 Open Access Article
Open Access ArticleAdvancing electrochemical N2 reduction: interfacial electrolyte effects and operando computational approaches
Lin
Jiang
 a,
Xiaowan
Bai
a,
Xiaowan
Bai
 a,
Xing
Zhi
b,
Kenneth
Davey
a,
Xing
Zhi
b,
Kenneth
Davey
 a and
Yan
Jiao
a and
Yan
Jiao
 *a
*a
aSchool of Chemical Engineering, The University of Adelaide, South Australia 5005, Australia. E-mail: yan.jiao@adelaide.edu.au
bSchool of Chemical Engineering and Light Industry, Guangdong University of Technology, Guangzhou, Guangdong 510006, P. R. China
First published on 19th November 2024
Abstract
The electrochemical N2 reduction reaction (eNRR) is a promising pathway for clean and sustainable production of ammonia, a compound essential for global industry. The challenges of the eNRR lie in the complexity of the electrode–electrolyte interface (EEI). While advances have been made in tuning the electrolyte compositions, the understanding of underlying atomic-level mechanisms remains limited. Operando computational techniques are emerging as instrumental tools to address relevant issues. In this review, we highlight a path forward by summarizing the recent advances in engineering strategies for direct-eNRR, including cations, organic solvents, ionic liquids; and for indirect-NRR with the incorporation of lithium-mediators. Additionally, we summarized relevant computational techniques that can investigate the interfacial dynamic properties associated with electrolyte modifications within N2 reduction. By promoting the application of these computational methodologies, this review contributes to the ongoing efforts towards the realization of highly efficient electrochemical N2 reduction.
Broader contextThis review highlights the critical role of electrolyte engineering in enhancing the efficiency of the electrocatalytic nitrogen reduction reaction (eNRR) for green ammonia (NH3) synthesis from N2. The electrolyte can significantly influence the reaction by affecting how reactants interact with the catalyst, stabilizing key intermediates, and optimizing the overall reaction environment. The review explores various electrolyte engineering strategies, including the use of organic solvents, ionic liquids, and modifications of alkali cations, which have shown promise in improving NH3 synthesis efficiency. However, despite experimental advancements, finding the ideal electrolyte composition remains a major challenge. Computational techniques, including density functional theory (DFT) and classical force field-based molecular dynamics (MD), microkinetic modelling (MKM), and machine learning (ML), are essential for overcoming challenges in electrolyte optimization for eNRR. These methods provide atomic-level insights into electrolyte behaviour and allow for fine-tuning compositions to enhance catalytic performance. By combining these computation tools, researchers can fine-tune electrolyte compositions and accelerate the discovery of more efficient systems. Computational techniques are vital for providing more insights and overcoming current challenges in electrolyte engineering for eNRR, therefore advancing sustainable ammonia synthesis at an industrial scale. |
1. Introduction
Ammonia (NH3) is a carbon-free energy carrier and is essential in agriculture and food industries. More than 95% of NH3 production relies on the traditional Haber–Bosch process that is energy intensive with significant greenhouse gas (i.e. CO2) emission because of intensive processing conditions such as temperature of 400–600 °C and pressure of 20–40 MPa.1 In contrast, electro-driven chemical reactions offer a promising alternative for sustainable, carbon-free green NH3 synthesis (gNH3s), leveraging the growing availability of renewable energy sources.Among the other nitrogen fixation pathways such as nitrate and nitrite reduction, electrochemical N2 reduction reaction (eNRR) offers distinct advantages.2–5 Firstly, eNRR relies on atmospheric N2, an abundant and readily available feedstock, whereas nitrate and nitrite sources are limited and would require additional sourcing. Additionally, eNRR offers a more streamlined reaction process, converting N2 directly to NH3 under mild conditions without requiring complex intermediates, whereas nitrate and nitrite reduction involve multistep reactions that challenge selectivity and control. eNRR can also be powered by renewable energy sources, lowering the environmental footprint and making it more sustainable compared to the environmental risks of using nitrate or nitrite as primary feedstocks. However, further development is necessary to achieve cost and efficiency competitiveness with the traditional Haber–Bosch process. Currently, there still persist significant hurdles in achieving efficient NH3 production.6
Importantly, electrochemical N2 reduction performance depends significantly on properties and characteristics of the electrode–electrolyte interface (EEI), which can be tuned via either, engineering of the electrode, or the electrolyte. A number of methods are reported to improve eNRR performance via regulating electrocatalytic material surfaces including, metal doping,7–9 alloying,10,11 defect engineering,12–14 and nanoscale structuring.15–22 Particularly, in recent years, transition-metal complexes have emerged as promising catalysts for eNRR due to their tuneable and coordination environments, which suggested to be capable of stabilizing key N2 intermediates.23–26 These studies underscore the potential of transition-metal complexes as state-of-the-art catalysts for eNRR, offering tailored active sites and ligand environments that significantly contribute to enhanced N2 reduction performance. Nevertheless, the strong competition from the hydrogen evolution reaction (HER) and the limited accessibility of interfacial N2 molecules result in sluggish kinetics for NH3 synthesis, posing challenges for industrial upscaling. Promising results for electrochemical N2 reduction have been reported by screening and tuning electrolyte compositions to create eNRR-favored interfacial microenvironments.27,28 However, a comprehensive understanding of the electrolyte's role remains limited, highlighting the need for deeper insights and guidelines to construct highly active and selective electrochemical systems. These include cations, solvent types, ionic liquids, and using mediators such as lithium. Among the use of these electrolyte contents, we provide an overview of two distinct reaction strategies for electrochemical N2 reduction, including (1) direct electrocatalytic N2 reduction reaction, direct-eNRR, where N2 is catalyzed/reduced directly on the electrode surface;29 and (2) indirect/mediated electrochemical N2 reduction using lithium, known as Li-NRR.
Computational techniques, especially density functional theory (DFT), are being widely used in material science and focus on describing physical properties i.e. electronic structure, from the computation of the ground-state energy for electrocatalyst materials and establishing the structure–activity relationship to identify the optimal catalysts for direct-eNRR.30,31 However, the dynamic mechanisms when electrolyte are considered remain unclear. Emerging computational techniques that incorporate the timescale and/or realistic electrochemical conditions (i.e. electrode potential and pH) are needed to establish understanding of the electrochemical EEI. Therefore, further design based on integration of material regulation and electrolyte engineering appears practical for realization of gNH3s.
Here we report a critical assessment of advances in electrolyte engineering beginning with an overview of both direct-eNRR and indirect Li-NRR. For direct-eNRR, we assess interfacial electrolyte compositions including the effect of, cation, solvent type, and ionic liquid on electrocatalytic N2 reduction. For Li-NRR, the fundamental changes in electrolyte composition focusing on lithium salt as anions and various proton sources are summarized. Based on these phenomena, we outline computations that could investigate electrolytes performance within eNRR including, first-principles integration for realistic conditions and kinetic modelling (mean field – microkinetic modelling and kinetic Monte Carlo simulation), ab initio modelling incorporated with explicit solvent molecules and machine learning-interatomic potential (ML-IAPs) for extended simulation time-scales. We provide a perspective on future computational contributions to this field and the likely outcomes in establishing interfacial dynamics for eNRR. We conclude that electrolyte engineering is important to advancing sustainable global gNH3s via electrochemical reduction of N2. Findings will benefit researchers and manufacturers in guiding computations for electrolyte engineering (Fig. 1).
 | ||
| Fig. 1 Scheme of electrolyte engineering for both direct electrocatalytic NRR (top panel) and lithium-mediated NRR (bottom panel), and associated operando computation techniques. | ||
2. Mechanisms for NRR
A critical step in reducing N2 to NH3 is breaking the highly stable N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond, a practical challenge evidenced by high negative electron affinity of N2 molecule of −1.8 eV and significant ionization potential of 15.06 V.32 There are two pathways for N2 reduction to activate the N
N triple bond, a practical challenge evidenced by high negative electron affinity of N2 molecule of −1.8 eV and significant ionization potential of 15.06 V.32 There are two pathways for N2 reduction to activate the N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bonds, (1) a conventional electrocatalytic process in an electrochemical interface encompassing electrode and electrolyte, defined as the direct strategy, (2) mediated process on electro-induced lithium/lithium-based decomposition, defined as the indirect strategy.
N bonds, (1) a conventional electrocatalytic process in an electrochemical interface encompassing electrode and electrolyte, defined as the direct strategy, (2) mediated process on electro-induced lithium/lithium-based decomposition, defined as the indirect strategy.
2.1 Mechanism for direct electrocatalytic N2 reduction
Direct eNRR on catalysts surface involves six proton-coupled electron transfers for every two NH3 molecules production (eqn (1)).| N2(g) + 6H+ + 6e− ↔ 2NH3(g) | (1) |
The difference between the dissociative pathway (ii) and associative pathway (iii)–(vi) is how the N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond is cleaved. In the dissociative pathway, the nitrogen molecule is initially adsorbed onto the catalyst surface, where the N
N triple bond is cleaved. In the dissociative pathway, the nitrogen molecule is initially adsorbed onto the catalyst surface, where the N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond is cleaved, resulting in two nitrogen atoms that are subsequently hydrogenated to form NH3. However, such bond cleavage requires a significant energy input due to the strength of the N
N triple bond is cleaved, resulting in two nitrogen atoms that are subsequently hydrogenated to form NH3. However, such bond cleavage requires a significant energy input due to the strength of the N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond, making the dissociative pathway challenging under ambient conditions. This dissociative mechanism, though energetically demanding, is characteristic of the Haber–Bosch process,12 which operates at high temperatures and pressures to overcome the energy barrier associated with N
N bond, making the dissociative pathway challenging under ambient conditions. This dissociative mechanism, though energetically demanding, is characteristic of the Haber–Bosch process,12 which operates at high temperatures and pressures to overcome the energy barrier associated with N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N cleavage. Catalysts such as iron (Fe), ruthenium (Ru), and molybdenum (Mo) based material are commonly used for ammonia production via Haber–Bosch process. Among these, specific catalytic sites, including Fe C7 and Ru B5, have been identified as particularly active, facilitating N2 dissociation more energetically favorably.40
N cleavage. Catalysts such as iron (Fe), ruthenium (Ru), and molybdenum (Mo) based material are commonly used for ammonia production via Haber–Bosch process. Among these, specific catalytic sites, including Fe C7 and Ru B5, have been identified as particularly active, facilitating N2 dissociation more energetically favorably.40
In contrast, the associative pathway is generally considered the most common mechanism for direct electrocatalytic N2 reduction reaction under ambient conditions, especially in aqueous electrochemical environments. In this pathway, the dissolved N2 molecule adsorbs onto the electrode surface without immediately breaking the N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond. It undergoes a series of continuous hydrogenation steps primarily from H2O molecules, forming intermediates such as N2H, N2H2, and eventually NH3. The first NH3 molecule desorbs concurrently with N–N bond cleavage, allowing for efficient ammonia production at lower energy requirements compared to the dissociative pathway. This associative mechanism can be further classified into four distinct sub-pathways based on the orientation of configurations: (iii) distal, and (iv) alternative pathways; and start from the side-on configuration; (v) consecutive pathway, and (vi) enzymatic pathway. Various electrocatalysts have shown promise in facilitating these pathways for direct-eNRR, including transition metals (TMs) and their derivatives, such as metal oxides, nitrides, and sulphides, etc.29 Additionally, metal-free catalysts like nitrogen- or boron-doped carbon materials and metal–organic frameworks (MOFs) exhibit notable reductive activity. These catalysts offer diverse electronic structures and active sites that can stabilize N2 adsorption and promote hydrogenation, thus enhancing direct-eNRR efficiency under ambient conditions.
N bond. It undergoes a series of continuous hydrogenation steps primarily from H2O molecules, forming intermediates such as N2H, N2H2, and eventually NH3. The first NH3 molecule desorbs concurrently with N–N bond cleavage, allowing for efficient ammonia production at lower energy requirements compared to the dissociative pathway. This associative mechanism can be further classified into four distinct sub-pathways based on the orientation of configurations: (iii) distal, and (iv) alternative pathways; and start from the side-on configuration; (v) consecutive pathway, and (vi) enzymatic pathway. Various electrocatalysts have shown promise in facilitating these pathways for direct-eNRR, including transition metals (TMs) and their derivatives, such as metal oxides, nitrides, and sulphides, etc.29 Additionally, metal-free catalysts like nitrogen- or boron-doped carbon materials and metal–organic frameworks (MOFs) exhibit notable reductive activity. These catalysts offer diverse electronic structures and active sites that can stabilize N2 adsorption and promote hydrogenation, thus enhancing direct-eNRR efficiency under ambient conditions.
Compared to the lithium-mediated NRR that's going to be introduced in the next session, the mechanism of direct eNRR offers unique advantages, particularly through its adaptable and streamlined design. Operating without complex intermediates, direct eNRR allows for simpler reaction pathways that facilitate efficient nitrogen reduction at lower energy costs. Its flexibility in electrolyte composition enables targeted tuning to optimize performance for nitrogen reduction. With these benefits, direct eNRR provides a straightforward, efficient solution well-suited to decentralized and on-site ammonia production, offering a sustainable approach that complements renewable energy applications for nitrogen fixation.
2.2 Mechanism for Li-NRR
Li-NRR (lithium mediated nitrogen reduction reaction) is established via introducing lithium salt to the non-aqueous electrolyte where lithium ions are mediators for N2 reduction under highly reducing potential (−3.04 V versus standard hydrogen electrode, SHE).41 This method is extensively reported and initially by Fichtner et al.42 in the 1930s, and later in the 1990s by Tsuneto et al.43,44 A fundamental component in Li-NRR electrolyte is lithium salt, proton sources (HA), and solvent (typically, tetrahydrofuran-THF). The reaction mechanism for Li-NRR involves the steps shown in eqn (2)–(4).45–47 The steps involve (1) electro-deposition of Li+ from a Li-salt in solution, (2) dissociation of N2 on the deposited metallic Li, and (3) protonation of activated nitrogen molecule(s) from a proton carrier (HA), which is capable of undergoing a deprotonation–reprotonation cycle.| Li+ + e− ↔ Li (s) | (2) |
| N2 ↔ 2*N | (3) |
| *N + 3AH ↔ 3A− + NH3 | (4) |
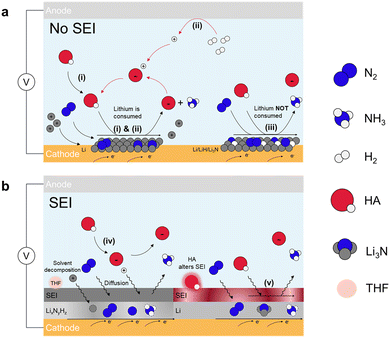 | ||
| Fig. 3 Indirect Li-NRR. Schematic for lithium-mediated NRR with (a) no SEI formed and (b) SEI formed. Five potential mechanisms are summarized.48 These include lithium cycling with (i) proton source from the electrolyte50 and (ii) proton shuttle model in which proton is from H2 oxidation.49 (iii) Electro-driven Li-NRR where lithium only provides the electron.46 SEI diffusion model for (iv) non-reactive SEI,47 and (v) adjusted permeable SEI layer through HA.51 | ||
Pathways (i) and (ii) exhibit the same mechanism of N2 splitting where N2 molecules undergo spontaneous dissociation and chemically react with the electrodeposited Li to form Li3N because of the strong electron donating ability of Li metal. The key differences between these two pathways lie in the proton source and the sustainability of the processes. In pathway (i), the proton source is ethanol. After ethanol donates a proton to the activated nitrogen (Li3N), the deprotonated ethanol remains, leading to its consumption and making the process less sustainable.46,50 In pathway (ii), the proton comes from H2 inserted at the anode side that undergoes the electrocatalytic oxidation process (HOR), offering a sustainable approach towards NH3 production. These two pathways are reported to follow CC model (chemical nitrogen splitting and chemical protonation) as they can proceed even if current is off.52 In pathway (iii), a key difference from the previous pathways is that lithium is electrodeposited but not returned to the solution, leading to excessive lithium buildup. This causes competing reactions, such as hydrogen evolution reaction (HER), which undermine the overall efficiency of the reaction. The pathway also involves the formation of multiple lithium-based components due to the high reactivity of metallic lithium, including Li, Li3N and LiH. About the reaction mechanism, on the layer of lithium-contained species, N2 is electrocatalytically dissociated and receives protons from the electrolyte to form NH3. DFT studies show that intermediates exhibit the capability of producing NH3 in the order of Li > Li3N > LiH.46 These three pathways operate without a solid electrolyte interphase (SEI), making the selectivity and activity for NH3 formation largely dependent on lithium electrodeposition, as shown in Fig. 3a.53–56
Beyond the pathways (i)–(iii), two other pathways for Li-NRR exist that involves the formation of solid electrolyte interphase (SEI). The SEI is a passivation layer that forms on the electrode surface, much like in traditional Li-ion batteries, where it develops from the decomposition of non-aqueous electrolytes under highly reductive potentials. This layer serves to protect the negative electrode by preventing further decomposition of the electrolyte. In the context of Li-NRR, the SEI plays a similar role, determining the stability and efficiency of nitrogen reduction, particularly in pathways (iv) and (v) as depicted in Fig. 3b. Once the SEI is formed, it facilitates the diffusion of reactants (N2, H+, and Li+) and products (NH3) through it during the reduction process.57 The major difference between pathway (v) and (iv) is that in pathway (v), the proton sources participate in SEI formation and can alter its permeability, depending on the main products formed from the interaction between the proton sources and active lithium.51 The rate at which these species diffuse through the SEI directly influences the overall performance of the reaction. The permeability of the SEI, influenced by the proton sources involved in its formation, is key to its effectiveness. Therefore, understanding how the SEI forms and functions, as well as modifying its physical and chemical properties, is crucial for enhancing the stability, efficiency, and long-term performance of the Li-NRR process.
The Li-NRR mechanism utilizes lithium's high reactivity to address the inherent difficulty of breaking the strong N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond, which allows nitrogen reduction to proceed efficiently, largely under ambient temperatures and pressures.53 Furthermore, Li-NRR benefits from excellent selectivity, with minimal competing side reactions, such as hydrogen evolution, which can otherwise reduce efficiency. This high selectivity contributes to improved faradaic efficiency, as more input energy is directed specifically toward ammonia formation, decreasing the need for post-reaction product separation and purification. Together, these attributes make Li-NRR a highly promising method for ammonia synthesis, offering a novel approach to activate the exceptionally stable N2 molecule and providing a viable alternative to traditional nitrogen fixation processes that are often energy-intensive and resource-demanding.
N bond, which allows nitrogen reduction to proceed efficiently, largely under ambient temperatures and pressures.53 Furthermore, Li-NRR benefits from excellent selectivity, with minimal competing side reactions, such as hydrogen evolution, which can otherwise reduce efficiency. This high selectivity contributes to improved faradaic efficiency, as more input energy is directed specifically toward ammonia formation, decreasing the need for post-reaction product separation and purification. Together, these attributes make Li-NRR a highly promising method for ammonia synthesis, offering a novel approach to activate the exceptionally stable N2 molecule and providing a viable alternative to traditional nitrogen fixation processes that are often energy-intensive and resource-demanding.
2.3 Competing hydrogen evolution reaction (HER)
HER occurs on the cathode with only two proton-coupled electron transfer, as shown in eqn (5)–(7). It is a dominating competing reaction in direct eNRR. HER has an equilibrium potential close to NH3 production, therefore leads to sluggish kinetics for eNRR and corresponding low current efficiency and yield for NH3. HER exists in both direct eNRR and (Section 2.1) and Li-NRR pathway (iii), because of the high reactivity of electrodeposited-lithium metal, providing facile electron transfer to the surface proton and forming H2.| Volmer: H+ + e− + * ↔ *H | (5) |
| Heyrovsky: H+ + e− + *H ↔ H2(g) | (6) |
| Tafel: *H + *H ↔ H2(g) | (7) |
3. Electrolyte engineering for direct electrocatalytic NRR
The general mechanism for direct-eNRR for NH3 synthesis requires a sequence of proton and electron transfers. At the EEI, while electrocatalysts facilitates electron transfer to N2, the electrolyte component plays multiple roles, including transporting reactants (N2) and products (NH3) and influencing the transfer rate of proton sources i.e. H2O. Electrolyte engineering is especially effective in addressing the challenge that the equilibrium potentials of eNRR and HER are close to each other, making it crucial to regulate the electrolyte for selective nitrogen reduction while minimizing competing hydrogen evolution. The reported approaches of electrolyte engineering for direct eNRR include cations, solvents including organic solvents and ionic liquids. Each of these methods plays a distinct role in influencing the overall efficiency and selectivity of eNRR, as presented in the following sections. Overall, a summary of the direct electrocatalytic NRR process of different metal active sites with employing various electrolytes is tabulated in Table 1.| Electrode | Electrolyte type | Electrolyte | NH3 yielda (nmol s−1) | FE (%) |
|---|---|---|---|---|
| a Units for ammonia yield rate converted to nmol s−1 with respect to the catalytic area (cm−2) or mass (mg−1). | ||||
| N-doped carbon nanospikes18 | SIW | 0.25 M LiClO4 (NaClO4/KClO4) | 1.59 ± 0.12 cm−2 | 11.56 ± 0.85 |
| Ag triangular nanoplates58 | SIW | 0.1 M KOH (LiOH/NaOH) | 0.954 mg−1 | 25 |
| Cu59 | SIW | 0.32 M KOH (LiOH/NaOH/RbOH/CsOH) | 0.86 cm−2 | 18 ± 3 |
| Cu56 | WIS | 5 M LiClO4 | 19.7 ± 3.9 × 10−2 cmGSA−2 | 12 |
| Rh1/MnO260 | WIS | 9 M K2SO4 | 4.43 mg−1 | 73.3 |
| TiO2/Au61 | WIS | 20 M LiTFSI | 0.33 cm−2 | 37.2 |
| Carbon62 | WIS | 10 M LiCl | (9.5 ± 0.4) × 10−1 cm−2 | 71 ± 1.9 |
| WSe2−x63 | WIS | 12 M LiClO4 | 2.96 mg−1 | 62.5 |
| Ni64 | Organic solvent | 0.1 M LiCl/EDA + 0.05 M H2SO4 | 3.58 × 10−2 cm−2 | 17.2 |
| Ni65 | Organic solvent | 2-Propanol/H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
1.54 × 10−1 cm−2 | 0.89 |
| FeOOH/CNTs66 | Organic solvent | Methanol/H2O (0.16 vol%) | 4.28 ± 0.12 mg−1 | 75.9 ± 4.1 |
| OV-rich α-Fe2O367 | Ionic liquid | n-Octylammonium formate | 0.52 mg−1 | 6.63 |
| Nano-Fe68 | Ionic liquid | [P6,6,6,14][F6P] | 0.1690 cm−2 | 20 |
| α-Fe@Fe3O469 | Ionic liquid | [C4mpyr][eFAP] + 1H,1H,5H-octafluoropentyl-1,1,2,2-tetrafluoro- ethyl ether (FPEE) | 3.71 × 10−4 cm−2 | 32 |
| Fe70 | Ionic liquid | [P6,6,6,14][eFAP] | 4.730 × 10−3 cm−2 | 60 |
3.1 Cation effect
Cations on a charged electrode surface accumulate and form the electrochemical double layer (EDL) that is essential for electrocatalytic reaction kinetics. These cations affect the interfacial potential, electric field, pH, and geometric microenvironment, leading to significant chemical interactions with adsorbed intermediates, affecting overall electrocatalytic performance.71,72 In particular, the presence of cations reduces proton donor ability due to solvation and steric effects, which slows down HER kinetics while increasing the selectivity for NH3 formation. For direct eNRR, the concentration of cations introduced to the electrolyte plays an important role in affecting the dynamics of the EEI. Depending on the salt concentration, salt-based aqueous electrolytes can be categorized into two types: (1) low-concentrated salt (salt-in-water, SIW) electrolyte and (2) high-concentrated salt (water-in-salt, WIS, salt concentration exceeds 5 M) electrolyte.73 | ||
| Fig. 4 Salt-in-water electrolyte. Faradaic efficiency (FE) and current density for (a) NH3, (b) H2. Reproduced with permission.59 Copyright 2020, American Chemical Society. (c) Effective current density, and (d) FE for eNRR and HER on AgTPs in electrolyte with different potassium cations concentrations. Reproduced with permission.58 copyright 2019, Royal Society of Chemistry. Electrolyte counterion effect of Li+ (grey-colour), Na+ (red), and K+ (blue) upon (e) formation rate and partial current density and (f) FE, which shows the order of Li+ > Na+ > K+. Reproduced with permission.80 Copyright 2018, American Association for the Advancement of Science. | ||
Despite these promising outcomes, there remains limited understanding of how cation affects the morphology of the EEI and eNRR dynamics. This knowledge gap highlights the need for further research to better understand how cations in SIW electrolyte could promote eNRR processes.
The reduced water activity in WIS electrolytes plays a crucial role in enhancing eNRR performance. By lowering the availability of active water molecules, these electrolytes increase the concentration of nitrogens at the electrode interface, thereby improving nitrogen delivery to the catalytic sites and boosting selectivity by suppressing competing reactions such as HER.56,58 As reported by Song et al.,18 the smaller cation induces a stronger electric field that contributes to an enriched N2 concentration at the interface, resulting in the ‘salting-out’ effect that, similarly, enriches the interfacial N2 concentration because of N2 precipitation, reported by Wang et al.62 This effect is accompanied by a disruption of the hydrogen bond network and an increased density of immobilized water molecules around the solute ions. As a result, the efficiency of these water molecules as proton sources and solvents decreases, thereby boosting the performance of eNRR. For example, a superior eNRR was achieved using 10 M LiCl as WIS electrolyte, which resulted in a faradaic efficiency (FE) of 71 ± 1.9% and NH3 yield of 9.5 ± 0.4 × 10−10 M s−1 cm−2 at −0.3 V vs. RHE (Fig. 5a and b). However, it is important to note that further increasing the salt concentration does not necessarily lead to improved eNRR performance, as the protonation step may be impeded by a deficiency of active H2O molecules, as suggested by Liu et al.61
 | ||
| Fig. 5 Water-in-salt electrolyte. (a) Yield rate and (b) corresponding NH3 faradaic efficiency in different salt concentrations (from 2 to 14 M). Reproduced with permission.62 Copyright 2021, Springer. The NH3 formation FE over (c) Rh1/MnO2, reproduced with permission,60 copyright 2022, Elsevier and (d) Se-vacancy-rich (WSe2−x) in both dilute electrolytes (DEs) and WISEs at selected potential. Reproduced with permission,63 Copyright 2022, American Chemical Society. | ||
Employing WIS electrolyte has reportedly been used and integrated with catalyst engineering including for e.g. vacancy, doping,56,60,61,63 to enhance its potential and applications. For example, Shen et al.60,63 demonstrated that using Rh1/MnO2 and WSe2−x electrodes in combination with a WIS electrolyte, resulted in highly significant FE of 73.3% and 62.5%, respectively (Fig. 5c and d). This remarkable selectivity performance is attributed to the synergistic effect arising from accumulation of delocalized electrons at vacancy sites or single atom doping sites, coupled with the rigid solvation sheaths formed in WIS environment. These factors collectively improve the transportation and activation kinetics of nitrogen while inhibiting proton mobility, leading to enhanced efficiency in the electrochemical N2 reduction.
3.2 Solvent effect
Non-aqueous solvents, including organic solvents and ionic liquids (ILs), enhance N2 solubility and improve eNRR performance compared to aqueous electrolytes. Organic solvents improve N2 solubility and reduce HER kinetics, but can form by-products. Mixing organic solvents with water balances solubility and stability, significantly improving NH3 production. ILs provide high conductivity and stability, also improve N2 solubility, especially when they are fluorinated. Combining ILs with aprotic solvents could further enhance N2 solubility and suppresses HER, leading to higher NH3 production and efficiency.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) and (2) pure de-ionized water, Fig. 6a. It was found that the mixed solvent significantly boosted FE for eNRR, exhibiting a 7 to 8-fold increase compared to pure water. However, despite this, FE remained relatively low (<1%) due to instability arising from alkoxides formation during 2-propanol reduction. To address this, LiCl was used as a supporting salt with ethylenediamine (EDA) as the electrolyte for NH3 synthesis (Fig. 6b), resulting in an overall FE of 17.2%. Therefore, pure organic solvents as electrolyte are not optimal because of the formation of non-sustainable by-products. A mixture of organic solvents with sustainable proton sources such as H2O, has reportedly emerged as a solution.64 This addresses low N2 solubility in aqueous solutions whilst maintaining overall stability. By combining organic solvents with water, it appears possible to leverage the benefits of both types of solvents, leading to a more sustainable electrochemical process. Ren et al.66 employed alcohol-water system (i.e., methanol, ethanol, n-propanol, n-butanol, dimethyl sulfoxide, and pure water) for electrolyte in eNRR, and reported a volcano-like relationship between the selected alcohols and their proton-donating ability. Amongst these, volumetric content of H2O in 0.16% in methanol–water electrolyte exhibited a record high eNRR FE of 75.9 ± 4.1% and NH3 yield of 262.5 ± 7.3 mg h−1 mg−1 on catalyst FeOOH/CNTs, an 8-fold increase compared with conventional aqueous electrolytes, Fig. 6c and d. In addition, the electrode potential for optimal eNRR is more negative with methanol–water electrolyte, providing a high degree of control of activity and selectivity for NH3 production. The synergistic effect of boosting N2 solubility and reducing HER kinetics leads to an expanded electrochemical potential window. This broadened range provides greater flexibility in fine-tuning the selectivity of the reaction, whilst concurrently preserving the efficiency of NH3 production. Moreover, the use of organic solvents has also been reported to offer advantages through the phenomenon of molecular crowding, as investigated by Guo et al. in their study which employed hydrophilic ploy(ethylene glycol) PEG400.82 The addition of PEG promoted the overall eNRR performances in both acidic and alkaline electrolytes, as shown in Fig. 6e and f. In particular, a maximum ammonia faradaic efficiency (FE) of 32.13% and a yield of 1.07 μM cm−2 h−1 were achieved in PEG-containing acidic electrolytes. This enhancement is attributed to the suppression of the Heyrovsky step in the HER, resulting from the sluggish proton diffusion caused by strong hydrogen-bonding interactions between electronegative oxygen atom in PEG and hydrogen atom in H3O+/H2O. This interaction effectively limits proton mobility, thereby favouring the nitrogen reduction reaction over competing HER processes.
1, v/v) and (2) pure de-ionized water, Fig. 6a. It was found that the mixed solvent significantly boosted FE for eNRR, exhibiting a 7 to 8-fold increase compared to pure water. However, despite this, FE remained relatively low (<1%) due to instability arising from alkoxides formation during 2-propanol reduction. To address this, LiCl was used as a supporting salt with ethylenediamine (EDA) as the electrolyte for NH3 synthesis (Fig. 6b), resulting in an overall FE of 17.2%. Therefore, pure organic solvents as electrolyte are not optimal because of the formation of non-sustainable by-products. A mixture of organic solvents with sustainable proton sources such as H2O, has reportedly emerged as a solution.64 This addresses low N2 solubility in aqueous solutions whilst maintaining overall stability. By combining organic solvents with water, it appears possible to leverage the benefits of both types of solvents, leading to a more sustainable electrochemical process. Ren et al.66 employed alcohol-water system (i.e., methanol, ethanol, n-propanol, n-butanol, dimethyl sulfoxide, and pure water) for electrolyte in eNRR, and reported a volcano-like relationship between the selected alcohols and their proton-donating ability. Amongst these, volumetric content of H2O in 0.16% in methanol–water electrolyte exhibited a record high eNRR FE of 75.9 ± 4.1% and NH3 yield of 262.5 ± 7.3 mg h−1 mg−1 on catalyst FeOOH/CNTs, an 8-fold increase compared with conventional aqueous electrolytes, Fig. 6c and d. In addition, the electrode potential for optimal eNRR is more negative with methanol–water electrolyte, providing a high degree of control of activity and selectivity for NH3 production. The synergistic effect of boosting N2 solubility and reducing HER kinetics leads to an expanded electrochemical potential window. This broadened range provides greater flexibility in fine-tuning the selectivity of the reaction, whilst concurrently preserving the efficiency of NH3 production. Moreover, the use of organic solvents has also been reported to offer advantages through the phenomenon of molecular crowding, as investigated by Guo et al. in their study which employed hydrophilic ploy(ethylene glycol) PEG400.82 The addition of PEG promoted the overall eNRR performances in both acidic and alkaline electrolytes, as shown in Fig. 6e and f. In particular, a maximum ammonia faradaic efficiency (FE) of 32.13% and a yield of 1.07 μM cm−2 h−1 were achieved in PEG-containing acidic electrolytes. This enhancement is attributed to the suppression of the Heyrovsky step in the HER, resulting from the sluggish proton diffusion caused by strong hydrogen-bonding interactions between electronegative oxygen atom in PEG and hydrogen atom in H3O+/H2O. This interaction effectively limits proton mobility, thereby favouring the nitrogen reduction reaction over competing HER processes.
 | ||
| Fig. 6 Solvent effect. (a) Effect of electrolyte medium of water or 2-propanol on NH3 synthesis rate and FE. Reproduced with permission.65 Copyright 2016, The Electrochemical Society. (b) Schematic for ethylenediamine (EDA)-based NH3 synthesis. Reproduced with permission.64 Copyright 2016, The Electrochemical Society. (c) Comparison of NRR performance achieved by commercial MoS2 in aqueous electrolytes and methanol–water electrolyte. The inset is the scanning electron micrograph image of used commercial MoS2. (d) NRR FE and NH3 yield rate over FeOOH/CNTs at the applied potential of −1.2 V vs. Ag/AgCl for 2 h in methanol–water electrolytes with water volumetric content of 0.04, 0.08, 0.16, 0.32, and 0.64%, respectively. Reproduced with permission.66 Copyright 2021, American Chemical Society. NH3 yield and FE of TiO2 nanoarray electrode in (e) 0.05 M H2SO4, and (f) 0.1 M NaOH electrolyte with/without 20 wt% poly(ethylene glycol) PEG400. Reproduced with permission.82 Copyright 2021, Wiley-VCH. | ||
Many studies have been conducted to investigate the solubility of gases in various ILs.84–89 Kang et al.90 reported synthesis of a series of phosphonium-based ILs with highly fluorinated anions. It was found that fluorine content is important to dissolving more N2. A binary system consisting of an IL and a fluorinated solvent mixture reportedly exhibits capability to dissolve a greater quantity of N2 molecules.91 Three fluorinated solvents were included in this work, namely trifluorotoluene (TFT), 1H,1H,2H-heptafluorocyclopentane (HFCP), and 1H,1H,5H-octafluoropentyl 1,1,2,2-tetrafluoroethyl ether (FPEE), in combination with the ionic liquid 1-butyl-1-methylpyrrolidinium tris(pentafluoroethyl)trifluorophosphate [C4mpyr][eFAP]. There was an increasing N2 solubility with increasing volume fraction of fluorinated solvent, being advantageous for eNRR. Therefore, based on the recent progress, two types of non-aqueous solvents are summarised which are pure IL, and IL – organic solvent-based mixtures.
The first application of ILs as electrolyte in electrocatalytic N2 reduction was reported in 2016.92 A gas diffusion electrode coated with Cp2TiCl2-supported hydrophobic IL, specifically 1-butyl-1-methylpyrrolidinium tris(pentafluoroethyl)tri fluorophosphate ([C9H20N][(C2F5)3PF3]), was used in a solid polymer electrolysis (SPE) cell for NH3 production. For this configuration, the NH3 yield per Cp2TiCl2 and current efficiency achieved are 27% and 0.2%, respectively. Inspired by this work, Zhou et al. in 2017 further advanced the use of IL.70 They employed [P6,6,6,14][eFAP] as the electrolyte, which has high N2 solubility, and achieved a conversion efficiency of up to 60% for NH3 using nanostructured iron-based catalysts. However, the production of NH3 remained low, at 10−12 M s−1 cm−2, due to the high viscosity of IL, which limited N2 mass transfer (Fig. 7a and b).
 | ||
| Fig. 7 eNRR performance using ionic liquid. (a) Faradaic efficiency and (b) NH3 production rate for electro-reduction of selected N2-saturated ionic liquids on selected electrodes at a constant potential −0.8 V vs. NHE. Findings for FTO and SS substrate are based on a 3 h experiment. Reproduced with permission.70 Copyright 2017, Royal Society of Chemistry. (c) Potential dependence of NH3 yield and FE (%) at IL mole fraction XIL = 0.16. (d) Constant potential electrolysis at −0.65 V vs. NHE in selected mixed electrolytes. Reproduced with permission.69 Copyright 2018, American Chemical Society. (e) Schematic for the one-step electrocatalytic synthesis of NH3 and acetone in ionic liquids. (f) NH3 yield and faradaic efficiency in this work, using Fe-modified graphene and Fe-modified stainless steel as electrode. Reproduced with permission.68 Copyright 2021, Royal Society of Chemistry. | ||
Recent advancements in eNRR have demonstrated the benefits of combining ionic liquids with other components to enhance eNRR performance. Suryanto et al.93 explored a mixture of an aprotic fluorinated solvent and ionic liquid mixture FPEE-[C4mpyr][eFAP] as electrolyte.91 This combination significantly boosted eNRR selectivity and activity when α-Fe nanorods were used as the electrode under ambient conditions. The optimized electrolyte mixture, with a mole fraction (XIL) of 0.23, achieved the highest ammonia yield (approximately 2.35 × 10−11 M s−1 cmGSA−2) and efficiency (32%), at −0.65 V vs. NHE (Fig. 7c and d).
Building on the advantages of these combinations, Zhang et al.68 reported a one-step electrochemical synthesis of NH3 from N2 in an ionic liquid using a nano-Fe catalyst in an electrolyte that combines ionic liquid and isopropanol. Isopropanol also serves as the proton source to limit proton supply, which not only enhanced nitrogen solubility but also suppressed the competitive HER. This innovative combination resulted in a high ammonia production efficiency of 60% and a production rate of 13.06 mg m−2 h−1 (Fig. 7e and f).
4. Electrolyte engineering for indirect Li-NRR
In Li-NRR, non-aqueous electrolytes were used, and lithium ions are introduced into the electrolyte as mediators for N2 reduction and might form part of the electrode surface. This is quite distinct to direct eNRR, where the electrode material does not directly participate in N2 reduction. Therefore, the overall electrochemical activity and selectivity is largely depend on the physical and chemical property of the electro-induced lithium or lithium-based deposition, along with the corresponding SEI formation.46–48,51 There are two main reasons for the use of electrolyte in Li-NRR, which are providing sources of Li+ and H+. THF is extensively reported as the solvent for Li-NRR, capable of dissolving significant amounts of N2.94 A common electrolyte system for Li-NRR is a mixture of Li-salt/THF/HA. The resemblance of metallic Li to the SEI layer in traditional Li-ion61 batteries is hypothesized to significantly impact the overall performance of Li-NRR.95 Therefore, regulating the chemical and physical property of SEI by varying electrolyte components can effectively improve Li-NRR for sustainable NH3 production.48 Here, we summarize the effect on the selection of various Li-salt with different anions (Section 4.1) and how proton sources impact Li-NRR (Section 4.2). A summary of the indirect Li-NRR process using various electrolytes is tabulated in Table 2.| Electrode | Electrolyte | Reaction conditions | NH3 yielda (nmol s−1) | FE (%) |
|---|---|---|---|---|
| a Units for ammonia yield rate converted to nmol s−1 with respect to the catalytic area (cm−2) or mass (mg−1). b Room temperature. c Averaged ammonia yield rate; stabilized yield rate after 24 h of operation is given 150 ± 8 cm−2. | ||||
| Ni96 | THF/LiNTf2/ethanol | 15 bar, RTb | 223 ± 8 cm−2 (ave.)c | ∼100 |
| Ni97 | THF/LiNTf2/ethanol | 15 bar, RT | 530 ± 20 cm−2 | 98 ± 2 |
| Ni97 | THF/LiNTf2/iso-propanol | 15 bar, RT | 430 ± 20 cm−2 | 96 ± 1 |
| Mo98 | THF/LiClO/ethanol | 20 bar, RT | 10.8 ± 0.2 cm−2 | 78 ± 1.3 |
| Stainless steel cloth (SSC)99 | THF/LiBF4/PhOLi | 1 bar, RT | — | 72 ± 3 |
| SSC100 | Diethylene glycol dimethyl ether/LiBF4/EtOH | 1 bar, RT | — | 64 ± 1 |
| SSC101 | THF/LiBF4/ethanol | 1 bar, RT | — | 61 ± 1 |
| Cu57 | THF/LiBF4/ethanol | 20 bar, RT | 2500 ± 100 cm−2 | 71 ± 3 |
| Cu49 | THF/LiBF4/tris(pentafluoroethyl)trifluorophosphate | 20 bar, RT | 53 ± 1 cm−2 | 69 ± 1 |
4.1 Anion effect
The selection of anions in the electrolyte significantly influences the performance and efficiency of the Li-NRR process. Common anions used in Li-NRR include perchlorate (ClO4−), tetrafluoroborate (BF4−), and triflate (OTf−). Several experiments have explored the effects of these Li-salts on Li-NRR performance.48,50,102 For instance, Li et al. performed a theoretical investigation for screening lithium salts;57 demonstrating through first-principle studies that fluorine-based electrolyte like LiHF2 and LiF, shows slower Li+ conductivity but higher surface mobility, which collectively led to a relatively stable phase of the enriched-SEI layer. Based on these theoretical insights, a high selectivity and NH3 production rate was reported using LiBF4 as the electrolyte, achieving approximately 71 ± 3% FE and 2.5 ± 0.1 μM s−1 cmgeo−2 at a current density of 1 A cmgeo−2 (Fig. 8a and b). Fig. 8c shows that the thinnest SEI layer was formed with LiBF4, resulting in less NH3 accumulated within the SEI compared with other lithium salts, but with higher selectivity.95 Additionally, Fu et al.103 assessed various lithium salts in a continuous-flow reactor, which was coupled with hydrogen oxidation at the anode. Among the investigated lithium salts (BOB−, PF6−, ClO4−, TFSI−, and BF4−), LiBF4 offered superior performance with a FE of up to 61% (Fig. 8d). This enhanced performance was attributed to the formation of a compact SEI layer. Their study also highlighted two critical principles for designing effective lithium salts for Li-NRR: ensuring reductive stability by avoiding anions with carbonyl or carboxyl groups, which could be reduced by metallic lithium, and addressing electrolyte poisoning due to polymerization. Moreover, Du et al.96 investigated the use of concentrated salt with bis(trifluoromethylsulfonyl)imide (NTf2−) and bis(fluorosulfonyl)imide (FSI−) anions for Li-NRR. These anions were found to be highly stable under lithium-reduction conditions and supported rapid lithium electro-deposition rates. Superior Li-NRR performance was achieved with an NH3 production rate of 150 ± 20 nM s−1 cm−2 and a current efficiency of 99%, using 2 M LiNTf2 in THF/ethanol with a Ni wire electrode at a fixed potential of −0.55 V vs. Li/Li+. The study revealed that a concentrated LiNTf2 electrolyte resulted in the greatest FE and NH3 production compared to LiFSI and other common electrolytes (Fig. 8e). LiNTf2 electrolyte reportedly creates a compact SEI layer that resulted in the superior FE. Further investigations showed that varying the concentration of LiNTf2 from 0.1 M to 3 M indicated that the optimal NH3 production rate occur between 1.5 M–2.0 M, as shown in Fig. 8f. These findings from the studies on Li-NRR provide valuable evidence of the practicality and effectiveness of different lithium salts in advancing gNH3 production. | ||
| Fig. 8 Lithium salt effect. (a) Faradaic efficiency and (b) NH3 production of porous Cu electrode using differing lithium salts at current density from −0.075 to −1.0 A cmgeo−2. (c) Accumulated NH3 amount in electrolyte, SEI, and gas phase using different lithium salts at a current density of −1.0 A cmgeo−2. Reproduced from permission.95 Copyright 2022, Elsevier. (d) Faradaic efficiencies of different lithium salts at 1 M and, 0.5 M for LiBOB. Reproduced with permission.103 Copyright 2024, American Chemical Society. (e) NRR faradaic efficiency and NH3 yield rate plotted against conductivity for different electrolytes at 2 M, a mixed 1 M LiNTf2 + 1 M LiBF4 system and 1 M LiNTf2. (f) Comparison of the Li-NRR faradaic efficiency with NH3 yield rate as a function of LiNTf2 concentration. Reproduced with permission.96 Copyright 2022 Springer. | ||
4.2 Proton sources
The selection of proton source is crucial for Li-NRR, not only it drives the protonation of Li3N, but also take part in the formation of SEI that has significant impact on the long-term reductive stability and the corresponding NH3 formation activity and selectivity. Ethanol (EtOH) is the earliest reported and has been the most extensively studied in Li-NRR electrochemical system, whereas the precise reasons for itswidespread use and effectiveness still remain unclear.43 Du et al.97 reported that it is irreversibly consumed due to THF oxidation, forming by-products of 2-ethoxytetrahydorfuran and pentanal diethyl acetal (Fig. 9a and b). In addition, the reduced metallic lithium is highly electrochemically and chemically unstable and forms lithium ethoxide (CH3LiO) with ethanol.50,94 Therefore, an increasing concentration of ethanol as proton source can lead to its consumption due to the interaction of Li, and further leads to the unstable electrochemical system. A major issue related to this is the sacrificial solvent mechanism, where protons are not produced from sustainable sources such as H2O or H2, thereby limiting the practical industrial application. Other types of alcohols have also been reported and shown some promising results, such as 1-butanol, iso-propanol, and etc.51,97,99 Iso-propanol has been proposed as an alternative proton source, showing similar high Li-NRR reaction metrics (Fig. 9c) without undergoing THF degradation.97 Additionally, Lazouski et al.51 and Krishnamurthy et al.104 reported the effect of chemical nature of proton donor via solvatochromic Kamlet–Taft parameters, reflecting the hydrogen bond donating (α) and accepting (β) capabilities. Findings showed that linear aliphatic alcohols exhibited the highest number of highly active proton donor groups for Li-NRR. Among the proton donors tested, including the commonly used ethanol, 1-butanol exhibited the greatest NH3 selectivity. Additionally, the NH3 FE exhibited a peak in response to concentration at 0.25 M, showing a correlation between NH3 FE and SEI permeability, which in turn effects relative mass-transfer coefficients for N2 and H2 through SEI. Optimal selection of both the lithium salt and proton carrier plays a synergistic role in enhancing Li-NRR performances. In addition to sacrificial proton source, a “proton shuttle” mechanism also exists, reported by Suryanto et al.,49 as shown in Fig. 3a for pathway (ii). Such a non-sacrificial proton mechanism, involving using proton source that can reversibly donate and accept protons without being consumed in the reaction. For example, phosphonium-based cations such as trihexyltetradecylphosphonium ([P6,6,6,14]+)70 have been shown to undergo reversible deprotonation, forming a stable ylide structure, confirmed through 31P nuclear magnetic resonance (NMR) and mass spectrometry. This provides a foundation for undergoing a complete cycle of protonation–deprotonation, for highly stable Li-NRR operations with minimal oxidation or reduction of the proton source. Consequently, such proton shuttle mechanism has led to highly stable long-term Li-NRR, achieving a constant NH3 formation rate of 53.7 ± 0.4 nM s−1 cm−2, and a constant current density of 22.5 mA cm−2 in one-compartment cell reactor, with a mean FE of 69%. However, a limitation of the proton shuttle mechanism is the limited mass-transfer in the batch cell reactors. | ||
| Fig. 9 Proton source effect. Most plausible side products (a) 2-ethoxytetrahydrofuran and (b) pentanal diethyl acetal with ethanol and tetrahydrofuran sources highlighted with, respectively, blue and green colour. (c) NH3 yield rate and Li-NRR FE plotted against Kamlet–Taft hydrogen bond donation parameter for the proton carrier (except NH4NTf2) added at 0.1 M concentration (except for the tetrahydrofuran data, which represent a solution with no additional proton carrier). Reproduced with permission.97 Copyright 2023, Royal Society of Chemistry. (d) Schematic proton-shuttling process for lithium-mediated ammonia synthesis in a continuous-flow electrolyzer. Faradaic efficiency changed with varying (e) phenol, and (f) lithium phenoxide concentrations. Reproduced with permission.99 Copyright 2024 Springer. | ||
More recently, an innovative approach for continuous NH3 production has emerged by incorporating the hydrogen oxidation reaction (HOR) at the anode of continues-flow electrolyser, which generates renewable H+ on the go (Fig. 9d).99,101 In this design, EtOH is initially employed to provide protons and form NH3. The resulting ethoxide EtO− acts as a proton shuttle mediator, which can combine with D2 (isotope-labelled deuterium) for further protonation. This is confirmed by the NDx (x = 1, 2, and 3) products through operando mass spectrometry tests. This setup achieved a FE for ammonia production of up to 61 ± 1% with an energy efficiency of 13 ± 1%, demonstrating the potential for highly stable continuous-flow electrochemical synthesis of ammonia by Li-NRR.
In addition, this research also raises the question of whether EtOH could be replaced by with other proton carriers. Further investigation revealed that while EtOH plays a crucial role in SEI formation, H+ generated from the anode can be shuttled with other species, even without EtOH.105 Experiments conducted by Fu et al. found that phenol (PhOH) exhibited the highest FE of 72 ± 3% in the Li-NRR process, attributed to the highly-stable deprotonated PhO− (Fig. 9e).99 Interestingly, protons in proton sources (HA) were found to be non-essential for ammonia formation, as demonstrated by the use of PhOLi (Fig. 9f), which achieved a FE of 74 ± 2%. Moving forward, understanding the mechanistic aspects of the proton shuttle mechanism is crucial for engineering electrolytes that can achieve high NH3 activity and selectivity in Li-NRR, ultimately contributing to the practical development of green ammonia synthesis (gNH3s).
Additionally, Fig. 10 compares ammonia yield rate and faradaic efficiency for both direct eNRR and Li-NRR, offering a clearer perspective on various electrolyte engineering strategies. As shown in Fig. 10a, Li-NRR generally exhibits higher NH3 selectivity and activity compared to direct eNRR in most experimental studies. Fig. 10b provides an enlarged view of different electrolyte engineering approaches in direct eNRR, highlighting the challenge of simultaneously achieving both high activity and selectivity.
5. Computational techniques for electrolyte modelling: dynamic and kinetic mechanism
Although electrolyte engineering can significantly enhance eNRR reductive performance, the underlying mechanism(s) are still not fully understood. Therefore, computation techniques will become powerful tools to reveal the reaction mechanisms and establish guidelines for electrolyte design. Modelling electrolytes requires consideration for both solvation and electrode potential effects, which impacts reaction intermediates, electrodes, and electrolytes. To model the solvation effect, there are three methods to consider this in calculations, in the order of increasing computation costs: (1) implicit solvation model, giving the dielectric and ionic response of electrolyte by creating a solvation cavity definition which treats the interface as a dielectric continuum; (2) explicit solvation model, which builds electrolyte components into the modelling process, and (3) hybrid implicit plus explicit solvation models, which combines the consideration in points (1) and (2), as depicted in Fig. 11.When treating electrode potential in simulations, the simplest and most common approach is the computational hydrogen electrode (CHE). This method accounts for electrode potential (U) by adding a constant term (−eU) to the free energy calculations for neutral system.101,106,107 While CHE has been widely accepted and used in the past, it has limitations when it comes to capturing the detailed effects of specific electrode conditions on the reaction.
To improve upon the limitations of the simplified CHE model, more advanced methods have been developed to simulate reactions under specific electrode potentials. These methods often involve introducing additional electrons into the system to alter the work function, thereby adjusting the electrode potential. In such cases, the computation unit cell will need to be neutralized to avoid artificial electrostatic interactions.108 This can be achieved through solvation models and specific approaches, such as constructing a uniform counter-charge background or placing a counter-charged explicit electrolyte/electrode system, which results in a realistic region of decaying electrostatic potentials.109,110 With these method in place, the system could accommodate an additional number of electrons (Ne). When the charge of a system is held constant, this approach is referred to as the constant-charge method.108 Alternatively, the charge of a system can be varied to maintain a constant electrode potential, known as the constant-potential method, where the electron's chemical potential (μ) of the system is kept in constant. In this approach, the surface potential is determined relative to the vacuum state for the work function, while the number of electrons is adjusted within the system in a self-consistent manner.111,112 The key difference between the constant charge and constant potential methods lie in whether electron exchange with an external source is allowed, which enables the potential to remain constant throughout the calculation. These advanced techniques enable us to conduct simulations not only for material design but also for capturing interfacial dynamics, which is crucial for advancing electrolyte design modelling in complex scenarios.
In the following sections, we present a comprehensive summary of electrolyte modelling approaches. We begin with constant electrode potential (CEP) with static first-principles calculations (Section 5.1.1). We then explore its application in macroscopic kinetic computations, including mean-field microkinetic modelling (MF-MKM) (Section 5.1.2), and kinetic Monte Carol simulation (kMC) (Section 5.1.3). Beyond this hybrid approach based on static calculation, we also discuss quantum mechanical molecular dynamics simulations, which employ fully explicit solvent and electrolyte molecules through an ab initio modelling approach (Section 5.2). While this method provides the most accurate depiction of solvation and electrification effects, it is limited by short time scales and high computational costs. To overcome these challenges, we also examine the use of machine learning-interatomic potentials (ML-IAPs) generated from DFT calculations, which extend molecular dynamics simulations to longer time scales while maintaining quantum mechanical accuracy (Section 5.3).
5.1 Microkinetic modelling of first-principle based computations
The constant electrode potential (CEP) method was then developed to address the dynamic potential/charge variations in catalyst surface and simulate the realistic electrocatalytic N2 reduction kinetics.28,113 In the CEP model, the Fermi energy level or μ is pre-set and remains consistent at all time. Computationally, this method relies on the implicit solvation model such that the electrode–electrolyte interface is treated as polarizable continuum model, where the electronic and continuum ionic charge are distributed via linear Poisson–Boltzmann model (LPB) or non-linear Poisson114 – Boltzmann model (NLPB), providing the fundamental treatment of the electrified system.115 Alternative methods are also available, such as the self-consistent continuum solvation (SCCS) model, where the ionic charge is distributed on a Gaussian-shaped plane.116 The LPB model is available through software such as VASPsol,117,118 implemented in the most widely used plane wave DFT code – Vienna Ab Initio Simulation Package (VASP). In addition, Mathew et al.118 developed the charge-asymmetric nonlocally determined local-electric (CANDLE) solvation model to determine asymmetrically charged solvents that was implemented in JDFTx code.119 Non-local cavity definition is used in the solvation model and prevented the possible “solvent leakage”.120 Most recently, Plaisance et al.121 developed “VASPsol++” solvation model based on the origin framework of VASPsol code that is implemented in VASP,93 capturing a non-local cavity and dielectric responses that is closer to the realistic electrochemical operating condition, which characterized a non-linear double hump shape of the differential capacitance curve.122
These computational codes provide a foundational base for simulating charged surface under applied electrode potentials. By incorporating ionic screening and a self-consistent field cycle, the number of electrons (Ne) can be determined and adjusted through an external charge flux (also known as the reference electrode), which operates in a self-consistent manner. This approach enables the exchange and optimization of Ne needed to maintain the equivalent Fermi energy in the grand canonical ensemble. Consequently, the overall charge of the system varies self consistently, establishing a constant potential framework instead of a constant charge. Liu's team developed a constant-potential model for ab initio molecular dynamics (AIMD) simulation, and implemented it into VASP.123–127 This model, referred to as the constant-potential hybrid-solvation dynamic model (CP-HS-DM), incorporates both explicit and implicit solvation models enabled by VASPsol. In this model, the ionic charge compensates the net electronic charges, maintaining the overall charge neutrality of the unit cell, while the Fermi level evolves according to the grand-canonical distribution at the target electrode potential. Specifically, the external electrochemical potential (U) is adjusted by computing the work function (Φ) through variations in Ne, which are required to achieve equivalent Fermi energy in the grand-canonical states, with reference to the standard hydrogen electrode (SHE):
 | (8) |
| Ω = Etot + ΦNe | (9) |
This foundation sets the stage for further advancements in microkinetic modelling strategies such as the MF-MKM and kMC, enabling more detailed and accurate predictions of electrochemical processes through time via engineering electrolyte and enhancing our understanding of complex catalytic systems.
In the past, MF-MKM has been used to develop efficient electrocatalyst materials. For an eNRR example, Singstock et al.137 assessed the mechanism on Fe–S–Mo surface which exhibited good N2 reduction.138 The computation of the effect of a realistic solvated environment was achieved via grand-canonical density functional theory (GC-DFT) using a hybrid solvation model (implicit + explicit). The GC-based nudged elastic band (NEB), combined with MF-MKM analysis showed good agreement with experimental results, as shown in Fig. 12a and b. This approach is instrumental in identifying effective electrocatalysts by modelling the kinetic performance in a realistic electrocatalytic environment. In addition to the material design, it is important to incorporate the electrolyte effect such as the cation effect, mass transfer, and local pH values. By using such method, Choi et al.139 resolved the discrepancy between experiment and theoretical computation for potential- and pH-dependent competition of NRR/HER. A cross-over potential was found between the N2 adsorption and H adsorption (Volmer-step) on Fe–N–C catalysts, leading to a different surface coverage for each species, which resulted in varied yield rates and FE. Such advanced techniques have achieved a similar experimental value (pH and electrode potential) for the maximum NH3 yield rate and selectivity (Fig. 12c–e). In addition, our recent work has provided atomic-level insight into the critical role of interfacial N2 concentration by employing MF-MKM combined with first-principle calculations.140 It is suggested that the sluggish N2 reductive activity cannot be addressed by merely regulating the electrode potential or material design alone. Instead, optimal eNRR conditions should ensure both strong N2 activation by the electrocatalysts and a high N2 availability at EEI, which can be improved through targeted electrolyte engineering strategies.
 | ||
| Fig. 12 Microkinetic modelling for eNRR. Heatmap for NH3 yield (coloured shading) and NRR FE (black and white shading) as pH and applied bias are varied in NRR microkinetic model with employing (a) CHE model, and (b) CEP model for Fe2Mo6S8. Reproduced with permission.137 Copyright 2022, American Chemical Society. MKM findings using CEP model at three pH, (c) 13, (d) 7.2 and (e) 0. The relative rNH3 is determined via dividing rNH3 by its maximum value. Dashed lines represent MKM without HER. rNH3 and coverage are shown in the upper and lower panels, respectively. Reproduced with permission.139 Copyright 2021 Springer. | ||
MF-MKM approach can be widely used for eNRR, including Li-NRR mechanism. Andersen et al.47 were the first established a simple microkinetic modelling model for Li-NRR. In this model, the diffusion of Li+, H+ and N2 molecules are explicitly considered and assumed to be the rate-limiting steps. Consequently, the selectivity for NH3 and H2 formation at steady state can be predicted and presented as function of diffusion rates of Li+, H+, and N2. The concentrations of H+ and N2 significantly contribute to the high selectivity of Li+ deposition and NH3 formation, which can be improved by employing gas diffusion electrode. In addition, the MF-MKM investigation revealed that the diffusion rates play a crucial role in forming the SEI layer, further determining the overall electrochemical performance.
pij(t) = kij![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−kijt) exp(−kijt) | (10) |
 | ||
| Fig. 13 kMC simulation for eNRR. (a) TOF value for eNRR on Ru(001) and bimetallic catalysts RuTi(110) and, RuV2(110) as a function of temperature, pressure and time. (b) H coverage change over time on Ru(001) and bimetallic Ru-based catalysts under mild conditions of 300 K and 1 bar. Reproduced with permission.150 Copyright 2022, American Chemical Society. (c) Schematic for electrode/electrolyte interface and where SEI growth reactions are expected to occur. (d) Evolution of SEI layer thickness with time following the ethylene carbonate decomposition reaction over Li2CO3(001). Reproduced with permission.151 Copyright 2022, American Chemical Society. | ||
In addition to modelling the reaction process, kMC simulation could also be used to model dynamic phenomena including formation of the SEI27,151–153 and ion diffusion.143 The understanding about SEI formation is important because its presence and characteristics significantly impact long-term stability. Although no specific example exists for eNRR, there are relevant studies in Li-metal battery field. To be specific, the SEI formation is a consequence of electrolyte reduction involving organic and inorganic layer, as shown in Fig. 13c. Initially, the formed SEI layer on the electrode surfaces acts as a protective barrier, preventing further reduction of the electrolyte during initial stages. However, SEI layer growth results in capacity loss and leads to the poor battery performance in a single cycle.151 Furthermore, kMC is capable of simulating evolution of the SEI layer thickness with time, as shown in Fig. 13d.151 Similarly, in the context of the Li-NRR electrochemical reaction, the presence and characteristics of the SEI layer on the electrode surface play a crucial role in determining the long-term stability and efficiency of the reaction. Understanding the SEI layer growth through modelling methods can enable the tuning of its growth behaviour, thereby enhancing Li-NRR performance. This makes it a focus area for improving the durability and effectiveness of these systems, and the understanding of this behaviour can benefit from kMC modelling.
5.2 Ab initio modelling: explicit solvation model
Molecular dynamic (MD) simulation is also a crucial computation strategy for electrolyte modelling with explicit solvent. It allows configuration of the realistic electrochemical reaction microenvironment, thus serving as a better bridge between computation and experiment.154,155 Within MD, there are mainly three categories: classic molecular dynamics (cMD); ab initio molecular dynamics (AIMD); machine learning based molecular dynamics (MLMD). In cMD simulations, particle motion is based on the empirical force fields in the form of classical mechanics equations. However, the accuracy of cMD in describing bond formation and breaking during electrocatalytic reactions at the EEI is limited, as it does not fully represent the electronic polarization behaviour in interactions between the electrolyte and electrode.156 Hence, in this section, we focus on the AIMD approach, and MLMD approach in the later section.There are two type of AIMD simulation methods: Born–Oppenheimer MD (BOMD) and Car–Parrinello MD (CPMD).157 Every MD step is quantum mechanically calculated by solving the Schrödinger equation based on adiabatic approximation (or BO approximation), which separates the wave function and Hamiltonian of the nucleus. In AIMD simulations, the solvent or electrolyte is explicitly modelled, revealing the interfacial microenvironment or EDL when an external potential is applied. This approach provides simulation trajectories at a quantum mechanical level, effectively capturing the interfacial structural dynamics.158–161 Factors including, the coordination number (Fig. 14a), hydrogen bonding net (Fig. 14b), water orientation (Fig. 14c) and EDL structures, making significant impact on the reaction energetic. Combining the sampling techniques (metadynamics and thermodynamic integration) with (constrained) AIMD modelling strategy, the free energy differences between two states can be obtained.162–165 Whilst this method has been developed and applied in theoretical study of electrocatalytic processes including HER and carbon dioxide reduction reaction (CO2RR), application to electrochemical nitrogen reduction reaction (eNRR) is limited.
 | ||
| Fig. 14 Ab initio modelling of electrochemical reactions including eNRR. (a) Cation coordination with CO2. Cation–oxygen bond length evolution with the presence of various cations by AIMD simulations at 300 K. Reproduced with permission.166 Copyright 2021 Springer. (b) Statistical distribution of the number of H bonds along the Pt(111) electrode surface in normal direction. Shaded areas represent gap zones of interfacial water and H-bond networks. Reproduced with permission.167 Copyright 2022 Springer. (c) Number of hydrogen bond donors and acceptors of interface water molecules on Pt(111) catalyst as a function of potential. A hydrogen bond is defined when the O–O distance is <3.5 Å and the O–O–H angle is <35°. Insets show the structural models of interface water at negative and positive potentials a.u., arbitrary units. Reproduced with permission.168 Copyright 2020, American Association for the Advancement of Science. Comparison of free energy for the first protonation of N2 with variation in electrode potential: (d) CHE model and (e) thermodynamic integration with full explicit solvent model (right). Reproduced with permission.169 Copyright 2022, American Chemical Society. | ||
Incorporating realistic aqueous environments and varying electrode potentials in AIMD simulations significantly enhances the accuracy of modelling electrochemical interfaces for eNRR. Qian et al.169 reported an AIMD simulation and considered the effect of electrode potential and aqueous environment and on Fe–N4 solid–liquid interface/EEI. The aqueous environment was simulated by including the explicit water molecules (ca. 141), whilst the electrode potential was modified via varying the number of H3O+ in the system. Constrained AIMD (c-AIMD) simulation and thermodynamic integration was applied to analyse the free energy profiles of N2 adsorption and its proton transfer. The free energy diagram for the hydrogenation of *N2 was determined by comparing the explicit solvent model and the CHE-based model, Fig. 14d and e. It was found that the explicit solvent model predicted a lower potential for the exothermic conversion of *N2 than the CHE-based model. Computed findings were in good agreement with experiment,139 evidencing accuracy and consistency of the modelling method, and establishing the significance of incorporating realistic aqueous environment and accounting for electrode potential. More recently, Mao et al.170 explored the role of alkali ions in modulating the activity and selectivity of NRR on Fe electrode via AIMD approach and evidenced by experiments. Four promotion effects of alkali cations on advancing NRR kinetics were identified: (i) N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond cleavage; (ii) intermediate stabilization; (iii) HER suppression; and (iv) charge redistribution on the interface. Additionally, among the selected alkali ions (Li+, Na+, and K+), the Li+ solvation shell mediates the NRR kinetics by receiving the lowest *NNH formation energy barrier −0.50 eV at constant potential condition U = −0.3 V vs. RHE. For Li-NRR, several detailed DFT studies have been conducted to assist experimental procedures. These include, computing the formation free energy of possible Li-contained compounds at selected voltage, modelling the free-energy barrier for surface mobility95 and determining the free energy diagram for N2 reduction intermediates on possible formed Li-contained SEI.46 Maniscalco et al.171 simulated the first step of Li-NRR via CPMD to show a new, unexplored mechanism, namely, nitrogen adsorption into the lithium bulk, nitrogen–hydrogen reaction and oxygen reactivity with the lithium surface. Hydrogen activation facilitated ready reaction with molecular nitrogen driven by the low density of lithium and the formation of oxide and nitride structures. Because of the complexity of the electrochemical environment of Li-NRR, the future optimal method for scale-up is likely in in modelling the potential electrolyte reduction, SEI formation and simulating the EDL on the electrified interface.27,152,155 With AIMD, it is difficult to maintain a constant electrode potential during computation because the potential/charges on the electrode are affected by the dipole variations on the EEI, thereby affecting the reaction rates, such limitation is reportedly obviated for HER and ORR,28,123,125 however there are no reports for eNRR.
N bond cleavage; (ii) intermediate stabilization; (iii) HER suppression; and (iv) charge redistribution on the interface. Additionally, among the selected alkali ions (Li+, Na+, and K+), the Li+ solvation shell mediates the NRR kinetics by receiving the lowest *NNH formation energy barrier −0.50 eV at constant potential condition U = −0.3 V vs. RHE. For Li-NRR, several detailed DFT studies have been conducted to assist experimental procedures. These include, computing the formation free energy of possible Li-contained compounds at selected voltage, modelling the free-energy barrier for surface mobility95 and determining the free energy diagram for N2 reduction intermediates on possible formed Li-contained SEI.46 Maniscalco et al.171 simulated the first step of Li-NRR via CPMD to show a new, unexplored mechanism, namely, nitrogen adsorption into the lithium bulk, nitrogen–hydrogen reaction and oxygen reactivity with the lithium surface. Hydrogen activation facilitated ready reaction with molecular nitrogen driven by the low density of lithium and the formation of oxide and nitride structures. Because of the complexity of the electrochemical environment of Li-NRR, the future optimal method for scale-up is likely in in modelling the potential electrolyte reduction, SEI formation and simulating the EDL on the electrified interface.27,152,155 With AIMD, it is difficult to maintain a constant electrode potential during computation because the potential/charges on the electrode are affected by the dipole variations on the EEI, thereby affecting the reaction rates, such limitation is reportedly obviated for HER and ORR,28,123,125 however there are no reports for eNRR.
Theoretically, AIMD can simulate all elements in the periodic table and provides accurate insights into bond cleavage and formation, charge transfer, and polarization effects. However, for electrolyte modelling, AIMD approach can be computationally expensive because it requires a significant number of energetically favourable solvent arrangements within a larger simulation cell to accurately describe dynamics within the EDL, which confines the overall simulation to a short time frame. Progress towards developing more affordable methods to accurately capture solvent and electrolyte dynamics is essential. Machine-learning techniques may play a crucial role in this effort, and will be discussed in the following section.
5.3 Machine learning-interatomic potentials (ML-IAP): extended time scale with ab initio accuracy
To address limitations of computational resources and constraints of atomic size and time length scale, development of machine learning-interatomic potential (ML-IAP) enables extended simulation time and atomic size scale together with ab initio accuracy.161,172 ML-IAPs represents the potential-energy surfaces (PES) by fitting large data sets from DFT-based computations via machine learning. A first step is to construct a set of data containing the energies and atomic forces corresponding to individual points on the PES. The structural inputs, e.g. atomic coordinates, are transformed onto suitable machine learning numerical descriptors e.g. atom centered symmetry functions, bispectrum of the neighbour density and Coulomb matrix after ‘training’ with equivalent atomic configurations such as permutation, translation and rotation(s). The goal of training is to optimize the parameters of a ‘smooth’ PES that best describes the structural input data for high-dimensional system. The ML-IAPs therefore is derived through selected machined-learned regressions to improve scalability of simulation model size and time span. Selected ML-IAPs reported include, neural network potential (NNP),173 spectral neighbour analysis potential (SNAP),174 Gaussian approximation potential (GAP),175 smooth overlap atomistic potential (SOAP)176 and deep-potential (DP).177At present, there are limited reports applying machine learning framework models to develop efficient and accurate MLPs for complex multiphase such as the interfacial solid–liquid system. Specifically, Schran et al.178 reported six complex aqueous systems i.e. F−–H2O, SO42−–H2O, CNT–H2O, BNNT–H2O, MoS2–H2O and TiO2–H2O through AIMD-driven active learning protocols relying on neural network potentials (Behler–Parrinello NNPs). This method reportedly gave high-accurate performance of >90% in reference to AIMD findings. These outcomes are derived from an array of features including, radial distribution functions (RDFs), vibrational density of states (VDOS) and the root mean square error (RMSE) of the force. A Behler–Parrinello NNPs, based active learning training workflow179 was used to characterize the pressure–temperature dependent behaviour of a single layer of water molecules within a graphene-like channel from a first-principle based understanding,180 to give insight into possible future development of nano-scaled materials and electrolyte design. However, there appears to be limited reports on the application of MLPs on N2 reductive process, which highlights a promising area for future research and development.
6. Conclusion and perspective
In this review, we explored two pathways for green NH3 synthesis: the conventional direct electrocatalytic NRR and the novel indirect Li-NRR. Both pathways have shown significant progress through electrolyte engineering approaches based on experimental results. To scale electrochemical ammonia synthesis for industrial viability, specific performance targets must be achieved. According to the US Department of Energy's REFUEL program (2016),181 practical electrochemical NH3 production requires high production rates of current density above 300 mA cm−2. Additionally, the Coulombic or faradaic efficiency should exceed 90%, dedicating the majority of the current to ammonia synthesis and minimizing side reactions such as hydrogen evolution. An energy efficiency of at least 60% is also required to ensure a substantial portion of electrical energy is directly converted into ammonia. Achieving these benchmarks would allow electrochemical methods to compete with the traditional thermochemical Haber–Bosch process.For direct eNRR, these methods include modifying alkali cations and concentrations in SIW and WIS electrolytes, utilizing organic solvents, and employing ionic-liquid-based electrolytes. Developing an optimal interfacial microenvironment is key to enhancing the electrocatalytic performance of the N2 reduction process. For Li-NRR, higher NH3 FE and yield rates have been reported by regulating lithium salts and proton sources. However, a deeper understanding is required to explore the formation of the SEI in relation to electrolyte compositions and its impact on the long-term stability of continuous NH3 synthesis. To achieve these, computational techniques offer a quantitative theoretical framework to understand interfacial dynamics at the atomic level, providing insights to fine-tune electrolyte compositions and enhance eNRR performances. For practical green NH3 synthesis (gNH3s), future developments in both reaction pathways are necessary.
For direct eNRR, future theoretical computations will need to incorporate both the electrode potential and solvation effect. Present understanding of the impact of electrolytes is insufficient, including ions, organic solvents, and ILs, as well as corresponding interfacial dynamics such as EDL structure, cation concentration, and local pH, on the electrified interface in electrocatalytic NRR. A judicious combination of first-principles-based microkinetic modelling, and ab initio modelling, together with advanced in situ/operando characterisations including, in situ Raman spectroscopy, in situ XRD and FTIR spectroscopy will therefore be needed to establish interfacial electrocatalytic dynamics and atomic-level understanding of eNRR.
For indirect Li-NRR, experimental results have achieved highly selective NH3 formation at the laboratory scale. However, high energy efficiency remains a significant challenge.182 One major reason is that the reduction of Li+ requires a very negative electrode potential of up to −3.04 V vs. SHE. Similarly, other metals such as calcium and magnesium have demonstrated the feasibility of mediated-NRR processes, but they also require high reduction potentials, with standard reduction potentials of −2.87 V and −2.37 V vs. SHE, respectively.183–185 It is important to explore other active metals or metal alloys to provide a strong foundation for mediating NRR under potential to achieve higher energy efficiency.186
Moreover, with the development of proton-shuttle mechanisms and reactor designs employing HOR to avoid the proton sacrificial phenomenon, the long-term stability of the electrochemical process has significantly improved. However, solvent degradation and volatility remain an issue in THF-based electrochemical systems, exploring alternative electrolyte solvents is needed for future development of gNH3s. Recent publications have aimed at assessing optimal solvents based on their conductivity, side reactions, and NH3 FE.100,187,188 For instance, compared to the cyclic ether-based solvents, chain-ether-based solvent is reported to exhibit higher stability in Li-NRR electrochemical system, attributed to the more complete solvation of conductive ions.188 300 h of stable operation is achieved employing diethylene glycol dimethyl ether by Li et al.100 Although significant progress has been made, the ultimate goal of industrial implementation to replace the Haber–Bosch (H–B) process still faces several challenges, including achieving higher energy efficiency, ensuring long-term stability, and addressing issues such as solvent decomposition in electrochemical systems.
To summarize, it is recognized that electrolytes significantly impact the electrochemical performance of NRR in both reaction pathways. To accelerate progress, advanced computation techniques incorporated with MD – based approach are efficient tools for exploring the underlying interfacial dynamics brought by the electrolyte contents. However, challenge still exists in accessing long time scales while maintaining quantum-level accuracy when employing full explicit solvation models. Machine learning interatomic potential-based forcefield simulations may be key to advancing electrolyte engineering by enabling longer simulation times and larger system sizes while maintaining ab initio accuracy.
Author contributions
L. J. conceptualized and contributed to writing – original draft. X. B. and X. Z. reviewed and contributed to conceptualizing and discussion. K. D. reviewed and contributed to writing the MS. Y. J. conceptualization, reviewing and supervision.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We gratefully acknowledge the financial support from Australian Research Council (ARC) Centre of Excellent project through CE230100032.Notes and references
- J. W. Erisman, M. A. Sutton, J. Galloway, Z. Klimont and W. Winiwarter, Nat. Geosci., 2008, 1, 636–639 CrossRef CAS.
- S. Paul, S. Sarkar, A. Adalder, S. Kapse, R. Thapa and U. K. Ghorai, ACS Sustainable Chem. Eng., 2023, 11, 6191–6200 CrossRef CAS.
- S. Sarkar, A. Adalder, S. Paul, S. Kapse, R. Thapa and U. K. Ghorai, Appl. Catal., B, 2024, 343, 123580 CrossRef CAS.
- R. Daiyan, T. Tran-Phu, P. Kumar, K. Iputera, Z. Tong, J. Leverett, M. H. A. Khan, A. Asghar Esmailpour, A. Jalili, M. Lim, A. Tricoli, R. S. Liu, X. Lu, E. Lovell and R. Amal, Energy Environ. Sci., 2021, 14, 3588–3598 RSC.
- A. Adalder, S. Paul, N. Barman, A. Bera, S. Sarkar, N. Mukherjee, R. Thapa and U. K. Ghorai, ACS Catal., 2023, 13, 13516–13527 CrossRef CAS.
- X. Fu, J. Zhang and Y. Kang, Chem Catal., 2022, 2, 2590–2613 CrossRef CAS.
- T. Wu, H. Zhao, X. Zhu, Z. Xing, Q. Liu, T. Liu, S. Gao, S. Lu, G. Chen, A. M. Asiri, Y. Zhang and X. Sun, Adv. Mater., 2020, 32, 2000299 CrossRef CAS.
- T. Wu, X. Zhu, Z. Xing, S. Mou, C. Li, Y. Qiao, Q. Liu, Y. Luo, X. Shi, Y. Zhang and X. Sun, Angew. Chem., Int. Ed., 2019, 58, 18449–18453 CrossRef CAS.
- H. M. Xu, H. R. Zhu, C. J. Huang, Z. J. Zhang, T. Y. Shuai, Q. N. Zhan and G. R. Li, Sci. China: Chem., 2024, 67, 1137–1160 CrossRef CAS.
- Z. H. Xue, S. N. Zhang, Y. X. Lin, H. Su, G. Y. Zhai, J. T. Han, Q. Y. Yu, X. H. Li, M. Antonietti and J. S. Chen, J. Am. Chem. Soc., 2019, 141, 14976–14980 CrossRef CAS PubMed.
- C. Kim, J. Y. Song, C. Choi, J. P. Ha, W. Lee, Y. T. Nam, D. M. Lee, G. Kim, I. Gereige, W. Bin Jung, H. Lee, Y. Jung, H. Jeong and H. T. Jung, Adv. Mater., 2022, 34, 2205270 CrossRef CAS.
- C. Yang, Y. Zhu, J. Liu, Y. Qin, H. Wang, H. Liu, Y. Chen, Z. Zhang and W. Hu, Nano Energy, 2020, 77, 105126 CrossRef CAS.
- W. Peng, M. Luo, X. Xu, K. Jiang, M. Peng, D. Chen, T. S. Chan and Y. Tan, Adv. Energy Mater., 2020, 10, 1–9 Search PubMed.
- Y. Wang, M. M. Shi, D. Bao, F. L. Meng, Q. Zhang, Y. T. Zhou, K. H. Liu, Y. Zhang, J. Z. Wang, Z. W. Chen, D. P. Liu, Z. Jiang, M. Luo, L. Gu, Q. H. Zhang, X. Z. Cao, Y. Yao, M. H. Shao, Y. Zhang, X. B. Zhang, J. G. Chen, J. M. Yan and Q. Jiang, Angew. Chem., Int. Ed., 2019, 58, 9464–9469 CrossRef CAS.
- L. Chen, C. Tang, Y. Jiao and S. Z. Qiao, ChemSusChem, 2021, 14, 671–678 CrossRef CAS.
- L. Chen, C. Tang, K. Davey, Y. Zheng, Y. Jiao and S.-Z. Qiao, Chem. Sci., 2021, 12, 8079–8087 RSC.
- Y. Jiao, Y. Zheng, M. Jaroniec and S. Z. Qiao, Chem. Soc. Rev., 2015, 44, 2060–2086 RSC.
- Y. Song, D. Johnson, R. Peng, D. K. Hensley, P. V. Bonnesen, L. Liang, J. Huang, F. Yang, F. Zhang, R. Qiao, A. P. Baddorf, T. J. Tschaplinski, N. L. Engle, M. C. Hatzell, Z. Wu, D. A. Cullen, H. M. Meyer, B. G. Sumpter and A. J. Rondinone, Sci. Adv., 2018, 4, 1700336 CrossRef.
- Y. Yang, S. Wang, H. Wen, T. Ye, J. Chen, C. Li and M. Du, Angew. Chem., Int. Ed., 2019, 58, 15362–15366 CrossRef CAS PubMed.
- P. Liu, Z. Huang, S. Yang, J. Du, Y. Zhang, R. Cao, C. Chen, L. Li, T. Chen, G. Wang, D. Rao, X. Zheng and X. Hong, ACS Catal., 2022, 12, 8139–8146 CrossRef CAS.
- X.-Y. Zhang, I. V. Zatovsky, J.-M. Cao and X.-L. Wu, Tungsten, 2024, 6, 657–662 CrossRef.
- L.-W. Chen, Y.-C. Hao, J. Li, L. Hu, Y. Guo, S. Li, D. Liu, Z. Zhu, S.-Q. Wu, H.-Z. Huang, A.-X. Yin, B. Wang and Y.-W. Zhang, Sci. China: Chem., 2022, 65, 2188–2196 CrossRef CAS.
- U. K. Ghorai, S. Paul, B. Ghorai, A. Adalder, S. Kapse, R. Thapa, A. Nagendra and A. Gain, ACS Nano, 2021, 15, 5230–5239 CrossRef CAS PubMed.
- A. Biswas, N. Barman, A. Nambron, R. Thapa, K. Sudarshan and R. S. Dey, Mater. Horiz., 2024, 11, 2217–2229 RSC.
- S. Murmu, S. Paul, S. Kapse, R. Thapa, S. Chattopadhyay, N. Abharana, S. N. Jha, D. Bhattacharyya and U. K. Ghorai, J. Mater. Chem. A, 2021, 9, 14477–14484 RSC.
- S. Paul, S. Sarkar, D. Dolui, D. Sarkar, M. Robert and U. K. Ghorai, Dalton Trans., 2023, 52, 15360–15364 RSC.
- J. W. Abbott and F. Hanke, J. Chem. Theory Comput., 2022, 18, 925–934 CrossRef CAS.
- X. Zhao, Z. H. Levell, S. Yu and Y. Liu, Chem. Rev., 2022, 122, 10675–10709 CrossRef CAS PubMed.
- G. Qing, R. Ghazfar, S. T. Jackowski, F. Habibzadeh, M. M. Ashtiani, C.-P. Chen, M. R. Smith and T. W. Hamann, Chem. Rev., 2020, 120, 5437–5516 CrossRef CAS PubMed.
- Y. Yang, J. Wang, Y. Shu, Y. Ji, H. Dong and Y. Li, Phys. Chem. Chem. Phys., 2022, 24, 8591–8603 RSC.
- X. Zhang, K. Ren, Y. Liu, Z. Gu, Z. Huang, S. Zheng, X. Wang, J. Guo, I. V. Zatovsky, J. Cao and X. Wu, Acta Phys.-Chim. Sin., 2023, 40, 2307057 Search PubMed.
- C. G. Zhan, J. A. Nichols and D. A. Dixon, J. Phys. Chem. A, 2003, 107, 4184–4195 CrossRef CAS.
- Y. Abghoui, A. L. Garden, J. G. Howalt, T. Vegge and E. Skúlason, ACS Catal., 2016, 6, 635–646 CrossRef CAS.
- Y. Abghoui and E. Skúlason, Catal. Today, 2017, 286, 78–84 CrossRef CAS.
- Y. Abghoui and E. Skúlason, Catal. Today, 2017, 286, 69–77 CrossRef CAS.
- Y. Abghoui and E. Skúlason, J. Phys. Chem. C, 2017, 121, 24036–24045 CrossRef CAS.
- Y. Abghoui and E. Skúlason, J. Phys. Chem. C, 2017, 121, 6141–6151 CrossRef CAS.
- Y. Abghoui, A. L. Garden, V. F. Hlynsson, S. Björgvinsdóttir, H. Ólafsdóttir and E. Skúlason, Phys. Chem. Chem. Phys., 2015, 17, 4909–4918 RSC.
- X. Yang, J. Nash, J. Anibal, M. Dunwell, S. Kattel, E. Stavitski, K. Attenkofer, J. G. Chen, Y. Yan and B. Xu, J. Am. Chem. Soc., 2018, 140, 13387–13391 CrossRef CAS PubMed.
- K. Honkala, A. Hellman, I. N. Remediakis, A. Logadottir, A. Carlsson, S. Dahl, C. H. Christensen and J. K. Nørskov, Science, 2005, 307, 555–558 CrossRef CAS PubMed.
- W. Chang, A. Jain, F. Rezaie and K. Manthiram, Nat. Catal., 2024, 7, 231–241 CrossRef CAS.
- F. Fichter, P.-L. Girard and H. Erlenmeyer, Helv. Chim. Acta, 1930, 13, 1228–1236 CrossRef CAS.
- A. Tsuneto, A. Kudo and T. Sakata, Chem. Lett., 1993, 851–854 CrossRef CAS.
- A. Tsuneto, A. Kudo and T. Sakata, J. Electroanal. Chem., 1994, 367, 183–188 CrossRef CAS.
- J. M. McEnaney, A. R. Singh, J. A. Schwalbe, J. Kibsgaard, J. C. Lin, M. Cargnello, T. F. Jaramillo and J. K. Nørskov, Energy Environ. Sci., 2017, 10, 1621–1630 RSC.
- J. A. Schwalbe, M. J. Statt, C. Chosy, A. R. Singh, B. A. Rohr, A. C. Nielander, S. Z. Andersen, J. M. McEnaney, J. G. Baker, T. F. Jaramillo, J. K. Norskov and M. Cargnello, ChemElectroChem, 2020, 7, 1542–1549 CrossRef CAS.
- S. Z. Andersen, M. J. Statt, V. J. Bukas, S. G. Shapel, J. B. Pedersen, K. Krempl, M. Saccoccio, D. Chakraborty, J. Kibsgaard, P. C. K. Vesborg, J. Nørskov and I. Chorkendorff, Energy Environ. Sci., 2020, 13, 4291–4300 RSC.
- X. Cai, C. Fu, H. Iriawan, F. Yang, A. Wu, L. Luo, S. Shen, G. Wei, Y. Shao-Horn and J. Zhang, iScience, 2021, 24, 103105 CrossRef CAS.
- B. H. R. Suryanto, K. Matuszek, J. Choi, R. Y. Hodgetts, H. L. Du, J. M. Bakker, C. S. M. Kang, P. V. Cherepanov, A. N. Simonov and D. R. MacFarlane, Science, 2021, 372, 1187–1191 CrossRef CAS.
- N. Lazouski, Z. J. Schiffer, K. Williams and K. Manthiram, Joule, 2019, 3, 1127–1139 CrossRef CAS.
- N. Lazouski, K. J. Steinberg, M. L. Gala, D. Krishnamurthy, V. Viswanathan and K. Manthiram, ACS Catal., 2022, 12, 5197–5208 CrossRef CAS.
- T. Wang, Y. Zhang, B. Huang, B. Cai, R. R. Rao, L. Giordano, S. G. Sun and Y. Shao-Horn, Nat. Catal., 2021, 4, 753–762 CrossRef CAS.
- M. Spry, O. Westhead, R. Tort, B. Moss, Y. Katayama, M.-M. Titirici, I. E. L. Stephens and A. Bagger, ACS Energy Lett., 2023, 8, 1230–1235 CrossRef CAS PubMed.
- R. Tort, O. Westhead, M. Spry, B. J. V. Davies, M. P. Ryan, M. M. Titirici and I. E. L. Stephens, ACS Energy Lett., 2023, 1003–1009 CrossRef CAS PubMed.
- L. Gao, Y. Cao, C. Wang, X. Yu, W. Li, Y. Zhou, B. Wang, Y. Yao, C. Wu, W. Luo and Z. Zou, Angew. Chem., Int. Ed., 2021, 133, 5317–5321 CrossRef.
- A. Guha, S. Narayanaru, N. M. Kaley, D. Krishna Rao, J. Mondal and T. N. Narayanan, Mater. Today Commun., 2019, 21, 100700 CrossRef CAS.
- S. Li, Y. Zhou, K. Li, M. Saccoccio, R. Sažinas, S. Z. Andersen, J. B. Pedersen, X. Fu, V. Shadravan, D. Chakraborty, J. Kibsgaard, P. C. K. Vesborg, J. K. Nørskov and I. Chorkendorff, Joule, 2022, 6, 2083–2101 CrossRef CAS.
- W. Y. Gao, Y. C. Hao, X. Su, L. W. Chen, T. A. Bu, N. Zhang, Z. L. Yu, Z. Zhu and A. X. Yin, Chem. Commun., 2019, 55, 10705–10708 RSC.
- N. C. Kani, A. Prajapati, B. A. Collins, J. D. Goodpaster and M. R. Singh, ACS Catal., 2020, 10, 14592–14603 CrossRef CAS.
- P. Shen, X. Li, Y. Luo, N. Zhang, X. Zhao and K. Chu, Appl. Catal., B, 2022, 316, 121651 CrossRef CAS.
- M. Liu, S. Zhang, M. Chen and L. Wu, Appl. Catal., B, 2022, 319, 121925 CrossRef CAS.
- M. Wang, S. Liu, H. Ji, T. Yang, T. Qian and C. Yan, Nat. Commun., 2021, 12, 3198 CrossRef CAS.
- P. Shen, X. Li, Y. Luo, Y. Guo, X. Zhao and K. Chu, ACS Nano, 2022, 16, 7915–7925 CrossRef CAS PubMed.
- K. Kim, C. Yoo, J. Kim, H. C. Yoon and J. Han, J. Electrochem. Soc., 2016, 163, F1523–F1526 CrossRef CAS.
- K. Kim, N. Lee, C.-Y. Yoo, J.-N. Kim, H. C. Yoon and J.-I. Han, J. Electrochem. Soc., 2016, 163, F610–F612 CrossRef CAS.
- Y. Ren, C. Yu, X. Han, X. Tan, Q. Wei, W. Li, Y. Han, L. Yang and J. Qiu, ACS Energy Lett., 2021, 6, 3844–3850 CrossRef CAS.
- C. Zhang, S. Liu, T. Chen, Z. Li and J. Hao, Chem. Commun., 2019, 55, 7370–7373 RSC.
- Q. Zhang, Y. Zhao, L. Yu, X. Zhang, Y. Bei and B. Tang, Green Chem., 2021, 23, 7685–7691 RSC.
- B. H. R. Suryanto, C. S. M. Kang, D. Wang, C. Xiao, F. Zhou, L. Miguel Azofra, L. Cavallo, X. Zhang and D. R. MacFarlane, ACS Energy Lett., 2018, 3, 1219–1224 CrossRef CAS.
- F. Zhou, L. M. Azofra, M. Ali, M. Kar, A. N. Simonov, C. McDonnell-Worth, C. Sun, X. Zhang and D. R. MacFarlane, Energy Environ. Sci., 2017, 10, 2516–2520 RSC.
- M. M. Waegele, C. M. Gunathunge, J. Li and X. Li, J. Chem. Phys., 2019, 151, 160902 CrossRef.
- B. Deng, M. Huang, X. Zhao, S. Mou and F. Dong, ACS Catal., 2022, 12, 331–362 CrossRef CAS.
- L. Suo, O. Borodin, T. Gao, M. Olguin, J. Ho, X. Fan, C. Luo, C. Wang and K. Xu, Science, 2015, 350, 938–943 CrossRef CAS PubMed.
- D. Strmcnik, K. Kodama, D. Van Der Vliet, J. Greeley, V. R. Stamenkovic and N. M. Marković, Nat. Chem., 2009, 1, 466–472 CrossRef CAS.
- Y.-C. Hao, Y. Guo, L.-W. Chen, M. Shu, X.-Y. Wang, T.-A. Bu, W.-Y. Gao, N. Zhang, X. Su, X. Feng, J.-W. Zhou, B. Wang, C.-W. Hu, A.-X. Yin, R. Si, Y.-W. Zhang and C.-H. Yan, Nat. Catal., 2019, 2, 448–456 CrossRef CAS.
- Y. Liu, M. Han, Q. Xiong, S. Zhang, C. Zhao, W. Gong, G. Wang, H. Zhang and H. Zhao, Adv. Energy Mater., 2019, 9, 1–9 Search PubMed.
- G. F. Chen, X. Cao, S. Wu, X. Zeng, L. X. Ding, M. Zhu and H. Wang, J. Am. Chem. Soc., 2017, 139, 9771–9774 CrossRef CAS.
- X. Zhu, Z. Liu, Q. Liu, Y. Luo, X. Shi, A. M. Asiri, Y. Wu and X. Sun, Chem. Commun., 2018, 54, 11332–11335 RSC.
- X. Sun, P. Wang, K. Davey, Y. Zheng and S. Z. Qiao, Small, 2023, 19, 1–7 Search PubMed.
- Y. Song, D. Johnson, R. Peng, D. K. Hensley, P. V. Bonnesen, L. Liang, J. Huang, F. Yang, F. Zhang, R. Qiao, A. P. Baddorf, T. J. Tschaplinski, N. L. Engle, M. C. Hatzell, Z. Wu, D. A. Cullen, H. M. Meyer, B. G. Sumpter and A. J. Rondinone, Sci. Adv., 2018, 4, 1–9 Search PubMed.
- C. B. Kretschmer, J. Nowakowska and R. Wiebe, Ind. Eng. Chem., 1946, 38, 506–509 CrossRef CAS.
- Y. Guo, J. Gu, R. Zhang, S. Zhang, Z. Li, Y. Zhao, Z. Huang, J. Fan, Z. Chen and C. Zhi, Adv. Energy Mater., 2021, 11, 2101699 CrossRef CAS.
- Y. Tian, Y. Liu, H. Wang, L. Liu and W. Hu, ACS Sustainable Chem. Eng., 2022, 10, 4345–4358 CrossRef CAS.
- J. C. Thermodynamics, S. Stevanovic and M. F. C. Gomes, J. Chem. Thermodyn., 2013, 59, 65–71 CrossRef.
- J. L. Anderson, J. K. Dixon and J. F. Brennecke, Acc. Chem. Res., 2007, 40, 1208–1216 CrossRef CAS PubMed.
- D. Almantariotis, T. Gefflaut, A. A. H. Pádua, J. Y. Coxam and M. F. Costa Gomes, J. Phys. Chem. B, 2010, 114, 3608–3617 CrossRef CAS.
- J. E. Bara, T. K. Carlisle, C. J. Gabriel, D. Camper, A. Finotello, D. L. Gin and R. D. Noble, Ind. Eng. Chem. Res., 2009, 48, 2739–2751 CrossRef CAS.
- P. Scovazzo, D. Camper, J. Kieft, J. Poshusta, C. Koval and R. Noble, Ind. Eng. Chem. Res., 2004, 43, 6855–6860 CrossRef CAS.
- D. Almantariotis, A. S. Pensado, H. Q. N. Gunaratne, C. Hardacre, A. A. H. Pádua, J. Y. Coxam and M. F. Costa Gomes, J. Phys. Chem. A, 2017, 121, 426–436 CrossRef CAS.
- C. S. M. Kang, X. Zhang and D. R. Macfarlane, J. Phys. Chem. C, 2018, 122, 24550–24558 CrossRef CAS.
- C. S. M. Kang, X. Zhang and D. R. MacFarlane, J. Phys. Chem. C, 2019, 123, 21376–21385 CrossRef CAS.
- A. Katayama, T. Inomata, T. Ozawa and H. Masuda, Electrochem. Commun., 2016, 67, 6–10 CrossRef CAS.
- B. H. R. Suryanto, C. S. M. Kang, D. Wang, C. Xiao, F. Zhou, L. M. Azofra, L. Cavallo, X. Zhang and D. R. MacFarlane, ACS Energy Lett., 2018, 3, 1219–1224 CrossRef CAS.
- G. R. Zhuang, K. Wang, Y. Chen and P. N. Ross, J. Vac. Sci. Technol., A, 1998, 16, 3041–3045 CrossRef CAS.
- S. Li, Y. Zhou, K. Li, M. Saccoccio, R. Sažinas, S. Z. Andersen, J. B. Pedersen, X. Fu, V. Shadravan, D. Chakraborty, J. Kibsgaard, P. C. K. Vesborg, J. K. Nørskov and I. Chorkendorff, Joule, 2022, 6, 2083–2101 CrossRef CAS PubMed.
- H.-L. Du, M. Chatti, R. Y. Hodgetts, P. V. Cherepanov, C. K. Nguyen, K. Matuszek, D. R. MacFarlane and A. N. Simonov, Nature, 2022, 609, 722–727 CrossRef CAS PubMed.
- H. L. Du, K. Matuszek, R. Y. Hodgetts, K. Ngoc Dinh, P. V. Cherepanov, J. M. Bakker, D. R. MacFarlane and A. N. Simonov, Energy Environ. Sci., 2023, 1082–1090 RSC.
- K. Li, S. Z. Andersen, M. J. Statt, M. Saccoccio, V. J. Bukas, K. Krempl, R. Sažinas, J. B. Pedersen, V. Shadravan, Y. Zhou, D. Chakraborty, J. Kibsgaard, P. C. K. Vesborg, J. K. Nørskov and I. Chorkendorff, Science, 2021, 374, 1593–1597 CrossRef CAS PubMed.
- X. Fu, A. Xu, J. B. Pedersen, S. Li, R. Sažinas, Y. Zhou, S. Z. Andersen, M. Saccoccio, N. H. Deissler, J. B. V. Mygind, J. Kibsgaard, P. C. K. Vesborg, J. K. Nørskov and I. Chorkendorff, Nat. Commun., 2024, 15, 2417 CrossRef CAS PubMed.
- S. Li, Y. Zhou, X. Fu, J. B. Pedersen, M. Saccoccio, S. Z. Andersen, K. Enemark-Rasmussen, P. J. Kempen, C. D. Damsgaard, A. Xu, R. Sažinas, J. B. V. Mygind, N. H. Deissler, J. Kibsgaard, P. C. K. Vesborg, J. K. Nørskov and I. Chorkendorff, Nature, 2024, 629, 92–97 CrossRef CAS.
- X. Fu, J. B. Pedersen, Y. Zhou, M. Saccoccio, S. Li, R. Sažinas, K. Li, S. Z. Andersen, A. Xu, N. H. Deissler, J. B. V. Mygind, C. Wei, J. Kibsgaard, P. C. K. Vesborg, J. K. Nørskov and I. Chorkendorff, Science, 2023, 379, 707–712 CrossRef CAS PubMed.
- N. Lazouski, M. Chung, K. Williams, M. L. Gala and K. Manthiram, Nat. Catal., 2020, 3, 463–469 CrossRef CAS.
- X. Fu, S. Li, N. H. Deissler, J. B. V. Mygind, J. Kibsgaard and I. Chorkendorff, ACS Energy Lett., 2024, 3790–3795 CrossRef.
- D. Krishnamurthy, N. Lazouski, M. L. Gala, K. Manthiram and V. Viswanathan, ACS Cent. Sci., 2021, 7, 2073–2082 CrossRef CAS.
- J. Bjarke Valbæk Mygind, J. B. Pedersen, K. Li, N. H. Deissler, M. Saccoccio, X. Fu, S. Li, R. Sažinas, S. Z. Andersen, K. Enemark-Rasmussen, P. C. K. Vesborg, J. Doganli-Kibsgaard and I. Chorkendorff, ChemSusChem, 2023, 16, 1–9 CrossRef.
- R. Y. Hodgetts, H. L. Du, T. D. Nguyen, D. Macfarlane and A. N. Simonov, ACS Catal., 2022, 12, 5231–5246 CrossRef CAS.
- J. K. Nørskov, J. Rossmeisl, A. Logadottir, L. Lindqvist, J. R. Kitchin, T. Bligaard and H. Jónsson, J. Phys. Chem. B, 2004, 108, 17886–17892 CrossRef.
- U. Benedikt, W. B. Schneider and A. A. Auer, Phys. Chem. Chem. Phys., 2013, 15, 2712 RSC.
- K. Schwarz and R. Sundararaman, Surf. Sci. Rep., 2020, 75, 100492 CrossRef CAS.
- A. Y. Lozovoi, A. Alavi, J. Kohanoff and R. M. Lynden-Bell, J. Chem. Phys., 2001, 115, 1661–1669 CrossRef CAS.
- C. D. Taylor, S. A. Wasileski, J.-S. Filhol and M. Neurock, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 165402 CrossRef.
- A. Y. Lozovoi and A. Alavi, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 68, 245416 CrossRef.
- J. A. Gauthier, S. Ringe, C. F. Dickens, A. J. Garza, A. T. Bell, M. Head-Gordon, J. K. Nørskov and K. Chan, ACS Catal., 2019, 9, 920–931 CrossRef CAS.
- D. Gunceler, K. Letchworth-Weaver, R. Sundararaman, K. A. Schwarz and T. A. Arias, Modell. Simul. Mater. Sci. Eng., 2013, 21, 074005 CrossRef.
- S. Ringe, N. G. Hörmann, H. Oberhofer and K. Reuter, Chem. Rev., 2022, 122, 10777–10820 CrossRef CAS PubMed.
- O. Andreussi, I. Dabo and N. Marzari, J. Chem. Phys., 2012, 136, 234117 CrossRef.
- K. Mathew, R. Sundararaman, K. Letchworth-Weaver, T. A. Arias and R. G. Hennig, J. Chem. Phys., 2014, 140, 0–8 CrossRef CAS.
- K. Mathew, V. S. C. Kolluru, S. Mula, S. N. Steinmann and R. G. Hennig, J. Chem. Phys., 2019, 151, 234101 CrossRef.
- R. Sundararaman, K. Letchworth-Weaver, K. A. Schwarz, D. Gunceler, Y. Ozhabes and T. A. Arias, SoftwareX, 2017, 6, 278–284 CrossRef.
- O. Andreussi, N. G. Hörmann, F. Nattino, G. Fisicaro, S. Goedecker and N. Marzari, J. Chem. Theory Comput., 2019, 15, 1996–2009 CrossRef CAS PubMed.
- S. M. R. Islam, F. Khezeli, S. Ringe and C. Plaisance, J. Chem. Phys., 2023, 159, 234117 CrossRef CAS PubMed.
- R. Sundararaman, K. Letchworth-Weaver and K. A. Schwarz, J. Chem. Phys., 2018, 148, 144105 CrossRef.
- X. Zhao and Y. Liu, J. Am. Chem. Soc., 2021, 143, 9423–9428 CrossRef CAS PubMed.
- Z. Levell, J. Le, S. Yu, R. Wang, S. Ethirajan, R. Rana, A. Kulkarni, J. Resasco, D. Lu, J. Cheng and Y. Liu, Chem. Rev., 2024, 124, 8620–8656 CrossRef CAS PubMed.
- X. Bai, X. Zhao, Y. Zhang, C. Ling, Y. Zhou, J. Wang and Y. Liu, J. Am. Chem. Soc., 2022, 144, 17140–17148 CrossRef CAS PubMed.
- X. Zhao and Y. Liu, J. Am. Chem. Soc., 2020, 142, 5773–5777 CrossRef CAS.
- S. Yu, Z. Levell, Z. Jiang, X. Zhao and Y. Liu, J. Am. Chem. Soc., 2023, 145, 25352–25356 CrossRef CAS.
- E. Skúlason, G. S. Karlberg, J. Rossmeisl, T. Bligaard, J. Greeley, H. Jónsson and J. K. Nørskov, Phys. Chem. Chem. Phys., 2007, 9, 3241–3250 RSC.
- A. H. Motagamwala, M. R. Ball and J. A. Dumesic, Annu. Rev. Chem. Biomol. Eng., 2018, 9, 413–450 CrossRef PubMed.
- J. Chen, M. Jia, P. Hu and H. Wang, J. Comput. Chem., 2021, 42, 379–391 CrossRef CAS.
- E. D. Hermes, A. N. Janes and J. R. Schmidt, J. Chem. Phys., 2019, 151, 014112 CrossRef PubMed.
- A. J. Medford, C. Shi, M. J. Hoffmann, A. C. Lausche, S. R. Fitzgibbon, T. Bligaard and J. K. Nørskov, Catal. Lett., 2015, 145, 794–807 CrossRef CAS.
- I. A. W. Filot, R. A. Van Santen and E. J. M. Hensen, Angew. Chem., Int. Ed., 2014, 53, 12746–12750 CrossRef CAS.
- A. Bajpai, K. Frey and W. F. Schneider, Langmuir, 2020, 36, 465–474 CrossRef CAS PubMed.
- A. H. Motagamwala and J. A. Dumesic, Chem. Rev., 2021, 121, 1049–1076 CrossRef CAS.
- B. W. J. Chen, L. Xu and M. Mavrikakis, Chem. Rev., 2021, 121, 1007–1048 CrossRef CAS PubMed.
- N. R. Singstock and C. B. Musgrave, J. Am. Chem. Soc., 2022, 144, 12800–12806 CrossRef CAS PubMed.
- K. Lu, F. Xia, B. Li, Y. Liu, I. B. Abdul Razak, S. Gao, J. Kaelin, D. E. Brown and Y. Cheng, ACS Nano, 2021, 15, 16887–16895 CrossRef CAS PubMed.
- C. Choi, G. H. Gu, J. Noh, H. S. Park and Y. Jung, Nat. Commun., 2021, 12, 4353 CrossRef CAS PubMed.
- L. Jiang, X. Bai, X. Zhi and Y. Jiao, Adv. Energy Mater., 2024, 14, 1–11 Search PubMed.
- M. Pineda and M. Stamatakis, J. Chem. Phys., 2022, 156, 120902 CrossRef CAS.
- M. Andersen, C. Panosetti and K. Reuter, Front. Chem., 2019, 7, 1–24 CrossRef.
- J. Li, I. Maresi, Y. Lum and J. W. Ager, J. Chem. Phys., 2021, 155, 164701 CrossRef CAS.
- T. P. Schulze, J. Comput. Phys., 2008, 227, 2455–2462 CrossRef.
- A. F. Voter, Radiat. Eff. Solids, 2007, 1–23 Search PubMed.
- R. Harris and M. Grant, J. Phys. A: Gen. Phys., 1990, 23, 2–7 CrossRef.
- M. Biehl, Multiscale Model. Ep. Growth, 2005, pp. 3–18 Search PubMed.
- D. Raabe, Acta Mater., 2000, 48, 1617–1628 CrossRef CAS.
- W. E. Frazier, S. Hu and V. V. Joshi, Comput. Mater. Sci., 2020, 185, 109945 CrossRef.
- C. H. Lee, S. Pahari, N. Sitapure, M. A. Barteau and J. S. I. Kwon, ACS Catal., 2022, 12, 15609–15617 CrossRef CAS.
- M. Bin Jassar, C. Michel, S. Abada, T. De Bruin, S. Tant, C. Nieto-Draghi and S. N. Steinmann, ACS Appl. Energy Mater., 2023, 6, 6934–6945 CrossRef CAS.
- Q. Wu, M. T. McDowell and Y. Qi, J. Am. Chem. Soc., 2023, 145, 2473–2484 CrossRef CAS PubMed.
- A. Wang, S. Kadam, H. Li, S. Shi and Y. Qi, npj Comput. Mater., 2018, 4, 15 CrossRef.
- G. Fan, W. Xu, J. Li, Y. Ni, M. Yu, F. Liu and F. Cheng, J. Phys. Chem. C, 2022, 126, 13629–13639 CrossRef CAS.
- S. Schnur and A. Groß, New J. Phys., 2009, 11, 125003 CrossRef.
- E. Cea-Klapp, J. M. Míguez, P. Gómez-Álvarez, F. J. Blas, H. Quinteros-Lama and J. M. Garrido, Phys. Chem. Chem. Phys., 2020, 22, 27121–27133 RSC.
- N. Yao, X. Chen, Z. H. Fu and Q. Zhang, Chem. Rev., 2022, 122, 10970–11021 CrossRef CAS PubMed.
- J. Le, M. Iannuzzi, A. Cuesta and J. Cheng, Phys. Rev. Lett., 2017, 119, 1–6 CrossRef.
- S. Surendralal, M. Todorova and J. Neugebauer, Phys. Rev. Lett., 2021, 126, 166802 CrossRef CAS.
- X. Y. Li, A. Chen, X. H. Yang, J. X. Zhu, J. B. Le and J. Cheng, J. Phys. Chem. Lett., 2021, 12, 7299–7304 CrossRef CAS PubMed.
- J. B. Le, X. H. Yang, Y. B. Zhuang, M. Jia and J. Cheng, J. Phys. Chem. Lett., 2021, 12, 8924–8931 CrossRef CAS PubMed.
- J. Cheng, X. Liu, J. VandeVondele, M. Sulpizi and M. Sprik, Acc. Chem. Res., 2014, 47, 3522–3529 CrossRef CAS.
- Encyclopedia of Applied and Computational Mathematics, ed. B. Engquist, Springer Berlin Heidelberg, Berlin, Heidelberg, 2015 Search PubMed.
- A. Laio and M. Parrinello, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 12562–12566 CrossRef CAS.
- A. Laio and F. L. Gervasio, Rep. Prog. Phys., 2008, 71, 126601 CrossRef.
- M. C. O. Monteiro, F. Dattila, B. Hagedoorn, R. García-Muelas, N. López and M. T. M. Koper, Nat. Catal., 2021, 4, 654–662 CrossRef CAS.
- P. Li, Y. Jiang, Y. Hu, Y. Men, Y. Liu, W. Cai and S. Chen, Nat. Catal., 2022, 5, 900–911 CrossRef CAS.
- J. B. Le, Q. Y. Fan, J. Q. Li and J. Cheng, Sci. Adv., 2020, 6, 1–8 Search PubMed.
- S.-J. Qian, H. Cao, J.-W. Chen, J.-C. Chen, Y.-G. Wang and J. Li, ACS Catal., 2022, 12, 11530–11540 CrossRef CAS.
- X. Mao, X. Bai, G. Wu, Q. Qin, A. P. O’Mullane, Y. Jiao and A. Du, J. Am. Chem. Soc., 2024, 146, 18743–18752 CrossRef CAS PubMed.
- D. Maniscalco, D. A. Rudolph, E. Nadimi and I. Frank, Nitrogen, 2022, 3, 404–413 CrossRef CAS.
- T. Mueller, A. Hernandez and C. Wang, J. Chem. Phys., 2020, 152, 050902 CrossRef CAS PubMed.
- J. Behler and M. Parrinello, Phys. Rev. Lett., 2007, 98, 1–4 CrossRef PubMed.
- A. P. Thompson, L. P. Swiler, C. R. Trott, S. M. Foiles and G. J. Tucker, J. Comput. Phys., 2015, 285, 316–330 CrossRef CAS.
- A. P. Bartók, M. C. Payne, R. Kondor and G. Csányi, Phys. Rev. Lett., 2010, 104, 1–4 CrossRef.
- A. P. Bartók, R. Kondor and G. Csányi, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 1–16 Search PubMed.
- T. Wen, L. Zhang, H. Wang, E. Winan and D. J. Srolovitz, Mater. Futures, 2022, 1, 022601 CrossRef CAS.
- C. Schran, F. L. Thiemann, P. Rowe, E. A. Müller, O. Marsalek and A. Michaelides, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, 1–8 CrossRef.
- V. Kapil, C. Schran, A. Zen, J. Chen, C. J. Pickard and A. Michaelides, Nature, 2022, 609, 512–516 CrossRef CAS PubMed.
- B. Lin, J. Jiang, X. C. Zeng and L. Li, Nat. Commun., 2023, 14, 4110 CrossRef CAS PubMed.
- G. Soloveichik, ARPA-e, 2016, pp. 1–16. https://arpa-e.energy.gov/?q=arpa-e-programs/refuel.
- D. Jin, A. Chen and B. L. Lin, J. Am. Chem. Soc., 2024, 146, 10–13 Search PubMed.
- X. Fu, V. A. Niemann, Y. Zhou, S. Li, K. Zhang, J. B. Pedersen, M. Saccoccio, S. Z. Andersen, K. Enemark-Rasmussen, P. Benedek, A. Xu, N. H. Deissler, J. B. V. Mygind, A. C. Nielander, J. Kibsgaard, P. C. K. Vesborg, J. K. Nørskov, T. F. Jaramillo and I. Chorkendorff, Nat. Mater., 2024, 23, 101–107 CrossRef CAS PubMed.
- M. Krebsz, R. Y. Hodgetts, S. Johnston, C. K. Nguyen, Y. Hora, D. R. MacFarlane and A. N. Simonov, Energy Environ. Sci., 2024, 17, 4481–4487 RSC.
- J. Gallagher, Nat. Energy, 2024, 9, 627 CrossRef.
- Y. Chen, C. Ye, N. Zhang, J. Liu, H. Li, K. Davey and S. Z. Qiao, Mater. Today, 2024, 73, 260–274 CrossRef CAS.
- X. Cai and J. Zhang, Nat. Energy, 2024, 9, 629–630 CrossRef CAS.
- X. Cai, X. Li, J. You, F. Yang, Z. Shadike, S. Qin, L. Luo, Y. Guo, X. Yan, S. Shen, G. Wei, Z. J. Xu and J. Zhang, J. Am. Chem. Soc., 2023, 145, 25716–25725 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |



