Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Tris(pentafluorophenyl)borane: leveraging historical and emerging work to identify alternatives for organic electronic applications
Kathryn M.
Wolfe
 ,
Michael J.
Grant
,
Michael J.
Grant
 ,
Irene E.
Park
,
Irene E.
Park
 and
Gregory C.
Welch
and
Gregory C.
Welch
 *
*
Department of Chemistry, University of Calgary, 2500 University Drive N.W., Calgary, Alberta T2N 1N4, Canada. E-mail: gregory.welch@ucalgary.ca; Tel: +1-403-210-7603
First published on 27th May 2025
Abstract
Tris(pentafluorophenyl)borane (B(C6F5)3) is a versatile Lewis acid now having played a key role in the development of boron-based Lewis acid chemistry. The borane has found utility in many applications ranging from a co-catalyst for olefin-polymerization to a dopant for organic (semi-)conductors. Highlighted within are historical advances of B(C6F5)3 utilization in the realms of molecular transformations and frustrated Lewis pairs (FLPs). Applications of B(C6F5)3 in various electronic devices is upcoming and presented, as well emerging dopant strategies for organic-based electronic applications are included. This perspective is completed by presenting other known Lewis acids with respective properties relevant for dopant applications in organic electronics.
1. Introduction
Tris(pentafluorophenyl)borane (B(C6F5)3; Fig. 1) is a popular boron-based Lewis acid that has seen applications in many branches of science and engineering. With a 3-coordinate neutral structure there is an empty p-orbital on boron which can accept lone pairs of electrons, thus enabling reactivity. The Lewis acidity of B(C6F5)3 is influenced by the three electron withdrawing pentafluorophenyl substituents that remove electron density away from boron and make the atom more acidic. The bulky pentafluorophenyl groups sterically encumber the boron center and introduces intermolecular interactions, which renders the compound a solid at room temperature with increased air and moisture stability compared to halide BX3 (X = F, Cl, Br) counterparts. These properties make B(C6F5)3 a potent yet stable Lewis acid, and therefore has been widely used both academically and industrially.1 First synthesized in the early 1960's,2 B(C6F5)3 received little attention until the early 1990's when the compound was employed as a co-catalyst for the polymerization of olefins.3–6 Following this, the volume of research and industrial application involving B(C6F5)3 as a catalyst for molecular transformations was exponential. Around this time B(C6F5)3 was also being applied in electronic devices, such as lithium batteries, with reports dating back as early as the 1990's.7 Then in the mid 2000's, the discovery of frustrated Lewis pairs (FLPs), based on the combination of B(C6F5)3 with sterically bulky phosphines, re-popularized the chemistry of this borane and led to the first reports of metal-free hydrogenation using hydrogen gas (H2) and the subsequent sequestration of many other small molecules (e.g. CO2, N2O, olefins).8–11 While the chemistry of B(C6F5)3 for molecular transformations1,6,12–14 and FLPs9–11,15 has been extensively reviewed, the use of B(C6F5)3 as a p-type dopant for organic (semi-)conductors with reports of increased stability and efficiency of organic electronic devices is emerging.Within we first highlight established applications of B(C6F5)3 in the realms of organic/organometallic molecular transformations and FLPs. The reported interactions, properties, and reactivity are critical to consider when applying B(C6F5)3 as a p-type dopant in organic electronics, as the role of the Lewis acid has been somewhat elusive and debated, and thus these accounts provide valuable insight. A variety of electronic devices, including batteries, organic transistors, solar cells, etc., are reviewed with relevant dopant mechanisms, emerging applications, and dopant strategies. Lastly, we present alternate borane- and borate-based Lewis acids with the respective properties relevant for dopant applications in organic electronics. Our perspective is that while the utility and scope of B(C6F5)3 is impressive, the variety of materials applied in organic electronics is so vast that alternate Lewis acids need to be considered as B(C6F5)3 will not harbour the optimal properties for every set of materials and device architectures that develop.
2. Molecular transformations using B(C6F5)3
2.1. Overview
The empty p-orbital on the boron of B(C6F5)3 can accept a pair of electrons and thus can activate Lewis basic sites on compounds to promote catalytic reactions & organic/organometallic transformations. Some important reactions & transformations involving B(C6F5)3 include olefin polymerization,1,5,6 aldol-type Michael reactions,16 hydrosilylations,17,18 Piers–Rubinsztajn reactions,17,19,20 allylstannations,21–23 and Diels–Alder reactions.24–27 Unlike other boron/halogen containing Lewis acids (BX3, X = F, Cl, Br), the pentafluorophenyl groups on B(C6F5)3 increase the sterics about the boron center, embodies B–C bonds that are less prone to hydrolysis, and promotes intermolecular interactions which allows for stable and isolable intermediates/products in a plethora of catalytic reactions. This resulted in mechanistic studies on Lewis acid catalyzed and co-catalysed reactions which were previously not achievable with traditional boron/halogen containing Lewis acids. Furthermore, the high thermal stability of B(C6F5)3 (stable up to 270 °C) is ideal for reactions that require higher temperatures in which traditional metal catalysts would degrade. There are comprehensive reviews on the various organic and organometallic transformations that B(C6F5)3 is capable of catalyzing and/or plays a key role in ref. 13 and 28, therefore, this section merely highlights enlightening studies using B(C6F5)3 in various molecular transformations and FLPs. Reactions involving B(C6F5)3 in the presence of water are also included as B(C6F5)3·H2O adducts are an attractive p-type dopant for organic electronic materials (vide infra).2.2. Historical molecular transformations using B(C6F5)3
In 1991, both the groups of Marks and Robinson separately demonstrated highly active and productive olefin polymerization using B(C6F5)3 as a co-catalyst with alkyl group-IV metallocenes, specifically dimethyl zirconocenes (Fig. 2a).3,29 When paired with metallocenes, the Lewis acidic B(C6F5)3 can abstract an alkyl group and is rendered anionic and the metal center cationic enabling coordination–insertion polymerization. Following this discovery, a plethora of reports using B(C6F5)3 as a co-catalyst for olefin polymerization emerged throughout the 1990's and beyond.3–6,29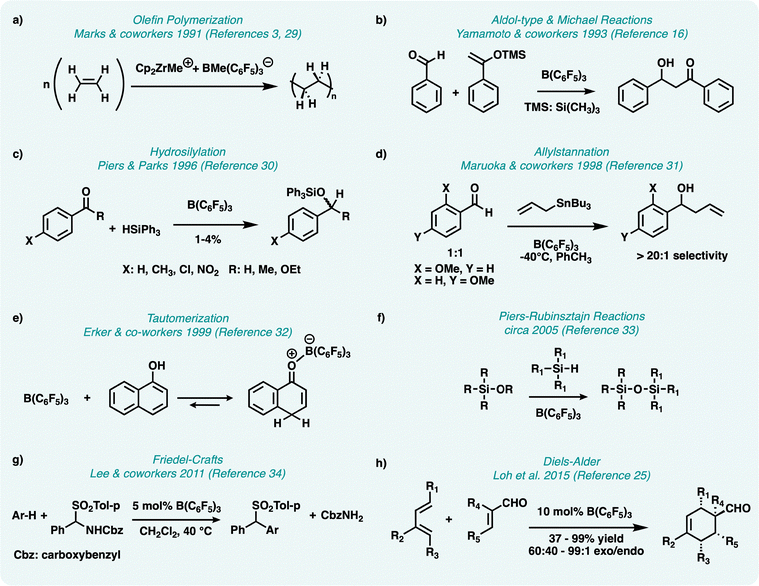 | ||
Fig. 2 Critical reactions involving B(C6F5)3 for catalysis & chemical transformations. (a) Olefin polymerization is achieved using Cp2ZrMe+ + BMe(C6F5)3−,3,29 (b) Adol-type and Michael reactions utilizing aldehydes and silyl enol ethers are catalyzed by B(C6F5)3,16 (c) hydrosilylation of aryl carbonyls are catalyzed by B(C6F5)3,30 (d) allylstannations of ortho-anisaldehydes are favoured over para-anisaldehydes by >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the presence of (B(C6F5)3),31 (e) tautomerization of aromatic enols can be done by coordination of the boron on B(C6F5)3 with the oxygen on the enol,32 (f) Piers–Rubinsztajn reactions see siloxane formation using hydrosilanes and alkoxysilanes in the presence of catalytic B(C6F5)3,33 (g) Friedel–Crafts reactions using 1,2,4-trimethoxy benzene was successfully alkylated with a N-benzyloxycarbonylamino phenyl p-tolylsulfone in the presence of catalytic B(C6F5)3,34 (h) Diels–Alder exo-selective isomers are formed open chain-dienes and α,β-unsaturated enals in the presence of catalytic B(C6F5)3.25 1 in the presence of (B(C6F5)3),31 (e) tautomerization of aromatic enols can be done by coordination of the boron on B(C6F5)3 with the oxygen on the enol,32 (f) Piers–Rubinsztajn reactions see siloxane formation using hydrosilanes and alkoxysilanes in the presence of catalytic B(C6F5)3,33 (g) Friedel–Crafts reactions using 1,2,4-trimethoxy benzene was successfully alkylated with a N-benzyloxycarbonylamino phenyl p-tolylsulfone in the presence of catalytic B(C6F5)3,34 (h) Diels–Alder exo-selective isomers are formed open chain-dienes and α,β-unsaturated enals in the presence of catalytic B(C6F5)3.25 | ||
In 1993, B(C6F5)3 was explored as a catalyst for aldol-type and Michael reactions, which were previously achieved with alternate Lewis acids, however, these reactions suffered from poor yields if even trace amounts of water were present.16 With B(C6F5)3 being somewhat water and air-stable, high yielding aldol-type and Michael reactions were achieved and resulted in the coupling of various silyl enol ethers and aldehydes (Fig. 2b). Hydrosilylation of carbonyls is used for the preparation of silylethers, and typically uses metal catalysts to do so. However, in 1996, Piers and Parks demonstrated the catalytic activity of B(C6F5)3 for the hydrosilylation of aryl carbonyls (Fig. 2c), to which B(C6F5)3 was reported as being comparable with traditional metal catalysts used in terms of selectivity and conversion rates.30 Later, hydrosilylation of imines using B(C6F5)3 was also demonstrated.35 In 1998, Maruoka and coworkers reported allylation and reduction of an ortho-anisaldehyde using B(C6F5)3 and an allyl tributyltin reagent in a competitive reaction with para-anisaldehyde, where the allylation of the ortho-anisaldehyde resulted in a >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 favour over the para-anisaldehyde (Fig. 2d).31 The authors suggested the reaction proceeds via pentacoordinate B(C6F5)3 adducts with the ortho-anisaldehyde, a highly unusual reaction as this requires chelation of a boron-based Lewis acid, something that is notoriously difficult to do. However, Piers and coworkers later elucidated the mechanism: B(C6F5)3 abstracts the allyl group from the tin reagent, producing the activated “SnBu3+” species which then catalyzes the reaction thus suggesting hypercoordination of the borane is not necessary.21,36
1 favour over the para-anisaldehyde (Fig. 2d).31 The authors suggested the reaction proceeds via pentacoordinate B(C6F5)3 adducts with the ortho-anisaldehyde, a highly unusual reaction as this requires chelation of a boron-based Lewis acid, something that is notoriously difficult to do. However, Piers and coworkers later elucidated the mechanism: B(C6F5)3 abstracts the allyl group from the tin reagent, producing the activated “SnBu3+” species which then catalyzes the reaction thus suggesting hypercoordination of the borane is not necessary.21,36
Developed in the 2000's, the Piers–Rubinsztajn reaction is one that involves the synthesis of alkoxysilanes and the respective polymers. It was first noted by Piers and Parks in 1996 that a small amount of silyl ethers were transformed into silyl alkanes in the presence of B(C6F5)3 when attempting to reduce aromatic carbonyl compounds.30 This lead to Rubinsztajn and Cella investigating the formation of siloxane polymers using B(C6F5)3.33 Shortly after, the Piers–Rubinsztajn reaction was formulated and has since been widely used for the synthesis of polymeric siloxanes (Fig. 2f).17,19,20 These polymers are composed of strong Si–O bonds, which provides the bulk material with high chemical resistance and thermal stability but at the consequence of requiring harsh conditions for degradation or recycling. However, the depolymerization of polydimethylsiloxane using reagents B(C6F5)3 and dimethyl carbonate under mild conditions was more recently reported.37 This demonstrates the versatility of B(C6F5)3 for both the synthesis and sustainable degradation of organosiloxanes.
Tautomerization of aromatic enols are difficult due to breaking aromaticity, however, due to stable B(C6F5)3-carbonyl adducts, the tautomerization of naphthol with B(C6F5)3 for isolation of the keto isomer was shown to be possible (Fig. 2e).32 Following this, several pyrrole based tautomers were made and put to use in Ziegler–Natta catalysis chemistry as Brønsted acid activators for highly efficient polymerizations.38–41 Due to the ability to form adducts with carbonyl and imine containing compounds, it was then discovered that B(C6F5)3 can catalyze other important C–C coupling reactions. Indeed, Diels–Alder reactions of 2-cycloalkenones can be catalyzed by B(C6F5)3, however, like other Lewis acids these reactions resulted in high ratios of the endo product.24 Then in 2015, Loh and coworkers demonstrated the use of B(C6F5)3 for exo-selective isomers using open chain-dienes and α,β-unsaturated enals as the dienophiles (Fig. 2h).25 It was hypothesized that due to the bulky nature of B(C6F5)3, sterics cause the favour of the exo-product with ratios ranging from 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 to 99
40 to 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was later supported by Soós and coworkers in 2018.26 Additionally, B(C6F5)3 is capable of catalyzing Friedel–Crafts reactions, showcasing yet another example of making C–C bond formation more efficient. A reaction of this type was first reported in 2011, where 1,2,4-trimethoxy benzene was successfully alkylated with a N-benzyloxycarbonylamino phenyl p-tolylsulfone in the presence of B(C6F5)3 with high yields (Fig. 2g).34
1, which was later supported by Soós and coworkers in 2018.26 Additionally, B(C6F5)3 is capable of catalyzing Friedel–Crafts reactions, showcasing yet another example of making C–C bond formation more efficient. A reaction of this type was first reported in 2011, where 1,2,4-trimethoxy benzene was successfully alkylated with a N-benzyloxycarbonylamino phenyl p-tolylsulfone in the presence of B(C6F5)3 with high yields (Fig. 2g).34
2.3. Frustrated Lewis pairs involving B(C6F5)3
Frustrated Lewis pairs (FLPs) can be described as compounds that contain Lewis base/acid pairs with sufficient steric hinderance to prevent Lewis adduct formation via dative bonding. The seminal discovery of FLPs (first publication in 2006) provided the next sequence of impactful research involving B(C6F5)3. The serendipitous discovery of FLPs came from a unusual reaction of B(C6F5)3 with the sterically bulky phosphine bismesitylphosphine ((C6H2Me3)2PH) to form the air & moisture stable white zwitterionic solid, (C6H2Me3)2PH(C6F4)BF(C6F5)2.8 Treatment of this compound with Me2SiHCl resulted in the exchange of a fluorine atom for a hydride at the boron and gave (C6H2Me3)2PH(C6F4)BH(C6F5)2. This compound contained both a proton (on the phosphorus) and hydride (on the boron) which when gently heated (∼100 °C) released H2 gas and gave the phosphino-borane (C6H2Me3)2P(C6F4)B(C6F5)2 (Fig. 3), which was bright orange in color owing to intramolecular charge transfer characteristics. The process of H2 liberation was found to be reversible and thus this resulted in the first ever report of metal-free hydrogen activation.8 By retaining the Lewis basicity and acidity within the (C6H2Me3)2P(C6F4)B(C6F5)2 molecule, electron density can be donated and accepted simultaneously and allowed for reversible heterolytic cleavage and liberation of H2 at moderate (25 °C) and high (>100 °C) temperatures, respectively. Upon further investigation of the interaction (or lack thereof) of bulky phosphines (PR3, R = i-Pr, Cy & PHR′2, R′ = t-Bu, C6H2Me3-2,4,6) with B(C6F5)3, the term FLP was coined.42 Subsequently a diverse and rapid emergence of research involving B(C6F5)3 based FLPs for many reactions, including metal-free hydrogenation, hydroamination, activation of various small molecules, and dehydrogenation has been extensively reviewed.9–11,15,43–45 Furthermore, many variations of FLPs have been made, where both the Lewis acid and Lewis base have been exchanged.10,15 This section aims to highlight select molecular transformations catalyzed by FLPs using B(C6F5)3 as the Lewis acid component. | ||
| Fig. 3 The seminal discovery of FLPs; (C6H2Me3)2PH(C6F4)BH(C6F5)2 liberates H2 when heated (to ∼100 °C) to give the phosphino-borane (C6H2Me3)2P(C6F4)B(C6F5)2, a process which is reversible upon cooling.8 | ||
Metal-free hydrogenation has positive implications for some important industries, such as the food & beverage and petrochemical industries, where the use of metal catalysts in these industries can be costly. For example, hydrogenation is responsible for the manufacturing of food products such as oils, shortenings, and spreads. Following the discovery of FLPs as a catalyst for metal-free hydrogenation, useful transformations such as the hydrogenation of alkenes/olefins/alkynes (Fig. 4a),46–49 aromatics,50 carbonyls (Fig. 4b),51–53 imines/nitriles/enamines (Fig. 4c and d),54–56 silyl enol ethers (Fig. 4f),57 and oximes (Fig. 4e)58 have been reported using B(C6F5)3 as the Lewis acid component.
 | ||
| Fig. 4 Metal-free hydrogenation using B(C6F5)3 FLPs. (a) B(C6F5)3 FLPs are capable of reducing alkynes to alkenes, and upon further treatment from alkenes to alkanes.46–49 (b) B(C6F5)3 based FLPs can reduce carbonyls to primary alcohols.51–53 (c) B(C6F5)3 based FLPs can reduce nitriles into imines, and then into amines.54,55 (d) B(C6F5)3 based FLPs can reduce enamines into amines.56 (e) B(C6F5)3 based FLPs can reduce oximes to hydroxylamines and then amines.58 (f) B(C6F5)3 based FLPs can reduce silyl enol ethers into silyl ethers.57 | ||
Hydroamination reactions are relevant for many synthetic chemists, as they can be employed using amines and alkenes/alkynes intermolecularly or intramolecularly, where the latter results in N-heterocycle formation. Stephan and coworkers demonstrated the first account of using B(C6F5)3 based FLPs for hydroamination reactions using aryl amines and terminal alkynes in an intermolecular fashion to produce an aryl enamine (Fig. 5).59 Later, the same authors expanded the scope of B(C6F5)3 based FLPs for hydroamination using aryl amines and terminal alkynes in both an inter- and intramolecular fashion, producing a plethora of compounds including a series of N-heterocycles.60 However, instead of forming the imine N-heterocycles, the intramolecular hydroamination reactions were performed under a H2 atmosphere, resulting in one-pot hydroamination/hydrogenation reactions further demonstrating versatility of B(C6F5)3 based FLPs.
 | ||
| Fig. 5 Hydroamination using B(C6F5)3 as an FLP.59 When the combination of B(C6F5)3 and an aryl amine is treated with alkynes it creates a zwitterionic FLP intermediate, and then a 1,3 proton transfer induces the release of an aryl enamine product and regeneration of B(C6F5)3. | ||
Other than activating H2 for metal-free hydrogenation, capturing and activating alternate small molecules such as CO,61–63 CO2,64–69 N2O,70 NO,71 and SO2,72 alkenes,43 alkynes,73–75 and disulphides76 using FLPs is highly sought after. We refer the reader to a recent review involving FLPs capturing and converting CO2.69 In brief, Stephan and Erker reported the first use of FLP chemistry for the sequestration of CO2, where the FLP PtBu3 + B(C6F5)3 and FLP complex (Me3C6H2)2PCH2CH2B(C6F5)2, can capture and liberate CO2 reversibly (Fig. 6a).64 Later, Kumacheva and coworkers determined the thermodynamics of the capture and liberation of CO2via FLPs by a novel micro fluidics approach that provided a means for future thermodynamic characterization of FLP CO2 sequestrations and other reactions of the like.77 The first conversion of CO2 to methanol via FLPs was reported by O’Hare and coworkers, however, thermolysis to produce free methanol resulted in the decomposition of B(C6F5)3.65 In 2010, Piers and coworkers reported CO2 conversion using a 2,2,6,6-tetramethylpiperidine/B(C6F5)3 FLP with triethylsilane for a deoxygenative reduction of CO2 to CH4.66 Other than CO2 activation, using the same PtBu3 & B(C6F5)3 FLP for CO (Fig. 6b),61 N2O (Fig. 6c),70 and SO2 (Fig. 6d)72 activation has been successful, where all reactions result in complexation of the small molecules with the PtBu3 & B(C6F5)3 pair by bridging them and producing solids.
 | ||
| Fig. 6 Small molecule activation using B(C6F5)3 FLPs. (a) CO2 activation & capture using PtBu3 and B(C6F5)3 at 25 °C results in a PtBu3CO2B(C6F5)3 complex, where CO2 can be liberated at 80 °C under vacuum.64 (b) CO activation & capture using PtBu3 and 2[B(C6F5)3] at room temperature in the presence of H2 to form a formyl borate derivative, which can be reversed to release CO upon placing under vacuum.61 (c) PtBu3, B(C6F5)3, & N2O form a bridged B(C6F5)3–N2O–PtBu3 species in a trans configuration upon combining the reagents and heating at 25 °C.70 (d) PtBu3, B(C6F5)3, & SO2 react at room temperature to form a bridged zwitterionic B−(C6F5)3–SO2–P+tBu3 adduct.72 | ||
2.4. Emerging applications of B(C6F5)3 in the presence of H2O for molecular transformations
Upon exposure to water, B(C6F5)3 forms oxygen bridged borate adducts and complexes (Fig. 7a) and can decompose into boronic acids and boroxines, especially upon heating.78 Therefore, chemists working with B(C6F5)3 typically ensure a moisture free environment for storage and reaction conditions. However, there are emerging applications of adducts involving B(C6F5)3 and water (B(C6F5)3·H2O; Fig. 7a) which transform B(C6F5)3 from a Lewis acid to a potent Brønsted acid. This results in altered reactivity and applications, including as a p-type dopant for organic electronics (vide infra).79 Note, considering the study done by Beljonne and coworkers, upon deprotonation of B(C6F5)3·H2O, it is likely that the resulting [B(C6F5)3·OH]− anion complexes with free B(C6F5)3 and B(C6F5)3·H2O to form the energetically favoured complexes [B(C6F5)3·OH·B(C6F5)3]− and [B(C6F5)3·OH·H2OB(C6F5)3]− (Fig. 7a), respectively.79 | ||
| Fig. 7 B(C6F5)3 in the presence of H2O for molecular transformations; (a) adducts of B(C6F5)3 with H2O: B(C6F5)3·H2O, [B(C6F5)3·OH·B(C6F5)3]−, and [B(C6F5)3·OH·H2O·B(C6F5)3]−,79 (b) C–F bond substitution of alkanes is catalyzed by B(C6F5)3·H2O (1 mol%) at room temperature to afford an array of C-, N-, O-, and S-substituted products,80 (c) B(C6F5)3 in H2O affords O–H bond insertion for α-diazoesters to form α-hydroxyesters at moderate temperatures (40 °C),81 (d) dehydration reactions involving allylation of electron-rich arenes and allyl alcohols using B(C6F5)3 in water,82 (e) syntheses of oligosiloxanes using H2O·B(C6F5)3 as a catalyst for the conversion of hydrosilanes and tethered hydrosilanes to make oligomeric siloxanes,83 (f) regio- and chemo-selective para-alkylation of anilines using H2O·B(C6F5)3.84 | ||
Efforts to break C–F bonds under benign conditions have persisted, one example being defluorination functionalization of tertiary aliphatic fluorides mediated by a H2O·B(C6F5)3 adduct. In 2017, Moran and coworkers reported that 1 mol% of the adduct was capable of catalyzing C–F bond substitution of aliphatic fluorides at room temperature using various heteroatomic nucleophiles to afford a range of S-, O-, N-, and C-substituted products in good yields (Fig. 7b).80 The authors previously reported a Friedel–Crafts autocatalytic mechanism, where initiation occurs by abstraction of the fluoride ion by a B(C6F5)3·H2O adduct (via F–H coordination) resulting in a aliphatic carbocation free to react with a plethora of nucleophiles, and is the proposed mechanism for this work.85
Molecular transformations using B(C6F5)3 and water as the solvent is emerging, in 2018 B(C6F5)3 was found to catalyze O–H bond insertion in α-diazoesters to form α-hydroxyesters using B(C6F5)3 in water (Fig. 7c).81 In 2020, Wang and coworkers reported the allylation of electron-rich arenes and allyl alcohols using B(C6F5)3 in water, and provided a series of indoles coupled to 1,3-diphenylallyl alcohol.82 These reactions are proposed to proceed by an FLP of B(C6F5)3 and 2,6-lutidine, which then reacts to abstract the hydroxyl group of 1,3-diphenylallyl alcohol to provide a reactive allyl cation and [B(C6F5)3:OH]− (Fig. 7d). In 2021, Wang and coworkers continued this work by allylation of 1,3-diketones or β-ketone esters with allyl alcohols in water.86 The authors propose this proceeds by adduct formation and thus activation of the ketone via adduct formation between the carbonyl and B(C6F5)3, which renders the –CH2 group bridging the diketone acidic and upon deprotonation forms a reactive carbocation.
While reactions involving B(C6F5)3 and water have been demonstrated, excess water can still be problematic as the Brønsted acidity is expected to decrease.78,79 This can be mediated by adding controlled amounts of water to a reaction mixture to form B(C6F5)3·H2O, or by adding the premade adduct directly. This has been demonstrated for the synthesis of oligosiloxanes using B(C6F5)3·H2O as a catalyst by Leitao and coworkers, who reported the conversion of hydrosilanes and tethered hydrosilanes to make oligomeric siloxanes using controlled mol percentages of B(C6F5)3·H2O (Fig. 7e).83 Pulis and coworkers reported on the para-alkylation of anilines, where the H2O·B(C6F5)3 adduct was added to the reaction directly for highly selective para-C-alkylation over the N-alkylation and ortho-C-alkylation pathways (Fig. 7f).84 The authors suggest this mechanism proceeds by protonation of the alkyl functional group by B(C6F5)3·H2O to form a reactive carbocation.
3. Applications of B(C6F5)3 in electronic devices
3.1. Overview
Society relies heavily on electronic devices, with an indication that dependence on such technology is forever increasing. Some examples of on-going research in this field are batteries/energy storage, energy conversion, lighting displays, and sensors. B(C6F5)3 has seen research and application in this field; however, the function of B(C6F5)3 varies significantly, further highlighting the vast scope and utilization of the compound. While the incorporation of boron into organic electronic materials is classic,87–90 the use of B(C6F5)3 is emerging and has proved to be fruitful. This section serves to highlight various roles of B(C6F5)3 in electronic devices, more specifically for its role in batteries, organic electronics, and emerging applications.3.2. Incorporating B(C6F5)3 in batteries
3.3. B(C6F5)3 as a dopant in organic electronics
Early applications using B(C6F5)3 for organic semi-conductor modification was carried out by Bazan and coworkers, where modulated optical properties and electronic energy levels was achieved without synthetic modification (Fig. 9b).128–130 Oligomers consisting of a dithienosilole (R = C12H25; DTSC12) core capped with two benzothiadiazole (BT) units were combined with stoichiometric amounts of B(C6F5)3, resulting in Lewis acid–base complexes involving the boron on B(C6F5)3 and the nitrogen of the BT units (Fig. 9a).128 This pulled electron density away from the π-system of the BT unit towards the Lewis acid, increasing donor–acceptor charge transfer character of the system, resulting in an overall narrowing of the energy gap and red-shifting of the optical absorption (Fig. 9b). The work was expanded on by complexing B(C6F5)3 with various Lewis basic polymers,129 with eventual applications in organic light emitting diodes (OLEDs).130
 | ||
| Fig. 9 Bazan and coworkers demonstrate how B(C6F5)3 can tune the electronic gap of benzothiadiazole (BT) & DTSC12 based oligomers.128 (a) When added in stoichiometric amounts, B(C6F5)3 forms a complex with the nitrogen on the BT unit of the BT/DTSC12 based oligomers. (b) Modified from Bazan and coworkers,128 increasing the stoichiometric equivalence of B(C6F5)3 (red line is neat B(C6F5)3) in relation to the BT/DTSC12 oligomers (black line is neat BT/DTSC12), a bathochromic shift is observed for the λmax of the BT/DTSC12 spectra. | ||
Nguyen and coworkers first reported on the p-doping effect of B(C6F5)3 on a pyridine-based copolymer for hole-only diode devices in 2014, and observed an enhancement of hole-mobility by two orders of magnitude compared to their pristine counterparts.125 Following this, many accounts of doping organics with B(C6F5)3 for use in electronics started to emerge, particularly for solution-processed organic electronic devices. Taking advantage of the unique synergistic effect of B(C6F5)3 doping on modifying optoelectronic properties and solid-state morphologies of organic materials, the application area has broadened to the bulk heterojunctions (BHJs) in organic photovoltaics (OPVs),131–135 hole transporting layers (HTLs) in perovskite solar cells (PSCs),136,137 organic thermoelectrics (OTEs),138–142 organic thin-film transistor (OTFT)-based sensors,143–147 and organic photodetectors (OPDs).143,145 This section includes a mini review of B(C6F5)3 as a p-type dopant for the active organic components in organic electronics, proposed mechanisms, emerging applications, and new dopant strategies. For more detailed reviews regarding the effect of doping active organic components, please refer to the reviews by Anthopoulos and coworkers,148 Koch and coworkers,123 and Baumgartner and coworkers.149
 | ||
| Fig. 10 Possible doping mechanisms in organic electronics by B(C6F5)3. (a) Integer charge transfer (ICT) works to abstract an electron from the organic compound, allowing for increased hole transport throughout the material. (b) Charge transfer complex formation (CTC) results in orbital hybridization and an electron transfer into a charge transfer hybrid orbital (c) Brønsted acid doping in organic materials can occur when in the presence of B(C6F5)3 and H2O, and occurs in three main steps: (i) protonation of a π-conjugated system in the presence of the proposed B(C6F5)3·OH2·B(C6F5)3 complex (ii) electron transfer from a neighbouring π-conjugated system, and (iii) formation of a neutral protonated radical species, [PCPDTBT-H]˙, and “p-doped” delocalized cationic radical species, [PCPDTBT]˙+, balanced with the counter ion, [B(C6F5)3·OH]−.151 | ||
Later, Beljonne and coworkers suggested that more energetically favorable bridged anion complexes, such as [B(C6F5)3·OH·B(C6F5)3]− and [B(C6F5)3·OH·OH2·B(C6F5)3]−, were more feasible to have formed than the monomeric species [B(C6F5)3·OH]−, as per DFT calculations.79 In addition, Brønsted acid doping processes are entropically driven by the loss of H2 gas to counter the highly endergonic process, where H2 gas was successfully detected by Beverina and coworkers in 2023.152 This mechanism accounted for the single signal seen in the EPR of these doped systems, as the result is a single spin-carrying species in the form of a radical cation. A follow up study on P3HT doped by B(C6F5)3 was done to include EPR experiments, where a single EPR signature was indeed observed.153 While Brønsted acid doping with B(C6F5)3 can occur in the presence of water, if the organic material being doped contains Lewis basic moieties, it should be noted that Brønsted acid and CTC doping compete, however, Brønsted acid doping has been deemed as the more effective doping approach.140 Therefore, in designing organic systems doped with B(C6F5)3, one must define a target doping mechanism and consider the presence of water and/or Lewis basic moieties. Note, many early studies reviewed within do not elucidate doping mechanisms at play and instead speculate with minimal supporting data.
 | ||
| Fig. 11 Application of B(C6F5)3 in OPVs. (a) Structure of an OPV used to harvest and convert solar radiation into current, B(C6F5)3 can be incorporated into the BHJ for increased charge mobilities and PCEs. (b) In the work done by Chen & coworkers,155 adduct formation between the boron of B(C6F5)3 with the nitrogen on the pyrazine-based polymer, PBP-Cl, is observed, which was applied to OPVs for enhanced performance. | ||
Both the BHJ and organic-based electron/hole transporting layers can undergo doping to enhance performance. Instances of using B(C6F5)3 to augment OPV device performance have been reported, specifically when utilized in the BHJ. One example is the work of Ma and coworkers, where the authors utilized Lewis basic donor polymers (PCE10) with fullerene (PC71BM) acceptors in the BHJ of OPVs,131 and then added B(C6F5)3 to induce doping in the donor PCE10 polymers. Not only did B(C6F5)3 dope the PCE10 polymers, albeit mechanisms unknown, highly ordered nanostructures were observed with increased charge transport and thereby device performance for an increase in power conversion efficiency (PCE) from 8.9% to 9.6%. Chen and coworkers designed and synthesized a pyrazine-based donor polymer (PBP-Cl) to induce intermolecular B–N coordination for CTC doping with B(C6F5)3 in BHJ of OPVs (Fig. 11b), where Y6 was used a non-fullerene acceptor.155 By adding a trace amount of B(C6F5)3 at 0.05 wt% relative to PBP-Cl in the BHJ, increased hole mobilities were observed with PCEs improving from 13.7% to 15.2%. Despite this, morphological alteration in the BHJ due to the presence of dopants can be detrimental, especially when the presence of dopants breaks up intermolecular interactions of the donor and/or acceptor species that are designed to induce favorable orientations that result in efficient charge transport in these types of devices.155–157 Realizing this, in 2019 Ma and coworkers applied a different approach by employing a planar heterojunction (PHJ), where the donor and acceptor materials are coated individually and subsequentially. In this work, the best device performance was observed when B(C6F5)3 was employed at the donor–acceptor interface, thereby residing between the donor and acceptor layers, and resulted an increase to PCE from 1.20% to 1.39%.132 The authors also doped a PCE10/PC71BM BHJ by vapor annealing which resulted in an overall improved photovoltaic performance, marking an increase to PCE from 8.6% to 9.4%.133 Various studies that utilize B(C6F5)3 as a dopant are continuously being published, with many of them putting an efforts into modifying the formation of favorable BHJ morphology.134,135 Notable is the doping of the electron transporting layers, where employing the H2O·B(C6F5)3 complex as a dopant for the known cathode interlayer material, (N,N-dimethyl-ammonium N-oxide)propyl perylene diimide (PDINO) resulted in high performance OPVs with a PCE of 17.7%. The authors utilized DFT to show that the complex coordinates to the N-oxide terminal unit of PDINO by the boron core, thus displacing the water moiety.158 Furthermore, Kelvin probe force microscopy and ultra-violet photoelectron spectroscopy of PDINO·B(C6F5)3 thin films determined the work function of the films were lowered upon increasing levels of the H2O·B(C6F5)3 complex, enabling a smaller energy mismatch with the Y6 BHJ acceptor material.
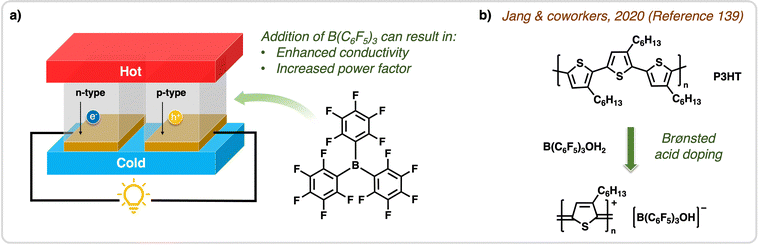 | ||
| Fig. 12 Application of B(C6F5)3 in OTEs. (a) Structure of an organic thermoelectric device used to convert heat into current. Using B(C6F5)3 to p-dope OTEs results in enhanced conductivity and increases the power factor of the devices. (b) In the work done by Jang & coworkers,139 Brønsted acid doping was observed when mixing B(C6F5)3 (in the presence of water) with P3HT, which was used for OTEs and resulted in enhanced conductivity. | ||
Jang and coworkers utilized B(C6F5)3 as a Lewis acid dopant in inert atmosphere and as a Brønsted acid dopant in air to p-dope poly(3-hexylthiophene) (P3HT) through a one-step solution mixing method.139 The Brønsted acid doping induced a conformational change in P3HT that resulted in a quinoidal structure, promoting backbone planarity (Fig. 12b). This structural change resulted in improved intra/inter-chain charge transport, reflected by the enhanced σ of 33.0 S cm−1 compared to Lewis acid-doped P3HT films at 0.020 S cm−1. This outcome showcases the doping efficiency and air-stability of B(C6F5)3 when employed as a Brønsted acid dopant. In the case of strong Lewis basic polymers, the competition between Brønsted acid and CTC doping in ambient atmosphere can limit doping efficiency. Consequently, in 2022 Jang and coworkers thermally annealed doped polymer films (PCDTPT) at 120 °C, transitioning from Lewis acid/base adducts (CTC) to Brønsted acid doping. This resulted in an increase in σ by three orders of magnitude reaching power factor of 8.48 μW m−1 K−2.140 They suggested that the Lewis acid–base interaction of B(C6F5)3 with PCDTPT can be thermally disrupted to form Brønsted acidic B(C6F5)3·H2O complexes, and when employed with the weak Lewis basic polymer (PCDTBT), CTC doping was effectively suppressed and Brønsted acid doping increased to achieve a high power factor of 49.6 μW m−1 K−2.
Despite the impressive increase in performance resulting from B(C6F5)3 by doping via CTC or Brønsted acid doping, B(C6F5)3 (and B(C6F5)3 water complexes) are bulky and can disrupt the solid-state morphology of organic thin films. Cho and coworkers addressed this challenge posed by the steric bulk of B(C6F5)3 dopants,142 where they employed sequential doping by dissolving B(C6F5)3 in the non-polar aliphatic solvent, hexanes, and then dipped their prepared organic semiconducting films into the solution. This method allowed B(C6F5)3 to diffuse into a semi-crystalline polymer film made up of poly[2,5-bis(3-tetradecylthiophen-2-yl)thieno[3,2-b]thiophene] (PBTTT) without disrupting the crystallinity of the film. The authors propose that hexanes can intercalate with the side chains of the PBTTT polymer but not the backbone, therefore, this method of subsequential doping resulted in deposition of the B(C6F5)3 dopant in the lamellar spacing of PBTTT. Compared to solution-mixed doping, this method effectively suppressed dopant-induced disorder, resulting in a narrower density of states and reduced charge traps. The outcome was an impressive electrical conductivity (σ) of 230 S cm−1 and power factor of 140 μW m−1 K−2, ranking among the highest values reported for B(C6F5)3-doped organic polymer films. OTE devices doping using B(C6F5)3 has therefore seen success,157 including noncontact mode (IR irradiation) OTEs,165 and self-healable and stretchable OTEs.166
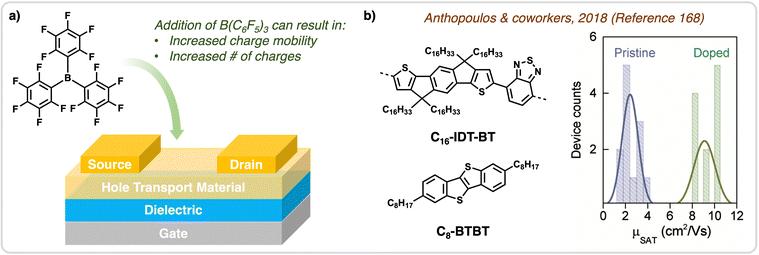 | ||
| Fig. 13 Application of B(C6F5)3 in OTFTs. (a) Structure of a type of OTFTs, specifically a bottom-gate top-contact OTFT. Doping hole transporting materials with B(C6F5)3 in OTFTs results in an improvement in charge mobility and increases the number of charges generated. (b) Modified from Anthopoulos & co-workers,168 when the mixture of hole transporting materials, C16-IDT-BT & C8-BTBT, were doped with B(C6F5)3 and applied in OTFTs hole mobilities increased to 11 cm2 V−1 s−1. | ||
Early work in this field was done by Heeney and coworkers who were able to achieve 11-fold enhancement of hole-mobility in pyrazine-based copolymer OTFTs.127 These devices were highly sensitive to dopant concentrations due to the bulky nature of B(C6F5)3 complex, which disrupts solid-state morphology thereby affecting charge transport properties.125,127 However, with careful optimization of the stoichiometric amount of B(C6F5)3 added, the organic materials can simultaneously undergo p-doping and induced long-range crystallization resulting in enhanced hole-mobility, which was observed by Anthopoulos and co-workers in 2018.168 This work explored several systems of organic molecular and polymeric materials for applications in OTFTs, more specifically they compared molecular and polymeric materials to blends of molecular polymeric materials in the presence of B(C6F5)3. The most notable system was that of the polymer C16-IDT-BT blended with the small molecule C8-BTBT, where upon doping with B(C6F5)3 the resulting OTFT devices reached a maximum of 11 cm2 V−1 s−1 (Fig. 13b).
OTFTs are commonly studied for their application as sensors, particularly gas sensors. In 2012, Katz and coworkers fabricated OTFT-based NH3 gas sensor using Lewis basic, copper phthalocyanine (CuPc) and B(C6F5)3 as an active layer.144 Upon the exposure to NH3 gas, which is a stronger Lewis base than CuPc, B(C6F5)3 effectively formed a Lewis acid–base complex with NH3 resulting in a pronounced changes to the current, achieving a low limit of detection (LOD) of 0.35 ppm. However, it should be noted as per the correction to this work that there is a possibility of B(C6F5)3 forming an adduct with trace water, which can act as a Brønsted acid p-dopant and thereby also affects the observed current in these devices.169 Another study done in 2020 by Kim and coworkers fabricating solution-processed OTFT-based NH3 gas sensor using poly(3-hexylthiophene) (P3HT) doped with B(C6F5)3 as an active layer showed enhancement of current response.147 This OTFT-based NH3 gas sensor was more selective towards NH3 compared to acetone, methanol, and dichloromethane, where they attributed to the strong Lewis acid (B(C6F5)3) and Lewis base (NH3) interaction for CTC type doping.
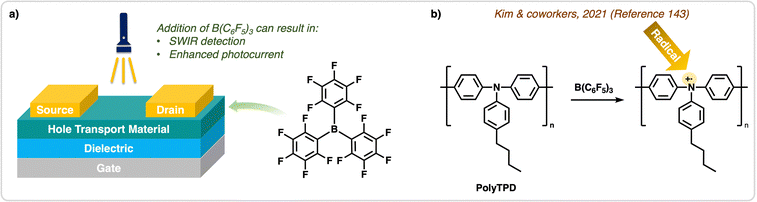 | ||
| Fig. 14 Application of B(C6F5)3 in OPTRs. (a) Structure of organic phototransistor devices used to detect various wavelengths of light. Addition of B(C6F5)3 in these types of devices can result in SWIR detection and enhanced photocurrent. (b) In the work done by Kim & coworkers,143 doping of PolyTPD with B(C6F5)3 to generate radicals was done for applications in OPTRs. | ||
Another example of using B(C6F5)3 as a dopant in OPDs was reported by Someya and Anthopoulos in 2021, where an organic photodiode with a bulk heterojunction consisting of a diketopyrrolopyrrole & thiophene based donor polymer (PMDPP3T) with a fullerene acceptor (PC61BM), and was doped by B(C6F5)3 alongside n-type organic dopants.145 The authors controlled doping concentration (0.02 wt%) to achieve enhanced photocurrent and NIR detectivity, without increasing dark current. This is notable as doping often increases dark current, which can result in rapid device degradation under ambient conditions.
In regard to emerging electrochemical device applications, Abe and coworkers reported a new method for enhancing electrochemiluminescence (ECL) by Lewis acid–base pairing complexation of B(C6F5)3 with organic donor–acceptor (D–A) diads. This complexation served to stabilize electrogenerated radicals, preventing the formation of unstable radicals which can lead to unwanted chemical reactions.173 In the study, pyridal (py) functionalized benzothienobenzothiophene (BTBT) D–A diads were used as the active component, forming dative B–N bonds with B(C6F5)3. They report that van der Waals interaction between H⋯F of py and –C6F5 groups restricted the rotational motion, maintaining a planar configuration during the excited intramolecular charge transfer state. This restriction effectively inhibited non-radiative decay. Additionally, the strong electron-withdrawing coordination of B(C6F5)3 facilitated efficient charge separation. Moreover, single crystals of py-BTBT diads revealed highly ordered 1D columnar π-stacks in the solid state, contributing to efficient singlet exciton delocalization. Therefore, the py-BTBT diads demonstrated improved radical stability with a 156-fold increase in ECL intensity. B(C6F5)3 was reported to not only dope the organic material and induce favorable morphology changes in the solid state, but also acted as an oxygen trap and thus increased the stability of the organic electrochemical transistor (OECT) devices over several cycles.174
Another promising usage of B(C6F5)3 was to enhance the photophysical properties of organic light-emitting films, which was done by Huang and coworkers in 2023.175 They employed supramolecular diarylfluorene compounds functionalized with pyridine as a host material and B(C6F5)3 as a guest material to explore B–N coordination and the effect on emission of the host material. By casting this material into films, they observed Förster resonance energy transfer (FRET) from the host material to the coordinated B(C6F5)3 which resulted in longer fluorescence lifetime. In addition, according to DFT, the HOMO and LUMO of the B(C6F5)3-coordinated molecule is highly delocalized, resulting in an intramolecular charge transfer state. Since the intramolecular charge transfer state is highly sensitive to polarity, changing the ratio of host to B(C6F5)3 has led to wide range of fluorescence emission that are highly tunable even in the film. In addition, the blending of coordinated molecule has also led to enhanced PLQY (∼30% to almost 80–90%) and conductivity (two orders of magnitude increase) of the films.
 | ||
| Fig. 15 Coordination of B(C6F5)3 with the nitrogen on the –CN functional groups of TCNQ and F4-TCNQ dramatically increases the EA and oxidation potential of the p-type dopants.177 | ||
Koch and co-workers investigated the utility of TCNQ·4B(C6F5)3 and derivatives as dopants for organic polymers: three p-type organic polymers were evaluated for doping with F4TCNQ·4B(C6F5)3, poly(3-hexylthiophene) (P3HT), methylated poly(para-phenylene) (MeLPPP), and poly(9,9-dioctylfluorene-alt-benzothiadiazole) (F8BT).177 For both P3HT and MeLPPP, thin film and solution spectroscopic studies showed significant doping with F4TCNQ·4B(C6F5)3, evident from the appearance of polaron-related absorption. At the same ratio of either B(C6F5)3 or TCNQ, neither polymer showed any evidence of doping. F8BT, a polymer with a very high ionization energy (circa 5.9 eV) was not doped by F4TCNQ·4B(C6F5)3, but F6TCNNQ·4B(C6F5)3 did show considerable doping at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 dopant ratio. Further, it was determined that at very low dopant ratios, 1
10 dopant ratio. Further, it was determined that at very low dopant ratios, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 or 1
100 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, F4TCNQ·4B(C6F5)3 dopes P3HT more effectively than F4TCNQ alone, improving conductivity by a factor of ∼1000 relative to F4TCNQ-doped P3HT. This work was eloquently expanded upon by Campoy-Quiles and co-workers, who investigated over 20 additional organic semiconductors.178 Nearly all these semiconductors demonstrated better conductivity when doped with F4TCNQ·4B(C6F5)3 compared to BCF or F4TCNQ alone, including notable examples Y6 and other NFAs and PM6, suggesting incorporation into the BHJ of OPV devices could be very promising. Lastly, owing to its large molecular volume, F4TCNQ·4B(C6F5)3 shows improved thermal de-doping performance and consequently improved stability at elevated temperatures.
50, F4TCNQ·4B(C6F5)3 dopes P3HT more effectively than F4TCNQ alone, improving conductivity by a factor of ∼1000 relative to F4TCNQ-doped P3HT. This work was eloquently expanded upon by Campoy-Quiles and co-workers, who investigated over 20 additional organic semiconductors.178 Nearly all these semiconductors demonstrated better conductivity when doped with F4TCNQ·4B(C6F5)3 compared to BCF or F4TCNQ alone, including notable examples Y6 and other NFAs and PM6, suggesting incorporation into the BHJ of OPV devices could be very promising. Lastly, owing to its large molecular volume, F4TCNQ·4B(C6F5)3 shows improved thermal de-doping performance and consequently improved stability at elevated temperatures.
4. Alternate Lewis acids for doping organics
4.1. Overview & perspective
The application of Lewis acids to enhance electronic device performance by doping requires specific structure–property relationships; upon reviewing molecular transformations and electronics to which B(C6F5)3 is applied several target properties stand out. First, the strength of the Lewis acid is critical for reactivity and performance, and in the case of doping increasing Lewis acidity is hypothesized to enhance p-doping mechanisms such as Brønsted acid doping, adduct formation/CTC doping, and complexation with other dopants. Second, the steric bulk and intermolecular interactions of B(C6F5)3 with organic materials prevents migration in the solid-state as a means of improving stability. Third, B(C6F5)3 is solution processable in a range of organic solvents, which is ideal for devices fabricated by coating/printing methods. Fourth, it is stable and has distinguishable properties that are easily analyzed with common instrumentation, such as NMR spectroscopy, for in depth structural and mechanistic studies. Each aforementioned property plays a key role; for example, while increasing Lewis acidity may lead to better doping efficiencies if a Lewis acid is incompatible with target organic compounds due to a lack of intermolecular interactions it may migrate in processed films and reduce stability and efficiency over time. There are no clear trends for applying Lewis acids as dopants for organic compounds as a whole, as it depends on the materials, device architecture, and role of the Lewis acid. Therefore, this section presents alternates to B(C6F5)3, and when available, relevant properties to encourage a more effective and systematic approach to selecting an appropriate dopant for niche systems.For notable alternatives to B(C6F5)3 presented within, Lewis acidity based on the Gutmann–Beckett (GB) method, fluoride ion affinity (FIA), and hydride ion affinity (HIA) is provided when available. Note, variability of these values across studies is common, therefore, relative strength to that of B(C6F5)3 for each compound/study is provided when available. The Gutmann–Beckett method involves complexation of a Lewis acid with a phosphine oxide, typically triethylphosphine oxide, and subsequent evaluation of the chemical shifts in 31P-NMR spectra. Lewis acidity can be determined via relative correlations of Δδ of P where a higher Δδ indicates a stronger Lewis acid. FIA is a computational method that determines the binding energy or enthalpy change when a fluoride ion (F−) binds to a Lewis acid and is typically reported in kJ mol−1. The stronger the Lewis acid, the larger the value, and if a Lewis acid has a FIA greater than that of antimony pentafluoride (SbF5, 493 kJ mol−1) it is considered to be a Lewis super acid (LSA).179,180 Similar to FIA is HIA, which computes the binding energy/enthalpy change when a hydride ion (H−) binds to a Lewis acid and is reported in kJ mol−1. Electronic characteristics such as calculated LUMO energy levels, formal reductions, and electron affinity are provided when available. Lastly, sterics are considered by including calculated buried volumes (%Vbur) of Lewis acid anions, typically fluoride ion adducts, where the %Vbur is the volume of the ligands that surround a boron center. Note, sterics need to be considered in the context of FLPs when selecting a dopant (for CTC), where using the method of Radius and coworkers181 may define any Lewis acid/base interactions present when doping various organic compounds and compositions. Within are select tri-substituted aryl boranes, carboranyl boranes, and borates and the relative aforementioned properties to be applied for (1) identifying appropriate boron-based Lewis acid for organic electronic applications, (2) to better understand the effect of these types of Lewis acid dopants, and (3) to encourage new dopant and/or active material component designs.
4.2. Tri-substituted aryl boranes & borates
Triphenyl borane (B(C6H5)3; Fig. 16a), being the non-fluorinated analog of B(C6F5)3, exhibits 65%,182 78%,183 and 74%183 relative Lewis acidity (RLA) in relation to B(C6F5)3 using the GB method, FIA, and HIA, respectively, and the LUMO energy level of B(C6H5)3 is destabilized by 1.52 eV183 relative to B(C6F5)3 (Table 1). Despite this, B(C6H5)3 has seen many applications in molecular transformations,184 and is less sterically encumbered than B(C6F5)3 by 5.8% (%Vbur). Increasing the degree of phenyl fluorination, as seen for B(C6H3-2,6-F2)3, B(C6H2-2,4,6-F3)3, and B(C6H-2,3,5,6-F4)3 (Fig. 16a), results in RLAs (by GB method) of 82%,185 88%,186 and 98%,186 respectively, with LUMO energy levels destabilized relative to B(C6F5)3 by 0.91 eV, 0.74 eV, and 0.21 eV, respectively (Table 1). The %Vbur reported for B(C6H3-2,6-F2)3 and B(C6H2-2,4,6-F3)3 indicates sterics about the boron center are near identical to that of B(C6F5)3, an expected result when having fluorine substituted 2-positions on all phenyls in these compounds.187 One method to increase withdrawing behaviour on triaryl borane phenyl groups beyond perfluorination is to install trifluoromethyl groups (–CF3), as is seen with BArF, BTolF, and B(FMes)3 (Fig. 16a). Indeed, RLAs (by GB method) of BArF, BTolF, and B(FMes)3 are 106%, 108%, and 122%, respectively (Table 1). However, the electron affinity of BArF relative to B(C6F5)3 is destabilized by 0.24 eV,188 while the LUMO energy level of BTolF relative to B(C6F5)3 is stabilized by 0.59 eV.189 For %Vbur, BArF is less encumbered relative to B(C6F5)3 by 5.2%,181 and B(Fmes)3 is greatly encumbered relative to B(C6F5)3 with an increase to %Vbur of 26.9%.181 Substituting perfluorinated phenyls with perchlorinated phenyls in B(C6F5)3 is seen for B(C6Cl5)(C6F5)2, B(C6Cl5)2(C6F5), and B(C6Cl5)3 (Fig. 16a). Here, the C6Cl5 substituents are more electron withdrawing than C6F5 substituents due to the decrease in π-back-donation from Cl relative to that of F, and is apparent by the formal reductions of B(C6Cl5)(C6F5)2, B(C6Cl5)2(C6F5), and B(C6Cl5)3 being −1.87 V, −1.55 V, and −1.48 V, respectively (Table 1).190 However, Lewis acidity is both electronic and steric dependent, where using the GB method the RLAs of B(C6Cl5)(C6F5)2, B(C6Cl5)2(C6F5), and B(C6Cl5)3 are 96%, 93%, and 0%, respectively.190 While the GB method for B(C6Cl5)3 is 0 due to a lack of complexation with triethylphosphine oxide, a RLA using FIA resulted in 89% relative to that of B(C6F5)3 which indicates B(C6Cl5)3 will complex with small Lewis bases.191 The RLAs are supported when considering %Vbur, where B(C6Cl5)(C6F5)2, B(C6Cl5)2(C6F5), and B(C6Cl5)3 are encumbered by 4.3%, 7.9%, and 11.3% relative to B(C6F5)3, respectively.190| Aryl Borane | Lewis Acidity | RLA | Electronics | Sterics |
|---|---|---|---|---|
| GB Δδ = Gutmann–Beckett Method 31P Δδ (ppm), FIA = fluoride ion affinity (kJ mol−1), HIA = hydride ion affinity (kJ mol−1), LUMO = calculated LUMO energy (eV), FR = formal reduction potential experimentally found using cyclic voltammetry (V), BV = buried volume of Lewis acids adducts with a fluoride ion [LA–F]−.a BV is calculated using F-ion adduct cartesian coordinates obtained from associated references and the SambVca 2.1 web application,199 EA = calculated electron affinity (eV), RLA = relative Lewis acidity compared to B(C6F5)3 (%).b Relative B(C6F5)3 data, NR = not reported or relevant data not available. | ||||
| B(C6F5)3 | Due to variability across studies, relative B(C6F5)3 data for each compound/study is reported | |||
| B(C6H5)3 | GB Δδ: 19.6 (C6D6), b30.2 (C6D6)182 | GB Δδ: 65 | ||
| FIA: 354, b452183 | FIA: 78 | LUMO: −2.02183 | BV: 53.1181 | |
| HIA: 360, b484183 | HIA: 74 | LUMO: −3.52183 | BV: 58.9181 | |
| B(C6H3-2,6-F2)3 | GB Δδ: 21.2 (CD2Cl2), b25.8 (CD2Cl2)185 | GB Δδ: 82 | LUMO: −2.39187 | BV: 58.0187 |
| FIA: 368, b457187 | FIA: 81 | LUMO: −3.30187 | , BV: 59.4187 | |
| B(C6H2-2,4,6-F3)3 | GB Δδ: 30.5 (CDCl3), b34.8 (CDCl3)186 | GB Δδ: 88 | LUMO: −2.56187 | BV: 58.0187 |
| FIA: 390, b457187 | FIA: 85 | LUMO: −3.30187 | , BV: 59.4187 | |
| B(C6H-2,3,5,6-F4)3 | GB Δδ: 34.0 (CDCl3), b34.8 (CDCl3)186 | GB Δδ: 98 | LUMO: −2.59192 | NR |
| LUMO: −2.80192 | ||||
| BArF3 | GB Δδ: 28.2 (CD2Cl2), b26.6 (CD2Cl2)193 | GB Δδ: 106 | EA: −2.79188 | BV: 53.7181 |
| EA: −3.03188 5/22/2025 10:05:00 AM | BV: 58.9181 | |||
| BTolF | GB Δδ: 31.9 (C6D6), 29.0 (CD2Cl2), b29.5 (C6D6), b26.5 (CD2Cl2)194 | GB Δδ: 108 (C6D6) & 109 (CD2Cl2) | LUMO: −4.09189bLUMO: −3.50189 | NR |
| B(FMes)3 | GB Δδ = 44.21 (calculated) b36.34 (calculated)195 | GB Δδ: 122 | NR | BV: 85.8181 |
| BV: 58.9181 | ||||
| B(C6Cl5)(C6F5)2 | GB Δδ: 32.5 (CD2Cl2), b33.7 (CD2Cl2)190 | GB Δδ: 96 | FR: −1.87190 | BV: 63.2181 |
| FR: −1.97190 | BV: 58.9181 | |||
| B(C6Cl5)2(C6F5) | GB Δδ: 31.2 (CD2Cl2), b33.7 (CD2Cl2)190 | GB Δδ: 93 | FR: −1.55190 | BV: 66.8181 |
| FR: −1.97190 | BV: 58.9181 | |||
| B(C6Cl5)3 | GB Δδ: 0 (CD2Cl2) b33.7 (CD2Cl2)191 | GB Δδ: 0 | FR: −1.48190 | BV: 70.2181 |
| FIA: 366, b413191 | FIA: 89 | FR: −1.97190 | BV: 58.9181 | |
| B(C12H8)(C6H5) | GB Δδ: 33.1 (C6D6), bNR196 | |||
| FIA: 366, b452183 | FIA: 81 | LUMO: −2.52183 | BV: 47.3181 | |
| HIA: 382, b484183 | HIA: 79 | LUMO: −3.52183 | BV: 58.9181 | |
| B(C12F8)(C6F5) | FIA: 457, b452183 | FIA: 101 | LUMO: −3.78183 | BV: 52.7183 |
| HIA: 491, b484183 | HIA: 101 | LUMO: −3.52183 | , BV: 58.1183 | |
| B2(C6F5)2(C6F4)2 | FIA: 477, b452197 | FIA: 105 | LUMO: −4.68197 | NR |
| HIA: 514, b484197 | HIA: 106 | LUMO: −3.93197 | ||
| B(C12F9)3 | FIA: 431, b452197 | FIA: 95 | LUMO: −3.95197 | BV: 82.9181 |
| HIA: 452, b484197 | HIA: 93 | LUMO: −3.93197 | BV: 58.9181 | |
| B(C10F7)3 | FIA: 483, b452197 | FIA: 106 | LUMO: −3.91197 | BV: 58.8181 |
| HIA: 519, b484197 | HIA: 107 | LUMO: −3.93197 | BV: 58.9181 | |
| B(OC6H5)3 | GB Δδ: 23.0 (C6D6)b30.2 (C6D6)182 | GB Δδ: 76 | ||
| FIA: 350, b454198 | FIA: 77 | LUMO: −0.39198 | BV: 49.2198 | |
| HIA: 323, b497198 | HIA: 65 | LUMO: −3.43198 | , BV: 59.7198 | |
| B(OC6F5)3 | GB Δδ = 34.5 (C6D6) b30.2 (C6D6)182 | GB Δδ: 114 | ||
| FIA: 431, b454198 | FIA: 95 | LUMO: −1.27198 | BV: 54.9181 | |
| HIA: 411, b497198 | HIA: 83 | LUMO: −3.43198 | BV: 58.9181 | |
| B(OoFC6H4)3 | GB Δδ = 23.8 (CDCl3) bNR198 | |||
| FIA: 351, b454198 | FIA: 77 | LUMO: −0.75198 | BV: 50.7198 | |
| HIA: 339, b497198 | HIA: 68 | LUMO: −3.43198 | , BV: 59.7198 | |
| B(OmFC6H4)3 | GB Δδ = 22.0 (CDCl3) bNR198 | |||
| FIA: 368, b454198 | FIA: 81 | LUMO: −0.76198 | BV: 43.3198 | |
| HIA: 340, b497198 | HIA: 68 | LUMO: −3.43198 | , BV: 59.7198 | |
| B(OpFC6H4)3 | GB Δδ = 14.1 (CDCl3) bNR198 | |||
| FIA: 359, b454198 | FIA: 79 | LUMO: −0.86198 | BV: 43.4 | |
| HIA: 329, b497198 | HIA: 66 | LUMO: −3.43198,199 | , BV: 59.7198 | |
Altering aryl substituents is another method to influence the Lewis acidity, electronics, and sterics about the boron in tri-substituted boranes. Borafluorene Lewis acids, such as B(C12H8)(C6H5) and B(C12F8)(C6F5) (Fig. 16b), contain antiaromatic five membered borole rings that influence the electronics, sterics, and thereby Lewis acidity relative to B(C6H5)3 and B(C6F5)3, respectively. Comparing B(C12H8)(C6H5) to B(C6H5)3 the RLA (by FIA), LUMO, and %Vbur is 103%,183 0.50 eV stabilized,183 and decreased by 5.8%,181 respectively (Table 1). While B(C6F5)3 is a stronger Lewis acid than B(C12H8)(C6H5), it is apparent that borafluorene substitution can slightly increase Lewis acidity while decreasing sterics. This is supported by comparing B(C12F8)(C6F5) to B(C6F5)3 the RLA (by FIA), LUMO, and %Vbur which is 101%, 0.26 eV stabilized, and decreased by 5.4%, respectively (Table 1).183 One example of a bridged borane is provided, B2(C6F5)2(C6F4)2 (Fig. 16b), which bears a six-membered central ring that bridges two C6F4 groups via two C6F5 substituted boranes. The RLA (by FIA) is 105% and the LUMO enegry level is 0.75 eV stabilized (Table 1).197 Expanding π-conjugation is another approach to afford alternate tri-substituted boranes, two examples being B(C12F9)3 and B(C10F7)3 (Fig. 16b). When comparing B(C12F9)3 to B(C6F5)3, the RLA (by FIA) is 95%,197 the LUMO is stabilized by 0.02 eV,197 and %Vbur is increased by 24%,181 indicating a substantial increase in sterics while maintaining Lewis acidity (Table 1). However, when comparing B(C10F7)3 with B(C6F5)3, the RLA (by FIA) is 106%,197 the LUMO is destabilized by 0.02 eV,197 and %Vbur is increased by 0.01%, indicating sterics are maintained while slightly increasing Lewis acidity (Table 1).181
Worth noting are select triaryl borates, which differ from boranes by an oxygen atom that links the central boron and carbon atoms of aryl substituents. B(OC6H5)3 (Fig. 16c) compared to B(C6F5)3 reveals a RLA (by FIA) of 77%, a LUMO energy level that is destabilized by 3.04 eV, and %Vbur that indicates 10.5% less encumbrance (Table 1).198 Further, B(OC6F5)3 (Fig. 16c) compared to B(C6F5)3 reveals a RLA (by FIA) of 95%,198 a LUMO energy level that is destabilized by 2.16 eV,198 and is 5.0% less encumbered (Table 1).181 While this suggests that both sterics and Lewis acidity is decreased when comparing borates to boranes, using the GB method to measure the Lewis acidity of B(OC6F5)3 relative to that of B(C6F5)3 affords a RLA of 114%, suggesting the borate counterpart is significantly more Lewis acidic. Furthermore, the RLA (by GB method) for B(C6H5)3 to B(C6F5)3 is 65%, which is lower than the RLA of B(OC6H5)3 to B(C6F5)3 at 77% and suggests that the presence of the bridging oxygen does increase Lewis acidity. Notable for triaryl borates is the variability in properties when considering ortho-, meta-, and para-substitution of fluorine on B(OC6H5)3 affording B(OoFC6H4)3, B(OmFC6H4)3, and B(OpFC6H4)3, respectively (Fig. 16c). In terms of RLA (to B(C6F5)3 by FIA), B(OoFC6H4)3, B(OmFC6H4)3, and B(OpFC6H4)3 afford values of 77%, 81%, and 79%, respectively (Table 1).198 In terms of LUMO energy levels relative to B(C6F5)3, B(OoFC6H4)3, B(OmFC6H4)3, and B(OpFC6H4)3 afford destabilizations of 2.68 eV, 2.67 eV, and 2.57 eV, respectively.198 Lastly, the %Vbur compared to that of B(C6F5)3 for B(OoFC6H4)3, B(OmFC6H4)3, and B(OpFC6H4)3 exhibits less encumbrance by 9.0%, 16.4%, and 16.3%, respectively.198 This suggests that meta-substitution results in the highest Lewis acidity while being the least sterically encumbered.
4.3. Carborane substituted boranes
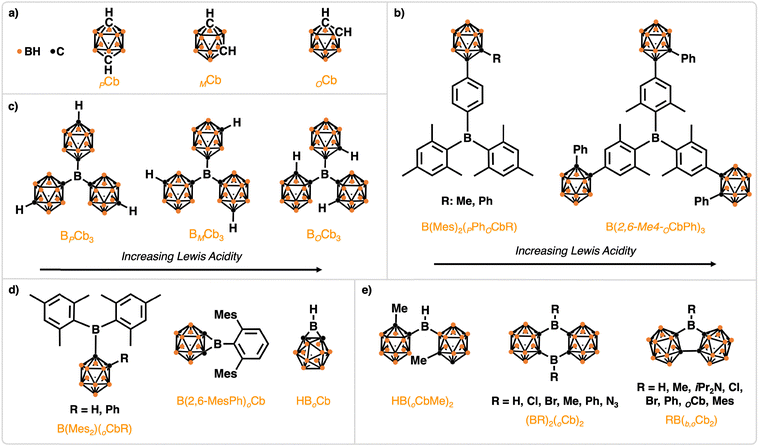
Carborane substituents on boranes are an emerging strategy for producing Lewis superacids (LSAs) with superior stability to their aryl counterparts. Carboranes are non-classically bonded polyhedral clusters of boron, carbon, and hydrogen atoms (Fig. 17a). Icosahedral closo-carboranes with the formula C2B10H12 are the most common and can exist in three isomers: 1,2-, 1,7-, and 1,12-, referred to as ortho (OCb), meta (MCb), and para (PCb), respectively (Fig. 17a). All three isomers are very strongly electron withdrawing, and unlike aryl substituents cannot act as π-donors, making them promising candidates for the synthesis of highly Lewis acidic species. Computational studies in the Martin group found that carboranes are superior to aryl groups in general at increasing the Lewis acidity of boranes; for primary, secondary and tertiary boranes it is demonstrated that Lewis acidity follows the trend BOCbXH3−X > BMCbXH3−X > BPCbXH3−X > B(C6F5)XH3−X > B(C6H5)XH3−X (X = 1, 2, 3; Fig. 17c).200 Early work with carboranyl substituents focused on perimeter substitution of triaryl boranes (B(Mes)2(PPhOCb) & B(2,6-Me4-OCb)3; Fig. 17b) which resulted in increased Lewis acidity.201,202 Towards applications, Park and coworkers applied these peripheral carboranyl-substituted triaryl boranes as fluoride ion sensors.203 Considering the increase in Lewis acidity, stability, and emerging work, we preset select directly-bound ortho-carboranyl boranes,189,204–220 many of which exceed the pentafluorophenyl analogues in terms of Lewis acidity and performance in chemical reactions.The use of carborane as a directly-bound functional group towards the synthesis of Lewis acidic boranes has been spearheaded by Martin and coworkers.189,200,218,221 Tris(ortho-carboranyl)borane (BOCb3, Fig. 17c), analogous to B(C6F5)3, exhibits substantial increases to Lewis acidity when probed by the GB method, FIA, and HIA for RLAs of 115%, 134%, and 129%, respectively (Table 2).200 Indeed, Lewis superacidity was confirmed computationally with an FIA of 605 kJ mol−1.180 In terms of electronics, relative to B(C6F5)3 the LUMO energy level is stabilized by 0.49 eV. In terms of sterics, relative to B(C6F5)3 BOCb3 sees an increase of 18.8% (%Vbur) and will preferentially forms FLPs over adducts.221 Notable is the high thermal stability, with no decomposition when heated to 250 °C.
| Borane | Lewis acidity | RLA | Electronics | Sterics |
|---|---|---|---|---|
| GB Δδ = Gutmann–Beckett Method 31P Δδ (ppm), FIA = fluoride ion affinity (kJ mol−1), HIA = hydride ion affinity (kJ mol−1), LUMO = calculated LUMO energy (eV), BV = buried volume of Lewis acids adducts with a fluoride ion [LA–F]−.a BV is calculated using F–ion adduct cartesian coordinates obtained from associated references and the SambVca 2.1 web application,199 RLA = relative Lewis acidity compared to B(C6F5)3 (%).b Relative B(C6F5)3 data.c Data is relative to SbF5 rather than B(C6F5), NR = not reported or relevant data not available. | ||||
| B(C6F5)3 | Due to variability across studies, relative B(C6F5)3 data for each compound/study is reported | |||
| BoCb3 | GB Δδ: 34.1 (C6D6), b29.7 (C6D6)200 FIA: 605, b452200 HIA: 622, b484200 | GB Δδ: 115 FIA: 134 HIA: 129 | LUMO: −3.99200bLUMO: −3.50200 | BV: 71.9200bBV: 53.1200 |
| B(Mes)2(OCbH) | FIA: 556204 | NR | LUMO: −2.13204 | BV: NR |
| B(Mes)2(OCbPh) | FIA: 533204 | NR | LUMO: −2.11204 | BV: NR |
| B(2,6-MesPh)OCb | FIA: 466, c474207 HIA: 535, b546207 | FIA: 98 HIA: 98 | LUMO: NR | BV: NR |
| HBOCb | FIA: 512, c474207 HIA: 580, b546207 | FIA: 108 HIA: 106 | LUMO: NR | BV: NR |
| HB(oCbMe)2 | GB Δδ: 35.8 (C6D6), b29.7 (C6D6)200 FIA: 527, b452200 HIA: 540, b484200 | GB Δδ: 121 FIA: 117 HIA: 112 | LUMO: −3.10200bLUMO: −3.50200 | BV: 64.7200bBV: 53.1200 |
| (BH)2oCb2 | FIA: 538, c474214 HIA: 615, b546214 | FIA: 114 HIA: 113 | LUMO: NR | BV: 55.6214 |
| (BCl)2oCb2 | GB Δδ: 36.8 (C6D6), b26.6 (C6D6)214 FIA: 535, c474214 HIA: 601, b546214 | GB Δδ: 138 cFIA: 113 HIA: 110 | LUMO: −1.50214 | BV: 60.6214 |
| (BBr)2oCb2 | GB Δδ: 40.8 (C6D6), b26.6 (C6D6)214 FIA: 537, c474214 HIA: 608, b546 214 | GB Δδ: 153 cFIA: 133 HIA: 111 | LUMO: −1.57214 | BV: 61.4214 |
| (BMe)2oCb2 | GB Δδ: 32.6 (C6D6), b26.6 (C6D6)214 FIA: 495, c474214 HIA: 562, b546214 | GB Δδ: 123 cFIA: 104 HIA: 103 | LUMO: −1.03214 | BV: 61.5214 |
| (BPh)2oCb2 | GB Δδ: 28.9 (C6D6), b26.6 (C6D6)214 FIA: 523, c474214 HIA: 592, b546214 | GB Δδ: 109 cFIA: 110 HIA: 108 | LUMO: −1.18214 | BV: 66.2214 |
| (BN3)2oCb2 | GB Δδ: 37.8 (C6D6), b26.6 (C6D6)216 FIA: 515, c474216 HIA: 578, b546216 | GB Δδ: 142 cFIA: 109 HIA: 106 | LUMO: −1.33216 | BV: 59.2216 |
| iPr2NB(b,OCb) | GB Δδ: 6.9 (C6D6)213 FIA: 414, c474217 HIA: 480, b546217 | FIA: 87 HIA:88 | LUMO: −2.05213 | BV: 67.7217 |
| ClB(b,OCb) | GB Δδ: 40.3 (C6D6), b29.6 (C6D6)217 FIA: 560, c474217 HIA: 625, b546217 | GB Δδ: 136 cFIA: 118 HIA: 114 | LUMO: NR | BV: 55.5217 |
| BrB(b,OCb) | GB Δδ: 41.5 (C6D6), b29.6 (C6D6)217 FIA: 565, c474217 HIA: 635, b546217 | GB Δδ: 140 cFIA: 119 HIA: 116 | LUMO: NR | BV: 56.3217 |
| PhB(b,OCb) | GB Δδ: 34.3 (C6D6), b29.6 (C6D6)217 FIA: 514, c474217 HIA: 585, b546217 | GB Δδ: 116 cFIA: 108 HIA: 107 | LUMO: −1.17217 | BV: 61.2217 |
| O CbB(b,OCb) | GB Δδ: 34.9 (C6D6), b29.6 (C6D6)212 FIA: 621, c475212 HIA: 632, b484212 | GB Δδ: 118 cFIA: 131 HIA: 131 | LUMO: −3.94212bLUMO: −3.52212 | BV: 67.7212 |
Two examples of mono-substituted carboranyl boranes include dimesityl(ortho-carboranyl)borane bearing either a H or Ph substituent on the carborane (B(Mes)2oCbH & B(Mes)2oCbPh; Fig. 17d). Computational investigation determined both were LSAs with FIAs of 556 kJ mol−1 for B(Mes)2oCbH and 533 kJ mol−1 for B(Mes)2oCbPh (Table 2).204 LUMO energy levels were also computed for values of −2.13 eV for B(Mes)2oCbH and −2.11 eV for B(Mes)2oCbPh. Notably, both derivatives are water stable when considering aqueous workups in the syntheses, and while long-term experiments revealed hydrolysis on exposure to air over multiple weeks the carboranyl substituent remained bound to central boron. Another example of a mono-substituted carboranyl borane is the borirane, HBOCb (Fig. 17d). While not a LSA, the FIA for HBOCb is reported as 512 kJ mol−1 (Table 2), which is higher than typical reports for B(C6F5)3 (∼450–480 kJ mol−1).207 Note, several works have explored the properties and reactivity of these types of boriranes, including N-heterocyclic carbene stabilization.205 For example, 5 and 7 membered ring-expansion products form when treated with aldehydes and ketones, respectively.206–208,222,223 Computational investigation found the carboranyl borirane has higher ring strain than borirane, where this combined with the lack of π-delocalisation from the carborane leads to increased Lewis acidity.
Bis(1-methyl ortho-carboranyl)borane (HB(oCbMe)2, Fig. 17e) is an example of a di-substituted carboranyl borane and is analogous to Piers’ borane. Lewis superacidity was confirmed computationally with FIA at 527 kJ mol−1 (exceeding SbF5 at 493 kJ mol−1; Table 2).200 Electronically, the LUMO energy level of HB(oCbMe)2 is destabilized by 0.40 eV relative to B(C6F5)3, and in terms of sterics is encumbered by 11.6% relative to B(C6F5)3 (%Vbur).200 Additional examples of di-substituted carboranyl boranes include carboranyl diboranthracene ((BR)2OCb2, R = H, Cl, Br, Me, Ph, N3), though (BH)2OCb2 was only observed in situ, and carboranyl borafluorene analogues (RB(b,O)Cb, R = iPr2N, Cl, Br, Ph, OCb; Fig. 17e). For the carboranyl diboranthracene analogues, RLAs (by FIA) compared to B(C6F5)3 are 114%, 113%, 133%, 104%, and 110% for R being H, Cl, Br, Me, Ph, and N3 (Table 2), respectively, showcasing the high Lewis acidity of these compounds.214,216 The LUMO energy levels are also reported, albeit no calculations were done for B(C6F5)3 so data cannot be compared, but are −1.50 eV, −1.57 eV, −1.03 eV, −1.18 eV, and −1.33 eV for R being Cl, Br, Me, Ph, and N3 (Table 2), respectively.214,216 In terms of sterics, again no %Vbur calculations (or relevant crystal structure data) for B(C6F5)3 was provided so this data cannot be compared, but %Vbur are 55.6%, 60.6%, 61.4%, 61.5%, and 66.2%, and 59.2% for R being H, Cl, Br, Me, Ph, and N3 (Table 2), respectively.214,216 Notable is the thermal stability of these compounds, all isolated compounds were stable to at least 235 °C, with (BMe)2OCb2 showing the highest melting point of 285 °C. The carboranyl borafluorene analogues exhibit increased Lewis acidity, except for the iPr2N substituted carborane, with RLAs (by FIA) to that of B(C6F5)3 being 87%, 118%, 119%, 108%, and 131% for R being iPr2N, Cl, Br, Ph, and OCb (Table 2), respectively.212,217 As is observed for classical borafluorenes, fusion of the substituents around the central boron atom results in an increase in Lewis acidity, resulting in an FIA increase of 39 kJ mol−1 for OCbB(b,OCb) relative to BOCb3. Only select LUMO energy levels are reported for these compounds for −2.05 eV, −1.17 eV, and −3.94 eV energies for the iPr2N, Ph, and OCb analogues, respectively.212,217 Furthermore, relative to B(C6F5)3, the LUMO energy level of OCbB(b,OCb) is stabilized by 0.42 eV. Lastly, the buried volumes of this series of carboranyl borafluorenes were 67.7%, 55.5%, 56.3%, 61.2%, and 67.7% for R being iPr2N, Cl, Br, Ph, and OCb, respectively.212,217
5. Conclusion
In summary, historical and emerging applications of B(C6F5)3 has been presented which showcase the Lewis acid to be highly versatile. Compared to its halide counterparts, B(C6F5)3 is unique in its stability and formation of analyzable crystalline structures appropriate for mechanistic studies, which has paved the way for improved chemical designs and applications. This is evident when reviewing the electronics field, where B(C6F5)3 has recently emerged as a popular dopant for the active materials leading to higher performance devices, however, little understanding of the mechanisms and material interactions at play is evident. For example, B(C6F5)3 is hygroscopic and is known to form water adducts, oxygen bridged borate complexes and can decompose into boronic acids and boroxines upon long-term exposure to water, oxygen, and heat. Such varied chemical species are likely to influence the long-term performance of organic electronic devices but have been rarely discussed within this field. Despite this, B(C6F5)3 has shown great promise to date for applications in materials science and is an area that is seemingly ripe for future research. However, an interdisciplinary approach involving both inorganic chemists and materials scientists is needed. To start bridging this gap, we presented select triaryl boranes, triaryl borates, and carboranyl boranes and relevant properties for doping applications in organic electronics. Aluminum, indium, and pentavalent pnictogen (antinomy, phosphorus, bismuth) based Lewis acids were not covered but are notable alternatives. Looking towards future work, collaborations between the materials science and inorganic communities alongside the application of machine learning to identify and screen additional alternatives, in terms of predicted properties and interactions, would be of immense value to the materials science community.Data availability
There is no original data. All work covered has been published prior.Conflicts of interest
There are no conflicts to declare.Acknowledgements
GCW acknowledges funding from the NSERC DG program (2019-04392), the Canada Foundation for Innovation, and the University of Calgary. KMW thanks NSERC for a CGS-D scholarship & IEP for a PGS-D scholarship. KMW, MJG, and IEP thank Alberta Student Aid for Alberta Graduate Excellence Scholarships.References
- W. E. Piers and T. Chivers, Pentafluorophenylboranes: From Obscurity to Applications, Chem. Soc. Rev., 1997, 26(5), 345, 10.1039/cs9972600345.
- A. G. Massey and A. J. Park, Tris(Pentafluorophenyl)Boron, J. Organometal. Chem., 1964, 2(3), 245–250, DOI:10.1016/S0022-328X(00)80518-5.
- X. Yang, C. L. Stern and T. J. Marks, Cation-like Homogeneous Olefin Polymerization Catalysts Based upon Zirconocene Alkyls and Tris(Pentafluorophenyl)Borane, J. Am. Chem. Soc., 1991, 113(9), 3623–3625, DOI:10.1021/ja00009a076.
- X. Yang, C. L. Stern and T. J. Marks, Cationic Zirconocene Olefin Polymerization Catalysts Based on the Organo-Lewis Acid Tris(Pentafluorophenyl)Borane. A Synthetic, Structural, Solution Dynamic, and Polymerization Catalytic Study, J. Am. Chem. Soc., 1994, 116(22), 10015–10031, DOI:10.1021/ja00101a022.
- M. Bochmann, Cationic Group 4 Metallocene Complexes and Their Role in Polymerisation Catalysis: The Chemistry of Well Defined Ziegler Catalysts, J. Chem. Soc., Dalton Trans., 1996,(3), 255, 10.1039/dt9960000255.
- E. Y.-X. Chen and T. J. Marks, Cocatalysts for Metal-Catalyzed Olefin Polymerization: Activators, Activation Processes, and Structure–Activity Relationships, Chem. Rev., 2000, 100(4), 1391–1434, DOI:10.1021/cr980462j.
- X. Sun, A Novel Lithium Battery Electrolyte Based on Lithium Fluoride and a Tris(Pentafluorophenyl) Borane Anion Receptor in DME, Electrochem. Solid-State Lett., 1998, 1(6), 239, DOI:10.1149/1.1390698.
- G. C. Welch, R. R. S. Juan, J. D. Masuda and D. W. Stephan, Reversible, Metal-Free Hydrogen Activation, Science, 2006, 314(5802), 1124–1126, DOI:10.1126/science.1134230.
- D. W. Stephan and G. Erker, Frustrated Lewis Pairs: Metal-Free Hydrogen Activation and More, Angew. Chem., Int. Ed., 2010, 49(1), 46–76, DOI:10.1002/anie.200903708.
- D. W. Stephan, Frustrated Lewis Pairs, J. Am. Chem. Soc., 2015, 137(32), 10018–10032, DOI:10.1021/jacs.5b06794.
- D. W. Stephan, Frustrated Lewis Pairs: From Concept to Catalysis, Acc. Chem. Res., 2015, 48(2), 306–316, DOI:10.1021/ar500375j.
- K. Ishihara and H. Yamamoto, Arylboron Compounds as Acid Catalysts in Organic Synthetic Transformations, Eur. J. Org. Chem., 1999,(3), 527–538, DOI:10.1002/(SICI)1099-0690(199903)1999:3
![[double bond splayed right]](https://www.rsc.org/images/entities/char_e00a.gif) 527::AID-EJOC527
527::AID-EJOC527![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) 3.0.CO;2-R.
3.0.CO;2-R. - G. Kumar, S. Roy and I. Chatterjee, Tris(Pentafluorophenyl)Borane Catalyzed C–C and C–Heteroatom Bond Formation, Org. Biomol. Chem., 2021, 19(6), 1230–1267, 10.1039/D0OB02478C.
- V. Nori, F. Pesciaioli, A. Sinibaldi, G. Giorgianni and A. Carlone, Boron-Based Lewis Acid Catalysis: Challenges and Perspectives, Catalysts, 2021, 12(1), 5, DOI:10.3390/catal12010005.
- Frustrated Lewis Pairs II: Expanding the Scope, in Topics in Current Chemistry, ed. G. Erker and D. W. Stephan, Springer Berlin Heidelberg, Berlin, Heidelberg, 2013, vol. 334 DOI:10.1007/978-3-642-37759-4.
- K. Ishihara, N. Hananki and H. Yamamoto, Tris(Pentafluorophenyl)Boron as a New Efficient, Air Stable, and Water Tolerant Catalyst in the Adol-Type and Michael Reactions, Syn. Lett., 1993, 8, 577–579, DOI:10.1055/s-1993-22535.
- H. Gao, A. Battley and E. M. Leitao, The Ultimate Lewis Acid Catalyst: Using Tris(Pentafluorophenyl) Borane to Create Bespoke Siloxane Architectures, Chem. Commun., 2022, 58(54), 7451–7465, 10.1039/D2CC00441K.
- T. Hackel and N. A. McGrath, Tris(Pentafluorophenyl)Borane-Catalyzed Reactions Using Silanes, Molecules, 2019, 24(3), 432, DOI:10.3390/molecules24030432.
- M. A. Brook, New Control Over Silicone Synthesis Using SiH Chemistry: The Piers–Rubinsztajn Reaction, Chem. – Eur. J., 2018, 24(34), 8458–8469, DOI:10.1002/chem.201800123.
- X. Chen, M. Yi, S. Wu, L. Tan, X. Ge, M. He and G. Yin, Synthesis of Structurally Precise Polysiloxanes via the Piers–Rubinsztajn Reaction, Materials, 2019, 12(2), 304, DOI:10.3390/ma12020304.
- J. M. Blackwell, W. E. Piers and M. Parvez, Mechanistic Studies on Selectivity in the B(C6F5)3-Catalyzed Allylstannation of Aldehydes: Is Hypercoordination at Boron Responsible?, Org. Lett., 2000, 2(5), 695–698, DOI:10.1021/ol0000105.
- D. J. Morrison, J. M. Blackwell and W. E. Piers, Mechanistic Insights into Perfluoroaryl Borane-Catalyzed Allylstannations: Toward Asymmetric Induction with Chiral Boranes, Pure Appl. Chem., 2004, 76(3), 615–623, DOI:10.1351/pac200476030615.
- D. J. Morrison and W. E. Piers, Weaker Lewis Acid, Better Catalytic Activity: Dual Mechanisms in Perfluoroarylborane-Catalyzed Allylstannation Reactions, Org. Lett., 2003, 5(16), 2857–2860, DOI:10.1021/ol034928i.
- M. Ge, B. M. Stoltz and E. J. Corey, Mechanistic Insights into the Factors Determining Exo–Endo Selectivity in the Lewis Acid-Catalyzed Diels–Alder Reaction of 1,3-Dienes with 2-Cycloalkenones, Org. Lett., 2000, 2(13), 1927–1929, DOI:10.1021/ol0060026.
- J.-H. Zhou, B. Jiang, F.-F. Meng, Y.-H. Xu and T.-P. Loh, B(C6F5)3: A New Class of Strong and Bulky Lewis Acid for Exo-Selective Intermolecular Diels–Alder Reactions of Unreactive Acyclic Dienes with α,β-Enals, Org. Lett., 2015, 17(18), 4432–4435, DOI:10.1021/acs.orglett.5b02066.
- M. Bakos, Z. Dobi, D. Fegyverneki, Á. Gyömöre, I. Fernández and T. Soós, Janus Face of the Steric Effect in a Lewis Acid Catalyst with Size-Exclusion Design: Steric Repulsion and Steric Attraction in the Catalytic Exo-Selective Diels–Alder Reaction, ACS Sustainable Chem. Eng., 2018, 6(8), 10869–10875, DOI:10.1021/acssuschemeng.8b02099.
- J. Wang, Z. Liu, J. Li, Z. Song, C. Hu and Z. Su, Exo/Endo Selectivity Control in Diels–Alder Reactions of Geminal Bis(Silyl) Dienes: Theoretical and Experimental Studies, J. Org. Chem., 2019, 84(7), 3940–3952, DOI:10.1021/acs.joc.8b03090.
- G. Erker, Tris(Pentafluorophenyl)Borane: A Special Boron Lewis Acid for Special Reactions, Dalton Trans., 2005, 1883–1890, 10.1039/b503688g.
- J. A. Ewen, M. J. Edler, R. L. Jones, L. Haspeslagh, J. L. Atwood, S. G. Bott and K. Robinson, Metallocene/Polypropylene Structural Relationships: Implications on Polymerization and Stereochemical Control Mechanisms, Macromol. Symp., 1991, 48–49(1), 253–295, DOI:10.1002/masy.19910480121.
- D. J. Parks and W. E. Piers, Tris(Pentafluorophenyl)Boron-Catalyzed Hydrosilation of Aromatic Aldehydes, Ketones, and Esters, J. Am. Chem. Soc., 1996, 118(39), 9440–9441, DOI:10.1021/ja961536g.
- T. Ooi, D. Uraguchi, N. Kagoshima and K. Maruoka, Hypercoordination of Boron and Aluminum: Synthetic Utility as Chelating Lewis Acids, J. Am. Chem. Soc., 1998, 120(21), 5327–5328, DOI:10.1021/ja9736828.
- D. Vagedes, R. Fröhlich and G. Erker, Treatment of Naphthols with B(C6F5)3: Formation and Characterization of the Lewis Acid Adducts of Their Keto Isomers, Angew. Chem., Int. Ed., 1999, 38(22), 3362–3365, DOI:10.1002/(SICI)1521-3773(19991115)38:22
![[double bond splayed right]](https://www.rsc.org/images/entities/char_e00a.gif) 3362::AID-ANIE3362
3362::AID-ANIE3362![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) 3.0.CO;2-N.
3.0.CO;2-N. - S. Rubinsztajn and J. A. Cella, A New Polycondensation Process for the Preparation of Polysiloxane Copolymers, Macromolecules, 2005, 38(4), 1061–1063, DOI:10.1021/ma047984n.
- P. Thirupathi, L. N. Neupane and K.-H. Lee, Tris(Pentafluorophenyl)Borane [B(C6F5)3]-Catalyzed Friedel–Crafts Reactions of Activated Arenes and Heteroarenes with α-Amidosulfones: The Synthesis of Unsymmetrical Triarylmethanes, Tetrahedron, 2011, 67(38), 7301–7310, DOI:10.1016/j.tet.2011.07.041.
- J. M. Blackwell, E. R. Sonmor, T. Scoccitti and W. E. Piers, B(C6F5)3-Catalyzed Hydrosilation of Imines via Silyliminium Intermediates, Org. Lett., 2000, 2(24), 3921–3923, DOI:10.1021/ol006695q.
- J. M. Blackwell, W. E. Piers and R. McDonald, Mechanistic Studies on the B(C6F5)3 Catalyzed Allylstannation of Aromatic Aldehydes with Ortho Donor Substituents, J. Am. Chem. Soc., 2002, 124(7), 1295–1306, DOI:10.1021/ja012028w.
- I. Protsak, V. Gun’ko, Y. Morozov, I. M. Henderson, D. Zhang, Z. Yinjun and V. Turov, Intermediates of Tris(Pentafluorophenyl)Borane and Dimethyl Carbonate Pave the Way for Deeper Organosiloxane Depolymerization Reactions, Polym. J., 2021, 53(4), 573–579, DOI:10.1038/s41428-020-00452-0.
- G. Kehr, R. Fröhlich, B. Wibbeling and G. Erker, (N-Pyrrolyl)B(C6F5)2-A New Organometallic Lewis Acid for the Generation of Group 4 Metallocene Cation Complexes, Chem. – Eur. J., 2000, 6(2), 258–266, DOI:10.1002/(SICI)1521-3765(20000117)6:2
![[double bond splayed right]](https://www.rsc.org/images/entities/char_e00a.gif) 258::AID-CHEM258
258::AID-CHEM258![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) 3.0.CO;2-E.
3.0.CO;2-E. - G. Kehr, R. Roesmann, R. Fröhlich, C. Holst and G. Erker, Protonation of the Heterocyclic Cp-Anion Equivalent [Pyrrolyl-B(C6F5)3]Li – Formation of a Useful Neutral Brønsted Acid for the Generation of Homogeneous Metallocene Ziegler Catalysts, Eur. J. Inorg. Chem., 2001,(2), 535–538, DOI:10.1002/1099-0682(200102)2001:2
![[double bond splayed right]](https://www.rsc.org/images/entities/char_e00a.gif) 535::AID-EJIC535
535::AID-EJIC535![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) 3.0.CO;2-6.
3.0.CO;2-6. - S. Guidotti, I. Camurati, F. Focante, L. Angellini, G. Moscardi, L. Resconi, R. Leardini, D. Nanni, P. Mercandelli, A. Sironi, T. Beringhelli and D. Maggioni, Synthesis and Reactivity of (C6F5)3B-N-Heterocycle Complexes. 1. Generation of Highly Acidic Sp3 Carbons in Pyrroles and Indoles, J. Org. Chem., 2003, 68(14), 5445–5465, DOI:10.1021/jo020647x.
- A. Bonazza, I. Camurati, S. Guidotti, N. Mascellari and L. Resconi, Synthesis and Reactivity of (C6F5)3B-N-Heterocycle Complexes, Macromol. Chem. Phys., 2004, 205(3), 319–333, DOI:10.1002/macp.200300132.
- G. C. Welch, L. Cabrera, P. A. Chase, E. Hollink, J. D. Masuda, P. Wei and D. W. Stephan, Tuning Lewis Acidity Using the Reactivity of “Frustrated Lewis Pairs”: Facile Formation of Phosphine–Boranes and Cationic Phosphonium–Boranes, Dalton Trans., 2007, 3407–3414, 10.1039/b704417h.
- J. S. J. McCahill, G. C. Welch and D. W. Stephan, Reactivity of “Frustrated Lewis Pairs”: Three-Component Reactions of Phosphines, a Borane, and Olefins, Angew. Chem., Int. Ed., 2007, 46(26), 4968–4971, DOI:10.1002/anie.200701215.
- J. Paradies, Mechanisms in Frustrated Lewis Pair-Catalyzed Reactions, Eur. J. Org. Chem., 2019,(2–3), 283–294, DOI:10.1002/ejoc.201800944.
- M. L. McGraw and E. Y.-X. Chen, Lewis Pair Polymerization: Perspective on a Ten-Year Journey, Macromolecules, 2020, 53(15), 6102–6122, DOI:10.1021/acs.macromol.0c01156.
- J. S. Reddy, B.-H. Xu, T. Mahdi, R. Fröhlich, G. Kehr, D. W. Stephan and G. Erker, Alkenylborane-Derived Frustrated Lewis Pairs: Metal-Free Catalytic Hydrogenation Reactions of Electron-Deficient Alkenes, Organometallics, 2012, 31(15), 5638–5649, DOI:10.1021/om3006068.
- L. Greb, P. Oña-Burgos, B. Schirmer, S. Grimme, D. W. Stephan and J. Paradies, Metal-Free Catalytic Olefin Hydrogenation: Low-Temperature H2 Activation by Frustrated Lewis Pairs, Angew. Chem., Int. Ed., 2012, 51(40), 10164–10168, DOI:10.1002/anie.201204007.
- J. A. Nicasio, S. Steinberg, B. Inés and M. Alcarazo, Tuning the Lewis Acidity of Boranes in Frustrated Lewis Pair Chemistry: Implications for the Hydrogenation of Electron-Poor Alkenes, Chem. – Eur. J., 2013, 19(33), 11016–11020, DOI:10.1002/chem.201301158.
- A. Willms, H. Schumacher, T. Tabassum, L. Qi, S. L. Scott, P. J. C. Hausoul and M. Rose, Solid Molecular Frustrated Lewis Pairs in a Polyamine Organic Framework for the Catalytic Metal-free Hydrogenation of Alkenes, ChemCatChem, 2018, 10(8), 1835–1843, DOI:10.1002/cctc.201701783.
- Y. Segawa and D. W. Stephan, Metal-Free Hydrogenation Catalysis of Polycyclic Aromatic Hydrocarbons, Chem. Commun., 2012, 48(98), 11963, 10.1039/c2cc37190a.
- T. Mahdi and D. W. Stephan, Enabling Catalytic Ketone Hydrogenation by Frustrated Lewis Pairs, J. Am. Chem. Soc., 2014, 136(45), 15809–15812, DOI:10.1021/ja508829x.
- D. J. Scott, M. J. Fuchter and A. E. Ashley, Nonmetal Catalyzed Hydrogenation of Carbonyl Compounds, J. Am. Chem. Soc., 2014, 136(45), 15813–15816, DOI:10.1021/ja5088979.
- T. Mahdi and D. W. Stephan, Facile Protocol for Catalytic Frustrated Lewis Pair Hydrogenation and Reductive Deoxygenation of Ketones and Aldehydes, Angew. Chem., Int. Ed., 2015, 54(29), 8511–8514, DOI:10.1002/anie.201503087.
- P. A. Chase, G. C. Welch, T. Jurca and D. W. Stephan, Metal-Free Catalytic Hydrogenation, Angew. Chem., Int. Ed., 2007, 46(42), 8050–8053, DOI:10.1002/anie.200702908.
- P. A. Chase, T. Jurca and D. W. Stephan, Lewis Acid-Catalyzed Hydrogenation: B(C6F5)3-Mediated Reduction of Imines and Nitriles with H2, Chem. Commun., 2008, 1701–1703, 10.1039/b718598g.
- P. Spies, S. Schwendemann, S. Lange, G. Kehr, R. Fröhlich and G. Erker, Metal-Free Catalytic Hydrogenation of Enamines, Imines, and Conjugated Phosphinoalkenylboranes, Angew. Chem., Int. Ed., 2008, 47(39), 7543–7546, DOI:10.1002/anie.200801432.
- H. Wang, R. Fröhlich, G. Kehr and G. Erker, Heterolytic Dihydrogen Activation with the 1,8-Bis(Diphenylphosphino)Naphthalene/B(C6F5)3 Pair and Its Application for Metal-Free Catalytic Hydrogenation of Silyl Enol Ethers, Chem. Commun., 2008, 5966–5968, 10.1039/b813286k.
- J. Mohr and M. Oestreich, B(C6F5)3-Catalyzed Hydrogenation of Oxime Ethers without Cleavage of the N–O Bond, Angew. Chem., Int. Ed., 2014, 53(48), 13278–13281, DOI:10.1002/anie.201407324.
- T. Mahdi and D. W. Stephan, Frustrated Lewis Pair Catalyzed Hydroamination of Terminal Alkynes, Angew. Chem., Int. Ed., 2013, 52(47), 12418–12421, DOI:10.1002/anie.201307254.
- T. Mahdi and D. W. Stephan, Stoichiometric and Catalytic Inter- and Intramolecular Hydroamination of Terminal Alkynes by Frustrated Lewis Pairs, Chem. – Eur. J., 2015, 21(31), 11134–11142, DOI:10.1002/chem.201501535.
- R. Dobrovetsky and D. W. Stephan, Stoichiometric Metal-Free Reduction of CO in Syn-Gas, J. Am. Chem. Soc., 2013, 135(13), 4974–4977, DOI:10.1021/ja401492s.
- M. Sajid, L. Elmer, C. Rosorius, C. G. Daniliuc, S. Grimme, G. Kehr and G. Erker, Facile Carbon Monoxide Reduction at Intramolecular Frustrated Phosphane/Borane Lewis Pair Templates, Angew. Chem., Int. Ed., 2013, 52(8), 2243–2246, DOI:10.1002/anie.201208750.
- D. W. Stephan, Frustrated Lewis Pair Chemistry of CO, Chem. Soc. Rev., 2023, 52(14), 4632–4643, 10.1039/D3CS00294B.
- C. M. Mömming, E. Otten, G. Kehr, R. Fröhlich, S. Grimme, D. W. Stephan and G. Erker, Reversible Metal-Free Carbon Dioxide Binding by Frustrated Lewis Pairs, Angew. Chem., Int. Ed., 2009, 48(36), 6643–6646, DOI:10.1002/anie.200901636.
- A. Ashley, A. Thompson and D. O’Hare, Non-Metal-Mediated Homogeneous Hydrogenation of CO2 to CH3OH, Angew. Chem., Int. Ed., 2009, 48(52), 9839–9843, DOI:10.1002/anie.200905466.
- A. Berkefeld, W. E. Piers and M. Parvez, Tandem Frustrated Lewis Pair/Tris(Pentafluorophenyl)Borane-Catalyzed Deoxygenative Hydrosilylation of Carbon Dioxide, J. Am. Chem. Soc., 2010, 132(31), 10660–10661, DOI:10.1021/ja105320c.
- I. Peuser, R. C. Neu, X. Zhao, M. Ulrich, B. Schirmer, J. A. Tannert, G. Kehr, R. Fröhlich, S. Grimme, G. Erker and D. W. Stephan, CO2 and Formate Complexes of Phosphine/Borane Frustrated Lewis Pairs, Chem. – Eur. J., 2011, 17(35), 9640–9650, DOI:10.1002/chem.201100286.
- M. Harhausen, R. Fröhlich, G. Kehr and G. Erker, Reactions of Modified Intermolecular Frustrated P/B Lewis Pairs with Dihydrogen, Ethene, and Carbon Dioxide, Organometallics, 2012, 31(7), 2801–2809, DOI:10.1021/om201076f.
- Md. N. Khan, Y. Van Ingen, T. Boruah, A. McLauchlan, T. Wirth and R. L. Melen, Advances in CO2 Activation by Frustrated Lewis Pairs: From Stoichiometric to Catalytic Reactions, Chem. Sci., 2023, 13661–13695, 10.1039/D3SC03907B.
- E. Otten, R. C. Neu and D. W. Stephan, Complexation of Nitrous Oxide by Frustrated Lewis Pairs, J. Am. Chem. Soc., 2009, 131(29), 9918–9919, DOI:10.1021/ja904377v.
- A. J. P. Cardenas, B. J. Culotta, T. H. Warren, S. Grimme, A. Stute, R. Fröhlich, G. Kehr and G. Erker, Capture of NO by a Frustrated Lewis Pair: A New Type of Persistent N-Oxyl Radical, Angew. Chem., Int. Ed., 2011, 50(33), 7567–7571, DOI:10.1002/anie.201101622.
- M. Sajid, A. Klose, B. Birkmann, L. Liang, B. Schirmer, T. Wiegand, H. Eckert, A. J. Lough, R. Fröhlich, C. G. Daniliuc, S. Grimme, D. W. Stephan, G. Kehr and G. Erker, Reactions of Phosphorus/Boron Frustrated Lewis Pairs with SO2, Chem. Sci., 2013, 4(1), 213–219, 10.1039/C2SC21161K.
- M. A. Dureen and D. W. Stephan, Terminal Alkyne Activation by Frustrated and Classical Lewis Acid/Phosphine Pairs, J. Am. Chem. Soc., 2009, 131(24), 8396–8397, DOI:10.1021/ja903650w.
- M. A. Dureen, C. C. Brown and D. W. Stephan, Addition of Enamines or Pyrroles and B(C6F5)3 “Frustrated Lewis Pairs” to Alkynes, Organometallics, 2010, 29(23), 6422–6432, DOI:10.1021/om1008346.
- M. A. Dureen, C. C. Brown and D. W. Stephan, Deprotonation and Addition Reactions of Frustrated Lewis Pairs with Alkynes, Organometallics, 2010, 29(23), 6594–6607, DOI:10.1021/om1009044.
- M. A. Dureen, G. C. Welch, T. M. Gilbert and D. W. Stephan, Heterolytic Cleavage of Disulfides by Frustrated Lewis Pairs, Inorg. Chem., 2009, 48(20), 9910–9917, DOI:10.1021/ic901590s.
- D. Voicu, M. Abolhasani, R. Choueiri, G. Lestari, C. Seiler, G. Menard, J. Greener, A. Guenther, D. W. Stephan and E. Kumacheva, Microfluidic Studies of CO2 Sequestration by Frustrated Lewis Pairs, J. Am. Chem. Soc., 2014, 136(10), 3875–3880, DOI:10.1021/ja411601a.
- D. C. Bradley, I. S. Harding, A. D. Keefe, M. Motevalli and D. H. Zheng, Reversible Adduct Formation between Phosphines and Triarylboron Compounds, J. Chem. Soc., Dalton Trans., 1996,(20), 3931, 10.1039/dt9960003931.
- P. S. Marqués, G. Londi, B. Yurash, T.-Q. Nguyen, S. Barlow, S. R. Marder and D. Beljonne, Understanding How Lewis Acids Dope Organic Semiconductors: A “Complex” Story, Chem. Sci., 2021, 12(20), 7012–7022, 10.1039/D1SC01268A.
- M. Dryzhakov, E. Richmond, G. Li and J. Moran, Catalytic B(C6F5)3H2O-Promoted Defluorinative Functionalization of Tertiary Aliphatic Fluorides, J. Fluorine Chem., 2017, 193, 45–51, DOI:10.1016/j.jfluchem.2016.11.005.
- H. H. San, S.-J. Wang, M. Jiang and X.-Y. Tang, Boron-Catalyzed O–H Bond Insertion of α-Aryl α-Diazoesters in Water, Org. Lett., 2018, 20(15), 4672–4676, DOI:10.1021/acs.orglett.8b01988.
- H. Zhang, X.-Y. Zhan, Y. Dong, J. Yang, S. He, Z.-C. Shi, X.-M. Zhang and J.-Y. Wang, Dehydration in Water: Frustrated Lewis Pairs Directly Catalyzed Allylization of Electron-Rich Arenes and Allyl Alcohols, RSC Adv., 2020, 10(29), 16942–16948, 10.1039/D0RA02912B.
- K. M. Rabanzo-Castillo, V. B. Kumar, T. Söhnel and E. M. Leitao, Catalytic Synthesis of Oligosiloxanes Mediated by an Air Stable Catalyst, (C6F5)3B(OH2), Front. Chem., 2020, 8, 477, DOI:10.3389/fchem.2020.00477.
- L. Winfrey, L. Yun, G. Passeri, K. Suntharalingam and A. P. Pulis, H2O·B(C6F5)3-Catalyzed Para-Alkylation of Anilines with Alkenes Applied to Late-Stage Functionalization of Non-Steroidal Anti-Inflammatory Drugs, Chem. – Eur. J., 2024, 30(13), e202303130, DOI:10.1002/chem.202303130.
- M. Dryzhakov and J. Moran, Autocatalytic Friedel–Crafts Reactions of Tertiary Aliphatic Fluorides Initiated by B(C6F5)3·H2O, ACS Catal., 2016, 6(6), 3670–3673, DOI:10.1021/acscatal.6b00866.
- G.-M. Zhang, H. Zhang, B. Wang and J.-Y. Wang, Boron-Catalyzed Dehydrative Allylation of 1,3-Diketones and β-Ketone Esters with 1,3-Diarylallyl Alcohols in Water, RSC Adv., 2021, 11(28), 17025–17031, 10.1039/D1RA01922H.
- F. Jäkle, Advances in the Synthesis of Organoborane Polymers for Optical, Electronic, and Sensory Applications, Chem. Rev., 2010, 110, 3985–4022, DOI:10.1021/cr100026f.
- M. J. D. Bosdet and W. E. Piers, B–N as a C–C Substitute in Aromatic Systems, Can. J. Chem., 2009, 87, 8–29, DOI:10.1139/V08-110.
- J.-Y. Wang and J. Pei, BN-Embedded Aromatics for Optoelectronic Applications, Chin. Chem. Lett., 2016, 27, 1139–1146, DOI:10.1016/j.cclet.2016.06.014.
- S. K. Mellerup and S. Wang, Boron-Based Stimuli Responsive Materials, Chem. Soc. Rev., 2019, 48, 3537, 10.1039/c9cs00153k.
- Y.-S. Duh, K. H. Lin and C.-S. Kao, Experimental Investigation and Visualization on Thermal Runaway of Hard Prismatic Lithium-Ion Batteries Used in Smart Phones, J. Therm. Anal. Calorim., 2018, 132(3), 1677–1692, DOI:10.1007/s10973-018-7077-2.
- J. Duan, X. Tang, H. Dai, Y. Yang, W. Wu, X. Wei and Y. Huang, Building Safe Lithium-Ion Batteries for Electric Vehicles: A Review, Electrochem. Energy Rev., 2020, 3(1), 1–42, DOI:10.1007/s41918-019-00060-4.
- B. Diouf and R. Pode, Potential of Lithium-Ion Batteries in Renewable Energy, Renewable Energy, 2015, 76, 375–380, DOI:10.1016/j.renene.2014.11.058.
- Y. Chen, Y. Kang, Y. Zhao, L. Wang, J. Liu, Y. Li, Z. Liang, X. He, X. Li, N. Tavajohi and B. Li, A Review of Lithium-Ion Battery Safety Concerns: The Issues, Strategies, and Testing Standards, J. Energy Chem., 2021, 59, 83–99, DOI:10.1016/j.jechem.2020.10.017.
- X. Wu, K. Song, X. Zhang, N. Hu, L. Li, W. Li, L. Zhang and H. Zhang, Safety Issues in Lithium Ion Batteries: Materials and Cell Design, Front. Energy Res., 2019, 7, 65, DOI:10.3389/fenrg.2019.00065.
- X. Liu, D. Ren, H. Hsu, X. Feng, G.-L. Xu, M. Zhuang, H. Gao, L. Lu, X. Han, Z. Chu, J. Li, X. He, K. Amine and M. Ouyang, Thermal Runaway of Lithium-Ion Batteries without Internal Short Circuit, Joule, 2018, 2(10), 2047–2064, DOI:10.1016/j.joule.2018.06.015.
- M. Herstedt, M. Stjerndahl, T. Gustafsson and K. Edström, Anion Receptor for Enhanced Thermal Stability of the Graphite Anode Interface in a Li-Ion Battery, Electrochem. Commun., 2003, 5(6), 467–472, DOI:10.1016/S1388-2481(03)00106-1.
- J. Xiang, Y. Zhang, B. Zhang, L. Yuan, X. Liu, Z. Cheng, Y. Yang, X. Zhang, Z. Li, Y. Shen, J. Jiang and Y. Huang, A Flame-Retardant Polymer Electrolyte for High Performance Lithium Metal Batteries with an Expanded Operation Temperature, Energy Environ. Sci., 2021, 14(6), 3510–3521, 10.1039/D1EE00049G.
- Y. M. Lee, J. E. Seo, N.-S. Choi and J.-K. Park, Influence of Tris(Pentafluorophenyl) Borane as an Anion Receptor on Ionic Conductivity of LiClO4-Based Electrolyte for Lithium Batteries, Electrochim. Acta, 2005, 50(14), 2843–2848, DOI:10.1016/j.electacta.2004.11.058.
- C.-C. Chang and T.-K. Chen, Tris(Pentafluorophenyl) Borane as an Electrolyte Additive for LiFePO4 Battery, J. Power Sources, 2009, 193(2), 834–840, DOI:10.1016/j.jpowsour.2009.04.033.
- Y. M. Lee, Y.-G. Lee, Y.-M. Kang and K. Y. Cho, Nature of Tris(Pentafluorophenyl)Borane as a Functional Additive and Its Contribution to High Rate Performance in Lithium-Ion Secondary Battery, Electrochem. Solid-State Lett., 2010, 13(5), A55, DOI:10.1149/1.3329703.
- G.-B. Han, J.-N. Lee, J. W. Choi and J.-K. Park, Tris(Pentafluorophenyl) Borane as an Electrolyte Additive for High Performance Silicon Thin Film Electrodes in Lithium Ion Batteries, Electrochim. Acta, 2011, 56(24), 8997–9003, DOI:10.1016/j.electacta.2011.07.136.
- S. Wu and A. Huang, Effects of Tris(Pentafluorophenyl) Borane (TPFPB) as an Electrolyte Additive on the Cycling Performance of LiFePO4 Batteries, J. Electrochem. Soc., 2013, 160(4), A684–A689, DOI:10.1149/2.074304jes.
- H. Yue, Y. Yang, Y. Xiao, Z. Dong, S. Cheng, Y. Yin, C. Ling, W. Yang, Y. Yu and S. Yang, Boron Additive Passivated Carbonate Electrolytes for Stable Cycling of 5 V Lithium–Metal Batteries, J. Mater. Chem. A, 2019, 7(2), 594–602, 10.1039/C8TA09380F.
- K. Xu, Y. Lam, S. S. Zhang, T. R. Jow and T. B. Curtis, Solvation Sheath of Li+ in Nonaqueous Electrolytes and Its Implication of Graphite/Electrolyte Interface Chemistry, J. Phys. Chem. C, 2007, 111(20), 7411–7421, DOI:10.1021/jp068691u.
- S. J. An, J. Li, C. Daniel, D. Mohanty, S. Nagpure and D. L. Wood, The State of Understanding of the Lithium-Ion-Battery Graphite Solid Electrolyte Interphase (SEI) and Its Relationship to Formation Cycling, Carbon, 2016, 105, 52–76, DOI:10.1016/j.carbon.2016.04.008.
- A. Wang, S. Kadam, H. Li, S. Shi and Y. Qi, Review on Modeling of the Anode Solid Electrolyte Interphase (SEI) for Lithium-Ion Batteries, npj Comput. Mater., 2018, 4(1), 15, DOI:10.1038/s41524-018-0064-0.
- Z. Chen and K. Amine, Tris(Pentafluorophenyl) Borane as an Additive to Improve the Power Capabilities of Lithium-Ion Batteries, J. Electrochem. Soc., 2006, 153(6), A1221, DOI:10.1149/1.2194633.
- T. Li, X.-Q. Zhang, N. Yao, Y.-X. Yao, L.-P. Hou, X. Chen, M.-Y. Zhou, J.-Q. Huang and Q. Zhang, Stable Anion-Derived Solid Electrolyte Interphase in Lithium Metal Batteries, Angew. Chem., Int. Ed., 2021, 60(42), 22683–22687, DOI:10.1002/anie.202107732.
- S. Li, W. Zhang, Q. Wu, L. Fan, X. Wang, X. Wang, Z. Shen, Y. He and Y. Lu, Synergistic Dual-Additive Electrolyte Enables Practical Lithium-Metal Batteries, Angew. Chem., Int. Ed., 2020, 59(35), 14935–14941, DOI:10.1002/anie.202004853.
- M. Wu, Y. Li, X. Liu, S. Yang, J. Ma and S. Dou, Perspective on Solid-Electrolyte Interphase Regulation for Lithium Metal Batteries, SmartMat, 2021, 2(1), 5–11, DOI:10.1002/smm2.1015.
- H. Gao, Y. Li, R. Guo and B. M. Gallant, Controlling Fluoride-Forming Reactions for Improved Rate Capability in Lithium-Perfluorinated Gas Conversion Batteries, Adv. Energy Mater., 2019, 9(21), 1900393, DOI:10.1002/aenm.201900393.
- M. Kartal, M. Uysal, A. Alp and H. Akbulut, Tris(Pentafluorophenyl) Borane as an Electrolyte Additive for Li–O2 Batteries, Int. J. Hydrogen Energy, 2016, 41(18), 7600–7608, DOI:10.1016/j.ijhydene.2016.02.092.
- T. Zhao, X. Zheng, D. Wang, L. Huang, B. Li, X. Liu, H. Yang, Y. Dai, Y. Huang and W. Luo, A Quasi-Solid-State Polyether Electrolyte for Low-Temperature Sodium Metal Batteries, Adv. Funct. Mater., 2023, 33(48), 2304928, DOI:10.1002/adfm.202304928.
- X. Zhou, X. Chen, Z. Yang, X. Liu, Z. Hao, S. Jin, L. Zhang, R. Wang, C. Zhang, L. Li, X. Tan and S. Chou, Anion Receptor Weakens ClO4− Solvation for High-Temperature Sodium-Ion Batteries, Adv. Funct. Mater., 2023, 34(5), 2302281, DOI:10.1002/adfm.202302281.
- A. Y. S. Eng, C. B. Soni, Y. Lum, E. Khoo, Z. Yao, S. K. Vineeth, V. Kumar, J. Lu, C. S. Johnson, C. Wolverton and Z. W. Seh, Theory-Guided Experimental Design in Battery Materials Research, Sci. Adv., 2022, 8(19), eabm2422, DOI:10.1126/sciadv.abm2422.
- C. K. Chiang, C. R. Fincher, Y. W. Park, A. J. Heeger, H. Shirakawa, E. J. Louis, S. C. Gau and A. G. MacDiarmid, Electrical Conductivity in Doped Polyacetylene, Phys. Rev. Lett., 1977, 39(17), 1098–1101, DOI:10.1103/PhysRevLett.39.1098.
- J. Blochwitz, M. Pfeiffer, T. Fritz and K. Leo, Low Voltage Organic Light Emitting Diodes Featuring Doped Phthalocyanine as Hole Transport Material, Appl. Phys. Lett., 1998, 73(6), 729–731, DOI:10.1063/1.121982.
- D. T. Duong, C. Wang, E. Antono, M. F. Toney and A. Salleo, The Chemical and Structural Origin of Efficient P-Type Doping in P3HT, Org. Electron., 2013, 14(5), 1330–1336, DOI:10.1016/j.orgel.2013.02.028.
- D. T. Duong, H. Phan, D. Hanifi, P. S. Jo, T. Nguyen and A. Salleo, Direct Observation of Doping Sites in Temperature-Controlled, p-Doped P3HT Thin Films by Conducting Atomic Force Microscopy, Adv. Mater., 2014, 26(35), 6069–6073, DOI:10.1002/adma.201402015.
- I. E. Jacobs, E. W. Aasen, J. L. Oliveira, T. N. Fonseca, J. D. Roehling, J. Li, G. Zhang, M. P. Augustine, M. Mascal and A. J. Moulé, Comparison of Solution-Mixed and Sequentially Processed P3HT:F4TCNQ Films: Effect of Doping-Induced Aggregation on Film Morphology, J. Mater. Chem. C, 2016, 4(16), 3454–3466, 10.1039/C5TC04207K.
- E. F. Aziz, A. Vollmer, S. Eisebitt, W. Eberhardt, P. Pingel, D. Neher and N. Koch, Localized Charge Transfer in a Molecularly Doped Conducting Polymer, Adv. Mater., 2007, 19(20), 3257–3260, DOI:10.1002/adma.200700926.
- I. Salzmann, G. Heimel, M. Oehzelt, S. Winkler and N. Koch, Molecular Electrical Doping of Organic Semiconductors: Fundamental Mechanisms and Emerging Dopant Design Rules, Acc. Chem. Res., 2016, 49(3), 370–378, DOI:10.1021/acs.accounts.5b00438.
- I. D. V. Ingram, D. J. Tate, A. V. S. Parry, R. Sebastian Sprick and M. L. Turner, A Simple Method for Controllable Solution Doping of Complete Polymer Field-Effect Transistors, Appl. Phys. Lett., 2014, 104(15), 153304, DOI:10.1063/1.4871096.
- P. Zalar, M. Kuik, Z. B. Henson, C. Woellner, Y. Zhang, A. Sharenko, G. C. Bazan and T. Nguyen, Increased Mobility Induced by Addition of a Lewis Acid to a Lewis Basic Conjugated Polymer, Adv. Mater., 2014, 26(5), 724–727, DOI:10.1002/adma.201303357.
- P. Pingel, M. Arvind, L. Kölln, R. Steyrleuthner, F. Kraffert, J. Behrends, S. Janietz and D. Neher, P-Type Doping of Poly(3-Hexylthiophene) with the Strong Lewis Acid Tris(Pentafluorophenyl)Borane, Adv. Electron. Mater., 2016, 2(10), 1600204, DOI:10.1002/aelm.201600204.
- Y. Han, G. Barnes, Y.-H. Lin, J. Martin, M. Al-Hashimi, S. Y. AlQaradawi, T. D. Anthopoulos and M. Heeney, Doping of Large Ionization Potential Indenopyrazine Polymers via Lewis Acid Complexation with Tris(Pentafluorophenyl)Borane: A Simple Method for Improving the Performance of Organic Thin-Film Transistors, Chem. Mater., 2016, 28(21), 8016–8024, DOI:10.1021/acs.chemmater.6b03761.
- G. C. Welch, R. Coffin, J. Peet and G. C. Bazan, Band Gap Control in Conjugated Oligomers via Lewis Acids, J. Am. Chem. Soc., 2009, 131(31), 10802–10803, DOI:10.1021/ja902789w.
- G. C. Welch and G. C. Bazan, Lewis Acid Adducts of Narrow Band Gap Conjugated Polymers, J. Am. Chem. Soc., 2011, 133(12), 4632–4644, DOI:10.1021/ja110968m.
- P. Zalar, Z. B. Henson, G. C. Welch, G. C. Bazan and T.-Q. Nguyen, Color Tuning in Polymer Light-Emitting Diodes with Lewis Acids, Angew. Chem., Int. Ed., 2012, 51(30), 7495–7498, DOI:10.1002/anie.201202570.
- H. Yan, J. Chen, K. Zhou, Y. Tang, X. Meng, X. Xu and W. Ma, Lewis Acid Doping Induced Synergistic Effects on Electronic and Morphological Structure for Donor and Acceptor in Polymer Solar Cells, Adv. Energy Mater., 2018, 8(19), 1703672, DOI:10.1002/aenm.201703672.
- H. Yan, Y. Tang, X. Sui, Y. Liu, B. Gao, X. Liu, S. F. Liu, J. Hou and W. Ma, Increasing Quantum Efficiency of Polymer Solar Cells with Efficient Exciton Splitting and Long Carrier Lifetime by Molecular Doping at Heterojunctions, ACS Energy Lett., 2019, 4(6), 1356–1363, DOI:10.1021/acsenergylett.9b00843.
- H. Yan, Y. Tang, X. Meng, T. Xiao, G. Lu and W. Ma, Achieving High Doping Concentration by Dopant Vapor Deposition in Organic Solar Cells, ACS Appl. Mater. Interfaces, 2019, 11(4), 4178–4184, DOI:10.1021/acsami.8b16162.
- Z. Chen, Y. Tang, B. Lin, H. Zhao, T. Li, T. Min, H. Yan and W. Ma, Probe and Control of the Tiny Amounts of Dopants in BHJ Film Enable Higher Performance of Polymer Solar Cells, ACS Appl. Mater. Interfaces, 2020, 12(22), 25115–25124, DOI:10.1021/acsami.0c06127.
- D. Zhang, Q. Li, J. Zhang, J. Wang, X. Zhang, R. Wang, J. Zhou, Z. Wei, C. Zhang, H. Zhou and Y. Zhang, Control of Nanomorphology in Fullerene-Free Organic Solar Cells by Lewis Acid Doping with Enhanced Photovoltaic Efficiency, ACS Appl. Mater. Interfaces, 2020, 12(1), 667–677, DOI:10.1021/acsami.9b17238.
- J. Luo, J. Xia, H. Yang, L. Chen, Z. Wan, F. Han, H. A. Malik, X. Zhu and C. Jia, Toward High-Efficiency, Hysteresis-Less, Stable Perovskite Solar Cells: Unusual Doping of a Hole-Transporting Material Using a Fluorine-Containing Hydrophobic Lewis Acid, Energy Environ. Sci., 2018, 11(8), 2035–2045, 10.1039/C8EE00036K.
- S. S. Reddy, V. M. Arivunithi, V. G. Sree, H. Kwon, J. Park, Y.-C. Kang, H. Zhu, Y.-Y. Noh and S.-H. Jin, Lewis Acid-Base Adduct-Type Organic Hole Transport Material for High Performance and Air-Stable Perovskite Solar Cells, Nano Energy, 2019, 58, 284–292, DOI:10.1016/j.nanoen.2019.01.041.
- Y. J. Jeong, J. Jung, E. H. Suh, D.-J. Yun, J. G. Oh and J. Jang, Self-Healable and Stretchable Organic Thermoelectric Materials: Electrically Percolated Polymer Nanowires Embedded in Thermoplastic Elastomer Matrix, Adv. Funct. Mater., 2020, 30(9), 1905809, DOI:10.1002/adfm.201905809.
- E. H. Suh, J. G. Oh, J. Jung, S. H. Noh, T. S. Lee and J. Jang, Brønsted Acid Doping of P3HT with Largely Soluble Tris(Pentafluorophenyl)Borane for Highly Conductive and Stable Organic Thermoelectrics Via One-Step Solution Mixing, Adv. Energy Mater., 2020, 10(47), 2002521, DOI:10.1002/aenm.202002521.
- E. H. Suh, S. B. Kim, H. S. Yang and J. Jang, Regulating Competitive Doping in Solution-Mixed Conjugated Polymers for Dramatically Improving Thermoelectric Properties, Adv. Funct. Mater., 2022, 32(46), 2207413, DOI:10.1002/adfm.202207413.
- H. J. Cheon, T. S. Lee, J. E. Lee, S. B. Kim, E. H. Suh, S.-K. Kwon, Y. J. Jeong, J. Jang and Y.-H. Kim, Design of Donor–Acceptor Polymer Semiconductors for Optimizing Combinations with Dopants to Maximize Thermoelectric Performance, Chem. Mater., 2023, 35(4), 1796–1805, DOI:10.1021/acs.chemmater.2c03739.
- J. Kim, D. Ju, S. Kim and K. Cho, Disorder-Controlled Efficient Doping of Conjugated Polymers for High-Performance Organic Thermoelectrics, Adv. Funct. Mater., 2024, 34(6), 2309156, DOI:10.1002/adfm.202309156.
- C. Lee, H. Kim and Y. Kim, Short-Wave Infrared-Sensing Organic Phototransistors with a Triarylamine-Based Polymer Doped with a Lewis Acid-Type Small Molecule, ACS Appl. Mater. Interfaces, 2021, 13(16), 19064–19071, DOI:10.1021/acsami.1c00472.
- W. Huang, K. Besar, R. LeCover, A. M. Rule, P. N. Breysse and H. E. Katz, Highly Sensitive NH 3 Detection Based on Organic Field-Effect Transistors with Tris(Pentafluorophenyl)Borane as Receptor, J. Am. Chem. Soc., 2012, 134(36), 14650–14653, DOI:10.1021/ja305287p.
- B. Wang, A. D. Scaccabarozzi, H. Wang, M. Koizumi, M. I. Nugraha, Y. Lin, Y. Firdaus, Y. Wang, S. Lee, T. Yokota, T. D. Anthopoulos and T. Someya, Molecular Doping of Near-Infrared Organic Photodetectors for Photoplethysmogram Sensors, J. Mater. Chem. C, 2021, 9(9), 3129–3135, 10.1039/D0TC05549B.
- Y. Kato, K. Fukuda, T. Someya and T. Yokota, An Ultra-Flexible Temperature-Insensitive Strain Sensor, J. Mater. Chem. C, 2023, 11(41), 14070–14078, 10.1039/D3TC02960C.
- A. A. Meresa and F. S. Kim, Selective Ammonia-Sensing Platforms Based on a Solution-Processed Film of Poly(3-Hexylthiophene) and p-Doping Tris(Pentafluorophenyl)Borane, Polymers, 2020, 12(1), 128, DOI:10.3390/polym12010128.
- A. D. Scaccabarozzi, A. Basu, F. Aniés, J. Liu, O. Zapata-Arteaga, R. Warren, Y. Firdaus, M. I. Nugraha, Y. Lin, M. Campoy-Quiles, N. Koch, C. Müller, L. Tsetseris, M. Heeney and T. D. Anthopoulos, Doping Approaches for Organic Semiconductors, Chem. Rev., 2022, 122(4), 4420–4492, DOI:10.1021/acs.chemrev.1c00581.
- C. R. Bridges and T. Baumgartner, Lewis Acids and Bases as Molecular Dopants for Organic Semiconductors, J. Phys. Org. Chem., 2020, 33(9), e4077, DOI:10.1002/poc.4077.
- H. Méndez, G. Heimel, A. Opitz, K. Sauer, P. Barkowski, M. Oehzelt, J. Soeda, T. Okamoto, J. Takeya, J.-B. Arlin, J.-Y. Balandier, Y. Geerts, N. Koch and I. Salzmann, Doping of Organic Semiconductors: Impact of Dopant Strength and Electronic Coupling, Angew. Chem., Int. Ed., 2013, 52(30), 7751–7755, DOI:10.1002/anie.201302396.
- B. Yurash, D. X. Cao, V. V. Brus, D. Leifert, M. Wang, A. Dixon, M. Seifrid, A. E. Mansour, D. Lungwitz, T. Liu, P. J. Santiago, K. R. Graham, N. Koch, G. C. Bazan and T.-Q. Nguyen, Towards Understanding the Doping Mechanism of Organic Semiconductors by Lewis Acids, Nat. Mater., 2019, 18(12), 1327–1334, DOI:10.1038/s41563-019-0479-0.
- F. Pallini, S. Mattiello, N. Manfredi, S. Mecca, A. Fedorov, M. Sassi, K. Al Kurdi, Y.-F. Ding, C.-K. Pan, J. Pei, S. Barlow, S. R. Marder, T.-Q. Nguyen and L. Beverina, Direct Detection of Molecular Hydrogen upon P- and n-Doping of Organic Semiconductors with Complex Oxidants or Reductants, J. Mater. Chem. A, 2023, 11(15), 8192–8201, 10.1039/D3TA00231D.
- C. E. Tait, A. Reckwitz, M. Arvind, D. Neher, R. Bittl and J. Behrends, Spin–Spin Interactions and Spin Delocalisation in a Doped Organic Semiconductor Probed by EPR Spectroscopy, Phys. Chem. Chem. Phys., 2021, 23(25), 13827–13841, 10.1039/D1CP02133H.
- D. M. Stoltzfus, J. E. Donaghey, A. Armin, P. E. Shaw, P. L. Burn and P. Meredith, Charge Generation Pathways in Organic Solar Cells: Assessing the Contribution from the Electron Acceptor, Chem. Rev., 2016, 116(21), 12920–12955, DOI:10.1021/acs.chemrev.6b00126.
- L. Guo, K. Liu, X. Tan, X. Wang, J. Huang, Z. Wei and G. Chen, B ← N Coordination Enables Efficient P-Doping in a Pyrazine-Based Polymer Donor Toward Enhanced Photovoltaic Performance, Macromolecules, 2021, 54(23), 10758–10766, DOI:10.1021/acs.macromol.1c01793.
- A. Markina, K.-H. Lin, W. Liu, C. Poelking, Y. Firdaus, D. R. Villalva, J. I. Khan, S. H. K. Paleti, G. T. Harrison, J. Gorenflot, W. Zhang, S. De Wolf, I. McCulloch, T. D. Anthopoulos, D. Baran, F. Laquai and D. Andrienko, Chemical Design Rules for Non-Fullerene Acceptors in Organic Solar Cells, Adv. Energy Mater., 2021, 11(44), 2102363, DOI:10.1002/aenm.202102363.
- Z. Li, K. Jiang, G. Yang, J. Y. L. Lai, T. Ma, J. Zhao, W. Ma and H. Yan, Donor Polymer Design Enables Efficient Non-Fullerene Organic Solar Cells, Nat. Commun., 2016, 7(1), 13094, DOI:10.1038/ncomms13094.
- Y. Li, Z. Zhang, X. Han, T. Li and Y. Lin, Fine-Tuning Contact via Complexation for High-Performance Organic Solar Cells, CCS Chem., 2022, 4(3), 1087–1097, DOI:10.31635/ccschem.021.202100832.
- Y. Zhang, W. Wang, F. Zhang, K. Dai, C. Li, Y. Fan, G. Chen and Q. Zheng, Soft Organic Thermoelectric Materials: Principles, Current State of the Art and Applications, Small, 2022, 18(12), 2104922, DOI:10.1002/smll.202104922.
- M. Mukherjee, A. Srivastava and A. K. Singh, Recent Advances in Designing Thermoelectric Materials, J. Mater. Chem. C, 2022, 10(35), 12524–12555, 10.1039/D2TC02448A.
- H. Wang and C. Yu, Organic Thermoelectrics: Materials Preparation, Performance Optimization, and Device Integration, Joule, 2019, 3(1), 53–80, DOI:10.1016/j.joule.2018.10.012.
- O. Bubnova, Z. U. Khan, A. Malti, S. Braun, M. Fahlman, M. Berggren and X. Crispin, Optimization of the Thermoelectric Figure of Merit in the Conducting Polymer Poly(3,4-Ethylenedioxythiophene), Nat. Mater., 2011, 10(6), 429–433, DOI:10.1038/nmat3012.
- T. Park, C. Park, B. Kim, H. Shin and E. Kim, Flexible PEDOT Electrodes with Large Thermoelectric Power Factors to Generate Electricity by the Touch of Fingertips, Energy Environ. Sci., 2013, 6(3), 788, 10.1039/c3ee23729j.
- G.-H. Kim, L. Shao, K. Zhang and K. P. Pipe, Engineered Doping of Organic Semiconductors for Enhanced Thermoelectric Efficiency, Nat. Mater., 2013, 12(8), 719–723, DOI:10.1038/nmat3635.
- W. Lee, H. Kim and Y. Kim, Contact/Noncontact-Mode Thermoelectric Characteristics of Polytriarylamine/Lewis Acid Complex Films in Horizontal Device Geometry, Adv. Energy Sustainable Res., 2023, 4(9), 2300009, DOI:10.1002/aesr.202300009.
- Y. J. Jeong, J. Jung, E. H. Suh, D. Yun, J. G. Oh and J. Jang, Self-Healable and Stretchable Organic Thermoelectric Materials: Electrically Percolated Polymer Nanowires Embedded in Thermoplastic Elastomer Matrix, Adv. Funct. Mater., 2020, 30(9), 1905809, DOI:10.1002/adfm.201905809.
- P. Lin and F. Yan, Organic Thin-Film Transistors for Chemical and Biological Sensing, Adv. Mater., 2012, 24(1), 34–51, DOI:10.1002/adma.201103334.
- J. Panidi, A. F. Paterson, D. Khim, Z. Fei, Y. Han, L. Tsetseris, G. Vourlias, P. A. Patsalas, M. Heeney and T. D. Anthopoulos, Remarkable Enhancement of the Hole Mobility in Several Organic Small-Molecules, Polymers, and Small-Molecule:Polymer Blend Transistors by Simple Admixing of the Lewis Acid p-Dopant B(C6F5)3, Adv. Sci., 2018, 5(1), 1700290, DOI:10.1002/advs.201700290.
- W. Huang, K. Besar, R. LeCover, A. M. Rule, P. N. Breysse and H. E. Katz, Correction to Highly Sensitive NH 3 Detection Based on Organic Field-Effect Transistors with Tris(Pentafluorophenyl)Borane as Receptor, J. Am. Chem. Soc., 2012, 134(43), 18149, DOI:10.1021/ja309862d.
- H. Ren, J. Chen, Y. Li and J. Tang, Recent Progress in Organic Photodetectors and Their Applications, Adv. Sci., 2021, 8(1), 2002418, DOI:10.1002/advs.202002418.
- A. E. Mansour, D. Lungwitz, T. Schultz, M. Arvind, A. M. Valencia, C. Cocchi, A. Opitz, D. Neher and N. Koch, The Optical Signatures of Molecular-Doping Induced Polarons in Poly(3-Hexylthiophene-2,5-Diyl): Individual Polymer Chains versus Aggregates, J. Mater. Chem. C, 2020, 8(8), 2870–2879, 10.1039/C9TC06509A.
- A. R. Jupp, Evidence for the Encounter Complex in Frustrated Lewis Pair Chemistry, Dalton Trans., 2022, 51(28), 10681–10689, 10.1039/D2DT00655C.
- T. Ikeda, K. Tahara, R. Ishimatsu, T. Ono, L. Cui, M. Maeda, Y. Ozawa and M. Abe, Lewis Pairing-Induced Electrochemiluminescence Enhancement from Electron Donor-Acceptor Diads Decorated with Tris(Pentafluorophenyl)Borane as an Electrochemical Protector, Angew. Chem., Int. Ed., 2023, 63(21), e202301109, DOI:10.1002/anie.202301109.
- T. C. Hidalgo Castillo, M. Moser, C. Cendra, P. D. Nayak, A. Salleo, I. McCulloch and S. Inal, Simultaneous Performance and Stability Improvement of a P-Type Organic Electrochemical Transistor through Additives, Chem. Mater., 2022, 34(15), 6723–6733, DOI:10.1021/acs.chemmater.2c00632.
- X. An, C. Wei, L. Bai, J. Zhou, L. Wang, Y. Han, L. Sun, J. Lin, H. Liu, J. Li, M. Xu, H. Ling, L. Xie and W. Huang, Photoexcitation Dynamics and Energy Engineering in Supramolecular Doping of Organic Conjugated Molecules, Light: Sci. Appl., 2023, 12(1), 30, DOI:10.1038/s41377-022-01062-6.
- P. A. Albrecht, S. M. Rupf, M. Sellin, J. Schlögl, S. Riedel and M. Malischewski, Increasing the Oxidation Power of TCNQ by Coordination of B(C6F5)3, Chem. Commun., 2022, 58(32), 4958–4961, 10.1039/D2CC00314G.
- A. E. Mansour, R. Warren, D. Lungwitz, M. Forster, U. Scherf, A. Opitz, M. Malischewski and N. Koch, Coordination of Tetracyanoquinodimethane-Derivatives with Tris(Pentafluorophenyl)Borane Provides Stronger p-Dopants with Enhanced Stability, ACS Appl. Mater. Interfaces, 2023, 15(39), 46148–46156, DOI:10.1021/acsami.3c10373.
- O. Zapata-Arteaga, A. Perevedentsev, M. Prete, S. Busato, P. S. Floris, J. Asatryan, R. Rurali, J. Martín and M. Campoy-Quiles, A Universal, Highly Stable Dopant System for Organic Semiconductors Based on Lewis-Paired Dopant Complexes, ACS Energy Lett., 2024, 9(7), 3567–3577, DOI:10.1021/acsenergylett.4c01278.
- L. O. Müller, D. Himmel, J. Stauffer, G. Steinfeld, J. Slattery, G. Santiso-Quiñones, V. Brecht and I. Krossing, Simple Access to the Non-Oxidizing Lewis Superacid PhF → Al(ORF)3(RF = C(CF3)3), Angew. Chem., Int. Ed., 2008, 47(40), 7659–7663, DOI:10.1002/anie.200800783.
- L. Greb, Lewis Superacids: Classifications, Candidates, and Applications, Chem. – Eur. J., 2018, 24(68), 17881–17896, DOI:10.1002/chem.201802698.
- L. Zapf, M. Riethmann, S. A. Föhrenbacher, M. Finze and U. Radius, An Easy-to-Perform Evaluation of Steric Properties of Lewis Acids, Chem. Sci., 2023, 14(9), 2275–2288, 10.1039/D3SC00037K.
- G. J. P. Britovsek, J. Ugolotti and A. J. P. White, From B(C6F5)3 to B(OC6F5)3: Synthesis of (C6F5)2BOC6F5 and C6F5B(OC6F5)2 and Their Relative Lewis Acidity, Organometallics, 2005, 24(7), 1685–1691, DOI:10.1021/om049091p.
- L. Xiang, J. Wang, A. Matler and Q. Ye, Structure-Constraint Induced Increase in Lewis Acidity of Tris(Ortho-Carboranyl)Borane and Selective Complexation with Bestmann Ylides, Chem. Sci., 2024, 15(43), 17944–17949, 10.1039/D4SC06144F.
- S. Mummadi and C. Krempner, Triphenylborane in Metal-Free Catalysis, Molecules, 2023, 28(3), 1340, DOI:10.3390/molecules28031340.
- L. Greb, C.-G. Daniliuc, K. Bergander and J. Paradies, Functional-Group Tolerance in Frustrated Lewis Pairs: Hydrogenation of Nitroolefins and Acrylates, Angew. Chem., Int. Ed., 2013, 52(22), 5876–5879, DOI:10.1002/anie.201210175.
- M. N. Bhagat, G.-F. Chang, C. K. Bennett, A. Raghuraman, M. E. Belowich, L. J. Broadbelt, S. T. Nguyen and J. M. Notestein, Improving and Stabilizing Fluorinated Aryl Borane Catalysts for Epoxide Ring-Opening, Appl. Catal., A, 2022, 636, 118601, DOI:10.1016/j.apcata.2022.118601.
- K. M. Marczenko, S. Jee and S. S. Chitnis, High Lewis Acidity at Planar, Trivalent, and Neutral Bismuth Centers, Organometallics, 2020, 39, 4287–4296, DOI:10.1021/acs.organomet.0c00378.
- F. Holtrop, C. Helling, M. Lutz, N. P. van Leest, B. de Bruin and J. C. Slootweg, Enhancement of London Dispersion in Frustrated Lewis Pairs: Towards a Crystalline Encounter Complex, Syn. Lett., 2023, 34, 1122–1128, DOI:10.1055/a-1928-4902 Art ID: ST-2022-07-0325-C.
- M. O. Akram, J. R. Tidwell, J. L. Dutton and C. D. Martin, Tris(Ortho-carboranyl)Borane: An Isolable, Halogen-Free, Lewis Superacid, Angew. Chem., Int. Ed., 2022, 61(46), e202212073, DOI:10.1002/anie.202212073.
- A. E. Ashley, T. J. Herrington, G. G. Wildgoose, H. Zaher, A. L. Thompson, N. H. Rees and T. Kr, Separating Electrophilicity and Lewis Acidity: The Synthesis, Characterization, and Electrochemistry of the Electron Deficient Tris(Aryl)Boranes B(C6F5)3−n(C6Cl5)n (n = 1–3), J. Am. Chem. Soc., 2011, 133, 14727–14740, DOI:10.1021/ja205037t.
- H. Zhao, J. H. Reibenspies and F. P. Gabbaï, Lewis Acidic Behavior of B(C6Cl5)3, Dalton Trans., 2013, 42(3), 608–610, 10.1039/C2DT31482G.
- M. Sultana, A. Paul and L. Roy, Computational Investigation of the Mechanism of FLP Catalyzed H2 Activation and Lewis Base Assisted Proton Transfer, ChemistrySelect, 2020, 5, 13397–13406, DOI:10.1002/slct.202003794.
- T. J. Herrington, A. J. W. Thom, A. J. P. White and A. E. Ashley, Novel H2 Activation by a Tris[3,5-Bis(Trifluoromethyl)Phenyl]Borane Frustrated Lewis Pair, Dalton Trans., 2012, 41(30), 9019, 10.1039/c2dt30384a.
- L. A. Körte, J. Schwabedissen, M. Soffner, S. Blomeyer, C. G. Reuter, Y. V. Vishnevskiy, B. Neumann, H. Stammler and N. W. Mitzel, Tris(Perfluorotolyl)Borane—A Boron Lewis Superacid, Angew. Chem., Int. Ed., 2017, 56(29), 8578–8582, DOI:10.1002/anie.201704097.
- M. Galdeano, F. Ruipérez and J. M. Matxain, Theoretical Characterization of New Frustrated Lewis Pairs for Responsive Materials, Polymers, 2021, 13(10), 1573, DOI:10.3390/polym13101573.
- S. Yruegas, J. J. Martinez and C. D. Martin, Intermolecular Insertion Reactions of Azides into 9-Borafluorenes to Generate 9,10-B,N-Phenanthrenes, Chem. Commun., 2018, 54(50), 6808–6811, 10.1039/c8cc01529e.
- H. Böhrer, N. Trapp, D. Himmel, M. Schleep and I. Krossing, From Unsuccessful H2-Activation with FLPs Containing B(Ohfip)3 to a Systematic Evaluation of the Lewis Acidity of 33 Lewis Acids Based on FLuoride, Chloride, Hydride and Methyl Ion Affinities, Dalton Trans., 2015, 44, 7489–7499, 10.1039/c4dt02822h.
- M. M. Alharbi, Y. Ingen, A. van; Roldan, T. Kaehler and R. L. Melen, Synthesis and Lewis Acidity of Fluorinated Triaryl Borates, Dalton Trans., 2023, 52(6), 1820–1825, 10.1039/D2DT04095F.
- L. Falivene, Z. Cao, A. Petta, L. Serra, A. Poater, R. Oliva, V. Scarano and L. Cavallo, Towards the Online Computer-Aided Design of Catalytic Pockets, Nat. Chem., 2019, 11(10), 872–879, DOI:10.1038/s41557-019-0319-5.
- M. O. Akram, C. D. Martin and J. L. Dutton, The Effect of Carborane Substituents on the Lewis Acidity of Boranes, Inorg. Chem., 2023, 62(33), 13495–13504, DOI:10.1021/acs.inorgchem.3c01872.
- J. O. Huh, H. Kim, K. M. Lee, Y. S. Lee, Y. Do and M. H. Lee, O-Carborane-Assisted Lewis Acidity Enhancement of Triarylboranes, Chem. Commun., 2010, 46(7), 1138–1140, 10.1039/B918263B.
- K. M. Lee, J. O. Huh, T. Kim, Y. Do and M. H. Lee, A Highly Lewis Acidic Triarylborane Bearing Peripheral O-Carborane Cages, Dalton Trans., 2011, 40(44), 11758, 10.1039/c1dt11064k.
- B. H. Choi, J. H. Lee, H. Hwang, K. M. Lee and M. H. Park, Novel Dimeric o-Carboranyl Triarylborane: Intriguing Ratiometric Color-Tunable Sensor via Aggregation-Induced Emission by Fluoride Anions, Organometallics, 2016, 35(11), 1771–1777, DOI:10.1021/acs.organomet.6b00172.
- J. Kahlert, L. Böhling, A. Brockhinke, H.-G. Stammler, B. Neumann, L. M. Rendina, P. J. Low, L. Weber and M. A. Fox, Syntheses and Reductions of C-Dimesitylboryl-1,2-Dicarba-Closo-Dodecaboranes, Dalton Trans., 2015, 44, 9766–9781, 10.1039/C5DT00758E.
- H. Wang, J. Zhang and Z. Xie, Reversible Photothermal Isomerization of Carborane-Fused Azaborole to Borirane: Synthesis and Reactivity of Carbene-Stabilized Carborane-Fused Borirane, Angew. Chem., Int. Ed., 2017, 56(31), 9198–9201, DOI:10.1002/anie.201704642.
- H. Zhang, J. Wang, W. Yang, L. Xiang, W. Sun, W. Ming, Y. Li, Z. Lin and Q. Ye, Solution-Phase Synthesis of a Base-Free Benzoborirene and a Three-Dimensional Inorganic Analogue, J. Am. Chem. Soc., 2020, 142(41), 17243–17249, DOI:10.1021/jacs.0c06538.
- Y. Wei, J. Wang, W. Yang, Z. Lin and Q. Ye, Boosting Ring Strain and Lewis Acidity of Borirane: Synthesis, Reactivity and Density Functional Theory Studies of an Uncoordinated Arylborirane Fused to o-Carborane, Chem. – Eur. J., 2022, 29(5), e202203265, DOI:10.1002/chem.202203265.
- J. Wang, P. Jia, W. Sun, Y. Wei, Z. Lin and Q. Ye, Synthesis of Iminoboryl O-Carboranes by Lewis Base Promoted Aminoborirane-to-Iminoborane Isomerization, Inorg. Chem., 2022, 61(23), 8879–8886, DOI:10.1021/acs.inorgchem.2c00944.
- J. Krebs, A. Häfner, S. Fuchs, X. Guo, F. Rauch, A. Eichhorn, I. Krummenacher, A. Friedrich, L. Ji, M. Finze, Z. Lin, H. Braunschweig and T. B. Marder, Backbone-Controlled LUMO Energy Induces Intramolecular C–H Activation in Ortho-Bis-9-Borafluorene-Substituted Phenyl and o-Carboranyl Compounds Leading to Novel 9,10-Diboraanthracene Derivatives, Chem. Sci., 2022, 13(47), 14165–14178, 10.1039/D2SC06057D.
- T. Bischof, X. Guo, I. Krummenacher, L. Beßler, Z. Lin, M. Finze and H. Braunschweig, Alkene Insertion Reactivity of a O-Carboranyl-Substituted 9-Borafluorene, Chem. Sci., 2022, 13(25), 7492–7497, 10.1039/D2SC02750J.
- A. Benton, J. D. Watson, S. M. Mansell, G. M. Rosair and A. J. Welch, The Lewis Acidity of Borylcarboranes, J. Organomet. Chem., 2020, 907, 121057, DOI:10.1016/j.jorganchem.2019.121057.
- L. Xiang, J. Wang, A. Matler and Q. Ye, Structure-Constraint Induced Increase in Lewis Acidity of Tris(Ortho-Carboranyl)Borane and Selective Complexation with Bestmann Ylides, Chem. Sci., 2024, 15(43), 17944–17949, 10.1039/D4SC06144F.
- S. Yruegas, J. C. Axtell, K. O. Kirlikovali, A. M. Spokoyny and C. D. Martin, Synthesis of 9-Borafluorene Analogues Featuring a Three-Dimensional 1,10-Bis(o-Carborane) Backbone, Chem. Commun., 2019, 55(20), 2892–2895, 10.1039/C8CC10087J.
- C. Zhang, J. Wang, W. Su, Z. Lin and Q. Ye, Synthesis, Characterization, and Density Functional Theory Studies of Three-Dimensional Inorganic Analogues of 9,10-Diboraanthracene—A New Class of Lewis Superacids, J. Am. Chem. Soc., 2021, 143(23), 8552–8558, DOI:10.1021/jacs.1c03057.
- J. Krebs, M. Haehnel, I. Krummenacher, A. Friedrich, H. Braunschweig, M. Finze, L. Ji and T. B. Marder, Synthesis and Structure of an o-Carboranyl-Substituted Three-Coordinate Borane Radical Anion, Chem. – Eur. J., 2021, 27(31), 8159–8167, DOI:10.1002/chem.202100938.
- C. Zhang, X. Liu, J. Wang and Q. Ye, A Three-Dimensional Inorganic Analogue of 9,10-Diazido-9,10-Diboraanthracene: A Lewis Superacidic Azido Borane with Reactivity and Stability, Angew. Chem., Int. Ed., 2022, 61(36), e202205506, DOI:10.1002/anie.202205506.
- C. Zhang, J. Wang, Z. Lin and Q. Ye, Synthesis, Characterization, and Properties of Three-Dimensional Analogues of 9-Borafluorenes, Inorg. Chem., 2022, 61(45), 18275–18284, DOI:10.1021/acs.inorgchem.2c03111.
- M. O. Akram, J. R. Tidwell, J. L. Dutton and C. D. Martin, Bis(1-Methyl-ortho-Carboranyl)Borane, Angew. Chem., Int. Ed., 2023, 62(34), e202307040, DOI:10.1002/anie.202307040.
- M. Diab, K. Jaiswal, D. Bawari and R. Dobrovetsky, The Chemistry of [1,1′-bis(o-Carboranyl)]Borane η2-σ-Silane Adduct, Isr. J. Chem., 2023, 63(7–8), e202300010, DOI:10.1002/ijch.202300010.
- L. Xiang, J. Wang, I. Krummenacher, K. Radacki, H. Braunschweig, Z. Lin and Q. Ye, Persistent and Predominantly Localized Boron Radical from the Reduction of a Three-Dimensional Analogue of NHC-Stabilized Borafluorenium, Chem. – Eur. J., 2023, 29(42), e202301270, DOI:10.1002/chem.202301270.
- K. Vashisth, S. Dutta, M. O. Akram and C. D. Martin, Examining the Reactivity of Tris(Ortho-Carboranyl)Borane with Lewis Bases and Application in Frustrated Lewis Pair Si–H Bond Cleavage, Dalton Trans., 2023, 52(28), 9639–9645, 10.1039/D3DT01557B.
- J. Wang, L. Xiang, X. Liu, A. Matler, Z. Lin and Q. Ye, Avenue to Novel O-Carboranyl Boron Compounds – Reactivity Study of o-Carborane-Fused Aminoborirane towards Organic Azides, Chem. Sci., 2024, 15(13), 4839–4845, 10.1039/D4SC00489B.
- J. Wang and Q. Ye, Borirenes and Boriranes: Development and Perspectives, Chem. – Eur. J., 2024, 30(11), e202303695, DOI:10.1002/chem.202303695.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2025 |