Metal co-doped cesium manganese chlorine nanocrystals with high efficiency and tunable red emission†
Shijia
Liu
a,
Xuebin
Zhang
a,
Qin
Xu
 *a,
Zhiheng
Xu
b,
Haibo
Zeng
*a,
Zhiheng
Xu
b,
Haibo
Zeng
 *c and
Dandan
Yang
*c and
Dandan
Yang
 *a
*a
aInstitute of Innovation Materials and Energy, School of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou, 225002, China. E-mail: dandanyang@yzu.edu.cn
bDepartment of Nuclear Science and Technology, Nanjing University of Aeronautics and Astronautics, Nanjing 211106, China
cMIIT Key Laboratory of Advanced Display Materials and Devices, Institute of Optoelectronics & Nanomaterials, College of Materials Science and Engineering, Nanjing University of Science and Technology, Nanjing 210094, China
First published on 30th January 2024
Abstract
Low photoluminescence efficiency and instability of lead-free halide perovskite nanomaterials are the main obstacles preventing their practical applications in the fields of optoelectronic devices and X-ray imaging. In this work, lanthanide-doped cesium manganese halide nanocrystals were synthesized by a simple hot injection method. The chemical composition and structural characteristics of the manganese-based perovskites were investigated in detail to prove that lanthanide ions successfully replaced partial manganese ions. As a result, red photoluminescence with a high photoluminescence quantum yield of 35% and a broad emission was obtained. More importantly, the roles of thulium and lead ions in enhancing the luminescence efficiency and tuning photoluminescence from 662 nm to 628 nm were studied in detail by modulating the molar ratios of thulium/lead. Furthermore, the thulium-doped NCs maintained a stable crystal phase and photoluminescence after two weeks. This work provides new insights into enhancing the photoluminescence and tuning the optical bandgap of a perovskite host with co-doping.
Introduction
In recent years, lead halide perovskite nanocrystals (NCs) have attracted much attention due to their outstanding optoelectronic properties, such as narrow emission linewidths,1–3 tunable bandgap,4,5 high photoluminescence quantum yield (PLQY)6,7 and promising applications in photoelectric detectors,8–10 light-emitting diodes (LEDs),11,12 and X-ray imaging.13,14 However, the toxicity and poor stability have hindered practical applications of lead halide perovskite NCs. To improve the chemical and photostability, a universal approach is to reduce the lead content by replacing Pb2+ ions with nontoxic and stable metal ions to form the lead-free or lead-less perovskite. For instance, Ge2+ or Sn2+-based perovskites were synthesized by replacing Pb2+ with Ge2+ or Sn2+. Unfortunately, the relatively low PLQY (<4%) and instability of these perovskites are still not satisfactory.15 Cu-based perovskite NCs exhibit excellent optical properties;16 however, they are easy to oxidize, resulting in a serious decline in optical properties. Huang et. al. demonstrated the solution-phase synthesis of cesium europium halide perovskite NCs but with PLQY a of only about 2%.17 Outsides, other lead-free metal halides, such as Mn-based halides,18 Cd-based halides,19 and double perovskites, featured by self-trapped exciton (STE), large Stokes shift, large PL bandwidth, and long lifetimes, have been considered as alternatives for optoelectronic applications.20 However, the synthesis of stable and highly efficient lead-free perovskite NCs is still a thorny issue.Recently, color-tunable emission has been realized in Mn-based halides by changing the Mn2+ coordination structure, which has also received special attention.21,22 In addition, Cs3MnBr5 NCs as crystal seeds were also used to synthesize CsPbBr3 NCs with special morphology and dimensions.23 Another noteworthy study is the improvement of optical properties and structural stability. For example, Guan et al. designed a low amount of Pb2+-doped CsMnCl3 NCs and improved the PLQY from 0.7% to 21% due to the effective energy transfer from [PbCl6]4− to Mn2+ ions.24 In contrast, the addition of Zn2+ ions serves as an obstacle to the energy transfer between Mn2+ ions, enhancing the PLQY of Zn2+-doped CsMnCl3 NCs with red emission at 654 nm from 56.3% to 77.1%.25 In addition to the ion doping mentioned above, lanthanide ions (Ln3+) can also promote luminescence conversion, but their progress as downshifters is limited by the narrow absorption widths and very small absorption cross-section of Ln3+ ions.26 For instance, CsMnBr3 NCs as an efficient sensitizer to transfer energy to Ln3+ ions, resulted in the decrease of Mn2+ red emission and generated vaying NIR emissions.26,27 However, during the emission by Ln3+-doped perovskite NCs, the PL efficiency of excitons emitted by the perovskite host increases, which has not attracted enough attention. Therefore, the selection and design of metal co-doping ions play an important role in exploring the PL properties of lead-free perovskites.
In this work, we prepared lanthanide-doped CsMnCl3 NCs with red emission at 662 nm by a hot injection method. Structure, composition, and chemical state studies confirmed that Ln3+ ions partially replaced Mn2+ ions, which were successfully introduced into CsMnCl3 NCs. As a result, the PL intensity is monotonously enhanced with the increase in the concentration of Ln3+ doping (7% Tm3+, 13% Yb3+, and 7% Er3+), and the highest PLQY of up to 35% was obtained for Ln3+-CsMnCl3 NCs. To further study the role of Ln3+ ions in enhancing the luminescence of CsMnCl3 NCs and optimizing their optical properties, the Pb/Tm co-doped CsMnCl3 NCs were synthesized. The energy levels of Tm3+ ions play a transitional role in the process of exciton transfer from the Pb2+ to the CsMnCl3 host, resulting in improving the PL efficiency of the CsMnCl3 host. Furthermore, modulating the Pb doping concentration can break the spin-forbidden transition of Pb/Tm co-doped CsMnCl3 NCs, resulting in tunable photoluminescence from 662 nm to 628 nm, and the highest PLQY of up to 52.2% was obtained. In addition, the good stability of Tm3+-CsMnCl3 NCs compared to CsMnCl3 NCs is discussed.
The synthesis process of colloidal CsMnCl3 and Ln3+-CsMnCl3 NCs is similar to the one used to prepare lead-based perovskite NCs by a simple hot-injection method (see the detailed Experimental section in the ESI,† S1). It is slightly different from our approach and the effects of different solvents on the solubility and luminescence of CsMnCl3 NCs are discussed. Four common solvents, including toluene, cyclohexane, n-hexane, octane, and tetradecane, were used to dissolve CsMnCl3 NCs and maintain their quality. Surprisingly, the octane solution of CsMnCl3 NCs maintained good solubility and strong fluorescence, whereas the NCs with strong luminescence in tetradecane solution showed serious agglomeration and the shape evolved from the hexagonal to rod structure (Fig. S2, ESI†), which may be related to the chain length of alkanes and intermolecular forces (Fig. S3, ESI†).28 In order to balance the luminescence and dispersion, octane is the best choice for CsMnCl3 NCs.
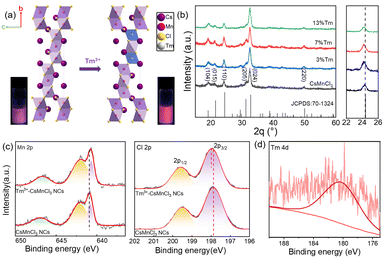
Fig. 1a exhibits the crystal structure of CsMnCl3 along an axis. CsMnCl3 NCs have a hexagonal structure formed by Mn3Cl12 chains (three face-sharing [MnCl6]4− octahedral unit) shared by corners and stabilized by cesium ions (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group). Taking Tm as an example, Tm3+ ions are doped to form [TmCl6]4−octahedron to substitute [MnCl6]4−, in principle, generating a cation vacancy (VMn).26 After doping with Tm3+ ions, a light pink transparent solution emits stronger red light under a 365 nm UV lamp, as shown in the illustration of Fig. 1a. Fig. 1b presents a series of X-ray diffraction (XRD) patterns of Tm3+-CsMnCl3 NCs (CsMnCl3 NCs with different Tm3+ doping concentrations), and all diffraction peaks correspond well to the standard diffractions from hexagonal CsMnCl3 NCs (PDF card 70-1324). After partial substitution of Mn2+ (0.67–0.83 Å, depending on the high- or low-spin coordination) by the larger Tm3+ (0.87 Å), the (110) diffraction peak gradually shifts to a smaller angle (from 24.4° to 24.2°) (Fig. 1b, right panel).25,29 This can be explained by the expansion of the crystal lattice after the incorporation of Tm3+, which in turn proves that Tm3+ ions were successfully introduced into the CsMnCl3 lattice without altering the structure.
m space group). Taking Tm as an example, Tm3+ ions are doped to form [TmCl6]4−octahedron to substitute [MnCl6]4−, in principle, generating a cation vacancy (VMn).26 After doping with Tm3+ ions, a light pink transparent solution emits stronger red light under a 365 nm UV lamp, as shown in the illustration of Fig. 1a. Fig. 1b presents a series of X-ray diffraction (XRD) patterns of Tm3+-CsMnCl3 NCs (CsMnCl3 NCs with different Tm3+ doping concentrations), and all diffraction peaks correspond well to the standard diffractions from hexagonal CsMnCl3 NCs (PDF card 70-1324). After partial substitution of Mn2+ (0.67–0.83 Å, depending on the high- or low-spin coordination) by the larger Tm3+ (0.87 Å), the (110) diffraction peak gradually shifts to a smaller angle (from 24.4° to 24.2°) (Fig. 1b, right panel).25,29 This can be explained by the expansion of the crystal lattice after the incorporation of Tm3+, which in turn proves that Tm3+ ions were successfully introduced into the CsMnCl3 lattice without altering the structure.
To confirm the chemical composition and electronic characteristics of Mn, Cl, and Tm elements in CsMnCl3 and Tm3+-CsMnCl3 NCs, X-ray photoelectron spectroscopy (XPS) measurements were performed. Fig. 1c shows the chemical states of the two samples of Mn and Cl. The main peaks in the Mn 2p XPS spectrum at 640.85 eV and 642.09 eV correspond to 2p3/2 and 2p1/2, respectively, as well as a satellite peak at around 646.9 eV, indicating the +2 valence state of Mn.27 Clear changes can be seen with the movement to low binding energy compared to that with CsMnCl3 NCs, which contribute to the fact that the electron absorption capacity of Tm is less than that of Mn, and increases the electron cloud density around Mn and shifts it to the low binding energy. In contrast, the peaks of Cl 2p shift slightly in the direction of high binding energy after Tm doping, indicating the interaction between Tm and Cl ions. In addition, Fig. 1d shows that the weak binding energy located at 178.9 eV of Tm 2p is due to a small amount of Tm doping, indicating that the Tm3+ ions were doped into CsMnCl3 NCs. The movement of Cs 3d (Fig. S4, ESI†) is not obvious, demonstrating that the incorporation of Tm3+ into the host lattice Mn2+, rather than that of Cs+ and Cl−.
Fig. S5 (ESI†) shows the transmission electron microscopy (TEM) images of CsMnCl3 NCs with different TmCl3 contents. All samples exhibited good monodispersity and homogeneous hexagonal shapes. Meanwhile, the average lengths are 14.35 nm, 16.89 nm, 18.16 nm, and 18.80 nm, respectively. Obviously, the size of pure hexagonal-doped NCs increases with the increase in the content of Tm3+ doping, which may be attributed to the partial substitution of Tm3+ ions with (0.87 Å) to Mn2+ (0.67–0.83 Å). Similar results have been reported for the hexagonal CsCdBr3 NCs.30 To further prove the effect of Tm3+ doping on the structure of CsMnCl3 NCs, high-resolution TEM (HR-TEM) images of CsMnCl3 NCs with different Tm3+ doping contents were conducted, as shown in Fig. 2a–d. With the increases of Tm3+ ions, the crystal plane spacing of CsMnCl3 increases continuously from 0.137 to 0.142 nm, which indicates that the Mn2+ ions are partially substituted by Tm3+ ions with larger ionic radii, leading to a slight lattice expansion. This result is consistent with the XRD variations (Fig. 1b). To confirm the existence of Tm3+ ions in CsMnCl3 NCs, energy dispersion spectroscopy (EDS) measurements of Tm3+-CsMnCl3 NCs were conducted, as shown in Fig. 2e. It can be seen from the EDS data that Cs, Mn, Cl, and Tm elements were uniformly distributed in these nanocrystals, and the Tm element was distributed scarcely compared to the other elements. Based on the above results, a small amount of Tm3+ ions can be doped into the CsMnCl3 lattice, partially replacing the Mn2+ ions, and no new morphology appeared.
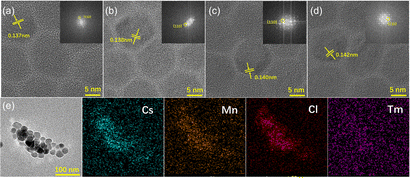 | ||
| Fig. 2 (a)–(d) HR-TEM images and corresponding FFT pattern of 0%, 3%, 7%, and 13% Tm doping of CsMnCl3 NCs. (e) TEM and EDS mapping images of Tm3+-CsMnCl3 NCs. | ||
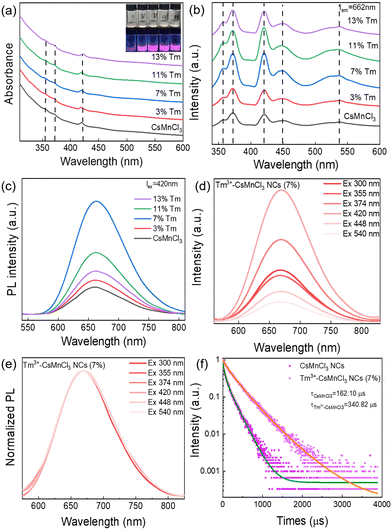
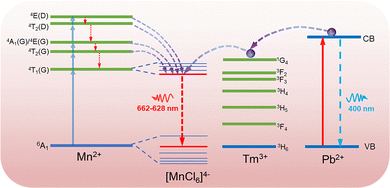
Fig. 3a and b show a comparison of the absorption spectra and PL excitation (PLE) spectra of CsMnCl3 and Tm3+-CsMnCl3 NCs, respectively. The materials showed similar peaks with five structured peaks at 355 nm, 374 nm, 420 nm, 448 nm, and 540 nm, which are ascribed to the electronic transition of Mn2+ ions from 6A1(S) to 4T1(P), 4E(D), 4T2(D), 4A1/4E(G), and 4T2(G), respectively.31 These peaks typically come from the d–d transitions in the octahedrally coordinated Mn2+ ions. The assignment of Mn2+ based on the d–d transitions has been studied in detail.20,32Fig. 3c presents the PL spectra of CsMnCl3 NCs under 420 nm excitation, and the impact of Tm3+ doping on their luminescence properties was studied. For the undoped sample, there is a PL peak at 662 nm, while for Tm3+-CsMnCl3 NCs, the red emission intensity initially increases and then decreases with the increase of the Tm3+ doping contents, among which, the PL intensity of 7% doped material was the highest and the peak position remained unchanged, which coincided with the luminescence under the ultraviolet lamp (the illustration in Fig. 3a). In addition, we studied the PL emission spectra at different excitation wavelengths selected from the absorption spectra of the undoped and 7% Tm3+-CsMnCl3 NCs, as well as the excitation wavelength at 300 nm and found that 7% Tm3+-CsMnCl3 NCs have the same emission peak at 662 nm, identical spectral shape, and FWHM (Fig. 3d and e). Thus, the red PL emission is attributed to the relaxation from the low energy excited state to the ground state of Mn2+ (from 4T1 to 6A1), as illustrated in Fig. 4, which is consistent with the assignment of the red emission in the octahedrally coordinated Mn2+. However, for CsMnCl3 NCs, the PL emission spectra show a little change at the six different excitation wavelengths (Fig. S6a, ESI† and Fig. 6b), which may be related to the defect state. Fig. 3f shows that the 662 nm emission band of the undoped and 7% Tm3+-CsMnCl3 NCs has an average lifetime of 162.10 μs and 340.82 μs after fitting with triple exponential decay, respectively. The relatively short lifetime of CsMnCl3 NCs indicates that the non-radiative recombination is dominant, which results in a low PLQY of 0.3%.22 In contrast, the 7% Tm3+-doped CsMnCl3 NCs were diluted to a certain concentration, and the measured PLQY was 35%. In addition, we attempted doping with other lanthanide dopants, such as Yb3+ and Er3+ ions. Fig. S7 (ESI†) shows that the PL intensity at 662 nm for the sample with 13% Yb is the strongest, while the PL intensity at 662 nm for the sample with 7% Er3+ was the highest. The enhanced luminescence of Ln3+-CsMnCl3 NCs may be due to the partial substitution of Ln3+ ions for Mn2+ ions, which effectively suppresses the energy transfer between Mn2+ and promotes the excitons to be confined to the [MnCl6]4− octahedron (Fig. 4). However, the high doping content of Ln3+ will lead to the concentration quenching effect due to the non-radiative energy transfer between the dopant and lattice.
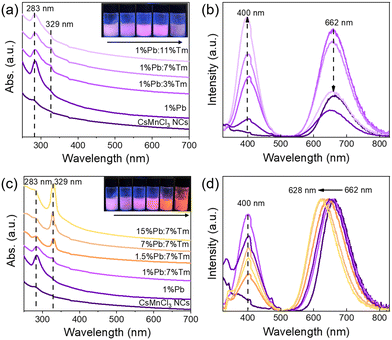
To gain more insights into the role of Ln3+ ions in enhancing the luminescence of CsMnCl3 NCs and optimizing their optical properties, Pb/Tm co-doped CsMnCl3 NCs were prepared by the introduction of a small amount of Pb2+ ions. The Pb-doped CsMnCl3 NCs were used as a reference sample, Fig. 5a shows that the absorption around 283 nm was greatly enhanced due to the parity allowed transition from 1S0 to 3P1 of [PbCl6]4− octahedrons.24,33 Tm was selected as the 1G4 energy level, which is the most perfect candidate for the formation of the gradient levels.34 With the increases in the doping content of Tm, the absorption peak at 283 nm decreased, and the emission spectra for Pb/Tm co-doped NCs revealed the presence of CsMnCl3 host and intrinsic Pb2+ ions (Fig. 5b). As the Tm3+ doping concentration increases to 3–7%, more excitons are transferred to the CsMnCl3 host and then released, accompanied by the emission of more photons. As a result, the PL intensity of the CsMnCl3 host increases significantly. The strong peak at 662 nm is mainly attributed to the emission of the CsMnCl3 host. These results show that a part of photoinduced exciton will be transferred from the Pb2+ host to the 1G4 energy level of Tm3+, and then these excitons will be transferred downward to the lower energy state 4T1 of Mn2+, promoting more excitons to be confined to the [MnCl6]4− octahedron. Finally, the exciton will be released with the emission spectra of 662–628 nm (Fig. 4). Thus, Tm3+ ions play a transitional role in the process of exciton transfer, making it easier and more efficient to transfer excitons from the Pb2+ host to the CsMnCl3 host. Interestingly, the PL intensity decreases with higher Tm doping contents, accompanied by a significant increase for the PL peak at 400 nm. The strong emission at 400 nm mainly belongs to the emission of intrinsic Pb2+ ions due to the non-radiative energy transfer between the Tm3+ dopant and the lattice.
To verify the role of Pb2+ ions, we further investigated the effect of Pb doping content on the optical properties of CsMnCl3 NCs. Both 7% Tm3+-doped and 1%Pb/7%Tm co-doped exhibited excellent optical properties, and found that they have an obvious absorption peak at 329 nm compared with the other samples; the NCs with 7% Tm3+ acted as a reference sample. Interestingly, when the Pb doping concentration increased from 1% to 15%, the absorption peak at 329 nm was significantly enhanced (Fig. 5c), which is attributed to the spin-forbidden 4T1(P) to 6A1(S) transition. The presence of an obvious absorption peak in Pb/Tm co-doped CsMnCl3 NCs implies that the spin-forbidden transition is further broken depending on the doping, leading to lower crystal symmetry.35,36 Correspondingly, the absorption peak at 283 nm gradually weakens. Hence, the tunable PL of Pb/Tm co-doped CsMnCl3 NCs may mainly be dependent on the broken spin-forbidden transition. Thus, Fig. 5d shows that the red emission showed a blue shift from 662 nm to 628 nm, which originated from the d–d transitions in the [MnCl6]4− octahedron rather than an isolated Mn2+ ion, and the emission peak at 400 nm remained unchanged. Moreover, the PL peak remained unchanged for CsMnCl3 NCs with Pb doping from 7% to 15%.
Fig. S8 (ESI†) is the Tauc plot curve converted by the Kubelka–Munk equation for the bandgap calculation. The bandgap of CsMnCl3 NCs is estimated to be 3.85 eV, which is consistent with that from a previous report.24 As the Pb/Tm doping ratio increases, the corresponding bandgap decreases sequentially. The band gap decreased to 3.39 eV when the doping of Pb was 15%. In addition, the optimum PLQY of the PL peak at 628 nm is 52.5%. Correspondingly, the PL decay lifetime of Pb/Tm co-doped CsMnCl3 NCs with the emission peak at 628 nm can be fitted with triple-exponential decay, and an average lifetime of 333.93 μs was obtained (Fig. S9, ESI†), which is similar to that of 7% Tm3+-CsMnCl3 NCs (340.82 μs). Table S1 (ESI†) summarizes in detail the parameters of PL decay lifetimes of the undoped, 7% Tm3+, and Pb/Tm co-doped CsMnCl3 NCs. Therefore, Pb2+ ions cannot play a similar effect as Tm3+ ions, and Pb2+ ions mainly regulate the PL emission of CsMnCl3 NCs due to the absence of the forbidden transition. When the Pb content increased to 50%, the absorption peak at 328 nm gradually disappeared, as shown in Fig. S10 (ESI†), and a new absorption peak at 384 nm appeared. As a result, the PL spectra of the Pb/Tm co-doped NCs show the yellow-orange emission at 606 nm and ultraviolet emission at 400 nm, which results from the isolated Mn2+ ions and CsPbCl3 NCs.37 The XRD pattern well corresponds to the cubic phase of CsPbCl3 NCs (Fig. S11, ESI†), indicating the formation of the CsPbCl3 host. Based on the above results, the tunable red emission of Tm3+-CsMnCl3 NCs with partial Pb doping breaks through the limitation of a single-emission peak of CsMnCl3 NCs, and Pb/Tm co-doped CsMnCl3 NCs with high efficiency are further realized.
The phase and storage stability of undoped and Tm3+-doped CsMnCl3 NCs were tested at 50% humidity and 25 °C under atmospheric pressure. Fig. 6a and b show the XRD patterns and PL spectra of the two thin film samples, the Tm3+-doped NCs with red emission maintained the hexagonal phase after two weeks, while undoped NCs were transferred from the red-emitting hexagonal phase to the blue emitting hexagonal Cs2MnCl4(H20)2 phase, and partially decomposed into CsCl and MnCl2 after one week (Fig. S12, ESI†). In addition, as the storage time increases, the Tm3+-doped NCs and undoped NCs maintained 63% and 17% of their PL intensities recorded at room temperature after two weeks (Fig. 6c and d).
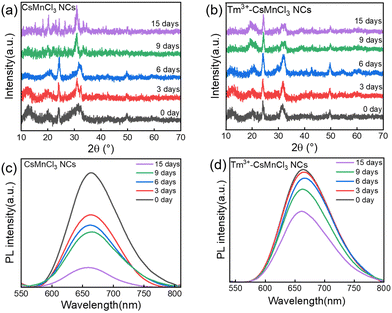 | ||
| Fig. 6 Time-dependent XRD patterns of (a) CsMnCl3 and (b) Tm3+-CsMnCl3 NCs. PL variation of (c) CsMnCl3 and (d) Tm3+-CsMnCl3 NCs after different storage times. | ||
Conclusions
In summary, we successfully developed various lanthanide-doped CsMnCl3 NCs via the hot-injection method and expanded the optical properties of the perovskite NCs. The substitution of Ln3+ ions for Mn2+ ions into the internal lattice of CsMnCl3 NCs was preliminarily demonstrated by XRD, TEM, and EDX measurements. Interestingly, the introduction of Ln3+ ions considerably improved the red emission centered at 662 nm for CsMnCl3 NCs. PLQY was successfully enhanced from 0.3% to 35%. In addition, the Pb/Tm co-doped CsMnCl3 NCs were synthesized, the energy levels of Tm3+ ions played a transitional role in the process of exciton transfer from the Pb2+ to the CsMnCl3 host while controlling the amount of Pb doping to obtain tunable PL emission from 662 nm to 628 nm due to the absence of the forbidden transition, and the PLQY was further optimized to 52.5%. Moreover, Tm3+-CsMnCl3 NCs maintained good phase and storage stability compared with undoped NCs after two weeks. We believe that this work provides a new approach to improving the PL properties, tuning the optical bandgap, and exploring the luminescent mechanism for lead-free perovskite NCs.Author contributions
D. Yang and Q. Xu supervised the study. D. Yang conceived the idea for the manuscript and designed the experiments. S. Liu and X. Zhang carried out the synthesis and characterizations of NCs. The manuscript was mainly written by D. Yang and S. Liu. Z. Xu, and H. Zeng revised and improved the manuscript. All authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (62204215 and 12275132), the Basic Science (Natural Science) Research Project of Colleges and Universities in Jiangsu Province (22KJB430012), and China Postdoctoral Science Foundation (2023M742946). Yangzhou University Testing Center was also thanked for the XPS and XRD characterizations.Notes and references
- D. Zhang, Y. Yang, Y. Bekenstein, Y. Yu, N. A. Gibson, A. B. Wong, S. W. Eaton, N. Kornienko, Q. Kong, M. Lai, A. P. Alivisatos, S. R. Leone and P. Yang, J. Am. Chem. Soc., 2016, 138, 7236–7239 CrossRef CAS PubMed.
- S. Wei, Y. Yang, X. Kang, L. Wang, L. Huang and D. Pan, Chem. Commun., 2016, 52, 7265–7268 RSC.
- Y. Tong, E. Bladt, M. F. Ayguler, A. Manzi, K. Z. Milowska, V. A. Hintermayr, P. Docampo, S. Bals, A. S. Urban, L. Polavarapu and J. Feldmann, Angew. Chem., Int. Ed., 2016, 55, 13887–13892 CrossRef CAS PubMed.
- X. Zhu, S. Meng, Y. Zhao, S. Zhang, J. Zhang, C. Yin and S. Ye, J. Phys. Chem. Lett., 2020, 11, 9587–9595 CrossRef CAS PubMed.
- C. Jang, K. Kim, H. W. Nho, S. M. Lee, H. Mubarok, J. H. Han, H. Kim, D. Lee, Y. Jang, M. H. Lee, O. H. Kwon, S. K. Kwak, W. B. Im, M. H. Song and J. Park, Small, 2023, 19, 2206668 CrossRef CAS PubMed.
- J. N. Yang, Y. Song, J. S. Yao, K. H. Wang, J. J. Wang, B. S. Zhu, M. M. Yao, S. U. Rahman, Y. F. Lan, F. J. Fan and H. B. Yao, J. Am. Chem. Soc., 2020, 142, 2956–2967 CrossRef CAS PubMed.
- F. Liu, Y. Zhang, C. Ding, S. Kobayashi, T. Izuishi, N. Nakazawa, T. Toyoda, T. Ohta, S. Hayase, T. Minemoto, K. Yoshino, S. Dai and Q. Shen, ACS Nano, 2017, 11, 10373–10383 CrossRef CAS PubMed.
- T. Yang, Y. Zheng, Z. Du, W. Liu, Z. Yang, F. Gao, L. Wang, K. C. Chou, X. Hou and W. Yang, ACS Nano, 2018, 12, 1611–1617 CrossRef CAS PubMed.
- Y.-Z. Shen, J. Guan, C. Ma, Y. Shu, Q. Xu and X.-Y. Hu, Anal. Chem., 2022, 94, 1742–1751 CrossRef CAS PubMed.
- Y. Shu, Q. Ye, T. Dai, J. Guan, Z. Ji, Q. Xu and X. Hu, J. Hazard. Mater., 2022, 430, 128360 CrossRef CAS PubMed.
- S. Zhang, H. Liu, X. Li and S. Wang, Nano Energy, 2020, 77, 105302 CrossRef CAS.
- J. Guan, M. Song, L. Chen, Y. Shu, D. Jin, G. Fan, Q. Xu and X.-Y. Hu, Carbon, 2021, 175, 93–100 CrossRef CAS.
- L. Wang, K. Fu, R. Sun, H. Lian, X. Hu and Y. Zhang, Nano-Micro Lett., 2019, 11, 52 CrossRef PubMed.
- J.-X. Wang, O. M. Bakr and O. F. Mohammed, Matter, 2022, 5, 2547–2549 CrossRef CAS.
- D. Yang, X. Zhang, S. Liu, Z. Xu, Y. Yang, X. Li, Q. Ye, Q. Xu and H. Zeng, Nanoscale, 2023, 15, 1637–1644 RSC.
- S. Liu, H. Liu, G. Zhou, X. Li and S. Wang, Chem. Eng. J., 2022, 427, 1314430 Search PubMed.
- J. Huang, T. Lei, M. Siron, Y. Zhang, S. Yu, F. Seeler, A. Dehestani, L. N. Quan, K. Schierle-Arndt and P. Yang, Nano Lett., 2020, 20, 3734–3739 CrossRef CAS PubMed.
- T. Chang, Y. Dai, Q. Wei, X. Xu, S. Cao, B. Zou, Q. Zhang and R. Zeng, ACS Appl. Mater. Interfaces, 2023, 15, 5487–5494 CrossRef CAS PubMed.
- X. Zhou, K. Han, Y. Wang, J. Jin, S. Jiang, Q. Zhang and Z. Xia, Adv. Mater., 2023, 35, 2212022 CrossRef CAS PubMed.
- A. M. Anthony, M. K. Pandian, P. Pandurangan and M. Bhagavathiachari, ACS Appl. Mater. Interfaces, 2022, 14, 29735–29743 CrossRef CAS PubMed.
- K. Li, W. Zhang, L. Niu, Y. Ye, J. Ren and C. Liu, Adv. Sci., 2022, 10(4), 2204843 CrossRef PubMed.
- Q. Kong, B. Yang, J. Chen, R. Zhang, S. Liu, D. Zheng, H. Zhang, Q. Liu, Y. Wang and K. Han, Angew. Chem., Int. Ed., 2021, 60, 19653–19659 CrossRef CAS PubMed.
- S. Bera, S. Banerjee, R. Das and N. Pradhan, J. Am. Chem. Soc., 2022, 144, 7430–7440 CrossRef CAS PubMed.
- L. Q. Guan, S. Shi, X. W. Niu, S. C. Guo, J. Zhao, T. M. Ji, H. Dong, F. Y. Jia, J. W. Xiao, L. D. Sun and C. H. Yan, Adv. Sci., 2022, 9, 2201354 CrossRef CAS PubMed.
- X. Hao, H. Liu, W. Ding, F. Zhang, X. Li and S. Wang, J. Phys. Chem. Lett., 2022, 13, 4688–4694 CrossRef CAS PubMed.
- H. Bahmani Jalali, A. Pianetti, J. Zito, M. Imran, M. Campolucci, Y. P. Ivanov, F. Locardi, I. Infante, G. Divitini, S. Brovelli, L. Manna and F. Di Stasio, ACS Energy Lett., 2022, 7, 1850–1858 CrossRef CAS PubMed.
- Y. Su, L. Yuan, B. Wang, S. Wu and Y. Jin, J. Colloid Interface Sci., 2022, 624, 725–733 CrossRef CAS PubMed.
- J. Zhao, G. Pan, W. Xu, S. Jin, H. Zhang, H. Gao, M. Kang and Y. Mao, Chem. Commun., 2020, 56, 14609–14612 RSC.
- E. Song, Z. Chen, M. Wu, S. Ding, S. Ye, S. Zhou and Q. Zhang, Adv. Opt. Mater., 2016, 4, 798–806 CrossRef CAS.
- J. Guo, Q. Hu, M. Lu, A. Li, X. Zhang, R. Sheng, P. Chen, Y. Zhang, J. Wu, Y. Fu, G. Sun, W. W. Yu and X. Bai, Chem. Eng. J., 2022, 427, 131010 CrossRef CAS.
- H. Xiao, P. Dang, X. Yun, G. Li, Y. Wei, Y. Wei, X. Xiao, Y. Zhao, M. S. Molokeev, Z. Cheng and J. Lin, Angew. Chem., Int. Ed., 2020, 60, 3699–3707 CrossRef PubMed.
- J. Almutlaq, W. J. Mir, L. Gutiérrez-Arzaluz, J. Yin, S. Vasylevskyi, P. Maity, J. Liu, R. Naphade, O. F. Mohammed and O. M. Bakr, ACS Mater. Lett., 2021, 3, 290–297 CrossRef CAS.
- W. Zhang, J. Wei, Z. Gong, P. Huang, J. Xu, R. Li, S. Yu, X. Cheng, W. Zheng and X. Chen, Adv. Sci., 2020, 7, 2002210 CrossRef CAS PubMed.
- C. Luo, W. Li, J. Fu and W. Yang, Chem. Mater., 2019, 31, 5616–5624 CrossRef CAS.
- Y. Li, C. Wang, G. Xu, G. Luo and Z. Deng, J. Phys. Chem. Lett., 2023, 14, 2006–2011 CrossRef CAS PubMed.
- X. Yang, C. Pu, H. Qin, S. Liu, Z. Xu and X. Peng, J. Am. Chem. Soc., 2019, 141, 2288–2298 CrossRef CAS PubMed.
- K. Xing, X. Yuan, Y. Wang, J. Li, Y. Wang, Y. Fan, L. Yuan, K. Li, Z. Wu, H. Li and J. Zhao, J. Phys. Chem. Lett., 2019, 10, 4177–4184 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental part, Absorbance and PL spectra, XRD and HR-TEM, EDS mapping, XPS spectra. See DOI: https://doi.org/10.1039/d3tc04539k |
| This journal is © The Royal Society of Chemistry 2024 |