Covalent modification and exfoliation of graphene oxide using ferrocene†
M. B.
Avinash
,
K. S.
Subrahmanyam
,
Y.
Sundarayya
and
T.
Govindaraju
*
New Chemistry Unit, Jawaharlal Nehru Centre for Advanced Scientific Research, Jakkur, Bangalore, 560064, India. E-mail: tgraju@jncasr.ac.in
First published on 7th July 2010
Abstract
Large scale preparation of single-layer graphene and graphene oxide is of great importance due to their potential applications. We report a simple room temperature method for the exfoliation of graphene oxide using covalent modification of graphene oxide with ferrocene to obtain single-layer graphene oxide sheets. The samples were characterized by FESEM, HRTEM, AFM, EDAX, FT-IR, Raman and Mössbauer spectroscopic studies. HRTEM micrograph of the covalently modified graphene oxide showed increased interlayer spacing of ∼2.4 nm due to ferrocene intercalation. The presence of single-layer graphene oxide sheets were confirmed by AFM studies. The covalently modified ferrocene–graphene oxide composite showed interesting magnetic behavior.
Introduction
Graphene has been the subject of intense research because of its remarkable properties and potential applications.1 Graphene is the mother of all graphitic forms. The massless Dirac fermions nature of carriers in single-layer graphene leads to an anomalous quantum Hall effect. Single-layer graphene possesses extraordinary electronic properties with a carrier mobility of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm2 V−1 s−1.2 Such carrier mobilities allow ballistic transport properties at room temperature. The electron mobility of graphene is nearly ten times greater than that of commercially available Si wafers.3,4 Therefore, graphene could be a potential substitute for Si in electronics. Theoretical studies predict a large surface area of ∼2600 m2 g−1 for a single-layer graphene.5 The highest surface area to volume ratio of graphene makes it one of the best 2D materials for chemical sensing applications. High values of Young's modulus (∼1100 GPa),6 fracture strength (125 GPa)6 and thermal conductivity (∼5000 W m−1 K−1)7 are among the other characteristic properties of graphene.
000 cm2 V−1 s−1.2 Such carrier mobilities allow ballistic transport properties at room temperature. The electron mobility of graphene is nearly ten times greater than that of commercially available Si wafers.3,4 Therefore, graphene could be a potential substitute for Si in electronics. Theoretical studies predict a large surface area of ∼2600 m2 g−1 for a single-layer graphene.5 The highest surface area to volume ratio of graphene makes it one of the best 2D materials for chemical sensing applications. High values of Young's modulus (∼1100 GPa),6 fracture strength (125 GPa)6 and thermal conductivity (∼5000 W m−1 K−1)7 are among the other characteristic properties of graphene.
Geim and coworkers for the first time obtained single and bi-layer graphene by micro-mechanical cleavage of highly oriented pyrolytic graphite (HOPG).8 Single-layer graphene was peeled off from graphite using a scotch tape. However the large scale preparation of graphene single-layers using this technique is limited. Large quantities of few layer graphenes are produced by chemical vapor deposition of camphor, conversion of nanodiamond and thermal exfoliation of graphitic oxide.9 Graphite on oxidation with mixture of oxidizing reagents yields graphitic oxide. The presence of epoxide, hydroxyl, carboxyl and other oxygen functionalities increases the interlayer spacing (d-spacing) of graphene layers from 3.35 Å to ∼7 Å. During thermal exfoliation CO2 produced on account of rapid heating expels the π-stacked graphitic oxide layers to single, bi or few layer graphene oxide sheets. The interlayer cohesive energy or exfoliation energy for pyrolytic graphite is found to be 61 meV per carbon atom.10 With the carbon–carbon bond length of 1.42 Å, 1 nm square of graphene consisting of 38 carbon atoms involves separation energy of 2 eV.11 Such large cohesive energy process can now be furnished by a simpler chemical means. Chemical functionalization serves as an excellent means to manipulate the properties of graphene for its effective usage in various applications. Chemical functionalization can facilitate hydrogen storage, spintronics, and decoration of the defect sites and to modify the electronic properties by varying the energy gap. The noncovalent functionalization involving the strong π–π interaction between graphene sheet and the functionalizing moiety facilitates stable dispersions. Noncovalent functionalization of graphene involving pyrenyl rings and sulfonated polyaniline have been reported.12
Graphene can either act as an electron donor or as an electron acceptor, which has a direct dependence on its electronic structure.13 The electronic properties of graphene can be controlled by means of doping,14 molecular charge transfer,15 functionalization or intercalation. Graphite intercalation compounds (GICs) have been studied extensively since its first report in 1841.16 In addition to enhancing the interlayer spacing the intercalation affects the electronic structure of the graphene layers. GICs have a wide range of tunable electronic properties.17 Intercalation of K and Rb extends the interlayer spacing to 5.35 Å (C8K) and 5.65 Å (C8Rb), respectively, from graphite's 3.35 Å. In some GICs the interlayer spacing can be more than 1 nm.13,17 Supermetallic conductivity in bromine-intercalated graphite and superconductivity in K, Ca intercalated graphite have been reported.18,19 Intercalation with AsF5 or SbF5 has shown metallic conductivities higher than that of copper.20 Bromine dopes the graphene sheets with holes simultaneously increasing the interlayer separation. It is also argued that the increase of mobility and resistance anisotropy with reduced diamagnetic susceptibility of intercalated samples suggests the parallel combination of weakly-coupled hole-doped graphene sheets responsible for supermetallic conductivity. In this regard the limit at which the inter-planar coupling is sufficiently weak to assure that the resulting in-plane conductivity is due to parallel contributions of doped graphene sheets. The charge transfer between the intercalate and the graphene sheet can result in a significant increase in free carriers per carbon.
Graphite intercalation with alkali metals, halogens, metal halides, etc. is mostly achieved by high temperature methods and is unstable at ambient conditions. Ferrocene is a sandwich compound composed of a pair of planar cyclopentadienyl ring of 6π-electrons and 6d-electrons on Fe(II) atom. Ferrocene is capable of engaging in electron transfer processes. The edge state induced magnetism in graphene coupled with ferrocene interaction can give rise to interesting magnetic properties.21 To the best of our knowledge neither graphene oxide (few layer) intercalation nor covalent functionalization with metallocene has been studied. Recently, with ferrocene playing a crucial role, the transformation of single-walled carbon nanotubes into few-layer graphene depending on the nanotube diameter and the annealing temperature has been reported.22 In this paper we report the covalent functionalization of graphene oxide with ferrocene which results in the intercalation, exfoliation, and molecular charge transfer. A simple green chemistry procedure involving the solid phase alumina and trifluoroacetic anhydride at room temperature has been employed to prepare the covalently linked ferrocene–graphene oxide. Since the potential use of graphene depends on the control of its properties, the ferrocene–graphene oxide involving intercalation and molecular charge transfer could be instrumental in understanding the properties of graphene and graphene oxide and their potential applications.
Experimental
Materials and methods
All solvents and reagents were obtained from Sigma-Aldrich unless otherwise mentioned. Graphite was obtained from Alfa Aesar, ferrocene from Spectrochem, acidic alumina from SD fine Chemicals. FESEM micrographs were obtained on aluminium surface. HRTEM micrographs were obtained on a 200 mesh holey carbon supported copper grids. Sonicated dilute dispersion of the sample taken on a fresh, ultra clean Si (111) substrate was used for AFM studies. Dry sample on a glass plate was used for Raman studies. FTIR spectra were recorded using KBr pellets.Preparation of graphene oxide (GO)
Graphitic oxide was prepared by reacting graphite (2–15 μm) with a mixture of concentrated nitric acid (2 ml), sulfuric acid (2 ml) and potassium chlorate at room temperature for 5 days. Thermal exfoliation of graphitic oxide was carried out in a long quartz tube at 1050 °C under an argon atmosphere to yield exfoliated graphene (EG). The EG (50 mg) was further treated with concentrated nitric acid (2 ml), concentrated sulfuric acid (2 ml) and water (16 ml), which was subsequently heated in a microwave oven for 10 min. under hydrothermal conditions. The sample was further heated at 100 °C for 12 h. The product was washed with deionized water to remove traces of acid. The resulting acid treated EG with hydroxyl, epoxide and carboxyl functional groups is termed as GO.Preparation of covalently linked ferrocene–graphene oxide (FGO)
Covalent functionalization of graphene oxide with ferrocene was achieved using the procedure reported by B. C. Ranu et al. for the selective Friedel–Crafts monoacylation of ferrocene (Scheme 1).23 A solution of ferrocene (186 mg, 1 mmol) in dichloromethane (5 ml) was adsorbed on the surface of activated (heated at 150 °C for 3 h under reduced pressure and then cooled under nitrogen) acidic alumina (3 g) and then the solvent was evaporated off completely under vacuum. A mixture of GO (20 mg) and trifluoroacetic anhydride (700 μl, 5 mmol) was added to the above reactants. The reaction mixture was allowed to stand at room temperature with occasional shaking under a moisture guard for 36 h. The product was taken into methanol as suspension and separated by filtration using Whatman 41 filter paper. The residue was washed with copious amounts of methanol to remove excess reagents. The sample was further washed with methanol, distilled water, ethanol and dried under vacuum to obtain pure FGO (12 mg) in good yield (∼50–60%). This preparative method can be easily extended for the large scale preparation of FGO using several hundred milligrams of GO without any further modifications to the procedure discussed above.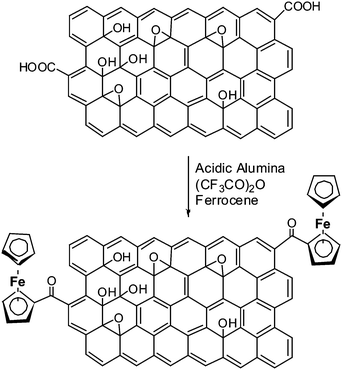 | ||
| Scheme 1 Covalent modification of graphene oxide with ferrocene by Friedel–Crafts monoacylation on an acidic alumina surface. | ||
Characterization
The FGO samples were characterized by field emission scanning electron microscopy (FESEM), high resolution transmission electron microscopy (HRTEM) and atomic force microscopy (AFM). FESEM measurements were performed by using FEI Nova nanoSEM-600 equipped with a field emission gun operating at 30 kV. Energy dispersive spectroscopy (EDS) analysis was performed with an EDAX Genesis instrument attached to the SEM column. HRTEM images were obtained from a JEOL JEM 3010 electron microscope fitted with a Gatan CCD camera operating at an accelerating voltage of 300 kV. AFM measurements were performed using Innova (Veeco) atomic force microscope. Infrared spectra were recorded on a Bruker IFS 66v/S FT-IR spectrometer. Magnetic measurements were carried out by using the vibrating sample magnetometer (VSM) in a physical property measurement system (PPMS) [Quantum Design, San Diego, CA, USA]. Raman spectra were recorded on LabRAM HR high resolution Raman microscope (Horiba Jobin Yvon) with 632 nm HeNe laser. Mössbauer spectra were recorded at room temperature in transmission mode using 57Co γ-ray source in a rhodium matrix and multi-channel analyzer. The calibrations for velocity and isomer shift were performed using α-iron (Fe) foil.Results and discussion
The interlayer spacing between the graphene layers play a key role in hydrogen adsorption and other applications. Insertion of spacer molecules can be employed for the controlled exfoliation of graphene layers. The process involves compensation of the weak van der Waals force of attraction between graphene layers by means of spacer–graphene layer interactions. The non-covalent interaction of C60 fullerene intercalated graphite layers has been reported.24 The interlayer distance in case of C60 intercalated graphite is ∼1.3 nm. We modified GO by appending ferrocene to obtain FGO. A green chemistry approach was used to covalently append ferrocene onto GO using a reported procedure.23 Solvent free procedure involved the Friedel–Crafts monoacyclation of ferrocene with carboxylic groups present on GO using trifluoroacetic anhydride on acidic alumina (Scheme 1). Presumably, the activated acidic alumina (Lewis acid) catalyzes the acylation of ferrocene with the aid of trifluoroacetic anhydride. The acidic alumina granules are of micrometre dimensions and were separated quite effectively. FT-IR spectroscopic studies on FGO further confirmed the presence of covalently attached ferrocene on GO (Fig. 1). The peak at 1725 cm−1 corresponding to vibrational frequency of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (–COOH) in GO shifted to 1734 cm−1 in FGO. This shift in IR frequency of C
O (–COOH) in GO shifted to 1734 cm−1 in FGO. This shift in IR frequency of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O is attributed to covalent functionalization of GO with ferrocene via a carboxyl group. The peaks at 1582 cm−1 and 1398 cm−1 are attributed to aromatic C
O is attributed to covalent functionalization of GO with ferrocene via a carboxyl group. The peaks at 1582 cm−1 and 1398 cm−1 are attributed to aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond stretching and C–H bending vibrational frequencies respectively. The Mössbauer spectrum of the FGO sample further confirmed the presence of Fe2+ (see the ESI†). The monoacylation of ferrocene in FGO introduces asymmetric chemical and electronic environment on cyclopentadienyl rings of ferrocene which is evident from the Mössbauer spectrum of FGO.
C double bond stretching and C–H bending vibrational frequencies respectively. The Mössbauer spectrum of the FGO sample further confirmed the presence of Fe2+ (see the ESI†). The monoacylation of ferrocene in FGO introduces asymmetric chemical and electronic environment on cyclopentadienyl rings of ferrocene which is evident from the Mössbauer spectrum of FGO.
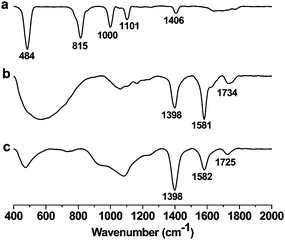 | ||
| Fig. 1 FT-IR spectra of (a) ferrocene; (b) ferrocene–graphene oxide (FGO); and (c) graphene oxide (GO). | ||
FGO samples were characterized by FESEM and HRTEM as shown in Fig. 2. The energy dispersive X-ray analysis (EDAX) performed on FGO did not show the presence of fluorine, and hence confirm the complete removal of trifluoroacetic anhydride during the work up (Fig. 2b). The peaks due to Al and Si in the EDAX spectrum are due to the substrate used for characterizing the sample. Moreover the sample on a Si(111) substrate showed no traces of Al. Covalently linked ferrocene on GO sample was further confirmed by EDAX analysis of FGO which showed the presence of Fe (Fig. 2b). A relative atomic percentage of 0.76%, 72.69% and 26.55% for Fe, C and O respectively were obtained by the EDAX analysis. Ferrocene, being a sandwich compound consisting of two cyclopentadienyl rings, is believed to stack in between the successive graphene sheets by means of π–π interaction. Theoretical studies have shown the interaction of ferrocene in a perpendicular orientation of its C5 axis with respect to the graphene sheet to be approximately isoenergetic for the stack and bridge positions.25 The binding energy of the ferrocene and graphene composite is similar to that of calculated benzene adsorption on graphene, suggesting the weak van der Waals π -stacking interaction. However the mere mixing of GO and ferrocene in dichloromethane resulted in stable dispersions with an interlayer spacing of ∼0.5 nm was observed (see the ESI†). Hence the role of solid phase alumina and trifluoroacetic anhydride is crucial to enhance d-spacing. An interlayer spacing of ∼2.4 nm was found in FGO uniformly throughout the sample and intercalation of ferrocene is believed to stabilize the GO sheets (Fig. 2c). The GO sheets are expected to be thicker than the individual pristine graphene sheets due to the presence of oxygen containing functionalities and the sp3 hybridized carbon atoms displaced from the original graphene plane. We found many large flat FGO sheet as shown in Fig. 2d and the crystalline nature of the FGO sheets was confirmed by selected area electron diffraction (SAED) pattern (Fig. 2d, inset). The well-defined hexagonal diffraction pattern is similar to that of single-layer graphene prepared by manual peeling off from graphite and also through a confined self-assembly approach.8b,26 To understand the effect of non-covalent interaction of ferrocene with graphene we subjected EG to similar reaction conditions used for the preparation of FGO. The d-spacing in the ferrocene treated EG was found to be ∼1 nm (see the ESI†).
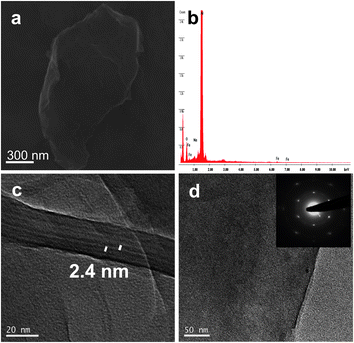 | ||
| Fig. 2 (a) FESEM micrograph of FGO. (b) EDAX analysis shows the presence of Fe in FGO. (c) HRTEM micrograph with an increased interlayer spacing of ∼2.4 nm. (d) HRTEM micrograph of FGO sheet, the inset shows the corresponding selected area electron diffraction (SAED) pattern. | ||
The interaction of ferrocene with the GO sheet in FGO was determined by Raman spectroscopic studies. The Raman spectra with a Lorentzian fit to the G-mode is shown in Fig. 3. The EG has a G-band at 1570 cm−1. The GO has a G-band at 1573 cm−1. I(D)/I(G) intensity ratio in case of GO is increased on converting EG to GO. The increased I(D)/I(G) intensity ratio indicates the formation of sp3 carbon on functionalization. FGO showed G-band at 1577 cm−1, and hence the G-band position has been blue shifted by 4 cm−1. This indicates the electron accepting feature of the ferrocene moiety as the electron acceptors are known to cause blue shift in the G-band position.15a,b The characteristic feature of charge-transfer induced changes in graphene is aided by theoretical proof.15c The I(D)/I(G) intensity ratio was found to increase, whereas the I(2D)/I(G) intensity ratio was found to decrease on forming the FGO covalent conjugate.
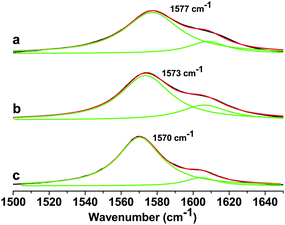 | ||
| Fig. 3 Raman spectra of (a) ferrocene–graphene oxide (FGO), (b) graphene oxide (GO) and (c) thermally exfoliated graphene (EG) showing the G-band along with a Lorentzian fit. | ||
Atomic force microscopy (AFM) studies revealed the presence of single-layer FGO sheets as shown in Fig. 4. The height profile in Fig. 4 shows the topographical thickness of the single-layer FGO sheet (see the ESI†). The topographical thickness of single-layer FGO is found to be ∼0.91 nm which is comparable to the reported single-layer thickness of GO.27 Recently, a chemically synthesized single-layer graphene using confined self-assembly approach has been reported to have a layer thickness of ∼0.6 nm.26 The topographical height of 0.91 nm is attributed to single-layer FGO sheet as the presence of oxygen functionalities and covalently linked ferrocene will distort the graphene planarity. The twisting and folding of the sheets due to chemical modifications on GO are believed to increase the height profile.
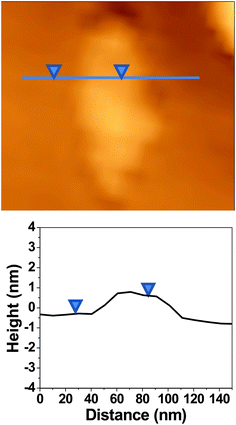 | ||
| Fig. 4 AFM image of single-layer ferrocene–graphene oxide (FGO) with the corresponding height profile. Topographical thickness of single-layer FGO is 0.91 nm. | ||
The temperature dependence of magnetization of GO was studied (Fig. S1, ESI†). The field cooled (FC) and zero field cooled (ZFC) data showed a divergence at 500 Oe. The same divergence almost disappears at higher field viz, application of 1 T. The dominance of the ferromagnetic domain over antiferromagnetic domain at higher field results in the disappearance of divergence observed.28 Ferrocene also has the similar divergence for FC and ZFC magnetic data at 500 Oe. The magnetization in ferrocene is nearly 40 times smaller than that of GO. Interestingly the FGO show little divergence between the FC and ZFC magnetic data. Moreover the magnetization was found to be more than that of both graphene oxide and ferrocene. Hence some kind of magnetic interaction due to ordering in FGO is believed to show such a magnetic behavior. Though intrinsic carbon defects or adatoms might contribute to the origin of the unexpected magnetism, theoretical studies suggest the metal-free magnetism in graphene samples is either due to localized states or edge states.29 The non-bonding edge states have localized spins resulting in spin magnetism. Thus intercalation of few-layer graphene with chemical species differs from that of GICs due to the significant contributions of edge state effects along with charge transfer interactions. In Fig. 5 the magnetic hysteresis of GO, ferrocene and FGO are shown, respectively. The magnetic hysteresis was measured at 5 K and 300 K. Ferrocene has very small magnetic hysteresis compared to GO and FGO (Fig. 5b). The remnant magnetization (Mr) and coercive field (Hc) for GO, ferrocene and FGO are given in Table S1 in the ESI.†
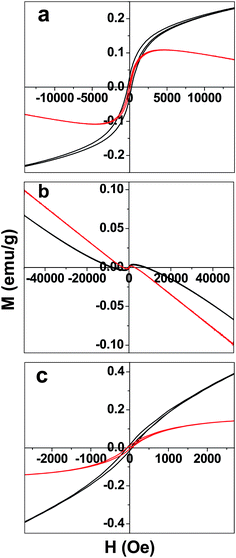 | ||
| Fig. 5 Magnetic hysteresis of (a) graphene oxide (GO), (b) ferrocene, and (c) ferrocene–graphene oxide (FGO) measured at 5 K (black trace) and 300 K (red trace). | ||
Conclusion
Intercalation and exfoliation of GO using covalently attached ferrocene was achieved. Covalently functionalized GO with ferrocene was obtained by a room temperature green chemistry approach on the solid phase alumina. The d-spacing was increased to ∼2.4 nm upon covalent modification of GO with ferrocene. AFM studies show the presence of single-layer FGO sheets. Raman spectroscopic studies show the molecular charge transfer between the intercalated ferrocene and the GO sheets. The covalently modified FGO composite showed interesting magnetic behavior. These results will have a significant impact on modifying the structure and properties of GO, which can be exploited for future applications.Acknowledgements
We thank Prof. C. N. R. Rao, FRS for constant support and encouragement, JNCASR for providing financial support, Prof. Sundaresan group for their help in magnetic measurements and VINL for AFM measurements.References
- C. N. R. Rao, A. K. Sood, K. S. Subrahmanyam and A. Govindaraj, Angew. Chem., Int. Ed., 2009, 48, 7752–7777 CrossRef CAS.
- K. I. Bolotin, K. J. Sikes, Z. Jiang, M. Klima, G. Fudenberg, J. Hone, P. Kim and H. L. Stormer, Solid State Commun., 2008, 146, 351–355 CrossRef CAS.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, M. I. Katsnelson, I. V. Grigorieva, S. V. Dubonos and A. A. Firsov, Nature, 2005, 438, 197–200 CrossRef.
- Y. Zhang, Y. Tan, H. L. Stormer and P. Kim, Nature, 2005, 438, 201–204 CrossRef CAS.
- M. D. Stoller, S. Park, Y. Zhu, J. An and R. S. Ruoff, Nano Lett., 2008, 8, 3498–3502 CrossRef CAS.
- C. Lee, X. Wei, J. W. Kysar and J. Hone, Science, 2008, 321, 385–388 CrossRef CAS.
- A. A. Balandin, S. Ghosh, W. Bao, I. Calizo, D. Teweldebrhan, F. Miao and C. N. Lau, Nano Lett., 2008, 8, 902–907 CrossRef CAS.
- (a) K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS; (b) J. C. Meyer, A. K. Geim, M. I. Katsnelson, K. S. Novoselov, T. J. Booth and S. Roth, Nature, 2007, 446, 60–63 CrossRef CAS.
- K. S. Subrahmanyam, S. R. C. Vivekchand, A. Govindaraj and C. N. R. Rao, J. Mater. Chem., 2008, 18, 1517–1523 RSC.
- R. Zacharia, H. Ulbricht and T. Hertel, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 69, 155406–7 CrossRef.
- S. Niyogi, E. Bekyarova, M. E. Itkis, J. L. McWilliams, M. A. Hamon and R. C. Haddon, J. Am. Chem. Soc., 2006, 128, 7720–7721 CrossRef CAS.
- (a) H. Bai, Y. Xu, L. Zhao, C. Li and G. Shi, Chem. Commun., 2009, 1667–1669 RSC; (b) Y. Xu, H. Bai, G. Lu, C. Li and G. Shi, J. Am. Chem. Soc., 2008, 130, 5856–5857 CrossRef CAS.
- T. Enoki, M. Suzuki and M. Endo, Graphite Intercalation Compounds and Applications, Oxford University Press, New York, 2003 Search PubMed.
- L. S. Panchakarla, K. S. Subrahmanyam, S. K. Saha, A. Govindaraj, H. R. Krishnamurthy, U. V. Waghmare and C. N. R. Rao, Adv. Mater., 2009, 21, 4726–4730 CAS.
- (a) B. Das, R. Voggu, C. S. Rout and C. N. R. Rao, Chem. Commun., 2008, 5155–5157 RSC; (b) R. Voggu, B. Das, C. S. Rout and C. N. R. Rao, J. Phys.: Condens. Matter, 2008, 20, 472204 CrossRef; (c) A. K. Manna and S. K. Pati, Chem.–Asian J., 2009, 4, 855–860 CrossRef CAS.
- P. J. Shaffault, J. Prakt. Chem., 1841, 21, 155 Search PubMed.
- M. S. Dresselhaus and G. Dresselhaus, Adv. Phys., 2002, 51, 1–186 CrossRef CAS.
- S. Tongay, J. Hwang, D. B. Tanner, H. K. Pal, D. Maslov and A. F. Hebard, 2009, arXiv:0907.1111v1, [Cond-mat. matrl-sci].
- (a) I. T. Belash, O. V. Zharikov and A. V. Palnichenko, Synth. Met., 1989, 34, 455–460 CAS; (b) T. E. Weller, M. Ellerby, S. S. Saxena, R. P. Smith and N. T. Skipper, Nat. Phys., 2005, 1, 39–41 CrossRef CAS; (c) N. Emery, C. Herold, M. d'Astuto, V. Garcia, C. Bellin, J. F. Mareche, P. Lagrange and G. Loupias, Phys. Rev. Lett., 2005, 95, 087003–4 CrossRef CAS; (d) N. B. Hannay, T. H. Geballe, B. T. Matthias, K. Andres, P. Schmidt and D. MacNair, Phys. Rev. Lett., 1965, 14, 225–226 CrossRef CAS.
- (a) G. M. T. Foley, C. Zeller, E. R. Falardeau and F. L. Vogel, Solid State Commun., 1977, 24, 371–375 CrossRef CAS; (b) F. L. Vogel, J. Mater. Sci., 1977, 12, 982–986 CrossRef CAS.
- (a) N. Tagmatarchis and M. Prato, Pure Appl. Chem., 2005, 77, 1675–1684 CrossRef CAS; (b) Y. F. Li1, R. Hatakeyama, T. Kaneko, T. Izumida, T. Okada and T. Kato, Nanotechnology, 2006, 17, 4143–4147 CrossRef.
- L. Guan and J. Li, J. Phys. Chem. C, 2009, 113, 7481–7483 CrossRef CAS.
- B. C. Ranu, U. Jana and A. Majee, Green Chem., 1999, 1, 33–34 RSC.
- V. Gupta, P. Scharff, K. Risch, H. Romanus and R. Muller, Solid State Commun., 2004, 131, 153–155 CrossRef CAS.
- E. L. Sceats and J. C. Green, J. Chem. Phys., 2006, 125, 154704–1 CrossRef.
- W. Zhang, J. Cui, C. Tao, Y. Wu, Z. Li, L. Ma, Y. Wen and G. Li, Angew. Chem., Int. Ed., 2009, 48, 5864–5868 CrossRef CAS.
- P. K. Ang, S. Wang, Q. Bao, J. T. L. Thong and K. P. Loh, ACS Nano, 2009, 3, 3587–3595 CrossRef CAS.
- H. S. S. R. Matte, K. S. Subrahmanyam and C. N. R. Rao, J. Phys. Chem. C, 2009, 113, 9982–9985 CrossRef CAS.
- T. Enoki and K. Takai, Solid State Commun., 2009, 149, 1144–1150 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Magnetic data; AFM images; TEM micrographs; and Mössbauer spectroscopic data. See DOI: 10.1039/c0nr00024h |
| This journal is © The Royal Society of Chemistry 2010 |
