MoS2 quantum dot decorated g-C3N4 composite photocatalyst with enhanced hydrogen evolution performance†
Xixiong Jinab,
Xiangqian Fanab,
Jianjian Tianab,
Ruolin Chengab,
Mengli Liab and
Lingxia Zhang*ab
aState Key Laboratory of High Performance Ceramics and Superfine Microstructure, Shanghai Institute of Ceramics, Chinese Academy of Sciences, 1295 Ding-xi Road, Shanghai 200050, People's Republic of China. E-mail: zhlingxia@mail.sic.ac.cn
bUniversity of Chinese Academy of Sciences, 19A Yuquan Road, Beijing 100049, People's Republic of China
First published on 26th May 2016
Abstract
A novel MoS2 quantum dot (QD) decorated g-C3N4 (MoS2-QDs/CN) heterostructured photocatalyst has been synthesized via a simple wetness impregnation method. The as-prepared MoS2-QDs/CN composite exhibited strong absorption in the visible light region. Its photocatalytic activity on hydrogen evolution was evaluated by splitting water under visible light irradiation (λ > 420 nm). The highest H2 yield of 19.66 μmol h−1 was achieved on 50 mg 1 wt% MoS2-QDs/CN photocatalyst (loaded with 1 wt% Pt), which was 6.6 and 1.84 times those on pure g-C3N4 and 1 wt% Pt loaded g-C3N4, respectively.
1 Introduction
The giant gap between the rapid development of industrialization and the efficiency to harness fossil fuel has led to the imminent energy crisis.1 Furthermore, the continuous consumption of fossil fuel has caused ever-increasing environmental pollution. Therefore, the search for clean energy has become the global research emphasis. Hydrogen, with the fuel value (∼143 kJ g−1) three times higher than gasoline and the characteristics of environment friendliness and transportation convenience, is a possible candidate to replace gasoline.2,3 In 1972, Fujishima and Honda discovered a novel photocatalytic hydrogen evolution method.4 Since then, photocatalysis has been considered as one of the most promising strategies to achieve green and low-cost energy.Most of the studies on photocatalysis have been focused on the preparation of efficient photocatalysts. TiO2 has been once considered as an option for efficient photocatalyst over the past decades. But the over-wide band-gap of TiO2 (3.2 eV) limits its absorption range in the ultra violet region. Then in 2009, Antonietti et al. first reported the visible light photocatalytic activity of graphitic carbon nitride (g-C3N4),5 making it a promising candidate for efficient photocatalysis.
The discovery of g-C3N4 can be dated back to 1834, when g-C3N4 was synthesized by Berzelius in 1834 and named as “melon” by Liebig.6 In 1922, Franklin did further research on melon and firstly introduced the concept of carbon nitride.7 g-C3N4 is the most stable form of carbon nitride, in which carbon and nitrogen atoms are sp2 bonded, leading to the π-conjugated, 2-dimensional graphite-like structure.8–10 Compared with other inorganic semiconductor photocatalysts, g-C3N4 is more stable with respect to thermal and chemical attack. Also, low health and environment risks make it safer to put into application. Beyond these, g-C3N4 has an appealing electronic structure (band gap = ∼2.7 eV, conduction band at ∼−0.8 eV and valence band at ∼1.9 eV), which enormously widens its light adsorption range to visible region.
However, g-C3N4 still suffers problems such as low quantum efficiency and high rate of carrier recombination, which have enormously affected the photocatalytic performance of pure g-C3N4. Series of methods have been tried to improve its photocatalytic performance, including metal doping,11,12 graphene13 and semiconductor14,15 coupling, copolymer constructing16,17 etc.
Molybdenum disulfide (MoS2) has recently become an attractive material in photodetector,18 photovoltaic cells,19 electrocatalyst20 and photocatalyst,15 due to its high mobility at room temperature (∼410 cm2 V−1 s−1) with on/off ratio of 108 and excellent optical absorption property. The S–Mo–S coordination in the crystal lattice generates unsaturated Mo and S atoms at the edge (Fig. S1†), leading to the unique “edge activity” of MoS2.21,22 MoS2 achieved large enhancement in electrocatalytic H2 evolution reaction (HER) when it was hybridized with Au,23 and graphene.24,25 MoS2 has been fabricated into different forms to achieve this goal, such as nanoparticles,26 nanosheets,27 and nanoflowers.28 Above them, the higher quantum confinement and small-size effect of MoS2 quantum dots (MoS2-QDs) leads to unsaturated bonds both on surfaces and edges, providing more opportunity to connect with other atoms and obtain higher activity toward HER.20 Also, MoS2-QDs can gather the photo-induced electron around the edges and efficiently prevent charge recombination. Therefore, MoS2-QD is a promising candidate as cocatalyst in photocatalytic hydrogen evolution.
Herein, we synthesized MoS2-QDs decorated g-C3N4 (MoS2-QDs/CN) via a facile impregnation route. MoS2-QDs were obtained via a simple sonication method from commercialized MoS2 powder,29 which is environment-friendly and cost-effective. The MoS2-QDs/CN composite showed much enhanced photocatalytic activity for hydrogen evolution by water-splitting under visible light irradiation.
2 Experimental
2.1 Synthesis of MoS2-QDs/CN photocatalyst
All reagents, including (NH2)2CO (AR, Sinopharm Chemical Reagent Corp, P. R. China), MoS2 powder (99%, <2 μm, Sigma Aldrich) and N-methyl-2-pyrrolidinone (98%, Sigma-Aldrich) were used directly without any further purification.The metal-free g-C3N4 powder was prepared by calcining 20 g of urea in an alumina crucible at 550 °C for 4 h with a ramp of 5 °C min−1. The product was collected and grinded into powder.
MoS2-QDs synthesis involves bath sonication and probe sonication of MoS2 powder.29 200 mg of MoS2 was added into 20 mL of N-methyl-2-pyrrolidinone (NMP) and sonicated continuously in ultrasonic bath for 3.5 h. Then, the dispersion was further sonicated with a sonic probe for 3.5 h. The as-prepared dispersion was kept undisturbed overnight to settle down bulk MoS2. The top two-thirds of the dispersion was sucked out and centrifuged at 5500 rpm for 90 min. Finally, the quantum dots were extracted and washed with distilled water and ethanol, and then re-dispersed in a certain amount of distilled water to obtain a 5 mg mL−1 MoS2-QDs suspension.
MoS2-QDs/CN composite photocatalysts were prepared as follows: 0.3 g bulk g-C3N4 was dispersed in 300 mL deionized water and sonicated for 30 min. Certain amount of MoS2-QDs suspension was added drop-wisely into the as-dispersed g-C3N4 and stirred for 24 h. After that, the product was collected by centrifugation, washed with ethanol, and dried at 70 °C for 24 h. Finally, the sample was calcined at 300 °C for 1 h and named as x% MoS2-QDs/CN, where x = 0.5, 1, 3 and 7 wt, representing the mass percentage of MoS2-QDs in the composite.
2.2 Characterizations
The crystal structure of MoS2-QDs/CN composite photocatalysts was investigated by X-ray diffraction (XRD; Rigaku D/Max 2200PC) with Cu Kα radiation operated at 40 kV of acceleration voltage and 40 mA of applied current. The scanning rate was maintained at 4° min−1 in the 2θ range of 10–80°. Infrared spectra were obtained on KBr pellets on a Thermofischer Nicolet IS50 spectrometer. The morphology of the samples was recorded by transmission electron microscopy (TEM; FEI Tecnai G2F20) operated at 200 kV. UV-Vis diffuse reflection spectroscopy was examined on a Shimadzu UV-3600 spectrometer, using BaSO4 as reference. X-ray photoelectron spectroscopy (XPS) measurements were performed on a Thermo Scientific Escalab 250 spectrometer with Al Kα radiation line source and the binding energies for the high resolution spectra were calibrated by setting C 1s to 285 eV. The photoluminescence (PL) spectra of the samples were obtained by Edinburgh Instruments FLSP-920 fluorescence spectrophotometer with an excitation wavelength of 400 nm at room temperature.2.3 Photocatalytic activity
The photocatalytic activity of MoS2-QDs/CN photocatalyst for water-splitting hydrogen evolution was conducted in a glass reactor. The reactor was connected with gas chromatography (Techcomp GC7900, high purity nitrogen as carrier gas), and 300 W Xe lamp (λ > 420 nm) was used as light source. 50 mg of photocatalyst was dispersed in 90 mL of deionized water, along with 10 mL of triethanolamine. As for loading 1 wt% Pt on the photocatalysts, 1 mL of 0.5 g L−1 H2PtCl6 was added into the dispersion. Then, the reactor was swept by nitrogen flow for 15 min to remove the dissolved oxygen. The dispersion was stirred and irradiated under 300 W Xe lamp for 1 h to reduce Pt species. Next, the reactor was swept again by nitrogen flow for 15 min to remove the additional hydrogen. During the photocatalytic experiment, 1.5 mL gas sample in the reactor was extracted and analyzed by gas chromatography every hour.2.4 Electrochemical characterizations
The electrochemical performance of MoS2-QDs/CN was tested on a CHI 660A electrochemical workstation (Chenhua Instrument, Inc.) in a standard three-electrode system consisting of Pt wire as counter electrode, Ag/AgCl (in saturated KCl) as reference electrode and sample-coated indium tin oxides (ITO) glass as working electrode. Na2SO4 (0.2 M) aqueous solution was used as the electrolyte. The working electrode was prepared as follows: 20 mg of the grinded sample was mixed with 40 mg iodine in a testing tube, followed by adding acetone to full and sonicating the slurry for 30 min. Then, the dispersion was transferred into a 20 mL beaker and two pieces of 2 cm × 0.5 cm ITO glass were sunk into the dispersion with 10 V applied voltage for 5 min. Finally, the ITO glass with sample film was calcined at 200 °C for 2 h. EIS curves were obtained at −0.4 V vs. Ag/AgCl. Irradiation used in the photocurrent tests was from a Xe lamp with a UV filter (λ > 420 nm) and obtained at 0.4 V vs. Ag/AgCl. Mott–Schottky plots were collected at a frequency of 962 Hz in dark.3 Results and discussion
3.1 Characterizations of MoS2-QDs/CN
The FT-IR spectra in Fig. 1 show that both the MoS2-QDs/CN composite and pure g-C3N4 have peaks at 810 cm−1, 1200–1690 cm−1 and 3000–3300 cm−1, which can be assigned to the graphite-like structure and stretching modes of C–N heterocylces.13,30 For MoS2-QDs/CN composite and MoS2-QDs, there is a peak at 669 cm−1 corresponding to MoS2, indicating the successful incorporation of MoS2 in the composite. The XRD patterns (Fig. 1c) also support this result. There are two diffraction peaks in the XRD pattern of pure g-C3N4. The strong peak at 27.6° represents the interlayer stacking of the conjugated aromatic compounds, indexing to the graphite-like material with the d-spacing of 0.323 nm. The weak one at 12.7° corresponds to the (100) plane of the melon structure with an interplanar separation of 0.696 nm. For MoS2-QDs/CN composite, one strong peak at (002) plane and six other small peaks that fit well with MoS2 (JCPDS 77-1716), can be observed. It can be concluded that the loading of MoS2 has not destroyed the graphite-like structure of g-C3N4. However, in the previous study of Gopalakrishnan et al.,29 only one peak of (002) plane was observed in the XRD pattern of MoS2-QDs. The possible reason is that in our composite, some MoS2-QDs form nanoclusters on the surface of g-C3N4 as observed by TEM (Fig. 2a). And yet considering the relatively stronger intensity of the (002) plane compared with the other six peaks, most MoS2-QDs are still well dispersed on the surface of g-C3N4.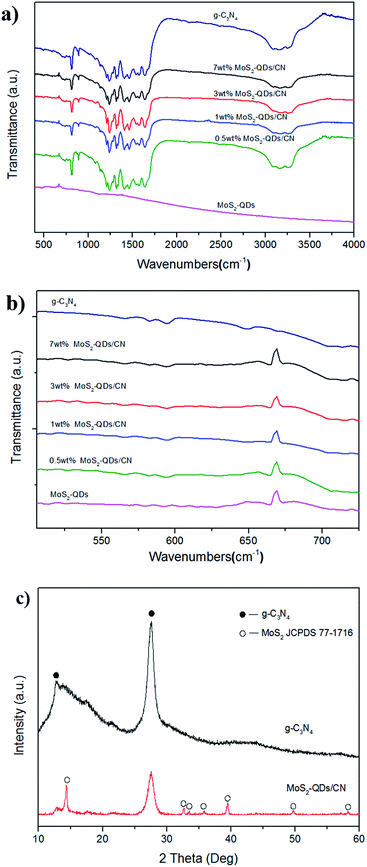 | ||
| Fig. 1 (a and b) The FT-IR spectra of g-C3N4, MoS2-QDs/CN and MoS2-QDs; (c) XRD patterns of g-C3N4 and MoS2-QDs/CN composite. | ||
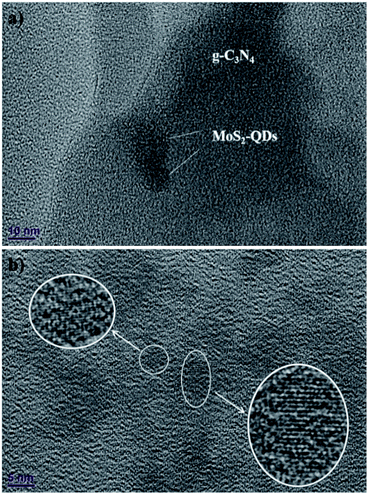
The TEM image in Fig. 2a exhibits the micrograph of MoS2-QDs/CN composite, where the sheet-like g-C3N4 is decorated with crystallized MoS2-QDs. The diameter of MoS2-QDs prepared by sonication has been well controlled at ∼10 nm and the crystallinity of MoS2 has been well preserved, as revealed by the striation observed in the partially amplified image inserted in Fig. 2b. However, the increasing introduction of MoS2-QDs has led to the agglomeration of MoS2-QDs as observed in Fig. S2.†
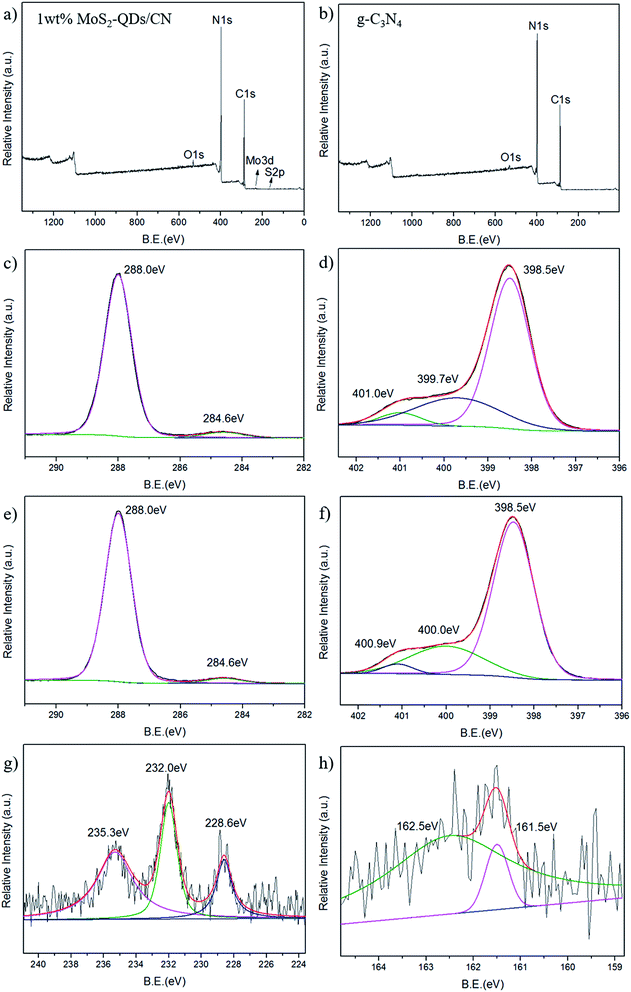
The chemical composition and elemental states in 1 wt% MoS2-QDs/CN composite and g-C3N4 was investigated by XPS (Fig. 3). The XPS survey spectrum of the composite is shown in Fig. 3a. Except for three peaks at 529.2 eV, 399.3 eV and 291.9 eV corresponding to O, N, and C, respectively, which can also be observed in survey spectrum of g-C3N4, two more peaks at the binding energies of 234.4 eV and 161.5 eV assigned to Mo and S can be observed.
The high-resolution C 1s and N 1s XPS spectra of the 1 wt% MoS2-QDs/CN composite and g-C3N4 are shown in Fig. 3c–f. The C 1s peak in Fig. 3c and e can be deconvoluted into two peaks with binding energies of ∼288.0 eV and ∼284.6 eV, which can be assigned to carbon sp2 bonded to the three nitrogen atoms in triazine rings38 and the surface graphitic or amorphous carbons39,40 respectively. Both N 1s spectra in Fig. 3d and f exhibit an asymmetrical peak, indicating the existence of nitrogen atoms in multiple chemical-environment. The N 1s peak of pure g-C3N4 can be deconvoluted into three fitting Gaussian peaks at 401.0 eV, 399.7 eV and 398.5 eV. The former two peaks can be attributed to the conjugated nitrogen atom41 and amino groups.42 The peak at 398.5 eV corresponds with the nitrogen atom sp2 bonded to two carbon atoms, further confirming the sp2-bonded graphite-like structure of g-C3N4.10 After MoS2-QDs is coupled with g-C3N4, the two peaks of the conjugated nitrogen atom and the nitrogen atom of amino groups shift to 400 and 400.9 eV, respectively, revealing the formation of substantial interface and the intimate interaction between MoS2 and g-C3N4. The peak at 398.5 eV is identically same as pure g-C3N4, suggesting that the triazine structure of g-C3N4 has been well-preserved after the introduction of MoS2-QDs.
In Mo 3d spectrum of 1 wt% MoS2-QDs/CN composite (Fig. 3g), three peaks at 235.3 eV, 232.0 eV and 228.6 eV can be observed. The Mo 3d3/2 peak at 235.3 eV is attributed to Mo6+ in MoO3.43 The latter Mo 3d5/2 peaks at 232.0 eV and 228.6 eV correspond to the Mo6+ in MoO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 and Mo4+ in MoS2,44 respectively. The S 2p spectrum in Fig. 3h shows a doublet located at 161.5 and 162.5 eV corresponding to the S 2p3/2 strongly adsorbed to MoS2 (110)45 and the S 2p3/2 in MoS2,44 respectively. Both the high resolution spectra of Mo 3d and S 2p confirm the existence of MoS2-QDs in the composite.
46 and Mo4+ in MoS2,44 respectively. The S 2p spectrum in Fig. 3h shows a doublet located at 161.5 and 162.5 eV corresponding to the S 2p3/2 strongly adsorbed to MoS2 (110)45 and the S 2p3/2 in MoS2,44 respectively. Both the high resolution spectra of Mo 3d and S 2p confirm the existence of MoS2-QDs in the composite.
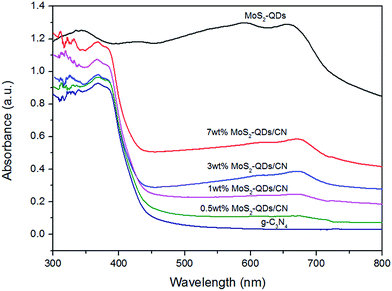
The optical absorption of g-C3N4, MoS2-QDs and MoS2-QDs/CN composite was investigated by UV-Vis absorption spectra (Fig. 4). The absorption edge of g-C3N4 is at ∼450 nm, corresponding to the band gap of 2.65 eV. And the absorption intensity of MoS2-QDs triumphs over all other samples. The composite samples' absorption edges slightly red-shift. The excitonic peak at 660 nm arising from the K point of the Brillouin zone of MoS2 can be clearly observed both in the composite samples and in MoS2-QDs, indicating MoS2-QDs has been successfully decorated on the surface of g-C3N4.29 The absorption of the composite samples intensifies with the increasing content of MoS2-QDs, suggesting the close connection between the introduction of MoS2-QDs and the improved light absorption of the MoS2-QDs/CN composite. And the band-gap results shown in Table S1† exhibit the decreased band-gap of the composite with the increasing content of MoS2-QDs. Previous studies exhibit that nanostructured semiconductor usually has larger band-gap than its bulk counterpart because of quantum confinement effect and consequently makes the adjusting of its absolute CB and VB potentials.31–34 According to TEM observation, higher content of MoS2-QDs is more likely to form nanoclusters, leading to narrower band-gap of the quantum dots and thus shortening the band-gap of the composite probably by shrinking the potential difference between the CB edge of MoS2-QDs and the VB edge of g-C3N4.
To further investigate the influence of MoS2-QDs incorporation on the band-gap of the composite, Mott–Schottky tests were performed to confirm the electronic potentials of the composite and MoS2-QDs. The measured potentials has been converted to the reversible hydrogen electrode (RHE) scale via the Nernst equation: ERHE = EAg/AgCl + 0.05916pH + E0Ag/AgCl, in which EAg/AgCl is the potential measured against the Ag/AgCl reference electrode while E0Ag/AgCl stands for the standard potential of Ag/AgCl electrode at 298 K (0.1976 V). The Mott–Schottky curves of MoS2-QDs and 1 wt% MoS2-QDs/CN are shown in Fig. 5a and b, respectively. The calculated CB edge of pure MoS2-QDs is at −1.20 eV. However, two obvious slopes can be observed in Fig. 5b, which can be assigned to g-C3N4 and MoS2-QDs in the composite, whose calculated conduction band edges are located at −1.55 eV and −1.40 eV, respectively. The result reveals that the nanocluster formation of MoS2-QDs causes the downshifting of its CB edge and thus the shrinkage of the band-gap of the composite, well supporting the above speculation on the relationship between the MoS2-QDs content and the change of the composite band-gap. The possible mechanism is illustrated in Fig. S4.†
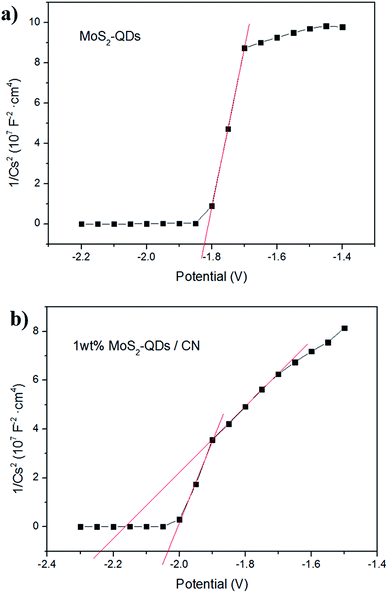 | ||
| Fig. 5 Mott–Schottky plots collected on (a) MoS2-QDs and (b) 1 wt% MoS2-QDs/CN composite at a frequency of 962 Hz in dark. | ||
The photoluminescence (PL) spectra of pure g-C3N4 and MoS2-QDs/CN composite samples are shown in Fig. 6. All the photocatalysts exhibit broad PL peak centered at ∼450 nm under the 400 nm excitation at room temperature. This emission with the photon energy equal to the band-gap of g-C3N4 could result from the recombination of the photogenerated charge carriers corresponding to intrinsic HOMO–LUMO transition. Comparing all the spectra, we can find that PL intensity decreases with the increased incorporation of MoS2-QDs. At ∼460 nm, there is a strong peak caused by charge transfer emission. In pure g-C3N4, the electron transfers from donor (the conjugated nitrogen) to acceptor (3-s-triazine ring),17 while in the composite, driven by the build-in electric field, the photogenerated electrons on the CB of g-C3N4 can immigrate to the CB of MoS2-QDs and meanwhile the holes generated on the VB of MoS2 can transfer to the VB of g-C3N4. Therefore, MoS2-QDs incorporation can effectively prevent the recombination of photogenerated electrons and holes, which will directly contribute to the improved photocatalytic performance of the composite.
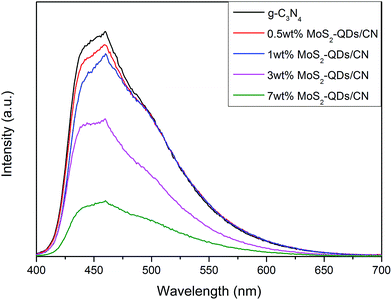 | ||
| Fig. 6 Photoluminescence spectra of pure g-C3N4 and MoS2-QDs/CN composite samples at an excitation wavelength of 400 nm. | ||
3.2 Photocatalytic activity
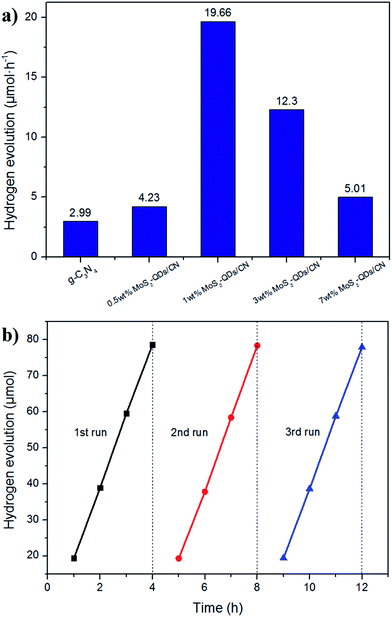
Photocatalytic hydrogen evolution over different MoS2-QDs/CN composite samples with 1 wt% Pt was evaluated under visible light irradiation (λ > 420 nm) using triethanolamine as sacrificial agent to consume photogenerated holes. Fig. 7a presents the hydrogen evolution of MoS2-QDs/CN photocatalysts loaded with different amount of MoS2 quantum dots.The pure g-C3N4 sample without loading cocatalysts evolves H2 at the rate of 2.99 μmol h−1, while the highest H2 evolution rate of 19.66 μmol h−1 has been achieved on 50 mg 1 wt% MoS2-QDs/CN, which is about 6.6 times higher than that on pure g-C3N4. On the other side, the further increased addition of MoS2-QDs leads to an obvious decrease in photocatalytic hydrogen production. Possible reason can be explained as follows: the increased introduction of MoS2-QDs leads to the agglomeration of quantum dots, namely the decrease of active sites on the edges, and consequently the decrease of H2 evolution.
The aggregates of MoS2-QDs also cause the gradual shrinkage of the quantum dots' band-gap, resulting in the gradual decrease of the composite band-gap as illustrated in Fig. S4.† The close distance between CB edges of MoS2-QDs and g-C3N4 would weaken the driven force and impede the transfer of charge carriers. Therefore, 1 wt% of MoS2-QDs incorporation into g-C3N4 could lead to a balance between the suitable band-gap structure, the high mobility of charge transfer and the low probability of charge recombination, thus achieving the best photocatalytic performance.
Control experiments were conducted to further determine the role of MoS2-QDs in photocatalytic H2 evolution (Fig. S5†). Pure g-C3N4 showed a relatively low H2 evolution rate (2.99 μmol h−1) than MoS2-QDs/CN (7.43 μmol h−1) and the H2 evolution rate on g-C3N4 loaded with Pt (10.71 μmol h−1) was much less than that on MoS2-QDs/CN loaded with Pt (19.66 μmol h−1), suggesting the contribution of MoS2-QDs to the improved H2 evolution.
A 12 h cycle experiment with intermittence in every 4 h was performed on 1 wt% MoS2-QDs/CN composite photocatalyst to examine its stability (Fig. 7b). Only a slight decrease of H2 evolution rate happened in the third run (19.48 μmol h−1) and the average H2 evolution rate maintained at 19.58 μmol h−1.
3.3 Electrochemical characterizations
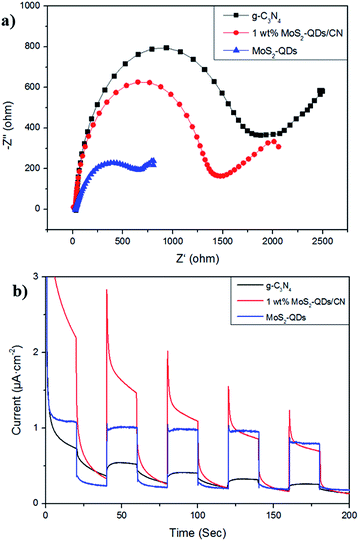
The charge transfer in g-C3N4, 1 wt% MoS2-QDs/CN and MoS2-QDs were studied by EIS in dark. As shown in Fig. 8a, the semicircular diameter of MoS2-QDs is the smallest among all the samples, demonstrating the excellent conductivity of the catalyst. And the electrochemical impedance of 1 wt% MoS2-QDs/CN is smaller than that of CN, suggesting the decoration of MoS2-QDs has improved the conductivity of g-C3N4 and further accelerated charge transfer. This phenomenon further proves the lowered recombination rate of photogenerated carriers in the composite and consequently the increased number of charge carriers evolved in photocatalytic reaction due to the introduction of MoS2-QDs, which agrees well with the above PL result in Fig. 6.The photocurrent responses of g-C3N4, 1 wt% MoS2-QDs/CN and MoS2-QDs were investigated for several on–off cycles of irradiation (Fig. 8b). It is clear that the photocurrent intensity of 1 wt% MoS2-QDs/CN is much higher than that of g-C3N4, which is in accordance with the improved photocatalytic H2 evolution rate on 1 wt% MoS2-QDs/CN. The spectra also suggest the improved photocatalytic activity of the composite can be attributed to the decoration of MoS2-QDs, which has greatly improved the photo-response of the photocatalyst and the separation/transfer ability of charge carriers in the composite.
3.4 Discussion on photocatalytic mechanism
Based on the above characterizations, the enhanced photocatalytic performance of the MoS2-QDs/CN composite can be attributed to the enhanced light absorption and the improved charge transfer ability resulting from the synergetic effect of MoS2-QDs and g-C3N4 together with Pt. The possible photocatalytic mechanism of the MoS2-QDs/CN composite was illustrated in Fig. 9.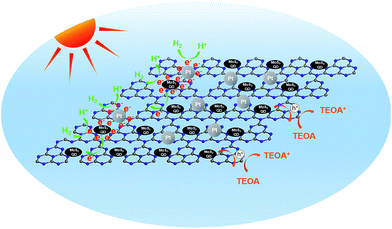 | ||
| Fig. 9 Schematic illustration of the generation, separation, and transfer of electron–hole pairs for photocatalytic hydrogen evolution in the MoS2-QDs/CN composite. | ||
Mott–Schottky plots (Fig. 5b) determine the CB potentials of g-C3N4 and MoS2 at −1.55 eV and −1.40 eV, respectively. In the composite, the top part of the VB is mainly dominated by g-C3N4, while the bottom part of the CB is mainly contributed by MoS2-QDs. Such band alignment results in the formation of a type-II heterojunction (Fig. S6†).35–37 When the composite is irradiated, electrons can be excited from CBs of both g-C3N4 and MoS2-QDs, which can easily transfer from the surface of g-C3N4 to the edges of MoS2-QDs, inciting HER at the active edges of MoS2-QDs. With sufficient chemical potential from the CB of MoS2-QDs to Pt, electrons are also inclined to accumulate on Pt to prolong their life-time and finally react with protons. On the other hand, the holes generated from MoS2-QDs can easily move to g-C3N4 due to the type-II band alignment, leading to the redistribution of electrons and holes so that the oxidation reaction is highly likely to take place and effectively prevent the electron–hole recombination process and resulting in the improved hydrogen evolution performance of the MoS2-QDs/CN composite photocatalyst.
As mentioned above, no H2 has been produced on MoS2-QDs. But the decoration of MoS2-QDs on g-C3N4 has greatly improved the H2 evolution from 2.99 μmol h−1 on pure g-C3N4 to 7.43 μmol h−1 on MoS2-QDs/CN in the absence of Pt, indicating the synergetic catalysis effect between MoS2-QDs and g-C3N4. Specifically, MoS2-QDs loading has enhanced the visible light absorption, and the well-matched band structure of MoS2-QDs and g-C3N4 facilitates the separation and transfer of charge carriers in the composite. Moreover, the photocatalytic performance of MoS2-QDs/CN is better than the previously reported g-C3N4 decorated with bulk MoS2,15 which is more likely to be attributed to the more prominent edge activity of MoS2-QDs. When Pt, as an electron trapper, was loaded on these catalysts, the H2 yields have been further increased to 10.71 and 19.66 μmol h−1 on Pt/CN and MoS2-QDs/Pt/g-C3N4, respectively. However, no obvious synergetic effect between MoS2-QDs and Pt has been observed. Comparing the H2 evolution amount on 1 wt% MoS2-QDs/CN (7.43 μmol h−1) and 1 wt% Pt/CN (10.71 μmol h−1), MoS2-QDs have great potential to replace noble metal as cocatalyst for photocatalytic HER.
4 Conclusions
In summary, a new MoS2-QDs/g-C3N4 heterostructured photocatalyst (MoS2-QDs/CN) has been synthesized via a facile impregnation method. The obtained composite photocatalyst exhibits enhanced absorption in visible light range compared with pure g-C3N4. The introduction of MoS2-QDs effectively prevents charge recombination and improves the photocatalytic H2 evolution activity. The highest H2 evolution rate of 19.66 μmol h−1 has been achieved on 50 mg of 1 wt% MoS2-QDs/CN photocatalyst, which is 6.6 times higher than that on pure g-C3N4. The band-structure of MoS2-QDs/CN composite results in a type-II heterostructure, which is preferable in preventing the recombination of photo-induced carriers. The photocatalytic performance of MoS2-QDs/CN is superior to the previously reported g-C3N4 decorated with bulk MoS2,15 because of the more prominent edge activity of MoS2-QDs. MoS2-QDs have great potential to replace noble metal cocatalyst for photocatalytic HER. The novel MoS2-QDs/CN composite is a promising candidate for visible light H2 evolution, and provides inspiration on the development of other heterostructured photocatalysts for solar fuel production.Acknowledgements
This work was financially supported by the National Key Basic Research Program of China (2013CB933200), National 863 plans projects (2012AA062703), Youth Innovation Promotion Association CAS (2012200).Notes and references
- N. Armaroli and V. Balzani, Angew. Chem., Int. Ed., 2007, 46, 52–66 CrossRef CAS PubMed
.
- N. S. Lewis and D. G. Nocera, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 15729–15735 CrossRef CAS PubMed
.
- J. A. Turner, Science, 1999, 285, 687–689 CrossRef CAS PubMed
.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS PubMed
.
- X. C. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76 CrossRef CAS PubMed
.
- J. Liebig, Ann. Pharm., 1834, 10, 10 Search PubMed
.
- E. C. Franklin, J. Am. Chem. Soc., 1922, 44, 486 CrossRef CAS
.
- A. Thomas, A. Fischer, F. Goettmann, M. Antonietti, J. O. Muller, R. Schlogl and J. M. Carlsson, J. Mater. Chem., 2008, 18, 4893–4908 RSC
.
- Y. Wang, J. Zhang, X. Wang, M. Antonietti and H. Li, Angew. Chem., Int. Ed., 2010, 49, 3356–3359 CrossRef CAS PubMed
.
- S. C. Yan, Z. S. Li and Z. G. Zou, Langmuir, 2010, 26, 3894–3901 CrossRef CAS PubMed
.
- Q. Liu and J. Zhang, Langmuir, 2013, 29, 3821–3828 CrossRef CAS PubMed
.
- B. Yue, Q. Y. Li, H. Iwai, T. Kako and J. H. Ye, Sci. Technol. Adv. Mater., 2011, 12, 034401 CrossRef
.
- Q. J. Xiang, J. G. Yu and M. Jaroniec, J. Phys. Chem. C, 2011, 115, 7355–7363 CAS
.
- L. Ge, F. Zuo, J. Liu, Q. Ma, C. Wang, D. Sun, L. Bartels and P. Feng, J. Phys. Chem. C, 2012, 116, 13708–13714 CAS
.
- Y. Tian, L. Ge, K. Wang and Y. Chai, Mater. Charact., 2014, 87, 70–73 CrossRef CAS
.
- X. C. Wang, X. F. Chen, A. Thomas, X. Z. Fu and M. Antonietti, Adv. Mater., 2009, 21, 1609–1612 CrossRef CAS
.
- X. Fan, L. Zhang, R. Cheng, M. Wang, M. Li, Y. Zhou and J. Shi, ACS Catal., 2015, 5, 5008–5015 CrossRef CAS
.
- O. Lopez-Sanchez, D. Lembke, M. Kayci, A. Radenovic and A. Kis, Nat. Nanotechnol., 2013, 8, 497–501 CrossRef CAS PubMed
.
- M. Fontana, T. Deppe, A. K. Boyd, M. Rinzan, A. Y. Liu, M. Paranjape and P. Barbara, Sci. Rep., 2013, 3, 1634 Search PubMed
.
- F. Li, J. Li, Z. Cao, X. Lin, X. Li, Y. Fang, X. An, Y. Fu, J. Jin and R. Li, J. Mater. Chem. A, 2015, 3, 21772–21778 CAS
.
- L. S. Byskov, J. K. Norskov, B. S. Clausen and H. Topsoe, J. Catal., 1999, 187, 109–122 CrossRef CAS
.
- P. Raybaud, J. Hafner, G. Kresse, S. Kasztelan and H. Toulhoat, J. Catal., 2000, 190, 128–143 CrossRef CAS
.
- T. F. Jaramillo, K. P. Jorgensen, J. Bonde, J. H. Nielsen, S. Horch and I. Chorkendorff, Science, 2007, 317, 100–102 CrossRef CAS PubMed
.
- Y. Li, H. Wang, L. Xie, Y. Liang, G. Hong and H. Dai, J. Am. Chem. Soc., 2011, 133, 7296–7299 CrossRef CAS PubMed
.
- L. Liao, J. Zhu, X. Bian, L. Zhu, M. D. Scanlon, H. H. Girault and B. Liu, Adv. Funct. Mater., 2013, 23, 5326–5333 CrossRef CAS
.
- B. Hinnemann, P. G. Moses, J. Bonde, K. P. Jorgensen, J. H. Nielsen, S. Horch, I. Chorkendorff and J. K. Norskov, J. Am. Chem. Soc., 2005, 127, 5308–5309 CrossRef CAS PubMed
.
- H. Zhao, Y. Dong, P. Jiang, H. Miao, G. Wang and J. Zhang, J. Mater. Chem. A, 2015, 3, 7375–7381 CAS
.
- M. L. Li, L. X. Zhang, X. Q. Fan, M. Y. Wu, Y. Y. Du, M. Wang, Q. L. Kong, L. L. Zhang and J. L. Shi, Appl. Catal., B, 2016, 190, 36–43 CrossRef CAS
.
- D. Gopalakrishnan, D. Damien and M. M. Shaijumon, ACS Nano, 2014, 8, 5297–5303 CrossRef CAS PubMed
.
- S. C. Yan, Z. S. Li and Z. G. Zou, Langmuir, 2009, 25, 10397–10401 CrossRef CAS PubMed
.
- H. L. Wang and J. A. Turner, J. Electrochem. Soc., 2010, 157, F173–F178 CrossRef CAS
.
- T. Z. Markus, M. Wu, L. Wang, D. H. Waldeck, D. Oron and R. Naaman, J. Phys. Chem. C, 2009, 113, 14200–14206 CAS
.
- F. A. Frame, E. C. Carroll, D. S. Larsen, M. Sarahan, N. D. Browning and F. E. Osterloh, Chem. Commun., 2008, 19, 2206–2208 RSC
.
- L. Liao, Q. Zhang, Z. Su, Z. Zhao, Y. Wang, Y. Li, X. Lu, D. Wei, G. Feng, Q. Yu, X. Cai, J. Zhao, Z. Ren, H. Fang, F. Robles-Hernandez, S. Baldelli and J. Bao, Nat. Nanotechnol., 2014, 9, 69–73 CrossRef CAS PubMed
.
- J. Wang, Z. Guan, J. Huang, Q. Li and J. Yang, J. Mater. Chem. A, 2014, 2, 7960–7966 CAS
.
- Z. I. Alferov, Semiconductors, 1998, 32, 1–4 CrossRef
.
- J. Tersoff, Phys. Rev. B: Condens. Matter Mater. Phys., 1984, 30, 4874 CrossRef CAS
.
- V. Wiertz and P. Bertrand, Identification of the N-containing functionalities introduced at the surface of ammonia plasma treated carbon fibres by combined ToF-SIMS and XPS, 1998 Search PubMed
.
- T. J. Moravec and T. W. Orent, J. Vac. Sci. Technol., 1981, 18, 226–228 CrossRef CAS
.
- S. D. Gardner, C. S. K. Singamsetty and G. L. Booth, Carbon, 1995, 3, 587–595 CrossRef
.
- Y. P. Wu, S. B. Fang and Y. Y. Jiang, Solid State Ionics, 1999, 120, 117–123 CrossRef CAS
.
- J. Lahaye, G. Nanse, A. Bagreev and V. Strelko, Carbon, 1999, 37, 585–590 CrossRef CAS
.
- G. Hopfengärtner, D. Borgmann, I. Rademacher, G. Wedler, E. Hums and G. W. Spitznagel, J. Electron Spectrosc. Relat. Phenom., 1993, 63, 91–116 CrossRef
.
- Handbook of X-ray photoelectron spectroscopy: a reference book of standard spectra for identification and interpretation of XPS data, ed. J. Chastain and R. C. King, Physical Electronics, Eden Prairie, MN, 1995 Search PubMed
.
- S. W. A. Galtayries and J. Grimblot, J. Electron Spectrosc. Relat. Phenom., 1997, 87, 31–44 CrossRef
.
- C. O. A. Olsson and S. E. Hörnström, Corros. Sci., 1994, 36, 141 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: The illustration of MoS2 crystal structure, HRTEM image of 7 wt% MoS2-QDs/CN, band-gaps of all samples, explanation of the band-gap shrinkage of MoS2-QDs/CN composite, control experiment results of photocatalytic HER and illustration of the electron–hole pair separation and transfer in the MoS2-QDs/CN composite. See DOI: 10.1039/c6ra07060d |
| This journal is © The Royal Society of Chemistry 2016 |