Recent progress in metal–organic frameworks for the separation of gaseous hydrocarbons
Jing-Hong
Li
,
Jun-Xian
Chen
 ,
Rui-Biao
Lin
,
Rui-Biao
Lin
 * and
Xiao-Ming
Chen
* and
Xiao-Ming
Chen
 *
*
Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, School of Chemistry, IGCME, Sun Yat-Sen University, Guangzhou 510275, China. E-mail: linruibiao@mail.sysu.edu.cn; cxm@mail.sysu.edu.cn
First published on 25th August 2023
Abstract
The separation of gaseous hydrocarbons is involved in many important industrial processes for manufacturing chemicals, polymers, plastics, and fuels, and is performed through cryogenic distillation, which is heavily energy-intensive. Adsorption-based gas separation technology by using adsorbent materials can potentially fulfill a much energy-efficient gas separation. As a new generation of adsorbent materials, metal–organic frameworks (MOFs) have been demonstrated to have great potential in addressing important gas separations of hydrocarbons. In this review, we outline the uniqueness of MOF adsorbents for their separation application for gaseous hydrocarbons. A variety of microporous MOFs have been developed for separating gaseous hydrocarbons, which have been achieved by fine-tuning their pore sizes for high molecular sieving effects and/or immobilizing binding sites on their pore surfaces for their specific recognition of small molecules. Herein, we highlight recent important progress in this very important topic, focusing on the purification of ethylene, propylene, and butadiene.
1. Introduction
Gaseous hydrocarbons involve not only essential fuels for modern society but also are applied as important feedstocks in the chemical industry. Hydrocarbons usually are volatile, invisible, and flammable, and tend to be highly dispersed, easily contain mixtures of low concentration, and thus are difficult to handle. The separation and purification of hydrocarbons are critical industrial chemical processes to produce bulk commodities for fuels, polymers, and plastics. Some of these separation processes are carried out on a very large industrial scale. For example, olefin/paraffin separation produces light olefins of over 200 million tonnes.1 The huge markets of hydrocarbon products make a large contribution (thousands of billions of US dollars) to the annual revenues of the oil and gas industry. Most of the industrial separations of hydrocarbons traditionally rely on thermal-driven technologies, mainly fractional distillation involving numerous evaporation–condensation cycles, which are a high energy-intensity process. In particular, to separate mixed hydrocarbons of similar volatilities for high purity, relevant industrial processes are operated in complicated facilities and under harsh conditions. For the separation and purification of industrial commodities, it is believed that energy-intensive processes, such as distillation consume 10–15% of the world's energy production. Adsorptive separation based on porous adsorbents is a promising technology to lower relevant separation energy use. For example, alternative technologies, such as adsorptive separation and/or membrane-based technologies, can be up to 10 times more energy-efficient than the traditional distillation methods, which have not been fully realized.1 These nonthermal technologies can separate hydrocarbon molecules according to their chemical affinities or sizes rather than differences in boiling points, and thus can be carried out under mild conditions, hence greatly reducing the energy inputs.2 The core of these candidate technologies is the separation performance of solid porous media or permeable membranes, which are often made of porous materials with nanosized voids and desired internal pore features.Metal–organic frameworks (MOFs, also known as porous coordination polymers) are an emerging type of crystalline porous solids, which are assembled by metal-containing nodes and organic linkers or struts through coordination bonds to have pore space. MOFs are unique owing to their exceptional porosity, diverse structures, tunable pore size, and ease of functionality.3–6 After two decades of intensive practices, MOF materials are well demonstrated for their exceptional capability for pore adjustment and interior modification. Today, about 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 of MOFs have been synthesized. MOFs can show ultrahigh porosity with the largest pore surface area of over 7000 m2 g−1, or with pore sizes ranging from 3 to 100 Å, the highest thermal stability of over 600 °C, or exceptional chemical stability in concentrated acidic/basic solutions.3,7,8 The high modularity of MOFs enables us to construct their isostructural analogues of different pore sizes/shapes and surfaces (such as functional organic groups, metal open sites, etc.) with the same coordination networks. The key design element for MOFs is thus to functionalize their pore surface with binding sites and/or adjust their pore size/shape for molecular separation. In this context, MOFs have shown great promise that is superior to conventional porous materials (activated carbon and zeolites) in addressing important separations of gaseous hydrocarbons.9–19
000 of MOFs have been synthesized. MOFs can show ultrahigh porosity with the largest pore surface area of over 7000 m2 g−1, or with pore sizes ranging from 3 to 100 Å, the highest thermal stability of over 600 °C, or exceptional chemical stability in concentrated acidic/basic solutions.3,7,8 The high modularity of MOFs enables us to construct their isostructural analogues of different pore sizes/shapes and surfaces (such as functional organic groups, metal open sites, etc.) with the same coordination networks. The key design element for MOFs is thus to functionalize their pore surface with binding sites and/or adjust their pore size/shape for molecular separation. In this context, MOFs have shown great promise that is superior to conventional porous materials (activated carbon and zeolites) in addressing important separations of gaseous hydrocarbons.9–19
For separation processes in physical adsorbents, their separation mechanism can be roughly categorized into either equilibrium or non-equilibrium adsorption. Equilibrium adsorption processes are thermodynamic and are mainly driven by binding affinity differences dominated by those functional sites (i.e., binding sites) through supramolecular interactions. Pore functionalization with strong binding sites can significantly boost such type of separation performance. For non-equilibrium adsorption processes,20 including kinetic separation and molecular sieving, the separation is achieved by differing the diffusivity, which is highly affected by the pore sizes and flexibility of adsorbents. The kinetic separation can be amplified after narrowing the pores of adsorbents. There are also cases that the separation is achieved by a combination of both thermodynamic and kinetic processes. In addition, there are gas separation processes that are usually performed with the collaboration of framework flexibility and structural transformation, namely, gate-opening or pore opening. Considering all aspects, it is very important to systematically control the pore size, pore surface and even the flexibility of adsorbents.
On the other hand, hydrocarbons are composed of the same elements with different carbon bonds and carbon/hydrogen ratios. Their physical properties and even chemical properties can be very similar (Table 1). The adsorptive separation by differing their molecular shapes or adsorption affinities in physical adsorbents is thus required to perform with high accuracy, which can be achieved by MOFs.13 For example, compared with the mixture counterpart propane, propylene, as one major petrochemical product, differs by just two hydrogen atoms, and there is around a 5 K difference in their boiling points. It has been demonstrated that MOFs with embedded binding sites (ranging from open metal sites to polar functional groups, such as anionic fluoride, hydroxyl and amino) can effectively separate olefins/paraffins owing to the difference in thermodynamic affinity. Another applicable approach is to adjust the pore sizes of MOFs for size-matching of gas molecules, which is expected to enhance kinetic separation or sieving effect and finally achieve high selectivity. If the pore aperture size of adsorbents is right between the molecular sizes of different gas components, an adsorption cut-off happens along with the inaccessibility of internal pore space to certain gas components, thus leading to molecular sieving. For some MOFs, the adjustment of pore size in MOFs can be done up to a precision level of 0.1 Å.13 Given that most hydrocarbon gases have their molecular sizes or kinetic diameters between 3 and 5 Å, microporous MOFs with comparable pore sizes have been intensively investigated for separating hydrocarbon mixtures. Usually, MOFs with large pores can show high sorption capacity but low separation selectivity for gas, whereas the ultramicroporous ones can exhibit high selectivity but low sorption capacity (termed sorption capacity versus selectivity trade off). By simultaneously tuning the pore size and incorporating binding sites, the internal pore space of MOFs can integrate molecular shape matching and preferential binding toward the targeted gas molecules, which is expected to address the trade-off for exceptional separation performance. The complicated effects of MOF flexibility for separation of hydrocarbons have also been noted.21
| Adsorbate | Normal boiling point/K | Density/g L−1 (101.325 kPa, 273.15 K) | Kinetic diameter/Å | Polarizability × 1025/cm3 | Dipole moment × 1018/esu cm | Quadruple moment × 1026/esu cm2 |
|---|---|---|---|---|---|---|
| a Density at 298.15 K. | ||||||
| CH4 | 111.66 | 0.717 | 3.758 | 25.93 | 0 | 0 |
| C2H4 | 169.42 | 1.261 | 4.163 | 42.52 | 0 | 1.50 |
| C2H6 | 184.55 | 1.355 | 4.443 | 44.3–44.7 | 0 | 0.65 |
| C2H2 | 188.40 | — | 3.3 | 33.3–39.3 | 0 | — |
| C3H6 | 225.46 | 1.914 | 4.678 | 62.6 | 0.366 | — |
| C3H8 | 231.02 | 2.011 | 4.3–5.118 | 62.9–63.7 | 0.084 | — |
| c-C3H6 | 240.34 | 1.920 | 4.23–4.807 | 56.6 | 0 | — |
| i-C4H10 | 261.34 | 2.689 | 5.278 | 81.4–82.9 | 0.132 | — |
| i-Butene | 266.25 | — | 4.84 | 8.14–8.29 | — | — |
| 1-Butene | 266.92 | — | 4.5 | 79.7–85.2 | 0.359–0.438 | — |
| 1,3-Butadiene | 268.62 | — | 5.2 | 86.4 | 0 | — |
| n-C4H10 | 272.66 | 2.704 | 4.687 | 82.0 | 0.05 | — |
| trans-2-Butene | 274.03 | — | — | 84.9 | 0 | — |
| cis-2-Butene | 276.87 | — | 4.23 | — | 0.253 | — |
| neo-C5H12 (2,2-dimethylpropane) | 282.65 | 2.334 (70.922 kPa) | 6.2–6.464 | 102.0 | 0 | — |
| i-C5H12 | 300.99 | 1.120 (34.543 kPa) | 5.0 | — | 0.13 | — |
| n-C5H12 | 309.22 | 0.789 (24.455 kPa) | 4.5 | 99.9 | 0 | — |
| neo-C6H14 (2,2-dimethylbutane) | 322.87 | — | 6.2 | — | — | — |
| i-C6H14 (2-methylpentane) | 333.40 | 0.343 (8.960 kPa) | 5.5 | — | 0.1 | — |
| 3-Methylpentane | 336.40 | — | 5.5 | — | — | — |
| n-C6H14 | 341.88 | 0.231 (6.041 kPa) | 4.3 | 119 | 0 | — |
| C6H6 | 353.24 | 0.403a (12.695 kPa) | 5.349–5.85 | 100–107.4 | 0 | — |
| c-C6H12 (cyclohexane) | 353.93 | 0.446a (13.019 kPa) | 6.0–6.182 | 108.7–110 | 0 | — |
| n-C7H16 | 371.57 | 0.067 (15.250 kPa) | 4.3 | 136.1 | 0 | — |
| i-C8H18 (2,2,4-trimethylpentane) | 372.39 | — | 6.2 | 154.4 | 0 | — |
| Toluene | 383.79 | 0.037 (0.906 kPa) | 5.25 | 118–123 | 0.375 | — |
| Ethylbenzene | 409.36 | — | 5.8 | 142 | 0.59 | — |
| p-Xylene | 411.53 | — | 5.8 | 137–149 | 0.1 | — |
| m-Xylene | 412.34 | — | 6.8 | 142 | 0.37 | — |
| o-Xylene | 417.59 | — | 6.8 | 141–149 | 0.640 | — |
By virtue of pore structural and chemical control of MOF materials, namely, tuning pore size, incorporating functional sites, or their combination, significant progress has been made for gaseous hydrocarbon separation. This review focuses on the research progress of MOF materials for separation of gaseous hydrocarbons in about recent five years. In particular, we summarize representative MOF materials with unique pores and functional sites for C2H2 and CO2 separation, C2H2/C2H4 separation, C2H4 and C2H6 separation, C3H4/C3H6 separation, C3H6/C3H8 separation, and purification of butadiene, xylenes and other volatile gaseous hydrocarbons.
2. Pore structure control of MOFs for separating gaseous hydrocarbons
The separation performance of adsorbents towards target molecules can be affected by various factors, mainly dominated by the adsorbent–adsorbate and adsorbate–adsorbate interactions in the pore space. The adsorbent–adsorbate interactions for specific gases can be adjusted by controlling the pore chemistry and pore size of porous materials for optimal binding affinity, whereas adsorbate–adsorbate interactions are dependent on the physical properties of gas molecules including molecular polarity or polarizability, sizes and shapes. Therefore, rationally tuning the pore sizes of MOFs and/or functionalizing their pore surface have been demonstrated as efficient approaches to boost the separation performance for gases. Both adsorbent–adsorbate and adsorbate–adsorbate intermolecular interactions for larger or heavier molecules with high polarizability are usually superior to those for small, light and non-polar ones, due to more supramolecular interactions (ranging from hydrogen bonding to electrostatic interactions and van der Waals forces). In this section, representative examples of MOFs for hydrocarbon separation were presented according to the sequence of the polarizability and boiling points of various gases (Table 2).| Materials | Gas separation | Strategies | Uptakea (mmol g−1) | Selectivityb | Ref. |
|---|---|---|---|---|---|
| a Uptake amount at 1 bar and room temperature. b Calculated by IAST at ambient temperature and 1 bar. c Kinetic selectivity at ambient temperature. d Equilibrium-kinetic combined selectivity at ambient temperature. e Uptake amount at 1.05 bar and 333 K. f Uptake amount at 1.05 bar and 393 K. g Uptake amount at 7.1 mbar and 333 K. | |||||
| ATC-Cu | CH4/N2 | Incorporating adjacent open copper sites | 2.9/0.75 | 9.7 | 23 |
| Ni(ina)2 | Optimizing pore sizes (5.0 × 4.8 Å2) | 1.82/0.53 | 15.8 | 24 | |
| DMOF-A2 | Optimizing pore sizes (5.3 Å) and incorporating parallel aromatic rings | 1.65/0.39 | 7.2 | 25 | |
| MAF-92 | CO2/CH4 | Optimizing pore sizes (5.0 × 4.8 Å2) | 2.06/0.06 | 1.3 × 107 | 27 |
| CuI@UiO-66-(COOH)2 | C2H4/C2H6 | Optimizing pore sizes (4.1 Å) and incorporating copper(I) ions onto the pore surfaces | 1.9/0.9 | 80.8 | 41 |
| UTSA-280 | Optimizing pore rigidity and pore size (3.8 Å) | 2.5/0.098 | >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
35 | |
| Co-gallate | Optimizing pore size (5.2 Å) | 3.37/0.31 | 52 | 36 | |
| ZnAtzPO4 | Optimizing pore structure (3.8 Å) and incorporating electronegative functional groups | 1.92/1.04 | 36.6c/12.4d | 39 | |
| ZU-901 | Optimizing pore size (3.4 × 4.2 Å2) | 1.55/0.26 | — | 40 | |
| MAF-49 | C2H6/C2H4 | Incorporating multiple hydrogen-bonding acceptors and dipole repulsion groups | 1.7/1.69 | 2.7 | 59 |
| Cu(Qc)2 | Optimizing pore size (3.3 Å) | 1.85/0.78 | 3.4 | 60 | |
| [Fe2(O2)(dobdc)] | Incorporating Fe-peroxo sites | 3.29/2.6 | 4.4 | 61 | |
| SIFSIX-2-Cu-i | C2H2/C2H4 | Optimizing pore size (4.4 Å) and incorporating SiF62− sites | 4.02/2.19 | 44.8 | 70 |
| UTSA-200a | Optimizing pore size (3.4 Å) and incorporating SiF62− sites | 3.65/0.63 | 6320 | 71 | |
| UTSA-300a | Optimizing pore size (1.3 × 2.8 Å2) and incorporating SiF62− sites | 3.08/0.04 | 27 | 72 | |
| NCU-100a | Optimizing pore size (1.4 × 3.0 Å2) and incorporating SiF62− sites | 4.57/0.32 | 7291 | 76 | |
| UTSA-74 | C2H2/CO2 | Incorporating open Zn sites | 4.82/3.03 | 9 | 101 |
| CuI@UiO-66-(COOH)2 | Incorporating copper(I) ions onto the pore surfaces | 2.44/0.85 | 185 | 107 | |
| JNU-4 | Incorporating open Cu sites | 9.82/7.1 | 8.2 | 108 | |
| CPL-1-NH2 | Incorporating amine sites | 1.84/0.21 | 119 | 114 | |
| CAU-10-H | Suitable pore sizes (4.7 Å) | 4.01/2.68 | 4 | 115 | |
| CAU-10-NH2 | Suitable pore sizes (3.8 Å) and incorporating amine sites | 3.57/2.08 | 10.8 | 116 | |
| FJU-90 | Pore space partition | 8.04/4.6 | 4.3 | 122 | |
| UTSA-300a | Optimizing pore size (1.3 × 2.8 Å2) and incorporating SiF62− sites | 3.08/0.18 | 860 | 72 | |
| NCU-100a | Optimizing pore size (1.4 × 3.0 Å2) and incorporating SiF62− sites | 4.57/0.49 | 1787 | 125 | |
| FJI-H36 | Suitable pore sizes (12.9 × 12.9 Å2 and 8.4 × 10.2 Å2), flexible pore structure, incorporating Ni and free N atoms sites | 6.46/4.20 | 3.5 | 127 | |
| SOFOUR-TEPE-Zn | Incorporating electronegative pore surfaces | 3.98/0.63 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 833 833 |
97 | |
| ZUL-330 | Optimizing pore size (3.3 × 3.7 Å2) and incorporating SiF62− sites | 7.33/1.1 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 086 086 |
98 | |
| Cu-F-pymo | CO2/C2H2 | Incorporating residual guest molecules blocking the priority site for C2H2 | 2.09/0.1 | >105 | 143 |
| Cd-NP | Suitable pore space (6.1 × 4.5 × 4.5 Å3), window size (3.2 Å) and incorporating complementary electrostatic potentials on the pore surface | 2.59/0.43 | 85 | 144 | |
| Y-bptc | Incorporating large pore cage space (pore diameter: 9.46 Å), suitable pore window (4.2 Å) and μ3-OH− anions sites | 2.48/1.17 | 4.1/114c | 136 | |
| MUF-4 | Suitable pore window size (2.2 Å) | 3.17/— | 3363c | 139 | |
| ALF | Incorporating electropositive surface and the hydrogen-confined pore cavities with appropriate dimensional size (4.1 × 5.3 Å2) | 3.85/0.15 | 6.5 × 105 | 148 | |
| MAF-23-O | C3H6/C3H8 | Incorporating carbonyl oxygen atoms sites | 1.34/1 | 8.8/71c | 149 |
| UTSA-400 | Optimizing pore sizes (3.0 Å) and incorporating WO2F42− sites | 1.84/0.05 | >107 | 151 | |
| Y-abtc | Optimizing pore sizes (4.7 Å) | 2/0.07 | — | 152 | |
| HIAM-301 | Optimizing pore sizes (4.6 Å) | 3.16/0.27 | 150 | 153 | |
| Co-gallate | Optimizing pore sizes (4.2 × 5.1 Å2) | 1.79/0.14 | 333 | 154 | |
| JNU-3a | Incorporating orthogonally arrayed dynamic apertures | 2.62/2.14 | 513 | 155 | |
| NTU-85 | Optimizing pore sizes (4.5 Å) | 0.45/0.003 | 1570 | 156 | |
| ZJU-75a | Suitable pore sizes (4.1 × 4.4 Å2) and incorporating high-density open Ni sites | 3.31/2.33 | 54.2 | 157 | |
| PCP-IPA | C3H8/C3H6 | Suitable pore sizes (4.7 × 5.6 Å2) and incorporating parallel-aligned aromatic-based units | 2.23/2.25 | 2.48 | 169 |
| FDMOF-2 | Optimizing pore sizes (3.0 Å) and incorporating –CF3 groups | 5.04/4.15 | 2.18 | 170 | |
| ELM-12 | C3H4/C3H6 | Suitable pore cavities (6.1 × 4.3 × 4.3 Å3 and 6.8 × 4.0 × 4.2 Å3) and incorporating dynamic dangling OTf− groups | 2.79/1.45 | 83 | 181 |
| UTSA-200 | Optimizing pore size (3.4 Å) and incorporating SiF62− sites | 3.58/1.20 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
182 | |
| sql-NbOFFIVE-bpe-Cu-AB | Optimizing pore size (3.96 × 5.56 Å2) and incorporating NbOF52− sites | 3.04/2.10 | 220 | 189 | |
| ZU-52 | C4H6/n-C4H8/i-C4H8 | Optimizing pore size (4.31 Å) and incorporating fluoroniobate sites | 2.64/2.26/0.48 | — | 192 |
| ZU-33 | Optimizing pore size (4.20 Å) and incorporating GeF62−sites | 2.67/0.57/0.42 | — | ||
| MAF-23 | i-C4H8/n-C4H8/C4H10/C4H6 | Incorporating flexible quasi-discrete pores | 2/2/2/2 | — | 193 |
| Zn-bzc-2CH3 | n-C4H10/i-C4H10 | Optimizing pore size (4.13 Å) | 2.42/0.03 | — | 195 |
| Zr-abtc | nHEX/3MP/23DMB | Suitable pore window size (4.5 Å) | 1.28/1.02/0.58 | — | 197 |
| HIAM-203 | nHEX/3MP/22DMB | Suitable pore window size (4.8 Å) | 1.7/1.43/0.07 | — | 198 |
| CopzNi | nHEX/2MP/22DMB | Incorporating open Ni sites | 2.17/1.46/0.08 | — | 199 |
| Mn-dhbq | pX/mX/oX | Incorporating multiple open Mn sites, rich π-electrons, and structural flexibility | 1.74/1.48/0.22e | — | 206 |
| 1.33/0.16/0.21f | |||||
| ZU-61 | Incorporating adaptable pore structure and NbOF52− sites | 3.44/3.37/3.2g | — | 207 | |
| ZUL-C3 | pX/mX/oX/EB | Incorporating nonaromatic low-polar pore environment | 3.25/3.35/3.41/3.27 | — | 208 |
| MAF-41 | EB/ST/Tol/Bz | Incorporating structural flexibility | 2.31/0.3 | — | 209 |
2.1. Methane purification
Natural gas is a cleanest fossil fuel and has been widely used due to its natural abundance and high energy intensity, and is usually contaminated with gas impurities such carbon dioxide (CO2) and nitrogen (N2). The upgrading of natural gas involves the purification of methane (CH4). Coal-bed methane (CBM) is an excellent complement to conventional natural gas; the recovery of CH4 from it can also reduce the emission of the greenhouse gas CH4 from CBM as well as enhance the safety of coal mineral.22Ma et al. used an alkyl MOF, Cu2(ATC) (ATC-Cu, H2ATC = 1,3,5,7-adamantane tetracarboxylic acid), as a methane nano-trap that features oppositely adjacent open metal sites (OMSs) and dense alkyl groups for capturing methane molecules.23 At 1 bar and 298 K, this MOF exhibits the highest methane uptake capacity (2.90 mmol g−1) among reported MOFs, showing a high CH4/N2 selectivity of 9.7 for an equimolar mixture under ambient conditions. ATC-Cu thus shows promising potential for capturing methane from CBM with low content of methane (<30%). Structural and computational modelling indicated that pairs of open Cu sites with a Cu⋯Cu distance of 4.43 Å between two neighboring Cu paddlewheels afford considerable dual Coulombic interactions and act as strong binding sites for methane molecules. Also, there are cavities with an aliphatic surface that can serve as the second binding sites for CH4, with an average interaction distance of ca. 3.5 Å.
Yang et al. studied four nickel-based coordination networks with functional sites (–NH2) or varied pore sizes for the separation of CH4/N2, which supports that the pore size and chemical environment of adsorbents play an important role during methane purification.24 Among those four MOFs, Ni(ina)2 showed the highest CH4/N2 selectivity (15.8) with an adsorption capacity of 1.82 mmol g−1 under ambient conditions. Ni(ina)2 shows good thermal and moisture stability as well, which can be easily scaled up at a low cost.
The influence of humidity on the separation performances of MOFs for CH4 separation has been noticed. Li et al. investigated a series of isostructural MOFs, [Zn2(1,4-NDC)2(DABCO)], [Zn2(ADC)2(DABCO)], [Ni2(ADC)2(DABCO)], and [Cu2(ADC)2(DABCO)] (namely, DMOF-N, DMOF-A1, DMOF-A2, DMOF-A3, respectively, H21,4-NDC = 1,4-naphthalenedicarboxylic acid, H2ADC = 9,10-anthracene dicarboxylic acid, and DABCO = 1,4-diazabicyclo[2.2.2]octane), for CH4 purification.25 Altering their aromatic moieties and/or metal centers results in varied pore sizes, hydrophobicity and stability. The hydrophobic pore space suppressed the water sorption and enhanced the moisture resistance of those MOFs, which show high CH4 uptakes and CH4/N2 selectivity. Breakthrough experiments for the CH4/N2 mixture under humid conditions indicated that their CH4/N2 separation performance can be retained even under high humidity (40% RH).26
Zhang et al. reported two isostructural flexible metal-azolate frameworks (abbreviated as MAFs, a subclass of MOFs with azolate ligands19) [Zn3(OH)2(pzdc)(tz)]·DMA and [Zn3(OH)2(pzdc)(atz)]·DMA (MAF-91·DMA and MAF-92·DMA, respectively, H3pzdc = 3,5-pyrazoledicarboxylic acid, Htz = 1,2,4-triazole, and Hatz = 3-amino-1,2,4-triazole), which possess quasi-discrete pores with analogous sizes (MAF-91·DMA: 3.6 × 2.4 and 4.0 × 2.1 Å2, and MAF-92·DMA: 3.7 × 1.3 and 4.0 × 2.1 Å2).27 Due to the stronger intra-framework hydrogen-bonding interaction in MAF-92, there is a higher gating energy than MAF-91. Thus, MAF-92 showed tremendous shrinkage of apertures (1.0 × 0.8 and 2.5 × 1.0 Å2) after guest removal, while those in MAF-91 (3.5 × 2.2 and 4.4 × 2.0 Å2) showed negligible changes (Fig. 1). Single-component gas adsorption revealed that MAF-92 can adsorb a large amount of CO2 (2.1 mmol g−1 at 273 K and 1 bar), but completely excludes N2 and CH4. The molecular sieving performance was further confirmed by breakthrough experiments for CO2/N2 and CO2/CH4, giving selectivities of >1500. This work revealed that the controlled gated barrier can be expected to achieve definite and ideal molecular sieve effect. In contrast, the very commonly encountered framework flexibility of MOFs could be an important drawback to size-dependent molecular sieving for separation of similar molecules.
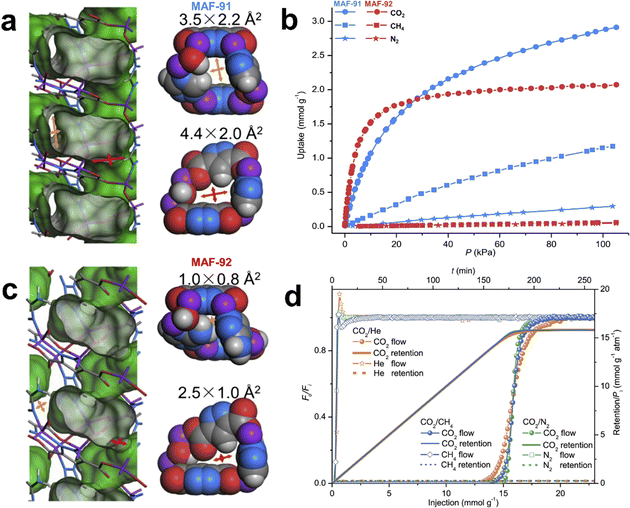 | ||
Fig. 1 (a) and (c) Crystal structures of MAF-91 and MAF-92. (b) CO2, CH4, and N2 adsorption isotherms of MAF-91 and MAF-92 at 298 K. (d) Column breakthrough behaviors of MAF-92 for 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 CO2/N2, 10 90 CO2/N2, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 CO2/CH4 and 10 90 CO2/CH4 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 CO2/He mixtures. Reprinted with permission from ref. 27. Copyright 2021 Cell Press. 90 CO2/He mixtures. Reprinted with permission from ref. 27. Copyright 2021 Cell Press. | ||
2.2. C2H4 and C2H6 separation
Ethylene (C2H4) is one of the most important chemical raw materials, which is widely used in the production of plastics, rubber, coatings, and other chemical products. It is mainly produced by cracking ethane (C2H6) or naphtha; thus, C2H6 is a major impurity in the raw mixture. However, the separation of C2H4 from the C2H4/C2H6 mixture is highly challenging due to their small molecular size difference (<0.5 Å) and boiling point difference (ca. 15 K). MOFs have been reported to exhibit significant progress in the separation of C2H4/C2H6.28–41Usually, the introduction of OMSs on the pore surface of MOFs enables preferential adsorption of C2H4 over C2H6.28–33,38,41–44 For example, HKUST-128 and M2(dobdc)29–31 (M-MOF-74; M = Mg, Mn, Fe, Co, Ni, and Zn) exhibit stronger binding enthalpies for olefins than alkanes due to the metal–π interactions. Altering the charge density of the metal centers can improve the olefin adsorption and selectivity as revealed by M2(dobdc) isomers.33 The appropriate aperture combined with OMSs of high density can also significantly improve the bonding affinity for olefins. In 2020, Qian et al. reported two microporous MOFs functionalized with different amounts of carboxylate groups, in which the carboxylate groups not only can adjust the aperture size, but also chelate with copper(I) ions via post-synthetic modification.41 Therefore, CuI@UiO-66-(COOH)2 exhibits optimal apertures and exposed Cu(I) centers to form a strong binding affinity for C2H4, while suppressing the adsorption of C2H6. Compared with other UiO-66 materials, CuI@UiO-66-(COOH)2 showed the highest ideal adsorbed solution theory (IAST) selectivity of 80.8 for an equimolar C2H4/C2H6 mixture at 298 K and 1 bar.
Molecular sieves can show the maximum C2H4/C2H6 selectivity via molecular size exclusion, though it is quite challenging due to their similar molecular sizes. In 2018, Chen et al. reported a rigid ultramicroporous MOF [Ca(C4O4)(H2O)] (UTSA-280, H2C4O4 = squaric acid) with one-dimensional (1D) pore channels for molecular sieving separation of C2H4/C2H6. UTSA-280, being assembled by calcium oxide chains and squarate linkers, shows 1D pores with the minimum cross-sectional area of 14.4 Å2, which falls precisely between the sizes of C2H4 and C2H6 (13.7 Å2 and 15.5 Å2, respectively). Therefore, UTSA-280 can adsorb C2H4 with an adsorption capacity of 2.5 mmol g−1 at 298 K and 1 bar while blocking the diffusion of the relatively large C2H6 molecules (Fig. 2).35 The C2H4/C2H6 selectivity of UTSA-280 was estimated to be over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. The adsorption heat (Qst) of UTSA-280 for C2H4 ranges from 20.5 to 35.0 kJ mol−1, which is lower than those of MOFs with OMSs (40–85 kJ mol−1).30,45 Breakthrough experiments confirmed that high purity (>99.2%) of C2H4 can be obtained from an equimolar C2H4/C2H6 mixture with a productivity of 1.86 mmol g−1. It is worth noting that UTSA-280 is capable of efficiently capturing C2H4 from a quaternary CH4/C2H4/C2H6/C3H8 mixture (45/25/25/5). Dispersion-corrected density functional theory (DFT-D) calculations and single-crystal X-ray diffraction experiments revealed that C2H4 molecules are adsorbed by UTSA-280 in a linear array with weak C–H⋯O hydrogen bonding, π⋯π stacking and van der Waals (vdW) interactions.
000. The adsorption heat (Qst) of UTSA-280 for C2H4 ranges from 20.5 to 35.0 kJ mol−1, which is lower than those of MOFs with OMSs (40–85 kJ mol−1).30,45 Breakthrough experiments confirmed that high purity (>99.2%) of C2H4 can be obtained from an equimolar C2H4/C2H6 mixture with a productivity of 1.86 mmol g−1. It is worth noting that UTSA-280 is capable of efficiently capturing C2H4 from a quaternary CH4/C2H4/C2H6/C3H8 mixture (45/25/25/5). Dispersion-corrected density functional theory (DFT-D) calculations and single-crystal X-ray diffraction experiments revealed that C2H4 molecules are adsorbed by UTSA-280 in a linear array with weak C–H⋯O hydrogen bonding, π⋯π stacking and van der Waals (vdW) interactions.
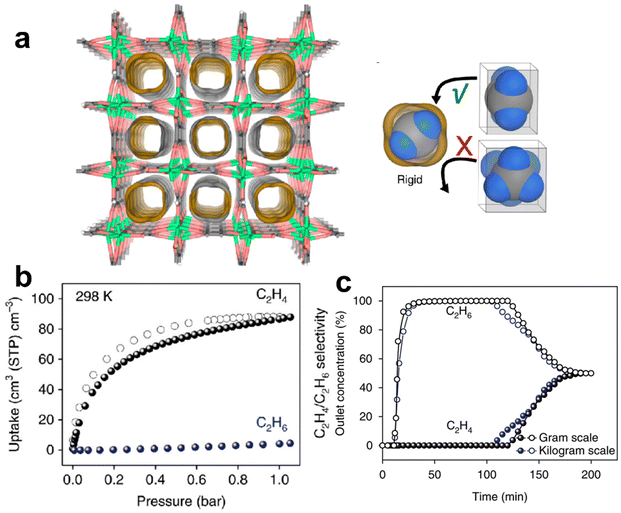 | ||
Fig. 2 (a) Structure of UTSA-280. (b) Sorption isotherms of UTSA-280 for C2H4 and C2H6 at 298 K. (c) Breakthrough curves for UTSA-280 from different scales for an equimolar mixture of C2H4/C2H6 at 298![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K and 1 K and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bar. Reprinted with permission from ref. 35. Copyright 2018 Nature Publishing Group. bar. Reprinted with permission from ref. 35. Copyright 2018 Nature Publishing Group. | ||
In 2018, Ren et al. reported a series of gallate-based MOFs, [M(C7O5H4)·2H2O] (termed M-gallates, M = Mg, Ni, and Co), for C2H4/C2H6 separation with high sieving effect.36 These MOFs contain 3D interconnected zigzag channels and have Brunauer–Emmett–Teller (BET) surfaces of ca. 424 (Ni), 559 (Mg), and 475 (Co) m2 g−1. The pore sizes (3.47–3.69 Å) of these MOFs are close to the molecular sizes of C2H4 (3.28 × 4.18 × 4.84 Å3) and C2H6 (3.28 × 4.18 × 4.84 Å3). Therefore, M-gallates could highly selectively adsorb C2H4 over C2H6. Among them, Co-gallate displays the highest performance for separation of C2H4/C2H6. The C2H4 uptake capacity of Co-gallate is much higher than that for C2H6 (3.37 mmol g−1vs. 0.31 mmol g−1), resulting in a high IAST selectivity of 52 at 298 K and 1 bar for an equimolar C2H4/C2H6 mixture. The IAST selectivity of Mg-gallate is about 37.3 for an equimolar C2H4/C2H6 mixture at 298 K and 1 bar. Breakthrough experiments for a 50/50 C2H4/C2H6 mixture further demonstrated the excellent C2H4/C2H6 separation performances of these low-cost M-gallates.
In 2020, Xing et al. reported a phosphate-pillared MOF, [Zn3(atz)3(PO4)]n (ZnAtzPO4, Hatz = 3-amino-1,2,4-triazole), for C2H4/C2H6 separation with high selectivity (12.4 at 298 K and 32.4 at 273 K).39 Single-component gas adsorption and kinetic studies revealed that ZnAtzPO4 has a higher C2H4 adsorption capacity (1.92 mmol g−1) than for C2H6 (1.04 mmol g−1) at 298 K and 1 bar, as well as a faster adsorption rate for C2H4 compared with C2H6, giving a kinetic selectivity of 36.6. DFT calculations demonstrated that electronegative groups decorated on the narrow pore apertures of ZnAtzPO4 can effectively capture C2H4 and inhibit the diffusion of C2H6, resulting in an efficient equilibrium-kinetic C2H4/C2H6 separation. A flexible ultra-microporous MOF, [Cu2(pyz-CH3)(pzdc)2] (ZU-901, pyz-CH3 = 2-methylpyrazine, and H2pzdc = 2,3-pyrazinedicarboxylic acid), with a pore size of 3.4 × 4.2 Å2 was recently reported to show a C2H4 adsorption–desorption curve in an S shape with high C2H4 working capacity and facile regeneration.40 Single-component sorption experiments showed that ZU-901 can adsorb 1.55 mmol g−1 C2H4 and much less C2H6 (0.26 mmol g−1) at 298 K and 1 bar. The adsorption selectivity of ZU-901 calculated for the pressure swing adsorption (PSA) process is 65. The Qst of ZU-901 for C2H4 was calculated to be ∼25 kJ mol−1, which indicates that ZU-901 can be mildly regenerated. The simulated two-bed PSA process revealed that polymer-grade ethylene (ca. 99.5%) can be obtained by ZU-901 with only 1/10 of the energy consumption compared to simulating cryogenic distillation (2.03 kJ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mol−1vs. 21.84 kJ
mol−1vs. 21.84 kJ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mol−1).
mol−1).
For C2H4-selective MOF materials, multiple adsorption and desorption cycles are typically required to achieve high-purity ethylene. In contrast, C2H6-selective adsorbents can directly give pure ethylene by one single separation operation, making it simple to operate and more energy efficient. Compared to the C2H4 molecule, the C2H6 molecule has a slightly larger polarizability and two additional hydrogen atoms. MOFs with OMSs usually interact strongly with C2H4 molecules via π complexation, whereas MOFs with relatively less polar sites, usually, can preferentially adsorb C2H6 over C2H4.46–66
In an earlier study, Gascon et al. firstly reported preferential capture of C2H6 over C2H4 by utilizing the gate-opening effect of ZIF-7 (also known as MAF-3).58 However, the separation efficiency was low due to the lack of active binding sites. In 2015, Zhang et al. reported a porous MAF [Zn(batz)] (MAF-49, H2batz = bis(5-amino-1H-1,2,4-triazol-3-yl)methane), which shows a high C2H6/C2H4 selectivity of 9 at 316 K.59 In MAF-49, there are quasi-discrete cages functionalized with a high density of electronegative nitrogen atoms on the surface, which are further interconnected by smaller paths into 1D channels (Fig. 3). DFT calculations and crystallographic study indicated that a C2H6 molecule confined in the cage can form stronger and more non-classical C–H⋯N hydrogen bonds or electrostatic interactions through better structural matching with the cage surface of MAF-49 as compared to the C2H4 molecule, which is beneficial for capture, or stronger binding of C2H6 compared with C2H4. MAF-49 showed, for the first time, an exceptional reversed selectivity compared with porous materials including MOFs, which conventionally bind more strongly with C2H4vs. C2H6. Breakthrough experiments for the C2H4/C2H6 (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture showed that MAF-49 can give highly pure C2H4 (99.95%+) within a single breakthrough operation under ambient conditions.
1) mixture showed that MAF-49 can give highly pure C2H4 (99.95%+) within a single breakthrough operation under ambient conditions.
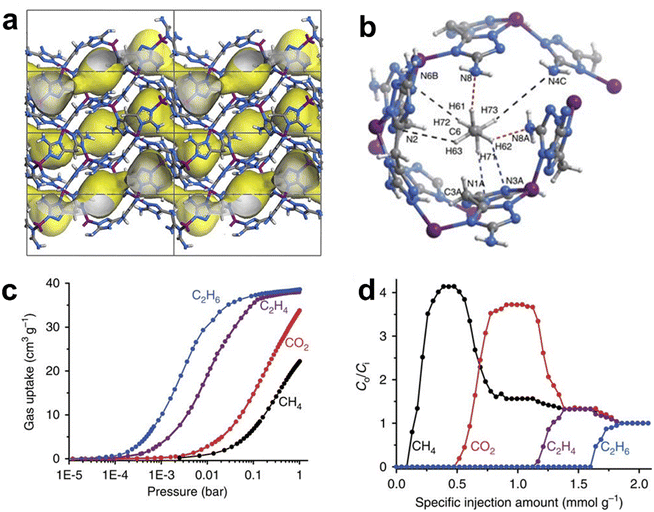 | ||
Fig. 3 (a) Crystal structure of MAF-49. (b) Preferential adsorption sites for C2H6 in MAF-49 revealed by DFT calculations. (c) Gas adsorption isotherms for C2H6, C2H4, CO2 and CH4 in MAF-49 at 316![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K. (d) Breakthrough curves of the CH4/CO2/C2H4/C2H6 mixture (1 K. (d) Breakthrough curves of the CH4/CO2/C2H4/C2H6 mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (vol)) for MAF-49 measured at 313 1 (vol)) for MAF-49 measured at 313![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K and 1 K and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bar. Reprinted with permission from ref. 59. Copyright 2018 Nature Publishing Group. bar. Reprinted with permission from ref. 59. Copyright 2018 Nature Publishing Group. | ||
An appropriate combination of pore sites and pore surface can maximize the weak host–guest interactions, thus highly enhancing the performance of MOFs for C2H6/C2H4 separation. In 2018, Chen et al. reported two isoreticular MOFs, [Cu(Qc)2] (HQc = quinoline-5-carboxylic acid) and [Cu(ina)2] (Hina = isonicotinic acid), featuring 1D channels with pore sizes of ∼4.1 and ∼3.3 Å, respectively, and an array of low-polarity aromatic surfaces with different contact areas on the channels (Fig. 4).60 Single-component sorption results demonstrated that [Cu(Qc)2] shows a higher C2H6 uptake than C2H4 (60.0 cm3 cm−3vs. 25.3 cm3 cm−3, i.e., 1.85 mmol g−1vs. 0.78 mmol g−1) at 298 K and 1 bar, thus giving a high C2H6/C2H4 selectivity of 3.4 for an equimolar C2H6/C2H4 mixture at 298 K and 1 bar. In contrast, [Cu(ina)2] shows comparable adsorption capacity for both gases (C2H4: 67.4 cm3 cm−3, and C2H6: 64.3 cm3 cm−3, i.e., 1.99 mmol g−1vs. 1.90 mmol g−1) due to the smaller polar surface. Neutron powder diffraction and DFT-D calculations showed that the high C2H6 selectivity in [Cu(Qc)2] can be attributed to the more C–H⋯π interactions formed between C2H6 and [Cu(Qc)2]. Breakthrough experiments demonstrated that high purity of C2H4 (> 99.9%) can be directly collected from a 50/50 C2H6/C2H4 mixture by a packed column bed of [Cu(Qc)2], with a separation productivity of 587 mmol L−1.
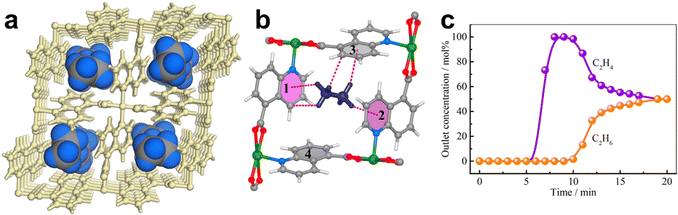 | ||
| Fig. 4 (a) Neutron diffraction crystal structure of [Cu(Qc)2]·0.41C2D6. (b) Preferential binding sites for the C2D6 molecule and the close vdW contacts within the rhombic cavity of aromatic rings, highlighting C–H⋯π interactions in pink dashed bonds. (c) Experimental column breakthrough curves for an equimolar C2H6/C2H4 mixture (298 K, 1 bar) in an adsorber bed packed with Cu(Qc)2. Reprinted with permission from ref. 60. Copyright 2018 American Chemical Society. | ||
The incorporation of the peroxo sites into MOFs can improve the affinity for C2H6. In 2018, Chen et al. reported a microporous MOF [Fe2(O2)(dobdc)] (dobdc4− = 2,5-dioxido-1,4-benzenedicarboxylate) decorated with Fe–peroxo sites that exhibits a high C2H6 affinity with a large Qst of 66.8 kJ mol−1 (Fig. 5).61 Single-component sorption isotherms showed that [Fe2(O2)(dobdc)] can adsorb a large amount of C2H6 (74.3 cm3 g−1, 3.32 mmol g−1) at 1 bar and 298 K and the IAST selectivity for 50/50 C2H6/C2H4 was calculated to be 4.4 under the same conditions. High-resolution neutron powder diffraction showed that C2D6 molecules can form strong non-classical C–D⋯O hydrogen bonds with very short D⋯O distances (∼2.17 to 2.22 Å). Therefore, [Fe2(O2)(dobdc)] exhibits excellent separation performance, yielding polymer-grade C2H4 (≥99.99%) from 50/50 C2H4/C2H6 mixtures in a single breakthrough operation and a productivity of 0.79 mmol g−1. This result further confirms the unique and important role of non-classical C–H⋯O hydrogen bonds in the molecular recognition and selective adsorption of ethane by MOFs.
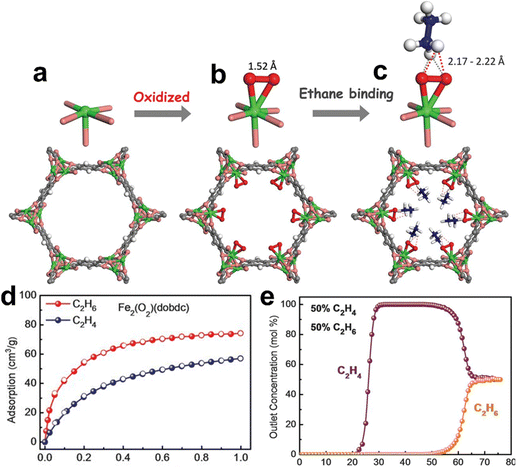 | ||
| Fig. 5 Crystal structures of (a) pristine [Fe2(dobdc)] and [Fe2(O2)(dobdc)] (b) after oxidization and (c) after final C2D6-loading. (d) Sorption isotherms of [Fe2(O2)(dobdc)] for C2H6 and C2H4 under ambient conditions. (e) Breakthrough curves of [Fe2(O2)(dobdc)] for an equimolar C2H6/C2H4 mixture under ambient conditions. Reprinted with permission from ref. 61. Copyright 2018 the American Association for the Advancement of Science. | ||
2.3. C2H2/C2H4 separation
In the petrochemical industry, the production of C2H4 involves trace amounts of acetylene (C2H2) impurities (∼1%). The removal of these impurities is crucial as even small amounts of acetylene during ethylene polymerization can cause catalyst poisoning and highly affect the production of polyethylene. Extensive research has been conducted on the adsorptive separation of MOFs for C2H2/C2H4 mixtures.67–78 Chen et al. first reported a series of M’MOFs for separation of C2H4 from C2H2/C2H4.67 However, these M’MOFs show modest selectivity for C2H2 over C2H4. In 2015, they further reported an amino-functionalized microporous MOF (UTSA-100) with 1D channels (4.3 Å) and small cages (4.0 Å), showing a moderately high C2H2 uptake of 95.6 cm3 g−1 (4.27 mmol g−1) at 296 K but with a much lower C2H4 uptake (37.2 cm3 g−1, 1.66 mmol g−1). The calculated IAST selectivity of UTSA-100 for a 1/99 C2H2/C2H4 mixture is 10.7. This work well demonstrates that suitable pore sizes and binding sites can highly improve the separation performance of MOFs for removing trace C2H2 from C2H4.68To obtain materials with high C2H2 adsorption capacity and high selectivity, in 2016, Chen et al. reported a series of SIFSIX MOFs, namely, SIFSIX-1-Cu, SIFSIX-2-Cu, SIFSIX-2-Cu-i, SIFSIX-3-Ni, and SIFSIX-3-Zn, which allow preferential capture of C2H2 from the C2H2/C2H4 mixture with high selectivity and adsorption capacity.70 These materials possess different pore sizes, which can be systematically fine-tuned by replacing ligands, metal nodes, inorganic anions or framework interpenetration. Among them, SIFSIX-2-Cu-i with a two-fold interpenetrated framework and pore size of 5.2 × 5.2 Å2 (BET surface area 503 m2 g−1) exhibits a high uptake for C2H2 (2.1 mmol g −1, i.e., 47 cm3 g−1) at a low pressure of 0.025 bar, indicating its strong binding affinity for C2H2 (Fig. 6). The IAST selectivity of SIFSIX-2-Cu-i for C2H2/C2H4 (1/99) is up to 44.54. SIFIX-2-Cu with a pore size of 8.0 × 8.0 Å2 (BET surface area: 1178 m2 g−1) shows the highest C2H2 uptake of 8.5 mmol g−1 and a moderate IAST selectivity for C2H2/C2H4 (1/99) of 10.6. Breakthrough experiments demonstrated that all these SIFSIX MOFs can produce polymer-grade C2H4 from C2H2/C2H4 (1/99 or 50/50 mixtures). DFT-D calculations and high-resolution neutron powder diffraction studies reveled that SiF62− pillars in the framework can form strong hydrogen-bonding interactions with C2H2, resulting in the high selectivity and adsorption capacity for C2H2. This work revealed that the combination of optimal pore size and multiple active sites can greatly improve the selectivity and adsorption capacity of the MOFs.
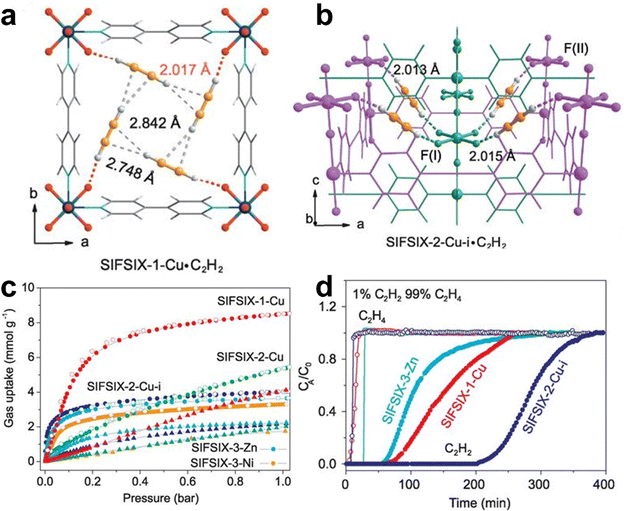 | ||
| Fig. 6 (a) Crystal structure of SIFSIX-1-Cu·C2H2. (b) DFT-D simulated structure of C2H2-loaded SIFSIX-2-Cu-i. (c) Single-component sorption isotherms of SIFSIX-1-Cu, SIFSIX-2-Cu, SIFSIX-2-Cu-i, SIFSIX-3-Zn, and SIFSIX-3-Ni for C2H2 and C2H4 under ambient conditions. (d) Breakthrough curves of SIFSIX-1-Cu, SIFSIX-2-Cu, and SIFSIX-3-Zn for a 1/99 C2H2/C2H4 mixture. Reprinted with permission from ref. 70. Copyright 2016 the American Association for the Advancement of Science. | ||
In 2017, Chen et al. reported a 2-fold interpenetrated MOF SIFSIX-14-Cu-i (UTSA-200) with a smaller pore size (3.4 Å) than SIFSIX-2-Cu-i (4.4 Å) and also functional as SiF62− sites that can enhance the binding affinity for C2H2 (Fig. 7).71 The pore size of the activated structure (UTSA-200a) ideally falls between the kinetic diameter of C2H4 (4.2 Å) and C2H2 (3.3 Å), endowing UTSA-200a with a high performance of molecular sieving separation of C2H2/C2H4. Single component equilibrium adsorption isotherms showed that UTSA-200a exhibits a high low-pressure uptake (58 cm3 cm−3) for C2H2 at 0.01 bar but a negligible uptake (∼0.25 mmol g−1) for C2H4 below 0.2 bar, which is significantly lower than the absorption of SIFSIX-2-Cu-i (2.28 mmol g−1). The IAST selectivity for a 1/99 C2H2/C2H4 mixture of UTSA-200a was calculated to be 6000 at 298 K and 1 bar. High-resolution neutron powder diffraction further revealed that the strong binding affinity of UTSA-200a for the C2D2 molecule was achieved by short C–D⋯F hydrogen-bonding interactions (1.921 Å). Breakthrough experiments revealed that UTSA-200a can efficiently separate C2H2 from a 1/99 C2H2/C2H4 mixture to give an extremely high purity C2H4 (99.9999%) with a productivity of 87.5 mmol g−1.
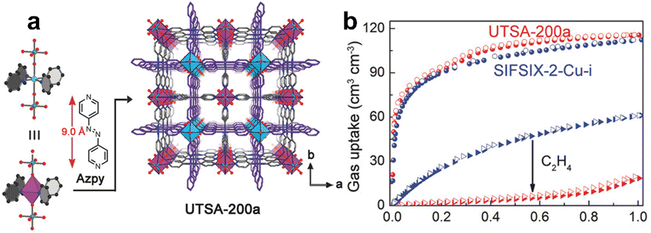 | ||
| Fig. 7 (a) Crystal structure of UTSA-200. (b) C2H2 and C2H4 adsorption isotherms of UTSA-200a at 298 K. Adapted from ref. 71 with permission from Wiley-VCH, Copyright 2018. | ||
In 2017, Chen et al. reported an ultramicroporous SIFSIX-MOF [Zn(dps)2(SiF6)] (UTSA-300, dps = 4,4′-dipyridylsulfide) with small apertures (2.4 × 3.3 Å2) for specific recognition of C2H2 from C2H4.72 The desolvated structure UTSA-300a (aperture size: 3.3 Å) exhibits large affinity toward C2H2 with a Qst of up to 57.6 kJ mol−1 and thus can be selectively gate opened by C2H2. It should be noted that UTSA-300 shows complete size exclusion of C2H4, giving IAST selectivity for the equimolar C2H2/C2H4 mixture up to >104 at 298 K. Breakthrough experiments confirmed its good separation performance for an equimolar C2H2/C2H4 mixture. Subsequently, an isostructural framework of UTSA-300 [Cu(dps)2(SiF6)] (NCU-100 or UTSA-300-Cu) was reported.76 In contrast to UTSA-300, NCU-100 possesses elongated Cu(II)–F bonds, showing a larger cavity size (3.6 × 4.3 × 4.2 Å3vs. 3.5 × 3.9 × 4.1 Å3) in the closed-pore phase. Single-component sorption experiments revealed that NCU-100a shows not only higher low-pressure uptake (0.73 mmol g−1vs. 0.04 mmol g−1) for C2H2 at 0.01 bar and 298 K but also a higher total uptake capacity (4.57 mmol g−1vs. 3.08 mmol g−1) at 298 K and 1 bar, resulting in an IAST selectivity for 1/99 C2H2/C2H4 up to 7291 (298 K and 1 bar). This excellent performance for C2H2/C2H4 separation can also be attributed to the combination of optimal pore size and strong binding sites, as confirmed by DFT calculations and crystallographic studies. Breakthrough experiments confirmed that high purity C2H4 (>99.99%) can be obtained from a 1/99 C2H2/C2H4 mixture with a productivity of 14.9 mmol g−1.
Recently, Zhai et al. synthesized a series of cluster-based MOFs, [M9(μ4-TAZ)6(μ3-HTAZ)x(μ3-TAZ)12−x(A)y] solvent (SNNU-98-M, M = Mn, Co, Ni, and Zn), for the purification of C2H2, in which the tetrazolate (TAZ) ligands coordinate with metal centers in tridentate (μ3-TAZ) and tetradentate (μ4-TAZ) bridging modes.78 These MOFs of acs topology show high framework densities and high stabilities, as well as small pore sizes of 5.2 Å (Mn), 4.8 Å (Co), 4.8 Å (Ni), and 4.2 Å (Zn), respectively, which are expected to increase the separation selectivity and volumetric storage capacity. Single-component gas adsorption revealed that SNNU-98-Mn exhibits the highest volumetric C2H2 uptake (222.9 cm3 cm−3) at 298 K and 1 bar, and a high uptake for C2H2 (175.3 cm3 cm−3) at 298 K and 0.1 bar, whereas SNNU-98-Co shows the highest C2H2/C2H4 IAST selectivity (2405.7) under room temperature and 1 bar. Column breakthrough experiments revealed that all these materials show good C2H2/C2H4 separation performance, with SNNU-98-Mn displaying a higher C2H4 productivity of 64.6 mmol g−1 from a 1/99 C2H2/C2H4 mixture and a longer breakthrough retention time (1362 min g−1 and 701 min g−1 at 273 K and 298 K, respectively, with a gas flow rate of 2 mL min−1).
The separation of C2H4 from multicomponent mixtures in one step is significant for obtaining polymer-grade C2H4 due to the presence of multiple impurities in the cracking gas. Recently, more MOFs have been revealed to be capable of such multicomponent separation.79–94 In 2018, Lu et al. reported the highly selective adsorption of C2H4 from a C2H2/C2H4/C2H6 mixture by a stable MOF, (Me2NH2)[Co3(DCPN)2(μ3-OH)(H2O)]·11H2O (TJT-100, DCPN = 5-(3′,5′-dicarboxylphenyl)nicotinate).79 TJT-100 exhibits a 1D channel (pore size: ∼8.0 Å) decorated with a high density of carboxylate oxygen atoms. Single-component adsorption showed that the adsorption capacities of TJT-100 for C2H2, C2H4, and C2H6 were 127.7 cm3 g−1, 98.1 cm3 g−1, and 105.4 cm3 g−1, respectively. The breakthrough experimental results of TJT-100 for the C2H2/C2H4/C2H6 mixture (0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99
99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5) confirmed the preferential capture of C2H2 and C2H6, giving C2H4 with an excellent purity of 99.997%. DFT calculations revealed that C2H2 and C2H6 have multiple electrostatic interactions with the framework, which facilitates preferential adsorption of C2H2 and C2H6 by TJT-100.
0.5) confirmed the preferential capture of C2H2 and C2H6, giving C2H4 with an excellent purity of 99.997%. DFT calculations revealed that C2H2 and C2H6 have multiple electrostatic interactions with the framework, which facilitates preferential adsorption of C2H2 and C2H6 by TJT-100.
In 2019, Zaworotko et al. reported a synergistic sorbent separation technique (SSST), which enables the one-step production of polymer-grade C2H4 from a ternary (C2H2/C2H6/C2H4) gas mixture, and even a quaternary (CO2/C2H2/C2H6/C2H4) gas mixture (Fig. 8).80 Three microporous MOFs, TIFSIX-2-Cu-I, SIFSIX-3-Ni, and Zn-atz-ipa, were placed in tandem on the sorbent bed, offering selective adsorption of C2H2, CO2, and C2H6, respectively. Notably, Zn-atz-ipa shows a rare, higher uptake of C2H6 than CO2, C2H2 and C2H4 at 0 to 0.4 bar. Breakthrough experiments showed that the packing order of the three MOFs in the sorbent bed has a significant impact on the separation performance, whereas the particle size and amount of the adsorbent have a minor effect. When the packing order is SIFSIX-3-Ni@Znatz-ipa@TIFSIX-2-Cu-i, high purity C2H4 can be gained from a 1/49.5/49.5 C2H2/C2H4/C2H6 or 1/33/33/33 C2H2/C2H4/C2H6/CO2 mixture and the working capacities are 0.32 and 0.10 mmol g−1, respectively. This work provides a new path for selective separation of a specific component molecule from a multicomponent gas mixture.
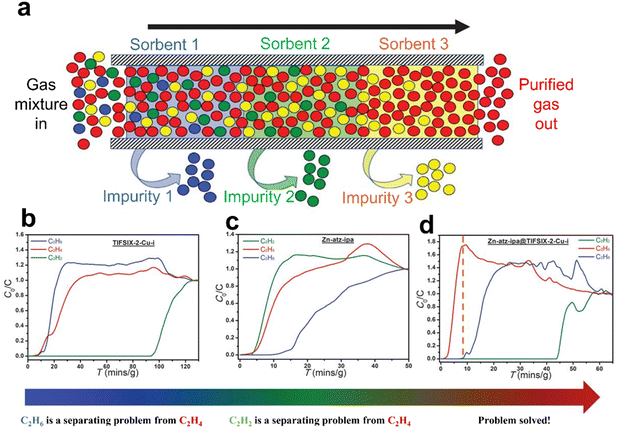 | ||
Fig. 8 (a) SSST involves an adsorption bed with three task-specific physisorbents to purify the commodity (red) with specific binding sites for each trace impurity (blue, green, and yellow). (b) and (c) Experimental column breakthrough curves for C2H2/C2H4/C2H6 separation (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture) on TIFSIX-2-Cu-i and Zn-atz-ipa at 298 K and 1 bar. Breakthrough experiments were conducted in a column (inner diameter, 8 mm) at a flow rate of 2.1 ml min−1. (d) Experimental column breakthrough curves for C2H2/C2H4/C2H6 separation (1 1 mixture) on TIFSIX-2-Cu-i and Zn-atz-ipa at 298 K and 1 bar. Breakthrough experiments were conducted in a column (inner diameter, 8 mm) at a flow rate of 2.1 ml min−1. (d) Experimental column breakthrough curves for C2H2/C2H4/C2H6 separation (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture) on a tandem-packed column of TIFSIX-2-Cu-i (∼250 mg) and Zn-atz-ipa (∼600 mg) at 298 K and 1 bar. Reprinted with permission from ref. 80. Copyright 2019 the American Association for the Advancement of Science. 1 mixture) on a tandem-packed column of TIFSIX-2-Cu-i (∼250 mg) and Zn-atz-ipa (∼600 mg) at 298 K and 1 bar. Reprinted with permission from ref. 80. Copyright 2019 the American Association for the Advancement of Science. | ||
2.4. C2H2 and CO2 separation
Acetylene (C2H2) is an important chemical raw material that is mainly produced by thermal cracking of hydrocarbons or the partial combustion of methane. The resulting gas stream contains various impurities, with carbon dioxide (CO2) being the major one. Thus, the separation of C2H2 from CO2/C2H2 mixtures is important in industry. Although CO2 and C2H2 have similar sizes (CO2: 3.18 × 3.33 × 5.36 Å3, and C2H2: 3.32 × 3.34 × 5.7 Å3) and physical properties (boiling point of CO2 = 194.7 K and of C2H2 = 189.3 K), the opposite quadrupole moments and slightly discrepant polarizabilities between C2H2 and CO2, as well as the stronger π-bonding ability with metal sites and hydrogen-bonding donor ability of C2H2, allow the realization of their separation by means of rational design of pore surfaces and flexible framework amplification.72,95–98In the early studies, Kitagawa et al. reported that a microporous MOF material, Cu2(pzdc)2(pyz), with non-coordinated oxygen atoms on the pore surface forms strong hydrogen-bonding interactions with C2H2, showing high C2H2/CO2 separation potential.99 This study firstly demonstrates the effective role of Lewis base sites in enhancing the affinity of a MOF for C2H2. In addition, MOFs with high density of OMSs can facilitate the recognition of acetylene.100–108 In 2016, Chen et al. reported a MOF-74 isomer, namely, UTSA-74, which has two different (octahedral and tetrahedral) metal coordination geometries along the 1D channel.101 Each octahedrally coordinated metal ion provides two OMSs upon removal of the coordinated water molecules, leading to enhanced C2H2/CO2 separation. The single-component adsorption isotherms showed that UTSA-74 has a comparable C2H2 uptake capacity (145 cm3 cm−3) to Zn-MOF-74 but a smaller CO2 uptake capacity (90 cm3 cm−3vs. 146 cm3 cm−3), resulting in UTSA-74 with a good C2H2/CO2 separation performance. This result can be attributed to that each open Zn site can bind with two C2H2 molecules, whereas two oxygen atoms of a CO2 molecule occupy two adjacent OMSs, as demonstrated by the single-crystal X-ray structures and molecular modeling studies. Breakthrough experiments for a 50/50 C2H2/CO2 mixture further demonstrate its good practical separation performance. This is also the first example of using breakthrough experiments to demonstrate the separation performance of MOFs for the C2H2/CO2 mixture.
Based on copper(I)-alkynyl chemistry, anchoring Cu(I) on the surface of a MOF can significantly improve its specific recognition for C2H2. Qian et al. reported a Cu(I)-modified porous MOF, Cu(I)@UiO-66-(COOH)2, which exhibits significantly enhanced C2H2/CO2 separation performance with a high C2H2/CO2 IAST selectivity of 185, compared to the prototype UiO-66-(COOH)2.107 The strong π-complexation between the π electrons on C2H2 and Cu ions enables Cu(I)@UiO-66-(COOH)2 to adsorb a large amount of C2H2 (0.9 mmol g−1) at low pressure (0.01 bar) and exhibit a high Qst of 74.5 kJ mol−1. Breakthrough experiments demonstrated its highly efficient separation performance for C2H2/CO2. In addition, optimal distribution of OMSs on the pore surface of MOFs can achieve the maximum utilization of these sites. Recently, Li et al. reported a microporous MOF (JNU-4) with high-density OMSs.108 These sites consist of square-planar copper centers separated by organic ligands, allowing each metal center to effectively bind with C2H2; thus, JNU-4 achieves a high C2H2 adsorption capacity (222 cm3 g−1, 9.91 mmol g−1) and a moderate IAST selectivity of 8.2 for an equimolar C2H2/CO2 mixture. Notably, this material absorbs C2H2 up to 200 cm3 g−1 (8.93 mmol g−1) at 298 K and 0.5 bar, but demonstrates a low Qst of 26.8 kJ mol−1. Grand Canonical Monte Carlo (GCMC) simulations demonstrated that C2H2 molecules can bind on both sides of OMSs, while CO2 molecules can only bind on one side. Breakthrough experiments revealed that it has a high C2H2 absorption capacity (160 cm3 g−1, 7.14 mmol g−1) from an equimolar C2H2/CO2 mixture and can provide fuel-grade C2H2 gas.
Although a high density of OMSs can improve the selectivity for C2H2, electrostatic interactions between CO2 and metal also exist simultaneously.109–111 This requires a rational distribution of metal sites or the combination of multiple functions such as the pore size and shape to achieve a better C2H2 selectivity.100,105,112 However, OMSs of high density may result in a high Qst, thus leading to an increase of the energy consumption for adsorbent regeneration. In addition to OMSs, the introduction of functional groups (–NH2, –F, –Cl, –Br, –CF3, etc.) on organic ligands is also an effective strategy to promote selective adsorption of C2H2.113–116 In 2021, Zhao et al. reported that an amine-functionalized flexible MOF (CPL-1-NH2) with a 1D channel (3.8 × 4.4 Å2) exhibits a high IAST selectivity of 119 for a 50/50 C2H2/CO2 mixture at 298 K and 1 bar.114 Theoretical calculations showed that the high selectivity of CPL-1-NH2 for C2H2 can be attributed to the NH2 functionalization that enhances C2H2 interaction with the framework, but blocks the stronger interaction between the non-coordinated oxygen atoms and CO2 molecules by occupying the adsorption site of CO2. In the same year, Qian et al. reported an Al-MOF (CAU-10-H) with a pore size of 4.7 Å, exhibiting a high C2H2 storage density (392 g L−1) and a separation factor (3.4).115 GCMC simulations showed that the suitable pore size not only enhances the interaction of C2H2 with high density oxygen atoms and aromatic rings on the pore surface, but also enables synergistic interaction between adjacent C2H2 molecules. Subsequently, CAU-10-H was functionalized with amine to furnish a new MOF (CAU-10-NH2).116 The later study showed that the amine groups can improve the uptake capacity of C2H2 at low pressure, and significantly enhance the stacking density (0.46 cm3 g−1) and C2H2/CO2 selectivity (10.8) compared with the prototype MOF. In addition, these materials have the advantages of high stability, low Qst, easy scale-up, and low cost.
It is well known that pore size and shape play an important role in the separation process, as an appropriate pore size can enhance the strength of interactions between the pore surface and adsorbate, as well as among adsorbates themselves.97,100,112,117–123 MOFs can be designed and modified to alter the pore sizes, even at the sub-nanometer scale, through substitutions of metal ions, organic ligands, or inorganic anions. Strategies such as building self-interpenetrated networks and pore space partition (PSP) can also be used for the pore size or space modulation.13,124 In 2019, Chen et al. reported a PSP approach for highly efficient C2H2/CO2 separation. The new porous MOF (FJU-90) was synthesized by inserting a triangular ligand (2,4,6-tris(4-pyridyl)pyridine) into the cylindrical channel of prototype FJU-88.122 The 1D channel of FJU-88 was separated into a number of aperture cavities with the aperture size decreasing from 12.0 × 9.4 to 5.4 × 5.1 Å2. Thus, the combination of optimized pore space and oxygen atom sites endows FJU-90a with a high C2H2 adsorption capacity (180 cm3 g−1, 8.04 mmol g−1). The separation performance of the activated MOF (FJU-90a) for a 50/50 C2H2/CO2 mixture was further confirmed by breakthrough experiments with a productivity of 1.87 mol kg−1.
In 2017, Chen et al. reported a SIFSIX-MOF UTSA-300 that exhibits not only efficient separation of C2H2/C2H4 but also a high selective uptake for C2H2 from the C2H2/CO2 mixture.72 Single-component adsorption isotherms revealed that the activated MOF (UTSA-300a) shows a high capacity for C2H2 adsorption (68.9 cm3 g−1, 3.08 mmol g−1) but adsorbs negligible CO2 (3.25 cm3 g−1, 0.14 mmol g−1) at 298 K and 1 bar, resulting in a high IAST C2H2/CO2 selectivity of 743 (298 K and 1 bar) (Fig. 9). Due to the strong C–H⋯F interactions formed between C2H2 molecules and SIF62−, C2H2 can easily diffuse into UTSA-300a, while CO2 is restricted because of electrostatic repulsion, as confirmed by DFT calculations and neutron powder diffraction studies. The separation performance of UTSA-300a for a 50/50 C2H2/CO2 mixture was further demonstrated by breakthrough experiment in a packed column bed of UTSA-300a. Subsequently, systematic fine-tuning of the pore size of UTSA-300 was performed by replacing the fluoride anionic linkers and metal ions.125 Three new SIFSIX-type MOFs, namely SIFSIX-dps-Cu (NCU-100), GeFSIX-dps-Cu, and NbOFFIVE-dps-Cu, were reported to exhibit different pore sizes (1.4 × 3.0 Å2, 1.5 × 3.0 Å2, and 2.2 × 2.7 Å2, respectively) and interlayer distances (4.10 Å, 4.06 Å, and 3.69 Å, respectively). Single-component adsorption isotherms indicated that SIFSIX-dps-Cu shows the highest C2H2 uptake (4.57 mmol g−1) with a high IAST C2H2/CO2 selectivity up to 1787 at 298 K and 1 bar. In addition, the gate-opening pressure of SIFSIX-dps-Cu for C2H2 is 0.035 bar, which is lower than those of GeFSIX-dps-Cu (0.05 bar), NbOFFIVE-dps-Cu (0.3 bar), and UTSA-300 (0.06 bar), probably because of its larger interlayer distance. DFT and grand canonical Monte Carlo simulations further revealed that C2H2 molecules have multiple host–guest interactions (H⋯F hydrogen-bonding interactions) in both inter- and intra-layer cavities. Breakthrough experiments confirmed that high purity C2H2 (≥99.9%) can be obtained from C2H2/CO2 (50/50) with a high productivity of 2.48 mmol g−1.
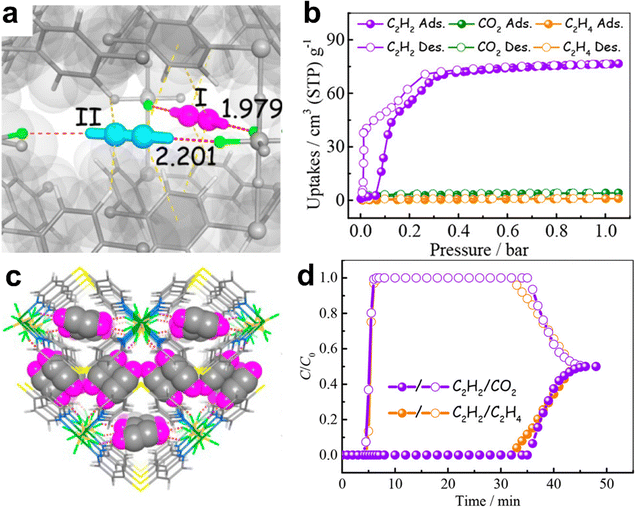 | ||
| Fig. 9 (a) and (c) Neutron diffraction crystal structure of UTSA-300⊃C2D2 and (b) C2H2, CO2, and C2H4 sorption isotherms for UTSA-300a at 273 K. (d) Experimental column breakthrough curves for equimolar C2H2/CO2 (purple) and C2H2/C2H4 (orange) mixtures (298 K, 1 bar) in a fixed-bed packed with UTSA-300a. Reprinted with permission from ref. 72. Copyright 2017 American Chemical Society. | ||
A suitable pore size combined with active sites and a flexible structure can greatly improve the adsorption capacity of a material for C2H2.95,106,114,125–127 Recently, Hong et al. reported that a flexible MOF, namely, [Ni(DTBDA)(MeOH)2(DMA)(H2O)2] (FJI-H36, DTBDA = 3′,5′-di(1H-1,2,4-triazol-1-yl)-[1,1′-biphenyl]-3,5-dicarboxylic acid), with adaptive channels shows efficient adsorption of C2H2.127 FJI-H36 contains two types of cavities with the size of 12.9 × 12.9 Å2 and 8.4 × 10.2 Å2, respectively, as well as high-density active sites of open NiII (4.52 mol L−1) and free N atoms (9.04 mol L−1). Single-component sorption experiments confirmed that FJI-H36 shows a high C2H2 uptake of 159.9 cm3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cm−3 at 298 K and 1 bar, and an ultra-high C2H2 storage density of 561 g L−1. Breakthrough experiments for a 50/50 C2H2/CO2 mixture further demonstrated its high adsorption capacity for C2H2 (3.82 mmol g−1). The structure of FJI-H36 adaptively changes with the adsorption of C2H2, leading to a dense packing of C2H2 in it, as demonstrated by the X-ray crystal structure of C2H2@FJI-H36.
cm−3 at 298 K and 1 bar, and an ultra-high C2H2 storage density of 561 g L−1. Breakthrough experiments for a 50/50 C2H2/CO2 mixture further demonstrated its high adsorption capacity for C2H2 (3.82 mmol g−1). The structure of FJI-H36 adaptively changes with the adsorption of C2H2, leading to a dense packing of C2H2 in it, as demonstrated by the X-ray crystal structure of C2H2@FJI-H36.
Recently, Wang et al. reported a sulfate-pillared MOF [Zn(tepb)(SO4)] (SOFOUR-TEPE-Zn, TEPB = tetra(4-pyridyl)benzene), which possesses dense electronegative pore surfaces to highly promote the separation of C2H2/CO2 (Fig. 10).97 SOFOUR-TEPE-Zn is an isostructural framework with SOFOUR-1-Zn,128 but with more electron-rich pore surfaces due to the higher electronegative ethylene groups in the TEPE ligand in contrast to the phenyl ring in the TEPB ligand. Single-component sorption results of SOFOUR-TEPE-Zn reveled a higher C2H2 uptake (89.1 cm3 g−1, 3.98 mmol g−1) than SOFOUR-1-Zn (69.4 cm3 g−1, 3.10 mmol g−1) at 1 bar and 298 K, but a much lower CO2 uptake (14.1 cm3 g−1, 0.63 mmol g−1), resulting in a very high IAST selectivity of 16833 for 50/50 C2H2/CO2. Breakthrough experiments for 50/50 C2H2/CO2 of SOFOUR-TEPE-Zn give a productivity of 60.1 cm3 g−1 (2.68 mmol g−1) of 99.5% purity or 33.2 cm3 g−1 (1.48 mmol g−1) of 99.99% purity in its desorption process by stepped helium purging and mild heating. Moreover, SOFOUR-TEPE-Zn also maintains a high C2H2 productivity of 75.5 cm3 g−1 (3.37 mmol g−1) of 99.5% purity with 99.82% C2H2 recovery in the simulated pressure swing adsorption processes. DFT-D and GCMC simulation studies revealed that the preferential binding of C2H2 in SOFOUR-TEPE-Zn can be mainly attributed to electron-rich pore surfaces, providing multiple optimal adsorption sites for C2H2.
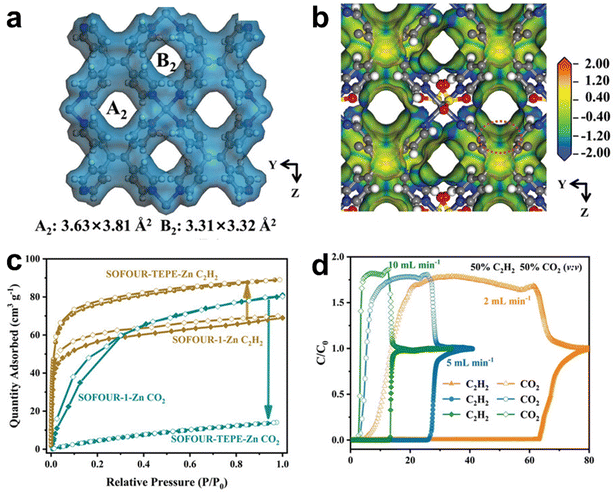 | ||
| Fig. 10 (a) and (b) The building blocks of SOFOUR-TEPE-Zn and electrostatic surface potential. (c) C2H2 and CO2 adsorption isotherms for SOFOUR-1-Zn and SOFOUR-TEPE-Zn at 298 K. (d) Breakthrough curves of SOFOUR-TEPE-Zn for C2H2/CO2 (50/50, v/v) at different flow rates at 298 K. Reprinted with permission from ref. 97. Copyright 2023 Wiley-VCH. | ||
CO2-selective adsorbents can yield high purity C2H2 in one step rather than multiple adsorption–desorption steps, making the operation simple and more energy efficient. Currently, only a limited number of CO2-selective MOF materials have been reported, manifesting the challenge in the design of CO2-selective materials.129–148 Fortunately, several strategies have been reported to promote the selective adsorption of CO2 in MOFs. For example, Chen et al. introduced hydroxyl functional groups into MOF frameworks, enabling selective capture of CO2 from C2H2, with a high IAST selectivity of 118.7 for CO2/C2H2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v
2, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) at 0.1 bar and ambient temperature.137 In 2021, Chen et al. reported an ultramicroporous MOF [Cu(F-pymo)2]·1.25H2O (Cu-F-pymo, F-pymo = 5-fluoropyrimidin-2-olate) with zeolitic gismondine (GIS) topology exhibiting a high selective adsorption of CO2 over C2H2, which depends on activation temperature (Fig. 11).143 Cu-F-pymo contains two distinct pore environments, namely, spherical cavities and 1D helical channels, both of which are occupied by removable water molecules. Single-component adsorption isotherms revealed that partially dehydrated Cu-F-pymo can adsorb 1.19 mmol g−1 C3H6 and show negligible C2H2 uptake (0.1 mmol g−1) at 298 K and 1 bar. Breakthrough experiments revealed that highly pure acetylene (>99.9%) can be obtained directly from a 50/50 CO2/C2H2 mixture by a single separation operation. Modeling studies demonstrated that CO2 can be preferentially adsorbed in the 1D channels, while C2H2 primarily occupies the spherical cavities. Therefore, the remaining water molecules in the spherical cavities blocked the preferential site, leading to the molecular sieving effect of Cu-F-pymo for CO2/C2H2.
v) at 0.1 bar and ambient temperature.137 In 2021, Chen et al. reported an ultramicroporous MOF [Cu(F-pymo)2]·1.25H2O (Cu-F-pymo, F-pymo = 5-fluoropyrimidin-2-olate) with zeolitic gismondine (GIS) topology exhibiting a high selective adsorption of CO2 over C2H2, which depends on activation temperature (Fig. 11).143 Cu-F-pymo contains two distinct pore environments, namely, spherical cavities and 1D helical channels, both of which are occupied by removable water molecules. Single-component adsorption isotherms revealed that partially dehydrated Cu-F-pymo can adsorb 1.19 mmol g−1 C3H6 and show negligible C2H2 uptake (0.1 mmol g−1) at 298 K and 1 bar. Breakthrough experiments revealed that highly pure acetylene (>99.9%) can be obtained directly from a 50/50 CO2/C2H2 mixture by a single separation operation. Modeling studies demonstrated that CO2 can be preferentially adsorbed in the 1D channels, while C2H2 primarily occupies the spherical cavities. Therefore, the remaining water molecules in the spherical cavities blocked the preferential site, leading to the molecular sieving effect of Cu-F-pymo for CO2/C2H2.
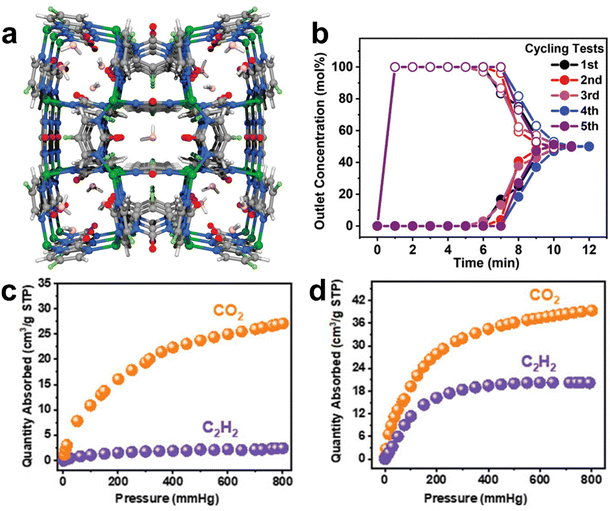 | ||
| Fig. 11 (a) Schematic structure of Cu-F-pymo MOF with residual water molecules. (b) Breakthrough cycling tests of the equimolar CO2/C2H2 mixture. (c) and (d) Separation performance toward CO2 and C2H2 in Cu-F-pymo under different activation conditions. Reprinted with permission from ref. 143 Copyright 2021 Wiley-VCH. | ||
Besides size-matching between gas molecules and the pore structure, reasonable charge distribution would also facilitate the selective recognition of CO2 over C2H2. Chen et al. reported an ultramicroporous porous material Cd[Fe(CN)5NO] (Cd-NP) that exhibits stronger affinity for CO2 in contrast to C2H2. Cd-NP contains quasi-discrete ellipsoidal cavities (6.1 × 4.5 × 4.5 Å3) connected by small apertures (3.2 Å), and its BET surface area is 305 m2 g−1 (Fig. 12).144 Thanks to suitable pore sizes and electrostatic distribution on pore surfaces, Cd-NP shows a high CO2 uptake of 58.0 cm3 g−1 (2.59 mmol g−1) but a lower C2H2 uptake of 9.7 cm3 g−1 (0.43 mmol g−1) at 1 bar and 298 K, thus showing a high IAST selectivity (85) for an equimolar CO2/C2H2 mixture. The Qst of CO2 was calculated to be 27.7 kJ mol−1, which would facilitate regeneration of the material under mild conditions. The breakthrough process showed that Cd-NP was capable of producing a high purity C2H2 (99.9%) directly from 50/50 CO2/C2H2 with a productivity of 2.34 mol L−1. GCMC simulations and neutron powder diffraction experiments showed that the preferential adsorption of CO2 in Cd-NP can be attributed to the confinement effect of the pore cavity and electrostatically complementary pore surface.
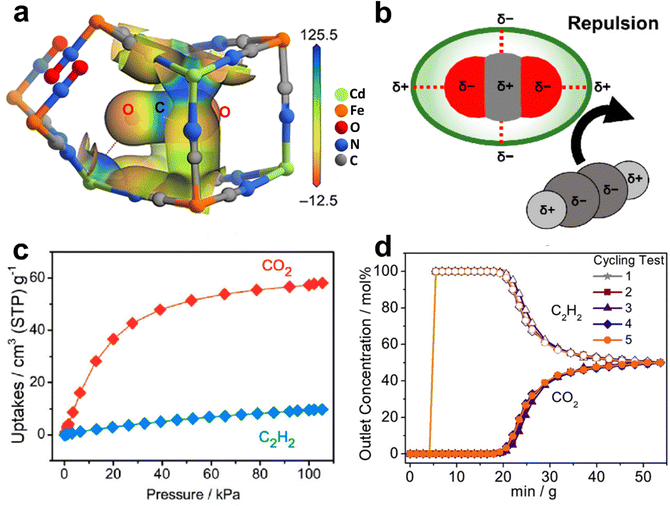 | ||
Fig. 12 (a) Electrostatic potential (ESP) of Cd-NP⊃CO2 mapped onto the 0.15 e−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Å−3 electron density isosurface. (b) Electrostatically driven adsorption mechanism towards CO2 and C2H2 molecules. (c) CO2 and C2H2 sorption isotherms for Cd-NP at 298 K. (d) Cycling tests of the equimolar CO2/C2H2 mixture in a column packed with Cd-NP at 298 K and 1 bar. Reproduced with permission from ref. 144. Copyright 2021 Wiley-VCH. Å−3 electron density isosurface. (b) Electrostatically driven adsorption mechanism towards CO2 and C2H2 molecules. (c) CO2 and C2H2 sorption isotherms for Cd-NP at 298 K. (d) Cycling tests of the equimolar CO2/C2H2 mixture in a column packed with Cd-NP at 298 K and 1 bar. Reproduced with permission from ref. 144. Copyright 2021 Wiley-VCH. | ||
CO2/C2H2 inverse separation can also be achieved using synergistic effects of thermodynamics and kinetics. Li et al. reported an ultramicroporous MOF, Y-bptc, to achieve one-step C2H2 purification from a CO2/C2H2 mixture. Y-bptc processes small windows (4.2 Å) interconnected with large cubic cages with ftw topology. Equilibrium and kinetic adsorption studies reveled that Y-bptc absorbs 55 cm3 g−1 (2.45 mmol g−1) CO2 and a lower C2H2 uptake at 298 K and 1 bar with an IAST selectivity of 4.1 for 50/50 CO2/C2H2.136 Moreover, CO2 diffuses faster than C2H2 in Y-bptc, and the calculated kinetic separation coefficient reaches 114 at 298 K. Breakthrough experiments confirmed that CO2 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CO2/C2H2 mixture can be readily removed by Y-bptc, giving C2H2 with a purity of >99% and productivity of 1.52 mmol g−1 in a one-step separation process. GCMC simulations further revealed the high affinity of Y-bptc for CO2 resulting from the formation of hydrogen-bonding interactions between μ3-OH− groups of the framework and CO2 molecules.
1 CO2/C2H2 mixture can be readily removed by Y-bptc, giving C2H2 with a purity of >99% and productivity of 1.52 mmol g−1 in a one-step separation process. GCMC simulations further revealed the high affinity of Y-bptc for CO2 resulting from the formation of hydrogen-bonding interactions between μ3-OH− groups of the framework and CO2 molecules.
In 2023, Wade et al. reported two isostructural MOFs [Zn5Cl4(bbta)3] (MUF-4, bbta2− = benzo-1,2,4,5-bistriazolate) and MUF-4-F, which exhibited completely opposite adsorption selectivity for CO2/C2H2. MUF-4-F can be derived by F− to Cl− ligand exchange of MUF-4.139 Both MUF-4-F and MFU-4 possess alternating large pore and small pore cavities but have different connecting windows (∼3.7 Å and ∼2.2 Å, respectively). C2H2 molecules can easily enter MFU-4-F. Equilibrium and kinetic adsorption studies revealed that MFU-4-F exhibits a higher C2H2 (6.66 mmol g−1) uptake than CO2 (3.24 mmol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) g−1) at 300 K and 1 bar and shows a fast adsorption rate for both gases. In contrast, MFU-4 exhibits high kinetic selectivity for uptake of CO2 over C2H2 up to 3360 at 300 K. Computational studies revealed that C2H2 was blocked by the smaller windows created by the Zn–Cl groups in MUF-4. Breakthrough experiments demonstrated that high purity (>98%) C2H2 can be obtained directly from a 50/50 CO2/C2H2 mixture in MUF-4.
g−1) at 300 K and 1 bar and shows a fast adsorption rate for both gases. In contrast, MFU-4 exhibits high kinetic selectivity for uptake of CO2 over C2H2 up to 3360 at 300 K. Computational studies revealed that C2H2 was blocked by the smaller windows created by the Zn–Cl groups in MUF-4. Breakthrough experiments demonstrated that high purity (>98%) C2H2 can be obtained directly from a 50/50 CO2/C2H2 mixture in MUF-4.
2.5. C3H6 and C3H8 separation
Propylene (C3H6) is also an essential industrial feedstock with large demand that is only lower than that of ethylene. The industrial production of C3H6 inevitably contains a certain amount of propane (C3H8) impurity. However, they have very similar molecular sizes (C3H6: 3.8 × 4.0 × 6.5 Å3, and C3H8: 3.8 × 4.2 × 6.8 Å3), kinetic diameters (C3H6: 4.0 Å, and C3H8: 4.2 Å) and physical properties (boiling points of 189.3 and 194.7 K, respectively). Therefore, it is more challenging to tune the pore size for sieving separation of C3H6/C3H8, because they have highly similar molecular sizes and kinetic diameters, whereas possible changes of the size and shapes of MOF apertures may occur in the adsorption process, as many MOFs are actually flexible. Fortunately, significant research progress about highly efficient separation of C3H6/C3H8 has been recently realized by MOFs through rational structural design and modification of the pore size and pore surface.149–168The incorporation of active sites in MOFs’ pore surface improves not only kinetic selectivity but also thermodynamic selectivity for gas separation. In 2019, Zhang et al. reported a MAF, MAF-23-O, which can be easily synthesized by heating the flexible prototype [Zn2(btm)2] (MAF-23, H2btm = bis(5-methy-1H-1,2,4-triazol-3-yl)methane) under oxygen gas flow (Fig. 13).149 Single-crystal structure analyses showed that MAF-23-O is isostructural to MAF-23 and contains half the amount of the oxidized btm2− ligands, namely, btk2− (H2btk = bis(5-methyl-1,2,4-triazol-3-yl)methanone). This in situ ligand oxidative modification makes the framework more rigid and more hydrophilic. The IAST selectivity for C3H6/C3H8 in MAF-23-O was calculated to be 8-9, which was higher than that of MAF-23 (3-4). Moreover, C3H6 and C3H8 exhibit similar diffusion rates in MAF-23, but show very different diffusion rates in MAF-23-O with a high kinetic selectivity of 71. Theoretical calculations demonstrated that the exposed oxygen atoms in the framework of MAF-23-O can form strong C–H⋯O/N interaction with C3H6, improving the thermodynamic selectivity of MAF-23-O for C3H6. Additionally, the decreased flexibility of the framework slows down the diffusion of C3H6, thus improving the kinetic selectivity. Breakthrough experiments revealed that MAF-23-O exhibits efficient separation for an equimolar C3H6/C3H8 mixture at 298 K and 1 bar with an adsorption selectivity of 15, which is 10 times that of MAF-23.
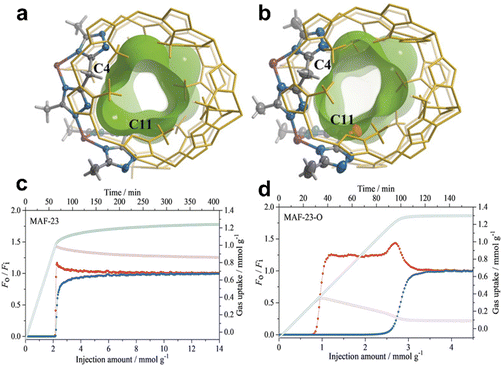 | ||
| Fig. 13 Crystal and pore structures of (a) MAF-23 and (b) MAF-23-O. Breakthrough curves (filled symbols) and adsorption kinetic curves (open symbols) for (c) MAF-23 and (d) MAF-23-O using an equimolar C3H6/C3H8 (blue/red) mixture at 298 K and 1 bar. Reprinted with permission from ref. 149. Copyright 2019 Wiley-VCH. | ||
In 2016, Eddaoudi et al. reported a oxyfluoride-based MOF [Ni(pyr)2(NbOF5)]·2H2O (NbOFFIVE-1-Ni, or KAUST-7, pyr = pyrazine) for selective adsorption of C3H6 from C3H8.150 KAUST-7 is isostructural to SIFSIX-3 and composed of Ni(II)-pyrazine square-grid layers and (NbOF5)2− pillars with pcu topology, exhibiting 1D channels with an aperture size of 3.0 Å and a periodic array of fluoride anions on the pore surface. Single-component sorption experiments revealed that KAUST-7 can adsorb ∼60 mg g−1 (1.43 mmol g−1) C3H6 but negligible C3H8 uptake at 298 K and 1 bar. Breakthrough experiments demonstrated that C3H8 of 97% purity can be obtained from C3H6/C3H8 50/50 mixed-gas by a packed column bed of KAUST-7, with a separation productivity of 0.6 mol g−1. KAUST-7 also can adsorb C3H8 by pore-opening sorption behavior at 273 K.
In 2023, Chen et al. reported another SIFSIX MOF [Ni(WO2F4)(pyz)2] (UTSA-400) featuring 1D channels that can exclude C3H8 molecules and adsorb C3H6 molecules with a high binding affinity (Fig. 14).151 UTSA-400 shows an isostructural framework with SIFSIX-3 and NbOFFIVE-1-Ni, but with highly tilted pyrazine linkers owing to larger WO2F42− in contrast to SiF62− and NbOF52−. The pore cavities in UTSA-400 are 6.7 × 5.5 × 3.7 Å3 with exposed oxide/fluoride pairs on the pore surface (BET surface area: 226 m2 g−1) that can serve as the binding sites for propylene molecules. Single-component sorption results of UTSA-400 indicated a much higher C3H6 capacity (92.1 cm3 cm−3, 2.62 mmol g−1) than NbOFFIVE-1-Ni (54.3 cm3 cm−3, 1.34 mmol g−1) by 63%, at 1 bar and 298 K, which is also higher than those of Y-abtc (64.6 cm3 cm−3, 1.98 mmol g−1) and Co-gallate (66.6 cm3 cm−3, 1.79 mmol g−1). Under the same conditions, UTSA-400 showed negligible C3H8 uptake because of inaccessible inward diffusion of propane molecules. The Qst of UTSA-400 for C3H6 is 60.5 kJ mol−1, being comparable to those of MOFs with OMSs (44–57 kJ mol−1).30,45 Breakthrough separation experiment demonstrated that polymer-grade (99.7%) propylene can be obtained from an equimolar C3H6/C3H8 mixture with a productivity of 56.7 L L−1. Besides size exclusion, the separation performance of UTSA-400 can also be attributed to strong C–H⋯O/F interactions, as confirmed by in situ infrared spectroscopy and DFT-D calculations.
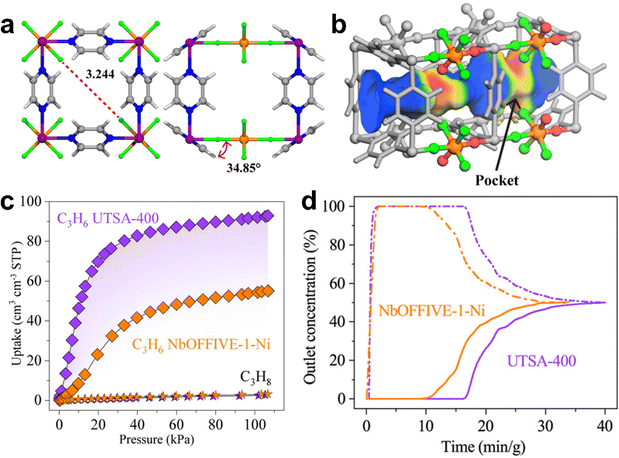 | ||
| Fig. 14 (a) Crystal structure of UTSA-400. (b) Connolly surface of UTSA-400 mapped with electrostatic potential with a probe of 1.2 Å. (c) Single-component adsorption isotherms of C3H6 and C3H8 for UTSA-400 and NbOFFIVE-1-Ni at 298 K. (d) Breakthrough curves for NbOFFIVE-1-Ni and UTSA-400 for an equimolar binary mixture of C3H6 (solid line)/C3H8 (dashed line) at 298 K and 1 bar. Reprinted with permission from ref. 151. Copyright 2023 American Chemical Society. | ||
In 2018, Li et al. studied four microporous MOFs, namely, Zr-bptc, Zr-abtc, Y-bptc, and Y-abtc, which were obtained by the combination of two analogous metal clusters (Zr6 and Y6 clusters) and two different ligands (abtc = 3,3′,5,5′-azobenzenetetracarboxylates, and bptc = 3,3′,5,5′-biphenyltetracarboxylates).152 Among these materials, Y-abtc has cage-like pores connected through small windows, exhibiting ftw topology and optimal pore size (4.72 Å) that enables it to adsorb small C3H6 molecules (4.48 Å) with fast kinetics but completely exclude larger C3H8 molecules (5.1 Å). Single-component sorption experiments revealed that Zr-bptc exhibits similar adsorption capacities for C3H6 and C3H8, whereas its isostructural Y-bptc excludes both gases, attributable to the presence of equilibrium cations (dimethylammonium) in the Y-bptc affecting its pore size. In contrast, Y-abtc with the optimal pore size shows a high adsorption capacity for C3H6 (∼2 mmol g−1) and a negligible C3H8 uptake at 298 K. Column breakthrough experiments indicated that polymer-grade C3H6 (99.5%) can be obtained from 5/95 C3H6/C3H8 mixtures by Y-abtc. Recently, the same group reported a new Y-based MOF, Y6(OH)8(eddi)3(DMA)2 (HIAM-301, H4eddi = 5,5′-(ethene-1,2-diyl)diisophthalic acid, and DMA = dimethylammonium), also exhibiting the molecular sieving separation of C3H6/C3H8 mixtures. HIAM-301 is composed of 12-connected Y6(OH)8(COO)12 clusters bridged by 4-connected eddi4− linkers to form a 3D framework with ftw topology and a pore size of 4.6 Å.153 It is isostructural to Y-abtc and possesses distorted cubic cages (size: 10 × 10 Å2), which provides better control over guest accessibility. Single-component adsorption isotherms revealed that HIAM-301 can adsorb 3.16 mmol g−1 C3H6 and exhibit minor C3H8 adsorption (<0.3 mmol g−1) at 298 K and 1 bar, resulting in a high IAST selectivity (>150) for equimolar C3H6/C3H8 under the same condition. The practical performance for separation of 5/95 C3H6/C3H8 was confirmed by dynamic breakthrough experiments with a high productivity of 46.4 cm3 g−1 (>99.5% purity).
In 2020, Chen et al. reported an ultramicroporous Co-gallate MOF [Co(C7O5H4)] (Co-gallate) with 3D channels, which exhibits efficient C3H6/C3H8 separation by molecular sieving effect.154 Co-gallate shows elliptical windows (size: 4.2 × 5.1 Å2), which are precisely between the sizes of C3H8 and C3H6 (Fig. 15). Single-component adsorption isotherms showed that Co-gallate has a high adsorption capacity of C3H6 (66.6 cm3 cm−3, 1.79 mmol g−1), whereas the adsorption of C3H8 (5.2 cm3 cm−3, 0.14 mmol g−1) is minor at 298 K. The IAST selectivity of Co-gallate for a 50/50 C3H6/C3H8 mixture was calculated up to 330 at 1 bar and 298 K. Fixed-bed breakthrough experiments further confirmed its molecular sieving separation performance for 50/50 C3H6/C3H8, with a C3H6 productivity of 36.4 cm3 cm−3, 0.98 mmol g−1 (97.7%+ purity).
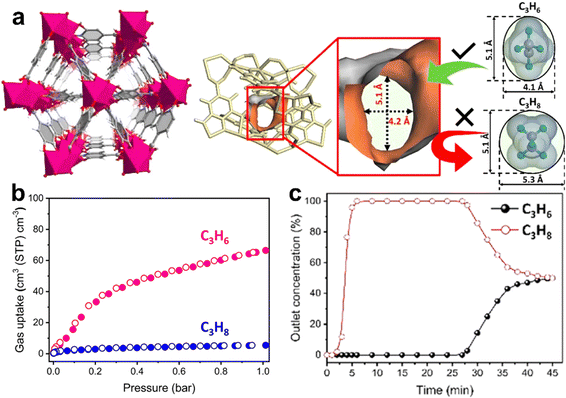 | ||
| Fig. 15 (a) Structure of the Co-gallate MOF and rationale for C3H6/C3H8 separation. (b) Gas sorption isotherms of propylene and propane at 298 K for Co-gallate. (c) The breakthrough experiments were carried out in a packed column. Reprinted with permission from ref. 154. Copyright 2020 American Chemical Society. | ||
In 2021, Li et al. reported a MOF (JNU-3a) featuring 1D channels (size ∼4.5 × 5.3 Å2) attached with small pockets arranged on both sides for C3H6/C3H8 separation, where 1D channels could facilitate fast adsorption–desorption kinetics (Fig. 16).155 In addition, the small pockets were connected with the 1D channel through small apertures (∼3.7 Å) that can undergo gate-opening for C3H6 and C3H8 at different partial pressures. JNU-3a exhibits a stepwise adsorption isotherm and shows temperature-dependent gate-opening for C3H6 and C3H8, where C3H8 exhibits a higher gate-opening pressure than C3H6. Therefore, the high selectivity of JNU-3a for C3H6/C3H8 can be obtained at optimal temperature (303 K). JNU-3a can adsorb 58.6 cm3 g−1 (2.62 mmol g−1) C3H6 at 303 K, and the C3H6 packing density inside JNU-3a was calculated to be 404 g L−1. The dynamic feature of pore aperture was further revealed by single-crystal X-ray diffraction and theoretical calculation studies. Breakthrough experiment revealed that high-purity C3H6 (≥99.5%) can be obtained from a 50/50 C3H6/C3H8 mixture by JNU-3a. After applying helium purge to those adsorbed gases, C3H6 was collected with a maximum productivity of 53.5 L kg−1. The unique pore structure reported in this work would inspire future design of novel MOFs for application in adsorptive separation.
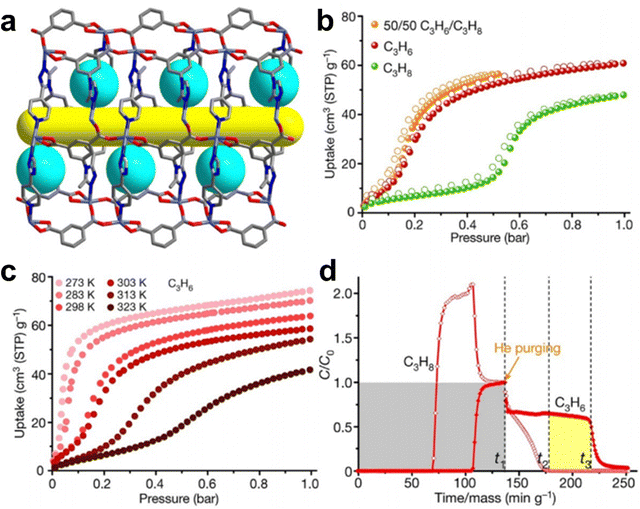 | ||
| Fig. 16 (a) Pore structure of JNU-3 viewed along the b axis showing the molecular pockets (turquoise) and 1D channels (yellow); (b) pure C3H8 (green), pure C3H6 (red), and an equimolar C3H6/C3H8 mixture (orange) adsorption/desorption isotherms of JNU-3a at 303 K; (c) C3H6 adsorption isotherms of JNU-3a at different temperatures; (d) breakthrough curves (starting at t = 0) of an equimolar C3H6/C3H8 mixture (1.0 mL min−1) on JNU-3a, followed by desorption curves (starting at t = t1) under helium gas (10.0 mL min−1) sweeping at 303 K. C3H8, open diamonds; C3H6, solid diamonds. C and C0 are the concentrations of each gas at the outlet and inlet, respectively. Grey area, mixed gas input; yellow area, C3H6 gas output; t1, the beginning of desorption; t2, starting point of collecting C3H6; t3, the end point of collecting C3H6. Reprinted with permission from ref. 155. Copyright 2021 Nature Publishing Group. | ||
In 2023, Bai et al. reported a supertetrahedral-cluster (Cu10O13)-based MOF (NTU-85) which possesses square-shaped 1D channels that host lattice water molecules.156 The partial lattice water molecules can be precisely removed to form a MOF (NTU-85-WNT) of pore surface decorated with water nanotubes (∼4.5 Å). Single-component sorption results of NTU-85-WNT indicated a rapid C3H6 uptake (20.9 mL mL−1, 0.45 mmol g−1), while the adsorption of C3H8 (0.13 mL mL−1, 0.003 mmol g−1) at 298 K can be neglected. The IAST selectivity of NTU-85-WNT for an equimolar C3H6/C3H8 mixture reaches up to 1570. Fixed-bed breakthrough experiments further confirmed its molecular sieving performance for an equimolar C3H6/C3H8 mixture, with a C3H6 productivity of 1.6 mL mL−1 (98.8%+ purity). It is worth noting that high purity C3H8 (>99.5%) can be obtained in one adsorption–desorption cycle due to the efficient sieving performance.
An optimal pore size combined with high-density binding sites can efficiently separate C3H6/C3H8, allowing for high-density stacking of C3H8 and maximizing its adsorption capacity. Recently, Chen et al. reported a robust Hofmann-type MOF, [Co(pyz-NH2)Ni(CN)4] (ZJU-75a, pyz-NH2 = 2-aminopyrazine), with high-density binding sites, showing excellent separation performance for C3H6/C3H8.157 ZJU-75a exhibits an isostructural framework with [Co(pyz)Ni(CN)4] (ZJU-74a, pyz = pyrazine), decorated with amino groups. ZJU-75a exhibits an appropriate pore size (4.1 × 4.4 Å2) and OMSs of high density (8.89 mmol cm−3). Single-component sorption isotherms showed that ZJU-75a and ZJU-74a exhibit comparable C3H6 uptakes of 104.3 cm−3 cm3 (3.31 mmol g−1) and 111.4 cm−3 cm3 (3.68 mmol g−1) at 1 bar and 296 K, respectively, whereas the C3H8 adsorption capacity of ZJU-75a (73.5 cm−3 cm3, 2.33 mmol g−1) is obviously smaller than that of ZJU-74a (103.6 cm−3 cm3, 3.42 mmol g−1). Thus, the IAST selectivity of ZJU-75a for C3H6/C3H8 was calculated to be 54.2 at 296 K and 1 bar, much higher than that of ZJU-74a (4.3). The breakthrough studies indicate that ZJU-75a can yield C3H6 of high-purity (99.5%+) with a productivity of 18.7 L kg−1 and separation factor of 14.7. Structural and computational studies indicated that high-density OMSs and nitrogen atoms (from [Ni(CN)4]2 units and -NH2 groups) in ZJU-75a contribute jointly to the strong adsorption of C3H6, thus resulting in a high C3H6 storage density (0.818 g mL−1).
C3H8-selective adsorptive separation would be a relatively simple and energy-efficient way to get pure propylene. However, the differences of the molecular size (∼0.5 Å) and polarizability (C3H6: 62.6 × 10−25, and C3H8: 62.9–63.7 × 10−25 cm3) between C3H6 and C3H8 are smaller than those between C2H4 and C2H6. So far, only a few C3H8-selective MOFs have been reported.169–180
In 2022, Xing et al. reported an ultramicroporous pillared layered MOF [Co(IPA)(DPG)]n (PCP-IPA, IPA = isophthalic acid, and DPG = meso-α,β-di(4-pyridyl)glycol) featuring 1D pores (size ∼4.7 × 5.6 Å2) and periodic parallel-aligned isophthalic acid units on the pore surface, exhibiting an excellent selectivity for C2H6 and C3H8.169 Although C2H6 and C3H8 have different molecular sizes, the suitable pore size of PCP-IPA facilitates their directional adsorption and maximizes the interaction between PCP-IPA and C3H8/C2H6. Therefore, PCP-IPA exhibits not only a C3H8/C3H6 (50/50) IAST selectivity of 2.48 but also a relatively high adsorption selectivity (2.80) for C2H6/C2H4 (50/50) at 1.0 bar and 298 K. Both C3H8 and C2H4 can be directly obtained with high purity (99.99%) through a fixed-bed column from C3H8/C3H6 (50/50) and C2H4/C2H6 (50/50) mixtures, respectively, affording a high C3H6 productivity (15.23 L kg−1) and excellent C2H4 productivity (26.2 L kg−1). Modeling simulation studies revealed that tighter and more multiple vdW interactions (C–H⋯O/C) can be formed between paraffins and PCP-IPA compared to olefins.
Very recently, Li et al. reported two isostructural MOFs (FDMOF-1 and FDMOF-2) functionalized with different amounts of fluorinated functional groups (–CF3), showing strong affinity for C3H8 over C3H6 (Fig. 17).170 Compared with the protype MOF Zn2(BDC)2(DABCO) (Zn-DMOF, BDC = 1,4-benzenedicarboxylate), the introduction of different amounts of –CF3 groups into a MOF not only increases its stability but also adjusts the pore size/shape. Therefore, FDMOF-2 with the maximal amount of –CF3 groups shows the smallest aperture (5.1 Å) and exhibits the optimal C3H8 affinity. Single-component sorption isotherms showed that FDMOF-2 displays a higher C3H8 uptake of 140 cm3 cm−3 (5.04 mmol g−1) but a lower C3H6 uptake of 115 cm3 cm−3 (4.14 mmol g−1), resulting in the IAST selectivity for 50/50 C3H8/C3H6 up to 2.18 at 298 K and 1 bar. Breakthrough experiments revealed that high purity (>99.99%) C3H6 can be directly produced from 50/50 C3H8/C3H6 mixtures, affording 0.501 mol L−1 production of C3H6. It is worth pointing out that the excellent separation performance of FDMOF-2 for C3H8/C3H6 can be maintained under 70% relative humidity conditions. Single-crystal X-ray diffraction and theoretical calculation studies confirmed that the strong affinity of UTSA-400 for C3H8 can be attributed to strong non-classical C–H⋯π/F hydrogen-bonding interactions, resulting in a stronger binding affinity for C3H8vs. C3H6 with an initial Qst value difference of −3.7 kJ mol−1.
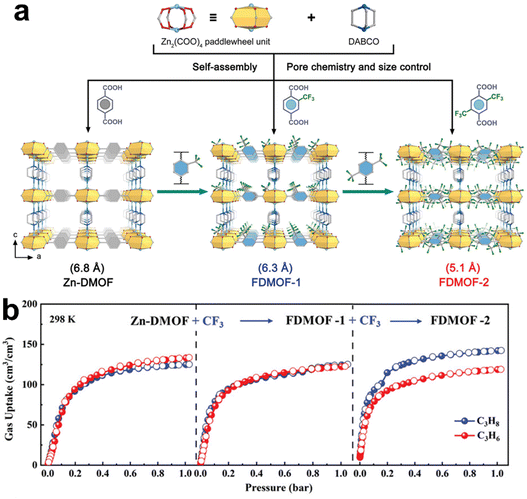 | ||
| Fig. 17 (a) Crystal structures of Zn-DMOF, FDMOF-1, and FDMOF-2. (b) Single-component gas adsorption isotherms of the samples at 298 K. Reprinted with permission from ref. 170. Copyright 2023 Wiley-VCH. | ||
2.6. C3H4/C3H6 separation
The removal of traces of propyne (C3H4 of ∼1%) from propylene is essential to obtain high purity propylene (C3H6). However, it is very difficult to separate trace amounts of C3H4 from C3H6, because their chemical/physical properties and molecular sizes (C3H4: 6.2 × 3.8 × 3.8 Å3; C3H6: 6.5 × 4.0 × 4.2 Å3) are highly similar. Nevertheless, some MOFs have been reported to show great potential for the separation of C3H4/C3H6.181–189In 2017, Chen et al. reported a flexible-robust MOF [Cu(bpy)2(OTf)2] (ELM-12, bpy = 4,4′-bipyridine, and OTf− = trifluoromethanesulfonate) which exhibits excellent performance for removing trace C3H4 from a 1/99 C3H4/C3H6 mixture. ELM-12 composed of 2D square-grid sheets with dynamic dangling OTf− groups exhibit two kinds of cavities of different shapes and sizes (6.1 × 4.3 × 4.3 Å3 and 6.8 × 4.0 × 4.2 Å3), which were comparable with the size and shape of C3H4 (6.2 × 3.8 × 3.8 Å3) (Fig. 18).181 Single-component adsorption isotherms revealed that ELM-12 shows a sharp increasing adsorption for C3H6 with an uptake of 1.83 mmol g−1 at 298 K and 1 bar, as well as a large Qst of 60.6 kJ mol−1 for C3H6, indicating its strong interaction with the C3H6 molecule. In contrast, both the uptake capacity (0.67 mmol g−1 at 0.1 bar and 298 K) and Qst (15.8 kJ mol−1) of ELM-12 for C3H8 are significantly lower. The multiple interactions, such as weak or non-classical C–D⋯O hydrogen bonds, between ELM-12 and C3D4 molecules were further confirmed by high-resolution neutron powder diffraction studies. Breakthrough experiments demonstrated that high purity (99.9998%) of C3H6 can be obtained from a 1/99 C3H4/C3H6 mixture.
 | ||
| Fig. 18 (a) Crystal structure of ELM-12. (b) C3H4 and C3H6 adsorption isotherms of ELM-12. Reprinted with permission from ref. 181. Copyright 2017 American Chemical Society. | ||
In 2018, Chen et al. studied a series of MOFs with different types of structures, functionalities, and pore sizes (Fig. 19).182 Besides for highly selective C2H2/C2H4 separation,70,71 SIFSIX-type MOFs such as SIFX-1-Cu, SIFSIX-2-Cu-i, SIFSIX-3-Ni, and UTSA-200 also exhibit strong binding affinities for C3H4 compared to C3H6 and show high C3H4 adsorption capacities at very low pressure. The former three SIFSIX-MOFs show slightly larger pore sizes than both C3H4 and C3H6, which lead to moderate C3H4/C3H6 selectivity. The activated UTSA-200 with the optimal pore size (3.4 Å) and strong binding sites has the best separation performance for C3H4/C3H6. Single-component adsorption isotherms revealed that UTSA-200 exhibits the highest C3H4 uptake capacity of 95 cm3 cm−3 (2.99 mmol g−1) at 0.01 bar and 298 K, while adsorbs negligible C3H6 (0.33 mmol g−1, at 298 K and 0.4 bar), resulting in an extremely high IAST selectivity of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 for 1/99 C3H4/C3H6 at 298 K and 1 bar. Breakthrough experiments demonstrated that high purity (99.9999%) C3H6 can be yielded from 1
000 for 1/99 C3H4/C3H6 at 298 K and 1 bar. Breakthrough experiments demonstrated that high purity (99.9999%) C3H6 can be yielded from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 and 0.1
99 and 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99.9 C3H4/C3H6 mixtures with a productivity of 62.9 mmol g−1 and 143.8 mmol g−1, respectively. Neutron powder diffraction studies and DFT-D calculations revealed that C3H4 molecules can open the pores of UTSA-200 and form strong C–H⋯F interactions with the framework.
99.9 C3H4/C3H6 mixtures with a productivity of 62.9 mmol g−1 and 143.8 mmol g−1, respectively. Neutron powder diffraction studies and DFT-D calculations revealed that C3H4 molecules can open the pores of UTSA-200 and form strong C–H⋯F interactions with the framework.
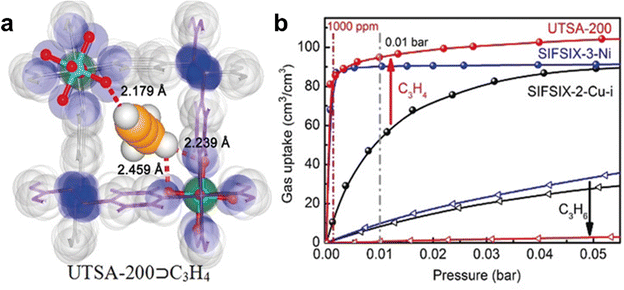 | ||
| Fig. 19 (a) DFT-D optimized structure and binding sites of UTSA-200⊃C3H4. (b) C3H4 and C3H6 adsorption isotherms of UTSA-200 at 298 K. Reprinted with permission from ref. 182. Copyright 2018 Wiley-VCH. | ||
In 2022, Xing et al. reported a flexible MOF GeFSIX-14-Cu-i (ZU-33, GeFSIX = hexafluorogermanate, and 14 = 4,4′- azopyridine), which exhibits guest/temperature-dependent structural dynamics and shows strong binding affinity towards acetylene and propadiene.188 ZU-33 featuring a 2-fold interpenetrated structure is composed of Cu(II)-4,4′-azobipyridne 2D layers and GeF62− pillars, and its pore size (3.08–5.04 Å) was dynamically adjustable by the rotational motion of GeF62− pillars and the organic ligands. Single-component gas adsorption experiments revealed that ZU-33 shows a steep uptake for alkynes (acetylene and propyne) and propadiene at low pressure (0.01 bar) and 303 K, while there is a threshold pressure for olefin adsorption and a size exclusion effect for alkanes. In addition, for C2H4 that is smaller than propyne and propadiene regarding the kinetic diameters, ZU-33 nearly inhibits C2H4 molecules to diffuse into its pores, suggesting the inverse size sieving below 0.5 bar at 303 K. Molecular simulations and single-crystal X-ray diffraction revealed that the interactions between the alkyne molecules (propyne and propadiene) and ZU-33 are more intense, thus requiring less input energy to overcome the energy barrier for the structural deformation. Breakthrough tests on a ZU-33 packed column verified that acetylene and propadiene can be directly removed from simulated cracking gases.
2.7. C4 hydrocarbon separation
C4 olefins including 1,3-butadiene (1,3-C4H6), 1-butene (n-C4H8) and isobutene (i-C4H8) are important raw materials for the production of synthetic rubbers and chemicals. However, C4 olefin separation represents one of the great challenges in hydrocarbon purification owing to the similar structures and physical properties.190,191Xing et al. reported several interpenetrated anion-pillared ultra-microporous MOFs, such as GeFSIX-2-Cu-i (ZU-32), NbFSIX-2-Cu-i (ZU-52) and GeFSIX-14-Cu-i (ZU-33), and realized highly efficient separation of C4 olefins through molecular recognition.192 This series of materials show ultrafine-tuning of the pore size/shape (4.20–4.83 Å) by replacing pillared inorganic anions with different bulks and different lengths of organic ligands. Among them, NbFSIX-2-Cu-i with a pore aperture size of 4.31 Å (F⋯F distance) exhibits high C4H6 (2.64 mmol g−1) and n-C4H8 (2.26 mmol g−1) uptake but negligible i-C4H8 uptake (0.48 mmol g−1), showing uptake selectivities of about 5.00 and 5.74 for n-C4H8/i-C4H8 and C4H6/i-C4H8, respectively. In contrast, GeFSIX-14-Cu-i shows a smaller pore size of 4.20 Å (F⋯F distance), resulting in negligible adsorption of n-C4H8 (0.57 mmol g−1) and i-C4H8 (0.42 mmol g−1), but still retaining large uptake for C4H6 (2.67 mmol g−1), accompanying a gate-opening adsorption behavior for C4H6. Breakthrough experiments demonstrated that these materials can efficiently separate C4H6 from C4H6/n-C4H8/i-C4H8/He (50/15/30/5) mixtures, as well as efficiently separate C4H6/n-C4H8 (50/50) and n-C4H8/i-C4H8 (50/50) mixtures.
Because of its high degree of unsaturation and strong coordination ability, 1,3-C4H6 is commonly adsorbed preferentially over other C4 hydrocarbons by MOFs with functional sites. It is thus energy-intensive and might induce undesired polymerization as the purification involves capture of 1,3-C4H6 and its subsequent release through heating in general. Zhang et al. noticed that the collaborative interactions between the host framework and guest flexibility can significantly change the conformation of certain C4 hydrocarbons and thus exhibit abnormal adsorption and reversed selectivity (Fig. 20).193 In particular, a hydrophilic pore of a MAF with free N atoms, namely, [Zn2(btm)]2 (MAF-23), with 1D quasi-discrete pores (aperture size 3.6 Å, and cage size ca. 6 Å) was employed for separation of four hydrocarbons, n-C4H8, i-C4H8, C4H10 and 1,3-C4H6. As revealed by experimental and DFT calculations, n-C4H8, the most similar one to 1,3-C4H6 among these gas molecules, adopts a metastable cis conformation to form stronger non-classical C–H⋯N interactions, and hence has a significantly stronger binding affinity, whereas 1,3-C4H6 adopts the stable trans conformation and has the weakest binding affinity because of the unfitting configuration for forming stronger non-classical hydrogen-bonding interactions. In other words, MAF-23 with a unique pore structure and surface serves as a guest conformation-controlling adsorbent to achieve preferential adsorption of n-C4H8, i-C4H8 and C4H10 over 1,3-C4H6. Therefore, 1,3-C4H6 can be first eluted during the breakthrough operation under ambient conditions, and directly purified for the desired purity (≥99.5%) to meet the industrial requirement in one single separation operation.
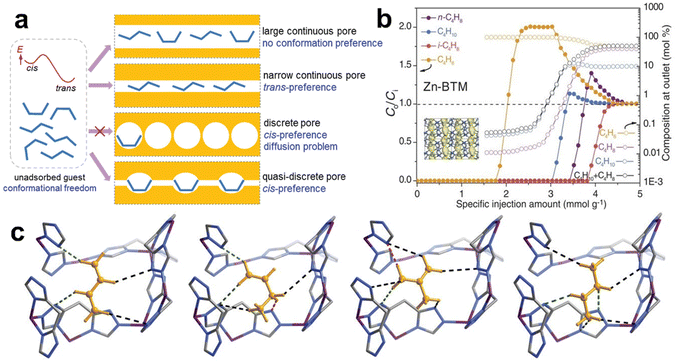 | ||
Fig. 20 (a) Schematic diagrams of controlling the guest conformation through the quasi-discrete pore in MAF-23. (b) Breakthrough curves of MAF-23 for a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 C4H6/n-C4H8/i-C4H8/C4H10 mixture. (c) Crystal structures of host–guest complexes of C4H6, n-C4H8, i-C4H8, and C4H10. Reprinted with permission from ref. 193. Copyright 2017 the American Association for the Advancement of Science. 1 C4H6/n-C4H8/i-C4H8/C4H10 mixture. (c) Crystal structures of host–guest complexes of C4H6, n-C4H8, i-C4H8, and C4H10. Reprinted with permission from ref. 193. Copyright 2017 the American Association for the Advancement of Science. | ||
The separation of C4 geometric isomers is also a challenging separation process, among which the separation of trans/cis-2-butene is of prime importance to increase the value of C4 olefins. Ren et al. reported that M-gallate (M = Ni, Mg, and Co), featuring oval-shaped pores, are ideally suitable for shape-selective separation of trans/cis-2-butene through their difference in the minimum molecular cross-section sizes, in addition to a highly efficient separation of 1,3-butadiene, 1-butene, and i-butene.194 Mg-gallate displays a narrow pore window size of 3.6 × 4.6 Å2, capturing the smaller trans-2-butene (3.5 × 4.6 Å2) while excluding the slightly larger cis-2-butene (3.6 × 4.9 Å2), affording a high trans/cis-2-butene uptake selectivity of 3.19 at 298 K and 1.0 bar in single-component adsorption isotherms. DFT-D study showed that Mg-gallate interacts with trans-2-butene and 1,3-butadiene through short distances of intermolecular C⋯H–O interactions (C⋯H distances 2.57–2.83 and 2.45–2.79 Å, respectively).
The separation of isomeric C4 paraffins is also an important task in the petrochemical industry. Zhong et al. regulated the pore aperture of the cage-like Zn-bzc by stepwise installation of methyl groups on its narrow aperture to achieve both molecular-sieving separation and a high n-C4H10 uptake.195 The resulting Zn-bzc-2CH3 is not only a new benchmark adsorbent featuring molecular sieving for n-C4H10/iso-C4H10 separation and a high n-C4H10 adsorption capacity (2.42 mmol g−1), but also hydrophobic to eliminate the negative effect of water vapor on gas separation under humid conditions. Breakthrough tests proved that high-purity i-C4H10 (99.99%) can be collected. The minimum energy path of n-C4H10 and i-C4H10 from cage to cage passing through the narrow aperture was determined for Zn-bzc-nCH3 (n = 0, 1, 2), and the results suggested easy transports of both n-C4H10 and i-C4H10 for the larger apertures of the parent Zn-bzc MOF and Zn-bzc-CH3 due to the low energy barrier together with a small barrier difference, while kinetic forbiddance with a dramatical increase of the diffusion energy barrier from 23.8 to 131.6 kJ mol−1 for n-C4H10 to migrate through Zn-bzc-2CH3.
2.8. Linear/branched alkane separation
The branched C5–C6 paraffins are major components in high-octane gasoline, which have relatively higher Research Octane Number (RON) values than their normal counterparts. To boost octane ratings in gasoline, the separation of linear alkanes from their branched isomers is very important in the petroleum industry, which is traditionally done by energy-intensive distillation processes.Eddaoudi et al. reported two new 12-connected rare-earth metal (Y3+ and Tb3+) fumarate based fcu-MOFs with both octahedral and tetrahedral cages that were solely interconnected with triangular windows of aperture size ca. 4.7 Å, and discovered that both of them can act as a adsorbate-size cut-off for the total sieving of C4 and C5 branched paraffins.196
In addition, Li et al. prepared two Zr-MOF compounds, Zr-bptc and Zr-abtc, which are highly stable with optimal pore structures for the separation of C6 alkane isomers (Fig. 21).197 For instance, Zr-abtc featuring an scu-type structure with 1D channels (d = 7 Å) accommodates all C6 alkane isomers (n-hexane, 3-methylpentane and 2,3-dimethylbutane), but favors n-hexane because of its stronger interactions with the pore surface, resulting in a mono/dibranched separation factor (∼1.3) in the breakthrough test.
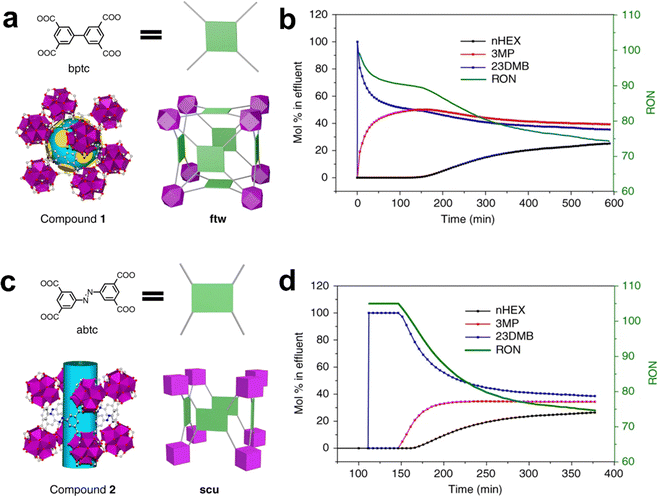 | ||
Fig. 21 (a) and (c) Organic ligands, crystal structures, and topologies of Zr-bptc and Zr-abtc. (b) and (d) Breakthrough curves of an equimolar ternary mixture of C6 alkane isomers at 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for Zr-bptc (top) and Zr-abtc (bottom). The green curve represents the real-time RON of the eluted products. Reprinted with permission from ref. 197. Copyright 2018 Nature Publishing Group. °C for Zr-bptc (top) and Zr-abtc (bottom). The green curve represents the real-time RON of the eluted products. Reprinted with permission from ref. 197. Copyright 2018 Nature Publishing Group. | ||
In 2020, Li et al. reported a new flexible MOF, calcium chloranilate (HIAM-203), which specially possesses chloro-decorated 1D channels.198 HIAM-203 exhibits structural flexibility upon adsorption of C6 alkanes with different branching, as well as similar temperature-dependent adsorption behavior toward alkane isomers. It can take up a plentiful amount of n-hexane and 3-methylpentane at 30 °C, but completely excludes 2,2-dimethylbutane, while at 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C 3-methylpentane is also excluded. This phenomenon may be rationalized by the significant difference in binding affinity near the pore aperture of HIAM-203. As the pore size of HIAM-203 (5.6 Å) is between the kinetic diameters of 3-methylpentane (5.5 Å) and 2,2-dimethylbutane (6.2 Å), it thus inhibits the diffusion of 2,2-dimethylbutane.
°C 3-methylpentane is also excluded. This phenomenon may be rationalized by the significant difference in binding affinity near the pore aperture of HIAM-203. As the pore size of HIAM-203 (5.6 Å) is between the kinetic diameters of 3-methylpentane (5.5 Å) and 2,2-dimethylbutane (6.2 Å), it thus inhibits the diffusion of 2,2-dimethylbutane.
In 2023, Bao et al. employed the Hofmann-type MOFs, [M(pz)Ni(CN)4] (M = Co and Ni) to demonstrate similar temperature-swing molecular exclusion for separation of hexane isomers.199 CopzNi displays excellent separation efficiency for linear/mono-branched and mono-branched/di-branched alkanes with the highest adsorption capacity to date. CopzNi exhibits lower-energy regeneration, scalability, recyclability and high stability, representing a promising candidate for relevant separation processes.
There are also needs for purification of valuable C5-C7 olefins. Isoprene, which accounts for 15–25% of C5 fractions including important olefins like 1-pentene and trans-2-pentene, is widely used in the production of synthetic rubber, pharmaceutical and pesticide intermediates.200 The regeneration and desorption efficiency should also be taken into account for separation.201 Cui et al. found that anion-pillared hybrid porous materials, namely, ZU-62 (also termed NbOFFIVE-2-Cu-i) and TIFSIX-2-Cu-i, exhibit a good separation performance for C5 olefin mixtures (trans-2-pentene, 1-pentene and isoprene).202 Owing to a contraction of pore aperture originating from the rotation of 4,4′-dipyridylacetylene ligand, ZU-62 excludes the relatively large molecule of isoprene in the low pressure range (0–6 kPa), while TIFSIX-2-Cu-i is able to distinguish the three C5 olefins with a high uptake of trans-2-pentene (3.1 mmol·g−1), superior to that of zeolite 5A (2.0 mmol·g−1). DFT-D calculations confirmed that the selective adsorption was achieved by a favorable electrostatic environment as well as suitable pore confinement. Also, the regeneration tests showed that C5 olefins can be easily desorbed from ZU-62 and TIFSIX-2-Cu-i at 298 K.
2.9. Aromatic isomer separation
The separation of C8 aromatic compounds (xylene isomers, ethylbenzene and styrene) is also one of the important separations.1 Tremendous efforts have been devoted to developing MOF adsorbents for relevant separations. Most of these adsorbents contain aromatic organic ligands, in which the phenyl or heterocycle rings allow strong π–π stacking interactions with C8 aromatic molecules to facilitate the adsorption.203,204MOFs featuring structural flexibility like breathing or gate-opening have been applied for separation of aromatic isomers.18,37,205 Li et al. reported that a stacked 1D manganese-based MOF [Mn(dhbq)(H2O)2] (Mn-dhbq, H2dhbq = 2,5-dihydroxy-1,4-benzoquinone) exhibits temperature-dependent discriminative adsorption of xylene isomers owing to reversible framework swelling.206 At 363 K, pX can be fully intercepted from the pX/mX/oX ternary mixture by a column of Mn-dhbq, whereas the effluent of the mX/oX mixture can be further separated by another column of Mn-dhbq at a lower temperature (303 K) where mainly oX finally flows out. The purity of pX is over 97% collected during the desorption cycle when using 1,4-diethylbenzene as an eluent at 433 K for 1 hour after liquid-phase adsorption of a quaternary mixture (pX/mX/oX/EB, 22/22/50/6) at 393 K. DFT calculations indicated that the temperature-dependent flexibility between 1D coordination chains (different degrees of swelling at different temperatures) endows such selective adsorption of xylene isomers through π–π stacking interactions with the aromatic ligand.
Xing et al. reported a flexible anion pillared MOF [Ni(bpy)2(NbOF5)] (ZU-61) with pcu topology and a pore size of 7.8 Å exhibiting efficient separation performance for xylene isomer.207 Single-component adsorption revealed that ZU-61 shows a higher low-pressure uptake for mX and oX than pX at 0.01 bar and 333 K, as well as a high capacity for mX (3.4 mmol g−1) and oX (3.2 mmol g−1). Breakthrough experiments for a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 pX/mX/oX mixture confirmed that high purity (>99.9%) pX can elute from the column first, and then followed by mX and oX. The excellent performance for xylene isomer separation can be attributed to the rotational NbOF52− anions, which allows adaptive host–guest interaction (C–H⋯F interactions) depending on the shape of the xylene isomer, as confirmed by DFT calculations and crystallographic studies. These results showed that ZU-61 exhibits great potential for the purification of pX.
1 pX/mX/oX mixture confirmed that high purity (>99.9%) pX can elute from the column first, and then followed by mX and oX. The excellent performance for xylene isomer separation can be attributed to the rotational NbOF52− anions, which allows adaptive host–guest interaction (C–H⋯F interactions) depending on the shape of the xylene isomer, as confirmed by DFT calculations and crystallographic studies. These results showed that ZU-61 exhibits great potential for the purification of pX.
Given that all xylene isomers contain phenyl rings for π–π stacking interactions, another approach to enhance the separation selectivity would be utilizing the difference of their alkyl groups while inhibiting the interactions with phenyl rings. Yang et al. constructed a pillar-layered MOF Cu(bpdc)(ted)0.5 (ZUL-C3) by using poly-cycloalkane-type ligands to show a non-aromatic pore environment for xylene separation.208 The pore space of the MOF magnifies the difference of host–guest interactions with xylene isomers and ethylbenzene owing to their different distribution of methyl (ethyl) groups. Liquid-phase batch experiment indicated that this MOF can separate xylene isomers and ethylbenzene from each other, showing separation potential for oX/pX and oX/mX separation.
A flexible MOF, [Cu2(fbdim)]·p-xylene (MAF-41·pX), showing a 3D hinged-fence-like framework and 1D channels (4.2 × 9.8 × 10.1 Å3 cavities and 3.9 × 6.7 Å2 apertures), was demonstrated by Zhang et al. to exhibit unprecedented inversed molecular sieving (so called intermediate-sized molecular sieving) for the purification of styrene (Fig. 22).209 The framework structure can transform to a nonporous one upon removal of template molecules. The activated MAF-41 can adsorb styrene (ST) to restore the as-synthesized structure while totally excluding ethylbenzene (EB), toluene (Tol), and benzene (Bz). Styrene with a purity of 99.9%+ can be obtained from the multicomponent mixture after one single adsorption–desorption cycle because the pores are individually opened and simultaneously occupied by the target guest. The aperiodic pore opening is believed to avoid co-adsorption of guest molecules smaller than the opened pores. Kinetic sorption studies revealed that the EB/Tol/Bz adsorptions stayed quite low all the time, while the ST uptake of MAF-41 significantly increased for both the single-component and mixtures, which confirms no EB/Tol/Bz co-adsorption or replacement of ST in MAF-41. Although Cu(I) complexes and Cu(I)-based MOFs may have poor stability in air and/or water, guest-free MAF-41 did not collapse not only at temperatures up to 500 °C, but also in boiling water for at least one week, and even remained stable in a solution of pH 3–14 at room temperature for at least 3 days.
 | ||
Fig. 22 (a) Crystal structure of guest-free MAF-41. (b) Adsorption isotherms of activated MAF-41 for ST (styrene, 298![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K), EB (ethylbenzene, 298 K), EB (ethylbenzene, 298![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K), Tol (toluene, 298 K), Tol (toluene, 298![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K), Bz (benzene, 298 K), Bz (benzene, 298![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K), N2 (77 K), N2 (77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K), and CO2 (195 K). Reprinted with permission from ref. 209. Copyright 2019 Nature Publishing Group. K), and CO2 (195 K). Reprinted with permission from ref. 209. Copyright 2019 Nature Publishing Group. | ||
In 2022, Li et al. reported a family of double-walled metal-dipyrazolate frameworks (BUT-53 to -58) composed of divalent metal ions and dipyrazolate ligands with different symmetries, lengths, and functionalities for trace benzene removal (Fig. 23).210 In this study, multiple merits, e.g., high stability, tunable pore, high adsorption capacity and selectivity, were integrated into these hydrophobic MOFs, which all exhibited high benzene uptakes (2.47–3.28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mmol
mmol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) g−1) at room temperature and ultra-low pressures (<10
g−1) at room temperature and ultra-low pressures (<10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pa). BUT-55 is the best-performing adsorbent for the capture of trace benzene among them. It shows an extremely long breakthrough time of ∼8000 h g−1 for a benzene-containing (10 ppm, 10 mL min−1) gas mixture under both dry and humid (relative humidity = 50%) conditions. When the relative humidity was 80%, the breakthrough time decreased to ∼6000 h g−1, which is still much higher than that of other previously reported materials. After adsorption of benzene, BUT-55 can be regenerated under mild heating. As revealed by both the single-crystal structure of benzene-loaded BUT-55 and DFT calculations, multiple non-classical hydrogen-bonding C–H⋯X (X = N, O, π) interactions are the key to its strong affinity and high sensitivity toward benzene. The high benzene binding energy of BUT-55 contributes to its high adsorption selectivity of benzene over water or other volatile organic compounds (VOCs), such as cyclohexane and ethanol. It should also be pointed out that the BUT-55 sample is able to work continuously around one year under the breakthrough experiment conditions. In other words, a certain amount of BUT-55 can be used to capture trace airborne benzene for a long time due to its high benzene adsorption capacity under ultra-low pressures and high adsorption selectivity. The performance of these MOFs demonstrates high potential in the removal of benzene from ambient air.
Pa). BUT-55 is the best-performing adsorbent for the capture of trace benzene among them. It shows an extremely long breakthrough time of ∼8000 h g−1 for a benzene-containing (10 ppm, 10 mL min−1) gas mixture under both dry and humid (relative humidity = 50%) conditions. When the relative humidity was 80%, the breakthrough time decreased to ∼6000 h g−1, which is still much higher than that of other previously reported materials. After adsorption of benzene, BUT-55 can be regenerated under mild heating. As revealed by both the single-crystal structure of benzene-loaded BUT-55 and DFT calculations, multiple non-classical hydrogen-bonding C–H⋯X (X = N, O, π) interactions are the key to its strong affinity and high sensitivity toward benzene. The high benzene binding energy of BUT-55 contributes to its high adsorption selectivity of benzene over water or other volatile organic compounds (VOCs), such as cyclohexane and ethanol. It should also be pointed out that the BUT-55 sample is able to work continuously around one year under the breakthrough experiment conditions. In other words, a certain amount of BUT-55 can be used to capture trace airborne benzene for a long time due to its high benzene adsorption capacity under ultra-low pressures and high adsorption selectivity. The performance of these MOFs demonstrates high potential in the removal of benzene from ambient air.
 | ||
| Fig. 23 (a) Structures of C6H6@BUT-55 and benzene–benzene interactions. (b) Logarithmic-scale plots of P/P0 to view the benzene adsorption of BUT-53 to BUT-58 at low partial pressure. Reprinted with permission from ref. 210. Copyright 2022 Nature Publishing Group. | ||
Very recently, Mo et al. reported a flexible MOF, [Sr2(BINDI)(H2O)2)] (WYU-62, H4BINDI = N,N′-bis(5-isophthalic acid)-naphthalenediimide) with electron-deficient NDI cores, which shows fast adsorption of trace benzene vapor at low pressure, accompanying a fluorescence-enhanced sensing (limit of detection = 0.133 mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L−1).211 WYU-62 could be easily obtained by immersing the as-synthesized MOF [Sr2(BINDI)(DMF)(H2O)] (MYU-61) in water, during which discrete 0D pores of MYU-61 have transformed to 1D channels (aperture size = 5.1 × 9.1 Å2). Aromatic hydrocarbon vapor adsorption isotherms revealed that WYU-62a can show high benzene uptake at very low pressure (P/P0 < 0.01). DFT calculations and crystallographic studies indicated that electron-rich benzene was tightly wrapped between the two electron-deficient NDI moieties with strong π–π and C−H⋯π interactions. Therefore, it has excellent benzene adsorption capacity, whereas the accompanying host–guest charge transfer enables visual detection of trace benzene vapor.
L−1).211 WYU-62 could be easily obtained by immersing the as-synthesized MOF [Sr2(BINDI)(DMF)(H2O)] (MYU-61) in water, during which discrete 0D pores of MYU-61 have transformed to 1D channels (aperture size = 5.1 × 9.1 Å2). Aromatic hydrocarbon vapor adsorption isotherms revealed that WYU-62a can show high benzene uptake at very low pressure (P/P0 < 0.01). DFT calculations and crystallographic studies indicated that electron-rich benzene was tightly wrapped between the two electron-deficient NDI moieties with strong π–π and C−H⋯π interactions. Therefore, it has excellent benzene adsorption capacity, whereas the accompanying host–guest charge transfer enables visual detection of trace benzene vapor.
3. MOF-based membranes for separating gaseous hydrocarbons
Though a variety of MOFs have been extensively studied for light-hydrocarbon purification in recent two decades, only a few have been fabricated into MOF-based mixed matrix membranes (MMMs), which may integrate the advantages of the separation performance of MOFs and processability of polymers for olefin/paraffin separation.212 For example, MAF-4 (or ZIF-8) and Zr-fum-fcu-MOF (or MOF-801) have been incorporated with membranes for C3H6/C3H8 separation.213,214Modification of MOF membranes with the composite ionic liquid/Ag+ (IL/Ag+) can efficiently improve their olefin/paraffin separation properties. Yang et al. synthesized layered Zn2(bim)4 membranes215 using the in situ interfacial assembly (ISIA)216 method and further modified them with varied IL/Ag+ composites.217 Adsorption isotherms for Zn2(bim)4 powder and its IL/Ag+ modified sample showed that the adsorption capacity for olefin can significantly improve after modification with IL/Ag+ but the adsorption for paraffin shows negligible changes, which is attributed to the strong π-complexation of olefins with Ag+. Further study revealed that the pristine Zn2(bim)4 membrane showed a C3H6/C3H8 separation factor of 1.14 with a C3H6 permeance of 106 GPU (SFC2H4/C2H6: 1.42, and PC2H4: 311) (Fig. 24). In contrast, the modified membranes exhibited a significant improvement in olefin/paraffin separation with the optimized C3H6/C3H8 separation factor of 28.8 ± 3.8 with the C3H6 permeance of 129.8 ± 40.4 GPU (SFC2H4/C2H6: 12.0 ± 2.2, and PC2H4: 45.6 ± 23.7 GPU). The preferential binding affinity of Ag+ in IL for olefins facilitates the delivery of olefins in the modified Zn2(bim)4 membranes. On the other hand, Ag+ filling in the space of laminated modified Zn2(bim)4 membranes further blocks paraffin penetration and thus improves the olefin/paraffin selectivity. In addition to the composite modification membrane strategy, intrinsic structural regulation of MOF membranes is also crucial. Recently, a series of Zn2(bim)4 membranes functionalized with different amounts of amino groups, namely, Nx-Zn2(bim)4(x represents the molar ratio of raw 5-aminobenzimidazole, bim) were also investigated for separation.218 Among them, the N10-Zn2(bim)4 membrane exhibits the highest H2/CO2 separation performance with a separation factor of 1158 and a H2 permeance of 1417 GPU. Moreover, its ideal selectivities for H2/CO2, H2/CH4 and H2/C3H8 were 829, 99, and 33, respectively, showing a distinct size exclusion effect for relatively large molecules.
 | ||
| Fig. 24 (a) C3H6/C3H8 separation performance of Zn2(bim)4 membranes modified by diversified composites, IL/Ag+(8-0), IL/Ag+(8-1), IL/Ag+(8-2) and IL/Ag+(8-5). (b) C2H4/C2H6 separation performance of Zn2(bim)4 membranes modified by diversified composites, IL/Ag+(8-1), IL/Ag+(8-2) and IL/Ag+(8-5). Reprinted with permission from ref. 217. Copyright 2021 Elsevier. | ||
Long et al. fabricated a series of membranes with M2(dobdc) (M = Co, Ni, Mg, and Mn) as fillers and 6FDA-DAM as the primary polymer by reducing the filler size to the sub-100 nm level to specially improve the filler-polymer compatibility and dispersion.219 Among them, Ni2(dobdc) showed the best C2H4/C2H6 separation performance without reduction in selectivity even under high-pressure owing to the presence of strong and multiple nanocrystal-polymer interactions, which suppresses plasticization by reducing polymer chain mobility.
Polycrystalline MOF membranes are less explored but also important. Liu et al.220 have recently prepared a polycrystalline Co-gallate membrane with a reported freezing contra-diffusion protocol221 that can enable more accurate control over the reaction kinetics between metal ions and ligands to effectively tune the MOF nucleation and growth in the bulk solution. Maintaining the frozen state of the metal precursor inside the macroporous α-Al2O3 substrate resulted in the formation of the preferred c-oriented and well-intergrown Co-gallate membrane with not only abundant open metal sites (at the increased missing-linker defects), but also reduced the thickness of the membrane. The multiscale structure endows the Co-gallate membrane with an outstanding C2H4/C2H6 separation performance superior to those of state-of-the-art membranes including polycrystalline MOF membranes, MOF-based MMMs, and polymeric membranes. The optimized C2H4/C2H6 selectivity is 8.3 with a C2H6 permeance of 72.6 GPU. The resultant membrane also shows a negative correlation with the operation temperature and high long-term stability.
4. Conclusion and outlook
MOF materials have made significant progress in the separation of gaseous hydrocarbons in the past few years, which was achieved by rational control over their pore chemistry/size, affording an alternative separation technology for relevant applications. The high crystallinity of MOFs facilitates systematic and deep investigation on the exact adsorbent–adsorbate interactions in the type of porous materials for high separation performance, as demonstrated by their crystallographic studies on the host–guest interactions. Controlling of the pore structure of MOF materials at high-accuracy level is applicable due to the high modularity and diverse functionality of MOFs, which facilitates highly efficient separation of methane, olefin/paraffin, alkyne/olefin, alkane isomers and aromatics. As outlined in this review, the collaborative control of the pore size and pore surface has been explored for the potential of MOFs for hydrocarbon separation. MOFs with high separation selectivities and large gas uptakes have been documented. The development of this realm has gradually turned to evaluate the potential of MOFs in practical separation processes, which required continuous research endeavors.There are many important issues remaining unaddressed before further industrial and commercial usage of MOFs. Shaping/pelleting of MOFs would be the first step in actual processes given engineering considerations, but that would lead to loss of adsorption capacity; thus, further study on mechanical stabilities of MOFs might be necessary. Simultaneously, the diffusion kinetics of MOF pellets should be comprehensively investigated. There are also concerns on current research for evaluations of their separation performances through swing adsorption or membrane separation processes, as these evaluations mainly focus on evaluating candidate materials under simulated conditions rather than the actual applications. In relevant simulated evaluations, the presence of contaminants such as water has gradually got involved. In contrast, the actual gas streams would be more complicated, while the separation condition can be even harsher (under elevated pressures/temperatures), which require the adsorbents to show good stability and durability, including good water resistance and impurity tolerance. The long-term durability and regeneration of MOFs during separation processes have been rarely involved in current research. Moreover, systematic evaluation of materials production, including capital and operating costs, should be performed prior to large-scale deployments of MOF-based adsorptive separation technology. Solvent-free and continuous synthesis methods have been demonstrated to be applicable for MOFs, which show great potential for large-scale production of MOF materials. In addition, new technical issues such as thermal management during the adsorption process would come out when sorption is scaled up. For membrane-based separation, there are also several challenges although it is capable of direct production of pure gas and can be simply operated under mild conditions. Several mixed-matrix MOF-based membranes have been developed, which show large permeance differences for hydrocarbon separation. However, the processability, defect, compatibility, and scaling up in membrane fabrication as well as the permeability-selectivity trade-off are still quite challenging. In terms of membrane separation, molecular sieving of hydrocarbons is still highly desirable. As the structure numbers of MOFs keep growing, there are also emerging technologies like machine learning under artificial intelligence for the computational design and discovery of novel MOF materials, while automated chemistry for high-throughput screening is also possible by combination with robotics.
As promising adsorbent materials for hydrocarbon separation, MOFs with high separation performance can be synthesized by combination of rational control of their pore size and pore surface. Continuous collaborative efforts among scientists, engineers and industrial partners will promote the application of MOF adsorbents to scientifically and technologically important industrial hydrocarbon separation, which would reap great benefits for society.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge supports from the National Natural Science Foundation of China (22090061, 22101307), the Hundred Talents Program of Sun Yat-Sen University and the Fundamental Research Funds for the Central Universities (Sun Yat-Sen University, 22qntd2301).Notes and references
- D. S. Sholl and R. P. Lively, Seven chemical separations to change the world, Nature, 2016, 532, 435–437 CrossRef.
- S. Chu, Y. Cui and N. Liu, The path towards sustainable energy, Nat. Mater., 2017, 16, 16–22 CrossRef.
- H. Furukawa, K. E. Cordova, M. O’Keeffe and O. M. Yaghi, The Chemistry and Applications of Metal-Organic Frameworks, Science, 2013, 341, 1230444 CrossRef PubMed.
- S. Kitagawa, R. Kitaura and S. I. Noro, Functional Porous Coordination Polymers, Angew. Chem., Int. Ed., 2004, 43, 2334–2375 CrossRef CAS PubMed.
- B. Chen, S. Xiang and G. Qian, Metal-Organic Frameworks with Functional Pores for Recognition of Small Molecules, Acc. Chem. Res., 2010, 43, 1115–1124 CrossRef CAS.
- Y. Cui, B. Li, H. He, W. Zhou, B. Chen and G. Qian, Metal-Organic Frameworks as Platforms for Functional Materials, Acc. Chem. Res., 2016, 49, 483–493 CrossRef CAS PubMed.
- I. M. Hönicke, I. Senkovska, V. Bon, I. A. Baburin, N. Bönisch, S. Raschke, J. D. Evans and S. Kaskel, Balancing Mechanical Stability and Ultrahigh Porosity in Crystalline Framework Materials, Angew. Chem., Int. Ed., 2018, 57, 13780–13783 CrossRef.
- Z.-S. Wang, M. Li, Y.-L. Peng, Z. Zhang, W. Chen and X.-C. Huang, An Ultrastable Metal Azolate Framework with Binding Pockets for Optimal Carbon Dioxide Capture, Angew. Chem., Int. Ed., 2019, 58, 16071–16076 CrossRef CAS PubMed.
- R.-B. Lin, S. Xiang, B. Li, Y. Cui, G. Qian, W. Zhou and B. Chen, Our journey of developing multifunctional metal-organic frameworks, Coord. Chem. Rev., 2019, 384, 21–36 CrossRef CAS.
- R.-B. Lin, S. Xiang, H. Xing, W. Zhou and B. Chen, Exploration of porous metal-organic frameworks for gas separation and purification, Coord. Chem. Rev., 2019, 378, 87–103 CrossRef CAS.
- K. Adil, Y. Belmabkhout, R. S. Pillai, A. Cadiau, P. M. Bhatt, A. H. Assen, G. Maurin, M. Eddaoudi and K. Adil, Gas/vapour separation using ultra-microporous metal-organic frameworks: insights into the structure/separation relationship, Chem. Soc. Rev., 2017, 46, 3402–3430 RSC.
- J.-R. Li, R. J. Kuppler, H.-C. Zhou and J.-R. Li, Selective gas adsorption and separation in metal-organic frameworks, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC.
- R.-B. Lin, S. Xiang, W. Zhou and B. Chen, Microporous Metal-Organic Framework Materials for Gas Separation, Chem, 2020, 6, 337–363 CAS.
- R.-B. Lin, Z. Zhang and B. Chen, Achieving High Performance Metal-Organic Framework Materials through Pore Engineering, Acc. Chem. Res., 2021, 54, 3362–3376 CrossRef CAS PubMed.
- H. Wang and J. Li, Microporous Metal-Organic Frameworks for Adsorptive Separation of C5–C6 Alkane Isomers, Acc. Chem. Res., 2019, 52, 1968–1978 CrossRef CAS PubMed.
- H. Wang, Y. Liu and J. Li, Designer Metal-Organic Frameworks for Size-Exclusion-Based Hydrocarbon Separations: Progress and Challenges, Adv. Mater., 2020, 32, 2002603 CrossRef CAS PubMed.
- F. Xie, L. Yu, H. Wang and J. Li, Metal-Organic Frameworks for C6 Alkane Separation, Angew. Chem., Int. Ed., 2023, 62, e202300722 CrossRef CAS.
- D.-D. Zhou and J.-P. Zhang, On the Role of Flexibility for Adsorptive Separation, Acc. Chem. Res., 2022, 55, 2966–2977 CrossRef CAS.
- J.-P. Zhang, Y.-B. Zhang, J.-B. Lin and X.-M. Chen, Metal Azolate Frameworks: From Crystal Engineering to Functional Materials, Chem. Rev., 2011, 112, 1001–1033 CrossRef.
- Y. Wang and D. Zhao, Beyond Equilibrium: Metal-Organic Frameworks for Molecular Sieving and Kinetic Gas Separation, Cryst. Growth Des., 2017, 17, 2291–2308 CrossRef CAS.
- J.-P. Zhang, H.-L. Zhou, D.-D. Zhou, P.-Q. Liao and X.-M. Chen, Controlling flexibility of metal-organic frameworks, Natl. Sci. Rev., 2018, 5, 907–919 CrossRef CAS.
- I. Karakurt, G. Aydin and K. Aydiner, Mine ventilation air methane as a sustainable energy source, Renewable Sustainable Energy Rev., 2011, 15, 1042–1049 CrossRef CAS.
- Z. Niu, X. Cui, T. Pham, P. C. Lan, H. Xing, K. A. Forrest, L. Wojtas, B. Space and S. Ma, A Metal-Organic Framework Based Methane Nano-trap for the Capture of Coal-Mine Methane, Angew. Chem., Int. Ed., 2019, 58, 10138–10141 CrossRef CAS.
- S.-M. Wang, M. Shivanna and Q.-Y. Yang, Nickel-Based Metal-Organic Frameworks for Coal-Bed Methane Purification with Record CH4/N2 Selectivity, Angew. Chem., Int. Ed., 2022, 61, e202201017 CrossRef CAS PubMed.
- T. Li, X. Jia, H. Chen, Z. Chang, L. Li, Y. Wang and J. Li, Tuning the Pore Environment of MOFs toward Efficient CH4/N2 Separation under Humid Conditions, ACS Appl. Mater. Interfaces, 2022, 14, 15830–15839 CrossRef CAS PubMed.
- M. Chang, Y. Zhao, D. Liu, J. Yang, J. Li and C. Zhong, Methane-trapping metal-organic frameworks with an aliphatic ligand for efficient CH4/N2 separation, Sustainable Energy Fuels, 2019, 4, 138–142 RSC.
- X.-W. Zhang, D.-D. Zhou and J.-P. Zhang, Tuning the gating energy barrier of metal-organic framework for molecular sieving, Chem, 2021, 7, 1006–1019 CAS.
- Q. Min Wang, D. Shen, M. Bülow, M. Ling Lau, S. Deng, F. R. Fitch, N. O. Lemcoff and J. Semanscin, Metallo-organic molecular sieve for gas separation and purification, Microporous Mesoporous Mater., 2002, 55, 217–230 CrossRef.
- Z. Bao, S. Alnemrat, L. Yu, I. Vasiliev, Q. Ren, X. Lu and S. Deng, Adsorption of Ethane, Ethylene, Propane, and Propylene on a Magnesium-Based Metal-Organic Framework, Langmuir, 2011, 27, 13554–13562 CrossRef CAS PubMed.
- E. D. Bloch, W. L. Queen, R. Krishna, J. M. Zadrozny, C. M. Brown and J. R. Long, Hydrocarbon Separations in a Metal-Organic Framework with Open Iron(II) Coordination Sites, Science, 2012, 335, 1606–1610 CrossRef CAS.
- S. J. Geier, J. A. Mason, E. D. Bloch, W. L. Queen, M. R. Hudson, C. M. Brown and J. R. Long, Selective adsorption of ethylene over ethane and propylene over propane in the metal-organic frameworks M2(dobdc) (M = Mg, Mn, Fe, Co, Ni, Zn), Chem. Sci., 2013, 4, 2054 RSC.
- G. Chang, M. Huang, Y. Su, H. Xing, B. Su, Z. Zhang, Q. Yang, Y. Yang, Q. Ren, Z. Bao and B. Chen, Immobilization of Ag(I) into a metal-organic framework with –SO3H sites for highly selective olefin–paraffin separation at room temperature, Chem. Commun., 2015, 51, 2859–2862 RSC.
- J. E. Bachman, M. T. Kapelewski, D. A. Reed, M. I. Gonzalez and J. R. Long, M2(m-dobdc) (M = Mn, Fe, Co, Ni) Metal-Organic Frameworks as Highly Selective, High-Capacity Adsorbents for Olefin/Paraffin Separations, J. Am. Chem. Soc., 2017, 139, 15363–15370 CrossRef CAS PubMed.
- S. Sen, N. Hosono, J.-J. Zheng, S. Kusaka, R. Matsuda, S. Sakaki and S. Kitagawa, Cooperative Bond Scission in a Soft Porous Crystal Enables Discriminatory Gate Opening for Ethylene over Ethane, J. Am. Chem. Soc., 2017, 139, 18313–18321 CrossRef CAS.
- R.-B. Lin, L. Li, H.-L. Zhou, H. Wu, C. He, S. Li, R. Krishna, J. Li, W. Zhou and B. Chen, Molecular sieving of ethylene from ethane using a rigid metal-organic framework, Nat. Mater., 2018, 17, 1128–1133 CrossRef CAS PubMed.
- Z. Bao, J. Wang, Z. Zhang, H. Xing, Q. Yang, Y. Yang, H. Wu, R. Krishna, W. Zhou, B. Chen and Q. Ren, Molecular Sieving of Ethane from Ethylene through the Molecular Cross-Section Size Differentiation in Gallate-based Metal-Organic Frameworks, Angew. Chem., Int. Ed., 2018, 57, 16020–16025 CrossRef CAS.
- C. Gu, N. Hosono, J.-J. Zheng, Y. Sato, S. Kusaka, S. Sakaki and S. Kitagawa, Design and control of gas diffusion process in a nanoporous soft crystal, Science, 2019, 363, 387–391 CrossRef CAS.
- M. H. Mohamed, Y. Yang, L. Li, S. Zhang, J. P. Ruffley, A. G. Jarvi, S. Saxena, G. Veser, J. K. Johnson and N. L. Rosi, Designing Open Metal Sites in Metal-Organic Frameworks for Paraffin/Olefin Separations, J. Am. Chem. Soc., 2019, 141, 13003–13007 CrossRef CAS PubMed.
- Q. Ding, Z. Zhang, C. Yu, P. Zhang, J. Wang, X. Cui, C.-H. He, S. Deng and H. Xing, Exploiting equilibrium-kinetic synergetic effect for separation of ethylene and ethane in a microporous metal-organic framework, Sci. Adv., 2020, 6, eaaz4322 CrossRef CAS PubMed.
- C. Yu, Z. Guo, L. Yang, J. Cui, S. Chen, Y. Bo, X. Suo, Q. Gong, S. Zhang, X. Cui, S. He and H. Xing, A Robust Metal-Organic Framework with Scalable Synthesis and Optimal Adsorption and Desorption for Energy-Efficient Ethylene Purification, Angew. Chem., Int. Ed., 2023, 62, e202218027 CrossRef CAS.
- L. Zhang, L. Li, E. Hu, L. Yang, K. Shao, L. Yao, K. Jiang, Y. Cui, Y. Yang, B. Li, B. Chen and G. Qian, Boosting Ethylene/Ethane Separation within Copper(I)-Chelated Metal-Organic Frameworks through Tailor-Made Aperture and Specific π-Complexation, Adv. Sci., 2020, 7, 1901918 CrossRef CAS.
- P. J. Bereciartua, Á. Cantín, A. Corma, J. L. Jordá, M. Palomino, F. Rey, S. Valencia, E. W. Corcoran, P. Kortunov, P. I. Ravikovitch, A. Burton, C. Yoon, Y. Wang, C. Paur, J. Guzman, A. R. Bishop and G. L. Casty, Control of zeolite framework flexibility and pore topology for separation of ethane and ethylene, Science, 2017, 358, 1068–1071 CrossRef CAS PubMed.
- B. Li, Y. Zhang, R. Krishna, K. Yao, Y. Han, Z. Wu, D. Ma, Z. Shi, T. Pham, B. Space, J. Liu, P. K. Thallapally, J. Liu, M. Chrzanowski and S. Ma, Introduction of π-Complexation into Porous Aromatic Framework for Highly Selective Adsorption of Ethylene over Ethane, J. Am. Chem. Soc., 2014, 136, 8654–8660 CrossRef CAS.
- Y. Yang, L. Li, R.-B. Lin, Y. Ye, Z. Yao, L. Yang, F. Xiang, S. Chen, Z. Zhang, S. Xiang and B. Chen, Ethylene/ethane separation in a stable hydrogen-bonded organic framework through a gating mechanism, Nat. Chem., 2021, 13, 933–939 CrossRef CAS PubMed.
- J. W. Yoon, Y.-K. Seo, Y. K. Hwang, J.-S. Chang, H. Leclerc, S. Wuttke, P. Bazin, A. Vimont, M. Daturi, E. Bloch, P. L. Llewellyn, C. Serre, P. Horcajada, J.-M. Grenèche, A. E. Rodrigues and G. Férey, Controlled Reducibility of a Metal-Organic Framework with Coordinatively Unsaturated Sites for Preferential Gas Sorption, Angew. Chem., Int. Ed., 2010, 49, 5949–5952 CrossRef CAS PubMed.
- C.-X. Chen, Z.-W. Wei, T. Pham, P. C. Lan, L. Zhang, K. A. Forrest, S. Chen, A. M. Al-Enizi, A. Nafady, C.-Y. Su and S. Ma, Nanospace Engineering of Metal-Organic Frameworks through Dynamic Spacer Installation of Multifunctionalities for Efficient Separation of Ethane from Ethane/Ethylene Mixtures, Angew. Chem., Int. Ed., 2021, 60, 9680–9685 CrossRef CAS PubMed.
- J. Pires, M. L. Pinto and V. K. Saini, Ethane Selective IRMOF-8 and Its Significance in Ethane–Ethylene Separation by Adsorption, ACS Appl. Mater. Interfaces, 2014, 6, 12093–12099 CrossRef CAS.
- H. Yang, Y. Wang, R. Krishna, X. Jia, Y. Wang, A. N. Hong, C. Dang, H. E. Castillo, X. Bu and P. Feng, Pore-Space-Partition-Enabled Exceptional Ethane Uptake and Ethane-Selective Ethane–Ethylene Separation, J. Am. Chem. Soc., 2020, 142, 2222–2227 CrossRef CAS PubMed.
- Z. Y. Di, C. P. Liu, J. D. Pang, S. X. Zou, Z. Y. Ji, F. L. Hu, C. Chen, D. Q. Yuan, M. C. Hong and M. Y. Wu, A Metal-Organic Framework with Nonpolar Pore Surfaces for the One-Step Acquisition of C2H4 from a C2H4 and C2H6 Mixture, Angew. Chem., Int. Ed., 2022, 61, e202210343 CrossRef CAS PubMed.
- S. Geng, E. Lin, X. Li, W. Liu, T. Wang, Z. Wang, D. Sensharma, S. Darwish, Y. H. Andaloussi, T. Pham, P. Cheng, M. J. Zaworotko, Y. Chen and Z. Zhang, Scalable Room-Temperature Synthesis of Highly Robust Ethane-Selective Metal-Organic Frameworks for Efficient Ethylene Purification, J. Am. Chem. Soc., 2021, 143, 8654–8660 CrossRef CAS.
- M. Kang, S. Yoon, S. Ga, D. W. Kang, S. Han, J. H. Choe, H. Kim, D. W. Kim, Y. G. Chung and C. S. Hong, High-Throughput Discovery of Ni(IN)2 for Ethane/Ethylene Separation, Adv. Sci., 2021, 8, 2004940 CrossRef CAS.
- Y.-P. Li, Y.-N. Zhao, S.-N. Li, D.-Q. Yuan, Y.-C. Jiang, X. Bu, M.-C. Hu and Q.-G. Zhai, Ultrahigh-Uptake Capacity-Enabled Gas Separation and Fruit Preservation by a New Single-Walled Nickel-Organic Framework, Adv. Sci., 2021, 8, 2003141 CrossRef CAS PubMed.
- W. Liu, S. Geng, N. Li, S. Wang, S. Jia, F. Jin, T. Wang, K. A. Forrest, T. Pham, P. Cheng, Y. Chen, J.-G. Ma and Z. Zhang, Highly Robust Microporous Metal-Organic Frameworks for Efficient Ethylene Purification under Dry and Humid Conditions, Angew. Chem., Int. Ed., 2023, 62, e202217662 CrossRef CAS.
- A. A. Lysova, D. G. Samsonenko, K. A. Kovalenko, A. S. Nizovtsev, D. N. Dybtsev and V. P. Fedin, A Series of Mesoporous Metal-Organic Frameworks with Tunable Windows Sizes and Exceptionally High Ethane over Ethylene Adsorption Selectivity, Angew. Chem., Int. Ed., 2020, 59, 20561–20567 CrossRef CAS PubMed.
- J. Pei, J.-X. Wang, K. Shao, Y. Yang, Y. Cui, H. Wu, W. Zhou, B. Li and G. Qian, Engineering microporous ethane-trapping metal-organic frameworks for boosting ethane/ethylene separation, J. Mater. Chem. A, 2020, 8, 3613–3620 RSC.
- O. T. Qazvini, R. Babarao, Z.-L. Shi, Y.-B. Zhang and S. G. Telfer, A Robust Ethane-Trapping Metal-Organic Framework with a High Capacity for Ethylene Purification, J. Am. Chem. Soc., 2019, 141, 5014–5020 CrossRef CAS PubMed.
- Y. Ye, Y. Xie, Y. Shi, L. Gong, J. Phipps, A. M. Al-Enizi, A. Nafady, B. Chen and S. Ma, A Microporous Metal-Organic Framework with Unique Aromatic Pore Surfaces for High Performance C2H6/C2H4 Separation, Angew. Chem., Int. Ed., 2023, 62, e202302564 CrossRef CAS PubMed.
- C. Gücüyener, J. van den Bergh, J. Gascon and F. Kapteijn, Ethane/Ethene Separation Turned on Its Head: Selective Ethane Adsorption on the Metal-Organic Framework ZIF-7 through a Gate-Opening Mechanism, J. Am. Chem. Soc., 2010, 132, 17704–17706 CrossRef PubMed.
- P.-Q. Liao, W.-X. Zhang, J.-P. Zhang and X.-M. Chen, Efficient purification of ethene by an ethane-trapping metal-organic framework, Nat. Commun., 2015, 6, 8697 CrossRef PubMed.
- R.-B. Lin, H. Wu, L. Li, X.-L. Tang, Z. Li, J. Gao, H. Cui, W. Zhou and B. Chen, Boosting Ethane/Ethylene Separation within Isoreticular Ultramicroporous Metal-Organic Frameworks, J. Am. Chem. Soc., 2018, 140, 12940–12946 CrossRef CAS PubMed.
- L. Li, R.-B. Lin, R. Krishna, H. Li, S. Xiang, H. Wu, J. Li, W. Zhou and B. Chen, Ethane/ethylene separation in a metal-organic framework with iron-peroxo sites, Science, 2018, 362, 443–446 CrossRef CAS PubMed.
- S. Jiang, J. Li, M. Feng, R. Chen, L. Guo, Q. Xu, L. Chen, F. Shen, Z. Zhang, Y. Yang, Q. Ren, Q. Yang and Z. Bao, Hydrophobic paraffin-selective pillared-layer MOFs for olefin purification, J. Mater. Chem. A, 2022, 10, 24127–24136 RSC.
- G. D. Wang, R. Krishna, Y. Z. Li, W. J. Shi, L. Hou, Y. Y. Wang and Z. H. Zhu, Boosting Ethane/Ethylene Separation by MOFs through the Amino-Functionalization of Pores, Angew. Chem., Int. Ed., 2022, 61, e202213015 CrossRef CAS PubMed.
- L. Yang, L. Yan, W. Niu, Y. Feng, Q. Fu, S. Zhang, Y. Zhang, L. Li, X. Gu, P. Dai, D. Liu, Q. Zheng and X. Zhao, Adsorption in Reversed Order of C2 Hydrocarbons on an Ultramicroporous Fluorinated Metal-Organic Framework, Angew. Chem., Int. Ed., 2022, 61, e202204046 CrossRef CAS PubMed.
- M. H. Yu, H. Fang, H. L. Huang, M. Zhao, Z. Y. Su, H. X. Nie, Z. Chang and T. L. Hu, Tuning the Trade-Off between Ethane/Ethylene Selectivity and Adsorption Capacity within Isoreticular Microporous Metal-Organic Frameworks by Linker Fine-Fluorination, Small, 2023, 19, 2300821 CrossRef CAS.
- H. Zeng, X.-J. Xie, M. Xie, Y.-L. Huang, D. Luo, T. Wang, Y. Zhao, W. Lu and D. Li, Cage-Interconnected Metal-Organic Framework with Tailored Apertures for Efficient C2H6/C2H4 Separation under Humid Conditions, J. Am. Chem. Soc., 2019, 141, 20390–20396 CrossRef CAS.
- S.-C. Xiang, Z. Zhang, C.-G. Zhao, K. Hong, X. Zhao, D.-R. Ding, M.-H. Xie, C.-D. Wu, M. C. Das, R. Gill, K. M. Thomas and B. Chen, Rationally tuned micropores within enantiopure metal-organic frameworks for highly selective separation of acetylene and ethylene, Nat. Commun., 2011, 2, 204 CrossRef.
- T.-L. Hu, H. Wang, B. Li, R. Krishna, H. Wu, W. Zhou, Y. Zhao, Y. Han, X. Wang, W. Zhu, Z. Yao, S. Xiang and B. Chen, Microporous metal-organic framework with dual functionalities for highly efficient removal of acetylene from ethylene/acetylene mixtures, Nat. Commun., 2015, 6, 7328 CrossRef CAS.
- S. Yang, A. J. Ramirez-Cuesta, R. Newby, V. Garcia-Sakai, P. Manuel, S. K. Callear, S. I. Campbell, C. C. Tang and M. Schröder, Supramolecular binding and separation of hydrocarbons within a functionalized porous metal-organic framework, Nat. Chem., 2015, 7, 121–129 CrossRef CAS.
- X. Cui, K. Chen, H. Xing, Q. Yang, R. Krishna, Z. Bao, H. Wu, W. Zhou, X. Dong, Y. Han, B. Li, Q. Ren, M. J. Zaworotko and B. Chen, Pore chemistry and size control in hybrid porous materials for acetylene capture from ethylene, Science, 2016, 353, 141–144 CrossRef CAS PubMed.
- B. Li, X. Cui, D. O'Nolan, H.-M. Wen, M. Jiang, R. Krishna, H. Wu, R.-B. Lin, Y.-S. Chen, D. Yuan, H. Xing, W. Zhou, Q. Ren, G. Qian, M. J. Zaworotko and B. Chen, An Ideal Molecular Sieve for Acetylene Removal from Ethylene with Record Selectivity and Productivity, Adv. Mater., 2017, 29, 1704210 CrossRef PubMed.
- R.-B. Lin, L. Li, H. Wu, H. Arman, B. Li, R.-G. Lin, W. Zhou and B. Chen, Optimized Separation of Acetylene from Carbon Dioxide and Ethylene in a Microporous Material, J. Am. Chem. Soc., 2017, 139, 8022–8028 CrossRef CAS PubMed.
- T. Ke, Q. Wang, J. Shen, J. Zhou, Z. Bao, Q. Yang and Q. Ren, Molecular Sieving of C2-C3 Alkene from Alkyne with Tuned Threshold Pressure in Robust Layered Metal-Organic Frameworks, Angew. Chem., Int. Ed., 2020, 59, 12725–12730 CrossRef CAS PubMed.
- S. Mukherjee, S. Chen, A. A. Bezrukov, M. Mostrom, V. V. Terskikh, D. Franz, S.-Q. Wang, A. Kumar, M. Chen, B. Space, Y. Huang and M. J. Zaworotko, Ultramicropore Engineering by Dehydration to Enable Molecular Sieving of H2 by Calcium Trimesate, Angew. Chem., Int. Ed., 2020, 59, 16188–16194 CrossRef CAS.
- J. Shen, X. He, T. Ke, R. Krishna, J. M. van Baten, R. Chen, Z. Bao, H. Xing, M. Dincă, Z. Zhang, Q. Yang and Q. Ren, Simultaneous interlayer and intralayer space control in two-dimensional metal-organic frameworks for acetylene/ethylene separation, Nat. Commun., 2020, 11, 6259 CrossRef CAS PubMed.
- J. Wang, Y. Zhang, P. Zhang, J. Hu, R.-B. Lin, Q. Deng, Z. Zeng, H. Xing, S. Deng and B. Chen, Optimizing Pore Space for Flexible-Robust Metal-Organic Framework to Boost Trace Acetylene Removal, J. Am. Chem. Soc., 2020, 142, 9744–9751 CrossRef CAS.
- Z. Zhang, S. B. Peh, Y. Wang, C. Kang, W. Fan and D. Zhao, Efficient Trapping of Trace Acetylene from Ethylene in an Ultramicroporous Metal-Organic Framework: Synergistic Effect of High-Density Open Metal and Electronegative Sites, Angew. Chem., Int. Ed., 2020, 59, 18927–18932 CrossRef CAS PubMed.
- J.-W. Wang, S.-C. Fan, H.-P. Li, X. Bu, Y.-Y. Xue and Q.-G. Zhai, De-Linker-Enabled Exceptional Volumetric Acetylene Storage Capacity and Benchmark C2H2/C2H4 and C2H2/CO2 Separations in Metal-Organic Frameworks, Angew. Chem., Int. Ed., 2023, 62, e202217839 CrossRef CAS PubMed.
- H.-G. Hao, Y.-F. Zhao, D.-M. Chen, J.-M. Yu, K. Tan, S. Ma, Y. Chabal, Z.-M. Zhang, J.-M. Dou, Z.-H. Xiao, G. Day, H.-C. Zhou and T.-B. Lu, Simultaneous Trapping of C2H2 and C2H6 from a Ternary Mixture of C2H2/C2H4/C2H6 in a Robust Metal-Organic Framework for the Purification of C2H4, Angew. Chem., Int. Ed., 2018, 57, 16067–16071 CrossRef CAS PubMed.
- K.-J. Chen, D. G. Madden, S. Mukherjee, T. Pham, K. A. Forrest, A. Kumar, B. Space, J. Kong, Q.-Y. Zhang and M. J. Zaworotko, Synergistic sorbent separation for one-step ethylene purification from a four-component mixture, Science, 2019, 366, 241–246 CrossRef CAS PubMed.
- Q. Dong, X. Zhang, S. Liu, R.-B. Lin, Y. Guo, Y. Ma, A. Yonezu, R. Krishna, G. Liu, J. Duan, R. Matsuda, W. Jin and B. Chen, Tuning Gate-Opening of a Flexible Metal-Organic Framework for Ternary Gas Sieving Separation, Angew. Chem., Int. Ed., 2020, 59, 22756–22762 CrossRef CAS PubMed.
- Z. Xu, X. Xiong, J. Xiong, R. Krishna, L. Li, Y. Fan, F. Luo and B. Chen, A robust Th-azole framework for highly efficient purification of C2H4 from a C2H4/C2H2/C2H6 mixture, Nat. Commun., 2020, 11, 3163 CrossRef CAS PubMed.
- J.-W. Cao, S. Mukherjee, T. Pham, Y. Wang, T. Wang, T. Zhang, X. Jiang, H.-J. Tang, K. A. Forrest, B. Space, M. J. Zaworotko and K.-J. Chen, One-step ethylene production from a four-component gas mixture by a single physisorbent, Nat. Commun., 2021, 12, 6507 CrossRef CAS PubMed.
- S. Mukherjee, N. Kumar, A. A. Bezrukov, K. Tan, T. Pham, K. A. Forrest, K. A. Oyekan, O. T. Qazvini, D. G. Madden, B. Space and M. J. Zaworotko, Amino-Functionalised Hybrid Ultramicroporous Materials that Enable Single-Step Ethylene Purification from a Ternary Mixture, Angew. Chem., Int. Ed., 2021, 60, 10902–10909 CrossRef CAS PubMed.
- Y. Wang, C. Hao, W. Fan, M. Fu, X. Wang, Z. Wang, L. Zhu, Y. Li, X. Lu, F. Dai, Z. Kang, R. Wang, W. Guo, S. Hu and D. Sun, One-step Ethylene Purification from an Acetylene/Ethylene/Ethane Ternary Mixture by Cyclopentadiene Cobalt-Functionalized Metal-Organic Frameworks, Angew. Chem., Int. Ed., 2021, 60, 11350–11358 CrossRef CAS PubMed.
- B. Zhu, J. W. Cao, S. Mukherjee, T. Pham, T. Zhang, T. Wang, X. Jiang, K. A. Forrest, M. J. Zaworotko and K. J. Chen, Pore Engineering for One-Step Ethylene Purification from a Three-Component Hydrocarbon Mixture, J. Am. Chem. Soc., 2021, 143, 1485–1492 CrossRef CAS PubMed.
- Q. Ding, Z. Zhang, Y. Liu, K. Chai, R. Krishna and S. Zhang, One-Step Ethylene Purification from Ternary Mixtures in a Metal-Organic Framework with Customized Pore Chemistry and Shape, Angew. Chem., Int. Ed., 2022, 61, e202208134 CrossRef CAS PubMed.
- X. W. Gu, J. X. Wang, E. Wu, H. Wu, W. Zhou, G. Qian, B. Chen and B. Li, Immobilization of Lewis Basic Sites into a Stable Ethane-Selective MOF Enabling One-Step Separation of Ethylene from a Ternary Mixture, J. Am. Chem. Soc., 2022, 144, 2614–2623 CrossRef CAS PubMed.
- G. D. Wang, Y. Z. Li, W. J. Shi, L. Hou, Y. Y. Wang and Z. Zhu, One-Step C2H4 Purification from Ternary C2H6/C2H4/C2H2 Mixtures by a Robust Metal-Organic Framework with Customized Pore Environment, Angew. Chem., Int. Ed., 2022, 61, e202205427 CrossRef CAS PubMed.
- Y. Wang, M. Fu, S. Zhou, H. Liu, X. Wang, W. Fan, Z. Liu, Z. Wang, D. Li, H. Hao, X. Lu, S. Hu and D. Sun, Guest-molecule-induced self-adaptive pore engineering facilitates purification of ethylene from ternary mixture, Chem, 2022, 8, 3263–3274 CAS.
- P. Zhang, Y. Zhong, Y. Zhang, Z. Zhu, Y. Liu, Y. Su, J. Chen, S. Chen, Z. Zeng, H. Xing, S. Deng and J. Wang, Synergistic binding sites in a hybrid ultramicroporous material for one-step ethylene purification from ternary C2 hydrocarbon mixtures, Sci. Adv., 2022, 8, eabn9231 CrossRef CAS PubMed.
- Y. Jiang, Y. Hu, B. Luan, L. Wang, R. Krishna, H. Ni, X. Hu and Y. Zhang, Benchmark single-step ethylene purification from ternary mixtures by a customized fluorinated anion-embedded MOF, Nat. Commun., 2023, 14, 401 CrossRef PubMed.
- H. Sun, F. Chen, R. Chen, J. Li, L. Guo, Y. Liu, F. Shen, Q. Yang, Z. Zhang, Q. Ren and Z. Bao, Customizing Metal-Organic Frameworks by Lego-Brick Strategy for One-Step Purification of Ethylene from a Quaternary Gas Mixture, Small, 2023, 19, e2208182 CrossRef PubMed.
- L. Wang, H. Huang, X. Zhang, H. Zhao, F. Li and Y. Gu, Designed metal-organic frameworks with potential for multi-component hydrocarbon separation, Coord. Chem. Rev., 2023, 484, 215111 CrossRef CAS.
- J.-P. Zhang and X.-M. Chen, Optimized Acetylene/Carbon Dioxide Sorption in a Dynamic Porous Crystal, J. Am. Chem. Soc., 2009, 131, 5516–5521 CrossRef CAS PubMed.
- C.-T. He, Z.-M. Ye, Y.-T. Xu, D.-D. Zhou, H.-L. Zhou, D. Chen, J.-P. Zhang and X.-M. Chen, Hyperfine adjustment of flexible pore-surface pockets enables smart recognition of gas size and quadrupole moment, Chem. Sci., 2017, 8, 7560–7565 RSC.
- X. Liu, P. Zhang, H. Xiong, Y. Zhang, K. Wu, J. Liu, R. Krishna, J. Chen, S. Chen, Z. Zeng, S. Deng and J. Wang, Engineering Pore Environments of Sulfate-pillared Metal-Organic Framework for Efficient C2H2/CO2 Separation with Record Selectivity, Adv. Mater., 2023, 35, 2210415 CrossRef CAS PubMed.
- X. Zhu, T. Ke, J. Zhou, Y. Song, Q. Xu, Z. Zhang, Z. Bao, Y. Yang, Q. Ren and Q. Yang, Vertex Strategy in Layered 2D MOFs: Simultaneous Improvement of Thermodynamics and Kinetics for Record C2H2/CO2 Separation Performance, J. Am. Chem. Soc., 2023, 145, 9254–9263 CrossRef CAS PubMed.
- R. Matsuda, R. Kitaura, S. Kitagawa, Y. Kubota, R. V. Belosludov, T. C. Kobayashi, H. Sakamoto, T. Chiba, M. Takata, Y. Kawazoe and Y. Mita, Highly controlled acetylene accommodation in a metal-organic microporous material, Nature, 2005, 436, 238–241 CrossRef CAS PubMed.
- J. Gao, X. Qian, R.-B. Lin, R. Krishna, H. Wu, W. Zhou and B. Chen, Mixed Metal-Organic Framework with Multiple Binding Sites for Efficient C2H2/CO2 Separation, Angew. Chem., Int. Ed., 2020, 59, 4396–4400 CrossRef CAS PubMed.
- F. Luo, C. Yan, L. Dang, R. Krishna, W. Zhou, H. Wu, X. Dong, Y. Han, T.-L. Hu, M. O’Keeffe, L. Wang, M. Luo, R.-B. Lin and B. Chen, UTSA-74: A MOF-74 Isomer with Two Accessible Binding Sites per Metal Center for Highly Selective Gas Separation, J. Am. Chem. Soc., 2016, 138, 5678–5684 CrossRef CAS PubMed.
- Z. Niu, X. Cui, T. Pham, G. Verma, P. C. Lan, C. Shan, H. Xing, K. A. Forrest, S. Suepaul, B. Space, A. Nafady, A. M. Al-Enizi and S. Ma, A MOF-based Ultra-Strong Acetylene Nano-trap for Highly Efficient C2H2/CO2 Separation, Angew. Chem., Int. Ed., 2021, 60, 5283–5288 CrossRef CAS PubMed.
- J. Pei, K. Shao, J.-X. Wang, H.-M. Wen, Y. Yang, Y. Cui, R. Krishna, B. Li and G. Qian, A Chemically Stable Hofmann-Type Metal-Organic Framework with Sandwich-Like Binding Sites for Benchmark Acetylene Capture, Adv. Mater., 2020, 32, 1908275 CrossRef CAS PubMed.
- Y.-L. Peng, T. Pham, P. Li, T. Wang, Y. Chen, K.-J. Chen, K. A. Forrest, B. Space, P. Cheng, M. J. Zaworotko and Z. Zhang, Robust Ultramicroporous Metal-Organic Frameworks with Benchmark Affinity for Acetylene, Angew. Chem., Int. Ed., 2018, 57, 10971–10975 CrossRef CAS PubMed.
- K. Shao, H.-M. Wen, C.-C. Liang, X. Xiao, X.-W. Gu, B. Chen, G. Qian and B. Li, Engineering Supramolecular Binding Sites in a Chemically Stable Metal-Organic Framework for Simultaneous High C2H2 Storage and Separation, Angew. Chem., Int. Ed., 2022, 61, e202211523 CrossRef CAS PubMed.
- H. Zeng, M. Xie, Y.-L. Huang, Y. Zhao, X.-J. Xie, J.-P. Bai, M.-Y. Wan, R. Krishna, W. Lu and D. Li, Induced Fit of C2H2 in a Flexible MOF Through Cooperative Action of Open Metal Sites, Angew. Chem., Int. Ed., 2019, 58, 8515–8519 CrossRef CAS PubMed.
- L. Zhang, K. Jiang, L. Yang, L. Li, E. Hu, L. Yang, K. Shao, H. Xing, Y. Cui, Y. Yang, B. Li, B. Chen and G. Qian, Benchmark C2H2/CO2 Separation in an Ultra-Microporous Metal-Organic Framework via Copper(I)-Alkynyl Chemistry, Angew. Chem., Int. Ed., 2021, 60, 15995–16002 CrossRef CAS PubMed.
- H. Zeng, X.-J. Xie, Y. Wang, D. Luo, R.-J. Wei, W. Lu and D. Li, Spatial disposition of square-planar mononuclear nodes in metal-organic frameworks for C2H2/CO2 separation, Chem. Sci., 2022, 13, 12876–12882 RSC.
- S. R. Caskey, A. G. Wong-Foy and A. J. Matzger, Dramatic Tuning of Carbon Dioxide Uptake via Metal Substitution in a Coordination Polymer with Cylindrical Pores, J. Am. Chem. Soc., 2008, 130, 10870–10871 CrossRef CAS PubMed.
- M. Fischer, F. Hoffmann and M. Fröba, New Microporous Materials for Acetylene Storage and C2H2/CO2 Separation: Insights from Molecular Simulations, ChemPhysChem, 2010, 11, 2220–2229 CrossRef CAS PubMed.
- S. Xiang, W. Zhou, J. M. Gallegos, Y. Liu and B. Chen, Exceptionally high acetylene uptake in a microporous metal-organic framework with open metal sites, J. Am. Chem. Soc., 2009, 131, 12415–12419 CrossRef CAS PubMed.
- Y.-P. Li, Y. Wang, Y.-Y. Xue, H.-P. Li, Q.-G. Zhai, S.-N. Li, Y.-C. Jiang, M.-C. Hu and X. Bu, Ultramicroporous Building Units as a Path to Bi-microporous Metal-Organic Frameworks with High Acetylene Storage and Separation Performance, Angew. Chem., Int. Ed., 2019, 58, 13590–13595 CrossRef CAS PubMed.
- W. Fan, S. Yuan, W. Wang, L. Feng, X. Liu, X. Zhang, X. Wang, Z. Kang, F. Dai, D. Yuan, D. Sun and H.-C. Zhou, Optimizing Multivariate Metal-Organic Frameworks for Efficient C2H2/CO2 Separation, J. Am. Chem. Soc., 2020, 142, 8728–8737 CrossRef PubMed.
- L. Yang, L. Yan, Y. Wang, Z. Liu, J. He, Q. Fu, D. Liu, X. Gu, P. Dai, L. Li and X. Zhao, Adsorption Site Selective Occupation Strategy within a Metal-Organic Framework for Highly Efficient Sieving Acetylene from Carbon Dioxide, Angew. Chem., Int. Ed., 2021, 60, 4570–4574 CrossRef CAS PubMed.
- J. Pei, H.-M. Wen, X.-W. Gu, Q.-L. Qian, Y. Yang, Y. Cui, B. Li, B. Chen and G. Qian, Dense Packing of Acetylene in a Stable and Low-Cost Metal-Organic Framework for Efficient C2H2/CO2 Separation, Angew. Chem., Int. Ed., 2021, 60, 25068–25074 CrossRef CAS PubMed.
- X. Zhang, R.-B. Lin, H. Wu, Y. Huang, Y. Ye, J. Duan, W. Zhou, J.-R. Li and B. Chen, Maximizing acetylene packing density for highly efficient C2H2/CO2 separation through immobilization of amine sites within a prototype MOF, Chem. Eng. J., 2022, 431, 134184 CrossRef CAS.
- Z. Di, C. Liu, J. Pang, C. Chen, F. Hu, D. Yuan, M. Wu and M. Hong, Cage-Like Porous Materials with Simultaneous High C2H2 Storage and Excellent C2H2/CO2 Separation Performance, Angew. Chem., Int. Ed., 2021, 60, 10828–10832 CrossRef CAS PubMed.
- W. Gong, H. Cui, Y. Xie, Y. Li, X. Tang, Y. Liu, Y. Cui and B. Chen, Efficient C2H2/CO2 Separation in Ultramicroporous Metal-Organic Frameworks with Record C2H2 Storage Density, J. Am. Chem. Soc., 2021, 143, 14869–14876 CrossRef CAS PubMed.
- J. Lee, C. Y. Chuah, J. Kim, Y. Kim, N. Ko, Y. Seo, K. Kim, T. H. Bae and E. Lee, Separation of Acetylene from Carbon Dioxide and Ethylene by a Water-Stable Microporous Metal-Organic Framework with Aligned Imidazolium Groups inside the Channels, Angew. Chem., Int. Ed., 2018, 57, 7869–7873 CrossRef CAS PubMed.
- L. Liu, Z. Yao, Y. Ye, Y. Yang, Q. Lin, Z. Zhang, M. O’Keeffe and S. Xiang, Integrating the Pillared-Layer Strategy and Pore-Space Partition Method to Construct Multicomponent MOFs for C2H2/CO2 Separation, J. Am. Chem. Soc., 2020, 142, 9258–9266 CrossRef CAS PubMed.
- L. Wang, W. Sun, Y. Zhang, N. Xu, R. Krishna, J. Hu, Y. Jiang, Y. He and H. Xing, Interpenetration Symmetry Control Within Ultramicroporous Robust Boron Cluster Hybrid MOFs for Benchmark Purification of Acetylene from Carbon Dioxide, Angew. Chem., Int. Ed., 2021, 60, 22865–22870 CrossRef CAS PubMed.
- Y. Ye, Z. Ma, R.-B. Lin, R. Krishna, W. Zhou, Q. Lin, Z. Zhang, S. Xiang and B. Chen, Pore Space Partition within a Metal-Organic Framework for Highly Efficient C2H2/CO2 Separation, J. Am. Chem. Soc., 2019, 141, 4130–4136 CrossRef CAS PubMed.
- Y. Ye, S. Xian, H. Cui, K. Tan, L. Gong, B. Liang, T. Pham, H. Pandey, R. Krishna, P. C. Lan, K. A. Forrest, B. Space, T. Thonhauser, J. Li and S. Ma, Metal-Organic Framework Based Hydrogen-Bonding Nanotrap for Efficient Acetylene Storage and Separation, J. Am. Chem. Soc., 2022, 144, 1681–1689 CrossRef CAS PubMed.
- Q.-G. Zhai, X. Bu, X. Zhao, D.-S. Li and P. Feng, Pore Space Partition in Metal-Organic Frameworks, Acc. Chem. Res., 2017, 50, 407–417 CrossRef CAS PubMed.
- J. Wang, Y. Zhang, Y. Su, X. Liu, P. Zhang, R.-B. Lin, S. Chen, Q. Deng, Z. Zeng, S. Deng and B. Chen, Fine pore engineering in a series of isoreticular metal-organic frameworks for efficient C2H2/CO2 separation, Nat. Commun., 2022, 13, 200 CrossRef CAS PubMed.
- M. Shivanna, K.-i Otake, B.-Q. Song, L. M. van Wyk, Q.-Y. Yang, N. Kumar, W. K. Feldmann, T. Pham, S. Suepaul, B. Space, L. J. Barbour, S. Kitagawa and M. J. Zaworotko, Benchmark Acetylene Binding Affinity and Separation through Induced Fit in a Flexible Hybrid Ultramicroporous Material, Angew. Chem., Int. Ed., 2021, 60, 20383–20390 CrossRef CAS PubMed.
- J. Tian, Q. Chen, F. Jiang, D. Yuan and M. Hong, Optimizing Acetylene Sorption through Induced-fit Transformations in a Chemically Stable Microporous Framework, Angew. Chem., Int. Ed., 2023, 62, e202215253 CrossRef CAS PubMed.
- D. Sensharma, D. J. O'Hearn, A. Koochaki, A. A. Bezrukov, N. Kumar, B. H. Wilson, M. Vandichel and M. J. Zaworotko, The First Sulfate-Pillared Hybrid Ultramicroporous Material, SOFOUR-1-Zn, and Its Acetylene Capture Properties, Angew. Chem., Int. Ed., 2022, 61, e202116145 CrossRef CAS PubMed.
- L.-Z. Cai, Z.-Z. Yao, S.-J. Lin, M.-S. Wang and G.-C. Guo, Photoinduced Electron-Transfer (PIET) Strategy for Selective Adsorption of CO2 over C2H2 in a MOF, Angew. Chem., Int. Ed., 2021, 60, 18223–18230 CrossRef CAS PubMed.
- K.-J. Chen, H. S. Scott, D. G. Madden, T. Pham, A. Kumar, A. Bajpai, M. Lusi, K. A. Forrest, B. Space, J. J. Perry and M. J. Zaworotko, Benchmark C2H2/CO2 and CO2/C2H2 Separation by Two Closely Related Hybrid Ultramicroporous Materials, Chem, 2016, 1, 753–765 CAS.
- D. S. Choi, D. W. Kim, D. W. Kang, M. Kang, Y. S. Chae and C. S. Hong, Highly selective CO2 separation from a CO2/C2H2 mixture using a diamine-appended metal-organic framework, J. Mater. Chem. A, 2021, 9, 21424–21428 RSC.
- J. Cui, Z. Qiu, L. Yang, Z. Zhang, X. Cui and H. Xing, Kinetic-Sieving of Carbon Dioxide from Acetylene through a Novel Sulfonic Ultramicroporous Material, Angew. Chem., Int. Ed., 2022, 61, e202208756 CrossRef CAS PubMed.
- M. L. Foo, R. Matsuda, Y. Hijikata, R. Krishna, H. Sato, S. Horike, A. Hori, J. Duan, Y. Sato, Y. Kubota, M. Takata and S. Kitagawa, An Adsorbate Discriminatory Gate Effect in a Flexible Porous Coordination Polymer for Selective Adsorption of CO2 over C2H2, J. Am. Chem. Soc., 2016, 138, 3022–3030 CrossRef CAS PubMed.
- Y. Gu, J.-J. Zheng, K.-I. Otake, M. Shivanna, S. Sakaki, H. Yoshino, M. Ohba, S. Kawaguchi, Y. Wang, F. Li and S. Kitagawa, Host-Guest Interaction Modulation in Porous Coordination Polymers for Inverse Selective CO2/C2H2 Separation, Angew. Chem., Int. Ed., 2021, 60, 11688–11694 CrossRef CAS PubMed.
- C. Hao, H. Ren, H. Zhu, Y. Chi, W. Zhao, X. Liu and W. Guo, CO2-favored metal-organic frameworks SU-101(M) (M = Bi, In, Ga, and Al) with inverse and high selectivity of CO2 from C2H2 and C2H4, Sep. Purif. Technol., 2022, 290, 120804 CrossRef CAS.
- C. He, P. Zhang, Y. Wang, Y. Zhang, T. Hu, L. Li and J. Li, Thermodynamic and kinetic synergetic separation of CO2/C2H2 in an ultramicroporous metal-organic framework, Sep. Purif. Technol., 2023, 304, 122318 CrossRef CAS.
- L. Li, J. Wang, Z. Zhang, Q. Yang, Y. Yang, B. Su, Z. Bao and Q. Ren, Inverse Adsorption Separation of CO2/C2H2 Mixture in Cyclodextrin-Based Metal-Organic Frameworks, ACS Appl. Mater. Interfaces, 2019, 11, 2543–2550 CrossRef CAS PubMed.
- X.-Y. Li, Y. Song, C.-X. Zhang, C.-X. Zhao and C. He, Inverse CO2/C2H2 separation in a pillared-layer framework featuring a chlorine-modified channel by quadrupole-moment sieving, Sep. Purif. Technol., 2021, 279, 119608 CrossRef CAS.
- Q. Liu, S. G. Cho, J. Hilliard, T.-Y. Wang, S.-C. Chien, L.-C. Lin, A. C. Co and C. R. Wade, Inverse CO2/C2H2 Separation with MFU-4 and Selectivity Reversal via Postsynthetic Ligand Exchange, Angew. Chem., Int. Ed., 2023, 62, e202218854 CrossRef CAS PubMed.
- B. Ma, D. Li, Q. Zhu, Y. Li, W. Ueda and Z. Zhang, A Zeolitic Octahedral Metal Oxide with Ultra-Microporosity for Inverse CO2/C2H2 Separation at High Temperature and Humidity, Angew. Chem., Int. Ed., 2022, 61, e202209121 CrossRef CAS PubMed.
- D. Ma, Z. Li, J. Zhu, Y. Zhou, L. Chen, X. Mai, M. Liufu, Y. Wu and Y. Li, Inverse and highly selective separation of CO2/C2H2 on a thulium-organic framework, J. Mater. Chem. A, 2020, 8, 11933–11937 RSC.
- O. T. Qazvini, R. Babarao and S. G. Telfer, Selective capture of carbon dioxide from hydrocarbons using a metal-organic framework, Nat. Commun., 2021, 12, 197 CrossRef CAS PubMed.
- Y. Shi, Y. Xie, H. Cui, Y. Ye, H. Wu, W. Zhou, H. Arman, R.-B. Lin and B. Chen, Highly Selective Adsorption of Carbon Dioxide over Acetylene in an Ultramicroporous Metal-Organic Framework, Adv. Mater., 2021, 33, 2105880 CrossRef CAS PubMed.
- Y. Xie, H. Cui, H. Wu, R.-B. Lin, W. Zhou and B. Chen, Electrostatically Driven Selective Adsorption of Carbon Dioxide over Acetylene in an Ultramicroporous Material, Angew. Chem., Int. Ed., 2021, 60, 9604–9609 CrossRef CAS PubMed.
- J. Yang, M. Tong, G. Han, M. Chang, T. Yan, Y. Ying, Q. Yang and D. Liu, Solubility-Boosted Molecular Sieving-Based Separation for Purification of Acetylene in Core–Shell IL@MOF Composites, Adv. Funct. Mater., 2023, 33, 2213743 CrossRef CAS.
- J. Yu, J. Zhang, P. Zhang, Y. Wang, S.-N. Li and Q.-G. Zhai, Controllable inverse C2H2/CO2 separation in ultra-stable Zn-organic frameworks for efficient removal of trace CO2 from acetylene, J. Mater. Chem. A, 2022, 10, 23630–23638 RSC.
- Z. Zhang, S. B. Peh, R. Krishna, C. Kang, K. Chai, Y. Wang, D. Shi and D. Zhao, Optimal Pore Chemistry in an Ultramicroporous Metal-Organic Framework for Benchmark Inverse CO2/C2H2 Separation, Angew. Chem., Int. Ed., 2021, 60, 17198–17204 CrossRef CAS PubMed.
- Z. Zhang, Z. Deng, H. A. Evans, D. Mullangi, C. Kang, S. B. Peh, Y. Wang, C. M. Brown, J. Wang, P. Canepa, A. K. Cheetham and D. Zhao, Exclusive Recognition of CO2 from Hydrocarbons by Aluminum Formate with Hydrogen-Confined Pore Cavities, J. Am. Chem. Soc., 2023, 145, 11643–11649 CrossRef CAS PubMed.
- Y. Wang, N.-Y. Huang, X.-W. Zhang, H. He, R.-K. Huang, Z.-M. Ye, Y. Li, D.-D. Zhou, P.-Q. Liao, X.-M. Chen and J.-P. Zhang, Selective Aerobic Oxidation of a Metal-Organic Framework Boosts Thermodynamic and Kinetic Propylene/Propane Selectivity, Angew. Chem., Int. Ed., 2019, 58, 7692–7696 CrossRef CAS PubMed.
- A. Cadiau, K. Adil, P. M. Bhatt, Y. Belmabkhout and M. Eddaoudi, A metal-organic framework-based splitter for separating propylene from propane, Science, 2016, 353, 137–140 CrossRef CAS PubMed.
- Y. Xie, Y. Shi, E. M. Cedeño Morales, A. El Karch, B. Wang, H. Arman, K. Tan and B. Chen, Optimal Binding Affinity for Sieving Separation of Propylene from Propane in an Oxyfluoride Anion-Based Metal-Organic Framework, J. Am. Chem. Soc., 2023, 145, 2386–2394 CrossRef CAS PubMed.
- H. Wang, X. Dong, V. Colombo, Q. Wang, Y. Liu, W. Liu, X.-L. Wang, X.-Y. Huang, D. M. Proserpio, A. Sironi, Y. Han and J. Li, Tailor-Made Microporous Metal-Organic Frameworks for the Full Separation of Propane from Propylene Through Selective Size Exclusion, Adv. Mater., 2018, 30, 1805088 CrossRef PubMed.
- L. Yu, X. Han, H. Wang, S. Ullah, Q. Xia, W. Li, J. Li, I. da Silva, P. Manuel, S. Rudić, Y. Cheng, S. Yang, T. Thonhauser and J. Li, Pore Distortion in a Metal-Organic Framework for Regulated Separation of Propane and Propylene, J. Am. Chem. Soc., 2021, 143, 19300–19305 CrossRef CAS PubMed.
- B. Liang, X. Zhang, Y. Xie, R.-B. Lin, R. Krishna, H. Cui, Z. Li, Y. Shi, H. Wu, W. Zhou and B. Chen, An Ultramicroporous Metal-Organic Framework for High Sieving Separation of Propylene from Propane, J. Am. Chem. Soc., 2020, 142, 17795–17801 CrossRef CAS PubMed.
- H. Zeng, M. Xie, T. Wang, R.-J. Wei, X.-J. Xie, Y. Zhao, W. Lu and D. Li, Orthogonal-array dynamic molecular sieving of propylene/propane mixtures, Nature, 2021, 595, 542–548 CrossRef CAS PubMed.
- Q. Dong, Y. Huang, J. Wan, Z. Lu, Z. Wang, C. Gu, J. Duan and J. Bai, Confining Water Nanotubes in a Cu10O13-Based Metal-Organic Framework for Propylene/Propane Separation with Record-High Selectivity, J. Am. Chem. Soc., 2023, 145, 8043–8051 CrossRef CAS PubMed.
- D. Liu, J. Pei, X. Zhang, X.-W. Gu, H.-M. Wen, B. Chen, G. Qian and B. Li, Scalable Green Synthesis of Robust Ultra-Microporous Hofmann Clathrate Material with Record C3H6 Storage Density for Efficient C3H6/C3H8 Separation, Angew. Chem., Int. Ed., 2023, 62, e202218590 CrossRef CAS PubMed.
- M.-H. Yu, B. Space, D. Franz, W. Zhou, C. He, L. Li, R. Krishna, Z. Chang, W. Li, T.-L. Hu and X.-H. Bu, Enhanced Gas Uptake in a Microporous Metal-Organic Framework via a Sorbate Induced-Fit Mechanism, J. Am. Chem. Soc., 2019, 141, 17703–17712 CrossRef CAS PubMed.
- X. Wang, R. Krishna, L. Li, B. Wang, T. He, Y.-Z. Zhang, J.-R. Li and J. Li, Guest-dependent pressure induced gate-opening effect enables effective separation of propene and propane in a flexible MOF, Chem. Eng. J., 2018, 346, 489–496 CrossRef CAS.
- S. Tu, L. Yu, Y. Wu, Y. Chen, H. Wu, L. Wang, B. Liu, X. Zhou, J. Xiao and Q. Xia, A new yttrium-based metal-organic framework for molecular sieving of propane from propylene with high propylene capacity, AlChE J., 2022, 68, e17551 CrossRef CAS.
- J. Peng, H. Wang, D. H. Olson, Z. Li and J. Li, Efficient kinetic separation of propene and propane using two microporous metal organic frameworks, Chem. Commun., 2017, 53, 9332–9335 RSC.
- L. Li, R.-B. Lin, X. Wang, W. Zhou, L. Jia, J. Li and B. Chen, Kinetic separation of propylene over propane in a microporous metal-organic framework, Chem. Eng. J., 2018, 354, 977–982 CrossRef CAS.
- K. Li, D. H. Olson, J. Seidel, T. J. Emge, H. Gong, H. Zeng and J. Li, Zeolitic Imidazolate Frameworks for Kinetic Separation of Propane and Propene, J. Am. Chem. Soc., 2009, 131, 10368–10369 CrossRef CAS PubMed.
- C. Y. Lee, Y.-S. Bae, N. C. Jeong, O. K. Farha, A. A. Sarjeant, C. L. Stern, P. Nickias, R. Q. Snurr, J. T. Hupp and S. T. Nguyen, Kinetic Separation of Propene and Propane in Metal-Organic Frameworks: Controlling Diffusion Rates in Plate-Shaped Crystals via Tuning of Pore Apertures and Crystallite Aspect Ratios, J. Am. Chem. Soc., 2011, 133, 5228–5231 CrossRef CAS PubMed.
- N. Lamia, M. Jorge, M. A. Granato, F. A. Almeida Paz, H. Chevreau and A. E. Rodrigues, Adsorption of propane, propylene and isobutane on a metal-organic framework: Molecular simulation and experiment, Chem. Eng. Sci., 2009, 64, 3246–3259 CrossRef CAS.
- A. Knebel, B. Geppert, K. Volgmann, D. I. Kolokolov, A. G. Stepanov, J. Twiefel, P. Heitjans, D. Volkmer and J. Caro, Defibrillation of soft porous metal-organic frameworks with electric fields, Science, 2017, 358, 347–351 CrossRef CAS PubMed.
- Q. Ding, Z. Zhang, C. Yu, P. Zhang, J. Wang, L. Kong, X. Cui, C.-H. He, S. Deng and H. Xing, Separation of propylene and propane with a microporous metal-organic framework via equilibrium-kinetic synergetic effect, AlChE J., 2021, 67, e17094 CrossRef CAS.
- Y. Chen, Z. Qiao, D. Lv, C. Duan, X. Sun, H. Wu, R. Shi, Q. Xia and Z. Li, Efficient adsorptive separation of C3H6 over C3H8 on flexible and thermoresponsive CPL-1, Chem. Eng. J., 2017, 328, 360–367 CrossRef CAS.
- P. Zhang, L. Yang, X. Liu, J. Wang, X. Suo, L. Chen, X. Cui and H. Xing, Ultramicroporous material based parallel and extended paraffin nano-trap for benchmark olefin purification, Nat. Commun., 2022, 13, 4928 CrossRef CAS PubMed.
- Y. Wang, T. Li, L. Li, R. B. Lin, X. Jia, Z. Chang, H. M. Wen, X. M. Chen and J. Li, Construction of Fluorinated Propane-Trap in Metal-Organic Frameworks for Record Polymer-Grade Propylene Production under High Humidity Conditions, Adv. Mater., 2023, 35, 2207955 CrossRef CAS PubMed.
- X. Li, J. Liu, K. Zhou, S. Ullah, H. Wang, J. Zou, T. Thonhauser and J. Li, Tuning Metal-Organic Framework (MOF) Topology by Regulating Ligand and Secondary Building Unit (SBU) Geometry: Structures Built on 8-Connected M6 (M = Zr, Y) Clusters and a Flexible Tetracarboxylate for Propane-Selective Propane/Propylene Separation, J. Am. Chem. Soc., 2022, 144, 21702–21709 CrossRef CAS PubMed.
- S.-Q. Yang, F.-Z. Sun, R. Krishna, Q. Zhang, L. Zhou, Y.-H. Zhang and T.-L. Hu, Propane-Trapping Ultramicroporous Metal-Organic Framework in the Low-Pressure Area toward the Purification of Propylene, ACS Appl. Mater. Interfaces, 2021, 13, 35990–35996 CrossRef CAS PubMed.
- A. N. Hong, H. Yang, T. Li, Y. Wang, Y. Wang, X. Jia, A. Zhou, E. Kusumoputro, J. Li, X. Bu and P. Feng, Pore-Space Partition and Optimization for Propane-Selective High-Performance Propane/Propylene Separation, ACS Appl. Mater. Interfaces, 2021, 13, 52160–52166 CrossRef CAS PubMed.
- C. He, Y. Wang, Y. Chen, X. Wang, J. Yang, L. Li and J. Li, Modification of the pore environment in UiO-type metal-organic framework toward boosting the separation of propane/propylene, Chem. Eng. J., 2021, 403, 126428 CrossRef CAS.
- M. Chang, J. Ren, Y. Wei, J.-X. Wang, Q. Yang, D. Liu and J.-F. Chen, A robust metal-organic framework with guest molecules induced splint-like pore confinement to construct propane-trap for propylene purification, Sep. Purif. Technol., 2021, 279, 119656 CrossRef CAS.
- L. Yang, X. Cui, Q. Ding, Q. Wang, A. Jin, L. Ge and H. Xing, Polycatenated Molecular Cage-Based Propane Trap for Propylene Purification with Recorded Selectivity, ACS Appl. Mater. Interfaces, 2020, 12, 2525–2530 CrossRef CAS PubMed.
- P. Iacomi, F. Formalik, J. Marreiros, J. Shang, J. Rogacka, A. Mohmeyer, P. Behrens, R. Ameloot, B. Kuchta and P. L. Llewellyn, Role of Structural Defects in the Adsorption and Separation of C3 Hydrocarbons in Zr-Fumarate-MOF (MOF-801), Chem. Mater., 2019, 31, 8413–8423 CrossRef CAS.
- E. Andres-Garcia, L. Oar-Arteta, J. Gascon and F. Kapteijn, ZIF-67 as silver-bullet in adsorptive propane/propylene separation, Chem. Eng. J., 2019, 360, 10–14 CrossRef CAS.
- E. Andres-Garcia, J. López-Cabrelles, L. Oar-Arteta, B. Roldan-Martinez, M. Cano-Padilla, J. Gascon, G. Mínguez Espallargas and F. Kapteijn, Cation influence in adsorptive propane/propylene separation in ZIF-8 (SOD) topology, Chem. Eng. J., 2019, 371, 848–856 CrossRef CAS.
- U. Böhme, B. Barth, C. Paula, A. Kuhnt, W. Schwieger, A. Mundstock, J. Caro and M. Hartmann, Ethene/Ethane and Propene/Propane Separation via the Olefin and Paraffin Selective Metal-Organic Framework Adsorbents CPO-27 and ZIF-8, Langmuir, 2013, 29, 8592–8600 CrossRef PubMed.
- L. Li, R.-B. Lin, R. Krishna, X. Wang, B. Li, H. Wu, J. Li, W. Zhou and B. Chen, Flexible-Robust Metal-Organic Framework for Efficient Removal of Propyne from Propylene, J. Am. Chem. Soc., 2017, 139, 7733–7736 CrossRef CAS PubMed.
- L. Li, H.-M. Wen, C. He, R.-B. Lin, R. Krishna, H. Wu, W. Zhou, J. Li, B. Li and B. Chen, A Metal-Organic Framework with Suitable Pore Size and Specific Functional Sites for the Removal of Trace Propyne from Propylene, Angew. Chem., Int. Ed., 2018, 57, 15183–15188 CrossRef CAS PubMed.
- H.-M. Wen, L. Li, R.-B. Lin, B. Li, B. Hu, W. Zhou, J. Hu and B. Chen, Fine-tuning of nano-traps in a stable metal-organic framework for highly efficient removal of propyne from propylene, J. Mater. Chem. A, 2018, 6, 6931–6937 RSC.
- L. Yang, X. Cui, Q. Yang, S. Qian, H. Wu, Z. Bao, Z. Zhang, Q. Ren, W. Zhou, B. Chen and H. Xing, A Single-Molecule Propyne Trap: Highly Efficient Removal of Propyne from Propylene with Anion-Pillared Ultramicroporous Materials, Adv. Mater., 2018, 30, 1705374 CrossRef PubMed.
- L. Yang, X. Cui, Z. Zhang, Q. Yang, Z. Bao, Q. Ren and H. Xing, An Asymmetric Anion-Pillared Metal-Organic Framework as a Multisite Adsorbent Enables Simultaneous Removal of Propyne and Propadiene from Propylene, Angew. Chem., Int. Ed., 2018, 57, 13145–13149 CrossRef CAS PubMed.
- Y.-L. Peng, C. He, T. Pham, T. Wang, P. Li, R. Krishna, K. A. Forrest, A. Hogan, S. Suepaul, B. Space, M. Fang, Y. Chen, M. J. Zaworotko, J. Li, L. Li, Z. Zhang, P. Cheng and B. Chen, Robust Microporous Metal-Organic Frameworks for Highly Efficient and Simultaneous Removal of Propyne and Propadiene from Propylene, Angew. Chem., Int. Ed., 2019, 58, 10209–10214 CrossRef CAS PubMed.
- Y. Jiang, J. Hu, L. Wang, W. Sun, N. Xu, R. Krishna, S. Duttwyler, X. Cui, H. Xing and Y. Zhang, Comprehensive Pore Tuning in an Ultrastable Fluorinated Anion Cross-Linked Cage-Like MOF for Simultaneous Benchmark Propyne Recovery and Propylene Purification, Angew. Chem., Int. Ed., 2022, 61, e202200947 CrossRef CAS PubMed.
- Q. Wang, J. Hu, L. Yang, Z. Zhang, T. Ke, X. Cui and H. Xing, One-step removal of alkynes and propadiene from cracking gases using a multi-functional molecular separator, Nat. Commun., 2022, 13, 2955 CrossRef CAS PubMed.
- M.-Y. Gao, A. A. Bezrukov, B.-Q. Song, M. He, S. J. Nikkhah, S.-Q. Wang, N. Kumar, S. Darwish, D. Sensharma, C. Deng, J. Li, L. Liu, R. Krishna, M. Vandichel, S. Yang and M. J. Zaworotko, Highly Productive C3H4/C3H6 Trace Separation by a Packing Polymorph of a Layered Hybrid Ultramicroporous Material, J. Am. Chem. Soc., 2023, 145, 11837–11845 CrossRef CAS PubMed.
- H. I. Mahdi and O. Muraza, An exciting opportunity for zeolite adsorbent design in separation of C4 olefins through adsorptive separation, Sep. Purif. Technol., 2019, 221, 126–151 CrossRef CAS.
- E. V. Makshina, M. Dusselier, W. Janssens, J. Degrève, P. A. Jacobs, B. F. Sels and E. V. Makshina, Review of old chemistry and new catalytic advances in the on-purpose synthesis of butadiene, Chem. Soc. Rev., 2014, 43, 7917–7953 RSC.
- Z. Zhang, Q. Yang, X. Cui, L. Yang, Z. Bao, Q. Ren and H. Xing, Sorting of C4 Olefins with Interpenetrated Hybrid Ultramicroporous Materials by Combining Molecular Recognition and Size-Sieving, Angew. Chem., Int. Ed., 2017, 56, 16282–16287 CrossRef CAS PubMed.
- P.-Q. Liao, N.-Y. Huang, W.-X. Zhang, J.-P. Zhang and X.-M. Chen, Controlling guest conformation for efficient purification of butadiene, Science, 2017, 356, 1193–1196 CrossRef CAS PubMed.
- J. Chen, J. Wang, L. Guo, L. Li, Q. Yang, Z. Zhang, Y. Yang, Z. Bao and Q. Ren, Adsorptive Separation of Geometric Isomers of 2-Butene on Gallate-Based Metal-Organic Frameworks, ACS Appl. Mater. Interfaces, 2020, 12, 9609–9616 CrossRef CAS PubMed.
- L. Wang, W. Xue, H. Zhu, X. Guo, H. Huang and C. Zhong, Stepwise Engineering the Pore Aperture of a Cage-like MOF for the Efficient Separation of Isomeric C4 Paraffins under Humid Conditions, Angew. Chem., Int. Ed., 2023, 62, e202218596 CrossRef CAS PubMed.
- A. H. Assen, Y. Belmabkhout, K. Adil, P. M. Bhatt, D.-X. Xue, H. Jiang and M. Eddaoudi, Ultra-Tuning of the Rare-Earth fcu-MOF Aperture Size for Selective Molecular Exclusion of Branched Paraffins, Angew. Chem., Int. Ed., 2015, 54, 14353–14358 CrossRef CAS PubMed.
- H. Wang, X. Dong, J. Lin, S. J. Teat, S. Jensen, J. Cure, E. V. Alexandrov, Q. Xia, K. Tan, Q. Wang, D. H. Olson, D. M. Proserpio, Y. J. Chabal, T. Thonhauser, J. Sun, Y. Han and J. Li, Topologically guided tuning of Zr-MOF pore structures for highly selective separation of C6 alkane isomers, Nat. Commun., 2018, 9, 1745 CrossRef PubMed.
- Y. Lin, L. Yu, S. Ullah, X. Li, H. Wang, Q. Xia, T. Thonhauser and J. Li, Temperature-Programmed Separation of Hexane Isomers by a Porous Calcium Chloranilate Metal-Organic Framework, Angew. Chem., Int. Ed., 2022, 134, e202214060 CrossRef.
- F. Zheng, L. Guo, R. Chen, F. Zhou, Z. Zhang, Q. Yang, Y. Yang, Q. Ren and Z. Bao, Temperature-swing molecular exclusion separation of hexane isomers in robust MOFs with double-accessible open metal sites, Chem. Eng. J., 2023, 460, 141743 CrossRef CAS.
- K. Jie, Y. Zhou, E. Li, R. Zhao, M. Liu and F. Huang, Linear Positional Isomer Sorting in Nonporous Adaptive Crystals of a Pillar[5]arene, J. Am. Chem. Soc., 2018, 140, 3190–3193 CrossRef CAS PubMed.
- T. Wang, J. Gu, Q. Cui and H. Wang, Study on Adsorption and Desorption Performances of Trace C4–C6 Alkane Mixture on MIL-101(Cr) and WS-480, Energy Fuels, 2019, 33, 7587–7594 CrossRef CAS.
- Y. Yu, L. Yang, B. Tan, J. Hu, Q. Wang, X. Cui and H. Xing, Remarkable separation of C5 olefins in anion-pillared hybrid porous materials, Nano Res., 2021, 14, 541–545 CrossRef CAS.
- R. Lyndon, Y. Wang, I. M. Walton, Y. Ma, Y. Liu, Z. Yu, G. Zhu, S. Berens, Y. S. Chen, S. G. Wang, S. Vasenkov, D. S. Sholl, K. S. Walton, S. H. Pang and R. P. Lively, Unblocking a rigid purine MOF for kinetic separation of xylenes, Chem. Commun., 2022, 58, 12305–12308 RSC.
- M. Shivanna, K.-I. Otake, J.-J. Zheng, S. Sakaki, S. Kitagawa and M. Shivanna, Control of local flexibility towards p-xylene sieving in Hofmann-type porous coordination polymers, Chem. Commun., 2020, 56, 9632–9635 RSC.
- H. Wang, X. Dong, E. Velasco, D. H. Olson, Y. Han, J. Li and H. Wang, One-of-a-kind: a microporous metal–organic framework capable of adsorptive separation of linear, mono- and di-branched alkane isomers via temperature- and adsorbate-dependent molecular sieving, Energy Environ. Sci., 2018, 11, 1226–1231 RSC.
- L. Li, L. Guo, D. H. Olson, S. Xian, Z. Zhang, Q. Yang, K. Wu, Y. Yang, Z. Bao, Q. Ren and J. Li, Discrimination of xylene isomers in a stacked coordination polymer, Science, 2022, 377, 335–339 CrossRef CAS PubMed.
- X. Cui, Z. Niu, C. Shan, L. Yang, J. Hu, Q. Wang, P. C. Lan, Y. Li, L. Wojtas, S. Ma and H. Xing, Efficient separation of xylene isomers by a guest-responsive metal–organic framework with rotational anionic sites, Nat. Commun., 2020, 11, 5456 CrossRef CAS PubMed.
- J. Zhou, T. Ke, Y. Song, H. Cai, Z. A. Wang, L. Chen, Q. Xu, Z. Zhang, Z. Bao, Q. Ren and Q. Yang, Highly Efficient Separation of C8 Aromatic Isomers by Rationally Designed Nonaromatic Metal-Organic Frameworks, J. Am. Chem. Soc., 2022, 144, 21417–21424 CrossRef CAS PubMed.
- D.-D. Zhou, P. Chen, C. Wang, S.-S. Wang, Y. Du, H. Yan, Z.-M. Ye, C.-T. He, R.-K. Huang, Z.-W. Mo, N.-Y. Huang and J.-P. Zhang, Intermediate-sized molecular sieving of styrene from larger and smaller analogues, Nat. Mater., 2019, 18, 994–998 CrossRef CAS PubMed.
- T. He, X.-J. Kong, Z.-X. Bian, Y.-Z. Zhang, G.-R. Si, L.-H. Xie, X.-Q. Wu, H. Huang, Z. Chang, X.-H. Bu, M. J. Zaworotko, Z.-R. Nie and J.-R. Li, Trace removal of benzene vapour using double-walled metal-dipyrazolate frameworks, Nat. Mater., 2022, 21, 689–695 CrossRef CAS PubMed.
- W.-B. Li, Y. Wu, X.-F. Zhong, X.-H. Chen, G. Liang, J.-W. Ye, Z.-W. Mo and X.-M. Chen, Fluorescence Enhancement of a Metal-Organic Framework for Ultra-Efficient Detection of Trace Benzene Vapor, Angew. Chem., Int. Ed., 2023, 62, e202303500 CrossRef CAS PubMed.
- R. Wei, X. Liu and Z. Lai, MOF or COF membranes for olefin/paraffin separation: Current status and future research directions, Adv. Membr., 2022, 2, 100035 CrossRef.
- C. Zhang, Y. Dai, J. R. Johnson, O. Karvan and W. J. Koros, High performance ZIF-8/6FDA-DAM mixed matrix membrane for propylene/propane separations, J. Membr. Sci., 2012, 389, 34–42 CrossRef CAS.
- Y. Liu, Z. Chen, G. Liu, Y. Belmabkhout, K. Adil, M. Eddaoudi and W. Koros, Conformation-Controlled Molecular Sieving Effects for Membrane-Based Propylene/Propane Separation, Adv. Mater., 2019, 31, 1807513 CrossRef PubMed.
- Y. Peng, Y. Li, Y. Ban, H. Jin, W. Jiao, X. Liu and W. Yang, Metal-organic framework nanosheets as building blocks for molecular sieving membranes, Science, 2014, 346, 1356–1359 CrossRef CAS PubMed.
- K. Yang, Y. Ban, A. Guo, M. Zhao, Y. Zhou, N. Cao and W. Yang, In situ interfacial assembly of ultra-H2-permeable metal-organic framework membranes for H2/CO2 separation, J. Membr. Sci., 2020, 611, 118419 CrossRef CAS.
- K. Yang, Y. Ban and W. Yang, Layered MOF membranes modified with ionic liquid/AgBF4 composite for olefin/paraffin separation, J. Membr. Sci., 2021, 639, 119771 CrossRef CAS.
- H. Song, Y. Peng, C. Wang, L. Shu, C. Zhu, Y. Wang, H. He and W. Yang, Structure Regulation of MOF Nanosheet Membrane for Accurate H2/CO2 Separation, Angew. Chem., Int. Ed., 2023, 62, e202218472 CrossRef CAS PubMed.
- J. E. Bachman, Z. P. Smith, T. Li, T. Xu and J. R. Long, Enhanced ethylene separation and plasticization resistance in polymer membranes incorporating metal-organic framework nanocrystals, Nat. Mater., 2016, 15, 845–849 CrossRef CAS PubMed.
- Y. Sun, T. Ji, Y. Gao, J. Yan, Y. He, G. Xu, F. Yan, Q. Bian and Y. Liu, Freezing Contra-diffusion: A New Protocol for Synthesizing Co-Gallate MOF Membranes toward Superior Ethylene/Ethane Separation Performance, ACS Mater. Lett., 2023, 5, 558–564 CrossRef CAS.
- L. Liu, Y. Song, T. Ji, Y. Sun, D. He, H. Hu and Y. Liu, Beyond Solution-Based Protocols: MOF Membrane Synthesis in Supercritical Environments for an Elegant Sustainability Performance Balance, ACS Mater. Lett., 2020, 2, 1142–1147 CrossRef CAS.
| This journal is © the Partner Organisations 2023 |




