Emerging membrane technologies and disinfection methods for efficient removal of waterborne pathogens during the COVID-19 pandemic and post-pandemic
Shadpour
Mallakpour
 *a,
Elham
Azadi
a and
Chaudhery Mustansar
Hussain
*a,
Elham
Azadi
a and
Chaudhery Mustansar
Hussain
 b
b
aOrganic Polymer Chemistry Research Laboratory, Department of Chemistry, Isfahan University of Technology, Isfahan, 84156-83111, Islamic Republic of Iran. E-mail: mallak@iut.ac.ir; mallak777@yahoo.com; Fax: +98-31-3391-2350; Tel: +98-31-3391-3267
bDepartment of Chemistry and Environmental Science, New Jersey Institute of Technology, Newark, NJ 07102, USA
First published on 16th November 2022
Abstract
Viruses and other microorganisms can enter water sources from different routes and cause pollution and irreparable damage. So, cost-effective and efficient systems for providing safe water are necessary. Efficient filtration systems based on antimicrobial materials have received a lot of attention in this regard. A wide range of materials play an important role in the production of efficient water filtration systems. Metal and metal oxide particles with anti-viral and antimicrobial properties comprising Cu, Cu2O, Ag, TiO2, and ZnO play a valuable role in the preparation of water filtration systems. Biopolymers such as cellulose or carbon nanomaterials like graphene or its derivatives have been reported to provide safe water. In this review, we summarize the use of diverse materials in the preparation of efficient filtration-based systems like membranes and paper filters for water treatment. Pathogen-containing water samples were effectively disinfected using the prepared water disinfection systems.
1. Introduction
Water is the main element on the earth required for the life of all living beings. Today, providing unpolluted and healthy water is a challenge for communities. In some parts of the earth, countless people still do not have access to safe drinking water. Furthermore, the surface water may be contaminated with microorganisms and its consumption can cause gastrointestinal infections and may even lead to death. The COVID-19 outbreak, which was caused by the SARS-CoV-2 virus, has resulted in great global concern in the scientific and health communities, and in the general public.1–3 In particular, more recently a new variant of this virus, the Delta variant, which was first identified in India, has been spreading rapidly throughout the world. To date, it has been reported in more than 80 countries. This has caused rising concern, as these variant types may be able to bypass vaccine-based immunity.4,5With the spread of the epidemic, the presence of SARS-CoV-2 in the environment is increasing. Recently, researchers reported the trace detection of this virus in medical wastewater, river water, municipal sewage, secondary-treated wastewater, and so on. Therefore, further studies are required in this regard. For virus-infected communities living in buildings and apartments, a sanitary (or wastewater) plumbing system might be a potential route for SARS-CoV-2 transmission (in addition to transmission through aerosols as well as surfaces such as the inner wall of the sink and toilet bowl) and environmental contamination and COVID-19 spread (Fig. 1a). Therefore, a series of suggestions have been reported in this regard to prevent the spread of this epidemic through piping systems. Furthermore, face masks discarded after use by the general public, patients, and health workers can enter the aqueous environment such as surface water/river water and cause water pollution, thus creating a new transmitting passageway (Fig. 1b). It is necessary to manage discarded masks that provide a possible transmission route for SARS-CoV-2, particularly in developing regions or areas that report high rates of the disease and infected patients.6–12
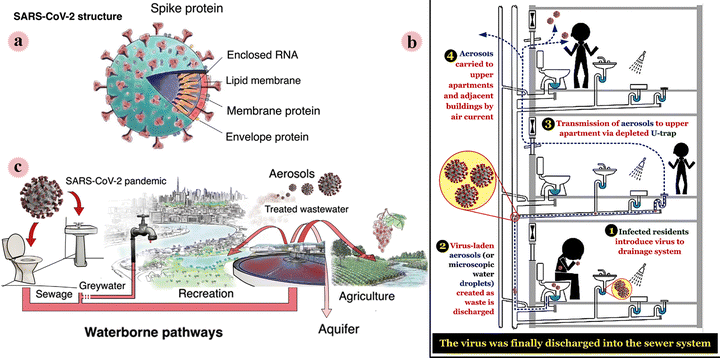 | ||
| Fig. 1 (a) Schematic illustration of the main structural features of SARS-CoV-2. (b) A transmission route of SARS-CoV-1 virus (possible for SARS-CoV-2 virus) in buildings through the sanitary (or wastewater) plumbing system. (c) Overview of potential SARS-CoV-2 dissemination via waterborne pathways in industrialized countries. This figure has been reproduced from ref. 6 and 12 with permission from Elsevier and Springer, copyright 2021 and 2020. | ||
According to studies, viruses can survive on plants irrigated with wastewater for a period of time (depending on conditions such as sunlight and heat). Therefore, irrigating golf courses and parks, as well as agricultural fields with sewage water that is contaminated with the virus may transmit the disease to players and even people and children in the parks. Fig. 2 shows the effects of using virus-containing wastewater for agricultural irrigation. Hence, during SARS-CoV-2 epidemics, wastewater, as a sustainable source of fresh water, is an indirect infection passageway to transmit disease. Therefore, this issue should be further investigated.13
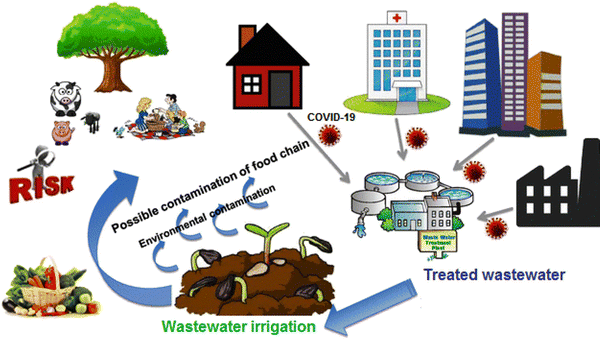 | ||
| Fig. 2 The potential implications of reclaimed wastewater reuse for irrigation on the agricultural environment. This figure has been reproduced from ref. 13 with permission from Elsevier, copyright 2021. | ||
Since infectious diseases can be transmitted through water and increase mortality, the need for water purification and the provision of safe water is essential, especially in developing countries, and countries where pollution is greater due to a lack of sanitation. Today, with the help of filtration technologies as well as water disinfection using materials with antimicrobial properties, safe water can be provided. Nanotechnology is expanding rapidly, and much progress has been made in providing antimicrobial materials for controlling water pollution. Diverse metals and metal oxides have been used in this regard. Furthermore, carbon nanomaterials and biopolymers are good candidates for fabrication filtration systems.14–21 This review outlines the use of diverse materials for water treatment to reduce contamination and infectious diseases.
2. Different materials for water treatment
2.1. Metal and metal oxide particles
For the purification of drinking water and to provide safe and healthy water for people existing in poor areas using affordable and effective technology, Yu et al. prepared a filtration membrane by employing zwitterionic Ag nanoparticles and PCBDA [poly(carboxybetaine acrylate-co-dopamine methacryamide)] copolymers.25 PCBDA copolymer was selected as a reducing/stabilizing agent (due to the robust coordination capability of catechol groups to the surface of the metal) to fabricate zwitterionic PCBDA@Ag nanoparticles in a facile one-step process. Then, via mussel-inspired chemistry, robust and uniform immobilization of zwitterionic PCBDA@Ag nanoparticles on polyamide membranes (interactions among amino and catechol groups) was performed. For this aim, a polyamide microfiltration membrane was placed in hydrochloric acid. After incubation (30 min) to expose more NH2 groups, the membrane was rinsed via water until pH = 7. After that, this membrane was soaked in PCBDA@Ag and finally, was washed with water. As can be observed in Fig. 3a, by mussel-inspired chemistry, catechol moieties related to PCBDA@Ag were reacted to amino groups of polyamide membrane via covalent and non-covalent interactions including Schiff base reaction/Michael-addition and robust H-bonding, respectively resulting in robust immobilization of Ag onto microfiltration. The contact killing efficiency and bacterial anti-adhesion ability of the prepared PCBDA@Ag microfiltration against E. coli, S. aureus, MRSA, and P. aeruginosa was evaluated. Owing to the antimicrobial performance of surface-immobilized Ag, the fabricated membrane showed anti-adhesive, good performance against microbes, and simple elimination of disabled microbes from the membrane surface. As illustrated in Fig. 3b, the released Ag+ induced bacterial membrane destruction, hinders ATP-making, and destroys the replication activities of DNA. Furthermore, excessive ROS generated via electron transfer between the substrate and immobilized Ag leads to peroxidation, membrane permeability, and oxidative damage. So, due to the synergistic effect of Ag+ and ROS, PCBDA@Ag microfiltration showed robust antibacterial activity (higher than 99.9%). Pathogen-containing water was effectively disinfected by the prepared water disinfection device. Kill and remove strategies of the membrane are shown in Fig. 3c. Via the ‘‘anti-adhesion-kill-remove” strategy (as illustrated in Fig. 3c), PCBDA@Ag microfiltration exhibited an anti-biofouling feature and successfully inhibited the formation of biofilm.
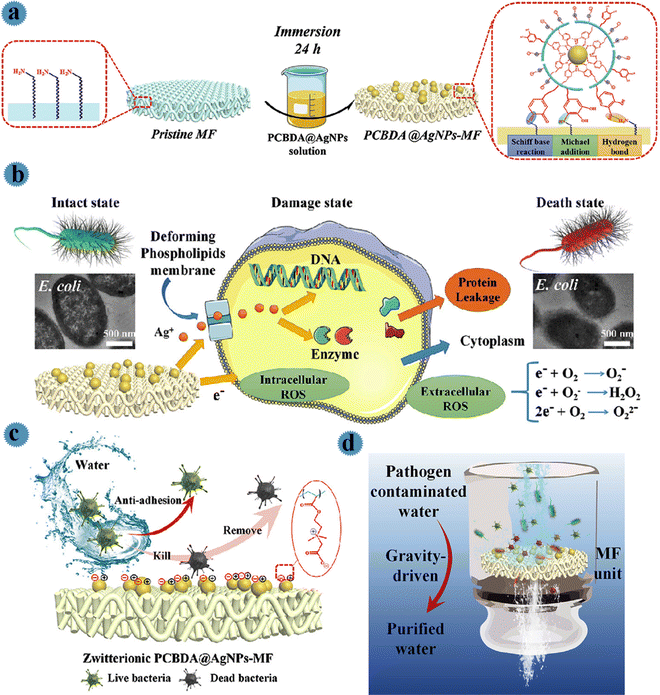 | ||
| Fig. 3 (a) Schematic illustration of the preparation processes of PCBDA@AgNPs-MF. (b) The schematic illustration of the mechanism of antibacterial activity of PCBDA@AgNPs-MF. (c) Chematic illustration of kill and remove strategies with the zwitterionic surface of PCBDA@AgNPs-MF. (d) Schematic water disinfection performance of PCBDA@AgNPs-MF. This figure has been reproduced from ref. 13 with permission from Elsevier, copyright 2021. | ||
The performance of a PCBDA@AgNPs microfiltration membrane for water disinfection was studied via a gravity-driven filtration process. For this aim, 100 mL of pathogen-contaminated feed water (high bacterial concentration: 106 CFU mL−1) was prepared. The prepared membrane with a diameter of 4 cm was placed in filter units, and the bacteria-contaminated water permeated through the surface of the membrane with a certain height of feed water (10 cm). During the filtration process, bacteria in water can be inactivated in contact with the membrane (Fig. 3d).
Yang and co-workers developed an antimicrobial membrane by Ag nanoparticles anchoring wood carbon, and used it as an affordable, high flux, and scalable filter for greatly effective water disinfection.26 In this study, cost-effective natural wood (balsa wood), with 3D porous and aligned channels, was used as an effective matrix for water disinfection. Besides, wood carbon channels contain carbonized nano-fibrils with outstanding biological/electronic features that provide distinctive nano-scaled tips construction for the disinfection process. Ag nanoparticles with great antimicrobial activity and bio-compatibility powerfully interacted with vital microorganisms and inactivated them. The fabrication process of the Ag/wood carbon-based filter is shown in Fig. 4. Via a simple approach, well-dispersed nano-Ag particles were in situ prepared in a wood carbon matrix (Fig. 4a). At first, balsa wood was soaked in AgNO3 solution for penetration of the precursor. After drying the AgNO3/wood composite, it was carbonized, and Ag+ was reduced to Ag by carbon (during carbonization), and Ag nanoparticles were homogeneously formed in wood channels. The electric field sterilization device and antimicrobial behavior of the designed membrane can be observed in Fig. 4c. A homemade device for water cleansing was prepared and its action was tested. It disinfected more than 5 log (99.999%) of diverse microorganisms comprising E. coli, E. faecalis, S. typhimurium, and B. subtilis in 4 V voltage. Its other advantages were fantastic durability (7 days), low energy consumption (2 J L−1 m−2), and high flux (3.8 × 103 L m−2 h−1). The great performance of the fabricating system was due to the synergism of Ag nanoparticles, carbon nano-fibrils, and the low tortuous construction of carbon channels.
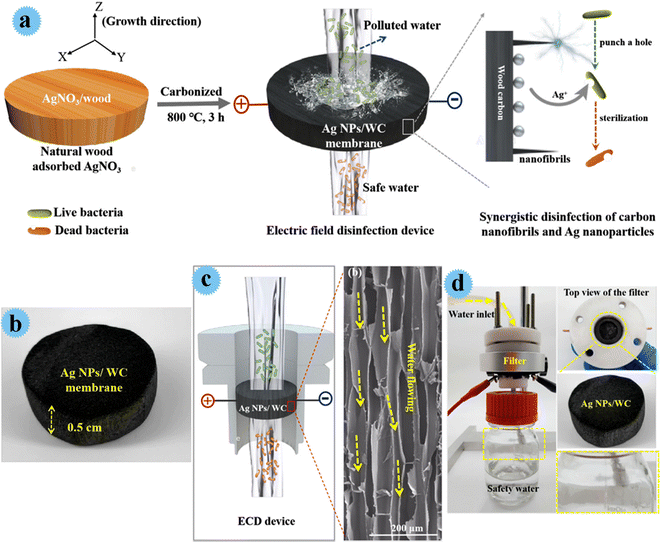 | ||
| Fig. 4 (a) A piece of balsa wood adsorbs the precursor of AgNO3 and the electric field disinfection device using a Ag NPs/WC membrane. (b) Photo image of the 3D Ag NPs/WC membrane. (c) Schematic of the electric field disinfection device and the high-resolution SEM image of the carbon-wood membrane, which shows long and arrayed channels in the radial section of the wood. (d) Photo image of the experimental setup for the electric field disinfection. This figure has been reproduced from ref. 26 with permission from Elsevier, copyright 2021. | ||
In another study, Ag nanoparticles were decorated on the nano-fibrous membrane including poly(vinyl alcohol-co-ethylene) via charge adsorption using the polydopamine/polyethyleneimine layer on the surface of the nano-fiber for great inactivation/interception of bacteria in water (Fig. 5).27 The antimicrobial, anti-fouling, mechanical, and filtration performances of the membrane were examined. Filtering and sterilizing of the bacteria can be observed in Fig. 5c, which was carried out on the benchtop. Moreover, after attaching the membrane with E. coli and S. aureus bacteria, they were inactivated successfully at a 99.99% rate due to the exceptional ability of Ag toward microorganisms. In comparison to control samples, the developed membrane presented an outstanding repetitive-use filtration performance after the back-washing test, which showed anti-bio-fouling features.
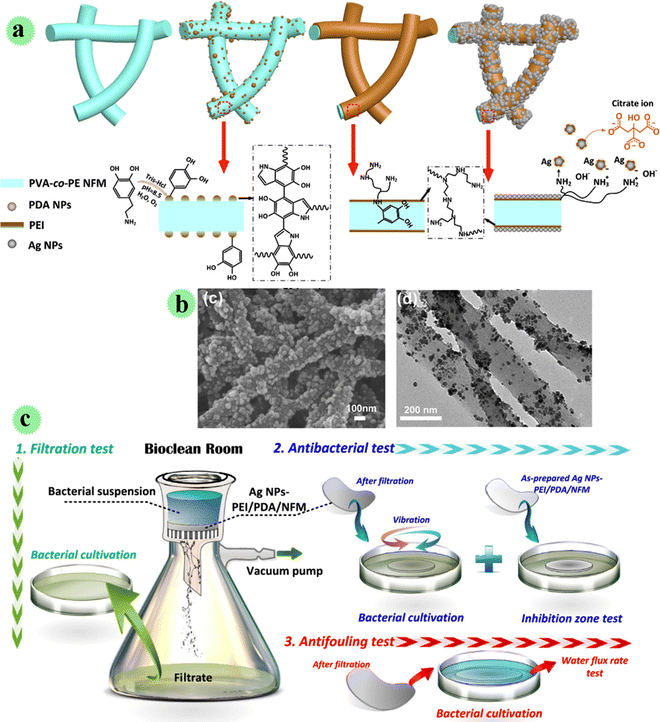 | ||
| Fig. 5 (a) Preparation of AgNPs-PEI/PDA/NFM and its serial precursors. (b) SEM microscopy and TEM microscopy. (c) Interception, inactivation and antifouling test of Ag/PEI/PDA/NFM on bacteria. This figure has been reproduced from ref. 27 with permission from Elsevier, copyright 2021. | ||
According to the WHO guidelines, drinking water should contain less than 100 CFU mL−1 pathogenic bacteria. However, in developing countries, due to poverty and lack of sanitation equipment, >30% of people are deprived of clean water, and this issue causes waterborne diseases. Recently, efficient, cost-effective, antimicrobial filtration-based point-of-use sterilization nano-systems have been developed to clean drinking water. In this regard, in 2020, Liu and co-workers prepared a biomass-based filter as an inexpensive bactericidal/anti-fouling filter to decontaminate water. In this study, gallic acid-protected Ag nanoparticles as water disinfectants were decorated onto flexible collagen fibers (as membrane-building materials with hydrophilic carboxyl, hydroxyl, and amino groups).28 The loading stability of Ag nanoparticles, antibacterial performance, anti-bio-fouling feature, and disinfection behavior of the prepared filter were examined in gravity-driven conditions. A thin filter with 3D porous network construction, respectable mechanical strength, strongly immobilized Ag particles, and excellent bactericidal activity was prepared by employing an Fe(III) cross-linker (Fig. 6). By formation of a complex between Fe3+ and carboxyl groups, as well as cross-linking of fibers/gallic acid/nano-Ag, a micro-porous system with good mechanical behavior was produced. Owing to the existence of anionic gallic acid, the prepared film showed a negatively charged surface. So, this film exhibited bactericidal activity and a bacterial anti-adhesive performance due to the slow release of Ag+ and negative surface. Approximately all microorganisms were killed within 20 min by the prepared safe water purification device. Also, the leaching of ions (Ag+ and Fe3+) was lower in drinking water standards.
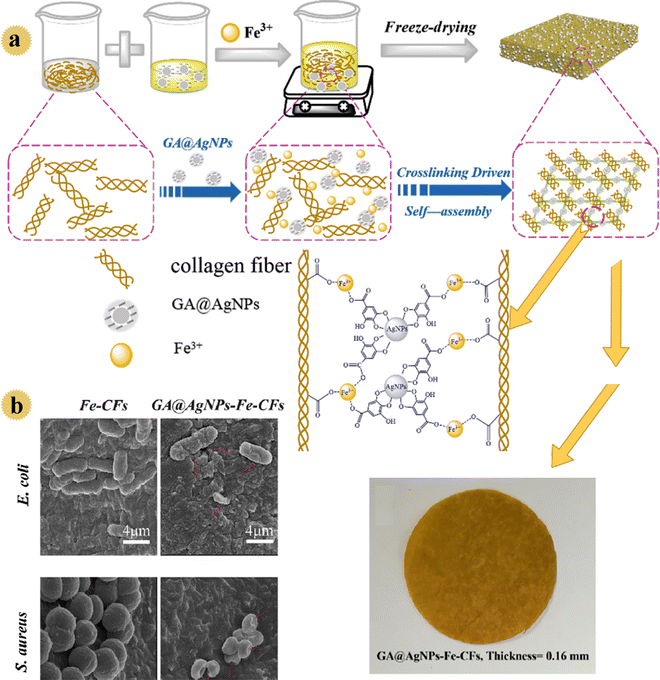 | ||
| Fig. 6 (a) Schematic illustration of the self-assembly of the GA@AgNPs-Fe-CF film with 3D interconnected porous network structures and photographs of the GA@AgNPs-Fe-CFs. (b) SEM images of E. coli and S. aureus cells in contact with the Fe-CF and GA@AgNPs-Fe-CF films. This figure has been reproduced from ref. 28 with permission from American Chemical Society, copyright 2020. | ||
Since Ag nanoparticles are a very hopeful antimicrobial agent, they have been broadly used for incorporation in nano-fibers for efficient water filtration and bio-fouling mitigation. The fabricated membranes by Ag nanoparticles are very effective toward a broad range of microorganisms, so, they show an improved anti-bio-fouling performance. Besides, the surface hydrophilicity of membranes is increased via the insertion of Ag nanoparticles, thus, a higher water flux, as well as enhanced antimicrobial performance, can be achieved. In a recent study, Pan et al. decorated an electrospun polyacrylonitrile nano-fiber mat with Ag nanoparticles for the preparation of an antimicrobial thin-film composite as a support of a forwarding osmosis membrane.29 For this aim, in a facile process, Ag nanoparticles were in situ synthesized on/in nano-fibers. Polyacrylonitrile was selected as a support due to many good features like good electrospinnability and great tensile strength after electrospinning. The fabrication process of the antimicrobial membrane is shown in Fig. 7; using an electrospinning method, AgNO3/polyacrylonitrile nano-fiber was prepared. After that, it was laminated and immersed in N2H4·H2O solution for the production of Ag0 from Ag+. Then, through interfacial polymerization, a polyamide layer was placed on Ag/polyacrylonitrile, and the membrane was obtained. The influence of Ag on antimicrobial activity, water flux, physicochemical features, and reverse salt flux was examined. The outcomes disclosed a scaffold-like structure of the Ag/polyacrylonitrile nano-fiber support. The prepared membrane exhibited great hydrophilicity and high water flux. Even after using low Ag content (0.5 wt%), excellent activity toward microorganisms [E. coli (96%) and S. aureus (92%)] was seen, in which the antimicrobial performance was mostly due to the released Ag+ species (Fig. 7b).
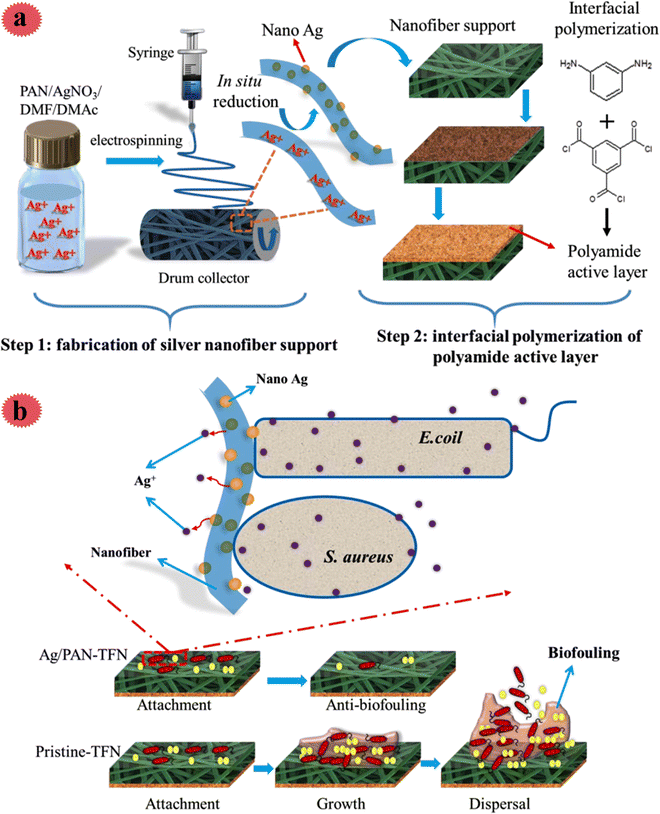 | ||
| Fig. 7 (a) Schematic diagram of the preparation of the silver/polyacrylonitrile nanofiber thin-film nanocomposite (Ag/PAN-TFN) FO membranes. (b) Schematic diagram of the antibacterial mechanism of the Ag/PAN-TFN FO membrane. This figure has been reproduced from ref. 29 with permission from American Chemical Society, copyright 2019. | ||
In order to provide safe and affordable water, Liu et al. made cellulose filter paper with antimicrobial properties.30 Ag nanoparticles were decorated onto the cellulose filter paper for the fabrication of a water sterilization system. For stable and robust immobilization of Ag onto the filter and to prevent leaching into the treated drinking water, the cellulose filter paper was modified with natural lipoic acid via esterification reaction among functional groups of lipoic acid (–COOH) and b-glucose units (–OH) of filter paper (Fig. 8a). Anionic Ag nanoparticles (Ag/galic acid) were strongly immobilized on the filter through strong chelation of the Ag surface and disulfide connections of lipoic acid. The prepared filtration system showed good anti-adhesion and strong performance for microorganisms that inhibited bio-film formation. Besides, the leached Ag+ from the filter was in the standard amount. The potential use of the fabricated antimicrobial filter for disinfection was tested by employing natural river water (Fig. 8e). 100 mL of river water was treated in 10 min by this filter. The resulting water quality was in line with WHO drinking water standards, which indicated the practical use for disinfection of drinking water.
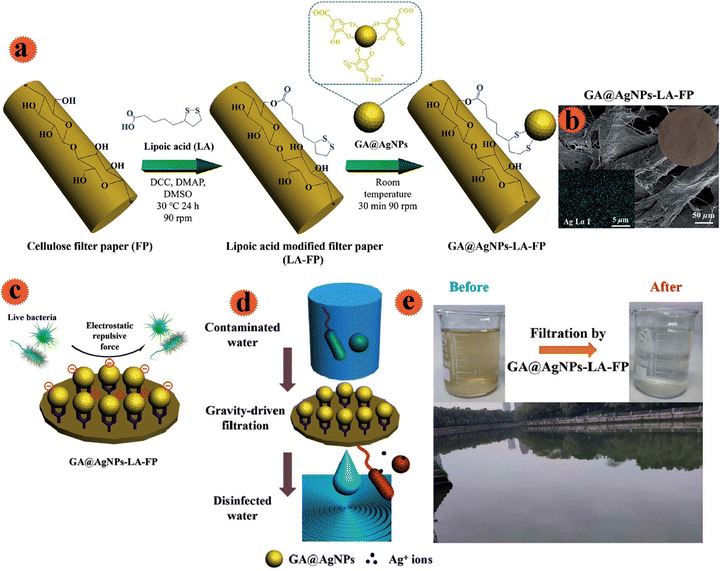 | ||
| Fig. 8 (a) Schematic illustration of the robust immobilization of GA@AgNPs on a lipoic acid-modified filter paper surface. (b) SEM images of the surface morphologies of GA@AgNPs-LA-FP. (c) Schematic of the anti-adhesive action of the GA@AgNPs-LA-FP. (d) Schematic POU water disinfection performance of GA@AgNPs-LA-FP. (e) Photographs showing the appearance of natural river water before and after treatment with GA@AgNPs-LA-FP. This figure has been reproduced from ref. 30 with permission from Royal Society of Chemistry , copyright 2021. | ||
Also for the disinfection of water, Fan et al. prepared porous chitosan membrane-covered Ag nanoparticle embedding filter paper.31 Ag nanoparticles were in situ loaded on the filter through thermal reduction by employing a green reducing agent (glucose). The chitosan coating provided benefits, including excellent improvement in the durability of the filter under wet conditions (tensile strength was 1.8 MPa which shows a 700% increase), as well as preventing the total Ag release into clean water. The total Ag in disinfected water was <45 μg L−1, which was half of the standard amount (<100 μg L−1). Ag nanoparticles on the filter paper improved meaningfully the bactericidal performance. After using the 0.5 wt% chitosan covering, the filter paper showed good bactericidal effectiveness toward E. coli and B. subtilis (4 and 3.6 log reduction, respectively). When the content of chitosan was increased (from 0.5 to 2.0 wt%), the performance against microorganisms was improved (E. coli and B. subtilis was 4.9 and 4.8 log reduction, respectively).
In another study, an emergency drinking water filter was prepared by coating cellulose paper with Ag nanoparticles.32 This system had many advantages like being highly portable, having efficient performance, and was easy to use. Fabrication of the filter was performed based on the chemical reduction procedure by employing NaBH4 and AgNO3. Cellulose papers were immersed in diverse solutions of Ag ions (0.025, 0.05, and 0.10 M) for different times (1, 2, and 3 h). After rinsing cellulose filter paper with ethanol to flush un-absorbed AgNO3 for placing in NaBH4 media, it was washed and dried, and the filter was prepared. E. coli deletion efficacy was examined to test the filtration behavior. Based on the Box–Behnken Design results, the optimal parameters for the best E. coli removal effectiveness percentage were 0.035 M AgNO3, and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio for NaBH4
1 ratio for NaBH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AgNO3.
AgNO3.
Jiang and co-workers reported the facile fabrication of chitosan-covered nano-Ag particles inserted in cotton fabric.33 This study was performed through in situ thermal reduction of Ag salt. Chitosan was used to avoid the extreme release of Ag and improve the bactericidal effectiveness, and cotton fabric with high porosity was used as a supporting substrate. In comparison with substrates based on filter paper, cotton fabrics have outstanding mechanical strength (in wet and dry media). The prepared antimicrobial membrane was applied for water disinfection toward E. coli and B. subtilis. After using 1.5 wt% chitosan and 50 mg L−1 Ag, the antimicrobial effectiveness toward E. coli and B. subtilis was 4 and 3 log reductions, respectively. In the treated water, the total Ag was <20 mg L−1, which was lower than 100 mg L−1 (standard), so the prepared filter showed potential for drinking water disinfection.
Mishra and co-workers studied the performance of antimicrobial membranes based on polyvinylidene fluoride/Ag/TiO2 for water purification and removal of E. coli.34 Nano-Ag and TiO2 particles were employed as antimicrobial and anti-fouling functions for the membrane. After the insertion of nanoparticles, pure water flux was three-fold higher than the pristine membrane (465.8 L m−2 h−1), and faster removal of E. coli was observed.
In addition to the above, further studies have been conducted in this regard and the results show the positive effect and effective role of Ag nanoparticles in the preparation of filters and antimicrobial membranes for the preparation of safe drinking water.35–39 For example, Qi et al. in situ synthesized the nano-Ag on the surface of the filtration membrane in a facile and effective method.40 The fabricated Ag-based membranes revealed exceptional antibacterial features with >90% effectiveness toward both E. coli and S. aureus microorganisms. Zhao et al. reported the fabrication of 3D woven filters comprising nano-Ag doped nano-fibers in a membrane bio-reactor.41 Antimicrobial filters were prepared by wrapping the weft yarn with electrospun nano-fibers including nano-Ag (2 wt%) as a powerful antimicrobial agent. The prepared filter showed high flux and high antimicrobial performance.
Indeed, public health and safe drinking water are closely related. Viral, bacterial, and parasitic infections cause infectious diseases and endanger human health. One of the methods for disinfecting water is electrochemical water disinfection and this has been used so far due to its non-toxic byproducts and irreversible inactivation. After exposing microorganisms to an intense pulsed electric field, electroporation occurs and complete in-activation is achieved. However, large-scale use of these methods is limited due to the huge energy input required. In order to solve this problem 1D nanostructures have been used to achieve local development of the electric field (at lower energy input). Based on the above-mentioned discussions, more recently, Cu-based nano-wires (Cu7S4 nano-wires) covered with N-doped carbon film were fabricated on Cu foam using an easy solvothermal technique and carbonization method and used as a filter for water treatment. Covering carbon film improved the nano-wire conductivity for achieving great sterilization proficiency and developed the long-term stability of disinfection features. The construction process of the Cu7S4/carbon film is shown in Fig. 9a. The Cu foam was submerged in alkaline media and Cu(OH)2 nano-wires were created on its surface. Then, Cu7S4 nano-wires were prepared by vulcanization and hydrothermal treatment. The sterilization behavior of the fabricated filter was tested toward S. aureus and E. coli microorganisms. As can be observed in Fig. 9b, two key mechanisms of the flow sterilization were bacteria electroporation and the production of ROS. The sterilization rate could retain >99% in 12 h working under 1500 mL min−1 flow and a voltage of 10 V. The resultant filter possessed long-term effectiveness and stability in water decontamination.43
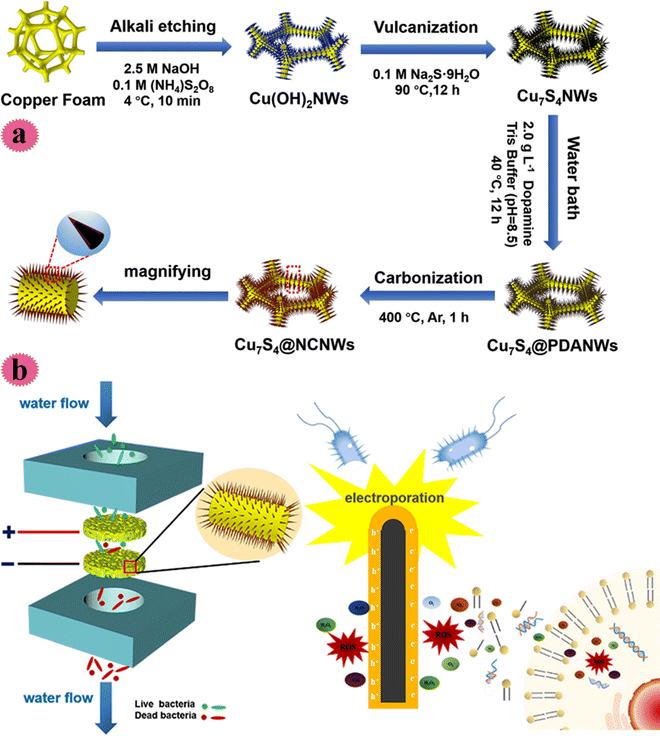 | ||
| Fig. 9 (a) Schematic synthesis process of the Cu7S4@NCNW electrode. (b) Synergistic mechanism of electroporation and ROS for the Cu7S4@NCNW electrode filtration system. This figure has been reproduced from ref. 43 with permission from American Chemical Society, copyright 2021. | ||
In a recent study, Wang et al. prepared branched CuO–Co3O4 nano-wires on Cu foam as a substrate.42 Then, a layer of carbon film was coated on nano-wires to obtain new filter CuO–Co3O4@C nano-wires for drinking water sterilization. Cu foam was selected due to its relatively low price, 3D-hole construction, and high electrical conductivity. CuO-based nano-wires with good features such as physicochemical features and high specific surface area were employed to improve the sterilization performance. Besides, Co3O4 is a low-cost, abundant, and important transition metal oxide, which has attracted extensive attention. Furthermore, the electrical conductivity and protection of the nano-wires can improve by using carbon film. Based on the outcomes from electroporation and ROS sterilization technology, the prepared filter showed good sterilization efficacy for S. aureus and E. coli (99.5%) (external voltage was 10 V and the flow rate was 1000 mL min−1). Also, outstanding bactericidal stability was observed for the filter, in which after continuous operation for 12 h, the effectiveness was >80%. The resultant disinfection filter showed rapid and long-time stability, which showed good potential for future applications.
Electroporation sterilization, as a low energy consumption and high-efficiency method with a considerable sterilization effect, has been presented for water disinfection, but its practical application is limited due to slow water treatment. So, Hao and co-workers organized a Ag/CuO nano-wire co-modified 3D nano-Cu composite for high-performance water disinfection. Through electrodeposition, Ag nanoparticles were homogeneously distributed on the CuO surface, and a cluster flower construction was formed to increase the nanowires' local density. Ag–CuO nano-wire nanocomposites enhanced the electric field strength and disinfection performance. It promoted an increase of active sites and ROS-based sterilization (indeed, more active sites cause more ROS for better sterilization) (Fig. 10). Owing to the synergism of high electric field intensity/ROS, the organized composite displayed a great performance (>99.9%) to inactivate bacterial and ultrahigh water flux under low voltage. The antibacterial action of the prepared Ag–CuO composite was studied using S. aureus and E. coli containing water. Also, a fluorescence microscope was used to measure the ROS level.46
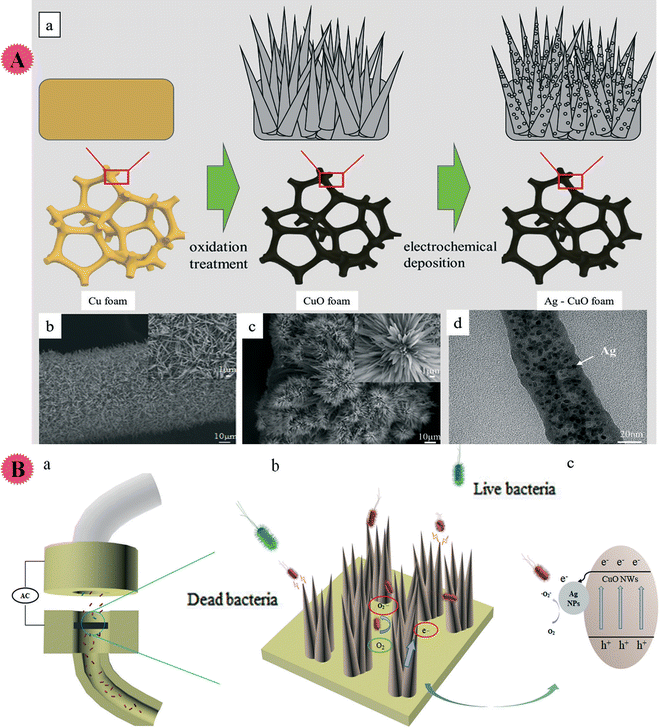 | ||
| Fig. 10 (A) (a) The Ag NP–CuO NW nanostructure synthetic process. Cu foam was primarily oxidized and turned into CuO foam; then, through the method of electrochemical deposition, Ag NPs coated the CuO surface. (b and c) The SEM images of CuO and Ag–CuO with the partially enlarged details on the right top corner respectively. (d) TEM image of the Ag NP–CuO NW nanostructure indicating that the diameter of the sharp nanowire is about 50 nm, and Ag NPs are uniformly distributed on the nanowire. (B) Illustrated summary of the mechanism of the process of electroporation disinfection with Ag–Cu NWs. (a) The structural composition of the electroporation disinfection model. (b) The electroporation model inactivation mechanism. When bacteria flow close to the tip of the nanowires, the high intensity electric field around the tip induces the formation of irreversible pores in the cell membrane; meanwhile, as the electrons flow along the material/solution interface, ROS (˙O2− and H2O2) are formed in the model (c). The two mechanisms promote the bacteria inactivation. This figure has been reproduced from ref. 46 with permission from Royal Society of Chemistry , copyright 2019. | ||
In a similar work, through in situ chemical oxidation, carbon/Cu2O–Ag nanoparticles were organized as an electrode for the fabrication of a water sterilization filter device.47 In this study, via a one-step impregnation process, nano-Ag and a layer of carbon were grown on Cu2O nano-wires. A water filter prepared with a carbon/Cu2O–Ag nanostructure displayed outstanding disinfection presentation (99.6% disinfecting speed) toward E. coli and S. aureus. Based on the outcomes, the effective disinfection with a high flow rate was due to the robust bond of Ag nanoparticles inserted in the carbon layer. Besides, improved electrode conductivity came from the developed electronic transmission between the connection of Ag nanoparticles and Cu2O nano-wires. The produced filter with rapid water disinfection showed great potential for practical use. The sterilization mechanism to kill bacteria was based on the synergism of bacteria electroporation and ROS production.
Szekeres et al. fabricated an efficient virus filter for water treatment. In this study, cheap organic materials were used to prepare high-performance point-of-use virus filters.48 By the papermaking method, well-organized porous Cu-covered nano-fibrillated cellulose was fabricated, and photos and microscopic micrographs of them can be observed in Fig. 11. Furthermore, the appropriate mass, the functionalization ratio of cellulose, and pure nano-fibrillated cellulose were optimized. As model human pathogenic virions, MS2 bacteriophages were tested. The outcomes showed verified the significant role of fixation of cellulose nano-fibers for virus retention degree and great enhancement in filtration efficiency. Three pH values (5.0, 7.5, and 9) were tested for flow experiments with 50 mg of Cu-functionalized filters of nano-fibrillated cellulose. Although these filters did not have high robustness, they showed virus retention of 3 and 4 magnitudes at pH = 7.5 and 9.0, and pH = 5.0, respectively. More promising results (at least 5 magnitudes) were observed for filters comprising double amounts of materials at all three pH values. So, the amount of Cu and nano-fibrillated cellulose played important roles in the fabrication of effective filters.
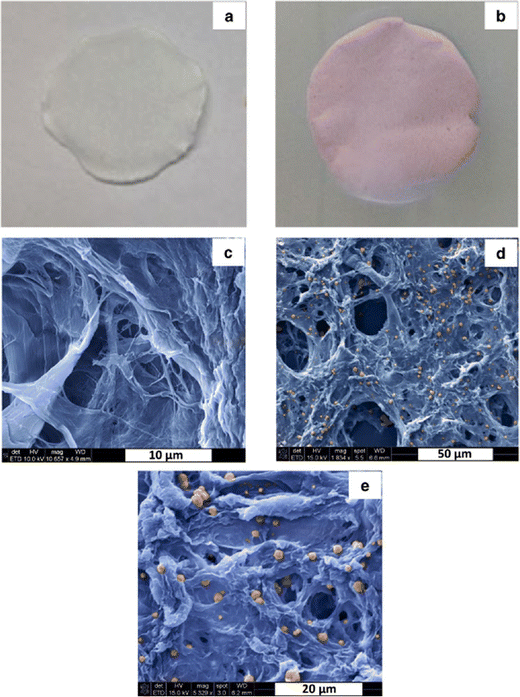 | ||
| Fig. 11 Photos and SEM micrographs of pure NFC paper (a and c) and Cu-NFC paper (b, d and e). This figure has been reproduced from ref. 48 with permission from American Chemical Society, copyright 2018. | ||
Like metals and metal oxides, MWCNTs (multi-walled carbon nanotubes) have shown good effectiveness for virus removal in drinking water uses.49 So, Domagała and co-workers coated Cu2O onto MWCNTs by three distinct production processes: Cu2+ ion attachment, Cu(OH)2 precipitation, and attachment of [Cu(NH3)4]2+ complex. Based on transmission electron microscopy Cu2O was distributed uniformly on the surface of the MWCNTs. The prepared filters based on nano-Cu2O/MWCNT composites were evaluated for virus removal (MS2 bacteriophages) before and after 24 h at pH 5-7. Based on the outcomes production method influenced on removal efficacy of the virus by Cu2O nanoparticle-coated MWCNTs. Besides, the virus removal mechanism was due to the electrostatic adsorption of the MS2, and the anti-viral features of copper (e.g., Cu2O nanoparticles) that endures the permeate act.
As a water disinfection process for the protection of humans from waterborne pathogens, Huo et al. developed an electroporation–disinfection cell with a maximum lifetime by employing a porous electrode based on polydopamine-protected Cu–phosphide nanowire-modified Cu foam to damage microorganisms.50 Cu foam with porous construction was selected due to its high conductivity, low price, and direct precursor for growth (in situ) of Cu–phosphide nano-wire. Indeed, this porous electrode meaningfully improved the disinfection effectiveness. The surface of the electrode was entirely covered with polydopamine and the electrode stability was improved, and the power source eliminated the electrochemical corrosion.
Deng et al. synthesized a Cu nanoparticle-decorated graphene sponge using a simplistic hydrothermal technique and used it as a novel bactericidal filter against E. coli (Gram-negative bacterium) for disinfection of drinking water.51 For this aim, graphene oxide was fabricated from graphite based on a modified Hummers' method. Its suspension was prepared, and Cu salt and glucose were added to it. After the ultrasonic process, the prepared suspension was kept at 120 °C (for 20 h) and finally, it was freeze-dried. Cu nanoparticles with an average size of 3–4 nm were supported, stabilized, and well-dispersed on a 3D porous graphene network surface. To elucidate the probable antibacterial mechanism of a Cu/graphene composite sponge, oxidative capability and intercellular ROS were determined. The outcomes displayed >99.9% inactivation of viable E. coli cells by the prepared composite, which revealed an efficient antibacterial performance. Flow cytometric analysis and morphological examinations specified the important role of the Cu/graphene composite sponge for the destruction of the cell membrane in antibacterial action. Besides, the determination of intercellular ROS, as well as oxidation of glutathione, proved the participation of oxidative stress produced by a Cu/graphene composites sponge in bactericidal behavior. Furthermore, the release of copper ions from Cu/graphene (standard amount) had a positive effect on the inactivation performance.
Shimabuku and co-workers reported excellent virus inactivation of a filter by employing Ag/CuO-modified activated carbon for water treatment.52 In fact, the adsorbent feature of activated carbon was combined with various features of Ag and CuO nanoparticles, and by this synergism, a promising material with a noteworthy lifetime for the removal of T4 bacteriophages from drinking water was prepared. The anti-viral performance was estimated by passing the bacteriophage suspension through the fabricated filter. Granular activated carbon/Ag0.5%Cu1.0% showed good performance in the inactivation of the virus. Besides, the level of Ag and Cu nanoparticles in filtered water was lower than the recommended limits in drinking water.
Mazurkow et al. developed anti-viral filter materials (CuxOy/Al2O3) for the removal of a model virus (bacteriophage MS2) from water. These materials were based on spray-dried alumina granules (plate-shaped alumina as support) modified with a CuxOy nanoparticles spray granulation method. The influence of the Cu oxidation state on the elimination ability of the virus was systematically examined. Large numbers of active sites for adsorption were provided by CuxOy nanoparticles. Al2O3 support was selected to avoid the accumulation of nano-sized CuxOy and to assist the porous granular construction. Flow experiments showed 99.9% removal of MS2 bacteriophages by metallic Cu and Cu2O, while in the presence of CuO, deletion of the virus was not observed. The surface charge of Cu species (based on zeta potential analysis) affected the virus removal properties. Based on these findings, the improved anti-viral performance of Cu and Cu2O was due to their positive surface charge characteristics for effective adsorption of MS2 bacteriophages (negatively charged virus).53
Megarajan and co-workers synthesized Cu nanoparticles with N-myristoyltaurine, and examined the antimicrobial and anti-biofilm of them toward waterborne pathogens including E. coli, P. aeruginosa, S. typhi, and S. flexneri. The fabricated Cu nanoparticles with wine red color (5–12 nm) and surface plasmon resonance feature (maximum: 590 nm), were well-dispersed with a spherical morphology. Based on the outcomes, waterborne pathogens were effectively eliminated by Cu nanoparticles. Also, they inhibited bacterial colonization for preventing the formation of bio-film. The filters based on these nanoparticles have good potential for water treatment.54
Ungur and Hruza fabricated antimicrobial polymeric nano-fibrous filters for water and air purification by employing CuO and polyurethane. By using micro- and nanoparticles of CuO, polyurethane nano-fibers were modified, and the effect of dimensional properties of CuO on the nano-fibers construction, antimicrobial effectiveness, as well as stability of particle fixation was examined. Diverse percentages of CuO (5; 7; 9.5 and 12%) were inserted into pre-electrospinning solutions and were carefully combined. Through industrial nanospider methods, composite layers were fabricated. The antimicrobial performance of the nano-fibrous mats containing nanoparticles was examined using E. coli and S. gallinarum microorganisms. Under the simulated situations of water filtration, the stability of nanoparticles' fixation into the construction of fiber was determined. This research proved that CuO microparticles are more suitable additives for the antibacterial modification of polyurethane filters compared to nanoparticles.55
In another study by Dankovich and Smith, Cu nanoparticles were incorporated in cellulosic filters for cleansing the water. Based on low-cost and environmentally benign techniques, Cu nanoparticles were in situ prepared in a paper by using a heat source and ascorbic acid as a mild reducing agent for copper ions. Nanoparticles were dispersed on the surfaces of paper fiber. The antimicrobial performance of the filter was examined by passing an E. coli microorganism suspension through the filters. The effluent was examined for Cu release and viable microorganisms. The prepared filter was effective in bacteria reduction. Besides, the released copper levels in the water were lower than the recommended limit of Cu in drinking water.56
In an investigation to kill microorganisms from aquaculture water, Sanito et al. used this biological disinfectant (nano-TiO2). In this examination, a novel composite filter based on TiO2 and polyaniline, as a disinfectant, was prepared to remove microorganisms (E. coli and S. aureus) from aquaculture water. For the fabrication of the filter, a non-woven fabric was cleaned, ultrasonicated, washed, and dried. Then, the fabric was soaked in TiO2/polyaniline composite. After ultrasonication for the removal of the unstable composite, it was dried, and a filter was prepared. Based on the outcomes, TiO2/polyaniline composite with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio showed 98.9 and 80% rates of elimination for E. coli and S. aureus respectively. The fabricated filter had a great removal efficiency (99%, best result) for microbes in aquaculture water after soaking the filter 3 times. OH radicals play a significant role in killing microorganisms by penetration of their wall cells. Furthermore, cell permeability was enhanced because of oxidative degradation and made the microorganisms decay. These destroyed the metabolism of the cell, and the cells died quickly.58
2 ratio showed 98.9 and 80% rates of elimination for E. coli and S. aureus respectively. The fabricated filter had a great removal efficiency (99%, best result) for microbes in aquaculture water after soaking the filter 3 times. OH radicals play a significant role in killing microorganisms by penetration of their wall cells. Furthermore, cell permeability was enhanced because of oxidative degradation and made the microorganisms decay. These destroyed the metabolism of the cell, and the cells died quickly.58
Nano-TiO2 has a wide range of applications in disinfection as well as purification of the environment due to its great bactericidal action. Xu et al. reported low-cost and efficient water microbiological purification technology by employing TiO2 nanoparticles. They developed a novel ceramic filter modified via nano-TiO2/chitosan composites for E. coli elimination from drinking water. Indeed, nano-TiO2 produces electron-hole pairs, which form OH radicals for the deactivation of microorganisms. Characterization outcomes showed the successful coating of nano-TiO2/chitosan composites on the filter surface. To produce the filter treated by TiO2/chitosan nanocomposites, the optimum conditions were determined which were 0.05, 0.2, and 0.04 g of nano-TiO2, chitosan, as well as coating amount, respectively. The outcomes of this study displayed effective deletion of E. coli from water because of irregular pores in the modified filter with TiO2/chitosan; attachment of chitosan/TiO2 nanocomposites cell membrane to damage, and generation of ROS by nano-TiO2 and oxidative stress in cells. This work was a cost-effective-feasible solution to resolve the water safety concerns.59
Furthermore, Zhao and co-workers effectively removed E. coli from drinking water by functionalization of a low-cost spongy filter covered with Fe/nano-TiO2 composite. After fabrication of the modified filter, the mass ratio of Fe and TiO2 was optimized in the Fe/nano-TiO2 composite and the covered weight of the composite and rice-husk ratio were also optimized. After that, the features of crystal and morphology were examined. Finally, the mechanical performance of the Fe/TiO2-covered filter for E. coli removal was revealed. The Rice-husk ratio showed an excessive effect on the rate of filtration, indeed, because the increased coated weight caused a reduction in holes. The microorganism reduction was due to the micropores and presence of Fe/TiO2 (synergistic bactericidal influence of nano-TiO2 and Fe to remove E. coli).60
In another investigation, nano-TiO2 was decorated on a ceramic disk filter as an inexpensive household water cleansing system to reduce E. coli. For the manufacture of a filter covered with nano-TiO2 particles, the mass fraction of nano-TiO2, heating hold time, the ratio of rice-husk, and particle size of rice-husk were optimized. The outcomes showed optimum conditions in using 2.21% mass fraction, 1.41 h heating time, 29.03% rice-husk ratio, and 0.28 mm particle size of rice-husk. This study showed the good potential of a filter impregnated with nano-TiO2 for the purification of water.61
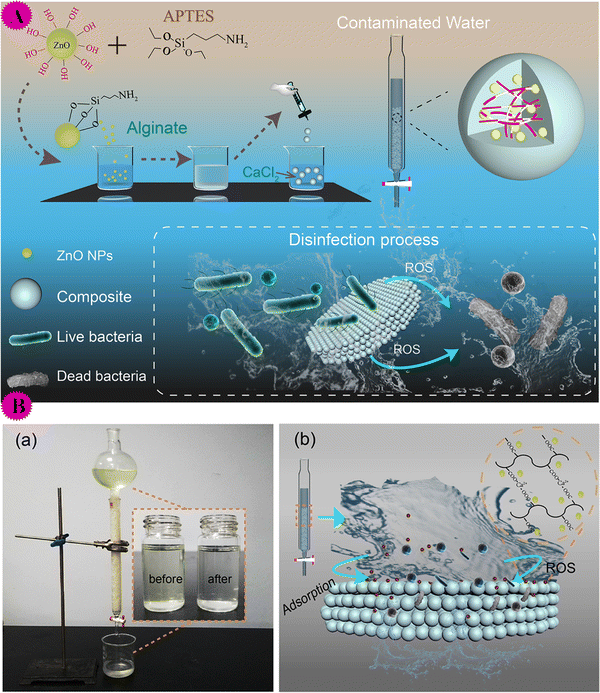 | ||
| Fig. 12 (A) Illustration of the synthesis of the ZnO NPs@Calcium alginate (top) and disinfection process for water purification (bottom). (B) (a) Water purifier based on the composite; inset is the image of the contaminated water before and after treatment by the purifier. (b) Schematic illustration of eliminating organic matter and killing bacteria by the composite. This figure has been reproduced from ref. 63 with permission from American Chemical Society, copyright 2020. | ||
To provide safe water in remote communities and small rural areas of low-income, Huang et al. coated ceramic disk filters with ZnO nanoparticles for E. coli elimination from water. The reduction of E. coli was tested by filter-coated nano ZnO. Several factors influenced the performance of the modified filtration system such as the pore size of the filters. The covering of nano-ZnO changed the surface and porosity of the filter. The reduction of microorganisms was due to the retention of the filter as well as the antibacterial behavior of ZnO nanoparticles.64 In a similar work, a nanocomposite of Ag/ZnO was prepared and coated on a ceramic disk filter to remove microorganisms (E. coli) from household drinking water. Through many tests according to the Box-Behnken strategy, the production of Ag/ZnO-coated filter was optimized. The microscopy outcomes specified the main Ag/ZnO distribution on the surface of Ag/ZnO coated filter. The antibacterial performance of the filter was examined via the detection of a difference in cell status as well as intracellular ROS. Photocatalytic and non-photocatalytic performances of Ag/ZnO were investigated. Considering the outcomes, filtration, photocatalytic, as well as non-photocatalytic antibacterial actions were key mechanisms for the removal of microorganisms by the prepared filter.65
Biron et al. reported successful disinfection of water by coating ZnO nanoparticles on tubular ceramic membranes of mullite and alumina. In this study by employing 3-aminopropyltriethoxysilane as a connection molecule, ZnO was connected to ceramics. Microfiltration tests for the investigation of antimicrobial action in water disinfection from S. aureus microorganisms by filters with and without ZnO covering were evaluated. The prepared membrane without ZnO nanoparticles indicated low effectiveness of the disinfection performance (35% for mullite-based membranes and 45% for alumina). The fabricated membranes with ZnO coating showed great disinfection membranes that showed efficacy (>99%).66
Munnawar et al. fabricated anti-fouling nanocomposite membranes based on chitosan/ZnO nanoparticles and a polyethersulfone matrix for water disinfection. For this aim, chitosan/ZnO was prepared by a chemical precipitation process, then, diverse amounts (5%, 10%, and 15% w/w) of chitosan/ZnO were incorporated into polyethersulfone via a phase inversion technique. The influence of chitosan/ZnO on water retention, roughness, morphology, and permeability flux was studied. The prepared membrane with 15% chitosan/ZnO showed the lowest contact angle and larger mean pore sizes compared to the pure polyethersulfone membrane. The fabricated membrane displayed important prevention against microbial fouling, water permeability, as well as hydrophilicity. Due to the synergistic influence of ZnO nanoparticles and chitosan, the fabricated membrane showed good performance toward microorganisms (E. coli, S. aureus, B. cereus, and fungi for example F. solani, S. typhi, and A. fumigatus).67
2.2. Biomaterials
In another study for drinking water purification uses, Mihranyan and co-workers estimated the virus removal performance of filter papers based on mille-feuille nanocellulose of a simulated wastewater matrix with varying thicknesses. Based on the achieved outcomes, the prepared filter paper including 100% nanocellulose, proficiently removed even the smallest viruses with 99.9980–99.9995% effectiveness. This filter showed excessive potential to develop inexpensive, robust, as well as sustainable systems for water purification.73
In Bangladesh, permanent access to clean drinking water is difficult and mortality is high due to infectious diseases transmitted through water such as waterborne diarrheal diseases. In order to address a global problem and provide clean water, Gustafsson and co-workers produced a filter paper with good pathogen removal features based on nano-cellulose extraction of Pithophora green macroalgae, which is developing in Bangladesh. For the fabrication of the filter, they prepared a 0.2 wt% distribution of Pithophora cellulose, and then, it was run many times in succession by employing a micro-fluidizer. Finally, a nylon filter membrane was drained by the resulting nano-cellulose dispersion (Fig. 13a). This research team showed the potential of filter paper for the elimination of all sorts of infectious bacteria as well as viruses from drinking water. The filter performance was tested for in vitro model viruses from real-life water samples (Dhanmondi Lake and Turag River in Bangladesh). The solution proposed by this research group could enhance the life quality of lots of people in Bangladesh and elsewhere. The prepared pathogen barrier filter showed efficient removal of bacteria and viruses. By employing a 300 mm flat sheet of the Pithophora filter, 3 L of high-safe drinking water in 25 min could be produced (Fig. 13b). So, it is a good region with poor access to drinking water.74
 | ||
| Fig. 13 (a) Cultivation of Pithophora algae at the University of Dhaka, Bangladesh, extracted algae cellulose and filter paper sheets made from it at Uppsala University, Sweden. (b) River water sample before and after filtration. This figure has been reproduced from ref. 74 with permission from American Chemical Society, copyright 2019. | ||
Most of the common filters used are non-renewable materials or they are expensive, time-consuming, and expensive to prepare, therefore, researchers around the world have turned their attention to the use of degradable and low-consumption materials. Heydarifard and co-workers developed a novel disinfectant filter based on foam-formed cellulose filter paper for drinking water applications. Indeed, cellulose foam paper is an appropriate material for use as a water filter due to its high porosity, non-uniform pore distribution, and great wet strength. The antimicrobial performance of the prepared filter toward many microorganisms including B. subtilis and B. cereus (Gram +ve), and P. aeruginosa, K. pneumonia, and E. coli (Gram −ve) was studied. The results showed excellent antimicrobial properties and high wet strength action provided by the addition of glutaraldehyde or 1,2,3,4-butanetetracarboxylic acid to cellulose paper pre-treated using cationic polymer (polyacrylamide).75
One of the highly efficient and robust methods for the removal of viruses is removal size-exclusion filtration. In a study for drinking water purification, Manukyan et al. developed a nanocellulose-based filter for virus removal filtration. For this aim, using a microfluidizer, cladophora nanocellulose was distributed in DI water, and then drained on a support membrane. After drying, the filter with 11 and 33 μm thickness was obtained. The virus removal capacity of the prepared filter was tested, and considering the outcomes, these filters exhibited a high (over 4 log10) small-size virus retention capacity and high (180 L m−2 h−1) flow rates at 3 bar pressure.76
To capture and deactivate the water-pathogenic microorganisms, Heydarifard and co-workers developed water-resistant cellulose foam paper to improve the antimicrobial performance of cellulose foam paper and water disinfection. For the preparation of the antimicrobial cellulose foam filter by conversion of a conventional cellulose filter, antibacterial thermoplastic starch as a functional wet-end additive was added to pulp slurries. Antibacterial thermoplastic starch-modified filter attained outstanding growth inhibition against E. coli. Before and after filtering, bacteria viability and construction of cellulose foam paper were examined and the outcomes confirmed the efficiency of antimicrobial cellulose foam for disabling pathogens.77
2.3. Carbon nanomaterials
According to the WHO organization, more than 600 million people worldwide suffer from a shortage of clean drinking water, most of them living in underdeveloped areas. Therefore, it is necessary to develop a healthy water supply technology with low cost and effective efficiency. Antimicrobial water filters based on Laser-induced graphene have been used for bacteria inactivation due to the antifouling as well as antimicrobial and electrochemical performance and texture of laser-induced graphene. In this regard, Barbhuiya et al. prepared filters using laser-induced graphene and examined their anti-viral and antibacterial performances. These filters were produced by using polyethersulfone ultrafiltration membranes. Based on the outcomes, high voltages were necessary for virus inactivation compared to bacteria. The surface electrical effects and ROS generation showed an important role in the inactivation of the virus. This work showed potential for use in water treatment and disinfection of various pathogenic microorganisms, comprising Vaccinia lister virus.78Li et al. developed the investigated graphene oxide-based filter for water treatment. Chitosan chloride/graphene oxide composite was prepared by employing a one-step solution blending process. The antimicrobial performance was tested toward two model microorganisms (E. coli and S. aureus). A composite-modified quartz sand filter was manufactured and tested (Fig. 14). The outcomes showed completely inactivated E. coli and S. aureus by 100 mg L−1 of filter in 15 min. After washing with filter media (3 times), the antibacterial rate remained >90%.79
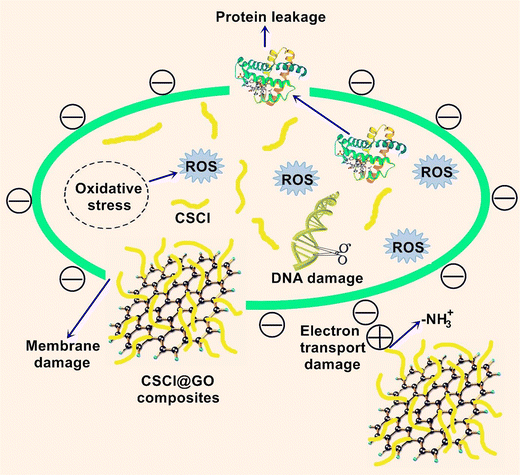 | ||
| Fig. 14 Schematic of the antibacterial mechanism of CSCl@GO composites. This figure has been reproduced from ref. 79 with permission from Elsevier, copyright 2019. | ||
In order to decontaminate the water from waterborne microorganisms, Jakubczak et al. planned a new multifunctional activated carbon-supported bioactive composite filtration bed. For this aim, a hybrid nanocomposite structure was prepared via simplistic in situ surface decoration through a sol–gel method by employing graphene oxide, activated carbon, and Al2O3/Ag nanocomposite components. Bacteria adsorption and biological tests demonstrated the great efficiency of the filter for eliminating waterborne microorganisms. Furthermore, this filtration system did not show nanoparticle release, which indicated the stability and safety of the materials. In short times of contact, the nanofiltration bed eliminated 100% of bacteria cells.80
2.4. Other materials
Harmless/simple technology and energy-effective water treatment are essential and very desirable to challenge water pollution produced via pathogenic contaminants, especially in remote industrial areas. To provide a household-level method for the disinfection of pathogens, Xu et al. engineered a multifunctional gravity-driven membrane system by employing polyphenols from plant sources for household water treatment (Fig. 15). Polyphenol was deposited on a micro-porous PVDF membrane in a simple one-step process. Through the co-deposition of gallic acid with catechol groups and polyethyleneimine, a positively charged membrane with favorable hydrophilicity was constructed. The fabricated membrane was applied in gravity-driven filtration of metal ion (Ag+)/E. coli contaminated water, and the outcomes showed >6 log pathogenic reduction (E. coli) in water. In this study, Ag+ was captured and reduced by polyphenol-containing catechol groups. Indeed, the prepared polyphenol-engineered membrane had a reducing ability. So, pathogens are effectively removed by electrostatic interaction and inactivated by a synergistic mechanism.81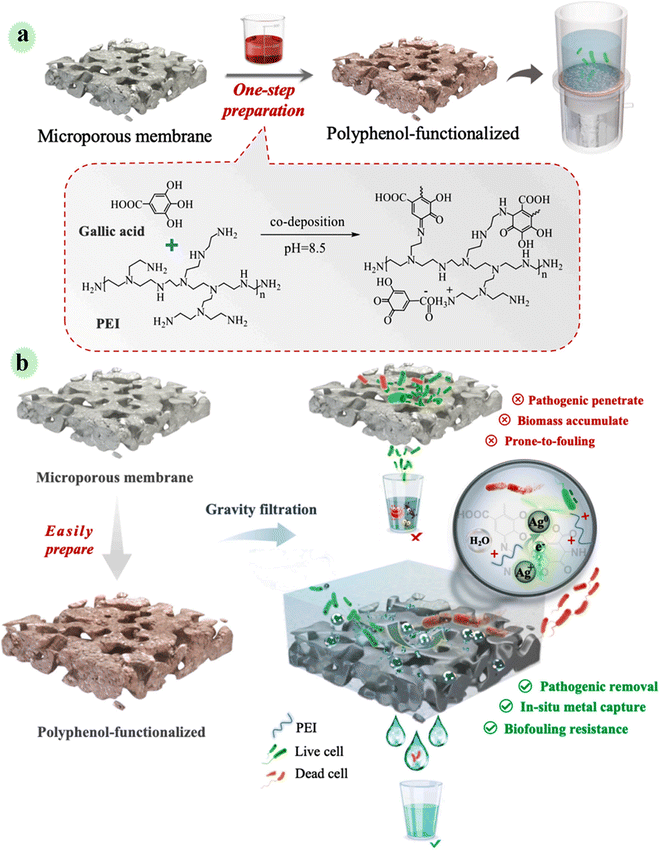 | ||
| Fig. 15 (a) Schematic showing one-step functionalization of microporous membrane by co-deposition of gallic acid and PEI, and its gravity filtration set-up for point of use applications. (b) A holistic mechanism for enhanced pathogenic removal and anti-biofouling. This figure has been reproduced from ref. 81 with permission from Elsevier, copyright 2021. | ||
In another study, Si and co-workers used N-halamine as an antimicrobial agent for the preparation of BNF membranes (biocidal and rechargeable nanofibrous membranes) for purification and disinfection of water contaminated by pathogenic microbes. N-Halamine with high durability as well as antimicrobial action was covalently incorporated into poly(vinyl alcohol-co-ethylene) nano-fibers. Fig. 16 shows the production and bactericidal routes. The fabricated BNF membranes displayed great features such as high bactericidal efficacy (99.999% contact-killing), great surface area, robust mechanical strength, high porosity, and great hydrophilicity. This membrane effectively disinfected microbes by direct filtration.82 In addition to these materials, various other materials have been developed in the preparation of antimicrobial membranes.83–87
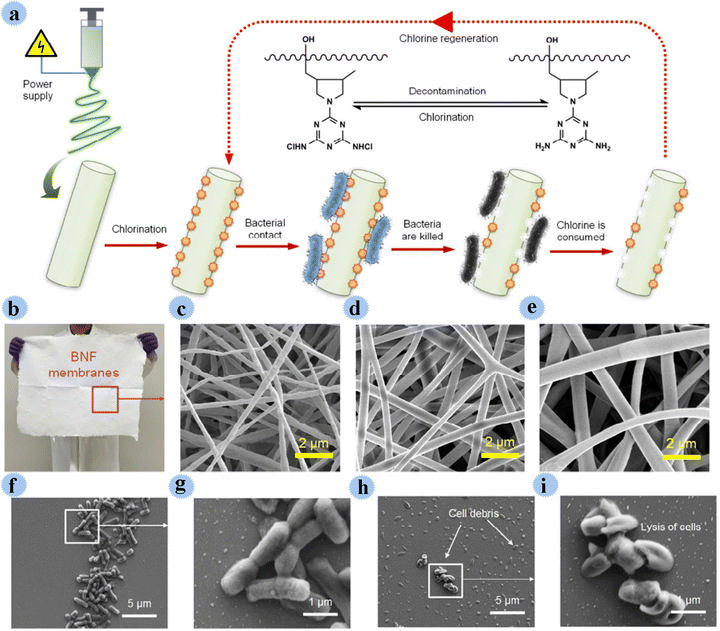 | ||
| Fig. 16 (a) Schematic of the design, processing, and bactericidal functions of the as-prepared BNF membranes. (b) An optical image of a BNF membrane on a large scale of 50 × 60 cm2. Microscopic nanofibrous architecture of (c) BNF-6, (d) BNF-8, and (e) BNF-10 membranes. FE-SEM images of E. coli cells in contact with control BNF-6 membranes (f and g) and charged BNF-6 membranes (h and i). This figure has been reproduced from ref. 82 with permission from American Chemical Society, copyright 2017. | ||
3. Conclusions and recommendations
Viral, bacterial, and parasitic infections can enter water, so, efficient filtration systems based on antimicrobial materials have received a lot of attention. As mentioned and can be seen in Table 1, a wide range of materials play an efficient role in the production of filtration systems for water purification. Metals and metal oxides including Cu, Cu2O, Ag, TiO2, and ZnO, play a role in this regard. Moreover, natural materials such as cellulose and carbon-based nanomaterials like graphene showed good performances in killing microorganisms. Besides, polyphenols and N-halamine as antimicrobial materials can be used for producing filtration systems for pathogen disinfection.| Different materials for water treatment | Used materials | Kind of microorganism | Performance and results |
|---|---|---|---|
| Ag nanoparticles | Zwitterionic Ag/PCBDA [poly(carboxybetaine acrylate-co-dopamine methacryamide)] | E. coli, S. aureus, MRSA, and P. aeruginosa | Microfiltration exhibited anti-biofouling, and inactivated microbes in contact with the membrane25 |
| Ag/wood carbon | E. coli, E. faecalis, S. typhimurium, and B. subtilis | 99.999% killing of diverse microorganisms26 | |
| Ag/poly(vinyl alcohol-co-ethylene) | E. coli and S. aureus | Successful inactivation with a 99.99% rate due to the exceptional ability of Ag toward microorganisms27 | |
| Gallic acid-protected Ag/collagen fibers | E. coli and S. aureus | Approximately all microorganisms were killed in 20 min by the prepared purification device28 | |
| Electrospun polyacrylonitrile/Ag nanoparticles | E. coli and S. aureus | Excellent activity toward microorganisms [E. coli (96%) and S. aureus (92%)] was seen29 | |
| Chitosan/Ag nanoparticles | E. coli and B. subtilis | With increasing the content of chitosan (from 0.5 to 2.0 wt%), the performance against microorganisms was improved31 | |
| Cellulose paper/Ag nanoparticles | E. coli | Under optimal conditions the best removal of E. coli was seen32 | |
| Chitosan/Ag particles/cotton fabric | E. coli, and B. subtilis | Using 1.5 wt% chitosan and 50 mg L−1 Ag, antimicrobial effectiveness for E. coli, and B. subtilis was 4 and 3 log reductions, respectively.33 | |
| Polyvinylidene fluoride/Ag/TiO2 | E. coli | Fast removal of E. coli was observed34 | |
| Cu/CuO | Cu7S4/N-doped carbon film | S. aureus and E. coli | The sterilization rate could retain >99% in 12 h working under a 1500 mL min−1 flow and 10 V voltage43 |
| CuO–Co3O4 nano-wires | S. aureus and E. coli | After continuous operation for 12 h, the effectiveness was >80%42 | |
| Ag/CuO nano-wires | S. aureus and E. coli | This composite displayed great performance (>99.9%) to inactivate bacterial and ultrahigh water flux under low voltage.46 | |
| Carbon/Cu2O–Ag nanoparticles | S. aureus and E. coli | Carbon/Cu2O–Ag nanostructure displayed outstanding disinfection presentation (99.6% disinfecting speed)47 | |
| Cu-Covered nano-fibrillated cellulose | Human pathogenic virions, MS2 bacteriophages | Great performance in filtration efficiency48 | |
| Cu2O/MWCNTs | MS2 bacteriophages | Effective action for virus removal in drinking water was obtained | |
| Cu nanoparticle-decorated graphene sponge | E. coli | >99.9% inactivation of E. coli cells49 | |
| CuxOy/Al2O3 | MS2bacteriophages | 99.9% removal of MS2 bacteriophages53 | |
| Cu nanoparticles with N-myristoyltaurine | E. coli, P. aeruginosa, S. typhi, and S. flexneri | Waterborne pathogens were effectively eliminated by Cu nanoparticles54 | |
| CuO and polyurethane | E. coli and S. gallinarum | Effective removal of microorganisms55 | |
| TiO2 particles | TiO2 and polyaniline | E. coli and S. aureus | The fabricated filter specified great removal efficiency (99%, best result) of microbes in aquaculture water after 3 times soaking the filter.58 |
| Nano-TiO2/chitosan composites | E. coli | Effective deletion of E. coli from water because of irregular pores in modified filter with TiO2/chitosan59 | |
| Fe/nano-TiO2 composite | E. coli | Microorganism reduction was due to the micropores and presence of Fe/TiO2 (synergistic bactericidal influence of nano-TiO2 and Fe to remove E. coli)60 | |
| ZnO particles | ZnO nanoparticles as the killer of microorganisms were incorporated into the calcium alginate matrix | S. aureus and E. coli | Killed microorganisms from the polluted water (over 99%) owing to exceptional sorption of 3D alginate and antibacterial feature of ZnO63 |
| Ag/ZnO | E. coli | Filtration, photocatalytic, and non-photocatalytic antibacterial actions were key mechanisms for the removal of E. coli by this filter65 | |
| ZnO nanoparticles on tubular ceramic membranes of mullite and alumina | S. aureus | The fabricated membranes showed great disinfection (>99% efficacy)66 | |
| Chitosan/ZnO nanoparticles and polyethersulfone | Microorganisms (E. coli, S. aureus, B. cereus, and fungi for example F. solani, S. typhi, and A. fumigatus) | Good performance toward microorganisms67 | |
| Cellulose | Cationic-modified nanocellulose | Qbeta and MS2 viruses | At pH = 7, the filtration efficiencies for removal of MS2 and Qbeta were 99.9% and 93.6% respectively.72 |
| Mille-feuille nanocellulose | Many viruses | 99.9980–99.9995% effectiveness73 | |
| Cellulose foam paper | B. subtilis, B. cereus, P. aeruginosa, K. pneumonia, and E. coli | Excellent antimicrobial properties75 | |
| Graphene and derivatives | Graphene oxide | E. coli and S. aureus | The outcomes showed completely inactivated E. coli and S. aureus by 100 mg L−1 of filter in 15 min79 |
| Activated carbon graphene oxide, and Al2O3/Ag nanocomposite | Bacteria cells | In short times of contact, the nanofiltration bed eliminated 100% of bacteria cells80 | |
| Other materials | Polyphenols from plant sources/PVDF membrane | E. coli | Outcomes showed >6 log pathogenic reduction (E. coli) in water81 |
| N-Halamine | E. coli | Great features such as high bactericidal efficacy (99.999% contact-killing)82 |
On the other hand, to date, most studies have shown that the transmission of SARS-CoV-2 is primarily through airborne pathways. However, the possibility of transmitting the virus through the fecal-oral route and aerosolization of infected sewage from urinals, toilets, and sewage pipelines is possible. So, more studies are needed in this area and more sampling of surface water and wastewater should be done. On the other hand, the release of nanomaterials like metals from filtration systems into the water and environment causing concern should be further investigated. Their toxicity should also be more investigated.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are immensely grateful for financial support from the Research Affairs Division of Isfahan University of Technology (IUT), Isfahan. I. R. Iran, and Iran Nanotechnology Initiative Council (INIC) Tehran, I. R. Iran. We would also like to show our gratitude to the National Elite Foundation (NEF), Tehran, I. R. Iran, and Center of Excellence in Sensors and Green Chemistry IUT.References
- B. Adelodun, K. Y. Kareem, P. Kumar, V. Kumar, K. S. Choi, K. K. Yadav, A. Yadav, A. El-Denglawey, M. Cabral-Pinto, C. T. Son, S. Krishnan and N. A. Khan, J. Cleaner Prod., 2021, 318, 128451 CrossRef CAS.
- M. Hossain, J. Cleaner Prod., 2021, 280, 124782 CrossRef CAS PubMed.
- A. Farooq, J. Lee, H. Song, C. H. Ko, I. H. Lee, Y. M. Kim, G. H. Rhee, S. Pyo and Y. K. Park, J. Hazard. Mater., 2022, 423, 127222 CrossRef CAS.
- S. V. Williams, A. Vusirikala, S. N. Ladhani, E. Fernandez Ruiz De Olano, N. Iyanger, F. Aiano, K. Stoker, G. Gopal Rao, L. John, B. Patel, N. Andrews, G. Dabrera, M. Ramsay, K. E. Brown, J. Lopez Bernal and V. Saliba, Eurosurveillance, 2021, 26, 1–5 Search PubMed.
- S. Alexandar, M. Ravisankar, R. S. Kumar and K. Jakkan, Int. J. Pharm. Clin. Res, 2021, 5, 83–85 Search PubMed.
- H. N. Tran, G. T. Le, D. T. Nguyen, R. S. Juang, J. Rinklebe, A. Bhatnagar, E. C. Lima, H. M. N. Iqbal, A. K. Sarmah and H. P. Chao, Environ. Res., 2021, 193, 110265 CrossRef CAS.
- M. Patel, A. K. Chaubey, C. U. Pittman, T. Mlsna and D. Mohan, Sci. Total Environ, 2021, 765, 142698 CrossRef CAS PubMed.
- A. Buonerba, M. V. A. Corpuz, F. Ballesteros, K. H. Choo, S. W. Hasan, G. V. Korshin, V. Belgiorno, D. Barceló and V. Naddeo, J. Hazard. Mater., 2021, 415, 125580 CrossRef CAS PubMed.
- C. Chen, L. Guo, Y. Yang, K. Oguma and L. Hou, Sci. Total Environ, 2021, 801, 149678 CrossRef CAS.
- M. V. A. Corpuz, A. Buonerba, G. Vigliotta, T. Zarra, F. Ballesteros, P. Campiglia, V. Belgiorno, G. Korshin and V. Naddeo, Sci. Total Environ, 2020, 745, 140910 CrossRef CAS PubMed.
- G. D. Bhowmick, D. Dhar, D. Nath, M. M. Ghangrekar, R. Banerjee, S. Das and J. Chatterjee, npj Clean Water, 2020, 3, 32, DOI:10.1038/s41545-020-0079-1.
- A. Bogler, A. Packman, A. Furman, A. Gross, A. Kushmaro, A. Ronen, C. Dagot, C. Hill, D. Vaizel-Ohayon, E. Morgenroth, E. Bertuzzo, G. Wells, H. R. Kiperwas, H. Horn, I. Negev, I. Zucker, I. Bar-Or, J. Moran-Gilad, J. L. Balcazar, K. Bibby, M. Elimelech, N. Weisbrod, O. Nir, O. Sued, O. Gillor, P. J. Alvarez, S. Crameri, S. Arnon, S. Walker, S. Yaron, T. H. Nguyen, Y. Berchenko, Y. Hu, Z. Ronen and E. Bar-Zeev, Nat. Sustain., 2020, 3, 981–990 CrossRef.
- S. Lahrich, F. Laghrib, A. Farahi, M. Bakasse, S. Saqrane and M. A. El Mhammedi, Sci. Total Environ, 2021, 751, 142325 CrossRef CAS PubMed.
- A. Ojha, Nanomaterials for removal of waterborne pathogens, Elsevier, 2020 Search PubMed.
- N. Verma, S. Vaidh, G. S. Vishwakarma and A. Pandya, Nanotoxicity, 2020, 365–383 CAS.
- A. Bhatt, P. Arora and S. K. Prajapati, J. Environ. Chem. Eng., 2020, 8, 104429 CrossRef CAS.
- A. Majumder, A. K. Gupta, P. S. Ghosal and M. Varma, J. Environ. Chem. Eng., 2021, 9, 104812 CrossRef CAS.
- K. P. Goswami and G. Pugazhenthi, J. Environ. Manage., 2020, 268, 110583 CrossRef CAS.
- Y. Ibrahim, M. Ouda, D. Kadadou, F. Banat, V. Naddeo, H. Alsafar, A. F. Yousef, D. Barceló and S. W. Hasan, J. Environ. Chem. Eng., 2021, 9, 105613 CrossRef CAS.
- L. Chen, Y. Deng, S. Dong, H. Wang, P. Li, H. Zhang and W. Chu, Chemosphere, 2021, 281, 130728 CrossRef CAS PubMed.
- A. S. Adeleye, J. R. Conway, K. Garner, Y. Huang, Y. Su and A. A. Keller, Chem. Eng. J., 2016, 286, 640–662 CrossRef CAS.
- J. Talapko, T. Matijević, M. Juzbašić, A. Antolović-Požgain and I. Škrlec, Microorganisms, 2020, 8, 1–13 CrossRef.
- A. K. Bhardwaj, S. Sundaram, K. K. Yadav and A. L. Srivastav, Environ. Technol. Innov., 2021, 23, 101721 CrossRef CAS.
- S. L. Loo, W. B. Krantz, A. G. Fane, Y. Gao, T. T. Lim and X. Hu, Environ. Sci. Technol., 2015, 49, 2310–2318 CrossRef CAS PubMed.
- R. Yu, R. Zhu, J. Jiang, R. Liang, X. Liu and G. Liu, J. Colloid Interface Sci., 2021, 598, 302–313 CrossRef CAS PubMed.
- Z. Yang, H. Ni, P. Liu, H. Liu, K. Yang, Z. Zhang, B. Wang, X. Li and F. Chen, Chin. Chem. Lett., 2021, 32, 3143–3148 CrossRef CAS.
- P. Cheng, X. Wang, Y. Liu, C. Kong, N. Liu, Y. Wan, Q. Guo, K. Liu, Z. Lu, M. Li and D. Wang, Appl. Surf. Sci., 2020, 506, 144664 CrossRef CAS.
- G. Liu, J. Jiang, R. Yu, H. Yan and R. Liang, Ind. Eng. Chem. Res., 2020, 59, 10857–10867 CrossRef CAS.
- S. F. Pan, X. X. Ke, T. Y. Wang, Q. Liu, L. Bin Zhong and Y. M. Zheng, Ind. Eng. Chem. Res., 2019, 58, 984–993 CrossRef CAS.
- G. Liu, R. Yu, J. Jiang, Z. Ding, J. Ma and R. Liang, RSC Adv., 2021, 11, 4873–4882 RSC.
- M. Fan, L. Gong, J. Sun, D. Wang, F. Bi and Z. Gong, ACS Appl. Mater. Interfaces, 2018, 10, 38239–38245 CrossRef CAS.
- S. M. Praveena, K. Karuppiah and L. T. L. Than, Cellulose, 2018, 25, 2647–2658 CrossRef CAS.
- S. Jiang, C. Tang, Z. Gong, Z. Zhang, D. Wang and M. Fan, Mater. Lett., 2020, 277, 128256 CrossRef CAS.
- J. R. Mishra, S. K. Samal, S. Mohanty and S. K. Nayak, Mater. Chem. Phys., 2021, 268, 124723 CrossRef CAS.
- J. Wu, C. Yu and Q. Li, J. Membr. Sci., 2017, 531, 68–76 CrossRef CAS.
- J. Rayner, H. Zhang, J. Schubert, P. Lennon, D. Lantagne and V. Oyanedel-Craver, ACS Sustainable Chem. Eng., 2013, 1, 737–745 CrossRef CAS.
- L. V. Garcia Peña, P. Petkova, R. Margalef-Marti, M. Vives, L. Aguilar, A. Gallegos, A. Francesko, I. Perelshtein, A. Gedanken, E. Mendoza, J. C. Casas-Zapata, J. Morató and T. Tzanov, Ind. Eng. Chem. Res., 2017, 56, 3599–3606 CrossRef.
- J. Wang, Y. Wu, Z. Yang, H. Guo, B. Cao and C. Y. Tang, Sci. Rep., 2017, 7, 3–10 CrossRef PubMed.
- J. Wen, X. Tan, Y. Hu, Q. Guo and X. Hong, Environ. Sci. Technol., 2017, 51, 6395–6403 CrossRef CAS.
- L. Qi, Z. Liu, N. Wang and Y. Hu, Appl. Surf. Sci., 2018, 456, 95–103 CrossRef CAS.
- F. Zhao, S. Chen, Q. Hu, G. Xue, Q. Ni, Q. Jiang and Y. Qiu, Sep. Purif. Technol., 2017, 175, 130–139 CrossRef CAS.
- H. Wang, M. Yu, S. Cui, L. Dong, S. Wang, S. Wei, H. Feng and S. Chen, J. Environ.: Chem. Eng., 2021, 9, 105629 CAS.
- S. Cui, S. Chen, H. Wang, L. Dong and S. Wang, ACS Appl. Nano Mater., 2021, 4, 6124–6134 CrossRef CAS.
- S. Mallakpour, E. Azadi and C. M. Hussain, New J. Chem., 2021, 45, 6167–6179 RSC.
- J. O. Ighalo, P. A. Sagboye, G. Umenweke, O. J. Ajala, F. O. Omoarukhe, C. A. Adeyanju, S. Ogunniyi and A. G. Adeniyi, Environ. Nanotechnol., Monit. Manage., 2021, 15, 100443 CAS.
- L. Yue, S. Chen, S. Wang, C. Wang, X. Hao and Y. F. Cheng, Environ. Sci.: Nano, 2019, 6, 2801–2809 RSC.
- S. Wang, W. Wang, L. Yue, S. Cui, H. Wang, C. Wang and S. Chen, Chem. Eng. J., 2020, 382, 122855 CrossRef CAS.
- G. P. Szekeres, Z. Nemeth, K. Schrantz, K. Nemeth, M. Schabikowski, J. Traber, W. Pronk, K. Hernadi and T. Graule, ACS Omega, 2018, 3, 446–454 CrossRef CAS PubMed.
- K. Domagała, C. Jacquin, M. Borlaf, B. Sinnet, T. Julian, D. Kata and T. Graule, Water Res., 2020, 179, 115879 CrossRef.
- Z. Y. Huo, H. Liu, W. L. Wang, Y. H. Wang, Y. H. Wu, X. Xie and H. Y. Hu, J. Mater. Chem. A, 2019, 7, 7347–7354 RSC.
- C. H. Deng, J. L. Gong, G. M. Zeng, P. Zhang, B. Song, X. G. Zhang, H. Y. Liu and S. Y. Huan, Chemosphere, 2017, 184, 347–357 CrossRef CAS PubMed.
- Q. L. Shimabuku, F. S. Arakawa, M. Fernandes Silva, P. Ferri Coldebella, T. Ueda-Nakamura, M. R. Fagundes-Klen and R. Bergamasco, Environ. Technol., 2017, 38, 2058–2069 CrossRef CAS.
- J. M. Mazurkow, N. S. Yüzbasi, K. W. Domagala, S. Pfeiffer, D. Kata and T. Graule, Environ. Sci. Technol., 2020, 54, 1214–1222 CrossRef CAS.
- M. Sengan, S. B. Subramaniyan, S. Arul Prakash, R. Kamlekar and A. Veerappan, Microb. Pathog., 2019, 127, 341–346 CrossRef CAS PubMed.
- G. Ungur and J. Hrůza, RSC Adv., 2017, 7, 49177–49187 RSC.
- T. A. Dankovich and J. A. Smith, Water Res., 2014, 63, 245–251 CrossRef CAS.
- D. John, J. Jose, S. G. Bhat and V. S. Achari, Heliyon, 2021, 7, e07451 CrossRef CAS PubMed.
- R. C. Sanito, T. H. Yeh, S. J. You and Y. F. Wang, Environ. Technol. Innov., 2021, 22, 101502 CrossRef CAS.
- Z. Xu, G. Huang, C. An, J. Huang, X. Chen, X. Xin, P. Song, R. Feng and Y. Li, Sep. Purif. Technol., 2020, 248, 116984 CrossRef CAS.
- Y. Zhao, G. Huang, C. An, J. Huang, X. Xin, X. Chen, Y. Hong and P. Song, J. Water Process Eng., 2020, 33, 101013 CrossRef.
- Y. He, G. Huang, C. An, J. Huang, P. Zhang, X. Chen and X. Xin, Sci. Total Environ., 2018, 616–617, 1628–1637 CrossRef CAS PubMed.
- M. Sheikh, M. Pazirofteh, M. Dehghani, M. Asghari, M. Rezakazemi, C. Valderrama and J. L. Cortina, Chem. Eng. J., 2020, 391, 123475 CrossRef CAS.
- W. B. Zhao, M. R. Du, K. K. Liu, R. Zhou, R. N. Ma, Z. Jiao, Q. Zhao and C. X. Shan, ACS Appl. Mater. Interfaces, 2020, 12, 13305–13315 CrossRef CAS.
- J. Huang, G. Huang, C. An, Y. He, Y. Yao, P. Zhang and J. Shen, Environ. Pollut., 2018, 238, 52–62 CrossRef CAS.
- J. Huang, G. Huang, C. An, X. Xin, X. Chen, Y. Zhao, R. Feng and W. Xiong, Chemosphere, 2020, 245, 125545 CrossRef CAS.
- D. da Silva Biron, V. dos Santos and C. P. Bergmann, Ceram. Int., 2021, 47, 27082–27090 CrossRef CAS.
- I. Munnawar, S. S. Iqbal, M. N. Anwar, M. Batool, S. Tariq, N. Faitma, A. L. Khan, A. U. Khan, U. Nazar, T. Jamil and N. M. Ahmad, Carbohydr. Polym., 2017, 175, 661–670 CrossRef CAS.
- M. N. F. Norrrahim, N. M. Nurazzi, M. A. Jenol, M. A. A. Farid, N. Janudin, F. A. Ujang, T. A. T. Yasim-Anuar, S. U. F. Syed Najmuddin and R. A. Ilyas, Mater. Adv., 2021, 2, 3538–3551 RSC.
- R. R. D. Thomas, E. Philip, S. A. Paul, A. Madhavan, R. Sindhu, P. Binod, A. Pugazhendhi, R. Sirohi, A. Tarafdar and A. Pandey, Chemosphere, 2021, 281, 130738 CrossRef.
- M. N. Faiz Norrrahim, N. A. Mohd Kasim, V. F. Knight, K. K. Ong, S. A. Mohd Noor, S. H. Jamal, N. A. Ahmad Shah, N. A. Halim, R. A. Ilyas and W. M. Z. W. Yunus, Appl. Sci. Eng. Prog., 2021, 1–8 Search PubMed.
- S. Mbakop, L. N. Nthunya and M. S. Onyango, Processes, 2021, 9, 611 CrossRef CAS.
- S. Watts, K. Maniura-Weber, G. Siqueira and S. Salentinig, Small, 2021, 17, 2100307 CrossRef CAS.
- O. Gustafsson, L. Manukyan and A. Mihranyan, Glob. Challenges, 2018, 2, 1800031 CrossRef.
- O. Gustafsson, L. Manukyan, S. Gustafsson, G. K. Tummala, S. Zaman, A. Begum, M. A. Alfasane, K. Siddique-E-Rabbani and A. Mihranyan, ACS Sustainable Chem. Eng., 2019, 7, 14373–14383 CrossRef CAS.
- S. Heydarifard, K. Taneja, G. Bhanjana, N. Dilbaghi, M. M. Nazhad, K. H. Kim and S. Kumar, Carbohydr. Polym., 2018, 181, 1086–1092 CrossRef CAS PubMed.
- L. Manukyan, J. Padova and A. Mihranyan, Biologicals, 2019, 59, 62–67 CrossRef CAS.
- S. Heydarifard, Y. Pan, H. Xiao, M. Nazhad and O. Shipin, Carbohydr. Polym., 2017, 163, 146–152 CrossRef CAS PubMed.
- N. H. Barbhuiya, S. P. Singh, A. Makovitzki, P. Narkhede, Z. Oren, Y. Adar, E. Lupu, L. Cherry, A. Monash and C. J. Arnusch, Materials, 2021, 14, 1–11 CrossRef.
- X. Li, J. Sun, Y. Che, Y. Lv and F. Liu, Int. J. Biol. Macromol., 2019, 121, 760–773 CrossRef CAS PubMed.
- M. Jakubczak, E. Karwowska, A. Fiedorczuk and A. M. Jastrzębska, RSC Adv., 2021, 11, 18509–18518 RSC.
- S. Xu, D. Lu, J. Qi, P. Wang, Y. Zhao, H. Zhang and J. Ma, Chem. Eng. J., 2021, 410, 128289 CrossRef CAS.
- Y. Si, J. Li, C. Zhao, Y. Deng, Y. Ma, D. Wang and G. Sun, ACS Biomater. Sci. Eng., 2017, 3, 854–862 CrossRef CAS.
- Z. Ma, Z. Yan, X. Yin, Y. Si, J. Yu and B. Ding, Compos. Commun., 2021, 27, 100875 CrossRef.
- M. Maliha, B. Tan, K. Wong, S. Miri, R. Brammananth, R. L. Coppel, M. Werrett, P. C. Andrews and W. Batchelor, Colloids Surf., A, 2021, 627, 127167 CrossRef CAS.
- C. Liu, H. Shan, X. Chen, Y. Si, X. Yin, J. Yu and B. Ding, ACS Appl. Mater. Interfaces, 2018, 10, 44209–44215 CrossRef CAS.
- Y. Ma, N. Wisuthiphaet, N. Nitin and G. Sun, ACS Appl. Mater. Interfaces, 2021, 13, 41056–41065 CrossRef.
- Y. Ma, J. Yi, B. Pan, N. Nitin and G. Sun, ACS Appl. Mater. Interfaces, 2020, 12, 51057–51068 CrossRef CAS.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2023 |



