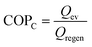Recent advances in metal–organic frameworks for water absorption and their applications
Lu
Cheng†
,
Yu
Dang†
,
Yu
Wang
 * and
Kai-Jie
Chen
* and
Kai-Jie
Chen
 *
*
Key Laboratory of Special Functional and Smart Polymer Materials of Ministry of Industry and Information Technology, Xi’an Key Laboratory of Functional Organic Porous Materials, School of Chemistry and Chemical Engineering, Northwestern Polytechnical University, Xi’an, Shaanxi 710072, China. E-mail: wychem@nwpu.edu.cn; ckjiscon@nwpu.edu.cn
First published on 30th November 2023
Abstract
Potable water and energy shortage have become global issues. With the limited water adsorption performance and regeneration conditions of traditional water adsorbents, innovation in water adsorbents has significant practical implications. Due to the abundant varieties of adsorption sites and their adjustable pore structures, metal–organic frameworks (MOFs) are considered promising water adsorbents with tailorable regeneration conditions, controllable absorption humidity range, and superior water capacity. Herein, we combed through the development of water-uptake MOFs. Their water absorption mechanisms and strategies are summarized through analysis of typical examples. In addition, the recent advances of MOFs focused on atmospheric water harvesting, indoor humidity control, adsorption-driven heat pumps, and industrial chemical dehydration are reviewed. Finally, the possible challenges in the evolving prospects are predicted and the corresponding solutions are proposed.
1. Introduction
Water is the most important survival resource for human beings. Although approximately 1.386 billion cubic kilometers of water exist on Earth, only about 0.0075% of the water can be directly used.1 Furthermore, the unequal geographical and temporal distribution of freshwater resources, such as water resources being severely scarce in deserts and some inland areas, render efficient water collection in these regions particularly crucial.2 As the atmosphere contains up to 12.9 × 109 million tons of water, the water scarcity issue in arid areas can be alleviated by collecting moisture from the atmosphere.3 In addition, water is an excellent adsorbate for adsorption heat pumps and refrigeration systems as it is one of the cleanest natural energy sources with a high enthalpy of evaporation (40.7 kJ mol−1).4 Therefore, finding suitable water adsorbents is an effective alternative to solve water harvesting and energy conversion problems. Meanwhile, water adsorbents are widely applied in chemical dehydration,5,6 indoor humidity maintenance,7,8 food preservation9 and other fields, and play an important role in many aspects of industrial production and daily life.Traditional desiccants include zeolites, silica, metal chlorides, and metal oxides. Specifically, zeolites loaded with Na+, K+, and Ca2+ ions can be used as high-temperature adsorbents due to their strong affinity and adsorption heat (3000–4500 kJ kg−1).10 However, the desorption temperature of zeolites needs to reach ca. 250–300 °C owing to the strong affinity, which is unfavourable for regeneration. Silica gels, composed of amorphous silica networks, characterized with high stability, can encapsulate water molecules relying on active silanol groups on the surface and inside the pores.11 Although the regeneration temperature of silica gel can be adjusted by the pore polarity,12 silica without being compounded with other adsorbents typically demonstrates low water uptake led by their relatively small surface area.13 Chemical adsorbents, such as metal chloride and metal oxides, generally achieve water absorption through chemical reactions or by forming complexes but it is unrecoverable due to the irreversible chemical interactions.14
Given the significance of water adsorbents, there is great potential for the development of water adsorption materials that can satisfy the specific requirements of water absorption performance and regeneration conditions. Metal–organic frameworks (MOFs) are long-range ordered organic–inorganic hybrid porous materials with adaptable pore size and chemistry based on the features of metal ions and ligands.22 Due to the essential attributes of ordered crystalline structure, tailorable hydrophilicity and adjustable pore size,23,24 MOFs have been implemented extensively in gas separation,25–27 catalysis,28–30 and sensing.31,32 They also have great application prospects in water adsorption with the advantages of tailorable regeneration temperature, controllable absorption humidity range and superior water capacity33 (Fig. 1).
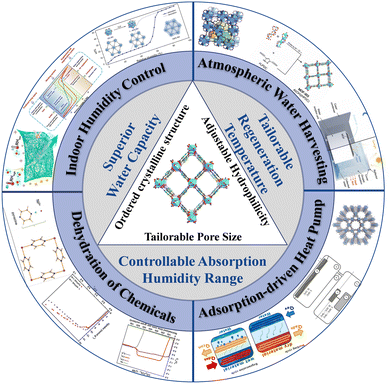 | ||
| Fig. 1 Schematic illustration of the structure, superiority and applications of water absorption MOFs. Reprinted with permissions from ref. 2, 8 and 15–21. Copyright 2017, American Association for the Advancement of Science. Copyright 2018, American Association for the Advancement of Science. Copyright 2018, Springer Nature. Copyright 2017, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. Copyright 2019, American Chemical Society. Copyright 2017, The American Association for the Advancement of Science. Copyright 2020, American Chemical Society. Copyright 2021 Wiley-VCH GmbH. Copyright 2018, Wiley-VCH Verlag GmbH & Co. | ||
In this review, we will introduce the design strategies of MOFs for water absorption and discuss the representative application of water absorption MOFs in detail including atmospheric water harvesting, indoor humidity control, adsorption-driven heat pump and industrial chemicals dehydration (Fig. 2).
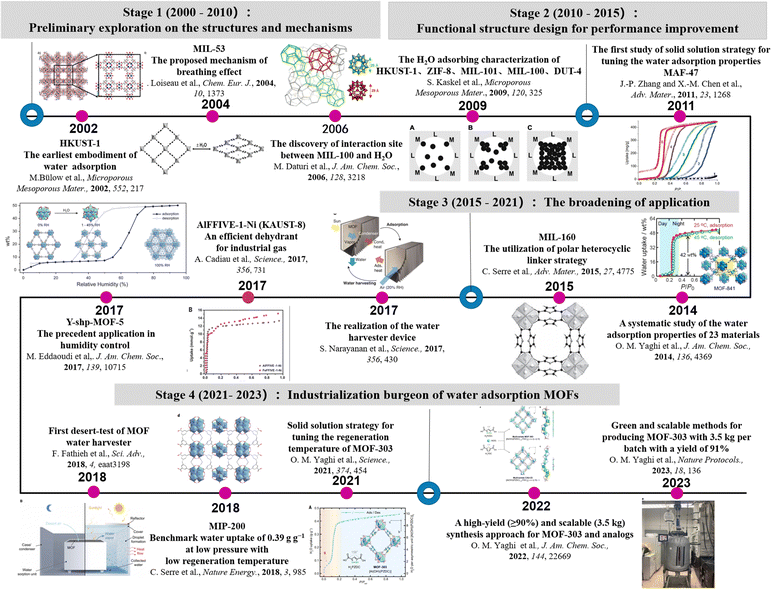 | ||
| Fig. 2 The timeline of MOF utilization in water absorption. Reprinted with permissions from ref. 2, 15–17, 20 and 34–43. Copyright 2013, American Chemical Society. Copyright 2004, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. Copyright 2006, American Chemical Society. Copyright 2009, Elsevier Inc. Copyright 2011, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim Copyright 2014, American Chemical Society. Copyright 2015, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. Copyright 2017, American Association for the Advancement of Science. Copyright 2017, The American Association for the Advancement of Science. Copyright 2018, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. Copyright 2018, American Association for the Advancement of Science. Copyright 2018, Springer Nature. Copyright 2021, The American Association for the Advancement of Science. Copyright 2022, American Chemical Society. Copyright 2022, Springer Nature Limited. | ||
2. Regulation strategies for water adsorption by MOFs
2.1 Classification and water absorption behavior of MOFs
Adsorption isotherms evaluate the water absorption performance of an adsorbent. The affinity of a material for water molecules and the adsorption process can be anticipated by analyzing the form of the isotherms and the position of the inflection point, which is vital to provide alternatives as water adsorbents in different application domains. As shown in Fig. 3, IUPAC suggests that physical adsorption isotherms can be divided into six types. Types I, II and III belong to reversible adsorption, with no hysteresis between adsorption and desorption.44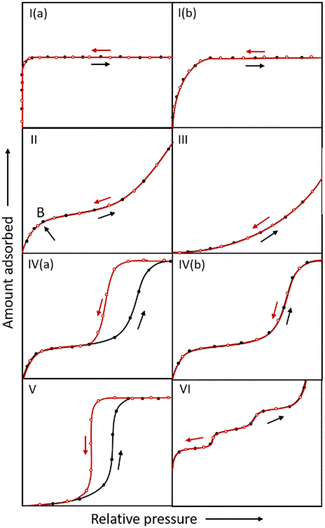 | ||
| Fig. 3 IUPAC classification of adsorption isotherms. The black and red lines represent the adsorption and desorption curves, respectively. | ||
(1) Microporous (pore width < 2 nm) adsorbents possess type I adsorption isotherms.32 In general, MOFs with open metal sites (OMSs, e.g. MOF-74,45 HKUST-146) or microporous MOFs with strongly hydrophilic pore channels (e.g. CAU-10-NH247), typically exhibit type I adsorption isotherms due to their strong affinity.
(2) MOFs absorbing water through a monolayer–multilayer adsorption mechanism usually show type II adsorption isotherms. A portion of the microporous MOF initially absorbs water using unsaturated metals or polarity sites on ligands, and then the primarily adsorbed water molecules continuously attract water to form water clusters or networks through hydrogen bonds, ultimately leading to pore filling. The adsorption of water via monolayer adsorption is relatively low and then rises abruptly due to multilayer adsorption with an obvious inflection point B. For instance, water molecules prefer to adhere to the μ3-OH located on the Zr-SBU in MOF-801 under low relative pressure,39 whereas in MOF-303, they prefer to adapt to the hydroxyl group of the ligand.41 All these primary adsorptions serve as binding sites for additional hydrogen bonding and water cluster formation.
(3) Since the interaction between adsorbent and adsorbate is relatively low, water molecules prefer surfaces with a high adsorption affinity, leading to the curve shown in type III isotherms.
(4) Type IV isotherms are generally found for mesoporous materials. At low relative pressures, the isotherms show a curve similar to type II, but the difference is that there is a desorption hysteresis at the middle-pressure section for type IV(a) isotherms. The mesoporous adsorbents undergo monolayer–multilayer adsorption, with the pore wall serving as the primary adsorption site. When the pressure rises, the pore diameter expands until it approaches the critical diameter, at which stage capillary condensation occurs and the adsorption amount rises dramatically. At room temperature, the critical diameter for capillary condensation is 2.076 nm when the adsorbate is water.48 Due to irreversible capillary condensation, which is regulated by the pore size of mesoporous materials, a hysteresis loop between adsorption and desorption exists. MIL-101, a typical mesoporous MOF with a high capacity for water absorption, undergoes capillary condensation at P/P0 ∼ 0.4. With two different-sized pores of 2.9 nm and 3.4 nm, water molecules first adsorb in the smaller cages before further filling in the larger cages, a phenomenon known as stepwise adsorption. The adsorption isotherm of MIL-101 roughly corresponds to type IV(a).49
When the pore size of mesoporous materials is smaller than the critical diameter of capillary condensation at the completion of monolayer adsorption, no capillary condensation occurs and the desorption curve is fully reversible. In the case of Co2Cl2(btdd), which has a pore size of 2.2 nm, monolayer adsorption is carried out firstly in the low relative pressure region and subsequently the pore size is reduced to 2 nm. Hence, pore filling occurs and is fully reversible, and the adsorption isotherm is represented as IV(b).50
(5) Microporous or mesoporous materials with hydrophobic pore channels generate type V adsorption isotherms, which are similar to type III in the low relative pressure region due to the weak adsorbent–adsorbate interaction at low pressures. When the relative pressure exceeds a particular value, a fast adsorptive uptake and a hysteresis loop exist due to the occurrence of irreversible phenomena such as capillary condensation.
(6) Materials with highly homogeneous non-porous surfaces result in type VI adsorption isotherms, which show a step-like appearance attributed to the sequential porous adsorption.
2.2 Water stability
Hydrolytic stability is the most important prerequisite for a water adsorbent. Although certain MOFs are unstable in the presence of water as they are assembled from relatively weak coordination bonds, numerous MOFs exhibit remarkable water stability due to their structural diversity and rational design.51 A reasonable structural design of MOFs enables stability under strong acidic or alkaline conditions for complex working conditions.52There are two pathways in MOF degradation in the presence of water: (i) hydrolysis and (ii) ligand–ligand substitution. During the hydrolysis process, the metal–ligand bond is attacked by the hydroxide anion and the proton dissociates from the water, forming the metal hydroxide and protonated ligand, as shown in eqn (1).53
| M–L + H2O → M–(OH) + LH | (1) |
| M–L + H2O → M–(OH)⋯LH | (2) |
In accordance with the hard–soft acid–base theory, high-valent metal ions (hard acids) with a high charge density have a strong affinity for the carboxylic acid ligands (hard bases), forming strong coordination bonds. Consequently, the MOFs composed of hard acids and hard bases can offer ultra-high water stability. By way of illustration, MIL-100 (Al, Fe) composed of M3+ and benzene-1,3,5-tricarboxylic acid, can maintain excellent crystallinity and porosity after the adsorption at 40 °C and desorption at 140 °C for 40 cycles.56 As a result of the high polarization of the coordination bond, MOFs constructed through soft acid and soft base, such as MAF-41 [Cu2(fbdim)], may also offer exceptional water stability.57
2.3 Factors affecting water absorption performance in MOFs
The water absorption property of MOFs is mainly controlled by adjusting the pore size, pore chemistry and flexibility. The characteristics associated with the performance of traditional water adsorption MOFs are summarised in Table 1.| MOF | Pore volume/nm | Pore size/nm | S BET/m2 g−1 | α | Water adsorption capacity/g g−1/cm3 g−1 | Desorption temperature/°C | Cycling stability | Potential applications | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| α refers to the relative humidity when it reaches half of the adsorption capacity at 95% relative humidity. Cycle stability refers to the number of cycles that maintain essentially constant performance tested in the reference. | |||||||||
| HKUST-1 | 0.62 | 0.9/0.6 | 1340 | 0.18 | 0.45/0.56 | 110 | Unstable over 40% RH | Dehydrating under low RH | 37 |
| Ni-MOF-74 | 0.46 | 1.1 | 1040 | 0.02 | 0.40/0.50 | 110 | 5 | Sea water desalination | 39 |
| Mg-MOF-74 | 0.53 | 1.1 | 1250 | 0.02 | 0.48/0.60 | 110 | 5 | Sea water desalination | 37 |
| Al-fumarate | 0.48 | 0.6/0.45 | 1021 | 0.25 | 0.47/0.59 | 65 | 4500 | Heat exchanger | 58 |
| UiO-66 | 0.49 | 1.1/0.8 | 1290 | 0.3 | 0.44/0.55 | 80 | 5 | Heat exchanger | 39 |
| UiO-66-NH2 | 0.52 | NR | 1123 | 0.15 | 0.27/0.34 | 65 | NR | Heat exchanger | 59 |
| UiO-66-NO2 | 0.4 | NR | 792 | 0.15 | 0.40/0.50 | 65 | NR | Heat exchanger | 59 |
| UiO-66-OH | 0.44 | 0.83/0.66 | 1099 | 0.22 | 0.39/0.49 | 65 | NR | Heat exchanger | 60 |
| UiO-66-N | 0.57 | 0.81/0.73 | 1413 | 0.15 | 0.49/0.61 | 80 | NR | Heat exchanger | 60 |
| DUT-52 | 0.66 | 1.01/0.8 | 2025 | 0.4 | 0.35/0.44 | 85 | NR | Heat exchanger | 60 |
| UiO-67 | 0.90 | 2.16/1.09 | 1639 | 0.5 | 0.18/0.22 | 85 | Poor stability | Heat exchanger | 60 |
| MOF-801 | 0.45 | 0.74/0.56/0.48 | 990 | 0.08 | 0.36/0.45 | 65 | 3000 | Water harvesting at low RH, heat exchanger | 39 |
| MOF-841 | 0.53 | 0.92 | 1390 | 0.23 | 0.48/0.60 | 70 | 5 | Exchanger | 39 |
| MOF-808 | 0.84 | 1.84/0.48 | 2060 | 0.30 | 0.60/0.75 | 60 | 5 | Heat exchanger | 39 |
| DUT-67 | 0.60 | 1.66/0.74 | 1560 | 0.49 | 0.5/0.62 | 100 | 20 | Heat exchanger | 61 |
| CAU-6 | 0.34 | 0.91 | 760 | 0.05 | 0.3/0.37 | NR | 5 | Heat exchanger | 39 |
| CAU-10 | 0.27 | 0.56 | 525 | 0.15 | 0.35/0.44 | 70 | 700 | Heat exchanger | 62 |
| CAU-10-NO2 | NR | NR | 440 | 0.33 | 0.16/0.20 | NR | NR | Heat exchanger | 47 |
| CAU-10-OH | NR | NR | NR | 0.12 | 0.31/0.39 | NR | NR | Heat exchanger | 47 |
| CAU-10-NH2 | NR | NR | NR | 0.12 | 0.22/0.27 | NR | NR | Heat exchanger | 47 |
| CAU-10-CH3 | NR | NR | NR | 0.45 | 0.18/0.22 | NR | NR | Heat exchanger | 47 |
| CAU-10-OCH3 | NR | NR | NR | 0.26 | 0.09/0.11 | NR | NR | Heat exchanger | 47 |
| MIL-160 | 0.398 | 0.5 | 1070 | 0.08 | 0.37/0.46 | 80 | 10 | Water harvesting at low RH, heat exchanger | 40 |
| MIL-125 | 0.68 | 0.61 | 1510 | 0.38 | 0.30/0.37 | 150 | NR | Heat exchanger | 63 |
| Al-MIL-53 | 0.51 | n.d. | NR | 0.30 | 0.09/0.11 | NR | NR | Heat exchanger | 64 |
| Al-MIL-53-OH | NR | n.d. | NR | 0.75 | 0.40/0.50 | NR | NR | Heat exchanger | 64 |
| Al-MIL-53-NH2 | NR | n.d. | NR | 0.02 | 0.09/0.11 | NR | NR | Heat exchanger | 64 |
| Al-MIL-53-(COOH)2 | NR | n.d. | NR | 0.30 | 0.13/0.16 | NR | NR | Heat exchanger | 65 |
| MIP-200 | 0.4 | 1.3/0.68 | 1000 | 0.18 | 0.39/0.49 (30 °C) | 70 | 50 | Heat exchanger | 17 |
| Y-shp-MOF-5 | 0.63 | 1.2 | 1550 | 0.37 | 0.50/0.62 | Room temperature under vacuum | 200 | Indoor humidity maintenance | 7 |
| MOF-303 | 0.5 | 0.94 | 1360 | 0.12 | 0.45/0.56 | 65 | 2000 | Water harvesting at low RH | 41 |
| MOF-333 | 0.5 | 0.94 | 1280 | 0.23 | 0.44/0.55 | 55 | 2000 | Water harvesting at low RH | 41 |
| ALP-MOF-1 | NR | 1.2/0.95 | 1900 | 0.80 | 0.59/0.73 | 75 | NR | Heat exchanger | 66 |
| ALP-MOF-2 | NR | 1.2/0.95 | 1860 | 0.32 | 0.38/0.47 | 120 | NR | Heat exchanger | 66 |
| MIL-100(Fe) | 1 | 2.9/2.5 | 1917 | 0.26 | 0.8/1.0 | 140 | 40 | Water harvesting at high RH | 67 |
| MIL-100(Al) | 1.14 | 2.9/2.5 | 1814 | 0.26 | 0.5/0.62 | 140 | 40 | Water harvesting at high RH | 56 |
| MIL-100(Cr) | 1.14 | 2.9/2.5 | 1848 | 0.26 | 0.8/1.0 | 140 | 40 | Water harvesting at high RH | 67 |
| MIL-101 | 1.58 | 3.4/2.9 | 3124 | 0.4 | 1.4/1.74 | 140 | 40 | Water harvesting at high RH | 49 |
| MIL-101-NH2 | 1.6 | n.d. | 2890 | 0.37 | 0.81/1.0 | NR | NR | Water harvesting at high RH | 68 |
| MIL-101-COOH | NR | n.d. | 2110 | 0.39 | 0.91/1.13 | NR | NR | Water harvesting at high RH | 68 |
| Co2Cl2btdd | NR | 2.2 | 1912 | 0.28 | 0.97/1.21 | 55 | 30 | Water harvesting at medium RH | 50 |
| Ni2Cl2btdd | NR | 2.3 | 1837 | 0.32 | 1.07/1.33 | 53 | 400 | Water harvesting at medium RH | 69 |
| Ni2F0.83Cl0.17btdd | NR | 2.3 | 1770 | 0.32 | 1.07/1.33 | 53 | Poor stability | Water harvesting at medium RH | 69 |
| Ni2Br2btdd | NR | 2.2 | 1530 | 0.24 | 0.75/0.93 | 58 | 400 | Water harvesting at medium RH | 69 |
| Cr-soc-MOF-1 | 2.2 | 1.7 | 4549 | 0.68 | 1.95/2.43 | Room temperature under vacuum | 100 | Indoor humidity maintenance | 70 |
| BIT-66 | 0.87 | 2.58/0.65 | 1417 | 0.60 | 0.71/0.88 | Room temperature under vacuum | 50 | Indoor humidity maintenance | 71 |
| UiO-67-4Me-NH2-38% | 0.69 | 1.6 | 1638 | 0.58 | 0.68/0.85 | Room temperature under vacuum | 10 | Indoor humidity maintenance | 21 |
| NU-1500-Cr | 1.24 | 1.4 | 3580 | 0.45 | 1.09/1.36 | Room temperature under vacuum | 20 | Indoor humidity maintenance | 72 |
| Zr-adip | 0.47 | 1.28/0.66 | 1214 | 0.13 | 0.43/0.54 | 70 | 300 | Heat exchanger | 73 |
| KMF-1 | 0.56 | 0.57 | 1130 | 0.14 | 0.39/0.49 | 80 | 50 | Heat exchanger | 74 |
| AlFFIVE-1-Ni | 0.102 | n.d. | 258 | 0.02 | 0.2/0.25 | 105 | 15 | Dehydration of chemicals | 20 |
| kag-MOF-1 | 0.12 | 0.38 | 210 | 0.05 | 0.14/0.17 | 120 | NR | Dehydration of chemicals | 75 |
| ZJNU-30 | 1.24 | 2.1/1.4/0.7 | 1570 | 0.25 | 1.2/1.49 | Room temperature under vacuum | NR | Heat exchanger | 19 |
(1) Absorption properties of microporous MOFs. The research on the water absorption performance of HKUST-1 ([Cu3(btc)2(H2O)x], H2btc = benzene-1,3,5-tricarboxylate) was published by Bülow and co-workers in 2002, as one of the earliest water adsorption studies of MOFs.76 HKUST-1 contains OMS and two types of pore channels: hydrophilic large pores with pore sizes of 9 Å and hydrophobic small pores surrounded by benzene rings with pore sizes of 6 Å. The water adsorption isotherm of HKUST-1 is approximately type I (Fig. 4). At low relative pressures (P/P0 = 0.1–0.3), water molecules preferentially adsorb near the OMS in the hydrophilic macropores to form coordination bonds, followed by pore filling, achieving a saturation adsorption of 0.45 g g−1 (45 wt%).37 Due to the strong coordination between water molecules and open copper ions, water molecules cannot be completely desorbed in the low-pressure region. Particularly at 0% relative humidity, around 9% of the water molecules cannot be desorbed without heating (up to 200 °C).46,77 HKUST-1 degraded severely when subjected to adsorption and desorption water cycling experiments, losing 53% of its capacity after 20 cycles.78
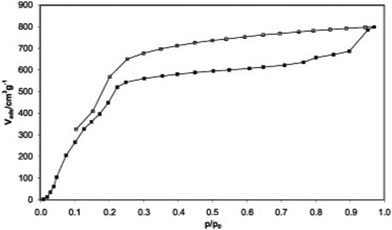 | ||
| Fig. 4 Water adsorption (solid symbols) and desorption (open symbols) isotherm of HKUST-1 at 25 °C. Reproduced with permission from ref. 37. Copyright 2009, Elsevier Inc. All rights reserved. | ||
UiO-66 ([Zr6O4(OH)4(bdc)12], H2bdc = 1,4-benzenedicarboxylic) has been extensively explored due to its superior water and chemical stability. At low pressure, water molecules selectively interact with the m3-OH of Zr clusters in UiO-66, which achieves less adsorption at this stage because of the comparatively hydrophobic pore surface. When the pressure reaches P/P0 = 0.35, continuous pore filling occurs related to the formation of hydrogen bonds, resulting in a saturation adsorption capacity of almost 0.44 g g−1. There is an irreversible hysteresis loop due to the limited flexibility of the backbone of the structure.79,80 In consequence, the water adsorption of UiO-66 generates a type V isotherm.81 With the same Zr6O4(OH)4(–CO2)12 SBUs, MOF-801 [Zr6O4(OH)4(fumarate)6] has similar absorption sites with UiO-66 (Fig. 5a–c). Due to the smaller sizes of cages, MOF-801 achieved a higher hydrophilicity and a tendency for the adsorption isotherm to move towards low relative pressure. In particular, at 25 °C, compared with UiO-66, MOF-801 embodies a steeper absorption at P/P0 = 0.08 going towards a lower relative pressure and a lower saturation adsorption quantity of nearly 0.36 g g−1, presenting a type II isotherm (Fig. 5d). Simultaneously, an increase in defects may enhance the hydrophilicity and specific surface area of MOF-801, resulting in a larger adsorption capacity of around 0.40 g g−1 and a propensity for isotherms to shift towards the low-pressure region.39
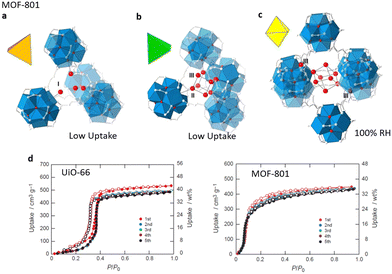 | ||
| Fig. 5 Water absorption steps in MOF-801 that is composed of tetrahedral cavities (a) and (b) and one octahedral cavity (c). (d) Cycle performance of MOF-801-P and UiO-66 at 25 °C. Reproduced with permission from ref. 39. Copyright 2014, American Chemical Society. | ||
With the longer 2,6-naphthalic acid, the cage diameters of DUT-52 rise to 8.0 Å and 10.1 Å, which leads to lower hydrophilicity and less water uptake, hence necessitating a greater pressure for pore filling. At 25 °C, the pore-filling pressure for DUT-52 is P/P0 = 0.4, which is larger than that of UiO-66. In conclusion, the pore size adjustment can alter the hydrophilicity of adsorbent, then influence its water absorption behaviors. With an increase in pore size, the stage of pore filling in a given topology often proceeds in the direction of increased pressure.60
CAU-10 ([Al(OH)(m-bdc)], m-H2bdc = 1,3-benzenedicarboxylic acid) is composed of isophthalate and helical chains assembled by cis vertex-sharing octahedral AlO6 units, with a square one-dimensional channel (ca. 5.6 Å).82 CAU-10 features a steep absorption trend at 0.15 < P/P0 < 0.20, because of the small pore size of 5.6 Å and hydrophilic pore surface. Water molecules preferentially engage with the hydroxyl group linked to the metal center, followed by multi-adsorption occurring based on the initially adsorbed water, resulting in a saturation adsorption capacity of around 0.32 g g−1 at 25 °C.16 Furthermore, CAU-10 can maintain its initial adsorption capacity after 700 cycles due to its stable one-dimensional chains formed by the Al–O bonds and negligible hysteresis loop.62,83
MIP-200 [Zr6(μ3-O)4(μ3-OH)4(mdip)2(formate)4], formed by a Zr6 oxocluster and linked by tetracarboxylic acid ligand (3,3′,5,5′-tetracarboxydiphenylmet-hane, H4mdip), contains two types of channel, 13 Å and 6.8 Å, respectively. Noticeably, at excessively low relative pressure (P/P0 = 0.01), water molecules are preferentially encapsulated between two Zr6 SBUs, interacting with the hydroxyl group and the metal center (Fig. 6a), yielding a strong zero-point heat of adsorption (70 kJ mol−1). Thus, a surge of water adsorption at the initial relative pressure was observed, implying the high degree of hydrophilicity. As the relative pressure attained was P/P0 = 0.1, the moisture content in the system was rationalized to pore filling. The majority of water was absorbed by MIP-200 at P/P0 < 0.25, as indicated in Fig. 6b. At 30 °C and P/P0 = 0.3, the equilibrium water uptake of 0.39 g g−1 is considerably higher than that of CAU-10 (0.30 g g−1) and MOF-801 (0.28 g g−1) (Fig. 6c). Most of the water molecules (25.1 wt%) can be removed below 65 °C, while more strongly bound water molecules (6.0 wt%) were released between 70 and 160 °C. As there is no hysteresis between the adsorption and desorption, the adsorption process was highly reversible with negligible energy loss (Fig. 6d).17
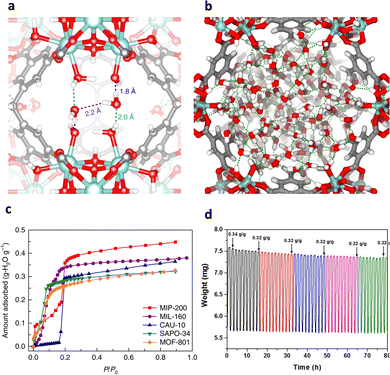 | ||
Fig. 6 (a) The initial adsorption sites for water molecules in MIP-200 at low pressure (P/P0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = = ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01). (b) Densely packed water molecules in the hexagonal channel at saturation (P/P0 0.01). (b) Densely packed water molecules in the hexagonal channel at saturation (P/P0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2). (c) Comparison of the water adsorption isotherms of MIP-200, MIL-160, CAU-10, SAPO-34 and MOF-801 at 30 °C. (d) Thermogravimetric analysis profile for multiple cycles of water adsorption–desorption of MIP-200. Reproduced with permission from ref. 17. Copyright 2018, Springer Nature. 0.2). (c) Comparison of the water adsorption isotherms of MIP-200, MIL-160, CAU-10, SAPO-34 and MOF-801 at 30 °C. (d) Thermogravimetric analysis profile for multiple cycles of water adsorption–desorption of MIP-200. Reproduced with permission from ref. 17. Copyright 2018, Springer Nature. | ||
Y-shp-MOF-5 [Y9(μ3-O)2(μ3-OH)12(OH)2(H2O)7(bteb)3] contains trigonal one-dimensional channels with diameters of 12 Å, with bteb (bteb = 1,2,4,5-tetrakis(4-carboxyphenyl)benzene) serving as a ligand. At low relative pressure (P/P0 < 0.1), water molecules are adsorbed on the open metal site and the bridging hydroxide ion by generating coordinate bonds and hydrogen bonds, respectively. Subsequently, the adsorbed molecules serve as secondary adsorption sites for additional water adsorption, after which the uptake reaches a platform due to the strong hydrophobicity of the ligand BTEB. Until the relative pressure approaches 0.5, the water molecules at the secondary adsorption sites are connected together by intermolecular hydrogen bonds to form superclusters, with rapid pore filling and a saturated adsorption quantity of 0.50 g g−1. Therefore, additional energy input is required to dissociate the superclusters during the desorption process, resulting in a significant hysteresis loop between 30% and 70% relative humidity. Strong contact between the water molecules and metal center of Y-shp-MOF-5 prevents around 5 to 6 wt% of water from desorbing at 25 °C. Following the second cycle, the water adsorption isotherm is nearly type V, with a saturation adsorption of 0.45 g g−1 which is unaffected after 200 cycles.7
ZrMOF-1 ([Zr6O4(OH)4(H2O)8(pep-1)]), constituted by 8-connected (8-c) cubic Zr6O4(OH)4(H2O)8 clusters and a rigid 8-c peripherally extended pentiptycene ligand, performed with high stability due to the high coordination numbers. ZrMOF-1 presents two types of cathedral cavities with pore sizes of 11 Å and 19 Å and a large BET surface area of 1877 m2 g−1. The water absorption isotherm of ZrMOF-1 is proximate to type IV(b). It shows a relatively low water uptake of 0.15 g g−1 at a low relative pressure stage (P/P0 = 0.2) owing to the hydrophobic structure provided by the ligand with a large number of benzene rings. The pore filling takes place at P/P0 = 0.3 with a water uptake of 0.65 g g−1. ZrMOF-1 has an excellent saturated adsorption capacity of 0.83 g g−1 that is only inferior to Ni2Cl2btdd, Co2Cl2btdd and Ni2F2btdd in microporous materials. In addition, ZrMOF-1 has ultra-high stability that can contain the crystallinity in strong acids/bases and maintains 94% of its original water-absorbing capacity after 500 cycles.84
(2) Adsorption properties of mesoporous MOFs. MIL-100 ([M3O(H2O)2OH(btc)2], M = Fe, Al, Cr), with mesopore sizes of 25 and 29 Å, is one of the most representative mesoporous MOFs for water adsorption (Fig. 7a).56,85 At low relative pressure (P/P0 < 0.25), the water molecules will first coordinate with metal sites, and then monolayer–multilayer adsorption occurs. When the pressure reaches 0.23 < P/P0 < 0.4, irreversible capillary condensation occurs with two water absorption steps sequentially corresponding to the smaller pores and larger pores, leading to the adsorption isotherm similar to the type IV(a). The saturation adsorption uptake of MIL-100(Cr) and MIL-100(Fe) would both be close to 0.8 g g−1,67 whereas MIL-100(Al) has a comparatively low adsorption capacity of approximately 0.50 g g−1 due to the lower pore volume caused by the incomplete removal of unreacted ligands. The pore sizes (29 Å and 34 Å) of MIL-101 ([Cr3X(H2O)2O(bdc)3], X = F, OH) are larger than that of MIL-100.86 This causes capillary condensation of MIL-101 to occur at a higher relative humidity, specifically, at P/P0 = 0.4 for small and P/P0 = 0.45 for large cages respectively (Fig. 7b). Because of the excellent thermal stability, the MIL-100 and MIL-101 series show exceptional cycling stability.87
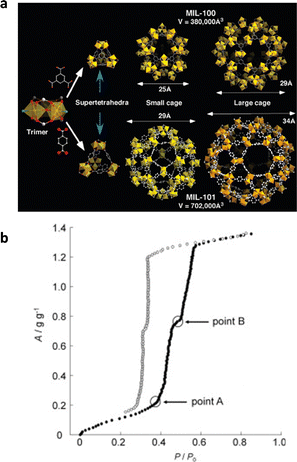 | ||
| Fig. 7 (a) Structures of the MIL-100 and MIL-101. Reproduced with permission from ref. 85. Copyright 2008, American Chemical Society. (b) Water adsorption (solid symbols) and desorption (open symbols) isotherms of MIL-101 at 25 °C. Reproduced with permission from ref. 86. Copyright 2012, Elsevier Inc. All rights reserved. | ||
However, capillary condensation does not always occur when the pore diameter is close to the critical diameter (2.076 nm for water at 25 °C).50 An exceptional example is Co2Cl2btdd (H2btdd = bis(1H-1,2,3-triazolo[4,5-b],[4′,5′-i])dibenzo[1,4]dioxin), also known as MAF-X27l-Cl,88 with honeycomb one-dimensional pore channels of 2.2 nm which are slightly bigger than 2.076 nm, and the structure of the channel is shown in Fig. 8a and b. At low relative pressure, water molecules first interact with coordination unsaturated Co2+ to form water chains by hydrogen bonding, which can reduce the pore width to 2.0 nm, below the critical diameter for capillary condensation.89 Due to the high hydrophilicity of the btdd2−, pore filling of the microporous pores occurred at a relatively low pressure of 0.28, with the adsorption capacity rising abruptly to 0.97 g g−1 (Fig. 8c). The adsorption isotherm approximates type IV(b) with no capillary coalescence. During 30 cycles from 25 °C to 120 °C, the capacity of Co2Cl2btdd reduced by 6.3 wt% without losing crystallinity. Co2Cl2btdd has a considerably greater water adsorption capacity at P/P0 = 0.3 (0.87 g g−1) than the mesoporous MIL-101, MIL-100, as well as the microporous CAU-10 and MOF-801.
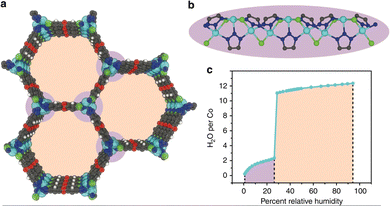 | ||
| Fig. 8 The honeycomb-like channels (a) and 1D metal chain (b) of Co2Cl2btdd viewed along the z-axis. (c) Water adsorption isotherm of Co2Cl2btdd at 20 °C. Reproduced with permission from ref. 89. Copyright 2019, The Author(s). | ||
Ni2Cl2btdd is more stable than Co2Cl2btdd due to the addition of inert Ni2+ to its structure, retaining 98% of its initial mass after 400 cycles. Due to the higher temperature and acid concentration employed in the synthesis of Ni2Cl2btdd, a better crystallinity can be obtained. Therefore, Ni2Cl2btdd achieved an impressive saturation adsorption capacity of 1.07 g g−1, which is the relatively high water adsorption capacity of all presently available materials with pore filling occurring within 35% RH.69
(1) Ligands. Generally, the polarity of pore channels can be altered by the polarity of the ligand, affecting the water absorption properties of MOFs. The water adsorption study of the MAF-47 series is one of the pioneering investigations in ligand regulation.38 Due to the high hydrophobicity of Hmim (2-methylimidazole), MAF-4, also known as ZIF-8, has an extremely low saturation of water absorption of 0.026 g g−1 at P/P0 < 0.9. Fortunately, MAF-7, which was synthesized by replacing the ligand with the more hydrophilic Hmtz (3-methyl-1,2,4-triazole), has a strong water affinity since the uncoordinated N atoms on Hmtz provide extra active sites for guest binding. Although MAF-4 and MAF-7 possess comparable pore volumes and specific surface areas, MAF-7 presents an increased water uptake of 0.443 g g−1 and the capillary compensation occurs near P/P0 = 0.3. MAF-47-x, the solid solutions consisting of different molar ratios of Hmim/Hmtz, had a higher pressure of pore filling with increasing proportions of Hmin and similar saturation water uptake with MAF-7, indicating that the H2O binding affinity can be decreased by reducing the density of active sites on the pore surface (Fig. 9a). Similar strategies were also used in the subsequent MOF-303 studies. MOF-303 [Al(OH)(pzdc)] (pzdc2− = 1-H-pyrazole-3,5-dicarboxylate) is isostructural with MOF-333 [Al(OH)(fdc)] (fdc2− = 2,4-furandicarboxylate). Interestingly, H2pzdc and H2fdc show different affinities with water molecules, thus the hydrophilicity of the pore can be efficiently regulated by altering the ratio of the two ligands, which enables control of the heats of adsorption (Qst) and desorption temperature. With an increasing degree of fdc2− incorporation, the Qst decreased continuously from 53 to 50 kJ mol−1 and the minimal desorption temperature decreased by 10 °C.41,42
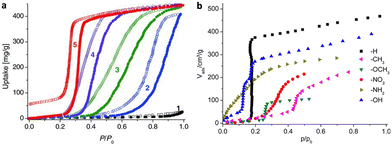 | ||
| Fig. 9 (a) Water adsorption (solid symbols) and desorption (open symbols) isotherms of MAF-4 (1), MAF-47-0.76 (2), MAF-47-0.49 (3), MAF-47-0.23 (4), and MAF-7 (5) at 25 °C. Reproduced with permission from ref. 38. Copyright 2011, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (b) Water adsorption isotherms of CAU-10-X at 25 °C. Reproduced with permission from ref. 47. Copyright 2013, American Chemical Society. | ||
The water absorption properties of CAU-10 derivatives were extensively explored. For example, CAU-10-X (X = CH3, OCH3, NO2, NH2 and OH) were synthesized through ligand functionalization to change the polarity, and exhibited a higher water affinity but low saturation adsorption of water due to the smaller effective pore volume.47 The presence of functional groups alters the polarity, which is principally reflected in the shift of the water adsorption isotherm and even in the change of the adsorption isotherm type. The hydrophilic groups like –NH2 and –OH may boost the polarity and the affinity for water molecules through hydrogen bonding, resulting in a shift of the adsorption isotherm towards the low-pressure region and an increase in capacity at this stage. Given the strong hydrogen bonding between –NH2 and water molecules, the isotherm of CAU-10-NH2 changes from type II to type I. The introduction of the hydrophobic groups –CH3, –OCH3, and –NO2 increases the hydrophobicity of the pore channel, hence inhibiting the interaction of the CAU-10 derivative with water at low pressure. Consequently, the adsorption isotherm shifts towards higher pressure, eventually increasing the pressure at which pore filling occurs (Fig. 9b). In addition to the modification of functional groups, adjusting the aromatic ring of the ligand may also impact the polarity of the derivative.90 Numerous different derivatives of CAU-10 were obtained by substituting the benzene ring of the ligand isophthalic acid with the more hydrophilic furan, pyridine, pyrazine, and thiophene, and the more hydrophobic pyrrole, respectively. In particular, the stronger polar hydrophilic aromatic ring increases the affinity for water, resulting in a larger uptake in the low-pressure region and a shift in the pore-filling stage towards lower pressures. The CAU-10 derivative with the highly hydrophilic furan, also known as MIL-160,91 expresses an approximate type I water adsorption isotherm. The hydrophobic pyrrole, on the other hand, enhances the hydrophobicity of the pore channel, decreasing the adsorption amounts in the low-pressure region and shifting the adsorption isotherm towards higher relative pressures.
Besides the organic ligands, inorganic bridging ligands coupled with metal centres also affect the water adsorption performance of MOFs. By exchanging the coordinated anions, the strength of the hydrogen bond between water and the pore wall can be regulated, which cannot be ascribed only to changes in pore wall polarity.69 By replacing the μ-Cl− in Ni2Cl2btdd with the more hydrophilic OH− and F−, for instance, the resultant Ni2(OH)2btdd and Ni2F0.83Cl0.17btdd demonstrate an increment in water adsorption at low relative pressure (P/P0 < 0.1). In contrast to the previously reported phenomenon of hydrophobic pore filling migrating to higher pressure, utilizing the more hydrophobic μ-Br− as opposed to μ-Cl− decreases the relative pressure of pore filling from 0.32 to 0.24. Infrared spectroscopy revealed that, due to the high hydrophilicity of F− and OH− groups, Ni2(OH)2btdd and Ni2F0.83Cl0.17btdd contain a significant proportion of ice-like water with saturated coordination H-bonding bound to the pore wall, providing fewer secondary adsorption sites than liquid-like water with unsaturated coordination H-bonding. With lower hydrophilicity and more liquid water, Ni2Br2btdd is more susceptible to pore filling, further implying that, in the presence of strong adsorption sites, the relative pressure for pore filling relies more on the interaction between the water molecules and the water bound to the pore wall than on the polarity of the pore wall itself.
(2) Central metal. Regulating the central metal can also affect the water adsorption behavior.92 Weak coordination interactions between coordination-unsaturated Zn2+ and water molecules in ALP-MOF-1(Zn) ([Zn2(μ-H2O)(alp)(bdc)], H2alp = allopurinol) contribute to almost negligible water adsorption at low relative pressure (P/P0 < 0.23). In contrast, the coordination between water molecules and Co2+ in ALP-MOF-2(Co) [Co2(μ-H2O)(alp)(bdc)] is much stronger than that with Zn2+, which markedly affects the water absorption behavior at low relative pressure (Fig. 10a). Individual water molecules initially coordinate with unsaturated Co2+ and form hydrogen bonds with the skeletal carbonyl oxygen atoms at a relative pressure of 0.04, above which, the adsorption continues with the coordinated water as the secondary adsorption sites until coordination saturation. By altering the Zn/Co metal, a variety of MOFs with distinct water absorption characteristics may be generated, suggesting that the polarity of the central metal can significantly impact the water absorption performance of the MOF, particularly in the low-pressure region (Fig. 10c and d).66
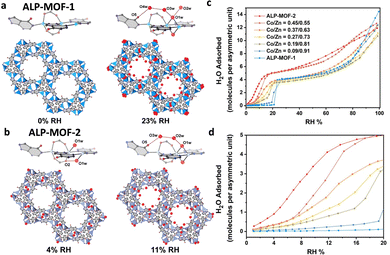 | ||
| Fig. 10 The stepwise water adsorptions of ALP-MOF-1 (a) and ALP-MOF-2 (b) at low relative humidity. Water adsorption isotherms of ALP-MOFs with different ratios of Co/Zn at (c) 0–100% RH and (d) 0–20% RH. Reproduced with permission from ref. 66. Copyright 2022, American Chemical. | ||
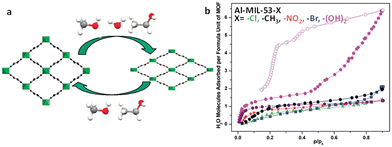 | ||
| Fig. 11 (a) Structural switching evolution of the MIL-53(Cr) upon adsorption and desorption. Reproduced with permission from ref. 97. Copyright 2010, American Chemical Society. (b) Water adsorption (solid symbols) and desorption (empty symbols) isotherms of the Al-MIL-53-X series at 25 °C. Reproduced with permission from ref. 99. Copyright 2011, American Chemical Society. | ||
Modifications of MIL-53 with functional groups lead to a more complex breathing behavior. In the case of the ligand functionalization of MIL-53(Al), a variety of MIL-53(Al) derivatives containing BDC-X ligands (X = Cl, Br, CH3, NO2, (OH)2) were synthesised.99 In particular, the modified MIL-53 with the BDC-X ligands (X = Cl, Br, CH3, NO2) behaved similarly to the unmodified MIL-53(Al), exhibiting a breathing effect with the capture of 1–2 water molecules per unit pore. Meanwhile, the dihydroxy-functionalized MIL-53(Al) collects a single water molecule per unit pore at P/P0 = 0.1 with subsequent pore shrinkage. Then, it captures an additional five water molecules per unit pore when the relative pressure increases (P/P0 = 0.4–0.9), which is accompanied by the pore expanding (Fig. 11b). The higher hydrophilicity of the modified backbone may account for this anomaly. Similarly, after the primary adsorption, MIL-53(Al)–(COOH)2 and MIL-53(Fe)–(COOH)2 expressed pore shrinkage, following which the water adsorption continues with further pore expansion.64,65 Due to the breathing effect, there arises spontaneously a distinct hysteresis loop between adsorption and desorption.
Recently Zaworotko and co-workers reported that a flexible 1D coordination network, [Cu(HQS)(TMBP)] (H2HQS = 8-hydroxyquinoline-5-sulfonic acid and TMBP = 4,4′-trimethylenedipyridine) can exhibit water vapor-induced reversible structural conformation, showing a stepped sorption profile (type F-IV) at <30% RH.100 Similarly, X-dia-2-Cd ([Cd(Imibz)2], HImibz = 4-((4-(1H-imidazol-1-yl)phenylimino)methyl)benzoic acid) exhibits a similar water-induced transformation. On the other hand, X-dia-2-Cd showed negligible hysteresis due to the very fast transient pore expansion.101
3. Application of water-absorbing MOF
3.1 Water harvesting devices
Deserts struggle with water constraint due to little precipitation.102 Water collection anywhere and anytime satisfies the immediate requirements of people living in extensively arid regions. However, traditional water treatment devices are typically large and intricate, making it difficult to collect water in the arid areas. Fortunately, the atmosphere contains a lot of water vapor, and directly extracting this vapor provides an effective way to solve these challenges.103 MOFs, with ultrahigh porosity and a tubular pore structure, have been suggested and exploited as absorbents for atmospheric water collection.104,105MOFs used for atmospheric water harvesting should fulfil the following conditions related to the operation environment and energy concerns of water harvesting devices: (1) strong chemical and water cycle stability for the complex conditions in the atmosphere. (2) High water absorption capacity under the operating conditions. (3) Low regeneration temperature. (4) Step-wise water absorption isotherms are favorable because of the large working capacity, but hysteresis loops should be avoided to reduce energy losses.106
Typically, the water collecting process consists of three steps: (1) the sorbent absorbs water vapor from the atmosphere at low temperatures and high relative humidity, (2) it releases it by raising the temperature or reducing the relative vapor pressure of the water inside the device, and (3) it then condenses the released water vapor to complete a water collection cycle. During this process, water vapor may be released and condensed naturally or with external energy.
In 2017, Yaghi and co-workers reported the first device for atmospheric water capture and collection using metal–organic frameworks (MOFs), specifically using MOF-801 as the water adsorbent and achieving water collection at a relative humidity of 20%. The device consists of a MOF coating that can be heated and a condensation unit (Fig. 12a and b), and begins water absorption at 25 °C and 20% relative humidity. The waste heat provided by a solar energy between 25 °C and 65 °C is used as the driving force for circulation (Fig. 12c and d). MOF-801 exhibits a steep adsorption isotherm at P/P0 = 0.08, with an adsorption capacity of up to 0.29 g g−1 at 25 °C and a relative humidity of 20% (Fig. 12e and f); therefore, the device is estimated to theoretically capture 0.24 L of water per kilogram of MOF per day. Due to the hydrophilicity of MOF-801, the regeneration temperature is as low as 65 °C, allowing for easy regeneration conditions such as using the low heat of natural sunlight, which negates the need for additional energy input.15
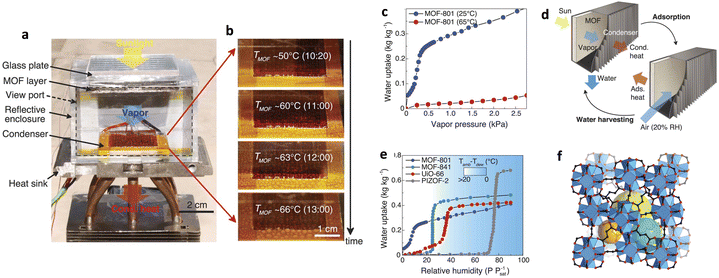 | ||
| Fig. 12 (a) Image of a water harvesting device with MOF-801 as the water adsorbent. (b) Formation and growth of water droplets as a function of MOF temperatures (TMOF) and local time of day. (c) Water-adsorption isotherms of MOF-801 at 25 °C and 65 °C, respectively. (d) A MOF water-harvesting system, composed of a MOF layer and a condenser, undergoing solar-assisted water-harvesting and adsorption processes. (e) Water adsorption isotherms of MOF-801, MOF-841, UiO-66, and PIZOF-2 at 25 °C. The background color map shows the minimum difference between the temperatures of the ambient air (Tamb) and the condenser (Tdew) required for dew collection with active cooling. (f) Crystal structure of MOF-801. Reproduced with permission from ref. 15. Copyright 2017, American Association for the Advancement of Science. | ||
Passive water collection devices employ solar energy for absorption and desorption processes without additional energy input, using air as the heat sink. The passive device operates only one cycle each day, with poor time efficiency, and water productivity mostly relies on the maximal adsorption capacity of MOFs. Due to the limitations of thermal conductivity and solar absorption capacity, a large number of adsorbents must be employed to satisfy the daily demands of an individual (3.5 liters),107 necessitating relatively expensive apparatus.
To increase apparatus productivity and decrease associated costs, it is preferable to design active water collection systems that can be repeatedly cycled and require minimal energy input. The active collection device absorbs and releases water with the aid of external energy sources, such as electricity for heating, refrigeration, and condensation. The optimal adsorbent for the active water collection system requires rapid water uptake and release kinetics and a low regeneration temperature. Among the various adsorbent options, MOF-303 stands out as the most promising candidate for the water collection system. At a temperature of 30 °C, MOF-303 exhibits a sharp adsorption uptake at a relative pressure of P/P0 = 0.12, with a maximum adsorption capacity of 0.40 g g−1 at a relative humidity of 30%. MOF-303 has a low adsorption enthalpy of approximately 52 kJ mol−1 and shows fast adsorption rates at both 30% and 40% relative humidity, outperforming SAPO-34, Zeolite 13X, and aluminum fumarate in this aspect. Furthermore, MOF-303 displays rapid desorption rates, with complete desorption achieved within one hour at 85 °C and even faster at higher temperatures, which is significantly shorter than the desorption times for SAPO-34 and Zeolite 13X. Notably, a 3 mm bed of MOF-303 can be fully dehydrated within a few minutes of mild heating (85–100 °C). An active water harvesting device was fabricated using MOF-303, which was subjected to adsorption at 27 °C and 32% relative humidity, followed by desorption at 85 °C. The device was cycled nine times over a 24 hour period, yielding a water production rate of 1.3 L kgMOF−1, which is significantly higher than that of passive water harvesting devices. In comparison, a device using aluminum fumarate as the adsorbent only produced 0.55 L kgMOF−1 under the same conditions, highlighting the superior dynamic water uptake performance of MOF-303 over other adsorbents.108
Besides passive and active water harvesting, a self-adaptive water harvesting system that optimizes the duration and efficacy of each cycle based on external environmental conditions has been developed. The system employs an algorithm to ensure the shortest possible cycle time is accomplished regardless of the environmental conditions. The adaptive water harvesting system combines the relative humidity and temperature of the external environment with the dew point value to configure the water collection device (Fig. 13a). The measured dew point is obtained in real-time, and the steady-state dew point value is chosen as the endpoint for adsorption, while the maximal dew point value is chosen as the endpoint for desorption. As a guide for adsorption and desorption, the external temperature corresponding to the maximal dew point value is used as the heating temperature. The system has the potential to considerably increase the efficacy and effectiveness of water harvesting devices, especially in arid regions with limited water resources. Compared to an active water harvesting device, a self-adaptive device with MOF-801 as the sorbent was shown to produce up to 26% more water (2.6 LH2O kgMOF-801−1 d−1) (3.52 LH2O kgMOF-801−1 d−1). In addition, the self-adaptive device reduces to 1.5 cycles per day, reducing energy consumption by 20–44% under the same climatic conditions. Furthermore, the self-adaptive device was verified to produce water continuously and consistently with no performance degradation after operating for over a year (Fig. 13b and e).109
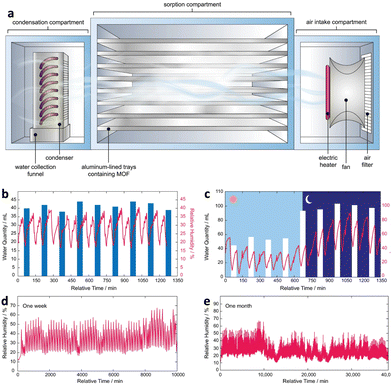 | ||
Fig. 13 (a) Schematic of the atmospheric water harvester. (b) Stress test for 24 ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h performed under a controlled environment (20% RH and 25 °C). (c) Assessment of real-world performance over a full day. (d) Real-environment continuous performance for one week. (e) Performance of self-adaptive water harvesting devices during continuous operation for a month. Reproduced with permission from ref. 109. Copyright 2022, The Author(s). h performed under a controlled environment (20% RH and 25 °C). (c) Assessment of real-world performance over a full day. (d) Real-environment continuous performance for one week. (e) Performance of self-adaptive water harvesting devices during continuous operation for a month. Reproduced with permission from ref. 109. Copyright 2022, The Author(s). | ||
Ni2Cl2btdd achieves an ultra-high water uptake of 0.9 g g−1 at 40% RH and shows great potential for atmospheric water absorption. Wang and Zhang et al. achieved striking progress in the passive water absorption device area.110 By using Ni2Cl2btdd and materializing the precise temperature zoning inside the suction unit, highly efficient water desorption and condensation was achieved with a small device. In the device, the heating temperature can barely reach 97 °C through direct solar radiation with the effect of double insulation layers and a photothermal plate. At the same time, the cooling part can achieve efficient condensation in the temperature difference of 60 °C due to the existence of vibrating fins. The device could obtain 0.495 LH2O kgMOF−1 after running one cycle (7 h) at 30 °C 40% RH and with 1000 W of light. Furthermore, the average unit volume and unit area water yield are 19.63 L m−3 and 717.25 g m−2 respectively, which are comparable to the previous device's use of 6.67 kg m−2 MOF and in the case of using only 1.45 kg m−2 MOF in the new device.
MOFs with water absorption stages greater than 30% RH can be utilized in arid water collection systems if a cooling source is incorporated into the adsorption device. When the temperature within the device is lowered, the relative humidity increases due to the reduction in saturated vapor pressure, which can exceed 30% RH compared to the external environment. Under these conditions, MOFs with water uptake steps at moderate relative humidity can be utilized for water harvesting. MIL-101 exhibits water absorption steps greater than 35% RH, with an adsorption capacity of 0.106 g g−1 at 30% RH and 25 °C in the external environment. When the cooling device is activated and the temperature within the device reaches 14 °C, the adsorption capacity of MIL-101 increases considerably to 1.05 g g−1 at a rapid rate. The majority of water can be desorbed from MIL-101 without additional heating after the cooling device has been deactivated. The average functional capacity per cycle is 0.77 g g−1 under conditions of 23 °C and 30% RH. Under optimal conditions, if the cooling device is activated for 20 minutes every 20 minutes, the predicted water collection rate can reach 27 L kg−1 day−1, which is significantly higher than current water harvesting devices made from MOF-303 and MOF-801.111 However, the high energy usage and maintenance requirements of the condenser apparatus are disadvantages of the improved condensation efficacy.
The development of MOFs for water collection has led to improvements in devices as well as the widespread development of MOF-based composite materials that can increase water absorption capacity and ease of regeneration through the synergistic effect of MOFs and other components, allowing them to adapt to various adsorption environments. Due to the outstanding photothermal property and thermal conductivity of carbon-based materials, the ability of MOFs complexed with carbon-based materials to speed up adsorption and analysis has been investigated. Du and co-workers have synthesized high water-absorbing photothermal composite materials composed of MOF-801 and carbon nanotubes (CNTs), which exhibit a good water absorption capacity and fast adsorption and desorption rates. This has contributed to the investigation of adding carbon materials to enhance the photothermal performance of water adsorbents.112
MOF-hydrogel composites have been applied to tropical regions with high relative humidity, in addition to water collection devices designed for arid regions. Using MIL-101(Cr) as a water-absorbing agent, which has a large adsorption capacity for water at high humidity (P/P0 = 0.9), and the hydrophilic polymer PNIPAM [poly(n-isopropylacrylate)] as a continuous water-absorbing matrix, a porous PC-MOF (polymer composite-MOF) water absorb-supply material with a strong outer wall was prepared (Fig. 14a). MIL-101 exhibited a precipitous water absorption trend between relative pressures of 0.4 and 0.5, and the saturation water uptake at 25 °C was as high as 1.4 g g−1. By combining the exceptional water absorption performance of MIL-101 with the continuous water absorption of PNIPAM, a sustainable process for atmosphere-water harvesting can be attained. MIL-101 captures water molecules from the environment, and since PNIPAM cannot expand to water absorption saturation due to the strength of the composite outer wall, it constantly absorbs and releases the water in MOFs (Fig. 14b). The system generates 6 grams of fresh water per gram sorbent per day with a total water delivery efficiency of 95% and an automated water delivery efficiency of 71% at 90% RH, the highest stated record for atmospheric water harvesting devices without heat sources.113 The related water absorption properties of the above devices are summarized in Table 2.
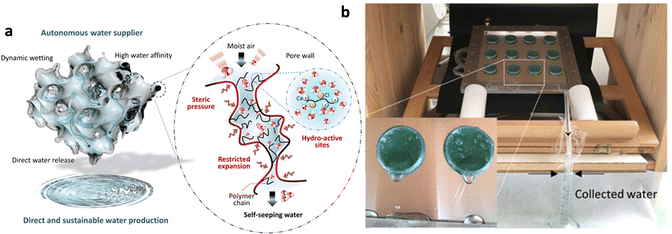 | ||
| Fig. 14 (a) Schematic illustration of polymer–MOF hybrid materials for self-seeping water. (b) Image of the proof-of-concept prototype for atmospheric water harvesting. Reproduced with permission from ref. 113. Copyright 2020, The Authors. | ||
| Types of MOF | Device type | Humidity and temperature for water absorption | Humidity and temperature for water desorption | Daily water output | Cycle times of one day | Ref. |
|---|---|---|---|---|---|---|
| MOF-801 | Passive water harvesting | 25 °C, 20% RH | 65 °C, 20% RH | 0.24 L kg−1 day−1 | 1 | 15 |
| MOF-303 | Positive water harvesting | 27 °C, 32% RH | 80–120 °C, 32% RH | 1.3 L kg−1 day−1 | 9 | 108 |
| MOF-801 | Self-adaptive water harvesting | 15–35 °C, 17–32% RH | Environment temperature corresponding to the dew point | 3.52 L kg−1 day−1 | Depends on the environmental | 109 |
| Conditions from 6 to >30 cycles daily | ||||||
| MIL-101 | Water harvesting by inner cooling device | Outside 25 °C, 30% RH, inside 14 °C | 30% RH, 25 °C | 27 L kg−1 day−1 | 35 | 111 |
| MIL-101 | Autonomous water collection | 25 °C, 90% RH | No desorption required | 6 L kg−1 day−1 | Autonomous self-supplement water harvesting | 113 |
3.2 Indoor humidity maintenance
In contemporary times, individuals tend to spend a significant portion of their diurnal activities and routines within enclosed indoor spaces. As recommended by the American Society of Heating, refrigerating and air-conditioning engineers (ASHRAE), maintaining indoor relative humidity levels between 45% and 65% is deemed optimal for ensuring human well-being and comfort.114 Deviations from this range, either in excess or deficiency, can adversely affect human health.115To regulate indoor RH levels, it is possible to utilize suitable water adsorption materials that can rapidly adsorb water vapor when the humidity level exceeds 65% RH and desorb it quickly when the RH level falls below 45% RH. Considering practical applications, the ideal MOF for indoor humidity control should exhibit a robust water absorption performance and high adsorption capacity, featuring an S-shaped adsorption curve with a steep absorption trend between 45% and 65% and an obvious hysteresis loop concentrated in 45–65%.
Y-shp-MOF-5 is the first MOF applied for indoor humidity control, which exhibits an S-shaped water adsorption isotherm at room temperature, with an adsorption capacity of 0.33 g g−1 at a RH of 65% and a saturated adsorption capacity of 0.50 g g−1. In a hermetically sealed environment with variable RH levels, when the RH level was slightly above 65%, Y-shp-MOF-5 adsorbed water vapor until the RH level dropped below 45% or the material reached its saturated adsorption capacity of 0.50 g g−1. Subsequently, when the humidity level fell below 45%, Y-shp-MOF-5 underwent desorption, releasing water molecules into the atmosphere and restoring the humidity to a level between 45% and 65%. Furthermore, Y-shp-MOF-5 exhibited excellent cycling performance, with no change in working capacity after 1000 cycles of adsorption at 85% RH and desorption at 25% RH. However, in the case where the relative humidity is drastically higher than 65% RH (e.g., 70–80% RH) and drastically lower than 45% RH (e.g., 35–25% RH), automatic dehumidification/humidification is required because the working capacity of the material is limited, and the sample is saturated by adsorption or desorbed completely.7
Cr-soc-MOF-1 ([Fe3(μ3-O)(H2O)2(tcpt)1.5Cl] H4tcpt = 3,3′′,5,5′′-tetrakis(4-carboxyphenyl)-p-terphenyl) exhibits an S-shaped water adsorption isotherm, with a steep adsorption trend between 60% and 70% RH and desorption occurs between 35% and 45% RH, satisfying the requirements of ASHRAE for indoor humidity control. Therefore, Cr-soc-MOF-1 can also be applied for indoor humidity control. Compared to Y-shp-MOF-5, Cr-soc-MOF-1 has a significantly higher surface area (BET specific surface area of 4549 m2 g−1) and water adsorption capacity (1.95 g g−1 at room temperature), which is approximately four times that of Y-shp-MOF-5. Additionally, Cr-soc-MOF-1 also exhibits excellent cycling stability, with no change in water adsorption performance observed after 100 adsorption–desorption cycles conducted between 25% and 85% RH.70
BIT-66 ([V3(O)3(H2O)(btb)2] H3btb = 1,3,5-tris(4-carboxyphenyl)benzene) demonstrates an S-shaped water adsorption isotherm, with adsorption primarily occurring between 55% and 65% RH and desorption occurring between 45–50% RH, accompanied by a noticeable hysteresis phenomenon. According to the working humidity range required by ASHRAE, BIT-66 shows a fairly high working capacity of approximately 0.45 g g−1, while the water capacity of Y-shp-MOF-5 was about 0.017 g g−1 within this working range. Although NU-1500-Cr exhibits a higher working capacity of approximately 0.86 g g−1, it has no significant hysteresis. Therefore, BIT-66 with a large hysteresis loop is better suited for maintaining indoor humidity. The indoor environment is also confronted by the intricate issue of mould, fungi and bacteria, especially under high humidity conditions. Fortunately, BIT-66 possesses both moisture tunability ability and bacteriostatic activity, which can effectively eliminate E. coli by producing reactive oxygen species under visible light irradiation.71
CAU-1-OH, built by functioning CAU-1, can be used as a humidity control agent with the bacteriostatic effect. The adsorption step of CAU-1-OH is mainly in the range of 40–60% RH, and the desorption process is mainly concentrated in the range of 30–50% RH, with a saturation adsorption capacity of ca. 0.45 g g−1. The working capacity of CAU-1-OH is significantly higher than Y-SHP-MOF-5 and Cr-soc-MOF-1. Importantly, CAU-1-OH is more applicable to daily life because of the high natural abundance and low toxicity of aluminum. Moreover, CAU-1-OH has a strong photocatalytic anti-bacterial ability, and the inactivation rate of E. coli reaches 99.94% after 2 hours of visible light irradiation.8
UiO-67-4Me-NH2-38% with a good adsorption ability was synthesized by ligand exchange on the basis of UiO-67. The introduction of methyl groups increased its hydrophobicity, resulting in an S-shaped adsorption isotherm with steep adsorption occurring between 55% and 65% and desorption concentrated between 45% and 55%. The inflection points of adsorption and desorption are entirely within the ASHRAE range, with a maximum adsorption capacity of approximately 0.65 g g−1. When the adsorption humidity is selected as 65% and 40% for the desorption humidity, the working capacity is approximately 0.52 g g−1, higher than that of BIT-66, Y-shp-MOF-5, and Cr-soc-MOF-1. And the related water absorption properties of indoor humidity maintenance are summarised in Table 3. In a 60 m3 room at 25 °C, increasing the humidity from 0 to 65% RH required 1.495 kg of UiO-67-4Me-NH2-38%, while decreasing the humidity from 100% to 65% RH required 0.805 kg of UiO-67-4Me-NH2-38%. In addition, UiO-67-4Me-NH2-38% preferentially captures formaldehyde, NH3, benzene, toluene, and n-hexane in the presence of both water and gaseous pollutants, achieving both humidity control and indoor air purification (Fig. 15).21
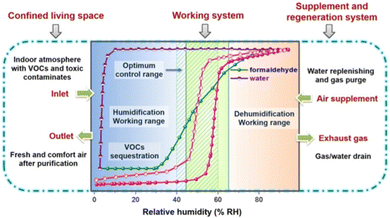 | ||
| Fig. 15 Indoor humidity maintenance isotherms and breakthrough curves with formaldehyde/water vapors of UiO-67-4Me-NH2-38%. Reproduced with permission from ref. 21. Copyright 2021, Wiley-VCH GmbH. | ||
3.3 Water adsorption heat exchanger
Due to frequent harsh weather, heating and cooling equipment demand has been rising. Conventional refrigerators and air conditioners utilize extremely combustible and poisonous chlorofluorocarbon refrigerants, which are environmentally hazardous and only 3–10% efficient. Therefore, developing eco-friendly and effective cooling and heating technology is crucial.116 Heat-driven adsorption heat exchangers, which use low-temperature waste heat or solar energy as the major energy source, may help solve energy and environmental issues.117 Water is an ecologically favorable adsorbate due to its high enthalpy of evaporation (40.7 kJ mol−1) and non-polluting nature.118,119Water adsorption heat exchanger operation is depicted in Fig. 16. In the process of regeneration or desorption of the adsorbent, the water in the pore channel is desorbed using external heat, and the liberated water vapor condenses in the condenser, releasing heat into the environment, as illustrated in Fig. 16a. In the adsorption process, adsorbents release heat by absorbing water vapor, which raises the temperature. The water container absorbs heat and lowers the ambient temperature owing to evaporation (Fig. 16b). The working conditions of the adsorption heat exchanger are determined by three temperatures, (1) high temperature (Thigh) for regeneration and desorption, (2) medium temperature (Tmedium) for condensation and adsorption of water by the adsorbent, and (3) low temperature (Tlow) for water evaporation. The energy utilization efficiency can be characterized by a coefficient of performance (COP) under the same working conditions. Typically, for refrigeration systems, the COPC is defined as the energy absorbed from the environment by the evaporation of the working fluid in the evaporation chamber (Qev) divided by the energy required to regenerate the adsorbent (Qregen), a value that is normally less than 1.119
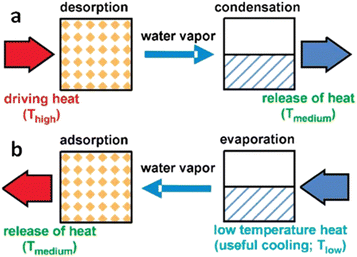 | ||
| Fig. 16 Principle process in a water adsorption heat exchanger: (a) In the regeneration or desorption process, the water in the pore channel is desorbed by external heat, and the released water vapor condenses in the condenser, which releases the condensed heat into the environment. (b) In the adsorption process, the adsorbent releases heat by absorbing water vapor, thus causing an increase in temperature, while the container containing water absorbs heat due to the evaporation of water, resulting in a decrease in ambient temperature. Reproduced with permission from ref. 119. Copyright 2009, American Chemical Society. | ||
Due to its availability and simplicity of manufacture, stable microporous aluminium fumarate is a good candidate for water adsorption heat exchangers. Microporous aluminum fumarate exhibits a characteristic steep S-shaped isotherm with a maximum adsorption capacity of 0.35 g g−1 at 0.20 < P/P0 < 0.35 and outstanding cycle stability.122 The thermal gradient technique allows it to develop directly on the metal substrate, and the excellent thermal coupling between the MOF and aluminium plate promotes heat transmission and remains stable after 4500 adsorption/desorption cycles.55 Afterward, microporous aluminium fumarate heat exchangers were investigated and fabricated. Microporous aluminium fumarate was directly synthesized with polysiloxane binders coated on aluminium plates to make water adsorption full-size heat exchangers. Under the operating conditions of the actual adsorption refrigerator, with the adsorption chamber at 90 °C (Thigh)/30 °C (Tmedium) and the evaporation chamber at 18 °C (Tlow), the maximum cooling power of the devices was 2900 W with an average of 690 W, and the maximum specific volume cooling power was 430 W L−1, with an average of 101 W L−1. The material may regenerate below 65 °C, enabling the system to be more efficient and energy-saving. At an evaporation chamber temperature of 22 °C, the device cooling efficiency (COPC) reaches 0.72 at a heat source of 65 °C and a heat dissipation temperature of 35 °C.123
Because of the high water uptake, low cost, and good water stability, CAU-10-H was used to make adsorption heat exchangers by Stock and co-workers (Fig. 17a). At 70 °C driving heat temperatures, the CAU-10-H coated heat exchanger had an excellent working capacity (0.256 g g−1) but only increased 0.018 g g−1 by raising the temperature to 150 °C. The adsorption heat exchanger works reliably at 70 °C (Thigh)/34 °C (Tmedium) in the adsorption chamber and 10 °C (Tlow) in the evaporation chamber (Fig. 17b), with a volumetric specific cooling power of 229 W L−1 and a specific cooling power of 1369 W kgAds−1. Its rapid adsorption kinetics of 0.6 g kgAds−1 s−1 took 430 s from 0 to 90% loading (Fig. 17c). The power output averaged 215 W, more than most silica-aluminate heat exchangers. The power of existing silica-aluminate water adsorption heat exchangers is in the range of 79–230 W. Furthermore, CAU-10-H only needs to be regenerative at 70 °C, which is lower than the driving temperature of water adsorbents such as silica-alumina phosphate and zeolite, enabling effective use of low-temperature heat sources such as waste heat and solar thermal collectors.18
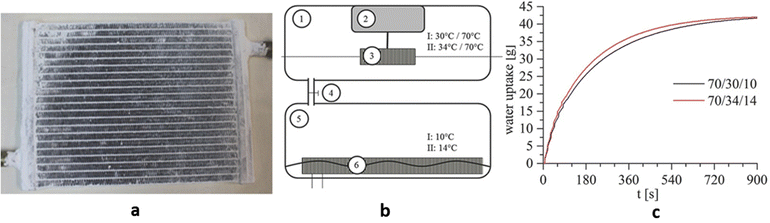 | ||
| Fig. 17 (a) Image of a coated heating exchanger. (b) Schematic setup of the measurement device with 1 adsorption chamber, 2 balance, 3 adsorber with hydraulic connections, 4 valve, 5 evaporator chamber, and 6 evaporator in water with hydraulic connections. (c) Overall water uptake of the coated heating exchangers. Reproduced with permission from ref. 18. Copyright 2017, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
MOF-841 ([Zr6O4(OH)4(mtb)2(HCOO)4(H2O)4], H4mtb = tetrahedral methanetetrabenzoic acid) exhibited high reversibility of water absorption with an S-shaped adsorption curve. Due to the lower Qst (50.4 kJ mol−1) of MOF-841 in contrast to the MOF-801 series, the COPC of MOF-841 can reach 0.83 at operating conditions of 70 °C (Thigh)/45 °C (Tmedium) for the adsorption chamber and 15 °C (Tlow) for the evaporation chamber.124
KMF-2 is synthesized by replacing half of the ipa2− ligand in CAU-10 with 3,5-pyridinedicarboxylate (pydc2−). Due to the more hydrophilic structure of KMF-2, the water absorption step moves to the lower pressure region and the water absorption capacity attains 0.32 g g−1 at P/P0 = 0.2. At 70 °C/30 °C in the adsorption chamber and 5 °C in the evaporation chamber, and KMF-2 performed a higher COPC of 0.75 exceeding CAU-10 (0.72).125
During experiments conducted under adsorption conditions of 30 °C/RH 35% and desorption conditions of 63 °C/RH 10%, MIP-200 demonstrated a steep adsorption curve at a P/P0 of 0.25 and a working capacity of 0.3 g g−1, higher than that of CAU-10-H (0.27 g g−1), MIL-160 (0.24 g g−1) and MOF-801 (0.14 g g−1). The COPC of MIP-200 increased with increasing desorption temperature within the range of 55–70 °C at an evaporation chamber temperature of 5 °C and a mid-temperature of 30 °C during adsorption. Furthermore, the COPC of MIP-200 was higher than that of commonly used solid adsorbents such as MIL-160, CAU-10, and MOF-801 at desorption temperatures of 60–100 °C.17
By replacing the bridging –CH2 on the MIP-200 ligand with –NH, the resulting MOF (Zr-adip) bearing Lewis base N sites shows increased hydrophilicity. Zr-adip achieved a high water uptake of 0.43 g g−1 at 30 °C and P/P0 = 0.25, which is higher than MIP-200 (0.39 g g−1), KMF-1 (0.39 g g−1) and MOF-303 (0.38 g g−1). And at 70 °C/30 °C in the adsorption chamber and 5 °C in the evaporation chamber, the COPc of Zr-adip (0.79) was slightly higher than MIP-200 (0.78), KMF-1 (0.75) and MOF-303 (0.72), showing a favourable energy utilization efficiency for the heat exchanger.73 Similarly, UiO-66-N, built by replacing the benzene ring of the UiO-66 ligand with pyridine, generated a higher water capacity (0.37 g g−1) and the adsorption step moved to lower pressure as the introduced N atom strengthens the hydrophilicity. At the adsorption temperature of 30 °C, evaporation temperature of 5 °C, and desorption temperature of 65 °C, the COPC of UiO-66-N was 0.71, much higher than that of UiO-66 (0.50), and better than well-performing CAU-23 (0.34), KMF-1 (0.42), MIL-160 (0.51), and MOF-303 (0.61).60 ZJNU-30 possessed steep adsorption at P/P0 of 0.22 and reached saturation rapidly without stepwise adsorption, achieving an exceedingly high working capacity of 1.2 g g−1, larger than that of MOF-841. Since ZJNU-30 ([Zr9O6(OH)6(PhCOO)6(L)4], L = 4,4′,4′′-benzene-1,3,5-triyl-1,1′,1′′-trinaphthoic acid) consists of three interconnected cages of different sizes (7 Å, 14 Å and 21 Å),126 the initial adsorption of water molecules occurs in the small cages at low relative pressures, which subsequently diffuses into the other connected cages, avoiding the capillary condensation that commonly occurs in mesoporous materials and ensuring low initial adsorption pressures. As ZJNU-30 showed no irreversible capillary condensation, it featured a particularly low desorption temperature of about 30 °C. At the operating conditions of 30 °C/25 °C in the adsorption chamber and 10 °C in the evaporation chamber, it provided the highest COPC reported to date, up to 0.948, showing great potential for application in the field of water adsorption heat exchange.19
3.4 Dehydration of chemicals
Natural gas is normally transported by pipeline systems, but the presence of water can cause corrosion of metal pipelines.127,128 Eddaoudi and co-workers reported the water adsorption MOFs of AlFFIVE-1-Ni (KAUST-8, [NiAlF5(H2O)(pyr)2]·2(H2O), pyr = pyrazine) and FeFFIVE-1-Ni (KAUST-9, [NiFeF5(H2O)(pyr)2]·4(H2O)) used for nature gas dehydration (Fig. 18a). The water uptakes of AlFFIVE-1-Ni and FeFFIVE-1-Ni increased sharply at relative humidity close to 0 due to the presence of OMS (Fig. 18b). At P/P0 = 0.05 the adsorption amounts of the two were 0.22 g g−1 and 0.18 g g−1, respectively, which were higher than most commercially used solid adsorbents such as 3A and 13X. Besides, the heats of adsorption for the two were 63 kJ mol−1 and 65 kJ mol−1, respectively, which were far lower than that of zeolites 3A, 4A and 5A (80–120 kJ mol−1), and thus possessed a lower regeneration temperature of ca. 105 °C (Fig. 18c). In addition, both AlFFIVE-1-Ni and FeFFIVE-1-Ni demonstrated excellent cycling stability, with no discernible change in performance after 15 cycles (Fig. 18d). Thus, it is evident that AlFFIVE-1-Ni and FeFFIVE-1-Ni satisfy the requirements for natural gas dehydration adsorbents, which was further demonstrated by the breakthrough experiment with a mixture of CO2/CH4 (v/v = 1/99) at RH of 75%. Changing the gas to CO2/N2 and increasing the CO2 concentration to 1, 10, and 50% did not significantly affect the water vapor retention time, indicating excellent dehydration performance (Fig. 18e–h).20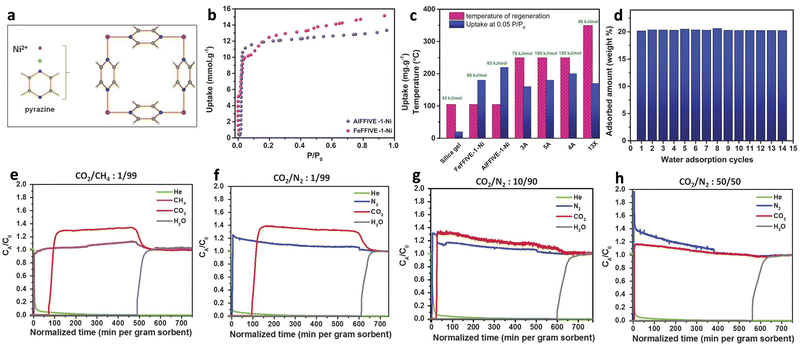 | ||
| Fig. 18 (a) The repetitive square pattern in the Ni-pyrazine (4,4′) square-grid layer. (b) Water adsorption isotherms of AlFFIVE-1-Ni (violet) and FeFFIVE-1-Ni (pink) at 35 °C. (c) Comparison and relationship between water uptake at P/P0 = 0.05, heat of sorption, and regeneration temperature of AlFFIVE-1-Ni and FeFFIVE-1-Ni, along with commercial desiccants. (d) Cycles for water absorption uptake of AlFFIVE-1-Ni (adsorption at 25 °C and desorption at 105 °C). (e) Breakthrough test with a humid (75% RH) CO2/CH4: 1/99 mixture. (f)–(h) Effect of CO2 concentration on water breakthrough tests (humid 75% RH). Reproduced with permission from ref. 20. Copyright, 2017, The American Association for the Advancement of Science. | ||
Kag-MOF-1 [Zn(HCN4)2,(H2O)2/9] possessed honeycomb one-dimensional pore channels with a pore size of approximately 3.8, while retaining fine crystallinity at pH = 4 and pH = 10. Fully activated kag-MOF-1 possesses metal sites that are accessible for coordination with water molecules. In the meantime, the N atoms in the ligand increase the hydrophilicity of the channel, resulting in the formation of hydrogen bonds with water molecules and a steep adsorption in the region of low relative pressure (P/P0 = 0.05). Although the water adsorption capacity of kag-MOF-1 is approximately 0.1 g g−1 when P/P0 = 0.05, which is lower than that of AlFFIVE-1-Ni and FeFFIVE-1-Ni, kag-MOF-1 requires less energy in the recycling process because its water adsorption heat is only 60 kJ mol−1. The dynamic breakthrough experiment was conducted at 25 °C and a RH of 75% in a nitrogen flow (8 cm3 min−1), giving a water retention duration of 1100 min g−1. Changing the gas to a CO2/N2 mixture (v/v = 10/90) resulted in a water retention time of 1050 min g−1, which was slightly less than that in the absence of CO2, indicating that kag-MOF-1 can concomitantly adsorb CO2 and H2O. Moreover, kag-MOF-1 exhibited acid resistance and the ability to assimilate H2S, enabling it to remove H2O, CO2, and H2S from natural gas in one step.75
Traditional water-absorbing MOF materials can also be applied in natural gas dehydration. UiO-66-NH2 and MIL-101 had higher saturated adsorption capacities (0.36 g g−1, 1.41 g g−1) compared to the commercial 3A molecular sieve, alumina and silica. UiO-66-NH2 (8.3 min g−1) and MIL-101 (18 min g−1) show a longer retention time than commercial adsorbents (<7.5 min g−1) in the dynamic breakthrough experiments carried out with a natural gas flow rate of 200 cm3 min−1 at 75% RH.6,129
Additionally, MOFs can be utilized for ethanol dehydration in order to produce high-purity ethanol. MAF-45 ([Cd4(CH3COO)4(H2dobdim)(H3dobdim)2], H4dobdim = 2,6-dihydroxymethyl-benzodiimidazole) and MAF-46 ([Zn4(OH)2(CH3COO)(Hdobdim)(H2dobdim)]) are flexible MOFs featuring high hydrophilicity, consisting of large cavities and minimally sized apertures. MAF-45 possesses a strong affinity to water and shows rigidity without obvious structural changes during water adsorption. For ethanol, the uptake of MAF-45 is low at a low relative pressure, but increases at P/P0 = 0.6 with 17% volume expansion. For MAF-46 with smaller aperture, the ethanol is hindered during the absorption. Although MAF-46 can gradually expand during the water absorption process, ethanol is still blocked after absorbing water which can occupy the pore apertures acting as a high diffusion barrier for ethanol. This guest-dominated gating adsorption process can avoid the co-adsorption problem effectively. Breakthrough experiments of H2O/EtOH vapor mixtures (5% and 10% water) were performed, and the two MOFs showed outstanding dehydration performance.130
3.5 Water purification
In addition to atmospheric water harvesting, MOFs also can be applied in sea water desalination and water pollution treatment to produce pure water.The principle of seawater desalination is homologous to harvesting water from the atmosphere. The adsorbent collected the evaporated seawater first and then regenerated it at low temperature, and the desorbed water condensed at the same time. To date, pure water produced by seawater desalination has achieved high-quality distilled water standards.131 Silica gels have been utilized for desalination, which means MOFs can also be potential candidates for seawater desalination due to the outstanding water absorption property. Simulink simulated seawater desalination and found COP-27(Ni) and aluminum fumarate can produce pure water of 4.3 m3 per (ton day) at 5 °C and 6 m3 per (ton day) at 20 °C, respectively.104 Moreover, aluminum fumarate can produce 6.8 m3 per (ton day) pure water in practical tests, which is higher than silica gels (5 m3 per (ton day)).132
Furthermore, various metal nanoparticles and fibers have been used for water pollution treatment to dislodge the chlorine,133,134 heavy metals135 and dyes.136 Apart from this, MOFs are widespread in polluted water treatment.137–140 For instance, Ce(III)-4,4′,4′′-((1,3,5-triazine-2,4,6-triyl)tris(azanediyl))tribenzoic acid-organic framework Ce-H3tatab-MOFs are available for removing fluoride from water. Under 318 K and pH = 4, the Langmuir adsorption capacity of Ce-H3tatab-MOFs for F− can attain 129.7 mg g−1 after a series of synthetic modifications.139 Jiang et al. produced WC-CO composites by carbonizing the ZIF-67/wood composite for dye removal (Fig. 19). The adsorption of CR and MB (Congo red (CR) and cationic dye methylene blue (MB)) by the composite was 1117.03 and 805.08 mg g−1, respectively. When the dye concentration reached 1200 mg L−1, 99.98% of dye can be removed by using WC-CO composites.138
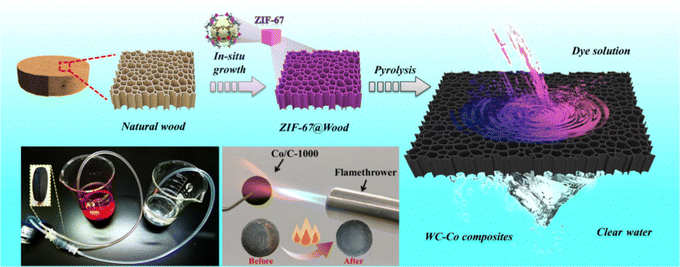 | ||
| Fig. 19 Schematic diagrams of the preparation of the WC-Co composites for water treatment. Reproduced with permission from ref. 138. Copyright 2022 Elsevier B.V. All rights reserved. | ||
4. Conclusions and outlook
MOFs have emerged as promising candidates in the field of water absorption because of their abundant adsorption sites and customizable pore structures. In this review, the water absorption characteristics and mechanisms for multiple MOFs were introduced, and the strategies for regulating water absorption properties were elaborated. In the meantime, the applications of water-absorption MOFs in atmospheric water harvesting, indoor humidity control, adsorption-driven heat pump and industrial chemicals dehydration are reviewed. Even though significant progress has been made in water-absorbent MOFs, there are still obstacles that should be overcome to promote the revolution in this field. Here, we proposed some obstacles and possible solutions:(1) First and foremost, the requests for high water stability of MOFs in specific applications are increasing. The suggested direction for the design and development of new water-absorbing MOFs is to increase the stability of MOFs in challenging circumstances in order to broaden their usage range under corresponding practical application scenarios.
(2) Although the thermodynamics of water adsorption has been widely investigated, studies on kinetics are still scarce. Note that the pore structure (geometry, size, and chemistry) of the coordination network and morphology of the adsorbent particles, as well as the device construction, can all influence the diffusion of water molecules. Therefore, additional efforts are suggested to optimize water adsorption kinetics. It is proposed that combined with computer simulation, the kinetics of water molecular diffusion should be fully studied on the molecular scale, to design better porous materials.
(3) In recent years, water-absorbing MOFs have demonstrated substantial commercial potential as a result of their extensive spectrum of applications, but the prerequisite is the sustainable large-scale production of MOFs with lower cost and low energy consumption. However, rapid and large-scale synthesis may have an impact on the water absorption performance and structural stability of MOFs, necessitating additional methodological innovations and performance testing to continue development. The development of emerging green synthesis approaches, such as mechanochemistry, chemical vapor deposition and aqueous phase synthesis, will be important ways to reduce costs. Moreover, compositing MOFs with other inexpensive functional adsorbents, such as hydrogels and hygroscopic salts, can also further decrease the costs.
(4) In order to collect enough water to meet the requirements of actual scenarios, not only must the development of adsorbents continue, but also the development of devices. For instance, modifying the closed geometry of passive water collecting devices to increase solar usage or improving the energy utilization of active water harvesting devices is highly desired. Consequently, productivity and stability should be monitored for an extended period in a specific application environment. Meanwhile, other applications, such as indoor humidity maintenance and chemical dehydration, have not yet been fully explored, as a result of which, it is urgent to promptly develop MOF devices for practical applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are thankful for the support from the National Natural Science Foundation of China (22071195, 22101231) and The Youth Innovation Team of Shaanxi Universities.Notes and references
- P. H. Gleick, Water in crisis: paths to sustainable water use, Ecol. Appl., 1998, 8, 571–579 CrossRef.
- M. J. Kalmutzki, C. S. Diercks and O. M. Yaghi, Metal–Organic Frameworks for Water Harvesting from Air, Adv. Mater., 2018, 30, e1704304 CrossRef PubMed.
- R. S. Schemenauer and P. Cereceda, A proposed standard fog collector for use in high-elevation regions, J. Appl. Meteorol., 1994, 33, 1313–1322 CrossRef.
- X. Liu, X. Wang and F. Kapteijn, Water and Metal–Organic Frameworks: From Interaction toward Utilization, Chem. Rev., 2020, 120, 8303–8377 CrossRef CAS PubMed.
- M. M. Ghiasi, A. Bahadori and S. J. F. Zendehboudi, Estimation of the water content of natural gas dried by solid calcium chloride dehydrator units, Fuel, 2014, 117, 33–42 CrossRef CAS.
- K. M. C. Santos, T. R. Menezes, M. R. Oliveira, T. S. L. Silva, K. S. Santos, V. A. Barros, D. C. Melo, A. L. Ramos, C. C. Santana, E. Franceschi, C. Dariva, S. M. Egues, G. R. Borges and J. F. De Conto, Natural gas dehydration by adsorption using MOFs and silicas: A review, Sep. Purif. Technol., 2021, 276, 119409 CrossRef CAS.
- R. G. AbdulHalim, P. M. Bhatt, Y. Belmabkhout, A. Shkurenko, K. Adil, L. J. Barbour and M. Eddaoudi, A Fine-Tuned Metal–Organic Framework for Autonomous Indoor Moisture Control, J. Am. Chem. Soc., 2017, 139, 10715–10722 CrossRef CAS PubMed.
- J. Zhang, P. Li, X. Zhang, X. Ma and B. Wang, Aluminum Metal–Organic Frameworks with Photocatalytic Antibacterial Activity for Autonomous Indoor Humidity Control, ACS Appl. Mater. Interfaces, 2020, 12, 46057–46064 CrossRef CAS PubMed.
- M. S. Rahman and T. P. Labuza, Handbook of food preservation, CRC Press, 2007, pp. 465–494 Search PubMed.
- A. Karmakar, V. Prabakaran, D. Zhao and K. J. Chua, A review of metal–organic frameworks (MOFs) as energy-efficient desiccants for adsorption driven heat-transformation applications, Appl. Energy, 2020, 269, 115070 CrossRef CAS.
- E.-P. Ng and S. Mintova, Nanoporous materials with enhanced hydrophilicity and high water sorption capacity, Microporous Mesoporous Mater., 2008, 114, 1–26 CrossRef CAS.
- H. Maher, T. H. Rupam, K. A. Rocky, R. Bassiouny and B. B. Saha, Silica gel-MIL 100(Fe) composite adsorbents for ultra-low heat-driven atmospheric water harvester, Energy, 2022, 238, 121741 CrossRef CAS.
- L. W. Wang, R. Z. Wang and R. G. Oliveira, A review on adsorption working pairs for refrigeration, Renewable Sustainable Energy Rev., 2009, 13, 518–534 CrossRef CAS.
- N. Bukovec, P. Bukovec and V. Arbanas, Tg and dsc investigation of CaCl2 6H2O, a phase change material for energy storage, Thermochim. Acta, 1989, 148, 281–288 CrossRef.
- H. Kim, S. Yang, S. R. Rao, S. Narayanan, E. A. Kapustin, H. Furukawa, A. S. Umans, O. M. Yaghi and E. N. Wang, Water harvesting from air with metal–organic frameworks powered by natural sunlight, Science, 2017, 356, 430–434 CrossRef CAS PubMed.
- F. Fathieh, M. J. Kalmutzki, E. A. Kapustin, P. J. Waller, J. Yang and O. M. Yaghi, Practical water production from desert air, Sci. Adv., 2018, 4, eaat3198 CrossRef PubMed.
- S. Wang, J. S. Lee, M. Wahiduzzaman, J. Park, M. Muschi, C. Martineau-Corcos, A. Tissot, K. H. Cho, J. Marrot, W. Shepard, G. Maurin, J.-S. Chang and C. Serre, A robust large-pore zirconium carboxylate metal–organic framework for energy-efficient water-sorption-driven refrigeration, Nat. Energy, 2018, 3, 985–993 CrossRef CAS.
- D. Lenzen, P. Bendix, H. Reinsch, D. Frohlich, H. Kummer, M. Mollers, P. P. C. Hugenell, R. Glaser, S. Henninger and N. Stock, Scalable Green Synthesis and Full-Scale Test of the Metal–Organic Framework CAU-10-H for Use in Adsorption-Driven Chillers, Adv. Mater., 2018, 30, 1705869 CrossRef PubMed.
- A. Luna-Triguero, A. Sławek, H. P. Huinink, T. J. H. Vlugt, A. Poursaeidesfahani, J. M. Vicent-Luna and S. Calero, Enhancing the Water Capacity in Zr-Based Metal–Organic Framework for Heat Pump and Atmospheric Water Generator Applications, ACS Appl. Nano Mater., 2019, 2, 3050–3059 CrossRef CAS.
- A. Cadiau, Y. Belmabkhout, K. Adil, P. M. Bhatt, R. S. Pillai, A. Shkurenko, C. Martineau-Corcos, G. Maurin and M. Eddaoudi, Hydrolytically stable fluorinated metal–organic frameworks for energy-efficient dehydration, Science, 2017, 356, 731–735 CrossRef CAS PubMed.
- N. X. Zhu, Z. W. Wei, C. X. Chen, X. H. Xiong, Y. Y. Xiong, Z. Zeng, W. Wang, J. J. Jiang, Y. N. Fan and C. Y. Su, High Water Adsorption MOFs with Optimized Pore-Nanospaces for Autonomous Indoor Humidity Control and Pollutants Removal, Angew. Chem., Int. Ed., 2022, 61, e202112097 CrossRef CAS PubMed.
- S. Kitagawa, R. Kitaura and S. Noro, Functional porous coordination polymers, Angew. Chem., Int. Ed., 2004, 43, 2334–2375 CrossRef CAS PubMed.
- Y. Duan, Y. Huang, C. Wang, Q. Wang, K. Ge, Z. Lu, H. Wang, J. Duan, J. Bai and W. Jin, Formation and fine-tuning of metal–organic frameworks with carboxylic pincers for the recognition of a C2H2 tetramer and highly selective separation of C2H2/C2H4, Chem. Sci., 2023, 14, 4605–4611 RSC.
- Q. Dong, Y. Huang, J. Wan, Z. Lu, Z. Wang, C. Gu, J. Duan and J. Bai, Confining Water Nanotubes in a Cu10O13-Based Metal–Organic Framework for Propylene/Propane Separation with Record-High Selectivity, J. Am. Chem. Soc., 2023, 145, 8043–8051 CrossRef CAS PubMed.
- A. U. Czaja, N. Trukhan and U. Muller, Industrial applications of metal–organic frameworks, Chem. Soc. Rev., 2009, 38, 1284–1293 RSC.
- Q. Dong, Y. Huang, K. Hyeon-Deuk, I. Y. Chang, J. Wan, C. Chen, J. Duan, W. Jin and S. Kitagawa, Shape-and size-dependent kinetic ethylene sieving from a ternary mixture by a trap-and-flow channel crystal, Adv. Funct. Mater., 2022, 32, 2203745 CrossRef CAS.
- J. Duan, M. Higuchi, J. Zheng, S.-I. Noro, I. Y. Chang, K. Hyeon-Deuk, S. Mathew, S. Kusaka, E. Sivaniah, R. Matsuda, S. Sakaki and S. Kitagawa, Density Gradation of Open Metal Sites in the Mesospace of Porous Coordination Polymers, J. Am. Chem. Soc., 2017, 139, 11576–11583 CrossRef CAS PubMed.
- J. R. Li, R. J. Kuppler and H. C. Zhou, Selective gas adsorption and separation in metal–organic frameworks, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC.
- X. Liu, J. Li, N. Li, B. Li and X. H. Bu, Recent advances on metal–organic frameworks in the conversion of carbon dioxide, Chin. J. Chem., 2021, 39, 440–462 CrossRef CAS.
- L. Su, Y. Zhang, X. Qiu, J. Han and Z. Tang, Photocatalytic Carboxylation of Phenyl Halides with CO2 by Metal–Organic Frameworks Materials, Chin. J. Chem., 2021, 39, 312–316 CrossRef CAS.
- S. Sen, N. N. Nair, T. Yamada, H. Kitagawa and P. K. Bharadwaj, High proton conductivity by a metal–organic framework incorporating Zn8O clusters with aligned imidazolium groups decorating the channels, J. Am. Chem. Soc., 2012, 134, 19432–19437 CrossRef CAS PubMed.
- Q. Wei, S. Xue, W. Wu, S. Liu, S. Li, C. Zhang and S. Jiang, Plasma Meets MOFs: Synthesis, Modifications, and Functionalities, Chem. Rec., 2023, 23, e202200263 CrossRef CAS PubMed.
- N. C. Burtch, H. Jasuja and K. S. Walton, Water Stability and Adsorption in Metal–Organic Frameworks, Chem. Rev., 2014, 114, 10575–10612 CrossRef CAS PubMed.
- A. S. Hall, A. Kondo, K. Maeda and T. E. Mallouk, Microporous brookite-phase titania made by replication of a metal–organic framework, J. Am. Chem. Soc., 2013, 135, 16276–16279 CrossRef CAS PubMed.
- T. Loiseau, C. Serre, C. Huguenard, G. Fink, F. Taulelle, M. Henry, T. Bataille and G. Ferey, A rationale for the large breathing of the porous aluminum terephthalate (MIL-53) upon hydration, Chem. – Eur. J., 2004, 10, 1373–1382 CrossRef CAS PubMed.
- A. Vimont, J.-M. Goupil, J.-C. Lavalley, M. Daturi, S. Surblé, C. Serre, F. Millange, G. Férey and N. Audebrand, Investigation of acid sites in a zeotypic giant pores chromium(III) carboxylate, J. Am. Chem. Soc., 2006, 128, 3218–3227 CrossRef CAS PubMed.
- P. Küsgens, M. Rose, I. Senkovska, H. Fröde, A. Henschel, S. Siegle and S. Kaskel, Characterization of metal–organic frameworks by water adsorption, Microporous Mesoporous Mater., 2009, 120, 325–330 CrossRef.
- J.-P. Zhang, A.-X. Zhu, R.-B. Lin, X.-L. Qi and X.-M. Chen, Pore Surface Tailored SOD-Type Metal–Organic Zeolites, Adv. Mater., 2011, 23, 1268–1271 CrossRef CAS PubMed.
- H. Furukawa, F. Gándara, Y.-B. Zhang, J. Jiang, W. L. Queen, M. R. Hudson and O. M. Yaghi, Water adsorption in porous metal–organic frameworks and related materials, J. Am. Chem. Soc., 2014, 136, 4369–4381 CrossRef CAS PubMed.
- A. Cadiau, J. S. Lee, D. Damasceno Borges, P. Fabry, T. Devic, M. T. Wharmby, C. Martineau, D. Foucher, F. Taulelle, C. H. Jun, Y. K. Hwang, N. Stock, M. F. De Lange, F. Kapteijn, J. Gascon, G. Maurin, J. S. Chang and C. Serre, Design of hydrophilic metal organic framework water adsorbents for heat reallocation, Adv. Mater., 2015, 27, 4775–4780 CrossRef CAS PubMed.
- N. Hanikel, X. Pei, S. Chheda, H. Lyu, W. Jeong, J. Sauer, L. Gagliardi and O. M. Yaghi, Evolution of water structures in metal–organic frameworks for improved atmospheric water harvesting, Science, 2021, 374, 454–459 CrossRef CAS PubMed.
- Z. Zheng, N. Hanikel, H. Lyu and O. M. Yaghi, Broadly Tunable Atmospheric Water Harvesting in Multivariate Metal–Organic Frameworks, J. Am. Chem. Soc., 2022, 144, 22669–22675 CrossRef CAS PubMed.
- Z. Zheng, H. L. Nguyen, N. Hanikel, K. K.-Y. Li, Z. Zhou, T. Ma and O. M. Yaghi, High-yield, green and scalable methods for producing MOF-303 for water harvesting from desert air, Nat. Protoc., 2023, 18, 136–156 CrossRef CAS PubMed.
- M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. S. W. Sing, Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report), Pure Appl. Chem., 2015, 87, 1051–1069 CrossRef CAS.
- P. D. Dietzel, R. E. Johnsen, R. Blom and H. Fjellvag, Structural changes and coordinatively unsaturated metal atoms on dehydration of honeycomb analogous microporous metal–organic frameworks, Chem. – Eur. J., 2008, 14, 2389–2397 CrossRef CAS PubMed.
- A. Domán, O. Czakkel, L. Porcar, J. Madarász, E. Geissler and K. László, Role of water molecules in the decomposition of HKUST-1: Evidence from adsorption, thermoanalytical, X-ray and neutron scattering measurements, Appl. Surf. Sci., 2019, 480, 138–147 CrossRef.
- H. Reinsch, M. A. van der Veen, B. Gil, B. Marszalek, T. Verbiest, D. de Vos and N. Stock, Structures, Sorption Characteristics, and Nonlinear Optical Properties of a New Series of Highly Stable Aluminum MOFs, Chem. Mater., 2012, 25, 17–26 CrossRef.
- J. Canivet, A. Fateeva, Y. Guo, B. Coasne and D. Farrusseng, Water adsorption in MOFs: fundamentals and applications, Chem. Soc. Rev., 2014, 43, 5594–5617 RSC.
- G. Akiyama, R. Matsuda, H. Sato, A. Hori, M. Takata and S. Kitagawa, Effect of functional groups in MIL-101 on water sorption behavior, Microporous Mesoporous Mater., 2012, 157, 89–93 CrossRef CAS.
- A. J. Rieth, S. Yang, E. N. Wang and M. Dinca, Record Atmospheric Fresh Water Capture and Heat Transfer with a Material Operating at the Water Uptake Reversibility Limit, ACS Cent. Sci., 2017, 3, 668–672 CrossRef CAS PubMed.
- M. Ding, X. Cai and H.-L. Jiang, Improving MOF stability: approaches and applications, Chem. Sci., 2019, 10, 10209–10230 RSC.
- K. Wang, Y. Li, L. H. Xie, X. Li and J. R. Li, Construction and application of base-stable MOFs: a critical review, Chem. Soc. Rev., 2022, 51, 6417–6441 RSC.
- J. J. Low, A. I. Benin, P. Jakubczak, J. F. Abrahamian, S. A. Faheem and R. R. Willis, Virtual high throughput screening confirmed experimentally: porous coordination polymer hydration, J. Am. Chem. Soc., 2009, 131, 15834–15842 CrossRef CAS PubMed.
- T. Y. Luo, S. Park, T. H. Chen, Prerna, R. Patel, X. Li, J. Ilja Siepmann, S. Caratzoulas, Z. Xia and M. Tsapatsis, Simultaneously Enhanced Hydrophilicity and Stability of a Metal–Organic Framework via Post-Synthetic Modification for Water Vapor Sorption/Desorption, Angew. Chem., Int. Ed., 2022, 61, e202209034 CrossRef CAS PubMed.
- H. Xu, Y. Wu, L. Yang, Y. Rao, J. Wang, S. Peng and Q. Li, Water-Harvesting Metal–Organic Frameworks with Gigantic Al(24) Units and their Deconstruction into Molecular Clusters, Angew. Chem., Int. Ed., 2023, 62, e202217864 CrossRef CAS PubMed.
- F. Jeremias, A. Khutia, S. K. Henninger and C. Janiak, MIL-100(Al, Fe) as water adsorbents for heat transformation purposes—a promising application, J. Mater. Chem. A, 2012, 22, 10148–10151 RSC.
- D. D. Zhou, P. Chen, C. Wang, S. S. Wang, Y. Du, H. Yan, Z. M. Ye, C. T. He, R. K. Huang, Z. W. Mo, N. Y. Huang and J. P. Zhang, Intermediate-sized molecular sieving of styrene from larger and smaller analogues, Nat. Mater., 2019, 18, 994–998 CrossRef CAS PubMed.
- F. Jeremias, D. Fröhlich, C. Janiak and S. K. Henninger, Advancement of sorption-based heat transformation by a metal coating of highly-stable, hydrophilic aluminium fumarate MOF, RSC Adv., 2014, 4, 24073–24082 RSC.
- G. E. Cmarik, M. Kim, S. M. Cohen and K. S. Walton, Tuning the adsorption properties of UiO-66 via ligand functionalization, Langmuir, 2012, 28, 15606–15613 CrossRef CAS PubMed.
- F.-F. Lu, X.-W. Gu, E. Wu, B. Li and G. Qian, Systematic evaluation of water adsorption in isoreticular UiO-type metal–organic frameworks, J. Mater. Chem. A, 2023, 11, 1246–1255 RSC.
- V. Bon, I. Senkovska, J. D. Evans, M. Wöllner, M. Hölzel and S. Kaskel, Insights into the water adsorption mechanism in the chemically stable zirconium-based MOF DUT-67 – a prospective material for adsorption-driven heat transformations, J. Mater. Chem. A, 2019, 7, 12681–12690 RSC.
- D. Frohlich, S. K. Henninger and C. Janiak, Multicycle water vapour stability of microporous breathing MOF aluminium isophthalate CAU-10-H, Dalton Trans., 2014, 43, 15300–15304 RSC.
- S.-N. Kim, J. Kim, H.-Y. Kim, H.-Y. Cho and W.-S. Ahn, Adsorption/catalytic properties of MIL-125 and NH2-MIL-125, Catal. Today, 2013, 204, 85–93 CrossRef CAS.
- A. Shigematsu, T. Yamada and H. Kitagawa, Wide control of proton conductivity in porous coordination polymers, J. Am. Chem. Soc., 2011, 133, 2034–2036 CrossRef CAS PubMed.
- N. Reimer, B. Gil, B. Marszalek and N. Stock, Thermal post-synthetic modification of Al-MIL-53–COOH: systematic investigation of the decarboxylation and condensation reaction, CrystEngComm, 2012, 14, 4119–4125 RSC.
- Y. Han, P. Das, Y. He, D. C. Sorescu, K. D. Jordan and N. L. Rosi, Crystallographic Mapping and Tuning of Water Adsorption in Metal–Organic Frameworks Featuring Distinct Open Metal Sites, J. Am. Chem. Soc., 2022, 144, 19567–19575 CrossRef CAS PubMed.
- G. Akiyama, R. Matsuda and S. Kitagawa, Highly porous and stable coordination polymers as water sorption materials, Chem. Lett., 2010, 39, 360–361 CrossRef CAS.
- N. Ko, P. G. Choi, J. Hong, M. Yeo, S. Sung, K. E. Cordova, H. J. Park, J. K. Yang and J. Kim, Tailoring the water adsorption properties of MIL-101 metal–organic frameworks by partial functionalization, J. Mater. Chem. A, 2015, 3, 2057–2064 RSC.
- A. J. Rieth, A. M. Wright, G. Skorupskii, J. L. Mancuso, C. H. Hendon and M. Dinca, Record-Setting Sorbents for Reversible Water Uptake by Systematic Anion Exchanges in Metal–Organic Frameworks, J. Am. Chem. Soc., 2019, 141, 13858–13866 CrossRef CAS PubMed.
- S. M. Towsif Abtab, D. Alezi, P. M. Bhatt, A. Shkurenko, Y. Belmabkhout, H. Aggarwal, Ł. J. Weseliński, N. Alsadun, U. Samin, M. N. Hedhili and M. Eddaoudi, Reticular Chemistry in Action: A Hydrolytically Stable MOF Capturing Twice Its Weight in Adsorbed Water, Chem, 2018, 4, 94–105 CAS.
- D. Ma, P. Li, X. Duan, J. Li, P. Shao, Z. Lang, L. Bao, Y. Zhang, Z. Lin and B. Wang, A Hydrolytically Stable Vanadium(IV) Metal–Organic Framework with Photocatalytic Bacteriostatic Activity for Autonomous Indoor Humidity Control, Angew. Chem., Int. Ed., 2020, 59, 3905–3909 CrossRef CAS PubMed.
- Z. Chen, P. Li, X. Zhang, P. Li, M. C. Wasson, T. Islamoglu, J. F. Stoddart and O. K. Farha, Reticular Access to Highly Porous acs-MOFs with Rigid Trigonal Prismatic Linkers for Water Sorption, J. Am. Chem. Soc., 2019, 141, 2900–2905 CrossRef CAS PubMed.
- B. Li, F. F. Lu, X. W. Gu, K. Shao, E. Wu and G. Qian, Immobilization of Lewis Basic Nitrogen Sites into a Chemically Stable Metal–Organic Framework for Benchmark Water-Sorption-Driven Heat Allocations, Adv. Sci., 2022, 9, e2105556 CrossRef PubMed.
- K. H. Cho, D. D. Borges, U. H. Lee, J. S. Lee, J. W. Yoon, S. J. Cho, J. Park, W. Lombardo, D. Moon, A. Sapienza, G. Maurin and J. S. Chang, Rational design of a robust aluminum metal–organic framework for multi-purpose water-sorption-driven heat allocations, Nat. Commun., 2020, 11, 5112 CrossRef CAS PubMed.
- M. I. H. Mohideen, R. S. Pillai, K. Adil, P. M. Bhatt, Y. Belmabkhout, A. Shkurenko, G. Maurin and M. Eddaoudi, A Fine-Tuned MOF for Gas and Vapor Separation: A Multipurpose Adsorbent for Acid Gas Removal, Dehydration, and BTX Sieving, Chem, 2017, 3, 822–833 CAS.
- Q. M. Wang, D. Shen, M. Bülow, M. L. Lau, S. Deng, F. R. Fitch, N. O. Lemcoff and J. Semanscin, Metallo-organic molecular sieve for gas separation and purification, Microporous Mesoporous Mater., 2002, 55, 217–230 CrossRef CAS.
- P. M. Schoenecker, C. G. Carson, H. Jasuja, C. J. J. Flemming and K. S. Walton, Effect of Water Adsorption on Retention of Structure and Surface Area of Metal–Organic Frameworks, Ind. Eng. Chem. Res., 2012, 51, 6513–6519 CrossRef CAS.
- S. K. Henninger, F. Jeremias, H. Kummer and C. Janiak, MOFs for Use in Adsorption Heat Pump Processes, Eur. J. Inorg. Chem., 2011, 2625–2634 Search PubMed.
- J. Canivet, J. Bonnefoy, C. Daniel, A. Legrand, B. Coasne and D. Farrusseng, Structure–property relationships of water adsorption in metal–organic frameworks, New J. Chem., 2014, 38, 3102–3111 RSC.
- F.-X. Coudert, A. Boutin, A. H. Fuchs and A. V. Neimark, Adsorption Deformation and Structural Transitions in Metal–Organic Frameworks: From the Unit Cell to the Crystal, J. Phys. Chem. Lett., 2013, 4, 3198–3205 CrossRef CAS.
- M. I. Hossain and T. G. Glover, Kinetics of Water Adsorption in UiO-66 MOF, Ind. Eng. Chem. Res., 2019, 58, 10550–10558 CrossRef CAS.
- D. Fröhlich, E. Pantatosaki, P. D. Kolokathis, K. Markey, H. Reinsch, M. Baumgartner, M. A. van der Veen, D. E. De Vos, N. Stock, G. K. Papadopoulos, S. K. Henninger and C. Janiak, Water adsorption behaviour of CAU-10-H: a thorough investigation of its structure–property relationships, J. Mater. Chem. A, 2016, 4, 11859–11869 RSC.
- M. F. de Lange, T. Zeng, T. J. H. Vlugt, J. Gascon and F. Kapteijn, Manufacture of dense CAU-10-H coatings for application in adsorption driven heat pumps: optimization and characterization, CrystEngComm, 2015, 17, 5911–5920 RSC.
- W. Liu, E. Wu, B. Yu, Z. Liu, K. Wang, D. Qi, B. Li and J. Jiang, Reticular Synthesis of Metal–Organic Frameworks by 8-Connected Quadrangular Prism Ligands for Water Harvesting, Angew. Chem., Int. Ed., 2023, 62, e202305144 CrossRef CAS PubMed.
- P. L. Llewellyn, S. Bourrelly, C. Serre, A. Vimont, M. Daturi, L. Hamon, G. De Weireld, J.-S. Chang, D.-Y. Hong and Y. Kyu, Hwang, High uptakes of CO2 and CH4 in mesoporous metal–organic frameworks mil-100 and mil-101, Langmuir, 2008, 24, 7245–7250 CrossRef CAS PubMed.
- G. Férey, C. Mellot-Draznieks, C. Serre and F. Millange, Crystallized frameworks with giant pores: are there limits to the possible?, Acc. Chem. Res., 2005, 38, 217–225 CrossRef PubMed.
- J. Ehrenmann, S. K. Henninger and C. Janiak, Water Adsorption Characteristics of MIL-101 for Heat-Transformation Applications of MOFs, Eur. J. Inorg. Chem., 2010, 471–474 Search PubMed.
- Y. Wang, N. Y. Huang, J. Q. Shen, P. Q. Liao, X. M. Chen and J. P. Zhang, Hydroxide Ligands Cooperate with Catalytic Centers in Metal–Organic Frameworks for Efficient Photocatalytic CO(2) Reduction, J. Am. Chem. Soc., 2018, 140, 38–41 CrossRef CAS PubMed.
- A. J. Rieth, K. M. Hunter, M. Dinca and F. Paesani, Hydrogen bonding structure of confined water templated by a metal–organic framework with open metal sites, Nat. Commun., 2019, 10, 4771 CrossRef PubMed.
- D. Damasceno Borges, G. Maurin and D. S. Galvão, Design of Porous Metal–Organic Frameworks for Adsorption Driven Thermal Batteries, MRS Adv., 2017, 2, 519–524 CrossRef CAS.
- A. Permyakova, O. Skrylnyk, E. Courbon, M. Affram, S. Wang, U. H. Lee, A. H. Valekar, F. Nouar, G. Mouchaham, T. Devic, G. De Weireld, J. S. Chang, N. Steunou, M. Frere and C. Serre, Synthesis Optimization, Shaping, and Heat Reallocation Evaluation of the Hydrophilic Metal–Organic Framework MIL-160(Al), ChemSusChem, 2017, 10, 1419–1426 CrossRef CAS PubMed.
- N. C. Burtch and K. S. Walton, Modulating adsorption and stability properties in pillared metal–organic frameworks: a model system for understanding ligand effects, Acc. Chem. Res., 2015, 48, 2850–2857 CrossRef CAS PubMed.
- Q. Dong, X. Zhang, S. Liu, R. B. Lin, Y. Guo, Y. Ma, A. Yonezu, R. Krishna, G. Liu, J. Duan, R. Matsuda, W. Jin and B. Chen, Tuning Gate-Opening of a Flexible Metal–Organic Framework for Ternary Gas Sieving Separation, Angew. Chem., Int. Ed., 2020, 59, 22756–22762 CrossRef CAS PubMed.
- Y. Huang, J. Wan, T. Pan, K. Ge, Y. Guo, J. Duan, J. Bai, W. Jin and S. Kitagawa, Delicate Softness in a Temperature-Responsive Porous Crystal for Accelerated Sieving of Propylene/Propane, J. Am. Chem. Soc., 2023, 145, 24425–24432 CrossRef CAS PubMed.
- J. Arinez-Soriano, J. Albalad, C. Vila-Parrondo, J. Perez-Carvajal, S. Rodriguez-Hermida, A. Cabeza, J. Juanhuix, I. Imaz and D. Maspoch, Single-crystal and humidity-controlled powder diffraction study of the breathing effect in a metal–organic framework upon water adsorption/desorption, Chem. Commun., 2016, 52, 7229–7232 RSC.
- S. Bourrelly, B. Moulin, A. Rivera, G. Maurin, S. Devautour-Vinot, C. Serre, T. Devic, P. Horcajada, A. Vimont and G. Clet, Explanation of the adsorption of polar vapors in the highly flexible metal organic framework MIL-53 (Cr), J. Am. Chem. Soc., 2010, 132, 9488–9498 CrossRef CAS PubMed.
- F.-X. Coudert, A. U. Ortiz, V. Haigis, D. Bousquet, A. H. Fuchs, A. Ballandras, G. Weber, I. Bezverkhyy, N. Geoffroy, J.-P. Bellat, G. Ortiz, G. Chaplais, J. Patarin and A. Boutin, Water Adsorption in Flexible Gallium-Based MIL-53 Metal–Organic Framework, J. Phys. Chem. C, 2014, 118, 5397–5405 CrossRef CAS.
- S. Devautour-Vinot, G. Maurin, F. Henn, C. Serre and G. Ferey, Water and ethanol desorption in the flexible metal organic frameworks, MIL-53 (Cr, Fe), investigated by complex impedance spectroscopy and density functional theory calculations, Phys. Chem. Chem. Phys., 2010, 12, 12478–12485 RSC.
- S. Biswas, T. Ahnfeldt and N. Stock, New functionalized flexible Al-MIL-53-X (X = –Cl, –Br, –CH3, –NO2, –(OH)2) solids: syntheses, characterization, sorption, and breathing behavior, Inorg. Chem., 2011, 50, 9518–9526 CrossRef CAS PubMed.
- M. Shivanna, A. A. Bezrukov, V. Gascón-Pérez, K.-I. Otake, S. Sanda, D. J. O’Hearn, Q.-Y. Yang, S. Kitagawa and M. Zaworotko, Flexible coordination network exhibiting water vapor-induced reversible switching between closed and open phases, ACS Appl. Mater. Interfaces, 2022, 14, 39560–39566 CrossRef CAS PubMed.
- A. Subanbekova, V. I. Nikolayenko, A. A. Bezrukov, D. Sensharma, N. Kumar, D. J. O'Hearn, V. Bon, S.-Q. Wang, K. Koupepidou, S. Darwish, S. Kaskel and M. J. Zaworotko, Water vapour and gas induced phase transformations in an 8-fold interpenetrated diamondoid metal–organic framework, J. Mater. Chem. A, 2023, 11, 9691–9699 RSC.
- M. M. Mekonnen and A. Y. Hoekstra, Four billion people facing severe water scarcity, Sci. Adv., 2016, 2, e1500323 CrossRef PubMed.
- J. Wang, L. Hua, C. Li and R. Wang, Atmospheric water harvesting: critical metrics and challenges, Energy Environ. Sci., 2022, 15, 4867–4871 RSC.
- E. Elsayed, R. Al-Dadah, S. Mahmoud, P. A. Anderson, A. Elsayed and P. G. Youssef, CPO-27(Ni), aluminium fumarate and MIL-101(Cr) MOF materials for adsorption water desalination, Desalination, 2017, 406, 25–36 CrossRef CAS.
- W. Xu and O. M. Yaghi, Metal–Organic Frameworks for Water Harvesting from Air, Anywhere, Anytime, ACS Cent. Sci., 2020, 6, 1348–1354 CrossRef CAS PubMed.
- N. Hanikel, M. S. Prevot and O. M. Yaghi, MOF water harvesters, Nat. Nanotechnol., 2020, 15, 348–355 CrossRef CAS PubMed.
- R. D. Stelmach and T. Clasen, Household water quantity and health: a systematic review, Int. J. Environ. Res. Public Health, 2015, 12, 5954–5974 CrossRef PubMed.
- N. Hanikel, M. S. Prevot, F. Fathieh, E. A. Kapustin, H. Lyu, H. Wang, N. J. Diercks, T. G. Glover and O. M. Yaghi, Rapid Cycling and Exceptional Yield in a Metal–Organic Framework Water Harvester, ACS Cent. Sci., 2019, 5, 1699–1706 CrossRef CAS PubMed.
- H. A. Almassad, R. I. Abaza, L. Siwwan, B. Al-Maythalony and K. E. Cordova, Environmentally adaptive MOF-based device enables continuous self-optimizing atmospheric water harvesting, Nat. Commun., 2022, 13, 4873 CrossRef CAS PubMed.
- Z. Shao, Y.-C. Tang, H. Lv, Z.-S. Wang, P. Poredoš, Y. Feng, R. Sun, X. Feng, Z. Chen, Z. Gao, D.-D. Zhou, J.-P. Zhang and R. Wang, High-performance solar-driven MOF AWH device with ultra-dense integrated modular design and reflux synthesis of Ni2Cl2 (BTDD), Device, 2023, 1, 100058 CrossRef.
- Y. Feng, T. Ge, B. Chen, G. Zhan and R. Wang, A regulation strategy of sorbent stepwise position for boosting atmospheric water harvesting in arid area, Cell Rep. Phys. Sci., 2021, 2, 100561 CrossRef CAS.
- J. Wu, Z. Sui, X. Du, Y. Zhang and T. Ma, A Study on the Improvement of the Photothermal Characteristics of the Adsorbent for Sorption-Based Atmospheric Water Harvesting Driven by Solar, Coatings, 2023, 13, 154 CrossRef CAS.
- G. Yilmaz, F. Meng, W. Lu, J. Abed, C. Peh, M. Gao, E. Sargent and G. Ho, Autonomous atmospheric water seeping MOF matrix, Sci. Adv., 2020, 6, eabc8605 CrossRef CAS PubMed.
- A. Persily, Challenges in developing ventilation and indoor air quality standards: The story of ASHRAE Standard 62, Build. Environ., 2015, 91, 61–69 CrossRef PubMed.
- A. V. Arundel, E. M. Sterling, J. H. Biggin and T. D. Sterling, Indirect health effects of relative humidity in indoor environments, Environ. Health Perspect., 1986, 65, 351–361 CAS.
- M. Isaac and D. P. Van Vuuren, Modeling global residential sector energy demand for heating and air conditioning in the context of climate change, Energy Policy, 2009, 37, 507–521 CrossRef.
- J. B. DeCoste, G. W. Peterson, B. J. Schindler, K. L. Killops, M. A. Browe and J. J. Mahle, The effect of water adsorption on the structure of the carboxylate containing metal–organic frameworks Cu-BTC, Mg-MOF-74, and UiO-66, J. Mater. Chem. A, 2013, 1, 11922 RSC.
- S. B. Riffat and X. Ma, Improving the coefficient of performance of thermoelectric cooling systems: a review, Int. J. Energy Res., 2004, 28, 753–768 CrossRef.
- F. Jeremias, D. Fröhlich, C. Janiak and S. K. Henninger, Water and methanol adsorption on MOFs for cycling heat transformation processes, New J. Chem., 2014, 38, 1846–1852 RSC.
- S. K. Henninger, H. A. Habib and C. Janiak, MOFs as adsorbents for low temperature heating and cooling applications, J. Am. Chem. Soc., 2009, 131, 2776–2777 CrossRef CAS PubMed.
- F. Xu, Z. F. Bian, T. S. Ge, Y. J. Dai, C. H. Wang and S. Kawi, Analysis on solar energy powered cooling system based on desiccant coated heat exchanger using metal–organic framework, Energy, 2019, 177, 211–221 CrossRef CAS.
- H. W. B. Teo, A. Chakraborty, Y. Kitagawa and S. Kayal, Experimental study of isotherms and kinetics for adsorption of water on Aluminium Fumarate, Int. J. Heat Mass Transfer, 2017, 114, 621–627 CrossRef CAS.
- H. Kummer, F. Jeremias, A. Warlo, G. Füldner, D. Fröhlich, C. Janiak, R. Gläser and S. K. Henninger, A Functional Full-Scale Heat Exchanger Coated with Aluminum Fumarate Metal–Organic Framework for Adsorption Heat Transformation, Ind. Eng. Chem. Res., 2017, 56, 8393–8398 CrossRef CAS.
- M. F. de Lange, K. J. Verouden, T. J. Vlugt, J. Gascon and F. Kapteijn, Adsorption-Driven Heat Pumps: The Potential of Metal–Organic Frameworks, Chem. Rev., 2015, 115, 12205–12250 CrossRef CAS PubMed.
- B. N. Truong, D. D. Borges, J. Park, J. S. Lee, D. Jo, J. S. Chang, S. J. Cho, G. Maurin, K. H. Cho and U. H. Lee, Tuning Hydrophilicity of Aluminum MOFs by a Mixed-Linker Strategy for Enhanced Performance in Water Adsorption-Driven Heat Allocation Application, Adv. Sci., 2023, 10, 2301311 CrossRef CAS PubMed.
- H. Liu, Y. He, J. Jiao, D. Bai, D. L. Chen, R. Krishna and B. Chen, A Porous Zirconium-Based Metal–Organic Framework with the Potential for the Separation of Butene Isomers, Chem. – Eur. J., 2016, 22, 14988–14997 CrossRef CAS PubMed.
- M. Netusil and P. Ditl, Comparison of three methods for natural gas dehydration, J. Nat. Gas Chem., 2011, 20, 471–476 CrossRef CAS.
- P. Gandhidasan, A. A. Al-Farayedhi and A. A. Al-Mubarak, Dehydration of natural gas using solid desiccants, Energy, 2001, 26, 855–868 CrossRef CAS.
- R. Mesgarian, A. Heidarinasab, A. Rashidi and Y. Zamani, High Performance MIL-101 Nanoadsorbent for the Natural Gas Dehydration, J. Nanostruct., 2022, 12, 983–998 CAS.
- Y. C. Tang, Z. S. Wang, H. Yi, M. Y. Zhou, D. D. Zhou, J. P. Zhang and X. M. Chen, Water-Stable Metal Azolate Frameworks Showing Interesting Flexibilities for Highly Effective Bioethanol Dehydration, Angew. Chem., Int. Ed., 2023, 62, e202303374 CrossRef CAS PubMed.
- Y.-D. Kim, K. Thu, M. E. Masry and K. C. Ng, Water quality assessment of solar-assisted adsorption desalination cycle, Desalination, 2014, 344, 144–151 CrossRef CAS.
- E. Elsayed, R. Al-Dadah, S. Mahmoud, P. Anderson and A. Elsayed, Experimental testing of aluminium fumarate MOF for adsorption desalination, Desalination, 2020, 475, 114170 CrossRef CAS.
- S. Jian, Y. Cheng, X. Ma, H. Guo, J. Hu, K. Zhang, S. Jiang, W. Yang and G. Duan, Excellent fluoride removal performance by electrospun La–Mn bimetal oxide nanofibers, New J. Chem., 2022, 46, 490–497 RSC.
- S. Jian, Y. Chen, F. Shi, Y. Liu, W. Jiang, J. Hu, X. Han, S. Jiang and W. Yang, Template-Free Synthesis of Magnetic La-Mn-Fe Tri-Metal Oxide Nanofibers for Efficient Fluoride Remediation: Kinetics, Isotherms, Thermodynamics and Reusability, Polymers, 2022, 14, 5417 CrossRef CAS PubMed.
- W. Yang, Y. Wang, Q. Wang, J. Wu, G. Duan, W. Xu and S. Jian, Magnetically separable and recyclable Fe3O4@PDA covalent grafted by l-cysteine core-shell nanoparticles toward efficient removal of Pb2+, Vacuum, 2021, 189, 110229 CrossRef CAS.
- S. Jian, Z. Tian, K. Zhang, G. Duan, W. Yang and S. Jiang, Hydrothermal Synthesis of Ce-doped ZnO Heterojunction Supported on Carbon Nanofibers with High Visible Light Photocatalytic Activity, Chem. Res. Chin. Univ., 2021, 37, 565–570 CrossRef CAS.
- J. Wang, Y. Sun, X. Zhao, L. Chen, S. Peng, C. Ma, G. Duan, Z. Liu, H. Wang, Y. Yuan and N. Wang, A poly(amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater, e-Polym., 2022, 22, 399–410 CrossRef CAS.
- X. Ma, S. Zhao, Z. Tian, G. Duan, H. Pan, Y. Yue, S. Li, S. Jian, W. Yang, K. Liu, S. He and S. Jiang, MOFs meet wood: Reusable magnetic hydrophilic composites toward efficient water treatment with super-high dye adsorption capacity at high dye concentration, Chem. Eng. J., 2022, 446, 136851 CrossRef CAS.
- J. Song, W. Yang, X. Han, S. Jiang, C. Zhang, W. Pan, S. Jian and J. Hu, Performance of Rod-Shaped Ce Metal–Organic Frameworks for Defluoridation, Molecules, 2023, 28, 3492 CrossRef CAS PubMed.
- J. Hu, J. Song, X. Han, Q. Wen, W. Yang, W. Pan, S. Jian and S. Jiang, Fabrication of Ce-La-MOFs for defluoridation in aquatic systems: A kinetics, thermodynamics and mechanisms study, Sep. Purif. Technol., 2023, 314, 123562 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2024 |