Graphene quantum dots for energy storage and conversion: from fabrication to applications
Qianwen
Liu†
,
Jianhan
Sun†
,
Kun
Gao
,
Nan
Chen
 *,
Xiaotong
Sun
,
Dan
Ti
,
Congcong
Bai
,
Ranran
Cui
and
Liangti
Qu
*,
Xiaotong
Sun
,
Dan
Ti
,
Congcong
Bai
,
Ranran
Cui
and
Liangti
Qu
 *
*
Key Laboratory of Photoelectronic/Electrophotonic Conversion Materials, Key Laboratory of Cluster Science, Ministry of Education of China, School of Chemistry and Chemical Engineering, Beijing Institute of Technology, Beijing 100081, P. R. China. E-mail: gabechain@bit.edu.cn; lqu@bit.edu.cn
First published on 4th November 2019
Abstract
As a new kind of zero-dimensional (0D) material, graphene quantum dots (GQDs) have broad prospects in energy storage and conversion due to their unique physical and chemical properties. In addition to the excellent properties of graphene, GQDs also have quantum confinement effects and edge effects. The size of GQDs and types of their edges determine their excellent properties. In this paper, the progress in the synthesis, doping and modification methods of GQDs in recent years is reviewed, and the significant advances of GQDs in energy conversion devices such as supercapacitors/microsupercapacitors, solar cells, batteries and LEDs are summarized. In addition, we rationally analysed the shortcomings of GQDs for energy storage and conversion, and predicted the future development trend of GQD research and its challenges and opportunities.
1 Introduction
Graphene is a two-dimensional (2D) honeycomb monatomic carbon molecular layer with large surface area, intrinsic carrier mobility (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm2 V−1 s) and excellent mechanical strength and ductility. However, the zero band gap and metal conductivity of graphene severely limited the application of 2D graphene layers in functional materials and devices.1,2 Fortunately, the emergence and successful preparation of GQDs in time fundamentally overcame these disadvantages. As a 0D carbon-based nanomaterial, GQDs not only exhibit the excellent properties of graphene, but their typical quantum confinement and edge effects benefit from their lateral dimension of less than 10 nm and thickness of less than 2 nm,3–5 which endows GQDs with broad application prospects in the field of nanomaterials and -devices. For example, GQDs can be used to prepare photoelectric detectors and light emitting diode devices owing to their strong size optical dependence.4 In addition, through the multiple hot carrier collection mechanism, the introduction of GQDs in silicon-based solar cells could effectively break through the Shockley–Queisser efficiency limit.5 Furthermore, GQDs inherit the good biocompatibility, excellent chemical inertness and non-toxicity properties of graphene.5–8 Besides this, GQDs also have a larger specific surface area and better surface grafting than graphene through π–π conjugate bonding.9 In particular, a multitude of functional groups in GQDs make them a good non-zero band gap and photoluminescent semiconductor material. Density functional theory found that the band gap of GQDs containing individual aromatic rings reaches about 7.4 eV.10 Consequently, the original GQDs can be added as a conductive agent to the electrode in energy storage devices such as supercapacitors due to their good conductivity, large specific surface area and ease of doping and modification. It has been reported that top-down and bottom-up approaches are the main methods of manufacturing GQDs.5 Within these approaches, various techniques for fabricating GQDs with various chemical, size, and luminescent properties have been reported.11 The modification of GQDs plays an important role in the manufacture of materials and devices, which prompts researchers to make great efforts in the exploration of the chemical functions or reactivity of materials. Compared with the luminescence properties of other semiconductor quantum dots (QDs), as the excitation wavelength increases, the luminescence peak of GQDs broadens and red-shifts, exhibiting fluorescence characteristics associated with the excitation wavelength.12,13 The prominent luminescent properties of GQDs stimulate their development in biological applications such as bioimaging, biosensors, and cell isolation techniques.14 Additionally, chemical groups and dopants endow GQDs with intrinsic catalytic properties through band gap alignment, synergistic cooperation, light adsorption enhancement or promotion of charge transfer.15 Meanwhile, the stability of catalysts is significantly improved owing to the chemical inertness of GQDs. To date, many review articles on the biomedical,16,17 photocatalytic,18,19 and environmental20 applications of GQDs have been published. However, the development of GQDs in energy conversion has rarely been reported.21 Hence, we introduce the synthesis of universal GQDs, performance tuning, and unique applications in the field of energy conversion and storage. We are committed to providing a comparative and balanced perspective and an in-depth understanding of why GQDs deliver superior applications in energy conversion and storage, including electronics, solar photovoltaic cells, and nanogenerators. We anticipate that this review article will benefit all interested researchers, especially those who are engaged in research utilizing GQDs. Finally, future challenges and possibilities in the field of GQDs are also identified and discussed.
000 cm2 V−1 s) and excellent mechanical strength and ductility. However, the zero band gap and metal conductivity of graphene severely limited the application of 2D graphene layers in functional materials and devices.1,2 Fortunately, the emergence and successful preparation of GQDs in time fundamentally overcame these disadvantages. As a 0D carbon-based nanomaterial, GQDs not only exhibit the excellent properties of graphene, but their typical quantum confinement and edge effects benefit from their lateral dimension of less than 10 nm and thickness of less than 2 nm,3–5 which endows GQDs with broad application prospects in the field of nanomaterials and -devices. For example, GQDs can be used to prepare photoelectric detectors and light emitting diode devices owing to their strong size optical dependence.4 In addition, through the multiple hot carrier collection mechanism, the introduction of GQDs in silicon-based solar cells could effectively break through the Shockley–Queisser efficiency limit.5 Furthermore, GQDs inherit the good biocompatibility, excellent chemical inertness and non-toxicity properties of graphene.5–8 Besides this, GQDs also have a larger specific surface area and better surface grafting than graphene through π–π conjugate bonding.9 In particular, a multitude of functional groups in GQDs make them a good non-zero band gap and photoluminescent semiconductor material. Density functional theory found that the band gap of GQDs containing individual aromatic rings reaches about 7.4 eV.10 Consequently, the original GQDs can be added as a conductive agent to the electrode in energy storage devices such as supercapacitors due to their good conductivity, large specific surface area and ease of doping and modification. It has been reported that top-down and bottom-up approaches are the main methods of manufacturing GQDs.5 Within these approaches, various techniques for fabricating GQDs with various chemical, size, and luminescent properties have been reported.11 The modification of GQDs plays an important role in the manufacture of materials and devices, which prompts researchers to make great efforts in the exploration of the chemical functions or reactivity of materials. Compared with the luminescence properties of other semiconductor quantum dots (QDs), as the excitation wavelength increases, the luminescence peak of GQDs broadens and red-shifts, exhibiting fluorescence characteristics associated with the excitation wavelength.12,13 The prominent luminescent properties of GQDs stimulate their development in biological applications such as bioimaging, biosensors, and cell isolation techniques.14 Additionally, chemical groups and dopants endow GQDs with intrinsic catalytic properties through band gap alignment, synergistic cooperation, light adsorption enhancement or promotion of charge transfer.15 Meanwhile, the stability of catalysts is significantly improved owing to the chemical inertness of GQDs. To date, many review articles on the biomedical,16,17 photocatalytic,18,19 and environmental20 applications of GQDs have been published. However, the development of GQDs in energy conversion has rarely been reported.21 Hence, we introduce the synthesis of universal GQDs, performance tuning, and unique applications in the field of energy conversion and storage. We are committed to providing a comparative and balanced perspective and an in-depth understanding of why GQDs deliver superior applications in energy conversion and storage, including electronics, solar photovoltaic cells, and nanogenerators. We anticipate that this review article will benefit all interested researchers, especially those who are engaged in research utilizing GQDs. Finally, future challenges and possibilities in the field of GQDs are also identified and discussed.
2 Fabrication of GQDs
Considering that GQDs are tiny graphite planes with 0D and atomic thickness (usually only 1 or 2 layers and less than 2 nm thick), the fabrication methods have typically been classified as “top-down” and “bottom-up” approaches.2.1 Top-down methods
The preparation methods of GQDs from top to bottom contained the following several methods, including hydrothermal and solvothermal, electrochemical, stripping, strong acid oxidation, and electron beam irradiation methods.22,23 Among them, the hydrothermal and solvothermal synthesis of GQDs is one of the most common methods with simple steps and high yields.As shown in Fig. 1a, a simple method for synthesizing GQDs is to cut carbon materials with bases such as sodium hydroxide (NaOH) and ammonia. Gong et al. obtained biocompatible GQDs with up-conversion properties by using graphene oxide as a raw material, dimethylformamide as a solvent and a nitrogen source through a simple solvothermal method.24 On the other hand, the electrochemical method is beneficial to obtaining superior GQDs by precise control of their size and shape. After that, high quality GQDs prepared by chemical vapour deposition (CVD) combined with the electrochemical method using 3D graphene were reported (Fig. 1b).25 GQDs have a narrow particle size distribution, a low molecular weight and an average thickness greater than 1.25 nm. Subsequently, many efforts have been devoted to controlling and regulating the size of GQDs. Using multi-walled carbon nanotubes (MWCNTs) as a carbon source, Pillai et al. prepared GQDs via electrochemical oxidation by using a solution of LiClO4 dissolved propylene carbonate as an electrolyte (Fig. 1c).26 The size of GQDs can be adjusted by controlling the conditions of the electrolysis reaction, such as the temperature and time. Acid oxidation is a method of destroying carbon–carbon bonds and decomposing graphene sheets into small-sized GQDs of less than 100 nm by strong oxidation with a strong acid such as concentrated sulfuric acid, concentrated nitric acid or a mixed acid composed of concentrated sulfuric acid and concentrated nitric acid. The acid oxidation method requires very simple and inexpensive raw materials, which provides a new strategy for the preparation of GQDs. For instance, Ajayan et al. synthesized bitumen carbon fiber-based micrometer-GQDs through chemical oxidation and cleavage, and the results show that most of the GQDs obtained have zigzag edge structures with semiconductor characteristics, and their particle size distribution ranges between 1 nm and 4 nm (Fig. 1d).14 Furthermore, in 2019, Tour et al. reported a general method for preparing GQDs by placing various coal sources in sulfuric and nitric acid and sonicating and heating them (Fig. 1e).27 The GQDs obtained by the improved synthesis method have high crystallinity and good solubility in both water and organic solvents. In 2016, Zhang et al. prepared GQDs with a grain diameter of 4–10 nm by using an ultrasonic-assisted chemical stripping method and using O3 and H2O2 to conduct oxidation cutting of GO.28
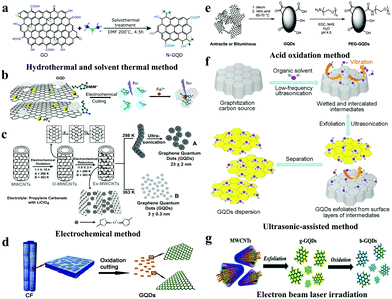 | ||
| Fig. 1 Top-down synthesis of GQDs. (a) The solvothermal method to prepare GQDs. (Reproduced with permission from ref. 24, copyright 2013 American Chemical Society.) (b) The electrochemical method to prepare GQDs. (Reproduced with permission from ref. 25, copyright 2014 Wiley-VCH.) (c) The preparation of photoluminescent GQDs from MWCNTs. (Reproduced with permission from ref. 26, copyright 2012 Wiley-VCH.) (d) The oxidation cutting of carbon fibers (CFs) to prepare GQDs. (Reproduced with permission from ref. 14, copyright 2012 American Chemical Society.) (e) The oxidation cutting of coal to prepare GQDs. (Reproduced with permission from ref. 27, copyright 2019 American Chemical Society.) (f) Large-scale synthesis of GQDs by ultrasonic-assisted liquid-phase exfoliation. (Reproduced with permission from ref. 29, copyright 2016 Elsevier B.V.) (g) GQDs synthesized via pulsed laser ablation. (Reproduced with permission from ref. 33, copyright 2016 the Royal Society of Chemistry.) | ||
Recently, various synthesis methods of GQDs have been reported. However, several limitations such as complicated synthesis procedures, low production yields, expensive equipment, extreme conditions and high cost have hampered their industrial application progress. The Pei group developed a method of ultrasonic-assisted exfoliation of raw graphitic carbon materials in the liquid-phase for the synthesis of GQDs (Fig. 1f),29 which enables rapid processing, is environmentally friendly, is low-cost, and can be effectively used for mass production of GQDs in industry. Moreover, the adjustable size and density of GQD defects provide a new strategy for large-scale synthesis of controllable GQDs. Pulsed laser ablation (PLA) is a top-down method with one-step reaction, a short synthesis time, good reproducibility, and a minimal experimental set-up, which has attracted great attention from researchers.30–32 Thus, the understanding of the photoluminescence (PL) mechanism for GQDs with different wavelengths is expected to be essential for their possible applications in biomedicine, photocatalysis, and optical devices. The Shen group synthesized a series of GQDs with tunable PL by controlling the PLA time.33 The GQDs implemented using PLA with carboxyl-functionalized MWCNTs exhibit emission colour change from green to blue (Fig. 1g).
2.2 Bottom-up methods
The bottom-up synthesis method refers to constructing from the bottom, that is, the gradual accumulation of atoms, molecules or clusters. The methods for synthesizing GQDs from bottom to up mainly include microwave and ultrasonic methods, solvent chemistry methods, and controlled thermal decomposition of polycyclic aromatic hydrocarbons. Kim et al. obtained nitrogen–sulfur double-doped GQDs by dissolving norepinephrine in DMSO and dialysing the solution after microwave irradiation, which showed good photocatalytic activity. Also, N-doped GQDs can be prepared in the same manner in an aqueous phase system (Fig. 2a).34 Yang et al. used salicylic acid to prepare GQDs by a free-radical polymerization process. These methods are also suitable for the preparation of heteroatom-doped GQDs, whose by-products are only H2O and CO2 (Fig. 2b).35 Pyrolytic citric acid is also an important monomer for the preparation of GQDs (Fig. 2c).36 Nitrogen–sulfur double-doped or N-doped GQDs can also be prepared by mixing citric acid with thiourea or urea (Fig. 2d).37 Nitrogen–sulfur double-doped GQDs have been synthesized via a one-pot pyrolysis approach using citric acid and L-cysteine as precursors.38 Wang et al. found that sodium-catalyzed reduction of methylbenzene and hexabromobenzene could produce hybrids of GQDs and carbon nanoribbons (Fig. 2e).39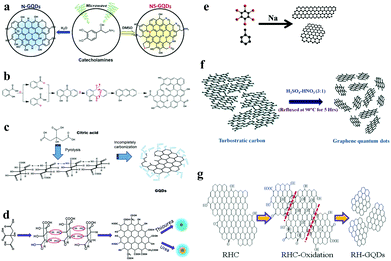 | ||
| Fig. 2 Bottom-up synthesis of GQDs. (a) Preparation of N-doped GQDs and N,S-doped GQDs by a microwave-assisted method. (Reproduced with permission from ref. 34, copyright 2016 Wiley-VCH.) (b) Ultraviolet irradiation induces free-radical polymerization to prepare GQDs. (Reproduced with permission from ref. 35, copyright 2017 American Chemical Society.) (c) Preparation of GQDs with citric acid as a monomer. (Reproduced with permission from ref. 36, copyright 2012 Elsevier B.V.) (d) Preparation of N-doped GQDs and N,S-doped GQDs from graphene and urea or thiourea. (Reproduced with permission from ref. 37, copyright 2013 the Royal Society of Chemistry.) (e) Hybrids of GQDs and carbon nanoribbons were synthesized from toluene and hexabromobenzene. (Reproduced with permission from ref. 39, copyright 2015 American Chemical Society.) (f) GQDs cut from graphitized fine carbon powder obtained by deciduous roasting. (Reproduced with permission from ref. 40, copyright 2014 the Royal Society of Chemistry.) (g) GQDs obtained by ultrasonically assisted cutting of graphitized fine carbon powder. (Reproduced with permission from ref. 41, copyright 2016 American Chemical Society.) | ||
In recent years, synthesis methods combining top-down and bottom-up methods have been studied in depth. For example, a graphitized carbon fine powder is obtained by calcining organic molecules in an inert gas atmosphere, which is a typical bottom-up synthesis concept. The graphitized fine carbon powder is then sheared to form GQDs, which is a typical top-down synthesis concept. After burning neem leaves in an inert gas atmosphere to obtain fine carbon powder, Ogale et al. converted the above carbon powder into GQDs using sulfuric acid and nitric acid. The GQDs prepared in this manner were used as a photoluminescence switch on-off-on probe for Ag+ ions (Fig. 2f).40 GQDs can also be prepared by mixing carbon powder with sulfuric acid in ultrasound and then reacting the mixture with nitric acid. At this time, the fine carbon powder in the synthesis method is obtained by baking rice husks (Fig. 2g).41
In summary, top-down and bottom-up methods are typical common methods of fabricating GQDs. Consequently, we briefly compared these two methods in terms of the differences in fabrication methods, sizes, quantum yields and applications in recent years. As can be seen in Table 1, the main advantages of the top-down method are the availability of raw materials, low price, and simple technological processes, with a high quantum yield, which can be used for large-scale production. The commonly used raw materials are mainly large molecular substances such as graphite. However, it is noticed that the size of GQDs is not adjustable based on top-down methods. Among the several top-down methods, the hydrothermal and solvothermal synthesis methods are relatively widely used, while the sizes of GQDs prepared by the molecular fusion method and the acid vapor cutting strategy are relatively small.
| Product type | Raw material(s) | Subclassification method | Lateral size of GQDs (nm) | Quantum yield (%) | Application(s) |
|---|---|---|---|---|---|
| N-GQDs42 | As-synthesized graphene oxide (GO) sheets | Hydrothermal cutting | 5–10 | — | Catalytic activity |
| Amino-N-GQDs43 | GO sheets | Ultrasonic shearing | 7.31 ± 0.50 | 33 | — |
| GQDs44 | XC-72 carbon black | Chemical oxidation | 4.1 ± 0.8 | — | Electrochemilu-minescence biosensors |
| GQDs45 | Graphite rods | Electrochemical method | 20 | 18.95 | Ion sensors and bioimaging |
| NP-GQDs46 | Citric acid and titanium butoxide | Solvothermal treatment | 3.03 ± 1.01 | 8.45 | Photocatalytic degradation of dyes |
| GQDs47 | Graphite | Acid vapor cutting | 3–5 | — | Electron transport layers for PSEs |
| N-O-GQDs48 | Pyrene | Molecular fusion | 2.4–4.8 | — | Micro-supercapacitors |
| N-GQDs49 | Glucosamine | Microwave-assisted hydrothermal method | 6 | 32 | Solar cells |
The bottom-up method is the second largest technique for preparing GQDs. In Table 2, it is worth noting that this method requires that appropriate carbon-containing precursors be selected, such as aromatic structural molecules, and the operation process is complex. Moreover, some carbon precursors are also difficult to obtain, which presents some obstacles to the preparation of GQDs. Furthermore, the quantum yield of GQDs is relatively low in most bottom-up synthesis methods. Since each coin has two sides, the advantage of the bottom-up method is that the precision of the product can be accurately controlled.
| Product type | Raw material(s) | Subclassification method | Lateral size of GQDs (nm) | Quantum yield (%) | Application |
|---|---|---|---|---|---|
| NS-GQDs34 | Norepinephrine | Microwave assisted solvothermal treatment | 4.1 | 17 | Photocatalysts |
| N-GQDs35 | Norepinephrine | Microwave assisted solvothermal treatment | 3.4 | 7 | Photocatalysts |
| GQDs36 | Salicylic acid | Stepwise organic synthesis | 1–5 | 81 | Fluorescence bioimaging |
| GQDs37 | Citric acid | Pyrolysis | 15 | 9.0 | Photoluminescence/photocatalytic |
| NS-GQDs39 | Citric acid and L-cysteine | Intermolecular condensation | 2.56–3.64 | 71 | Photocatalysts |
| GQDs40 | Hexabromobenzene | Stepwise organic synthesis | 5 | — | Oxygen reduction reaction |
| GQDs41 | Dead neem leaves | Carbonization of organic precursors | 5–6 | 1% taking quinine sulphate as a reference | Photoluminescence switch on-off-on probe for Ag+ ions |
3 Modification of GQDs
3.1 Heteroatom doping
Heteroatom doping has become an effective method for modulating the properties of nanomaterials. N-type or P-type doping can effectively change the electronic structure in a semiconductor material. For example, the electron density of graphene layers can be altered by doping the electron donor and acceptor structures. Doped GQDs are an example of doped semiconductor materials, in which the main element C in GQDs is partially replaced with some miscellaneous elements such as N, S, Se, B, P, I and Si.24,50,51 This substitution will change the properties of GQDs; for example, the doping with S and Se will increase the visible absorption of GQDs.52 The introduction of heteroatoms, a common modification method, has been shown to greatly improve even some of the physical and chemical properties of innovative GQDs. Most non-metallic heteroatoms including N, B and other elements in the structural spatial grid of graphene effectively replace carbon atoms and form electron cloud migration to generate electron holes, which will help doped GQDs play a great role in fuel cells, catalysts and electrochemical capacitors. For the chemical doping with heteroatoms, N and C atoms have similar radii and compact electronegativity. The N donor atom plays a crucial role in the electronic modification of graphene, including improving the conductivity, catalysis and fluorescence of graphene. At present, the N doping methods of GQDs mainly include chemical vapor deposition (CVD),53,54 the thermolysis method55 and plasma treatment.56In 2012, the Qu group successfully integrated N-doping and quantum effects, and obtained N-doped GQDs (N-GQDs) for the first time.57 N-GQDs with excellent luminescent properties and high efficiency oxygen reduction catalysis were prepared by introducing the N atom into the central position of acetone triol sing tetrabutylammonium perchlorate as electrolyte. Currently, functional materials are of important scientific and technological significance due to their valuable applications in photodetectors, solar cells, and bioimaging. However, GQDs with NIR emission have not been reported. In 2014, Lau reported a facile one-step synthesis of polyacrylonitrile (PAN)-based CFs to obtain N-doped GQDs.58 Noteworthily, the N/C and O/C atomic ratios and N bond configurations in the prepared GQDs can be easily adjusted by changing the heat treatment temperature of CFs. As shown in Fig. 3a, monodisperse N-GQDs having different particle sizes can be prepared by the microwave-assisted hydrothermal method (MAH) using glucose and ammonia as starting materials. Due to the hierarchical structure of N-GQDs, a wide π-conjugated system is generated, resulting in an optical response range of 365 to 980 nm for N-GQDs and a response rate of 325 V W−1 at 405 nm.
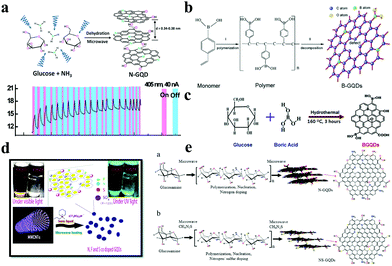 | ||
| Fig. 3 Heteroatom doping of GQDs. (a) N-GQDs synthesized by a microwave-assisted method and their photoresponses (Φ = 4.0 nm) when irradiated with 405 nm. (Reproduced with permission from ref. 58, copyright 2014 American Chemical Society.) (b) One-pot synthesis procedure for GH-BGQDs using glucose. (Reproduced with permission from ref. 60, copyright 2017 Wiley-VCH.) (c) The synthesis procedure for B-GQDs with glucose. (Reproduced with permission from ref. 61, copyright 2017 the Royal Society of Chemistry.) (d) The formation of co-doped GQDs; the inset shows a comparison of the same concentration of co-doped GQDs in DMF under visible light and UV light respectively. (Reproduced with permission from ref. 63, copyright 2018 the Royal Society of Chemistry.) (e) The growth mechanism of N-GQDs and NS-GQDs with microwaves. (Reproduced with permission from ref. 62, copyright 2015 Wiley-VCH.) | ||
In the periodic table, B and C are adjacent to each other and have similar atomic radii. Compared to the C atom, the B atom has one less electron in its outermost shell. People are very interested in exploring the properties of B-doped GQDs (B-GQDs), because if some C atoms in graphene are replaced with B atoms, some electronic defects will appear in the products which exhibit some unique properties.59 Metal-free B-GQDs were prepared by introducing vacancies and elemental boron molecules as substitutional defects. For example, Zhang et al. demonstrated in 2017 that defects of GQDs provide several active sites by decomposition of 4-vinylphenylboronic acid (VPBA) and boric acid, thereby triggering the formation of B-GQDs (Fig. 3b).60 The B-GQDs were synthesized from VPBA using boric acid as a precursor at 200 °C. Additionally, in the presence of boric acid, B-GQDs can be synthesized by a one-step method with simple operation, low temperature and hydrothermal treatment of glucose (Fig. 3c).61 B-GQDs possess relatively uniform nano-size and a high B-doping level, the content of which is about 4.25%. In addition to monoatomic doping, many GQDs are modified by diatomic or triatomic doping. Doping atoms can change the energy band gap and surface properties of GQDs, resulting in enhancement of the photoelectric properties and an increase in the quantum yield of GQDs. Assembled N and S co-doped GQDs (NS-GQDs) obtained by microwave treatment of glucosamine show great potential in organic light-emitting devices as optoelectronic devices.62 In 2015, also by microwave treatment of CNTs in ionic liquids, S, N and F co-doped GQDs (NFS-GQDs) were successfully obtained (Fig. 3d).63 Further, Pillai et al. modified a TiO2 mesoporous network with NSF-GQDs, which improved the light capture efficiency of the product. As the band gap of TiO2 was reduced by N doping, the PCE value reached 11.7%. In addition, S was used to promote the electron transfer process, and F played an effective role in binding to the surface of TiO2.64 N or O doping is beneficial to increase the interface wettability of the carbon electrode to the aqueous electrolyte, thereby enhancing the faradaic reaction dynamics.48 Inspired by this, the Wu group reported the first application of heavily GQDs co-doped with N and O in MSCs, with improved molecular melting methods, where the N and O contents were as high as 17.8 at% and 21.3 at%, respectively (Fig. 3e).62
3.2 Functionalization
The functionalization of GQDs will effectively improve the performance of GQDs, especially their optical properties. The main mechanism is to improve the electronic properties of graphene by adding electron-accepting groups and electron-donating groups. At the same time, the acidic functionalization of GQDs sometimes endows them with catalytic properties, which can be used as acidic solid catalysts for organic reactions. In oxidized GQDs, the type and amount of oxygen-containing functional groups will affect their properties. Qu et al. found that graphene oxide quantum dots containing carbonyl and carboxyl groups can be used as peroxidase mimics (Fig. 4a).65 Mohammad-Rezaei et al. oxidized rGO with nitric acid and sulfuric acid under microwave conditions.66 The carboxyl functionalized GQDs they obtained were used to catalyse the coupling reaction of 2-naphthol with a benzaldehyde derivative (Fig. 4b). Chen et al. obtained sulfonated GQDs by reacting chlorosulfonic acid with GQDs at 70 °C for 24 h under nitrogen protection.67 The sulfonated GQDs show good biomass catalytic capabilities, such as catalyzing the dehydration of fructose to form 5-HMF (Fig. 4c).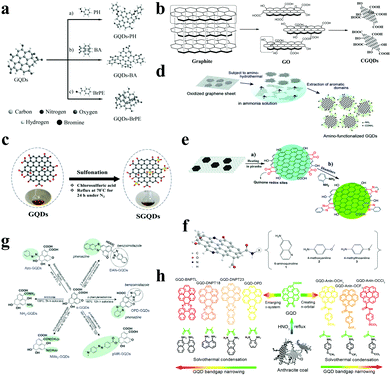 | ||
| Fig. 4 Functionalization of GQDs. (a) Modification of (a) carbonyl, (b) hydroxy and (c) carboxyl groups on oxidized GQDs. (Reproduced with permission from ref. 65, copyright 2015 Wiley-VCH.) (b) Preparation of carboxylic acid functionalized GQDs. (Reproduced with permission from ref. 66, copyright 2015 the Royal Society of Chemistry.) (c) Preparation of sulfonated GQDs. (Reproduced with permission from ref. 67, copyright 2018 Elsevier B.V.) (d) Preparation of amino-functionalized GQDs. (Reproduced with permission from ref. 68, copyright 2012 Wiley-VCH.) (e) Preparation of amino-functionalized GQDs from graphene oxide sheets. (Reproduced with permission from ref. 69, copyright 2015 the Royal Society of Chemistry.) (f) Synthesis of chemically functionalized GQDs using aniline derivatives and GQDs. (Reproduced with permission from ref. 70, copyright 2016 Nature.) (g) Synthesis of N-functionalized GQDs. (Reproduced with permission from ref. 71, copyright 2016 Wiley-VCH.) (h) Adding a large conjugated functional group to expand the conjugated structure of GQDs. (Reproduced with permission from ref. 72, copyright 2018 American Chemical Society.) | ||
Okamoto et al. synthesized amino-functionalized GQDs by hydrothermally mixing ammonia solution and graphene oxide sheets at 70–150 °C for 5 h,68 the amino groups of which are mainly located at the edge of the sheet, which facilitates precise control of the optical properties of GQDs (Fig. 4d). Pang et al. produced amino-functionalized GQDs by acid-catalyzed dehydration of 1,2-diamine and graphene oxide sheets (Fig. 4e).69 Rhee et al. also chemically functionalized GQDs using a variety of aniline derivatives that can be used as light sources to produce high purity red-green and orange LEDs (Fig. 4f).70 A study by Matsui et al. showed that the N-containing groups in N-functionalized GQDs can systematically modify the electronic structure to cause effective orbital resonance of the HOMO and LUMO levels of the GQDs.71 Further, the prepared N-functionalized GQDs exerted an excellent effect in the field of perovskite solar cells and phototransistors (Fig. 4g). Chen et al. found that the band gap of GQDs can be narrowed by extending the π-conjugated system or functionalizing the electron-donating groups of GQDs,72 so that the narrow band gap GQDs can be applied to photocatalytic water splitting and high performance CO2 reduction (Fig. 4h).
4 Applications of GQDs in energy conversion and storage devices
4.1 Supercapacitors/micro-supercapacitors
As energy storage devices, supercapacitors/micro-supercapacitors (SCs/MSCs) are receiving more attention due to their high power density, small size, short charging and discharging processes, and long lifespans. Basically, SCs/MSCs are divided into electrochemical double layer capacitors and pseudocapacitors because of their different charge storage mechanisms. Generally, SCs/MSCs having GQDs as electrode materials belong to an electrochemical double layer type, and its mechanism is charge adsorption on the surface of the electrode. Xue et al. electrodeposited GQDs on a gold finger electrode; the obtained MSC has a specific capacitance of 534.7 μF cm−2, a rate performance of up to 1000 V s−1, and a specific capacitance of 97.8% after 5000 cycles relative to its initial specific capacitance.73 At the same time, the MSC also has a short relaxation time constant as shown in Fig. 5a (τ0 = 103.6 μs in aqueous electrolyte and τ0 = 53.8 μs in ionic liquid electrolyte). Lee et al. developed a flexible transparent MSC using chelated graphene and GQDs with a specific capacity of 9.09 μF cm−2, which has a high transmittance of 92.97% at a wavelength of 550 nm and highly bendable characteristics.74 In the case of the bend, it is still possible to maintain long cycle stability (approximately 100% retention of its initial specific capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 continuous cycles) (Fig. 5b).
000 continuous cycles) (Fig. 5b).
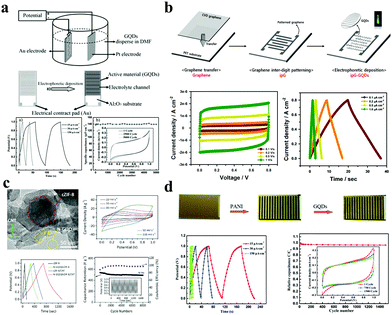 | ||
| Fig. 5 GQDs as SC/MSC electrodes. (a) GQD interdigitated MSC. (Reproduced with permission from ref. 73, copyright 2013 Wiley-VCH.) (b) Flexible transparent MSC using chelated graphene and GQDs. (Reproduced with permission from ref. 74, copyright 2016 Elsevier B.V.) (c) SC developed with N-GQD@cZIF-8/CNT electrodes. (Reproduced with permission from ref. 75, copyright 2018 Wiley-VCH.) (d) GQD/PANI asymmetric MSC. (Reproduced with permission from ref. 76, copyright 2013 the Royal Society of Chemistry.) | ||
The capacitive mechanism of pseudocapacitors is the reversible faradaic reactions occurring on the electrodes. SCs/MSCs made of carbon quantum dots doped with elements such as N and S are typical pseudocapacitors. Wu et al. used carbonized ZIF-8 as a self-sacrificing template, CNTs as a conductive network, and N-doped GQDs as a highly pseudocapacitive material to fabricate SCs. Their SCs have a high specific capacitance of 540 F g−1 at 0.5 A g−1 in 1 M H2SO4 electrolyte and have excellent cycle stability (approximately 90.9% retention of its initial specific capacitance after 8000 continuous cycles). The assembled SC has an energy density of 18.75 W h kg−1 and a power density of 108.7 W kg−1 (Fig. 5c).75 Asymmetric SCs/MSCs are also a large class of pseudocapacitors. Among them, GQD/polymer material asymmetric SCs are extremely representative. For example, Xue et al. developed a GQD/PANI asymmetric MSC, which has a specific capacity of 667.5 μF cm−2, a rate capability of up to 1000 V s−1, a very short relaxation time (τ0 = 115.9 μs in aqueous electrolyte), and great cycle stability (67.8% of its initial value after 1500 cycles).76 Moreover, the energy density of the SC was 0.093 μW h cm−2 and the power density was as high as 7.52 μW cm−2 (Fig. 5d). In addition, due to their excellent conductivity, GQDs can also be used as an auxiliary in SCs to enhance the properties of the electrode. Fan et al. embedded GQDs into an electrode made of high specific surface area carbon material to improve the conductivity of the electrode.77 The electrical double layer SC has a specific capacitance of 388 F g−1 at 1 A g−1 with excellent rate performance (τ0 = 0.68 s) and cycle stability in a two-electrode system (100% of its initial value after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles). At a high current density of 100 A g−1, the SC has a capacitance retention of 60% (Fig. 6a). Transition metal compounds are a class of materials with high pseudocapacitance, but it is difficult to use them alone as electrodes because of their poor electrical conductivity, structural stability, rate performance, and cycle stability. However, the addition of GQDs will effectively enhance the above performance. Wang et al. synthesized a multi-layer NiO@Co3O4 hollow sphere decorated with GQDs as the cathode of SCs, and activated carbon as the anode to form asymmetric SCs.78 The asymmetric SCs have a specific capacitance of 1361 F g−1 and an energy density of 38.44 W h kg−1 and high cycle stability (76.4% retention after 3000 cycles) (Fig. 6b). In conclusion, Tables 3 and 4 display different properties and performances of GQDs in MSCs (Table 3) and SCs (Table 4).
000 cycles). At a high current density of 100 A g−1, the SC has a capacitance retention of 60% (Fig. 6a). Transition metal compounds are a class of materials with high pseudocapacitance, but it is difficult to use them alone as electrodes because of their poor electrical conductivity, structural stability, rate performance, and cycle stability. However, the addition of GQDs will effectively enhance the above performance. Wang et al. synthesized a multi-layer NiO@Co3O4 hollow sphere decorated with GQDs as the cathode of SCs, and activated carbon as the anode to form asymmetric SCs.78 The asymmetric SCs have a specific capacitance of 1361 F g−1 and an energy density of 38.44 W h kg−1 and high cycle stability (76.4% retention after 3000 cycles) (Fig. 6b). In conclusion, Tables 3 and 4 display different properties and performances of GQDs in MSCs (Table 3) and SCs (Table 4).
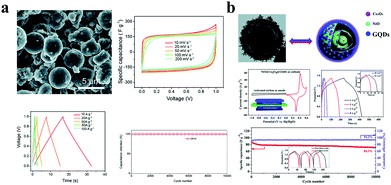 | ||
| Fig. 6 GQDs as supercapacitor additives. (a) GQDs as a conductive agent added to ultra-high specific surface area activated carbon SCs. (Reproduced with permission from ref. 77, copyright 2019 the Royal Society of Chemistry.) (b) GQDs as a conductive agent added to transition metal oxide pseudocapacitive SCs. (Reproduced with permission from ref. 78, copyright 2019 the Royal Society of Chemistry.) | ||
| Electrode material | Electrolyte | Specific capacity (μF cm−2) | Rate performance (V s−1) | Energy density (μW h cm−2) | Power density (μW cm−2) | Operating voltage (V) | Cycle stability |
|---|---|---|---|---|---|---|---|
| GQD//GQD73 | 0.5 M Na2SO4 | 534.7 | 1000 | 0.074 | 7.5 | 1 | 97.8%, 5000 cycles |
| GQD//GQD73 | 2 M EMIMBF4/AN | 468.1 | — | 0.474 | 56.7 | 2.7 | 94%, 5000 cycles |
| GQD//MnO273 | 0.5 M Na2SO4 | 1107.4 | — | 0.154 | 7.51 | 1 | 93.3%, 5000 cycles |
| N-O-GQD//N-O-GQD48 | 1 M H2SO4 | 5790 | — | 8.0 | 75 | 1 | 82.6%, 5000 cycles |
| GQD//MnO279 | 0.5 M Na2SO4 | 2980 | — | 0.414 | 15.01 | 1 | — |
| GQD//PANI76 | 0.5 M Na2SO4 | 667.5 | 1000 | 0.093 | 7.52 | 0.9 | 97.3%, 1500 cycles |
| Electrode material | Electrolyte | Specific capacity (F g−1) | Rate performance (V s−1) | Energy density (W h kg−2) | Power density (W kg−2) | Operating voltage (V) | Cycle stability |
|---|---|---|---|---|---|---|---|
| N-GQD@cZIF-8/CNT//N-GQD@cZIF-8/CNT75 | H2SO4/PVA | 400 | 1000 | 14 | 89.5 | 1 | 82%, 5000 cycles |
| GEAC//GEAC77 | Alkaline electrolyte | 388 | — | 13.47 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1 | 100%, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
| NiO@Co3O4@GQD//AC78 | KOH/PVA | 123 | — | 38.44 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 251 251 |
1.6 | 84.3%, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
| CoDC-0.5//CoDC-0.580 | 6 M KOH | 240 | — | 9.38 | 1250 | 1 | 90%, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
| Amine-GQD-TNA//amine-GQD-TNA81 | 1 M H2SO4 | 595 | — | 21.8 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1 | 90%, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
| N-GQD@Fe3O4-HNT electrode (three-electrode test system)82 | 1 M Na2SO4 | 418 | 1000 | 29 | 5200 | 1 | 82%, 3000 cycles |
| GQD/NiCo2S4 electrode (three-electrode test system)83 | 3 M KOH | 678.22 | — | — | — | 0.46 | 92%, 5000 cycles |
| GQD-3DG//GQD-3DG84 | 1 M H2SO4 | 268 | — | — | — | 0.8 | 90%, 5000 cycles |
4.2 Solar cells
Incorporation of GQDs into nanomaterials offered the opportunity for the picturesque charge carrier extraction capability for the enhancement of solar cell efficiency, owing to their unique optoelectronic characteristics, up-conversion field luminescence characteristics and band gap tunability and low application cost. GQDs have broad application prospects in high-performance photovoltaic devices, such as perovskite solar cells,85,86 organic and inorganic hybrid solar cells,87 and dye-sensitized solar cells.88,89 GQDs have a large specific surface area, high electron mobility and an excellent ability to absorb ultraviolet light and convert it into visible light, and are widely used as a sensitizer, an electron/hole transport layer, or an active layer additive for photovoltaic devices.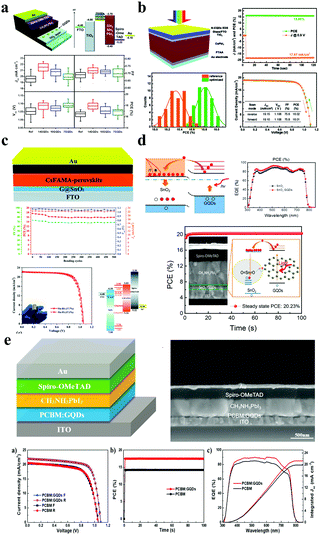 | ||
| Fig. 7 GQDs for perovskite solar cell devices. (a) GQD modified TiO2 layer for efficient planar perovskite solar cells. (Reproduced with permission from ref. 94, copyright 2013 the Royal Society of Chemistry.) (b) NN-GQDs for inorganic γ-CsPbI3 perovskite solar cells. (Reproduced with permission from ref. 97, copyright 2019 the Royal Society of Chemistry.) (c) GQD/SnO2 composites for flexible perovskite photovoltaics. (Reproduced with permission from ref. 102, copyright 2019 the Royal Society of Chemistry.) (d) The perovskite solar cells with the structure of SnO2:GQD PSCs. (Reprinted with permission from ref. 108, copyright 2017 American Chemical Society.) (e) Perovskite solar cells with a GQD doped PCBM electron transport layer. (Reproduced with permission from ref. 112, copyright 2017 Elsevier B.V.) | ||
It has been reported that significant electron accumulation caused by the charge barrier at the TiO2/perovskite interface always exists, resulting in increased hysteresis and reduced efficiency.98,99 Moreover, TiO2 suffers from low electron mobility (ca. 10−4 cm2 V−1 s−1), and requires sintering at a high temperature (ca. 400 °C), which limits the establishment of flexible PSCs.100,101 Inspired by this, in 2018, the Lin group designed a facile way to prepare GQD and SnO2 nanoparticle composites as electron transport layers (ETLs) for highly efficient rigid and flexible PSCs (Fig. 7c).102 As is known to all, low temperature SnO2 films can be processed via various methods such as spin-coating solution,103–105 chemical-bath deposition,106 and atomic layer deposition (ALD).107 However, several devices based on low-temperature solution suffer from serious hysteresis, making it difficult to determine their real PCEs.103–105 Consequently, the Yang group exploited high performance and very little hysteresis N–I–P planar PSCs with SnO2:GQDs as ETLs.108 In Fig. 7d, the Fermi level (EF) of the SnO2:GQDs decreases from 4.35 to 4.01 eV because the electron concentration increased through illumination. Moreover, the electron mobility of the SnO2:GQDs has improved from 6.72 × 10−4 to 1.01 × 10−3 cm2 V−1 s after illumination. Conclusively, the planar PSCs based on SnO2:GQDs ETLs achieve a maximum steady-state efficiency of 20.23% with very little hysteresis. This research provides a simple and valid method to increase device efficiency by enhancing the electronic properties of the ETLs. Similarly, organic transport layers such as [6,6]-phenyl C61 butyric acid methyl ester PCBM109 and PCBDAN110 have attracted considerable attention due to their simple and low-temperature production process. Unfortunately, the intrinsic low electrical conductivity and electron mobility of PCBM still hinder the promotion of PSCs.111 In 2017, the Yang group reported a powerful method to enhance planar PSCs’ performance by using GQDs as a planar PCBM ETL with additional forward PSCs not only to dramatically increase the PCE of PSCs, but also to increase the light stability of the devices.112 Their PCE increased from 14.68 to 17.56, while the PCBM:GQD device maintained an original maximum of approximately 80% over a continuous full-spectrum sunshine time of more than 300 h (Fig. 7e). Then, in order to better verify the impact of GQD doping on PCBM performance, the authors used PCBM and PCBM:GQDs as ETLs to obtain sandwich structure devices. The results show that the conductivity is one order of magnitude higher than that of pure PCBM under GQD doping conditions. In summary, the introduction of GQDs into the perovskite thin film not only effectively passivates large chunks of grain boundaries and eliminates electron traps, thereby reducing charge recombination, but also promotes electron transfer in the perovskite layer.
4.3 Li+/Na+ batteries
In Li+ or Na+ batteries, GQDs can effectively alleviate volume expansion,116 increase the diffusion speeding of Li+/Na+,117,118 increase electron transfer,119 and improve electrochemical performance.120,121 Therefore, when GQDs are used as an anode material or mixed with an active material as an auxiliary agent, the electrochemical performance will be improved.Due to its advantages of large capacity, low cost, and abundant sources, VOx is considered to be a promising lithium ion battery electrode material.122,123 So far, various forms of VO2 nanostructures have been prepared for use as lithium-ion battery (LIB) cathode materials. However, most of these materials show rapid capacity decay and poor high-speed performance, and the resistance tends to increase rapidly during cycling. In 2015, the Fan group first reported the application of GQDs in batteries (VO2@GQD) as an efficient surface “sensitizer” and “stabilizer” to coat the VO2 surface by electrophoresis.124 It is well known that GQDs can improve electrochemical performance and separate VO2 nanomaterials from each other, thereby avoiding agglomeration and minimizing the dissolution of active substances.122 As shown in Fig. 8a, after 1500 cycles at 18 A g−1, the GVG electrode has a capacity exceeding 420 mA h g−1 and a capacity retention rate of 94%. Moreover, VO2@GQD exhibits a high capacity of 306 mA h g−1 and superior rate tolerance and a lower capacity decay (12% after 1500 cycles at 18 A g−1) with a power density of 42 kW kg−1 at an energy density of more than 100 W h kg−1. Recently, great efforts have been made towards exploring new applications for high-performance LIBs.125–127 The existing LIBs have short cycle lives and low power density, which cannot meet the efficient storage requirements of renewable energy and/or electric vehicles or HEVs.128 Therefore, the current work is to improve the energy density, power density and cycle life of electrode materials. In 2019, the Kim group reported a fresh LTO/N-GQD/super-hierarchical anode material for LIBs.129 Compared to pure LTO (Li4Ti5O12), the LTO with N-GQDs increases the specific capacity by 23% and exhibits better electrical properties, such as discharge capacity maintained at about 170 mA h g−1 at 20 °C for over 200 cycles as shown in Fig. 8b. The development and utilization of porous materials have received extensive attention in energy storage and conversion.130,131 Among them, metal organic frameworks (MOFs) have the advantages of colossal surface area, anisotropic structure, multifunctional pores and high carbon content of organic ligands, which are beneficial for preparing porous materials.132,133 However, the poor conductivity and instability of porous materials still limit their widespread use in batteries. In order to solve these shortcomings, the Yuan group synthesized porous carbon derived from ZIF-8@GQDs and the electrode material showed excellent electrochemical performance.134 The electrode material of lithium battery has a long cycle stability on account of that GQDs were loaded onto the surface of the MOF derivative ZIF-8 to form a cathode material of LIBs.
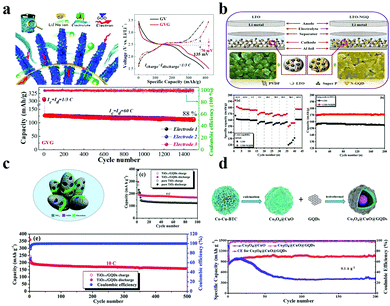 | ||
| Fig. 8 Electrochemical characterization for batteries with GQDs. (a) The GVG electrode with GQDs in Li+ and Na+ batteries and the electrochemical performance. (Reproduced with permission from ref. 124, copyright 2015 American Chemical Society.) (b) The electrochemical performance of LTO-NGQ20 with rate capability from 0.2C to 50C and long cycling performance electrodes. (Reproduced with permission from ref. 129, copyright 2019 Elsevier B.V.) (c) The electrochemical reaction process of TiO2−x/GQDs. (Reproduced with permission from ref. 137, copyright 2018 the Royal Society of Chemistry.) (d) The preparation of Co3O4@CuO@GQDs and the long-term cycling performance. (Reproduced with permission from ref. 141, copyright 2019 Elsevier B.V.) | ||
Among the various anode candidates, TiO2 is widely used because of its environmental friendliness, safety, low cost, and good chemical stability.135,136 It is regrettable that the poor capacity and low rate performance of a TiO2 anode limit its practical application. Thus, increasing the electrochemical performance of the TiO2 anode is the key problem. In 2018, the Wei group successfully designed and fabricated a TiO2−x/GQD hybrid.137 TiO2 embedded with GQDs has higher electronic conductivity and higher specific surface area. Moreover, as displayed in Fig. 8c, the TiO2−x/GQD anode exhibited an excellent Li-storage capacity of 168.5 mA h g−1 at 5 °C and a long-term cycling performance of 160.1 mA h g−1 over 500 cycles at 10 °C.
Recently, transition metal oxides (TMO) or sulfides (TMS) appeared as potential competitors for the next generation of electrode materials in LIBs due to their large theoretical capacities (500–1000 mA h g−1) and abundant reserves and low cost.138–140 Taking this into account, the Wang group prepared yolk–shell Co3O4@CuO microspheres, which was followed by surface modification of carboxyl functionalized GQDs.141 The results are shown in Fig. 8d; as an anode material in LIBs, NiO@Co3O4@GQDs provide a relatively large reversible capacity of about 1158 mA h g−1 after 250 cycles and a current density of 0.1 A g−1, while the reversible capacity of NiO@Co3O4 is only about 1327 mA h g−1. In addition, the GQDs wrapped on the outer surfaces of the Co3O4@CuO microspheres effectively maintain the durability of anodes, making the anode material GQDs@Co3O4@CuO have better cycle capacity and exhibit superior lithium storage performance, yielding a high initial specific capacity (816 mA h g−1) and a high reversible charging capacity of 1054 mA g−1 after 200 cycles and 0.1 mA g−1 (Fig. 8d). On the other hand, transition metal disulfides have become the focus of research and application owing to their specific 2D layered feature, electronic structure, and unique physical and chemical properties.142,143 Among them, molybdenum disulfide (MoS2) exhibits excellent performance in energy storage and conversion. It is a pity that the practical application of a MoS2 anode is hindered by its intrinsic pulverization, which in turn induces rapid capacity degradation and poor cycling performance. In order to overcome this obstacle, the Zhang group first reported a facile synthesis of GQD doped MoS2 nanosheets via a solvothermal process.116 The obtained GQD/MoS2 material manifests remarkably improved electrochemical lithium storage performance compared to the original MoS2, such as high reversible capacity (1099 mA h g−1 at 100 mA g−1), good cycle stability, and excellent rate performance (660 mA h g−1 at 5000 mA g−1). Silicon is well known as the most promising anode material for high-capacity lithium ion batteries.144 To date, several methods have been successfully used for improving the electrochemical properties of silicon anodes.145,146 However, the development of silicon particles is limited by their size and electrical conductivity. With this in mind, the Li group reported the use of GQDs in a silicon anode.119 The effect of the coating layer on the diffusion of lithium ions between the silicon surface and the electrolyte will be reduced to a very low level. This work provides an easy method to design and prepare silicon-based anode materials for next-generation high performance lithium ion batteries.
4.4 Light-emitting diodes
Since graphene was discovered by Geim and Novoselov,147 its high carrier mobility and linear dispersion band structure make it a promising candidate for optoelectrical applications. However, as bulk graphene does not have a band gap, it has limitations in further applications. Compared to bulk graphene, GQDs have quantum confinement and edge effects, so the band gap that changes carrier behaviour exists in GQDs and makes their applications in photodetection,58,148 photovoltaics,149,150 light-emitting diodes (LEDs)151,152 and plasmonics153,154 more versatile. Among these applications, LEDs have a bright future due to their energy saving, environmental protection and long cycling lives. The basic principle of GQD-based LED illumination is as follows: an external electric field excites carriers and then emits light after immediate recombination. In LEDs, GQDs can act as the electroluminescent phosphor,155–157 dopant,151 gap tuner70 or color converter.158 In the area of electroluminescent phosphors, Kim et al. fabricated highly-efficient LEDs based on octadecylamine (ODA) GQDs.156 The amount of oxygen components in the ODA-GQDs was lower than that in pure GQDs due to nucleophilic substitution between the amine and the epoxy functional groups. The current efficiency of the ODA-GQD-based LEDs is 6.51 cd A−1 (Fig. 9a) and is the highest among the efficiencies reported for GQD-based LEDs.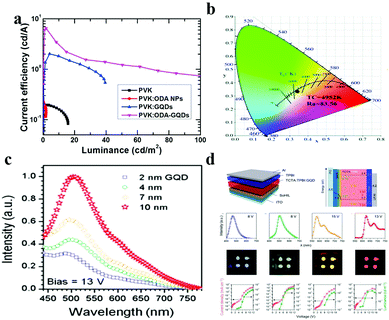 | ||
| Fig. 9 GQDs for light-emitting diode applications. (a) Current efficiency–luminance for LEDs with PVK, PVK:ODA NPs, PVK:GQDs and PVK:ODA-GQDs. (Reproduced with permission from ref. 156, copyright 2017 Elsevier B.V.) (b) The CIE color coordinates, CRI values, and CCT values for white LED lamps fabricated by integrating a 450 nm blue chip with GQD@ZIF-8. (Reproduced with permission from ref. 157, copyright 2018 the Royal Society of Chemistry.) (c) Electroluminescence spectra of an OLED using GQDs as a light emitter at a bias of 13 V. (Reproduced with permission from ref. 151, copyright 2014 American Chemical Society.) (d) LED structure, energy levels, electroluminescence spectra, photographs of blue, green, orange and red LEDs using aniline derivative functionalized GQDs as band gap tuners. (Reproduced with permission from ref. 148, copyright 2014 Nature.) | ||
In addition, a new luminescent material (GQD@ZIF-8) was synthesized by incorporating edge-sulfonated GQDs as active fluorescent species into a zeolitic imidazolate framework (ZIF-8) as a stabilizer and carrier.157 In white LEDs, GQD@ZIF-8 was used as a novel yellow phosphor based on a blue-light LED chip with proximate CIE coordinates of (0.33, 0.33) of highly pure white light and a larger color-rendering index (Fig. 9b). In the field of dopants, Rhee et al. first reported the electroluminescence of GQDs and organic LEDs with GQDs, which exhibited white light emission with an external quantum efficiency of ∼0.1%.151 They demonstrated OLEDs employing 4,4′-bis(carbazol-9-yl)biphenyl (CBP) as a host and a series of GQDs which have advantages such as appropriate energy-band structures and excellent organic solubility as dopants. As shown in Fig. 9c, with the increasing size of the GQDs, the electroluminescence (EL) intensity is also increased at a fixed bias. This is because the lower band-gaps of large size GQDs facilitate energy transfer from the host to the GQDs, which is the most important process for achieving bright electroluminescence. In addition, as the size of the GQDs increases from 2 nm to 10 nm, the Commission Internationale de l’Éclairage chromaticity diagram indicates that the EL color shifts from red to blue. When GQDs are used as a band gap tuner in the active layer, the problems of poor color purity and color tunability of the LEDs are perfectly solved. An aniline derivative is used by Rhee et al. to chemically functionalize GQDs, which causes GQDs to generate new extrinsic energy levels and very narrow linewidths of light, resulting in a red-shifted electroluminescence effect and improved color purity.70 The maximum current efficiency of the LED is 3.47 cd A−1 and the external quantum efficiency is 1.28%, which is the highest value for carbon nanoparticle phosphor-based LEDs. Lau and his co-workers synthesized a GQD–agar composite, as a color converter, which works in color conversion materials in blue LEDs to achieve white light emission (Fig. 9d). It also exhibits excellent optical stability and no observable luminescence quenching. The white LED has a luminous efficiency of 42.2 lm W−1, and its light conversion efficiency (61.1%) for continuous operation over 100 h is very stable.
4.5 Other applications
GQDs, an advanced multifunctional material, have been introduced into the development of energy conversion devices due to their unique optical and photoelectric properties. GQD-based energy converters have the ability to convert heat, light, moisture, pressure and other forms of energy into electrical energy.Taking advantage of the fact that GQD fillers can improve the charge capture effect at the material phase interface, the Bakar group established a nanogenerator that achieves a higher output voltage and produces a positive polarity output signal of 4.6 V wide and 48 ms wide.159 The results show that the nanogenerator could successfully light up an LED when connected to an external circuit, and work steadily for up to 60 h (Fig. 10a). These superior properties are primarily dependent on the ability of GQDs to increase sensitivity to mechanical stimulations. In the Kim group, researchers applied GQDs on Ag nanowires to increase the sensitivity of external mechanical stimulation of the material.160 As shown in Fig. 10b, GQDs significantly increase the output current of the as-prepared product. Normally, when the electronic skin is under a pressure of 10 N, the GQDs (G-Ag-NWs) can output a short-circuit current density of ∼10 mA cm−2, which is 20 times that without GQDs. On the other hand, GQDs as an active material can produce a high-performance moisture-triggered nanogenerator. Ion concentration gradient induced electricity is a new form of energy conversion.161,162 Here, GQDs act as an effective additive to improve the performance of moisture production and power generation. The Berry group established the first GQD-based electronic humidity sensor that produces approximately 60 nA at 0.4 V when the atmosphere changes from humid air to helium.163 The results showed that due to the GQD additive, the tunnel barrier width can be reduced by 0.36 nm and the conductivity of the device can be increased by 43 times (Fig. 10c). The Qu group demonstrated a GQD-based electricity nanogenerator, in which electrochemical polarization treatment could generate a high voltage of up to 0.27 V under 70% change in relative humidity.164 After optimizing the load resistance, the power density is 1.86 mW cm−2 (Fig. 10d). Therefore, by self-assembling GQDs onto polymer microfibers to form a permeable network, it is possible to construct a humidity and pressure sensor that operates by electronic tunnel modulation using the hygroscopic nature of the polymer. In 2017, the Pan group proposed a novel recipe to fabricate Bi2Te3/GQD hybrid nanosheets, in which GQDs can be uniformly embedded in a Bi2Te3 nanosheet matrix, revealing that GQDs may affect carrier transport behavior. Importantly, the thermoelectric performance of Bi2Te3/GQD hybrid nanostructures could be further enhanced by the optimization of the density and dispersion manner of GQDs in the Bi2Te3 matrix.165 Recently, the entry of GQDs in the field of photocatalysis for solar energy harvesting and conversion attracted increasing attention. In 2017, highly-ordered metal/GQD nanocomposites with fine structural control were fabricated by the Xiao group.166 These (M/GQDs)n (M = Au, Ag, Pt) multilayer thin films exhibited highly-efficient and versatile catalytic performance under ambient conditions. Most importantly, the authors found that the catalytic performances of the (M/GQDs)n multilayer thin films can be optimized by tuning the assembly cycle and sequence, as well as by selecting different types of metal NPs. After that, some prominent photoelectrochemical works based on GQDs and nanoclusters have been reported.167,168 These studies open new technical methods for high-efficiency solar energy harvesting and conversion.
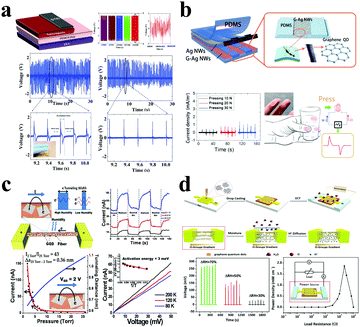 | ||
| Fig. 10 GQDs for other energy conversion applications. (a) GQDs mixed with barium titanate for a nanogenerator device. (Reproduced with permission from ref. 159, copyright 2018 Elsevier B.V.) (b) Triboelectric electronic skin based on GQDs. (Reproduced with permission from ref. 160, copyright 2018 Elsevier B.V.) (c) The device with a GQD network and humidity/pressure sensors. (Reproduced with permission from ref. 163, copyright 2013 American Chemical Society.) (d) Moisture-triggered nanogenerator based on GQDs. (Reproduced with permission from ref. 164, copyright 2017 American Chemical Society.) | ||
5 Summary and prospects
GQDs have important applications in the field of energy storage and conversion due to their special structure and intrinsic properties. In recent years, the research on SCs/MSCs using simple GQDs as electrodes has become less and less. Instead, studies of pseudocapacitive SCs with GQDs as conductive agents have increased vigorously. This trend is in line with the pursuit of greater capacity. However, the doping and modification of GQDs results in a decrease in their conductivity. Doping some heteroatoms can make GQDs possess pseudocapacitive characteristics. Due to their unique optoelectronic properties, upconversion field luminescence properties and band gap tunability, the incorporation of GQDs into nanomaterials increases the ability of charge carrier extraction to improve solar cell efficiency. GQDs have a large specific surface area, high electron mobility and excellent UV absorption and the ability to convert into visible light. Therefore, GQDs are widely used as sensitizers, electron/hole transport layers or active layer additives for photosensitive devices. In Li+/Na+ batteries, GQDs can effectively alleviate volume expansion, increase the ion diffusion rate and electron transfer, improve the interfacial electrical double layer and enhance electrochemical performance. Since bulk graphene does not have a band gap, it shows great limitations in further applications. Compared with bulk graphene, GQDs have quantum confinement and edge effects, so band gaps which modify carrier behavior exist in GQDs and enlarge their applications in LEDs. GQD-based energy converters can collect heat and convert it into electricity or convert it into energy such as electricity through light, moisture, pressure and other forms of energy.Although GQDs and their applications have made great progress in recent years, there are still many problems to be solved. In general, the first problem is that there is no clear definition of the actual size of GQDs, which has a great impact on the subsequent research of GQDs. For small-scale research, tiny differences will be amplified, even if the difference is only a few nanometers. The lack of size definition results in a very broad size range of graphene quantum dots. This problem also makes the physical and chemical properties of graphene quantum dots unclear. The industrial production and green synthesis of GQDs is another problem that needs to be addressed urgently. The inability to industrialize production has made it difficult to reduce the manufacturing cost of GQDs, which seriously affects the practical application of GQDs. Fortunately, the production of GQDs by green chemistry has received more attention in recent years. The synthesis of GQDs is progressing towards low pollution, low toxicity and low energy consumption, and there is still room for further improvement in these methods. In particular, there is still great room for development in the combination of green chemical synthesis and low-cost industrial production. We believe that GQD-based research will show great potential in these directions.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key R&D Program of China (2017YFB1104300 and 2016YFA0200200), NSFC (No. 21671020, 51433005 and 51673026), NSFC-MAECI (51861135202), and the Beijing Natural Science Foundation (2172049).Notes and references
- Y. Zhu, S. Murali, W. Cai, X. Li, J. W. Suk, J. R. Potts and R. S. Ruoff, Adv. Mater., 2010, 22, 3906–3924 CrossRef CAS PubMed.
- M. J. Sweetman, S. M. Hickey, D. A. Brooks, J. D. Hayball and S. E. Plush, Adv. Funct. Mater., 2019, 29, 1808740 CrossRef.
- X. Wang, G. Sun, N. Li and P. Chen, Chem. Soc. Rev., 2016, 45, 2239–2262 RSC.
- X. T. Zheng, A. Ananthanarayanan, K. Q. Luo and P. Chen, Small, 2015, 11, 1620–1636 CrossRef CAS.
- L. Li, G. Wu, G. Yang, J. Peng, J. Zhao and J.-J. Zhu, Nanoscale, 2013, 5, 4015–4039 RSC.
- D. Pan, J. Zhang, Z. Li and M. Wu, Adv. Mater., 2010, 22, 734–738 CrossRef CAS.
- X. Zhou, Y. Zhang, C. Wang, X. Wu, Y. Yang, B. Zheng, H. Wu, S. Guo and J. Zhang, ACS Nano, 2012, 6, 6592–6599 CrossRef CAS.
- H. Sun, L. Wu, W. Wei and X. Qu, Mater. Today, 2013, 16, 433–442 CrossRef CAS.
- X. Hai, J. Feng, X. Chen and J. Wang, J. Mater. Chem. B, 2018, 6, 3219–3234 RSC.
- N. Fuyuno, D. Kozawa, Y. Miyauchi, S. Mouri, R. Kitaura, H. Shinohara, T. Yasuda, N. Komatsu and K. Matsuda, Adv. Opt. Mater., 2014, 2, 983–989 CrossRef CAS.
- K. Li, W. Liu, Y. Ni, D. Li, D. Lin, Z. Su and G. Wei, J. Mater. Chem. B, 2017, 5, 4811–4826 RSC.
- S. Kim, D. H. Shin, C. O. Kim, S. S. Kang, S. S. Joo, S.-H. Choi, S. W. Hwang and C. Sone, Appl. Phys. Lett., 2013, 102, 053108 CrossRef.
- S. Kim, D. H. Shin, C. O. Kim, S. S. Kang, J. M. Kim, S.-H. Choi, L.-H. Jin, Y.-H. Cho, S. W. Hwang and C. Sone, Appl. Phys. Lett., 2012, 101, 163103 CrossRef.
- J. Peng, W. Gao, B. K. Gupta, Z. Liu, R. Romero-Aburto, L. Ge, L. Song, L. B. Alemany, X. Zhan, G. Gao, S. A. Vithayathil, B. A. Kaipparettu, A. A. Marti, T. Hayashi, J.-J. Zhu and P. M. Ajayan, Nano Lett., 2012, 12, 844–849 CrossRef CAS.
- Y. Yan, J. Gong, J. Chen, Z. Zeng, W. Huang, K. Pu, J. Liu and P. Chen, Adv. Mater., 2019, 31, 1808283 CrossRef.
- P. Zheng and N. Wu, Chem. – Asian J., 2017, 12, 2343–2353 CrossRef CAS.
- M. Li, T. Chen, J. J. Gooding and J. Liu, ACS Sens., 2019, 4, 1732–1748 CrossRef CAS.
- X. Li, J. Yu, S. Wageh, A. A. Al-Ghamdi and J. Xie, Small, 2016, 12, 6640–6696 CrossRef CAS.
- Z. Zeng, S. Chen, T. T. Y. Tan and F.-X. Xiao, Catal. Today, 2018, 315, 171–183 CrossRef CAS.
- E. Haque, J. Kim, V. Malgras, K. R. Reddy, A. C. Ward, J. You, Y. Bando, M. S. A. Hossain and Y. Yamauchi, Small Methods, 2018, 2, 1800050 CrossRef.
- X. Li, M. Rui, J. Song, Z. Shen and H. Zeng, Adv. Funct. Mater., 2015, 25, 4929–4947 CrossRef CAS.
- S. Zhu, Q. Meng, L. Wang, J. Zhang, Y. Song, H. Jin, K. Zhang, H. Sun, H. Wang and B. Yang, Angew. Chem., Int. Ed., 2013, 125, 4045–4049 CrossRef.
- Y. Xu, X.-H. Jia, X.-B. Yin, X.-W. He and Y.-K. Zhang, Anal. Chem., 2014, 86, 12122–12129 CrossRef CAS.
- Q. Liu, B. Guo, Z. Rao, B. Zhang and J. R. Gong, Nano Lett., 2013, 13, 2436–2441 CrossRef CAS.
- A. Ananthanarayanan, X. Wang, P. Routh, B. Sana, S. Lim, D.-H. Kim, K.-H. Lim, J. Li and P. Chen, Adv. Funct. Mater., 2014, 24, 3021–3026 CrossRef CAS.
- D. B. Shinde and V. K. Pillai, Chem. – Eur. J., 2012, 18, 12522–12528 CrossRef CAS.
- L. Nilewski, K. Mendoza, A. S. Jalilov, V. Berka, G. Wu, W. K. A. Sikkema, A. Metzger, R. Ye, R. Zhang, D. X. Luong, T. Wang, E. McHugh, P. J. Derry, E. L. Samuel, T. A. Kent, A.-L. Tsai and J. M. Tour, ACS Appl. Mater. Interfaces, 2019, 11, 16815–16821 CrossRef CAS.
- J. Wen, M. Li, J. Xiao, C. Liu, Z. Li, Y. Xie, P. Ning, H. Cao and Y. Zhang, Mater. Today. Commun., 2016, 8, 127–133 CrossRef CAS.
- L. Lu, Y. Zhu, C. Shi and Y. T. Pei, Carbon, 2016, 109, 373–383 CrossRef CAS.
- V. Amendola and M. Meneghetti, Phys. Chem. Chem. Phys., 2009, 11, 3805–3821 RSC.
- G. W. Yang, Prog. Mater. Sci., 2007, 52, 648–698 CrossRef CAS.
- J. Pal, M. Ganguly, C. Mondal, Y. Negishi and T. Pal, Nanoscale, 2015, 7, 708–719 RSC.
- S. R. M. Santiago, T. N. Lin, C. T. Yuan, J. L. Shen, H. Y. Huang and C. A. J. Lin, Phys. Chem. Chem. Phys., 2016, 18, 22599–22605 RSC.
- S.-J. Jeon, T.-W. Kang, J.-M. Ju, M.-J. Kim, J. H. Park, F. Raza, J. Han, H.-R. Lee and J.-H. Kim, Adv. Funct. Mater., 2016, 26, 8211–8219 CrossRef CAS.
- J. Zhu, Y. Tang, G. Wang, J. Mao, Z. Liu, T. Sun, M. Wang, D. Chen, Y. Yang, J. Li, Y. Deng and S. Yang, ACS Appl. Mater. Interfaces, 2017, 9, 14470–14477 CrossRef CAS.
- Y. Dong, J. Shao, C. Chen, H. Li, R. Wang, Y. Chi, X. Lin and G. Chen, Carbon, 2012, 50, 4738–4743 CrossRef CAS.
- D. Qu, M. Zheng, P. Du, Y. Zhou, L. Zhang, D. Li, H. Tan, Z. Zhao, Z. Xie and Z. Sun, Nanoscale, 2013, 5, 12272–12277 RSC.
- R. Zhang, J. R. Adsetts, Y. Nie, X. Sun and Z. Ding, Carbon, 2018, 129, 45–53 CrossRef CAS.
- H. Jin, H. Huang, Y. He, X. Feng, S. Wang, L. Dai and J. Wang, J. Am. Chem. Soc., 2015, 137, 7588–7591 CrossRef CAS.
- A. Suryawanshi, M. Biswal, D. Mhamane, R. Gokhale, S. Patil, D. Guin and S. Ogale, Nanoscale, 2014, 6, 11664–11670 RSC.
- Z. Wang, J. Yu, X. Zhang, N. Li, B. Liu, Y. Li, Y. Wang, W. Wang, Y. Li, L. Zhang, S. Dissanayake, S. L. Suib and L. Sun, ACS Appl. Mater. Interfaces, 2016, 8, 1434–1439 CrossRef CAS PubMed.
- R. Riaz, M. Ali, I. A. Sahito, A. A. Arbab, T. Maiyalagan, A. S. Anjum, M. J. Ko and S. H. Jeong, Appl. Surf. Sci., 2019, 480, 1035–1046 CrossRef CAS.
- W.-S. Kuo, Y.-T. Shao, K.-S. Huang, T.-M. Chou and C.-H. Yang, ACS Appl. Mater. Interfaces, 2018, 10, 14438–14446 CrossRef CAS.
- F. Zuo, C. Zhang, H. Zhang, X. Tan, S. Chen and R. Yuan, Electrochim. Acta, 2019, 294, 76–83 CrossRef CAS.
- Y. Fu, G. Gao and J. Zhi, J. Mater. Chem. B, 2019, 7, 1494–1502 RSC.
- Y. Guo, F. Cao and Y. Li, Sens. Actuators, B, 2018, 255, 1105–1111 CrossRef CAS.
- D. Shen, W. Zhang, F. Xie, Y. Li, A. Abate and M. Wei, J. Power Sources, 2018, 402, 320–326 CrossRef CAS.
- Z. Li, L. Cao, P. Qin, X. Liu, Z. Chen, L. Wang, D. Pan and M. Wu, Carbon, 2018, 139, 67–75 CrossRef CAS.
- M. T. Hasan, R. Gonzalez-Rodriguez, C. Ryan, K. Pota, K. Green, J. L. Coffer and A. V. Naumov, Nano Res., 2019, 12, 1041–1047 CrossRef CAS.
- S. Yang, J. Sun, P. He, X. Deng, Z. Wang, C. Hu, G. Ding and X. Xie, Chem. Mater., 2015, 27, 2004–2011 CrossRef CAS.
- Y. Zhang, J. Zhao, H. Sun, Z. Zhu, J. Zhang and Q. Liu, Sens. Actuators, B, 2018, 266, 364–374 CrossRef CAS.
- L. S. Walekar, M. Zheng, L. Zheng and M. Long, Microchim. Acta, 2019, 186, 278 CrossRef.
- D. Wei, Y. Liu, Y. Wang, H. Zhang, L. Huang and G. Yu, Nano Lett., 2009, 9, 1752–1758 CrossRef CAS.
- C.-P. Han, C.-J. Chen, C.-C. Hsu, A. Jena, H. Chang, N.-C. Yeh, S.-F. Hu and R.-S. Liu, Catal. Today, 2019, 335, 395–401 CrossRef CAS.
- H. Safardoust-Hojaghan and M. Salavati-Niasari, J. Cleaner Prod., 2017, 148, 31–36 CrossRef CAS.
- J. Moon, J. An, U. Sim, S.-P. Cho, J. H. Kang, C. Chung, J.-H. Seo, J. Lee, K. T. Nam and B. H. Hong, Adv. Mater., 2014, 26, 3501–3505 CrossRef CAS.
- Y. Li, Y. Zhao, H. Cheng, Y. Hu, G. Shi, L. Dai and L. Qu, J. Am. Chem. Soc., 2012, 134, 15–18 CrossRef CAS.
- L. Tang, R. Ji, X. Li, G. Bai, C. P. Liu, J. Hao, J. Lin, H. Jiang, K. S. Teng, Z. Yang and S. P. Lau, ACS Nano, 2014, 8, 6312–6320 CrossRef CAS.
- T. V. Tam, S. G. Kang, M. H. Kim, S. G. Lee, S. H. Hur, J. S. Chung and W. M. Choi, Adv. Energy Mater., 2019, 9, 1900945 CrossRef.
- H. Wang, R. Revia, K. Wang, R. J. Kant, Q. Mu, Z. Gai, K. Hong and M. Zhang, Adv. Mater., 2017, 29, 1605416 CrossRef.
- T. Van Tam, S. G. Kang, K. F. Babu, E.-S. Oh, S. G. Lee and W. M. Choi, J. Mater. Chem. A, 2017, 5, 10537–10543 RSC.
- M. T. Hasan, R. Gonzalez-Rodriguez, C. Ryan, N. Faerber, J. L. Coffer and A. V. Naumov, Adv. Funct. Mater., 2018, 28, 1804337 CrossRef.
- S. Kundu, R. M. Yadav, T. N. Narayanan, M. V. Shelke, R. Vajtai, P. M. Ajayan and V. K. Pillai, Nanoscale, 2015, 7, 11515–11519 RSC.
- S. Kundu, P. Sarojinijeeva, R. Karthick, G. Anantharaj, G. Saritha, R. Bera, S. Anandan, A. Patra, P. Ragupathy, M. Selvaraj, D. Jeyakumar and K. V. Pillai, Electrochim. Acta, 2017, 242, 337–343 CrossRef CAS.
- H. Sun, A. Zhao, N. Gao, K. Li, J. Ren and X. Qu, Angew. Chem., Int. Ed., 2015, 54, 7176–7180 CrossRef CAS.
- A. Shomali, H. Valizadeh, A. Banan and R. Mohammad-Rezaei, RSC Adv., 2015, 5, 88202–88208 RSC.
- K. Li, J. Chen, Y. Yan, Y. Min, H. Li, F. Xi, J. Liu and P. Chen, Carbon, 2018, 136, 224–233 CrossRef CAS.
- H. Tetsuka, R. Asahi, A. Nagoya, K. Okamoto, I. Tajima, R. Ohta and A. Okamoto, Adv. Mater., 2012, 24, 5333–5338 CrossRef CAS.
- B.-P. Qi, H. Hu, L. Bao, Z.-L. Zhang, B. Tang, Y. Peng, B.-S. Wang and D.-W. Pang, Nanoscale, 2015, 7, 5969–5973 RSC.
- W. Kwon, Y.-H. Kim, J.-H. Kim, T. Lee, S. Do, Y. Park, M. S. Jeong, T.-W. Lee and S.-W. Rhee, Sci. Rep., 2016, 6, 24205 CrossRef CAS PubMed.
- H. Tetsuka, A. Nagoya, T. Fukusumi and T. Matsui, Adv. Mater., 2016, 28, 4632–4638 CrossRef CAS.
- Y. Yan, J. Chen, N. Li, J. Tian, K. Li, J. Jiang, J. Liu, Q. Tian and P. Chen, ACS Nano, 2018, 12, 3523–3532 CrossRef CAS.
- W.-W. Liu, Y.-Q. Feng, X.-B. Yan, J.-T. Chen and Q.-J. Xue, Adv. Funct. Mater., 2013, 23, 4111–4122 CrossRef CAS.
- K. Lee, H. Lee, Y. Shin, Y. Yoon, D. Kim and H. Lee, Nano Energy, 2016, 26, 746–754 CrossRef CAS.
- Z. Li, X. Liu, L. Wang, F. Bu, J. Wei, D. Pan and M. Wu, Small, 2018, 14, 1801498 CrossRef.
- W. Liu, X. Yan, J. Chen, Y. Feng and Q. Xue, Nanoscale, 2013, 5, 6053–6062 RSC.
- Y. Qing, Y. Jiang, H. Lin, L. Wang, A. Liu, Y. Cao, R. Sheng, Y. Guo, C. Fan, S. Zhang, D. Jia and Z. Fan, J. Mater. Chem. A, 2019, 7, 6021–6027 RSC.
- X. Yin, C. Zhi, W. Sun, L.-P. Lv and Y. Wang, J. Mater. Chem. A, 2019, 7, 7800–7814 RSC.
- B. Shen, J. Lang, R. Guo, X. Zhang and X. Yan, ACS Appl. Mater. Interfaces, 2015, 7, 25378–25389 CrossRef CAS.
- S. Zhang, J. Zhu, Y. Qing, L. Wang, J. Zhao, J. Li, W. Tian, D. Jia and Z. Fan, Adv. Funct. Mater., 2018, 28, 1805898 CrossRef.
- Z. Li, P. Qin, L. Wang, C. Yang, Y. Li, Z. Chen, D. Pan and M. Wu, Electrochim. Acta, 2016, 208, 260–266 CrossRef CAS.
- A. B. Ganganboina, A. D. Chowdhury and R.-a. Doong, Electrochim. Acta, 2017, 245, 912–923 CrossRef CAS.
- Y. Huang, T. Shi, Y. Zhong, S. Cheng, S. Jiang, C. Chen, G. Liao and Z. Tang, Electrochim. Acta, 2018, 269, 45–54 CrossRef CAS.
- Q. Chen, Y. Hu, C. Hu, H. Cheng, Z. Zhang, H. Shao and L. Qu, Phys. Chem. Chem. Phys., 2014, 16, 19307–19313 RSC.
- S. H. Shin, D. H. Shin and S.-H. Choi, ACS Sustainable Chem. Eng., 2019, 7, 13178–13185 CrossRef CAS.
- L. Najafi, B. Taheri, B. Martín-García, S. Bellani, D. Di Girolamo, A. Agresti, R. Oropesa-Nuñez, S. Pescetelli, L. Vesce, E. Calabrò, M. Prato, A. E. Del Rio Castillo, A. Di Carlo and F. Bonaccorso, ACS Nano, 2018, 12, 10736–10754 CrossRef CAS.
- K. D. Lee, M. J. Park, D.-Y. Kim, S. M. Kim, B. Kang, S. Kim, H. Kim, H.-S. Lee, Y. Kang, S. S. Yoon, B. H. Hong and D. Kim, ACS Appl. Mater. Interfaces, 2015, 7, 19043–19049 CrossRef CAS.
- D. Torres, D. Sebastián, M. J. Lázaro, J. L. Pinilla, I. Suelves, A. S. Aricò and V. Baglio, Electrochim. Acta, 2019, 306, 396–406 CrossRef CAS.
- T. Liu, K. Yu, L. Gao, H. Chen, N. Wang, L. Hao, T. Li, H. He and Z. Guo, J. Mater. Chem. A, 2017, 5, 17848–17855 RSC.
- H. Sung, N. Ahn, M. S. Jang, J.-K. Lee, H. Yoon, N.-G. Park and M. Choi, Adv. Energy Mater., 2016, 6, 1501873 CrossRef.
- Z.-K. Wang, X. Gong, M. Li, Y. Hu, J.-M. Wang, H. Ma and L.-S. Liao, ACS Nano, 2016, 10, 5479–5489 CrossRef CAS.
- G. S. Han, Y. H. Song, Y. U. Jin, J.-W. Lee, N.-G. Park, B. K. Kang, J.-K. Lee, I. S. Cho, D. H. Yoon and H. S. Jung, ACS Appl. Mater. Interfaces, 2015, 7, 23521–23526 CrossRef CAS.
- J. Ryu, E. Lee, S. Lee and J. Jang, Chem. Commun., 2014, 50, 15616–15618 RSC.
- J. Ryu, J. W. Lee, H. Yu, J. Yun, K. Lee, J. Lee, D. Hwang, J. Kang, S. K. Kim and J. Jang, J. Mater. Chem. A, 2017, 5, 16834–16842 RSC.
- T. Mahmoudi, Y. Wang and Y.-B. Hahn, ACS Energy Lett., 2019, 4, 235–241 CrossRef CAS.
- Y. Wang, T. Mahmoudi, W.-Y. Rho, H.-Y. Yang, S. Seo, K. S. Bhat, R. Ahmad and Y.-B. Hahn, Nano Energy, 2017, 40, 408–417 CrossRef CAS.
- H. Bian, Q. Wang, S. Yang, C. Yan, H. Wang, L. Liang, Z. Jin, G. Wang and S. Liu, J. Mater. Chem. A, 2019, 7, 5740–5747 RSC.
- M. Tai, X. Zhao, H. Wei, G. Wang, F. Hao, X. Li, X. Yin, Y. Zhou, J. Han, Y. Wei, K. Jiang and H. Lin, ACS Appl. Mater. Interfaces, 2018, 10, 26206–26212 CrossRef CAS.
- G. Xing, B. Wu, S. Chen, J. Chua, N. Yantara, S. Mhaisalkar, N. Mathews and T. C. Sum, Small, 2015, 11, 3606–3613 CrossRef CAS.
- W. Tress, N. Marinova, T. Moehl, S. M. Zakeeruddin, M. K. Nazeeruddin and M. Grätzel, Energy Environ. Sci., 2015, 8, 995–1004 RSC.
- H. Tan, A. Jain, O. Voznyy, X. Lan, F. P. García de Arquer, J. Z. Fan, R. Quintero-Bermudez, M. Yuan, B. Zhang, Y. Zhao, F. Fan, P. Li, L. N. Quan, Y. Zhao, Z.-H. Lu, Z. Yang, S. Hoogland and E. H. Sargent, Science, 2017, 355, 722–726 CrossRef CAS.
- Y. Zhou, S. Yang, X. Yin, J. Han, M. Tai, X. Zhao, H. Chen, Y. Gu, N. Wang and H. Lin, J. Mater. Chem. A, 2019, 7, 1878–1888 RSC.
- W. Ke, G. Fang, Q. Liu, L. Xiong, P. Qin, H. Tao, J. Wang, H. Lei, B. Li, J. Wan, G. Yang and Y. Yan, J. Am. Chem. Soc., 2015, 137, 6730–6733 CrossRef CAS.
- X. Liu, K.-W. Tsai, Z. Zhu, Y. Sun, C.-C. Chueh and A. K.-Y. Jen, Adv. Mater. Interfaces, 2016, 3, 1600122 CrossRef.
- W. Ke, D. Zhao, C. Xiao, C. Wang, A. J. Cimaroli, C. R. Grice, M. Yang, Z. Li, C.-S. Jiang, M. Al-Jassim, K. Zhu, M. G. Kanatzidis, G. Fang and Y. Yan, J. Mater. Chem. A, 2016, 4, 14276–14283 RSC.
- E. H. Anaraki, A. Kermanpur, L. Steier, K. Domanski, T. Matsui, W. Tress, M. Saliba, A. Abate, M. Grätzel, A. Hagfeldt and J.-P. Correa-Baena, Energy Environ. Sci., 2016, 9, 3128–3134 RSC.
- J. P. Correa Baena, L. Steier, W. Tress, M. Saliba, S. Neutzner, T. Matsui, F. Giordano, T. J. Jacobsson, A. R. Srimath Kandada, S. M. Zakeeruddin, A. Petrozza, A. Abate, M. K. Nazeeruddin, M. Grätzel and A. Hagfeldt, Energy Environ. Sci., 2015, 8, 2928–2934 RSC.
- J. Xie, K. Huang, X. Yu, Z. Yang, K. Xiao, Y. Qiang, X. Zhu, L. Xu, P. Wang, C. Cui and D. Yang, ACS Nano, 2017, 11, 9176–9182 CrossRef CAS.
- J. H. Kim, C.-C. Chueh, S. T. Williams and A. K. Y. Jen, Nanoscale, 2015, 7, 17343–17349 RSC.
- J. Xie, X. Yu, X. Sun, J. Huang, Y. Zhang, M. Lei, K. Huang, D. Xu, Z. Tang, C. Cui and D. Yang, Nano Energy, 2016, 28, 330–337 CrossRef CAS.
- Z. Bin, J. Li, L. Wang and L. Duan, Energy Environ. Sci., 2016, 9, 3424–3428 RSC.
- Z. Yang, J. Xie, V. Arivazhagan, K. Xiao, Y. Qiang, K. Huang, M. Hu, C. Cui, X. Yu and D. Yang, Nano Energy, 2017, 40, 345–351 CrossRef CAS.
- M.-L. Tsai, W.-C. Tu, L. Tang, T.-C. Wei, W.-R. Wei, S. P. Lau, L.-J. Chen and J.-H. He, Nano Lett., 2016, 16, 309–313 CrossRef CAS.
- M.-L. Tsai, W.-R. Wei, L. Tang, H.-C. Chang, S.-H. Tai, P.-K. Yang, S. P. Lau, L.-J. Chen and J.-H. He, ACS Nano, 2016, 10, 815–821 CrossRef CAS.
- M.-L. Tsai, D.-S. Tsai, L. Tang, L.-J. Chen, S. P. Lau and J.-H. He, ACS Nano, 2017, 11, 4564–4570 CrossRef CAS.
- J. Guo, H. Zhu, Y. Sun, L. Tang and X. Zhang, J. Mater. Chem. A, 2016, 4, 4783–4789 RSC.
- Y.-M. Chen, S.-T. Hsu, Y.-H. Tseng, T.-F. Yeh, S.-S. Hou, J.-S. Jan, Y.-L. Lee and H. Teng, Small, 2018, 14, 1703571 CrossRef.
- J. Park, J. Moon, C. Kim, J. H. Kang, E. Lim, J. Park, K. J. Lee, S.-H. Yu, J.-H. Seo, J. Lee, J. Heo, N. Tanaka, S.-P. Cho, J. Pyun, J. Cabana, B. H. Hong and Y.-E. Sung, NPG Asia Mater., 2016, 8, e272 CrossRef CAS.
- K. Lijuan, Y. Yongqiang, L. Ruiyi and L. Zaijun, Electrochim. Acta, 2016, 198, 144–155 CrossRef.
- Y. Ji, J. Hu, J. Biskupek, U. Kaiser, Y.-F. Song and C. Streb, Chem. – Eur. J., 2017, 23, 16637–16643 CrossRef CAS.
- C. Zhu, D. Chao, J. Sun, I. M. Bacho, Z. Fan, C. F. Ng, X. Xia, H. Huang, H. Zhang, Z. X. Shen, G. Ding and H. J. Fan, Adv. Mater. Interfaces, 2015, 2, 1400499 CrossRef.
- J. Liu, H. Xia, D. Xue and L. Lu, J. Am. Chem. Soc., 2009, 131, 12086–12087 CrossRef CAS.
- D. Chao, X. Xia, J. Liu, Z. Fan, C. F. Ng, J. Lin, H. Zhang, Z. X. Shen and H. J. Fan, Adv. Mater., 2014, 26, 5794–5800 CrossRef CAS.
- D. Chao, C. Zhu, X. Xia, J. Liu, X. Zhang, J. Wang, P. Liang, J. Lin, H. Zhang, Z. X. Shen and H. J. Fan, Nano Lett., 2015, 15, 565–573 CrossRef CAS.
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359–367 CrossRef CAS.
- A. S. Aricò, P. Bruce, B. Scrosati, J.-M. Tarascon and W. van Schalkwijk, Nat. Mater., 2005, 4, 366–377 CrossRef.
- K. Kang, Y. S. Meng, J. Bréger, C. P. Grey and G. Ceder, Science, 2006, 311, 977–980 CrossRef CAS.
- H.-G. Jung, M. W. Jang, J. Hassoun, Y.-K. Sun and B. Scrosati, Nat. Commun., 2011, 2, 516 CrossRef.
- F. Khan, M. Oh and J. H. Kim, Chem. Eng. J., 2019, 369, 1024–1033 CrossRef CAS.
- J.-K. Sun and Q. Xu, Energy Environ. Sci., 2014, 7, 2071–2100 RSC.
- S. J. Yang, T. Kim, J. H. Im, Y. S. Kim, K. Lee, H. Jung and C. R. Park, Chem. Mater., 2012, 24, 464–470 CrossRef CAS.
- J. Hu, H. Wang, Q. Gao and H. Guo, Carbon, 2010, 48, 3599–3606 CrossRef CAS.
- J. Li, Y. Chen, Y. Tang, S. Li, H. Dong, K. Li, M. Han, Y.-Q. Lan, J. Bao and Z. Dai, J. Mater. Chem. A, 2014, 2, 6316–6319 RSC.
- H. Yu, W. Zhu, H. Zhou, J. Liu, Z. Yang, X. Hu and A. Yuan, RSC Adv., 2019, 9, 9577–9583 RSC.
- B. Qiu, M. Xing and J. Zhang, J. Am. Chem. Soc., 2014, 136, 5852–5855 CrossRef CAS PubMed.
- Y. Zhang, C. W. Foster, C. E. Banks, L. Shao, H. Hou, G. Zou, J. Chen, Z. Huang and X. Ji, Adv. Mater., 2016, 28, 9391–9399 CrossRef CAS PubMed.
- W. Zhang, T. Xu, Z. Liu, N.-L. Wu and M. Wei, Chem. Commun., 2018, 54, 1413–1416 RSC.
- D. Sun, Y. Tang, D. Ye, J. Yan, H. Zhou and H. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 5254–5262 CrossRef CAS.
- L. Qi, Y. Xin, Z. Zuo, C. Yang, K. Wu, B. Wu and H. Zhou, ACS Appl. Mater. Interfaces, 2016, 8, 17245–17252 CrossRef CAS.
- H. Wang, J. Wang, D. Cao, H. Gu, B. Li, X. Lu, X. Han, A. L. Rogach and C. Niu, J. Mater. Chem. A, 2017, 5, 6817–6824 RSC.
- M. Wu, H. Chen, L.-P. Lv and Y. Wang, Chem. Eng. J., 2019, 373, 985–994 CrossRef CAS.
- R. Lv, J. A. Robinson, R. E. Schaak, D. Sun, Y. Sun, T. E. Mallouk and M. Terrones, Acc. Chem. Res., 2015, 48, 56–64 CrossRef CAS.
- H. Wang, H. Yuan, S. Sae Hong, Y. Li and Y. Cui, Chem. Soc. Rev., 2015, 44, 2664–2680 RSC.
- I. Kovalenko, B. Zdyrko, A. Magasinski, B. Hertzberg, Z. Milicev, R. Burtovyy, I. Luzinov and G. Yushin, Science, 2011, 334, 75–79 CrossRef CAS PubMed.
- M. Heurlin, M. H. Magnusson, D. Lindgren, M. Ek, L. R. Wallenberg, K. Deppert and L. Samuelson, Nature, 2012, 492, 90 CrossRef CAS.
- S. Kawakami, H. Kaidou, Y. Akita, H. Munakata and K. Kanamura, Electrochemistry, 2015, 83, 445–451 CrossRef CAS.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666 CrossRef CAS.
- C. O. Kim, S. W. Hwang, S. Kim, D. H. Shin, S. S. Kang, J. M. Kim, C. W. Jang, J. H. Kim, K. W. Lee, S.-H. Choi and E. Hwang, Sci. Rep., 2014, 4, 5603 CrossRef CAS PubMed.
- K. J. Williams, C. A. Nelson, X. Yan, L.-S. Li and X. Zhu, ACS Nano, 2013, 7, 1388–1394 CrossRef CAS.
- V. Gupta, N. Chaudhary, R. Srivastava, G. D. Sharma, R. Bhardwaj and S. Chand, J. Am. Chem. Soc., 2011, 133, 9960–9963 CrossRef CAS PubMed.
- W. Kwon, Y.-H. Kim, C.-L. Lee, M. Lee, H. C. Choi, T.-W. Lee and S.-W. Rhee, Nano Lett., 2014, 14, 1306–1311 CrossRef CAS.
- S. H. Song, M.-H. Jang, J. Chung, S. H. Jin, B. H. Kim, S.-H. Hur, S. Yoo, Y.-H. Cho and S. Jeon, Adv. Opt. Mater., 2014, 2, 1016–1023 CrossRef CAS.
- P. Alonso-González, A. Y. Nikitin, F. Golmar, A. Centeno, A. Pesquera, S. Vélez, J. Chen, G. Navickaite, F. Koppens, A. Zurutuza, F. Casanova, L. E. Hueso and R. Hillenbrand, Science, 2014, 344, 1369 CrossRef.
- H. Yan, X. Li, B. Chandra, G. Tulevski, Y. Wu, M. Freitag, W. Zhu, P. Avouris and F. Xia, Nat. Nanotechnol., 2012, 7, 330 CrossRef CAS.
- R. Sekiya, Y. Uemura, H. Murakami and T. Haino, Angew. Chem., Int. Ed., 2014, 53, 5619–5623 CrossRef CAS.
- D. H. Kim and T. W. Kim, Nano Energy, 2017, 32, 441–447 CrossRef CAS.
- D. Pan, L. Wang, Z. Li, B. Geng, C. Zhang, J. Zhan, L. Yin and L. Wang, New J. Chem., 2018, 42, 5083–5089 RSC.
- C. M. Luk, L. B. Tang, W. F. Zhang, S. F. Yu, K. S. Teng and S. P. Lau, J. Mater. Chem., 2012, 22, 22378–22381 RSC.
- E. A. Bakar, M. A. Mohamed, P. C. Ooi, M. F. M. R. Wee, C. F. Dee and B. Y. Majlis, Org. Electron., 2018, 61, 289–295 CrossRef.
- Z. Xu, C. Wu, F. Li, W. Chen, T. Guo and T. W. Kim, Nano Energy, 2018, 49, 274–282 CrossRef CAS.
- Y. Zhao, R.-j. Tong, M.-Q. Chen and F. Xia, Sens. Actuators, B, 2019, 284, 96–102 CrossRef CAS.
- P. Qi, T. Zhang, J. Shao, B. Yang, T. Fei and R. Wang, Sens. Actuators, A, 2019, 287, 93–101 CrossRef CAS.
- T. S. Sreeprasad, A. A. Rodriguez, J. Colston, A. Graham, E. Shishkin, V. Pallem and V. Berry, Nano Lett., 2013, 13, 1757–1763 CrossRef CAS.
- Y. Huang, H. Cheng, G. Shi and L. Qu, ACS Appl. Mater. Interfaces, 2017, 9, 38170–38175 CrossRef CAS.
- S. Li, T. Fan, X. Liu, F. Liu, H. Meng, Y. Liu and F. Pan, ACS Appl. Mater. Interfaces, 2017, 9, 3677–3685 CrossRef CAS.
- Z. Zeng, F.-X. Xiao, H. Phan, S. Chen, Z. Yu, R. Wang, T.-Q. Nguyen and T. T. Yang Tan, J. Mater. Chem. A, 2018, 6, 1700–1713 RSC.
- Z. Zeng, Y.-B. Li, S. Chen, P. Chen and F.-X. Xiao, J. Mater. Chem. A, 2018, 6, 11154–11162 RSC.
- Z. Zeng, T. Li, Y.-B. Li, X.-C. Dai, M.-H. Huang, Y. He, G. Xiao and F.-X. Xiao, J. Mater. Chem. A, 2018, 6, 24686–24692 RSC.
Footnote |
| † Qianwen Liu and Jianhan Sun contributed equally to this work. |
| This journal is © the Partner Organisations 2020 |