Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
ESIPT-based fluorescence probe for the rapid detection of hypochlorite (HOCl/ClO−)†
Luling
Wu
 a,
Qingye
Yang
b,
Liyuan
Liu
a,
Adam C.
Sedgwick
a,
Qingye
Yang
b,
Liyuan
Liu
a,
Adam C.
Sedgwick
 *a,
Alexander J.
Cresswell
*a,
Alexander J.
Cresswell
 *a,
Steven D.
Bull
*a,
Steven D.
Bull
 *a,
Chusen
Huang
*a,
Chusen
Huang
 *b and
Tony D.
James
*b and
Tony D.
James
 *ac
*ac
aDepartment of Chemistry, University of Bath, Bath, BA2 7AY, UK. E-mail: t.d.james@bath.ac.uk; s.d.bull@bath.ac.uk; a.c.sedgwick@bath.ac.uk; a.j.cresswell@bath.ac.uk
bThe Education Ministry Key Laboratory of Resource Chemistry, Shanghai Key Laboratory of Rare Earth Functional Materials, and Shanghai Municipal Education Committee Key Laboratory of Molecular Imaging Probes and Sensors, Department of Chemistry, Shanghai Normal University, 100 Guilin Road, Shanghai 200234, China. E-mail: huangcs@shnu.edu.cn
cDepartment of Materials and Life Sciences, Faculty of Science and Technology, Sophia University, 7-1 Kioi-cho, Chiyoda-ku, Tokyo 102-8554, Japan
First published on 4th July 2018
Abstract
ESIPT-based fluorescence probes are emerging as an attractive tool for the detection of biologically relevant analytes owing to their unique photophysical properties. In this work, we have developed an ESIPT-based fluorescence probe (TCBT-OMe) for the detection of HClO/ClO− through the attachment of a bioorthogonal dimethylthiocarbamate linker. TCBT-OMe was shown to rapidly detect HClO/ClO− (<10 s) at biologically relevant concentrations (LoD = 0.16 nM) and have an excellent selectivity towards others ROS/RNS and amino acids. Therefore, TCBT-OMe was tested in live cells and was successfully shown to be able to detect endogenous and exogenous HClO/ClO− in HeLa cells. Additionally, TCBT-OMe acts as a dual input logic gate for Hg2+ and H2O2. Interestingly, Hg2+ alone gradually causes a fluorescence response but requires >30 min to produce a fluorescence response. Test strips containing TCBT-OMe were prepared and were demonstrated as an effective way to detect HClO/ClO− in water. Furthermore, TCBT-OMe was shown to detect exogenously added HClO/ClO− in three different water samples with little interference thus demonstrating the effectiveness as a method for the detection of HClO/ClO− in drinking water samples.
Hypochlorous acid (HOCl) is a biologically important reactive oxygen species (ROS), which partially dissociates to form its hypochlorite anion (ClO−) under physiological conditions. In biological systems, myeloperoxidase, an enzyme found in leukocytes produces HOCl/ClO− by catalysing the reaction between Cl + H2O2 → HOCl.1 This vital ROS is used in immune defence systems due to its microbicidal properties.1 Unfortunately, excessive production of HOCl/ClO− can lead to the damage of a range of biological targets such as amino acids, proteins, carbohydrates and lipids.2,3 As a consequence, HOCl/ClO− has been associated with a number of diseases causing cell and tissue damage.4
In addition to its role in biological systems, HOCl/ClO− is produced by the chlorination of water (Cl2 + H2O → HOCl), which is the most common method for the treatment of water especially in public swimming pools.5 NaOCl (Bleach) is also extensively used as a disinfectant for both domestic and industrial purposes. Unfortunately, over-exposure to HOCl/ClO−, results in swimming pool-associated asthma, irritation to the oesophagus, throat and spontaneous vomiting (http://www.who.int/water_sanitation_health/dwq/chlorine.pdf).6 Additionally, there is an increased risk of bladder cancer associated with chlorinated by-products produced from chlorinated water.7,8 Therefore, given the potential health hazard towards animals and humans, the development of an effective method for HOCl/ClO− detection is required.
Within our research group, we are interested in developing reaction-based fluorescence sensors for the detection of biologically important analytes.9–13 Small-molecule fluorescence probes are a particular attractive tool owing to their high sensitivity, selectivity and high spatial and temporal resolution.14 In particular, we are interested in using Excited State Intramolecular Proton Transfer (ESIPT)-based fluorescence probes due to their excellent photophysical properties, which include intense luminescence, photostability and a large stokes shift.15,16 Previously, we reported an ESIPT-based fluorescence probe for the detection of peroxynitrite (ONOO−) through the use of a benzyl boronic ester protecting group (Scheme 1).15 This protecting group blocked the ESIPT process and therefore a low fluorescence intensity was observed. The addition of ONOO−, resulted in the fluorophore's deprotection and an increase in fluorescence intensity was observed.
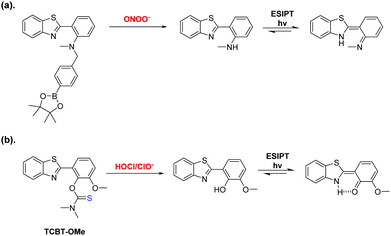 | ||
| Scheme 1 (a) Our previously reported ESIPT probe for the detection of ONOO−. (b) This work – a thiocarbamate linker-based ESIPT TCBT-OMe for the detection of HOCl/ClO−. | ||
In this work, we believed a methoxy-hydroxybenzothiazole (HBT-OMe) fluorophore would provide an effective ESIPT fluorescence probe for the detection of HOCl/ClO− (see ESI,† S1).17,18
To obtain TCBT-OMe, we first prepared HBT-OMe by the addition of a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 H2O2–(30% in H2O)/HCl solution to 2-aminothiophenol and O-vanillin in EtOH. This reaction proceeded quickly and smoothly, in a good yield (68%). With HBT-OMe in hand, four equivalents of dimethylthiocarbamoyl chloride was then added slowly to a solution of HBT-OMe in DCM. DIPEA was subsequently added dropwise to the reaction, which produced TCBT-OMe in excellent yield (72%).
1 H2O2–(30% in H2O)/HCl solution to 2-aminothiophenol and O-vanillin in EtOH. This reaction proceeded quickly and smoothly, in a good yield (68%). With HBT-OMe in hand, four equivalents of dimethylthiocarbamoyl chloride was then added slowly to a solution of HBT-OMe in DCM. DIPEA was subsequently added dropwise to the reaction, which produced TCBT-OMe in excellent yield (72%).
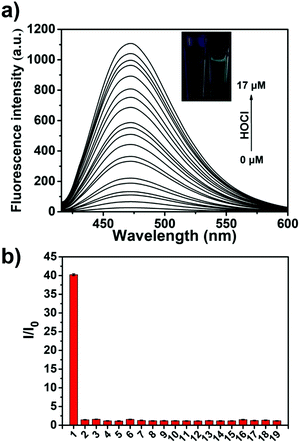
We then evaluated the UV-Vis of TCBT-OMe with the addition of HOCl/ClO− (10 μM), which resulted in the formation of a UV absorption peak at ∼310 nm (see ESI,† Fig. S1). Bhattacharyya et al. have reported that the fluorescence emission of the ESIPT process can be effected by intermolecular hydrogen bonding.19,20 Therefore, evaluation of ESIPT-based fluorescence probes are commonly carried out in the presence of the surfactant cetyl trimethylammonium bromide (CTAB, 1 mM) or by using a large ratio of organic solvent.19,21–23 It is believed that the formation of a micellar environment creates a hydrophobic pocket that aids the ESIPT process. Therefore, we evaluated the ability of TCBT-OMe to detect HOCl/ClO− by fluorescence in the presence of CTAB, 1 mM. As shown in Fig. 1a, TCBT-OMe was found to be very sensitive towards HOCl/ClO− reacting with micromolar concentrations to produce a large increase in fluorescence (∼42 fold – Fig. S3, ESI†). TCBT-OMe was shown to rapidly react with HOCl/ClO− producing a fluorescence response within less than 10 s (see ESI,† Fig. S4) and have a very low Limit of Detection (LoD) of 0.16 nM (see ESI,† Fig. S5). HOCl/ClO− (35 μM) was added to TCBT-OMe at different pH values and a bell-shaped curve was observed. The largest fluorescence response was seen at the pKa of HOCl/ClO− = 7.53 (Fig. S5, ESI†) suggestive of general acid–base catalysis being in operation. (see ESI,† Scheme S1 for proposed mechanism).
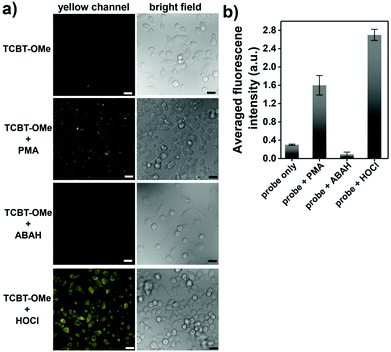
We then evaluated the selectivity of TCBT-OMe towards other reactive oxygen/nitrogen species (ROS/RNS) and amino acids (Fig. 1b). Remarkably, TCBT-OMe had an excellent selectivity towards HOCl/ClO− therefore permitting its use as a fluorescence probe for the detection of HOCl/ClO− in live cells. As shown in Fig. 2, TCBT-OMe was successfully used to visualise endogenously stimulated HOCl/ClO− in HeLa cells using phorbol 12-myristate 13-acetate (PMA, which is a ROS stimulant that induces the production of HOCl/ClO−). Separately, HeLa cells were also pretreated with 4-aminobenzoic acid hydrazide (ABAH, which is a specific inhibitor of MPO which suppressed the generation of HOCl) and as expected only weak fluorescence was observed. TCBT-OMe was also able to detect HOCl/ClO− added exogenously to the HeLa cells.
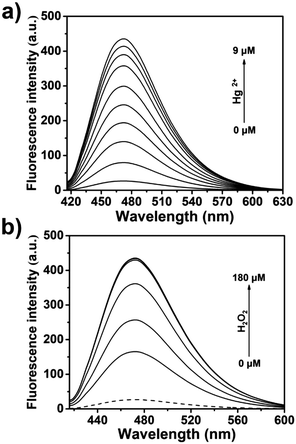
The dimethylthiocarbamate linker of TCBT-OMe has previously been used in the construction of dual input molecular logic gate24 for the detection of Hg2+ ‘AND’ H2O2 (see ESI† Scheme S2 for proposed mechanism).25,26 Therefore, we evaluated the ability of TCBT-OMe to perform molecular logic with the input of Hg2+ and H2O2. The presence of solely H2O2 (120 μM) led to a small increase in fluorescence intensity (dashed line), however, with subsequent additions of Hg2+ (0–9 μM) a large fluorescence response was observed (Fig. 3a). To demonstrate that both analytes are required, Hg2+ was added first, followed by the addition of H2O2 (0–180 μM). As shown in Fig. 3b, the subsequent addition of H2O2 rapidly led to an increase in fluorescence intensity. TCBT-OMe was shown to be selective towards Hg2+ over other metal cations in the presence of H2O2 (see ESI,† Fig. S9). Interestingly, Hg2+ alone resulted in a slow increase in fluorescence intensity (see ESI,† Fig. S10). This is believed to be due to the instability of the dimethylcarbonate formed from the reaction of TCBT-OMe with Hg2+.
Despite this interesting dual responsive reactivity of TCBT-OMe, this ‘AND’ logic requires minutes to fully react, whereas HOCl/ClO− reacts with TCBT-OMe within seconds. Therefore, due to the significantly greater reactivity of TCBT-OMe towards HOCl/ClO− over Hg2+, we believed we could use it as an effective method for the detection of HOCl/ClO− in drinking water sources.
We produced test strips by simply soaking a commercially available test strip in water containing TCBT-OMe (0.8 mM). After drying, test strips impregnated with TCBT-OMe were placed in water containing HClO/ClO− (0–200 μM). As shown in Fig. 4, there is a clear colour/intensity difference in the test strips that have been dipped into water containing various concentrations of HClO/ClO−.
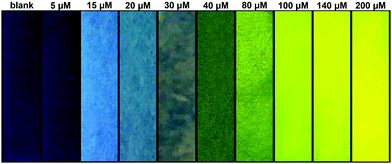 | ||
| Fig. 4 Photograph showing the colour changes of TCBT-OMe impregnated test strips after addition to water samples containing different concentrations of HClO/ClO− under UV light (365 nm). | ||
In addition to detecting HClO/ClO− in water, TCBT-OMe was added into three different water samples containing 1 mM CTAB (Sample A, tap water from University of Bath; Sample B, water from the Avon River (Bath); Sample C, water from Roman spa in Bath). Interestingly, little interference was observed for the exogenous addition of HClO/ClO− to each water sample (>95% recovery) – see ESI,† Table S1.
In summary, we have developed an ESIPT-based fluorescence TCBT-OMe for the detection of HClO/ClO−. TCBT-OMe was shown to have a very high sensitivity and selectivity towards HClO/ClO− fully reacting within 10 s and having a LoD of 0.16 μM. Significantly, TCBT-OMe was able to detect endogenous and exogenous HClO/ClO− in HeLa cells. Additionally, TCBT-OMe was shown as a dual input logic gate with Hg2+ and H2O2 as inputs. Interestingly, Hg2+ alone gradually produced a fluorescence response but required >30 min to produce a significant fluorescence response. Test strips containing TCBT-OMe were developed and shown to be an effective way to detect HClO/ClO− in water. Furthermore, TCBT-OMe was shown to detect exogenously added HClO/ClO− in three different water samples with little interference demonstrating its effectiveness as a method to detect HClO/ClO− in drinking water samples.
LW wishes to thank China Scholarship Council and the University of Bath for supporting his PhD work in the UK. We would like to thank the EPSRC and the University of Bath for funding. ACS thanks the EPSRC for a studentship. AJC wishes to thank the Royal Society for a University Research Fellowship. TDJ wishes to thank the Royal Society for a Wolfson Research Merit Award and Sophia University for a visiting professorship. NMR characterisation facilities were provided through the Chemical Characterisation and Analysis Facility (CCAF) at the University of Bath (http://www.bath.ac.uk/ccaf). The EPSRC UK National Mass Spectrometry Facility at Swansea University is thanked for analyses. All data supporting this study are provided as supplementary information accompanying this paper (ESI†).
Conflicts of interest
No conflicts of interest.Notes and references
- A. Strzepa, K. A. Pritchard and B. N. Dittel, Cell. Immunol., 2017, 317, 1–8 CrossRef PubMed.
- M. J. Davies, J. Clin. Biochem. Nutr., 2011, 48, 8–19 CrossRef PubMed.
- S. J. Klebanoff, J. Leukocyte Biol., 2005, 77, 598–625 CrossRef PubMed.
- B. S. van der Veen, M. P. J. de Winther and P. Heeringa, Antioxid. Redox Signaling, 2009, 11, 2899–2937 CrossRef PubMed.
- L. Kunigk, R. Gedraite and C. J. Kunigk, Environ. Eng. Manage. J., 2018, 17, 711–720 Search PubMed.
- C. Zwiener, S. D. Richardson, D. M. De Marini, T. Grummt, T. Glauner and F. H. Frimmel, Environ. Sci. Technol., 2007, 41, 363–372 CrossRef PubMed.
- K. P. Cantor, R. Hoover, P. Hartge, T. J. Mason, D. T. Silverman, R. Altman, D. F. Austin, M. A. Child, C. R. Key, L. D. Marrett, M. H. Myers, A. S. Narayana, L. I. Levin, J. W. Sullivan, G. M. Swanson, D. B. Thomas and D. W. West, J. Natl. Cancer Inst., 1987, 79, 1269–1279 Search PubMed.
- C. M. Villanueva, K. P. Cantor, S. Cordier, J. J. K. Jaakkola, W. D. King, C. F. Lynch, S. Porru and M. Kogevinas, Epidemiol., 2004, 15, 357–367 CrossRef.
- A. C. Sedgwick, R. S. L. Chapman, J. E. Gardiner, L. R. Peacock, G. Kim, J. Yoon, S. D. Bull and T. D. James, Chem. Commun., 2017, 53, 10441–10443 RSC.
- C. M. Lopez-Alled, A. Sanchez-Fernandez, K. J. Edler, A. C. Sedgwick, S. D. Bull, C. L. McMullin, G. Kociok-Kohn, T. D. James, J. Wenk and S. E. Lewis, Chem. Commun., 2017, 53, 12580–12583 RSC.
- A. C. Sedgwick, H. H. Han, J. E. Gardiner, S. D. Bull, X. P. He and T. D. James, Chem. Commun., 2017, 53, 12822–12825 RSC.
- D. Wu, A. C. Sedgwick, T. Gunnlaugsson, E. U. Akkaya, J. Yoon and T. D. James, Chem. Soc. Rev., 2017, 46, 7105–7123 RSC.
- E. V. Lampard, A. C. Sedgwick, X. L. Sun, K. L. Filer, S. C. Hewins, G. Kim, J. Yoon, S. D. Bull and T. D. James, ChemistryOpen, 2018, 7, 262–265 CrossRef PubMed.
- J. Chan, S. C. Dodani and C. J. Chang, Nat. Chem., 2012, 4, 973–984 CrossRef PubMed.
- A. C. Sedgwick, X. L. Sun, G. Kim, J. Yoon, S. D. Bull and T. D. James, Chem. Commun., 2016, 52, 12350–12352 RSC.
- J. S. Wu, W. M. Liu, J. C. Ge, H. Y. Zhang and P. F. Wang, Chem. Soc. Rev., 2011, 40, 3483–3495 RSC.
- B. C. Zhu, P. Li, W. Shu, X. Wang, C. Y. Liu, Y. Wang, Z. K. Wang, Y. W. Wang and B. Tang, Anal. Chem., 2016, 88, 12532–12538 CrossRef PubMed.
- B. C. Zhu, L. Wu, M. Zhang, Y. W. Wang, Z. Y. Zhao, Z. K. Wang, Q. X. Duan, P. Jia and C. Y. Liu, Sens. Actuators, B, 2018, 263, 103–108 CrossRef.
- N. Sarkar, K. Das, S. Das, A. Datta, D. Nath and K. Bhattacharyya, J. Phys. Chem., 1995, 99, 17711–17714 CrossRef.
- K. Das, N. Sarkar, A. K. Ghosh, D. Majumdar, D. N. Nath and K. Bhattacharyya, J. Phys. Chem., 1994, 98, 9126–9132 CrossRef.
- D. P. Murale, H. Kim, W. S. Choi and D. G. Churchill, Org. Lett., 2013, 15, 3946–3949 CrossRef PubMed.
- H. R. Zheng, L. Y. Niu, Y. Z. Chen, L. Z. Wu, C. H. Tung and Q. Z. Yang, Chin. Chem. Lett., 2016, 27, 1793–1796 CrossRef.
- R. Hu, J. A. Feng, D. H. Hu, S. Q. Wang, S. Y. Li, Y. Li and G. Q. Yang, Angew. Chem., Int. Ed., 2010, 49, 4915–4918 CrossRef PubMed.
- S. Erbas-Cakmak, S. Kolemen, A. C. Sedgwick, T. Gunnlaugsson, T. D. James, J. Yoon and E. U. Akkaya, Chem. Soc. Rev., 2018, 47, 2228–2248 RSC.
- D. P. Murale, H. Liew, Y. H. Suh and D. G. Churchill, Anal. Methods, 2013, 5, 2650–2652 RSC.
- W. Shu, L. G. Yan, J. Liu, Z. K. Wang, S. Zhang, C. C. Tang, C. Y. Liu, B. C. Zhu and B. Du, Ind. Eng. Chem. Res., 2015, 54, 8056–8062 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8cc03717e |
| This journal is © The Royal Society of Chemistry 2018 |