Safety assessment in development and operation of modular continuous-flow processes
Norbert
Kockmann
 *a,
Philipp
Thenée
b,
Christoph
Fleischer-Trebes
b,
Gabriele
Laudadio
c and
Timothy
Noël
*a,
Philipp
Thenée
b,
Christoph
Fleischer-Trebes
b,
Gabriele
Laudadio
c and
Timothy
Noël
 *c
*c
aLaboratory of Equipment Design, Department of Biochemical and Chemical Engineering, TU Dortmund, Germany. E-mail: kockmann@bci.tu-dortmund.de; Fax: +49(0)231 755 8084; Tel: +49(0)231 755 8077
bINVITE GmbH, Geb. W32, Kaiser-Wilhelm-Allee 50, D-51373 Leverkusen, Germany
cDepartment of Chemical Engineering and Chemistry, Micro Flow Chemistry and Process Technology, Eindhoven University of Technology, PO BOX 513, 5600 MB Eindhoven, The Netherlands. E-mail: t.noel@tue.nl; Web: www.NoelResearchGroup.com
First published on 30th March 2017
Abstract
Improved safety is one of the main drivers for microreactor application in chemical process development and small-scale production. Typical examples of hazardous chemistry are presented indicating potential risks also in miniaturized equipment. Energy balance and kinetic parameters describe the heat production potential and, together with heat transfer capability, the temperature development in a continuous flow reactor. Besides these calculation procedures, checklists for laboratory safety and risk assessment are the basis for improved laboratory work as well as for equipment-related safety discussions. For complete and larger chemical plants, hazard and operation (HAZOP) studies are the appropriate method of handling hazardous processes and their scale-up.
1 Introduction and motivation
Safety issues are dominant in chemical process engineering due to the high risk potential from often toxic substances as well as the harsh process conditions of extreme pressure and temperature. Early equipment and processes were often overdesigned due to unknown material behavior and less stringent cost restrictions.1 Better knowledge of material behavior as well as process know-how and prediction led to improved geometrical design with thin vessel walls. Their limits are more tested and sensible behavior was provoked. In the early 1970s learning by accident was not tolerable anymore. Triggered by heavy accidents in chemical plants (e.g. Flixborough in 1974 or Seveso in 1976), chemists and engineers developed and refined safety assessment methodologies, such as HAZOP (HAZard and OPerability study). One of the pioneers, Trevor Kletz, stated that the method is important, including human errors, flaws and mistakes, violations (for whatever reason), mismatches or low attention. Most of the incidents are very simple and most incidents started with minor flaws.2 “A daily commitment from all individuals in an institution is required for an effective safety and security program. It is important for individuals at all levels to work together to eliminate the risk of exposure to hazardous materials and conditions in the laboratory.”3 This is valid not only in the laboratory but also in pilot plants and operations; safety has the highest priority in the chemical industry.One of the main drivers for the chemical industry to carry out chemical processes in microreactors is the apparently increased safety aspects.4 The dimensions of such reactors are very small and thus the total inventory of hazardous material is quite low. For explosion hazards, the power of an explosion is proportional with the total mass of explosive material to the power of 1/3.5,6 This explains immediately that the severity of a potential explosion is very low in a microreactor.7 However, this does not mean that microreactors are inherently safe under every condition. Explosions can still occur and these explosions can be propagated to the neighboring vessels containing typically a large inventory of hazardous starting materials or products. Therefore, it is important that the right safety measures are taken to prevent explosion propagation.8,9 This can be achieved by using check valves to avoid back flow and by incorporating a suitable quench stream to smother the hazardous reaction mixture (e.g. diluting oxygen with a nitrogen stream). Explosion propagation can occur if the channel diameter is larger than λ/π, where λ denotes the detonation cell width which can be calculated from the induction length.10 For example, for an ethene/oxygen reaction mixture, it was found that the diameter had to be lower than 0.1 mm to avoid explosion propagation. This indicates that microreactors cannot be considered to be inherently safe under every circumstance. However, it should be noted that the safe operating window can be substantially broadened in continuous-flow reactors due to a lowering of the upper explosion limit (Fig. 1).10
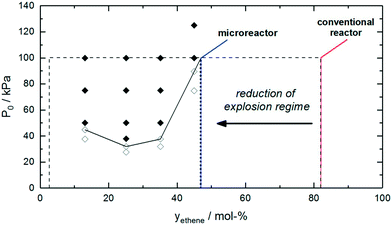 | ||
| Fig. 1 Explosive behavior of ethene/oxygen reaction mixtures at different pressures. The use of continuous-flow microreactors allows reduction of the explosion regime. Adapted from ref. 10. | ||
This can be mainly attributed to the continuous operation mode of flow reactors which allow minimizing the occurrence of local hot spots which serve as an ignition source for explosive reaction mixtures. Such thermal explosions are caused by exponential increases of the reaction rate with increasing reaction temperatures as dictated by the Arrhenius equation. This can lead to runaway reactions which eventually cause a reactor rupture and thus an explosion. This phenomenon is also called parametric sensitivity which indicates that a small deviation of a given parameter can lead to a chain of events resulting in an uncontrollable increase of reaction rate and temperature.11 Hot spot formation can be minimized in microreactors due to the excellent heat transfer characteristics and by using reactors made of materials with a high thermal conductivity (e.g. silicon carbide). Hot spot formation in microreactors is often found in catalyst beds.9,12–15 In general, a higher flow rate, lower pressure and lower reaction temperature will allow for an increase in the minimum critical oxygen concentration, above which explosion propagation becomes possible.
The reactor material is not only of importance to ensure high thermal conductivity (to avoid thermal explosions) but also plays a role in radical processes.16,17 Stainless steel can catalyze the decomposition and isomerization of peroxides. In addition, stainless steel reactors can also trigger the radical formation of explosive hydroperoxide intermediates during the oxidation of hydrocarbons with molecular oxygen.18 Kinetic explosions can occur when the formation of radicals occurs exponentially (uncontrolled radical initiation and propagation). Such explosions can be quenched via a radical chain termination, which typically happens at the reactor surface. Due to the high surface-to-volume ratios, the occurrence of kinetic explosions can be better controlled in a microreactor. For the platinum-catalyzed oxidation of hydrogen, Veser observed that the explosive behavior for a conventional reactor (with 1 m diameter) was at 420 °C at atmospheric pressure.19 However, in a microreactor (with a diameter of 1 mm), the explosive behavior was observed only at 750 °C and a further reduction of the diameter could expand the safe processing window even more. The suppression of the explosion behavior was explained by an adequate kinetic quenching of the radical chain mechanism at the reactor walls.
For these reasons, microreactors are often used to enable safe processing of hazardous reactions. An exhaustive overview of all the different reactions is beyond the scope of this review and we direct the reader to more specialized accounts on this topic.20,21 Herein, we focus to give some representative examples of certain safety hazards and how they can be overcome via continuous-flow microreactor technology. This includes
(i) gas–liquid reactions;
(ii) hazardous reagents generated in flow, which are immediately consumed in a follow-up reaction;
(iii) extreme reaction conditions (high temperature and high pressure);
(iv) use of organometallic reagents.
For each reaction, the hazards associated with the toxic reagents are given along with the NFPA 704 standard. NFPA 704, also called the fire diamond, is the standard system for the rapid identification of hazardous materials during emergency situations and is maintained by the National Fire Protection Association. The designation of the different divisions and the scales used are explained in Fig. 2.22
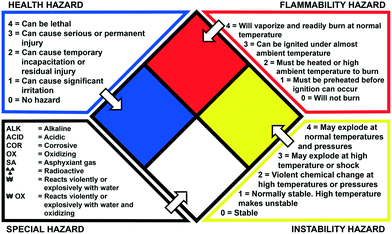 | ||
| Fig. 2 The fire diamond or NFPA 704 standard for rapid identification of hazardous materials.22 | ||
This minireview covers safety issues in laboratory chemical synthesis in flow reactors, in scale-up to production plants as well as practical issues and benefits from modular plant setup. Basic correlations on heat transfer and reaction kinetics are given for treatment of thermal stability of chemical reactors. Guidelines for laboratory protocols are given with a storage matrix for dangerous chemicals and process risk assessment in low, moderate and high risk with related standard operating procedures (SOPs). During the EU-funded F3 project,23 a safety analysis methodology for modular process design was developed and is presented here for scale-up guidance of small-scale production processes. This contribution not only gives some aid and help at hand but also aims to contribute to sharpening one's mindset, opening the eyes and giving a safe feeling.
2 Hazardous reaction in flow chemistry
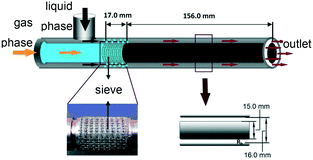
Miniaturized equipment, e.g. microreactors or intensified separation equipment, can handle harsh process conditions, but must be carefully designed or chosen for safe operation conditions. After a short description of the mass and heat balance in a tubular reactor, typical chemical reactions with hazardous potential are presented. Safety assessment in microstructure devices is followed by the description of thermal stability and parametric sensitivity in chemical reactors. A checklist at the end of this chapter can guide safe process design for the laboratory environment.2.1 Mass and energy balance
The reactor performance and safety behavior is governed by the fluid dynamics with mixing, dispersion and heat transfer as well as by reaction kinetics and thermodynamics. The mean residence time of the reacting fluid is given by the volume flow rate and internal volume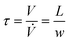 | (1) |
Temperature development along the tube is described by the following differential equation
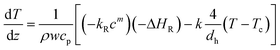 | (2) |
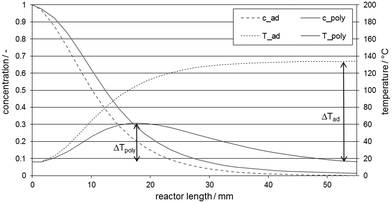 | ||
| Fig. 3 Concentration and temperature development along the microchannel for a rapid model reaction, adiabatic and polytropic (with external heat transfer) case.11kR = 8 s−1; EA/R = 20 K; ΔHR = 200 kJ mol−1; UV = 27 MW m−3 K−1, ΔTad = 110 K, tR = 0.134 s. | ||
The above discussion highlights already reveal the important process parameters for safe operation. Flow velocity w, heat capacity ρcp, and heat transfer coefficient with specific surface 4k/dh = UV determine the heat removal potential in a reactor, while the reaction time tR ∝ (kRc0m−1)−1 and reaction enthalpy (−ΔHR) determine the heat release potential. Since the temperature increase of a chemical reaction determines the potential of reaction runaway or solvent evaporation, adiabatic temperature rise is often given with ΔTad = (−ΔHR)c0(ρcp)−1. The role of these parameters in the thermal stability of chemical reactors will be discussed in more detail in one of the next sections.
2.2 Overview of typical chemical reactions (lab stage)
Typical reactions in microstructured equipment include organometallic reactions, nitration, oxidation, halogenation or handling of unstable intermediates. In the following, a selection of typical hazardous reactions is presented with their risk potential in tabular form.(i) dilution of oxygen in nitrogen to stay below the limiting oxygen concentration;
(ii) working under low gas pressures and reaction temperatures;
(iii) limiting the stoichiometry of the gaseous compound.
It is immediately clear that such restrictions result in a sub-optimal operation of the reactor and add significant complexity to the overall process.
Continuous-flow reactors, and more specifically microreactors, provide a well-defined gas–liquid interfacial area and avoid the risks associated with headspaces (Table 1). This means that the diffusion of the gaseous reactant into the reaction mixture is not rate limiting anymore, resulting in high reaction rates compared to the batch counterparts.26 One of the most studied gaseous reactants in continuous-flow microreactors is oxygen.27,28 While being the greenest oxidation reagent, its use in industry has been limited due to severe safety risks associated with this gas. However, in flow, oxygen is often used in an undiluted form, which allows boosting the reaction kinetics significantly. In other cases, a dilute oxygen source ensures that the reaction mixture never gets into the explosive regime (Table 1, entry 1).29 An important form of oxygen is singlet oxygen which is generated upon light irradiation in the presence of a photosensitizer (Table 1, entries 2 and 3).30,31 Such photochemical reactions are ideally carried out in microreactors which enable a homogeneous irradiation of the entire reaction mixture.32–34 An intriguing example of oxidation chemistry in flow is the synthesis of the anti-malarial artemisinin which involves both a singlet oxygen and a triplet oxygen oxidation strategy (Table 1, entry 3).31 Ozonolysis reactions constitute another very important transformation in organic chemistry. This transformation uses ozone as a powerful oxidizer to cleave double bonds (Table 1, entries 4a and b).35,36 In most cases, ozone is generated on-demand from an oxygen stream and the corresponding ozone is directly consumed in the flow reactor (e.g. O-Cube®, a commercially available device to generate ozone for flow applications). Another powerful gas-phase reactant is hydrogen gas (Table 1, entries 5a and b) which is required to enable a myriad of different reduction reactions.37,38 Hydrogen is an extremely flammable gas and is often generated electrochemically from water. Interestingly, the same equipment can also be used to generate deuterium gas from D2O.39–41 Direct fluorination reactions using F2 gas is an interesting approach to introduce fluorine into an organic molecule. However, the gas is highly corrosive and toxic and requires special precautions with regard to the reaction setup. As an example, 10% F2 in N2 allows efficient fluorination of diketones which in a subsequent reaction with hydrazine are converted to the corresponding 4-fluoropyrazoles (Table 1, entry 6).42 Chlorination reactions pose similar challenges to the reactor design. If traces of moisture are present, highly corrosive HCl is formed which can corrode the entire rig. By using a strict dry zone for the chlorine chemistry these challenges can be overcome (Table 1, entry 7).43 Also, carbon monoxide is a very toxic substance and is challenging to use on a laboratory scale due to its limited solubility in most organic solvents. However, its use in flow has been demonstrated repeatedly, allowing, e.g. palladium-catalyzed carbonylative coupling chemistry in a safe way.44
| Entry | Reaction class | Scheme | NFPA 704 | Hazards | Ref. |
|---|---|---|---|---|---|
| 1 | Oxygen oxidation |

|
Oxygen: |
Oxygen (O2):• Powerful oxidizer• May cause or intensify fire | 20, 29 |
| 2 | Singlet oxygen oxidation |
|
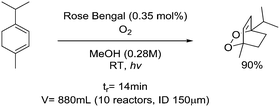
Singlet or triplet oxygen (1O2, 3O2):• Highly reactive, explosive species• May form explosive peroxides• Photosensitizer, skin contact can cause photodermatitis | 27, 30 | |
| 3 | Singlet–triplet oxygen oxidation |
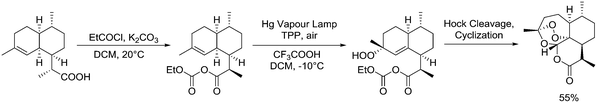
|
20, 31 | ||
| 4a | Ozonolysis |
|
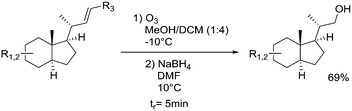
Ozone: |
Ozone (O3):• Powerful and toxic oxidizer• May cause or intensify fire• May cause serious skin and eye irritations | 20, 35 |
| 4b | Ozonolysis |
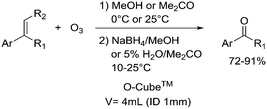
|
20, 27, 36 | ||
| 5a | Hydrogenation |
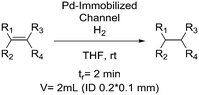
|
Hydrogen: |
Hydrogen (H2):• Extremely flammable gas• High pressure is often required for demanding reactions | 20, 37 |
| 5b | Hydrogenation |

|
38 | ||
| 6 | Fluorination |
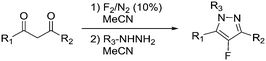
|
Fluorine: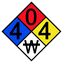 |
Fluorine (F2):• Corrosive and toxic gas• Powerful oxidizer• May cause or intensify fire• Fatal if inhaled• May cause skin burns and eye damage | 20, 21, 42 |
| 7 | Carbonylation |
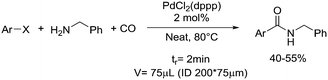
|
Carbon monoxide: |
Carbon monoxide (CO): • Extremely toxic and flammable gas• May cause damage to organs through prolonged/repeated exposure | 20, 43, 44 |
| 8 | Chloride substitution of alcohols |
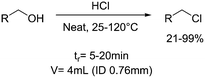
|
Hydrogen chloride: |
Hydrogen chloride (HCl):• Corrosive gas• Toxic if inhaled• May cause skin burns and eye damage• May cause respiratory irritations | 45 |
One particularly hazardous chemical is diazonium salt, which can decompose upon heating to generate nitrogen. Explosions by diazonium salts are dependent on the counter anion, e.g. halides and perchlorates are more prone to explosive decomposition than tetrafluoroborate salts. As a representative example, amino acids can be diazotized and displaced to generate chiral α-hydroxy acid (Table 2, entry 1).46 The compound was next purified in a series of liquid–liquid extractions/phase separations to obtain the pure target compound on a multigram scale. An extremely useful, yet very dangerous, diazo compound is diazomethane. This C1 building block is gaseous in nature and is acutely toxic by inhalation, carcinogenic and, in addition, is very explosive. Anhydrous diazomethane can be prepared in a so-called tube-in-tube reactor, which is a commercialized membrane reactor with a gas permeable membrane. To generate the diazomethane in flow, Diazald® (N-methyl-N-nitroso-p-toluenesulfonamide) is combined with potassium hydroxide in a methanol/water mixture. Diazomethane can subsequently diffuse through the membrane and can be engaged in various anhydrous diazomethane reactions, e.g. Arndt–Eistert reactions to prepare α-halo ketones (Table 2, entry 2).47 Another hazardous nitrogen-containing compound, which has been of great synthetic value, is hydrazoic acid. This volatile product has a pungent smell and is extremely poisonous (maximum nonfatal limit is 10 mg). This compound has been used to prepare tetrazoles in flow (Table 2, entry 3).48 In the first step, hydrazoic acid is prepared from sodium azide and acetic acid. Hydrazoic acid is subsequently converted into the corresponding tetrazole by reaction with a nitrile. Any residual hydrazoic acid after the reaction is immediately quenched by sodium nitrite to avoid its exposure in the environment. Carbonylation chemistry can be done by directly using the gas form (see previous section). However, CO can also be generated by dehydration of the CO-precursor formic acid in sulfuric acid at elevated temperatures. This procedure was carried out in a tube-in-tube reactor. The CO gas can subsequently permeate through the membrane to the outer tube where, e.g. palladium-catalyzed aminocarbonylation is carried out (Table 2, entry 4).49 The generation of chlorine gas can also be achieved by mixing less hazardous precursors. Chlorine can be prepared by reaction of HCl with NaOCl in flow. The chlorine gas can be subsequently extracted into an organic solvent where an organic transformation is carried out, e.g. chlorination of silanes (Table 2, entry 5).50 Another notorious gas that is best generated in situ is phosgene. This gas was even used in World War I as a more potent alternative for chlorine gas. Phosgene is particularly dangerous as it has an odor detection threshold of 0.4 ppm which is four times the threshold limit value. It can be safely produced in flow starting from its precursor triphosgene by mixing it with a nitrogen base (Table 2, entry 6).51 The generated phosgene was used for the generation of acid chlorides which can be subsequently coupled with amines to produce amide bonds. Epimerization of chiral centers could be suppressed by reducing the residence time of the acid chloride in flow (1.5 s). Hydrogen cyanide or “blue acid” is another poisonous substance with an infamous history, i.e. it was used as Zyklon B in Nazi extermination camps. Hydrogen cyanide can be safely produced in flow by combining potassium cyanide with acetic acid (Table 2, entry 7).52 The generated hydrogen cyanide is subsequently used in a Strecker reaction to prepare 3,4-diamino-1H-isochromen-1-ones.
| Entry | Reaction class | Scheme | NFPA 704 | Hazards | Ref. |
|---|---|---|---|---|---|
| 1 | Diazotization |
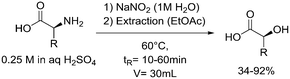
|
Diazonium salts:• Unstable intermediate• Explosive at high temperature | 20, 46 | |
| 2 | Arndt–Eistert reaction |
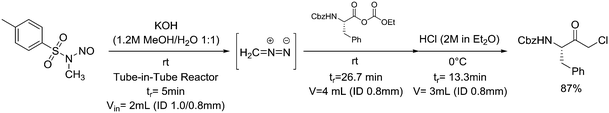
|
Diazomethane: |
Diazomethane (CH2N2):• Extremely toxic and reactive gas• May cause cancer | 20, 47 |
| 3 | Huisgen cycloaddition |
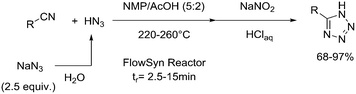
|
Hydrazoic acid: |
Hydrazoic acid (HN3):• Extremely explosive substance• May cause respiratory irritations | 20, 21, 48 |
| 4 | Carbonylation |
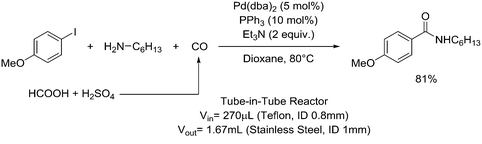
|
Carbon monoxide: |
Carbon monoxide (CO):• Extremely toxic and flammable gas• May cause damage to organs through prolonged/repeated exposure | 20, 49 |
| 5 | Chlorination |
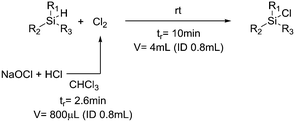
|
Chlorine: |
Chlorine (Cl2):• Powerful oxidizer• May cause or intensify fire• Toxic if inhaled• Toxic to aquatic life• May cause serious respiratory, skin and eye irritations | 50 |
| 6 | Phosgene-mediated reaction |
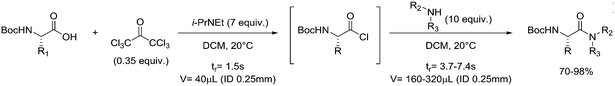
|
Phosgene: |
Phosgene (COCl2):• Fatal if inhaled• May cause skin burns and eye damage | 20, 51 |
| 7 | Strecker reaction |
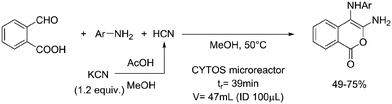
|
Hydrogen cyanide: |
Hydrogen cyanide (HCN):• Dangerous poison• Fatal if inhaled• Extremely flammable liquid and vapour• Extremely toxic to aquatic life | 52 |
| Entry | Reaction class | Scheme | NFPA 704 | Synthetic issue | Ref. |
|---|---|---|---|---|---|
| NFPA 704 legend: blue = health; red = fire; yellow = reactivity; white = special (oxidizer or water reactive substances). Numbers from 0 (minimum) to 4 (extreme). | |||||
| 1 | Lithiation metal–metal exchange |
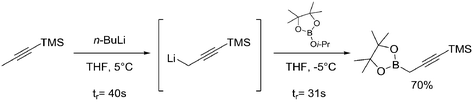
|
Organolithium reagent: |
Organolithium reagent (RLi):• Pyrophoric liquid• On contact with water releases flammable gases• Fatal if inhaled or swallowed;• May cause damage to organs through prolonged/repeated exposure• May cause skin burns and eye damage• Extremely toxic for aquatic life | 20, 58 |
| 2 | Organometallic reagents, addition reaction |
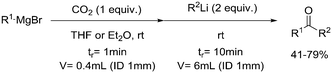
|
21, 59 | ||
| 3 | Tamoxifen synthesis |
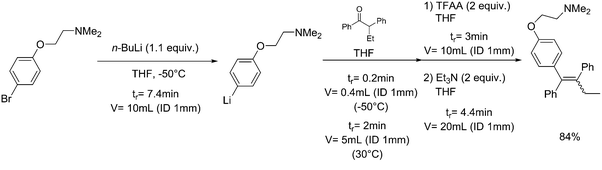
|
Grignard reagent: |
Grignard reagent (RMgX):• Pyrophoric liquid• On contact with water releases flammable gases• May form explosive peroxides• May cause serious respiratory, skin and eye irritations | 20, 21, 61 |
| 4 | Amitriptyline synthesis |
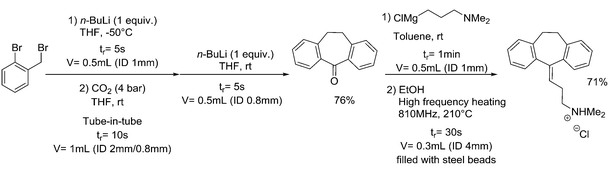
|
20, 21, 60 | ||
| Entry | Separation step | Scheme | NFPA 704 | Hazards | Ref. |
|---|---|---|---|---|---|
| 1 | Distillation |
 Stagewise evaporation and condensation, adapted from ref. 62 Stagewise evaporation and condensation, adapted from ref. 62 |
Xylene: |
Solvent mixtures• Acetone/n-butanol• Toluene/o-xylene• i-Octane/n-octane• o-Xylene/p-xylene• n-Hexane/cyclohexane | 62–64 |
| 2 | Distillation |
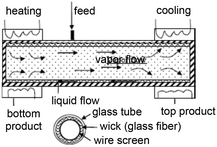 Zero-gravity distillation column, adapted from ref. 65 Zero-gravity distillation column, adapted from ref. 65 |
Ethanol: |
Solvent mixtures• Ethanol/water• Methanol/water• Alkanes, jet fuel, fuel additives with sulfur | 65, 66 |
| 3 | Distillation |
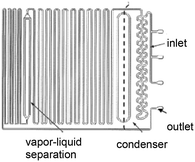 Meandering channel on-chip evaporation and phase separation, adapted from ref. 67 Meandering channel on-chip evaporation and phase separation, adapted from ref. 67 |
Toluene: |
Solvent mixtures• Methanol/toluene• Dichloromethane/toluene | 67 |
| 4 | Distillation |
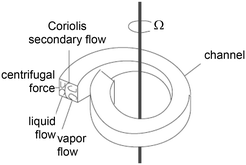 Spiral channel with on-chip evaporation and phase separation, adapted from ref. 68 Spiral channel with on-chip evaporation and phase separation, adapted from ref. 68 |
Xylene: |
Solvent mixtures• 2,2-Dimethylbutane/2-methyl-2-butene• Acetone/n-butanol• Toluene/o-xylene• i-Octane/n-octane• o-Xylene/p-xylene | 68 |
| 5 | Extraction |
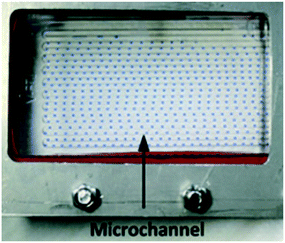 Microchannel chip with 2-phase flow, adapted from ref. 78 Microchannel chip with 2-phase flow, adapted from ref. 78 |
Toluene, butyl acetate: |
Solvent mixtures• Al3+–DHAB chelate from aq. solution/1-butanol• Co–nitroso-DMAP complex from aq. solution/toluene• Ion-pairs from aq. solution/butylacetate• Radioactive waste from NHO3 aq./tri-n-butylphosphate (TBP)• Co–nitroso-DMAP complex from aq. solution/toluene• Progesterone from water/ethyl acetate• Vanillin from toluene/water• Extraction of radionuclides from different extract solvents• Copper ions from water/oil• Acetic acid from n-nonane/water | 69–78 |
Acetic acid + radioactivity: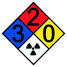 |
|||||
| 6 | Extraction |
 Microreactor with membrane Microreactor with membrane |
Diethyl ether: |
Solvent mixtures• Butyl-rhodamine B from aq. solution/isobutanol• N,N Dimethylformamide (DMF) from aq. solution/dichloromethane DCM or diethylether DEE• Penicillin G from potassium carbonate solution/n-butylacetate | 79–81 |
| 7 | Extraction |
 Mixer–settler module, consisting of T-mixer, capillary and settler, adapted from ref. 82 Mixer–settler module, consisting of T-mixer, capillary and settler, adapted from ref. 82 |
Succinic acid: |
Solvent mixtures• Iodine from aq. solution/kerosene• Succinic acid from n-butanol/water• Acetic acid from kerosene/water | 82 |
| 8 | Extraction |
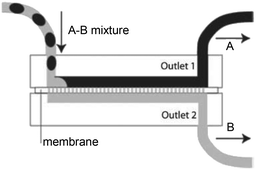
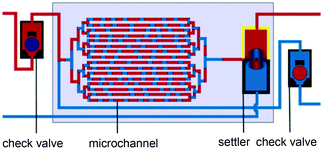 Pulsating counter-current flow with check valves and settling device between microchannel plates, adapted from ref. 83 Pulsating counter-current flow with check valves and settling device between microchannel plates, adapted from ref. 83 |
Nitric acid: |
Solvent mixtures•HNO3–water solution/tributyl phosphate (TBP)–kerosene | 83 |
| 9 | Synthesis + extraction | Tubular extractor with membrane separator; quenching the reaction by extraction | Phenyl-hydrazine: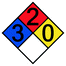 |
Solvent mixtures• HCl aqueous solution/Et3N/toluene• H3PO4 aqueous solution/phenylhydrazine/hydrazone DCM solution• Hexane/diphenhydramine NaCl aqueous solution• MIBK/HMF/fructose dehydration reaction solution | 84–87 |
| Miniaturized stirred mixer and gravity settler; excess reactant separation | |||||
| Tubular extractor with membrane separator; product separation following a reaction | |||||
| Tubular extractor and gravity settler; protection of an unstable intermediate | |||||
| 10 | Absorption |
 Absorption of gas component into liquid phase, explosive atmosphere (H2 and CO), reaction mixture determines the safety measures, image adapted from ref. 90 Absorption of gas component into liquid phase, explosive atmosphere (H2 and CO), reaction mixture determines the safety measures, image adapted from ref. 90 |
Hydrogen sulfide: |
Solvent mixtures• CO2 into monoethanolamine solution• H2S absorption in N-methyldiethanolamine (MDEA) | 89, 90 |
For distillation, solvents are partly evaporated by addition of heat. This high temperature and vapor phase causes additional risks by forming an explosive atmosphere (entry 1 and 4), further reaction (entry 2), or potential decomposition (entry 3). To avoid high temperature, vacuum may be applied to the distillation device. Small internal volume and good ventilation in fume hoods may prevent the formation of an explosive atmosphere. Inertization, dilutions or well-cooled equipment sections can lower the risk further. Separation by extraction also needs low or ambient temperatures leading to lower risk and energy consumption. For liquid–liquid extraction, an additional phase with a second solvent is formed, often accompanied by additional risks. The solvent may be more toxic or dangerous than the original one(s) (entry 5). Some of the products are toxic or radioactive and must be treated with special care (entries 5 and 6). Solubility heat release must be considered for strong acids or bases (entry 9). The dilution of components and solvent mixtures, which often occur during research,88 have to be discussed during risk analysis. The third major separation unit shown in Table 5 is selective gas phase absorption to remove components from a gaseous mixture (entry 10), often under elevated pressure. The gas can form an explosive or toxic atmosphere; hence, the reaction mixture determines necessary safety measures. Leak tightness and pressure stability must be checked in advance.
During adsorption or chromatographic separation, solid supported scavengers concentrate molecules of interest,91 which might result in contamination risk. Eventually, higher concentration may occur when regenerating the scavengers. The regeneration solvent must also be included in the safety analysis. Non-conventional scavengers and solvents are given in a review by O'Brien et al.92
Solid formation in crystallization and precipitation leads to a high concentration of hazardous molecules, substances, and mixtures. Agglomeration of hazardous material has to be treated with care. Some examples of continuous flow crystallization are given in the literature, handling pharmaceuticals93,94 or amino acids.95 Similarly, follow-up steps such as filtration and drying can be dangerous due to agglomeration and atmospheric contact. Nitrogen purge is a possible measure to avoid oxygen or moisture contact. Often, pre-concentration by evaporation96 is combined with crystallization and must be checked carefully due to higher temperature and concentration as with prior process steps.
Accumulation of unreacted material can also cause reaction runaway, but now in separation vessels, equipment headers or similar process spaces. Waste stream treatment can also lead to unwanted accumulation of reactive material. A temperature sensor in these areas or volumes is very helpful in controlling unwanted reaction in sensible places. In summary, separation steps have to be treated with care, often in more detail than the predecessor reactions due to higher volume, hold-up and additional components.
2.3 Graphical safety assessment
The risk of thermal runaway can be graphically identified in the so-called Semenov diagram by comparing the heat release from a chemical reaction with heat transfer towards the cooling medium. The heat generation per volume from a first-order chemical reaction can be calculated by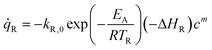 | (3) |
This equation is represented by the bold curve in the Semenov diagram (Fig. 4). To determine the volume specific heat transfer of a microstructured device, estimation with the volumetric heat transfer coefficient UV is given in ref. 97.
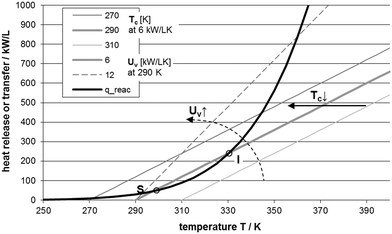 | ||
| Fig. 4 Semenov diagram for exothermic reaction, adiabatic calculation adopted from ref. 10, T in K and UV in 106 W m−3 K−1 or kW L−1 K−1, first-order reaction, k0 = 0.1 s−1, c0 = 0.5 mol L−1, EA/R = 5000 K, (−ΔHR) = −200 kJ mol−1, adapted from ref. 97. | ||
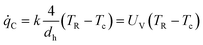
| (4) |
The Semenov diagram is a useful tool to find the suitable cooling temperature for low risk of reaction runaway by considering typical heat transfer rates and reaction kinetics.97 Please note that the reactor inlet temperature may vary from the cooling temperature and can lead to a slightly different curve of the release reaction heat. According to Klais,12 this assumption “fails, as the heat flux is mainly controlled by the molecular thermal conductivity of the reaction medium inside the channels and only partly by turbulent mixing of the reaction mixture.” For microreactors, the relation of the different contributions to the entire heat transfer, i.e. reactor channel, reactor wall, and cooling channel, has to be investigated in more detail in critical cases. With this investigation, a possible improvement potential can be identified and realized in an optimized reactor design and operation, e.g. reactor wall material with high thermal conductivity, increased heat transfer in cooling channel or lower reactor cooling temperature.
2.4 Parametric sensitivity and thermal runaway
An exothermal reaction accelerates itself if the heat production of the chemical reaction is larger than the heat transfer to the cooling medium. The exponential increase of the reaction rate with rising temperature leads to an exponential increase in heat production, too. This rapid heat release due to small temperature (or concentration, heat exchange, flow rate, etc.) is called parametric sensitivity. Small changes of system parameters lead to disproportionately large temperature fluctuations. Fig. 5 shows the simulated temperature profile within an ideal plug flow reactor for different inlet concentrations.98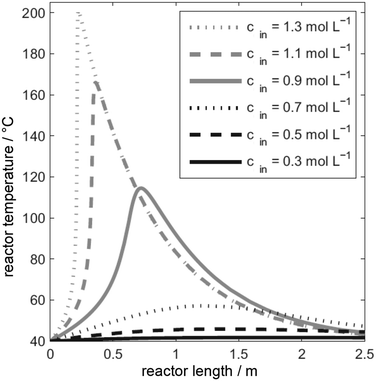 | ||
| Fig. 5 Temperature profile of an exothermic reaction over the length of an ideal flow tube at different inlet concentrations, adapted from ref. 98. | ||
For low concentrations between 0.3 and 0.7 mol L−1 the temperature increases only slightly, while the reactor responds very sensitively to higher concentrations between 0.7 and 0.9 mol L−1 with a drastically increased maximum temperature of approx. 70 K and probable solvent evaporation (aqueous system). Different mathematical approaches exist to describe the parametric sensitivity.99 To obtain a first estimation, the Semenov diagram was already described above with important process parameters. From the Arrhenius equation the exponential coefficient with activation energy and cooling temperature, often the reactor inlet temperature too determines the heat generation rate.
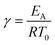 | (5) |
The heat production potential S′ is described by the adiabatic temperature rise with the Arrhenius coefficient γ
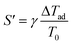 | (6) |
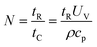 | (7) |
The ratio of heat production potential and heat removal capacity determines the potential of thermal runaway of an exothermic reaction.
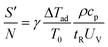 | (8) |
Large values of this ratio lead to high heat production and thermal runaway. Semenov100 determined the limiting value of this ratio, above which the reaction becomes unstable, to be
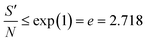 | (9) |
Another approach was proposed by Frank-Kamenetskii101 for the thermal runaway of gas-phase reactions. He determined a minimum capillary diameter, below which the heat conduction in the gas with thermal conductivity af is high enough to limit the heat generation.
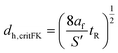 | (10) |
The equation is often used for the design of flame barriers in process plants. In continuous-flow reactors, convective heat transfer is dominant and allows for another approach for critical hydraulic diameter.
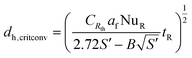 | (11) |
The coefficient B in eqn (11) depends on the reaction order and was determined to be 2.60, 3.37, and 4.57 for a reaction order of 0.5, 1 and 2, respectively.11,102,103 The entire heat transfer is determined from the internal Nusselt number NUR and the correction factor CRth with the external thermal resistance Rth,
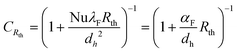 | (12) |
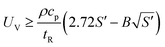 | (13) |
A focus on dimensioning microreactor channels with regard to the heat management and reaction classes is given by Westermann et al.104 Using a short-cut approach the authors provide a method to select channel diameters and to determine if isothermal operation in a microreactor is feasible. This approach may help if a scale-up of microreactors is desirable by enlarging the channel diameter.
In summary, a detailed discussion of mixing, heat transfer and residence time characteristics together with reaction kinetics and thermodynamics is necessary to determine safe operation and scale-up of continuous-flow reactors and equipment. Further discussion on safety is guided by checklists.
2.5 Checklist for process safety
The following lists provide key words, which can be taken as suggestions for further safety discussions from our experience.105 Concerning important process parameters, the following topics should be addressed:• Data collection (MSDS material safety data sheets, experience, literature, etc.) with identification of the substances: reactants, intermediates, products and waste
• Hazard characterization such as fire and explosion hazards, environmental hazards, exposure risk
• Pressure (operating pressure, pressure increase, vapor pressure, safeguards with pressure limit, test pressure), maximum allowable pressure, material selection (apparatus, piping, containers), geometry and wall thickness, mechanical strength or stiffness
• Temperature control, material selection, heating/cooling system (media, power, operation window, failure), phase change
• Concentration: accumulation of reagents, mix-up of components, feeding sequence, contamination, mixing, corrosion and material compatibility, precipitation
• Flow rate, reverse flow, pressure build-up, blocking
Concerning reaction kinetics, the following topics should be discussed:
• Thermal stability (autocatalysis, deflagration), reaction heat and adiabatic temperature rise, reaction kinetics including activation energy and reaction order
• Control of reaction rate, accumulation of components
• Decomposition risk including heat and onset temperature of decomposition with maximum handling temperatures; potential deflagration of a substance with rate of gas generation
• Heat accumulation, explosion/detonation (gas, dust, explosion limits, critical ignition energy)
• Gas evolution (gas type, velocity), solid formation, deposition
• Oxidation potential, flammability, flash point, ignition temperature (temperature class), friction and impact sensitivity
•Catalyst, deactivation, poisoning, incomplete conversion
The entire process needs a discussion of the following topics:
• Start-up/shut-down, interruption, emergency cooling, power loss
• Operation without staff and control, automation
Environmental concerns are the following:
• Electrostatics, electrical conductivity, flow velocity, explosion detection
• Type of storage and transport vessels (size, heat accumulation, electrostatics)
• Storage, interim storage (temperature, humidity, time, catalysis)
• Disposal and waste treatment
Process safety is always accompanied by occupational health measures. The risk assessment starts with data collection such as physical and chemical properties of all substances, heat production rate of all reactions (desired and undesired reactions), rate of gas generation, fire and explosion hazards. A risk assessment has to be conducted through the entire process development from lab to production plant.
3 Transfer from lab to production
During the scale-up from lab to pilot plant or production scale, the increasing production rate leads to higher content of hazardous chemicals within the process and therefore to critical safety aspects. Inadequate scale-up procedures may lead to dangerous operation conditions, and hence, it is crucial to apply methodologies which enable the identification of safety risks during scale-up.Scale-up of microreactors can be achieved mainly in two ways: parallel setup of miniaturized devices or smart scale-up of the intensified process conditions. The plate-type microreactors allow for the compact combination of mixing and heat transfer. A further possible way to increase productivity and throughput of microstructured devices is the long time operation in continuous mode.20 Despite many approaches to scale-up microreactors in the literature, consistent methodologies for conducting safety assessment during the scale-up are rather rare for microstructured continuous processes. Kockmann and Roberge11 presented a scale-up concept for modular microstructured reactors focusing on mixing, heat transfer and reactor safety and derived a checklist which guides the safe scale-up of microreactors. Klais et al. proposed guidance on safety and health for process intensification including microchannel design with a focus on reaction hazards,12 explosion hazards13 and risk analysis.14 The Royal Society of Chemistry (RSC) published a note to provide guidance on safety issues raised by the scale-up of conventional batch reactor equipment.106 The main hazards are identified to be runaway and over pressurization. These hazards arise mainly from the change of heat removal and production rates of conventional reactor equipment. According to the Royal Society of Chemistry (RSC), the risk assessment of the proposed chemical process is the first and most critical step during the scale-up procedure.106
With the use of process intensified equipment different issues need to be considered during the risk assessment, such as the connection of conventional equipment with microstructured devices. This motivated Klais et al. to focus their publication on the risk assessment covering the characteristics of microstructured devices.14 Klais et al. propose the HAZOP-like method to carry out the risk assessment. The traditional HAZOP study was therefore expanded with guide words and parameters, which are more appropriate regarding microstructured equipment. Characteristics such as short residence times, parametric sensitivity, high surface-to-volume ratio and small diameters are covered by the developed method.
3.1 Temperature management
Leonhardt et al. presented an early risk assessment of chemical processes on pilot plant scale with a worst-case approach.107 The presented approach focuses on safety-related workflows for a typical scale-up process of batch reactions. Nevertheless, the general approach can be guidance for continuous processes as well. The workflow consists of detailed analysis of the chemical lab process including estimation of reaction mass, heat capacity and adiabatic temperature rise. The thermal stability of all relevant species and their maximum handling temperature THM is derived from differential thermal analysis/differential scanning calorimetry (DTA/DSC) screening tests and the observed decomposition temperature TZ (THM is usually determined as THM = TZ − 80 to 100 K).107 This temperature difference is a conservative estimation and should be revised for microstructured reactors with emphasis on the small internal volume. To identify the main risks for the future pilot plant, a worst case for each relevant step of the chemical process is derived. Examples for worst case scenarios would be the full accumulation of reactants in larger vessels, the breakdown of cooling or a blocked venting valve. Subsequently, Leonhardt et al. classify the main risks regarding thermal potential, gas evolution, hazardous material properties and increased reactivity. The classification of thermal risk levels are exemplarily shown in Fig. 6.107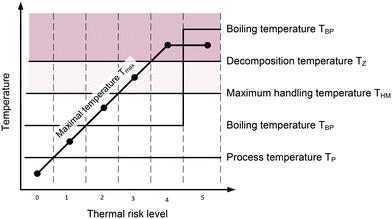 | ||
| Fig. 6 Thermal risk levels derived by comparing temperature levels: process temperature TP, boiling point of reaction mixture TBP under atmospheric conditions, maximum achievable temperature Tmax = TP + ΔTad, maximum handling temperature THM and temperature range of decomposition (Tmax reaches decomposition temperature TZ). Adapted from ref. 107. | ||
The maximum temperature Tmax is calculated by adding the adiabatic temperature rise ΔTad to the process temperature Tp. The boiling point of the reaction mixture is represented by TBP. The thermal risk ranges from level 0, where no thermal risk is given, to level 5, where runaway will most likely occur. The definition of risk levels will promote a common understanding about thermal risks.
The next step of the workflow is the compatibility check between the process and the pilot plant equipment. For example, it has to be checked if the temperature and pressure limits of the plant equipment could be exceeded in the defined worst-case scenarios. For too high values, it has to be investigated if appropriate safety measures can be installed. Leonhardt et al. note that it is hard to install technical process-safety facilities, which are capable of preventing all worst cases.107 Here the important role of safety experts is emphasized who are involved throughout the entire development process.
Other examples in the literature do not focus on the scale-up procedure itself but may give input on how to conduct risk assessment regarding microstructured devices. Benaïssa et al. present a methodology in order to evaluate the inherently safer characteristics of a continuous-flow intensified reactor.108 First, a HAZOP study of the so-called heat-exchanger reactor in a pilot plant setup was carried out. Potential hazards were identified and serve as a starting point for further simulations in order to estimate the potential risks. Ebrahimi et al. proposed a checklist approach for safety analysis of an intensified process.109 The checklist is based on the concept of layers of protection (LOPA) for a chemical process. Schwolow et al. showed a systematic scale-up approach for an exothermic Michael addition.110 Kinetic studies in lab experiments were carried out in a coiled stainless steel capillary. The scale-up was then carried out in a modular plate reactor (ESK production reactor, 3 M, Kempten, Germany). The residence time distribution and the mixing behavior were characterized together with the heat removal potential of the production reactor. In combination with simulations it was possible to predict the reaction yield in the production reactor so that safe scale-up was feasible.
3.2 Guideline for adequate operating procedures in the laboratory
Most recently, a guideline was published on setting up appropriate standard operating procedures (SOPs) for chemical laboratories.3 The operating procedure should focus on processes with operational ranges and conditions, emergency shutdown, and individual hazardous chemicals as well as classes of hazardous chemicals. Furthermore it should include management and use of chemical equipment by authorized users and lab-specific safety and security risks based on surroundings and environmental factors. A decision tree for risk assessment is presented resulting in management and storage SOPs with a storage matrix (see Table 6) and process risk mitigation SOPs differentiating the possible risk classes. For low risk chemicals and processes, laboratory standards are sufficient. Moderate risks should be on a tolerable, preferably acceptable, level, while the costs of such risk reduction measures should be accounted for. Processes of moderate risk may include neutralization of a carbonate by acid or chemical reactions with moderate heat release without risk of solvent evaporation. The moderate risk SOP is a condensed version of the high risk SOP.| Storage options and considerations | Chemical characteristics |
|---|---|
| Ventilated storage cabinets; typically the default storage location and of a size that allows enough space to segregate potentially cross-reactive compounds | Toxicity, volatility, odor |
| Flammable storage cabinets; generally non-ventilated. Fire codes may govern quantities or require fire suppression | Flammable liquids |
| Desiccant storage and inert atmosphere storage | Air- and/or water-sensitive chemicals |
| Below ambient temperature storage: refrigerator or freezer | Stability with temperature |
| Designated areas and protocols for cylinder storage | Compressed gases |
For high risk processes and chemicals, substantial efforts should be made to minimize the risk. Processes of high risk may include the use of toxic materials, explosives, pyrophorics, metal–organic reagents, infrequently performed activities with a high potential for failure, and/or first-time activities with less experience. The high amount of flammable solvents may also require high risk SOPs. The experience level of laboratory workers or researchers is also decisive for the risk assessment. The High Risk Standard Operating Procedure Form includes a detailed description of the process parameters and materials, process equipment description, special handling prescriptions, emergency actions, important controls and monitoring measures. Personal protection and work practice controls must be described, together with training activities and their verification and review process. Special shutdown and cleaning requirements with waste treatment have to be considered together with storage and transportation requirements. Senior staff will review the SOP for completeness and accuracy. A modification procedure of the SOP has to be established for the laboratory including responsibilities of supervisors. To illustrate the laboratory safety procedure the guideline3 gives four scenarios on typical situations. Neutralization of carbonate by an acid is slightly exothermic and exposes the operator to a moderate risk, particularly for students in the lab. Use and filtration of a pyrophoric catalyst can lead to air contact and possible hazard in the presence of a flammable solvent. Here, the detailed SOP has to be elaborated and trained with the persons concerned. Handling diazomethane as toxic and explosive gases also needs an exhaustive SOP as well as special training and precautions.
3.3 Checks for safe operation
To ensure a safe transfer from the lab scale to the pilot plant, the Royal Society of Chemistry (RSC), UK, gives the following recommendations:• If possible, carry out trials with water or inert substances in the intended plant to determine unknown heat and mass transfer rates.
• Use the same apparatus, materials and chemicals at all stages of the scale-up that accurately reflect those that will be used in the final plant.
• Take into account that laboratory chemicals and solvents are often of higher purity than bulk chemicals. Impurities may lead to blockages or can catalyze undesirable side reactions.
• Humidity and fine particles in solvents and larger plant and equipment compartments have to be considered.
• Consider filtering, drying, or additional purging steps during start-up and during long-term operation.
In comparison to conventional reactor equipment microstructured devices are more sensible to impurities and solid formation. Nevertheless, the small internal volume and closed containment help to reduce risks, especially with improved temperature control and heat management:
• Continuous-flow lab experiments can provide sufficient data, such as reaction time and adiabatic temperature rise, possible side reactions, and an estimation of the heat production potential.
• The change in heat removal rates can be limited during scale-up with small steps in equipment enlargement.
After successful laboratory campaign and in preparation of the scale-up, the following steps and checks are recommended:11,105
• Determine and fix the channel element hydraulic diameter to element length dh/L ratio for geometrical similarity of different reactor scale.
• Check flow velocity and related Re number for flow regime in the mixing and residence channels.
• Determine the necessary length and volume of the reactor related to volumetric flow rate and residence time with characteristic reaction time.
• Determine mixing characteristics by pressure loss in the reactor channel, energy dissipation rate and mixing time scale tm.
• Calculate laminar, transitional and turbulent heat transfer correlations for a given geometry, fluid properties and flow conditions. Estimate, calculate, or measure the single heat transfer resistances Rth from geometry, employed materials and flow conditions, or alternatively, the volumetric heat transfer coefficient UV from the energy balance and temperatures at inlet and outlet.
• Determine calorimetric data as important reaction parameters such as adiabatic temperature rise ΔTad and activation energy EA.
• Check the approach for critical hydraulic diameter (eqn (11)) or minimum volumetric heat transfer (eqn (13)).
• Fit rate data to m-order rate expressions in the interested temperature range.
• Check the S′ approach with the estimated heat transfer regime.
• In ambiguous or critical cases of parametric sensitivity, check further models from the literature with higher numerical effort.107
The above mentioned criteria give only a preliminary view and should be enlarged to embrace the complexity of a real chemical system. This may include further pressure and temperature limits, possible polymerization or precipitation, decomposition, or gas evolution. With the gained specific experience, the checklists above should be enlarged for preparation of future risk assessments.
3.4 Checklist for occupational safety
The US Department of Labor, Occupational Safety and Health Administration (OSHA) provides multiple checklists for process safety management (PSM)111 including checks for process safety information available, process hazard analysis (e.g. HAZOP, or “what if …” questions), standard operating procedures (SOPs), training, contractors (performing maintenance and repair), and others. Further lists can be found in ref. 112. Occupational safety concerns not only particularly personal protection in the plant, building and vicinity, but also has implications for the plant and components as well as on the plant operation.105• Open handling (e.g. dust, gas, vapor), filling, sampling, transport, leakage, special influences (noise, vibration, pressure, temperature, radiation, humidity)
• Risk of accident (e.g. tripping hazard, accessibility, hot surfaces), mechanical hazards, squeezing, slip hazard, thermal hazard by contact
• Toxicological, pharmatoxicological, biological media properties, safety clothing, work rules, contact with chemical/biological substances (breath, skin, eyes), biosafety (e.g. effect of microorganisms)
• Cleaning (e.g. decontamination, deactivation, disinfection, sterilization), decontamination after accident, disinfection (person/vessels/instruments)
• Emergency plan, communication, interfaces (analytic, waste management, medical service, fire department)
The above list describes a few points regarding the production plant. The entire plant environment needs to be examined for the holistic view and comprehensive treatment.
4 Safety concepts for small scale production plants
Modern chemical production tends to transfer batch processes to a continuous operation mode. Modularization of small scale production plants in combination with continuous-flow chemistry aims for flexible and fast production to face the challenges from global markets.113,114 This is realized by the re-use of engineering information within a standardized planning workflow. In particular, risk assessment is often time-consuming and labor-intensive. Hence, it is necessary to adapt conventional risk assessment methods to the characteristics of the modular design approach. A module in process engineering and in our approach is a functional unit represented by an equipment assembly and related information database with related process and equipment data on various scales from first lab studies to operation and maintenance.115 This includes data from conceptual design, process steps, involved media and reactions, as well as operation conditions and safety data. The modular approach enables the re-use of engineering data and knowledge from a related database during the entire period of process development and scale-up. A module based planning approach, which allows structured planning of new modular plants in a time-efficient way is described by Fleischer-Trebes et al. in ref. 116. Hence, the safety analysis is also organized according to the modular approach.4.1 Overview of risk analysis methods
Various risk analysis methods are established within the process industry and reviewed by many authors. Villa et al. describe the progress of quantitative risk assessment during the past decade and focus on the development towards dynamic risk analysis.117 Marhavilas et al. give a broad overview of risk analysis and assessment methodologies for multiple industry sectors.118 The progress in the development of methods and models in process safety and risk management is discussed by Khan et al.119 Important risk assessment methodologies are compared in Fig. 7 for selected criteria.120 Qualitative methods such as the HAZOP study and the failure mode, effects, and criticality analysis (FMECA) are very well suited for identifying failures and potential hazards. Popular methods using Boolean logic are fault tree analysis (FTA), event tree analysis (ETA), and bow-tie analysis (BTA).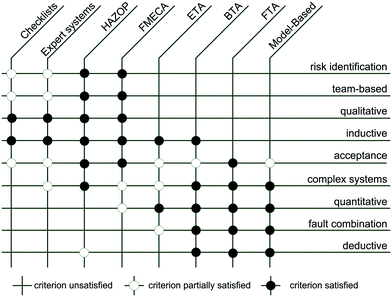 | ||
| Fig. 7 Comparison of risk assessment methodologies.120 | ||
Summarizing the diagram in Fig. 7, a HAZOP study embraces the most criteria with 6 + 1 followed by FMECA and FTA with 5 fully fulfilled criteria and 2 or 1 partially fulfilled, respectively. The following parts focus on the HAZOP study, but other elements can also be included if applicable and appropriate.
4.2 HAZOP study
The HAZOP study is one of the most frequently used safety analysis methods.121 The method describes a structured and systematic approach to identify potential hazards and operability issues and assess the consequences and defines appropriate countermeasures. The success of the HAZOP study comes with the systematic approach to follow process flow diagrams (PFDs) and piping and instrumentation diagrams (P&IDs). Based on these diagrams a complex system can be divided into manageable sections, which are analyzed by a multi-disciplinary team.121 The study starts with the definition of target functions including process parameters for each segment. Guide words (more, less, none, etc.) are applied to the process parameters (temperature, pressure, etc.). The combination of guide words and target functions leads to deviations in process conditions, from which the possible causes are evaluated. Safety relevant consequences from the deviations are discussed and countermeasures are defined. The international standard IEC 61882:2016 provides a guide for HAZOP studies.122The HAZOP study was often modified to the relevant systems. An overview of modified HAZOP studies is given by Khan et al.119 Seligmann et al.123 developed a blended hazard identification called BLHAZID. The BLHAZID methodology combines the function-driven HAZOP with the component-driven failure mode and effect analysis FMEA approach. Argenti et al.124 integrated the HAZOP into a layer of protection LOPA approach to limit the role played by expert judgement. Klais et al.14 developed a HAZOP-like study to cover the characteristics of microstructured equipment, such as short residence times or parameter sensitivity. Boonthum et al.125 combine an automatic HAZOP analysis with a structural model to build guidance for hazard and mal-operation identification. The HAZOP studies are modified in order to overcome several limitations such as the adaptation to batch processes, to study the human and management factors or to enable programmable electronic systems for efficient support. Baybutt et al.126 analyze the weaknesses of the HAZOP study.
In recent years much research regarding automated HAZOP analysis was carried out. Dunjó et al.121 estimated in a literature review that about 40% of the research is focused on HAZOP automation. Recently, Zhang et al.127 published an approach for automatic HAZOP analysis with special regard to unsteady operation, such as startup and shutdown. Mass transfer and relationships among process variables are analyzed by a Petri net directed graph model which is based on fuzzy logic. With the use of guidewords possible operation deviations can be identified by qualitative simulation. Zhang et al. validated their method with a rectification column system. Janošovský et al. presented a model-based HAZOP study using Aspen HYSYS.128 The results of the numerical simulations are collected and transformed into standard HAZOP tables. Detailed overviews of former approaches can be found elsewhere.119,121
A central element in the risk assessment is the definition of risk and its recognition. The concept of “productive vagueness” is nearly 20 years old and gave the possibility of scenario-based identification of hazards and succeeding evaluation of dangers, assisted by probabilities.129 This concept is similar to today's discussion of recognition heuristics,130 a more general form of risk treatment in public discussions. Recently, Bock and Haferkamp published a method combining hazard analysis with graphical display and semi-quantitative assessment.131,132
4.3 Safety assessment for modular continuous plants
Modular production plants are currently developed and operated by many engineering and chemical companies, e.g. INVITE.133 These plants are designed for continuous production within a standardized 20 foot transport container.134 A continuous process is therefore divided into different modules. One module represents at least one unit operation including the peripheral components, such as pumps and piping. The engineering information for each module (P&ID, cost estimation, etc.) is stored in process equipment design (PED) modules. The PEDs therefore ensure the documentation during the engineering and plant life cycle and enable the re-use of engineering work. If a module is built, the information is transferred to a physical module, the so-called process equipment assemblies (PEAs). A detailed description of the modular concept can be found in the work of Fleischer-Trebes et al.116 The idea of continuously operated modular production plants within a transport container was implemented in the F3 Factory project funded by the EU.23 The technical feasibility was shown for seven different case studies, which are described in several publications, and the final report. The follow-up project CONSENS (Integrated Control and Sensing) is currently focusing to advance the continuous production by introducing novel online sensing equipment and closed loop control of key process and product parameters.135A modular production plant has to fulfil the same safety requirements as conventional production plants, i.e. the Seveso III directive (2012/18/EU136) in the EU and OSHA PSM Rule (Occupational Safety & Health Administration – Process Safety Management of Highly Hazardous Chemicals standard) or the Risk Management Plan (RMP) in the USA. They all have in common that the process hazards have to be analyzed; thus hazard identification and evaluation is possible. A comparison of the different regulatory requirements is given in ref. 137. The mentioned regulations ensure that systems are installed to manage and report risks and emergency plans are established.
On the equipment level in particular, the directive for pressure equipment (2014/68/EC), the machinery directive (2006/42/EC), the low voltage directive (2014/35/EU) and the directive for electromagnetic compatibility (2014/30/EU) regulate the equipment design and operation in the EU. There is no direct equivalent for each of the directives in the USA. The pressure vessel code according to the American Society of Mechanical Engineers (ASME) is comparable to the pressure equipment directive in the EU and is a worldwide accepted standard, but it is not legally binding. Moreover, production plants are often operated in an environment with potential explosive atmospheres. The corresponding standards and regulations can be found in the European ATEX directive (2014/34/EU138) or by IECEx (international commission system for certification139).
4.4 Risk assessment
In general, a risk assessment has to be conducted for every plant in order to identify safety relevant risks, to evaluate the risk and to define countermeasures to prevent potential accidents. The conventional risk assessment procedure for newly developed plants is embedded in the process development phase. During the conceptual process design only little information on technical equipment is available. Nevertheless, safety aspects need to be addressed because the selection of process substances and conditions has a high impact on the overall safety for the developed plant. In this phase a preliminary safety analysis is performed with the objective of showing the feasibility of a safety concept for the following development steps. In the engineering phase, the selection of process equipment has a major influence on safety. The basic safety review is carried out in the final stage of the basic engineering phase. The process design phase closes with a detailed safety review. Detailed process information such as PI&D diagrams and detailed specifications of equipment as well as piping and installation plans are available now. Based on the detailed information risk assessment methodologies such as HAZOP, analysis is carried out. After the construction phase a pre-start-up safety review is accomplished. Through a plant inspection and checks it is tested if all previously defined safety measures are implemented and work appropriately.The planning process for modular plants can rely on several advantages from the information pool of single modules from prior projects or earlier development phase. Modular design offers the flexibility of changing single modules within the plant and may lead to shorter general risk assessment of the entire revised plant. A standardized safety assessment allows for re-using safety relevant information during the whole development phase.
Risk assessment methods, which can be applied in an early process development step, are checklists and heuristics. For modular plants it is very useful to separate the safety assessment into two stages.120 In an early development phase a flexible risk assessment is conducted with checklists and heuristics on a module level, the intra-modular risk assessment. Later, the HAZOP analysis is performed to address the interdependencies between the modules (inter-modular risk assessment) for the entire plant.
• The module is well suited for the selected operation conditions with regard to safety aspects.
• The module is generally suitable but additional safety measures are necessary.
• The module is not suitable. The adaptation of the process or the design, assembly, and selection of an alternative or even a new module has to be considered.
• The risk assessment cannot be completed due to missing information.
If there is a lack of information in the early development phase, potential risk areas cannot be excluded and have to be considered at a later time as part of the overall risk assessment. The checklist approach is suitable for a first systematic risk assessment during the conceptual design phase.
Modules are flexible to handle substances and chemical reactions or separation steps which have their special operation conditions. To gain an overview of which module is applicable for which conditions, each module is characterized for suitable substances, reactions, separations, and operation windows. An important attribute is the assignment of a temperature class to a module. The temperature class of the module defines which substances may be processed without the risk of ignition or material failure.
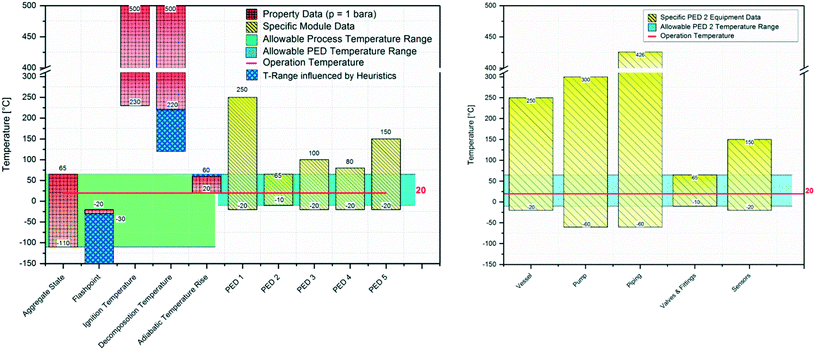
Fig. 8 shows the comparison between substance specific temperature data such as ignition or decomposition temperature, or adiabatic temperature rise and the acceptable temperature range of the PEDs, which consist of the single devices and components built in every PED. If all relevant data are available in a database, the comparison can be conducted automatically. An acceptable operation window for the selected module can be derived. For this example the maximum temperature is defined by a valve in PED 2 to 65 °C and the minimal temperature due to the same valve −10 °C. During a failure it would be possible that the temperature rises above 65 °C due to a wrong process temperature and the adiabatic temperature rise. Thus safety measures need to be integrated to avoid such high temperatures or the limited component must be replaced by another one with a larger temperature range.
4.5 Optimization potential from modular risk assessment
The intra-modular risk assessment ensures an early re-use of available information such as P&ID, component lists, and 3D-CAD drawings and defines safe operation conditions with regard to temperature, pressure, substances, and reaction conditions. The HAZOP analysis during the inter-modular risk assessment profits from the available module data with routine information, while the study can be accelerated and focus on important aspects. The approach of dividing the risk assessment into a part which is focusing on the modules itself and a part which is focusing on the entire plant contributes to the concept of modular designed plants. The information gained during the intra-modular risk assessment is independent of the latter process and can be re-used in several projects. New processes can benefit from already successfully discussed and implemented modules. Storage of the safety information in the PED database allows for rapid selection of suitable modules and reduction of lead time. This method can be extended by the selection methodology of technical reactor equipment according to Krasberg et al.141 Further benefits for and from modular plants can be found in a recently published white paper.142 Major challenges for safety management in continuous flow processes were identified with the following.• Operation and safety experience must be combined with module database in frequent update cycles.
• Safety certificates and related data are necessary for combined PEDs and modules.
• PEDs should be designed inherently safe; nevertheless module/module interactions have to be observed closely.
• Safety concepts and strategies of module vendors, owner/operators, and regulatory agencies have to be harmonized.
Since the economic and safety potential are visible, common efforts should be promoted to step further in modular design, planning, construction, and operation of future chemical plants.
5 Conclusion and outlook
Safe processing of hazardous, rapid, and strongly exothermic reactions in microchannels is one of the main drivers for successful application of microreactors. Novel process windows enable often harsh process conditions, which can only be handled in small devices with a low internal volume. Good results from laboratory studies must be brought to pilot and production scale for concise process and product development. This contribution gives an overview of typical reactions, methods, standard operating procedures (SOPs), and checklists for a consistent pathway from lab development to pilot plant production of continuous-flow processes in relation to safety issues. The scale-up of microstructured devices often follows constant mixing time or heat transfer characteristics. For strong exothermic reactions, the parametric sensitivity and reactor stability is a crucial issue, which is described with three different approaches. Based on dimensionless numbers for reaction kinetics and thermodynamics, a correlation is given including the convective heat transfer for safe operation conditions. With a modular plant setup, simple and robust scale-up with equipment modularity for multipurpose applications will give new drive for microreactor development and applications. Equipment modules are treated with checklist safety analysis, pumps, vessels, reactors, and devices with a larger internal volume, while the entire plant is treated in a HAZOP study. Due to modular design and re-use of engineering effort and information, time savings and improved safety assessment are achieved for small-scale production plants. Further improvement due to the modular planning process and plant setup has to be evaluated with more modularly constructed plants under operation.Acknowledgements
The research leading to these results has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No. 636942. Financial support is also provided by the Dutch Science Foundation (NWO) via a VIDI grant for T. N. (Grant No. 14150).References
- D. J. Smith, Reliability, Maintainability and Risk: Practical Methods for Engineers including Reliability Centred Maintenance and Safety-Related Systems, Elsevier Science, Burlington, 2011 Search PubMed.
- T. A. Kletz, What went wrong?: Case histories of process plant disasters and how they could have been avoided, Gulf Professional Publishing, Burlington, MA, 2009 Search PubMed.
- National Academies of Sciences, Engineering, and Medicine, Chemical Laboratory Safety and Security: A Guide to Developing Standard Operating Procedures, The National Academies Press, Washington, DC, 2016 Search PubMed.
- T. Noël, Y. Su and V. Hessel in Organometallic Flow Chemistry, ed. T. Noël, Springer International Publishing, Cham, 57, 2016, vol. 1, pp. 1–41 Search PubMed.
- W. E. Baker, P. A. Cox, P. S. Westine, J. Kulesz and R. A. Strehlow, Explosion hazards and evaluation, Elsevier Scientific Pub. Co, Amsterdam, 1983 Search PubMed.
- F. A. Williams and R. A. Strehlow, AIAA J., 1985, 23(70), 1139 CrossRef.
- M. Gödde, C. Liebner and H. Hieronymus, Chem. Ing. Tech., 2009, 81(1–2), 73 CrossRef.
- C. Liebner, J. Fischer, S. Heinrich, T. Lange, H. Hieronymus and E. Klemm, Process Saf. Environ. Prot., 2012, 90(2), 77 CrossRef CAS.
- S. Heinrich, F. Edeling, C. Liebner, H. Hieronymus, T. Lange and E. Klemm, Chem. Eng. Sci., 2012, 84, 540 CrossRef CAS.
- J. Fischer, C. Liebner, H. Hieronymus and E. Klemm, Chem. Eng. Sci., 2009, 64(12), 2951 CrossRef CAS.
- N. Kockmann and D. M. Roberge, Chem. Eng. Process., 2011, 50(10), 1017 CrossRef CAS.
- O. Klais, F. Westphal, W. Benaïssa and D. Carson, Chem. Eng. Technol., 2009, 32(11), 1831 CrossRef CAS.
- O. Klais, F. Westphal, W. Benaissa and D. Carson, Chem. Eng. Technol., 2009, 32(12), 1966 CrossRef CAS.
- O. Klais, F. Westphal, W. Benaissa, D. Carson and J. Albrecht, Chem. Eng. Technol., 2010, 33(3), 444 CrossRef CAS.
- O. Klais, J. Albrecht, D. Carson, M. Kraut, P. Lö, C. Minnich, F. Olschewski, C. Reimers, A. Simoncelli and M. Uerdingen, Chem. Eng. Technol., 2010, 33(7), 1159 CrossRef CAS.
- J. Alagy, P. Trambouze and H. van Landeghem, Ind. Eng. Chem. Process Des. Dev., 1974, 13(4), 317 CAS.
- J. Hao, H. Cheng, H. Wang, S. Cai and F. Zhao, J. Mol. Catal. A: Chem., 2007, 271(1–2), 42 CrossRef CAS.
- N. Theyssen, Z. Hou and W. Leitner, Chem. – Eur. J., 2006, 12(12), 3401 CrossRef CAS PubMed.
- G. Veser, Chem. Eng. Sci., 2001, 56(4), 1265 CrossRef CAS.
- B. Gutmann, D. Cantillo and C. O. Kappe, Angew. Chem., Int. Ed., 2015, 54(23), 6688 CrossRef CAS PubMed.
- M. Movsisyan, E. I. P. Delbeke, J. K. E. T. Berton, C. Battilocchio, S. V. Ley and C. V. Stevens, Chem. Soc. Rev., 2016, 45(18), 4892 RSC.
- Fire Diamond, http://chemwatch.net/ (last accessed January 2017) Search PubMed.
- F3 Factory: Flexible, Fast and Future Production Processe-Final Report, http://www.f3factory.com/scripts/pages/en/newsevents/F3_Factory_final_report_to_EC.pdf (last accessed February 2017) Search PubMed.
- C. J. Mallia and I. R. Baxendale, Org. Process Res. Dev., 2016, 20(2), 327 CrossRef CAS.
- T. Noël and V. Hessel, ChemSusChem, 2013, 6(3), 405 CrossRef PubMed.
- Y. Su, V. Hessel and T. Noël, AIChE J., 2015, 61(7), 2215 CrossRef CAS.
- H. P. L. Gemoets, Y. Su, M. Shang, V. Hessel, R. Luque and T. Noël, Chem. Soc. Rev., 2016, 45(1), 83 RSC.
- A. Gavriilidis, A. Constantinou, K. Hellgardt, K. K. Hii, G. J. Hutchings, G. L. Brett, S. Kuhn and S. P. Marsden, React. Chem. Eng., 2016, 1(6), 595 CAS.
- X. Ye, M. D. Johnson, T. Diao, M. H. Yates and S. S. Stahl, Green Chem., 2010, 12(7), 1180 RSC.
- K. S. Elvira, R. C. R. Wootton, N. M. Reis, M. R. Mackley and A. J. DeMello, ACS Sustainable Chem. Eng., 2013, 1(2), 209 CrossRef CAS.
- J. Turconi, F. Griolet, R. Guevel, G. Oddon, R. Villa, A. Geatti, M. Hvala, K. Rossen, R. Göller and A. Burgard, Org. Process Res. Dev., 2014, 18(3), 417 CrossRef CAS.
- Y. Su, N. J. W. Straathof, V. Hessel and T. Noël, Chem. – Eur. J., 2014, 20(34), 10562 CrossRef CAS PubMed.
- D. Cambié, C. Bottecchia, N. J. W. Straathof, V. Hessel and T. Noël, Chem. Rev., 2016, 116(17), 10276 CrossRef PubMed.
- M. B. Plutschack, C. A. Correia, P. H. Seeberger and K. Gilmore, Top. Organomet. Chem., 2015, 48, 43–76 CrossRef.
- S. Hübner, U. Bentrup, U. Budde, K. Lovis, T. Dietrich, A. Freitag, L. Küpper and K. Jähnisch, Org. Process Res. Dev., 2009, 13(5), 952 CrossRef.
- M. Irfan, T. N. Glasnov and C. O. Kappe, Org. Lett., 2011, 13(5), 984 CrossRef CAS PubMed.
- J. Kobayashi, Science, 2004, 304(5675), 1305 CrossRef CAS PubMed.
- Z. Amara, M. Poliakoff, R. Duque, D. Geier, G. Franciò, C. M. Gordon, R. E. Meadows, R. Woodward and W. Leitner, Org. Process Res. Dev., 2016, 20(7), 1321 CrossRef CAS.
- E. Habraken, P. Haspeslagh, M. Vliegen and T. Noël, J. Flow Chem., 2015, 5(1), 2 CrossRef CAS.
- S. B. Ötvös, I. M. Mándity and F. Fülöp, Mol. Diversity, 2011, 15(3), 605 CrossRef PubMed.
- S. Chandrasekhar, B. V. D. Vijaykumar, B. M. Chandra, C. Raji and P. Naresh, Tetrahedron Lett., 2011, 52(30), 3865 CrossRef CAS.
- J. R. Breen, G. Sandford, D. S. Yufit, J. A. K. Howard, J. Fray and B. Patel, Beilstein J. Org. Chem., 2011, 7, 1048 CrossRef CAS PubMed.
- P. W. Miller, N. J. Long, A. J. de Mello, R. Vilar, J. Passchier and A. Gee, Chem. Commun., 2006, 546 RSC.
- T. Fukuyama, T. Totoki and I. Ryu, Green Chem., 2014, 16(4), 2042 RSC.
- S. Borukhova, T. Noël and V. Hessel, Org. Process Res. Dev., 2016, 20(2), 568 CrossRef CAS.
- D. X. Hu, M. O'Brien and S. V. Ley, Org. Lett., 2012, 14(16), 4246 CrossRef CAS PubMed.
- V. D. Pinho, B. Gutmann, L. S. M. Miranda, R. O. M. A. de Souza and C. O. Kappe, J. Org. Chem., 2014, 79(4), 1555 CrossRef CAS PubMed.
- B. Gutmann, D. Obermayer, J.-P. Roduit, D. M. Roberge and C. O. Kappe, J. Flow Chem., 2012, 2(1), 8 CrossRef CAS.
- C. Brancour, T. Fukuyama, Y. Mukai, T. Skrydstrup and I. Ryu, Org. Lett., 2013, 15(11), 2794 CrossRef CAS PubMed.
- F. J. Strauss, D. Cantillo, J. Guerra and C. O. Kappe, React. Chem. Eng., 2016, 1(5), 472 CAS.
- S. Fuse, N. Tanabe and T. Takahashi, Chem. Commun., 2011, 47(47), 12661 RSC.
- D. R. J. Acke and C. V. Stevens, Green Chem., 2007, 9(4), 386 RSC.
- V. Hessel, D. Kralisch, N. Kockmann, T. Noël and Q. Wang, ChemSusChem, 2013, 6(5), 746 CrossRef CAS PubMed.
- S. Borukhova, T. Noël, B. Metten, E. de Vos and V. Hessel, ChemSusChem, 2013, 6(12), 2220 CrossRef CAS PubMed.
- D. Cantillo and C. O. Kappe, J. Org. Chem., 2013, 78(20), 10567 CrossRef CAS PubMed.
- A. Nagaki and J.-I. Yoshida, Top. Organomet. Chem., 2015, 137–175 CrossRef.
- J.-I. Yoshida, A. Nagaki and T. Yamada, Chem. – Eur. J., 2008, 14(25), 7450 CrossRef CAS PubMed.
- D. R. Fandrick, F. Roschangar, C. Kim, B. J. Hahm, M. H. Cha, H. Y. Kim, G. Yoo, T. Kim, J. T. Reeves, J. J. Song, Z. Tan, B. Qu, N. Haddad, S. Shen, N. Grinberg, H. Lee, N. Yee and C. H. Senanayake, Org. Process Res. Dev., 2012, 16(5), 1131 CrossRef CAS.
- J. Wu, X. Yang, Z. He, X. Mao, T. A. Hatton and T. F. Jamison, Angew. Chem., Int. Ed., 2014, 53(32), 8416 CrossRef CAS PubMed.
- L. Kupracz and A. Kirschning, Adv. Synth. Catal., 2013, 355(17), 3375 CrossRef CAS.
- P. R. D. Murray, D. L. Browne, J. C. Pastre, C. Butters, D. Guthrie and S. V. Ley, Org. Process Res. Dev., 2013, 17(9), 1192 CrossRef CAS.
- A. Ziogas, V. Cominos, G. Kolb, H. J. Kost, B. Werner and V. Hessel, Chem. Eng. Technol., 2012, 35(1), 58 CrossRef CAS.
- A. L. Tonkovich, W. W. Simmons, L. J. Silva, D. Qiu, S. T. Perry and T. Yuschak, Distillation process using microchannel technology, US Pat., 7305850B2, 2007 Search PubMed.
- A. Tonkovich, K. Jarosch, R. Arora, L. Silva, S. Perry, J. Mcdaniel, F. Daly and B. Litt, Chem. Eng. J., 2008, 135, S2–S8 CrossRef CAS.
- D. R. Seok and S.-T. Hwang, AIChE J., 1985, 31(12), 2059 CrossRef CAS.
- X. Huang, D. King, F. Zheng, V. Stenkamp, W. Tegrothenhuis and B. Roberts, Catal. Today, 2008, 136(3–4), 291 CrossRef CAS.
- R. L. Hartman, H. R. Sahoo, B. C. Yen and K. F. Jensen, Lab Chip, 2009, 9(13), 1843 RSC.
- J. M. MacInnes, J. Ortiz-Osorio, P. J. Jordan, G. H. Priestman and R. Allen, Chem. Eng. J., 2010, 159(1–3), 159 CrossRef CAS.
- H.-B. Kim, K. Ueno, M. Chiba, O. Kogi and N. Kitamura, Anal. Sci., 2000, 16(8), 871 CrossRef CAS.
- M. Tokeshi, T. Minagawa and T. Kitamori, J. Chromatogr. A, 2000, 894(1–2), 19 CrossRef CAS PubMed.
- H. Hisamoto, T. Horiuchi, M. Tokeshi, A. Hibara and T. Kitamori, Anal. Chem., 2001, 73(6), 1382 CrossRef CAS.
- H. Hotokezaka, M. Tokeshi, M. Harada, T. Kitamori and Y. Ikeda, Prog. Nucl. Energy, 2005, 47(1–4), 439 CrossRef CAS.
- A. Aota, M. Nonaka, A. Hibara and T. Kitamori, Angew. Chem., Int. Ed., 2007, 46(6), 878 CrossRef CAS PubMed.
- P. Znidarsic-Plazl and I. Plazl, Lab Chip, 2007, 7(7), 883 RSC.
- D. M. Fries, T. Voitl and P. R. von Rohr, Chem. Eng. Technol., 2008, 31(8), 1182 CrossRef CAS.
- G. Hellé, C. Mariet and G. Cote, Procedia Chem., 2012, 7, 679 CrossRef.
- L. Yang, Y. Zhao, Y. Su and G. Chen, Chem. Eng. Technol., 2013, 36(6), 985 CrossRef CAS.
- A. Holbach and N. Kockmann, Green Process. Synth., 2013, 2, 2 Search PubMed.
- Z.-X. Cai, Q. Fang, H.-W. Chen and Z.-L. Fang, Anal. Chim. Acta, 2006, 556(1), 151 CrossRef CAS PubMed.
- J. G. Kralj, H. R. Sahoo and K. F. Jensen, Lab Chip, 2007, 7(2), 256 RSC.
- Y. Wu, W. Li, H. Gao, Q. Li, Y. Li, K. Wang, I. Mahmood and H. Liu, J. Membr. Sci., 2010, 347(1–2), 17 CrossRef CAS.
- M. N. Kashid, Y. M. Harshe and D. W. Agar, Ind. Eng. Chem. Res., 2007, 46(25), 8420 CrossRef CAS.
- W. Lan, S. Jing, S. Li and G. Luo, Ind. Eng. Chem. Res., 2016, 55(20), 6006 CrossRef CAS.
- T. Noël, S. Kuhn, A. J. Musacchio, K. F. Jensen and S. L. Buchwald, Angew. Chem., Int. Ed., 2011, 50(26), 5943 CrossRef PubMed.
- M. O'Brien, P. Koos, D. L. Browne and S. V. Ley, Org. Biomol. Chem., 2012, 10(35), 7031 Search PubMed.
- D. R. Snead and T. F. Jamison, Chem. Sci., 2013, 4(7), 2822 RSC.
- T. Shimanouchi, Y. Kataoka, T. Tanifuji, Y. Kimura, S. Fujioka and K. Terasaka, AIChE J., 2016, 62(6), 2135 CrossRef CAS.
- K. Wang and G. Luo, Chem. Eng. Sci., 2016 DOI:10.1016/j.ces.2016.10.025.
- C. Ye, M. Dang, C. Yao, G. Chen and Q. Yuan, Chem. Eng. J., 2013, 225, 120 CrossRef CAS.
- M.-Y. Pan, T. Li, Y. Zhou, Z. Qian, L. Shao, J.-X. Wang and J.-F. Chen, Chem. Eng. Process., 2015, 95, 135 CrossRef CAS.
- S. V. Ley, I. R. Baxendale, R. N. Bream, P. S. Jackson, A. G. Leach, D. A. Longbottom, M. Nesi, J. S. Scott, R. I. Storer and S. J. Taylor, J. Chem. Soc., Perkin Trans. 1, 2000, 23, 3815 RSC.
- M. O'Brien, R. Denton and S. Ley, Synthesis, 2011, 2011(08), 1157 CrossRef.
- R. J. P. Eder, S. Schrank, M. O. Besenhard, E. Roblegg, H. Gruber-Woelfler and J. G. Khinast, Cryst. Growth Des., 2012, 12(10), 4733 CAS.
- S. Lawton, G. Steele, P. Shering, L. Zhao, I. Laird and X.-W. Ni, Org. Process Res. Dev., 2009, 13(6), 1357 CrossRef CAS.
- L. Hohmann, R. Gorny, O. Klaas, J. Ahlert, K. Wohlgemuth and N. Kockmann, Chem. Eng. Technol., 2016, 39(7), 1268 CrossRef CAS.
- B. J. Deadman, C. Battilocchio, E. Sliwinski and S. V. Ley, Green Chem., 2013, 15(8), 2050 RSC.
- N. Kockmann and D. M. Roberge, Chem. Eng. Technol., 2009, 32(11), 1682 CrossRef CAS.
- M. G. Gelhausen, S. K. Kurt and N. Kockmann, Chem. Ing. Tech., 2015, 87(6), 781 CrossRef CAS.
- A. Varma, M. Morbidelli and H. Wu, Parametric sensitivity in chemical systems, Cambridge University Press, Cambridge, U.K., New York, NY, 1999 Search PubMed.
- N. N. Semenov and J. E. S. Bradley, Some problems in chemical kinetics and reactivity, Pergamon Press, London, 1959 Search PubMed.
- D. A. Frank-Kamenetskii, Diffusion and heat transfer in chemical kinetics, New York Plenum Press, 1969 Search PubMed.
- N. Kockmann, Chem. Ing. Tech., 2012, 84(5), 646 CrossRef CAS.
- M. Baerns, A. Behr, A. Brehm, J. Gmehling, K.-O. Hinrichsen, H. Hofmann, U. Onken, R. Palkovits and A. Renken, Technische Chemie, Wiley-VCH, Weinheim, 2013 Search PubMed.
- T. Westermann and L. Mleczko, Org. Process Res. Dev., 2016, 20(2), 487 CrossRef CAS.
- N. Kockmann, Chem. Ing. Tech., 2012, 84(5), 715 CrossRef CAS.
- EHSC Note on Safety Issues in the Scale-Up of Chemical Reactions, http://www.rsc.org/globalassets/04-campaigning-outreach/realising-potential-of-scientists/regulations-health-safety/safety-issues-in-the-scaleup-of-chemical-reactions.pdf (last accessed February 2017) Search PubMed.
- J. Leonhardt, M. Nau, M. Wolter and C. Rau, J. Loss Prev. Process Ind., 2008, 21(4), 400 CrossRef CAS.
- W. Benaïssa, N. Gabas, M. Cabassud, D. Carson, S. Elgue and M. Demissy, J. Loss Prev. Process Ind., 2008, 21(5), 528 CrossRef.
- F. Ebrahimi, T. Virkki-Hatakka and I. Turunen, Chem. Eng. Process., 2012, 52, 28 CrossRef CAS.
- S. Schwolow, B. Heikenwälder, L. Abahmane, N. Kockmann and T. Röder, Org. Process Res. Dev., 2014, 18(11), 1535 CrossRef CAS.
- Auditing Checklist for 29 CFR 1910.119, https://www.osha.gov/dte/grant_materials/fy09/sh-19479-09/08_PSM_Auditing_Checklist.pdf (last accessed February 2017) Search PubMed.
- Preventing Chemical Accidents, https://www.osha.gov/dte/grant_materials/fy08/sh-17813-08/compliance_checklist.pdf (last accessed February 2017) Search PubMed.
- C. Bramsiepe and G. Schembecker, Chem. Ing. Tech., 2012, 84(5), 581 CrossRef CAS.
- Ł. Hady and G. Wozny, Chem. Ing. Tech., 2012, 84(5), 597 CrossRef.
- L. Hohmann, K. Kössl, N. Kockmann, G. Schembecker and C. Bramsiepe, Chem. Eng. Process., 2017, 111, 115 CrossRef CAS.
- C. Fleischer-Trebes, N. Krasberg, C. Bramsiepe and N. Kockmann, Chem. Ing. Tech., 2016 DOI:10.1002/cite.201600083.
- V. Villa, N. Paltrinieri, F. Khan and V. Cozzani, Safety Science, 2016, 89, 77–93 CrossRef.
- P. K. Marhavilas, D. Koulouriotis and V. Gemeni, J. Loss Prev. Process Ind., 2011, 24(5), 477 CrossRef.
- F. Khan, S. Rathnayaka and S. Ahmed, Process Saf. Environ. Prot., 2015, 98, 116 CrossRef CAS.
- C. Fleischer, J. Wittmann, N. Kockmann, T. Bieringer and C. Bramsiepe, Chem. Ing. Tech., 2015, 87(9), 1258 CrossRef CAS.
- J. Dunjó, V. Fthenakis, J. A. Vilchez and J. Arnaldos, J. Hazard. Mater., 2010, 173(1–3), 19 CrossRef PubMed.
- International Electrotechnical Commission, Hazard and operability studies (HAZOP studies) - Application guide, IEC 61882:2016, 2016 Search PubMed.
- B. J. Seligmann, E. Németh, K. M. Hangos and I. T. Cameron, J. Loss Prev. Process Ind., 2012, 25(4), 746 CrossRef CAS.
- F. Argenti, E. Brunazzi and G. Landucci, Chem. Eng. Trans., 2015, 43, 2383 Search PubMed.
- N. Boonthum, U. Mulalee and T. Srinophakun, Reliability Engineering & System Safety, 2014, 121, 152–163 Search PubMed.
- P. Baybutt, J. Loss Prev. Process Ind., 2015, 33, 52 CrossRef.
- Y. Zhang, W. Zhang and B. Zhang, Chin. J. Chem. Eng., 2015, 23(12), 2065 CrossRef CAS.
- J. Janošovský, J. Labovský and L. Jelemenský, Chemical Engineering Transactions, 2016, 48, 505 Search PubMed.
- K. Eichendorf, E. Guntrum, C. Jochum and K.-J. Niemitz, Chem. Eng. Technol., 2002, 25(4), 439 CrossRef CAS.
- G. Gigerenzer, Risiko: Wie man die richtigen Entscheidungen trifft, Bertelsmann, München, 2013 Search PubMed.
- F.-J. Bock and K. Haferkamp, Chem. Ing. Tech., 2015, 87(1–2), 103 CrossRef CAS.
- F.-J. Bock and K. Haferkamp, Chem. Ing. Tech., 2015, 87(1–2), 95 CrossRef CAS.
- Forschungszentrum INVITE, www.invite-research.com (last accessed February 2017).
- T. Bieringer, S. Buchholz and N. Kockmann, Chem. Eng. Technol., 2013, 36(6), 900 CrossRef CAS.
- Consens-Integrated Control and Sensing, www.consens-spire.eu (last accessed June 2016).
- European Union, Directive 2012/18/EU of the European Parliament and of the Council of 4 July 2012 on the control of major-accident hazards involving dangerous substances, amending and subsequently repealing Council Directive 96/82/EC, 2012 Search PubMed.
- J. Dunjó Denti, J. Arnaldos and J. A. Vílchez Sánchez, New trends for conducting hazard and operability (HAZOP) studies in continuous chemical processes, Universitat Politècnica de Catalunya, Barcelona, DL, 2010 Search PubMed.
- Equipment for potentially explosive atmospheres (ATEX), http://ec.europa.eu/growth/sectors/mechanical-engineering/atex/ (last accessed February 2017).
- System for certification to standards relating to equipment for use in explosive atmospheres (IECEx systems), http://www.iecex.com/standards.htm (last accessed February 2017).
- C. Jochum, Gefahrenanalyse zur Bewertung des Gefahrenpotentials von prozessbezogenen Anlagen, Wirtschaftsverl, NW, Bremerhaven, 2000 Search PubMed.
- N. Krasberg, L. Hohmann, T. Bieringer, C. Bramsiepe and N. Kockmann, Processes, 2014, 2(1), 265 CrossRef CAS.
- Temporärer ProcessNet-Arbeitskreis Modulare Anlagen and Modular Plants, Flexible chemical production by modularization and standardization - status quo and future trends, Frankfurt am Main, 2016 Search PubMed.
| This journal is © The Royal Society of Chemistry 2017 |