Arai, Q. Wu, C. Zhang and F. Zhao, Green Chem., 2020, 22, 8140–8168 RSC
.
- A. Yamaguchi, Y. Murakami, K. Yamazaki, M. Shirai and N. Hiyoshi, Catal. Today, 2024, 425, 114356 CrossRef CAS
.
- H. Yang, X. Zhu, H. W. Amini, B. Fachri, M. Ahmadi, G. H. Brink, P. J. Deuss and H. J. Heeres, Appl. Catal., A, 2023, 654, 119062 CrossRef CAS
.
- R. Beerthuis, G. Rothenberg and N. R. Shiju, Green Chem., 2015, 17, 1341–1361 RSC
.
- J. Rios, J. Lebeau, T. Yang, S. Li and M. D. Lynch, Green Chem., 2021, 23, 3172–3190 RSC
.
- Y. Nakagawa, M. Yabushita and K. Tomishige, Asian J. Org. Chem., 2023, 12, e202300409 CrossRef CAS
.
- J. Rios, J. Lebeau, T. Yang, S. Li and M. D. Lynch, Green Chem., 2021, 23, 3172–3190 RSC
.
- K. Hatakeyama, Y. Nakagawa, M. Tamura and K. Tomishige, Green Chem., 2020, 22, 4962–4974 RSC
.
- G. Xu, J. Guo, Y. Zhang, Y. Fu, J. Chen, L. Ma and Q. Guo, ChemCatChem, 2015, 7, 2485–2492 CrossRef CAS
.
- H. Chen and J. Sun, J. Ind. Eng. Chem., 2021, 94, 78–91 CrossRef CAS
.
- Y. Liao, S. Koekewijn, G. V. Bossche, J. V. Aelst, S. V. D. Bosch, T. Renders, K. Navare, T. Nicokai, K. V. Aelst, M. Maesen, H. Matsusima, J. M. Thevelein, K. V. Acker, B. Lagrain, D. Verboekend and B. F. Sels, Science, 2020, 367, 1385 CrossRef CAS PubMed
.
- H. Wang, W. Wang, R. Wang, X. Jiang, W. Li, Z. H. He, K. Wang, Y. Yang, Q. Li and Z. T. Liu, Appl. Catal., A, 2024, 670, 119520 CrossRef CAS
.
- J. Zhang, H. Zhao, L. Yang, H. Jiang, Y. Du and R. Chen, Appl. Catal., A, 2023, 666, 119428 CrossRef CAS
.
- Z. Wang, B. Ye, R. Zhou, Z. Zhong, P. Chen and Z. Hou, Appl. Catal., A, 2023, 657, 119144 CrossRef CAS
.
- D. Yin, R. Ji, J. Zhang, S. Yu, L. Li, S. Liu, L. Jiang, Z. Song and Y. Liu, Fuel, 2023, 333, 126481 CrossRef CAS
.
- D. Yin, R. Ji, F. Lv, L. Jiang, J. Zhang, M. Liu, Z. Jia, S. Yu, R. Zhao and Y. Liu, Fuel, 2023, 332, 126060 CrossRef CAS
.
- X. Zhu, J. Zhang, H. Jiang and R. Chen, Appl. Catal., A, 2022, 634, 118538 CrossRef CAS
.
- C. Yang, K. Li, J. Wang and S. Zhou, Appl. Catal., A, 2021, 610, 117961 CrossRef CAS
.
- Y. Wang, J. Yao, H. Li, D. Su and M. Antonietti, J. Am. Chem. Soc., 2011, 133, 2362–2365 CrossRef CAS PubMed
.
- Y. Shao, J. Zhang, H. Jiang and R. Chen, Ind. Eng. Chem. Res., 2021, 60, 5806–5815 CrossRef CAS
.
- H. Li, T. She, G. Chen, M. Sun, L. Niu and G. Bai, Mol. Catal., 2021, 504, 111493 CrossRef CAS
.
- S. Xu, J. Du, Q. Zhou, H. Li, C. Wang and J. Tang, Colloid Interface Sci., 2021, 604, 876–884 CrossRef CAS PubMed
.
- M. Aliahmadi, M. Davoudi and A. N. Kharat, React. Kinet., Mech. Catal., 2020, 131, 819–828 CrossRef CAS
.
- J. Zhang, C. Zhang, H. Jiang, Y. Liu and R. Chen, Ind. Eng. Chem. Res., 2020, 59, 10768–10777 CrossRef CAS
.
- M. Ishikawa, M. Tamura, Y. Nakagawa and K. Tomishige, Appl. Catal., B, 2016, 182, 193–203 CrossRef CAS
.
- Y. Nakagawa, M. Ishikawa, M. Tamura and K. Tomishige, Green Chem., 2014, 16, 2197–2203 RSC
.
- C. Li, Y. Nakagawa, M. Tamura, A. Nakayama and K. Tomishige, ACS Catal., 2020, 10, 14624–14639 CrossRef CAS
.
- C. R. Lee, J. S. Yoon, Y. W. Suh, J. W. Choi, J. M. Ha, D. J. Suh and Y. K. Park, Catal. Commun., 2012, 17, 54–58 CrossRef CAS
.
- L. Huang, F. Tang, P. Liu, W. Xiong, S. Jia, F. Hao, Y. Lv and H. Luo, Fuel, 2022, 327, 125115 CrossRef CAS
.
- S. Wang, L. Yang, T. Zhu, N. Jiang, F. Li, H. Wang, C. Zhang and H. Song, React. Chem. Eng., 2022, 7, 170–180 RSC
.
- F. Zhang, C. Wu, S. Wang, S. Wang, T. Li, L. Zou, H. Yu and H. Yin, Catal. Sci. Technol., 2022, 12, 2257–2264 RSC
.
- P. Yan, P. Tian, K. Li, M. A. C. Stuart, J. Wang, X. Yu and S. Zhou, Chem. Eng. J., 2020, 397, 125484 CrossRef CAS
.
- H. Zhang, A. Han, K. Okumura, L. Zhong, S. Li, S. Jaenicke and G. K. Chuah, J. Catal., 2018, 364, 354–365 CrossRef CAS
.
- A. Chen, G. Zhao, J. Chen, L. Chen and Y. Yu, RSC Adv., 2013, 3, 4171–4175 RSC
.
- H. Zhou, H. Wang, A. D. Sadow and I. I. Slowing, Appl. Catal., B, 2020, 270, 118890 CrossRef CAS
.
- L. L. Sheu, Z. Karpinski and W. M. H. Sachtler, J. Phys. Chem., 1989, 93, 4890–4894 CrossRef CAS
.
-
G. Bergeret and P. Gallezot, Particle Size and Dispersion Measurements, in Handbook of Heterogeneous Catalysis, ed. G. Ertl, H. Knözinger, F. Schüth and J. Weitkamp, Wiley-VCH, Weinheim, 2008, pp. 738–765 Search PubMed
.
- G. Li, J. Han, H. Wang, H. Zhu and Q. Ge, ACS Catal., 2015, 5, 2009–2016 CrossRef CAS
.
- H. Zhou, B. Han, T. Liu, X. Zhong, G. Zhuang and J. Wang, Green Chem., 2017, 19, 3585–3594 RSC
.
- S. Fehn, M. Zaheer, C. E. Denner, M. Friedrich and R. Kempe, New J. Chem., 2016, 40, 9252–9256 RSC
.
- Y. Yoon, R. Rousseau, R. S. Weber, D. Mei and J. A. Lercher, J. Am. Chem. Soc., 2014, 136, 10287–10298 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4gc03793f |
| This journal is © The Royal Society of Chemistry 2024 |