Metal-free near-infrared-induced radical-promoted cationic RAFT polymerization for high penetration photocuring†
Shiliang
He
,
Ying
Zhu
,
Yuxin
Lu
,
Zhenwei
Shi
,
Yuan
Wang
,
Jiajia
Li
 *,
Jian
Zhu
*,
Jian
Zhu
 * and
Na
Li
*
* and
Na
Li
*
State and Local Joint Engineering Laboratory for Novel Functional Polymeric Materials, Jiangsu Key Laboratory of Advanced Functional Polymer Design and Application, Department of Polymer Science and Engineering, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, China. E-mail: chemjjli@suda.edu.cn; chemzhujian@suda.edu.cn; chemlina@suda.edu.cn
First published on 7th March 2025
Abstract
The application of cationic RAFT polymerization in photocuring has enabled the fabrication of stimuli-responsive materials. However, these systems mainly rely on UV light, limiting their broader applications. In this study, we introduce a metal-free, NIR-sensitive radical-promoted cationic RAFT polymerization system using IR-780 as the photocatalyst. The system offers excellent control over polymer molecular weights and narrow molecular weight distributions, enabling precise polymerization even under deep curing conditions. By employing a combination of cationic RAFT agents and NIR light, we achieve controlled polymerization of a range of vinyl ethers, demonstrating the versatility and efficiency of the approach. Additionally, the method is applied to photocuring applications, demonstrating its potential for fabricating complex 3D structures and welding applications. This work provides a strategy for deep photocuring with spatial and temporal control, expanding the potential applications of RAFT polymerization in biocompatible and large-scale manufacturing systems.
Introduction
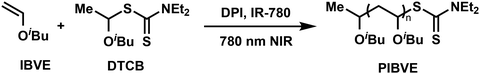
Living cationic polymerization (LCP) has long been established as a reliable technique for producing well-defined polymers with controlled molecular weight and narrow molecular weight distribution (MWD).1–4 Meanwhile, photoinduced cationic polymerization has found extensive use in photocuring applications due to its simplicity and efficiency.5,6 However, these non-living photopolymerization systems lack precise control over the polymerization process, often resulting in polymers with uncontrolled molecular weight and broad MWDs. In recent years, photo-controlled cationic polymerization has garnered significant attention for its ability to provide excellent spatial and temporal control, enabling the synthesis of polymers with tailored structures.7–11 For instance, Chiu's group demonstrated the use of a photoswitchable Brønsted acid initiator to manipulate polymer dispersity in the cationic polymerization of vinyl ethers.12 Our group developed the latent mediator or initiator strategy, which can generate initiator or mediator on-demand in situ, enabling fine control of polymer dispersity by modulating light on–off cycles during polymerization.13,14 Fors et al. developed a dual-catalyst system that alternates between cationic and radical polymerization via different light sources, allowing for precise control over polymer sequences and structures.15 This strategy is used for spatial control of the physical properties of thermosets, enabling the fabrication of multimaterial thermosets.16,17Despite these advancements, the light wavelengths used in controlled cationic polymerization are predominantly limited to UV and visible light. These wavelengths, while effective, suffer from limited penetration depth, posing challenges for applications requiring deep curing. In contrast, near infrared (NIR) light offers superior penetration and is ideal for in-depth photocuring.18–24 Yagci and Liu reported NIR-induced radical-promoted cationic polymerization using upconverting materials,25–27 showcasing its potential for fabricating macroscale and multi-scalable structures.28–30 However, these NIR systems typically rely on non-living polymerization, lacking the molecular weight and dispersity control critical for advanced polymeric materials. Our group has previously addressed this limitation by employing cationic RAFT agents in radical-promoted cationic polymerization, achieving well-controlled poly(vinyl ether)s.31–33 This system demonstrates compatibility with a wide range of photocatalysts across UV to NIR wavelengths. For instance, we utilized Fe(Cp)2(CO)4 as an NIR initiator,33,34 but its limited NIR absorption and the residual metal in the resulting polymers restricted its applicability, particularly in biocompatible systems. To overcome these challenges, we selected a NIR-sensitive organic dye, IR-780,35 to develop a metal-free, NIR-induced radical-promoted cationic RAFT polymerization system (Fig. 1). This study introduces a versatile and efficient strategy for synthesizing well-defined polymers with controlled molecular weights and MWDs, extending the application of cationic RAFT polymerization to deep photocuring and biocompatible systems.
Results and discussion
The polymerization was performed by using S-1-isobutoxyethyl N,N-diethyl dithiocarbamate (DTCB) as the cationic RAFT agent, diphenyliodonium hexafluorophosphate (DPI) as the oxidant to generate initiating protons, and IR-780 as the NIR-sensitive photocatalyst. A solvent mixture of toluene and dichloromethane (DCM) (v/v = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was employed to dissolve IR-780. The influence of each component on the polymerization of isobutyl vinyl ether (IBVE) was first explored. The polymerization rate decreased with reduced amounts of DPI (Table 1, entries 1–3), and no polymerization occurred in its absence (Table 1, entry 4), confirming that DPI is essential for generating initiating cations. Increasing the amount of IR-780 accelerated polymerization (Table 1, entry 5), likely due to an enhanced photo electron/energy transfer (PET) process, whereas reducing IR-780 slowed polymerization (Table 1, entry 6). Interestingly, polymerization still occurred slowly without IR-780 or in the dark (Table 1, entries 7 and 8), attributed to the weak Lewis acidity of DPI.36 The molecular weight of the obtained polymers was tunable by adjusting the IBVE-to-DTCB molar ratio (Table 1, entries 9–11). In the absence of DTCB, uncontrolled polymerization occurred, yielding polymers with broad MWDs (Table 1, entry 12), highlighting the critical role of the RAFT process in achieving controlled polymerization. The proposed polymerization mechanism is illustrated in Scheme S1† according to our previous work.31 Upon NIR light irradiation, IR-780 reaches its excited state and undergoes oxidation by DPI via the PET process, reducing DPI and generating an aryl radical. This radical adds to the monomer or RAFT agent to form a vinyl ether-type radical, which reacts further with DPI to generate the initiating cation necessary for cationic RAFT polymerization. Proton nuclear magnetic resonance (1H NMR) confirmed the chain-end structure of the obtained PIBVE (Fig. S2†). A characteristic peak at 6 ppm, corresponding to the proton attached to the carbon bonded to the sulfur of the dithiocarbamate-terminated chain end, verified the incorporation of the RAFT agent. Additionally, the peak at ∼7.2 ppm (peak j) indicated the radical addition of the aryl radical to the monomer.
1) was employed to dissolve IR-780. The influence of each component on the polymerization of isobutyl vinyl ether (IBVE) was first explored. The polymerization rate decreased with reduced amounts of DPI (Table 1, entries 1–3), and no polymerization occurred in its absence (Table 1, entry 4), confirming that DPI is essential for generating initiating cations. Increasing the amount of IR-780 accelerated polymerization (Table 1, entry 5), likely due to an enhanced photo electron/energy transfer (PET) process, whereas reducing IR-780 slowed polymerization (Table 1, entry 6). Interestingly, polymerization still occurred slowly without IR-780 or in the dark (Table 1, entries 7 and 8), attributed to the weak Lewis acidity of DPI.36 The molecular weight of the obtained polymers was tunable by adjusting the IBVE-to-DTCB molar ratio (Table 1, entries 9–11). In the absence of DTCB, uncontrolled polymerization occurred, yielding polymers with broad MWDs (Table 1, entry 12), highlighting the critical role of the RAFT process in achieving controlled polymerization. The proposed polymerization mechanism is illustrated in Scheme S1† according to our previous work.31 Upon NIR light irradiation, IR-780 reaches its excited state and undergoes oxidation by DPI via the PET process, reducing DPI and generating an aryl radical. This radical adds to the monomer or RAFT agent to form a vinyl ether-type radical, which reacts further with DPI to generate the initiating cation necessary for cationic RAFT polymerization. Proton nuclear magnetic resonance (1H NMR) confirmed the chain-end structure of the obtained PIBVE (Fig. S2†). A characteristic peak at 6 ppm, corresponding to the proton attached to the carbon bonded to the sulfur of the dithiocarbamate-terminated chain end, verified the incorporation of the RAFT agent. Additionally, the peak at ∼7.2 ppm (peak j) indicated the radical addition of the aryl radical to the monomer.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 25 °C under a 780 nm NIR light
1) at 25 °C under a 780 nm NIR light
| Entry | [IBVE]0/[DTCB]0/[DPI]0/[IR-780]0 | Time | Conv. (%) |
M
n,th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (g mol−1) a (g mol−1) |
M n,SEC (g mol−1) | Đ |
|---|---|---|---|---|---|---|
| a Calculated based on conversion (Mn,th = [M]0/[CTA]0 × MIBVE × conversion + MDTCB). b Determined by tetrahydrofuran (THF) SEC using polystyrene (PS) calibration. c Polymerization in the dark. | ||||||
| 1 | 100/1/0.1/0.01 | 30 min | 89.1 | 9200 | 9100 | 1.13 |
| 2 | 100/1/0.05/0.01 | 60 min | 88.6 | 9100 | 9200 | 1.16 |
| 3 | 100/1/0.01/0.01 | 3.5 h | 59.2 | 6200 | 6400 | 1.23 |
| 4 | 100/1/0/0.01 | 24 h | — | — | — | — |
| 5 | 100/1/0.1/0.05 | 10 min | 90.0 | 9200 | 9200 | 1.14 |
| 6 | 100/1/0.1/0.001 | 5 h | 88.9 | 9100 | 9000 | 1.07 |
| 7 | 100/1/0.1/0 | 6 h | 88.6 | 9100 | 9200 | 1.16 |
| 8c | 100/1/0.1/0.01 | 6 h | 78.2 | 8000 | 7600 | 1.25 |
| 9 | 25/1/0.1/0.01 | 10 min | 88.5 | 2500 | 2700 | 1.18 |
| 10 | 50/1/0.1/0.01 | 10 min | 98.6 | 5200 | 5200 | 1.12 |
| 11 | 200/1/0.1/0.01 | 40 min | 89.3 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.17 |
| 12 | 100/0/0.1/0.01 | 10 min | 98.3 | 9800 | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3.46 |
Kinetics experiments were conducted to investigate the polymerization behavior. As depicted in Fig. 2A, a first-order linear relationship was observed in the semilogarithmic plot of monomer concentration versus polymerization time, indicating a constant cation concentration during the polymerization. Higher IR-780 concentrations accelerated polymerization, likely by increasing initiating cation production. An induction period was observed, especially at lower IR-780 concentrations, due to the relatively slow interaction between IR-780 and DPI. Thanks to the high penetration ability of NIR light, polymerization was carried out successfully with 3 mm thick pig skin as a translucent barrier, demonstrating efficient polymerization. The molecular weight of PIBVE increased linearly with monomer conversion, while the MWDs remained narrow (Fig. 2B). SEC traces displayed monomodal, symmetrical distributions that shifted smoothly toward higher molecular weights with increasing conversion (Fig. 2D–F). An in situ chain-extension experiment further validated the living nature of the polymerization. A clear shift in the SEC trace after the addition of a second monomer (Fig. 2C) demonstrated successful chain extension.
The monomer scope was screened using the optimized conditions ([Monomer]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [DTCB]0
[DTCB]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [DPI]0
[DPI]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [IR-780]0 = 100
[IR-780]0 = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1
0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
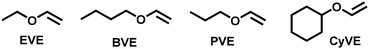
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01). The results are summarized in Table 2. Controlled molecular weights and narrow MWDs were observed for the polymerization of ethyl vinyl ether (EVE), butyl vinyl ether (BVE) and propoxy ethylene (PVE) (Table 2, entries 1–3). The polymerization of cyclohexyl vinyl ether (CyVE) showed relatively broad MWDs, likely due to a steric effect, which is consistent with a previous report (Table 2, entries 4).37 These results demonstrated the broad applicability of this method for synthesizing well-defined polymers.
0.01). The results are summarized in Table 2. Controlled molecular weights and narrow MWDs were observed for the polymerization of ethyl vinyl ether (EVE), butyl vinyl ether (BVE) and propoxy ethylene (PVE) (Table 2, entries 1–3). The polymerization of cyclohexyl vinyl ether (CyVE) showed relatively broad MWDs, likely due to a steric effect, which is consistent with a previous report (Table 2, entries 4).37 These results demonstrated the broad applicability of this method for synthesizing well-defined polymers.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) under various conditions at 25 °C under a 780 nm NIR light
1) under various conditions at 25 °C under a 780 nm NIR light
| Entry | M | Time (min) | Conv. (%) | M n,th (g mol−1) | M n,SEC (g mol−1) | Đ |
|---|---|---|---|---|---|---|
| a Calculated based on conversion (Mn,th = [M]0/[CTA]0 × MM × conversion + MDTCB). b Determined by tetrahydrofuran (THF) SEC using polystyrene (PS) calibration. | ||||||
| 1 | EVE | 15 | 95.6 | 7100 | 7100 | 1.13 |
| 2 | BVE | 20 | 73.6 | 7600 | 7300 | 1.25 |
| 3 | PVE | 20 | 86.2 | 7700 | 7400 | 1.16 |
| 4 | CyVE | 15 | 80.0 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
9800 | 1.50 |
Finally, we applied this polymerization method for photocuring. As depicted in Fig. 3, a photomask with the transparent slit word ‘RAFT’ was placed on a glass container filled with printing resins consisting of diethylene glycol divinyl ether (DDE), CyVE, DTCB, DPI, and IR-780 at a molar ratio of [DDE]0/[CyVE]0/[DTCB]0/[DPI]0/[IR-780]0 = 70/30/1/0.5/0.01 under 780 nm NIR light (λmax = 780 nm, 1.8 W cm−2). The photomask was then illuminated for 1 minute. After removing the photomask and unreacted solution, the clear 3D word ‘RAFT’ was observed (Fig. 3A). Subsequently, photocuring was performed with a sheet of A4 paper positioned between the light source and the mask. The 3D word ‘RAFT’ was successfully constructed after 6 minutes (Fig. 3B), demonstrating the excellent penetrating ability of the NIR light. Moreover, a successful polymer welding experiment was conducted by painting the resin containing DDE, DPI and IR-780 between two ‘F’ letters, followed by NIR light irradiation for 1 minute (Fig. 3C), showcasing great potential of this system for deep photocuring.
Conclusion
In conclusion, we have developed a versatile and efficient NIR-induced radical-promoted cationic RAFT polymerization system that offers exceptional control over polymer molecular weight and dispersity. The use of the NIR-sensitive organic dye IR-780 as a photocatalyst in combination with a cationic RAFT agent provides a metal-free and effective approach for polymer synthesis, even under deep curing conditions. The successful polymerization of various vinyl ethers with well-defined molecular characteristics demonstrates the broad applicability of this method. Additionally, the ability to conduct photocuring in a range of applications, including 3D printing and polymer welding, highlights the potential of this system for advanced manufacturing and biocompatible systems. Future work will focus on expanding the monomer scope and optimizing the system for even deeper curing processes, potentially opening new avenues in industrial applications requiring high precision and biocompatibility.Author contributions
Shiliang He performed the experimental work and prepared the draft. Ying Zhu, Zhenwei Shi and Yuan Wang helped in synthesizing the RAFT agent. Jiajia Li, Jian Zhu and Na Li conceived and designed the experiments, analysed the data and revised the manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Detailed experimental procedures, characterization methods, and ESI figures (Scheme S1 and Fig. S1–S10†) are presented.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 22371199), Suzhou Cutting-edge Technology Research Project (SYG202350), the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions and the Program of Innovative Research Team of Soochow University.References
- S. Aoshima and S. Kanaoka, A Renaissance in Living Cationic Polymerization, Chem. Rev., 2009, 109(11), 5245–5287, DOI:10.1021/cr900225g.
- E. J. Goethals and F. D. Prez, Carbocationic polymerizations, Prog. Polym. Sci., 2007, 32(2), 220–246, DOI:10.1016/j.progpolymsci.2007.01.001.
- Y. N. Chen, L. Zhang, Y. Jin, X. R. Lin and M. Chen, Recent Advances in Living Cationic Polymerization with Emerging Initiation/Controlling Systems, Macromol. Rapid Commun., 2021, 42, e2100148, DOI:10.1002/marc.202100148.
- L. Q. Wu, B. Rondon, S. Dym, W. Q. Wang, K. R. Chen and J. Niu, Regulating cationic polymerization: from structural control to life cycle management, Prog. Polym. Sci., 2023, 145, 101736, DOI:10.1016/j.progpolymsci.2023.101736.
- H. W. Lai, J. Zhang, F. Y. Xing and P. Xiao, Recent advances in light-regulated non-radical polymerisations, Chem. Soc. Rev., 2020, 49(6), 1867–1886, 10.1039/C9CS00731H.
- S. Q. Shi, C. Croutxé-Barghorn and X. Allonas, Photoinitiating systems for cationic photopolymerization: Ongoing push toward long wavelengths and low light intensities, Prog. Polym. Sci., 2017, 65, 1–41, DOI:10.1016/j.progpolymsci.2016.09.007.
- R. J. Sifri, Y. Ma and B. P. Fors, Photoredox Catalysis in Photocontrolled Cationic Polymerizations of Vinyl Ethers, Acc. Chem. Res., 2022, 55(14), 1960–1971, DOI:10.1021/acs.accounts.2c00252.
- C. Aydogan, G. Yilmaz, A. Shegiwal, D. M. Haddleton and Y. Yagci, Photoinduced Controlled/Living Polymerizations, Angew. Chem., Int. Ed., 2022, 61(23), e202117377, DOI:10.1002/anie.202117377.
- Q. Michaudel, V. Kottisch and B. P. Fors, Cationic Polymerization: From Photoinitiation to Photocontrol, Angew. Chem., Int. Ed., 2017, 56(33), 9670–9679, DOI:10.1002/anie.201701425.
- X. Zhang, Y. Jiang, Q. Ma, S. Hu and S. H. Liao, Metal-Free Cationic Polymerization of Vinyl Ethers with Strict Temporal Control by Employing an Organophotocatalyst, J. Am. Chem. Soc., 2021, 143(17), 6357–6362, DOI:10.1021/jacs.1c02500.
- Z. Yang, Y. Liao, Z. Y. Zhang, J. X. Chen, X. Zhang and S. H. Liao, Asymmetric Ion-Pairing Photoredox Catalysis for Stereoselective Cationic Polymerization under Light Control, J. Am. Chem. Soc., 2024, 146(10), 6449–6455, DOI:10.1021/jacs.3c12694.
- D. Liu, A. D. Sponza, D. Yang and M. Chiu, Modulating Polymer Dispersity with Light: Cationic Polymerization of Vinyl Ethers Using Photochromic Initiators, Angew. Chem., Int. Ed., 2019, 58(45), 16210–16216, DOI:10.1002/anie.201908775.
- M. Zhang, J. J. Li, M. Chen, X. Q. Pan, Z. B. Zhang and J. Zhu, Combination of the Photoinduced Atom Transfer Radical Addition Reaction and Living Cationic Polymerization: A Latent Initiator Strategy toward Tailoring Polymer Molecular Weight Distributions, Macromolecules, 2021, 54(13), 6502–6510, DOI:10.1021/acs.macromol.1c00332.
- M. Chen, J. J. Li, K. Q. Ma, G. Q. Jin, X. Q. Pan, Z. B. Zhang and J. Zhu, Controlling Polymer Molecular Weight Distribution through a Latent Mediator Strategy with Temporal Programming, Angew. Chem., Int. Ed., 2021, 60(36), 19705–19709, DOI:10.1002/anie.202107106.
- V. Kottisch, Q. Michaudel and B. P. Fors, Photocontrolled Interconversion of Cationic and Radical Polymerizations, J. Am. Chem. Soc., 2017, 139(31), 10665–10668, DOI:10.1021/jacs.7b06661.
- Y. Ma, R. J. Dreiling, E. A. Recker, J.-W. Kim, S. L. Shankel, J. Hu, A. D. Easley, Z. A. Page, T. H. Lambert and B. P. Fors, Multimaterial Thermoset Synthesis: Switching Polymerization Mechanism with Light Dosage, ACS Cent. Sci., 2024, 10(11), 2125–2131, DOI:10.1021/acscentsci.4c01507.
- Y. Ma, V. Kottisch, E. A. McLoughlin, Z. W. Rouse, M. J. Supej, S. P. Baker and B. P. Fors, Photoswitching Cationic and Radical Polymerizations: Spatiotemporal Control of Thermoset Properties, J. Am. Chem. Soc., 2021, 143(50), 21200–21205, DOI:10.1021/jacs.1c09523.
- Y. Zhu, D. D. Xu, Y. C. Zhang, Y. F. Zhou, Y. Yagci and R. Liu, Phenacyl Phenothiazinium Salt as A New Broad-wavelength-absorbing Photoinitiator for Cationic and Free Radical Polymerizations, Angew. Chem., Int. Ed., 2021, 60, 16917–16921, DOI:10.1002/anie.202104531.
- C. Tian, P. Wang, Y. Y. Ni, L. F. Zhang, Z. P. Cheng and X. L. Zhu, Photocontrolled Iodine-Mediated Reversible-Deactivation Radical Polymerization: Solution Polymerization of Methacrylates by Irradiation with NIR LED Light, Angew. Chem., Int. Ed., 2020, 59(10), 3910–3916, DOI:10.1002/anie.201914835.
- S. Allison-Logan, Q. Fu, Y. K. Sun, M. Liu, J. J. Xie, J. W. Tang and G. G. Qiao, From UV to NIR: A full spectrum metal-free photocatalyst for efficient polymer synthesis in aqueous conditions, Angew. Chem., Int. Ed., 2020, 59, 21392–21396, DOI:10.1002/anie.202007196.
- Z. Q. Li, X. C. Zou, F. Shi, R. Liu and Y. Yagci, Highly efficient dandelion-like near-infrared light photoinitiator for free radical and thiol-ene photopolymerizations, Nat. Commun., 2019, 10, 3560, DOI:10.1038/s41467-019-11522-0.
- C. Kutahya, C. Schmitz, V. Strehmel, Y. Yagci and B. Strehmel, Near-Infrared Sensitized Photoinduced Atom-Transfer Radical Polymerization (ATRP) with a Copper(II) Catalyst Concentration in the ppm Range, Angew. Chem., Int. Ed., 2018, 57(26), 7898–7902, DOI:10.1002/anie.201802964.
- S. Shanmugam, J. T. Xu and C. Boyer, Light-Regulated Polymerization under Near-Infrared/Far-Red Irradiation Catalyzed by Bacteriochlorophyll α, Angew. Chem., Int. Ed., 2016, 55(3), 1036–1040, DOI:10.1002/anie.201510037.
- Z. Wu and C. Boyer, Near-Infrared Light-Induced Reversible Deactivation Radical Polymerization: Expanding Frontiers in Photopolymerization, Adv. Sci., 2023, 10(33), e2304942, DOI:10.1002/advs.202304942.
- X. Y. Meng, H. Q. Lu, Z. Q. Li, C. Wang, R. Liu, X. Guan and Y. Yagci, Near-infrared light induced cationic polymerization based on upconversion and ferrocenium photochemistry, Polym. Chem., 2019, 10(41), 5574–5577, 10.1039/C9PY01262A.
- Z. Q. Li, J. Z. Zhu, X. Guan, R. Liu and Y. Yagci, Near-Infrared-Induced Cationic Polymerization Initiated by Using Upconverting Nanoparticles and Titanocene, Macromol. Rapid Commun., 2019, 40, e1900047, DOI:10.1002/marc.201900047.
- A. Kocaarslan, S. Tabanli, G. Eryurek and Y. Yagci, Near-Infrared Free-Radical and Free-Radical-Promoted Cationic Photopolymerizations by In-Source Lighting Using Upconverting Glass, Angew. Chem., Int. Ed., 2017, 56(46), 14507–14510, DOI:10.1002/anie.201707944.
- Y. Q. Zhao, J. Z. Zhu, W. Y. He, Y. Liu, X. X. Sang and R. Liu, 3D printing of unsupported multi-scale and large-span ceramic via near-infrared assisted direct ink writing, Nat. Commun., 2023, 14, 2381, DOI:10.1038/s41467-023-38082-8.
- P. Hu, H. Xu, Y. Pan, X. X. Sang and R. Liu, Upconversion particle-assisted NIR polymerization enables microdomain gradient photopolymerization at inter-particulate length scale, Nat. Commun., 2023, 14, 3653, DOI:10.1038/s41467-023-39440-2.
- J. Z. Zhu, Q. Zhang, T. Q. Yang, Y. Liu and R. Liu, 3D printing of multi-scalable structures via high penetration near-infrared photopolymerization, Nat. Commun., 2020, 11, 3462, DOI:10.1038/s41467-020-17251-z.
- S. L. He, X. R. Yang, Y. B. Zhao, Y. Liu, B. W. Zhao, X. Q. Pan, J. J. Li, J. Zhu and N. Li, Radical promoted cationic RAFT polymerization by photo electron transfer reaction, Polym. Chem., 2024, 15(38), 3847–3853, 10.1039/D4PY00774C.
- B. W. Zhao, J. J. Li, G. L. Li, X. R. Yang, S. P. Lu, X. Q. Pan and J. Zhu, Fast Living 3D Printing via Free Radical Promoted Cationic RAFT Polymerization, Small, 2023, 19(50), 2207637, DOI:10.1002/smll.202207637.
- J. J. Li, M. Chen, X. Lin, Q. L. Li, W. Zhang, G. Q. Jin, X. Q. Pan, J. Zhu and X. L. Zhu, Near-Infrared, Light-Induced Cationic and Radical RAFT Polymerization Catalyzed by Iron Complex, ACS Macro Lett., 2020, 9(12), 1799–1805, DOI:10.1021/acsmacrolett.0c00794.
- B. W. Zhao, J. J. Li, X. Q. Pan, Z. B. Zhang, G. Q. Jin and J. Zhu, Photoinduced Free Radical Promoted Cationic RAFT Polymerization toward “Living” 3D Printing, ACS Macro Lett., 2021, 10(10), 1315–1320, DOI:10.1021/acsmacrolett.1c00555.
- A. H. Bonardi, F. Dumur, T. M. Grant, G. Noirbent, D. Gigmes, B. H. Lessard, J.-P. Fouassier and J. Lalevéet, High Performance Near-Infrared (NIR) Photoinitiating Systems Operating under Low Light Intensity and in the Presence of Oxygen, Macromolecules, 2018, 51(4), 1314–1324, DOI:10.1021/acs.macromol.8b00051.
- R. Haraguchi, T. Nishikawa, A. Kanazawa and S. Aoshima, Metal-Free Living Cationic Polymerization Using Diaryliodonium Salts as Organic Lewis Acid Catalysts, Macromolecules, 2020, 53(11), 4185–4192, DOI:10.1021/acs.macromol.0c00823.
- S. W. Spring, C. S. Cerione, J. H. Hsu, S. L. Shankel and B. P. Fors, Scalable, Green Chain Transfer Agent for Cationic RAFT Polymerizations, Chin. J. Chem., 2022, 41, 399–404, DOI:10.1002/cjoc.202200557.
Footnote |
| † Electronic supplementary information (ESI) available: NMR spectrum and SEC of polymers. See DOI: https://doi.org/10.1039/d5py00117j |
| This journal is © The Royal Society of Chemistry 2025 |