Open Access Article
Open Access ArticleMolecular design of near-infrared (NIR) fluorescent probes targeting exopeptidase and application for detection of dipeptidyl peptidase 4 (DPP-4) activity†
Yuki
Hoshino
a,
Kenjiro
Hanaoka
 *b,
Kei
Sakamoto
c,
Masahiro
Yasunaga
d,
Takashi
Kojima
e,
Daisuke
Kotani
e,
Ayumu
Nomoto
a,
Eita
Sasaki
*b,
Kei
Sakamoto
c,
Masahiro
Yasunaga
d,
Takashi
Kojima
e,
Daisuke
Kotani
e,
Ayumu
Nomoto
a,
Eita
Sasaki
 b,
Toru
Komatsu
a,
Tasuku
Ueno
b,
Toru
Komatsu
a,
Tasuku
Ueno
 a,
Hiroyuki
Takamaru
f,
Yutaka
Saito
f,
Yasuyuki
Seto
c and
Yasuteru
Urano
*ag
a,
Hiroyuki
Takamaru
f,
Yutaka
Saito
f,
Yasuyuki
Seto
c and
Yasuteru
Urano
*ag
aGraduate School of Pharmaceutical Sciences, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan. E-mail: uranokun@m.u-tokyo.ac.jp
bGraduate School of Pharmaceutical Sciences, Keio University, 1-5-30 Shibakoen, Minato-ku, Tokyo 105-8512, Japan. E-mail: khanaoka@keio.jp
cDepartment of Gastrointestinal Surgery, Graduate School of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan
dDivision of Developmental Therapeutics, Exploratory Oncology Research & Clinical Trial Center, National Cancer Center, 6-5-1 Kashiwanoha, Kashiwa, Chiba 277-8577, Japan
eDepartment of Gastroenterology and Gastrointestinal Oncology, National Cancer Center Hospital East, 6-5-1, Kashiwanoha, Kashiwa-shi, Chiba 277-8577, Japan
fEndoscopy Division, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045, Japan
gGraduate School of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan
First published on 24th March 2022
Abstract
Monitoring the activities of proteases in vivo is an important requirement in biological and medical research. Near-infrared (NIR) fluorescent probes are particularly useful for in vivo fluorescence imaging, due to the high penetration of NIR and the low autofluorescence in tissue for this wavelength region, but most current NIR fluorescent probes for proteases are targeted to endopeptidase. Here, we describe a new molecular design for NIR fluorescent probes that target exopeptidase by utilizing the >110 nm blueshift of unsymmetrical Si–rhodamines upon amidation of the N atom of their xanthene moiety. Based on this molecular design, we developed Leu-SiR640 as a probe for leucine amino peptidase (LAP). Leu-SiR640 shows a one order of magnitude larger fluorescence increment (669-fold) upon reaction with LAP than existing NIR fluorescent probes. We similarly designed and synthesized EP-SiR640, a NIR fluorescent probe that targets dipeptidyl peptidase 4 (DPP-4). We show that this probe can monitor DPP-4 activity not only in living cells but also in mouse organs and tumors. This probe could also detect esophageal cancer in human clinical specimens, based on the overexpression of DPP-4 activity.
Introduction
Proteases play important roles in a wide range of biological phenomena and their activities are deeply related to many diseases, including cancer and neurodegenerative, inflammatory and vascular diseases.1–4 Proteases are also effective drug targets for the treatment of diseases such as hypertension (angiotensin-converting enzyme inhibitor),5 HIV (HIV protease inhibitor),6 and malignant melanoma (proteasome inhibitor).7 Therefore, detecting the activities of proteases in vivo is important both in biological research to understand disease mechanisms and in medicine for clinical diagnosis.8,9Activatable fluorescent probes are useful as chemical tools for detecting protease activities with high sensitivity and high temporal and spatial resolution.10 Among them, near-infrared (NIR) fluorescent probes have recently attracted attention,11 because NIR light (650–900 nm) shows high tissue penetration due to low absorption by biomolecules inside the body, and also because biological samples show low autofluorescence in this wavelength region.12,13 Thus, NIR fluorescent probes enable the detection of the target protease activity even in the living body.
Many NIR fluorescent probes for detecting protease activities have been developed by conjugating NIR fluorescent dyes and dark quenchers such as BHQ3 and QSY21 via a protease-recognition peptide.14,15 Their molecular design is based on the Förster resonance energy transfer (FRET) mechanism as a fluorescence-controlling off/on mechanism, and this approach can be applied to various NIR fluorophores. However, the molecular design can be applied only to probes for the enzymatic activity of endopeptidases that recognize a central region of the peptide sequence. In other words, these probes cannot detect the enzymatic activity of exopeptidases that recognize a terminal amino acid residue. Recently, a few NIR fluorescent probes for detecting exopeptidase activity have been developed by directly conjugating an amino acid to a NIR fluorophore.16–18 However, these probes do not show a dramatic fluorescence increment when cleaved via the enzymatic reaction (less than 30-fold). Therefore, new approaches to the molecular design of NIR fluorescent probes for exopeptidase activity are still needed.
In recent years, we have established a synthetic scheme for a group of unsymmetrical Si–rhodamines, which are NIR fluorophores.19 Rhodamines are widely used for fluorescence imaging because of their high fluorescence quantum yield, excellent photobleaching resistance, and high water solubility. In particular, unsymmetrical Si–rhodamines emit fluorescence in the far-red to NIR region, and it is possible to precisely control their absorption and emission wavelengths by means of alkyl substitution on the amino groups at the 3- and 6-positions of the xanthene ring. Here, we describe our discovery that unsymmetrical Si–rhodamine dyes showed very large blueshifts (>110 nm) of the absorption maximum when an amino group is converted to an amide group on the xanthene moiety. Although our previously reported symmetrical Si–rhodamine, SiR600, similarly showed a large blueshift, its fluorescence does not extend to the NIR wavelength region (∼650 nm), and a longer fluorescence wavelength is needed for in vivo imaging.20 Focusing on this photophysical property, we have developed a novel molecular design for NIR fluorescent probes that target exopeptidase activity. The developed probes show a fluorescence increase one order of magnitude higher than previously reported probes. As a proof of concept, we designed and synthesized Leu-SiR640 as a NIR fluorescent probe for leucine aminopeptidase (LAP) activity. This probe showed a 669-fold fluorescent increase after the enzymatic reaction. We also developed EP-SiR640 as a NIR fluorescent probe for dipeptidyl peptidase 4 (DPP-4) activity.
DPP-4 is a transmembrane exopeptidase that cleaves X-proline or X-alanine dipeptides from the peptide N-terminal. It hydrolyzes various biological peptides, including chemokines, neuropeptides, and bioactive peptides,21,22 and is involved in various biological processes and diseases. For instance, glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1), which are important for glucose homeostasis, are substrates of DPP-4, so DPP-4 inhibitors are widely used as therapeutic agents for type-2 diabetes.23 In recent years, DPP-4 has also attracted attention as a drug target for cancer immunotherapy and many studies have confirmed its antitumor effect and examined the mechanism involved.24–26 Furthermore, our group has found that fluorescent probes that target DPP-4 enable the rapid detection of esophageal cancer in resected tissue from patients,27,28 and it has also been reported that DPP-4 is overexpressed in esophageal cancer by other researchers.29 NIR fluorescent probes are expected to detect esophageal cancer in deeper regions compared with the previously reported green and red fluorescent probes for DPP-4. Therefore, we set out to develop a NIR fluorescent probe that targets DPP-4 activity, since we anticipated that this would be a powerful tool for studies of DPP-4-related biology and diseases, as well as a candidate for the clinical diagnosis of esophageal cancer. Indeed, EP-SiR640 was able to detect DPP-4 activity in living cells and mice, as well as in tumor-bearing mice and specimens from esophageal cancer patients.
Results and discussion
Photophysical properties of unsymmetrical Si–rhodamines
First, we designed and synthesized three unsymmetrical Si–rhodamines that contain an amino group (2,6-diMe SiR640, 2,6-diMe JuloSiR, and 2,6-diMe QuinoSiR) as dye scaffolds for exopeptidase probes, as well as their acetylated derivatives (Ac-2,6-diMe SiR640, Ac-2,6-diMe JuloSiR and Ac-2,6-diMe QuinoSiR) (Fig. 1a). A 2,6-dimethylbenzene moiety was introduced into the probe structure to prevent the attack of bionucleophiles such as reduced glutathione (GSH) at the 9-position of the Si–xanthene moiety of each probe. Interestingly, all the unsymmetrical Si–rhodamines showed very large blueshifts (more than 110 nm) of the absorption maximum upon amidation of the amino group on the xanthene ring (Fig. 1b and Fig. S1, ESI†). Moreover, the molar extinction coefficients and the fluorescence quantum yields of these unsymmetrical Si–rhodamines were also reduced by the amidation (Fig. 1a).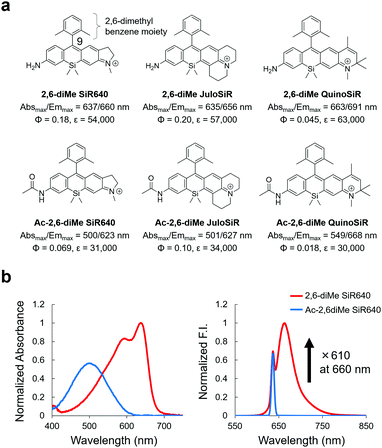 | ||
| Fig. 1 Changes in photophysical properties upon amidation of unsymmetrical Si–rhodamines. (a) Chemical structures and photophysical properties in phosphate-buffered saline (PBS; pH = 7.4) of the synthesized unsymmetrical Si–rhodamines and their acylated derivatives. The fluorescence quantum yields were determined using a Hamamatsu Photonics Quantaurus-QY spectrometer, and the photophysical properties of 2,6-diMe SiR640 are taken from ref. 19. (b) Normalized absorption (left) and emission (right) spectra of 1 μM 2,6-diMe SiR640 and Ac-2,6-diMe SiR640 in PBS (pH = 7.4) containing 0.1% DMSO as a co-solvent. The excitation wavelength was 637 nm. The sharp fluorescence peak of Ac-2,6-diMe SiR640 is the Rayleigh scattering of the excitation light. | ||
Due to the changes in the photophysical properties, all the acetylated unsymmetrical Si–rhodamines showed almost no fluorescence when excited at the absorption maximum before acetylation. Therefore, all compounds having an amino group showed a more than 100-fold greater fluorescence intensity than their acetylated forms (Fig. 1b and Fig. S1, ESI†). Among them, 2,6-diMe SiR640 showed the largest fluorescence intensity change (610-fold). Furthermore, the absorption and fluorescence spectra of all these compounds did not change in the range of pH 3–10 (Fig. S2, ESI†). Based on these results, it was expected that 2,6-diMe SiR640 would be a good basic scaffold for highly sensitive NIR fluorescent probes that target exopeptidase activity.
To investigate in detail the mechanism of this large absorption spectral change, we investigated the molecular orbitals of the synthesized unsymmetrical Si–rhodamines using TD-DFT (time-dependent density-functional theory) calculations. As a result, the transition from S0 to S1 of all compounds was assigned as the transition from the HOMO to the LUMO (Fig. S3d, ESI†), and the decrease of the HOMO energy level of each compound was larger than that of the LUMO energy level after acetylation of the N atom on the xanthene ring (Fig. S3, ESI†). Thus, we consider that the HOMO–LUMO energy gap of each compound was increased by acetylation, resulting in the observed large blueshift of the absorption maximum.
We further examined whether or not this large blueshift is observed in solvents other than water. Interestingly, the blueshift of Ac-2,6-diMe SiR640 in the absorption spectrum became larger (up to 123 nm) as the solvent polarity was increased, while 2,6-diMe SiR640 showed almost no blueshift regardless of the solvent polarity (Fig. S4, ESI†). Such fluorescent dyes that show a solvent polarity dependence of the absorption spectrum are known as environment-sensitive dyes. In general, the dipole moment of such dyes changes greatly between the ground state and the excited state, and is affected by the different degrees of solvent stabilization.30 The large blueshift of the absorption maximum in a polar solvent, as observed in Ac-2,6-diMe SiR640, can be explained as negative solvatochromism.31,32 Thus, it is considered that the acetylated unsymmetrical Si–rhodamines have a large dipole moment in the ground state, which is probably derived from the electron-withdrawing acetyl group, so that the blueshift of the absorption maximum in a polar solvent is larger than that in a less polar solvent (Fig. S5, ESI†). Thus, the large absorption wavelength difference between 2,6-diMe SiR640 and Ac-2,6-diMe SiR640 probably arises because 2,6-diMe SiR640 is not environment-sensitive, like typical rhodamines, but becomes environment-sensitive after acetylation.
Design and synthesis of a NIR fluorescent probe for leucine aminopeptidase (LAP)
Next, we examined if 2,6-diMe SiR640 could be used as a scaffold for NIR fluorescent probes for exopeptidase activity. As a proof of concept, we designed and synthesized Leu-SiR640 in which a recognition site for LAP, a leucine residue, was introduced into 2,6-diMe SiR640 (Fig. 2a), and we examined the absorption and fluorescence spectral changes after the enzymatic reaction. It was confirmed via HPLC analysis that the leucine residue of Leu-SiR640 was cleaved by the enzymatic reaction with LAP to produce 2,6-diMe SiR640 (Fig. S6, ESI†). Concomitantly, we observed a large redshift of the absorption maximum (139 nm) and a large fluorescence increase (up to 670-fold) (Fig. 2a and b). This fluorescence increase was suppressed by a LAP inhibitor, bestatin (Fig. 2c). Furthermore, Leu-SiR640 had a two orders of magnitude lower Km and a one order of magnitude higher kcat/Km compared with the fluorescent probe based on 7-amino-4-methylcoumarin (AMC) (Km = 189 μM, kcat/Km = 6.33 × 105 M−1 s−1),33 which is a widely used substrate for LAP (Fig. 2d). Furthermore, Leu-SiR640 showed a lower Km and a one order of magnitude higher fluorescence increase than existing NIR fluorescent probes for the activity of LAP (Fig. S7, ESI†). These results suggest that our molecular design for NIR fluorescent probes based on unsymmetrical Si–rhodamines is valid, and our scaffold is indeed suitable for developing NIR fluorescent probes with extremely high fluorescence activation in response to exopeptidase activity.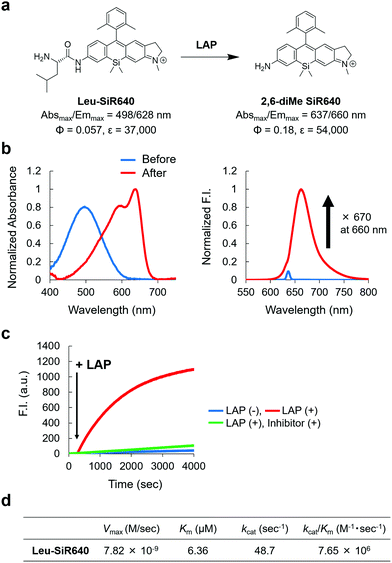 | ||
| Fig. 2 NIR fluorescent probe for LAP activity based on the unsymmetrical Si–rhodamine scaffold. (a) The reaction scheme of Leu-SiR640 with LAP and the photophysical properties. The data of 2,6-diMe SiR640 are taken from ref. 19. (b) Normalized absorption (left) and fluorescence (right) spectra of 1 μM Leu-SiR640 upon addition of LAP (11 ng) in 10 mM HEPES buffer (pH = 7.4). The excitation wavelength was 637 nm. The sharp small fluorescence peak is the Rayleigh scattering of the excitation light. (c) Time course of the fluorescence intensity change of 1 μM Leu-SiR640 upon addition of LAP (11 ng) at 300 s in 10 mM HEPES buffer (pH = 7.4). The excitation and emission wavelengths were 637 nm and 660 nm, respectively. The concentration of the LAP inhibitor, bestatin, was 100 μM. (d) Kinetic parameters of Leu-SiR640 with LAP. | ||
Design and synthesis of a NIR fluorescent probe for DPP-4
We next applied this molecular design to develop a NIR fluorescent probe for DPP-4 activity. The second proline from the N-terminal is essential for DPP-4 substrate recognition, and glycine–proline is a typical recognition sequence for DPP-4. So, we designed and synthesized GP-SiR640, EP-SiR640, DP-SiR640 and SP-SiR640 in which different DPP-4 recognition sequences were introduced into the 2,6-diMe SiR640 scaffold, and examined the changes of their photophysical properties upon enzymatic reaction with DPP-4. HPLC analysis indicated that all these probes afford 2,6-diMe SiR640 upon enzymatic reaction with DPP-4 (Fig. S8, ESI†), and showed a large redshift of the absorption maximum (about 140 nm) and a >200-fold fluorescence increase, similar to the probe for LAP activity (Fig. 3b and Fig. S9, ESI†). These fluorescence increases were suppressed by the DPP-4 inhibitor sitagliptin (Fig. 3c and Fig. S10, ESI†). Furthermore, the enzymatic reaction constants of each fluorescent probe were determined (Fig. 3d). The Km and kcat/Km values of each fluorescent probe were almost the same as those of a fluorescent probe based on AMC, GP-AMC (Km = 34 μM, kcat/Km = 7.6 × 105 M−1 s−1),34 which is a widely used fluorescent substrate. It is interesting that the kcat/Km value of DP-SiR640 is about one-third that of EP-SiR640, and the slight structural difference between these probes may affect their enzymatic recognition by DPP-4.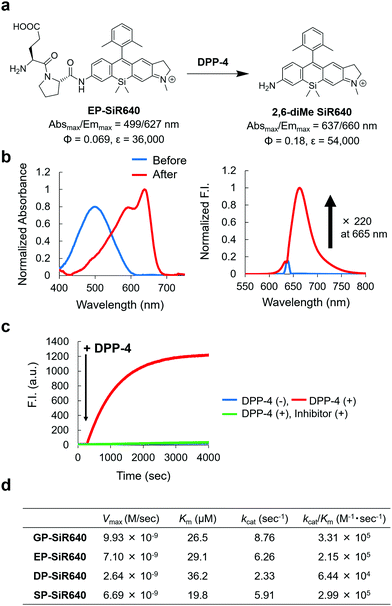 | ||
| Fig. 3 NIR fluorescent probe for DPP-4 activity based on the unsymmetrical Si–rhodamine scaffold. (a) Reaction scheme of EP-SiR640 with DPP-4 and the photophysical properties. The data of 2,6-diMe SiR640 are taken from ref. 19. (b) Normalized absorption (left) and fluorescence (right) spectra of 1 μM EP-SiR640 upon addition of DPP-4 (0.24 μg) in 10 mM HEPES buffer (pH = 7.4). The excitation wavelength was 637 nm. The sharp small fluorescence peak is the Rayleigh scattering of the excitation light. (c) Time course of the fluorescence intensity change of 1 μM EP-SiR640 upon addition of DPP-4 (0.24 μg) at 300 s in 10 mM HEPES buffer (pH = 7.4). The excitation and emission wavelengths were 637 nm and 660 nm, respectively. The concentration of the DPP-4 inhibitor, sitagliptin, was 1.8 μM. (d) Kinetic parameters of GP-SiR640, EP-SiR640, DP-SiR640 and SP-SiR640 with DPP-4. | ||
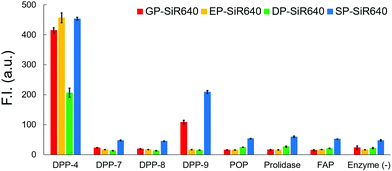
We then examined the selectivity of these probes for DPP-4 versus various other prolyl peptidases. There are many prolyl peptidases other than DPP-4 in the human body, and among them, DPP-8, DPP-9, and fibroblast active protein (FAP) are reported to release N-terminal proline dipeptides.23,35 So, we measured the fluorescence increase when various prolyl peptidases were added to each probe solution (Fig. 4). All the fluorescent probes showed a large fluorescence increase upon addition of DPP-4. Interestingly, EP-SiR640 and DP-SiR640 showed almost no fluorescence increase upon addition of DPP-9, whereas GP-SiR640 and SP-SiR640 showed a relatively large fluorescence increase. All the probes showed essentially no fluorescence increase when prolyl peptidases other than DPP-4 and DPP-9 were added. Among the probes, EP-SiR640 showed the highest selectivity and reactivity to DPP-4, and we thought that it would be suitable for the selective detection of DPP-4 activity in living samples. The structure at the active site is highly conserved among dipeptidyl peptidases, including DPP-4 and DPP-9. For example, the hydrogen bonds between the N-terminal amino group of the substrate peptide and Glu248 and Glu249 residues are essential for substrate recognition.36 On the other hand, the active site of DPP-9 has a narrower space around the N-terminal amino acid residue of the substrate than that of DPP-4.36 So, we assumed that EP-SiR640 and DP-SiR640 showed a low affinity for DPP-9 due to repulsion involving the negative charges of a carboxylic acid group of the probe and two glutamic acid residues at the active site of DPP-9.
Live-cell fluorescence imaging
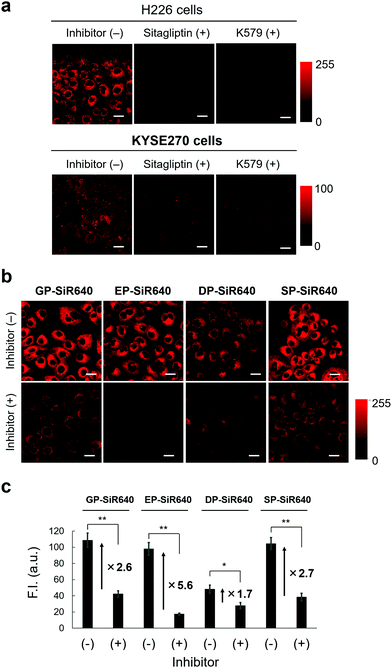
We then applied EP-SiR640 for live-cell fluorescence imaging of two different cell lines (H226 and KYSE270). First, the expression of DPP-4 in both cells was confirmed via western blotting (Fig. S11, ESI†). Then, EP-SiR640 was applied to both cell lines with or without the DPP-4 inhibitor (sitagliptin or K579). A fluorescence intensity increase of EP-SiR640 was observed in both cell lines within 10 min after addition of the probe, and this fluorescence increase was significantly suppressed in the presence of the DPP-4 inhibitor (Fig. 5a and Fig. S12, ESI†). In addition, EP-SiR640 was mainly localized in the mitochondria and partly in lysosomes and showed high intracellular retention, so that strong fluorescence was still observed even after three washing procedures (Fig. S13 and S14, ESI†). We also performed live-cell fluorescence imaging with four developed NIR fluorescent probes (GP-SiR640, EP-SiR640, DP-SiR640 and SP-SiR640) to compare their fluorescence activation in living cells. These fluorescent probes were applied to H226 cells with or without the DPP-4 inhibitor. A fluorescence increase was observed with all the probes, and was suppressed in the presence of the DPP-4 inhibitor (Fig. 5b and Fig. S15, ESI†). Among them, EP-SiR640 showed the largest fluorescence increase ratio after incubation for 60 min (Fig. 5c), in accordance with the in vitro experiments shown in Fig. 4. There are two possible reasons for this: (i) EP-SiR640 shows high selectivity for DPP-4 among the various prolyl peptidases, as shown in Fig. 4; (ii) EP-SiR640 shows lower membrane permeability than the other probes due to the negatively charged carboxylic acid group of the glutamic acid residue. DPP-4 is the membrane protein, and if the probe can easily enter the cells, an intracellular fluorescence increase would be observed independently of DPP-4 activity due to the enzymatic activation of the probe by intracellular enzymes other than DPP-4. The idea that EP-SiR640 and DP-SiR640, which both have a carboxylic acid group, show low cell-membrane permeability is consistent with the observation that their fluorescence intensity in A549 cells, which have a low level of DPP-4 expression, is low compared to that of GP-SiR640 (Fig. S16, ESI†).Furthermore, we investigated whether or not EP-SiR640 can detect differences of intracellular DPP-4 activity using various living cells. First, cell lysates of four different cell lines (H226, KYSE270, A549 and HEK293) were prepared, and the DPP-4 activity of each cell lysate was measured with EP-SiR640. Based on the fluorescence intensity increase of EP-SiR640 in the cell lysates, it was confirmed that H226 and KYSE270 cells, which express DPP-4 as determined via western blotting (Fig. S11, ESI†), had a higher DPP-4 activity than A549 and HEK293 cells (Fig. S17a, ESI†). Then, fluorescence imaging of each cell line with EP-SiR640 was performed. A fluorescence increase was observed in H226 and KYSE270 cells, while no fluorescence increase was observed in A549 and HEK293 cells, in accordance with the cell lysate experiments (Fig. S17, ESI†). When we examined the expression of DPP-4 in A549 and HEK293 cells via western blotting, we observed the expression of DPP-4 in A549 and HEK293 cells as well as in H226 and KYSE270 cells (Fig. S11, ESI†). From the result, we think that the lower fluorescence intensity of A549 and HEK293 cells in the experiments with the cell lysate and the live-cell fluorescence imaging shown in Fig. S17 (ESI†) may be due to the low expression of the active form of DPP-4 in these cells. Thus, the enzymatic activity level of DPP-4 in the living cells could be monitored through fluorescence imaging with EP-SiR640.
In vivo fluorescence imaging
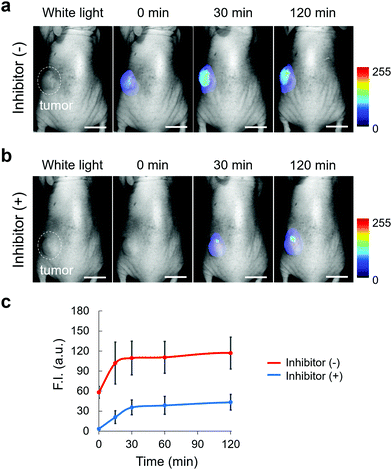
We next applied EP-SiR640 to in vivo fluorescence imaging to examine whether or not this probe can detect endogenous DPP-4 activity in living mice. All animal procedures were approved by the Animal Care and Use Committee of the University of Tokyo. Saline or DPP-4 inhibitor (sitagliptin or linagliptin) in saline was orally administered to mice every day for one week, then EP-SiR640 was intravenously administered, and fluorescence imaging was performed under anesthesia. Note that these two inhibitors are used in clinical practice to treat type-2 diabetes. We measured the time-dependent fluorescence increase of each exposed organ inside the body after the resection of the skin. When we performed the fluorescence imaging of mice orally administered with saline for a week after intravenous injection with 100 μM EP-SiR640via the tail vein, large and definite fluorescence increases were observed in the kidney, and the liver and the intestine, respectively (Fig. S18a, ESI†). So, it is considered that EP-SiR640 was rapidly metabolized to 2,6-diMe SiR640 and excreted mainly via the kidney within an hour, while 2,6-diMe SiR640 had a high intracellular retention as shown in Fig. S14 (ESI†). On the other hand, the fluorescence intensity increase seen in living mice given saline was suppressed by the administration of DPP-4 inhibitor (Fig. S18, ESI†). In addition, after oral administration of saline or various concentrations of the DPP-4 inhibitor (sitagliptin or linagliptin) in saline to mice every day for 3 days, the mice were sacrificed and their organs were removed. Each organ was incubated with EP-SiR640 aqueous solution, and ex vivo fluorescence imaging was performed. The fluorescence intensity increase was suppressed in a concentration-dependent manner by the DPP-4 inhibitors, and the DPP-4-inhibitory activity could be evaluated at the organ level (Fig. S19 and S20, ESI†). Both inhibitors inhibited the DPP-4 activity in all organs, especially the digestive organs such as the stomach and intestines, which may account for their therapeutic effect in type-2 diabetes. Linagliptin suppressed the DPP-4 activity in all organs at a lower dose (1 mg kg−1 day−1) than sitagliptin, and this is consistent with the much lower dissociation constant (Kd) of linagliptin than that of sitagliptin.37 Thus, this probe enables monitoring of the DPP-4 activity (not the biodistribution of the DPP-4 inhibitor) inside the body, with all the advantages of a fluorescent probe compared to a radioactive probe.Finally, we applied EP-SiR640 to tumor imaging in mice and clinical specimens. First, we applied EP-SiR640 to H226 tumor-bearing mice. Saline or DPP-4 inhibitor (sitagliptin) was intratumorally administered, and then EP-SiR640 was also intratumorally administered. We observed a time-dependent increase of fluorescence in the tumor, and the fluorescence increase was suppressed in the presence of the DPP-4 inhibitor (Fig. 6 and Fig. S21, ESI†). Strong fluorescence was maintained even 2 hours after the administration of the fluorescent probe, suggesting that EP-SiR640 is well retained in the tumors. The slight fluorescence observed in the tissues surrounding the tumor was considered to be due to leakage of the probe injected into the tumor or of the activated probe after enzymatic reaction in the tumor. Thus, EP-SiR640 could fluorescently detect DPP-4 activity in the tumor in vivo.
Fluorescence imaging in clinical specimens
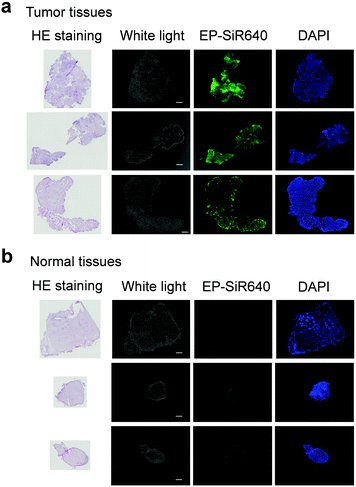
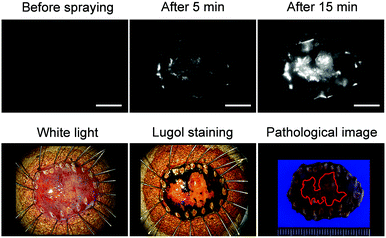
Next, we examined whether or not EP-SiR640 could detect human esophageal tumors that express DPP-4. Our group has previously reported that green and red fluorescent probes that target DPP-4 are effective for detecting esophageal tumors in clinical specimens obtained via ESD (endoscopic submucosal dissection).27,28 So, we examined if EP-SiR640 could also detect esophageal tumors in human ESD specimens. First, EP-SiR640 was applied to sections of human biopsy clinical specimens containing esophageal tumor and normal tissues. All samples were confirmed to contain tumor or normal tissue through hematoxylin/eosin (HE) staining (Fig. 7). We observed strong fluorescence from the tumor tissues after the addition of EP-SiR640, while no such fluorescence increase was seen in normal tissues (Fig. 7). This result suggested that EP-SiR640 can clearly distinguish between tumor and normal tissue sites in esophageal cancer biopsy specimens.Finally, we applied EP-SiR640 to the fluorescence imaging of esophageal tumor tissues in freshly resected ESD specimens. When EP-SiR640 was applied to the esophageal tumor specimens, regions of squamous cell carcinoma (SCC) could be detected within 5 minutes based on the increased fluorescence (Fig. 8 and Fig. S22, ESI†). Furthermore, the boundary between the tumor and normal tissues was clearly demarcated, and was in good agreement with the boundary seen after Lugol staining and with the pathological diagnosis of the tumor. We further applied EP-SiR640 to 6 ESD specimens of esophageal cancer. Although 2 small specimens showed a fluorescence increase in both normal and tumor regions, the others showed fluorescence images that coincided well with the Lugol staining images (Fig. S23 and Table S1, ESI†). Thus, we succeeded in rapidly detecting esophageal tumors in freshly resected ESD specimens using EP-SiR640.
Conclusions
We have established a new molecular design strategy for NIR fluorescent probes that target exopeptidase activity and show a large fluorescence enhancement. This design is based on the large blueshift of unsymmetrical Si–rhodamines triggered by N-acetylation on their xanthene moiety. The synthesized unsymmetrical Si–rhodamines showed blueshifts of more than 110 nm upon acetylation of the N atom on the xanthene ring (Fig. 1a). We chose 2,6-diMe SiR640 as a scaffold, and developed Leu-SiR640, which showed a fluorescence increase of more than 600-fold upon enzymatic reaction with LAP, based on the large blueshift in the absorption spectrum of this scaffold. This fluorescence increase is an order of magnitude larger than that of existing NIR fluorescent probes for LAP activity. We also observed small fluorescence increases due to the non-enzymatic reaction in Fig. 2c and performed stability experiments for the synthesized probes (Fig. S24, ESI†). As a result, the stability of the probe was dependent on the cleaved group of the probe and further stabilization of the probe may be possible by designing the structure of the cleaved group. We believe our approach utilizing this unique blueshift of unsymmetrical Si–rhodamines provides a flexible platform for NIR fluorescent probes that target various exopeptidase activities. The large absorption change may also be useful for the detection of exopeptidase activity via photoacoustic imaging, which is a novel imaging modality that utilizes ultrasonic waves emitted after the photoexcitation of molecules.38 Since ultrasonic waves have a higher tissue penetration than light,38,39 these probes should be useful for the photoacoustic imaging of exopeptidase activity deep inside the body.To demonstrate the usefulness of this molecular design strategy, we developed NIR fluorescent probes for detecting DPP-4 activity. By optimizing the DPP-4 recognition sequence, we succeeded in developing EP-SiR640, which detected DPP-4 activity in terms of a large fluorescence increase in vitro and was also applicable for the detection of DPP-4 activity in living cells. Furthermore, we could monitor the inhibitory activity of DPP-4 inhibitors towards endogenous DPP-4 inside the mouse body, as well as performing the in vivo fluorescence imaging of tumors in tumor-bearing mouse models. EP-SiR640 generated strong fluorescence in tumor tissues, but not in normal tissues in human biopsy clinical specimens, as well as freshly resected ESD esophageal cancer specimens. An esophageal tumor could be detected within 5 min (Fig. 8). Since DPP-4 is involved in various biological phenomena and diseases, including cancer and type-2 diabetes, EP-SiR640 is expected to be useful as a research tool for probing DPP-4-related biological phenomena and diseases, in addition to its clinical potential as a diagnostic aid.
Author contributions
Y. H. and K. H. planned the experiments and wrote the manuscript. Y. H., A. N. and E. S. synthesized compounds, measured photophysical data and performed fluorescence imaging of live cells and mouse models. K. S., Y. Seto, H. T. and Y. Saito performed the fluorescence imaging of ESD samples. M. Y., T. K. and D. K. performed the fluorescence imaging of biopsy samples. Y. H. and K. H. performed the TD-DFT study. K. H. and Y. U. planned the project. T. K. and T. U. advised on the experiments. All authors edited the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported in part by JSPS KAKENHI Grant Numbers JP20H02701, JP21H05262, JP20H04767, and JP18H04609, to K.H., JST SENTAN to K.H., Hoansha Foundation to K.H., Mochida Memorial Foundation for Medical and Pharmaceutical Research to K.H., Astellas Foundation for Research on Metabolic Disorders to K.H., The Tokyo Biochemical Research Foundation to K.H. and Nakatani Foundation for Advancement of Measuring Technologies in Biomedical Engineering to K.H. This work was also supported by AMED under Grant Number JP21ak0101182h0001 and JP21wm0325046s0101 to K.H. by a Grant-in-Aid for Scientific Research on Innovative Areas “Singularity Biology (No. 8007)” (JP19H05414 to K.H.) from The Ministry of Education, Culture, Sports, Science, and Technology, Japan.References
- M. Drag and G. S. Salvesen, Nat. Rev. Drug Discovery, 2010, 9, 690–701 CrossRef CAS PubMed.
- M. Egeblad and Z. Werb, Nat. Rev. Cancer, 2002, 2, 161–174 CrossRef CAS PubMed.
- J. Hu, P. E. Van den Steen, Q. X. A. Sang and G. Opdenakker, Nat. Rev. Drug Discovery, 2007, 6, 480–498 CrossRef CAS PubMed.
- D. E. Bredesen, Mol. Neurodegener., 2009, 4, 27 CrossRef.
- F. H. Messerli, S. Bangalore, C. Bavishi and S. F. Rimoldi, J. Am. Coll. Cardiol., 2018, 71, 1474–1482 CrossRef CAS PubMed.
- J. Mallolas, AIDS Rev., 2017, 19, 105–112 Search PubMed.
- S. Gandolfi, J. P. Laubach, T. Hideshima, D. Chauhan, K. C. Anderson and P. G. Richardson, Cancer Metastasis Rev., 2017, 36, 561–584 CrossRef CAS.
- P. Cheng and K. Pu, Nat. Rev. Mater., 2021, 6, 1095–1113 CrossRef CAS.
- Z. Zeng, S. S. Liew, X. Wei and K. Pu, Angew. Chem., Int. Ed., 2021, 60, 26454–26475 CrossRef CAS PubMed.
- H. W. Liu, L. Chen, C. Xu, Z. Li, H. Zhang, X. B. Zhang and W. Tan, Chem. Soc. Rev., 2018, 47, 7140–7180 RSC.
- J. Bin, Li, H. W. Liu, T. Fu, R. Wang, X. B. Zhang and W. Tan, Trends Chem., 2019, 1, 224–234 CrossRef PubMed.
- R. Weissleder, Nat. Biotechnol., 2001, 19, 316–317 CrossRef CAS PubMed.
- J. V. Frangioni, Curr. Opin. Chem. Biol., 2003, 7, 626–634 CrossRef CAS PubMed.
- T. Myochin, K. Hanaoka, T. Komatsu, T. Terai and T. Nagano, J. Am. Chem. Soc., 2012, 134, 13730–13737 CrossRef CAS PubMed.
- M. Verdoes, K. Oresic Bender, E. Segal, W. A. Van Der Linden, S. Syed, N. P. Withana, L. E. Sanman and M. Bogyo, J. Am. Chem. Soc., 2013, 135, 14726–14730 CrossRef CAS PubMed.
- X. He, L. Li, Y. Fang, W. Shi, X. Li and H. Ma, Chem. Sci., 2017, 8, 3479–3483 RSC.
- W. Zhang, F. Liu, C. Zhang, J. G. Luo, J. Luo, W. Yu and L. Kong, Anal. Chem., 2017, 89, 12319–12326 CrossRef CAS PubMed.
- B. Wu, Y. Lin, B. Li, C. Zhan and S. Wu, Anal. Chem., 2018, 90, 9359–9365 CrossRef CAS PubMed.
- K. Hanaoka, Y. Kagami, W. Piao, T. Myochin, K. Numasawa, Y. Kuriki, T. Ikeno, T. Ueno, T. Komatsu, T. Terai, T. Nagano and Y. Urano, Chem. Commun., 2018, 54, 6939–6942 RSC.
- Y. Kushida, K. Hanaoka, T. Komatsu, T. Terai, T. Ueno, K. Yoshida, M. Uchiyama and T. Nagano, Bioorg. Med. Chem. Lett., 2012, 22, 3908–3911 CrossRef CAS PubMed.
- E. E. Mulvihill and D. J. Drucker, Endocr. Rev., 2014, 35, 992–1019 CrossRef CAS PubMed.
- D. Röhrborn, N. Wronkowitz and J. Eckel, Front. Immunol., 2015, 6, 386 Search PubMed.
- D. Drucker, Diabetes Care, 2007, 30, 1335–1343 CrossRef CAS PubMed.
- K. Ohnuma, R. Hatano and C. Morimoto, Nat. Immunol., 2015, 16, 791–792 CrossRef CAS PubMed.
- R. Barreira Da Silva, M. E. Laird, N. Yatim, L. Fiette, M. A. Ingersoll and M. L. Albert, Nat. Immunol., 2015, 16, 850–858 CrossRef CAS PubMed.
- A. Munitz and S. P. Hogan, Nat. Immunol., 2019, 20, 250–252 CrossRef CAS PubMed.
- H. Onoyama, M. Kamiya, Y. Kuriki, T. Komatsu, H. Abe, Y. Tsuji, K. Yagi, Y. Yamagata, S. Aikou, M. Nishida, K. Mori, H. Yamashita, M. Fujishiro, S. Nomura, N. Shimizu, M. Fukayama, K. Koike, Y. Urano and Y. Seto, Sci. Rep., 2016, 6, 26399 CrossRef CAS PubMed.
- A. Ogasawara, M. Kamiya, K. Sakamoto, Y. Kuriki, K. Fujita, T. Komatsu, T. Ueno, K. Hanaoka, H. Onoyama, H. Abe, Y. Tsuji, M. Fujishiro, K. Koike, M. Fukayama, Y. Seto and Y. Urano, Bioconjugate Chem., 2019, 30, 1055–1060 CrossRef CAS PubMed.
- K. Augoff, A. Hryniewicz-Jankowska, R. Tabola, L. Czapla, P. Szelachowski, J. Wierzbicki, K. Grabowski and A. F. Sikorski, Oncol. Rep., 2014, 31, 2820–2826 CrossRef CAS PubMed.
- A. S. Klymchenko and Y. Mely, Prog. Mol. Biol. Transl. Sci., 2013, 113, 35–58 CAS.
- C. Reichardt, Chem. Rev., 1994, 94, 2319–2358 CrossRef CAS.
- P. Suppan, J. Photochem. Photobiol., A, 1990, 50, 293–330 CrossRef CAS.
- R. E. Morty and J. Morehead, J. Biol. Chem., 2002, 277, 26057–26065 CrossRef CAS PubMed.
- M. Kawaguchi, T. Okabe, T. Terai, K. Hanaoka, H. Kojima, I. Minegishi and T. Nagano, Chem. – Eur. J., 2010, 16, 13479–13486 CrossRef CAS PubMed.
- J. S. Rosenblum and J. W. Kozarich, Curr. Opin. Chem. Biol., 2003, 7, 496–504 CrossRef CAS PubMed.
- B. Ross, S. Krapp, M. Augustin, R. Kierfersauer, M. Arciniega, R. Geiss-Friedlander and R. Huber, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, E1437–E1445 CAS.
- G. Schnapp, T. Klein, Y. Hoevels, R. A. Bakker and H. Nar, J. Med. Chem., 2016, 59, 7466–7477 CrossRef CAS PubMed.
- L. V. Wang and J. Yao, Nat. Methods, 2016, 13, 627–638 CrossRef CAS PubMed.
- L. V. Wang and S. Hu, Science, 2012, 335, 1458–1462 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic details, spectra, supporting figures, and experimental details. See DOI: https://doi.org/10.1039/d1cb00253h |
| This journal is © The Royal Society of Chemistry 2022 |