Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Toxicity of metal–organic framework nanoparticles: from essential analyses to potential applications
Romy
Ettlinger
*a,
Ulrich
Lächelt
bc,
Ruxandra
Gref
d,
Patricia
Horcajada
e,
Twan
Lammers
f,
Christian
Serre
g,
Patrick
Couvreur
h,
Russell E.
Morris
a and
Stefan
Wuttke
*ij
aSchool of Chemistry, University of St. Andrews, St. Andrews, UK. E-mail: rle6@st-andrews.ac.uk
bDepartment of Pharmacy and Center for NanoScience (CeNS), Ludwig-Maximilians-Universität München, Munich, Germany
cDivision of Pharmaceutical Technology and Biopharmaceutics, University of Vienna, Vienna, Austria
dInstitut de Sciences Moléculaires d’Orsay, Université Paris Saclay, Paris, France
eMadrid Institute for Advanced Studies, Madrid, Spain
fInstitute for Experimental Molecular Imaging, RWTH Aachen University Clinic, Aachen, Germany
gDépartement de Chimie, Ecole Normale Supérieure de Paris, Paris, France
hInstitut Galien Paris-Sud, Université Paris Saclay, Paris, France
iIkerbasque, Basque Foundation for Science, Bilbao, Spain
jBasque Center for Materials, UPV/EHU Science Park, Leioa, Spain. E-mail: stefan.wuttke@bcmaterials.net
First published on 19th January 2023
Abstract
Correction for ‘Toxicity of metal–organic framework nanoparticles: from essential analyses to potential applications’ by Romy Ettlinger et al., Chem. Soc. Rev., 2022, 51, 464–484, https://doi.org/10.1039/D1CS00918D.
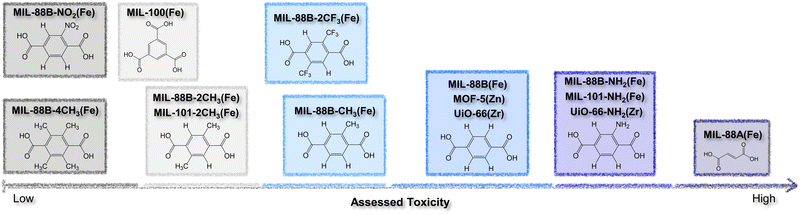
The authors regret that the information presented in Fig. 12 and discussed in the text on p. 478 may have been unclear, or inconsistent with the information derived from ref. 26. The corrected version of Fig. 12 is presented here. The corresponding sentence on p. 478, beginning, “Herein, endogenous fumaric acid…”, should also be revised as follows:
“Herein, nitro- and tetramethyl-terephthalic acid modifications turned out to be the most biofriendly organic linkers, followed by exogenous trimesic acid, amino-terephthalic acid, and terephthalic acid, and the endogenous fumaric acid had the lowest assessed IC50 value.”
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2023 |