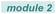Open Access Article
Open Access ArticlePhotoinduced iron-catalyzed C–H alkylation of polyolefins†
Zongnan
Zhang
,
Yanfeng
Zhang
 and
Rong
Zeng
and
Rong
Zeng
 *
*
School of Chemistry, Xi'an Jiaotong University, Xi'an 710049, P. R. China. E-mail: rongzeng@xjtu.edu.cn
First published on 4th August 2023
Abstract
Chemically introducing diverse polar groups into polyolefins via carbon–hydrogen bond alkylation with polar olefins is of substantial value in the synthesis of next-generation lightweight thermoplastics, which is still underdeveloped. In this work, we report a new approach for efficient carbon–hydrogen bond alkylation in commodity polyolefins using photoinduced iron catalysis. Various polyolefins could be functionalized with broad scope. Polar groups could be incorporated in a single step. The controllable synthesis of multi-polar functional polyolefins could be achieved by a designed module-assembled process. Remarkably, even low levels of functionalization could upcycle the polyolefin materials to exhibit unusual physical properties, such as enhancement of the transparencies, strains, stresses at break of the materials, and hydrophilicity.
Introduction
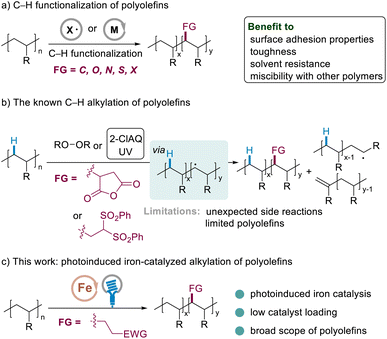
Polyolefins, comprising more than half of plastics, are ubiquitous in human daily life, contributing significantly to a large number of applications such as packaging, food containers, housing, medicine, automotive parts, and electronic products.1 Most polyolefins possess only inert C–H and C–C bonds; not surprisingly, the lack of polar functional groups in the backbone of these polymers makes the materials highly hydrophobic and difficult to interface with other materials, drastically limiting their applications. Incorporating even small amounts of polar groups into these hydrocarbons would enhance properties including surface adhesion properties, toughness, solvent resistance, and miscibility with other polymers, rendering these materials even more versatile and providing a novel opportunity for the synthesis of next-generation lightweight thermoplastics.2Efficient strategies involved the free-radical or transition metal catalyzed copolymerization of simple olefin precursors with polar vinyl monomers.3 However, while radical copolymerization suffered from a high polydispersity index and a high degree of branching, the later one had to use precious metal catalysts. Moreover, different catalytic systems have been typically developed in order to be feasible for different polar alkenes.3b,c
Alternatively, the C–H bond functionalization of commodity polyolefins has drawn chemist's significant attention since it could install polar groups directly into readily available polyolefins.1 This post-polymerization modification would also render these materials more versatile by forming C–C,2 C–O,4a–f C–N,4g C–S,5 and C–X6 bonds, providing new opportunities for the synthesis of next generation lightweight plastics (Scheme 1a).
The radical alkylation of polyolefins with polar olefins is an ideal protocol since polar groups could be installed atom-economically by forming robust C–C single bonds from bulk chemicals. The traditional method involves the use of free radicals generated by the thermal decomposition of peroxides (>150 °C).2a,b Subsequent C–H bond homolysis through hydrogen atom abstraction and radical trapping with unsaturated units, such as maleic anhydride, leads to functionalized polymers. In 2019, the Chen group reported an alternative method by using 2-chloroanthraquinone as an organic photoredox catalyst under UV light to promote the alkylation of polyethylene and polypropylene with 1,1-bis(phenylsulfonyl)ethylene.2d However, both the traditional methods and Chen's protocol suffer from limited polar alkenes, high loading of the radical promotor/catalyst, and unexpected side reactions (Scheme 1b), which would hugely limit the following applications. The development of new catalysis for the radical alkylation of polyolefins with diverse polar olefins remains a challenging issue, in particular using low loading of catalysts.7 Recently, we developed iron catalysis for the C–H alkylation of polyethers, in which degradation could be suppressed significantly using a low loading of the catalyst.8f Herein, as a new direction, we report efficient iron catalysis for the incorporation of polar groups into polyolefins (Scheme 1c).8 The mild visible light induced9 conditions might facilitate the alkylation of polyolefins with a series of readily available polyolefins, while the degradation is not observed significantly. The efficient access to many (multi)functionalized polyolefins exemplifies the strategic power of this method.5 Furthermore, the preliminary examination of the physical properties of the prepared materials exemplifies the untapped potential of this method in synthetic organic and polymer chemistry.
Results and discussion
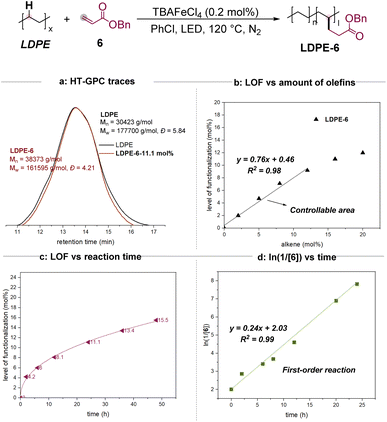
We began our studies by evaluating the reaction using the most prevalent PE (low-density polyethylene, LDPE) and benzyl acrylate 6 as starting materials. The use of TBAFeCl4 and PhCl at 120 °C under a blue LED for 24 h would result in the desired alkylation product LDPE-6 successfully. The incorporation of the functional group in the polymer changed the physical properties significantly. When LDPE could not be dissolved in CDCl3, LDPE-6 presented a much better solubility (see the ESI for detailed images†). The product was characterized by methods that reveal the presence and level of functionalization (LOF) including the 1H NMR (nuclear magnetic resonance), 13C NMR, heteronuclear multiple bond correlation (HMBC), and diffusion ordered NMR spectroscopy (DOSY) spectra in CDCl3. And then, the examined Mn, Mw, and Đ values by GPC indicated that these transformations presented excellent selectivity for C–H functionalization since no significant C–C bond degradation was observed (Scheme 2a). As a comparison, the reaction using benzoyl peroxide (BPO) as a radical initiator obtained only 2.2 mol% of LOF and observed significant degradation (see the ESI for more details†). Moreover, to address the concern of whether the oligomers of the acrylate were attached to the main chain of the polymer, the model reactions of cyclohexane or dodecane with methyl or benzyl acrylate were then conducted. The isolation and characterization of the mono- and di-alkylation products highly suggested the acrylate groups be incorporated into the main chain individually, although we cannot rule out the possibility of a dimer or oligomer-grafted process (see the ESI for more details†).The level of functionalization (LOF) could be controlled by the amounts of the alkenes (Scheme 2b). When less than 12 mol% of 6 was used in the 24-hour reactions, the LOFs depended on the amount of alkene (mol%) in terms of a linear function y = 0.76x + 0.46 with R2 = 0.98. The LOF could reach a maximum of 11.1% by using 20 mol% of 6. Moreover, the reaction was monitored by 1H NMR. When 20 mol% of 6 was used initially, the LOFs were 4.2% in 2 h, 6.0% in 6 h, 13.4% in 36 h, and 15.5% in 48 h, respectively (Scheme 2c). A linear relationship was observed for ln(1/[6]) vs. reaction time (Scheme 2d), indicating a first order dependence of the reaction rate with alkene 6.
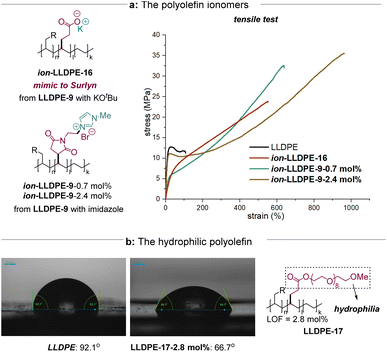
We next investigated the scope of electron-deficient alkenes with different polar functional groups by modifying the C–H bonds in LDPE based on the optimal conditions (Table 1). A series of polar alkenes could be converted via iron catalysis. Polar groups, such as ester (5–6) and sulfonyl groups (7), were able to be incorporated into the LDPE matrix. When 20 mol% of the starting alkene was used, the level of functionalization could reach up to 11.1%. Maleimide and its derivatives are able to graft LDPE successfully (8–10), which might provide a new reactive site for the additional functional group transformation. For example, the maleimide derivative 9 with a properly reactive site for nucleophilic substitution was converted to obtain the corresponding product LDPE-9 with 10.0 mol% LOF, while the terminal bromo group was remained. Pleasantly, the maleic anhydride 10, well known as a bulk chemical as well as a significant intermediate, is also a good substrate for C(sp3)–H bond functionalization of LDPE, albeit observing β-scission in the GPC test. The difunctional groups containing olefins, such as 11–13, are tolerated well to afford the corresponding polar polyolefins with 4.1–8.2% LOFs. Notably, no chlorination product is observed as determined using NMR spectra.3d
| a All levels of functionalization (LOFs) (given as a percentage) are determined by 1H NMR. b Experiments were typically run with a polyolefin (2.0 mmol monomer), polar alkene (20 mol%), iron catalyst (0.2 mol%) and PhCl (3 mL) in a 35 mL sealed tube with 390 nm LED photo-irradiation at 120 °C for 24 h. c LLDPE (100.0 mmol monomer), 5 (5 mol%), iron catalyst (0.1 mol%) and PhCl (40 mL) in a 100 mL sealed tube with 390 nm LED photo-irradiation at 120 °C for 24 h. d At rt. |
|---|
|
Furthermore, various polyolefins could be tolerated (Table 1b). A 2.8 gram-scale reaction of LLDPE with 5.0 mol% of 5 was able to obtain the corresponding product with a good LOF (4.7 mol%). The readily available atactic polypropylene (PP) was efficiently incorporated with an ester group with 5.7 mol% of LOF under these conditions without observing significant degradation. Polystyrene (PS) presented a better solubility and such a reaction was able to undergo at room temperature with 5 to obtain the corresponding product, albeit with lower efficiency (1.9 mol% of LOF). Moreover, the C–H bond functionalization of PIB also proceeded with benzyl acrylate 6 in moderate efficiency (3.3 mol% of LOF). The regioselectivity over the C–H functionalization of PP, PS, and PIB could be determined as a mixture of 1°, 2°, and 3° C–H alkylation by the model reactions and the corresponding 1H–1H COSY spectra (see the ESI†).
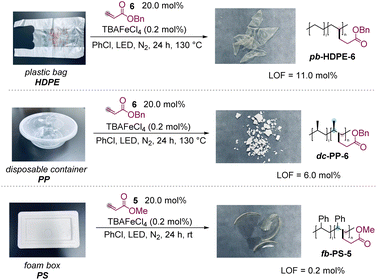
The polyolefin waste from our daily life was then investigated to demonstrate the utility of the protocol, which could also convert the polymer waste into new valuable materials (Scheme 3). First, a plastic bag from market waste, made of HDPE, was able to react with 6 smoothly to obtain the desired product in excellent LOF (11.0 mol%). A PP-made disposable container and PS-made package foam were also modified successfully with 6 and 5, producing polar products with 6.0 mol% and 0.2 mol% LOF, respectively.
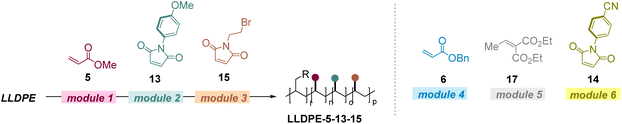
The broad scope of this protocol then drove us to conduct the modular incorporation of multiple polar groups into the polyolefins. We chose prevalent LLDPE as the starting material and various electron deficient olefins as modules. First, the LOFs of the polar groups are readily controllable (Table 2). When LLDPE was stepwise reacted with 2 mol% of 5, 2 mol% of 14, and 2 mol% of 9, a triple functionalized product was able to be obtained and the LOFs for each polar group were 1.9%, 1.6%, and 1.7%, respectively (entry 1). Notably, the multiple polar alkenes could also react with the polyolefin in one-pot, and similar efficiencies were obtained (entry 1 vs. entry 2). When 5 mol% of each alkene was used, the LOFs in the obtained product were 4.7%, 3.7%, and 4.6%, respectively (entry 3). In addition, products with diverse LOFs could be readily obtained by further controlling the ratio of each alkene. When 5 mol% of 5, 1 mol% of 14, and 2 mol% of 9 were used in the reaction sequence, products with 4.7%, 1.0%, and 1.4% LOFs could be obtained successfully (entry 4). The stepwise incorporation of the polar groups could be evidenced by determining the resonance signals of OMe, Ph, and methylene groups in the 1H NMR (proton nuclear magnetic resonance) spectra in CDCl3 (see the ESI†). Furthermore, LLDPE can react with four different alkenes in one pot successfully to obtain the desired multi-polar product (entries 5–7).
| Entry | LOF of LLDPE-FG/mol% | Reaction sequence | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2.0 mol% | 2.0 mol% | 2.0 mol% | — | — | — | 1.9/1.6/1.7 | Stepwise |
| 2 | 2.0 mol% | 2.0 mol% | 2.0 mol% | — | — | — | 1.2/1.8/1.9 | One-pot |
| 3 | 5.0 mol% | 5.0 mol% | 5.0 mol% | — | — | — | 4.7/3.7/4.6 | Stepwise |
| 4 | 5.0 mol% | 1.0 mol% | 2.0 mol% | — | — | — | 4.7/1.0/1.4 | 5, then 13 and 15 in one-pot |
| 5 | 2.0 mol% | 2.0 mol% | 2.0 mol% | 2.0 mol% | — | — | 0.6/1.7/1.4/1.0 | One-pot |
| 6 | 2.0 mol% | 2.0 mol% | 2.0 mol% | — | 2.0 mol% | — | 1.1/1.7/1.6/0.3 | One-pot |
| 7 | 2.0 mol% | 2.0 mol% | 2.0 mol% | — | — | 2.0 mol% | 0.4/1.7/1.2/1.2 | One-pot |
The quantum yield was determined to be 0.131, ruling out the potential radical chain processing mechanism.
Based on our previous studies and the known literature,8 a plausible mechanism is proposed in Scheme 4. Excitation of the photo-sensitive Fe(III) catalyst is known to generate a Cl-radical and Fe(II).8 This Cl-radical is a strong hydrogen abstraction agent and should readily abstract a hydrogen atom from polyolefin 1 to produce HCl and the polyolefin radical 2. This species would then undergo subsequent radical addition to the electron-withdrawing olefin to generate alkyl radical 3, which couples with HCl in the presence of Fe(II) to simultaneously complete the catalytic cycle by regenerating the Fe(III) catalyst and producing the alkylated polyolefin 4. The polar groups could be incorporated when polar vinyl precursors are used.
The properties of the polar functionalized polyethylene were next examined. First, the thermal transitions and thermal stabilities were evaluated by differential scanning calorimetry (DSC) and using a thermogravimetric analyzer (TGA) (see the ESI for more details†). Taking LLDPE-5, LLDPE-8, and LLDPE-9 as typical examples, while the decomposition onset temperatures (Td) of such materials remain high (above 300 °C), the incorporation of the functional group was proven to be responsive to the changing of the melting temperatures. The melting temperatures of such materials are 108, 102, and 98 °C, respectively, which are much lower than that of LLDPE (122 °C). These relationships are consistent with the fact that the existence of larger functional groups causes them to impart enthalpic penalties when incorporated into crystalline domains of polyethylenes.
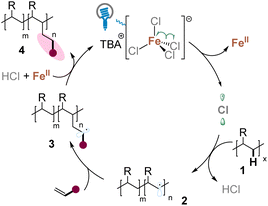
Covalent incorporation of ionized units as pendant groups into polyolefin backbones is an efficient tool to increase the toughness of the overall system including coatings, adhesives, impact modification, and thermoplastics.10 One of the best known examples among these ionomers is the Surlyn resin.6 Two high-value polyethylene ionomers were then synthesized (Scheme 5a). While treating the prepared polar polyolefin LLDPE-16 with tBuOK produced the ionomers ion-LLDPE-16, and the reaction of LLDPE-9 with 1-methylimidazole afforded the organic ionomers ion-LLDPE-9 successfully. The introduction of the ionic site significantly enhanced the transparencies, strains, and stresses at break of the materials. The tensile tests indicated that the strains at break (εb) of the ionomers were more than >550% and the stresses at break (σb) of ionomers were >20 MPa, which are over tenfold and almost treble compared with those of LLDPE, respectively. The εb and σb of ion-LLDPE-9 – 2.4 mol% even reached 963% and 36 MPa, respectively, which are comparable with those of the Surlyn resin (Scheme 5a).6
Finally, hydrophobic LLDPE was modified with acrylate 17 containing a hydrophilic PEG (polyethylene glycol) chain to obtain LLDPE-17. After incorporating 2.8 mol% of the hydrophilic group, the water contact angles decreased significantly from 92.1° to 66.7°, which is comparable with that of commercial PVA (polyvinyl alcohol) (66°),11 presenting excellent potential in synthesis of novel polymers (Scheme 5b).
Conclusions
In summary, utilizing a low-cost photoinduced iron-catalytic system, we have achieved the efficient C–H bond modification of commodity polyolefins with broad deficient alkenes. A series of polar functional groups could be installed into various polyolefins, such as LLDPE, PP, PVC, PS, PIB, even PVDF, etc. via this broad reaction platform. Plastic waste including a packaging LDPE bag, disposable PP container, and PS foam box could be used as raw materials to undergo such a modification directly. The powerful iron catalysis with tolerance towards various functional groups further pointed us toward the concept that multi-polar polyolefins could be synthesized through modularly polar group assembly through C–H bond modification. And the concept was validated successfully by the stepwise or one-pot multiple installations of the functional groups. Moreover, the polyolefin wastes could be upcycled by introducing even low levels of polar functionalization. The ionomers, which are comparable with Surlyn resin, and a hydrophilic material could be prepared readily from the commodity polyethylene, demonstrating the great potential in the construction of novel materials.Data availability
All experimental and characterization data, as well as NMR spectra are available in the ESI.†Author contributions
R. Z. conceptualized and supervised. R. Z. and Z. Z. designed the experiments. Z. Z. performed and analysed the experiments. Z. Z., Y. Z., and R. Z. prepared this manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
R. Z. is grateful for the financial support from the National Natural Science Foundation of China (21901197), the Xiaomi Young Talents Program, and the startup funds from Xi'an Jiaotong University (XJTU). We thank Prof. Yilong Cheng from XJTU for the helpful discussion and Dr Chao Feng and Dr Gang Chang from the Instrument Analysis Center of XJTU for assistance with NMR analysis. We also thank Mr Guoxiang Zhang from this group for reproducing the results of LLDPE-5-13-15.Notes and references
- (a) R. Geyer, J. R. Jambeck and K. L. Law, Sci. Adv., 2017, 3, e1700782 CrossRef PubMed; (b) C. Jehanno, J. W. Alty, M. Roosen, S. D. Meester, A. P. Dove, E. Y. X. Chen, F. A. Leibfarth and H. Sardon, Nature, 2022, 603, 803–814 CrossRef CAS PubMed; (c) C. W. S. Yeung, J. Y. Q. Teo, X. J. Loh and J. Y. C. Lim, ACS Mater. Lett., 2021, 3, 1660–1676 CrossRef CAS; (d) N. K. Boaen and M. A. Hillmyer, Chem. Soc. Rev., 2005, 34, 267–275 RSC; (e) J. B. Williamson, S. E. Lewis, R. R. Johnson, I. M. Manning and F. A. Leibfarth, Angew. Chem., Int. Ed., 2019, 58, 8654–8668 CrossRef CAS PubMed; (f) I. Vollmer, M. J. F. Jenks, M. C. P. Roelands, R. J. White, T. van Harmelen, P. de Wild, G. P. van der Laan, F. Meirer, J. T. F. Keurentjes and B. M. Weckhuysen, Angew. Chem., Int. Ed., 2020, 59, 15402–15423 CrossRef CAS PubMed; (g) C. M. Plummer, L. Li and Y. Chen, Polym. Chem., 2020, 11, 6862–6872 RSC; (h) M. G. Rodriguez, M. M. Díaz-Requejo and P. J. Pérez, Macromolecules, 2021, 54, 4971–4985 CrossRef.
- (a) G. Moad, Prog. Polym. Sci., 1999, 24, 81–142 CrossRef CAS; (b) P. Ghosh, B. Chattopadhyay and A. K. Sen, Polymer, 1998, 39, 193–201 CrossRef CAS; (c) M. M. Díaz-Requejo, P. Wehrmann, M. D. Leatherman, S. Trofimenko, S. Mecking, M. Brookhart and P. J. Pérez, Macromolecules, 2005, 38, 4966–4969 CrossRef; (d) C. M. Plummer, H. Zhou, S. Li, H. Zhong, Z. Sun, C. Bariashir, W.-H. Sun, H. Huang, L. Liu and Y. Chen, Polym. Chem., 2019, 10, 3325–3333 RSC.
- (a) The Elements of Polymer Science and Engineering, ed. A. Rudin and P. Choi, Academic Press, 3rd edn, 2013 Search PubMed; (b) L. S. Boffa and B. M. Novak, Chem. Rev., 2000, 100, 1479–1493 CrossRef CAS PubMed; (c) N. M. G. Franssen, J. N. H. Reeka and B. Bruin, Chem. Soc. Rev., 2013, 42, 5809 RSC; (d) L. Guo, S. Dai, X. Sui and C. Chen, ACS Catal., 2016, 6, 428–441 CrossRef CAS; (e) Z. Chen and M. Brookhart, Acc. Chem. Res., 2018, 51, 1831–1839 CrossRef CAS PubMed; (f) C. Chen, Nat. Rev. Chem., 2018, 2, 6–18 CrossRef CAS; (g) S. L. J. Luckham and K. Nozaki, Acc. Chem. Res., 2021, 54, 344–355 CrossRef CAS PubMed.
- (a) Y. Kondo, D. G. Cuadrado, J. F. Hartwig, N. K. Boaen, N. L. Wagner and M. A. Hillmyer, J. Am. Chem. Soc., 2002, 124, 1164–1165 CrossRef CAS PubMed; (b) C. Bae, J. F. Hartwig, N. K. Boaen Harris, R. O. Long, K. S. Anderson and M. A. Hillmyer, J. Am. Chem. Soc., 2005, 127, 767–776 CrossRef CAS PubMed; (c) C. Bae, J. F. Hartwig, H. Chung, N. K. Harris, K. A. Switek and M. A. Hillmyer, Angew. Chem., Int. Ed., 2005, 44, 6410–6413 CrossRef CAS PubMed; (d) A. Bunescu, S. Lee, Q. Li and J. F. Hartwig, ACS Cent. Sci., 2017, 3, 895–903 CrossRef CAS PubMed; (e) L. Chen, K. G. Malollari, A. Uliana, D. Sanchez, P. B. Messersmith and J. F. Hartwig, Chem, 2021, 7, 137–145 CrossRef CAS; (f) L. Chen, K. G. Malollari, A. Uliana and J. F. Hartwig, J. Am. Chem. Soc., 2021, 143, 4531–4535 CrossRef CAS PubMed; (g) H. Zhou, S. Wang, H. Huang, Z. Li, C. M. Plummer, S. Wang, W. Sun and Y. Chen, Macromolecules, 2017, 50, 3510–3515 CrossRef CAS.
- (a) J. B. Williamson, W. L. Czaplyski, E. J. Alexanian and F. A. Leibfarth, Angew. Chem., Int. Ed., 2018, 57, 6261–6265 CrossRef CAS PubMed; (b) J. B. Williamson, C. G. Na, R. R. Johnson, W. F. M. Daniel, E. J. Alexanian and F. A. Leibfarth, J. Am. Chem. Soc., 2019, 141, 12815–12823 CrossRef CAS PubMed.
- T. J. Fazekas, J. W. Alty, E. K. Neidhart, A. S. Miller, F. A. Leibfarth and E. J. Alexanian, Science, 2022, 375, 545–550 CrossRef CAS PubMed.
- (a) A. Vogler and H. Kunkely, Coord. Chem. Rev., 2006, 250, 1622–1626 CrossRef CAS; (b) O. S. Wenger, J. Am. Chem. Soc., 2018, 140, 13522–13533 CrossRef CAS PubMed.
- (a) G. Zhang, Z. Zhang and R. Zeng, Chin. J. Chem., 2021, 39, 3225–3230 CrossRef CAS; (b) Y. Jin, Q. Zhang, L. Wang, X. Wang, C. Meng and C. Duan, Green Chem., 2021, 23, 6984–6989 RSC; (c) Y. C. Kang, S. M. Treacy and T. Rovis, ACS Catal., 2021, 11, 7442–7449 CrossRef CAS PubMed; (d) G. Feng, X. Wang and J. Jin, Eur. J. Org. Chem., 2019, 6728–6732 CrossRef CAS; (e) N. Xiong, Y. Li and R. Zeng, Org. Lett., 2021, 23, 8968–8972 CrossRef CAS PubMed; (f) Z. Zhang, X. Li, D. Zhou, S. Ding, M. Wang and R. Zeng, J. Am. Chem. Soc., 2023, 145, 7612–7620 CrossRef CAS PubMed.
- (a) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322–5363 CrossRef CAS PubMed; (b) Y. Chen, L. Lu, D. Yu, C. Zhu and W. Xiao, Sci. China: Chem., 2019, 62, 24–57 CrossRef CAS.
- B. P. Grady, Polym. Eng. Sci., 2008, 48, 1029–1051 CrossRef CAS.
- S. Hong, K. Kim, Y. Lee, S. Cho, J. Ko, S. Hong and H. Park, J. Appl. Polym. Sci., 2009, 113, 2988–2996 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3sc03252c |
| This journal is © The Royal Society of Chemistry 2023 |