A novel approach towards hydrazine sensor development using SrO·CNT nanocomposites
Abstract
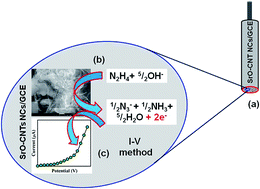
Strontium oxide nanoparticle decorated carbon nanotube nanocomposites (SrO·CNT NCs) were prepared in alkaline medium using a wet-chemical technique at low temperature. The SrO·CNT NCs were investigated using Fourier transform infrared (FT-IR) spectroscopy, UV/visible spectroscopy, field emission scanning electron microscopy (FESEM) coupled with electron dispersive spectroscopy (EDS), X-ray photoelectron spectroscopy (XPS), and X-ray powder diffraction (XRD) methods. A selective hydrazine sensor on a glassy carbon electrode (GCE) was fabricated with a thin-layer of the NCs using conducting coating binders. Improved electrochemical responses, a higher sensitivity including a large dynamic range, and long-term stability towards hydrazine were acquired using the fabricated SrO·CNT NCs/GCE sensor. The calibration curve was found to be linear (r2: 0.7311) over a wide range of hydrazine concentrations (0.2 nM to 0.2 M). The detection limit and sensitivity were calculated as 0.036 nM and ∼26.37 μA mM−1 cm−2 respectively. In this approach, hydrazine was detected using a current vs. voltage (I–V) method and a SrO·CNT NC modified GCE electrode with very high sensitivity compared to various nanomaterials. The synthesis of SrO·CNT NCs using a wet-chemical process is a unique way of establishing sensor based nanocomposites for toxic and carcinogenic agents.

 Please wait while we load your content...
Please wait while we load your content...