Open Access Article
Open Access ArticleNaphthalimide-based nonionic sulfonate photoacid generators: structure–property relationship and sub-30 nm resolution lithography
Changchang
Zhuang†
 a,
Jiao
Chen†
a,
Tao
Wang
a,
Jun
Zhao
b,
Haoyuan
Li
a,
Jiao
Chen†
a,
Tao
Wang
a,
Jun
Zhao
b,
Haoyuan
Li
 a,
Hanshen
Xin
a,
Hanshen
Xin
 *a and
Jianhua
Zhang
*a and
Jianhua
Zhang
 *a
*a
aSchool of Microelectronics, Shanghai Engineering Research Center for Integrated Circuits and Advanced Display Materials, Shanghai University, Shanghai, China. E-mail: xinhanshen@shu.edu.cn; jhzhang@oa.shu.edu.cn
bShanghai Synchrotron Radiation Facility, Shanghai Advanced Research Institute, Chinese Academy of Sciences, Shanghai, China
First published on 17th November 2025
Abstract
This study reports the design and synthesis of five novel nonionic photoacid generators (PAGs) based on a naphthalimide–benzenesulfonate backbone. Comprehensive evaluations were conducted to assess their solubility, thermal stability, photophysical properties, acid generation quantum yields (Φa), and lithographic performance. Ultraviolet-visible (UV-vis) spectroscopy revealed that the optical properties of the PAGs could be finely tuned through structural modifications, with all compounds exhibiting strong absorption at key lithographic wavelengths (248, 254, and 365 nm). Measurements of Φa ranged from 6.2% to 17.6%, establishing clear structure–property relationships. Notably, PAG 1d exhibited the highest Φa (17.6%) due to its optimal donor–π–acceptor (D–π–A) configuration, where the synergistic combination of electron-donating and electron-withdrawing groups enhanced photosensitivity and facilitated efficient acid release. Density functional theory (DFT) calculations corroborated these findings, confirming that the established structure–property relationships provide a robust foundation for the molecular engineering of PAGs with high Φa. When formulated into photoresists, all PAGs produced high-quality patterning across multiple lithographic platforms, including i-line (365 nm), deep-UV (254 nm), and electron-beam lithography (EBL), with their sensitivity directly correlating to the Φa of the PAGs. Remarkably, the PR-1d formulation exhibited exceptional performance in EBL, attaining the highest sensitivity (E0 = 54.4 μC cm−2) and a high resolution of 28 nm, thereby establishing its potential for sub-30 nm lithography applications. This work provides fundamental insights into the structure–property relationships of nonionic sulfonate PAGs and advances resist technology for next-generation lithography.
Keywords: Nonionic photoacid generators; Naphthalimide-benzenesulfonate; Acid generation quantum yield; Structure–property relationship; Sub-30 nm lithography.
1 Introduction
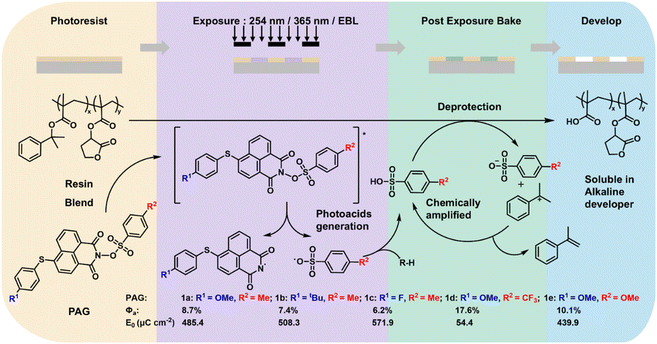
With the continuous advancement of lithography processes, the demands for the resolution and sensitivity of photoresists are gradually increasing.1–6 The chemically amplified photoresist (CAR) developed by Ito et al.7–9 significantly enhances the efficiency of photochemical reactions, effectively addressing the challenge of high sensitivity faced by photoresists. The CAR system consists of polymer resins, PAGs, basic quenchers, solvents, and other additives.10–12 During the exposure process, the PAGs in the photoresist formulation undergoes photolysis to generate acid. In the subsequent post-exposure baking process, acid-catalyzed transformations of polymer functional groups (such as depolymerization, intramolecular dehydration, crosslinking, and deprotection of acidic groups) lead to changes in the polarity and solubility of the polymer. After the catalytic reaction is completed, the released acid continues to catalyze the chain reaction.13–18 A small amount of PAGs can catalyze the entire chemical reaction of the photoresist, greatly reducing the required exposure dose and significantly increasing the sensitivity of the photoresist, making it an indispensable material in CARs.From a structural perspective, PAGs can be categorized into ionic and nonionic types. Ionic PAGs, such as sulfonium and iodonium salts, were the workhorses in the early development of CARs, owing to their high thermal stability and the ability to modulate acid strength through selection of counter anions.19–22 However, their application is often limited by the need for thicker films to achieve sufficient radiation absorption, a suboptimal out-of-band response, and solubility issues in certain formulations, which can adversely affect imaging performance.23–25 In contrast, nonionic PAGs, typically derived from arylsulfonates, exhibit superior solubility in organic solvents. They are also characterized by a broad absorption spectrum, straightforward synthesis, and ease of purification. These advantages collectively make them highly attractive for applications in photoresists and polymer photoinitiation.26–33 The core performance parameter for any PAG is acid generation quantum yields (Φa), which directly govern photoresist sensitivity. Simultaneously, the photoluminescence quantum yield (Φp) acts as a key competitive parameter; it reflects the extent to which excited-state energy is dissipated via radiation, thereby intrinsically reducing Φa.34–36 Beyond these, PAG performance is co-determined by several additional key parameters, including absorption characteristics (such as maximum absorption wavelength (λmax) and molar extinction coefficient (ε)), thermal stability, and compatibility with the photoresist matrix. For nonionic PAGs, molecular structure design plays a decisive role in precisely tuning these parameters and optimizing overall performance. Sulfonate ester PAGs based on the naphthalimide backbone have attracted widespread attention over the past decade due to their high stability, good solubility, and efficient acid generation capability.34,37–41 Previous studies have shown that in such nonionic naphthalimide-based sulfonate ester PAGs, the structure of substituent groups on the sulfonate ester unit plays an important role in regulating Φa.23,36,42 However, the constitutive relationship between the type of functional groups (e.g., electron-donating and electron-withdrawing groups) on the naphthalimide backbone and the Φa has not been systematically investigated. Deeper insight in this regard is essential for the rational design of next-generation high-performance PAGs. Furthermore, although nonionic PAGs have been extensively studied and applied in deep ultraviolet (DUV) lithography, their adoption in more advanced sub-30 nm lithography nodes has not been extensively documented and presents new challenges and opportunities that warrant further exploration.
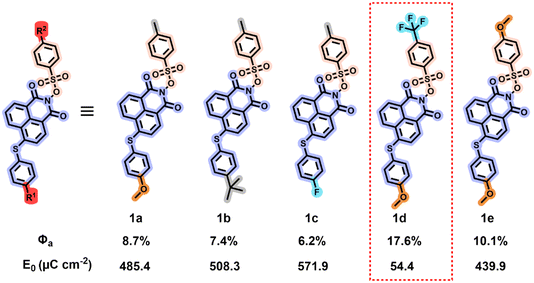
Here, we designed and synthesized five nonionic sulfonate ester PAGs (1a–e) based on the naphthalimide backbone. By individually adjusting the electronegativity of the substituent groups on the naphthalimide units and the benzene sulfonate units, we altered the frontier molecule orbital (FMO) energies of the PAGs to regulate their acid generation capabilities. Rhodamine B (RB) was used as an acid indicator, and the acid generation ability of the PAGs was determined via UV-visible spectrophotometry. Additionally, we performed theoretical calculations on the PAGs to further understand the relationship between their structure, FMO energy, and acid generation efficiency. These PAGs were subsequently mixed with a polymer (a laboratory-synthesized resin PG, poly(2-phenyl-2-propyl methyl methacrylate-co-γ-butyrolactone methyl methacrylate))43 to prepare the photoresists. The lithographic performance of these photoresists was evaluated using 365 nm and 254 nm UV exposure, as well as EBL. The results indicated that by independently adjusting the electron-donating/withdrawing abilities of the substituent groups on the naphthalimide units and the benzene sulfonate units, it is indeed possible to regulate the acid generation capacity of the PAGs, thereby tuning the sensitivity of the photoresists. As shown in Fig. 1, reducing the electron-donating ability of the substituents at the 4-position on the naphthalimide backbone gradually decreased the acid quantum yield of the PAG molecules. Conversely, increasing the electron-withdrawing ability of the substituents at the para-position on the benzene sulfonate units gradually enhanced the acid quantum yield of the PAG molecules. Consequently, PAG 1d, which possesses electron-donating groups at the 4-position on the naphthalimide backbone and electron-withdrawing groups at the para-position on the benzene sulfonate units, exhibited the highest Φa (17.6%). All five PAG-based photoresists produced high-quality patterns on various lithographic platforms (365 nm, 254 nm, and EBL), with their sensitivity directly related to their Φa values. Furthermore, the photoresist based on PAG 1d exhibits highly competitive performance, achieving a combination of the highest E0 (54.4 μC cm−2), high resolution (28 nm), and low line edge roughness (LER = 1.3 nm) under EBL among reported photoresists employing nonionic PAGs.31,44,45
2 Results and discussion
2.1 Synthesis and characterization of nonionic sulfonate ester PAGs 1a–1e
The designed nonionic sulfonate ester PAGs 1a–1e can be synthesized through a three-step reaction sequence, as depicted in Scheme 1. First, substituted phenylthiol derivatives (S1a–S1c) bearing different 4-position functional groups undergo a substitution reaction with 4-bromo-1,8-naphthalenedicarboxylic anhydride (S2) under alkaline conditions at 75 °C to generate intermediates S3a–S3c. Without isolation, intermediates S3a–c are then treated with hydroxylamine aqueous solution to afford 4-substituted N-hydroxy-1,8-naphthalenedicarboximide derivatives S4a–c. The resulting S4a–c intermediates, without further purification, subsequently react with substituted benzene sulfonyl chlorides to yield the corresponding naphthalene dicarboximide benzene sulfonate esters 1a–1e. The three-step synthesis achieves overall yields of 83.6–91.2%. The chemical structures of PAGs 1a–1e were fully characterized by 1H and 13C NMR spectroscopy, and high-resolution mass spectrometry. Detailed experimental procedures and characterization data for all PAGs can be found in the SI (Fig. S1–S17). This synthetic route adheres to the principles of green chemistry. It utilizes commercially available starting materials, demonstrates excellent atom economy and scalable process potential, and requires no precious metal catalysts or highly polluting reagents throughout the entire process. The fluorination-free/low-fluorine PAG system constructed based on this strategy avoids the use of persistent pollutants such as perfluorooctane sulfonate (PFOS) at the molecular design source, and its non-ionic sulfonate ester architecture also exhibits good environmental stability.2.2 Solubility
Table S1 summarizes the solubility of PAGs 1a–1e in commonly used solvents. These compounds exhibit good solubility in non-protonic polar solvents (e.g., DMF) and moderate-polarity solvents (e.g., DCE, 1,4-dioxane, and THF). However, they show comparatively poor solubility in highly polar or protonic solvents such as methanol and acetonitrile. This reduced solubility may be attributed to the presence of multiple aromatic rings in the molecular structure of PAGs, which enhances the hydrophobicity of the molecules. Consequently, they exhibit a stronger affinity for solvents with higher hydrophobicity, further diminishing their solubility in highly polar or protonic media.2.3 Thermal stability
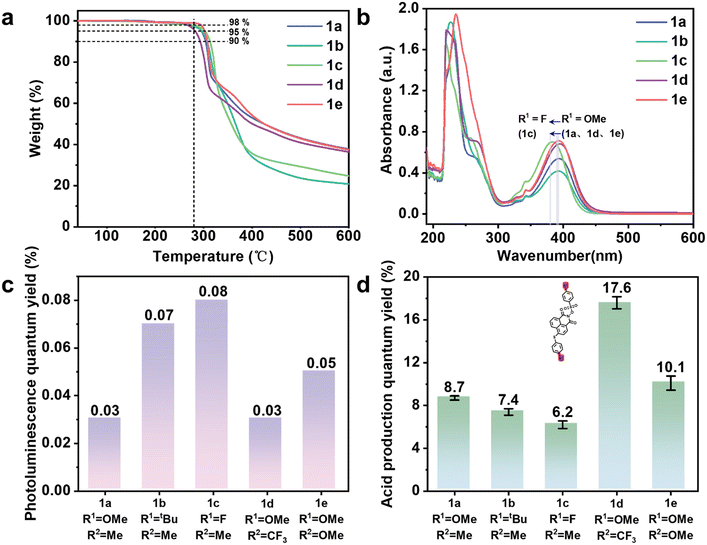
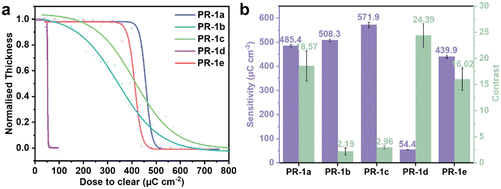
The thermal stability of PAGs is a critical factor determining their applicability in photoresist formulations, where typical pre-baking and PEB temperatures range from 85 °C to 130 °C. To systematically evaluate the thermal stability of PAGs 1a–1e, TGA and DSC analyses were performed. Fig. 2a and S18 present the TGA and DSC curves for PAGs 1a–1e, respectively, with corresponding thermal stability data summarized in Table S2. The experimental results demonstrate that the 5% weight loss temperature (T5wt%) for these PAGs ranges from 280 °C to 305 °C, while their melting points (Tm) fall between 160 °C and 216 °C, significantly higher than the typical baking temperatures used in photoresist processing. These findings confirm that all investigated PAGs exhibit excellent thermal stability, fully meeting the practical requirements for photoresist applications.2.4 Photophysical properties
The acid generation in PAG occurs only when the molecule absorbs photons and transitions to an excited state, leading to chemical bond cleavage and acid production. Inadequate UV absorption at specific wavelengths will hinder effective acid generation. Therefore, UV absorption testing of PAGs is crucial. Through UV-visible spectroscopy analysis of PAGs, the maximum absorption wavelength and suitable light source range can be determined. The UV absorption spectra of PAGs 1a–1e were measured at a concentration of 2.0 × 10−5 M in acetonitrile as the solvent, as illustrated in Fig. 2b. Table S3 presents a summary of the primary UV absorption statistics, where λmax indicates the wavelength of maximum absorption and ε represents the molar absorption coefficient. The results showed that PAGs 1a–1e exhibit intense absorption bands near 220 nm, attributed to the π–π* transitions of the naphthalimide units in their molecular structure. Additionally, significant absorption is observed near 390 nm, which is characteristic of intramolecular charge transfer (ICT) between the electron-donating phenylthio units and the electron-withdrawing naphthalimide units. Notably, the substituent structure (Scheme 1, R1) at the 4-position of the phenylthio unit significantly affects the ICT absorption peak. When the substituent is an electron-donating methoxy group (R1 = OMe), the absorption peaks of PAGs 1a, 1d, and 1e are essentially at the same position, whereas when the substituent is changed to an electron-withdrawing fluorine (R1 = F), the absorption of PAG 1c is markedly blueshifted by about 10 nm. The rigidity of the naphthalimide backbone ensures strong π-conjugation, while substituent-related intramolecular charge transfer demonstrates tunable optical properties through systematic structural modifications. To evaluate the potential application of PAGs 1a–1e at critical lithography wavelengths, we examined their ε at 248 nm, 254 nm, and 365 nm, as detailed in Table 1. The results indicate that at these key wavelengths, the ε values of PAGs 1a–1e exceed 104 M−1 cm−1. Therefore, all PAGs demonstrate significant potential for lithography applications at 248 nm, 254 nm, and 365 nm.| PAG | Φ p | 254 nm | Pattern performance | ||||
|---|---|---|---|---|---|---|---|
| Φ a | I (W cm−2) | Photoresist | 365 nm (min) | 254 nm (min) | EBL (μC cm−2) | ||
| 1a (R1 = OMe, R2 = Me) | 0.03% | 8.7% | 8.27 × 10−4 | PR-1a | >1 | 7 | 485.4 ± 4.60 |
| 1b (R1 = tBu, R2 = Me) | 0.07% | 7.4% | 8.22 × 10−4 | PR-1b | >1 | 10 | 508.3 ± 5.40 |
| 1c (R1 = F, R2 = Me) | 0.08% | 6.2% | 8.43 × 10−4 | PR-1c | >1 | 11 | 571.9 ± 11.45 |
| 1d (R1 = OMe, R2 = CF3) | 0.03% | 17.6% | 8.48 × 10−4 | PR-1d | 30 s | 2 | 54.4 ± 1.60 |
| 1e (R1 = OMe, R2 = OMe) | 0.05% | 10.1% | 8.23 × 10−4 | PR-1e | >1 | 5 | 439.9 ± 6.45 |
Based on the acid generation mechanism of PAGs, when excited molecules do not undergo dissociation but instead release energy through fluorescence, the efficiency of acid generation may be adversely affected. Consequently, the fluorescence spectra of the synthesized PAGs were evaluated. Fluorescence spectra of PAGs 1a–1e were measured at a concentration of 2.0 × 10−5 M, using an excitation wavelength of 254 nm and acetonitrile as the solvent, as depicted in Fig. S19. The fluorescence spectra revealed that all PAGs exhibited weak fluorescence emission upon excitation at 254 nm. Additionally, the Φp of all PAG molecules were determined (Fig. 2c and Table 1). The Φp values for PAGs 1a–1e were 0.03%, 0.07%, 0.08%, 0.03%, and 0.05%, respectively, indicating that the probability of these PAG molecules returning to the ground state via fluorescence emission is minimal. This low fluorescence emission favors bond cleavage and enhances Φa.
2.5 Acid generation quantum yield
Among the performance parameters of PAGs, the Φa is the most critical, as it directly impacts photoresist sensitivity. We accurately calculated the Φa of PAGs 1a–1e, and the detailed test procedures can be found in the SI. As depicted in Fig. 2d and Table 1, the measured Φa values of PAGs 1a–1e are 8.7%, 7.4%, 6.2%, 17.6%, and 10.1%, respectively, all of which are greater than the reported value of naphthalimide sulfonate PAG 13 (Φa = 4%) by Tsuguo Yamaoka's group.46 The experimental results demonstrate that adjusting the electron-donating and electron-withdrawing abilities of the substituents (R1 and R2) on the naphthalimide–benzenesulfonate backbone can significantly influence the Φa of PAGs. When R2 is fixed as methyl (Me, PAGs 1a–1c), PAG 1a, which possesses an electron-donating group (methoxy, OMe) at the R1 position, exhibits the highest Φa (8.7%), outperforming PAG 1b (R1 = tert-butyl, tBu, 7.4%) and PAG 1c (R1 = F, 6.2%). This indicates that when R1 is an electron-donating group, it can enhance the electron density of the naphthalimide, promote charge separation upon photoexcitation, and thereby increase Φa. Conversely, when R1 is fixed as methoxy (OMe, PAGs 1a, 1d, and 1e), the introduction of a strong electron-withdrawing group (trifluoromethyl, CF3) at the R2 position in PAG 1d achieves a significantly higher Φa (17.6%), far exceeding those of PAG 1a (R2 = Me, 8.7%) and PAG 1e (R2 = OMe, 10.1%). This suggests that when R2 is an electron-withdrawing substituent, it can reduce the electron density of the benzenesulfonate moiety, destabilize the N–O bond, and thereby substantially increase Φa. The outstanding performance of PAG 1d exemplifies an optimal D–π–A system, where the donor (R1 = OMe) enhances the photosensitization ability, and the acceptor (R2 = CF3) facilitates efficient acid release, maximizing intramolecular charge transfer efficiency and achieving Φa. This establishes a clear structure–property relationship for designing high-performance PAGs through rational substituent engineering.2.6 Theoretical calculations
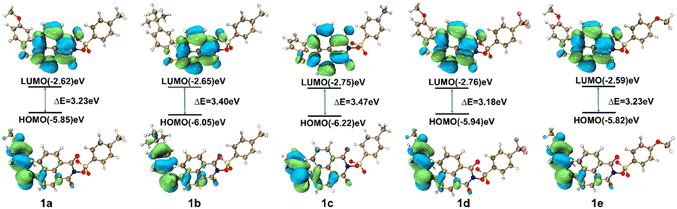
FMO theory serves as a critical tool for elucidating the electronic and photophysical properties of organic compounds.47,48 The calculation of FMO energy levels is essential for evaluating molecular photochemical processes and stability. The energy gap between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) reflects the charge transfer characteristics in excited states, with a larger HOMO–LUMO gap (ΔE) correlating with lower chemical reactivity.Through systematic density functional theory (DFT) calculations on a series of PAGs 1a–1e combined with experimental characterization, this study provides an in-depth understanding of the structure–activity relationship between molecular orbital energy levels, electronic transition properties, and Φa. The ground-state geometric structures of all molecules were optimized using the B3LYP/6-31G(d,p) method. Excited-state properties were calculated via time-dependent DFT (TD-DFT) at the B3LYP/6-311G(d,p) level, supplemented with FMO analysis and visualization using Multiwfn49 and Visual Molecular Dynamics software (Fig. 3). Table 2 summarizes the data on the HOMO, LUMO, ΔE, cutoff wavelengths (λcutoff = hc/ΔE), N–O bond lengths, and excitation energy of PAGs 1a–1e. Computational results indicate that the ΔE of the PAGs shows a significant negative correlation with the experimentally measured Φa (determined under 254 nm irradiation). FMO analysis reveal that the LUMO is primarily localized on the naphthalimide core, while the HOMO mainly resides on the phenylthio moiety attached to the naphthalimide. Therefore, altering the electron-donating/electron-withdrawing ability of the substituent (R1) at the 4-position of the phenylthio group can significantly influence the HOMO energy level of the PAG molecules, with relatively minor changes to the LUMO energy level. Specifically, when the substituent (R1) is changed from the electron-donating methoxy group (OMe) to the electron-withdrawing fluorine atom (F), the HOMO energy level of PAG 1c (−6.22 eV) is reduced by 0.37 eV, while its LUMO energy level (−2.75 eV) decreases by only 0.13 eV compared to those of PAG 1a (HOMO/LUMO = −5.85/−2.62 eV). Consequently, compared to PAG 1a, the ΔE of PAG 1c widens by 0.24 eV, resulting in a lower Φa. On the other hand, modifying the substituent (R2) at the 4-position of the phenylsulfonate unit influences both the HOMO and LUMO energy levels through inductive effects, with a more pronounced effect on the LUMO than on the HOMO. As a result, the ΔE of PAG 1d (3.18 eV) is narrow relative to PAG 1e (3.23 eV), resulting in a higher Φa. The λcutoff values of PAGs 1a–1e show excellent correlation with the experimental UV absorption spectra in the 300–400 nm range. Notably, PAG 1d has the longest N–O bond length (1.3747 Å), indicating a higher susceptibility to bond cleavage, which further supports its higher Φa (17.6%). Furthermore, the energy order of the strongest oscillator strength transition (S0 → S2) is: 1d (3.6020 eV) < 1e (3.6145 eV) < 1a (3.6172 eV) < 1b (3.6280 eV) < 1c (3.6471 eV). This order aligns perfectly with the experimental Φa measured at 254 nm, indicating that a lower excitation energy favors the initiation of the photochemical reaction. This result clarifies that rational design of the electronic properties of substituents R1 and R2 enables fine-tuning of the HOMO and LUMO energy levels, thereby influencing the energy gap and photochemical reactivity.
| PAG | HOMO (eV) | LUMO (eV) | ΔE (eV) | λ cutoff (nm) | BLN–O (Å) | Excitation energy (eV) |
|---|---|---|---|---|---|---|
| 1a (R1 = OMe, R2 = Me) | −5.85 | −2.62 | 3.23 | 384 | 1.3738 | 3.6172 |
| 1b (R1 = tBu, R2 = Me) | −6.05 | −2.65 | 3.40 | 365 | 1.3738 | 3.6280 |
| 1c (R1 = F, R2 = Me) | −6.22 | −2.75 | 3.47 | 357 | 1.3735 | 3.6471 |
| 1d (R1 = OMe, R2 = CF3) | −5.94 | −2.76 | 3.18 | 390 | 1.3747 | 3.6020 |
| 1e (R1 = OMe, R2 = OMe) | −5.82 | −2.59 | 3.23 | 384 | 1.3737 | 3.6145 |
2.7 Lithographic performance
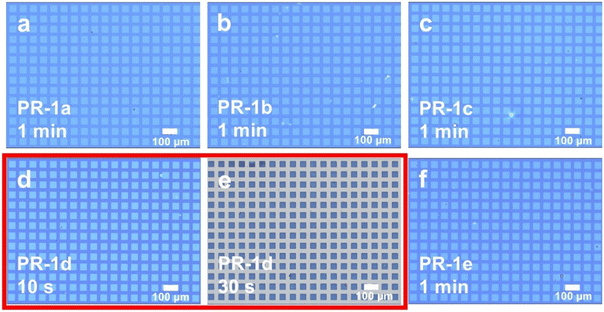
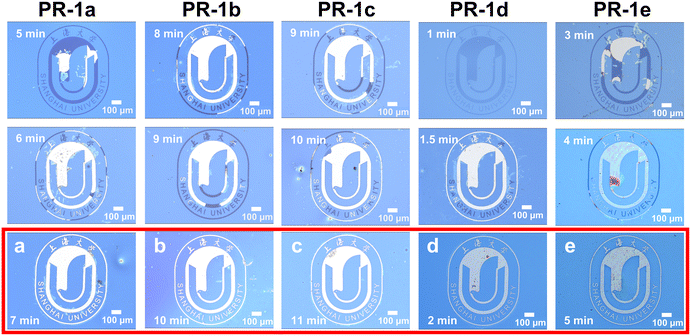
Fig. 4 presents a comparative analysis of lithographic patterns obtained using different photoresists (PR-1a to PR-1e) under 365 nm exposure conditions. The most notable observation is that PR-1d produces discernible pattern features within just 10 seconds (see Fig. 4d) and achieves a complete and clear lithographic pattern within 30 seconds (see Fig. 4e). This exceptional performance is directly correlated with the higher Φa of PAG 1d (see Table 1). Additionally, lithographic patterns are observable in PR-1a, PR-1b, PR-1c, and PR-1e after an exposure time of 1 minute, indicating that PAGs 1a–1e can be directly used in 365 nm lithography without the addition of sensitizers. This provides a new structural design strategy for PAGs suitable for 365 nm lithography and also offers new possibilities for further enhancing the performance of 365 nm photoresists.
As shown in Fig. 5, under a 254 nm light source with an exposure power of 0.8 mW cm−2, the five photoresists (PR-1a to PR-1e) gradually revealed and fully developed the school emblem pattern with increasing exposure time. Notably, the exposure results demonstrate a clear correlation between the Φa of PAGs and the sensitivity of the photoresists. Optical micrographs reveal that PR-1d achieves complete pattern formation within just 2 minutes of exposure time (Fig. 5d)—the shortest among all formulations—indicating the highest sensitivity. This is followed by PR-1e (5 minutes), PR-1a (7 minutes), PR-1b (10 minutes), and PR-1c (11 minutes), respectively. This sensitivity ranking (PR-1d > PR-1e > PR-1a > PR-1b > PR-1c) perfectly aligns with the Φa trend of their respective PAG components (see Table 1), providing direct experimental evidence that enhanced acid generation efficiency translates to superior photoresist sensitivity. The highly sensitive patterning capability of PR-1d highlights its potential for high-throughput deep UV lithography applications. These results not only validate the structure–performance relationship between PAG design and photoresist performance, but also demonstrate the practical feasibility of these formulations in semiconductor manufacturing processes.
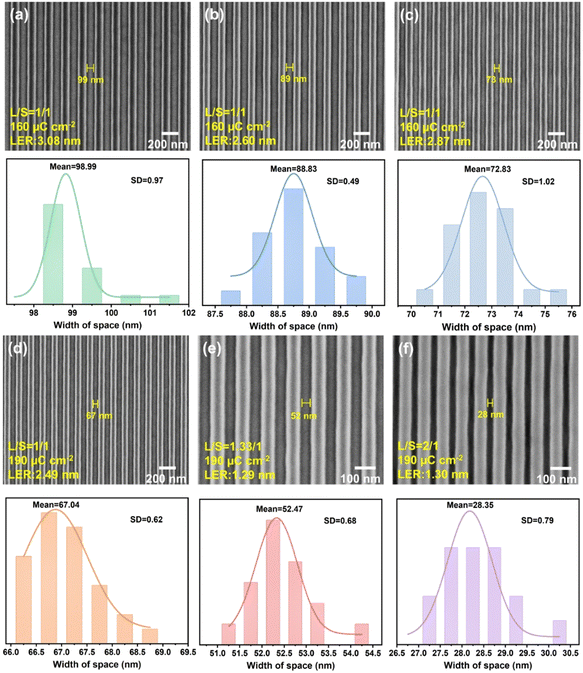
The combined results from sensitivity and contrast measurements position PR-1d as a particularly promising candidate for advanced lithographic processes requiring both high sensitivity and high resolution. Therefore, we further selected PR-1d for subsequent high-resolution line exposure experiments. Fig. 7 presents a comprehensive analysis of line/space (L/S) patterns fabricated with the PR-1d photoresist under optimized conditions, integrating SEM images with quantitative spatial distribution statistics. Fig. 7a–c, exposed at 160 μC cm−2, exhibit resolutions of 99 nm (LER = 3.08 nm), 89 nm (LER = 2.60 nm), and 73 nm (LER = 2.87 nm), respectively. Moreover, Fig. 7d–f, exposed at 190 μC cm−2, show higher resolutions of 67 nm (LER = 2.49 nm), 52 nm (LER = 1.29 nm), and 28 nm (LER = 1.30 nm), respectively. The histogram quantifies the spacing distribution, revealing that all patterns exhibit narrowly defined normal distributions. The calculated average values of the voids based on normal distribution analysis are 98.99 nm, 88.83 nm, 72.83 nm, 67.04 nm, 52.47 nm, and 28.35 nm, respectively, with corresponding standard deviations (SDs) of 0.97, 0.49, 1.02, 0.62, 0.68, and 0.79, respectively. Additionally, a clear dependence of the patterned space width on the exposure dose was observed in Fig. S26, confirming the dose as a key parameter for dimensional control in the CAR. Notably, even at the 28 nm resolution with a small LER value of 1.3 nm (Fig. 7f), the standard deviation remains as low as 0.79. These results conclusively demonstrate that PR-1d achieves both high resolution (28 nm) and exceptional uniformity (SD < 1) in nanopatterning. To objectively evaluate the performance of this work in the context of similar studies, we have provided Table S5 in the SI, which systematically compares the key performance parameters of the PAGs and corresponding photoresists reported in this study with those described in the recent literature. The analysis demonstrates that the advantages of our work are reflected in two aspects: systematically, this study covers a comprehensive performance evaluation ranging from molecular design and photophysical properties (such as Φa) to 365 nm/254 nm lithography and EBL, whereas most reports focus only on individual aspects. In terms of performance, the developed photoresist not only exhibits multi-wavelength compatibility, but also achieves a high resolution of 28 nm (compared to the 50–90 nm resolution typical of most reported nonionic PAG-based CARs) and a low LER of 1.30 nm (versus approximately 3.5 nm for similar materials) under EBL. These key metrics significantly outperform those reported in the existing literature, demonstrating the strong competitiveness of this material for high-end patterning applications.
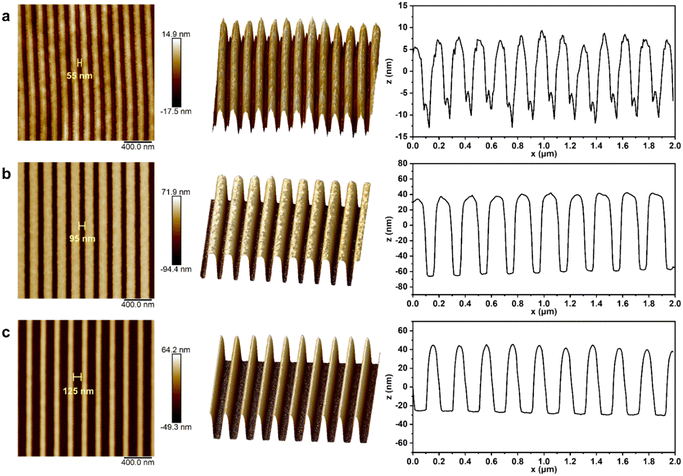
Through multi-mode characterization via Atomic Force Microscopy (AFM), the nanoscale patterning performance of the PR-1d photoresist under different electron beam exposure doses was systematically investigated (Fig. 8). At the lower dose (110 μC cm−2, Fig. 8a), the two-dimensional morphology revealed blurred line edges, with a measured space width of 55 nm, indicating incomplete pattern development. The three-dimensional topography displayed irregular and sharp nanostructures. Although the sidewall inclination angle was relatively large, the overall structure height was significantly insufficient. Line profile analysis confirmed a height variation of only about 20 nm, far below the initial photoresist film thickness (approximately 90 nm), demonstrating insufficient photoacid generation efficiency under this condition, which led to incomplete development of the pattern. Under the optimized dose (160 μC cm−2, Fig. 8b), the patterning quality was significantly enhanced. The two-dimensional morphology showed clear, complete, and uniform line/space structures, with a measured space width of 95 nm that closely matched the design target (minor deviations may originate from AFM tip convolution effects). The three-dimensional view further confirmed features with a high aspect ratio, steep sidewall morphology, and flat top surface. Line profile analysis indicated nearly vertical sidewall angles and well-controlled feature dimensions, achieving high-fidelity linewidth patterning. At the excessive dose (210 μC cm−2, Fig. 8c), although the two-dimensional morphology maintains the overall continuity of the pattern, overexposure causes excessive diffusion of the photoacid, resulting in significant graphic distortion and ultimately leading to a space width of 125 nm. The three-dimensional topography exhibited top surface erosion, reduced structure height, and edge rounding. Line profile results further confirmed the presence of top loss and linewidth shrinkage, indicating loss of critical dimension control due to overexposure. The study results demonstrate that the PR-1d photoresist, at the optimized dose, can simultaneously achieve high resolution, good sidewall morphology and structural uniformity in patterning performance. In addition, we performed AFM multimodal characterization on the patterns of the PR-1d photoresist at smaller feature sizes (Fig. S27). The measurement results show that even if the feature size is further reduced, all patterns still maintain good morphology quality: the visible contour of the two-dimensional morphology image is clear and complete, without obvious edge roughness or fracture phenomenon. The 3D view further indicates that the structure has a high aspect ratio and shape fidelity, with sharp pattern edges and steep sidewalls. The quantitative display of uniform structure height through line profile analysis further confirms the potential application of the PR-1d photoresist in advanced lithography.
Fig. 9 presents the lithographic process flow of the photoresist used in this study and its photochemical imaging mechanism.23,25,34 The photoresist film consists of polymer bearing acid-labile groups and PAGs. Upon exposure to 254 nm/365 nm/EBL, the PAGs absorb energy and reach an excited state, leading to the cleavage of their N–O bond. This homolytic dissociation generates radical pairs, which subsequently abstract protons from the surrounding environment to produce a strong acid. During the PEB step, the generated acid acts as a catalyst, triggering deprotection of the acid-labile groups on the polymer backbone. This reaction converts hydrophobic groups into hydrophilic carboxylic groups, rendering the exposed regions soluble in an alkaline developer. The acid is then regenerated, propagating a chain reaction and markedly improving efficiency. Finally, during development using a 2.38 wt% tetramethylammonium hydroxide (TMAH) aqueous solution, the chemically transformed exposed regions are dissolved and removed, while the unexposed areas remain, thereby forming clear positive-tone spatial patterns on the substrate. From the lithographic imaging mechanism, it is evident that the acid-generation efficiency of the PAG is the most critical factor governing resist sensitivity: the higher the Φa, the higher the lithographic sensitivity. In this study, PAG 1d features electron-withdrawing groups (–CF3) at the para position of the benzene sulfonate units and an electron-donating group (–OMe) at the para position of the phenylthio substituent located at the 4-position on the naphthalimide backbone. It exhibited the highest Φa (17.6%) and therefore showed markedly higher E0 (54.4 μC cm−2) than the other PAGs.
3 Conclusions
This study designed and synthesized five nonionic PAGs based on the naphthalimide–benzenesulfonate backbone, and systematically investigated their solubility, thermal stability, photophysical properties, Φp, Φa, and lithographic performance. The experimental results demonstrate that PAGs 1a–1e exhibit excellent solubility in common photoresist solvents and remarkable thermal stability, fully meeting lithographic processing requirements. The UV spectra demonstrate that the optical properties of the PAG can be adjusted by structural modifications. Φa measurements established a clear structure–property relationship for PAGs, where the superior performance of PAG 1d (Φa = 17.6%) reflects the optimal D–π–A system, in which the synergistic combination of electron-donating and electron-withdrawing groups enhances photosensitivity, while an electron-withdrawing substituent efficiently releases the acid, thereby achieving a high acid generation quantum yield. DFT calculations further confirm that the established structure–property relationship provides a solid foundation for the molecular engineering of PAG molecules with high Φa. Our comprehensive evaluation demonstrates that all five PAG-based positive-tone photoresists successfully achieve high-quality patterning across multiple lithographic platforms (365 nm, 254 nm, and EBL), with their sensitivity performance directly correlating with the Φa values of PAGs. Notably, the PR-1d formulation containing PAG 1d demonstrated exceptional performance in EBL evaluation, showing both the highest sensitivity (E0 = 54.4 μC cm−2) and achieving an industry-leading resolution of 28 nm at a 190 μC cm−2 exposure dose, representing state-of-the-art performance for nonionic PAG-based resists. This work provides fundamental insights into the structure–property relationships of nonionic sulfonate PAGs, as well as demonstrates their potential for sub-30 nm lithography applications and making them a promising resist for advanced node semiconductor fabrication.4 Experimental section
4.1 Photoresist preparation and spin-coating procedures
The polymer (poly(2-phenyl-2-propyl methacrylate-co-γ-butyrolactone-3-yl methacrylate), PG43) (47.5 mg) and PAG (5 mmol) were dissolved in 2 ml DCE and filtered twice through a 0.1 μm polytetrafluoroethylene membrane filter to prepare the photoresist solution. The silicon substrates were first pretreated with hexamethyldisilazane (HMDS) in an HMDS-6090 system at 150 °C for 600 s.50 The photoresist solution was then spin-coated onto the pretreated substrates at 2000 rpm or 6000 rpm for 60 s, resulting in film thicknesses of 180 ± 7 nm and 90 ± 5 nm, respectively. The coated substrates are then pre-baked at 90 °C for 60 s. The 180 nm-thick films are exposed to 365 nm or 254 nm UV light, while the 90 nm-thick films are patterned via EBL.4.2 Lithography experiment
The wafer was exposed to 365 nm, 254 nm, or EBL (using a CABL-9500C system operating at an acceleration voltage of 50 kV and a beam current of 100 pA). After exposure, the wafer was placed in a post-exposure bake (PEB) for 30 s at 110 °C. Subsequently, a 2.38 wt% tetramethylammonium hydroxide (TMAH) aqueous solution was used to develop it for 60 s followed by rinsing with water for 60 s and blowing with nitrogen gas.Author contributions
Changchang Zhuang was responsible for the synthesis of photoresist materials, the design of experiments, and the drafting of the manuscript. Jiao Chen was responsible for the synthesis of photoacid generators, organized the data and contributed to the writing and editing of the manuscript. Tao Wang participated in the EBL experiments. Jun Zhao offered guidance for the EBL experiments. Haoyuan Li provided financial support. Hanshen Xin and Jianhua Zhang provided financial support, supervised the experiments and revised the manuscript.Conflicts of interest
The authors declare no conflict of interest.Data availability
The data supporting this article have been included as part of the Supplementary information. Supplementary information: the SI contains additional experimental data, methodological details, and supporting figures referenced in the main text. See DOI: https://doi.org/10.1039/d5im00286a.Acknowledgements
The authors thank the BL08U1B beamline of the Shanghai Synchrotron Radiation Facility for providing the EBL. The National Natural Science Foundation of China (grant numbers: 22090013) and the Shanghai Committee of Science and Technology (grant number: YDZX20213100002672 and 21QA1402900) provided financial support for this study.References
- C. K. Ober, F. Käfer and C. Yuan, Recent developments in photoresists for extreme-ultraviolet lithography, Polymer, 2023, 280, 126020 CrossRef CAS.
- X. Wang, P. Tao, Q. Wang, R. Zhao, T. Liu, Y. Hu, Z. Hu, Y. Wang, J. Wang and Y. Tang, Trends in photoresist materials for extreme ultraviolet lithography: A review, Mater. Today, 2023, 67, 299–319 CrossRef CAS.
- H. Xu, V. Kosma, E. P. Giannelis and C. K. Ober, In pursuit of Moore's Law: Polymer chemistry in action, Polym. J., 2018, 50, 45–55 CrossRef CAS.
- M. Li and E. Aqad, Key Challenges and opportunities for advanced extreme ultraviolet lithography photoresist materials, Adv. Funct. Mater., 2025, 35, 2420962 CrossRef CAS.
- H. Chen, W. Li, Y. Zhao, X. Huang, J. Zhang, P. Ji, J. Zhao, P. Chen and X. Peng, Hybrid alkyl-ligand tin-oxo clusters for enhanced lithographic patterning performance via intramolecular interactions, Ind. Chem. Mater., 2025, 3, 543–552 RSC.
- R. Peng, P. Lian, J. Chen, T. Yu, Y. Zeng, S. Wang, X. Guo, R. Hu, J. Zhao, Y. Wu, G. Yang and Y. Li, Lithographic performances of aryl sulfonate ester-modified polystyrenes as nonchemically amplified resists, Ind. Chem. Mater., 2025, 3, 553–566 RSC.
- H. Ito, Dissolution behavior of chemically amplified resist polymers for 248-, 193-, and 157-nm lithography, IBM J. Res. Dev., 2001, 45, 683–695 CAS.
- H. Ito, Chemical amplification resists: Inception, implementation in device manufacture, and new developments, J. Polym. Sci., Part A: Polym. Chem., 2003, 41, 3863–3870 CrossRef CAS.
- H. I. H. Ito, Advances in chemical amplification resist systems, Jpn. J. Appl. Phys., 1992, 31, 4273 CrossRef CAS.
- E. Reichmanis, F. Houlihan, O. Nalamasu and T. Neenan, Chemical amplification mechanisms for microlithography, Chem. Mater., 1991, 3, 394–407 CrossRef CAS.
- E. Reichmanis, F. Houlihan, O. Nalamasu and T. Neenan, Chemically amplified resists: Chemistry and processes, Adv. Mater. Opt. Electron., 1994, 4, 83–93 CrossRef CAS.
- H. Ridaoui, A. Dirani, O. Soppera, E. Ismailova, C. Brochon, G. Schlatter, G. Hadziioannou, R. Tiron, P. Bandelier and C. Sourd, Chemically amplified photoresists for 193-nm photolithography: Effect of molecular structure and photonic parameters on photopatterning, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 1271–1277 CrossRef CAS.
- S. L. Ablaza, J. F. Cameron, G. Xu and W. Yueh, The effect of photoacid generator structure on deep ultraviolet resist performance, J. Vac. Sci. Technol., B: Microelectron. Nanometer Struct.--Process., Meas., Phenom., 2000, 18, 2543–2550 CrossRef CAS.
- C. M. Bottoms, T. Terlier, G. E. Stein and M. Doxastakis, Ion diffusion in chemically amplified resists, Macromolecules, 2021, 54, 1912–1925 CrossRef CAS.
- S.-Y. Moon and J.-M. Kim, Chemistry of photolithographic imaging materials based on the chemical amplification concept, J. Photochem. Photobiol., C, 2007, 8, 157–173 CrossRef CAS.
- M. Shirai and H. Okamura, i-Line sensitive photoacid generators for UV curing, Prog. Org. Coat., 2009, 64, 175–181 CrossRef CAS.
- M. Shirai and M. Tsunooka, Photoacid and photobase generators: Chemistry and applications to polymeric materials, Prog. Polym. Sci., 1996, 21, 1–45 CrossRef CAS.
- Y. Zhang, H. Yu, L. Wang, X. Wu, J. He, W. Huang, C. Ouyang, D. Chen and B. E. Keshta, Advanced lithography materials: From fundamentals to applications, Adv. Colloid Interface Sci., 2024, 329, 103197 CrossRef CAS PubMed.
- H. Wu and K. Gonsalves, Novel positive-tone chemically amplified resists with photoacid generator in the polymer chains, Adv. Mater., 2001, 13, 670–672 CrossRef CAS.
- Q. Wang, C. Yan, F. You and L. Wang, A new type of sulfonium salt copolymers generating polymeric photoacid: Preparation, properties and application, React. Funct. Polym., 2018, 130, 118–125 CrossRef CAS.
- M. Wang, N. D. Jarnagin, C.-T. Lee, C. L. Henderson, W. Yueh, J. M. Roberts and K. E. Gonsalves, Novel polymeric anionic photoacid generators (PAGs) and corresponding polymers for 193 nm lithography, J. Mater. Chem., 2006, 16, 3701–3707 RSC.
- M. Wang, W. Yueh and K. E. Gonsalves, Novel ionic photoacid generators (PAGs) and corresponding PAG bound polymers, J. Photopolym. Sci. Technol., 2007, 20, 751–755 CrossRef CAS.
- J. Deng, S. Bailey, S. Jiang and C. K. Ober, High-performance chain scissionable resists for extreme ultraviolet lithography: Discovery of the photoacid generator structure and mechanism, Chem. Mater., 2022, 34, 6170–6181 CrossRef CAS.
- T. Tsuchimura, Recent progress in photo-acid generators for advanced photopolymer materials, J. Photopolym. Sci. Technol., 2020, 33, 15–26 CrossRef CAS.
- Y. Liu, D. Wang, H. Wang, H. Chen, Q. Wang and W. Kang, Enhanced lithography performance with imino/imido benzenesulfonate photoacid generator-bound polymer resists, Small, 2025, 21, 2412297 CrossRef CAS.
- N. Zivic, P. K. Kuroishi, F. Dumur, D. Gigmes, A. P. Dove and H. Sardon, Recent advances and challenges in the design of organic photoacid and photobase generators for polymerizations, Angew. Chem., Int. Ed., 2019, 58, 10410–10422 CrossRef CAS PubMed.
- C. J. Martin, G. Rapenne, T. Nakashima and T. Kawai, Recent progress in development of photoacid generators, J. Photochem. Photobiol., C, 2018, 34, 41–51 CrossRef CAS.
- R. A. Lawson and C. L. Henderson, Mesoscale simulation of molecular resists: The effect of PAG distribution homogeneity on LER, Microelectron. Eng., 2009, 86, 741–744 CrossRef CAS.
- W. Xu, T. Li, G. Li, Y. Wu and T. Miyashita, Novel polymeric nonionic photoacid generators and corresponding polymer Langmuir-Blodgett (LB) films for photopatterning, J. Photochem. Photobiol., A, 2011, 219, 50–57 CrossRef CAS.
- N. A. Kuznetsova, G. V. Malkov and B. G. Gribov, Photoacid generators. Application and current state of development, Russ. Chem. Rev., 2020, 89, 173 CrossRef CAS.
- Y. Liu, D. Wang, Q. Wang and W. Kang, Polymerizable nonionic perfluorinated photoacid generators for high-resolution lithography, Small Methods, 2024, 8, 2400112 CrossRef CAS.
- C. J. Martin, G. Rapenne, T. Nakashima and T. Kawai, Recent progress in development of photoacid generators, J. Photochem. Photobiol., C, 2018, 34, 41–51 CrossRef CAS.
- E. Torti, G. Della Giustina, S. Protti, D. Merli, G. Brusatin and M. Fagnoni, Aryl tosylates as non-ionic photoacid generators (PAGs): Photochemistry and applications in cationic photopolymerizations, RSC Adv., 2015, 5, 33239–33248 RSC.
- L. Zhang, B. Feng, S. Pang, H. Xin, K. Li and Y. Jin, Synthesis and performance study of nonionic photoacid generators based on Norbornene-imide, J. Mol. Struct., 2024, 1304, 137653 CrossRef CAS.
- Y. Wang, G. Sheng, J. Xie, D. Wan and M. Jin, Push–pull biphenyl–based iodonium salts: Highly sensitive one-component photoinitiators for photopolymerization under UV–visible LEDs, Prog. Org. Coat., 2024, 188, 108209 CrossRef CAS.
- Q. Sun, B. Feng, Z. Sun, R. Liu, H. Ding and Y. Jin, Synthesis and properties of a series of sulfonate ester photoacid generators, React. Chem. Eng., 2024, 9, 630–641 RSC.
- J. Andraos, G. Barclay, D. Medeiros, M. Baldovi, J. Scaiano and R. Sinta, Model studies on the photochemistry of phenolic sulfonate photoacid generators, Chem. Mater., 1998, 10, 1694–1699 CrossRef CAS.
- T. Endo, S. Suzuki, N. Miyagawa and S. Takahara, Multifunctional photo acid generator for fluorescence imaging based on self-contained photoreaction, J. Photochem. Photobiol., A, 2008, 200, 181–186 CrossRef CAS.
- M. Ikbal, R. Banerjee, S. Atta, D. Dhara, A. Anoop and N. P. Singh, Synthesis, photophysical and photochemical properties of photoacid generators based on N-hydroxyanthracene-1, 9-dicarboxyimide and their application toward modification of silicon surfaces, J. Org. Chem., 2012, 77, 10557–10567 CrossRef CAS.
- E. Torti, S. Protti, D. Merli, D. Dondi and M. Fagnoni, Photochemistry of N-arylsulfonimides: An easily available class of nonionic photoacid generators (PAGs), Chemistry, 2016, 22, 16998–17005 CrossRef CAS.
- J.-P. Malval, F. Morlet-Savary, X. Allonas, J.-P. Fouassier, S. Suzuki, S. Takahara and T. Yamaoka, On the cleavage process of the N-trifluoromethylsulfonyloxy-1, 8-naphthalimide photoacid generator, Chem. Phys. Lett., 2007, 443, 323–327 CrossRef CAS.
- M. Oelgemoeller and W. H. Kramer, Synthetic photochemistry of naphthalimides and related compounds, J. Photochem. Photobiol., C, 2010, 11, 210–244 CrossRef.
- Z. Changchang, C. Jiao, W. Tao, Z. Jun, L. Haoyuan, X. Hanshen and Z. Jianhua, Anionic photoacid generator bound polymer photoresists with improved performance for advanced lithography patterning, J. Appl. Polym. Sci., 2024, 142, e56622 Search PubMed.
- L.-Y. Peng, S.-L. Xiang, J.-D. Huang, Y.-Y. Ren, P. Hong, C. Li, J. Liu and M.-Q. Zhu, Dual nonionic photoacids synergistically enhanced photosensitivity for chemical amplified resists, Chem. Eng. J., 2024, 482, 148810 CrossRef CAS.
- L. T. Zhou, Z. X. Tang, H. Y. Li, H. S. Xin and J. H. Zhang, Enhancing 365 nm photoresist performance using 2-isopropylthioxanthone as a photosensitizer, ACS Appl. Mater. Interfaces, 2025, 17, 39542–39551 CrossRef CAS.
- J.-P. Malval, S. Suzuki, F. Morlet-Savary, X. Allonas, J.-P. Fouassier, S. Takahara and T. Yamaoka, Photochemistry of naphthalimide photoacid generators, J. Phys. Chem. A, 2008, 112, 3879–3885 CrossRef CAS PubMed.
- I. Shafiq, I. Amanat, M. Khalid, M. A. Asghar, R. Baby, S. Ahmed and S. M. Alshehri, Influence of azo-based donor modifications on nonlinear optical amplitude of D-π-A based organic chromophores: A DFT/TD-DFT exploration, Synth. Met., 2023, 297, 117410 CrossRef CAS.
- M. Khalid, A. Ali, R. Jawaria, M. A. Asghar, S. Asim, M. U. Khan, R. Hussain, M. F. ur Rehman, C. J. Ennis and M. S. Akram, First principles study of electronic and nonlinear optical properties of A–D–π–A and D–A–D–π–A configured compounds containing novel quinoline–carbazole derivatives, RSC Adv., 2020, 10, 22273–22283 RSC.
- T. Lu, A comprehensive electron wavefunction analysis toolbox for chemists, Multiwfn, J. Chem. Phys., 2024, 8, 082503 CrossRef.
- T. Wang, C. Zhuang, G. Yang, H. Xin, L. Jiang and J. Zhang, Interface engineering of underlayer of chemically-amplified EUV photoresists to enhance the photolithographic performance, Mater. Sci. Eng., B, 2024, 307, 117539 CrossRef CAS.
Footnote |
| † These authors contributed equally. |
| This journal is © Institute of Process Engineering of CAS 2026 |