A comprehensive review on singlet oxygen generation in nanomaterials and conjugated polymers for photodynamic therapy in the treatment of cancer
Neetika
Singh
 ,
Ria
Sen Gupta
,
Ria
Sen Gupta
 and
Suryasarathi
Bose
and
Suryasarathi
Bose
 *
*
Department of Materials Engineering, Indian Institute of Science, Bangalore, Karnataka - 560012, India. E-mail: sbose@iisc.ac.in
First published on 24th January 2024
Abstract
A key role in lessening humanity's continuous fight against cancer could be played by photodynamic therapy (PDT), a minimally invasive treatment employed in the medical care of a range of benign disorders and malignancies. Cancerous tissue can be effectively removed by using a light source-excited photosensitizer. Singlet oxygen and reactive oxygen species are produced via the photosensitizer as a result of this excitation. In the recent past, researchers have put in tremendous efforts towards developing photosensitizer molecules for photodynamic treatment (PDT) to treat cancer. Conjugated polymers, characterized by their efficient fluorescence, exceptional photostability, and strong light absorption, are currently under scrutiny for their potential applications in cancer detection and treatment through photodynamic and photothermal therapy. Researchers are exploring the versatility of these polymers, utilizing sophisticated chemical synthesis and adaptable polymer structures to create new variants with enhanced capabilities for generating singlet oxygen in photodynamic treatment (PDT). The incorporation of photosensitizers into conjugated polymer nanoparticles has proved to be beneficial, as it improves singlet oxygen formation through effective energy transfer. The evolution of nanotechnology has emerged as an alternative avenue for enhancing the performance of current photosensitizers and overcoming significant challenges in cancer PDT. Various materials, including biocompatible metals, polymers, carbon, silicon, and semiconductor-based nanomaterials, have undergone thorough investigation as potential photosensitizers for cancer PDT. This paper outlines the recent advances in singlet oxygen generation by investigators using an array of materials, including graphene quantum dots (GQDs), gold nanoparticles (Au NPs), silver nanoparticles (Ag NPs), titanium dioxide (TiO2), ytterbium (Yb) and thulium (Tm) co-doped upconversion nanoparticle cores (Yb/Tm-co-doped UCNP cores), bismuth oxychloride nanoplates and nanosheets (BiOCl nanoplates and nanosheets), and others. It also stresses the synthesis and application of systems such as amphiphilic block copolymer functionalized with folic acid (FA), polyethylene glycol (PEG), poly(β-benzyl-L-aspartate) (PBLA10) (FA-PEG-PBLA10) functionalized with folic acid, tetra(4-hydroxyphenyl)porphyrin (THPP-(PNIPAM-b-PMAGA)4), pyrazoline-fused axial silicon phthalocyanine (HY-SiPc), phthalocyanines (HY-ZnPcp, HY-ZnPcnp, and HY-SiPc), silver nanoparticles coated with polyaniline (Ag@PANI), doxorubicin (DOX) and infrared (IR)-responsive poly(2-ethyl-2-oxazoline) (PEtOx) (DOX/PEtOx-IR NPs), particularly in NIR imaging-guided photodynamic therapy (fluorescent and photoacoustic). The study puts forward a comprehensive summary and a convincing justification for the usage of the above-mentioned materials in cancer PDT.
1. Introduction
Cancer is increasingly a global health issue, characterized by uncontrollable cell growth and division. Cancer has become one of the most important health concerns in modern times due to its significant impact on human well-being. Despite enormous efforts, there has been limited success in developing clinical treatments, and fatality rates remain high. The International Agency for Research on Cancer estimates that 10 million malignant tumour deaths and 19.3 million new cancer diagnoses will occur globally in 2020.1,2 Over the past decade, conventional therapies like chemotherapy, surgery and radiotherapy had a reasonable impact. Chemotherapy is currently the primary and most successful mode of tumor therapy in the clinic.3 In this context, phototherapies like photodynamic treatment (PDT) and photothermal treatment (PTT) have visible encouraging outcomes in the treatment of cancer. Treatment methods that integrate laser light with photosensitizers (PSs) provide great spatial-temporal controllability, low invasiveness, and little medication resistance.4 When a photosynthetic system is photoexcited, its ground state (S0) rapidly transitions to the lowest excited singlet state (S1). Subsequently, as the excited state follows non-radiative pathways, it may decay back to the ground state, potentially generating heat in the process. Notably, irreversible cell necrosis occurs when the temperature reaches 42 °C. Elevated temperatures can expedite cell death through mechanisms like ischemia, vascular thrombosis, and the degradation of cell membranes and proteins. Furthermore, the excited energy of the triplet state can lead to the production of extremely reactive oxygen species (ROS) if the excited state decays back to the triplet state (T1) via intersystem crossover (ISC).5,6 ROS is a collective term representing a family of molecules, and the specific molecular identity of each ROS is crucial for understanding its chemical reactivity and biological responses. Nonetheless, the term ROS remains valuable for providing a general description of downstream phenotypes. Major ROS in living systems encompass superoxide ([O2]˙), hydrogen peroxide (H2O2), hypochlorous acid (HOCl), singlet oxygen (1O2), lipid peroxides (ROOH), ozone (O3), and the hydroxyl radical ([OH]˙). Determining whether a particular ROS is present at sufficiently high concentrations to engage in productive chemistry in a given scenario is fundamental for comprehending its impact on biology.7 Singlet oxygen (1O2) has been shown to oxidize chromogenic substrates when created by photosensitization without H2O2 oxidation or enzymes.8 For the formation of singlet oxygen, a photosensitizer, dissolved molecular oxygen, and a suitable wavelength of light are required.9 Excitation of photosensitizers should be capable of efficient intersystem crossing, allowing for energy transfer to ground-state molecular oxygen.10 Singlet oxygen is vital in photodynamic treatment (PDT), which is based on the idea that cancer cells are eliminated by deadly reactive oxygen species (ROS). Treatments for a range of conditions, such as dermatological, ophthalmology, cardiovascular, and oncological conditions, have been addressed with this therapy, which is named as a prospective treatment. PDT uses light, molecules of oxygen, and photosensitizers (PS).11 This review centres on the latest developments in PDT that target cancer cells. The review also looks at the existing methods and strategies for producing singlet oxygen in PDT as described by other authors.12 The review concludes with an overview of the work of other authors on the design and development of fluorescent probes, conjugated polymers and their nanoparticles, and fluorescent conjugated polymers used in PDT for singlet oxygen generation.2. Background and mechanism of singlet oxygen generation
Less stable than molecular oxygen in its electronic ground state, “singlet oxygen” is the electronically excited state in which molecules of oxygen exist (Fig. 1). Two unpaired electrons make up the ground state of molecular oxygen. This is because its HOMO (3∑g−, Fig. 2a) has two degenerate orbitals. Three states arranged vertically in ascending energy sequence are shown in Fig. 2b. In the case of oxygen, the energy of the two excited singlet (electron-coupled) states is 150 kJ mol−1 for 1∑g+ and 95 kJ mol−1 for 1Δg.13,14 The bulk of singlet oxygen photophysicochemical events are identified from the 1Δg state because, under typical experimental circumstances, the 1∑g+ (higher excited) state rapidly relaxes to the lower energy 1Δg state. The process of producing singlet oxygen often involves transferring energy from a photosensitizer's excited state to the oxygen molecule (Fig. 2b).13 Triplet energy transfer (T1,PS S0,PS) to triplet oxygen (T1,O2 S1,O2) is a well-organized spin-coupled process that is triggered by photoexcitation of a high ISC rate PS molecule. As a result, singlet oxygen is produced, which is significantly more reactive than other forms of oxygen and capable of redox chemistry and moderate infrared phosphorescence.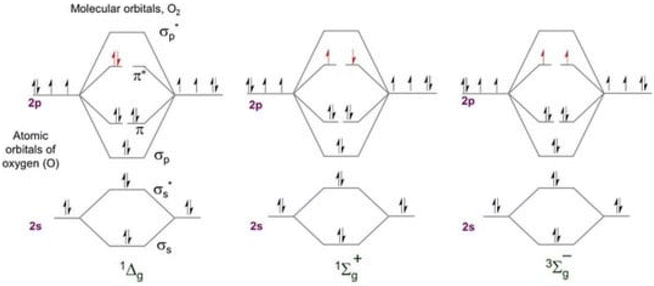 | ||
| Fig. 1 Molecular orbital energy diagram for the two excited singlet states of oxygen and the triplet ground state. | ||
 | ||
| Fig. 2 (a) Jablonski diagram showing the formation of singlet oxygen via the transfer of triplet energy from a photosensitizer; (b) relative configuration of molecular oxygen's electronic states.15 | ||
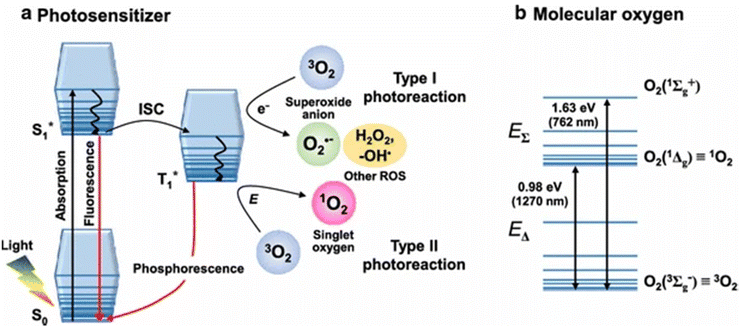
When light in the visible spectrum is absorbed by a sensitizer (often a dye molecule), it is stimulated to its first singlet state and then rapidly intersystem crosses to its first triplet state (S1, PS T1, PS) in photosensitized singlet oxygen generation (SOG). Spin-coupled triplet energy (T1, PS S0, PS)/(T1,O2 S1,O2) is exchanged between the excited PS and molecular oxygen during this phase. Subsequent developments in laser technology made it possible to directly excite the 3g state to 1g+ when excited at 1064 nm, leading to the advent of powerful YAG lasers. Rapid 3∑g−, 1g relaxation is the result of the spin-allowed transition (singlet to singlet) and the energy proximity between the two excited states, ruling out any potential physicochemical processes in this species. Conversely, the most effective, useful, and convenient method of producing 1O2 is photosensitized production.16
Considering that 1O2 is included in photodynamic therapy (PDT), a newly developed anticancer treatment that combines photoirradiation with photosensitizers (Sens), it is particularly interesting in biological and medical research.17 In photodynamic therapy (PDT), 1O2 is the first molecule formed under aerobic conditions immediately following photoirradiation. This leads to the direct oxidation of surrounding biomolecules, the shut-down of the circulatory system, and ultimately an immune or inflammatory reaction to cancer tissue. As a result, studying the generation and diffusion dynamics of 1O2 is critical for understanding the anticancer processes of PDT at the molecular level.18
Photodynamic therapy (PDT) involves using safe photosensitizing compounds, or photodynamic dyes, in combination with light to achieve a therapeutic effect. These photosensitizers tend to accumulate in cancer tissue. When they are exposed to the appropriate light for their excitation, they become activated when their concentration reaches a specific level. This activation leads to the production of singlet oxygen (1O2) through a process where an electron from molecular triplet oxygen (3O2) is transferred to a higher-energy orbital, resulting in a short-lived (fl = 106–109 s) electronically excited state of singlet oxygen (Fig. 3(a)). Singlet oxygen, with a lifespan of only 0.04 to 4 seconds, plays a crucial role in PDT. It can suppress cancer cells by blocking tumor blood vessels and enhancing the host's immune response. This suppression can have either a direct effect, such as necrosis and apoptosis in cancer cells, or an indirect effect, such as the development of blood clots (thrombus). PDT is considered a minimally invasive treatment because it selectively targets cells containing the photosensitizer while sparing the surrounding normal tissues19 (Fig. 3b).
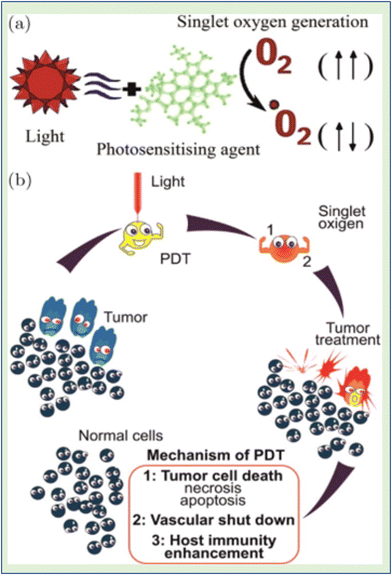 | ||
| Fig. 3 (a) Electronically excited state (singlet oxygen) in the process of 1O2 production. (b) PDT is regarded as a minimally invasive therapy that targets only tumor cells.19 | ||
3. Detection techniques and probes for singlet oxygen generation
Numerous direct and indirect approaches for 1O2 detection have been recognized over the last three decades, as shown in Fig. 4. These techniques can be used in point-spectroscopic or imaging modes.20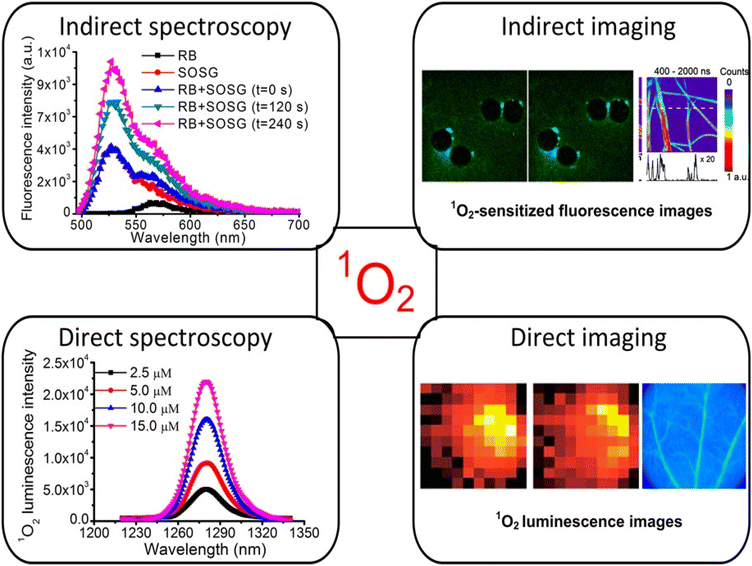 | ||
| Fig. 4 Methods of 1O2 measurement using both direct and indirect detection.20 | ||
3.1 Indirect approaches for singlet oxygen generation
Indirect methods for measuring oxygen levels rely on “reporter” or “probe” molecules that change when they interact with oxygen. Various spectroscopic techniques, such as EPR, absorption, fluorescence, and chemiluminescence, are used to detect these changes in the probe's properties. Commercial probes like diphenylisobenzofuran (DPBF), singlet oxygen sensor green (SOSG), fluoresceinyl cypridin luciferin analogue (FCLA), and 2,2,6,6-tetramethylpiperidine (TEMP) are readily accessible and commonly used for these purposes.21 The benefit is that there is high sensitivity because the “signal” from these probes is quite simple to sense. However, because the lifetimes and therefore diffusion distances of 1O2 in cells and tissue are so tiny, the PS and probe's degree of colocalization at the time of PDT is crucial for producing accurate and understandable data.One way to lessen the photodynamic effect is to limit the rivalry between the photodynamic reactions of O2 with the probe and the target cells/tissues. The fact that these probes enable wide-field imaging of 1O2 generation in tumors in vivo and quantitative studies of 1O2 generation in homogeneous systems (such as PS solution for comparing different PSs or treatment parameters) makes them still very useful in research settings.
3.2 Direct approaches for singlet oxygen generation
Direct exposure of the NIR luminescence emission of 1O2 at approximately 1270 nm may be considered the “gold standard” for PDT dosimetry;22 however, due to O2's enormously high reactivity, which results in a very low emission probability and short lifetime (<1 μs maximum studies) in typical biological microenvironments, there are significant technical tasks. It is extremely faint (1270 emission) because only 1 in 108 1O2 molecules produce luminescence. The current generation of NIR detectors on the market also do not have particularly high quantum efficiency. Several groups worldwide have developed time and wavelength resolved detection systems that are used to measure 1O2 lifetimes and quantum yields in solution, in vitro in cells, and in vivo in tissues. High-sensitivity NIR detectors, such as extended-wavelength PMT, short-pulsed (ns) laser sources with high (10 kHz) repetition rates for excitation, and electronics for time-resolved single-photon counting are some of these innovations.23–26Fig. 5 displays a common system architecture for 1O2 luminescence detection with time and wavelength resolution. The excitation light source used to observe the time-resolved 1O2 luminescence was a pulse laser. A filter wheel can be used to hold different BP filters (such as 1190, 1230, 1270, 1310, and 1350 nm) in order to provide wavelength-resolved 1O2 luminescence observations with better collection efficiency. This is in contrast to the spectral discrimination that a monochromator provides. For the purpose of measuring and counting the NIR luminescence, the best commercial PMT (H10330-45, Hamamatsu, Japan) and the fastest photon counter (MSA-300 multichannel, Becker & Hickl GmbH, Germany) were used.27
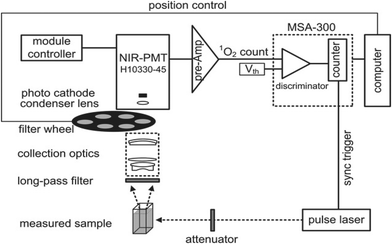 | ||
| Fig. 5 Schematic diagram of the time- and wavelength-resolved 1O2 luminescence detection system.20 | ||
Numerous additional techniques for singlet oxygen (1O2) detection are based on an examination of the interaction between singlet oxygen molecules and a component that is susceptible to the chemical. Different spectroscopic methods, such as NMR, EPR, UV-vis absorption, or fluorescence, can be used to monitor the change in a sensor molecule's structure, depending on the precise nature of the interaction.28
Several probes have been employed for singlet oxygen generation.
Some of the prominent ones include:
3.3 Spectrophotometric probes for singlet oxygen generation
An easier method for finding excited oxygen molecules is spectrophotometry. Singlet oxygen is typically trapped using a chemical probe, after which identification and quantification can be accomplished using an absorbance measurement (Fig. 6).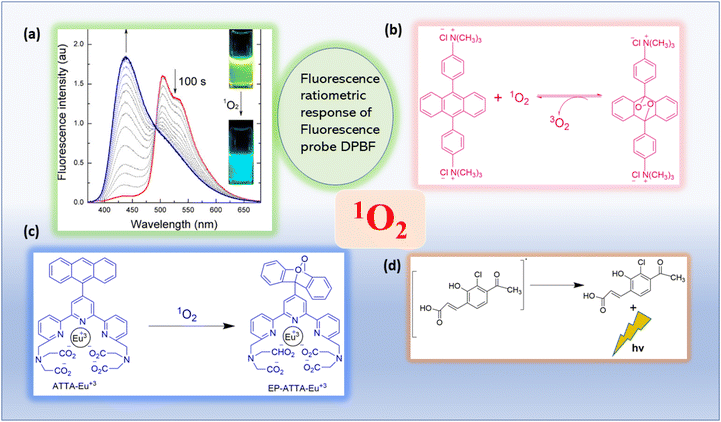 | ||
| Fig. 6 Absorbance or luminescence-based photophysical singlet oxygen detection methods are illustrated.29 (a) The inset image displays the fluorescence emission. Reproduced with permission.30 Copyright 2013, American Chemical Society. (b) UV–Vis absorption based spectrophotometric probe (bis-9,10-anthracene-(4-trimethylphenylammonium) dichloride). Adapted from ref. 31. (c) Reaction of ATTA-Eu3+ with 1O2. Adapted from ref. 32. (d) The mechanism of action of the 1O2 chemiluminescent probe SOCL upon interaction with 1O2 and its chemiexcitation route. Adapted from ref. 33. | ||
 | ||
| Fig. 7 ADPA's reactivity in the presence of 1O2. Reproduced with permission.36 Copyright 2016, Elsevier. | ||
 | ||
| Fig. 8 Schematic representation of the photosensitized oxidation of DMA using SF/SiO2 composite. This image was taken from ref. 40. | ||
 | ||
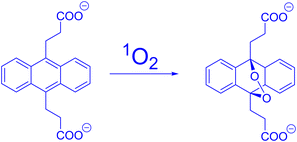
| Fig. 9 Singlet oxygen oxidation of DPA to its endoperoxide (this scheme was taken from ref. 41). | ||
3.4 Fluorescence probes for singlet oxygen generation
Since the detection of DPA derivatives depends on the measurement of absorbance, they are not very sensitive as probes. As a result, we created brand-new fluorometric probes for 1O2 to increase sensitivity. Fluorescence measurement is typically more sensitive and is therefore simpler to utilize in imaging research.The probe was stated to have a good selectivity for singlet oxygen because its response to hydroxyl radicals or superoxide was minimal.45 He et al. generated singlet oxygen via DPBF by using their developed multidipyridylanthracene-bridged organoplatinum(II) metallacycle46 (Fig. 10).
 | ||
| Fig. 10 1O2 produced by thermolyzing M-EPO reacts with DPBF. Reproduced with permission.46 Copyright 2020, American Chemical Society. | ||
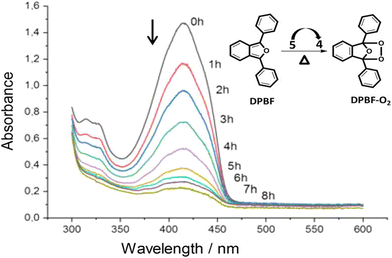
Phenazine and phenazine-containing photosensitizers KI-1 to KI-5 with varying functional groups were fabricated by Imato et al. They investigated their photophysical characteristics and photosensitizing potential in order to generate 1O2 using the trapping agent DPBF. By employing DPBF and homochromatic light (509 nm, 300 W cm2) or constant light (>510 nm, 30 mW cm2) in THF (air-saturated), the photosensitizing capacity of phenazine derivative KI-6 was evaluated. Formyl groups were nearly totally changed to hydroxyl groups via NaBH4. This was done to demonstrate that phenazine derivatives have superior photosensitizing properties (Fig. 11). The lowering caused the reaction rate (kobs) to drop from 0.144 to 0.009 min−1 and the KI-2 value to drop substantially from 0.48 to 0.05. As a result, formyl groups significantly increase the 1O2 generation of phenazine-based photosensitizers.47
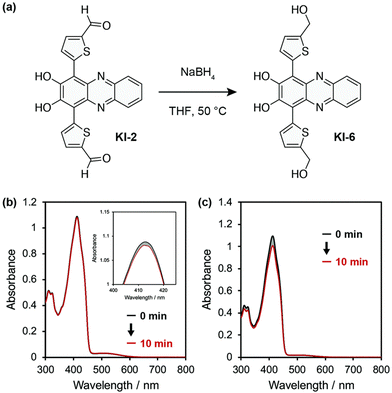 | ||
| Fig. 11 (a) KI-6 is created by reducing the formyl groups in the phenazine photosensitizer KI-2. (b) After exposure to monochromatic light (509 nm, 300 μW cm−2) in THF, the photoabsorption spectra of DPBF (Abs.@413 nm = ca. 1) are recorded in the presence of KI-6 (Abs.@509 nm = ca. 0.03). Magnified image of the peaks at 410 nm is shown in the inset. (c) DPBF (50 μM) photoabsorption spectra with KI-6 (5 μM) after continuous light exposure (>510 nm, 30 mW cm−2) in THF.47 | ||
3.5 Luminescence probes for singlet oxygen generation
Song and colleagues developed a novel europium(III) complex, [4′-(9-methyl-10-anthryl)-2,2′:6′,2′terpyridine-6,6′′-diyl] bis(methylenenitrilo) tetrakis(acetate)Eu3+, with the purpose of serving as a highly precise and targeted time-gated luminescence sensor for singlet oxygen (1O2). This new probe exhibits exceptional selectivity in reacting with 1O2 to form its endoperoxide (EP-MTTAEu3+) at an impressive rate of 1010 M−1 s−1. The reaction also results in notable enhancements in both luminescence lifetime (increasing from 0.80 to 1.29 ms) and luminescence quantum yield (rising from 0.90% to 13.8%). This probe demonstrates versatility by functioning effectively over a broad pH range spanning from 3 to 10 and exhibits remarkable water solubility. Furthermore, it boasts a significant stability constant of approximately ∼1021, making it a valuable tool for studying singlet oxygen in various research applications51 (Fig. 12).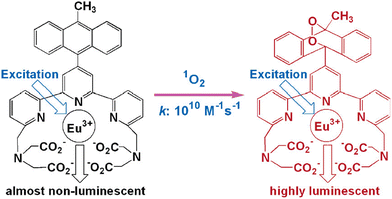 | ||
| Fig. 12 Reaction of MTTA−Eu3++ with 1O2. Reproduced with permission.51 Copyright 2006, American Chemical Society. | ||
3.6 Chemiluminescence probes for singlet oxygen generation
Chemiluminescence is considered one of the most effective methods for detecting singlet oxygen. Unlike fluorescence, chemiluminescence does not require an excitation light source, which eliminates issues related to background fluorescence and scattered light interference. This approach also reduces the risk of UV irradiation causing harm to living cells during fluorescence measurements while enhancing the signal-to-noise ratio. In recent years, the pursuit of singlet oxygen detection has led to the development of various chemiluminescence sensors. Among them, the most commonly used chemiluminescence probes for singlet oxygen include 2-methyl-6-phenyl-3,7-dihydroimidazo [1,2-a] pyrazin-3-one (CLA) and its derivatives, namely MCLA and FCLA (Fig. 13a–c). These compounds emit light on their own in the presence of singlet oxygen, making them valuable tools for singlet oxygen detection.52,53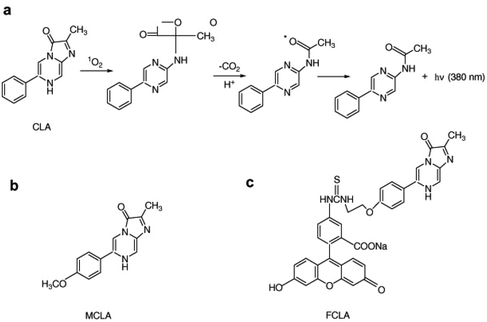 | ||
| Fig. 13 (a) Chemical structure of CLA and its reaction mechanism with singlet oxygen. Chemical structures of (b) FCLA and (c) MCLA. Reproduced with permission.49 Copyright 2010, Elsevier. | ||
4. Nanomaterials for singlet oxygen generation in PDT
Photodynamic therapy (PDT) has been instrumental towards the effective treatment of certain types of cancer. However, to date its true potential has not been explored clinically owing to several practical bottlenecks such as the dearth of photosensitizers (PSs) with high selectivity towards tumors, efficiency in ROS generation, etc.Although PSs with porphyrin structures, natural products and synthetic dyes have shown clinical applicability, inherent deficiencies including poor levels of water solubility, diminished cancer cell selectivity, ineffective photostability and 1O2 quantum yield, still pose major roadblocks.54–56
A quantum leap in terms of performance advancement of PDT has been achieved in the recent past due to technical advancements in the field of nanotechnology. Candidates including but not limited to carbon, metal and polymer nanomaterials have been dynamically established as nanomaterial-based PSs. Herein, we summarize how a wide range of nano-PSs with enhanced and distinctive photodynamic as well as photochemical properties can be deployed for augmenting singlet oxygen generation efficacy. This in turn can be further extrapolated for usage in PDT.
4.1 Metal-based nanoparticles
Recent studies suggest the possibility of directly sensitizing metal-based nanomaterials (NMs) for the generation of extremely cytotoxic 1O2. They act as a potential and viable tool for observing the therapeutic efficacy of PDT.Amongst the metal NMs, Au NMs have garnered immense research interest compared to other metal nanoparticles. Their ultrahigh chemical inertness, immense biocompatibility, tailorable optical characteristics, easy and tunable surface modifications and huge extinction coefficients make them versatile and potential candidates for PDT. Strong localized surface plasmon resonance features enable easy light absorption and subsequently generate heat, leading to effective photothermal characteristics. Additionally, their ability to be directly sensitized for SOG have also helped them to carve out a niche for themselves.
In line with cancer PDT, Au NPs stabilized via chemicals were found to be extremely effective at SOG. Several studies have reported the efficacy of chemically conjugating and modifying Au NPs for such treatment procedures. Chadwick et al. reported citrate stabilized Au NPs for SOG.57 The photogeneration process was actuated via laser irradiation, which was pulsed, and the operation progressed via equilibrated hot electrons. They also corroborated the fact that the size of Au NPs had a profound effect on the efficiency of SOG. Following this, Gao et al. fabricated DNA-driven shell–satellite Au–Ag plasmonic NPs for PDT. The fabricated assemblies exhibited chiro-plasmonic features in the visible range. Additionally, very high ROS generation efficacy was reported for the synthesized particles.58 Gold NPs conjugated with pectin and folic acid were synthesized via a facile chemical reduction method by Kumari et al. Pectin was meant to be a stabilizer and folic acid functioned as an effective cancer cell receptor.59 The presence of the folic acid component helped in the development of conjugations inside and outside the pectin matrix on top of the Au NP surface which in turn augmented the generation of 1O2. Similarly, Au NCs stabilised by 11-mercaptoundecanoic acid were reported by Hwang and his group in 2015.60 These NCs (nanoclusters) were conjugated with TAT-peptide to form nucleus targeting NCs. Gene transfection exhibited via these nanomaterials was about 81% in HeLa cells and TAT-Au NCs could sensitize SOG, which in turn augments intracellular ROS and causes cell death. Kawasaki and Miyata et al. worked along similar lines where they stabilized Au NCs with thiol and captotil.61,62 The synthesized Au NCs could emit red fluorescence and sensitize SOG. Such generation efficiency could effectively cause the death of cancer and microbial cells.
Architecture variation of the Au NPs also affected their SOG efficiency. Chan et al. developed core–shell structures with mesoporous silica shells which were coated with gold NRs (AuNR@mS). The fabrication was actuated by the seed crystal growth method. Herein ROS generation was affected by integrating Merocyanine 540 PS in the mesoporous silica core. The surface plasma resonance effect of Au NRs further augmented ROS production.63 Along similar lines, Farooq et al. optimized a nanoshell architecture with an Au shell and silica core for maximizing SOG efficiency via deploying a methylene blue (MB) PS.64 The presence of gold nanoshells greatly enhanced SOG in MB solutions by a huge margin of 320%.
Apart from core–shell architectures, Au NPs were often reported in other forms too. For example, Au nanorods, another form of Au NP, were researched by several scientists across the globe. Au NRs coated with PVP (polyvinylpyrrolidone) were reported by Zhao et al. Here, the NRs could be precisely sensitized for SOG via two-photon excitation (TPE).65 They varied the aspect ratios of the fabricated NRs and it was found that all the NRs facilitated the absorption of TPE for oxygen sensitization. It was observed that the fabricated Au NRs could effectively kill nearly 85% of HeLa cells. The same group later demonstrated the fact that the efficacy of TPE induced singlet oxygen generation could be further enhanced for aggregated NRs.66
Along similar lines, Au NRs, when coated with Lipofectamine 2000 (cationic lipid), were found to produce 1O2 when irradiated with a long wavelength of NIR light.67 Within a period of a few years, the same group led by Vankayala reported a new gold NP architecture, i.e. nano-echinus (Au NE). The seed growth technique using the surfactant double-chain cetyltrimethylammonium bromide was used to realise NP formation.68 The particles were formed with a hydrated radius of 350 ± 50 nm. It was meant to act as a dual nanomaterial in the second biological window for both PDT and PTT.
Besides, core–shell, NRs, and NEs, a new class of Au NP structure, i.e. nanocluster (NC), was delineated by many research groups. For example, polyvinyl acetate films impregnated with HAuCl4 and a radical precursor were duly irradiated with UV light for the preparation of Au nanoclusters (NCs).69 Single-molecule fluorescence spectroscopy (SMS) was employed to reveal the material's photoreactivity for the first time. The fabricated Au NCs were found to possess tailorable photochemical attributes which were beneficial for PDT. Spin multiplicity and the size of the NC were found to be major contributing factors towards the photochemical activity of the NCs.
With this idea in mind, Das et al. established the fact that the fluorescence intensity was greatly affected by the orientation of the oxygen moieties which got adsorbed onto the Au NC surface of different sizes.70 Au NCs of smaller sizes stabilized with BSA protein molecules had a superoxo orientation of oxygen, which in turn enhanced the formation of SOG in the presence of O2.
Hybrid types of NPs with Au were also explored by researchers. Saito et al. were amongst the first to detect O2− (superoxide) radicals and 1O2 on Au NP-desposited-TiO2. The samples were prepared by using 13 commercially available powders of TiO2 and subsequently SOG and superoxide radical generation were detected71 (Fig. 14). Due to the mixed crystalline phase effect of TiO2, such high generation efficiencies were observed and Zhou et al. reported the synthesis of Au (gold) nanorod/ZnO (zinc oxide) nanostructures. The nanoparticles were synthesized with different ZnO shell thicknesses. The hybrid structure was found to exhibit enhanced SOG efficacy when compared to Au nanorods or ZnO nanoparticles. The core–shell architecture was helpful at preventing the contact of Au nanoparticles with cell lines, thus reducing cytotoxicity. The SOG efficiency was found to scale linearly with the thickness of ZnO shells.72
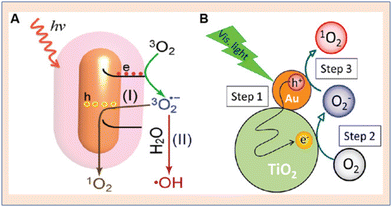 | ||
| Fig. 14 Schematic representation of singlet oxygen generation from gold nanoparticles. (A) Au nanorod. Reproduced with permission.73 Copyright 2014, Royal Society of Chemistry. (B) Au NP–TiO2 core–shell architecture for enhanced SOG. Reproduced with permission.71 Copyright 2014, American Chemical Society. | ||
It was Pasparakis et al. who used a continuous wave laser as well as pulsed laser sources and studied their respective effects on SOG from Au NPs.74 The pulsed laser was found to be more efficient at SOG. This visible difference was probably due to the different mechanistic pathways taken in sensitizing oxygen by Au NP irradiation.
Mostly Au NPs have been well reported and studied in the literature for their SOG efficacy. However, the exploration of Ag (silver) NPs for controlling SOG is still in its infancy and one of the major roadblocks in their applicability is posed by the troublesome reduction of Ag+ to Ag and the reduced stability of the NPs in an ambient environment.
Nevertheless, a novel yet facile approach was reported by Yu et al. for the fabrication of BSA-templated radically small Au NCs which demonstrated enhanced efficiency at SOG for PDT.75
On similar grounds, Aiello et al. prepared pectin coated silver-coated NPs and used riboflavin (Rb) as a sensitizer.76 This led to enhanced SOG, and the superoxide radical significantly contributed to PDT (Fig. 15a). Similarly, in 2016, it was Yu's group who reported a facile synthetic pathway for fabricating BSA-templated Ag nanoclusters which were ultrasmall in size.75 The Ag NCs exhibited enhanced SOG for treating cancer cells via PDT (Fig. 15b).
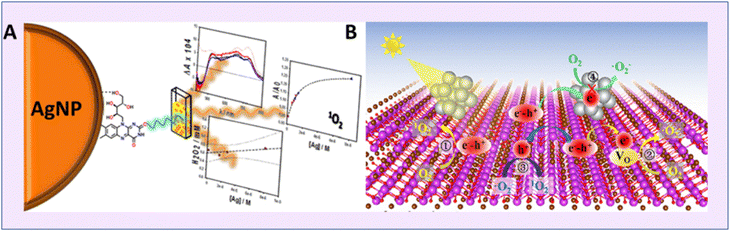 | ||
| Fig. 15 Schematic representation of singlet oxygen generation from silver nanoparticles. (A) Pectin-stabilized Ag NP. Reproduced with permission.76 Copyright 2016, American Chemical Society. (B) Defect-free Au NP from FL-BiOBr-NS for enhanced SOG. Reproduced with permission.77 Copyright 2022, American Chemical Society. | ||
4.2 Ceramic, metal oxide, and metal-sulfide-based nanoparticles
Ceramic-based NPs including metal oxide NPs have advantageous factors such as particle size, porosity, shape and easy tunable mono-dispersibility.78–80 These NPs are mostly water soluble and are highly biocompatible in nature. They are reported to be resistant towards microbial attack and their inherent characteristics such as swelling or porosity are independent of changes in pH.In this area ground-breaking research was conducted by Prasad and his group, who synthesized silica-based NPs with a diameter of 30 nm. These particles were entrapped within a PS-enabled anticancer drug.81 The SOG efficiency of these particles was found to be extremely high upon irradiation with light of 532 and 650 nm.
TiO2 is deemed to be extremely photostable and biocompatible. Such characteristics make it an ideal candidate to be NPs for PDT. Nosaka and his team reported SOG from titanium dioxide NPs in 2004.82 SOG was facilitated via laser irradiation in pulse mode using the gated photon counting method. The augmented photocatalytic oxidation of oxygen at the surface of the NPs was presumed to be the mechanism behind such efficient singlet oxygen generation (SOG).
From the perspective of cancer treatment, it was understood that NIR rays (650–1300 nm) had greater penetration depth inside tissues when compared to the usual UV irradiation. Hence it was thought that the basic integration of a light transducer into the fabricated TiO2 NPs would convert NIR into UV and hence would prove extremely beneficial for in vivo PDT.83 With this idea in mind, upconversion NPs or UCNPs have gained huge impetus for being incorporated into TiO2 NPs for treating tumors which are deep seated.
To explore UCNPs, Zhang et al. designed a nanocomposite consisting of UCNP–TiO2 core–shell type particles. A UCNP core was coated with a homogeneous layer of TiO284 (Fig. 16b). With 980 nm laser excitation, the fabricated UCNP could convert the laser source wavelength to UV and visible light, which in turn would activate the TiO2 layer for the production of reactive oxygen species. With the ability to kill nearly 50% of oral squamous carcinoma cells, these NPs emerged as potential candidates for cancer PDT.
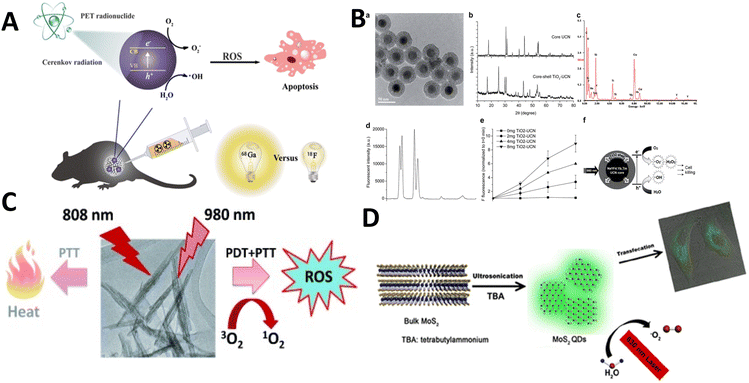 | ||
| Fig. 16 (A) Activated TiO2 NPs for PDT. Reproduced with permission.89 Copyright 2018, American Chemical Society. (B) Titania coated core-shell upconversion NPs and its suitable characterizations (a–f).84 Copyright 2014, Royal Society of Chemistry. (C) Photodynamic therapeutic nature of PEGylated W18O49 Nanowires. Reproduced with permission.87 Copyright 2013, Wiley Online Library. (D) Metal sulphide (MoS2) quantum dots for PDT. Reproduced with permission.90 Copyright 2016, American Chemical Society. | ||
Along similar lines, Wu and his team fabricated composites decorated with folic acid and the composites were abbreviated as FA-Gd-Si-Ti based nanocomposites.85 Such systems were designed for dual application of MRI (magnetic resonance imaging) and PDT. The presence of folic acid helped in selective binding at folate-based receptors on the cancerous cells thus helping the NPs to gain smooth entry into the cells. With this, 88.6% reduction in the growth inhibition ratio was observed for the tumors. Analogous NPs were synthesized by Lin and his group termed the nanocomposites as ((NaYF4:Yb3+,Tm3+@NaGdF4:Yb3+)@TiO2). These NPs were to be used for imaging in vivo cancer PDT.86 The hydrophilic PVP polymer assisted protocol was adopted for coating anatase grade titanium dioxide on top of the Yb/Tm-co-doped cores of the UCNPs. Endocytosis was the main mechanism for the take up of NPs by the cancer cells. This would subsequently produce ROS and lead to cell apoptosis.
Although TiO2 is the major focus for metal oxide-based NPs, tungsten and bismuth oxyhalide (BiOCl) have also been explored by researchers. Tungsten oxide nanowires (NWs) were reported for cancer PDT by Hwang et al. PEGylated W18O49 NWs were synthesized which could absorb in the NIR region (up to 1200 nm)87 (Fig. 16c). SOG via these nanoparticles was exemplary when irradiated with 980 nm NIR light. ROS levels were found to be elevated inside HeLa cells when treated with such NPs.
BiOCl possesses a tailorable band gap which facilitates its usage as an emerging photocatalyst that can be well exploited for PDT. Xu et al. prepared BiOCl nanoplates and nanosheets via a hydrothermal technique and functionalized them with PEI.88 These NPs were found to significantly reduce MCF cell viability: 70% and 35% for nanosheets and 35% for nanoplates. Their unique morphological features combined with their intrinsic band gaps were responsible for such efficient PDT performance.
Metal sulphide based NPs also contributed to the generation of reactive oxygen species. MOS2 quantum dots were synthesized through a sonication approach based on a tetrabutylammonium-assisted procedure90 (Fig. 16d). When irradiated with 630 nm light, the fabricated molybdenum sulphide NPs were found to be much more efficacious at SOG than any other organic photosensitizer.
4.3 Porous-framework, carbon-based and hybrid nanoparticles
Distinct and tuneable optical as well as physicochemical properties have made carbon nanomaterials (NMs) an emerging potent class of materials for biomedical applications. Enhanced photostability, effective biocompatibility, chemical resistance, and tailorable fluorescence over a wide range of the visible to NIR-II spectrum make these NMs ideal candidates for in vivo molecular level imaging. Additionally, in accordance with recent findings it has been observed that Buckminsterfullerenes91 and GQDs can be efficiently deployed as NPs for PDT which in turn could sensitize the generation of ROS.Safar and his team of researchers came up with hybrid systems consisting of porphyrins and chirally enriched single walled carbon nanotubes (6,5-SWCNT). These systems proved to be ideal candidates for PDT.92 The reported system was found to be much more efficient in terms of SOG when compared to neat, isolated porphyrins or E-SWCNT. This augmented performance stemmed from energy transfer from E-SWCNT to porphyrin which was finally transferred to O2 molecules. Gao et al. prepared carbon nanodots which were passivated by using cetyltrimethylammonium ammonium bromide (CTAB-CDs). The optical properties of the nanodots were excellent and they were prepared by hydrothermally treating fullerene.93 The CDs were found to significantly enhance and amplify the luminol–H2O2 chemiluminescence based signals. The CTAB-CDs could catalytically decompose hydrogen peroxide into OH˙ and O2˙−. Subsequently, they recombined forming an avalanche of singlet oxygen on the surface of the fabricated CTAB-CDs.
Similarly, Knoblauch and his team reported the synthesis of bromine functionalized CDs.94 These CDs were simple and inexpensive byproducts of hydrogen combustion. Metal-enhanced or amplified photosensitization of singlet oxygen species were reported with the usage of these brominated dots.
Blacha-Grzechnik et al. tailored a dyad structure via adopting electrochemical polymerization, thus forming layers of covalently conjugated photoactive poly(terthiophene) and fullerene.95 SOG via the synthesized moieties was tested in the presence of a certain singlet oxygen quencher (2,3,4,5-tetraphenylcyclopentadienone) via a process known as α-terpinene oxidation.
Apart from fullerenes, graphene quantum dots have also gained immense momentum for PDT. GQDs have the innate ability to induce a synergistic effect of apoptosis as well as autophagy which in turn could effectively kill cancer cells.96 GQDs generate oxidative stress and are highly efficient at SOG, thus causing the death of human glioma cell lines.
Kuo and his group fabricated TPE based GQDs with the help of a shearing reaction (ultrasonic). Such GQDs under TPE excitation for a mere 15 s produce huge amounts of singlet oxygen as well as O2 molecules, thus eliminating both Gram positive and Gram negative bacteria.97
Chemically reduced GOQDs (rGOQDs) were also found to enhance and augment the ROS generation capacity yielding high amounts of 1O2, O2−, and H2O2 under irradiation of white light.98 These rGOQDs possessed a greater number of electron–hole pairs owing to a low band gap and valence band compared to GOQDs. This resulted in a significant enhancement of ROS generation and higher efficacy for PDT.
Wang and his team functionalized and modified GQDs with adenine99 and Xing's group covalently conjugated a rhodamine derivative (TRITC) along with UCNP-GQDs.100 Both the modified GQDs were found to exhibit efficient in vivo PDT by SOG.
In the recent past researchers reported the usage of graphitic carbon nitride (g-C3N4) nanosheets for SOG in cancer PDT. Such nanoparticles showed significantly higher ROS generation when irradiated with LED and induced the death of HeLa cells.101 Just like the nanosheets, HA modified graphitic hollow C3N4 nanospheres showed immense potential for stimuli-responsive chemotherapy as well as cancer PDT.102 From this perspective Feng and his group conjugated UCNPs with g-C3N4 for the conversion of NIR into UV/Vis light that would match well with the absorption of g-C3N4 alone.103 This could kill HeLa cells via an in vitro strategy.
Besides the techniques exemplified above, reticular chemistry has emerged as a promising area to develop nanomaterials for PDT. Porous and crystalline frameworks including covalent organic frameworks (COFs) and metal–organic frameworks (MOFs) represent a class of nanoagents that have proved to be extremely beneficial in the recent times.104 Enhanced biocompatibility, tailorable porosity, design and structural flexibility, all add up to make these framework structures promising materials for PDT.105 A Hf–porphyrin based nanoscale MOF was reported by Lin and his group. The MOF was composed of structurally regular moieties of Hf–oxo clusters and 5,15-di(p-benzoato)porphyrin bridging ligands.106 The thin and uniform geometry of the material helped SOG through which cytotoxic effects were generated for the cancer cells. Similar MOFs were prepared by the same group, wherein the ligand H2DBP was reduced to 5,15-di(p-benzoato)chlorine.107
Lang and his team reported the fabrication of hexagonal PCN-222 in 2017. The MOF prepared could significantly induce apoptosis of tumor cells108via ROS generation upon visible light irradiation.
At the same time, a nanoscale MOF was reported by Tang et al. The nano-MOF was composed of zinc-metalated 5,10,15,20-tetrakis(4 methoxycarbonylphenyl) porphyrin and Cu2+. The MOF termed NP-1 could act as a controlled PS for SOG via triggering of H2S.109 Motivated by this, numerous research groups around the globe have made significant contributions towards designing nanoscale MOFs for cancer PDT.110–118
In tandem with MOFs, nanoscale COFs also play a major role in cancer PDT. Although the research in this direction is still in its infancy, several groups have made notable progress in this area. In general porphyrin and its derivatives are usually utilized for SOG and they have been extensively reported for PDT. However, their poor solubility and agglomeration effects hinder their ready usability. To remedy such issues, COFs can be designed using a porphyrin derivative as the starting material.
Xie and his team fabricated a nanoscale composite (MOF@POP) termed UNM. It was made by the controlled growth of an imine-linked COF on the surface of an amine-functionalized MOF.119 The system was found to be highly efficient in terms of SOG and thus was deployed for PDT. Deng et al. made use of species which were intrinsically incapable of SOG for the construction of a photosensitive COF (4,4′,4′′-(1,4-phenylene)-bis([2,2′:6′,2′′-terpyridine]-5,5′′-dicarbaldehyde)). The ROS generation efficiency of the designed COF was found to be high owing to its band gap of 1.96 eV which linearly overlapped with the band gap of superoxide radicals.120 Similarly, Zhang et al. synthesized an imine-based COF termed COFTTA–DHTA which had exceptional PDT efficacy via remodelling the extracellular matrix.121
4.4 Polymer-based nanoparticles
Contemporary research pertaining to PDT has gained a huge impetus in deploying biocompatible and biodegradable NPs based on polymeric materials.122 Such polymeric species have the ability to selectively target specific organs and hence suitably control the release of photosensitizers. Emulsion as well as interfacial polymerizations have been mainly reported for the preparation of polymer-based nanoparticles. These nanoparticles can be nanocapsules or nanospheres, solely depending on the synthesis pathway adopted. Phthalocyanines are one of the most important and reported materials for PDT. However, one of the major bottlenecks associated with them is their inherent affinity towards aggregation which significantly reduces the SOG efficacy. As a remedy, several modifications have been carried out.In 1991, tetrasulfonated zinc phthalocyanine (ZnPcS4) and aluminium naphthalocyanine were synthesized and entrapped in nanocapsules made of poly(isobutylcyanoacrylate) or poly(ethylbutylcyanoacrylate).123 Interfacial polymerization was employed to carry out the fabrication. The nanomaterial recorded near 100% efficiency for PS encapsulation. A novel “salt-out” method was employed for formulating a second-generation PS, i.e., hexadecafluoro zinc phthalocyanine (ZnPcF16) and subsequently encapsulating them in PEG-coated-PLA NPs.124 With very low percentages of loading, high efficiency for PDT was observed for the material. Moreno and his team used polyacrylamide (PAA) and amine functionalized PAA as a matrix and loaded it with disulfonated 4,7-diphenyl-1,10-phenanthroline ruthenium [Ru(dpp(SO3)2)3]. Such particles were able to exhibit high efficiency in SOG.125 In a slightly different work, PS-loaded NPs were synthesized using a precursor which contained the PS unit as a copolymer. The copolymer, i.e. poly(phthalocyanine-co-sebacic anhydride) (Pc-SA), was formulated using the monomeric units of sebacic anhydride and Zn(II) phthalocyanine.126 Here, with the PS loading being comparatively higher, quenching and entrapment of produced SOG is avoided, just leading to high SOG and hence greater usability in cancer PDT.
5. Utilizing conjugated polymers, copolymers, and nanocomposites, with a focus on enhancing singlet oxygen generation through dye-based and near-infrared (NIR) conjugated nanopolymers
Researchers are actively investigating novel approaches to photodynamic therapy (PDT), including the use of nanocomposites, copolymers, and conjugated polymers to increase the production of singlet oxygen. The strategic combination of dye-based and near-infrared (NIR) conjugated nanopolymers is the main focus, to increase the production efficiency of singlet oxygen.127 This strategy, which demonstrates the development of PDT methodologies through the design and use of cutting-edge polymeric materials, is a sophisticated and focused approach. The synergistic combination of these elements shows a promising path toward the creation of more specialized and effective treatments for a range of illnesses, especially when it comes to the treatment of cancer.One important family of organic molecules is conjugated polymers, which comprise considerable conjugated backbones and useful side chains.128,129 Delocalized electrons migrate along the conjugated framework of conjugated polymers, allowing the energy collected by the backbone to be converted into optical signals via electronic transitions. This results in high fluorescence quantum yields and remarkable light-harvesting properties.
Developing dual-modal therapeutic systems for chemotherapy and PDT, the majority of conjugated polymers currently utilized as PSs are physically combined with medications. Yet, unreliable drug leakage could endanger healthy tissues more severely and reduce the effectiveness of the combined anticancer effects.130 In addition to improving the effectiveness of ROS generation, CPs can play beneficial roles in disease therapy. For example, a large number of side chains are commonly linked to the CP backbones, which may facilitate drug dispersion and responsive drug release. Another illustration is the photothermal effect, whereby CPs frequently function as good photothermal agents due to their large molar extinction coefficients, provided that the near-infrared (NIR) absorption is regulated. For this reason, synergistic therapy in conjunction with photothermal therapy (PTT) or chemotherapy is another strategy to improve CP-based PDT.
Therefore, we discuss here the most recent studies of singlet oxygen generation for PDT employing conjugated polymers, copolymers, nanocomposites, or nano-conjugated polymers. Numerous studies reported in the literature are provided here.
Battah et al.131 reported the synthesis of hydroxypyridinone and 5-aminolaevulinic acid conjugates while Wang et al.132 synthesized hemoglobin (Hb) conjugates with polymeric micelles formed by triblock copolymers of poly(ethylene glycol)-block-poly(acrylic acid)-block-polystyrene (PEG-b-PAA-b-PS) through chemical conjugation. In vitro, HeLa cells were more susceptible to photocytotoxicity and increased the generation of 1O2 by the Hb-coupled photosensitizer carrier (Fig. 17).
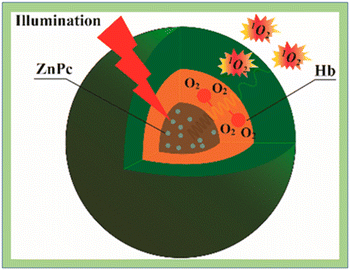 | ||
| Fig. 17 Singlet oxygen generation in ZnPC loaded HbMs. Reproduced with permission.132 Copyright 2015, American Chemical Society. | ||
Yesilgul et al.10 synthesized an erythrosine–luminol conjugate for singlet oxygen generation with chemical excitation. Spada et al.133 created conjugated poly(9,9-dioctylfluorene-altbenzothiadiazole) (F8BT) doped with platinum octaethyl porphyrin (PtOEP) which was able to efficiently generate singlet oxygen. According to Cheng et al.,134 mesoporous silica nanoparticles (MSNs) were functionalized with Pd–porphyrins to produce MSN–PdTPP, which might be used as phosphorescence probes for oxygen detection and tomography. Poly[(9,9-dioctylfluorenyl-2,7-diyl)-alt-co-(1,4-benzo-{2,1′,3}-thiadiazole)] was developed by Chang et al.135 Porphyrin was integrated into the π-conjugated backbone to enable photodynamic treatment that was sensitive to polymer dots.
Islam et al. synthesized a nano-formulation of 5-aminolevulinic acid (5-ALA) for tumour-targeted photodynamic treatment. P-ALA was obtained by coupling 5-ALA with the biocompatible polymer N-(2-droxypropyl)methacrylamide (HPMA) via the hydrazone link. P-ALA functions as a nanoscale molecule in an aqueous solution due to its average size of 5.5 nm. P-ALA was discovered to be non-cytotoxic up to 0.1 mg ml−1; but, at an IC50 of 20–30 g mL−1, it was observed to cause a significant degree of cell death when exposed to light. Most significantly, they found that 5-ALA, which profited from its nano-size by utilizing the enhanced permeability and retention (EPR) effect, was less likely to significantly accumulate in tumours than P-ALA. According to the authors, P-ALA exposure greatly enhanced in vivo anticancer activity without exhibiting any observable negative effects. As such, they hope to employ P-ALA as a nano-engineered photosensitizer for photodynamic treatment of cancer.136
A modest number of red-emitting TBT units doped with poly[9,9′-bis(2-(2-(2-bromoethoxy) ethoxy)ethyl)fluorene-2,7-ylene vinylene] was used by Huang et al. to develop novel zwitterionic red-emitting water-soluble conjugated polymers P1′ and P1′′. Through the use of TPE FRET, red emission from the poly(fluorene-2,7-ylene vinylene) segments (donor) to the TBT units (acceptor) was detected for both P1′ and P1′′. The authors reported that fluorescence resonance energy transfer (FRET) was used by P1′ and P1′′ to accomplish TPE red emission. They discovered that P1′′ had a significantly greater 1O2 quantum yield (61%) than P1′, which was just 39%. They proposed that zwitterionic P1′′ had been verified to exhibit promise as a superior TPE red-emitting photosensitizer for PDT guided by two-photon imaging.137
Shen and colleagues synthesised tetraphenylporphyrin (TPP) doped poly[9,9-dibromohexylfluorene-2,7-ylenethylene-alt-1,4-(2,5-dimethoxy)phenylene] nanoparticles (PFEMO). In living cancer cells, conjugated polymer nanoparticles doped with photosensitizers exhibit improved singlet oxygen generation and potent photodynamic treatment efficacy. The two-photon light-harvesting complex and the host material were made from the conjugated polymer PFEMO. Under one- and two-photon stimulation, enhanced singlet oxygen generation was reported138 (Fig. 18).
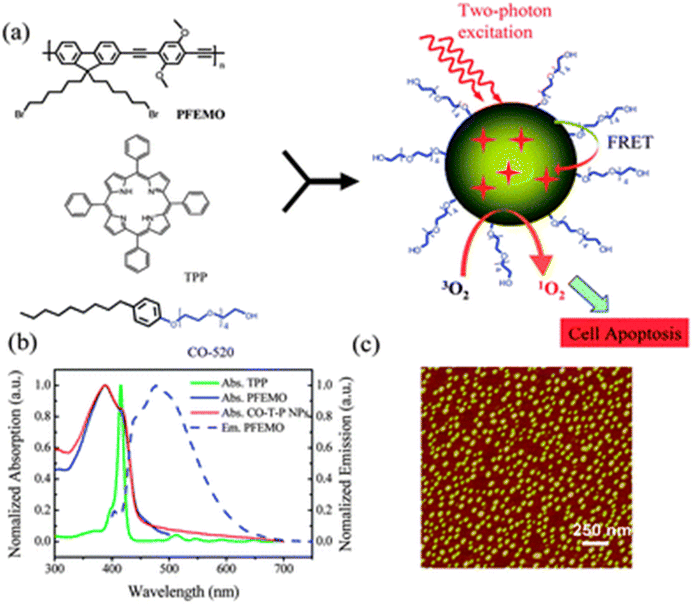 | ||
| Fig. 18 (a) Diagram showing the process of conjugated polymer nanoparticle formation for two-photon photodynamic therapy; (b) normalized absorption spectra of TPP, PFEMO and CO-T-P NPs (solid lines), and emission spectra of PFEMO (dashed line); (c) AFM imaging of CO-T-P NPs on a mica substation. Reproduced with permission.138 Copyright 2011, Royal Society of Chemistry. | ||
Ren et al. carefully planned and developed a new fully organic UIM of THPP-4PMMA-b-4P(PEGMA-co-APMA)@NIR-800 having a dual PTT/PDT purpose. MMA and PEGMA/BAPMA were sequentially polymerized using the photocontrolled BIT-RDRP technique after four-armed porphyrin-based initiators were first created. Due to the ability of ketocyanine to undergo photothermal conversion and the release of singlet oxygen from the porphyrin segment, the produced star-shaped block copolymer could produce UIMs with a diameter of approximately 13.1 nm in water at a concentration lower than CAC. Through PDT and/or PTT of the UIMs, excellent biocompatibility, minimal cytotoxicity, and great tumour therapy were revealed. More effective behavior was suggested through utilizing the PDT/PTT synergistic effect.139
A three-dimensional (3D) organic–inorganic photosensitizer made of a stiff POSS cage with dodecyl alkyl chains on its globular exterior and a porphyrin core was claimed to have been synthesized by Bao et al. Then, using a co-precipitation technique, PorPOSSC12 was encapsulated in the polymer matrix using two different types of semiconducting polymers with various fluorescence colours. In addition to efficiently preventing the photosensitizer from aggregating, the hydrophobic semiconducting polymer backbones can also be partially separated by the 3D nanostructure of POSS and the covalently attached to dodecyl alkyl, which helps to lessen fluorescence quenching caused by aggregation. The scientists speculate that as a result, the PorPOSSC12-doped SPNs may function effectively as photodynamic nanoagents for the treatment of cancer and as fluorescent probes for bioimaging140 (Fig. 19).
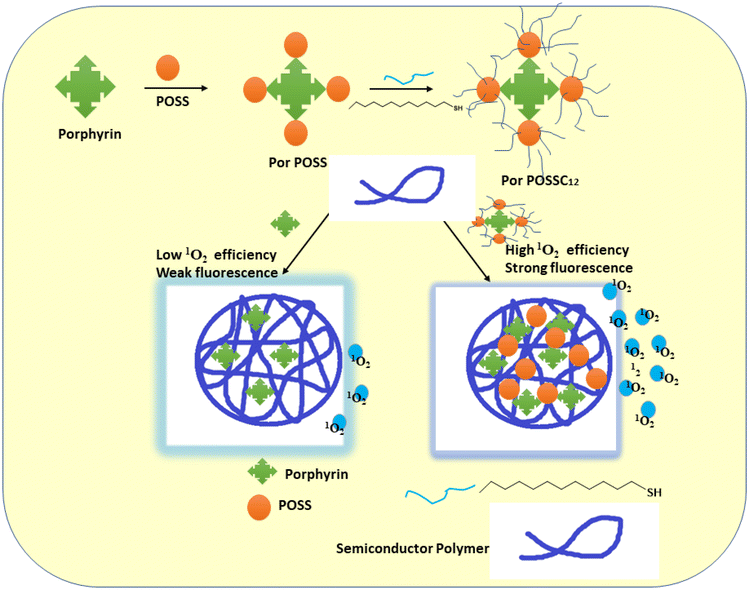 | ||
| Fig. 19 A schematic representation of the PorPOSSC12-doped SPN architecture, together with the way that fluorescence imaging is improved and its suitability for application in PDT is increased (this image was taken from ref. 140). | ||
Tetraphenylethylene-1-phenyvinyl-pyridine-phenylboronic acid (TPEPy-BA) and tetraphenylethylene-1-phenyvinyl-pyridine-phenylboronic acid pinacol ester (TPEPy-BE), two novel aggregation-induced emission-based photosensitizers, were developed and synthesized by Sauraj and his colleagues. In order to increase the photosensitizers’ water stability and cellular take up, they were also encapsulated within a copolymer (DSPE-PEG). The synthesized photosensitizer nanoparticles established a high intracellular reactive oxygen species generation efficacy. They observed that, when exposed to white light, tetraphenylethylene-1-phenyvinyl-pyridine-phenylboronic acid pinacol ester nanoparticles significantly outperformed tetraphenylethylene-1-phenyvinyl-pyridine-phenylboronic acid nanoparticles in terms of the photodynamic ablation of MCF-7 cells.141
Wang et al. rationally developed and produced mono- and tetra-nuclear Ir(III) complex–porphyrin conjugates, with [TPP-4Ir]4+ showing blatant aggregation-induced emission (AIE) properties. PSs made of Ir(III) complex–porphyrin conjugates were successfully achieved as nanoparticles (NPs). Moreover, [TPP-4Ir]4+ NPs display strong cytotoxicity against cancer cells, decent biocompatibility, high 1O2 generation capacity, low half-maximal inhibitory concentration (IC50) (0.47 106 M), and improved cellular take up when exposed to white light. The claim that transition metal complex PSs have exciting potential applications in medicine is expanded by that work142 (Fig. 20).
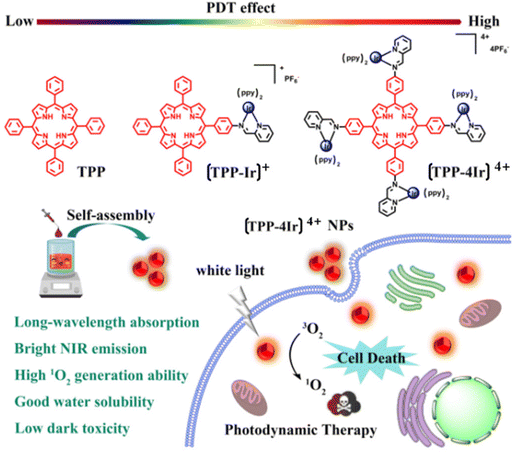 | ||
| Fig. 20 Chemical structures of TPP, [TPP-Ir]+ and [TPP-4Ir]4+ and design principle of using [TPP-4Ir]4+ as PS for PDT.142 | ||
Chen and coworkers developed aza-boron-dipyrromethene (Aza-BODIPY) with 2,6-diiodo-dipyrromethene (2,6-Diiodo-BODIPY) to create a near-infrared (NIR) chemical agent. Aza/I-BDP nanoparticles (NPs) were formed by enclosing them in DSPE-mPEG5000, an amphiphilic biocompatible copolymer. They noticed that the produced NPs showed an amazing photostability, a high quantum yield (ΦΔ = 59%) of 1O2 generation, and good photothermal conversion efficiency143 (Fig. 21).
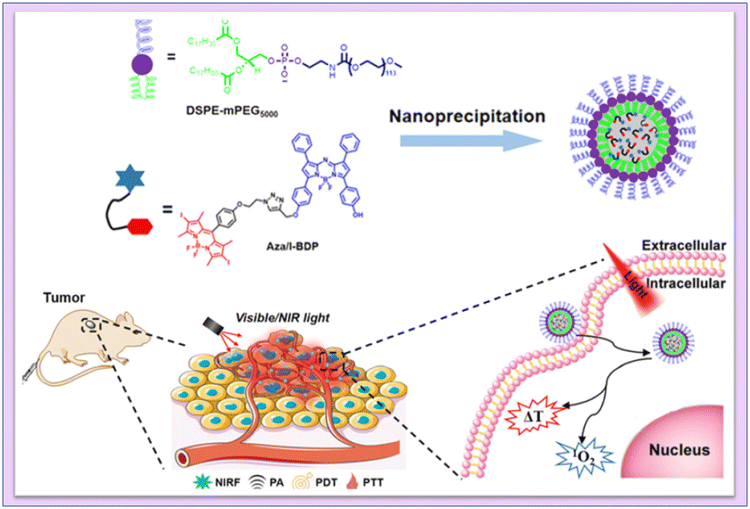 | ||
| Fig. 21 Schematic illustration of the mechanism of fluorescence/PA imaging-guided tumor PDT/PTT by Aza/I-BDP NPs. Reproduced with permission.143 Copyright 2023, American Chemical Society. | ||
Tetratroxaminobenzene porphyrin-loaded biodegradable silk nanospheres (NSs) were synthesized by Cheng et al. using the emulsion–solvent–evaporation method. In order to achieve a greater photoconversion efficiency and a quicker synthesis of active oxygen, TAPP was chosen as the fundamental unit of the photosensitizer and then allowed to self-assemble into biodegradable silk. Furthermore, these self-assembled structures display increased singlet oxygen-generating capability and PDT performances, according to the authors144 (Fig. 22).
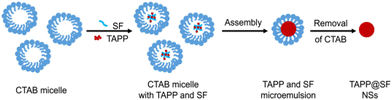 | ||
| Fig. 22 Schematic representation of preparing TAPP NSs. Reproduced with permission.144 Copyright 2023, Elsevier. | ||
Vinita et al. designed a surface charge augmented nanoconjugate system that decreased Pi3K/AKT indicating and decreased cell survival. To create the nanoconjugate system, the S–H group in triphenylphosphonium (TPP) was conjugated with both cationic and anionic AuNTs. The surface charge increased AuNTs combined with TPP exhibit cytotoxicity under 5-ALA-based PDT. Due to the generation of ROS species and the deregulation of mitochondria, DNA damage causes the induction of apoptosis. Together, 5-ALA and PDT with gold nanoconjugates help breast cancer cells undergo apoptosis. The created gold nanoconjugate system, according to the author, is an effective anti-cancer medication that targets mitochondria and causes cellular apoptosis in breast cancer.145
A unique triarylamine (TPA)-modified hemithioindigo (HTI)-based aggregation-induced emission (AIE) photosensitizer, 6Br-HTI-TPA-OMe, was successfully developed by Wang et al., who found that reversible control over 1O2 generation was possible due to the covalent link between the triarylamine AIE photosensitizing moiety and the HTI switch unit. The nanoparticles (NPs) synthesized from amphiphilic phospholipids also displayed photochromic activity in water. After receiving 520 nm light from an LED, the Z-NPs started to produce 1O2, but when switching to the E-NPs, viable energy transfer prevented 1O2 production. The authors suggested that reversible Z/E isomerization could photocontrol 1O2 production. The ability of 6Br-HTI-TPA-OMe to function as a photoswitchable AIE photosensitizer was confirmed by an in vitro anti-tumor experiment146 (Fig. 23).
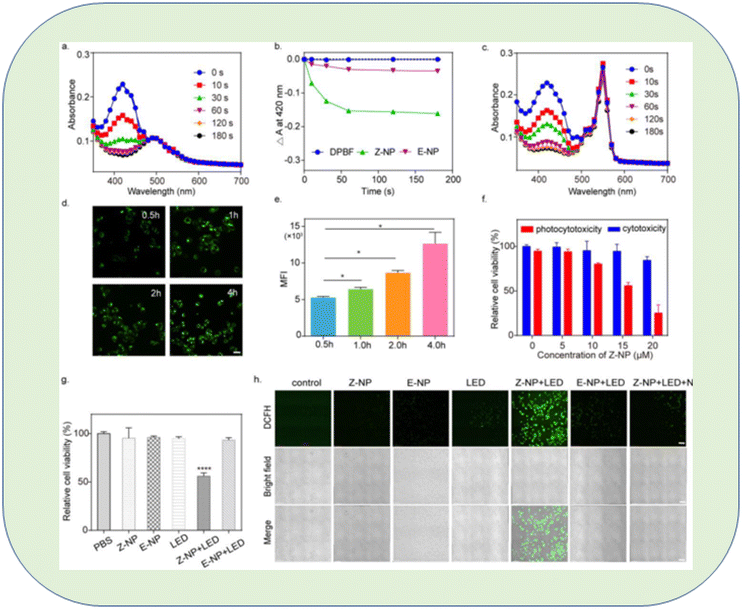 | ||
| Fig. 23 In vitro evaluation of 6Br-HTI-TPA-OMe NPs for reversible control of 1O2 generation. (a) Absorption spectra of DPBF (30 μM) with Z-NP (Z isomer concentration: 10 μM) in water at different irradiation times. (b) Photodegradation rate of DPBF with or without different nano-photosensitizers. (c) Absorption spectra of DPBF (30 μM) with RB (10 μM) in water at different irradiation times. Real-time take up images (d) and flow cytometry MFI value (e) of Z-NPs (15 μM) in 4T1 cells at different time points (0.5 h, 1 h, 2 h, 4 h). (f) MTS assay of Z-NPs at various concentrations co-cultured with 4T1 cells with or without LED irradiation (LED: 520 nm, 40 mW cm−2, 5 min). (g) MTS assay of 4T1 cells incubated with different treatments. (h) Confocal images of 4T1 cells stained with DCFH-DA after different treatments. The treatments included PBS, Z-NPs, E-NPs, LED, Z-NP + LED, E-NP + LED, Z-NP + LED + NaN3 (Z isomer concentration: 15 μM, NaN3 concentration: 20 mM, 520 nm, 40 mW cm−2, 5 min, scale bar: 100 μm). Significant differences between the groups are labelled for * p < 0.05, and **** for p < 0.0001.146 | ||
Zhao et al. synthesized a conjugated oligomer called UF-TTOEH-2Cl, which had a photoactive acceptor (A)–donor (D)–acceptor (A) structure. They used UF-TTOEH-2Cl to create a folic acid-functionalized amphiphilic block copolymer named FA-PEG-PBLA10 through a nanoprecipitation technique. This material exhibited a high photothermal conversion efficiency of 42% and a singlet oxygen (1O2) quantum yield (ΦΔ) of 17.2% when exposed to an 880 nm laser. Furthermore, they demonstrated the effectiveness of photodynamic therapy (PDT) and photothermal therapy (PTT) synergistic treatment for malignancies using UF-TTOEH-2Cl-based nanoparticles in an in vitro setting. They tested this approach on HeLa and SiHa cells, which are commonly used as models in cancer research. This research suggests that these nanoparticles (UTNPs) have potential applications in combined PDT and PTT for cancer treatment147 (Fig. 24).
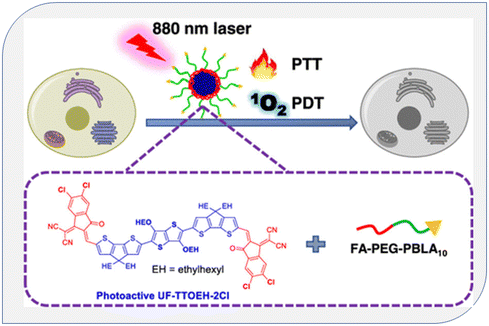 | ||
| Fig. 24 Diagram of singlet oxygen generation from conjugated polymers in PDT. Reproduced with permission.147 Copyright 2023, American Chemical Society. | ||
Diaxial ethyl vanillin, a bioactive molecule substituted for silicon phthalocyanine, was designed by Karanlak et al. Photophysicochemical and sono-photochemical tests were carried out. In contrast to the photodynamic application, the sono-photodynamic application, which combines the synergetic action of light and ultrasound together as a stimulation approach, increased singlet oxygen production through photochemical measures from 0.50 to 0.81. It is therefore appropriate as an excellent sensitizing agent in both PDT and SPDT given the molecule's photostability and high singlet oxygen production ability for both techniques.148
In a similar way, pyrazoline-fused axial silicon phthalocyanine (HY-SiPc), peripheral zinc phthalocyanine (HY-ZnPcp), and non-peripheral zinc phthalocyanine (HY-ZnPcnp) were all designed and synthesized by Yalazan and colleagues. They looked at and researched the roles that silicon phthalocyanine compounds with methoxylated pyrazoline groups played in both photophysical and photochemical processes. The authors suggested that, the HY-ZnPcp molecule, ΦΔ 0.73%, which produced the most singlet oxygen among the three produced phthalocyanines (HY-ZnPcp, HY-ZnPcnp, and HY-SiPc), could be regarded as a photosensitizer candidate for PDT.149
Liu et al. used an innovative reversible addition–fragmentation chain transfer (RAFT) polymerization method to create a thermosensitive star-shaped double hydrophilic polymer called THPP-(PNIPAM-b-PMAGA)4. This polymer consists of poly(N-isopropylacrylamide) (PNIPAM) and poly(methylacrylamide glucose) (PMAGA) block copolymers. The values of ΦΔ for different molecular weights of THPP-(PNIPAM-b-PMAGA)4 in dimethylformamide (DMF) were found to be 0.41 and 0.37. In summary, THPP-(PNIPAM-b-PMAGA)4 holds promise as a potential photodynamic therapy (PDT) reagent. It can be used as a photosensitizer for PDT. However, it is worth noting that this compound exhibits significant toxicity when exposed to light in the presence of HeLa cells. This research paper presents a novel approach that combines chemotherapy and PDT for tumor targeting, aiming to advance cancer treatment strategies.150
In order to enhance phototherapy and enable real-time 1O2 self-detection and O2 self-supply, Yang et al. developed a nano-sensor known as PAPD, which blended dual-channel ratiometric sensing with oxygen-augmenting approaches. An anthracene-based 1O2 sensitive fluorophore (DPA) was encapsulated in porphyrin metal–organic frameworks (PCN-224) and gold nanoparticles (AuNPs) were used as nano-enzymes to create the PAPD nano-sensor. The Au–S link was used to cover polyethylene glycol thiol (PEG-SH). PCN-224 is utilized as a photosensitizer and 1O2 reference fluorescence (FL) agent. After PCN-224-induced 1O2 is synthesized, the dual-channel ratio-metric FL signal of PAPD generates a dynamic, sensitive, and accurate 1O2 visualization and offers real-time therapeutic data related to the therapeutic procedure. Furthermore, the catalase-like activity of PAPD produces O2in situ by intracellular H2O2 oxidation and increases the 1O2 yield, which increases the effectiveness of destroying tumour cells. They claimed that the rationally designed nanosensor PAPD offered a paradigm for clinically precise hypoxic tumour treatment and real-time therapeutic evaluation151 (Fig. 25).
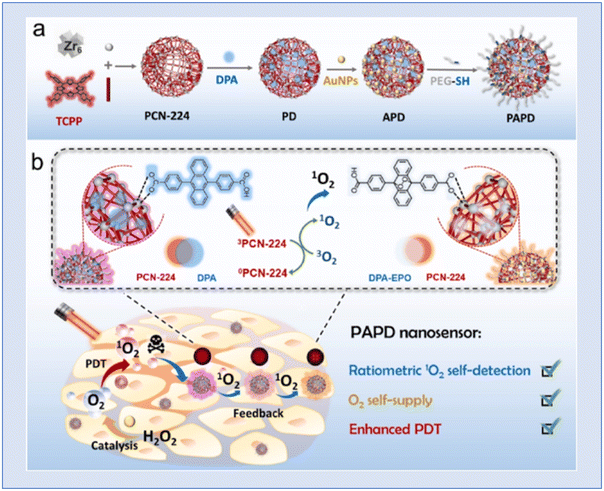 | ||
| Fig. 25 Representation of (a) PAPD preparation and (b) multifunctional integration of ratiometric 1O2 self-detection, O2 self-supply and enhanced PDT. Reproduced with permission.151 Copyright 2023, Elsevier. | ||
Tan et al. developed a synthesis method to create a conjugate called HES-SeSe-DOX with a high loading of the anticancer drug DOX. These conjugates, when accumulated in the form of nanoparticles (NPs) along with Ce6 (a photosensitizer), can respond to stimuli that affect diselenide bonds. When these bonds are disrupted, it leads to the cascade release of DOX and Ce6. Simultaneously, the seleninic acid formed from the broken diselenide bonds can work in conjunction with DOX and Ce6 to enhance the inhibition of tumor cell growth. These HES-SeSe-DOX/Ce6 NPs, which have received limited attention from researchers thus far, hold the potential to be a novel class of nanomedicine. To assess whether these NPs can enhance chemo-photodynamic anticancer therapy, comprehensive investigations of their in vitro and in vivo performances have been conducted. This research aims to explore their effectiveness as a promising approach for cancer treatment.152
The evolution of conjugated polymers for photodynamic therapy (PDT) constitutes a dynamic trajectory in cancer treatment research. Initially recognized for their advantageous properties, such as high fluorescence efficiency, remarkable photostability, and potent light absorption, conjugated polymers have emerged as promising candidates for PDT applications. Researchers have systematically delved into the development of these polymers, employing sophisticated chemical synthesis techniques and flexible polymer architectures to engineer variants with heightened singlet-oxygen generation capabilities, a crucial factor for the success of PDT. Beyond isolated examples, the overarching trend involves the strategic design of conjugated polymers tailored for optimal performance in PDT. The incorporation of photosensitizers into conjugated polymer nanoparticles is particularly noteworthy, as it enhances singlet-oxygen formation through efficient energy transfer. Moreover, the integration of nanotechnology has opened new avenues, offering innovative solutions to enhance photosensitizer performance and address challenges in cancer PDT. In summary, the development of conjugated polymers for PDT reflects a comprehensive and systematic approach, capitalizing on their inherent qualities and utilizing advanced synthesis methods to propel the field towards more effective cancer treatment modalities.
Dyes have been widely used in fluorophore moieties because of their outstanding photophysical properties and because of their ability to demonstrate good singlet oxygen production that is successfully applicable in PDT. Researchers have been exploring various types of dyes incorporated into conjugated polymers for singlet oxygen generation in PDT.
Singh et al. reported on singlet oxygen generation and the anticancer activity of polymerizing 1,4-benzoquinone (BQ), Amido Black 10B (AB-10B), and Alizarin Red (AR) with o- and p-phenylenediamines using ultrasound assistance for the first time.153 According to Singh and colleagues, lawsone (LW) was integrated into conjugated polymers of pyrrole, thiophene, p-phenylenediamine, and 1-naphthylamine using an ultrasound-assisted technique. The 1O2 generation investigation, according to the authors, was conducted using the DPBF test, which showed the highest quantum yield value of 0.11 for Ppy-LW. Additionally, they proposed that LW might be used in photodynamic therapy because it was an easy way to adjust fluorescence emission and singlet oxygen formation in conjugated polymers.154 The same group also designed ortho-phenylenediamine with 1,4-benzoquinone, which enabled the efficient production of singlet oxygen (1O2). According to the researchers, 1O2 generation investigations showed that the oligomer with the highest quantum yield value of 0.057 was composed of 70% benzoquinone. They proposed that their findings could be useful in the application of engineered oligomers as photosensitive probes for photodynamic treatment.155 Singh et al. also reported novel azo benzene functionalized aniline, 1-naphthylamine, luminol and o-phenylenediamine prepared by using an ultrasound technique. The researchers suggested that the synthesized polymers also showed high singlet oxygen generation characteristics.156
From 700 to 1100 nm, biological systems are transparent to NIR light. It is possible to manipulate light in this optical window while causing the least amount of harm to tissues and organs. A possible noninvasive cancer treatment method is photothermal therapy (PTT). By stabilizing the quinoid resonance structure, extending the large conjugated region, or employing D–A (donor–acceptor) exchanges in conjugated molecules, one is able to accomplish a low band gap in conjugated molecules, which satisfies the need for identifying the absorption in the biosafety optical window.157 Many groups are exploring the use of conjugated polymers (CPs) for photothermal cancer treatment because many of these polymers have high near-infrared (NIR) absorbance, making them promising candidates for this purpose.158
In image-guided photodynamic cancer therapy, using a photosensitizer that operates in the far-red (FR) or near-infrared (NIR) range is highly desirable. Wu et al. achieved this by employing a one-pot Suzuki polymerization process to create a polymer known as PTPEAQ. This polymer contains anthraquinone (AQ) as an electron acceptor and tetraphenylethylene (TPE), a well-known aggregation-induced emission (AIE) active group, as an electron donor. They further developed PTPEAQ-NP-HER2 by encapsulating PTPEAQ within a block copolymer and modifying its surface with an anti-Her2 antibody. PTPEAQ-NP-HER2 exhibits impressive AIE-active emission in the FR/NIR range and can efficiently generate singlet oxygen when exposed to visible light. This unique property has been successfully harnessed for the photodynamic ablation of cancer cells. They specifically demonstrated its effectiveness using SKBR-3 cells, a type of breast cancer cell characterized by overexpression of the HER2 receptor on the cell membrane159 (Fig. 26). Two cationic AIE active polymers, DCPN-1 and DCPN-2, were formed and synthesized by Cong et al. using the ring opening polymerization method. The majority of the visible light spectrum was covered by the intense emissions that DCPN-2 emits in the NIR region (>650 nm), and it efficiently produces reactive oxygen species (ROS) when exposed to light. Prominently, it was shown both in vitro and in vivo that DCPN-2 administration during light irradiation inhibited tumour growth.160
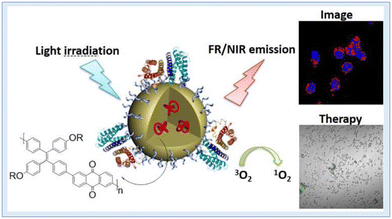 | ||
| Fig. 26 NIR conjugated polymers showing singlet oxygen generation and PDT. Reproduced with permission.159 Copyright 2016, American Chemical Society. | ||
Recent studies have concentrated on dual-modal imaging-guided phototherapy employing near-infrared fluorescence (NIRF) and photoacoustic (PA) techniques. One of the main challenges is creating a theranostic tool that is easy to use and efficient at improving photodynamic therapy (PDT) and imaging-guided photothermal therapy (PTT). In the study by Tan et al., Ag@PANI nanocomposites loaded with ICG as a theranostic agent for imaging-guided phototherapy are a promising way to advance cancer therapy, as their work suggests. These nanocomposites have the potential to be useful in solving cancer therapeutic issues due to their unique combination of features, which include excellent NIR absorption, improved photostability, efficient development of hyperthermia, and maintained ROS production. These results open up new possibilities for the theranostic treatment of cancer, where it can be used to fight tumours with more accuracy and efficacy.161
Sun and colleagues created and produced thiophene (TPA-TDPP), diketopyrrolopyrrole (DPP) core functionalized benzene (PDDP), and derivatives of benzene (TPA-PDDP). To prove the heavy atom effect, they ran electrochemistry experiments. TPA-TDPP's energy level dropped when triphenylamine (TPA) was added, and a 50% quantum yield increase in the synthesis of singlet oxygen (1O2) was observed. Additionally, they increased the power conversion efficiency (PCE) of TPA-TDPP to 38.7% by adding thiophene, triphenylamine, and long alkyl chains in the aggregated form. This was achieved by encouraging a twisting action and lowering intermolecular contact. TPA-TDPP nanoparticles’ enhanced permeability and retention (EPR) effect allowed for the targeting of tumour regions, as demonstrated by in vivo fluorescence imaging. These nanoparticles showed remarkable photodynamic/photothermal synergy.162
Nave et al. created a novel, straightforward technique for creating NIR light-responsive nano-systems that were utilized in cancer chemo-photodynamic/photothermal treatment. They accomplished this by creating an amphiphilic conjugate of poly(2-ethyl-2-oxazoline)-IR780 that, when combined, might encase doxorubicin (DOX/PEtOx-IR NPs) and have photodynamic/photothermal characteristics. The size and surface charge of the DOX/PEtOx-IR NPs were advantageous for applications linked to cancer. When exposed to NIR light, the DOX/PEtOx-IR NPs boosted the release of DOX by 1.7 times while producing singlet oxygen and a lower thermic impact. DOX/PEtOx-IR NPs and NIR light were able to entirely eradicate breast cancer cells (viability 4%) in in vitro experiments; this is representative of the improved outcomes from the chemo-photodynamic/photothermal treatment presented by the nanomaterials.163
Chen and colleagues effectively synthesized an NIR coumarin-BODIPY photosensitizer BDP-C with iodine for photodynamic treatment using a Knoevenagel condensation process. The maximum wavelengths of BDP-C's emission and absorption were 732 nm and 808 nm, respectively. 2.13 × 105 cm−1 M1 was found to be the highest molar absorption coefficient. After being exposed to radiation, BDP-C showed an outstanding long-term cell imaging capability, strong photostability, and good biocompatibility. It also had a moderate singlet oxygen quantum yield. According to in vitro research, BDP-C can be selectively localized in lysosomes where it can effectively destroy cancer cells when exposed to radiation. Overall, they demonstrated an NIR organic PDT photosensitizer, and BDP-C could serve as a viable substitute for any PDT photosensitizer based on BODIPY used in medical settings in the future.164
Owing to the benefits of two-photon excited phototherapy, as demonstrated by Wu and his coworkers, immense research interest have developed in the scientific community in this direction. The lack of a high-performance photosensitizer with broad two-photon absorption cross-sections and specialized targeting capabilities renders phototherapy ineffective in the treatment of cancer. Here, a new photosensitizer, 6DBF2, derived from BODIPY was developed with two-photon photosensitization for in vivo two-photon stimulated photodynamic treatment. Following near-infrared laser activation, 6DBF2 exhibits strong two-photon absorption and effective 1O2 production. The exceptional therapeutic effectiveness of 6DBF2 in two-photon stimulated photodynamic treatment was demonstrated by in vivo tumour ablation inside mouse models and in vitro cancer cell ablation following NIR two-photon irradiation. The authors reported a unique instance of lipid droplets that were targeted by photodynamic radiation allowing deep tumor cell imaging and treatment with near-infrared light irradiation.165
Wu and colleagues created novel organic photothermal nano-agents in the near-infrared (NIR) range by utilizing conjugated boron dipyrromethene (BODIPY) oligomers as a support. These nanomaterials showed outstanding photostability, tunable NIR absorptions, and a very effective conversion of NIR light to heat. Under NIR laser light, they may efficiently accumulate in tumour tissues and accomplish total tumour ablation. This strategy, known as “excited-state rotation, ground-state conjugation”, offers a state-of-the-art framework for creating sophisticated theranostic compounds with near-infrared absorption for a range of therapeutic uses.166
Recent years have seen an increase in phototherapeutic techniques like photodynamic therapy (PDT) and photothermal treatment (PTT) that have attracted significant attention in biological and medicinal fields. The key to maximizing therapeutic effectiveness is to discover potential compounds with beneficial PDT and PTT interaction effects, particularly those brought on by single-wavelength NIR light. A low-bandgap fluorene-based donor–acceptor–donor (D–A–D) conjugated oligomer, synthesized in a green pathway showed a broad absorption in both the visible and NIR ranges, according to Peng et al. The authors demonstrated that the oligomer had a good photothermal capacity with a conversion efficiency of 37.7% after being exposed to an 808 nm laser, along with concurrent photodynamic behavior that led to the production of reactive oxygen species. Additionally, its green fluorescence, which was stimulated by 420 nm light, offers the chance for imaging-guided therapy.167
6. Outlook and future prospects
Photodynamic therapy (PDT) involves interactions between reactive 1O2, light, and photosensitizers. Treatment responses vary significantly within and between lesions due to differences in vascular status and the microenvironment. Therefore, it appears to be challenging to accurately determine the required light dose and photosensitizer dose. Over the past 10 years, there have been notable and significant advancements in research towards the creation of nanomaterials that elicit the production of singlet oxygen (1O2) with a high quantum yield. Due to their extensive usefulness in photodynamic therapy (PDT), these compounds have garnered significant attention within the scientific community. The utilization of nanomaterials has become increasingly prevalent in various technological domains. The characteristics of these materials, such as their high surface area-to-volume ratio, enable their application in areas such as catalysis, energy storage, and drug delivery. It is anticipated that novel nano-photosensitizers (NPSs) will emerge as a promising avenue for scientific exploration with multifaceted and multifunctional properties. The envisioned outcome of this endeavour lies in uncovering significant and revolutionary discoveries in the field of cancer research. The concept of translating scientific discoveries from laboratory research to clinical applications, commonly referred to as bench to bedside, has thus gained significant attention in academic discourse.Conjugated polymers are fluorescent probes with high fluorescence efficiency, remarkable photostability, and potent light absorption, among their many advantageous qualities. The potential uses of these polymers in photodynamic and photothermal therapy for the detection and treatment of cancer are being investigated. Through sophisticated chemical synthesis and flexible polymer architectures, researchers can produce new conjugated polymers with high singlet-oxygen generation capabilities for photodynamic treatment (PDT). Conjugated polymer nanoparticles that contain photosensitizers improve the formation of singlet oxygen because they transfer energy effectively. Nonetheless, there are still issues to be resolved, like reduced singlet-oxygen generation efficiency in the near-infrared (NIR) wavelength region and restrictions on light penetration. Clinical safety of the conjugated polymers is another crucial factor, along with PDT effectiveness. Any organic or inorganic nanoparticle that is ingested into the body could have negative health effects. The purpose of this article is to provide an overview of singlet oxygen generation for PDT as well as highlight the most recent developments in conjugated polymers and polymers based on nanomaterials. With an emphasis on the idea of conjugated polymers and nano-conjugated polymers as photosensitizers, we give an overview of recent discoveries for SOG in PDT research and discuss the mechanisms important to SOG and PDT cancer therapy. In order to create singlet oxygen for photodynamic therapy, dye-based conjugated polymers have been studied as photosensitized materials. We have also mentioned the effects of dye-based conjugated polymers on singlet oxygen generation in PDT because they have not been well studied in the literature.
Author contributions
NS took the lead in conceptualization, writing and reviewing of the review article. RS contributed towards writing of the article and SSB was involved in proof-reading and reviewing of the article.Conflicts of interest
The authors declare no competing interests.Acknowledgements
Prof. Suryasarathi Bose would like to acknowledge DST-SERB for a Swarnajayanti fellowship. Dr Neetika Singh wishes to acknowledge the SERB-National Postdoctoral Fellowship (NPDF) (File No. PDF/2022/000556) scheme, DST, India, for providing funding to conduct this research work, and Ria Sen Gupta is grateful to MHRD for a Prime Minister's Research Fellowship (PMRF).References
- Y. Pang, C. Li, H. Deng and Y. Sun, Dalton Trans., 2022, 51, 16428–16438 RSC.
- H. Li, Y. Kim, H. Jung, J. Y. Hyun and I. Shin, Chem. Soc. Rev., 2022, 51, 8957–9008 RSC.
- P.-Y. Wu, Z.-C. Shen, J.-L. Jiang, B.-C. Zhang, W.-Z. Zhang, J.-J. Zou, J.-F. Lin, C. Li and J.-W. Shao, Biomater. Sci., 2022, 10, 6267–6281 RSC.
- J.-O. Yoo and K.-S. Ha, Int. Rev. Cell Mol. Biol., 2012, 295, 139–174 CAS.
- B. Lu, Y. Huang, Z. Zhang, H. Quan and Y. Yao, Mater. Chem. Front., 2022, 6, 2968–2993 RSC.
- Q. Wang, D. K. P. Ng and P.-C. Lo, J. Mater. Chem. B, 2018, 6, 3285–3296 RSC.
- B. C. Dickinson and C. J. Chang, Nat. Chem. Biol., 2011, 7, 504–511 CrossRef CAS PubMed.
- H. Hu, J. Zhang, Y. Ding, X. Zhang, K. Xu, X. Hou and P. Wu, Anal. Chem., 2017, 89, 5101–5106 CrossRef CAS PubMed.
- K.-X. Teng, L.-Y. Niu and Q.-Z. Yang, J. Am. Chem. Soc., 2023, 145, 4081–4087 CrossRef CAS PubMed.
- N. Yesilgul, T. B. Uyar, O. Seven and E. U. Akkaya, ACS Omega, 2017, 2, 1367–1371 CrossRef CAS PubMed.
- S. Jeong, W. Park, C.-S. Lee and K. Na, Macromol. Biosci., 2014, 14, 1688–1695 CrossRef CAS PubMed.
- R. A. Ponzio, L. E. Ibarra, E. E. Achilli, E. Odella, C. A. Chesta, S. R. Martínez and R. E. Palacios, J. Photochem. Photobiol., B, 2022, 234, 112510 CrossRef CAS PubMed.
- M. Bregnhøj, M. Westberg, B. F. Minaev and P. R. Ogilby, Acc. Chem. Res., 2017, 50, 1920–1927 CrossRef PubMed.
- P. R. Ogilby, Acc. Chem. Res., 1999, 32, 512–519 CrossRef CAS.
- J. W. Leem, S.-R. Kim, K.-H. Choi and Y. L. Kim, Nano Convergence, 2018, 5, 8 CrossRef PubMed.
- A. Kashyap, E. Ramasamy, V. Ramalingam and M. Pattabiraman, Molecules, 2021, 26, 2673 CrossRef CAS PubMed.
- S. Bonnet, J. Am. Chem. Soc., 2023, 145, 23397–23415 CrossRef CAS PubMed.
- S. Kim, T. Tachikawa, M. Fujitsuka and T. Majima, J. Am. Chem. Soc., 2014, 136, 11707–11715 CrossRef CAS PubMed.
- A. Abdurashitov, V. Tuchin and O. Semyachkina-Glushkovskaya, J. Innovative Opt. Health Sci., 2020, 13, 2030004 CrossRef CAS.
- B. Li, L. Lin, H. Lin and B. C. Wilson, J. Biophotonics, 2016, 9, 1314–1325 CrossRef CAS PubMed.
- B. Li, H. Lin, D. Chen, B. C. Wilson and Y. Gu, J. Innovative Opt. Health Sci., 2013, 06, 1330002 CrossRef.
- M. Niedre, M. S. Patterson and B. C. Wilson, Photochem. Photobiol., 2002, 75, 382–391 CrossRef CAS PubMed.
- M. T. Jarvi, M. J. Niedre, M. S. Patterson and B. C. Wilson, Photochem. Photobiol., 2006, 82, 1198 CrossRef CAS PubMed.
- A. Jiménez-Banzo, X. Ragàs, P. Kapusta and S. Nonell, Photochem. Photobiol. Sci., 2008, 7, 1003–1010 CrossRef PubMed.
- H.-J. Laubach, S. K. Chang, S. Lee, I. Rizvi, D. Zurakowski, S. J. Davis, C. R. Taylor and T. Hasan, J. Biomed. Opt., 2008, 13, 050504 CrossRef PubMed.
- P. R. Ogilby, Chem. Soc. Rev., 2010, 39, 3181 RSC.
- H. Lin, D. Chen, M. Wang, J. Lin, B. Li and S. Xie, J. Opt., 2011, 13, 125301 CrossRef.
- J. Krajczewski, K. Rucińska, H. E. Townley and A. Kudelski, Photodiagn. Photodyn. Ther., 2019, 26, 162–178 CrossRef CAS PubMed.
- B. Song, G. Wang, M. Tan and J. Yuan, J. Am. Chem. Soc., 2006, 128, 13442–13450 CrossRef CAS PubMed.
- D. Song, S. Cho, Y. Han, Y. You and W. Nam, Org. Lett., 2013, 15, 3582–3585 CrossRef CAS PubMed.
- J. M. Aubry, B. Cazin and F. Duprat, J. Org. Chem., 1989, 54, 726–728 CrossRef CAS.
- B. Song, G. Wang and J. Yuan, Chem. Commun., 2005, 3553 RSC.
- N. Hananya, O. Green, R. Blau, R. Satchi-Fainaro and D. Shabat, Angew. Chem., Int. Ed., 2017, 56, 11793–11796 CrossRef CAS PubMed.
- H. K. Yoon, X. Lou, Y.-C. Chen, Y.-E. Koo Lee, E. Yoon and R. Kopelman, Chem. Mater., 2014, 26, 1592–1600 CrossRef CAS PubMed.
- B. A. Lindig, M. A. J. Rodgers and A. P. Schaap, J. Am. Chem. Soc., 1980, 102, 5590–5593 CrossRef CAS.
- R. Bresolí-Obach, J. Nos, M. Mora, M. L. Sagristà, R. Ruiz-González and S. Nonell, Methods, 2016, 109, 64–72 CrossRef PubMed.
- D. Gao, R. R. Agayan, H. Xu, M. A. Philbert and R. Kopelman, Nano Lett., 2006, 6, 2383–2386 CrossRef CAS PubMed.
- Z. Wang, X. Hong, S. Zong, C. Tang, Y. Cui and Q. Zheng, Sci. Rep., 2015, 5, 12602 CrossRef CAS PubMed.
- E. Albiter, S. Alfaro and M. A. Valenzuela, Int. J. Photoenergy, 2012, 2012, 1–8 CrossRef.
- E. Albiter, S. Alfaro and M. A. Valenzuela, Photochem. Photobiol. Sci., 2015, 14, 597–602 CrossRef CAS PubMed.
- M. I. Burguete, F. Galindo, R. Gavara, S. V. Luis, M. Moreno, P. Thomas and D. A. Russell, Photochem. Photobiol. Sci., 2009, 8, 37–44 CrossRef CAS PubMed.
- X.-F. Zhang and X. Li, J. Lumin., 2011, 131, 2263–2266 CrossRef CAS.
- T. Entradas, S. Waldron and M. Volk, J. Photochem. Photobiol., B, 2020, 204, 111787 CrossRef CAS PubMed.
- T. Ohyashiki, M. Nunomura and T. Katoh, Biochim. Biophys. Acta, Biomembr., 1999, 1421, 131–139 CrossRef CAS PubMed.
- M. Price, J. J. Reiners, A. M. Santiago and D. Kessel, Photochem. Photobiol., 2009, 85, 1177–1181 CrossRef CAS PubMed.
- Y.-Q. He, W. Fudickar, J.-H. Tang, H. Wang, X. Li, J. Han, Z. Wang, M. Liu, Y.-W. Zhong, T. Linker and P. J. Stang, J. Am. Chem. Soc., 2020, 142, 2601–2608 CrossRef CAS PubMed.
- K. Imato, K. Ohira, M. Yamaguchi, T. Enoki and Y. Ooyama, Mater. Chem. Front., 2020, 4, 589–596 RSC.
- E. Y. Zhou, H. J. Knox, C. Liu, W. Zhao and J. Chan, J. Am. Chem. Soc., 2019, 141, 17601–17609 CrossRef CAS PubMed.
- H. Wu, Q. Song, G. Ran, X. Lu and B. Xu, TrAC, Trends Anal. Chem., 2011, 30, 133–141 CrossRef CAS.
- K. Tanaka, T. Miura, N. Umezawa, Y. Urano, K. Kikuchi, T. Higuchi and T. Nagano, J. Am. Chem. Soc., 2001, 123, 2530–2536 CrossRef CAS PubMed.
- B. Song, G. Wang, M. Tan and J. Yuan, J. Am. Chem. Soc., 2006, 128, 13442–13450 CrossRef CAS PubMed.
- D. Zhu, D. Xing, Y. Wei, X. Li and B. Gao, Luminescence, 2004, 19, 278–282 CrossRef CAS PubMed.
- Y. He, D. Xing, G. Yan and K. Ueda, Cancer Lett., 2002, 182, 141–145 CrossRef CAS PubMed.
- J. Ge, M. Lan, B. Zhou, W. Liu, L. Guo, H. Wang, Q. Jia, G. Niu, X. Huang, H. Zhou, X. Meng, P. Wang, C.-S. Lee, W. Zhang and X. Han, Nat. Commun., 2014, 5, 4596 CrossRef CAS PubMed.
- R. Vankayala, C.-L. Kuo, A. Sagadevan, P.-H. Chen, C.-S. Chiang and K. C. Hwang, J. Mater. Chem. B, 2013, 1, 4379 RSC.
- D. Bechet, P. Couleaud, C. Frochot, M.-L. Viriot, F. Guillemin and M. Barberi-Heyob, Trends Biotechnol., 2008, 26, 612–621 CrossRef CAS PubMed.
- S. J. Chadwick, D. Salah, P. M. Livesey, M. Brust and M. Volk, J. Phys. Chem. C, 2016, 120, 10647–10657 CrossRef CAS PubMed.
- F. Gao, M. Sun, W. Ma, X. Wu, L. Liu, H. Kuang and C. Xu, Adv. Mater., 2017, 29, 1606864 CrossRef PubMed.
- G. V. Kumari, M. A. JothiRajan and T. Mathavan, Mater. Res. Express, 2018, 5, 085027 CrossRef.
- R. Vankayala, C.-L. Kuo, K. Nuthalapati, C.-S. Chiang and K. C. Hwang, Adv. Funct. Mater., 2015, 25, 5934–5945 CrossRef CAS.
- H. Kawasaki, S. Kumar, G. Li, C. Zeng, D. R. Kauffman, J. Yoshimoto, Y. Iwasaki and R. Jin, Chem. Mater., 2014, 26, 2777–2788 CrossRef CAS.
- S. Miyata, H. Miyaji, H. Kawasaki, M. Yamamoto, E. Nishida, H. Takita, T. Akasaka, N. Ushijima, T. Iwanaga and T. Sugaya, Int. J. Nanomed., 2017, 12, 2703–2716 CrossRef CAS PubMed.
- M.-H. Chan, S.-P. Chen, C.-W. Chen, Y.-C. Chan, R. J. Lin, D. P. Tsai, M. Hsiao, R.-J. Chung, X. Chen and R.-S. Liu, J. Phys. Chem. C, 2018, 122, 2402–2412 CrossRef CAS.
- S. Farooq and R. E. de Araujo, Photodiagn. Photodyn. Ther., 2021, 35, 102466 CrossRef CAS PubMed.
- T. Zhao, X. Shen, L. Li, Z. Guan, N. Gao, P. Yuan, S. Q. Yao, Q.-H. Xu and G. Q. Xu, Nanoscale, 2012, 4, 7712 RSC.
- C. Jiang, T. Zhao, P. Yuan, N. Gao, Y. Pan, Z. Guan, N. Zhou and Q.-H. Xu, ACS Appl. Mater. Interfaces, 2013, 5, 4972–4977 CrossRef CAS PubMed.
- R. Vankayala, Y.-K. Huang, P. Kalluru, C.-S. Chiang and K. C. Hwang, Small, 2014, 10, 1612–1622 CrossRef CAS PubMed.
- P. Vijayaraghavan, C.-H. Liu, R. Vankayala, C.-S. Chiang and K. C. Hwang, Adv. Mater., 2014, 26, 6689–6695 CrossRef CAS PubMed.
- M. Sakamoto, T. Tachikawa, M. Fujitsuka and T. Majima, Langmuir, 2009, 25, 13888–13893 CrossRef CAS PubMed.
- T. Das, P. Ghosh, M. S. Shanavas, A. Maity, S. Mondal and P. Purkayastha, Nanoscale, 2012, 4, 6018 RSC.
- H. Saito and Y. Nosaka, J. Phys. Chem. C, 2014, 118, 15656–15663 CrossRef CAS.
- N. Zhou, H. Zhu, S. Li, J. Yang, T. Zhao, Y. Li and Q.-H. Xu, J. Phys. Chem. C, 2018, 122, 7824–7830 CrossRef CAS.
- C. Fang, H. Jia, S. Chang, Q. Ruan, P. Wang, T. Chen and J. Wang, Energy Environ. Sci., 2014, 7, 3431–3438 RSC.
- G. Pasparakis, Small, 2013, 9, 4130–4134 CrossRef CAS PubMed.
- Y. Yu, J. Geng, E. Y. X. Ong, V. Chellappan and Y. N. Tan, Adv. Healthcare Mater., 2016, 5, 2528–2535 CrossRef CAS PubMed.
- M. B. Rivas Aiello, J. J. Romero, S. G. Bertolotti, M. C. Gonzalez and D. O. Mártire, J. Phys. Chem. C, 2016, 120, 21967–21975 CrossRef CAS.
- Z. Yuan, Z.-J. Jiang, Y. Wang, Z. Jiang and B. Deng, J. Phys. Chem. C, 2022, 126, 13191–13201 CrossRef CAS.
- D. M. Willard, R. E. Riter and N. E. Levinger, J. Am. Chem. Soc., 1998, 120, 4151–4160 CrossRef CAS.
- I. Roy, T. Y. Ohulchanskyy, H. E. Pudavar, E. J. Bergey, A. R. Oseroff, J. Morgan, T. J. Dougherty and P. N. Prasad, J. Am. Chem. Soc., 2003, 125, 7860–7865 CrossRef CAS PubMed.
- M. Lal, L. Levy, K. S. Kim, G. S. He, X. Wang, Y. H. Min, S. Pakatchi and P. N. Prasad, Chem. Mater., 2000, 12, 2632–2639 CrossRef CAS.
- R. W. M. Allen, C. Templeton, M. J. Hostetler and C. T. Kraft, J. Am. Chem. Soc., 1998, 120, 1906–1911 CrossRef.
- Y. Nosaka, T. Daimon, A. Y. Nosaka and Y. Murakami, Phys. Chem. Chem. Phys., 2004, 6, 2917 RSC.
- M.-K. Tsang, G. Bai and J. Hao, Chem. Soc. Rev., 2015, 44, 1585–1607 RSC.
- N. M. Idris, S. S. Lucky, Z. Li, K. Huang and Y. Zhang, J. Mater. Chem. B, 2014, 2, 7017–7026 RSC.
- L. Zhang, L. Zeng, Y. Pan, S. Luo, W. Ren, A. Gong, X. Ma, H. Liang, G. Lu and A. Wu, Biomaterials, 2015, 44, 82–90 CrossRef CAS PubMed.
- Z. Hou, Y. Zhang, K. Deng, Y. Chen, X. Li, X. Deng, Z. Cheng, H. Lian, C. Li and J. Lin, ACS Nano, 2015, 9, 2584–2599 CrossRef CAS PubMed.
- P. Kalluru, R. Vankayala, C.-S. Chiang and K. C. Hwang, Angew. Chem., Int. Ed., 2013, 52, 12332–12336 CrossRef CAS PubMed.
- Y. Xu, Z. Shi, L. Zhang, E. M. B. Brown and A. Wu, Nanoscale, 2016, 8, 12715–12722 RSC.
- D. Duan, H. Liu, Y. Xu, Y. Han, M. Xu, Z. Zhang and Z. Liu, ACS Appl. Mater. Interfaces, 2018, 10, 5278–5286 CrossRef CAS PubMed.
- H. Dong, S. Tang, Y. Hao, H. Yu, W. Dai, G. Zhao, Y. Cao, H. Lu, X. Zhang and H. Ju, ACS Appl. Mater. Interfaces, 2016, 8, 3107–3114 CrossRef CAS PubMed.
- H. W. Kroto, J. R. Heath, S. C. O'Brien, R. F. Curl and R. E. Smalley, Nature, 1985, 318, 162–163 CrossRef CAS.
- G. A. M. Sáfar, R. N. Gontijo, C. Fantini, D. C. S. Martins, Y. M. Idemori, M. V. B. Pinheiro and K. Krambrock, J. Phys. Chem. C, 2015, 119, 4344–4350 CrossRef.
- D. M. Wang, M. X. Gao, P. F. Gao, H. Yang and C. Z. Huang, J. Phys. Chem. C, 2013, 117, 19219–19225 CrossRef CAS.
- R. Knoblauch, A. Harvey and C. D. Geddes, Plasmonics, 2021, 16, 1765–1772 CrossRef CAS.
- A. Blacha-Grzechnik, M. Krzywiecki, R. Motyka and M. Czichy, J. Phys. Chem. C, 2019, 123, 25915–25924 CrossRef CAS.
- Z. M. Markovic, B. Z. Ristic, K. M. Arsikin, D. G. Klisic, L. M. Harhaji-Trajkovic, B. M. Todorovic-Markovic, D. P. Kepic, T. K. Kravic-Stevovic, S. P. Jovanovic, M. M. Milenkovic, D. D. Milivojevic, V. Z. Bumbasirevic, M. D. Dramicanin and V. S. Trajkovic, Biomaterials, 2012, 33, 7084–7092 CrossRef CAS PubMed.
- W.-S. Kuo, C.-Y. Chang, H.-H. Chen, C.-L. L. Hsu, J.-Y. Wang, H.-F. Kao, L. C.-S. Chou, Y.-C. Chen, S.-J. Chen, W.-T. Chang, S.-W. Tseng, P.-C. Wu and Y.-C. Pu, ACS Appl. Mater. Interfaces, 2016, 8, 30467–30474 CrossRef CAS PubMed.
- Y. Zhang, C. Yang, D. Yang, Z. Shao, Y. Hu, J. Chen, L. Yuwen, L. Weng, Z. Luo and L. Wang, Phys. Chem. Chem. Phys., 2018, 20, 17262–17267 RSC.
- Z. Luo, D. Yang, C. Yang, X. Wu, Y. Hu, Y. Zhang, L. Yuwen, E. K. L. Yeow, L. Weng, W. Huang and L. Wang, Appl. Surf. Sci., 2018, 434, 155–162 CrossRef CAS.
- D. Zhang, L. Wen, R. Huang, H. Wang, X. Hu and D. Xing, Biomaterials, 2018, 153, 14–26 CrossRef CAS PubMed.
- L.-S. Lin, Z.-X. Cong, J. Li, K.-M. Ke, S.-S. Guo, H.-H. Yang and G.-N. Chen, J. Mater. Chem. B, 2014, 2, 1031 RSC.
- C. Liu, Z. Chen, Z. Wang, W. Li, E. Ju, Z. Yan, Z. Liu, J. Ren and X. Qu, Nanoscale, 2016, 8, 12570–12578 RSC.
- L. Zhou, Y. Xu, W. Yu, X. Guo, S. Yu, J. Zhang and C. Li, J. Mater. Chem. A, 2016, 4, 8000–8004 RSC.
- M. Lismont, L. Dreesen and S. Wuttke, Adv. Funct. Mater., 2017, 27, 1606314 CrossRef.
- Z. Li and Y.-W. Yang, J. Mater. Chem. B, 2017, 5, 9278–9290 RSC.
- K. Lu, C. He and W. Lin, J. Am. Chem. Soc., 2014, 136, 16712–16715 CrossRef CAS PubMed.
- K. Lu, C. He and W. Lin, J. Am. Chem. Soc., 2015, 137, 7600–7603 CrossRef CAS PubMed.
- D. Bůžek, J. Zelenka, P. Ulbrich, T. Ruml, I. Křížová, J. Lang, P. Kubát, J. Demel, K. Kirakci and K. Lang, J. Mater. Chem. B, 2017, 5, 1815–1821 RSC.
- Y. Ma, X. Li, A. Li, P. Yang, C. Zhang and B. Tang, Angew. Chem., Int. Ed., 2017, 56, 13752–13756 CrossRef CAS PubMed.
- Q. Guan, L.-L. Zhou, Y.-A. Li and Y.-B. Dong, Inorg. Chem., 2018, 57, 10137–10145 CrossRef CAS PubMed.
- F. Hu, D. Mao, Kenry, Y. Wang, W. Wu, D. Zhao, D. Kong and B. Liu, Adv. Funct. Mater., 2018, 28, 1707519 CrossRef.
- T. Luo, K. Ni, A. Culbert, G. Lan, Z. Li, X. Jiang, M. Kaufmann and W. Lin, J. Am. Chem. Soc., 2020, 142, 7334–7339 CrossRef CAS PubMed.
- Y.-T. Qin, H. Peng, X.-W. He, W.-Y. Li and Y.-K. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 34268–34281 CrossRef CAS PubMed.
- Y. Li, Z. Di, J. Gao, P. Cheng, C. Di, G. Zhang, B. Liu, X. Shi, L.-D. Sun, L. Li and C.-H. Yan, J. Am. Chem. Soc., 2017, 139, 13804–13810 CrossRef CAS PubMed.
- S.-Z. Ren, B. Wang, X.-H. Zhu, D. Zhu, M. Liu, S.-K. Li, Y.-S. Yang, Z.-C. Wang and H.-L. Zhu, ACS Appl. Mater. Interfaces, 2020, 12, 24662–24674 CrossRef CAS PubMed.
- H. S. Jung, P. Verwilst, A. Sharma, J. Shin, J. L. Sessler and J. S. Kim, Chem. Soc. Rev., 2018, 47, 2280–2297 RSC.
- J. Feng, W.-X. Ren, F. Kong and Y.-B. Dong, Inorg. Chem. Front., 2021, 8, 848–879 RSC.
- T. Luo, G. T. Nash, Z. Xu, X. Jiang, J. Liu and W. Lin, J. Am. Chem. Soc., 2021, 143, 13519–13524 CrossRef CAS PubMed.
- X. Zheng, L. Wang, Q. Pei, S. He, S. Liu and Z. Xie, Chem. Mater., 2017, 29, 2374–2381 CrossRef CAS.
- L. Zhang, S. Wang, Y. Zhou, C. Wang, X. Zhang and H. Deng, Angew. Chem., Int. Ed., 2019, 58, 14213–14218 CrossRef CAS PubMed.
- S.-B. Wang, Z.-X. Chen, F. Gao, C. Zhang, M.-Z. Zou, J.-J. Ye, X. Zeng and X.-Z. Zhang, Biomaterials, 2020, 234, 119772 CrossRef CAS PubMed.
- L. Yang and P. Alexandridis, Curr. Opin. Colloid Interface Sci., 2000, 5, 132–143 CrossRef CAS.
- A. Labib, V. Lenaerts, F. Chouinard, J. C. Leroux, R. Ouellet and J. E. van Lier, Pharm. Res., 1991, 8, 1027–1031 CrossRef CAS PubMed.
- E. Allémann, J. Rousseau, N. Brasseur, S. V. Kudrevich, K. Lewis and J. E. van Lier, Int. J. Cancer, 1996, 66, 821–824 CrossRef.
- M. J. Moreno, E. Monson, R. G. Reddy, A. Rehemtulla, B. D. Ross, M. Philbert, R. J. Schneider and R. Kopelman, Sens. Actuators, B, 2003, 90, 82–89 CrossRef CAS.
- J. Fu, X. Li, D. K. P. Ng and C. Wu, Langmuir, 2002, 18, 3843–3847 CrossRef CAS.
- Q. Zheng, Z. Duan, Y. Zhang, X. Huang, X. Xiong, A. Zhang, K. Chang and Q. Li, Molecules, 2023, 28, 5091 CrossRef CAS PubMed.
- L. Liu, X. Wang, S. Zhu and L. Li, ACS Appl. Bio Mater., 2021, 4, 1211–1220 CrossRef CAS PubMed.
- Y. Jiang and J. McNeill, Chem. Rev., 2017, 117, 838–859 CrossRef CAS PubMed.
- C. Zhang, Q. Yuan, Z. Zhang and Y. Tang, Molecules, 2023, 28, 399 CrossRef CAS PubMed.
- S. Battah, R. C. Hider, A. J. MacRobert, P. S. Dobbin and T. Zhou, J. Med. Chem., 2017, 60, 3498–3510 CrossRef CAS PubMed.
- S. Wang, F. Yuan, K. Chen, G. Chen, K. Tu, H. Wang and L.-Q. Wang, Biomacromolecules, 2015, 16, 2693–2700 CrossRef CAS PubMed.
- R. M. Spada, L. P. Macor, L. I. Hernández, R. A. Ponzio, L. E. Ibarra, C. Lorente, C. A. Chesta and R. E. Palacios, Dyes Pigm., 2018, 149, 212–223 CrossRef CAS.
- S.-H. Cheng, C.-H. Lee, C.-S. Yang, F.-G. Tseng, C.-Y. Mou and L.-W. Lo, J. Mater. Chem., 2009, 19, 1252 RSC.
- K. Chang, Y. Tang, X. Fang, S. Yin, H. Xu and C. Wu, Biomacromolecules, 2016, 17, 2128–2136 CrossRef CAS PubMed.
- R. Islam, K. Kotalík, V. Šubr, S. Gao, J.-R. Zhou, K. Yokomizo, T. Etrych and J. Fang, Nanomedicine, 2023, 48, 102636 CrossRef CAS PubMed.
- Y.-Q. Huang, S.-S. Jiang, L.-X. Pan, R. Zhang, K.-L. Liu, X.-F. Liu, Q.-L. Fan, L.-H. Wang and W. Huang, New J. Chem., 2021, 45, 15607–15617 RSC.
- X. Shen, L. Li, H. Wu, S. Q. Yao and Q.-H. Xu, Nanoscale, 2011, 3, 5140 RSC.
- S. Ren, H. Li, X. Xu, H. Zhao, W. He, L. Zhang and Z. Cheng, Biomater. Sci., 2023, 11, 509–517 RSC.
- B. Bao, X. Zhai, T. Liu, P. Su, L. Zhou, Y. Xu, B. Gu and L. Wang, Polym. Chem., 2020, 11, 7035–7041 RSC.
- Sauraj, J. H. Kang, Oh. Lee and Y. T. Ko, Nanoscale, 2023, 15, 4882–4892 RSC.
- Z. Wang, L. Li, W. Wang, R. Wang, G. Li, H. Bian, D. Zhu and M. R. Bryce, Dalton Trans., 2023, 52, 1595–1601 RSC.
- Z. Chen, Y. Chen, Y. Xu, X. Shi, Z. Han, Y. Bai, H. Fang, W. He and Z. Guo, ACS Appl. Bio Mater., 2023, 6, 3406–3413 CrossRef CAS PubMed.
- S. Cheng and J. Li, Biochem. Biophys. Res. Commun., 2023, 652, 55–60 CrossRef CAS PubMed.
- N. M. Vinita, U. Devan, S. Durgadevi, S. Anitha, D. Prabhu, S. Rajamanikandan, M. Govarthanan, A. Yuvaraj, M. Biruntha, A. Antony Joseph Velanganni, J. Jeyakanthan, P. A. Prakash, M. S. Mohamed Jaabir and P. Kumar, Sci. Rep., 2023, 13, 2230 CrossRef CAS PubMed.
- J. Wang, J. Wei, Y. Leng, Y. Dai, C. Xie, Z. Zhang, M. Zhu and X. Peng, Biosensors, 2023, 13, 324 CrossRef CAS PubMed.
- L. Zhao, M. Chang, Z. He, Y. Zhao, J. Wang and Y. Lu, ACS Appl. Polym. Mater., 2023, 5, 1530–1538 CrossRef CAS.
- C. C. Karanlık, G. Y. Atmaca and A. Erdoğmuş, J. Mol. Struct., 2023, 1274, 134498 CrossRef.
- H. Yalazan, H. Kantekin and M. Durmuş, New J. Chem., 2023, 47, 7849–7861 RSC.
- C. Liu, Y. Wang, S. Wang, P. Xu, R. Liu, D. Han and Y. Wei, Polymers, 2023, 15, 509 CrossRef CAS PubMed.
- Y. Yang, N. Li, Y. Zhu, J. Li, S. Li and X. Hou, Talanta, 2023, 259, 124493 CrossRef CAS PubMed.
- R. Tan, J. Ge, C. Wang, Y. Wan and X. Yang, Carbohydr. Polym., 2023, 311, 120748 CrossRef CAS PubMed.
- N. Singh, P. Kumar, R. Kumar and U. Riaz, Ind. Eng. Chem. Res., 2019, 58, 14044–14057 CrossRef CAS.
- N. Singh, E. S. Aazam and U. Riaz, J. Mol. Struct., 2021, 1240, 130533 CrossRef CAS.
- N. Singh, S. Singh, S. M. Ashraf and U. Riaz, Colloid Polym. Sci., 2020, 298, 1443–1453 CrossRef CAS.
- N. Singh, M. Arish, P. Kumar, A. Rub and U. Riaz, Sci. Rep., 2020, 10, 57 CrossRef CAS PubMed.
- L. Zhou, F. Lv, L. Liu and S. Wang, Acc. Chem. Res., 2019, 52, 3211–3222 CrossRef CAS PubMed.
- L. Cheng, C. Wang, L. Feng, K. Yang and Z. Liu, Chem. Rev., 2014, 114, 10869–10939 CrossRef CAS PubMed.
- W. Wu, G. Feng, S. Xu and B. Liu, Macromolecules, 2016, 49, 5017–5025 CrossRef CAS.
- Z. Cong, S. Xie, Z. Jiang, S. Zheng, W. Wang, W. Wang and H. Song, Chem. Eng. J., 2022, 431, 133748 CrossRef CAS.
- X. Tan, J. Wang, X. Pang, L. Liu, Q. Sun, Q. You, F. Tan and N. Li, ACS Appl. Mater. Interfaces, 2016, 8, 34991–35003 CrossRef CAS PubMed.
- W. Sun, X. Wang, Z. Cheng, X. Wang, N. Fan, P. Dong, M. q. Tong, Y. Liu and W. Sun, Biomed. Pharmacother., 2023, 158, 114071 CrossRef CAS PubMed.
- M. Nave, F. J. P. Costa, C. G. Alves, R. Lima-Sousa, B. L. Melo, I. J. Correia and D. de Melo-Diogo, Eur. J. Pharm. Biopharm., 2023, 184, 7–15 CrossRef CAS PubMed.
- L. Chen, X. Li, M. Xiong, Y. Zhao, S. Liu, C. Li and K. Wang, Mater. Des., 2023, 225, 111532 CrossRef CAS.
- T. Wu, X. Lu, Z. Yu, X. Zhu, J. Zhang, L. Wang and H. Zhou, J. Mater. Chem. B, 2023, 11, 1213–1221 RSC.
- Q. Wu, Y. Zhu, X. Fang, X. Hao, L. Jiao, E. Hao and W. Zhang, ACS Appl. Mater. Interfaces, 2020, 12, 47208–47219 CrossRef CAS PubMed.
- R. Peng, Y. Luo, Q. Cui, J. Wang and L. Li, ACS Appl. Bio Mater., 2020, 3, 1305–1311 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |
