Tailoring 6FDA-based click cross-linked membranes: modular synthesis and tunable gas separation†
Jieun
Park‡
,
Chang Oh
Lee‡
,
Ki Jung
Kim
,
Won Seok
Chi
* and
Hyungwoo
Kim
 *
*
School of Polymer Science and Engineering, Chonnam National University, 77 Yongbong-ro, Buk-gu, Gwangju 61186, Korea. E-mail: wschi@jnu.ac.kr; kimhw@jnu.ac.kr
First published on 6th December 2022
Abstract
A click covalent cross-linking approach for polyimide membranes has been demonstrated. The cross-linked membranes particularly exhibit systematic changes in gas permeability for H2 (30–237 Barrer) and CO2 (13–199 Barrer) as well as in physical properties, depending on the thiol cross-linkers embedded.
Design, System, ApplicationThis conceptual study demonstrates the feasibility of a covalent clickable cross-linking approach to control the macroscopic properties of polyimide membranes on a molecular level. With post-casting thiol-ene cross-linking, we newly develop an allylation procedure for the mother polymer 6FDA–DAM:DABA and facilely fabricate cross-linked membranes via photopolymerization in the presence of select thiol cross-linkers. After the thiol-ene reaction, the designed molecular changes in the membranes are reflected in their mechanical strength, gas transport behavior, and plasticization resistance; in particular, the gas-separation properties systematically vary depending on the type of cross-linker used. We believe that our design can be advanced by incorporating various thiol molecules and the further functionalization of allyl moieties to not only achieve the properties required for target applications but also to delineate the general structure-to-property relationships for gas separation membranes. |
Membrane technology offers energy-efficient and environmentally friendly gas separation with a small footprint and a compact module.1–3 Commercially available polymer membranes are cost-effective options for use as industrial gas separation membranes due to their scalability and excellent processability.1,4 However, there is a trade-off relationship between permeability and selectivity when conventional polymer membranes are employed. This is defined by the Robeson upper bound limit, which dictates that an increase in permeability causes a decrease in selectivity and vice versa.5,6 Thus, most polymeric membranes offer either high permeability or high selectivity, the choice of which depends on the target application and whether high flux or high purity is required.7,8 It is also necessary to develop versatile methods for the control of the molecular structure of polymer membranes, which would allow for the fine-tuning of their gas transport characteristics based on a clear understanding of the associated structure–property relationships while also producing the desired physicochemical properties for gas separation.
From a molecular design perspective, the chemical cross-linking of polymer chains represents an accessible method for the adjustment of the macroscopic properties of membranes, including their gas transport properties.9–12 For example, the inter-chain distance, internal free volume, and chain rigidity can be rationally controlled after cross-linking, all of which directly affect the permeability and selectivity of a membrane. Simultaneously, the densification of a membrane can be adjusted depending on the cross-linking density, while the embedding of a diverse range of potential cross-linkers opens up the possibility of further engineering the molecular structure of a membrane. Of particular note is that the plasticization resistance to condensable gases such as CO2 at high pressures can be improved by forming a network structure,13 which is required for the industrial use of polymer membranes.14
To date, a number of covalent cross-linking reactions using specifically designed building blocks15–17 or post-polymerization reactions—as represented by decarboxylation-induced cross-linking11,18–24—have been developed (Table S1†). Both non-covalent approaches, e.g., forming semi-interpenetrating networks or the clustering of polymer chains, and the use of cross-linking agents such as inorganic components and small molecules have been widely demonstrated.9,25–28 However, research into cross-linking reactions for membranes has been limited, especially considering the broad library of candidate chemical reactions for cross-linking. In particular, clickable cross-linking reactions that exhibit a high yield under benign conditions have rarely been employed in membrane science despite their potential for use in the engineering of the macroscopic properties of cross-linked membranes at a molecular level.
In the present study, we demonstrate a design principle for membranes of the well-known polyimide 6FDA–DAM:DABA, in which 6FDA is 4,4′-(hexafluoroisopropylidene)diphthalic anhydride, DAM is 2,4,6-trimethyl-1,3-diaminobenzene, and DABA is 3,5-diaminobenzoic acid (hereafter, referred to as 6FDD), in conjunction with thiol-ene clickable cross-linking reactions. This class of polyimide contains bulky –CF3 and –CH3 groups on the 6FDA and DAM units, respectively, which reduce local segmental chain mobility and lead to high and rigid fractional free volumes and excellent gas transport properties.29,30 The carboxylate groups on DABA also provide additional cross-linking reactions, for instance, radical generation, condensation, vinyl polymerization, metal coordination, acetalization, and urethane formation.18,31–38 For the clickable cross-linking reaction, we substituted the carboxylate groups on 6FDD with allyl moieties and investigated the resulting systematic changes in the mechanical strength, gas transport behavior, and plasticization resistance of the cross-linked membranes after post-casting the thiol-ene reaction with select thiol cross-linkers. Our conceptual approach could be advanced by incorporating other thiol linkers or by functionalizing the allyl moieties further, thus outlining general design principles for membranes to be used for specific applications.
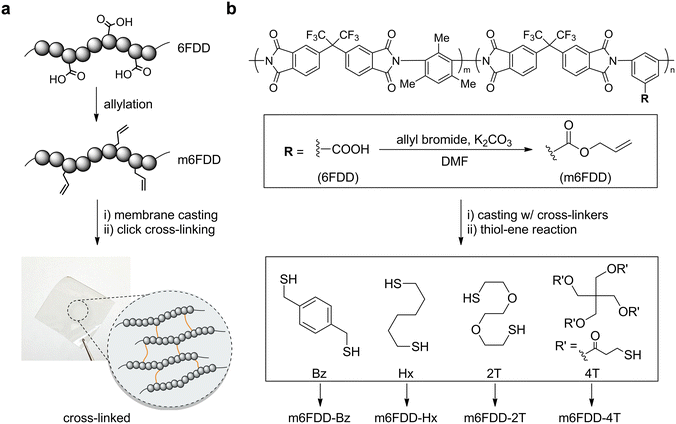
Fig. 1a presents a summary of the facile functionalization of 6FDD and the formation of cross-linked polyimide membranes. In this straightforward design, the dangling carboxylic acids on the backbone of the 6FDD were completely converted into allyl groups via nucleophilic substitution at room temperature, leading to modified 6FDD (m6FDD). The mother polymer formed a membrane after solvent casting in the presence of a cross-linker, which was further cross-linked in film form through click photopolymerization. As an example, after incorporating 1,4-benzenedimethanethiol (Bz) and running the post-casting thiol-ene reaction, a cross-linked membrane (m6FDD-Bz) was obtained as shown in the bottom photograph of Fig. 1a (size: 3 cm × 3 cm × 70 μm). The m6FDD-Bz membrane was glossy, transparent, and resilient, with a strong similarity to the membrane prepared from pristine 6FDD. After dissolution tests, the cross-linked membrane was found to be resistant to various organic solvents such as N,N-dimethylformamide (DMF), tetrahydrofuran (THF), methanol, and water.
Details of the synthetic process are presented in Fig. 1b. The acid functionalities in 6FDD (Mw: 210 kDa; Đ: 2.5; m/n ratio: 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) were substituted with allyl bromide in the presence of K2CO3 in DMF at 25 °C. As confirmed by a nuclear magnetic resonance (NMR) spectrometer, the yield of the reaction was fairly high at 87%, so most of the acids were substituted with allyl moieties when compared with pristine 6FDD (Fig. S1†). In the 1H NMR spectrum, new proton peaks for the allyl groups appeared at 6.03, 5.44–5.26, and 4.85 ppm. Of note is the integration ratio between the most shielded methyl protons (1.92 ppm) and the allylic proton (4.85 ppm), supporting the high substitution yield (Fig. S2†). In addition, the 13C NMR spectrum of m6FDD supports the presence of allylic carbons at 66.1 ppm, as shown in Fig. S3.† As revealed by Fourier-transform infrared spectroscopy (FTIR), the stretching adsorption of the vinylic carbons in the allyl moieties was also observed at 1665 cm−1 for m6FDD, unlike for 6FDD (Fig. S4†).
2) were substituted with allyl bromide in the presence of K2CO3 in DMF at 25 °C. As confirmed by a nuclear magnetic resonance (NMR) spectrometer, the yield of the reaction was fairly high at 87%, so most of the acids were substituted with allyl moieties when compared with pristine 6FDD (Fig. S1†). In the 1H NMR spectrum, new proton peaks for the allyl groups appeared at 6.03, 5.44–5.26, and 4.85 ppm. Of note is the integration ratio between the most shielded methyl protons (1.92 ppm) and the allylic proton (4.85 ppm), supporting the high substitution yield (Fig. S2†). In addition, the 13C NMR spectrum of m6FDD supports the presence of allylic carbons at 66.1 ppm, as shown in Fig. S3.† As revealed by Fourier-transform infrared spectroscopy (FTIR), the stretching adsorption of the vinylic carbons in the allyl moieties was also observed at 1665 cm−1 for m6FDD, unlike for 6FDD (Fig. S4†).
After functionalization, m6FDD was then used to fabricate solvent-casted membranes that included four different types of thiol cross-linker—aromatic Bz, aliphatic Hx, amphiphilic 2T, and tetra-functional 4T—in stoichiometry (a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of thiol to alkene). Their chemical structures are presented at the bottom of Fig. 1b. The sequential in situ thiol-ene reaction resulted in cross-linked membranes denoted as m6FDD-Bz, m6FDD-Hx, m6FDD-2T, and m6FDD-4T based on the cross-linkers used. Photographs of all of the cross-linked membranes except for m6FDD-Bz are displayed in Fig. S5.†
1 ratio of thiol to alkene). Their chemical structures are presented at the bottom of Fig. 1b. The sequential in situ thiol-ene reaction resulted in cross-linked membranes denoted as m6FDD-Bz, m6FDD-Hx, m6FDD-2T, and m6FDD-4T based on the cross-linkers used. Photographs of all of the cross-linked membranes except for m6FDD-Bz are displayed in Fig. S5.†
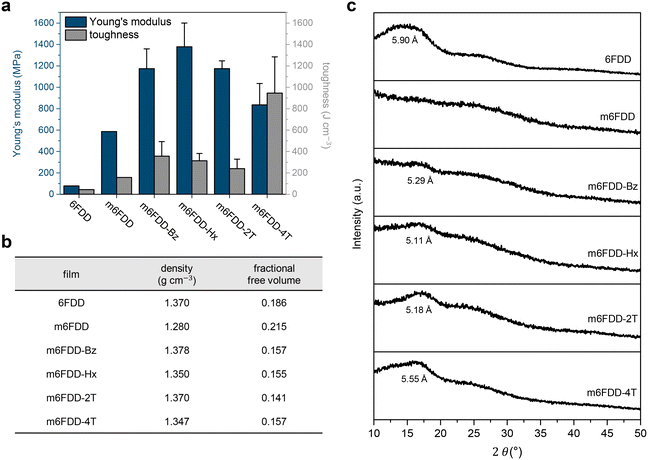
The mechanical properties of the membranes were significantly affected by the cross-linking (Fig. 2a). The tensile Young's modulus and toughness of the m6FDD membrane without any cross-linker were 0.59 GPa and 158 J cm−1, respectively, representing a 7.5- and 3.6-fold increase over the pristine 6FDD membrane, respectively. It was assumed that the introduction of the allyl pendants promoted the random chain entanglement of m6FDD, attributable to the enhanced flexibility of the chains due to the absence of inter-chain hydrogen bonds between the carboxylates.39–41
After post-casting chemical cross-linking, the elastic modulus and toughness of the membranes increased further. For example, m6FDD-Bz and m6FDD-Hx had an elastic modulus of 1.17 and 1.38 GPa and a toughness of 356 and 314 J cm−1, respectively. Both the covalent cross-linking points and the inter-chain interactions induced by the cross-linkers together appeared to affect the overall mechanical strength of the samples. The hydrocarbon cross-linkers Bz and Hx generate aromatic stacking and van der Waals forces, respectively, while 2T and 4T were chosen for their bi- and tetra-functionalities with ether or ester linkages. Interestingly, the modulus of m6FDD-2T was larger than that with tetra-functional 4T, though the latter had considerably higher toughness than the former. This could be ascribed to the lower cross-linking density of m6FDD-4T, possibly due to steric hindrance during photopolymerization, increasing the ductility of the material. The tensile stress–strain curves for all of the 6FDD-based membranes are presented in Fig. S6.†
The morphology of the membranes was also investigated using scanning electron microscopy (SEM). Overall, all of the samples had a smooth surface, indicating that the post-casting cross-linking did not lead to film shrinkage or induce a rough texture (Fig. S7†). The water contact angle of the membranes was similar at around 71–80°, except for m6FDD-2T (∼51°), which was attributed to the even distribution of amphiphilic 2T with ether linkages (Fig. S8†).
The fractional free volume (FFV), which is strongly associated with the gas permeation properties of a membrane, was investigated for all samples (Fig. 2b). In general, the FFV is determined based on the density and intrinsic free volume, each can be measured using the buoyancy method in water and calculated from the Bondi's group contribution theory.42 It was found that the m6FDD membrane was lighter than the 6FDD membrane. Thus, the FFV increased after substitution, indicating the generation of additional free volume due to the inefficient chain-packing of m6FDD. In contrast, the cross-linking reaction led to the efficient compact chain-packing of the polyimides, leading to a higher density and a lower FFV compared with m6FDD.
These observations were supported by X-ray diffraction (XRD) measurements (Fig. 2c). A broad halo was found at a 2θ of ∼14°, corresponding to a d-spacing of 5.90 Å for 6FDD. However, this peak completely disappeared for m6FDD because of the lack of the short-range ordering of the polymer chains presumably due to an increase in amorphous region. However, the halo peaks reappeared after cross-linking and smaller d-spacing values were observed, which supports the previous findings.
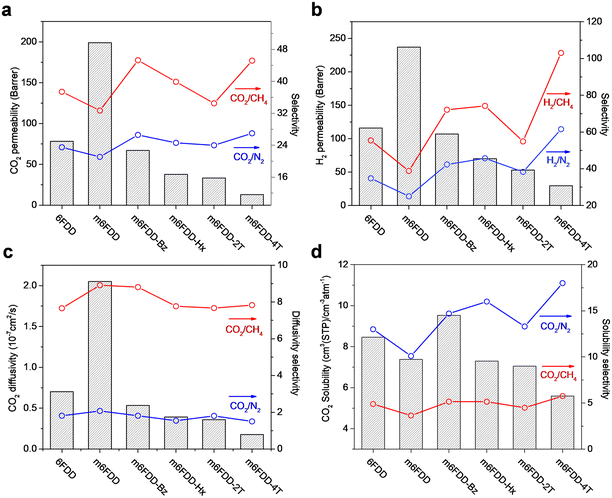
We analyzed the gas permeation properties of the cross-linked membranes for H2, N2, CH4, and CO2 at 150 psi and 35 °C. The 6FDD and m6FDD membranes were included as a comparison. Fig. 3a and b present the CO2 and H2 permeability and selectivity paired with larger gas molecules such as N2 and CH4 (i.e., CO2/N2, CO2/CH4, H2/N2, and H2/CH4) for the membranes. The pristine 6FDD membrane had a CO2 and H2 permeability of 78.2 and 116 Barrer, respectively, whereas much higher permeabilities were found in the less ordered m6FDD sample (i.e., 199 Barrer for CO2; 237 Barrer for H2). However, after cross-linking, the m6FDD-based membranes exhibited a lower permeability due to the greater compactness of the polymer chains, which is consistent with the reduced d-spacing observed in the XRD analysis. The CO2 and H2 gas permeability of the cross-linked membranes followed the order m6FDD-Bz > m6FDD-Hx > m6FDD-2T > m6FDD-4T. The reduced permeability of the glassy samples cross-linked with Bz, Hx, and 2T can be attributed to their FFVs, which decreased in the same order. This was accompanied by enhanced CO2 and H2 selectivity over larger gas molecules when compared to the 6FDD membrane. In contrast, the m6FDD-4T membrane had a much lower permeability but a higher selectivity even though it had a relatively high FFV and d-spacing compared to the other cross-linked membranes. This may be the result of its loosely cross-linked characteristics based on the sterically bulky tetra-directional cross-linker structure, leading to the transport-pathway obstruction of free uncross-linked chains of the cross-linker between the main polyimide chains. The corresponding gas permeation parameters for the membranes are summarized in Table S2.†
To understand the gas transport mechanisms, the CO2, N2, and CH4 permeability was de-convoluted into diffusivity and solubility (the H2 gas permeability exhibited an extremely short time lag, which prevented us from obtaining reliable diffusivity and selectivity data). The CO2 diffusivity and solubility of the pure 6FDD, m6FDD, and cross-linked membranes are displayed in Fig. 3c and d, respectively, together with their selectivity over larger gas molecules (CO2/CH4 and CO2/N2). Before cross-linking, m6FDD with the high internal free volume showed the highest CO2 diffusion coefficient. Meanwhile, the decrease in the CO2 permeability of the cross-linked membranes was associated with the lower CO2 diffusion coefficients. These results were in accordance with the lower FFVs and d-spacing of polymer chains in the cross-linked membranes. On the other hand, CO2 solubility was the main contributor to the CO2/CH4 and CO2/N2 selectivity of the cross-linked membranes rather than CO2 diffusivity. It was assumed that the high selectivity of the m6FDD-4T membrane was due to the dangling thiol groups from the partially reacted 4T. The corresponding diffusivity and solubility parameters are summarized in Tables S3 and S4.†
Fig. S9† shows the normalized CO2 permeability for the membranes as a function of the CO2 feed pressure. The CO2 permeability at each feed pressure was normalized by dividing it by the initial CO2 permeability at the lowest feed pressure (15 psi). Although all samples had a CO2 plasticization pressure point at 75 psi, some of the cross-linked membranes exhibited enhanced CO2 plasticization resistance at a relatively high gas feed pressure compared with the pristine 6FDD membrane. Given that the plasticization behavior of polymer chains is a persistent transition phenomenon, high CO2 plasticization resistance in response to the gradual increase in the feed pressure indirectly reflects membrane stability even under harsh pressure conditions. The hydrocarbon cross-linkers Bz and Hx clearly increased the stability of the membranes because they provided a more rigid polymer chain structure. In contrast, the use of 2T led to lower plasticization resistance due to the ether linkages preferentially sorbing the CO2 penetrants and the relatively mobile polymer chains.43,44 Likewise, the loosely cross-linked, ester-linkage-containing m6FDD-4T membrane was characterized by lower plasticization resistance than that of the 6FDD membrane.
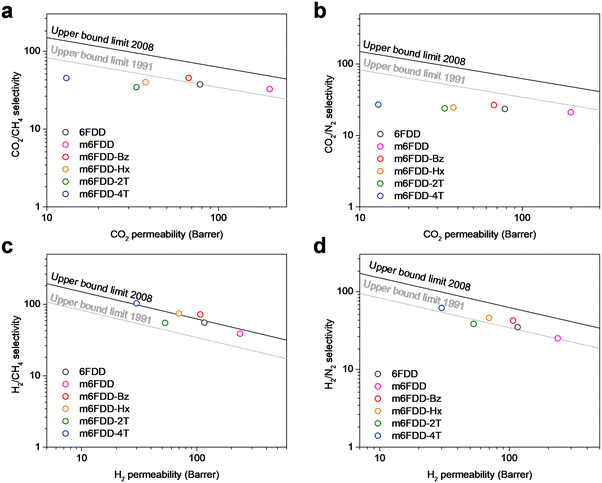
Fig. 4 presents the CO2/CH4, CO2/N2, H2/CH4, and H2/N2 gas-separation performance of the cross-linked membranes at 150 psi and 35 °C with Robeson upper bound limits. Notably, the membranes exhibited a predictable linear change in gas permeation in parallel with the Robeson upper bound limits. This observation supported the effect of the cross-linkers and illustrated the potential control of the gas-separation performance of the membranes, which can be selectively used depending on the target application with either high gas permeability (productivity) or high gas selectivity (efficiency).
Conclusions
In conclusion, we demonstrated a modular design for cross-linked 6FDD-based membranes with tunable gas-separation performance based on the post-casting thiol-ene reaction with select cross-linkers. The 6FDD polymer was functionalized with allyl groups to achieve the facile cross-linking reaction. The cross-linked membranes exhibited enhanced mechanical properties and modified polymer chain-packing structures depending on the cross-linker used. In particular, the rationally designed cross-linked membranes exhibited controlled gas separation properties; tunable gas permeability of H2 (30–237 Barrer) and CO2 (13–199 Barrer) was found and their selectivity over large gases increased 30–300%, subject to the cross-linkers. This proposed design strategy would be developed further by employing other forms of click chemistry or other cross-linkers to delineate the general polymer structure-to-transport principles for gas separation membranes.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant, funded by the Korean Government (MSIT, Ministry of Science and ICT) (No. 2022R1C1C1003149, 2020R1A5A8018367, and 2020R1F1A1075098).Notes and references
- M. Galizia, W. S. Chi, Z. P. Smith, T. C. Merkel, R. W. Baker and B. D. Freeman, Macromolecules, 2017, 50, 7809–7843 CrossRef CAS.
- Q. Qian, P. A. Asinger, M. J. Lee, G. Han, K. M. Rodriguez, S. Lin, F. M. Benedetti, A. X. Wu, W. S. Chi and Z. P. Smith, Chem. Rev., 2020, 120, 8161–8266 CrossRef CAS PubMed.
- D. S. Sholl and R. P. Lively, Nature, 2016, 532, 435–437 CrossRef PubMed.
- D. F. Sanders, Z. P. Smith, R. Guo, L. M. Robeson, J. E. McGrath, D. R. Paul and B. D. Freeman, Polymer, 2013, 54, 4729–4761 CrossRef CAS.
- L. M. Robeson, J. Membr. Sci., 2008, 320, 390–400 CrossRef CAS.
- L. M. Robeson, J. Membr. Sci., 1991, 62, 165–185 CrossRef CAS.
- J. W. Barnett, C. R. Bilchak, Y. Wang, B. C. Benicewicz, L. A. Murdock, T. Bereau and S. K. Kumar, Sci. Adv., 2020, 6, eaaz4301 CrossRef CAS PubMed.
- W. Qiu, L. Xu, C.-C. Chen, D. R. Paul and W. J. Koros, Polymer, 2013, 54, 6226–6235 CrossRef CAS.
- J. D. Wind, C. Staudt-Bickel, D. R. Paul and W. J. Koros, Ind. Eng. Chem. Res., 2002, 41, 6139–6148 CrossRef CAS.
- K. Vanherck, G. Koeckelberghs and I. F. J. Vankelecom, Prog. Polym. Sci., 2013, 38, 874–896 CrossRef CAS.
- C. Zhang, P. Li and B. Cao, J. Membr. Sci., 2017, 528, 206–216 CrossRef CAS.
- R. Shindo, S. Amanuma, K. Kojima, S. Sato, S. Kanehashi and K. Nagai, Polym. Eng. Sci., 2014, 54, 1089–1099 CrossRef CAS.
- H. Wang, K. Zhang, J. P. H. Li, J. Huang, B. Yuan, C. Zhang, Y. Yu, Y. Yang, Y. Lee and T. Li, Chem. Sci., 2020, 11, 4687–4694 RSC.
- MarketsandMarkets, Gas Separation Membranes Market by Material type (Polyimide & Polyaramide, Polysulfone, and Cellulose Acetate), Application (Nitrogen Generation & Oxygen Enrichment, Carbon Dioxide Removal, and Hydrogen Recovery), and Region – Global Forecast to 2024, 2019, report code CH 5549 Search PubMed.
- D. J. Harrigan, J. A. Lawrence III, H. W. Reid, J. B. Rivers, J. T. O'Brien, S. A. Sharber and B. J. Sundell, J. Membr. Sci., 2020, 602, 117947 CrossRef CAS.
- Y. Chen, J. Qin, T. Tong, H. Zhou, X. Cao and W. Jin, Sep. Purif. Technol., 2020, 252, 117401 CrossRef CAS.
- L. Kwisnek, S. Heinz, J. S. Wiggins and S. Nazarenko, J. Membr. Sci., 2011, 369, 429–436 CrossRef CAS.
- J. D. Wind, C. Staudt-Bickel, D. R. Paul and W. J. Koros, Macromolecules, 2003, 36, 1882–1888 CrossRef CAS.
- W. Qiu, C.-C. Chen, L. Xu, L. Cui, D. R. Paul and W. J. Koros, Macromolecules, 2011, 44, 6046–6056 CrossRef CAS.
- J. D. Wind, S. M. Sirard, D. R. Paul, P. F. Green, K. P. Johnston and W. J. Koros, Macromolecules, 2003, 36, 6433–6441 CrossRef CAS.
- A. M. Kratochvil and W. J. Koros, Macromolecules, 2008, 41, 7920–7927 CrossRef CAS.
- C. Zhang, B. Cao and P. Li, J. Membr. Sci., 2018, 546, 90–99 CrossRef CAS.
- J. S. Park, K. L. Gleason, K. E. Gaines, S. J. Mecham, J. E. McGrath and B. D. Freeman, Energy Procedia, 2014, 63, 210–216 CrossRef CAS.
- S. Li, H. McGinness, T. Wang and R. Guo, Polymer, 2021, 237, 124323 CrossRef CAS.
- G. K. Kline, J. R. Weidman, Q. Zhang and R. Guo, J. Membr. Sci., 2017, 544, 25–34 CrossRef CAS.
- X. Chen, Y. Fan, L. Wu, L. Zhang, D. Guan, C. Ma and N. Li, Nat. Commun., 2021, 12, 6140 CrossRef CAS PubMed.
- D. Gnanasekaran, P. A. Walter, A. A. Parveen and B. S. R. Reddy, Sep. Purif. Technol., 2013, 111, 108–118 CrossRef CAS.
- C.-Y. Wu, C.-C. Hu, L.-K. Lin, J.-Y. Lai and Y.-L. Liu, J. Membr. Sci., 2016, 499, 20–27 CrossRef CAS.
- Z. Liu, Y. Liu, W. Qiu and W. J. Koros, Angew. Chem., Int. Ed., 2020, 59, 14877–14883 CrossRef CAS PubMed.
- M. Etxeberria-Benavides, O. David, T. Johnson, M. M. Łozińskac, A. Orsi, P. A. Wright and S. Mastel, J. Membr. Sci., 2018, 550, 198–207 CrossRef CAS.
- H. Eguchi, D. J. Kim and W. J. Koros, Polymer, 2015, 58, 121–129 CrossRef CAS.
- B. Kraftschik and W. J. Koros, Macromolecules, 2013, 46, 6908–6921 CrossRef CAS.
- V. P. Babu, B. E. Kraftschik and W. J. Koros, J. Membr. Sci., 2018, 558, 94–105 CrossRef CAS.
- Z. Liu, W. Qiu, W. Quan, Y. Liu and W. J. Koros, J. Membr. Sci., 2021, 635, 119474 CrossRef CAS.
- C.-C. Chen, W. Qiu, S. J. Miller and W. J. Koros, J. Membr. Sci., 2011, 382, 212–221 CrossRef CAS.
- M. E. Dose, M. Chwatko, I. Hubacek, N. A. Lynd, D. R. Paul and B. D. Freeman, Polymer, 2019, 161, 16–26 CrossRef CAS.
- Y. Shi, Z. Wang, Y. Shi, S. Zhu, Y. Zhang and J. Jin, Macromolecules, 2022, 55, 2970–2982 CrossRef CAS.
- R. Thür, V. Lemmens, D. V. Havere, M. V. Essen, K. Nijmeijer and I. F. J. Vankelecom, J. Membr. Sci., 2020, 610, 118195 CrossRef.
- D.-C. Kong, M.-H. Yang, X.-S. Zhang, Z.-C. Du, Q. Fu, X.-Q. Gao and J.-W. Gong, Macromol. Mater. Eng., 2021, 306, 2100536 CrossRef CAS.
- M. E. Lamm, L. Song, Z. Wang, M. A. Rahman, B. Lamm, L. Fu and C. Tang, Macromolecules, 2019, 52, 8967–8975 CrossRef CAS.
- J. Park, C. O. Lee, J. W. Kim, J. H. Jo, W. S. Chi and H. Kim, Chem. Commun., 2022, 58, 4364–4367 RSC.
- A. X. Wu, S. Lin, K. M. Rodriguez, F. M. Benedetti, T. Joo, A. F. Grosz, K. R. Storme, N. Roy, D. Syar and Z. P. Smith, J. Membr. Sci., 2021, 636, 119526 CrossRef CAS.
- H. Lin, E. V. Wagner, J. S. Swinnea, B. D. Freeman, S. J. Pas, A. J. Hill, S. Kalakkunnath and D. S. Kalika, J. Membr. Sci., 2006, 276, 145–161 CrossRef CAS.
- H. Lin, E. V. Wagner, B. D. Freeman, L. G. Toy and R. P. Gupta, Science, 2006, 311, 639–642 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, synthetic procedures, NMR spectra, supplementary graphs and images and gas permeation data. See DOI: https://doi.org/10.1039/d2me00215a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |