A metal-free synthesis of diaryl-1,2-diketones by C–C triple bond cleavage of alkynones†
Xuesong
Wang
,
Guolin
Cheng
,
Jinhai
Shen
,
Xifa
Yang
,
Ming-e
Wei
,
Yadong
Feng
and
Xiuling
Cui
*
Key Laboratory of Xiamen Marine and Gene Drugs, Institutes of Molecular Medicine and School of Biomedical Sciences, Huaqiao University & Engineering Research Center of Molecular Medicine, Ministry of Education, Xiamen, 361021, China. E-mail: cuixl@hqu.edu.cn
First published on 18th August 2014
Abstract
A novel and environmentally benign protocol for diaryl-1,2-diketones was developed. Various diaryl-1,2-diketones were afforded in moderate to excellent yields by C–C triple bond cleavage of alkynones using molecular oxygen as an oxidant. A plausible reaction mechanism was proposed that accounts for all the experimental results. The products are important building blocks in organic synthesis and could be converted to various synthons via diverse transformations.
Diaryl-1,2-diketones represent an important structural moiety that appears in numerous bioactive compounds1 and is broadly utilized for constructing various complex and highly valuable molecules.2 Therefore, substantial efforts for the development of efficient synthetic strategies towards such a structure have been undertaken. The direct oxidation of internal alkynes, which could be accessible via the Sonogashira coupling reaction, appears to be the most straightforward method to synthesize the diaryl-1,2-diketones.3 The oxidation of benzoins or hydrobenzoins in the presence of metal catalysts, such as gold,4 palladium,5 nickel,6 vanadium,7 ruthenium,8 thymine iron(III),9 molybdenum,10 and chromium trioxide,11 has also been reported. Recently, building diaryl-1,2-diketones from 1,3-diaryldiketones through the C–C bond cleavage has been explored as an alternative strategy.12 However, the drawbacks associated with these procedures, such as the requirement of transition metals and toxic and/or expensive starting materials, low chemo-selectivity, and harsh reaction conditions, limit their wide application in chemical industries.
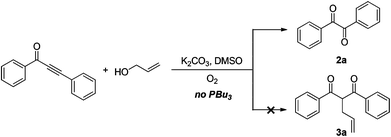
In the past few years, alkynones have emerged as versatile building blocks for the construction of complicated heterocyclic rings, such as triazoles,13 indoles,14 quinolines,15 chromones,16 furans,17 and isoxazoles.18 Recently, we have developed a tandem condensation of o-halo/methoxyarylynones with allylic alcohols to build 3-allyl-chromones catalyzed by PBu3 under metal-free conditions.20 However, the cleavage of the C–C triple bond19 in alkynones, one of the most challenging subjects in synthetic organic chemistry, has not been reported in the literature. Herein, we present a novel and environmentally friendly method for the preparation of diaryl-1,2-diketones by oxidative C–C triple bond cleavage of alkynones using molecular oxygen as an oxidant (Scheme 1).
The reaction of 1,3-diphenylprop-2-yn-1-one 1a with an allyl alcohol was initially conducted at 90 °C in the presence of K2CO3 as a base in DMSO under an O2 (1 atm) atmosphere. The expected product 3a was not observed, but diphenyl-1,2-diketone 2a was obtained in 63% yield (Scheme 1 and Table 1, entry 1). Further studies were focused on screening of the additives (entries 2–6). 1-Butanol, benzyl alcohol, and H2O gave similar results. H2O was chosen as an additive because of its advantages in terms of economy and environment (entry 6). Increasing or decreasing the ratio of H2O–DMSO resulted in decreasing the yield of benzil 2a (entries 7–10). The bases were also screened (entries 11–22). Cs2CO3, Na2CO3, and K3PO4 provided 63%, 48%, and 50% yields, respectively (entries 11–13), while weaker bases showed low efficiency (entries 14 and 15) and stronger bases did not exhibit reactivity (entries 16–22). The solvent also played a crucial role in this transformation (entries 6 and 23–30). DMSO, DMF, and NMP afforded the desired product 2a in 65%, 55%, and 48% yields, respectively. Only a trace amount of 2a was observed for other solvents, such as THF, 1,4-dioxane, DCE, toluene, EtOH, and water (entries 25–30). Further optimization of the reaction parameters revealed that the combination of DMSO as a solvent and O2 as an oxidant were necessary to get diaryl-1,2-diketone derivatives successfully. On the basis of the screening reactions above, the optimal reaction conditions were identified as follows: K2CO3 as a base and DMSO–H2O (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the solvent under an oxygen atmosphere at 90 °C for 8 h.
1) as the solvent under an oxygen atmosphere at 90 °C for 8 h.
| Entry | Additive | Solvent | Base | Yieldb (%) |
|---|---|---|---|---|
a Reaction conditions: 1a (0.5 mmol), additive/DMSO (40 μl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ml), base (0.5 mmol), under an O2 atmosphere at 90 °C for 8 h.
b Isolated yield based on 1a.
c H2O–DMSO (50 μl 2 ml), base (0.5 mmol), under an O2 atmosphere at 90 °C for 8 h.
b Isolated yield based on 1a.
c H2O–DMSO (50 μl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ml).
d H2O–DMSO (60 μl 2 ml).
d H2O–DMSO (60 μl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ml).
e H2O–DMSO (30 μl 2 ml).
e H2O–DMSO (30 μl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ml).
f H2O–DMSO (20 μl 2 ml).
f H2O–DMSO (20 μl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ml).
g 60 °C.
h 70 °C. DMSO = dimethylsulfoxide, NMP = 1-methylpyrrolidin-2-one. 2 ml).
g 60 °C.
h 70 °C. DMSO = dimethylsulfoxide, NMP = 1-methylpyrrolidin-2-one.
|
||||
| 1 | Allyl alcohol | DMSO | K2CO3 | 63 |
| 2 | Methanol | DMSO | K2CO3 | 25 |
| 3 | Ethanol | DMSO | K2CO3 | 30 |
| 4 | 1-Butanol | DMSO | K2CO3 | 62 |
| 5 | Benzyl alcohol | DMSO | K2CO3 | 50 |
| 6 | H 2 O | DMSO | K 2 CO 3 | 65 |
| 7 | H2O | DMSO | K2CO3 | 55c |
| 8 | H2O | DMSO | K2CO3 | 50d |
| 9 | H2O | DMSO | K2CO3 | 58e |
| 10 | H2O | DMSO | K2CO3 | 53f |
| 11 | H2O | DMSO | Cs2CO3 | 63 |
| 12 | H2O | DMSO | Na2CO3 | 48 |
| 13 | H2O | DMSO | K3PO4 | 50 |
| 14 | H2O | DMSO | Et3N | 15 |
| 15 | H2O | DMSO | Li2CO3 | 20 |
| 16 | H2O | DMSO | EtONa | None |
| 17 | H2O | DMSO | NaH | None |
| 18 | H2O | DMSO | KOtBu | None |
| 19 | H2O | DMSO | NaOtBu | None |
| 20 | H2O | DMSO | LiOtBu | None |
| 21 | H2O | DMSO | NaOH | None |
| 22 | H2O | DMSO | KOH | None |
| 23 | H2O | DMF | K2CO3 | 55 |
| 24 | H2O | NMP | K2CO3 | 48 |
| 25 | H2O | THF | K2CO3 | Traceg |
| 26 | H2O | Dioxane | K2CO3 | Trace |
| 27 | H2O | DCE | K2CO3 | Trace |
| 28 | H2O | Toluene | K2CO3 | Trace |
| 29 | H2O | EtOH | K2CO3 | Traceh |
| 30 | — | H2O | K2CO3 | Trace |
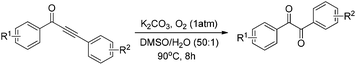
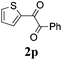
With the optimized reaction conditions in hand, the generality and scope for the substrates were investigated as illustrated in Table 2. Generally, alkynones with electron-donating substituents (OMe, Me, tBu) (entries 2–6) provided the corresponding products in higher yields than those bearing electron-withdrawing groups (F, Cl) (entries 7–10). As for regioisomeric substrates, alkynones with substituents in Ar–R1 (entries 3, 5, 8, and 10) could be transformed into the corresponding diaryl-1,2-diketone products in similar yields as those with substituents in Ar–R2 (entries 2, 4, 7, and 9). Additionally, alkynones with substituents in Ar–R1 and Ar–R2 were also investigated under the standard reaction conditions. The corresponding diaryl-1,2-diketones were obtained in moderate to excellent yields regardless of the electron-donating or electron-withdrawing groups at benzene rings (entries 11–15). It is worth noting that alkynone with two methoxyl groups provided an excellent yield because of the strong electron-donating effect (entry 11). However, when the substituents were at the ortho position of the aromatic ring, the yield decreased maybe due to the steric hindrance (entries 16–18). Moreover, alkynones possessing a naphthyl and thienyl ring were tolerated in this oxidation reaction system and provided the desired products 2o and 2p in 60% and 51% yields, respectively (entries 19 and 20). The transformation of alkynones with the aliphatic group was also attempted, but no desired products were observed.
| Entry | Substrate | Product | Yieldb (%) |
|---|---|---|---|
a Reaction conditions: 1 (0.5 mmol), H2O–DMSO (40 μl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ml), K2CO3 (0.5 mmol), under an O2 atmosphere for 8 h.
b Isolated yield on 1. 2 ml), K2CO3 (0.5 mmol), under an O2 atmosphere for 8 h.
b Isolated yield on 1.
|
|||
| 1 |
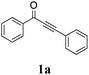
|
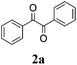
|
65 |
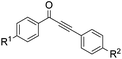
|
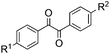
|
||
| 2 | R1 = OMe, R2 = H, 1b | 2b | 70 |
| 3 | R1 = H, R2 = OMe, 1b′ | 72 | |
| 4 | R1 = Me, R2 = H, 1c | 2c | 67 |
| 5 | R1 = H, R2 = Me, 1c′ | 69 | |
| 6 | R1 = t-Bu, R2 = H, 1d | 2d | 66 |
| 7 | R1 = Cl, R2 = H, 1e | 2e | 38 |
| 8 | R1 = H, R2 = Cl, 1e′ | 45 | |
| 9 | R1 = F, R2 = H, 1f | 2f | 40 |
| 10 | R1 = H, R2 = F, 1f′ | 44 | |
| 11 | R1 = OMe, R2 = OMe, 1g | 2g | 93 |
| 12 | R1 = Me, R2 = Me, 1h | 2h | 60 |
| 13 | R1 = t-Bu, R2 = OMe, 1i | 2i | 65 |
| 14 | R1 = F, R2 = OMe, 1j | 2j | 53 |
| 15 | R1 = F, R2 = F, 1k | 2k | 40 |
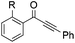
|
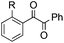
|
||
| 16 | R = OMe, 1l | 2l | 52 |
| 17 | R = Me, 1m | 2m | 50 |
| 18 | R = F, 1n | 2n | 35 |
| 19 |
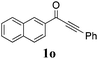
|
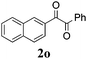
|
60 |
| 20 |
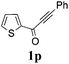
|
|
51 |
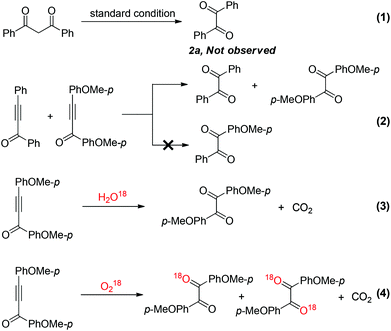
The initially proposed mechanism suspected that 1,3-diaryldiketone might serve as an intermediate in this transformation. Unexpectedly, no desired product 2a was detected when 1,3-diphenyldiketone was subjected under the optimal reaction conditions (Scheme 2, eqn (1)). To gain more insight of the reaction mechanism, 1,3-diphenylprop-2-yn-1-one and 1,3-bis(4-methoxyphenyl)prop-2-yn-1-one were treated in one pot under the standard reaction conditions (Scheme 2, eqn (2)). The potential crossover products were not detected by GC-MS, which indicated that this transformation probably occurred through an intramolecular process. In order to confirm the source of the oxygen atom of diaryl-1,2-diketone, the controlled experiments were conducted involving H2O18 and O218, respectively. The results showed that the oxygen atom of the diaryl-1,2-diketone derived from O2, but not from H2O. Furthermore, CO2 was generated and caused the clear limewater to become cloudy (Scheme 2, eqn (3) and (4)).
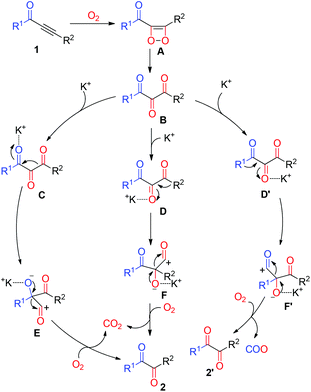
According to the results obtained and the literature,21 the mechanistic pathways of this oxidative cleavage metathesis are proposed and presented in Scheme 3. The initial step of the reaction involved the formation of 1,2-dioxetene A from the alkynone 1 with molecular oxygen. Then thermally inducing the transformation of intermediate A gave 1,2,3-tricarbonyl compound B. Subsequently, activation of the β-keto or the α-keto moiety of compound B in the presence of K2CO3 led to intermediates C, D, and D′, followed by C–C bond cleavage and the carbon immigration to intermediates E, F and F′, respectively. Finally, the elimination of carbon monoxide (CO) from intermediates E, F and F′ provided the desired diaryl-1,2-diketone 2 and 2′. Simultaneously, the gas carbon dioxide (CO2) was formed by the oxidation of CO with molecular oxygen.
In conclusion, we have developed a novel and environmentally benign method for the synthesis of diaryl-1,2-diketones with high chemo-selectivity by C–C triple bond cleavage of alkynones. The oxidation of triple bond, cleavage of C–C bond and carbon immigration were involved in this procedure.
Acknowledgements
Financial support was provided by NSF of China (21202048), Program for Minjiang Scholar program (10BS216), Xiamen Southern Oceanographic Center (13GYY003NF16), NSF of Fujian Province, China (2013J01050) and Fundamental Research Funds of Huaqiao University.Notes and references
- R. Maurya, R. Singh, M. Deepak, S. S. Handa, P. P. Yadav and P. K. Mishra, Phytochemistry, 2004, 65, 915 CrossRef CAS PubMed.
- (a) X. Deng and N. S. Mani, Org. Lett., 2006, 8, 269 CrossRef CAS PubMed; (b) A. Cabrera, P. Sharma, M. Ayala, L. Rubio-Perez and M. Amézquita-Valencia, Tetrahedron Lett., 2011, 52, 6758 CrossRef CAS PubMed.
- (a) S. Baskaran, J. Das and S. Chandrasekaran, J. Org. Chem., 1989, 54, 5182 CrossRef CAS; (b) Y. Ishii and Y. Sakata, J. Org. Chem., 1990, 55, 5545 CrossRef CAS; (c) Y. Sawama, M. Takubo, S. Mori, Y. Monguchi and H. Sajiki, Eur. J. Org. Chem., 2011, 3361 CrossRef CAS PubMed; (d) M. Tingoli, M. Mazzella, B. Panunzi and A. Tuzi, Eur. J. Org. Chem., 2011, 399 CrossRef CAS PubMed; (e) S. Kobayashi, H. Miyamura, R. Akiyama and T. Ishida, J. Am. Chem. Soc., 2005, 127, 9251 CrossRef CAS PubMed; (f) W. Ren, Y. Xia, S. J. Ji, Y. Zhang, X. Wan and J. Zhao, Org. Lett., 2009, 11, 1841 CrossRef CAS PubMed; (g) Z. Wan, C. D. Jones, D. Mitchell, J. Y. Pu and T. Y. Zhang, J. Org. Chem., 2006, 71, 826 CrossRef CAS PubMed; (h) K. Sakthivel and K. Srinivasan, Eur. J. Org. Chem., 2011, 2781 CrossRef CAS PubMed; (i) C.-F. Xu, M. Xu, Y.-X. Jia and C.-Y. Li, Org. Lett., 2011, 13, 1556 CrossRef CAS PubMed; (j) S. Trosien and S. R. Waldvogel, Org. Lett., 2012, 14, 2976 CrossRef CAS PubMed; (k) C.-M. Che, W.-Y. Yu, P.-M. Chan, W.-C. Cheng, S.-M. Peng, K.-C. Lau and W.-K. Li, J. Am. Chem. Soc., 2000, 122, 11380 CrossRef CAS.
- B. Karimi and F. K. Esfahani, Chem. Commun., 2009, 5555 RSC.
- (a) B. Karimi, S. Abedi, J. H. Clark and V. Budarin, Angew. Chem., Int. Ed., 2006, 45, 4776 CrossRef CAS PubMed; (b) N. Kakiuchi, Y. Maeda, T. Nishimura and S. Uemura, J. Org. Chem., 2001, 66, 6620 CrossRef CAS PubMed; (c) Y. Uozumi and R. Nakao, Angew. Chem., Int. Ed., 2003, 42, 194 CrossRef CAS PubMed; (d) K. Mori, T. Hara, T. Mizugaki, K. Ebitani and K. Kaneda, J. Am. Chem. Soc., 2000, 126, 10657 CrossRef PubMed.
- B. M. Choudary, M. L. Kantam, A. Rahman, Ch. V. Reddy and K. K. Rao, Angew. Chem., Int. Ed., 2001, 40, 763 CrossRef CAS.
- S. Velusamy and T. Punniyamurthy, Org. Lett., 2004, 6, 217 CrossRef CAS PubMed.
- (a) I. E. Markó, P. R. Giles, M. Tsukazaki, I. Chellé-Regnaut, C. J. Urch and S. M. Brown, J. Am. Chem. Soc., 1997, 119, 12661 CrossRef; (b) N. Komiya, T. Nakae, H. Sato and T. Naota, Chem. Commun., 2006, 4829 RSC; (c) E. Choi, C. Lee, Y. Na and S. Chang, Org. Lett., 2002, 4, 2369 CrossRef CAS PubMed.
- A. Al-Hunaiti, T. Niemi, A. Sibaouih, P. Pihko, M. Leskelä and T. Repo, Chem. Commun., 2010, 46, 9250 RSC.
- S. Velusamy, M. Ahamed and T. Punniyamurthy, Org. Lett., 2004, 6, 4821 CrossRef CAS PubMed.
- J.-D. Lou, N. Vatanian, C. Zhang and G.-Q. Wang, Synth. React. Inorg., Met.-Org., Nano-Met. Chem., 2009, 39, 121 CAS.
- (a) Y. Yuan and H. Zhu, Eur. J. Org. Chem., 2012, 329 CrossRef CAS PubMed; (b) L. Huang, K. Cheng, B. Yao, Y. Xie and Y. Zhang, J. Org. Chem., 2011, 76, 5732 CrossRef CAS PubMed; (c) N. Tada, M. Shomura, H. Nakayama, T. Miura and A. Itoh, Synlett, 2010, 1979 CAS.
- G. Cheng, X. Zeng, J. Shen, X. Wang and X. Cui, Angew. Chem., Int. Ed., 2013, 52, 13265 CrossRef CAS PubMed.
- (a) J. Shen, G. Cheng and X. Cui, Chem. Commun., 2013, 49, 10641 RSC; (b) R. Bernini, G. Fabrizi, A. Sferrazza and S. Cacchi, Angew. Chem., Int. Ed., 2009, 48, 8078 CrossRef CAS PubMed; (c) S. Cacchi, G. Fabrizi and E. Filisti, Org. Lett., 2008, 10, 2629 CrossRef CAS PubMed.
- (a) G. Abbiati, A. Arcadi, F. Marinelli and E. Rossi, Eur. J. Org. Chem., 2003, 1423 CrossRef CAS PubMed; (b) R. P. Korivi and C. H. Cheng, J. Org. Chem., 2006, 71, 7079 CrossRef CAS PubMed; (c) H. Zhou, L. Liu and S. Xu, J. Org. Chem., 2012, 77, 9418 CrossRef CAS PubMed.
- (a) M. Yoshida, Y. Fujino and T. Doi, Org. Lett., 2011, 13, 4526 CrossRef CAS PubMed; (b) C. Zhou, A. V. Dubrovsky and R. C. Larock, J. Org. Chem., 2006, 71, 1626 CrossRef CAS PubMed; (c) M. Yoshida, K. Saito, Y. Fujino and T. Doi, Chem. Commun., 2012, 48, 11796 RSC.
- (a) J. Fu, H. Shang, Z. Wang, L. Chang, W. Shao, Z. Yang and Y. Tang, Angew. Chem., Int. Ed., 2013, 52, 4198 CrossRef CAS PubMed; (b) H. Harkat, A. Blanc, J.-M. Weibel and P. Pale, J. Org. Chem., 2008, 73, 1620 CrossRef CAS PubMed; (c) H. Jiang, W. Yao, H. Cao, H. Huang and D. Cao, J. Org. Chem., 2010, 75, 5347 CrossRef CAS PubMed.
- (a) Z. She, D. Niu, L. Chen, M. A. Gunawan, X. Shanja, W. H. Hersh and Y. Chen, J. Org. Chem., 2012, 77, 3627 CrossRef CAS PubMed; (b) M. Ueda, S. Sugita, A. Sato, T. Miyoshi and O. Miyata, J. Org. Chem., 2012, 77, 9344 CrossRef CAS PubMed; (c) J. P. Waldo and R. C. Larock, J. Org. Chem., 2007, 72, 9643 CrossRef CAS PubMed; (d) M. Ueda, A. Sato, Y. Ikeda, T. Miyoshi, T. Naito and O. Miyata, Org. Lett., 2010, 12, 2594 CrossRef CAS PubMed.
- (a) P. Bisseret, G. Duret and N. Blanchard, Org. Chem. Front., 2014, 1, 825 RSC; (b) Q. Liu, P. Chen and G. Liu, ACS Catal., 2013, 3, 178 CrossRef CAS; (c) D.-Y. Lee, B.-S. Hong, E.-G. Cho, H. Lee and C.-H. Jun, J. Am. Chem. Soc., 2003, 125, 6372 CrossRef CAS PubMed; (d) Y. Liu, F. Song and S. Guo, J. Am. Chem. Soc., 2006, 128, 11332 CrossRef CAS PubMed; (e) A. Wang and H. Jiang, J. Am. Chem. Soc., 2008, 130, 5030 CrossRef CAS PubMed; (f) J. Sun, F. Wang, H. Hu, X. Wang, H. Wu and Y. Liu, J. Org. Chem., 2014, 79, 3992 CrossRef CAS PubMed; (g) Q. Jiang, A. Zhao, B. Xu, J. Jia, X. Liu and C. Guo, J. Org. Chem., 2014, 79, 2709 CrossRef CAS PubMed.
- X. Wang, G. Cheng and X. Cui, Chem. Commun., 2014, 50, 652 RSC.
- (a) A. Stergiou, A. Bariotaki, D. Kalaitzakis and I. Smonou, J. Org. Chem., 2013, 78, 7268 CrossRef CAS PubMed; (b) W. J. Baader and E. L. Bastos, Sci. Synth., 2009, 38, 335 Search PubMed; (c) K. A. Zaklika, B. Kaskar and A. P. Schaap, J. Am. Chem. Soc., 1980, 102, 386 CrossRef CAS; (d) A. Krebs, H. Schmalstieg, O. Jarchow and K.-H. Klaska, Tetrahedron Lett., 1980, 21, 3171 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details See DOI: 10.1039/c4qo00174e |
| This journal is © the Partner Organisations 2014 |