Open Access Article
Open Access ArticleTo flow or not to flow. A perspective on large-scale stationary electrochemical energy storage
Anukriti
Pokhriyal
 a,
Daniel
Rueda-García
a,
Daniel
Rueda-García
 a and
Pedro
Gómez-Romero
a and
Pedro
Gómez-Romero
 *ab
*ab
aCatalan Institute of Nanoscience and Nanotechnology, ICN2 (CSIC, BIST) Campus UAB, Bellaterra, 08193 Barcelona, Spain. E-mail: pedro.gomez@icn2.cat; Fax: +34 936917640; Tel: +34 937373608
bConsejo Superior de Investigaciones Científicas (CSIC), Spain
First published on 31st October 2023
Abstract
Energy storage is experiencing a renaissance as a result of the growing number of vital applications such as internet of things, smart grids, electric vehicles, renewable energy storage, etc. In particular, stationary energy storage must be urgently deployed at a large-scale to support full deployment of renewables and a sustainable grid. Electrochemical energy storage systems (EESS) will be key in this pursuit. Yet, present mature technologies are all sub-optimal. A myriad of new battery chemistries are being developed in research labs, each with specific strengths and drawbacks. EESS technologies such as lithium-ion batteries, lithium–sulfur, metal–air and other post-lithium technologies, but also supercapacitors, hybrid devices and redox flow batteries (RFBs), could benefit from collaborative development between top-down systems engineering and bottom-up material science. Lithium and post-lithium systems have been extensively researched and reviewed. This perspective article examines the energy storage landscape that goes from state-of-the-art flow cells to novel flowing and stationary technologies. While there is a wide scope for improvement of first generation RFBs, a wealth of novel concepts such as ambipolar electrolytes or shuttle cells to semisolid electrodes are also emerging. This could boost the energy density of these flowing systems. In our analysis we also propose other new concepts which could eventually lead to non-flowing large-scale electrochemical energy storage.
Introduction
True security of energy supply is the final and decisive reason to accelerate our transition to a sustainable energy model (as if the climate emergency, depletion of fossil fuels, and illnesses derived from polluting vehicles were not enough). The share of renewables in our energy mix must keep growing, both concerning centralized and distributed generation. This growth will only be possible if a concerted effort for energy storage takes place simultaneously in order to compensate for solar and wind power intermittency. However, energy storage has been a neglected piece of our global energy cycle, until the previous decade.The turning of the century marked the use of lithium-ion batteries (LIBs) as the ultimate technology to power consumer electronics. But now we are witnessing a Cambrian explosion of energy storage variety as present-day demands range from tiny set-and-forget supercapacitors in electronic devices, to electric vehicle batteries, renewable energy storage as well as supercapacitors and batteries for grid balancing. Therefore, as our requirements become more varied and our reliance on stored energy increases, a creative effort will be needed to properly match these emerging storage needs with storage technologies. This has resulted in a feverish search for better LIBs and beyond. Thus, lithium–sulphur, metal–air, Na, K, Mg, Ca, Zn or Al rechargeable batteries, as well as other technologies, such as supercapacitors, hybrid devices and various types of flow cells are being intensely researched and developed.
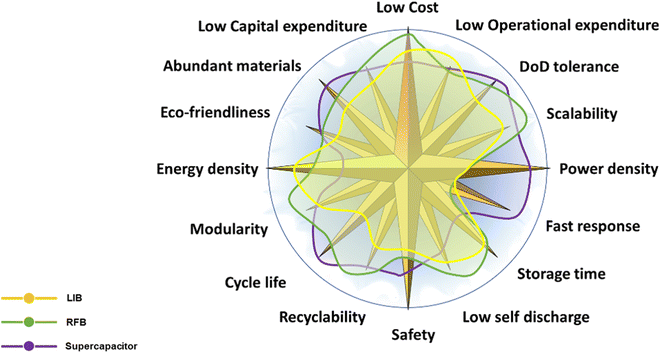
Nevertheless, the uptake of batteries in our power systems still remains quite low. We believe that this is primarily due to the current high cost of electrochemical energy storage systems (EESS) (although the prices have dropped sharply in recent years), but also due to the fact that there is not yet an ideal chemistry that meets all the requirements for large-scale storage. Fig. 1 shows key performance parameters of EESS and the relative performance of three main technologies. While we wait for a silver-bullet technology properly addressing all of them, we could work on co-developing application-specific technologies by improving specific design features.
 | ||
| Fig. 1 Multiple target features demanded from energy storage technologies and the capabilities of different EESS. | ||
At the moment, two technologies prevail when it comes to EESSs, namely conventional LIBs and redox flow batteries (RFBs). LIBs had a head start benefitting from a widening of their vertical applications (from consumer electronics to EVs to stationary storage). However, RFBs are catching up due to their specific suitability for large-scale renewable energy storage, associated with their decoupling of energy and power and scalability.
In 2019, 8.8 GWh of LIB capacity was installed for stationary energy storage vs. 0.25 GWh of RFBs.1 How this picture will evolve in the near and mid-term future will depend on the potential of each technology to lower costs and their potential to improve performance. Presently the per kWh installation cost of a 1 MW/4 h LIB system is approximately 448 USD per kWh and that for a 10 MW/4 h system would be 411 USD per kWh. On the other hand, the installation costs of Vanadium RFBs for 1 MW/4 h and 10 MW/4 h would be 601 USD per kWh and 554 USD per kWh, respectively. By 2030, these costs are expected to grow by at least 100 USD per kWh.2
Economy of scale would benefit all of these technologies, but the challenge remains matching technical improvements with cost-effectiveness. For instance, as discussed below, RFBs should improve energy density per volume of pumped electrolyte, whereas a great improvement on the cyclability of LIBs will be a must if they are to be employed for storing electricity from renewable plants, which are capable of producing electricity for decades. Moreover, both these technologies will need to keep lowering their price tag in order to be massively deployed. On the other hand, new EESS competing technologies are being developed which could challenge these two technologies. But how are they being developed and how long will it take them to hit the market?
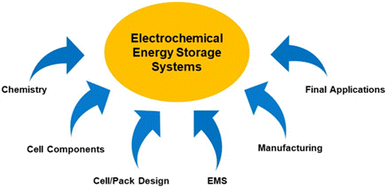
The development of any electrochemical storage technology is a complex process and requires convergence of multiple areas for their large-scale implementation, as shown in Fig. 2. For any EESS technology to be successful, the most important factors are researching its chemistry and testing it on small and large-scale for characterisation. Moreover, cell components such as separators, current collectors, or the electrodes themselves, need to be fabricated, modified, and improved to ensure optimal integration. In addition, a reliable design and assembly of the final device would be required to achieve high performance and efficiency for the transition from research laboratory to the real world. Parallelly, the practical or final applications for the technology should be explored, energy storage management algorithms should be developed, and the new device should be tested through prototypes as well as simulations. Finally, manufacturing and operating costs need to be assessed to incentivise consumers towards investing in the particular technology.
Conventional EESS development has largely followed a one-way bottom-up approach: from chemistry to cell design to eventual applications. Consequently, application developers have to use off-the-shelf technologies, missing opportunities for custom design. We believe that these conventional orthogonal paths require an overhaul coming from collaborative efforts among all stakeholders. A top-down approach that starts with identifying the requirements from an EESS and proceeds towards co-design and co-development with materials chemistry and device engineering stakeholders, will have a greater impact towards a universal development and uptake of EESS.
This article focuses on the connection between chemistries performances and final applications requirements in a key strategic area: large-scale stationary energy storage. Numerous studies have been carried out to identify possible applications for available energy storage technologies3–5 and techno-economic analyses have been conducted to evaluate their costs and benefits.6–8 Nonetheless, the uptake of EESS, specifically batteries, remains quite low. We believe that this is not just due to the current high costs of EESS or the challenges with energy policies but also due to the fact that all of the mature chemistries currently available have significant drawbacks. Therefore, this perspective analyses the two main present contenders for bulk energy storage: LIBs and RFBs. We discuss the factors hindering their use in large-scale energy storage applications. Finally, we examine the latest promising research beyond these technologies and propose possible paths for their co-development from materials to final applications.
Stationary energy storage: one technology fits all vs. custom chemistries for various final applications
As most countries move towards cleaner ways of generating energy, renewable energy technologies such as solar and wind will be at the forefront of combatting climate change. However, these can cause large fluctuations in the grid due to their variable and intermittent nature. Energy storage (particularly EESS) will be essential for storing the excess energy generated and releasing it during times of higher demand or lower generation. At the end of 2020, renewables accounted for 2799 GW of capacity worldwide, of which solar and wind combined had a share of 52%.9 Yet the installed capacity of EESS technologies was only 10 GW,10 clearly showing the huge gap between generation and storage.The question is not only to get more storage but also the appropriate type of storage. The growing complexity of our grid has led to an increasing variety of final applications, most of them resting on a stable power supply. Depending on these applications, the requirements demanded from the energy storage technology would also differ. Therefore, before an in-depth assessment of EESS, a study of possible applications for these technologies should be carried out. The US department of energy (DoE) defines use-cases for stationary grid connected energy storage on the basis of applications and the sector (that is, utility, residential, industrial, etc).11Table 1 summarizes and groups them for the purpose of our perspective.
| Final application | Description |
|---|---|
| Ancillary services | To provide power for a short duration of time to maintain the balance between electricity supply and demand as well as the frequency of the power grid. That is, the battery serves as a reserve for services such as frequency regulation |
| Energy shifting or energy arbitrage | Store energy when utility prices are low and feed it back into the grid (or the consumer directly) when the utility prices are high |
| Renewable energy storage | One of the most common applications is to store excess energy generated by either large wind or solar power plants or small rooftop solar units. This energy can then be used when generation is not available or not sufficient. For example, to power a house after sunset |
| Transmission and distribution line deferral | When electricity demand rises, there is a need to upgrade the transmission and distribution (T&D) line network. Batteries could instead be used to meet a short-term incremental increase in power demand instead of upgrading the entire T&D network |
| Uninterrupted power source | Several essential services such as telecommunication network towers and data centres require power back-up in case of a power cut. Batteries can replace the diesel generators that are commonly used for this purpose |
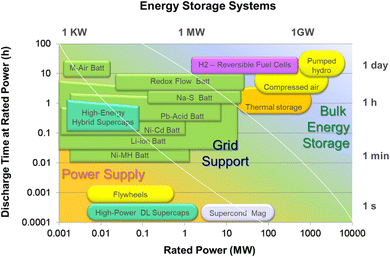
Each of these applications will require specific storage characteristics which will also depend on the sector and the project scale. Fig. 3 depicts a variety of energy storage technologies on a plot that underscores the nominal power and discharge time for each of them.12 Pumped hydro is the only energy storage technology with practical implementation in the GW-scale and can be ideal for the application of renewable energy storage but only in large-scale and in the utility sector. However, it is highly dependent of the topography of the area and thus cannot be set up easily everywhere.
EESS are catching up, and are boosted by the growth of a distributed energy model because they are best suited for the intermediate 1–100 MW area labelled as “Grid Support”, as shown in Fig. 3. Within the category of EESS, a large number of technologies are available that serve specific storage needs. However, in view of the growing number of alternative battery chemistries and storage technologies being developed, an even greater variety of such technologies will soon be available.
Sodium–sulfur (NaS) batteries were initially deployed for medium-scale stationary storage applications. However, due to safety issues with short circuiting through beta-alumina separators, as well as lack of scalable systems, NaS battery use remains very limited. At present, LIB is the most widely used technology. LIBs have paved the way for wearable consumer electronics and are now being used to power electric vehicles (EVs) as well as to store increasing amounts of renewable energy. Essentially, the same technology that powers our cell-phones is massively used in series and parallel cells configurations to store several hundred MWh of renewable energy as well as to power EVs. This might have been adequate as a practical starting point but settling for universal use of LIBs in the long run is not wise given their intrinsically limited cycle life.
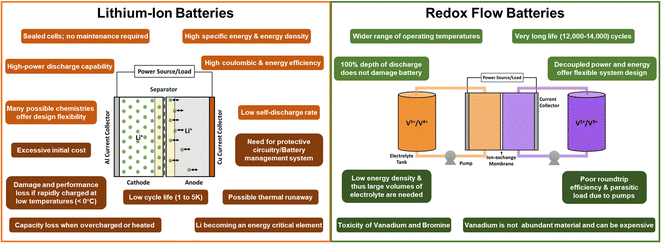
A feasible contender of LIBs for large-scale energy storage are Flow Batteries, especially RFBs. As described by the name, RFBs rely on redox reactions and the electrolytes (containing the active materials) are stored in external tanks, separated by a membrane and pumped into the cell. Fig. 4 describes the advantages and drawbacks of LIBs and RFBs.
LIB cells use solid electrode materials in a “material-intensive” design whereas RFBs make use of large volumes of lower-concentration active material solutions in “material-extensive” systems. These characteristics bring about advantages and drawbacks for each of them. LIBs have intrinsically coupled energy and power and are handicapped by high costs and short lives for large-scale applications. On the other hand, RFBs' inherent design decouples energy and power of the electrochemical cell by externalizing the storage of the active solutions. RFBs have lower costs but provide much lower energy density. Nonetheless, both technologies face the challenge of recycling materials. Lithium and vanadium are neither easily available nor environmentally friendly and require appropriate recycling and disposal methods.
Therefore, there are many opportunities for new concepts, new materials and new devices in the field of electrochemical energy storage, both among post-lithium chemistries as well as within the field of RFBs. Concerning the former, a wide variety of systems have been extensively researched and reviewed, including Li–S or metal–air batteries, as well as Na, K, Mg, Ca, Zn, or Al-ion systems. However, despite the greater suitability of RFBs for bulk energy storage, less discussion has been devoted to their novel developments and related emerging technologies. For this reason, we will focus on these new concepts in the next section. New materials but also new types of materials have been reported to go beyond well-established state-of-the-art-RFBs. From semisolid flow batteries to nanofluids or 2D-confined liquids, the field is blooming with new concepts and ideas as well as new designs of flow cells.
From flow batteries to flow supercapacitors and beyond
Electrolytes are important in every electrochemical storage device. They provide ionic conductivity in conventional batteries and supercapacitors. But in RFBs electrolytes are the essential core components as they contain active storage materials. So along with the properties marked by the solvent, the cell performance is determined by the different electroactive species dissolved in the negolytes (in contact with the negative) and posolytes (in contact with the positive current collector). An electrolyte can also provide additional capacity in non-flowing cells. For example, electrolytes containing redox active species such as iodide or hexacyanoferrate or even quinones, have been used to increase the energy density of supercapacitors.13–15Conventional RFBs
Iron–chromium (Fe/Cr) RFBs were first published by Thaller, L. H. in 1974.16 The technology had a specific energy of 15 W h kg−1 with good reversibility and fast kinetics of Fe3+/Fe2+ redox couple in an acidic electrolyte. However, the slow kinetics of Cr3+/Cr2+ require an elevated operational temperature (∼65 °C)17 or catalysts like Bi o Bi/Pb on carbon,18 which significantly increases the cost. In addition, the low redox potential of Cr3+/Cr2 may cause H2 evolution, which limits the coulombic efficiency and cycle life.17,18Halogen RFBs are based on a soluble metal ion in the anodic side and in general bromine (Br) in the cathodic side for example, Zn/Br and V/Br. However, Br2 vapor generation can cause corrosion problems in these batteries. Many other redox couples have been tried over time standing out: polysulfide/Br, Zn/Cr, Zn/Mn, or Zn/halogen, but none of them could improve the performance of the vanadium RFB.18
Vanadium RFBs (VRFBs) are the most mature and predominant chemistries for flow batteries. Many VRFB projects have already been implemented for storing large amounts of energy and in 2016, China approved the largest VRFB project (200 MW/800 MWh) for grid stability and energy arbitrage applications.11 However, VRFBs have low energy densities (25–35 Wh L−1) due to solubility limits.19 They also suffer from corrosion issues, and the high price of vanadium. Recent research on VRFBs is largely focused on cell optimization i.e. (i) development and performance of the membrane to improve internal resistance, (ii) cell improvement: effects of corrosion, temperature and cell voltage.19
Alternative RFB chemistries: aqueous vs. non-aqueous RFBs
The aqueous or non-aqueous nature of a flow cell is determined by the electrolyte solvent. In addition to this classification, RFBs can be classified as organic or inorganic if the electroactive solute is an organic or inorganic species, respectively. For example, all-vanadium systems are inorganic aqueous RFBs whereas water-soluble sulfonated quinones can be classified as organic aqueous RFBs.Aqueous electrolytes are the most commonly used because of their safety and low cost. They can also provide high power densities due to their greater ion mobility, however the low voltage restriction (∼1.2 V) is detrimental to achieving high power densities. On the other hand, the use of metal ions, such as vanadium, as electroactive redox materials leads to extended cyclability when compared with conventional solid-electrode batteries. However, they are burdened both by thermodynamic and kinetic limitations, which calls for novel materials to be introduced. The drawbacks of VRFBs have promoted research on alternative flow chemistries. For example, “All-Iron” flow chemistries have experienced a very recent positive development and are now available for large-scale use. These are being pioneered as the new-generation of RFBs for large-scale energy storage since iron could resolve the high-cost challenges associated with vanadium.11 The technology involves plating ductile Fe on the negative electrode. The company, ESS INC claims its iron flow technology can provide over 6–12 hours of storage and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles without capacity degradation.20
000 cycles without capacity degradation.20
Another area of improvement deals with the use of non-aqueous organic electrolytes. Organic redox-active molecules soluble in organic solvents are a relatively recent topic in RFB research but have gained enormous importance and relevance because of their wider window of electrochemical stability and flexibility leading to higher energy densities. Conversely organic solvents have higher costs, safety issues and lower power densities.21,22 Furthermore, in certain cases they have also shown low stability and poor cyclability.23–25 This could be attributed to high concentrations causing unwanted irreversible and spurious reactions26 as well as a dramatic reduction in the energy density upon repeated cycling.
Despite the poor solubility of organic compounds in water, water soluble organic compounds can be promising since water is cheap, abundant, eco-friendly, safe and has a great ionic conductivity. In recent years, significant developments in “organic RFBs” and “hybrid inorganic/organic systems” have taken place with the introduction of new groups of highly soluble organic molecules which are capable of providing superior cell voltages and charge capacity comparable to conventional metal-based systems or even better. However, their performance is negatively impacted by very high viscosities as the charge carrier mobility is reduced while the energy needed for electrolyte circulation is increased.27 However, recent works have significantly improved performance with good cycling and high capacity retention capabilities.28–31
Metal–organic RFBs and organometallic compounds and have also been studied to demonstrate stability at hundreds of cycles with high volumetric capacities, improved solubility, shift the potential of the active redox species and to increase the potential window.32 For example, Robb et al. successfully demonstrated chromium-ion based electrolyte with 97% efficiency.33
Redox active ambipolar electrolytes are one of the most promising solutions for RFB technology. An ambipolar electrolyte is one which contains in itself species able to charge and discharge in each of the electrodes of the device. Therefore, the electrolyte, eliminates the need for non-active counter ions. These electrolytes allow an increase in total active material inside the battery which would theoretically help increase the energy density of the flow batteries. As an example, ZnI2 would allow for the harnessing of the Zn− → Zn2+ and the I− → I3− redox couples simultaneously. An RFB developed with ZnI2 couple as an ambipolar electrolyte demonstrated an energy density of 167 Wh L−1.34 Another study showed a (ZnI2 + ZnCl2) based water-in-salt ambipolar redox electrolyte in a micro supercapacitor to show high energy densities and long-term cycling stability.35 However, precipitation challenges and high viscosity limit the scaling-up of these systems.
The energy density vs. current density of various RFB systems is shown in Fig. 5. Aqueous systems (black and green) have higher current densities, and subsequently higher power densities. Meanwhile, non-aqueous or organic electrolyte systems (orange and red) are concentrated in the low current density region regardless of whether they have low medium or high energy density.27
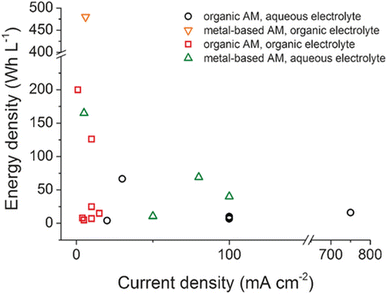 | ||
| Fig. 5 Energy density versus current density of selected RFB systems (AM = active material). Reprinted with permission from ref. 27, copyright 2016, Wiley. | ||
So how can the energy densities of RFBs be further improved? Increasing the working voltage and specific capacity are two possible lines of action. Higher voltages can be achieved by a proper choice of electrolyte and by electrode engineering through molecular design. On the other hand, favourable equivalent weights, with a high charge/mass ratio, as well as higher concentrations of active species would lead to greater capacities.36
From solutions to dispersions
Nanofluids are stable dispersions of nanoparticles in a base liquid medium which can “flow” as a primary characteristic due to low viscosities. Thus, electroactive nanofluids (ENFs) are promising for increasing the effective concentration of electroactive species, while maintaining lower viscosity for efficient flowing.37 2D-Materials with nanoconfined liquids, could be a first step for developing materials with an “internal electrolyte”.38 However, the primary challenge is to push forward the “solubility limit” of dispersed nanoparticles and ensure total utilization of all electroactive particles dispersed (through flowing or enhanced electron-transfer).39–41 ENFs have shown both capacitive and faradaic properties with full utilization of the active material, fast charge transfer and low viscosity.42 In fact, performance of conventional VRFBs can also be improved by adding graphene derivates.43 On the contrary, faradaic nanofluids can be formulated with just redox-active nanoparticles or clusters or with multiple redox couples.44,45Semisolid Redox Flow Batteries (SSFBs) were first proposed to skip solubility limits and improving energy densities of RFBs. Viscous carbon slurries (Fig. 6) have been proven to improve electrical conductivity within flow cells.46 Yet-Ming Chiang and col. developed the first SSFB in 2011 (ref. 47) based on Ketjen Black (KB) delivering maximum 397 Wh L−1 and 168 Wh kg−1. Their spin-off company, 24M, was created to commercialize this new concept.48 Similarly, the concept has been translated to Electrochemical Flow Capacitors (EFCs) to demonstrate carbon slurries for storing energy through a purely capacitive mechanism.49
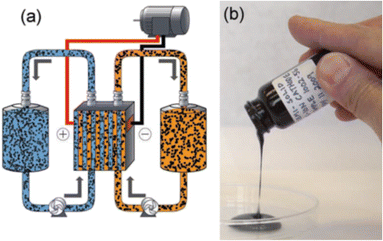 | ||
| Fig. 6 (a) Schematic diagram of a SSFB and (b) picture of typical electroactive paste. Reprinted with permission from ref. 47, copyright 2011, Wiley. | ||
The research into SSFBs and EFCs is relatively new and ongoing. It has been observed that the performance can vary significantly depending on carbon particle size, shape and concentration as well as activation, surfactants and flow mechanism.47,50–53 Along with carbons, several types of battery materials have been tested for SSFBs, such as lithium47,54–60 Mn–sulphur, Zn/polyaniline, MnO2/polypyrrole54 and Zn slurries.61 Proof of concept studies for sodium based SSFBs have also been carried out but they have lower energy densities.62 Alternatively, another emerging field of study is SSFBs without carbon slurries using conductive polymer microparticles.63
SSFBs are very promising for resolving the dilemma of storing large amounts of energy without compromising on the energy density. Nevertheless, more research is needed to gain a better understanding of the properties and interactions of different carbon particles and conducting polymers. At the same time, precipitation challenges and possible surfactants need to be evaluated for static and flow conditions.
Liquid metal batteries follow a new line of research to overcome the challenges of conventional solid state and flow batteries. The battery comprises molten metals separated by a molten salt to allow high voltage efficiencies at high current densities. These batteries have fast charge transfer kinetics and high rate capabilities owing to their liquid–liquid electrolyte interface.64 The MIT spin-off Ambri uses low-cost and commercially materials such as calcium and antimony based electrodes and calcium chloride electrolytes.65 However, liquid metal batteries need to be operated at higher temperatures (>200 °C) and have low theoretical specific energy density. Moreover, they can suffer from self-discharge due to solubility of the electrode species in the molten salt electrolyte.64
Perspective
When it comes to storing large amounts of charge in a battery to satisfy the new demands of our power grids, we can apparently rely either on systems using large amounts of active material externalized out of the electrochemical cell or choose a highly concentrated active material. The first approach is the one taken in RFBs whereas the second is characteristic of conventional (i.e. LIB) batteries, where about 80% of the electrode mass corresponds to active materials. But, why not both? What prevents the design of cells with “extensive” and at the same time “intensive” electrode materials?Top down approach to match applications with chemistries
It is clear that most stationary applications require EESS to have high power and energy densities, several years of lifespan, ability to respond within milliseconds and high efficiencies. Therefore, as batteries become an integral part of our evolving energy systems, there is an urgent need to move away from conventional Li-ion based batteries for all end-user applications. RFBs have undergone a relatively modest evolution from initial Fe/Cr systems into the now mature, all-vanadium and the emerging all-iron technologies. But further breakthroughs similar to those provided by ambipolar electrolytes or highly concentrated solutions are still needed to overcome the poor energy density associated with low solubility limits. Isn't there any highly soluble electroactive species to play with in this respect? Or even better, wouldn't it be great to have a bulk electroactive liquid? A pure flowing substance capable of reversibly exchanging one or maybe two electrons per molecule? As a matter of fact, such a compound exists. We call it water. But again, reversible Fuel Cells are far from being problem-free. Poor kinetics derived from the need of a triple-phase-boundary (electrode–electrolyte–gas) negatively impact the thermodynamics. Another unsolved problem is that low temperature Fuel Cells still need to break away from their well-known dependence on platinum.In reality, none of the electrochemical energy storage technologies explored so far are problem-free and have complementary advantages and drawbacks. As a result, new research needs to explore different designs and systems somewhat in-between conventional RFBs and conventional solid-state batteries (LIBs) such as the flowing shuttle concept66 SSFBs, nanofluids, fluid-confining 2-D materials or water-soluble redox-active polymers. In the flowing shuttle concept, a soluble electroactive species is “charged” in the corresponding electrode and then flowed as a “shuttle” to transfer that charge to a solid (non-flowable) redox material that can accumulate large capacities. Of course, the redox potential of the shuttle must be adequate for matching the redox potential of the solid phase. Nonetheless, currently all of the other new technologies also present serious drawbacks. For example, 2D-confined liquids could suffer from over-confinement of liquid electrolyte in the bulk solid electrode structure, whereas electronic conductivity of nanofluids could be hindered by low concentration of solid nanoparticles dispersed in liquid phase. On the bright side, electroactive fluids and slurries have advantages of fast ion diffusion, high specific power and tolerance for volume expansion compared to conventional solid electrodes.
The way forward: From flowing solutions to flowing dispersions…. To non-flowing dispersions?
Increasing electrolyte accessibility to the active electrode sites without losing electrical conductivity would be the most important factor for enhancing power and cyclability without losing capacity and energy density. But this is easier said than done, and unexpected difficulties frequently arise. For instance, the nanoconfinement of liquids in 2D solids was proposed to improve ionic transport; but it facilitates electrode exfoliation on repeated cycling.38 However, this apparent detrimental consequence could be turned into an advantage within the framework of novel designs of electrodes and devices.In this context, the field could evolve towards the design of electroactive bulk “nanopastes” (Fig. 7) which could work as effectively as thick solid electrodes and which would not need to be pumped. Napptilus Battery Lab has provided proof of concept studies for non-flowable nanopastes.67,68 This alternative design could strongly contribute towards lower battery manufacturing costs and would be most important within the upcoming framework of battery giga-factories. After all, “pastes” are well known by the industry when it comes to primary conventional cells. Why not then for large-scale rechargeable batteries?
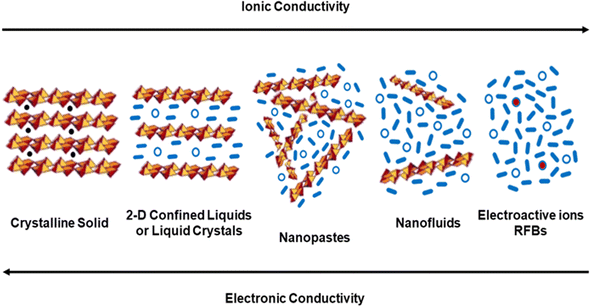 | ||
| Fig. 7 Schematic comparative diagram of the stages involved in moving from compact solid electrode materials to dilute nanofluids and RFB solutions. | ||
Flowing systems could keep breaking records for the energy density of their fluids while nanopaste electrodes could be designed as extra-thick or massive electrodes initiating a battery revolution which rests equally on the integration of materials and components in the devices as well as on the nature of the materials and components. However, nanopastes are affected by their dependence on pressure of the system or the need to fine tune a compatible electron-conducting network with an electrolyte transport network. This means there are plenty of opportunities for interdisciplinary research involving materials chemistry, surface science, nanoscience, electrochemistry, and engineering. Table 2 summarizes all the technologies discussed in this paper.
| Mechanism | Characteristics | Example | |
|---|---|---|---|
| Aqueous RFBs | Reversible redox cycling of electroactive species in solution | Flowable and pumped | All iron RFBs20 |
| Non-aqueous RFBs | Reversible redox cycling of electroactive species in organic electrolyte | Flowable and pumped | Li/Fc-TFSI in EC/PC/EMC mix23 Li/TEMPO in EC/PC/EMC mix25 |
| Hybrid organic/inorganic RFBs | Reversible redox active organic species in aqueous solutions | Flowable and pumped | MV/4-HO-TEMPO organic aqueous RFB28 |
| Organometallic and metal–organic RFBs | Reversible redox active organometallics or coordination compounds in aqueous solutions | Flowable and pumped | Aqueous CrPDTA RFB33 |
| Ambipolar electrolyte RFBs | Reversible redox ambipolar species in aqueous solutions | Flowable and pumped | Aqueous ZnI2 electrolyte34 |
| Liquid metal batteries | Reversible redox reactions of molten metals separated by a molten salt | Non flowable and non-pumped | Ca/Sb with CaCl2 base salt electrolyte65 |
| Semi solid flow battery | Viscous slurries using reversible redox active compounds typical of batteries | Flowable with continuous or intermittent pumping | LiCoO2 + KB in 1.3 M LiPF6 alkyl carbonate blend/Li4Ti5O12 + KB in 1 M LiPF6 in DMC47 |
| Electrochemical flow capacitors | Capacitive charging of suspended carbon particles | Flowable with continuous or intermittent pumping | Spherical carbon beads in 1 M Na2SO4 & 1 M TEABF4 in PC49 |
| Nanofluids | Highly concentrated reversible redox nanoparticles in low viscosity fluids | Flowable and pumped | rGO-PMo12 and rGO-PW12 in 1 M H2SO4 (ref. 37) |
| Nanopastes | Very high viscosity nano dispersions in an electrolyte for capacitive or reversible redox energy storage | Flowable and non-flowable, non-pumped | Capacitive (AC) and hybrid systems (i.e. AC-POMs) from Napptilus Battery Labs67 |
The race is on for the development of the ultimate solution in order to make massive energy storage possible. While none of the contenders seem to have reached optimal performance, both flowing and non-flowing designs could make it, with the various chemistries and cell designs possibly addressing combined needs of the niche applications. That is, high power densities with many hours of energy storage capabilities, extremely fast response time as well as high efficiency, low costs and environmentally friendly materials. Fig. 1 summarized the requirements demanded by end user applications from EESS. Getting all these performance features converging in various EESS technologies is certainly a difficult challenge, which will be most efficiently tackled by united efforts of the different sectors involved, as shown in Table 3.
| Application | Novel technology | Properties |
|---|---|---|
| Ancillary services | EFCs ENFs | Fast response (ms), high power density |
| Renewable energy storage and energy shifting or arbitrage | RFBs with organic electrolytes, metal organic compounds, redox active ambipolar electrolytes, iron flow, liquid metal, SSFBs, nanopastes | Large energy density, lower cost |
| Transmission and distribution line deferral | Iron flow, RFBs with organic electrolytes, nanopastes | Lower cost, low self-discharge, large energy density |
| Uninterrupted power source | Iron flow, RFBs with organic electrolytes, nanopastes | Lower cost, low self-discharge, large energy density |
For example, ancillary services require high power in a very short time period for grid balancing, which can be fulfilled by EFCs or ENFs. However, applications requiring large amounts of storage over longer time periods, such as RE storage, need high energy densities which are possible through RFBs with organic electrolytes. Thus, we note the need for a wide variety of different technologies able to address specific application demands, both in flowing as well as in non-flowing systems. But in addition to the present portfolio of researched technologies, we believe it is important to stress the need for further innovation. The future large-scale energy systems will undoubtedly need to move beyond the conventional solid state or flow regimes to incorporate not only new materials but also new types of materials, devices and systems to solve the grand challenge of ubiquitous energy storage.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
A. P. acknowledges her fellowship funded by H2020-MSCA-COFUND-2016, DOC-FAM (75439). The authors acknowledge funding from AEI (projects PID2021-128390OB-I00, TED2021-130205B-C21 and PLEC2022-009328). ICN2 is funded by the CERCA programme/Generalitat de Catalunya and supported by the Severo Ochoa Centres of Excellence programme, Grant CEX2021-001214-S, funded by MCIN/AEI/10.13039.501100011033.References
- E. Sánchez-Díez, et al., Redox flow batteries: Status and perspective towards sustainable stationary energy storage, J. Power Sources, 2021, 481, 228804 CrossRef.
- K. Mongird, et al., 2020 Grid Energy Storage Technology Cost and Performance Assessment, Energy Storage Grand Challenge Cost and Performance Assessment 2020, 2020, DOE/PA-0204 Search PubMed.
- B. Dunn, H. Kamath and T. Jean-Marie, Electrical Energy Storage for the Grid: A Battery of Choices, Science, 2011, 334, 928–935 CrossRef CAS PubMed.
- M. Aneke and M. Wang, Energy storage technologies and real life applications – A state of the art review, Appl. Energy, 2016, 179, 350–377 CrossRef.
- K. C. Divya and J. Østergaard, Battery energy storage technology for power systems—An overview, Electr. Power Syst. Res., 2009, 79, 511–520 CrossRef.
- D. Rastler, Electric Energy Storage Technology Options: A White Paper Primer on Applications, Costs, and Benefits, 2010, p. 1020676, https://www.epri.com/research/products/000000000001022261 Search PubMed.
- M. S. Ziegler, et al., Storage Requirements and Costs of Shaping Renewable Energy Toward Grid Decarbonization, Joule, 2019, 3, 2134–2153 CrossRef.
- P. Poonpun and W. T. Jewell, Analysis of the Cost per Kilowatt Hour to Store Electricity, IEEE Trans. Energy Convers., 2008, 23, 529–534 Search PubMed.
- Renewable capacity highlights, 2021, https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2021/Apr/IRENA_-RE_Capacity_Highlights_2021.pdf?la=en%26hash=1E133689564BC40C2392E85026F71A0D7A9C0B91 Search PubMed.
- Grid-Scale Storage, 2022, https://www.iea.org/reports/grid-scale-storage Search PubMed.
- Energy Storage Grand Challenge Energy Storage Market Report, 2020, https://www.energy.gov/sites/prod/files/2020/12/f81/EnergyStorageMarketReport2020_0.pdf Search PubMed.
- S. Ugarte, et al., Energy Storage: Which Market Designs and Regulatory Incentives Are Needed?, 2015, http://www.europarl.europa.eu/RegData/etudes/STUD/2015/563469/IPOL_STU(2015)563469_EN.pdf Search PubMed.
- G. Lota and E. Frackowiak, Striking capacitance of carbon/iodide interface, Electrochem. Commun., 2009, 11, 87–90 CrossRef CAS.
- B. Nagar, D. P. Dubal, L. Pires, A. Merkoçi and P. Gómez-Romero, Design and Fabrication of Printed Paper-Based Hybrid Micro-Supercapacitor by using Graphene and Redox-Active Electrolyte, ChemSusChem, 2018, 11, 1849–1856 CrossRef CAS PubMed.
- K. Jayaramulu, et al., Ultrathin Hierarchical Porous Carbon Nanosheets for High-Performance Supercapacitors and Redox Electrolyte Energy Storage, Adv. Mater., 2018, 30, 1705789 CrossRef PubMed.
- L. H. Thaller, Electrically Rechargeable Redox Flow Cells, 1974 Search PubMed.
- M. Park, J. Ryu and W. Wang, et al., Material design and engineering of next-generation flow-battery technologies, Nat. Rev. Mater., 2017, 2, 16080 CrossRef CAS.
- G. L. Soloveichik, Flow Batteries: Current Status and Trends, Chem. Rev., 2015, 115, 11533–11558 CrossRef CAS PubMed.
- A. Z. Weber, et al., Redox flow batteries: a review, J. Appl. Electrochem., 2011, 41, 1137 CrossRef CAS.
- C. E. Evans and Y. Song, Electrolytes for Iron Flow Battery, 2017 Search PubMed.
- R. Chen, Toward High-Voltage, Energy-Dense, and Durable Aqueous Organic Redox Flow Batteries: Role of the Supporting Electrolytes, ChemElectroChem, 2019, 6, 603–612 CrossRef CAS.
- J. Cao, Z. Zhu, J. Xu, M. Tao and Z. Chen, Nitrogen-doped porous graphene as a highly efficient cathodic electrocatalyst for aqueous organic redox flow battery application, J. Mater. Chem. A, 2017, 5, 7944–7951 RSC.
- X. Wei, et al., Materials and Systems for Organic Redox Flow Batteries: Status and Challenges, ACS Energy Lett., 2017, 2, 2187–2204 CrossRef CAS.
- J. Huang, et al., A Two-Electron Storage Nonaqueous Organic Redox Flow Battery, Adv. Sustainable Syst., 2018, 2, 1700131 CrossRef.
- X. Wei, et al., TEMPO-based catholyte for high-energy density nonaqueous redox flow batteries, Adv. Mater., 2014, 26, 7649–7653 CrossRef CAS PubMed.
- D. Rueda-García, D. P. Dubal, F. Hugenin and P. Gómez-Romero, Hurdles to organic quinone flow cells. Electrode passivation by quinone reduction in acetonitrile Li electrolytes, J. Power Sources, 2017, 350, 9–17 CrossRef.
- J. Winsberg, T. Hagemann, T. Janoschka, M. D. Hager and U. S. Schubert, Redox-Flow Batteries: From Metals to Organic Redox-Active Materials, Angew. Chem., Int. Ed., 2017, 56, 686–711 CrossRef CAS PubMed.
- S. Gentil, D. Reynard and H. H. Girault, Aqueous organic and redox-mediated redox flow batteries: a review, Curr. Opin. Electrochem., 2020, 21, 7–13 CrossRef CAS.
- T. Hagemann, et al., 2,2,6,6-Tetramethylpiperidin-1-yl)oxyl-Containing Zwitterionic Polymer as Catholyte Species for High-Capacity Aqueous Polymer Redox Flow Batteries, Chem. Mater., 2019, 31, 7987–7999 CrossRef CAS.
- Y. Zhu, et al., Enhanced cyclability of organic redox flow batteries enabled by an artificial bipolar molecule in neutral aqueous electrolyte, J. Power Sources, 2019, 417, 83–89 CrossRef CAS.
- L. Hu, et al., Electrochemical Double-Layer Capacitor Energized by Adding an Ambipolar Organic Redox Radical into the Electrolyte, Angew. Chem., Int. Ed., 2018, 57, 8214–8218 CrossRef CAS PubMed.
- D. P. Trudgeon, et al., Screening of effective electrolyte additives for zinc-based redox flow battery systems, J. Power Sources, 2019, 412, 44–54 CrossRef CAS.
- B. H. Robb, J. M. Farrell and M. P. Marshak, Chelated Chromium Electrolyte Enabling High-Voltage Aqueous Flow Batteries, Joule, 2019, 3, 2503–2512 CrossRef CAS.
- B. Li, et al., Ambipolar zinc-polyiodide electrolyte for a high-energy density aqueous redox flow battery, Nat. Commun., 2015, 6, 1–8 Search PubMed.
- C. Meng, et al., Water-in-Salt Ambipolar Redox Electrolyte Extraordinarily Boosting High Pseudocapacitive Performance of Micro-supercapacitors, ACS Energy Lett., 2022, 7, 1706–1711 CrossRef CAS.
- Y. Ding, C. Zhang, L. Zhang, Y. Zhou and G. Yu, Molecular engineering of organic electroactive materials for redox flow batteries, Chem. Soc. Rev., 2018, 47, 69–103 RSC.
- D. P. Dubal, D. Rueda-Garcia, C. Marchante, R. Benages and P. Gomez-Romero, Hybrid Graphene-Polyoxometalates Nanofluids as Liquid Electrodes for Dual Energy Storage in Novel Flow Cells, Chem. Rec., 2018, 18, 1076 CrossRef CAS PubMed.
- V. Augustyn and Y. Gogotsi, 2D Materials with Nanoconfined Fluids for Electrochemical Energy Storage, Joule, 2017, 1, 443–452 CrossRef CAS.
- S. Sen, C. M. Chow, E. Moazzen, C. U. Segre and E. V. Timofeeva, Electroactive nanofluids with high solid loading and low viscosity for rechargeable redox flow batteries, J. Appl. Electrochem., 2017, 47, 593–605 CrossRef CAS.
- F. Y. Fan, et al., Polysulfide flow batteries enabled by percolating nanoscale conductor networks, Nano Lett., 2014, 14, 2210–2218 CrossRef CAS PubMed.
- D. Rueda-garcia, et al., Battery and supercapacitor materials in flow cells . Electrochemical energy storage in a LiFePO4/reduced graphene oxide aqueous nano fluid, Electrochim. Acta, 2018, 281, 594–600 CrossRef CAS.
- D. P. Dubal and P. Gomez-Romero, Electroactive graphene nanofluids for fast energy storage, 2D Mater., 2016, 3, 031004 CrossRef.
- J. Lobato, E. Mena and M. Millán, Improving a Redox Flow Battery Working under Realistic Conditions by Using of Graphene based Nanofluids, ChemistrySelect, 2017, 2, 8446–8450 CrossRef CAS.
- S. Park, H. J. Lee, H. Lee and H. Kim, Development of a Redox Flow Battery with Multiple Redox Couples at Both Positive and Negative Electrolytes for High Energy Density, J. Electrochem. Soc., 2018, 165, A3215–A3220 CrossRef CAS.
- J. Friedl, et al., Asymmetric polyoxometalate electrolytes for advanced redox flow batteries, Energy Environ. Sci., 2018, 11, 3010–3018 RSC.
- Z. Qi and G. M. Koenig, Review Article: Flow battery systems with solid electroactive materials, J. Vac. Sci. Technol., B: Nanotechnol. Microelectron.: Mater., Process., Meas., Phenom., 2017, 35, 040801 Search PubMed.
- M. Duduta, et al., Semi-solid lithium rechargeable flow battery, Adv. Energy Mater., 2011, 1, 511–516 CrossRef CAS.
- W. C. Carter, Y.-M. Chiang, M. Duduta and P. Limthongkul, High Energy Density Redox Flow Device, 2017 Search PubMed.
- V. Presser, et al., The electrochemical flow capacitor: a new concept for rapid energy storage and recovery, Adv. Energy Mater., 2012, 2, 895–902 CrossRef CAS.
- J. W. Campos, et al., Investigation of carbon materials for use as a flowable electrode in electrochemical flow capacitors, Electrochim. Acta, 2013, 98, 123–130 CrossRef CAS.
- Q. Huang, H. Li, M. Grätzel and Q. Wang, Reversible chemical delithiation/lithiation of LiFePO 4 : towards a redox flow lithium-ion battery, Phys. Chem. Chem. Phys., 2013, 15, 1793–1797 RSC.
- M. Boota, et al., Activated Carbon Spheres as a Flowable Electrode in Electrochemical Flow Capacitors, J. Electrochem. Soc., 2014, 161, A1078–A1083 CrossRef CAS.
- L. Madec, et al., Surfactant for enhanced rheological, electrical, and electrochemical performance of suspensions for semisolid redox flow batteries and supercapacitors, Chempluschem, 2015, 80, 396–401 CrossRef CAS.
- K. B. Hatzell, M. Boota and Y. Gogotsi, Materials for suspension (semi-solid) electrodes for energy and water technologies, Chem. Soc. Rev., 2015, 44, 8664–8687 RSC.
- H. Liu, L. B. Liao, Y. C. Lu and Q. Li, High Energy Density Aqueous Li-Ion Flow Capacitor, Adv. Energy Mater., 2017, 7, 1601248 CrossRef.
- S. Hamelet, et al., Non-Aqueous Li-Based Redox Flow Batteries, J. Electrochem. Soc., 2012, 159, A1360–A1367 CrossRef CAS.
- H. Chen, N. C. Lai and Y. C. Lu, Silicon-Carbon Nanocomposite Semi-Solid Negolyte and Its Application in Redox Flow Batteries, Chem. Mater., 2017, 29, 7533–7542 CrossRef CAS.
- S. Li, et al., A Semiliquid Lithium Metal Anode, Joule, 2019, 3, 1637–1646 CrossRef CAS.
- I. Ruggeri, C. Arbizzani and F. Soavi, Carbonaceous catholyte for high energy density semi-solid Li/O2 flow battery, Carbon, 2018, 130, 749–757 CrossRef CAS.
- F. Soavi, A. Brilloni, F. De Giorgio and F. Poli, Semi-solid lithium/oxygen flow battery: an emerging, high-energy technology, Curr. Opin. Chem. Eng., 2022, 37, 100835 CrossRef.
- F. Chu, L. Guo, S. Wang and Y. Cheng, Semi-solid zinc slurry with abundant electron-ion transfer interfaces for aqueous zinc-based flow batteries, J. Power Sources, 2022, 535, 231442 CrossRef CAS.
- E. Ventosa, et al., Non-aqueous semi-solid flow battery based on Na-ion chemistry. P2-type Na x Ni 0.22 Co 0.11 Mn 0.66 O 2 –NaTi 2 (PO 4 ) 3, Chem. Commun., 2015, 51, 7298–7301 RSC.
- W. Yan, et al., All-polymer particulate slurry batteries, Nat. Commun., 2019, 10, 2513 CrossRef PubMed.
- S. Zhang, et al., Liquid metal batteries for future energy storage, Energy Environ. Sci., 2021, 14, 4177–4202 RSC.
- Ambri, https://ambri.com/solution/.
- Q. Wang, S. M. Zakeeruddin, D. Wang, I. Exnar and M. Grätzel, Redox Targeting of Insulating Electrode Materials: A New Approach to High-Energy-Density Batteries, Angew. Chem., Int. Ed., 2006, 45, 8197–8200 CrossRef CAS PubMed.
- V. Fabián Puerta, et al., Hybrid Paste Electrodes and Cell Stacking for High Power Electrical Energy Storage Devices Search PubMed.
- P. Gomez-Romero, et al., Hybrid Nanogel Electrodes and Cell Stacking in Distributed Architectures for High Power Electrical Energy Hybrid Storage Systems Search PubMed.
| This journal is © The Royal Society of Chemistry 2023 |