Recent advances in non-stoichiometric multicomponent hydrogen-bonded organic frameworks
He
Zhao
a,
Baiyang
Fan
a,
Guiyan
Liu
a,
Xingliang
Liu
 b and
Pengchong
Xue
b and
Pengchong
Xue
 *a
*a
aTianjin Key Laboratory of Structure and Performance for Functional Molecules, College of Chemistry, Tianjin Normal University, No. 393, Binshui West Road, Tianjin, 300387, P. R. China. E-mail: xuepengchong@126.com; hxxyxpc@tjnu.edu.cn
bSchool of Chemical Engineering Qinghai University, No. 251, Ningda Road, Xining, 810016, P. R. China. E-mail: liuxl1219@163.com
First published on 13th November 2025
Abstract
Non-stoichiometric multicomponent hydrogen-bonded organic frameworks (NS-HOFs) represent an emerging class of porous materials characterized by their continuously tunable properties. The most distinctive feature of NS-HOFs is the ability to precisely modulate material performance—such as fluorescence emission and gas adsorption selectivity—by adjusting the relative proportions of the constituent components within the framework. Despite the challenges posed by the reversible nature of hydrogen bonds, significant progress has been made in recent years, particularly in the fields of tunable luminescence and toxic gas capture. This review systematically summarizes recent advances in NS-HOFs, focusing on rational design strategies, structural characterization techniques, heterogeneity in component distribution, and their profound influence on key functionalities including light emission, pore environment polarity, and selective adsorption. By highlighting representative studies, this review aims to provide theoretical insights and practical examples to guide the future development of multifunctional NS-HOFs.
Introduction
HOFs, as a novel class of porous materials, exhibit distinct characteristics compared to metal–organic frameworks (MOFs), covalent organic frameworks (COFs), and crystalline porous organic salts (CPOs). Benefiting from their exceptional stability and designability, HOFs have experienced rapid and extensive development over the past decade.1–4 The HOFs are self-assembled via intermolecular hydrogen bonds5,6 (H-bonds) and other non-covalent interactions, such as van der Waals forces, π–π stacking, and electrostatic attractions between cations and anions.7–12 They have demonstrated remarkable performance in various applications, including gas adsorption and separation,13–21 catalysis,22–27 proton conduction,28–30 and chromism.17,31–35The majority of reported HOFs are single-component, assembled from one type of molecule assisted by various weak intermolecular forces. To engineer a HOF with a specific property, one must design and synthesize a building unit bearing the requisite functional groups. For instance, conferring catalytic activity for organic reactions, chiral recognition capability, or unique fluorescence emission requires the incorporation of active coordination sites, chiral moieties, or specific luminescent groups into the building unit, respectively. However, optimizing the properties of a single-component HOF is challenging; achieving desirable performance often necessitates molecular structure optimization, which relies on screening a large library of building units—a typically protracted process. Furthermore, the relatively weak energy of hydrogen bonds often renders HOF structures less robust compared to MOFs, COFs, or porous polymers. During applications such as catalysis or molecular/ion recognition, if the interactions between the unit molecules on the HOF pore surface and guest molecules are too numerous or too strong, the pore structure can be compromised. An additional limitation is that certain functionalities are difficult or impossible to achieve with a single type of molecule, examples include tunable luminescence, high electrical conductivity, long-persistent luminescence, and light harvesting with efficient energy transfer. Therefore, overcoming the inherent drawbacks of single-component HOFs represents a significant challenge at the forefront of materials science. It is well-established that introducing additional components into functional materials can enhance specific properties or even impart entirely new functionalities, as exemplified by alloys, doped OLEDs36,37 with high luminescent efficiency, and rare-earth-doped afterglow phosphors.38,39 Thus, incorporating guest molecules possessing unique functional groups into the host framework of a single-component HOF presents a viable strategy to address this challenge. Although some molecules can form two-component HOFs via electrostatic attraction between opposite charges40 and/or hydrogen bonds,41 their molar ratios are typically fixed (e.g., 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 2
1 or 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1);42–45 then the main challenge, therefore, lies in introducing variable amounts of other functional molecules into the framework structure to construct non-stoichiometric multicomponent HOFs.
1);42–45 then the main challenge, therefore, lies in introducing variable amounts of other functional molecules into the framework structure to construct non-stoichiometric multicomponent HOFs.
Numerous studies indicate that organic molecules with significantly divergent structures seldom form co-assemblies in the absence of strong intermolecular interactions. Conversely, molecules exhibiting structural similarity or comparable sizes are more prone to mutual substitution or co-assembly. For example, two ligands of identical length but differing emission wavelengths can substitute for one another within the same MOF structure, and energy transfer between the ligands can facilitate the emission of light of different colors from the MOF (Fig. 1a).46 We have also observed that in a supramolecular gel system, two gelators sharing identical hydrogen-bonding units and similar fluorophores can form co-assemblies with tunable properties. The concentration of the energy acceptor gelator not only modulates the fluorescence color of the system but also endows the two-component gel with responsiveness to acidic gases.47 Organic molecules with similar structures can also form non-stoichiometric co-crystals, and these co-crystals can possess even better properties.48
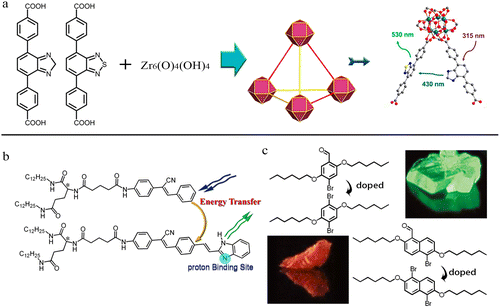 | ||
| Fig. 1 Examples of (a) a dual-ligand MOF with energy transfer. Reproduced from ref. 46. Copyright 2020 American Chemical Society (ACS), (b) a two-component supramolecular gel with energy transfer and acid response. Reproduced from ref. 47. Copyright 2013 American Chemical Society (ACS), and (c) doped room-temperature phosphorescent crystals. Reproduced from ref. 48. Copyright 2011 Springer Nature. | ||
Based on the aforementioned examples, a principle can be deduced: if two molecular building units capable of forming HOFs share an identical molecular backbone configuration and hydrogen-bonding groups, they may co-assemble into a two-component framework structure with readily adjustable molar ratios. This approach allows the generation of a large library of two-component HOFs with varying properties from two building units, facilitating the efficient screening of high-performance functional materials. Furthermore, if novel functionalities emerge from their co-assembly, the application scope of HOFs could be substantially broadened. Guided by this concept, we conducted proof-of-concept experiments in 2022,49 synthesizing two dumbbell-shaped tetra-arm fluorescent molecules (TPAD and BTAD, Fig. 2a) possessing identical backbones and hydrogen-bonding units but differing linkers. BTAD was found to function as an energy acceptor, doping into the host HOF formed by TPAD to form NS-HOFs. Subsequently, other research groups also verified the correctness of this strategy and demonstrated that the composition ratio in the system could indeed regulate the functionality of HOF materials. Therefore, this feature paper aims to summarize recent works on NS-HOFs.
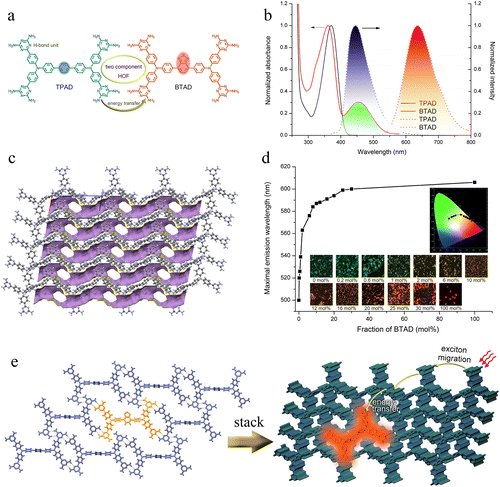 | ||
| Fig. 2 (a) Molecular structures of TPAD and BATD. (b) Normalized absorption and fluorescence spectra of TPAD and BTAD in DMF. The green section shows overlap between the absorption of BTAD and the emission of TPAD. (c) 3D framework structure with 1D channels. (d) Plot of emission maxima vs. the fraction of BTAD. Insets are photographs of cocrystals and their CIE dots in CIE images. (e) Schematic of the co-assembly process and energy transfer in the TC-HOF with a low content of BTAD. Solvent molecules are ignored for clarity. Reproduced from ref. 49. Copyright 2022 American Chemical Society (ACS). | ||
Construction and application of NS-HOFs
To facilitate the confirmation of the successful construction of HOFs with a fluorescence microscope and to observe the distribution of molecules within HOFs, our group designed and synthesized49 two dumbbell-shaped molecules: TPAD and BTAD. Both compounds share an identical molecular skeleton featuring four 2,4-diaminotriazine (DAT) groups, which serve as hydrogen-bonding units. The key difference lies in the central bridge group: TPAD contains a phenyl ring, while BTAD incorporates a strong electron-withdrawing benzothiadiazole (BTD) unit. This modification red-shifts the absorption and emission spectra of BTAD compared to TPAD, making it suitable as an energy acceptor.Spectroscopic studies in solution confirmed that TPAD exhibits strong cyan fluorescence (λem = 447 nm), while BTAD emits orange-red light (λem = 638 nm). Crucially, there is significant spectral overlap between the emission of TPAD and the absorption of BTAD, with a large overlap integral J(λ) = 4.08 × 1014 M−1 cm−1 nm4, indicating a high potential for Förster resonance energy transfer (FRET).50 Electrochemical and quantum chemical computational analyses revealed that both compounds have similar HOMO energy levels, but BTAD has a lower LUMO due to the electron-deficient BTD unit, rationalizing its red-shifted optical properties.
Single-crystal X-ray diffraction showed that TPAD forms a porous HOF (named X-HOF-2) with a triclinic structure (space group P1). The framework features 2D layers formed via double hydrogen bonds between DAT groups, which further stack into a 3D structure with interconnected S-shaped channels (Fig. 2b). The material demonstrated permanent porosity, as evidenced by CO2 adsorption measurements. The core innovation lies in the construction of TC-HOFs by co-crystallizing TPAD and BTAD from solution. Due to their nearly identical molecular geometries and hydrogen-bonding motifs, BTAD molecules can homogeneously replace TPAD within the framework without disrupting the crystal structure. Powder XRD patterns of mixed crystals with up to 30 mol% BTAD were identical to those of pure TPAD crystals, confirming the formation of a solid solution rather than phase-separated domains.
Fluorescence spectroscopy of the TC-HOFs revealed that the emission color could be continuously tuned from cyan to orange as the BTAD content increased from 0 to 30 mol% (Fig. 2d). Energy transfer efficiencies reached up to 97% with only 1.0 mol% BTAD, and complete quenching of TPAD emission was observed at 2.0 mol%, indicating that a single BTAD molecule can quench approximately 50 excited TPAD molecules. This amplification effect is attributed to efficient exciton migration within the HOF scaffold.51,52 Further red-shifting at higher BTAD concentrations was attributed to the formation of BTAD aggregates within the framework. Time-resolved fluorescence decay measurements provided direct evidence of energy transfer. The average lifetime of TPAD emission decreased with increasing BTAD content, while the acceptor emission showed a rise time consistent with energy transfer from TPAD to BTAD. These results confirm non-radiative energy transfer within the HOF matrix. The proposed mechanism involves exciton migration through the TPAD network until the energy is transferred to a nearby BTAD molecule (Fig. 2e), which then emits at a longer wavelength.53,54 The directionality of energy transfer is ensured by the lower energy gap of BTAD, preventing back-transfer. This work demonstrates a general strategy for constructing compositionally tunable TC-HOFs using building blocks with identical molecular skeletons. The efficient energy transfer within the framework enables precise control over fluorescence color through simple variation of the acceptor concentration.
In the above example, good co-crystals suitable for crystallographic analysis were not obtained, so the detailed arrangement of TPAD and BTAD in the crystal could not be accurately observed. In November of the same year, Hisaki et al. reported a series of new NS-HOFs (CP-Hp, CP-Py) (Fig. 3a) using a similar co-assembly strategy.55 They achieved breakthrough achievements - obtaining co-crystals suitable for single crystal analysis, obtaining an accurate structural representation, and being the first example to reveal the specific distribution of non-stoichiometric components in co-crystal NS-HOFs.
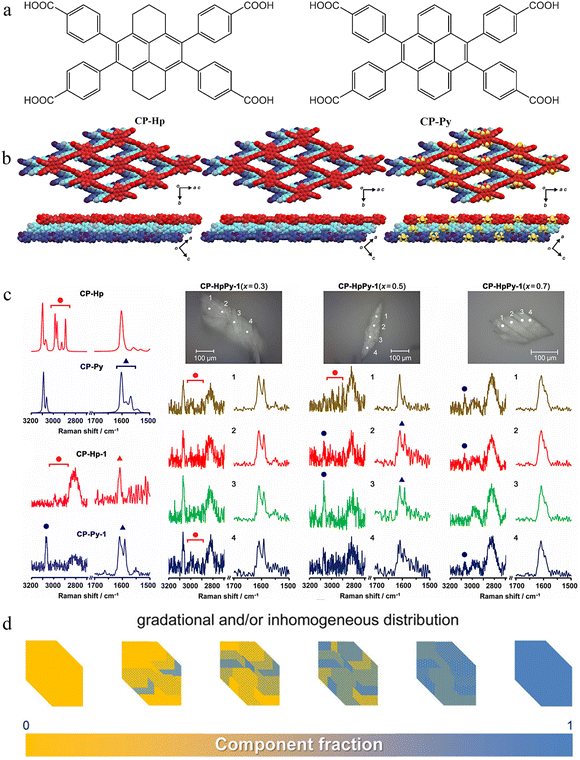 | ||
| Fig. 3 (a) The molecular formulas of CP-Hp and CP-Py. (b) Crystal structures of (left) CP-Hp-1, (middle) CP-Py-1, and (right) cocrystal CP-HpPy-1(0.5, 0.506), where yellow-colored parts indicate disordered propylene moieties of hexahydropyrene. (c) Microscopic Raman spectroscopic analysis of single crystals of the co-crystalline framework CP-HpPy-1. (upper left) Theoretical spectra for molecules of CP-Hp and CP-Py calculated at the DFT B3LYP/6-31G(d) level. (bottom left) Observed spectra of single crystals of CP-Hp-1 and CP-Py-1. Observed spectra of single crystals of CP-HpPy-1 (x = 0.3), CP-HpPy-1 (x = 0.5), and CP-HpPy-1 (x = 0.7), where spectra 1, 2, 3, and 4 were recorded at different positions numbered on the photographs of the single crystal shown at the top. (d) Schematic of the cocrystal with gradational and/or inhomogeneous distribution of components. Reproduced from ref. 55. Copyright 2022 Wiley-VCH. | ||
Both building blocks are equipped with four peripheral carboxy groups, which form robust and directional hydrogen-bonded dimers, facilitating the assembly into two-dimensional layered frameworks with sql-topology (Fig. 3b). The individual HOFs, CP-Hp-1 and CP-Py-1, are isostructural and host solvent molecules within rhombic pores, providing an ideal foundation for solid-solution formation.
Through co-crystallization from solutions with varying molar ratios of CP-Hp and CP-Py, the authors obtained single crystals of mixed frameworks, denoted as CP-HpPy-1(x, y), where x and y represent the molar fraction of CP-Hp in solution and its occupancy in the crystal, respectively. Single-crystal X-ray diffraction (SCXRD) revealed that the frameworks maintain structural integrity across a broad compositional range (0 < y < 1), with unit cell parameters evolving continuously between the two end members. The molecular arrangement of CP-HpPy-1 is basically the same as that of CP-Hp-1 and CP-Py-1 (Fig. 3b). Hisaki et al. analyzed a total of 26 single crystals with different mixing ratios or different batches of the same mixing ratio. These results suggest that co-crystals may grow in an uneven manner.
To determine whether CP-Hp and CP-Py are uniformly or ununiformly distributed in the co-crystals, Raman spectroscopy was performed on the co-crystals. The characteristic peaks of CP-Hp and CP-Py can be clearly observed (Fig. 3c). Raman spectra were recorded at different positions of the co-crystals. The spectra recorded at the four positions of CP-HpPy-1 (x = 0.5) showed significant differences. The spectrum at position 1 was mainly attributed to CP-Hp, while the spectrum at position 3 mainly showed the characteristic peak of CP-Py. Therefore, CP-Hp and CP-Py are not uniformly distributed within the co-crystal skeleton CP-HpPy-1, but have a gradient or non-uniform composition ratio (Fig. 3d). This finding underscores the complex kinetics of framework assembly and suggests that local composition variations may arise during crystal growth.
In 2024, they selected two fluorescent BTTA and NTTA and investigated the fluorescent NS-HOFs.56 The selection of two molecules is strategic for two primary reasons: (1) the molecular shapes and sizes of BTTA and NTTA are highly similar, promoting isostructurality and facilitating their incorporation into a single continuous framework without phase separation; and (2) their distinct photophysical properties. NTTA is a blue emitter, while BTTA exhibits charge-transfer-based green-yellow emission, enabling the direct visualization and quantification of component distribution via fluorescence microscopy. The parent frameworks, BTTA-1 and NTTA-1, both form isostructural sql-topological 2D networks sustained by robust carboxylic acid dimer hydrogen bonds. These 2D sheets undergo slip-stacked assembly, creating 1D porous channels. The NS-HOFs, denoted BxNγ-1, were synthesized by slow evaporation from mixed solutions of BTTA and NTTA in DMF/1,2,4-trichlorobenzene (TCB) at various initial molar fractions (x).
SCXRD of the mixed crystals revealed that the BxNγ-1 series maintains the same underlying sql topology. The key structural feature is the disordered occupancy of the benzothiadiazole and naphthalene cores within the same crystallographic site (Fig. 4b). By refining the occupancies, the authors could determine the precise molar fraction (y) of BTTA within each analyzed crystal. They discovered a non-linear, sigmoidal relationship between the initial solution composition (x) and the final crystalline composition (y), which was attributed to the vastly different solubilities of the two components (BTTA being much less soluble than NTTA) (Fig. 4c). Furthermore, the unit cell parameters (a, b, c, β) and the π-stacking distances between the core moieties were found to vary continuously and approximately linearly with the composition y, behaving as a classic Vegard's law-like solid solution and confirming the formation of a true mixed crystal rather than a mechanical mixture of phases.
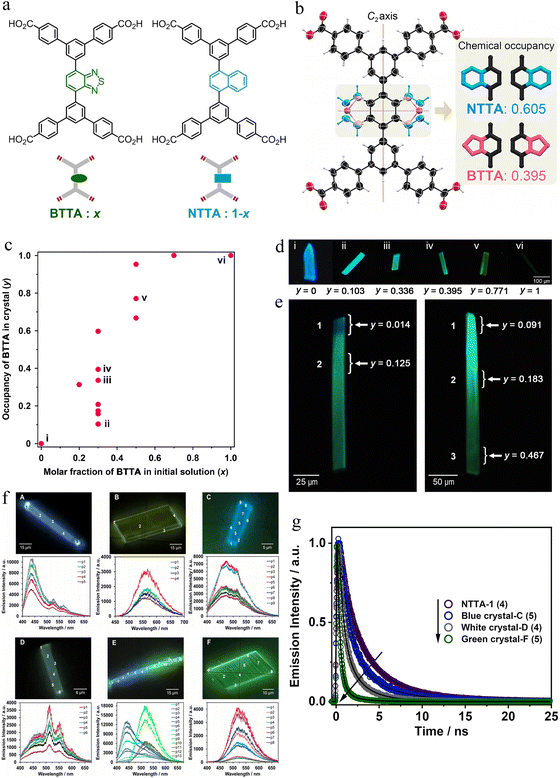 | ||
| Fig. 4 (a) Molecular structures of BTTA and NTTA. (b) Crystal structure of BTNT-1 (0.3, 0.395), in which the core moieties are disordered in two structures corresponding to BTTA and NTTA, with occupancies of 0.395 and 0.605, respectively. (c) Composition in single-crystals of BTNT-1(x, y), where x and y denote the molar fraction and occupancy of BTTA in the initial solution and the resultant single crystal, respectively. (d) Fluorescence photographs of single crystals whose composition ratios were determined by SCXRD. (e) Determination of composition ratio for several positions in inhomogeneous single crystals showing (left) blue-green and (right) light green-dark yellow by local SCXRD analysis. Y denotes the crystallographically-determined occupancy of BTTA. (f) Emission photographs and spectra obtained of representative single crystals of the synthesized and studied HOFs: (A) NTTA-1, (B) BTTA-1 and (C to F) BTNT-1(0.3): blue (crystal-C), white (crystal-D), blue-green (crystal-E) and green (crystal-F). The points on the crystal indicate the position from where the emission spectra were recorded. (g) Fluorescence emission decays of excited single crystals of NTTA-1, BTTA-1 and BTNT-1 (crystals-C, -D and -F) shown in Fig. 4f. The solid lines are from the best fits using a multiexponential function. The number in brackets indicates the interrogated point on the crystal shown in Fig. 4f. Reproduced from ref. 56. Copyright 2024 Wiley-VCH. | ||
Fig. 4d shows representative fluorescence photographs of bulk crystals of HOFs NTTA-1, BTTA-1, and BTNT-1(x) (x = 0.1, 0.3, 0.5, 0.7). The fluorescence color ranges from blue to dark yellow via green upon increasing the x value. In addition, the authors found that crystals of BTNT-1(x) with different emission color were formed even in the same crystallization batch. Furthermore, some single crystals with different fluorescence colors in different parts were concomitantly formed in the same batch too (Fig. 4e). Therefore, the distribution of the two components on the NS-HOF crystals is either homogeneous or heterogeneous. Using a highly focused synchrotron X-ray beam (∼1 µm diameter), the authors performed local SCXRD on crystals that exhibited visually heterogeneous fluorescence under a microscope. This groundbreaking approach allowed them to directly correlate local structure with local property. For instance, in a crystal with blue and green regions, the blue edge was found to contain only 1.4% BTTA (y = 0.014), while the adjacent green region contained 12.5% BTTA (y = 0.125). Another crystal showed a gradient from y = 0.091 (light green) to y = 0.467 (dark yellow) (Fig. 4e). This demonstrated that the non-stoichiometry is not always uniform and can manifest as compositionally graded single crystals. This heterogeneity was spectacularly visualized through fluorescence microscopy. The emission color of the BxNγ-1 crystals could be tuned systematically across the visible spectrum—from purple and blue to green, white, and yellow—by varying the global composition y (Fig. 4f). More remarkably, single crystals could exhibit multiple discrete colors or smooth color gradients, providing a direct visual map of the component distribution. This phenomenon is unique to non-stoichiometric systems and is unachievable in perfectly ordered, stoichiometric materials. The spectral overlap between the emission of NTTA and the absorption of BTTA facilitated Förster resonance energy transfer (FRET) from NTTA (donor) to BTTA (acceptor). This was evidenced by the quenching of the NTTA monomer fluorescence and shortened fluorescence lifetimes of the donor in mixed crystals (Fig. 4g). Fluorescence decay analysis also revealed multi-exponential kinetics, indicating the presence of multiple emitting species (monomers, excimers, charge-transfer states) and efficient energy transfer processes, all occurring within the highly ordered yet compositionally varied crystalline environment.
Although the three examples above demonstrate that co-crystallization can be used to construct binary HOFs, they do not address the influence of composition on the properties of HOFs. In 2023, Li Peng's research group studied this issue by preparing 11 isoreticular NS-HOFs with four building units,57 H4TBAPy-R (R = H, CH3, NH2, F) (Fig. 5a). These groups were chosen for their varying electronic properties, sizes, and potential to modulate host–guest interactions, while the core pyrene backbone and carboxylic acid locations ensured conformational and topological consistency. The synthesis was remarkably versatile. Single crystals of MTV-HOF-101 were obtained by dissolving any combination of two, three, or all four tectons in an equimolar ratio in DMF, followed by vapor diffusion of ethyl acetate. This straightforward method yielded a family of 11 distinct MTV-HOFs, named based on their constituent functional groups (e.g., HOF-101-HC from H and CH3; HOF-101-HCNF from H, CH3, NH2, and F).
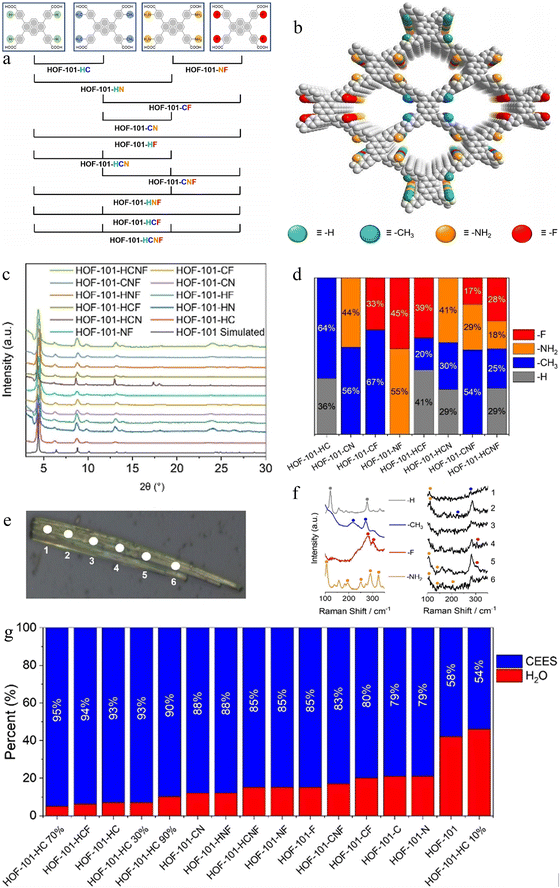 | ||
| Fig. 5 (a) Four H4TBAPy-R (R = H, CH3, NH2, F) tectons and MTVHOF-101 synthesized with each other. (b) Schematic diagram of the structure of MTV-HOF-101. (c) PXRD spectrum of MTV-HOF-101. (d) The proportion of each tecton in MTV-HOF-101 obtained by 1H NMR comparison. (e) Locations of points taken during laser confocal Raman spectroscopy of a single crystal of HOF-101-HCNF. (f) Raman spectra of H4TBAPy-R (R = H, CH3, NH2, and F) under laser excitation with a wavelength of 785 nm. (g) Comparison of the water and CEES adsorption of MTV-HOF-101 at 0.8 kPa. HOF-101-C, HOF-101-N and HOF-101-F means HOF-101-R (R = CH3, NH2 and F). Reproduced from ref. 57. Copyright 2023 Wiley-VCH. | ||
A critical aspect of this work was the rigorous confirmation that the resulting materials were true solid solutions and not mechanical mixtures of separate phases. Single-crystal and powder X-ray diffraction analysis unambiguously confirmed that all MTV-HOFs were isostructural with the parent HOF-101, crystallizing in the same space group with nearly identical unit cell parameters (Fig. 5b and c). The functional groups (-R) were found to be disordered over two positions on the peripheral phenyl rings, a clear signature of statistical mixing within a single crystalline phase rather than segregated domains. In order to determine the actual proportion of each component in MTV-HOF-101, MTV-HOF-101 was tested by 1H NMR. Interestingly, these ratios did not always match the initial stoichiometry in the synthesis solution (Fig. 5d). For instance, in HOF-101-CF, the content of H4TBAPy-CH3 was twice that of H4TBAPy-F. This deviation was attributed to differences in tecton's solubility and intermolecular interaction energies, providing a handle for understanding the crystallization dynamics.
Confocal Raman Spectroscopy was used to detect the distribution of different tectons within a single crystal. By scanning a focused laser (785 nm) across a needle-like crystal of HOF-101-HCNF, they observed spatial variation in the intensities of the characteristic Raman peaks for each functional group. Signals for –NH2 were stronger near the crystal's ends, while –F signals dominated in the center (Fig. 5e and f), indicating a heterogeneous, non-uniform distribution likely influenced by the crystallization kinetics and the differential interaction energies between tectons, as supported by DFT calculations.
The primary function of introducing multiple functional groups was to finely tune the pore microenvironment, particularly its hydrophobicity. Water vapor adsorption isotherms were a key diagnostic tool. The behavior varied dramatically based on the combination and distribution of functional groups. Homogeneous pore environment: MTV-HOFs like HOF-101-HCF, HOF-101-CNF, and HOF-101-HC (70% CH3) exhibited smooth, type V isotherms with a single, sharp inflection point at a relative pressure (P/P0) of ∼0.67–0.75. This indicates a uniform pore surface with a well-defined hydrophobicity intermediate between that of the pure components. Heterogeneous pore environment: frameworks such as HOF-101-NF and HOF-101-HCNF displayed distinct step-like isotherms with multiple inflection points, mirroring the adsorption profiles of physical mixtures of the pure HOFs. This was direct evidence of pore environment heterogeneity, corroborating the Raman mapping data and suggesting the presence of more hydrophilic (NH2-rich) and more hydrophobic (F-rich) domains within the same crystal. This ability to precisely engineer water uptake thresholds (from 50% to 80% RH) by simply selecting the type and ratio of functional groups is a profound demonstration of the power of the NS-HOF approach.
The practical utility of these tailored materials was demonstrated in the challenging capture of 2-chloroethyl ethyl sulfide (CEES), a simulant for sulfur mustard gas (HD). The critical challenge in this application is achieving high selectivity for CEES over water vapor, as real-world scenarios involve high humidity that can poison adsorbents like activated carbon or many MOFs. The CEES adsorption isotherms revealed that NS-HOFs generally exhibited higher affinity (steeper uptake at low pressure) and greater capacity than their single-component counterparts. HOF-101-HC and HOF-101-CF showed the highest capacities (∼97 and 80 cm3 g−1, respectively). By comparing the adsorbed quantities of CEES and water at a pressure of 0.8 kPa, the authors calculated selectivity values. HOF-101-HC and HOF-101-HCF achieved unprecedented selectivities of 93% and 94%, respectively, far exceeding those of the pure-component HOFs and benchmark MOFs like UiO-66 and MOF-808. This suggests a powerful synergistic effect where the multivariate pore surface optimally interacts with the hydrophobic CEES molecule while effectively repelling water. The demonstration that pore functionality, hydrophobicity, and adsorption selectivity can be exquisitely tuned through the rational combination of functional groups opens up vast possibilities for the design of next-generation porous materials.
Due to the weak strength of hydrogen bonds, when the guest molecules in the framework are removed or exchanged with other guests, the framework structure will change in many cases, demonstrating the property of responding to external stimuli, and some characteristics of HOFs will also alter, such as adsorption capacity and luminescent properties. However, how the guest molecules affect the luminescent properties and stimulus-responsive performance of NS-HOF materials has not been reported yet. Based on this consideration, in 2024, our group ingeniously prepared two dumbbell-shaped molecules, TMB and TMBT to construct the first bulk heterojunction (BHJ) HOF exhibiting white light emission by co-growth, and further demonstrate the groundbreaking fabrication of triblock heterojunction (THJ) HOF microrods by epitaxial co-growth or a replacement approach (Fig. 6a),58 and found that these materials showed exceptional stimuli-responsive luminescence and served as a sophisticated platform for multi-level information encryption and anti-counterfeiting applications.
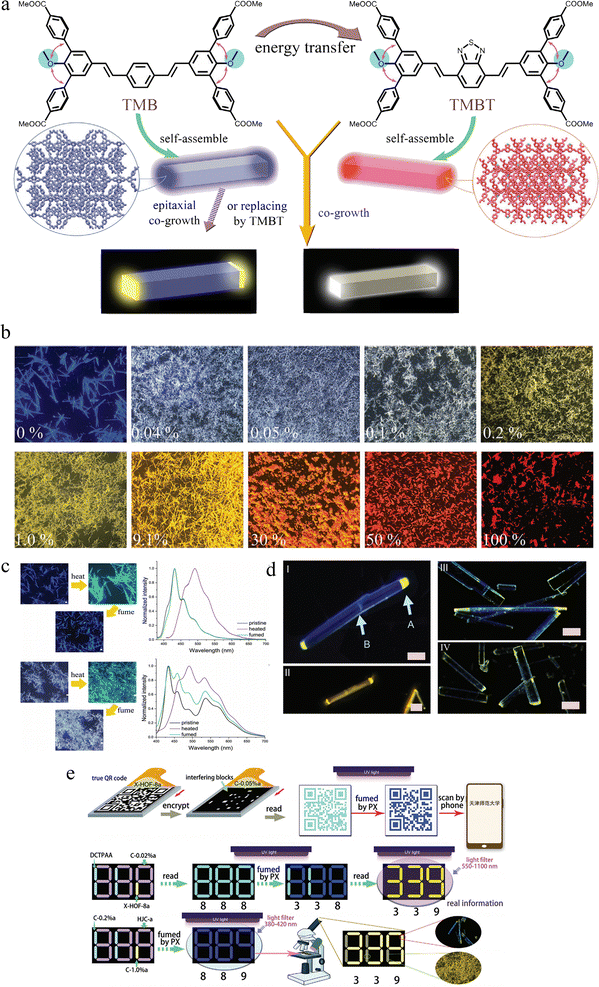 | ||
| Fig. 6 (a) Molecular structure of TMB and TMBT, and schematic diagram of energy transfer, self-assembly, the NS-HOFs co-crystal and triblock heterojunction microrods. (b) Fluorescence microscopic images of NS-HOFs with different contents of TMBT. (c) Photos of X-HOF-8 under heating at 80 °C for 2 h, and then fuming by PX vapor for 2 h, and corresponding fluorescence spectra (up). Photos of C-0.04% under heating and fuming processes and corresponding fluorescence (bottom). The bar size is 50 µm. (d) Photos of heterojunction crystals through (I and II) replacement and (III and IV) epitaxial co-growth methods. Growth time is 6 h for I and III, and 24 h for II. Photo (IV) of fractured microrods in the TMBT solution for 2 h. (e) The preparation process of anticounterfeiting labels and information reading process for the system of (top) X-HOF-8 and C-0.05%, (middle) DCTPAA, X-HOF-8, and C-0.02%, and (bottom) C-0.2%, C-1.0%, and HJC. Reproduced from ref. 58. Copyright 2024 Wiley-VCH. | ||
Both molecules feature peripheral methyl benzoate groups as hydrogen-bonding units and methoxy groups to induce steric hindrance, ensuring a non-planar conformation conducive to forming porous frameworks. The critical distinction lies in their central cores: TMB possesses a divinylbenzene unit, while TMBT incorporates an electron-deficient benzothiadiazole (BTD) unit. This design ensures three key outcomes: (1) structural compatibility. Their similar shapes and peripheral functional groups promote co-assembly into a single, isostructural crystalline phase rather than phase separation. (2) Photophysical complementarity: TMB acts as a blue-emitting energy donor, while TMBT serves as a yellow-orange emitting energy acceptor. The significant spectral overlap between TMB's emission and TMBT's absorption fosters highly efficient Förster resonance energy transfer (FRET), which is the fundamental mechanism for achieving white light emission in their mixed assemblies. (3) Stimulus response. After the loss of the guests, the weak hydrogen bond cannot maintain the original intermolecular packing, promoting HOFs to respond to external stimuli.
TMB could form rod-like HOF crystals, X-HOF-8, from p-xylene (PX), with a porous framework with 1D solvent channels accommodating PX molecules. The large intermolecular distances (∼4.48 Å) between the TMB cores result in bright blue fluorescence (ΦF = 21.4%) similar to its solution state. The crystals also exhibited optical waveguide properties, funneling light to their tips. On the other hand, TMBT constructed a rigid and non-porous framework (X-HOF-9), and emitted orange fluorescence (λem = 566 nm, ΦF = 15.2%) because of strong π–π stacking between the planar BTD cores.
By evaporating a PX solution containing a small molar fraction of TMBT (0.04–0.05%), the microrods that emitted intense white light under UV excitation were obtained (Fig. 6b). Fluorescence microscopy under a long-pass filter showed uniform yellow emission throughout the crystal confirming the molecular-level dispersion of TMBT acceptors within the TMB (donor) matrix. Time-resolved fluorescence measurements showed a decrease in the average lifetime of the TMB donor (from 1.13 ns to 0.98 ns) upon incorporation of TMBT, providing direct evidence of FRET. More importantly, a fascinating aspect of this system is its dynamic responsiveness. The parent X-HOF-8 undergoes a reversible single-crystal-to-single-crystal transformation upon guest loss and uptake. Heating X-HOF-8 at 80 °C removes the PX guests, triggering a phase transition to a new and non-porous phase, dubbed X-HOF-8a. This transformation is accompanied by an emissive color change from blue to cyan fluorescence, caused by closer π–π stacking in the guest-free structure. Exposing X-HOF-8a to PX vapor fully restores the original porous structure (X-HOF-8) and its blue emission. This cycle is repeatable multiple times (Fig. 6c). Crucially, this stimuli-responsiveness is retained in the BHJ HOFs. The white-emitting NS-HOF (C-0.04%) reversibly switched to a grey-emitting state (C-0.04%a) upon desolvation and back to white upon PX fuming. This property is foundational for their application in encryption. Moving beyond statistical BHJ HOFs, two innovative methods were used to create spatially defined triblock heterojunction HOFs (THJ HOFs). Molecular replacement: soaking pre-formed X-HOF-8 microrods in a TMBT solution led to a preferential replacement of TMB molecules with TMBT at the crystal tips, where molecules are more labile. This resulted in microrods with blue-emitting bodies and yellow-emitting BHJ ends (Fig. 6d(I and II)). Epitaxial co-growth: by adding TMBT solution during the growth of X-HOF-8 crystals, we achieved microrods with a distinct tri-block architecture: two yellow-emitting BHJ ends and a blue-emitting middle segment (Fig. 6d(III and IV)). These THJ microrods also exhibited reversible fluorescence changes upon desolvation/resolvation. The combination of composition-tunable emission, stimuli-responsive color switching, and complex spatial heterostructures makes these HOFs exceptionally powerful for anti-counterfeiting, for instance, QR code encryption, filter-based decryption, and heterojunction-based coding. THJ microrods themselves can be used as ink. Their complex fluorescence pattern (e.g., blue body with yellow ends) and response to solvent vapor create a high-security feature that is extremely difficult to replicate. This research provides a comprehensive blueprint for the bottom-up design of multifunctional, multi-component molecular crystals. It establishes NS-HOF-based heterostructures not merely as porous materials but as intelligent photonic systems with tailored energy landscapes and dynamic responses, opening vast possibilities for applications in solid-state lighting, photonic logic, and ultra-secure anti-counterfeiting technologies.
Summary and perspectives
The concept of non-stoichiometric incorporation of multiple components is not unique to HOFs and has also been explored in other porous frameworks, such as MOFs, COFs, and PAFs. In MOFs, mixed-metal or mixed-ligand strategies have been widely used to tune porosity, stability, and catalytic activity. For example, multivariate MOFs (MTV-MOFs) allow the integration of multiple functional groups within a single structure, similar to NS-HOFs, though often with higher structural rigidity due to stronger coordination bonds. COFs, on the other hand, typically exhibit more ordered structures due to covalent linkages, and non-stoichiometric COFs are less common but have been realized through mixed-linker synthesis to modulate electronic properties or pore size. PAFs, with their highly cross-linked aromatic frameworks, offer exceptional stability but limited compositional tunability. In contrast, NS-HOFs stand out for their mild synthesis conditions, facile component exchange, and dynamic responsiveness, though they generally exhibit lower surface areas and mechanical strength compared to MOFs and COFs.Compared to single-component HOFs, which often face challenges in property optimization and structural stability due to their limited functional versatility and weaker hydrogen-bonding interactions, non-stoichiometric multicomponent HOFs (NS-HOFs) offer a promising alternative. While single-component systems require extensive molecular screening to achieve desired functionalities, NS-HOFs enable continuous tuning of properties—such as fluorescence, gas adsorption selectivity, and pore environment polarity—through simple variation of component ratios. However, the reversible nature of hydrogen bonds still poses challenges for the long-term stability and recyclability of NS-HOFs, particularly under practical operating conditions. Nevertheless, the ability to integrate multiple functional units into a single framework significantly expands the application scope of HOFs, offering enhanced performance in areas such as tunable luminescence and selective guest capture.59 The representative systems discussed in this review demonstrate that structurally similar molecules with complementary photophysical characteristics can be successfully integrated into crystalline NS-HOFs through co-crystallization strategies, enabling efficient energy transfer, stimuli-responsive luminescence, and selective guest capture.
Despite these promising advances, the field of NS-HOFs is still in its infancy. Several challenges remain: firstly, the distribution of components within NS-HOFs is often non-uniform, and the relationship between crystallization kinetics and compositional homogeneity is not fully understood; secondly, the role of guest molecules in modulating framework properties—especially in stimulus-responsive behaviors—requires further investigation; and finally, the structural stability and recyclability of NS-HOFs under practical conditions needs improvement.
Future research should focus on the following directions: (1) developing in situ and real-time characterization techniques to elucidate the evolution of component distribution during nucleation and crystal growth. (2) Designing more rigid and multifunctional building blocks to improve framework stability and functional versatility. (3) Expanding the applications of NS-HOFs into areas such as optoelectronics, biosensing, and multi-level information encryption. (4) Exploring the practical utility of NS-HOFs under complex conditions, including high humidity and multi-component atmospheres.
In summary, NS-HOFs lie at the intersection of supramolecular chemistry, crystal engineering, and materials science, offering a versatile platform for the design of intelligent porous materials. Continued efforts in understanding and optimizing these systems will not only advance fundamental science but also accelerate the development of next-generation functional materials for a wide range of technological applications.
Conflicts of interest
There are no conflicts to declare.Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.Acknowledgements
This work was supported by the Scientific Research Foundation of Tianjin Normal University (No. 5RL151), the Tianjin Research Innovation Project for Postgraduate Students (No. 2022SKY252), the National Natural Science Foundation of China (NNSFC, No. 22265026 and 22002108), and the Project of Qinghai Science & Technology Department (No. 2024-ZJ-935).References
- R.-B. Lin, Y. He, P. Li, H. Wang, W. Zhou and B. Chen, Chem. Soc. Rev., 2019, 48, 1362–1389 RSC.
- I. Hisaki, X. Chen, K. Takahashi and T. Nakamura, Angew. Chem., Int. Ed., 2019, 58, 14794 CrossRef CAS PubMed.
- H. Yamagishi, H. Sato, A. Hori, Y. Sato, R. Matsuda, K. Kato and T. Aida, Science, 2018, 361, 1242–1246 CrossRef CAS PubMed.
- B. Wang, R.-B. Lin, Z. Zhang, S. Xiang and B. Chen, J. Am. Chem. Soc., 2020, 142, 14399–14416 CrossRef CAS.
- I. Hisaki, Y. Suzuki, E. Gomez, B. Cohen, N. Tohnai and A. Douhal, Angew. Chem., Int. Ed., 2018, 57, 12650–12655 CrossRef CAS.
- X. Chen, T. Zhang, Y. Han, Q. Chen, C. Li and P. Xue, J. Mater. Chem. C, 2021, 9, 9932–9940 RSC.
- C. Chen, L. Shen, H. Lin, D. Zhao, B. Li and B. Chen, Chem. Soc. Rev., 2024, 53, 2738–2760 RSC.
- J. Li and B. Chen, Chem. Sci., 2024, 15, 9874–9892 RSC.
- Y. Suzuki and I. Hisaki, Polym. J., 2024, 56, 1–16 CrossRef CAS.
- X. Song, Y. Wang, C. Wang, X. Gao, Y. Zhou, B. Chen and P. Li, J. Am. Chem. Soc., 2024, 146, 627–634 CrossRef CAS PubMed.
- X. Song, Y. Wang, C. Wang, D. Wang, G. Zhuang, K. O. Kirlikovali, P. Li and O. K. Farha, J. Am. Chem. Soc., 2022, 144, 10663–10687 CrossRef CAS PubMed.
- Z. Zhang, Y. Ye, S. Xiang and B. Chen, Acc. Chem. Res., 2022, 55, 3752–3766 CrossRef CAS PubMed.
- H. Zhao, B. Fan, S. Hu, X. Liu, B. Tang and P. Xue, Chin. Chem. Lett., 2025, 36, 111005 CrossRef CAS.
- Y. Yang, L. Li, R.-B. Lin, Y. Ye, Z. Yao, L. Yang, F. Xiang, S. Chen, Z. Zhang, S. Xiang and B. Chen, Nat. Chem., 2021, 13, 933–939 CrossRef CAS PubMed.
- M. Liang, S. Hu, N. Zhou, Z. Liu, Q. Chen, X. Chen, X. Liu, C.-P. Li, J. Hao and P. Xue, Small, 2023, 19, 2304340 CrossRef CAS PubMed.
- X.-J. Xi, Y. Li, F.-F. Lang, L. Xu, J. Pang and X.-H. Bu, Giant, 2023, 16, 100181 CrossRef CAS.
- F. Yuan, G. Han, Y. Li, W. Wei, L. He, Y. Chen, C. Chen, G. Lan, S. Xiang, B. Chen and Z. Zhang, Angew. Chem., Int. Ed., 2025, 64, e202513288 CrossRef CAS PubMed.
- Z. Ji, Y. Zhou, R. Krishna, M. Hong and M. Wu, Angew. Chem., Int. Ed., 2025, 64, e202513398 CrossRef CAS.
- S. Lin, P. Ke, L. Wang, Y. Liang, J. Hu, L. Zhang, Z. Zhang, Y. He and B. Chen, Angew. Chem., Int. Ed., 2025, 64, e202511773 CrossRef CAS.
- S. Lin, Y. Liang, P. Ke, L. Wang, D. Chen, L. Zhang and Y. He, Angew. Chem., Int. Ed., 2025, 64, e202500970 CrossRef CAS PubMed.
- Y.-B. Wang, Y.-X. Lin, J.-X. Wang, X. Zhang, H. Wu, W. Zhou, B. Chen, G. Qian and B. Li, J. Am. Chem. Soc., 2025, 147, 24403–24412 CrossRef CAS PubMed.
- A.-A. Zhang, Z.-X. Wang, Z.-B. Fang, J.-L. Li and T.-F. Liu, Angew. Chem., Int. Ed., 2024, 63, e202412777 CrossRef CAS PubMed.
- H. Zhao, Z. Zhou, X. Feng, C. Liu, H. Wu, W. Zhou and H. Wang, Nano Res., 2023, 16, 8809–8816 CrossRef CAS.
- W. Gong, D. Chu, H. Jiang, X. Chen, Y. Cui and Y. Liu, Nat. Commun., 2019, 10, 600 CrossRef CAS PubMed.
- B. Han, H. Wang, C. Wang, H. Wu, W. Zhou, B. Chen and J. Jiang, J. Am. Chem. Soc., 2019, 141, 8737–8740 CrossRef CAS PubMed.
- J.-H. Mei, Y.-R. Zeng, Y.-N. Gong, W.-J. Shi, D.-C. Zhong and T.-B. Lu, Angew. Chem., Int. Ed., 2025, 64, e202507332 CrossRef CAS PubMed.
- Y. Hou, H.-R. Fang, Y.-L. Li, A.-A. Zhang, X.-S. Huang, L. Cai, Q. Yin, R. Wang, J.-L. Li and T.-F. Liu, Angew. Chem., Int. Ed., 2025, 64, e202510614 CrossRef CAS PubMed.
- M. Lupa-Myszkowska, M. Oszajca and D. Matoga, Chem. Sci., 2023, 14, 14176–14181 RSC.
- S. C. Pal, D. Mukherjee, R. Sahoo, S. Mondal and M. C. Das, ACS Energy Lett., 2021, 6, 4431–4453 CrossRef CAS.
- Y.-L. Li, J.-F. Lu, Q. Yin, L. Cai, H.-J. Jiang, C. Liu, G. Xu and T.-F. Liu, Angew. Chem., Int. Ed., 2025, 64, e202504396 CrossRef CAS PubMed.
- Q. Huang, X. Chen, W. Li, Z. Yang, Y. Zhang, J. Zhao and Z. Chi, Chem, 2023, 9, 1241–1254 CAS.
- H. Zhao, X. Wang, S. Hu, M. Liang and P. Xue, Cryst. Growth Des., 2024, 24, 7504–7513 CrossRef CAS.
- Y. Lv, X. Liu, J. Liang, L. Dong, Y. Zhang, C. Lin, S. Xiang, B. Chen and Z. Zhang, Adv. Mater., 2025, 37, 2420486 CrossRef CAS PubMed.
- B. Zhou, L.-H. Cao, M.-F. Huang, Y. Yang, S. Qi, X.-J. Cao and X.-Y. Chen, Angew. Chem., Int. Ed., 2025, 64, e202504645 CrossRef CAS PubMed.
- K. Zhu and B. Yan, Adv. Mater., 2025, 37, 2508676 CrossRef CAS PubMed.
- J. Xin, P. Sun, F. Zhu, Y. Wang and D. Yan, J. Mater. Chem. C, 2021, 9, 2236–2242 RSC.
- S. Yan, M. Qin, C. Shen, L. Niu and Y. Zhang, Synth. Met., 2020, 263, 116368 CrossRef CAS.
- Y. Xiao, D. Zhang and C. Chang, RSC Adv., 2019, 9, 27386–27390 RSC.
- X. Shen, T. Fang, B. Guo, C. Shi, H. Wang, B. Yang and Y. Jin, Fibers Polym., 2025, 26, 1029–1037 CrossRef CAS.
- W. Xiao, C. Hu and M. D. Ward, J. Am. Chem. Soc., 2014, 136, 14200–14206 CrossRef CAS PubMed.
- J. Lü, C. Perez-Krap, M. Suyetin, N. H. Alsmail, Y. Yan, S. Yang, W. Lewis, E. Bichoutskaia, C. C. Tang, A. J. Blake, R. Cao and M. Schröder, J. Am. Chem. Soc., 2014, 136, 12828–12831 CrossRef PubMed.
- M. Morshedi, M. Thomas, A. Tarzia, C. J. Doonan and N. G. White, Chem. Sci., 2017, 8, 3019–3025 RSC.
- T. Adachi and M. D. Ward, Acc. Chem. Res., 2016, 49, 2669–2679 CrossRef CAS PubMed.
- Y.-H. Luo, X.-T. He, D.-L. Hong, C. Chen, F.-H. Chen, J. Jiao, L.-H. Zhai, L.-H. Guo and B.-W. Sun, Adv. Funct. Mater., 2018, 28, 1804822 CrossRef.
- R. Liang, J. Samanta, B. Shao, M. Zhang, R. J. Staples, A. D. Chen, M. Tang, Y. Wu, I. Aprahamian and C. Ke, Angew. Chem., Int. Ed., 2021, 60, 23176–23181 CrossRef CAS PubMed.
- J. Jia, L. Gutiérrez-Arzaluz, O. Shekhah, N. Alsadun, J. Czaban-Jóźwiak, S. Zhou, O. M. Bakr, O. F. Mohammed and M. Eddaoudi, J. Am. Chem. Soc., 2020, 142, 8580–8584 CrossRef CAS PubMed.
- P. Xue, R. Lu, P. Zhang, J. Jia, Q. Xu, T. Zhang, M. Takafuji and H. Ihara, Langmuir, 2013, 29, 417–425 CrossRef CAS PubMed.
- O. Bolton, K. Lee, H.-J. Kim, K. Y. Lin and J. Kim, Nat. Chem., 2011, 3, 205–210 CrossRef CAS PubMed.
- Q. Chen, T. Zhang, X. Chen, M. Liang, H. Zhao, P. Yuan, Y. Han, C.-P. Li, J. Hao and P. Xue, ACS Appl. Mater. Interfaces, 2022, 14, 24509–24517 CrossRef CAS.
- Z. Gao, K. Wang, Y. Yan, J. Yao and Y. S. Zhao, Natl. Sci. Rev., 2021, 8, nwaa162 CrossRef CAS PubMed.
- P. Xue, J. Ding, Y. Shen, H. Gao, J. Zhao, J. Sun and R. Lu, J. Mater. Chem. C, 2017, 5, 11532–11541 RSC.
- Y. Lv, Z. Xiong, Z. Yao, Y. Yang, S. Xiang, Z. Zhang and Y. S. Zhao, J. Am. Chem. Soc., 2019, 141, 19959–19963 CrossRef CAS PubMed.
- S. Kuila and S. J. George, Angew. Chem., Int. Ed., 2020, 59, 9393–9397 CrossRef CAS PubMed.
- F. Kang, L. Li, J. Han, D. Y. Lei and M. Peng, J. Mater. Chem. C, 2017, 5, 390–398 RSC.
- T. Hashimoto, R. Oketani, M. Nobuoka, S. Seki and I. Hisaki, Angew. Chem., Int. Ed., 2023, 62, e202215836 CrossRef CAS PubMed.
- T. Hashimoto, M. de la Hoz Tomás, R. Oketani, B. Cohen, M. Naruoka, N. Tohnai, A. Douhal and I. Hisaki, Angew. Chem., Int. Ed., 2025, 64, e202419992 CrossRef CAS PubMed.
- X. Y. Gao, Y. Wang, E. Wu, C. Wang, B. Li, Y. Zhou, B. Chen and P. Li, Angew. Chem., Int. Ed., 2023, 62, e202312393 CrossRef PubMed.
- H. Zhao, S. Hu, M. Liang and P. Xue, Adv. Opt. Mater., 2025, 13, 2402321 CrossRef CAS.
- S. Liu, L. Li, X.-Y. Gao, R. Cao, Y.-B. Zhang and T.-F. Liu, ACS Cent. Sci., 2025, 11, 1984–1992 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |





