Synthesis and antifreeze activity of fish antifreeze glycoproteins and their analogues
Raoul
Peltier
a,
Margaret A.
Brimble
*a,
Joanna M.
Wojnar
a,
David E.
Williams
a,
Clive W.
Evans
b and
Arthur L.
DeVries
c
aDepartment of Chemistry, The University of Auckland, Private Bag 92019, Auckland 1142, New Zealand. E-mail: m.brimble@auckland.ac.nz
bSchool of Biological Sciences, The University of Auckland, Private Bag 92019, Auckland 1142, New Zealand
cDepartment of Animal Biology, University of Illinois at Urbana-Champaign, 524 Burrill Hall, 407 S Goodwin, Urbana, IL 61801, USA
First published on 30th June 2010
Abstract
Fishes from both Arctic and Antarctic waters produce antifreeze glycoproteins (AFGPs) that modify and inhibit the growth of ice crystals, allowing them to survive in extreme cold conditions. These glycoproteins exhibit thermal hysteresis activity, i.e. they work in a non-colligative manner, separating the melting and freezing points of a solution. Such compounds have many potential applications; unfortunately their development is hampered by the difficulty of obtaining pure material. The synthesis of AFGPs is therefore a challenge that numerous groups have been tackling. The AFGPs consist predominantly of a repetitive three amino acid unit (Ala-Ala-Thr)n with the disaccharide β-D-galactosyl-(1–3)-α-D-N-acetylgalactosamine attached to the hydroxyl oxygen of each threonine residue. A large number of analogues have also been synthesized in order to find compounds that exhibit the same activity but that are easier to prepare. Starting from the early years of the AFGP discovery and including the more recent research, this perspective summarizes the different routes used to synthesize native AFGPs and lists the most relevant analogues synthesized, along with some information on their synthesis and their antifreeze activity, if evaluated. In this perspective we have taken special care to differentiate compounds that induce thermal hysteresis, compounds that modify the normal growth habit of ice crystals, and compounds that exhibit recrystallization inhibition properties.
 Raoul Peltier | Raoul Peltier was born in Tarbes, France. He received his MSc in chemistry at The University Paul Sabatier, Toulouse (France) in collaboration with the Polymer Research Group of Professor Zhu at the University of Montreal (Canada). He is presently completing a PhD at the University of Auckland (New Zealand) under the supervision of Professor David E. Williams, Professor Margaret A. Brimble and Associate Professor Clive W. Evans. His research is based on the biomimetic templating of crystal growth using the antifreeze glycoprotein as a model. |
 Margaret A. Brimble | Margaret Brimble was born in Auckland, New Zealand. She completed an MSc in chemistry at The University of Auckland. She was awarded a UK Commonwealth Scholarship to undertake her PhD studies at Southampton University. She currently holds the Chair of Organic and Medicinal Chemistry at the University of Auckland where her research program focuses on the synthesis of spiroacetal-containing natural products, pyranonaphthoquinone antibiotics, alkaloids and peptidomimetics for the treatment of neurodegenerative disorders, the synthesis of fish antifreeze neoglycopeptides and neoglycopeptides as vaccine components. |
 Joanna M. Wojnar | Joanna Wojnar completed her PhD in natural products chemistry at Victoria University of Wellington (New Zealand). She then undertook postdoctoral research in the lab of Steve Kent, at the University of Chicago, focusing on the chemical synthesis of proteins. Returning to New Zealand, she has taken up a postdoctoral position with Margaret Brimble at the University of Auckland, where she is working on the synthesis of antifreeze glycopeptide probes. |
 David E. Williams | David Williams is a graduate of the University of Auckland. He developed his research career in electrochemistry and chemical sensors at the UK Atomic Energy Research Establishment, Harwell, in the 1980s. He was Thomas Graham Professor of Chemistry at University College London from 1991–2002 and co-founded two companies to exploit his work on gas sensors. He was Chief Scientist of Inverness Medical Innovations, based at Unipath Ltd, Bedford, UK, from 2002–2005 where he developed his interests in biomedical diagnostic devices, microfluidics, immunoassays and biorecognition reagents. He joined the faculty of the Chemistry Department at Auckland University in February 2006. |
 Clive W. Evans | Clive Evans completed his PhD in zoology at the University of Auckland in 1975 and undertook postdoctoral research in the Department of Cell Biology at the University of Glasgow, focusing on immune cell interactions. He then moved to the University of St Andrews where he worked on aspects of cell behavior underpinning the metastatic spread of cancer cells, until returning to the University of Auckland in 1988. Here he shifted his attention to molecular aspects of fish development and physiology, and commenced an Antarctic research program primarily addressing functional aspects of fish antifreeze. |
 Arthur L. DeVries | Art DeVries is a native of the state of Montana in the US. He received a degree in zoology at the University of Montana and a PhD in biology from Stanford University in 1968. He spent six years as a research associate in the Physiological Research Laboratory, Scripps Institute of Oceanography, and then accepted a position in the Department of Physiology at the University of Illinois, Urbana-Champaign. Presently he is a professor in the Department of Animal Biology and Molecular and Integrative Physiology. His research focus is in cold adaptation of polar fishes and is centered around understanding the role that antifreeze proteins play in their survival. His research interests include structure, function, origin and evolution of the antifreeze proteins. |
1. Introduction
The extreme conditions experienced in both the Arctic and Antarctic polar ocean environments, where seawater temperature can reach down to −1.9 °C, would cause any temperate fish species to instantly freeze as their equilibrium freezing point is near −0.7 °C.1–3 Fishes that can survive under these freezing conditions have been found to produce a range of protective molecules, either antifreeze proteins (AFPs) or antifreeze glycoproteins (AFGPs), depending on the species.4–9 The antifreeze (glyco)proteins produced by these fishes have the capacity to lower the freezing point of the blood by inhibiting the growth of ice crystals that enter the body of the fishes, thus preventing them from freezing to death. Antifreeze (glyco)proteins are believed to operate by adsorbing onto specific faces of ice crystals, usually the prism faces, thereby inhibiting crystal growth predominantly along the a-axis.10–13 This alters the growth habit of the ice crystal from a hexagonal plate, creating a hexagonal bipyramid in which the now stepped prism faces are covered by a network of antifreeze (glyco)proteins.14 Once the crystal reaches this typical geometry, its growth is stopped until the temperature further decreases below a new depressed freezing point, also called the “hysteresis freezing point” at which crystal growth accelerates. The difference between the melting point and the depressed freezing point is referred to as the thermal hysteresis. When the temperature decreases further below the depressed freezing point, the ice crystals grow quickly along the c-axis, giving rise to needle-shaped, bipyramidal crystals.15The expression of thermal hysteresis behavior during freezing point depression is implicit in the original definition of “antifreeze activity”.16,17 Although AFGPs and AFPs modify the growth habit of ice crystals as explained above, not all ice crystal growth modifiers have thermal hysteresis activity. Another characteristic of the antifreeze (glyco)proteins is their capacity to inhibit ice recrystallization. Recrystallization is a process whereby large ice crystals grow larger at the expense of small ones when a frozen solution is held at a temperature below its equilibrium freezing point; its inhibition results in the conservation of the initial crystal size distribution.18 Again, although many AFGPs and AFPs have also been shown to act as ice recrystallization inhibitors,19–21 not all recrystallization inhibitors possess thermal hysteresis activity.22–24 Adsorption of antifreeze (glyco)proteins onto ice crystals is another property to consider. The observation of ice crystals faceting during freezing as well as the superheating of the ice in the presence of AFPs25,26 implies that antifreeze (glyco)proteins bind to ice crystals. Given this, the properties of thermal hysteresis, growth habit modification and recrystallization inhibition can thus be considered as consequences of different degrees of adsorption of the compounds onto ice.
Thermal hysteresis can be measured using microcapillary thermal hysteresis technology27 or a microscope equipped with a nanolitre osmometer,28 in which case the resulting crystal shape is observed during the experiment.29 Recrystallization inhibition activity, which can now be quantified,30 is usually measured using assays derived from the “splat cooling” method described by Knight and DeVries.31,32 A few methods allow the characterization of AF(G)P adsorption onto ice. Ice hemisphere etching, a method that looks at the mark left by AF(G)Ps on a single ice crystal grown in a dilute solution, is used to determine on which ice crystal plane the AF(G)Ps adsorb.33 This adsorption can be quantified using ellipsometry measurements on a single ice crystal.10
Despite extensive literature on the topic, the detailed molecular-level mechanism of ice growth modification and inhibition by antifreeze (glyco)proteins is still unclear. Two major models exist: the mattress button model which describes a 2D inhibition of the ice growth perpendicular to its surface26,34 and the step-pinning model which involves a 3D inhibition of a growing step on the ice surface.15,33 According to the step-pinning model, ice crystal growth occurs by steps advancing across the adsorption plane and growth inhibition occurs when antifreeze (glyco)proteins “pinned” to the surface inhibit this step-growth process. In the mattress button model, individual antifreeze (glyco)proteins are visualized as buttons that bind to the ice crystal surface, limiting ice lattice growth to the spaces between them, thus creating a mattress-like (puckered) surface. Ice crystal growth is presumed to be inhibited in both models through the Kelvin (Gibbs–Thompson) effect arising as a consequence of interface curvature. These models imply an adsorption-inhibition mechanism that occurs with strong adsorption of the protein. Recently, a study by Celik et al.25 observed and quantified the superheating of ice in the presence of hyperactive AFP. This observation, along with the earlier qualitative observation of superheated ice in the presence of AFP by Knight and DeVries,26 clearly argues for an irreversible binding of at least some of the AFPs. However, researchers have expressed doubt that adsorption is necessarily completely irreversible.35–39 Hall and Lips,40 for example, proposed a mechanism in which the labile adsorption of antifreeze (glyco)proteins provides a barrier for 2D nucleation by increasing the line tension of the ice surface.
As many reviews have covered research on antifreeze proteins,19,41–43 the present review will focus on the antifreeze glycoproteins, which are found in fish such as the Antarctic notothenioids and the northern cods.4,5,44 The AFGPs consist predominantly of a repetitive three amino acid unit (Ala-Ala-Thr)n with the disaccharide β-D-galactosyl-(1–3)-α-D-N-acetylgalactosamine attached to the hydroxyl oxygen of each threonine residue (Fig. 1).44,45 Their molar mass varies between 2.6 and 33.7 kDa, which corresponds to 4 to 50 repetitions of the glycosylated tripeptide unit.17 These isoforms are conveniently grouped into eight size classes based on their electrophoretic properties, with AFGP1 representing the largest and AFGP8 the smallest of the notothenioid antifreeze glycoproteins.46 Some minor sequence variations have been identified in the AFGPs; Ala-Ala-Thr units, for example, are occasionally replaced by Pro-Ala-Thr in the smaller AFGPs.47–49
 | ||
| Fig. 1 Native AFGP molecule; n varies between 4 and 50. | ||
The conformation of the AFGPs in the presence of ice has not been elucidated to date, although most researchers agree on an amphipathic structure with a hydrophilic face containing exposed hydroxyl groups of the sugars and a hydrophobic face containing the exposed methyl groups of both the amino acids and the N-acetyl groups.50–62 The nature of the interactions involved in the ice-binding process also remains uncertain, but the creation of many hydrogen-bonds between the hydroxyls of the sugars and the ice lattice remains to date the major hypothesis to explain the strong bonding of AFGPs to ice.63–65 Interestingly, recent studies with AFPs suggest that these molecules bind onto ice crystals via their hydrophobic faces66–70 rather than their hydrophilic ones as initially suggested.17,35,71 The difficulties in understanding the mechanism of action of AFGPs are mainly due to the problem of obtaining a large quantity of pure material to enable detailed studies, as extracting pure isoforms of AFGPs from the fish blood is a long and expensive process.72 Such problems slow down the development of potential AFGP-based medical and industrial applications. For these reasons, the synthesis of natural AFGPs and analogues is a challenge which numerous research groups are currently tackling. Descriptions of some of the synthetic work done on the native AFGPs can be found in existing reviews.64,73 However, a detailed review covering the large amount of synthetic work done on the AFGPs and listing the AFGP analogues prepared to date has not been published. Such a review is necessary in order to highlight the difficulties encountered during synthesis, what solutions exist today, and how these can be used to make new active compounds more efficiently. Here we provide, first, a summary of the different routes used to synthesize native AFGPs and, secondly, a compilation of the most relevant analogues synthesized to date, with some important details about their syntheses and their antifreeze activity if tested. Since the term “antifreeze activity” loosely covers the three distinct effects as discussed above, special care has been taken in order to differentiate compounds that exhibit thermal hysteresis, compounds that modify the normal growth habit of ice, or compounds that exhibit recrystallization inhibition properties.
2. Synthesis of native AFGP
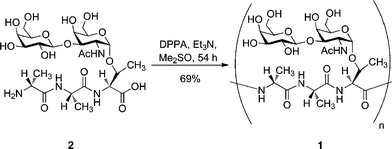
The first synthesis of the natural tripeptide building block containing the disaccharide β-D-galactosyl-(1–3)-α-D-N-acetylgalactosamine was reported by Tsuda and Nishimura in 1996.74 They synthesized the monomer 2 (Scheme 1) in 17% yield from the commercially available 1,3,4,6-tetra-O-acetyl-2-azido-2-deoxy-α/β-D-galactopyranose over six steps. Solution-phase synthesis was then used to polymerize the monomer and give 1 as a mix of polymers with an estimated molecular mass of 6000–7300 Da (i.e.n = 10–12). The solution phase linear polymerization was carried out using diphenylphosphorylazide (DPPA) as the polymerization promoter, which polymerized monomer 1 with a yield of 69% without the need for protecting groups. DPPA was chosen because of its ability to selectively activate a peptide carboxyl group even in the presence of unprotected hydroxyl groups on the complex carbohydrate moieties. The final products were not tested for any of their thermal hysteresis, ice growth habit modification or recrystallization inhibition properties. Six years later, Tachibana et al. published an improved solution-phase synthesis of native AFGP (Scheme 2).75 The amino acid sequence of the building block was modified from Ala-Ala-Thr to Ala-Thr-Ala in order to reduce steric hindrance of the sugars during the activation of the terminal carboxyl groups for the polymerization. The positioning of the threonine in the middle of the tripeptide sequence led to better polymerization yields. Acetyl protecting groups were converted into benzyl protecting groups to prevent racemisation or β-elimination reactions due to O-deacylation of the intermediates under alkaline conditions. Synthesis of the building block 4 was then successfully achieved through eight steps with a yield of 23% from 3. Polymerization of 4 was achieved using DPPA to give 5 as a mixture of glycopeptides with an estimated molecular mass of 6600 to 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 Da (i.e.n = 8–15) in quantitative yield. The resulting products showed a thermal hysteresis activity comparable to the native AFGP (about 0.5 °C) with the observation of hexagonal-bipyramidal shaped crystals. The solution phase polymerization was later improved by replacing DPPA by 1-isobutoxycarbonyl-2-isobutoxy-1,2-dihydroquinoline (IIDQ) or 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMT-MM) as the polymerization promoter.76 Either of these promoters gave the O-acetylated glycopeptide target efficiently with yields close to 90%, whereas DPPA gave good yields but with a complex mixture of acyl-migrated compounds. This synthetic method was used to study, for each glycoprotein, the influence of the molecular mass on the thermal hysteresis induced. A range of different lengths of the analogue 5 was synthesized (n = 1–7) and the resultant analogues tested. The results showed that a two-unit repeat of the glycosylated tripeptide sequence is enough to observe a small thermal hysteresis. Between two and five repeat-units, an increase in the thermal hysteresis value was observed along with the increase of the glycopeptide molecular mass. Between five and seven repeat-units however, the antifreeze activity values remained similar. These results need to be considered carefully as the longer native AFGPs extracted from fish (up to n = 50) induce a thermal hysteresis twice as pronounced as the small five unit-repeat form, suggesting that antifreeze activity increases with glycopeptide length.12,77,78
650 Da (i.e.n = 8–15) in quantitative yield. The resulting products showed a thermal hysteresis activity comparable to the native AFGP (about 0.5 °C) with the observation of hexagonal-bipyramidal shaped crystals. The solution phase polymerization was later improved by replacing DPPA by 1-isobutoxycarbonyl-2-isobutoxy-1,2-dihydroquinoline (IIDQ) or 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMT-MM) as the polymerization promoter.76 Either of these promoters gave the O-acetylated glycopeptide target efficiently with yields close to 90%, whereas DPPA gave good yields but with a complex mixture of acyl-migrated compounds. This synthetic method was used to study, for each glycoprotein, the influence of the molecular mass on the thermal hysteresis induced. A range of different lengths of the analogue 5 was synthesized (n = 1–7) and the resultant analogues tested. The results showed that a two-unit repeat of the glycosylated tripeptide sequence is enough to observe a small thermal hysteresis. Between two and five repeat-units, an increase in the thermal hysteresis value was observed along with the increase of the glycopeptide molecular mass. Between five and seven repeat-units however, the antifreeze activity values remained similar. These results need to be considered carefully as the longer native AFGPs extracted from fish (up to n = 50) induce a thermal hysteresis twice as pronounced as the small five unit-repeat form, suggesting that antifreeze activity increases with glycopeptide length.12,77,78
 | ||
| Scheme 1 First synthesis of a native AFGP described by Tsuda and Nishimura in 1996 (n = 10–12). | ||
 | ||
| Scheme 2 Improved synthesis of a native AFGP described by Tachibana et al. in 2002 (n = 8–15). | ||
In 1999, Jiaang et al. reported the synthesis of a similar glycosylated tripeptide building block 6 (Fig. 2) in which they planned to use solid-phase synthesis to prepare the native AFGPs.79 Solid-phase synthesis was preferred because of its better control over the peptide sequence and length compared to solution-polymerization methods. To facilitate the peptide coupling step, the sequence Ala-Thr-Ala was synthesized instead of Ala-Ala-Thr. A Moz-protecting group for N-terminus protection was chosen instead of the usual Fmoc-group, mainly because the TFA used in Fmoc-chemistry to cleave the peptide from the resin can eventually lead to the cleavage of the O-glycosidic linkage, which is sensitive to acidic and basic conditions. However, the Moz-protected building block 6, the deprotection of which can be achieved under photolytic conditions, was never used to prepare an antifreeze glycoprotein. Instead, the solid-phase synthesis of AFGPs was published by Tseng et al. two years later,80 using a different approach. The glycosylated amino acid 8 was synthesized in 60% yield from saccharide 7 (Scheme 3). Then, using classic Fmoc solid phase synthesis, glycopeptide 1 was synthesized in two different lengths (n = 4, 8). Cleavage was carried out with 95% TFA but this did not result in the cleavage of the O-glycosidic linkage as the authors anticipated in their previous paper.79 The final 14 residue AFGP 1 was obtained in 40% yield from 8 and the 26 residue peptide in 22% yield. The final products were not tested for any thermal hysteresis, ice growth habit modification or recrystallization inhibition properties.
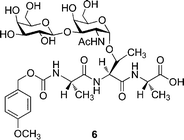 | ||
| Fig. 2 Building block synthesized by Jiaang et al. in 1999. | ||
 | ||
| Scheme 3 Synthesis of native AFGPs (n = 4, 8) by Tseng et al. | ||
3. Synthesis of AFGP analogues
The first examples of AFGP analogues were obtained by modifying the structure of natural AFGPs extracted from fish. These analogues were mainly obtained by degradation of the disaccharides. β-Elimination of the saccharides led to a loss of thermal hysteresis, as did periodate oxidation of 80% of the terminal galactose residues.5,6,81 Acetylation of 35% or more of the carbohydrate hydroxyl groups as well as complexation of the terminal galactose hydroxyls with borate also resulted in the loss of hysteresis properties.6,16,82,83 Oxidation of the C6 hydroxyls on the sugars did not appear to affect the thermal hysteresis activity except in the case where a negative charge was introduced.16,81,83,84 These studies highlight the importance of the carbohydrate structure, especially the necessity to have the hydroxyl groups intact (except for the hydroxyl group in position C6) in order to observe thermal hysteresis.Anisuzzaman et al. were among the first to attempt the synthesis of an AFGP analogue, which was simplified by removal of the N-acetyl group.85 They reported the synthesis of a tripeptide unit Ala-Thr-Ala in which a β-D-galactosyl-(1–3)-α-D-galactopyranose was attached to the threonine of 13 (Scheme 4). The synthesis was fully convergent as the coupling between the respective disaccharide and the tripeptide unit was carried out in the last step. The disaccharide was prepared by selectively protecting the hydroxyl groups of the galactoside except for the C3 hydroxy group. The glycosyl donor 9 was then coupled with α-bromogalactoside 10 to afford the disaccharide 11, which was then converted into the respective chloride 12. In parallel with the carbohydrate, the tripeptide was prepared by typical peptide sequential coupling. Glycosylation of the protected tripeptide with the disaccharide 12 was finally carried out in the presence of silver triflate. The fully protected disaccharide tripeptide was obtained with good α-stereoselectivity in 60% yield. The final compound 13 was obtained after deprotection of the carboxyl and hydroxyl groups. Unfortunately, this compound has not been polymerized or used in solid-phase synthesis; neither have its thermal hysteresis, ice growth habit modification or recrystallization inhibition properties been investigated.
 | ||
| Scheme 4 First analogue building block synthesized by Anisuzzaman et al. | ||
In 1990, Filira et al. and Meldal and Jensen were the first to use solid-phase synthesis to create AFGP analogues.86,87 However, their analogues were highly simplified versions of the native AFGPs, lacking the terminal galactose and the N-acetyl group. In the work by Filira et al., N-α-(fluoren-9-ylmethoxycarbonyl)-3-O-(2,3,4,6-tetra-O-acetyl-α-D-galactopyranosyl)-L-threonine was used as a symmetrical anhydride in continuous-flow solid-phase peptide synthesis to produce a range of glycopeptides 14 (Fig. 3a) with lengths varying between 6 and 21 residues (i.e.n = 2–7). Meldal et al. synthesized a similar analogue, but with a serine instead of a threonine (Fig. 3b) and using benzoyl instead of acetyl groups to protect the hydroxyls. During the peptide synthesis, the use of a pentafluorophenyl ester to protect the C-terminus of the building block is noteworthy as the protecting group is stable to the glycosylation conditions but also reactive enough to be used in solid-phase peptide synthesis. Three hydroxyl protected glycopeptides 15, consisting of three, six and nine residues respectively, were synthesized using this method. None of these compounds were tested for their thermal hysteresis, ice growth habit modification or recrystallization inhibition properties.
 | ||
| Fig. 3 AFGP analogues prepared by (a) Filira et al. (n = 2–7); (b) Meldal and Jensen (n = 1, 2, 3). | ||
Tachibana et al. used the method described in their first paper to synthesize numerous analogues of AFGPs (Table 1).75,76,88 They modified both the structure of the sugar moieties (16–23, 25–27) and the peptide backbone (24). All the analogues were synthesized using the same solution-phase polymerization method that was used to prepare 5. Observation of the antifreeze activity induced by these analogues gave some important information about the mechanism of interaction of the AFGPs with ice. Analogues 19 and 20 induced the formation of hexagonal-bipyramidal ice crystals and exhibited a low or very weak thermal hysteresis, respectively. Compounds 17, 18, 23–26 induced hexagonal-shaped ice crystals, which suggests weak interactions of the glycopeptides with ice; the other analogues synthesized did not modify the ice growth habit. A few conclusions can be drawn from these results. The terminal galactose does not seem to be necessary for observing thermal hysteresis; more likely it acts as an enhancer of the ice growth inhibition process. However, the conformation of the sugar attached to the threonine appears to be crucial, as replacing it with β-D-N-acetylgalactosamine (17) or α-D-N-acetyllactosamine (20) leads to a critical decrease or loss of the thermal hysteresis. The removal of the N-acetyl group of the α-D-N-acetylgalactosamine (21) also leads to the loss of thermal hysteresis, as does modification of the peptide backbone by replacing the threonine residues by serine residues (24), highlighting the importance of the methyl group of the threonines.
|
|
||||||
|---|---|---|---|---|---|---|
| Compound | R1 | R2 | M n | M w | Thermal hysteresis | Ice crystal shape |
| 16 |

|
CH3 | 1690 | 1690 | No |

|
| 17 |

|
CH3 | 4020 | 4020 | No |

|
| 18 |

|
CH3 | 6370 | 6370 | No |

|
| 19 |

|
CH3 | 3000 | 3000 | Yes (lower than for 5) |

|
| 20 |

|
CH3 | 3660 | 3660 | Yes (very weak) |

|
| 21 |

|
CH3 | 4100 | 4100 | No |

|
| 22 |

|
CH3 | 3180 | 3180 | No |

|
| 23 |

|
CH3 | 4700 | 4700 | No |

|
| 24 |

|
H | 4410 | 4410 | No |

|
| 25 |

|
CH3 | 1900 | 3500 | No |

|
| 26 |
|
CH3 | 4000 | 7700 | No |
|
| 27 |

|
CH3 | 8200 | 10000 | Not evaluated for antifreeze activity | |
The influence of peptide backbone modification on antifreeze activity was also investigated in three recent studies. Hachisu et al.89 described the synthesis of cyclic analogues of the AFGPs (28–30) (Fig. 4), containing the same peptide sequence repetition (Ala-Ala-Thr) and the same disaccharides. The building blocks were first prepared following the method used by Tachibana et al.75 to make the native AFGP. The solution polymerization of the linear small AFGPs and their cyclisation was then conducted in one-pot in the presence of DPPA and triethylamine. The desired cyclic compounds (2, 3 and 4 repetitions of building block 5) were then separated and purified by ion-exchange chromatography. Despite a major difference in conformation compared to the linear AFGPs (as observed by CD), the cyclic analogues still induced the formation of bipyramidal ice crystals and exhibited thermal hysteresis. Interestingly, the thermal hysteresis activity of these analogues did not appear to be dependent on the molar mass, with the smaller analogue (28) inducing a higher thermal hysteresis value than the larger ones (29, 30). The retention of activity of the AFGPs through cyclisation suggests that the C- and N-termini of the peptide sequences do not play essential roles in their mechanism of action, and that the conformation of AFGPs can be modified without losing their activity.
 | ||
| Fig. 4 Cyclic analogues of AFGP. | ||
In 2010, Heggemann et al. synthesized a range of AFGP analogues in which the native β-D-galactosyl-(1–3)-α-D-N-acetylgalactosamine was substituted with α-D-N-acetylgalactosamine.90 Synthesis of the building block 31 was achieved in 45% yield from 3 (Scheme 5). Assembly of the glycopeptides was performed by microwave-assisted solid-phase synthesis, which allowed the authors to introduce variation in the peptide sequence (glycopeptides 32–36). The recrystallization inhibition potential of these analogues was systematically studied and showed that the irregular substitution of alanine by proline (33), glycine (34) or serine (35) leads to a loss of recrystallization inhibition properties, whereas the regular substitution of alanine by proline (36) does not significantly affect the activity. This study highlights the importance of having periodic turns in the peptide sequence to observe recrystallization inhibition activity, but also shows that the terminal galactose of the native AFGP is not necessary to observe such activity. Unfortunately, none of these analogues were evaluated for modification of ice growth habit or thermal hysteresis.
 | ||
| Scheme 5 Synthesis of the building block used to prepare AFGP analogues described by Heggemann et al. in 2010. T* is equivalent to α-D-N-acetylgalactosamine in the peptide sequence. | ||
Recently, Peltier et al. reported the synthesis of similar AFGP analogues.91 These analogues were prepared using a similar method to that used by Heggemann et al.90 (Scheme 5) with the modification that 1,3,4,6-tetra-O-acetyl-2-azido-2-desoxy-α/β-D-galactopyranose was used for the glycosylation of Fmoc-threonine-O-allyl, which was performed using the Lewis acid BF3·OEt2. Further reductive acetylation of the azide group and deprotection of the allyl ester provided the Fmoc protected building block 31 in good yield. Glycopeptides 37 and 38 were then synthesized using the microwave-assisted solid-phase peptide synthesis method. The building block used to synthesize analogue 39 was made by coupling 1,2,3,4,6-penta-O-acetyl-β-D-galactopyranose to Fmoc-threonine-O-allyl followed by removal of the acetyl protecting groups. These analogues were examined for the nature of ice crystal shape that they induced as well as thermal hysteresis activity. The binding of the compounds onto ice was also characterized using a 1H NMR kinetic melting method that examines the water signal of the solution during the melting of ice. The results, summarized in Table 2, showed that 37 and 38 interact with ice crystals and modify their normal growth, whereas 39 and 40 did not show any signs of interaction with ice or ice growth habit modification at the studied concentration. Substitution of the alanine by proline does not seem to affect the interaction of the glycopeptide with ice in this system. The important role of the N-acetyl group as well as the role of the α-linkage of the sugar onto the peptide bond is also highlighted in this study. 37 and 38 showed bipyramidal shaped crystals and an observable thermal hysteresis only above 80 mg ml−1, which supports the hypothesis that the terminal galactose acts as an enhancer of the antifreeze activity.
| Compound | Peptide sequence | R | M W | Thermal hysteresis | Evidence for binding onto ice | Ice crystal shape (20 mg L−1) |
|---|---|---|---|---|---|---|
| 37 |

|
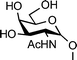
|
1998 | Only above 80 mg ml−1 | Yes |

|
| 38 |

|

|
1946 | Only above 80 mg ml−1 | Yes |

|
| 39 |

|
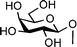
|
1833 | No | No |

|
| 40 |

|
— | 1185 | No | No |

|
Over the years, Ben's research group has focused on developing C-linked analogues of the antifreeze glycoproteins, arguing that the glycosidic O-linkage attaching the sugar to the peptide backbone is a labile bond that is susceptible to hydrolysis under acidic or basic conditions.64 Hence, using a C-linkage instead of the O-linkage would lead to more stable antifreeze glycoprotein analogues. The synthesis of the first C-linked AFGP analogue was completed in 1999 (Scheme 6).92 The terminal galactose and the N-acetyl group from the native AFGPs were removed to leave only α-D-galactose which was attached to lysine residues. Lysine was chosen because of the structural similarity to arginine which occasionally replaces a threonine in the smaller native peptides.93 Alanine residues were replaced by glycine in order to avoid racemisation encountered during the solid-phase synthesis. The protected saccharide 42 was coupled to the tripeptide 44 to give 45. The building block 46 was finally obtained in 40% yield from β-D-galactose pentaacetate 41 in six steps. Solid-phase synthesis was then used to prepare the C-linked AFGP analogues 47, 48 and 49 with lengths of n = 3, 6 and 9 respectively. These analogues were tested for their thermal hysteresis, ice growth habit modification and recrystallization inhibition properties.21 Both 48 and 49 induced the formation of hexagonal shaped ice crystals and a thermal hysteresis of about 0.06 °C. Both also induced recrystallization inhibition, with 49 being more effective than 48. While the hysteresis activity is relatively weak (AFGP8 is about 90 times more effective than 48 in terms of thermal hysteresis), it demonstrates that the O-linkage of the AFGPs can be modified and substituted by other linkages with still some thermal hysteresis or recrystallization inhibition properties retained. Analogue 47 was inactive for both recrystallization inhibition and thermal hysteresis.
 | ||
| Scheme 6 Synthesis of C-linked analogues. | ||
In 2001, Eniade and Ben published a fully convergent synthesis of AFGP analogues similar to 47 and 48, in which the preformed peptide backbone was glycosylated in the final step of the synthesis, using the appropriate saccharide.94 This synthesis included fewer steps and was more efficient than the linear synthesis reported in 1999. The tripeptide unit 43 was assembled first and then the full peptide backbone was prepared by solid-phase synthesis. A base labile resin (HMBA) was chosen in order to selectively remove the Boc protecting group from the lysine residues without cleaving the peptide from the resin. Glycosylation was then carried out on-resin using four or eight equivalents of the protected saccharide 42 in the presence of HATU and DIPEA in DMF. Cleavage with hydrazine and concomitant deprotection of the sugar hydroxyls afforded the 12 and 21 residue C-linked AFGP analogues in 44% and 26% yield, respectively. In the same year, Eniade et al. synthesized a range of glycosylated building blocks 50–54 that could be used to synthesize new C-linked AFGP analogues via solid-phase synthesis (Fig. 5).95 The syntheses of the glycopeptides themselves and their properties have not been reported to date.
 | ||
| Fig. 5 C-linked AFGP building blocks. | ||
Two studies evaluated the importance of the distance between the carbohydrate moieties and the peptide backbone for the C-linked analogues.20,96 AFGP mimics were synthesized with different C-linker chain lengths (Fig. 6, 55–61). These analogues were prepared by synthesizing the glycosylated amino acid first, then incorporating it into the peptide chain by solid-phase synthesis using Fmoc-chemistry. Analogues 55–57 failed to induce thermal hysteresis. However, analogue 55 exhibited recrystallization inhibition properties; 56 and 57 showed no activity. It is noteworthy that the linker in 55 includes the same number of atoms as the linker in the native AFGP. A second series of analogues prepared (58–61) contained an amide functional group in the linker. Similarly to the previous analogues, none of these compounds exhibited thermal hysteresis. However, analogue 60 showed some recrystallization inhibition activity and a weak dynamic ice shaping, establishing the presence of interactions between the compound and ice. The other analogues (58, 59 and 61) showed no sign of such activity. Molecular dynamic simulations showed that 60 supposedly adopts a structure different from the others, in which the hydrophobic face of the carbohydrate is folded onto the peptide backbone and hence is kept away from the solvent. The length of the C-linked glycopeptide also appears to be important as 48 and 49 are active for both recrystallization inhibition and thermal hysteresis whereas 61 is inactive for both. The importance of hydration on the ice recrystallization inhibition activity was also investigated.97 The same method as illustrated in Scheme 6 was used to synthesize C-linked analogues containing different types of sugars (Fig. 6, 60, 62–64). None of these analogues induced thermal hysteresis but, as described previously, weak dynamic ice shaping and recrystallization inhibition properties were observed in the presence of 60, whereas 62–64 did not show any such activity. Despite a simplification of the synthetic analogues compared to the native AFGPs, this study shows that the structure of the sugars is crucial for observing activity, with the native galactose being the most active of all the sugars investigated.
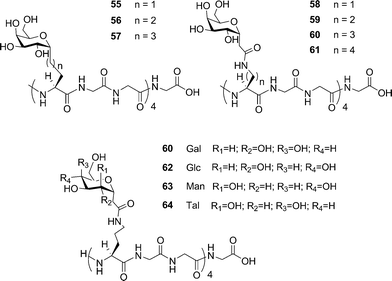 | ||
| Fig. 6 C-linked AFGP analogues used to study the influence of the side chain length and the influence of hydration. | ||
In 2009, in order to overcome the low yield of the glycosylation step, two studies focused on the synthesis of AFGP analogues using click chemistry as an alternative. Miller et al. describe the synthesis of two “click” building blocks (Fig. 7).98 Clicking of the furanose form of α-propargylated D-N-acetylgalactose onto azidoalanine using microwave radiation with a catalyst loading of 2 mol% CuSO4·5H2O and 15 mol% sodium ascorbate gave building block 65 in good yield. Microwave-assisted solid-phase peptide synthesis was then used to successfully synthesize glycopeptides 67 and 68. These compounds were tested for activity but unfortunately none of them induced any thermal hysteresis or ice growth modification at the studied concentration (20 mg ml−1). The inactivity could be due either to the nature of the click linker which is longer than the native linker, or to the substitution of the native pyranose sugar by a furanose. Clicking of the pyranose form of α-propargylated D-N-acetylgalactose and azidoalanine under the same conditions afforded building block 66, but the associated glycopeptide has not been synthesized to date.
 | ||
| Fig. 7 “Click” building block and “click” analogues. | ||
Norgren et al. describe the use of click chemistry to synthesize peptoid analogues of AFGPs.99 The analogues were prepared by first building the peptoid backbone using an Fmoc-protected alkyne-substituted peptoid monomer. Glycosylation was then carried out on the resin-bound full length peptoid using click chemistry. CuI, sodium ascorbate and DIPEA were used in DMF to afford analogues 69–75 (Fig. 8) in good yield. The recrystallization inhibition properties of analogues 72, 74 and 75 were measured but none of them showed any significant activity. This could be due either to the nature of the click linker, or to a difference in the conformation of the peptoid compared to the original peptide sequence.
 | ||
| Fig. 8 Peptoid oligomers synthesized by Norgren et al. | ||
As seen previously, the quest for finding a highly active analogue of AFGPs is often limited by the size of the glycopeptide that can be synthesized. Solution-phase polymerization allows the preparation of very large peptides, but the control over size and peptide sequence is poor. Solid-phase peptide synthesis, on the other hand, allows total control over the peptide sequence but the length of the glycopeptides synthesized rarely exceeds 20 residues. One solution to the problem could be to use native chemical ligation. This technique, developed in 1994 by Kent's group,100 allows the linking of two pre-existing (glyco)peptides by reaction of a C-terminal thioester of one peptide with the N-terminal cysteine residue of another. Recently, the first example of an AFGP analogue synthesized using native chemical ligation has been reported.101 This methodology was used to synthesize glycopeptides in which the native β-D-galactosyl-(1–3)-α-D-N-acetylgalactosamine was substituted with α-D-N-acetylgalactosamine (lacking the terminal galactose) with length of 9, 12 and 21 residues respectively. Ligation of the two glycopeptides, each comprising six residues, gave the 12 residue glycopeptide in 44% yield over three steps; further ligation with another six residue glycopeptide gave the 21 residue glycopeptide in 32% yield over three steps. Native chemical ligation of two glycopeptides gave rise to a cysteine residue at the linkage point. Desulfurization of these cysteine residues (90% yield) to yield alanine residues gave the final AFGP analogues. These analogues have not been tested for their thermal hysteresis, ice growth habit modification or recrystallization inhibition properties.
The most recent work on AFGP analogues to date uses molecular dynamic simulations, in addition to NMR, to study the influence of variations on the amino-acid carrying the sugar.102 The tripeptide Ala-Ala-Thr with an α-D-N-acetylgalactosamine attached to the hydroxyl of the threonine was synthesized (76), along with similar compounds for which the threonine was replaced by α-methylserine (77) or α-methylthreonine (78) (Fig. 9). These analogues have not been tested for their thermal hysteresis, ice growth habit modification or recrystallization inhibition properties. However, the molecular dynamic simulations show that the rigidity observed for 76 can structure the water molecules surrounding the tripeptide, whereas 77 and 78 are too flexible to have such an effect, highlighting the importance of the threonine residue in the AFGP conformation.
 | ||
| Fig. 9 AFGP analogues used to study the influence of non-natural amino acid introduction. | ||
4. Perspective
Antifreeze (glyco)proteins help to control the growth and the recrystallization of ice; these properties are unique and therefore find many potential applications. In the medical field for example, the AF(G)Ps can help to improve low-temperature preservation processes because of their ability to protect cells and their membranes from hypothermic damage.103–105 In the food industry, the use of AF(G)Ps as food additives would help reduce food damage during freezing, storage and transport.106,107 Today, AFP analogues are already used as an additive in frozen food such as ice cream to inhibit recrystallization and therefore improve the texture of the product.22 A more detailed description of the potential applications of AFPs and AFGPs can be found in several reviews.43,73,93 The development of these applications is closely linked, however, to the necessity of having a reliable source of active compounds. As a consequence, the development of an efficient and cost effective synthesis of the native AFGPs, or the development of a simpler analogue showing similar (or improved) properties, is a challenge that needs to be addressed before any medical or industrial applications become a reality. As the issue of the glycopeptide length can be addressed with native chemical ligation, the major problem in the synthesis of both the native AFGPs and their analogues seems to be the efficiency of the building block preparation. Analogues having only an α-D-N-acetylgalactosamine instead of the native disaccharide show reasonable activity both for thermal hysteresis as well as recrystallization inhibition. This already decreases the number of steps necessary to make the building block from eight to ten for the native AFGP down to five steps. However, the yields are still not optimal, especially for the amino-acid glycosylation step. A solution to this could be the use of click chemistry as described previously; the structure of the compounds obtained so far, however, does not seem to retain the features necessary for thermal hysteresis or ice growth habit modification. A deeper understanding of the mechanism of action of the AFGPs is now necessary to overcome such problems. To achieve this, new tools such as computational modeling could be particularly useful and should help to develop new, simpler and more active analogues.1025. Conclusion
Two different approaches to native AFGP synthesis, one polymerizing the building block in solution75 and the other using the glycosylated amino-acid in solid-phase peptide synthesis,80 have opened the way to the synthesis of numerous AFGP analogues, thereby allowing a better understanding of the AFGP structure/activity relationship. Whereas the terminal galactose is not absolutely necessary to observe thermal hysteresis activity, the structure of the α-D-N-acetylgalactosamine attached to the peptide backbone appears to be important, especially the presence of the N-acetyl group at C2. Substitution of alanine in the peptide backbone is tolerated, but the threonine residue has to be intact for the glycoprotein to exhibit thermal hysteresis or ice growth habit modification properties. Although they do not induce any thermal hysteresis, C-linked analogues appear as interesting alternatives to the native O-linked AFGPs in inhibiting recrystallization and modifying ice crystal shape.AFGPs show a number of potential applications in medicine, biotechnology and the food industry. To develop those applications, the problem of high cost due to low yield has to be resolved. This illustrates the importance of finding an efficient way to produce synthetic AFGPs, or of finding simpler analogues that exhibit the same activity. To date, no synthetic AFGP analogue has shown substantially better thermal hysteresis activity than the native glycoproteins. Evolution has resulted in AFGPs that are well adapted to their role, and mimicking, understanding and improving these properties remains a considerable challenge.
6. Abbreviations
| AFGP | antifreeze glycoprotein |
| AFP | antifreeze protein |
| Ala | alanine |
| aq | aqueous |
| Boc | tert-butyloxycarbonyl |
| CD | circular dichroism |
| CDI | 1,1′-carbonyldiimidazole |
| CSA | (±)-camphor-10-sulfonic acid |
| DIPEA | N,N-diisopropylethylamine |
| DMAP | 4-dimethylaminopyridine |
| DMF | N,N-dimethylformamide |
| DMT-MM | 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride |
| DPPA | diphenylphosphorylazide |
| Fmoc | 9-fluorenylmethoxycarbonyl chloride |
| Gal | galactose |
| GalNAc | N-acetylgalactose |
| HATU | 2-(7-aza-1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate |
| HMBA | 4-hydroxymethylbenzoic acid |
| IIDQ | 1-isobutoxycarbonyl-2-isobutoxy-1,2-dihydroquinoline |
| Mn | number-average molecular weight |
| Moz | p-methoxybenzyl carbonyl |
| MS | molecular sieves |
| Mw | weight-average molecular weight |
| NIS | N-iodosuccinimide |
| Pro | proline |
| pTsOH | p-toluenesulfonic acid |
| TBS | tert-butyldimethylsilyl |
| TBSOTf | tert-butyldimethylsilyl triflate |
| tBu | tert-butyl |
| tert | tertiary |
| THF | tetrahydrofuran |
| TMS | trimethylsilyl |
| TMU | tetramethylurea |
| TFA | trifluoroacetic acid |
| Thr | threonine |
| TMSOTf | trimethylsilyl trifluoromethanesulfonate |
| Z | benzyloxycarbonyl |
Acknowledgements
We thank the Human Frontiers Science Program, the MacDiarmid Institute for Advanced Materials and Nanotechnology and the Maurice Wilkins Center for Molecular Biodiscovery for financial support.References
- P. F. Scholander and L. Van Dam, J. Cell. Comp. Physiol., 1957, 49, 1–4 CrossRef CAS.
- P. F. Scholander, L. Van Dam, J. W. Kanwisher, H. T. Hammel and M. S. Gordon, J. Cell. Comp. Physiol., 1957, 49, 5–24 CrossRef CAS.
- P. F. Scholander and J. E. Maggert, Cryobiology, 1971, 8, 371–374 CAS.
- A. L. DeVries and D. E. Wohlschlag, Science, 1969, 163, 1073–1075 CAS.
- A. L. DeVries, S. K. Komatsu and R. E. Feeney, J. Biol. Chem., 1970, 245, 2901–2908 CAS.
- S. K. Komatsu, A. L. DeVries and R. E. Feeney, J. Biol. Chem., 1970, 245, 2909–2913 CAS.
- R. E. Feeney and Y. Yeh, Adv. Protein Chem., 1978, 32, 191–282 CAS.
- D. T. Osuga, R. E. Feeney, Y. Yeh and C. L. Hew, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 1980, 65, 403–406 CrossRef.
- C. L. Hew, G. L. Fletcher and V. S. Ananthanarayanan, Biochem. Cell Biol., 1980, 58, 377–383 CrossRef CAS.
- P. W. Wilson, D. Beaglehole and A. L. DeVries, Biophys. J., 1993, 64, 1878–1884 CrossRef CAS.
- J. A. Raymond, P. Wilson and A. L. DeVries, Proc. Natl. Acad. Sci. U. S. A., 1989, 86, 881–885 CAS.
- C. A. Knight, A. L. DeVries and L. D. Oolman, Nature, 1984, 308, 295–296 CAS.
- R. E. Feeney, T. S. Burcham and Y. Yeh, Annu. Rev. Biophys. Biophys. Chem., 1986, 15, 59–78 CrossRef CAS.
- A. L. DeVries, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 1988, 90, 611–621 CrossRef.
- J. A. Raymond and A. L. DeVries, Proc. Natl. Acad. Sci. U. S. A., 1977, 74, 2589–2593 CAS.
- A. L. DeVries, Science, 1971, 172, 1152–1155 CrossRef CAS.
- A. L. DeVries, Annu. Rev. Physiol., 1983, 45, 245–260 CrossRef CAS.
- V. R. Bouvet and R. N. Ben, in Glycomimetics: Modern Synthetic Methodologies, ed. R. Roy, O.U.P. American Chemical Society, Washington, D.C., 2004, p. 151 Search PubMed.
- M. M. Harding, L. G. Ward and A. D. J. Haymet, Eur. J. Biochem., 1999, 264, 653–665 CrossRef CAS.
- R. Y. Tam, C. N. Rowley, I. Petrov, T. Zhang, N. A. Afagh, T. K. Woo and R. N. Ben, J. Am. Chem. Soc., 2009, 131, 15745–15753 CrossRef CAS.
- A. Eniade, M. Purushotham, R. N. Ben, J. B. Wang and K. Horwath, Cell Biochem. Biophys., 2003, 38, 115–124 CrossRef CAS.
- S. Damodaran, J. Agric. Food Chem., 2007, 55, 10918–10923 CrossRef CAS.
- C. Budke and T. Koop, ChemPhysChem, 2006, 7, 2601–2606 CrossRef CAS.
- M. I. Gibson, C. A. Barker, S. G. Spain, L. Albertin and N. R. Cameron, Biomacromolecules, 2009, 10, 328–333 CrossRef CAS.
- Y. Celik, L. A. Graham, Y. F. Mok, M. Bar, P. L. Davies and I. Braslavsky, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 5423–5428 CrossRef CAS.
- C. A. Knight and A. L. DeVries, Science, 1989, 245, 505–507 CAS.
- J. A. Ramsay and R. H. J. Brown, J. Sci. Instrum., 1955, 32, 372–375 CrossRef CAS.
- A. Chakrabartty and C. L. Hew, Eur. J. Biochem., 1991, 202, 1057–1063 CrossRef CAS.
- M. E. Houston, H. Chao, R. S. Hodges, B. D. Sykes, C. M. Kay, F. D. Sonnichsen, M. C. Loewen and P. L. Davies, J. Biol. Chem., 1998, 273, 11714–11718 CrossRef CAS.
- C. Budke, C. Heggemann, M. Koch, N. Sewald and T. Koop, J. Phys. Chem. B, 2009, 113, 2865–2873 CrossRef CAS.
- M. Smallwood, D. Worrall, L. Byass, L. Elias, D. Ashford, C. J. Doucet, C. Holt, J. Telford, P. Lillford and D. J. Bowles, Biochem. J., 1999, 340, 385–391 CrossRef CAS.
- C. A. Knight, J. Hallett and A. L. DeVries, Cryobiology, 1988, 25, 55–60 CrossRef CAS.
- C. A. Knight, C. C. Cheng and A. L. DeVries, Biophys. J., 1991, 59, 409–418 CAS.
- P. W. Wilson, CryoLetters, 1993, 14, 31–36 Search PubMed.
- Y. Yeh and R. E. Feeney, Chem. Rev., 1996, 96, 601–617 CrossRef CAS.
- C. L. Hew and D. S. C. Yang, Eur. J. Biochem., 1992, 203, 33–42 CAS.
- T. S. Burcham, D. T. Osuga, Y. Yeh and R. E. Feeney, J. Biol. Chem., 1986, 261, 6390–6397 CAS.
- S. Zepeda, E. Yokoyama, Y. Uda, C. Katagiri and Y. Furukawa, Cryst. Growth Des., 2008, 8, 3666–3672 CrossRef CAS.
- E. Kristiansen and K. E. Zachariassen, Cryobiology, 2005, 51, 262–280 CrossRef CAS.
- D. G. Hall and A. Lips, Langmuir, 1999, 15, 1905–1912 CrossRef CAS.
- Z. Jia and P. L. Davies, Trends Biochem. Sci., 2002, 27, 101–106 CrossRef CAS.
- I. Grunwald, K. Rischka, S. M. Kast, T. Scheibel and H. Bargel, Philos. Trans. R. Soc. London, Ser. A, 2009, 367, 1727–1747 CrossRef CAS.
- S. Venketesh and C. Dayananda, Crit. Rev. Biotechnol., 2008, 28, 57–82 CrossRef CAS.
- A. L. DeVries, J. Vandenheede and R. E. Feeney, J. Biol. Chem., 1971, 246, 305–308 CAS.
- W. T. Shier, Y. Lin and A. L. DeVries, Biochim. Biophys. Acta, 1972, 263, 406–413 CAS.
- R. E. Feeney, Am. Sci., 1974, 62, 712–719 CAS.
- K. F. Geoghegan, D. T. Osuga, A. I. Ahmed, Y. Yeh and R. E. Feeney, J. Biol. Chem., 1980, 255, 663–667 CAS.
- C. L. Hew, D. Slaughter, G. L. Fletcher and S. B. Joshi, Can. J. Zool., 1981, 59, 2186–2192 CrossRef CAS.
- Y. Lin, A. L. DeVries and J. G. Duman, Biochem. Biophys. Res. Commun., 1972, 46, 87–92 CrossRef CAS.
- Y. Mimura, Y. Yamamoto, Y. Inoue and R. Chûjô, Int. J. Biol. Macromol., 1992, 14, 242–248 CrossRef CAS.
- B. N. N. Rao and C. A. Bush, Biopolymers, 1987, 26, 1227–1244 CrossRef CAS.
- V. R. Bouvet, G. R. Lorello and R. N. Ben, Biomacromolecules, 2006, 7, 565–571 CrossRef CAS.
- A. N. Lane, L. M. Hays, N. Tsvetkova, R. E. Feeney, L. M. Crowe and J. H. Crowe, Biophys. J., 2000, 78, 3195–3207 CrossRef CAS.
- A. N. Lane, L. M. Hays, R. E. Feeney, L. M. Crowe and J. H. Crowe, Protein Sci., 1998, 7, 1555–1563 CrossRef CAS.
- C. A. Bush, S. Ralapati, G. M. Matson, R. B. Yamasaki, D. T. Osuga, Y. Yeh and R. E. Feeney, Arch. Biochem. Biophys., 1984, 232, 624–631 CrossRef CAS.
- A. I. Ahmed, R. E. Feeney, D. T. Osuga and Y. Yeh, J. Biol. Chem., 1975, 250, 3344–3347 CAS.
- F. Franks and E. R. Morris, Biochim. Biophys. Acta, Gen. Subj., 1978, 540, 346–356 Search PubMed.
- J. A. Drewes and K. L. Rowlen, Biophys. J., 1993, 65, 985–991 CrossRef CAS.
- E. Berman, A. Allerhand and A. L. DeVries, J. Biol. Chem., 1980, 255, 4407–4410 CAS.
- J. A. Raymond, W. Radding and A. L. DeVries, Biopolymers, 1977, 16, 2575–2578 CrossRef CAS.
- V. V. Krishnan, W. H. Fink, R. E. Feeney and Y. Yeh, Biophys. Chem., 2004, 110, 223–230 CrossRef CAS.
- N. M. Tsvetkova, B. L. Phillips, V. V. Krishnan, R. E. Feeney, W. H. Fink, J. H. Crowe, S. H. Risbud, F. Tablin and Y. Yeh, Biophys. J., 2002, 82, 464–473 CrossRef CAS.
- D. Wen and R. A. Laursen, Biophys. J., 1992, 63, 1659–1662 CAS.
- R. N. Ben, ChemBioChem, 2001, 2, 161–166 CrossRef CAS.
- C. A. Knight, E. Driggers and A. L. DeVries, Biophys. J., 1993, 64, 252–259 CAS.
- J. Baardsnes, L. H. Kondejewski, R. S. Hodges, H. Chao, C. Kay and P. L. Davies, FEBS Lett., 1999, 463, 87–91 CrossRef CAS.
- D. R. Nutt and J. C. Smith, J. Am. Chem. Soc., 2008, 130, 13066–13073 CrossRef CAS.
- A. Wierzbicki, P. Dalal, T. E. Cheatham, J. E. Knickelbein, A. D. J. Haymet and J. D. Madura, Biophys. J., 2007, 93, 1442–1451 CrossRef CAS.
- A. D. J. Haymet, L. G. Ward and M. M. Harding, J. Am. Chem. Soc., 1999, 121, 941–948 CrossRef CAS.
- A. Jorov, B. S. Zhorov and D. S. C. Yang, Protein Sci., 2004, 13, 1524–1537 CrossRef CAS.
- H. Chao, F. D. Sonnichsen, C. I. Deluca, B. D. Sykes and P. L. Davies, Protein Sci., 1994, 3, 1760–1769 CrossRef CAS.
- Y. Wu, J. Banoub, S. V. Goddard, M. H. Kao and G. L. Fletcher, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 2001, 128, 265–273 CrossRef CAS.
- M. M. Harding, P. I. Anderberg and A. D. J. Haymet, Eur. J. Biochem., 2003, 270, 1381–1392 CrossRef CAS.
- T. Tsuda and S.-I. Nishimura, Chem. Commun., 1996, 2779–2780 RSC.
- Y. Tachibana, N. Matsubara, F. Nakajima, T. Tsuda, S. Tsuda, K. Monde and S.-I. Nishimura, Tetrahedron, 2002, 58, 10213–10224 CrossRef CAS.
- Y. Tachibana, K. Monde and S.-I. Nishimura, Macromolecules, 2004, 37, 6771–6779 CrossRef CAS.
- M. H. Kao, G. L. Fletcher, N. C. Wang and C. L. Hew, Can. J. Zool., 1986, 64, 578–582 CrossRef CAS.
- T. S. Burcham, M. J. Knauf, D. T. Osuga, R. E. Feeney and Y. Yeh, Biopolymers, 1984, 23, 1379–1395 CrossRef CAS.
- W.-T. Jiaang, K.-F. Hsiao, S.-T. Chen and K.-T. Wang, Synthesis, 1999, 1687–1690 CrossRef CAS.
- P.-H. Tseng, W.-T. Jiaang, M.-Y. Chang and S.-T. Chen, Chem.–Eur. J., 2001, 7, 585–590 CrossRef CAS.
- A. I. Ahmed, D. T. Osuga and R. E. Feeney, J. Biol. Chem., 1973, 248, 8524–8527 CAS.
- A. I. Ahmed, Y. Yeh, D. T. Osuga and R. E. Feeney, J. Biol. Chem., 1976, 251, 3033–3036 CAS.
- J. R. Vandenheede, A. I. Ahmed and R. E. Feeney, J. Biol. Chem., 1972, 247, 7885–7889 CAS.
- D. T. Osuga, M. S. Feather, M. J. Shah and R. E. Feeney, J. Protein Chem., 1989, 8, 519–528 CrossRef CAS.
- A. K. M. Anisuzzaman, L. Anderson and J. L. Navia, Carbohydr. Res., 1988, 174, 265–278 CrossRef.
- M. Meldal and K. J. Jensen, J. Chem. Soc., Chem. Commun., 1990, 483–485 RSC.
- F. Filira, L. Biondi, B. Scolaro, M. T. Foffani, S. Mammi, E. Peggion and R. Rocchi, Int. J. Biol. Macromol., 1990, 12, 41–49 CrossRef CAS.
- Y. Tachibana, G. L. Fletcher, N. Fujitani, S. Tsuda, K. Monde and S.-I. Nishimura, Angew. Chem., Int. Ed., 2004, 43, 856–862 CrossRef CAS.
- M. Hachisu, H. Hinou, M. Takamichi, S. Tsuda, S. Koshida and S.-I. Nishimura, Chem. Commun., 2009, 1641–1643 RSC.
- C. Heggemann, C. Budke, B. Schomburg, Z. Majer, M. Wißbrock, T. Koop and N. Sewald, Amino Acids, 2010, 38, 213–222 CrossRef CAS.
- R. Peltier, A. J. Dingley, C. W. Evans, M. A. Brimble and D. E. Williams, submitted.
- R. N. Ben, A. A. Eniade and L. Hauer, Org. Lett., 1999, 1, 1759–1762 CrossRef CAS.
- V. Bouvet and R. N. Ben, Cell Biochem. Biophys., 2003, 39, 133–144 CrossRef CAS.
- A. Eniade and R. N. Ben, Biomacromolecules, 2001, 2, 557–561 CrossRef CAS.
- A. Eniade, A. V. Murphy, G. Landreau and R. N. Ben, Bioconjugate Chem., 2001, 12, 817–823 CrossRef CAS.
- S. Liu and R. N. Ben, Org. Lett., 2005, 7, 2385–2388 CrossRef CAS.
- P. Czechura, R. Y. Tam, E. Dimitrijevic, A. V. Murphy and R. N. Ben, J. Am. Chem. Soc., 2008, 130, 2928–2929 CrossRef CAS.
- N. Miller, G. M. Williams and M. A. Brimble, Org. Lett., 2009, 11, 2409–2412 CrossRef CAS.
- A. S. Norgren, C. Budke, Z. Majer, C. Heggemann, T. Koop and N. Sewald, Synthesis, 2009, 488–494 CAS.
- P. E. Dawson, T. W. Muir, I. Clark-Lewis and S. B. H. Kent, Science, 1994, 266, 776–779 CrossRef CAS.
- J. Garner, K. A. Jolliffe, M. M. Harding and R. J. Payne, Chem. Commun., 2009, 6925–6927 RSC.
- F. Corzana, J. H. Busto, M. García de Luis, A. Fernández-Tejada, F. Rodríguez, J. Jiménez-Barbero, A. Avenoza and J. M. Peregrina, Eur. J. Org. Chem., 2010, 3525–3532 CrossRef CAS.
- B. Rubinsky, A. Arav and G. L. Fletcher, Biochem. Biophys. Res. Commun., 1991, 180, 566–571 CrossRef CAS.
- J.-H. Wang, Cryobiology, 2000, 41, 1–9 CrossRef CAS.
- C. Y. Lee, B. Rubinsky and G. L. Fletcher, CryoLetters, 1992, 13, 59–66 Search PubMed.
- M. Griffith and K. V. Ewart, Biotechnol. Adv., 1995, 13, 375–402 CrossRef CAS.
- R. E. Feeney and Y. Yeh, Trends Food Sci. Technol., 1998, 9, 102–106 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |

