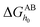Antifouling behaviours of PVDF/nano-TiO2 composite membranes revealed by surface energetics and quartz crystal microbalance monitoring†
Qiaoying Wang,
Zhiwei Wang*,
Jie Zhang,
Jie Wang and
Zhichao Wu
State Key Laboratory of Pollution Control and Resource Reuse, School of Environmental Science and Engineering, Tongji University, Shanghai 200092, P.R. China. E-mail: zwwang@tongji.edu.cn; Fax: +86 (21)65980400; Tel: +86 (21)65980400
First published on 5th September 2014
Abstract
Poly(vinylideneflouride) (PVDF)/nano-titanium dioxide (TiO2) composite membranes were prepared via a phase inversion method by dosing different amounts of TiO2 nanoparticles in PVDF casting solution to improve the antifouling ability. The extended Derjaguin–Landau–Verwey–Overbeek (XDLVO) theory and the quartz crystal microbalance with dissipation (QCM-D) monitoring were adopted to clarify the antifouling behaviours of the composite membranes. The results showed that the addition of nano-TiO2 could improve the membrane surface porosity, volume porosity, hydrophilicity and permeability. The electron donor monopolarity of the composite membranes was evidently enhanced, and the repulsive interaction energy barrier between foulants and membrane surfaces was increased by adding TiO2 nanoparticles, thus improving the antifouling ability. The optimal dosage of TiO2 nanoparticles was 0.05 wt% for the composite membranes. It was also found that when the TiO2 concentration was higher than 0.05 wt%, the aggregated TiO2 nanoparticles dispersed inside the membrane increased the roughness of the pore wall and lowered the energy barrier between foulants and the membrane inner surface, which allowed more foulants to adsorb into the membrane pores.
1. Introduction
Although membrane separation technology has been commonly applied worldwide for water and wastewater treatment,1,2 membrane fouling is still a critical factor hindering the practical application of membrane technology. Therefore, to improve membrane antifouling properties has attracted much attention in the past decades.3 Many strategies such as surface graft polymerization, chemical grafting, surface coating and additive dosing have been adopted to modify membrane characteristics.4 Among these methods, introduction of inorganic nanoparticles into polymeric membranes to form organic/inorganic composite membranes have shown significant effectiveness in improving membrane hydrophilicity, changing pore structure, and enhancing membrane antifouling ability.5Various inorganic nanomaterials including TiO2,6,7 SiO2,8 ZrO2,9 Al2O3,10 zeolite nanoparticles,11 graphene oxides,12 and carbon nanotubes13 have been used to fabricate composite membranes. Among these inorganic nanoparticles, nano-sized TiO2 has received much attention due to its high hydrophilicity, great chemical stability, and antibacterial property.14 Oh et al.15 modified poly(vinylideneflouride) (PVDF) ultrafiltration (UF) membrane by dispersing nano-sized TiO2 in a PVDF solution, and their results indicated that PVDF membrane fouling was reduced due to the enhancement of hydrophilicity after TiO2 addition. Cao et al.16 found that the membrane surface became smoother through the addition of nanoparticles. Li et al.6 reported that thermal stability of the composite membrane had been improved by the addition of TiO2 nanopaticles. They also found that the composite membrane had a top surface with high porosity at low loading amount of TiO2; however, at higher loading, the skin layer became much looser because of a significant aggregation of TiO2 nanopaticles. The work of Yang et al.17 also showed that the water permeability, hydrophilicity, mechanical strength and antifouling ability of the composite membrane was significantly enhanced by the addition of TiO2 nanoparticles.
Although the addition of TiO2 nanoparticles into the casting solution has been proven to improve the antifouling ability, a systematical investigation of mechanisms improving membrane antifouling behaviours of the composite membranes is still lacking. It is mainly attributed to that the presence of TiO2 nanoparticles can result in the changes of hydrophilicity, zeta potential, roughness and porosity simultaneously. All of those factors have their positive or negative influences on membrane fouling behaviours. It is of significance to establish a comprehensive understanding of the antifouling behaviours for the composite membranes by taking all of factors into consideration.
In this study, TiO2 nanoparticles with a series of dosages were introduced into PVDF materials to prepare PVDF/TiO2 composite membranes. The physicochemical properties of the composite membranes were characterized by scanning electron microscope (SEM), energy-diffusive X-ray analyzer (EDX), atomic force microscopy (AFM) and attenuated total reflectance Fourier transform infrared (ATR-FTIR). The membrane surface energetics (free energy and interaction energy between membranes and foulants) was assessed by extended Derjaguin–Landau–Verwey–Overbeek (XDLVO) theory in order to unifying the overall influences of membrane physicochemical factors. The antifouling behaviours were further determined by quartz crystal microbalance with dissipation (QCM-D) technology. The obtained results are expected to provide a sound understanding on the antifouling behaviours of PVDF/TiO2 composite membranes.
2. Materials and methods
2.1. Materials
Commercial grade PVDF (FR904) was purchased from Shanghai 3F New Materials Ltd. (Shanghai, China). Dimethylacetamide (DMAC) and polyethylene glycol (PEG, 400 Da) used as the solvent and pore-forming additive, respectively, were supplied by Sinopharm (Shanghai, China). TiO2 reagent with diameters of 10–50 nm and purity of 99.8% was obtained from Hangzhou Wanjing New Materials Co., Ltd. (Hangzhou, China). Soluble microbial products (SMP) have been reported to be the major membrane foulants in membrane bioreactors.18,19 SMP mainly contains polysaccharides, proteins, humic acids, nucleic acids, and other polymeric compounds, among which polysaccharides are known as the dominant foulants due to their macromolecular characteristics.20,21 In this study, sodium alginate (SA) supplied by Sigma Aldrich was adopted as model SMP-foulants at a concentration of 500 mg L−1. NaCl was used to adjust the ionic strength of the SA solution. pH was controlled with HCl and NaOH. Deionized water was used throughout this study.2.2. Membrane preparation
The pristine PVDF membrane and PVDF/TiO2 composite membranes were prepared by the phase inversion method. Pre-determined dosages of TiO2 nanoparticles were dispersed in 85 g DMAC in a beaker and bath-sonicated for 1 h. PVDF was dried at 80 °C for 24 h to eliminate the absorbed water molecules before the preparation, and then 8 g PVDF and 7 g PEG were completely dissolved in the DMAC solvent. PEG has been widely used as a good pore former during the fabrication of ultrafiltration/microfiltration membrane due to its high hydrophilicity.22,23 The casting solutions were dissolved at 80 °C for 48 h to form homogeneous solutions. The homogeneous casting solutions were subsequently casted on a glass plate and the scraper clearance was controlled at 250 μm, and then were shortly (generally 30 s) exposed to ambient air (20 ± 1 °C, 30 ± 5% relative humidity) to allow partial evaporation of the solvents. After that, these solution films together with the glass plate were moved toward the non-solvent bath for immersion precipitation at room temperature. In this study, water was used as the non-solvent bath media and the water temperature was maintained at 25 ± 1 °C. Phase inversion started immediately, and then the solid membrane was detached from the glass plate. The obtained membranes were then repeatedly washed with water and immersed in distilled water for the subsequent analysis. The PVDF/TiO2 membranes made from the casting solutions with 0.02, 0.05, 0.10 and 0.50 wt% TiO2 were named as T-0.02, T-0.05, T-0.1 and T-0.5, respectively. The pristine PVDF membrane was termed T-0 as a control.2.3. Membrane properties
The SEM and EDX analyses were carried out with an SEM and its adjunct EDX analyzer (Model XL-30, Philips, Netherlands) to characterize the membrane morphologies and elemental compositions of membrane samples, respectively. The membrane topography was also determined by AFM technology (Multimode IV, Bruker Nano Surface, USA) and the Nanoscope® control software was used for image acquisition. The average roughness (Ra), root mean-square roughness (Rq) and maximum roughness (Rm) were determined to quantitatively compare the variations. Ra is the average deviation of the measured z-values from the basic plane, which is thought of as half the average peak-to-valley depth. Rq indicates the standard deviation of an entire distribution of z-values for a large sample size, and Rm represents the difference between the largest positive and negative z-values. To study the surface chemical composition changes of the PVDF membranes, ATR-FTIR spectra were collected using a Nicolet 5700 spectrometer (Thermo Electron Corporation, USA) set at a 4 cm−1 resolution. The spectra were measured at wavenumbers in the range of 1500–700 cm−1. The measurements of the membrane volume porosity and pure water flux were conducted according to our previous report.24 The membrane volume porosity is defined as the pores' volume divided by the total volume of the membrane and was determined by gravimetric method. 20 mm × 20 mm membrane specimens were used and three measurements for each sample were carried out. Pure water flux was determined by dead-end filtration method. All membrane samples were subject to the compaction under a trans-membrane pressure (TMP) of 1.0 bar until the flux was stable. Then, the filtrate of 17.3 cm2 membrane sample was collected for 2 min under the TMP of 0.03 bar to calculate the pure water flux. The pure water flux was reported by averaging three measurements for each membrane at room temperature (25 ± 1 °C).Contact angle was measured using an optical contact angle measurement system (OCA 15 Plus, Data physics GmbH, Germany). Each reported value was expressed by averaging five measurements of different positions for each sample.
2.4. XDLVO theory
In this study, XDLVO theory was employed to assess the interaction energetics between foulants and membrane surfaces. According to van Oss,25 the energy balances for aqueous system are determined by the sum of Lifshitz–van der Waals (LW), electrostatic (EL) and acid–base (AB) interaction energy, which can be expressed by eqn (1).| UXDLVOmlc = ULWmlc + UELmlc + UABmlc | (1) |
In eqn (1), ULWmlc, UELmlc and UABmlc are the individual components of the total interfacial energy associated with LW, EL and AB forces, respectively. The subscripts m, l and c refer to membrane, bulk liquid (i.e., water in this study) and foulants (i.e., alginate in this study), respectively. These components' interfacial energy could be calculated as follows.
 | (2) |
 | (3) |
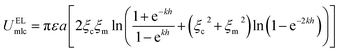 | (4) |
In the above equations, a is the radius of foulant, h the separation distance between membrane and foulants, λ the decay length of AB interactions (0.6 nm in aqueous systems).26 ε is the dielectric permittivity of the suspending fluid, κ the inverse Debye screening length, and ξm and ξc are the surface potentials of the membrane and foulant, respectively. 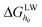 and
and  are the Liftshitz–van der Waals and acid–base interaction free energy components at the separation distance of h0, respectively, whereas the subscript h0 refers to the minimum separation distance of 0.158 nm.25
are the Liftshitz–van der Waals and acid–base interaction free energy components at the separation distance of h0, respectively, whereas the subscript h0 refers to the minimum separation distance of 0.158 nm.25  and
and  can be worked out by eqn (5) and (6), respectively.26
can be worked out by eqn (5) and (6), respectively.26
 | (5) |
 | (6) |
 | (7) |
| γTOT = γLW + γAB | (8) |
The apolar LW component shows a single electrodynamic property of a given material, whereas the polar AB component comprises two non-additive electron acceptor and electron donor parameters.26,31 The polar AB component of a material's surface free energy is expressed by eqn (9).32
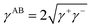 | (9) |
The specific measurement and calculation procedure of surface energetics parameters for XDLVO theory can be found in ESI (the text and Tables S1–S4†).
2.5. QCM-D measurements
QCM-D was used to dynamically examine the adsorption behaviors of the model SMP-foulants on the PVDF and PVDF/TiO2 composite membranes by monitoring the change of the oscillation frequency (Δf) when SA was flowing above the polymer-coated QCM-D crystal with a Q-Sense E4 unit (Q-Sense AB, Göteborg, Sweden).Firstly, 10-fold dilutions of the homogeneous casting solutions of T-0–T-0.5 membranes were performed with DMAC. The gold-coated crystal sensors with a fundamental resonant frequency of 4.95 MHz and a diameter of 14 mm were pre-cleaned using 10 wt% sodium dodecyl sulfate (Sinopharm, Shanghai, China), and then rinsed thoroughly with deionized water and dried with pure nitrogen. Then, the sensors were spin-coated with the diluted polymer solutions at 6000 rpm for 60 s (KW-4A, Chemat Technology, USA), followed by drying on a hot plate (DB-3, Changzhou Guohua Electric Appliance Co., Ltd, China) at 353 K for 30 min. 500 mg L−1 SA solution with 10 mM NaCl under pH = 6.5 was used as model foulants, and 10 mM NaCl under pH = 6.5 was used as background solution. The aqueous media was injected into the QCM-D flow-cell at 150 μL min−1 flow rate with a peristaltic pump (IsmaTec pump, IDEX). The stages for applying aqueous media to the QCM-D flow-cell include the following 5 steps: (A) deionized water; (B) background solution; (C) SA solution; (D) background solution; (E) deionized water. Each step was maintained for 15 min at constant temperature (25 °C) after acquiring a stable baseline with deionized water for several hours. The variation of frequency at the seventh overtone was observed to analyze the adsorption rate.
3. Results and discussion
3.1. Physicochemical properties of the composite membranes
According to the membrane formation mechanisms, once the casting solution and nonsolvent comes into contact, a skin layer forms immediately because the mutual diffusion between solvent in the casting solution and non-solvent in the coagulation bath. When the amount of nonsolvent diffused into the casting solution is sufficient to destabilize thermodynamically stability somewhere, the casting solution reduces its free energy from dividing into two liquid phases of different composition, i.e., a nucleus of the polymer-poor phase that forms the nascent pore and a polymer-rich phase that surrounds the pore. In other words, the polymer precipitation takes place at this moment. When more non-solvent enters the different sites of the skin layer, more pores appear. Since nano-TiO2 has the higher affinity to water compared to that of PVDF, penetration velocity of water (non-solvent) into the nascent membrane can be enhanced by the addition of TiO2 during the phase inversion, which is supposed to accelerate the formation of surface pores. In addition, due to the fact that the interaction between polymers and solvent molecules could be hindered by the obstruction of nanoparticles, it can therefore increase the solvent (DMAC) diffusion velocity from the membrane to water,34 which also contributed to the formation of micropores on the membrane surface. Therefore, the average pore size and porosity of PVDF/nano-TiO2 composite membranes was higher than that of the original PVDF membrane.
In order to further examine the effects of TiO2 nanoparticles on the membrane surface roughness, AFM analyses were performed for all the membranes. The three-dimensional AFM images are shown in Fig. S1 of the ESI† and the roughness is illustrated in Fig. 2. It could be observed that all the membranes have similar magnitude of asperity in their surfaces. The Ra and Rq values of all the membranes were about 72.2–78.6 nm and 58.0–59.7 nm, respectively, showing that the membrane surface roughness was not obviously changed with the addition of TiO2 nanoparticles in this study. However, Rm was generally enlarged with the increase of TiO2 dosages. This result indicates that the existence of insoluble aggregated TiO2 particles in the matrix might turn into a bump in the sub-layer during membrane preparation and increase the peak value of the asperity.
Fig. 4 shows the ATR-FTIR spectra of PVDF membrane and PVDF/TiO2 composite membranes. The absorption peak at 1180 cm−1 is assigned to the stretching vibration of CF2 groups, and the deformed vibration of CH2 groups appears at the frequency of 1402 cm−1.35 FTIR spectra of two major phases (α and β phases) of PVDF have been intensively investigated. In this study, the vibrational bands at 765 cm−1 (CF2 bending and skeletal bending), 795 cm−1 (CH2 rocking), 874 cm−1 and 974 cm−1 are associated with α-phase,36–38 and the vibrational bands at 840 cm−1 (CH2 rocking) correspond to β-phase. It was also visible that the absorption peak of α-phase was gradually reduced with the addition of TiO2, and disappeared when the amount of TiO2 was 0.5 wt%. On the contrary, the adsorption peak of β-phase was enhanced by the addition of TiO2. These results showed that the addition of TiO2 nanoparticles increased the content of β-phase crystallize of PVDF, and in the meantime reduced that of α-phase. Andrew and Clarke39 detected the melting temperature (Tm) of PVDF electrospun samples with different crystalline phase by measuring the melting enthalpy using differential scanning calorimetry (DSC). They reported that the variation of Tm was mainly due to the change in the relative amounts of the β and α phases, and an increased contribution from the lower melting α-phase could induce the decrease in Tm, i.e., the decrease of thermal stability. According to their results, it can be inferred that the thermal stability of composite membrane has been enhanced by the addition of TiO2 nanoparticles in this study.
3.2. XDLVO analysis
The surface tension parameters and the free energy of cohesion for all the membranes as calculated by eqn (7)–(9) are shown in Table 1. It can be seen that all the membranes have the larger γLW value compared with the γAB value, showing that all the membranes feature strong apolar properties. The electron donor component (γ−) was much higher than the electron acceptor component (γ+) for all the membranes, suggesting that all the membranes exhibited high electron donor monopolarity, which is in accordance with a previous study.43 Furthermore, the addition of nano-TiO2 obviously enhanced the electron donor monopolarity, and γ− value increased from 5.0 mJ m−2 to 12.8 mJ m−2 by adding 0.05 wt% nano-TiO2. It could be also observed from Table 1 that T-0.5 has the highest electron acceptor components (γ+) and polar components (γAB) value compared with other membranes, leading to the relatively strong polar properties. In general, surfaces with high electron acceptor capability will interact favorably with surfaces that possess electron-donor functionality, thus causing potentially attractive acid–base interaction.44| γLWs | γ+s | γ−s | γAB | γTOT | ΔGSWS | |||
|---|---|---|---|---|---|---|---|---|
| a Values are given as average ± standard deviation (n = 4). | ||||||||
| T-0 | 27.8 ± 1.7 | 0.5 ± 0.4 | 5.0 ± 2.9 | 2.6 ± 0.7 | 30.4 ± 1.7 | −0.8 ± 0.4 | −55.3 ± 9.1 | −56.1 ± 9.1 |
| T-0.02 | 30.6 ± 0.6 | 0.4 ± 0.5 | 11.2 ± 4.8 | 3.2 ± 1.9 | 33.8 ± 1.9 | −1.5 ± 0.2 | −37.3 ± 14.1 | −38.8 ± 14.1 |
| T-0.05 | 34.2 ± 1.5 | 0.1 ± 0.1 | 12.8 ± 4.8 | 1.9 ± 1.1 | 35.9 ± 2.2 | −2.7 ± 0.6 | −32.0 ± 8.7 | −34.8 ± 8.5 |
| T-0.1 | 31.0 ± 1.0 | 0.3 ± 0.2 | 10.2 ± 2.7 | 3.5 ± 0.7 | 34.5 ± 1.5 | −1.6 ± 0.3 | −40.6 ± 7.5 | −42.2 ± 7.8 |
| T-0.5 | 33.4 ± 1.0 | 1.2 ± 0.2 | 9.1 ± 2.2 | 6.6 ± 0.9 | 40.0 ± 0.5 | −2.5 ± 0.4 | −45.5 ± 4.2 | −48.0 ± 4.4 |
The LW and AB components of the surface free energy were calculated using eqn (5) and (6), and the sum of LW and AB free energy components for a given material yielded the free energy of cohesion (ΔGSWS). ΔGSWS represents the interaction energy when two surfaces of the same material (for instance, membrane–membrane or foulant–foulant) are immersed in a solvent (i.e., water in this study).25,42 The negative ΔGSWS value of the membrane surfaces suggests that the membranes are thermodynamically unstable in water, and the more negative the ΔGSWS value is, the stronger the hydrophobicity is. In this study,  was obvious larger than
was obvious larger than 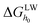 for all the membranes, implying that the contribution of AB free energy component was more significant compared to LW free energy component. The ΔGSWS value of all the membranes was negative, suggesting that all the membranes were of hydrophobic properties. However, the ΔGSWS value of T-0 was the lowest (−56.1 mJ m−2), and ΔGSWS value of the composite membranes was elevated by adding TiO2 nanoparticles, which manifests that TiO2 nanopaticles improved the free energy of cohesion of the membranes, and the improvement was most significant when the concentration of TiO2 was 0.05 wt% (i.e., T-0.05 membrane). However, with further increase of nano-TiO2 in the polymer matrix, the aggregation happened, causing the reduction of the free energy of cohesion compared to T-0.05 membrane.
for all the membranes, implying that the contribution of AB free energy component was more significant compared to LW free energy component. The ΔGSWS value of all the membranes was negative, suggesting that all the membranes were of hydrophobic properties. However, the ΔGSWS value of T-0 was the lowest (−56.1 mJ m−2), and ΔGSWS value of the composite membranes was elevated by adding TiO2 nanoparticles, which manifests that TiO2 nanopaticles improved the free energy of cohesion of the membranes, and the improvement was most significant when the concentration of TiO2 was 0.05 wt% (i.e., T-0.05 membrane). However, with further increase of nano-TiO2 in the polymer matrix, the aggregation happened, causing the reduction of the free energy of cohesion compared to T-0.05 membrane.
The surface tension parameters of alginate solution with 10 mM NaCl concentration under pH = 6.5 were used to calculate the total interfacial energy versus separation distance between alginate and different membranes with eqn (1)–(4). UXDLVOmlc is supposed to determine the alginate-membrane interactions when the alginate is approaching to the membrane surfaces to form fouling. Fig. 6 depicts that the alginate would be subject to the repulsive interaction when getting as close as 30 nm from all the membrane surfaces, and in order to reach the membrane surfaces the foulant must overcome the repulsion interaction energy. The higher the energy barrier is, the harder the initial adsorption is. In this study, the energy barrier between alginate (pH = 6.5, ionic strength = 10 mM NaCl) and T-0–T-0.5 was 0.6, 1.6, 2.4, 1.4 and 1.0 KT, respectively, demonstrating that the addition of TiO2 nanoparticles improved the interaction energy between foulants and membrane. Therefore, the initial adsorption of the foulants could be mitigated for T-0.02–T-0.5 membranes compared to T-0 membrane.
 | ||
| Fig. 6 Variations of interaction energy between membranes and alginate (ionic strength 10 mM NaCl, pH = 6.5). | ||
3.3. QCM-D analysis
QCM-D provides real-time measurements of molecular adsorption and/or interactions taking place on various surfaces. In this study, QCM-D was used to dynamically verify the deposition of foulants onto different membranes. Fig. 7 displays the real-time frequency shifts of the QCM-D sensor coated with the T-0–T-0.5 recipes as a function of the exposure time. It could be observed that when the foulant solution was injected into the sensor (Phase C), the frequency of all the crystals decreased instantly and the one casted with T-0.5 recipe showed a maximum decline, implying the highest adsorption amount of foulants. The order of frequency decrease during phase C for the five sensors with different membranes coated on is: T-0.5 > T-0.1 > T-0 > T-0.02 > T-0.05, which is unexpectedly inconsistent with that of interaction energy between membranes and alginate solution. The order of repulsive interaction energy barrier between alginate sodium and membranes is: T-0.05 > T-0.02 > T-0.1 > T-0.5 > T-0, which means that even though the interaction energy barrier is higher the membranes are still more favorable of deposition once nano-TiO2 aggregation happens in the membrane matrix. Washing with background solution and deionized water was performed after the alginate sodium adsorption experiment (Phase D and E) and the frequency of all the sensors increased about 1–5 Hz, indicating that desorption of alginate partly occurred from the membranes. However, the hydrodynamic washing could not remove all the deposited foulants and the initial membrane fouling had formed.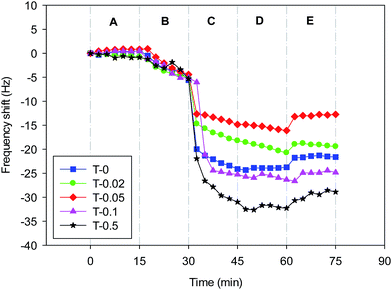 | ||
| Fig. 7 Representative frequency shifts (7th overtone) by the deposition of alginate sodium onto PVDF and PVDF/TiO2 coated QCM-D crystals. | ||
Based on the XDLVO and QCM-D analysis, there is an unexpected result that T-0.1 and T-0.5 has obtained the highest alginate fouling rate rather than T-0, since T-0 had been determined to have the lowest energy barrier with the foulants. Hoek et al.44 has reported that the repulsive interaction energy barrier between foulant and membrane calculated by DLVO theory could be impacted by the membrane roughness, and the repulsive interaction energy barrier between a foulant and a rough membrane is lower than the corresponding barrier for a smooth membrane. Although all the membrane in current study had similar membrane surface roughness, it could be observed from cross-sectional SEM image (Fig. 1) that TiO2 nanoparticles were aggregated into 0.2–1.0 μm particles and dispersed on the pore wall of T-0.1 and T-0.5, which resulted in increasing the roughness of internal membrane surface. However, the roughness of internal membrane surface could not be detected. Chen et al.45 reconstructed the membrane surface topology based on the statistical parameters obtained from AFM and adopted surface element integration technique to calculate the interaction energy between SMP and the reconstructed rough membrane surfaces in the framework of the XDLVO theory. Their results showed that the great influence of protrusion on the membrane surface could reduce the primary energy barrier height, and an attractive energy region was immediately surrounded by each protrusion as demonstrated in the roughness engendered interaction energy maps. Therefore, in this study, the aggregated TiO2 particles dispersed on the pore wall of T-0.1 and T-0.5 are supposed to reduce the interaction energy between foulants and membrane inner surface, inducing more foulants deposition on the membrane pore wall.
4. Conclusions
In this study, physicochemical properties and antifouling behaviours of composite membranes were investigated. The results showed that the addition of TiO2 could improve the membrane surface porosity, volume porosity, hydrophilicity and permeability. The addition of TiO2 nanoparticles obviously enhanced the electron donor monopolarity of the composite membranes and increased the repulsive interaction energy barrier between foulants and membrane surfaces, thus improving antifouling ability. The membrane topology obtained from AFM image showed that the incorporation of TiO2 did not change the average membrane surface roughness. However, the aggregated TiO2 particles with diameter about 0.2–1.0 μm were dispersed on the pore wall of membrane cross-sections, which was supposed to increase the roughness of membrane inner surface and reduce the repulsive interaction energy barrier between foulants and membrane inner surface. This could result in the deposition of more foulants. In other words, even though the addition of TiO2 nanoparticles could improve the interaction energy barrier between foulants and membrane surface, the aggregated TiO2 particles in the membrane matrix could aggravate the membrane pore fouling rate once the concentration of nanoparticles was high enough to form aggregations. Therefore, in this study, the composite membrane with 0.05% TiO2 nanoparticles had the best performance.List of symbols
Nomenclature
Greek letters
| εrε0 | Dielectric permittivity of the suspending fluid (F m−1) |
| ξm | Surface potentials of the membrane (mV) |
| ξc | Surface potentials of the foulant (mV) |
| θ | Contact angle (°) |
| κ | Inverse Debye screening length (m−1) |
| λ | Decay length of AB interactions (0.6 nm) |
Subscripts
| c | Foulants |
| l | Bulk liquid |
| m | Membrane |
| s | Solid surface |
Acknowledgements
This Project is financially supported by China Postdoctoral Science Foundation (2013M540389), National Natural Science Foundation of China (51378371&51308400) and Shanghai Rising-Star Program (14QA1403800).References
- M. Elimelech and A. W. Phillip, Science, 2011, 333, 712–717 CrossRef CAS PubMed.
- F. G. Meng, S. R. Chae and H. S. Shin, Environ. Eng. Sci., 2012, 29, 139–160 CrossRef CAS.
- P. Le-Clech, V. Chen and T. A. G. Fane, J. Membr. Sci., 2006, 284, 17–53 CrossRef CAS PubMed.
- G. R. Guillen, Y. J. Pan, M. H. Li and E. M. V. Hoek, Ind. Eng. Chem. Res., 2011, 50, 3798–3817 CrossRef CAS.
- M. M. Pendergast and E. M. V. Hoek, Energy Environ. Sci., 2011, 4, 1946–1971 CAS.
- J. F. Li, Z. L. Xu, H. Yang, L. Y. Yu and M. Liu, Appl. Surf. Sci., 2009, 255, 4725–4732 CrossRef CAS PubMed.
- W. Y. Li, X. L. Sun, C. Wen, H. Lu and Z. W. Wang, Front. Environ. Sci. Eng., 2013, 7, 492–502 CrossRef CAS PubMed.
- L. Y. Yu, Z. L. Xu, H. M. Shen and H. Yang, J. Membr. Sci., 2009, 337, 257–265 CrossRef CAS PubMed.
- P. Aerts, S. Kuypers, I. Genne, R. Leysen, J. Mewis, I. F. J. Vankelecom and P. A. Jacobs, J. Phys. Chem. B, 2006, 110, 7425–7430 CrossRef CAS PubMed.
- L. Yan, S. Hong, M. L. Li and Y. S. Li, Sep. Purif. Technol., 2009, 66, 347–352 CrossRef CAS PubMed.
- B. Moermans, W. D. Beuckelaer, I. F. J. Vankelecom, R. Ravishankar, J. A. Martens and P. A. Jacobs, Chem. Commun., 2000, 2467–2468 RSC.
- Z. H. Wang, H. R. Yu, J. F. Xia, F. F. Zhang, F. Li, Y. Z. Xia and Y. H. Li, Desalination, 2012, 299, 50–54 CrossRef CAS PubMed.
- L. Brunet, D. Y. Lyon, K. Zodrow, J. C. Rouch, B. Caussat, P. Serp, J. C. Remigy, M. R. Wiesner and P. J. J. Alvarez, Environ. Eng. Sci., 2008, 25, 565–575 CrossRef CAS.
- U. Diebold, Surf. Sci. Rep., 2003, 48, 53–229 CrossRef CAS.
- S. J. Oh, N. Kim and Y. T. Lee, J. Membr. Sci., 2009, 345, 13–20 CrossRef CAS PubMed.
- X. C. Cao, J. Ma, H. Shi and Z. F. Ren, Appl. Surf. Sci., 2006, 253, 2003–2010 CrossRef CAS PubMed.
- Y. N. Yang, H. X. Zhang, P. Wang, Q. Z. Zheng and J. Li, J. Membr. Sci., 2007, 288, 231–238 CrossRef CAS PubMed.
- Z. W. Wang, X. J. Mei, Z. C. Wu, S. F. Ye and D. H. Yang, Chem. Eng. J., 2002, 193–194, 77–87 Search PubMed.
- S. Hong and M. Elimelech, J. Membr. Sci., 1999, 132, 159–181 CrossRef.
- C. S. Laspidou and B. E. Rittmann, Water Res., 2002, 36, 2711–2720 CrossRef CAS.
- B. Q. Liao, D. M. Bagley, H. E. Kraemer, G. G. Leppard and S. N. Liss, Water Environ. Res., 2004, 76, 425–436 CrossRef CAS.
- A. Moriya, T. Maruyama, Y. Ohmukai, T. Sotani and H. Matsuyama, J. Membr. Sci., 2009, 342, 307–312 CrossRef CAS PubMed.
- G. Arthanareeswaran, D. Mohan and M. Raajenthiren, J. Membr. Sci., 2010, 350, 130–138 CrossRef CAS PubMed.
- Q. Y. Wang, Z. W. Wang and Z. C. Wu, Desalination, 2012, 297, 79–86 CrossRef CAS PubMed.
- C. J. van Oss, Interfacial Forces in Aqueous Media, Marcel Dekker, Inc., New York, 1994 Search PubMed.
- C. J. van Oss, Colloids Surf., A, 1993, 78, 1–49 CrossRef CAS.
- L. Gourley, M. Britten, S. F. Gauthier and Y. Pouliot, J. Membr. Sci., 1994, 97, 283–289 CrossRef CAS.
- C. R. Bouchard, J. Jolicoeur, P. Kouadio and M. Britten, Can. J. Chem. Eng., 1997, 75, 339–345 CrossRef CAS PubMed.
- C. J. van Oss and R. J. Good, J. Protein Chem., 1988, 7, 179–183 CrossRef CAS.
- G. Wolanksky and A. Marmur, Langmuir, 1998, 14, 5292–5297 CrossRef.
- M. Greiveldinger and M. E. R. Shanahan, J. Colloid Interface Sci., 1999, 215, 170–178 CrossRef CAS PubMed.
- S. Bhattacharjee, A. Sharma and P. K. Bhattacharya, Langmuir, 1994, 10, 4710–4720 CrossRef CAS.
- V. Vatanpour, S. S. Madaeni, A. R. Khataee, E. Salehi, S. Zinadini and H. A. Monfared, Desalination, 2012, 292, 19–29 CrossRef CAS PubMed.
- T. H. Bae and T. M. Tak, J. Membr. Sci., 2005, 249, 1–8 CrossRef CAS PubMed.
- F. M. Shi, Y. X. Ma, J. Ma, P. P. Wang and W. X. Sun, J. Membr. Sci., 2012, 389, 522–531 CrossRef CAS PubMed.
- Y. Wei, H. Q. Chu, B. Z. Dong, X. Li, S. J. Xia and Z. M. Qiang, Desalination, 2011, 272, 90–97 CrossRef CAS PubMed.
- M. Benz, W. B. Euler and O. J. Gregory, Macromolecules, 2002, 35, 2682–2688 CrossRef CAS.
- A. Salimi and A. A. Yousefi, Polym. Test., 2003, 22, 699–704 CrossRef CAS.
- J. S. Andrew and D. R. Clarke, Langmuir, 2008, 24, 670–672 CrossRef CAS PubMed.
- A. Rahimpour, S. S. Madaeni, A. H. Taheri and Y. Mansourpanah, J. Membr. Sci., 2008, 313, 158–169 CrossRef CAS PubMed.
- Z. X. Ji, X. Jin, S. George, T. Xia, H. Meng, X. Wang, E. Suarez, H. Y. Zhang, E. M. V. Hoek, H. Godwin, A. E. Nel and J. I. Zink, Environ. Sci. Technol., 2010, 44, 7309–7314 CrossRef CAS PubMed.
- J. A. Brant and A. E. Childress, J. Membr. Sci., 2002, 203, 257–273 CrossRef CAS.
- F. Shen, X. F. Lu, X. K. Bian and L. Q. Shi, J. Membr. Sci., 2005, 265, 74–84 CrossRef CAS PubMed.
- E. M. V. Hoek, S. Bhattacharjee and M. Elimelech, Langmuir, 2003, 19, 4836–4847 CrossRef CAS.
- L. Chen, Y. Tian, C. Q. Cao, J. Zhang and Z. N. Li, Water Res., 2012, 46, 2693–2704 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra07274j |
| This journal is © The Royal Society of Chemistry 2014 |