Understanding the mechanism of non-enzymatic glycation inhibition by cinnamic acid: an in vitro interaction and molecular modelling study†
Faizan Abul Qaisa,
Md. Maroof Alamb,
Imrana Naseemb and
Iqbal Ahmad*a
aDepartment of Agricultural Microbiology, Aligarh Muslim University, Aligarh, 202002, India. E-mail: ahmadiqbal8@yahoo.co.in; Fax: +91-571-2703516; Tel: +91-571-2703516 Tel: +91-9897902936
bDepartment of Biochemistry, Faculty of Life Sciences, Aligarh Muslim University, Aligarh, 202002, India
First published on 11th July 2016
Abstract
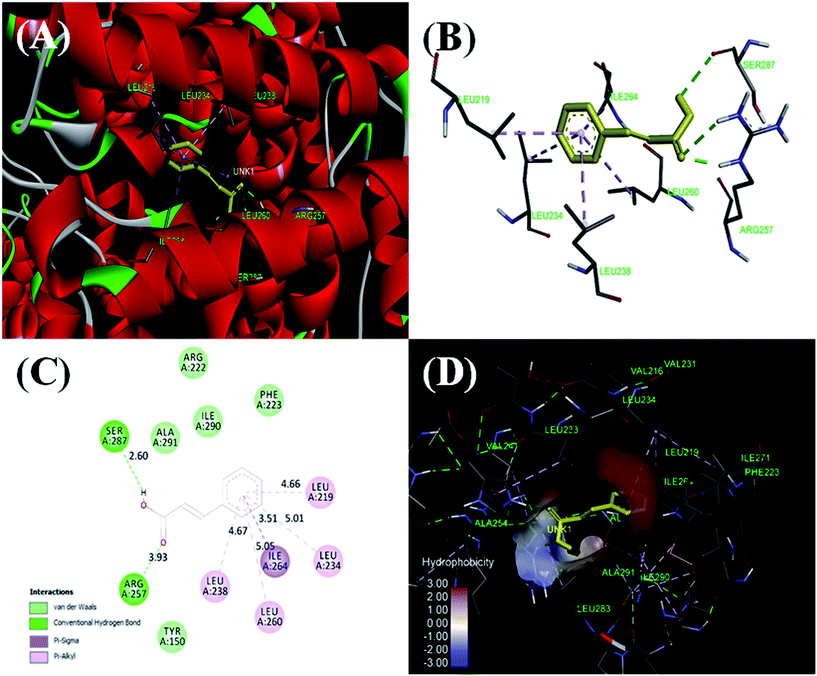
Under hyperglycaemic conditions non-enzymatic glycation of proteins gives rise to advanced glycation end products (AGEs). The AGEs thus formed generate free radicals, which foster the development of diabetes and its associated complications. Inhibition of glycation is expected to play a role in controlling diabetes. Plant derived antioxidants like cinnamic acid (CA) are known for limiting AGE formation, however, the mechanism involved is poorly understood. Therefore, we aimed to investigate the possible mechanism of inhibition of AGEs formation by CA through various experimental approaches. Glycation of HSA was achieved by incubating the reaction mixture with glucose for 30 days at 37 °C. The protein samples were tested for levels of free lysine & thiol groups, carbonyl content and reactive oxygen species (ROS). Interaction between CA and HSA was also studied through various biophysical techniques. Thermodynamic studies showed a strong exothermic interaction between CA and HSA. The positive value of TΔS° and negative value of ΔH° indicates that the HSA–CA complex is mainly stabilized by a hydrophobic interaction and hydrogen bond. Further, molecular docking reveals that CA binds to HSA subdomain IIA (Sudlow's site I) with a binding energy of −7.0 kcal mol−1, nearly the same as obtained in isothermal titration calorimetry (ITC) and fluorescence spectroscopy. The results of various spectroscopic techniques along with molecular docking and examination of many biomarkers highlights the role of CA in preventing disease progression.
1. Introduction
The reaction of reducing sugars such as fructose and glucose with proteins, gives rise to Amadori products.1 These further undergo a complex cascade of repeated rearrangements, condensations, oxidative modifications and cause the abnormal cross-linking of proteins to form advanced glycation end products (AGEs).2 The major AGEs in vivo appear to be formed from highly reactive intermediate carbonyl groups, known as oxoaldehydes or -dicarbonyls, including 3-deoxyglucosone, methylglyoxal and glyoxal.3,4 These can accumulate in the body and cause massive damage to the tissues by stimulating reactive oxygen species (ROS) production. AGEs directly stimulated by NADPH oxidase (NOX) after interaction with AGE receptor (RAGE) are present on the cell surface in several tissues or may indirectly increase ROS levels by altering antioxidant proteins. Of note, the expression of both NOX and antioxidant enzymes might also be affected by AGEs and thereby trigger age-related disorders and even diabetic complications such as neuropathy, nephropathy and retinopathy.5Diabetes is a heterogeneous metabolic disorder characterized by hyperglycaemia and altered carbohydrate, fat and protein metabolism.6 The mechanism of diabetes in humans is subjected to wide scrutiny on a physiological, molecular and genetics basis. Despite advances in understanding the disorder, mortality and the morbidity due to this disease is still high. One mechanism increasingly considered as a fundamental cause of diabetic tissue damage is glycation (non-enzymatic glycosylation).7 Although many drugs and interventions are available to manage diabetes, they are increasingly expensive and have inherent adverse effects. As such, there is a growing interest among researchers to use natural products with combined anti-glycation and antioxidant properties to prevent tissue damage. A primary approach has been the use of plant derived products to control disease progression. Various plant herbal medicine and phytocompounds have shown antidiabetic activities in vitro and in vivo. Due to their safety, and lesser side effects a wide variety of plant products have been explored as therapeutic intervention options.8
It is well established that dietary micronutrients contain bioactive compounds, that inhibit AGEs formation in vitro and in vivo.9,10 One such component is cinnamic acid. It is derived from cinnamon which has been used in several cultures as a traditional medicine. Many studies have shown antioxidant and antidiabetic properties of cinnamic acid and its derivatives.11,12 It has been reported to modulate gluconeogenesis, glycogenesis and accelerate the insulin sensitivity in diabetic rats.13,14 However the mechanism involved in still unclear. In our previous study, our group demonstrated the inhibitory effect of plant flavonoid, quercetin in the formation of AGEs.15 To the best of our knowledge, the mechanism of antiglycation of cinnamic acid is not yet fully explored. Therefore, the aim of this study, was to determine the inhibitory effect of cinnamic acid against glycation using human serum albumin (HSA) as a protein model and to understand its possible mode of interference through in vitro interaction studies and molecular modelling.
2. Material and methods
2.1. Materials
Fatty acid-free human serum albumin (HSA) (A3782), TNBSA, ibuprofen and gluconolactone (G2164) were purchased from Sigma-Aldrich, Chemical Company, USA. Cinnamic acid extrapure (034812), aminoguanidine (AG), DMSO, glucose warfarin and DTNB were obtained from SRL chemicals (India). All other chemicals and reagents used were of analytical grade.2.2. Human serum albumin (HSA) in vitro glycation assay
To determine the antiglycation activity of test compound, the method by Alam et al. was adopted with minor modifications.15 Human serum albumin (300 μM) was incubated with glucose (165 mM) in 10 mM PBS (pH 7.4) containing 0.02% NaN3 to avoid microbial contamination, at 37 °C for 30 days. Aminoguanidine (10 mM) incubated with HSA (300 μM) in presence of glucose (165 mM) was taken as positive control. HSA was incubated with glucose in the same concentration as mentioned above in the presence of varying concentration of cinnamic acid (25, 50, 100, 200 and 500 μM) for test group. On completion of incubation, all samples were dialyzed overnight against PBS to remove excess amount of glucose. All samples were stored at −20 °C for further examination and protein concentration in each sample were estimated by Lowry method.16
 | (1) |
The amount of free amino groups in all samples were estimated by using 2,4,6-trinitrobenzene sulfonic acid (TNBSA).21 Briefly, all protein samples were diluted to 0.2 mg mL−1 in 0.1 M sodium bicarbonate buffer (pH 8.5). To all diluted samples (0.5 mL), 0.25 Ml of the 0.01% (w/v) TNBSA solution was added and then the reaction mixture was incubated for 2 h at 37 °C. After incubation, 0.25 mL SDS (10%) and 0.125 mL of 1 N HCl was added to each sample. For blank, distilled was added instead of protein. The absorbance of each sample was recorded at 335 nm against blank.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1. All the results are expressed in nmol per mg protein.
000 M−1 cm−1. All the results are expressed in nmol per mg protein.2.3. Interaction studies
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
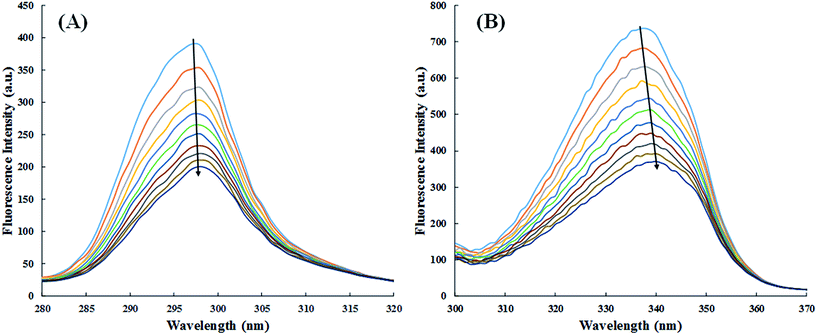
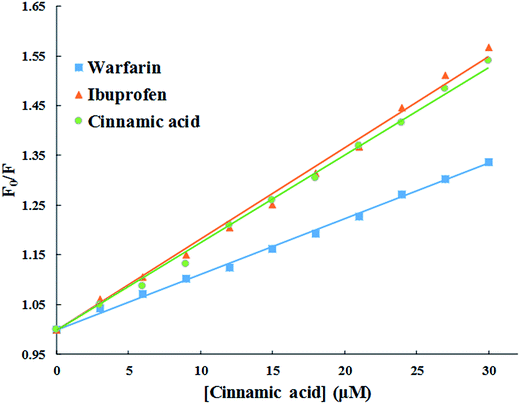
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The HSA-site marker complex was excited at 280 nm and emission spectra was recorded from 285 nm to 600 nm both in absence and presence of cinnamic acid (3–30 μM).
2. The HSA-site marker complex was excited at 280 nm and emission spectra was recorded from 285 nm to 600 nm both in absence and presence of cinnamic acid (3–30 μM).3. Results and discussion
3.1. Antiglycation activity of cinnamic acid
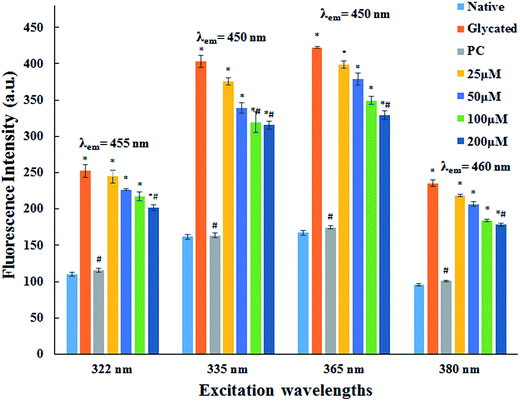
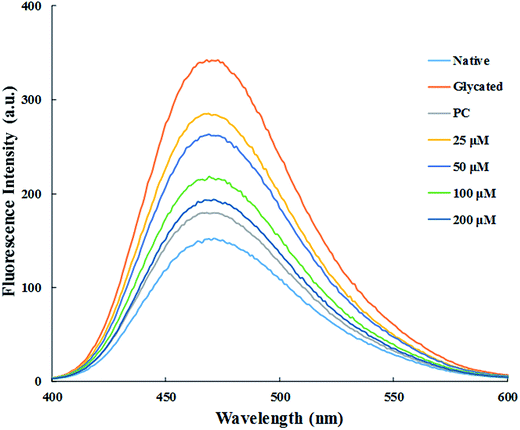
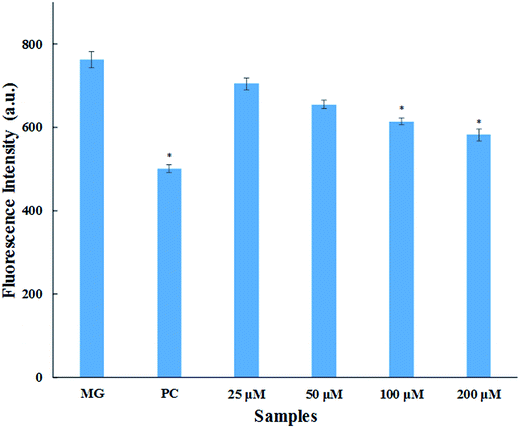
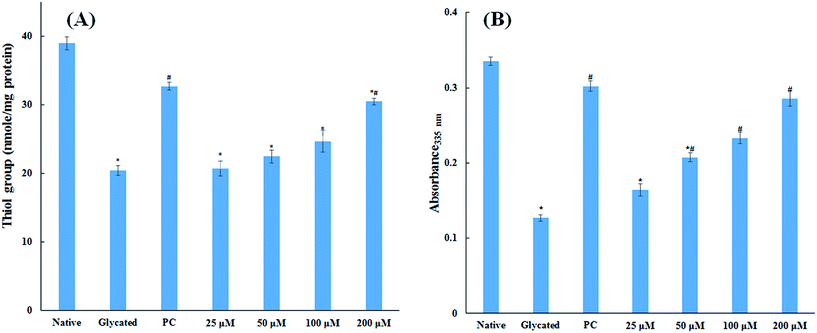
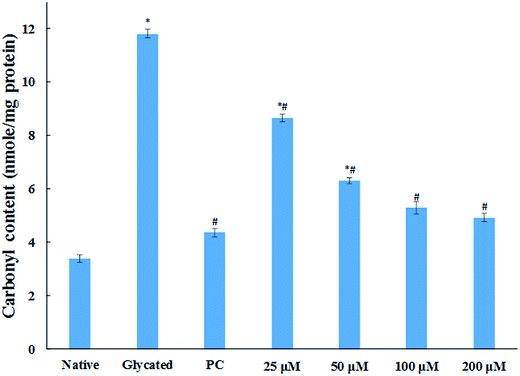
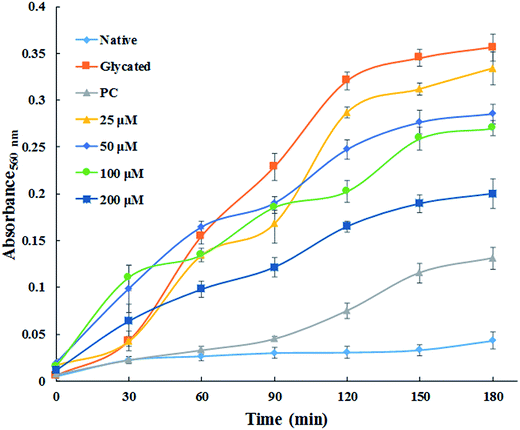
The major site for non-enzymatic glycation of proteins is free amino group present in lysine, but arginine, cysteine and histidine have also found to be involved in this phenomenon.23 In this process, free ε-NH2 group of arginine and lysine react with carbonyl group of various sugars to form Amadori product and finally leading to the formation of heterogeneous class of advanced glycated end products such as carboxymethyllysine (CML), vesperlysine (VESP) and carboxyethyllysine (CEL) etc.35 One of the most common mechanism involved in antiglycation process is the masking of lysine and arginine residues by small molecule inhibitors. It is clear from Fig. 4B that glycation of HSA with glucose for 28 days lead to 58.75% decrease in free amino group of lysine. The treatment of 25, 50, 100 and 200 μM of cinnamic acid to HSA showed 29.1%, 63.2%, 83.7% and 124.9% increment in availability of free ε-NH2 group of lysine as compared to glycated HSA. This result reveals that cinnamic acid has potent antiglycation activity which might be due to the masking of free amino groups in HSA.
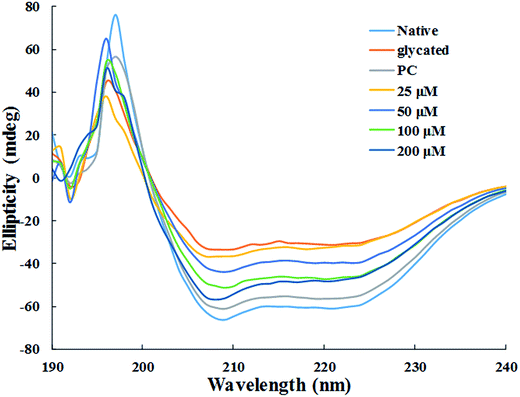 | ||
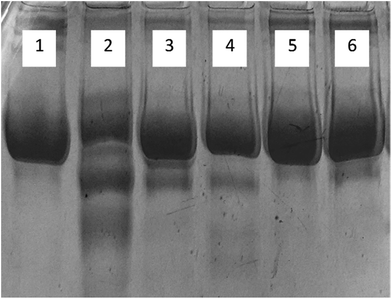
| Fig. 7 Far UV-CD spectra of native, glycated and cinnamic acid treated HSA at various concentrations. All the data have been expressed in mean ± SEM for three independent experiments. | ||
| Samples | Helix 1 (%) | Helix 2 (%) | Strand 1 (%) | Strand 2 (%) | Turns (%) | Unordered (%) |
|---|---|---|---|---|---|---|
| a Helix 1, Helix 2, Strand 1 and Strand 2 indicate a regular α-helix, distorted α-helix, regular β-strand and distorted β-strand, respectively. | ||||||
| Native | 30.2 | 24.3 | 07.1 | 0.90 | 12.9 | 24.5 |
| Glycated | 17.3 | 18.5 | 09.2 | 02.0 | 17.0 | 36.0 |
| Aminoguanidine | 28.8 | 25.4 | 04.9 | 0.40 | 14.0 | 26.4 |
| CA (200 μM) | 26.3 | 27.8 | 00.6 | 0.60 | 17.9 | 26.8 |
| CA (100 μM) | 25.5 | 25.7 | 01.8 | 0.20 | 16.2 | 30.6 |
| CA (50 μM) | 22.6 | 25.4 | 03.8 | 0.9 | 16.9 | 30.4 |
| CA (25 μM) | 16.1 | 18.1 | 08.4 | 02.8 | 19.4 | 35.2 |
3.2. Interaction studies
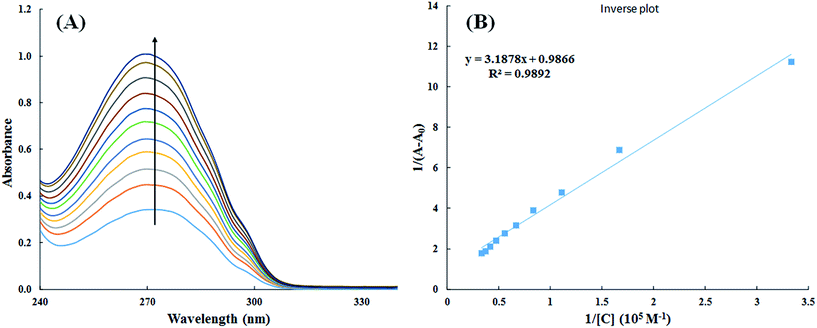 | ||
| Fig. 9 UV-visible absorption spectra of HSA in absence and presence of varying concentration of cinnamic acid (A) and plot of 1/(A − A0) vs. 1/[cinnamic acid] (B). | ||
The value of binding constant was calculated using eqn (2).41
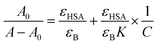 | (2) |
The double reciprocal plot of 1/(A − A0) vs. 1/C (Fig. 9B) is linear. The value of binding constant was estimated from the ratio of the intercept to that of slope of the above mentioned plot. The binding constant for the interaction of cinnamic acid with HSA was found to be 3.09 × 104 M−1 which signifies strong interaction between HSA and cinnamic acid.
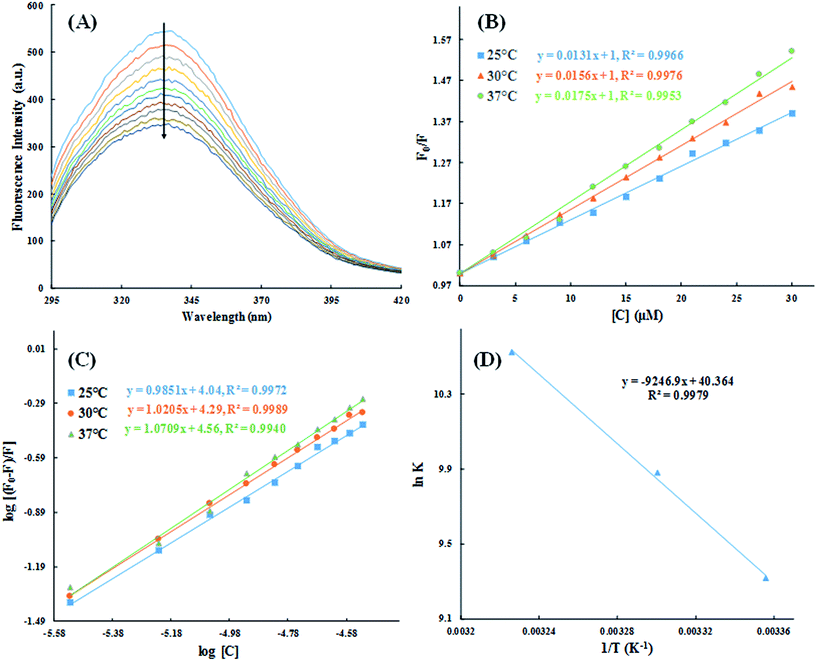
The quantitative analysis of fluorescence quenching data at different temperatures (298, 303 and 310 K) was performed through Stern–Volmer equation.42
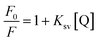 | (3) |
| pH | Temp (K) | Ksv (×104 M−1) | Kq (×1012 M−1 s−1) | R2 |
|---|---|---|---|---|
| 7.4 | 298 | 1.310 | 2.267 | 0.9966 |
| 303 | 1.560 | 2.699 | 0.9976 | |
| 310 | 1.819 | 3.146 | 0.9953 |
Generally, there are two types of fluorescence quenching mechanism i.e. dynamic and static which are governed by the way of interaction between quencher and HSA.43 If any quencher molecules possess sufficient amount of energy to collide with the excited state fluorophore of HSA (Trp-214) and take it back to the ground state, then this is due to the dynamic quenching mechanism. While in case of static quenching, there is formation of non-fluorescent ground state complex between quencher and fluorophore.44
To examine whether interaction of cinnamic acid with HSA is either static or dynamic quenching, eqn (4) was deployed.
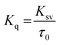 | (4) |
Fluorescence quenching of HSA in presence of quencher are also used to quantitate the binding constant (K) and the number of binding sites (n) by modified Stern–Volmer eqn (5):
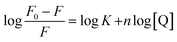 | (5) |
The values of n and K are obtained from the slope and Y-axis intercept from a linear regression of the plot of log[(F0 − F)/F)] vs. log[Q] (Fig. 10C). These values, as a function of temperature, are mentioned in Table 3. The values of K increases with increase in temperature, while the value of n was found to be approximately one at different temperatures. These results suggest the formation of a stable HSA–CA complex which might be more stable at 310 K (physiological temperature) as compared to lower temperature.
| pH | Temp (K) | K (×104 M−1) | n | R2 | ΔG° (kcal mol−1) | ΔH° (kcal mol−1) | TΔS° (kcal mol−1) |
|---|---|---|---|---|---|---|---|
| 7.4 | 298 | 1.111 | 0.985 | 0.9972 | −5.527 | 18.373 | 23.901 |
| 303 | 1.949 | 1.033 | 0.9989 | −5.928 | 24.301 | ||
| 310 | 3.711 | 1.046 | 0.9940 | −6.489 | 24.863 |
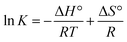 | (6) |
| ΔG° = ΔH° − TΔS° | (7) |
The nature of forces involved in the formation of HSA–cinnamic acid complex can be depicted from the magnitude and sign of various thermodynamic parameters.48 Therefore, we plotted the van't Hoff's plot to know the involvement of molecular forces in the HSA–cinnamic acid complex from the binding constants earlier obtained at their respective temperatures (Fig. 10D). The values of ΔH° and TΔS° mentioned in Table 3 were determined from the slope and Y-axis intercept of linear regression of van't Hoff's plot using eqn (6). The negative value of ΔG obtained from the eqn (7) suggest that the formation of HSA–cinnamic acid complex in spontaneous. Non-covalent interactions, mainly van der Waal's forces and hydrogen bonds played major role in cinnamic acid–HSA interactions.48 The positive TΔS° is also a strong manifestation that water molecules have been excluded from the interface of binding site of HSA, because the presence of water at binding site mimic nature of forces responsible for protein–ligand interaction. The positive value of TΔS° is routinely is regarded as quintessential affirmation of hydrophobic interactions.49
The synchronous fluorescence spectra of HSA in absence and presence of cinnamic acid is shown in Fig. 11. It is evident from the Fig. 11A that there is negligible shift (∼1 nm) on maximum emission wavelength of tyrosine residues, stating that the local environment around tyrosine residue did not had any significant change. While the synchronous fluorescence spectra of tryptophan (Fig. 11B) changed remarkably (3–4 nm) indicating the increase in polarity around the tryptophan residue thereby lowering the hydrophobicity of the residue.52
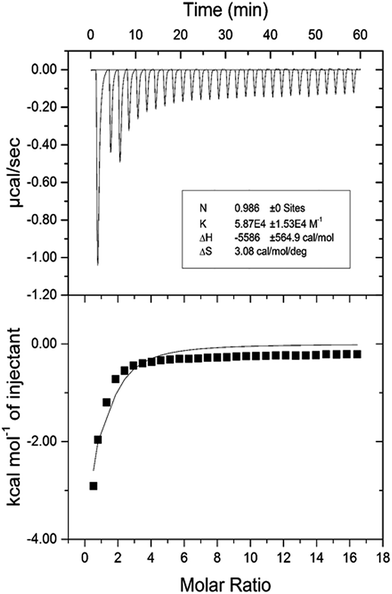
| pH | Temp. (K) | Kb (×104 M−1) | n | ΔG° (kcal mol−1) | ΔH° (kcal mol−1) | TΔS° (kcal mol−1) |
|---|---|---|---|---|---|---|
| 7.4 | 298 | 5.87 | 0.986 | −6.503 | −5.586 | 0.917 |
The negative value of ΔH° confirms that the formation of HSA–cinnamic acid complex is exothermic. The value on n close to unity deciphers that HSA has single binding site for cinnamic acid. The positive value of TΔS° and negative value of ΔH° also indicates that HSA–cinnamic acid complex is mainly stabilized by hydrophobic interaction and hydrogen bond.54 The values of binding constant and Gibb's energy change (ΔG°) obtained from spectroscopy and calorimetry (Tables 3 and 4) are comparable and found to in same order. In contrast, the values of TΔS° and ΔH° differ significantly from that of obtained by the fluorescence spectroscopy. This is due the fact that the value of ΔH° is temperature dependent while fluorescent spectroscopic technique calculates it as temperature-independent parameter.45,56–58 Furthermore, the differences in the values of thermodynamic parameters obtained by fluorescence spectroscopy and ITC is due to the fact that ITC measures a global change in the thermodynamic property, whereas the fluorescence spectroscopy only measures the local changes around the fluorophore (Trp-214).59,60
4. Conclusion
It is concluded that cinnamic acid strongly inhibits the formation of AGEs and acts as a potent antiglycating agent. The values of binding constants obtained both from UV-visible and fluorescence spectroscopy show a strong interaction between HSA and CA. While The negative value of Gibb's free energy change demonstrated that the process of HSA–CA complex formation is spontaneous, ITC results confirm that the reaction is exothermic in nature. Further, molecular docking studies showed that the interaction between HSA and CA are stabilised mainly through hydrophobic and hydrogen bond. The binding site of CA was at one of the major glycating sites in HSA. In the stable HSA–CA complex, reduced numbers of glycating sites are available to react further with free glucose, thereby inhibiting glycation. Evaluation of secondary structure by far-UV CD shows that treatment with CA, allows HSA to retained its native conformation required for proper function even in the presence of high concentration of glucose. Furthermore, the estimation of various biomarkers such as free lysine group, carbonyl content, free sulfhydryl group, reactive oxygen species etc. depicts that cinnamic acid is a quencher of free radical and could prove beneficial for diabetes and associated complications. Reckoning with these facts, the study may be valuable to compare the effect of different concentrations of CA on HSA–glucose structure and conformation upon glycation. However, most of the investigations in this area are limited to in vitro experimental studies and therefore, further investigations should examine the in vivo efficacy of cinnamic acid in suitable animal model.Conflict of interest
The authors declare there are no conflicts of interest.Acknowledgements
We are grateful to Prof. Mohd. Shakir, Chairman, Department of Chemistry, and Prof. Javed Musarrat, Department of Agricultural Microbiology Aligarh Muslim University, Aligarh for providing Instrumentation facilities and encouragement.References
- F. Akhter, M. S. Khan, U. Shahab and S. Ahmad, Bio-physical characterization of ribose induced glycation: a mechanistic study on DNA perturbations, Int. J. Biol. Macromol., 2013, 58, 206–210 CrossRef CAS PubMed.
- T. J. Lyons, S. R. Thorpe and J. W. Baynes, Glycation and autoxidation of proteins in aging and diabetes, in Hyperglycemia, Diabetes, and Vascular Disease, Springer, New York, 1992, pp. 197–217 Search PubMed.
- M. Brownlee, Biochemistry and molecular cell biology of diabetic complications, Nature, 2001, 414(6865), 813–820 CrossRef CAS PubMed.
- P. J. Thornalley, Advanced glycation and the development of diabetic complications. Unifying the involvement of glucose, methylglyoxal and oxidative stress, Endocrinol. Metab., 1996, 3, 149–166 CAS.
- S. Adisakwattana, W. Sompong, A. Meeprom, S. Ngamukote and S. Yibchok-anun, Cinnamic acid and its derivatives inhibit fructose-mediated protein glycation, Int. J. Mol. Sci., 2012, 13(2), 1778–1789 CrossRef CAS PubMed.
- L. Kardeşler, N. Buduneli, B. Bıyıkoğlu, Ş. Çetinkalp and N. Kütükçüler, Gingival crevicular fluid PGE 2, IL-1β, t-PA, PAI-2 levels in type 2 diabetes and relationship with periodontal disease, Clin. Biochem., 2008, 41(10), 863–868 CrossRef PubMed.
- V. P. Singh, A. Bali, N. Singh and A. S. Jaggi, Advanced glycation end products and diabetic complications, Korean J. Physiol. Pharmacol., 2014, 18(1), 1–4 CrossRef CAS PubMed.
- A. A. Chinchansure, A. M. Korwar, M. J. Kulkarni and S. P. Joshi, Recent development of plant products with anti-glycation activity: a review, RSC Adv., 2015, 5(39), 31113–31138 RSC.
- D. Cervantes-Laurean, D. D. Schramm, E. L. Jacobson, I. Halaweish, G. G. Bruckner and G. A. Boissonneault, Inhibition of advanced glycation end product formation on collagen by rutin and its metabolites, J. Nutr. Biochem., 2006, 17(8), 531–540 CrossRef CAS PubMed.
- S. Sang, X. Shao, N. Bai, C. Y. Lo, C. S. Yang and C. T. Ho, Tea polyphenol (−)-epigallocatechin-3-gallate: a new trapping agent of reactive dicarbonyl species, Chem. Res. Toxicol., 2007, 20(12), 1862–1870 CrossRef CAS PubMed.
- F. Natella, M. Nardini, M. Di Felice and C. Scaccini, Benzoic and cinnamic acid derivatives as antioxidants: structure–activity relation, J. Agric. Food Chem., 1999, 47(4), 1453–1459 CrossRef CAS PubMed.
- I. M. Liu, F. L. Hsu, C. F. Chen and J. T. Cheng, Antihyperglycemic action of isoferulic acid in streptozotocin-induced diabetic rats, Br. J. Pharmacol., 2000, 129(4), 631–636 CrossRef CAS PubMed.
- D. W. Huang and S. C. Shen, Caffeic acid and cinnamic acid ameliorate glucose metabolism via modulating glycogenesis and gluconeogenesis in insulin-resistant mouse hepatocytes, J. Funct. Foods, 2012, 4(1), 358–366 CrossRef CAS.
- W. Arlt, P. Neogi, C. Gross and W. L. Miller, Cinnamic acid based thiazolidinediones inhibit human P450c17 and 3beta-hydroxysteroid dehydrogenase and improve insulin sensitivity independent of PPARgamma agonist activity, J. Mol. Endocrinol., 2004, 32(2), 425–436 CrossRef CAS PubMed.
- M. M. Alam, I. Ahmad and I. Naseem, Inhibitory effect of quercetin in the formation of advance glycation end products of human serum albumin: an in vitro and molecular interaction study, Int. J. Biol. Macromol., 2015, 79, 336–343 CrossRef CAS PubMed.
- O. Classics Lowry, N. Rosebrough, A. Farr and R. Randall, Protein measurement with the Folin phenol reagent, J. Biol. Chem., 1951, 193, 265–275 Search PubMed.
- M. Bohlooli, M. Ghaffari-Moghaddam, M. Khajeh, Z. Aghashiri, N. Sheibani and A. A. Moosavi-Movahedi, Acetoacetate promotes the formation of fluorescent advanced glycation end products (AGEs), J. Biomol. Struct. Dyn., 2016, 1–9 Search PubMed.
- N. Sattarahmady, A. A. Moosavi-Movahedi, F. Ahmad, G. H. Hakimelahi, M. Habibi-Rezaei, A. A. Saboury and N. Sheibani, Formation of the molten globule-like state during prolonged glycation of human serum albumin, Biochim. Biophys. Acta, Gen. Subj., 2007, 1770(6), 933–942 CrossRef CAS PubMed.
- C. Lee, M. B. Yim, P. B. Chock, H. S. Yim and S. O. Kang, Oxidation-reduction properties of methylglyoxal-modified protein in relation to free radical generation, J. Biol. Chem., 1998, 273(39), 25272–25278 CrossRef CAS PubMed.
- B. Bouma, L. M. Kroon-Batenburg, Y. P. Wu, B. Brünjes, G. Posthuma, O. Kranenburg, P. G. de Groot, E. E. Voest and M. F. Gebbink, Glycation induces formation of amyloid cross-β structure in albumin, J. Biol. Chem., 2003, 278(43), 41810–41819 CrossRef CAS PubMed.
- M. L. Kakade and I. E. Liener, Determination of available lysine in proteins, Anal. Biochem., 1969, 27(2), 273–280 CrossRef CAS PubMed.
- R. L. Levine, J. A. Williams, E. P. Stadtman and E. Shacter, [37] Carbonyl assays for determination of oxidatively modified proteins, Methods Enzymol., 1994, 233, 346–357 CAS.
- Neelofar, J. Ahmad and K. Alam, Impact of in vitro non-enzymatic glycation on biophysical and biochemical regimes of human serum albumin: relevance in diabetes associated complications, RSC Adv., 2015, 5(78), 63605–63614 RSC.
- M. A. Husain, T. Sarwar, S. U. Rehman, H. M. Ishqi and M. Tabish, Ibuprofen causes photocleavage through ROS generation and intercalates with DNA: a combined biophysical and molecular docking approach, Phys. Chem. Chem. Phys., 2015, 17, 13837–13850 RSC.
- O. Trott and A. J. Olson, AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading, J. Comput. Chem., 2010, 31(2), 455–461 CAS.
- P. Pandya, L. K. Agarwal, N. Gupta and S. Pal, Molecular recognition pattern of cytotoxic alkaloid vinblastine with multiple targets, J. Mol. Graphics Modell., 2014, 54, 1–9 CrossRef CAS PubMed.
- G. M. Morris, D. S. Goodsell, R. S. Halliday, R. Huey, W. E. Hart, R. K. Belew and A. J. Olson, Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function, J. Comput. Chem., 1998, 19(14), 1639–1662 CrossRef CAS.
- J. Leclère and I. Birlouez-Aragon, The fluorescence of advanced Maillard products is a good indicator of lysine damage during the Maillard reaction, J. Agric. Food Chem., 2001, 49(10), 4682–4687 CrossRef.
- M. E. Westwood and P. J. Thornalley, Molecular characteristics of methylglyoxal-modified bovine and human serum albumins. Comparison with glucose-derived advanced glycation endproduct-modified serum albumins, J. Protein Chem., 1995, 14(5), 359–372 CrossRef CAS PubMed.
- D. Matulis and R. Lovrien, 1-Anilino-8-naphthalene sulfonate anion-protein binding depends primarily on ion pair formation, Biophys. J., 1998, 74(1), 422–429 CrossRef CAS PubMed.
- S. A. Bhat, A. Sohail, A. A. Siddiqui and B. Bano, Effect of non-enzymatic glycation on cystatin: a spectroscopic study, J. Fluoresc., 2014, 24(4), 1107–1117 CrossRef CAS PubMed.
- T. A. Khan, M. Saleemuddin and A. Naeem, Partially folded glycated state of human serum albumin tends to aggregate, Int. J. Pept. Res. Ther., 2011, 17(4), 271–279 CrossRef CAS.
- R. Nagai, K. Matsumoto, X. Ling, H. Suzuki, T. Araki and S. Horiuchi, Glycolaldehyde, a reactive intermediate for advanced glycation end products, plays an important role in the generation of an active ligand for the macrophage scavenger receptor, Diabetes, 2000, 49(10), 1714–1723 CrossRef CAS PubMed.
- R. Gomes, M. S. Silva, A. Quintas, C. Cordeiro, A. Freire, P. Pereira, A. Martins, E. Monteiro, E. Barroso and A. P. Freire, Argpyrimidine, a methylglyoxal-derived advanced glycation end-product in familial amyloidotic polyneuropathy, Biochem. J., 2005, 385(2), 339–345 CrossRef CAS PubMed.
- S. Awasthi and N. T. Saraswathi, Vanillin restrains non-enzymatic glycation and aggregation of albumin by chemical chaperone like function, Int. J. Biol. Macromol., 2016, 87, 1–6 CrossRef CAS PubMed.
- M. Khajehpour, J. L. Dashnau and J. M. Vanderkooi, Infrared spectroscopy used to evaluate glycosylation of proteins, Anal. Biochem., 2006, 348(1), 40–48 CrossRef CAS PubMed.
- M. F. Beal, Oxidatively modified proteins in aging and disease 1, 2, Free Radicals Biol. Med., 2002, 32(9), 797–803 CrossRef CAS PubMed.
- N. Sreerama and R. W. Woody, A self-consistent method for the analysis of protein secondary structure from circular dichroism, Anal. Biochem., 1993, 209(1), 32–44 CrossRef CAS PubMed.
- J. Jaumot and R. Gargallo, Experimental methods for studying the interactions between G-quadruplex structures and ligands, Curr. Pharm. Des., 2012, 18(14), 1900–1916 CrossRef CAS PubMed.
- H. Sun, J. Xiang, Y. Liu, L. Li, Q. Li, G. Xu and Y. Tang, A stabilizing and denaturing dual-effect for natural polyamines interacting with G-quadruplexes depending on concentration, Biochimie, 2011, 93(8), 1351–1356 CrossRef CAS PubMed.
- S. Tabassum, W. M. Al-Asbahy, M. Afzal, F. Arjmand and R. H. Khan, Interaction and photo-induced cleavage studies of a copper based chemotherapeutic drug with human serum albumin: spectroscopic and molecular docking study, Mol. BioSyst., 2012, 8(9), 2424–2433 RSC.
- M. R. Eftink, Fluorescence quenching reactions, in Biophysical and biochemical aspects of fluorescence spectroscopy, Springer, US, 1991, pp. 1–41 Search PubMed.
- J. Mariam, P. M. Dongre and D. C. Kothari, Study of interaction of silver nanoparticles with bovine serum albumin using fluorescence spectroscopy, J. Fluoresc., 2011, 21(6), 2193–2199 CrossRef CAS PubMed.
- A. Bhogale, N. Patel, P. Sarpotdar, J. Mariam, P. M. Dongre, A. Miotello and D. C. Kothari, Systematic investigation on the interaction of bovine serum albumin with ZnO nanoparticles using fluorescence spectroscopy, Colloids Surf., B, 2013, 102, 257–264 CrossRef CAS PubMed.
- M. Ishtikhar, S. Khan, G. Badr, A. O. Mohamed and R. H. Khan, Interaction of the 5-fluorouracil analog 5-fluoro-2′-deoxyuridine with ‘N’and ‘B’isoforms of human serum albumin: a spectroscopic and calorimetric study, Mol. BioSyst., 2014, 10(11), 2954–2964 RSC.
- Q. Saquib, A. A. Al-Khedhairy, S. A. Alarifi, S. Dwivedi, J. Mustafa and J. Musarrat, Fungicide methyl thiophanate binding at sub-domain IIA of human serum albumin triggers conformational change and protein damage, Int. J. Biol. Macromol., 2010, 47(1), 60–67 CrossRef CAS PubMed.
- S. Monti, S. Ottani, F. Manoli, I. Manet, F. Scagnolari, B. Zambelli and G. Marconi, Chiral recognition of 2-(3-benzoylphenyl) propionic acid (ketoprofen) by serum albumin: an investigation with microcalorimetry, circular dichroism and molecular modelling, Phys. Chem. Chem. Phys., 2009, 11(40), 9104–9113 RSC.
- P. D. Ross and S. Subramanian, Thermodynamics of protein association reactions: forces contributing to stability, Biochemistry, 1981, 20(11), 3096–3102 CrossRef CAS PubMed.
- M. Ishtikhar, G. Rabbani and R. H. Khan, Interaction of 5-fluoro-5′-deoxyuridine with human serum albumin under physiological and non-physiological condition: a biophysical investigation, Colloids Surf., B, 2014, 123, 469–477 CrossRef CAS PubMed.
- N. Ibrahim, H. Ibrahim, S. Kim, J. P. Nallet and F. Nepveu, Interactions between antimalarial indolone-N-oxide derivatives and human serum albumin, Biomacromolecules, 2010, 11(12), 3341–3351 CrossRef CAS PubMed.
- J. N. Miller, Recent advances in molecular luminescence analysis, in Proc Anal Div Chem Soc, 1979, vol. 16, no. 7, pp. 203–208 Search PubMed.
- U. Kragh-Hansen, Molecular aspects of ligand binding to serum albumin, Pharmacol. Rev., 1981, 33(1), 17–53 CAS.
- T. Chakraborty, I. Chakraborty, S. P. Moulik and S. Ghosh, Physicochemical and conformational studies on BSA–surfactant interaction in aqueous medium, Langmuir, 2009, 25(5), 3062–3074 CrossRef CAS PubMed.
- G. D. Sudlow, D. J. Birkett and D. N. Wade, The characterization of two specific drug binding sites on human serum albumin, Mol. Pharmacol., 1975, 11(6), 824–832 CAS.
- M. W. Freyer and E. A. Lewis, Isothermal titration calorimetry: experimental design, data analysis, and probing macromolecule/ligand binding and kinetic interactions, Methods Cell Biol., 2008, 84, 79–113 CAS.
- M. Ishtikhar, G. Rabbani and R. H. Khan, Interaction of 5-fluoro-5′-deoxyuridine with human serum albumin under physiological and non-physiological condition: a biophysical investigation, Colloids Surf., B, 2014, 123, 469–477 CrossRef CAS PubMed.
- A. A. Thoppil, R. Sharma and N. Kishore, Complexation of β-lactam antibiotic drug carbenicillin to bovine serum albumin: energetics and conformational studies, Biopolymers, 2008, 89(10), 831–840 CrossRef CAS PubMed.
- E. Ahmad, G. Rabbani, N. Zaidi, S. Singh, M. Rehan, M. M. Khan, S. K. Rahman, Z. Quadri, M. Shadab, M. T. Ashraf and N. Subbarao, Stereo-selectivity of human serum albumin to enantiomeric and isoelectronic pollutants dissected by spectroscopy, calorimetry and bioinformatics, PLoS One, 2011, 6(11), e26186 CAS.
- H. Watanabe, S. Tanase, K. Nakajou, T. Maruyama, U. Kragh-Hansen and M. Otagiri, Role of Arg-410 and Tyr-411 in human serum albumin for ligand binding and esterase-like activity, Biochem. J., 2000, 349(3), 813–819 CrossRef CAS PubMed.
- M. T. Rehman, H. Shamsi and A. U. Khan, Insight into the binding mechanism of imipenem to human serum albumin by spectroscopic and computational approaches, Mol. Pharmaceutics, 2014, 11(6), 1785–1797 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra12321j |
| This journal is © The Royal Society of Chemistry 2016 |