Bottom-up chemical synthesis of three-dimensional conjugated carbon nanostructures: from carbon nanocages to carbon nanotubes
Wei
Wang
,
Yu-Xuan
Wang
and
Hai-Bo
Yang
*
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, Department of Chemistry, East China Normal University, Shanghai 200062, P. R. China. E-mail: hbyang@chem.ecnu.edu.cn; Fax: +86 021 62235137
First published on 25th July 2014
Abstract
In this highlight, we present recent advances in the rational bottom-up chemical synthesis of three-dimensional (3-D) conjugated carbon nanostructures including carbon nanocages and carbon nanotubes. Derived from their efficient strategies for the construction of cycloparaphenylenes (CPPs), Itami et al. and Yamago et al. have successfully established bottom-up chemical syntheses for the construction of 3-D conjugated carbon structures, respectively. Two similar all-benzene carbon nanocages have been successfully prepared, which show intriguing photophysical properties. In addition, the initiation of carbon nanotube (CNT) growth from the selected CPPs was investigated by Itami et al., which provided a promising approach to the preparation of highly uniform CNTs.
1. Background
Since the discovery of fullerenes and carbon nanotubes (CNTs),1 investigation of these curved three-dimensional (3-D) conjugated carbon materials has become one on-going hot topic within modern chemistry. Indeed, some appealing progresses on these conjugated carbon nanostructures have deeply influenced the development of chemistry, and further affected our daily life. Apart from their structural beauty, these molecules have also exhibited wide applications in materials science. For instance, because of their nanoscale dimensions, these star molecules have been extensively applied in many fields of nanotechnology such as nanophotonic device fabrication.2 However, the highly efficient preparation of such conjugated carbon materials with high purity still remains a great challenge, which has limited further rapid development of the related fields in materials science. For example, even though the development of separation techniques has made some fullerenes like C60 and C70 commercially available, the preparation of other types of fullerenes with high purity on a large scale is still far from complete. In addition, in the case of CNTs, all known preparation methods always afford mixtures of different types of CNTs, which are virtually impossible to separate into their discrete forms on a large scale. Thus the development of a novel synthesis strategy towards the preparation of conjugated carbon materials with high efficiency and purity is still necessary.With the aim of resolving these problems, from a synthetic chemist's point of view, the bottom-up chemical synthesis strategy may provide a possible chance to achieve the preparation of such conjugated carbon materials with high purity in good yield. More importantly, this strategy may provide access to novel 3-D conjugated carbon structures that cannot be obtained through regular preparation processes, thus further enriching the family of fullerenes or CNTs.3 Scott et al. pioneered the bottom-up chemical synthesis of conjugated carbon materials. By employing polycyclic aromatic precursors bearing chlorine substituents at key points, followed by a flash vacuum pyrolysis (FVP) technique, C60 and a short [5,5] CNTs have been successfully prepared by Scott and co-workers, respectively (Fig. 1).4 However, successful examples of the bottom-up synthesis of other types of 3-D conjugated carbon nanostructures, such as conjugated carbon nanocages and nanotubes, are still rare.
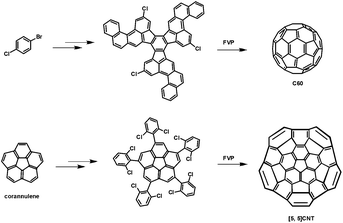 | ||
| Fig. 1 Chemical routes for the synthesis of C60 (top) and a short [5,5] CNT (bottom) by Scott et al. | ||
In 2008, a new type of nanoring, cycloparaphenylenes (CPPs),5 which could serve as the synthetic precursors of CNTs, were first synthesized by Jasti and Bertozzi et al. After this inspiring work, a full range of CPPs containing different phenyl groups were prepared by Jasti,6 Itami,7 and Yamago8et al. (Fig. 2). In order to overcome the strong strain energies of the bent aromatic system in CPPs, a series of different strain buffering moieties were employed. For instance, in Jasti's approach, the 3,6-syn-dimethoxy-cyclohexa-1,4-diene moiety was chosen as both the strain buffering moiety and the masked aromatic ring to ensure the formation of the less-strained macrocyclic precursors. The final aromatization reaction afforded the designated CPPs (Fig. 2B). Similarly, Itami and co-workers employed the cis-1,4-diphenylcyclohexane moiety as the strain buffering moiety to prepare CPPs (Fig. 2C). Different from the above mentioned approach, Yamago and co-workers selected the cis-platinum links as the strain buffering corners of the rigid metallacycles, which were further eliminated to afford the final CPPs (Fig. 2D).
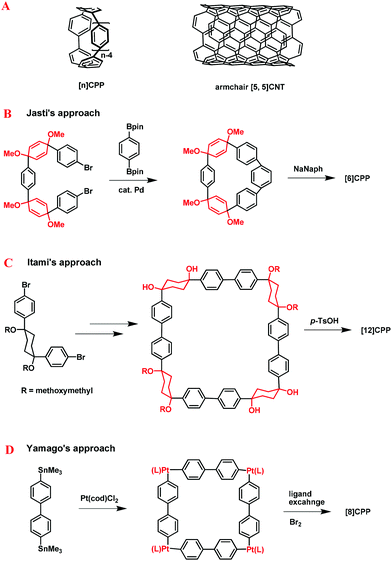 | ||
| Fig. 2 Schematic representation of the CPPs and CNTs (A). Three efficient approaches for the synthesis of CPPs developed by Jasti et al. (B), Itami et al. (C), and Yamago et al. (D), respectively. | ||
With the successful preparation of these two-dimensional (2-D) hoop-shaped conjugated molecules, a question immediately arose: can these strategies be applied to the rational synthesis of 3-D conjugated carbon materials? In this highlight, we present recent advances in the rational bottom-up chemical synthesis of 3-D conjugated carbon nanostructures. Two similar all-benzene carbon nanocages, which may serve as the synthetic precursor of junction CNTs, were successfully prepared by employing the bottom-up chemical synthesis strategy. In addition, the CNT growth from the selected CPPs was investigated by Itami et al., which provides a possible solution to the preparation of the uniform CNTs.
2. Bottom-up chemical synthesis of 3-D conjugated carbon nanostructures
It should be noted that, different from the widely investigated supramolecular cages connected by reversible bonds (i.e. noncovalent interactions or dynamic covalent bonds),9 the synthesis of carbon conjugated nanocages mainly relies on irreversible covalent bonds. Different from thermodynamically controlled supramolecular self-assembly processes, the lack of self-repairing and self-correcting abilities in the formation of a certain number of covalent bonds makes the formation of well-defined covalent carbon nanocages quite challenging. Based on their successful approaches for the synthesis of CPPs, Itami and Yamago further expanded their research to the synthesis of 3-D conjugated carbon nanostructures. Two similar conjugated carbon nanocages have been successfully prepared by these two groups independently. Both reports provided new insights into the bottom-up chemical synthesis of 3-D carbon nanostructures, thus representing inspiring examples for future investigations.In Segawa, Kamada, and Itami's work,10 in order to obtain the less-strained cage precursor, a new building block 1 containing three L-shaped strain buffering moieties on the benzene core was designed and synthesized. The three-fold homocoupling of this C3 symmetric building block via a Ni(0)-mediated Yamamoto coupling reaction led to the formation of the triangular prism-like cage precursor 2, in which the strain buffering moieties acted as the six corners. The sequential aromatization reaction of these corners resulted in the preparation of the expected conjugated carbon nanocage 3, possessing twenty benzene units, as shown in Scheme 1. This design approach relies on the direct coupling reaction of individual building blocks with special structural features to afford the main skeleton, which can be classified as the direct coupling approach.
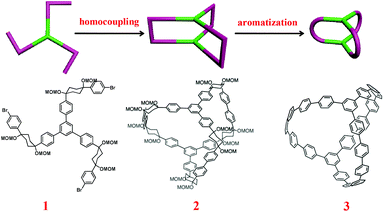 | ||
| Scheme 1 Schematic representation of Itami's direct coupling approach for the synthesis of conjugated carbon nanocages.10 (Copyright © 2013 Royal Society of Chemistry.) | ||
Previously, Yamago and co-workers presented the assembly/reductive elimination strategy for the synthesis of CPPs, in which the organoplatinum complexes served as the links for the skeleton formation. This strategy was initially inspired by the formation of discrete metallacycles via coordination-driven self-assembly. Since coordination-driven self-assembly has emerged as a powerful approach not only for the construction of 2-D polygons but also 3-D polyhedra with well-defined shape and size,11 researchers developing the syntheses of 3-D conjugated carbon nanocages may learn some lessons from this rapidly developing area. For instance, by employing the paneling approach, Fujita et al.12 realized the construction of a series of metallocages or metallocavitands, which were famous as “molecular flasks”, derived from multi-pyridyl ligands and organopalladium(II) acceptors. Inspired by Fujita's M4L6 type metallocages (Scheme 2A), Yamago et al. employed a similar paneling coupling approach to deal with the synthesis of 3-D conjugated carbon nanocages, as shown in Scheme 2B.
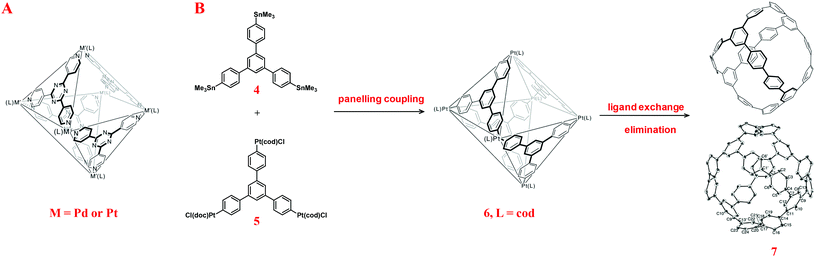 | ||
| Scheme 2 Schematic representation of Fujita's M4L6 cages (A). Yamago's panelling coupling approach for the synthesis of conjugated carbon nanocages and its crystal structure (B).13 (Copyright © 2013 Macmillan Publishers Limited.) | ||
In Yamago's approach,13 two different panelling building blocks 4 and 5 with D3h symmetry were designed and prepared. The coupling reaction of the equimolar 4 and 5 afforded the formation of octahedron-like cage precursor 6, in which six platinum complexes served as the buffering vertexes. The preparation of 6 was in a delightfully high yield (81%), which might be attributed to the reversible C–Pt bond formation during the transmetallation reaction. Sequential ligand exchange and elimination afforded the expected nanocage 7 with sixteen benzene units. Notably, the structure of a 3-D conjugated carbon nanocage was unambiguously confirmed by X-ray crystallographic analysis.
In both reports, the rational design of the building blocks played a crucial role. In order to afford the well-defined 3-D nanocage precursors, individual building blocks with special structural features (i.e. symmetry, geometry and the locations of the reaction sites etc.) were necessary. Moreover, the choice of the key reactions to afford the nanocage precursors deeply influenced the synthetic efficiency. Notably, in Yamago's approach, the preparation of the nanocage precursor 6 was mainly dependent on the reversible C–Pt bond formation, thus allowing for the high efficiency of the cage formation.
Based on the above-mentioned successful preparation of diverse CPPs, investigation of the growth of CNTs from CPPs was carried out by Itami et al.14 (Scheme 3). Two selected CPPs, i.e. [9]CPP or [12]CPP, were employed as the seed molecules to afford the corresponding CNTs by simply heating the CPPs with ethanol. The TEM analysis clearly reveals the CPP-template effect. For instance, the CNT growth from [9]CPP or [12]CPP were distributed to a diameter range of 1.2 nm or 1.3–1.7 nm, respectively, which is close to the diameter size of [9]CPP or [12]CPP. Furthermore, according to this study, a new mechanism, which could be named as the CPP radical mechanism, for CNT growth was proposed. However, this work also suffered from some limitations such as the limited reaction scale and the lack of perfect control of the diameter and chirality of the resultant CNTs. Nevertheless, this inspiring study provided a promising solution for the preparation of uniform CNTs, thus opening up a new route for the bottom-up synthesis of 3-D conjugated carbon nanostructures.
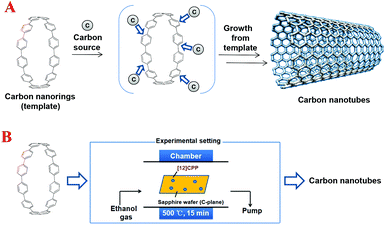 | ||
| Scheme 3 Schematic representation of the ‘growth-from-template’ strategy for the bottom-up synthesis of structurally uniform CNTs (A). The detailed growth experiments developed by Itami et al. (B). | ||
3. Summary
In this highlight, the recent advances in the rational bottom-up chemical synthesis of 3-D conjugated carbon nanostructures were presented. Based on their successful results for the construction of CPPs, Itami et al. and Yamago et al. further expanded their investigation to the synthesis of 3-D conjugated molecules. Two similar all-benzene carbon nanocages have been successfully constructed, which showed intriguing photophysical properties such as high fluorescence quantum yield. In addition, the initiation of CNT growth from the selected CPPs was investigated by Itami et al., which provided a possible solution for the creation of uniform CNTs. According to these reports, the rational bottom-up chemical synthesis of 3-D conjugated carbon nanostructures was proven to be feasible and practical. By using approaches similar to those described here, the construction of analogous structures with high-level complexity and functionality could be further realized. Although there is still a long way to go to achieve the ultimate goal of controllable chemical synthesis of fullerenes or CNTs with a uniform structure, the reports highlighted herein do inject a new design philosophy into this hot research area. However, the development of a new design philosophy and relevant methodologies are still urgently required.Acknowledgements
The work was financially supported by the National Natural Science Foundation of China (no. 21322206, 91027005, and 21132005) and the Key Basic Research Project of Shanghai Science and Technology Commission (no. 13JC1402200).Notes and references
- (a) H. W. Kroto, J. R. Heath, S. C. Obrien, R. F. Curl and R. E. Smalley, Nature, 1985, 318, 162 CrossRef CAS; (b) S. Iijima, Nature, 1991, 354, 54 CrossRef.
- (a) E. Sheka, Fullerenes: Nanochemistry, Nanomagnetism, Nanomedicine, Nanophotonics, CRC Press, 2011 Search PubMed; (b) M. Dresselhaus, G. Dresselhaus and P. Avouris, Carbon Nanotubes: Synthesis, Properties and Applications, Springer, 2001 Search PubMed.
- U. H. F. Bunz, S. Menning and N. Martín, Angew. Chem., Int. Ed., 2012, 51, 7094 CrossRef CAS PubMed.
- (a) L. T. Scott, M. M. Boorum, B. J. McMahon, S. Hagen, J. Mack, J. Blank, H. Wegner and A. de Meijere, Science, 2002, 295, 1500 CrossRef CAS PubMed; (b) L. T. Scott, E. A. Jackson, Q. Zhang, B. D. Steinberg, M. Bancu and B. Li, J. Am. Chem. Soc., 2012, 134, 107 CrossRef CAS PubMed.
- R. Jasti, J. Bhattacharjee, J. B. Neaton and C. R. Bertozzi, J. Am. Chem. Soc., 2008, 130, 17646 CrossRef CAS PubMed.
- E. S. Hirst and R. Jasti, J. Org. Chem., 2012, 77, 10473 Search PubMed.
- H. Omachi, Y. Segawa and K. Itami, Acc. Chem. Res., 2012, 45, 1378 Search PubMed.
- S. Yamago, E. Kayahara and T. Iwamoto, Chem. Rec., 2014, 14, 84 CrossRef CAS PubMed.
- (a) M. Mastalerz, Angew. Chem., Int. Ed., 2010, 49, 5042 CrossRef CAS PubMed; (b) F. Hof, S. L. Craig, C. Nuckolls and J. Rebek Jr., Angew. Chem., Int. Ed., 2002, 41, 1488 CrossRef CAS; (c) P. Mal, B. Breiner, K. Rissanen and J. R. Nitschke, Science, 2009, 324, 1697 CrossRef CAS PubMed.
- K. Matsui, Y. Segawa, T. Namikawa, K. Kamada and K. Itami, Chem. Sci., 2013, 4, 84 RSC.
- (a) S. Leininger, B. Olenyuk and P. J. Stang, Chem. Rev., 2000, 100, 853 CrossRef CAS PubMed; (b) R. Chakrabarty, P. S. Mukherjee and P. J. Stang, Chem. Rev., 2011, 111, 6810 CrossRef CAS PubMed.
- M. Yoshizawa, J. K. Klosterman and M. Fujita, Angew. Chem., Int. Ed., 2009, 48, 3418 CrossRef CAS PubMed.
- E. Kayahara, T. Iwamoto, H. Takaya, T. Suzuki, M. Fujitsuka, T. Majima, N. Yasuda, N. Matsuyama, S. Seki and S. Yamago, Nat. Commun., 2013, 4, 2694 Search PubMed.
- H. Omachi, T. Nakayama, E. Takahashi, Y. Segawa and K. Itami, Nat. Chem., 2013, 5, 572 CAS.
| This journal is © the Partner Organisations 2014 |



