Open Access Article
Open Access ArticleRecent advancements in the fabrication and photocatalytic applications of graphitic carbon nitride-tungsten oxide nanocomposites
Muhammad Ikram
Nabeel
a,
Dilshad
Hussain
 *a,
Naseer
Ahmad
a,
Muhammad
Najam-ul-Haq
b and
Syed Ghulam
Musharraf
*a,
Naseer
Ahmad
a,
Muhammad
Najam-ul-Haq
b and
Syed Ghulam
Musharraf
 *a
*a
aHEJ Research Institute of Chemistry, International Center for Chemical and Biological Sciences, University of Karachi, Karachi-75270, Pakistan. E-mail: dilshadhussain@iccs.edu; musharraf1977@yahoo.com
bInstitute of Chemical Sciences, Bahauddin Zakariya University, Multan 60800, Pakistan
First published on 21st August 2023
Abstract
The present review focuses on the widely used graphitic carbon nitride (g-C3N4)-tungsten oxide (WO3) nanocomposite in photocatalytic applications. These catalysts are widely employed due to their easy preparation, high physicochemical stability, nontoxicity, electron-rich properties, electronic band structure, chemical stability, low cost, earth-abundance, high surface area, and strong absorption capacity in the visible range. These sustainable properties make them predominantly attractive and unique from other photocatalysts. In addition, graphitic carbon nitride (g-C3N4) is synthesized from nitrogen-rich precursors; therefore, it is stable in strong acid solutions and has good thermal stability up to 600 °C. This review covers the historical background, crystalline phases, density-functional theory (DFT) study, synthesis method, 0-D, 1-D, 2-D, and 3-D materials, oxides/transition/nontransition metal-doped, characterization, and photocatalytic applications of WO3/g-C3N4. Enhancing the catalytic performance strategies such as composite formation, element-doping, heterojunction construction, and nanostructure design are also summarized. Finally, the future perspectives and challenges for WO3/g-C3N4 composite materials are discussed to motivate young researchers and scientists interested in developing environment-friendly and efficient catalysts.
Introduction
In the last few years, with the rapid growth in population, textile, pharmaceutical, food, paper, agriculture, pulp, and cosmetic industries have grown more rapidly, and the use of synthetic chemicals to fulfill the population demands have also increased considerably.1–3 Petroleum product consumption has also increased since all these industries directly or indirectly rely on fossil fuels to produce electricity.4 Moreover, marine oil spill pollution risk has also increased due to rapid maritime oil drilling. These petroleum hydrocarbons are toxic to marine biodiversity and indirectly affect human health.3,5 Similarly, pharmaceuticals' active compound consumption is increasing daily, and many expired, unused, and residual drugs are discharged into the aquatic environment.6–8 The situation has become severe, and drug residues have been detected in significant amounts in soil, wastewater, and even in drinking water globally.9,10 Several projects worldwide are looking for strategies to limit this ecotoxicological risk of pharmaceutical products.7,11Dyes and pigments are aromatic, nondegradable, toxic, and widely used in pharmaceuticals, textile industries, food colorants, cosmetics products, plastics farms, photographic film, and paper.12,13 During the finishing and dyeing operations, approximately 0.2 million tons of dyes are released into the environment each year from textile industries.12–14 Dye removal from water bodies is significant for the environment since a minute quantity (<1 mg L−1) can be highly toxic for aquatic life, decreasing the photosynthetic activity, hindering sunlight passage, affecting the symbiotic process, and reducing water oxygenation.15
Since the world is facing pollution problems, several methods (ion exchange, aerobic process, membrane separation, coagulation, sorption, photocatalysis, sonolysis, ozonation, electrocatalysis, electro-Fenton, photo-Fenton, etc.) have been used to purify wastewater.16,17 However, these techniques are complicated, expensive, time-consuming, and require additional operational costs.15,18,19 Among these, advanced oxidation processes (AOPs) are fast, most favorable, ecofriendly, and efficient for effectively removing pollutants.
AOPs are chemical methods used to remove various organic and inorganic contaminants from wastewater and are employed as alternatives to conventional treatment methods (filtration or biological treatment) to meet the required water quality standards.20 AOPs involve generating and utilizing hydroxyl radicals (·OH) by radiation in the presence of a catalyst. UV irradiation, ozone, and H2O2 improve advanced oxidation processes for photodegradation and nonselectively react with most organic pollutants due to the in situ production of highly reactive hydroxyl radicals (·OH). The advantages of AOPs are as follows: (1) AOPs are highly effective in degrading and removing a wide range of pollutants (organic, inorganic, pesticides, pharmaceuticals, dyes, and certain types of metals). They break down these pollutants into simple compounds that are not easily treatable by other methods.20 (2) AOPs have high oxidation potential, which allows them to oxidize and mineralize pollutants into nontoxic substances (carbon dioxide, water), promoting the complete degradation of contaminants and reducing their toxicity.21 (3) They generate highly reactive OH radicals, which attack and breakdown a broad spectrum of dyes, drugs, and other related pollutants, making them versatile and applicable to various contaminants. Besides, they can be applied to different water sources (municipal, industrial effluents, groundwater, and contaminated surface waters).22 (4) AOPs operate under mild conditions and achieve high treatment efficiencies in a relatively short time, which makes them suitable for practical applications.
AOPs include both homogeneous and heterogeneous photocatalysis. Fenton's reagent is a type of homogeneous photocatalyst that includes a mixture of Fe2+ salt (using FeSO4 as an iron source) and hydrogen peroxide that produces hydroxyl radicals (·OH) under ultraviolet (UV) irradiation (<320 nm). Heterogeneous photocatalysts include semiconductor materials such as g-C3N4, CdS ZnO, TiO2, SnO2, WOx, Fe2O3, ZnS, and BiOI.18,23,24 In heterogeneous photocatalysis, the contaminants are in a different phase (e.g., dissolved in water or adsorbed on the surface) and come in contact with the photocatalyst, where the oxidation reactions occur. (1) The photocatalyst absorbs light energy and creates electron–hole pairs, generating reactive species capable of initiating oxidation reactions.25 (2) Photocatalysis exhibits selectivity toward organic pollutants, targeting their chemical structures and functional groups, and efficiently degrading the pollutants.26 (3) Photocatalysts are widely available and relatively inexpensive compared to other AOPs, and this accessibility makes them a practical option for water treatment.27 (4) The optimization and customization of the process parameters are essential for photocatalysis.28 Photocatalysis can occur at mild reaction conditions, making it energy efficient and suitable for water treatment. Factors such as the choice of catalyst, pH, temperature, light source, and contaminants' nature can influence the catalytic efficiency.
There are various photocatalysts used in AOPs. The most frequently studied materials are g-C3N4, TiO2, ZnO, CdS, SnO2, WOx, Fe2O3, and ZnS. Due to the strong oxidation potential of these nanomaterials, they have a high activity of (·OH) radical generation. Photocatalysis based on AOP involves the generation of reactive species through the interaction of a photocatalyst with light energy and reactive species, such as hydroxyl radicals (OH·), superoxide radicals (O2˙−), and singlet oxygen (1O2), possesses high oxidation potentials, and plays a crucial role in the degradation of the pollutant. The mechanism of photocatalysis using AOP is shown in Fig. 1. The photocatalyst absorbs photons from a light source, usually ultraviolet (UV) or visible light. Electrons in the valence band (VB) gain sufficient energy for transition to the conduction band, leaving behind positively charged holes in the valence band and generating electron–hole pairs (e−/h+). The photogenerated electrons and holes participate in the redox reactions with adsorbed species on the surface of the photocatalyst or in the surrounding environment, and holes have a strong oxidizing power and can directly react with organic compounds, while the electrons can reduce oxidants or other species. The photogenerated h+ reacts with water molecules adsorbed on the photocatalyst surface, producing OH radicals. These hydroxyl radicals are highly reactive and can initiate the degradation of organic pollutants. The organic compounds are broken down into smaller, less harmful molecules, such as water, carbon dioxide, and other byproducts. The electrons from the conduction band recombine with the holes from the valence band, restoring the catalyst's initial state and allowing the photocatalytic process. The drawback of photocatalysis is that the fast recombination of photogenerated electron/hole (e−/h+) reduces the catalytic efficiency of the catalyst and (·OH) radical generation. Various surface modifications and structural changes have improved the activities of TiO2, ZnO, CdS, SnO2, WOx, Fe2O3, ZnS, and BiOI-based photocatalysts.29–35
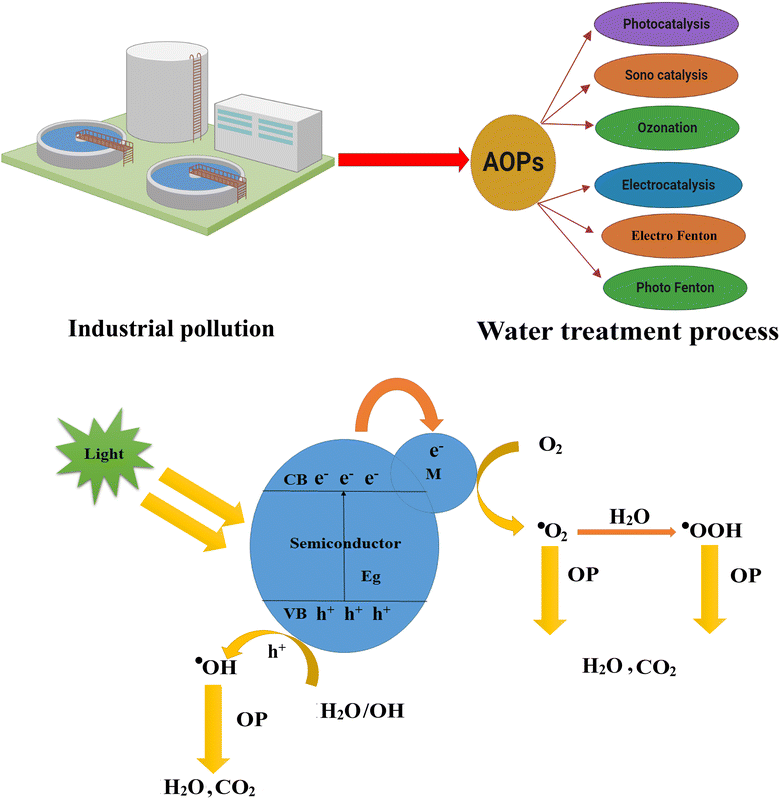 | ||
| Fig. 1 The water treatment method and photocatalysis (schematic diagram of the photoredox mechanism of different water pollutants). | ||
G-C3N4 has been a widely used nanocatalyst due to its easy preparation, nontoxicity, electron-rich properties, chemical stability, cost efficiency, and narrow bandgap (2.7 eV).24,36 In addition, g-C3N4 can easily be synthesized from urea, thiourea, melamine, cyanamide, and dicyandiamide, and it is stable in strong acid solutions and up to 600 °C in air.37,38 These sustainable properties of g-C3N4 make it predominantly elegant for solar energy utilization.39,40 However, the limitation of pristine g-C3N4 is the fast recombination of photogenerated electron/hole (e−/h+) that reduces the photocatalytic efficiency and hydroxyl radical (·OH) generation.38,41 The efficiency of pristine g-C3N4 photocatalysts can be improved by various surface modifications and structural changes.23,24,40,41 Similarly, the yield of g-C3N4 is very low. Hence, using g-C3N4 as the support material is preferred over using it alone.40,42
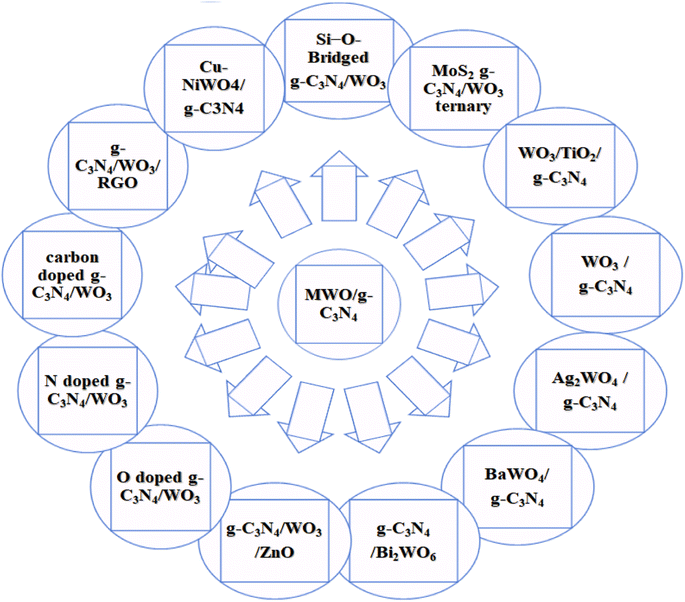
Metal oxide (MOx) materials improve the photocatalytic and electronic device efficiency. The role of tungsten oxide (WOx) is very prominent among many metal oxides.43,44 WOx is a semiconductor transition metal oxide with a bandgap of 2.4–2.8 eV, which can absorb the blue region of the solar spectrum; thus, it has attracted the interest of researchers due to its wide applications in several fields, such as semiconductors, solar energy devices, gas sensors, and photocatalysts.45,46 WO3/gC3N4, Ag2WO4@g-C3N4, BaWO4/g-C3N4, WO3/TiO2/g-C3N4, g-C3N4/WO3/ZnO, gC3N4/Bi2WO6, and graphene-WO3/TiO2 have been reported for various applications and perspective of MWO/g-C3N4 doped with various elements shown below in Fig. 2.40,43,45,47–51 Combining metal WOx and g-C3N4 has resulted in higher photocatalytic activity than WOx and pure g-C3N4.43 Recently, there has been significant progress in developing g-C3N4 and WO3 materials due to their unique features for various applications, including photocatalytic water splitting, pollutant degradation, energy conversion, and remarkable photochemical stability.
Tungsten trioxide has excellent photocatalytic activity in the visible light region. By integrating WOx and g-C3N4, the composite can leverage the advantages of both materials, leading to enhanced photocatalytic efficiency and a broader light absorption range. Similarly, after composite formation, the bandgap reduces to 2.5 eV.
Numerous reviews exist on the synthesis, modification, and applications of g-C3N4 and WO3 for photocatalysis and environmental application. For example, Jiang et al.52 wrote a review on doped g-C3N4 for photocatalysis, including metal doping, nonmetal doping, codoping, and heterojunction formation using g-C3N4.53 Similarly, WO3-based catalysts have been reported for the photocatalytic and photoelectrocatalytic removal of organic pollutants from water.54 This article comprehensively reviews WO3 and focuses on its properties, various synthesis and preparation methods, and strategies to enhance its performance. Similarly, a WO3-based photocatalyst for environmental remediation has been reported,55 presenting a comprehensive analysis of low-cost and environment-friendly methods for the synthesis of WO3 and investigating the correlation between morphology control and key strategies to enhance the photocatalytic performance, including elemental doping, cocatalyst hybridization, and heterojunction formation. Previous reviews focused only on either WO3 single materials or g-C3N4. In this review, we briefly discuss all the graphitic carbon nitride (g-C3N4)-tungsten oxide (WO3)-based nanocomposites, their derivatives (0D, 1D, 2D, 3D, transition metal/nonmetal doping, and ternary composite) and their photocatalytic applications. We have specifically focused on the versatile properties and rational design to precisely adjust the optical, electronic, luminescent, structural morphology, Z-scheme, and other physical properties of graphitic carbon nitride (g-C3N4)-tungsten oxide (WO3)-based nanocomposite. In photocatalysis, incorporating metals, transition metals, non metals, oxides, and their codoping has shown excellent potential for bandgap engineering and nanocomposite formation, leading to enhanced light absorption and redox band potentials tailored for specific photocatalytic applications. Moreover, this review also provides a summary of recent progress in the development of efficient and cost-effective graphitic carbon nitride (g-C3N4)-tungsten oxide (WO3)-based nanocomposite in photocatalysis and the removal of contaminants from the environment.
Graphitic carbon nitride-tungsten oxide composites have shown promising properties and applications in photocatalysis. However, some disadvantages are associated with their fabrication and use. Fabricating g-C3N4/WO3 nanocomposites is challenging, and achieving a uniform distribution and strong interaction between WO3 and g-C3N4 requires precise control over the synthesis conditions (temperature, time, precursor ratios). Large-scale synthesis with consistent properties requires complex and costly fabrication processes. The stability of g-C3N4/WO3 is another concern, especially under harsh photocatalytic conditions, and can lead to aggregation during prolonged use, resulting in a decline in the photocatalytic activity over time. This review highlights the composite's physical and chemical properties, shape, DFT structure, optical bandgap, and photocatalytic properties under UV/visible light. This review also focuses on the methods for composite formation to enhance the photocatalytic properties of MWOx/g-C3N4 by reducing the bandgap. Our survey shows that no previous review has been written on fabricated graphitic carbon nitride-based tungstate nanostructured materials (MWOx/g-C3N4) and their photocatalytic applications. Therefore, we hope that this review will provide the researcher with ideas to construct new materials and elaborate their multifunctional applications.
Applications of WO3/g-C3N4
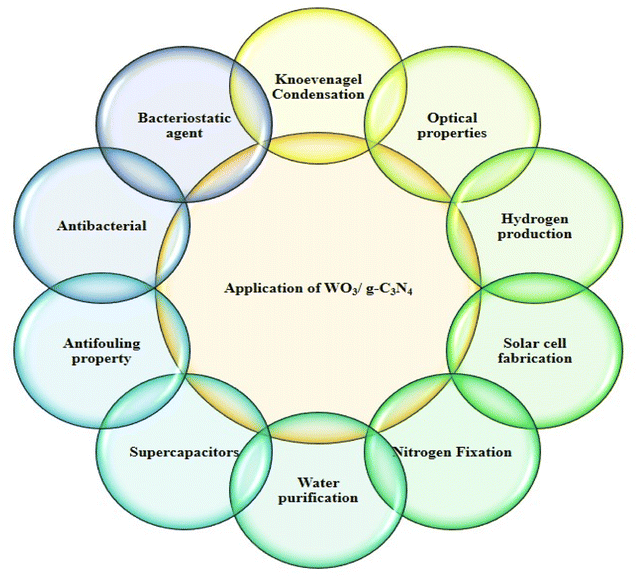
Nanotechnology is a growing field of research due to its properties linked with small particle sizes. Many properties of nanomaterials, such as electrical, optical, and mechanical, can be explained as a function of composition, size, and structural order. The synthesis of nanostructured materials is a complicated process since a small change in the parameters can affect the properties of the end products. However, the nanostructured materials synthesized by various methods under controlled conditions have a high surface-to-volume ratio and more atoms at the grain boundary. These nanostructured materials are used for many applications such as biomedical research, engineering applications, drug delivery, cancer therapy, environment-related application, sensing (biomolecules, pesticides, gas molecules, inorganic anions, and organic molecules), and cell imaging. Nanotechnology replaced old studies and technology to improve the lifestyle and environment for life, resolving many issues and miniaturizing the devices. Tungsten oxide-based graphitic carbon nitride nanostructured materials are semiconductors synthesized by numerous methods. Doping with other materials can adjust their electrical, magnetic, and optical properties. WO3/g-C3N4-based doped materials can be designed in dimensions such as 0D, 1D, 2D, and 3D. WO3/g-C3N4-based pure and doped materials are multifunctional due to their inherent properties.Below is a short view of the overall applications of doped WO3/g-C3N4 (Fig. 3) and photocatalytic applications against organic pollutants.
WO3/g-C3N4 materials purify air and water and efficiently degrade pollutants and harmful gases. This application can contribute to cleaner air and water resources.56
These materials are used in energy storage devices such as batteries, supercapacitors, and electrochemical performance. Their unique properties and high surface area make them suitable for energy storage and sensitive and selective sensing applications.57
These materials are also applied to produce chemicals through selective catalytic reactions. For example, the selective oxidation of alcohol into a carboxylic acid can enable environment-friendly synthesis routes throughout photocatalysis.58
WO3/g-C3N4 make them suitable for harnessing solar energy and are utilized in solar fuel production systems (photocatalytic water splitting) to generate clean energy. These materials can also produce solar fuels such as methane, methanol, or even hydrocarbon and are used as alternatives to traditional fossil fuels.59
Structure of graphitic carbon nitride and its different phases
There are seven allotropes of graphitic carbon nitride, including α-C3N4 (bandgap 5.5 eV), β-C3N4 (bandgap 4.85 eV), pseudocubic-C3N4 (bandgap 4.13 eV), cubic-C3N4 (bandgap 4.3 eV), g-o-triazine (bandgap 0.93 eV), g-h-triazine (bandgap 2.97 eV), and g-h-heptazine (bandgap 2.88 eV) and g-C3N4, predicted by Teter and Hemley in 1996, as shown in Fig. 2a.62–66 All these are hard crystalline forms of carbon nitride except g-C3N4. Among these, beta carbon nitride (β-C3N4) is a super hard material (pure carbon nitride bulk modulus 4.27 ± 0.15 Mbar) predicted to be harder than diamond (bulk modulus 4.43 Mbar). It has the same crystal structure as β-Si3N4.62,67 Corkill and Cohen calculated the bandgap of β-C3N4 to be 6.4 ± 0.5 eV in 1993.68
In 1993, Chen et al. synthesized thin C3N4 films (by magnetron snorting) on Si medium and polycrystalline zirconium substrate in purely nitrogen medium and investigated the structure of C3N4 using electron microscopy and Raman spectroscopy.67 Except for g-C3N4, all other phases of CN are super hard.70 In 2009, Wang and their coworker first reported applying g-C3N4 in photocatalysis. These carbon materials (g-C3N4) gained importance in the scientific area when breakthrough research was published in 2009, describing the water splitting to hydrogen gas.71
Moreover, g-C3N4 can be obtained from cheap precursors such as nitrogen-rich compounds cyanamide, dicyandiamide, thiourea, guanidinium chloride, guanidine thiocyanate urea, and melamine, and its large-scale production is easy by low-cost synthesis.66,67 It is easy to modify the structures and morphology of the g-C3N4; thus, it has become a hot topic for researchers.72
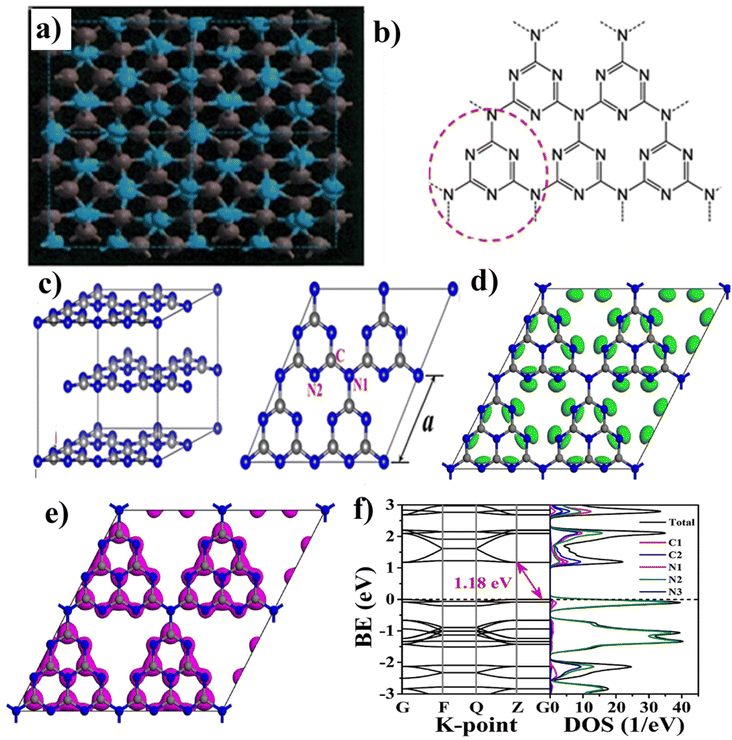 | ||
| Fig. 4 (a) Cubic-C3N4 structures down the [00l] axis, showing grey spheres (C atoms) and blue (N atoms), (b) s-triazine, heptazine structures of g-C3N4 (reproduced with permission from ref. 66 Copyright 2017, Springer Nature). (c) Geometric structures of g-C3N4 bulk and g-C3N4 monolayer, DFT structure (reproduced with permission from ref. 75 Copyright 2019, Wiley Online Library). (d) Calculated HOMO, (e) LUMO, (f) band structure and corresponding DOS of monolayer g-C3N4 (G, F, Q, and Z are high symmetry points in the reciprocal space) (reproduced with permission from ref. 76 Copyright 2017, Elsevier). | ||
Moreover, the bulk structure of g-C3N4 is formed through multilayer stacking, known as ‘AB-stacking’. The interaction energy between adjacent layers measures 0.036 eV Å−2, indicating the presence of van der Waals forces (weak π–π interactions) between the layers. The semiconductors' energy bandgap (Eg) was calculated using the GGA-PBE (generalized gradient approximation Perdew–Burke–Ernzerhof) function, predicting an Eg of 1.2 eV for monolayer g-C3N4 lower than the experimental value of 2.7 eV. The HSE06 functional provides a more accurate prediction, suggesting an Eg of 2.7 eV. In both cases, g-C3N4 exhibits an indirect bandgap, with the valence band maximum (VBM) and conduction band minimum (CBM) located at different K points in the Brillouin zone. The calculated band structure and corresponding DOS of monolayer g-C3N4 (G, F, Q, and Z are high symmetry points in the reciprocal space).77 The lowest unoccupied molecular orbital (LUMO) and highest occupied molecular orbital (HOMO) of monolayer g-C3N4 were calculated, as shown in Fig. 4(d–f). Following the electronic density of states (DOS), the HOMO covers all N2 atoms, while the LUMO is primarily found on C1 and C2 atoms with some N1 and N2 atoms. The tri-coordinated bridge N (N3) atoms do not contribute to the VB or CB edge, and in essence, N3 atoms do not produce excited electrons when exposed to light, and they do not allow electron migration through N3 atoms or transfer between heptazine (C6N7) units. Consequently, the photogenerated electron–hole pairs remain localized within each heptazine unit, resulting in inefficient separation and poor photocatalytic performance.
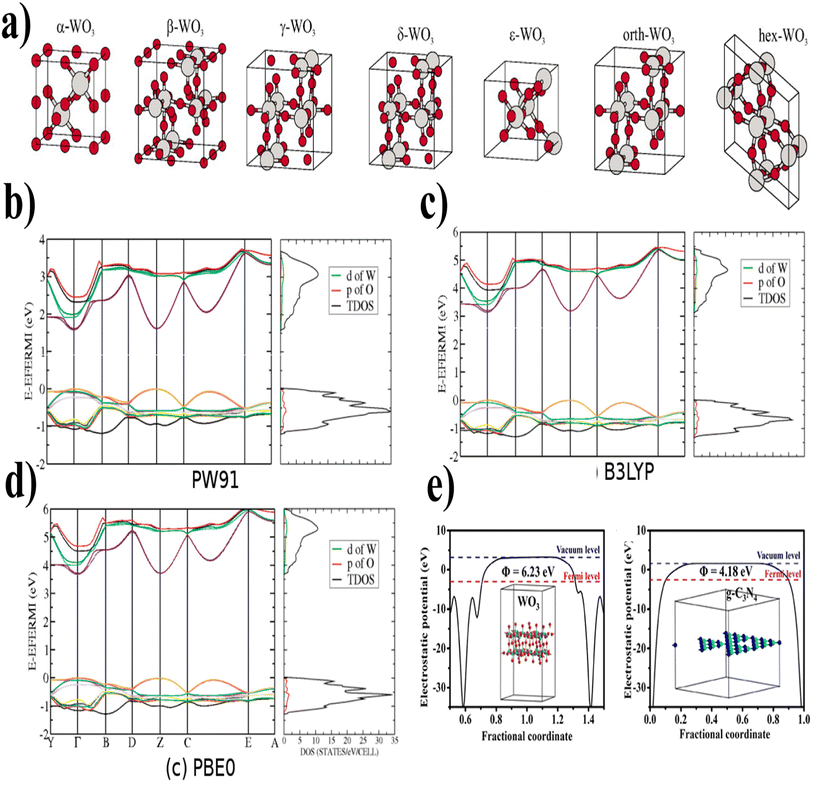 | ||
| Fig. 5 (a) The unit cells of WO3 at different temperatures show different phases (reproduced with permission from ref. 79 Copyright 2010, AIP Publishing). (b) PW91, (c) B3LYP, (d) PBE0 (band structure and density of states (DOS) of room-temperature monoclinic WO3 by different kinds of functionals) (reproduced with permission from ref. 81 Copyright 2011, American Chemical Society). (e) Electrostatic potentials WO3 (001) surface and g-C3N4 (001) surface (reproduced with permission from ref. 82 Copyright 2018, Elsevier). | ||
Fabrication strategies for graphitic carbon nitride–tungsten oxide
Various techniques, such as in situ growth, impregnation, thermal treatment, precipitation, ultrasonication, and calcination, have been explored to combine the two components effectively. The design principles of fabrication and strategies involve the meticulous control of synthesis parameters, interfacial engineering, morphology and composition control, and performance optimization through various strategies. By advancing these design principles and strategies, nanocomposites hold great promise for applications in photocatalysis, environmental remediation, and other fields where efficient and sustainable materials are required. Here, we describe a few methods of synthesis and fabrication of WO3/g-C3N4 and their doped composites.Fu and coworkers82 designed 2D–2D ultrathin WO3/g-C3N4 from electrostatic-assisted ultrasonic exfoliation of WO3 and two-step thermal etching of g-C3N4 (Fig. 6(a)). Fig. 6(b) indicates the zeta potentials at pH 4 of bulk WO3 (−9.7 mV) and WO3 nanosheets (−22.8 mV), indicating that the higher zeta potential value for WO3 nanosheets relative to the bulk WO3 and nanosheet of g-C3N4 show positive zeta potential (+10.3 mV) at the same pH. The opposite value of zeta potential results in strong electrostatic attraction, which is beneficial for charge transfer in WO3 and g-C3N4. The work function of WO3 is greater than the g-C3N4 value, inducing a charge transfer from g-C3N4 to WO3. Three factors, namely, Coulomb interaction, band edges, and internal electric field, play a major role in stopping the recombination of electrons from the conduction band of WO3 and holes (h+) from the valence band (VB) of g-C3N4. These factors also stop the recombination power of electrons from the conduction band of the g-C3N4 and holes (h+) from the valence band of WO3. Praus et al.83 first synthesized the WO3/g-C3N4 using zeta potential values by dispersing both components (exfoliated g-C3N4 and WO3) in an aqueous solution at pH = 2. In the pH range of 1.4–4.0, WO3 shows negative zeta potentials, while exfoliated g-C3N4 exhibited positive zeta potential. X. Han et al.84 developed the WO3/g-C3N4 (2D) named CNW composite through the hydrothermal process for H2 generation using the following precursors: melamine, CTAB, hexadecyl-trimethyl-ammonium-bromide, and WCl6. 2D WO3/g-C3N4 (10 wt% WO3) caused 1853 (mmol h−1 g−1) H2 generation, and the BET of CNW-10 was 86 m2 g−1. This value is 20 and 2.5 times greater than that of pristine WO3 and g-C3N4 NSs, respectively. Similarly, the average pore diameter and volumes for g-C3N4 NSs (2.0 nm, 0.24 cm3 g−1), CNW-10 (2.0 nm, 0.44 cm3 g−1), and WO3 (8.1 nm, 0.02 cm3 g−1) were determined. Similarly, Zhuang et al.85 synthesized the 2D–2D-WO3/g-C3N4 2D–2D heterostructure by an in situ preparation strategy from dicyandiamide, Na2WO4·2H2O, and NH4F for RhB degradation. Sample CNW-13 (13%, WO3) completely degraded RhB within 40 min. The photocatalyst showed efficient photocatalytic properties and good stability after several cycles. However, synthesizing 2D–2D WO3/g-C3N4 can be complex and challenging. Achieving the desired morphology, composition control, and interfacial properties in these hybrid structures requires precise synthesis techniques. This complexity can limit these materials' scalability and real-life implementation. Similarly, 2D–2D structures may have time-limited light absorption capabilities (particularly in certain range wavelengths). Thus, they might not efficiently utilize the whole solar spectrum of visible light, leading to lower photocatalytic efficiency.
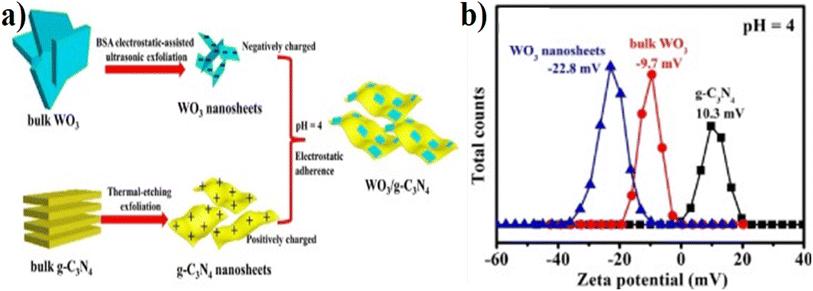 | ||
| Fig. 6 (a) Systematic synthesis scheme of 2D–2D heterojunction WO3/g-C3N4 through electrostatic interaction (Coulomb interaction), (b) pH = 4 value of zeta potentials of bulk and nanoscale sheets of WO3 and g-C3N4 (reproduced with permission from ref. 82 Copyright 2018, Elsevier). | ||
The elegant WO3/g-C3N4 composite hollow microspheres (CHMs), inorganic–organic 2D–2DWO3/g-C3N4 nanosheets (NS), and WO3 nanosheets NS/g-C3N4 nanosheets (NS) were synthesized by various researchers (Fig. 7(a–e)).86 A strategy was developed to enhance the heterostructure system by modulating the electronic and surface properties. A thin 2D/2D WO3/g-C3N4 heterojunction with carbon doping and the bridge were constructed with anionic polyacrylamide (APAM act as functions as the assistant template and carbon source). WO3 and APAM were inserted into the g-C3N4 nanosheet, as shown in Fig. 7(a). APAM is a hydrophilic polymer used for its carbonization potential, and the amide group forms hydrogen bonds on the g-C3N4 surface. In the above study, APAM was utilized as a template to fabricate a carbon-decorated 2D/2D WO3/g-C3N4 heterostructure based on Z-scheme photocatalysts and compared to individual WO3, g-C3N4, and their binary composites. The synthesized composite demonstrated significantly enhanced the degradation activity for tetracycline under visible light irradiation, attributed to the reduced bandgap, fast charge transfer rate, and enhanced quantum efficiency.
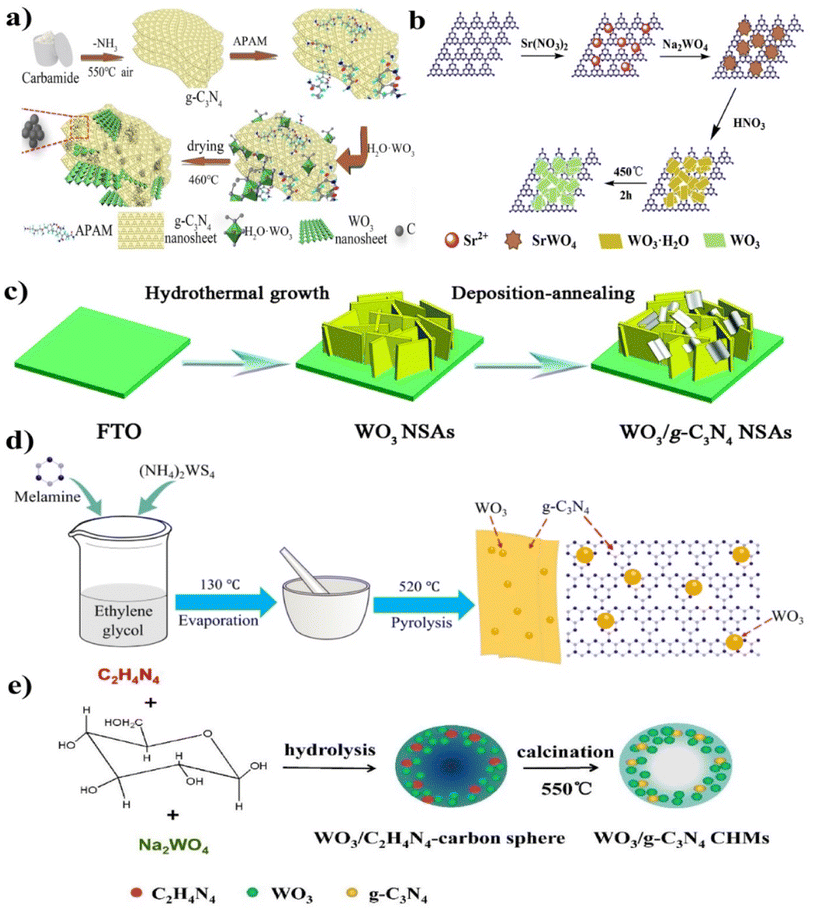 | ||
| Fig. 7 (a–e) Synthesis processes for the 2D–2DWO3/g-C3N4 heterojunction through different methods ((a) reproduced with permission from ref. 86 Copyright 2020, Elsevier, (b) reproduced with permission from ref. 87 Copyright 2018, Elsevier, (c) reproduced with permission from ref. 88 Copyright 2018, Royal Society of Chemistry, (d) reproduced with permission from ref. 89 Copyright 2017, IOP Publishing Ltd (e) reproduced with permission from ref. 90 Copyright 2017, Elsevier). | ||
Chen et al.87 synthesized a novel hierarchical sheet-on-sheet composite, successfully fabricated by a simple calcination method using acid-treated SrWO4/g-C3N4 as the precursors, as shown in Fig. 7(b). The synthesized WOCN composites demonstrated significantly enhanced photocatalytic activity in RhB degradation under simulated sunlight irradiation. The improved photocatalytic performance was attributed to forming a hierarchical heterostructure, which provided a larger specific surface area, improved visible-light absorption capability, reduced recombination of electron–hole pairs, and enhanced charge carrier separation efficiency. Li et al.88 synthesized the WO3 nanosheet arrays (NSAs) using a hydrothermal growth method, and g-C3N4 NSAs on an FTO substrate were prepared through a deposition–annealing process. Specifically, the WO3 NSAs grown on FTO were immersed in g-C3N4 NS dispersions for 1 h and then dried using a nitrogen stream. After repeating this cycle three times, the resulting sample was annealed at 400 °C for 1 h to enhance the adhesion between g-C3N4 NS and WO3 NSAs, facilitating the formation of WO3/g-C3N4 heterojunction arrays (Fig. 7(c)).
Cheng et al.89 synthesized the WO4/g-C3N4 by the one-pot pyrolysis method (Fig. 7(d)). The resulting product was labeled as WO4/g-C3N4. During the high-temperature synthesis, the interaction between WO3 and g-C3N4 modified the composite textural structures, thus improving the photocatalytic activity.90 The elegant Z-scheme hollow microspheres WO3/g-C3N4 composite was synthesized by precisely controlling the in situ hydrolysis, followed by the polymerization process consecutively, and the synthesis mechanism is shown in Fig. 7(e). The hollow structure gained significant attention due to its unique structural, physical, and chemical properties. These structures possessed characteristics such as low density, high specific surface area, and efficient mass transfer and photon utilization, and were used for photocatalytic reduction purposes. Also, the specific hollow structure enabled prolonged light trapping and enhanced photon utilization efficiency.91,92
Similarly, a few methods reported to synthesize transition metal-doped WO4/g-C3N4 are given in Fig. 8(a–c) to fabricate codoped WO4/g-C3N4, flowerlike MnWO4 loading g-C3N4 surface, and FeWO4/g-C3N4 nanosheet composites, respectively. Prabavathi et al.93 successfully synthesized CoWO4 nanoparticles assembled on g-C3N4 nanosheets by the hydrothermal method, followed by ultrasonication. The photocatalytic activity of fabricated CoWO4/g-C3N4 was investigated by the degradation of norfloxacin under visible light, demonstrating higher photocatalytic activity than pristine materials (Fig. 8(a)). The excellent photocatalytic activity of these materials was due to a bivalent metal tungstate semiconductor with excellent thermal and chemical stability; combining this metal tungstate with g-C3N4 showed enhanced photocatalytic activity. The binary metal oxide cobalt tungstate (CoWO4) demonstrated remarkable catalytic performance due to its feasible redox couple states (Co2+/Co3+) and high electrical conductivity ranging from 10−7 to 10−3 S cm−2. Incorporating tungsten (W) atoms in cobalt oxide significantly improved its conductivity compared to pristine cobalt oxide materials.94 Liu et al.95 reported the direct Z-scheme MnWO4/g-C3N4 composite fabricated by a facile hydrothermal method. Forming a direct Z-scheme photocatalytic system is a promising and effective approach to minimize the recombination of photogenerated electrons and holes while maintaining the redox ability of each component.96 The energy level and band structure of MnWO4 (narrow bandgap (2.7 eV)) were well-matched with those of g-C3N4, making it suitable for the formation of MnWO4/g-C3N4 heterojunction with excellent photocatalytic activity under visible light.
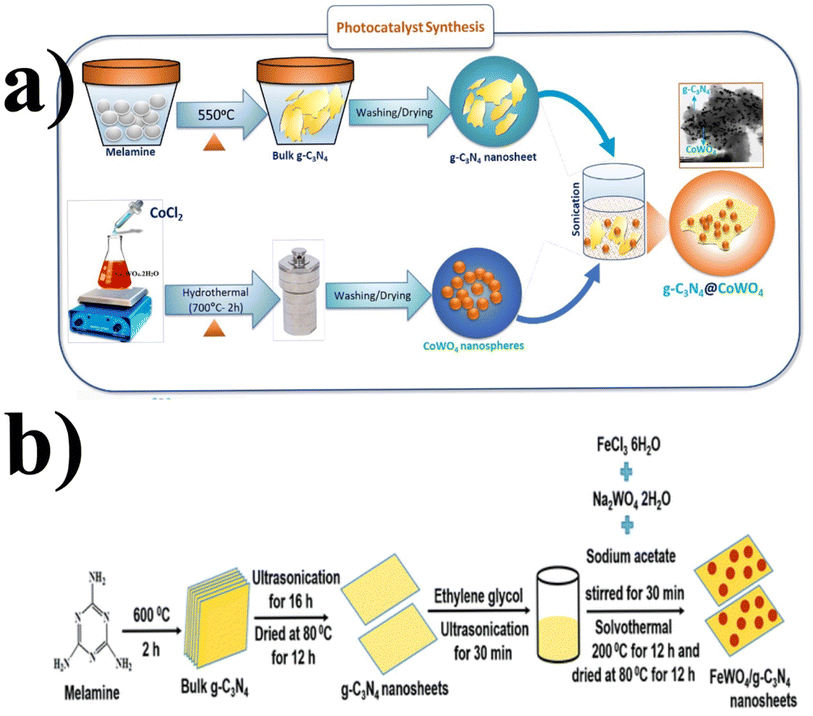 | ||
| Fig. 8 (a) Fabrication of codoped WO4/g-C3N4-based photocatalytic material (reproduced with permission from ref. 93 Copyright 2019, Elsevier). Schematic representation (b) of the FeWO4/g-C3N4 nanosheet composites preparation (reproduced with permission from ref. 97. Copyright 2018, Royal Society of Chemistry). | ||
Dadigala et al.97 synthesized a novel, stable, highly efficient, and visible light active hybrid photocatalytic system for FeWO4/g-C3N4 nanosheets by the in situ self-assembly solvothermal approach. These photocatalysts exhibited superior photocatalytic activity for RhB and TC compared with the pure FeWO4, g-C3N4 nanosheets (Fig. 8(b)). The FeWO4/g-C3N4 composite showed excellent photocatalytic activity because the band level alignment between FeWO4 and g-C3N4 allowed the formation of a direct Z-scheme photocatalytic system, enabling the efficient separation and transfer of photogenerated electron–hole pairs. Besides, FeWO4 and g-C3N4 are visible light-driven photocatalysts, and their combination as the FeWO4/g-C3N4 composite holds promise for achieving high photocatalytic activity.
The design of graphitic carbon nitride–tungsten oxide involves the careful consideration of several key factors. Firstly, the choice of synthesis method is crucial in achieving a well-controlled and homogeneous dispersion of tungsten species. Another important design principle is the tuning of the interface and the interfacial interactions. The interface facilitates efficient charge transfer and promotes synergistic effects between the two materials. Surface modification, functionalization, and heterojunction formation can enhance the interfacial contact and optimize the interfacial charge transfer kinetics. Bandgap engineering to expand the light absorption range, surface modification to enhance the catalytic activity, and cocatalyst deposition to improve charge separation and transfer. The morphology and composition composites are essential to design aspects and can be tailored by controlling the reaction conditions and precursor compositions to obtain desired structures, such as nanoparticles, nanosheets, or hierarchical architectures. Furthermore, efforts can be directed toward optimizing the composition ratios, improving the crystallinity, and exploring novel hybridization to unlock additional functionalities and applications.
The fabrication and photocatalytic applications of g-C3N4/WO3 pose several scientific challenges. One of the challenges is the effective synthesis of well-defined nanocomposite structures with controlled morphology and composition of g-C3N4/WO3. Achieving a uniform dispersion of WO3 on g-C3N4 and maintaining their intimate contact is crucial for maximizing the synergistic effects between the two materials.56,59 Strategies such as metal doping, nonmetal doping, codoping, and heterojunction formation have been explored to modulate the crystallographic, textural, and electronic properties of nanocomposites to enhance their photocatalytic efficiency. However, the cost of these metals is very high; thus, they are not used on a larger scale.98,99 Developing efficient catalysts for the degradation of pollutants, H2 evolution, and CO2 using g-C3N4/WO3 requires a comprehensive understanding of photocatalytic mechanisms and reaction pathways. Exploring the fundamental principles governing the photocatalytic mechanism and elucidating the roles of each component is essential for designing and optimizing a photocatalyst. The fabrication and photocatalytic applications of g-C3N4/WO3 nanocomposites face synthesis, performance optimization, and mechanistic understanding challenges. Addressing these challenges will contribute to advancing g-C3N4/WO3 nanocomposites for various environmental applications.
Electron-rich graphitic nitride materials transfer partial electrons to WO3 due to the lone pair electrons on nitrogen, thus increasing the electron density of WO3. A stable g-C3N4/WO3 composite structure formed by strong interactions leads to stable activity. For strong interaction between g-C3N4 and WO3, the interfacial charge transfer reduces the recombination of the e−/h+ pair, leading to improved efficiency. When weak interactions are formed between two components, decreased photocatalytic activity is observed since the g-C3N4/WO3 composite can split into a mixture of the g-C3N4 and WO3.101,102 Huang et al.101 first synthesized g-C3N4/WO3 nanomaterial from precursor dicyandiamide and (NH4)5H5[H2(WO4)6]·H2O for the degradation of 4-chlorophenol (4-CP) and methylene blue (MB), as shown in Fig. 9(e and f). The result indicates that under visible light irradiation, the activity of WO3/g-C3N4 nanomaterial was 4.2 and 2.9 times higher than that of pristine WO3 and g-C3N4, respectively. The TGA results in Fig. 9(a) reveal that the weight loss of WO3/g-C3N4 occurs in the 510–670 °C temperature range. WO3/g-C3N4 composites show stability before 510 °C (Fig. 9(b)). The SEM images of WO3 indicate that it has obvious edges with particle sizes of approximately 50–200 nm. The TEM images in Fig. 9(e and f) show bright particles of about 100–200 nm diameters (red circles) attached to the g-C3N4 surface. WO3/g-C3N4 showed 97% photocatalytic degradation efficiency for MB degradation within 2 h and 43% for 4-CP within 6 h under visible light irradiation (heterojunction formation due to interparticle electron transfer between component semiconductors). In the 4-CP structure, Cl and oxygen atom are strongly covalently bonded to the aryl or phenyl group, and there is no attacking side for the catalyst, while methylene blue has many atoms in the ring such as sulfur and nitrogen available for attack. These bonds easily break down in methylene blue. This issue can be resolved if the pH is optimized for this reaction.
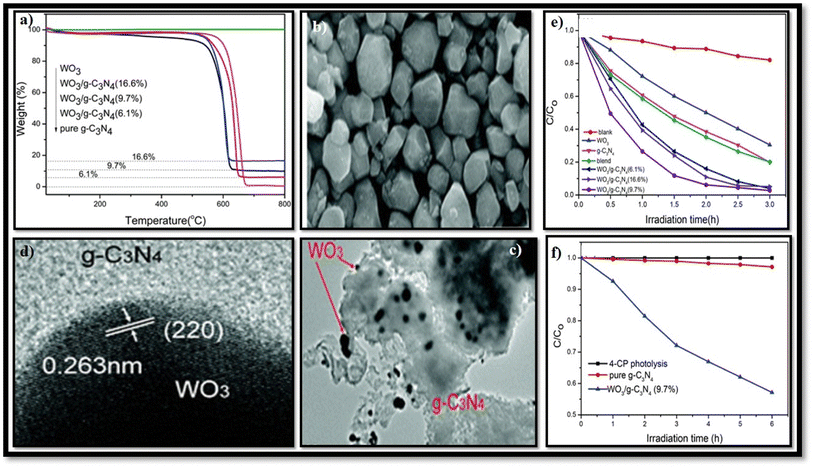 | ||
| Fig. 9 (a–f) (a) TGA of WO3/g-C3N4, (b) SEM of WO3, (c) TEM of WO3/g-C3N4, (d) HRTEM of the WO3/g-C3N4 (9.7%) composite, (e) degradation rate of MB by g-C3N4, WO3, and WO3/g-C3N4, and (f) degradation rate of 4-CP by g-C3N4 and WO3/g-C3N4 (reproduced with permission from ref. 103 Copyright 2013, Royal Society of Chemistry). | ||
Chen et al.104 prepared the WO3/g-C3N4 composite by heat treatment and ball milling procedures. They prepared different samples by varying the g-C3N4 and WO3 to check the methylene blue (MB) and fuchsin (BF) degradation efficiency. The photocatalytic efficiencies of pure g- and g-C3N4 and WO3 for MB (35.6%, 40.0%) and BF (30.9%, 42.4%) were determined. When the quantity of WO3 is 5.0 wt% in g-C3N4, the photocatalyst displays the greatest photocatalytic activity, with the photocatalytic efficiencies for MB and BF of 87.9% and 75.6%, respectively. Yang et al.43 manufactured the WO3/g-C3N4 materials through three different methods such as ultrasonic dispersion, calcination, and hydrothermal method, and the prepared samples by these processes were named CNW(U), CNW(C), and CNW(H), respectively. They used the synthesized materials for Rhodamine B degradation. The rate constant for CNW(U), CNW(C), and CNW (H) was 0.034 min−1, 0.022 min−1, and 0.048 min−1, respectively. CNW(H) showed a rate constant 4 and 2.6 times higher than that of WO3 (0.012 min−1) and g-C3N4 (0.018 min−1), respectively. The BET surface area of WO3, g-C3N4, CNW(U), CNW(C), and CNW (H) was 60.6, 11.2, 14.7, 14.3, and 15.1 m2 g−1, respectively. The degradation efficiency decreased in the order CNWH (95.3%) > CNWU (85.7%) > CNWC (77.2%). To examine the stability, efficiency, and morphological changes of g-C3N4/WO3 with respect to temperature, Doan et al.105 prepared the g-C3N4/WO3 composite at different temperatures (500, 600, and 700 °C) and named them WM(500), WM(600), and WM(700), respectively. At 600 °C and 700 °C, the decomposition of g-C3N4 occurred, and N-doped WO3 formation was realized. The maximum degradation was shown by WM(500) for 80% MB compared to other materials synthesized at different temperature ranges. The above experimental data show that the synthesis method affects the interaction between WO3 and g-C3N4. For example, the hydrothermal method shows the maximum efficiency since it can disperse WO3 on the g-C3N4 surface better than other methods. This strong interaction enhanced interfacial charge transfer, efficient separation of electron–hole pairs (photogenerated), and the highest photocatalytic activity. In addition, the stable composite structure (WO3 and g-C3N4) formed by the strong interaction in CNW(H) contributed to its superior stability compared to other photocatalyst composites.
Similar photocatalytic degradation was performed by Gondal et al.52 They synthesized g-C3N4/WO3 with dissimilar ratios and degraded Rhodamine B (RhB) and methyl tertiary-butyl (MTBE). Almost all WO3/g-C3N4 composites exhibited higher photocatalytic efficiency than pure WO3 or g-C3N4. The photocatalyst g-C3N4/WO3−x−0.1 (combination ratio of WO3 of 0.1) exhibited the highest photocatalytic activity (62%) of RhB under visible light irradiation in 90 min, while under the same conditions, pristine WO3 and g-C3N4 only showed a 42% and 27% photocatalytic efficiency, respectively. Similarly, the highest photodegradation efficiency of WO3/g-C3N4-0.2 (combination ratio of WO3 0.2) for MTBE (96.7%) was reported, while under the same conditions, the photodegradation proficiency attained on the pure phase of WO3 and g-C3N4 was 65.9% and 45.3%, respectively. The photocatalytic activity gradually reduced when the combination ratio of WO3 from 0.1 to 0.9 increased in g-C3N4. The reason could be the accumulation of WO3 on pure g-C3N4. Increasing WO3 on the graphitic carbon nitride decreased the separation efficiency of photoinduced electron–hole pairs and the photocatalytic activity effect.
As reported by Karimi and coworkers,48 the WO3/g-C3N4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) nanocomposite (W1G3) showed the best photocatalytic activity at pH 2.2 for the degradation of RB5 (99%) under solar light irradiation in 90 min. A greater amount of catalyst results in light scattering due to the reduction and aggregation of the irradiation field since higher concentration causes the solution to be more turbid, thus decreasing the light penetration ability and ultimately reducing the photocatalytic performance. Under proper light exposure, a maximum number of electrons were excited, and their efficiency increased. The acidic medium is most favorable for dye degradation instead of the basic medium. By changing the pH of the solution, the surface properties of the WO3/g-C3N4 nanocomposite changed, thus reducing the number of positively charged species. In the basic solution, excess OH ions decreased the photodegradation of RhB5.
3) nanocomposite (W1G3) showed the best photocatalytic activity at pH 2.2 for the degradation of RB5 (99%) under solar light irradiation in 90 min. A greater amount of catalyst results in light scattering due to the reduction and aggregation of the irradiation field since higher concentration causes the solution to be more turbid, thus decreasing the light penetration ability and ultimately reducing the photocatalytic performance. Under proper light exposure, a maximum number of electrons were excited, and their efficiency increased. The acidic medium is most favorable for dye degradation instead of the basic medium. By changing the pH of the solution, the surface properties of the WO3/g-C3N4 nanocomposite changed, thus reducing the number of positively charged species. In the basic solution, excess OH ions decreased the photodegradation of RhB5.
Zhang et al.106 synthesized the same photocatalyst through direct calcination method from raw materials (ammonium tungstate, melamine) at 550 °C and applied it for the selective oxidation (photocatalytic) of 5-hydroxymethyl furfural (HMF) to 2,5-diformylfuran (DFF) in UV and visible light. Compared to visible light, UV light irradiation easily caused overoxidation and resulted in a lower DFF selectivity, and the highest DFF selectivity (85.6%) was achieved with HMF conversion (31.2%) in the presence of oxygen. They also studied the charge transfer/separation efficiency of photogenerated e−/h+ pairs of WO3/g-C3N4, further confirmed by the EIS Nyquist circle. The Nyquist circle of WO3 (4.7%)/g-C3N4 was smaller than that of the pristine. The larger diameters indicate greater interfacial resistance, resulting in a higher current loss. Due to its low conductivity, the g-C3N4 electrode exhibited a large ohmic serial resistance (Rs). The doping of g-C3N4 with WO3 decreased the Rs value, demonstrating a faster interfacial charge transfer, reduced recombination, and enhanced photocatalytic efficiency. In addition, the photocatalytic oxidation of HMF to DFF on the g-C3N4 catalyst was higher in the presence of ACN and PhCF3 as solvents. The choice of the reaction solvent commonly influences the efficiency of a chemical reaction. Each solvent possesses distinct characteristics such as steric hindrance effect, dielectric constant, polarity, and acid–base properties. This enhanced performance could be attributed to its lower polarity and superior oxygen dissipation. Conversely, polar solvents may lead to reduced conversion rates due to their potential competition with the reactant for the active catalytic site.
Yan et al.107 reported the in situ coupling of WO3 with g-C3N4 (WO3/g-C3N4) using single-source precursor melamine and tungstate for MO and TC degradation under visible light in 120 min. MO degradation using the pristine catalyst g-C3N4 and WO3 was 38% and 26%, respectively, while the coupling of WO3 with g-C3N4 (W-CN-5) improved the best photocatalytic performance up to 93% for MO and 97% for TC. The mineralization (TOC) of MO (at 120 min was 52.9%) and TC (at 120 min was 56.6%) over the photocatalysts (W-CN-5) was much larger than that of pristine WO3 (15.7%) and g-C3N4 (19.3%) for MO and 3 times more than that (18.2%) for g-C3N4 for TC, with the highest reaction rate (0.0213 min−1), 5.6 times greater than that of pristine g-C3N4 (0.0038 min−1) for MO using W-CN-5. These results favor the coupling of g-C3N4 and WO3, which promote the photocatalytic activity and mineralization to degrade organic pollutants. MO degradation compared to TC using the same catalyst may be due to the different molecular structures of MO (organic dye) and TC (antibiotic compound) and can influence their degradation kinetics rates. The presence of specific functional groups, aromatic rings, or chemical bonds in TC may make it more susceptible to degradation than MO.
Chang F.108 also synthesized various combinations of WO3/g-C3N4 binary composites by a facile one-step calcination procedure and applied them for RhB and MB photocatalytic degradation. The UV DRS spectra of the pristine and composite are shown in Fig. 10(a). The band energies of WO3/g-C3N4-90% and g-C3N4 were estimated to be 2.48 eV and 2.78 eV, respectively. As shown in Fig. 10(b), the slopes of pristine g-C3N4 and WO3/g-C3N4-90% were calculated as 1 and 0.07, respectively, suggesting indirect and direct transitions. The photoluminescence (PL) spectra in Fig. 10(d) demonstrate the suppressed recombination rate of charge carriers in WO3/g-C3N4 compared to the pristine form. Suppressed recombination rate indicates higher degradation efficiency. Significantly, all binary hybrids composite showed higher photocatalytic efficiencies over RhB and MB than bare WO3 and g-C3N4, as shown in Fig. 10(e and f) under visible light (λ ≥ 420 nm). The photocatalytic performance of composites increased with the WO3 contents by up to 15% but decreased beyond that. This behavior was attributed to the optical competition between WO3 and g-C3N4 components or the potential disruption of heterojunction structures. WO3/g-C3N4 (15%) can degrade about 91.3% of RhB after 150 min and exhibits the largest k value of about 0.0127 min−1. Similarly, MB degradation under visible light irradiation was also checked by WO3/g-C3N4, demonstrating significantly improved photocatalytic efficiency and relatively large k value; the trapping agent detection was also performed, as shown in Fig. 10(g and h). The first limitation of this synthesized photocatalyst is its 150 min degradation time. The second limitation is that this catalyst completely degrades RhB but does not completely degrade MB simultaneously. The possible reason because the degradation of dyes (Rhodamine B and Methylene Blue) depends on various factors (chemical structures, environmental conditions, degradation mechanisms). RhB has a more complex structure with multiple functional groups, making it more susceptible to degradation by environmental factors (reactive chemicals, light, heat). At the same time, MB has a relatively simpler structure (which may contribute to its higher stability). RhB is sensitive to light, especially in the presence of oxygen, and can undergo photobleaching (results in a loss of color). MB is also susceptible to photodegradation; however, its degradation rate may be slower than RhB.
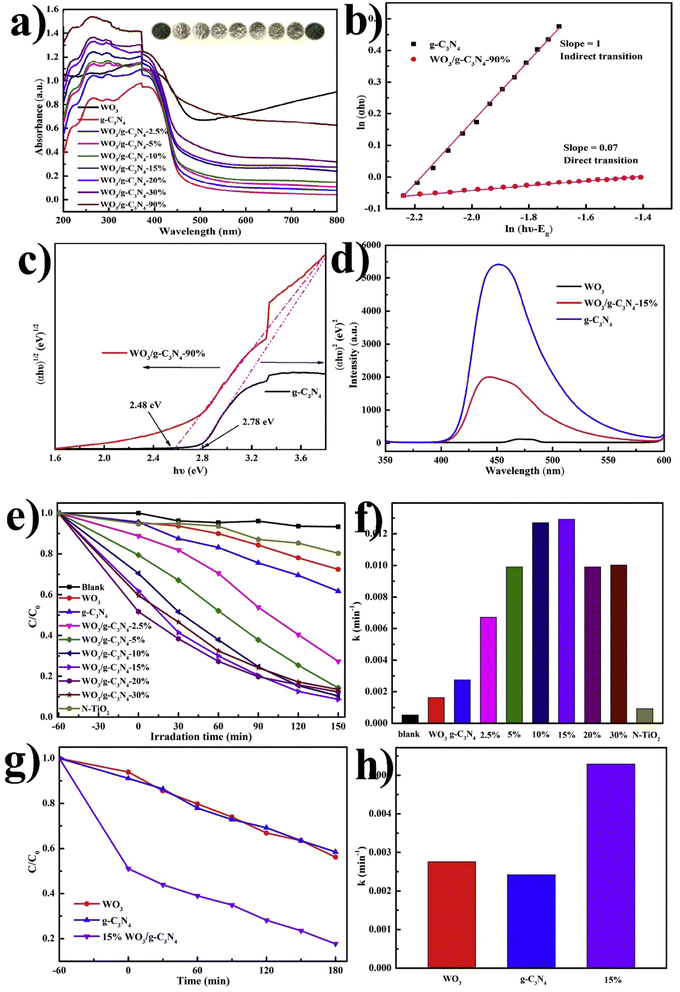 | ||
| Fig. 10 (a) UV-Vis DRS spectrum of bare WO3, g-C3N4, and binary series, (b) color variation in samples in the inset, ln(αhν) vs. ln(hν − Eg) plot, (c) (αhν)2 or (αhν)1/2versus Eg plot, (d) photoluminescence spectra of pristine WO3, g-C3N4, and composite WO3/g-C3N4 (e) photocatalytic degradation of RhB, (f) apparent rate constants (k) for RhB, (g) photocatalytic degradation of MB, and (h) corresponding k values (reproduced with permission from ref. 108 Copyright 2018, Elsevier). | ||
Researchers also used WO3/g-C3N4 for the degradation of other drugs and dyes, desulfuration, and selective oxidation: Ma et al.109 designed a WO3/few-layer g-C3N4 photocatalyst through the in situ calcination method for the oxidative desulfurization activity of Model oil (DBT, 4,6-DMDBT, 4-MDBT, and 3-MBT) at different temperatures from 0 °C to 60 °C. The sulfur removal efficiency was 100% at 50 °C at different intervals. The order of desulfurization activity can be listed as DBT > 4-MDBT > 4,6-DMDBT. Priya et al.110 successfully applied the same catalyst, 3 wt% WO3/g-C3N4, for acid orange 7 (AO7) degradation. The degradation efficiency was 100% using 3 wt% WO3/g-C3N4. Reactive species were detected by introducing the radical scavengers into the reaction solution. Ammonium oxalate (AO) employs holes (h+) detection, and to remove benzoquinone (BQ) and hydroxyl radicals, isopropanol was used to reduce the level of oxygen radicals (O2−). The result shows that the holes (h+) do not significantly affect photodegradation. However, IPA and AO exhibited a quick decrease in the degradation efficiency, revealing the role of superoxide (O2−) and hydroxyl radicals in the degradation using 3 wt% WO3/g-C3N4. Zhu Ma et al.56 developed and employed WO3/g-C3N4 using a facile hydrothermal method. (WCN) heterogeneous antibiotics degradation of sulfamethoxazole (SMX) increased to 91.7% when the optimized material WCN8 was applied at a concentration of 1.0 g L−1. Moreover, the photocatalytic performance inhibited both low pH (pH at 3) and higher pH (pH at 7 and 9), while better photocatalytic activity was obtained without pH adjustment. Another study was successfully carried out by Zhao et al.111 using WO3/g-C3N4 to desulfurize model oil thiophene and dibenzothiophene using an ionic liquid with the assistance of oxidizing agent H2O2 and observed that the 36% WO3/g-C3N4 composite showed improved performance and crystallinity. The removal rate of sulfur for model oil comprising DBT reached 91.2% with H2O2 at 60 °C for 180 min by the 36% WO3/g-C3N4 composite. Navarro et al.112 used WO3/g-C3N4 for the degradation of Orange G (OG) dye and ciprofloxacin (CF). 5% WO3/g-C3N4 degraded 98% (OG) and 100% (CF) as compared to bare g-C3N4 (40% degradation of OG, 60% degradation of CF) and bare WO3 (14% degradation of OG, 19% degradation of CF).
The g-C3N4/WO3 composite degrades when synthesized and combined in appropriate amounts since an optimum content of g-C3N4 into WO3 can facilitate the partition of the electron-opening pair. On the other hand, the doping of g-C3N4 ends up higher than the limits, the space-charge region winds up thin, and the irradiation of light on WO3 greatly surpasses the space-charge layer. This impact may induce prompt and less-demanding recombination of electron openings, bringing down photocatalytic degradation. Despite the improved photocatalytic activity of WO3/g-C3N4, this photocatalyst may still have limitations regarding quantum efficiency, synthesis, and cost. During charge transfer processes or recombination of photoinduced electrons and holes, some energy loss may occur, reducing the overall efficiency of WO3/g-C3N4. Synthesis of the WO3/g-C3N4 composite may involve complex procedures and sometimes requires specific conditions, making its production challenging and time-consuming. This type of complexity can limit the scalability and widespread application of the WO3/g-C3N4 composite. The cost of WO3/g-C3N4 and raw materials used for this composite can be relatively high, and this cost factor can hinder its large-scale commercial applications.
| h+ + H2O → *OH + H+ | (i) |
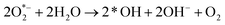 | (ii) |
| O2 + H+ + 2e− → H2O2 | (iii) |
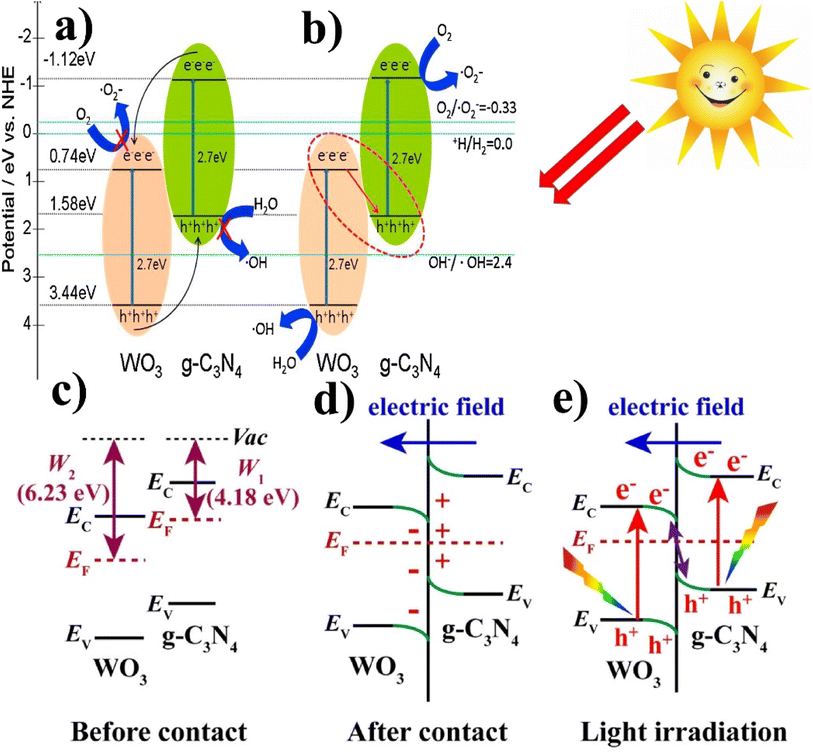 | ||
| Fig. 11 (a and b) Generalized mechanism of the photoexcited e−/h+ separation process of WO3/g-C3N4 (reproduced with permission from ref. 116 Copyright 2014, Elsevier). (c) Before contact with the work functions, g-C3N4 and WO3; (d) after contact at the internal electric field/band edge bending at the interface of WO3/g-C3N4. (e) Under light irradiation, the S-scheme charge transfer mechanism between WO3 and g-C3N4 (reproduced with permission from ref. 82 Copyright 2018, Elsevier). | ||
Katsumata et al.114 also proposed a photocatalytic mechanism for WO3/g-C3N4 composite, as shown in Fig. 11(a and b). In pristine g-C3N4, the photogenerated electrons/holes tend to recombine, which results in low activity because only a small amount (fraction) of them participate in the photocatalytic degradation reaction. Contrary to the pristine from, when the composite of WO3/g-C3N4 contains an optimized content of WO3 (10 wt%), WO3 covered a portion of the g-C3N4 surface, thus reducing electrons/holes recombination and forming a Z-scheme photocatalytic system.
Aslam et al.113 and Gondal et al.115 favored generic band–band transfer over Z-scheme mechanism. The bandgap value for the conduction and valence band of g-C3N4 is 1.57 eV and −1.12 eV, and the bandgap value of VB and CB for WO3 is 3.18 eV and 0.41 eV, respectively. The potential of the g-C3N4 conduction band is lower than the WO3 CB, which results from the migration of photoexcited electrons from the CB of WO3 to the CB of g-C3N4. In addition, the band structure of the WO3/g-C3N4 composite used in this study can be estimated using the following empirical equations.
| EVB = χ − Ee + 0.5Eg | (iv) |
| ECB = EVB − Eg | (v) |
Nidheesh et al.82 also investigated the photocatalytic mechanism of WO3/g-C3N4 by S-scheme heterojunction. Fig. 11 shows the mechanism (a) before contact with the work functions g-C3N4 and WO3, (b) after contact at the interface of WO3/g-C3N4, internal electric field, and band edge bending, and (c) under light irradiation S-scheme charge transfer mechanism between WO3 and g-C3N4. The band edge of g-C3N4 bends upward (loss of electrons), and WO3 bends the edge downward (accumulation of electrons). After light irradiation, electrons are excited from both photocatalysis (VB to CB). Due to the internal electric field, Coulomb interaction, and band edge (bending), the recombination of a few electrons from the conduction band of WO3 and holes (h+) from the valence band (VB) of g-C3N4 occurs and also inhibits the recombination of few electrons from the conduction band of g-C3N4 and holes (h+) from the valence band of WO3. This charge carrier transfer provides supreme redox capacity and helps to carry out the splitting of organic pollutants by providing strong force (S-scheme heterojunction).
Based on the above discussion, it can be inferred that the photocatalytic mechanism of the WO3/g-C3N4 composite does not align with the conventional charge separation process. Specifically, a conventional Z-scheme photocatalyst is preferred for the photocatalytic degradation of organic pollutants. Other researchers have also documented the Z-scheme photocatalytic mechanism of WO3/g-C3N4.114,117Table 1 shows a summary of the tungsten-based graphitic carbon nitride composites for several different applications.
| Precursor | Synthesis method | Parameters | Pollutants | Degradation efficiency | Time | Ref. |
|---|---|---|---|---|---|---|
| (NH4)5H5[H2(WO4)6]·H2O and dicyandiamide | Calcination, 450 °C | 300 W Xe lamp | Methylene blue (MB), 4-chlorophenol (4-CP) | 97% (MB), 43% (4-CP) | 2 h for MB and 6 h for 4-CP | 101 |
| Melamine/Na2WO4·2H2O | Hydrothermal method | 250 W Na lamp | Rhodamine B (RhB) | 95.3% | 2 h | 43 |
| Melamine powder, ammonium tungstate | Ball milling, heat treatment | 500 W xenon lamp | Methylene blue (MB), fuchsin (BF) | 87.9% (MB), 75.6% (BF) | — | 104 |
| Melamine, NaWO4·2H2O | Hydrothermal | 500 W xenon lamp | Rhodamine B (RhB) | 99% | 30 min | 113 |
| Melamine, WCl6 | Calcination | 300 W xenon lamp | Methyl orange (MO) | 60% | 180 min | 118 |
| Melamine, WO3 | Thermal decomposing | Fluorescent lamp | Methylene blue (MB) | 80% | — | 105 |
| Melamine and H2WO3 | Pyrolysis method | 500 W xenon lamp | Rh B and MTBE | 62% (RhB), 96.7% (MTBE) | 90 min | 115 |
| Melamine, H3PW12O40 | Calcination | 60 °C | Dibenzothiophene (DBT) | 91.2% | 180 min | 111 |
| Melamine, urea, Na2WO4·2H2O | Ultrasonic method | Solar light/400 W xenon lamp | Reactive black 5 (RB5) | 98% | 48 | |
| [(C16H33)2N(CH3)2]2 W2O11, urea | In situ calcination | Thermal (heating) | Dibenzothiophene (DBT) | 100% | 30 min | 109 |
| Urea, H2WO3 | Wet-impregnation | 450 W xenon lamps | Acid orange 7 (AO7) | 99% | 75 min | 110 |
| Melamine (NH4)10W12O41·xH2O | Calcination method | 300 W xenon lamp | 5-Hydroxymethyl furfural, 2,5-diformylfuran (DFF) | 85.6% | 10 h | 106 |
Modified WO3/g-C3N4 composites
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) structure is similar to coral and has crystallographic particle spacing (0.20 nm and 0.39 nm) and close interface (g-C3N4 and WO3) that allows accelerating the separation of photoexcited carriers. The coral-like structure (irregular and rougher surface) of the composite enhanced its adsorption capacity for pollutant degradation (MB (98%) and RbX (92%)). Fig. 12(b) shows the UV DRS of WO3/g-C3N4 nanocomposites, pure g-C3N4, WO3, and WO3/g-C3N4-1
1) structure is similar to coral and has crystallographic particle spacing (0.20 nm and 0.39 nm) and close interface (g-C3N4 and WO3) that allows accelerating the separation of photoexcited carriers. The coral-like structure (irregular and rougher surface) of the composite enhanced its adsorption capacity for pollutant degradation (MB (98%) and RbX (92%)). Fig. 12(b) shows the UV DRS of WO3/g-C3N4 nanocomposites, pure g-C3N4, WO3, and WO3/g-C3N4-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, showing the absorption range up to 500 nm, 504 nm, and 575 nm, respectively. The WO3/g-C3N4-1
1, showing the absorption range up to 500 nm, 504 nm, and 575 nm, respectively. The WO3/g-C3N4-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 composite extended the absorption range, appreciably improving the visible light adsorption assisting effective degradation and showing that WO3/g-C3N4-1
1 composite extended the absorption range, appreciably improving the visible light adsorption assisting effective degradation and showing that WO3/g-C3N4-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 absorbs visible light more effectively and generates more electron–hole pairs, thus effectively enhancing its efficiency. The possible reason could be the combination ratio of 1
1 absorbs visible light more effectively and generates more electron–hole pairs, thus effectively enhancing its efficiency. The possible reason could be the combination ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which may result in a synergistic effect arising from improved charge transfer and optimized band structure, where two materials work together more effectively to enhance the degradation efficiency. Deviating from this optimal stoichiometry ratio, such as in the cases of WO3/g-C3N4 (1
1, which may result in a synergistic effect arising from improved charge transfer and optimized band structure, where two materials work together more effectively to enhance the degradation efficiency. Deviating from this optimal stoichiometry ratio, such as in the cases of WO3/g-C3N4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and WO3/g-C3N4 (3
3) and WO3/g-C3N4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), could disrupt the balance of active sites or alter the electronic properties, leading to a decrease in the degradation efficiency. The WO3/g-C3N4-(1
1), could disrupt the balance of active sites or alter the electronic properties, leading to a decrease in the degradation efficiency. The WO3/g-C3N4-(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite may have a higher surface area and more favorable porosity than other compositions, allowing for more efficient adsorption and reaction of the target pollutants, as shown in Fig. 12(c–f).
1) composite may have a higher surface area and more favorable porosity than other compositions, allowing for more efficient adsorption and reaction of the target pollutants, as shown in Fig. 12(c–f).
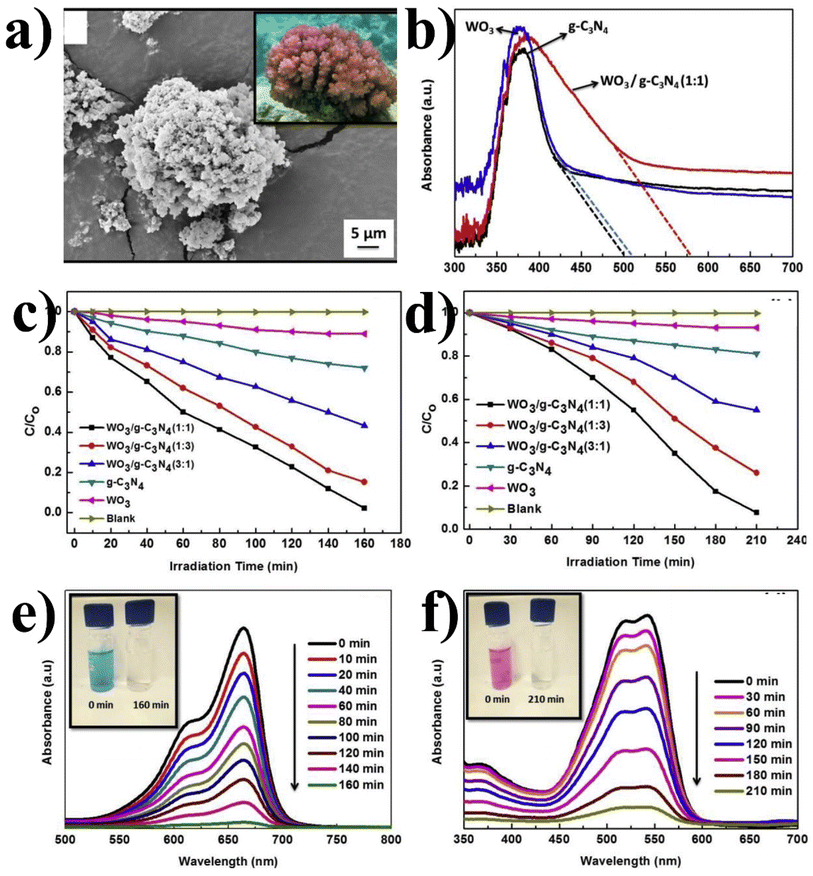 | ||
Fig. 12 (a) SEM image of WO3/g-C3N4 (−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), (b) DRS of WO3/g-C3N4 using various photocatalysts (c) C/C0 MB and (d) C/C0 RbX. (e) UV-Vis spectra at different time intervals under visible light for the degradation of MB and (f) RbX using WO3/g-C3N4 (−1 1), (b) DRS of WO3/g-C3N4 using various photocatalysts (c) C/C0 MB and (d) C/C0 RbX. (e) UV-Vis spectra at different time intervals under visible light for the degradation of MB and (f) RbX using WO3/g-C3N4 (−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (reproduced with permission from ref. 120 Copyright 2019, Elsevier). 1) (reproduced with permission from ref. 120 Copyright 2019, Elsevier). | ||
Lou et al.121 prepared 1D WO3−x nanowires on 2D g-C3N4-NS through a simple solvothermal process. WO3−x nanowires and g-C3N4-NS facilitated the charge transfer in WO3−x and g-C3N4 (beyond WO3−x/g-C3N4-30). Free WO3−x nanowires were created when W(CO)6 concentration in the precursor solution was too high, aggregating the nanowires with no contact with g-C3N4 surfaces and lowering the photocatalytic activity. The highest efficiency photocatalyst was applied for the methyl orange (MO) degradation with 90% efficiency in 30 min. 1D/2D g-C3N4-NS does not completely degrade MO due to certain structural characteristics or functional groups that make it more resistant or may be due to the presence of electron-withdrawing groups in the molecular structure, which can contribute to its photostability. The intermediate byproducts had lower reactivity and required different reaction conditions for complete degradation, leading to incomplete degradation. Firstly, the abundant oxygen vacancies on WO3−x nanowires contributed to a variation in free electrons and improved the conductivity of WO3−x. Secondly, the WO3−x nanowires exhibited a higher capacity for O2 adsorption compared to g-C3N4 due to the presence of surface oxygen vacancies, thereby promoting charge separation on g-C3N4.
Pan and coworker122 prepared for the first time the novel 2D/2D WO3/g-C3N4 thin photocatalyst through the S-scheme combining carbon (C) with anionic polyacrylamide (APAM) through bridge construction and assistant template, which was named as C-W/N and applied for tetracycline (TC) photodegradation. pH slightly affected the degradation efficiency (82.96%, 84.69%, 87.52%, and 86.33% at pH 3, 5, 7, and 9, respectively). The most efficient photocatalyst C-W/N exhibited 91% photodegradation compared to the other synthesized photocatalysts after 1 h irradiation. WO3 exhibited the lowest TC degradation, up to 12.14%, due the fast recombination of electron–hole pairs. By adjusting APAM, the photocatalytic efficiency of WO3 and g-C3N4 was promoted. As expected, when WO3 was combined with g-C3N4, it showed better photocatalytic activities than the pristine one. Although constructing 2D–2D WO3/g-C3N4 is an effective approach for photocatalytic degradation, its practical application is still limited due to its stability and durability. Fei Z. et al.123 prepared the photocatalyst WO3−x/2D g-C3N4 heterostructure by a single-step calcination process and studied its photocatalytic activity against the degradation and removal of tetracycline (TC), Rhodamine B (RhB), and S. aureus under an LED light. Doping with nonmetal oxygen atoms increased the surface area, reduced the bandgap, increased the light-harvesting power, and promoted the transport of charged ions.
Antoniadou and a coworker124 prepared the novel g-C3N4/WO3 thin films for methylene blue dye removal and hexavalent chromium (Cr6+) reduction. After 2 h under UV-A, almost 70% degradation of MB, 92.87% Cr6+ reduction was achieved by g-C3N4/WO3, and 65% degradation of MB, Cr6+ reduction was 6.17% realized by WO3, respectively. C. Xu et al.125 synthesized leaf-like WO3 nanoflakes decorated on g-C3N4 by the facile impregnation and annealing method for benzyl alcohol (BzOH) to benzaldehyde (BzH) conversion, suggesting that no significant conversion was obtained when the experiment was performed at a low temperature (30–50 °C); however, on increasing the temperature up to 80 °C, 98.6% BzOH conversion was observed. Xiao et al.126 constructed WO3/g-C3N4 composite hollow microspheres (CHMs) through the hydrothermal method for the degradation of ceftiofur sodium (CFS) and tetracycline hydrochloride (TC-HCl). The degradation efficiency was 82% for TC-HCl and 70% for CFS within 2 h.
0D, 1D, 2D, and 3D are important in photocatalytic applications.127 0D modifications can improve the stability of materials by creating additional active sites, resulting in enhanced catalytic activity. One-dimensional nanomaterials (1D) can enhance the electron transport within the material, leading to improved conductivity and performance, increasing the material's surface area, providing more contact sites for chemical reactions, and enhancing the catalytic activity and efficiency. In two-dimensional nanomaterials (2D), these two dimensions are outside the nanoscale,128 and 2D modifications (such as graphene-based materials) have a high aspect ratio, which allows for improved mechanical strength and electrical conductivity. Three-dimensional nanomaterials (3D) include bulk powders, dispersions of nanoparticles, bundles of nanowires, and nanotubes, as well as multinano layers that do not have any dimension to the nanoscale and can provide structural support and integrity to the material, making it more robust and resistant to degradation. The 3D structure allows for the efficient mass transport of reactants and products, enabling the better utilization of active sites and improved overall performance. A few disadvantages of these materials include the following: the synthesis techniques may require more complex procedures to obtain well-defined 1D, 2D, and 3D structures, and the synthesis methods for the production of large-scale uniform 2D and 3D structures (control over the morphology and interconnected pore structure in bulk materials) have complex procedures and higher costs. Aggregation in 2D/3D sheets may reduce the active surface area, thus limiting the photocatalytic efficiency. The separation and recovery of 1D, 2D/3D materials from the reaction medium can be difficult, thus impacting the recyclability (Table 2).
| Precursor | Synthesis method | Parameters | Pollutant/application | Degradation efficiency | Time | Trapping agent | Ref. |
|---|---|---|---|---|---|---|---|
| Urea, W(CO)6 | Solvothermal method | 300 W Xe lamp | Orange (MO) degradation | 90% | 35 min | ·OH, ·O2− | 121 |
| Urea, Na2WO3, APAM | Calcination | 300 W xenon lamp | Tetracycline (TC) | 91% | 60 min | ·O2−, h+ | 122 |
| Dicyandiamide, Na2WO4·2H2O, NH4F | In situ strategy | 70 W metal halide lamp | RhB | 100% | 40 min | ·O2− | 85 |
| Urea, Pluronic P123, WCl6 | Calcination process | 1000 W xenon lamp | Rhodamine B | 75% | 100 min | ·OH, ·O2− | 129 |
| Melamine, H4Na2O6W | Thermally exfoliated | 450 W xenon lamp | Phenol | 100% | 35 min | — | 83 |
| Melamine tungsten powder | Calcination | UVA lamps 15 W/BLB | Methylene blue (MB) | 70% | 120 min | ·O2−, h+ | 124 |
| Urea, Na2WO4 | Wet chemical process | 65 W CFL lamp | MB, BR | 98% (MB), 92% (BR) | 160 min 210 min | OH, ·O2− | 119 |
| Melamine, Na2WO4·2H2O | Impregnation and annealing | — | Benzyl alcohol (BA) | 89% | 7 h | — | 125 |
| Melamine, (NH4)10(H2W12O42)·4H2O | Thermal polymerization | 300 W Xe lamp | MO, TC | 93% (MO), 97% (TC) | 120 min | ·O2−, h+ | 107 |
| Melamine, Na2WO4·2H2O, Sr(NO3)2 | Calcination method | XG500 xenon long-arc lamp | Rhodamine B (RhB) | 96% | 100 min | OH, ·O2− | 130 |
| Dicyandiamide, Na2WO4·2H2O | Hydrothermal method | 300 W xenon lamp | Tetracycline hydrochloride | 70% | 120 min | ·OH, h+ | 126 |
Generally, introducing transition metals can form new energy levels and extend the visible light response from UV to visible region, suppressing the recombination rate of electron–hole (e−/h+) charges. In this section, we discuss the transition metal (Ag, Cd, Co, Fe, Cu, Mn, Ni, Zn)-doped WO4/g-C3N4 and their photocatalytic application, and also investigate the binding energies, bandgap reduction, porosity, changes in nanomaterials appearance, and degradation rate after doping transition metals.
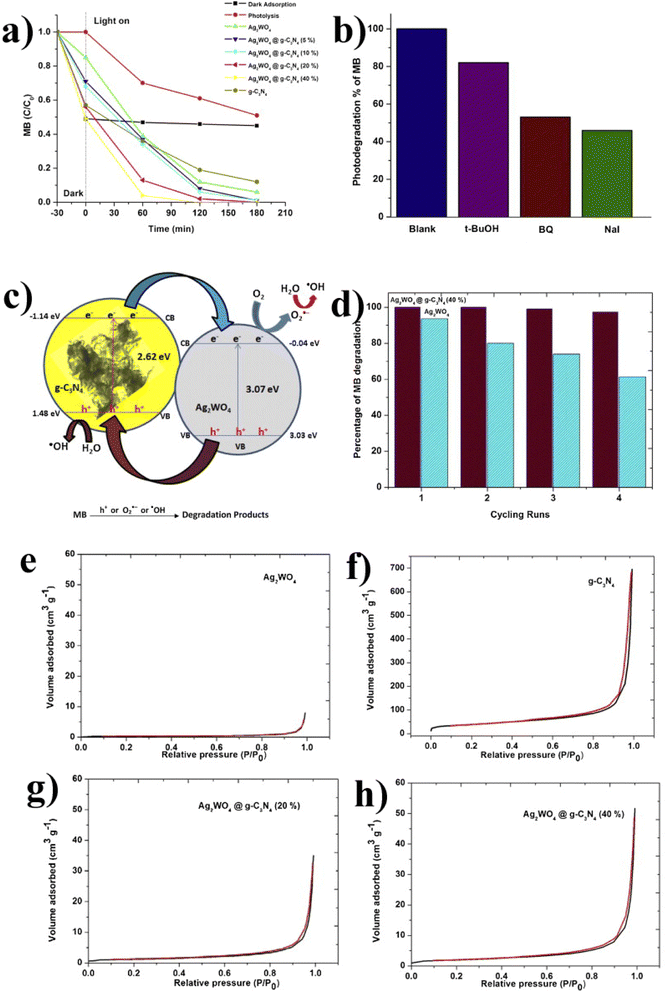 | ||
| Fig. 14 (a) C/C0 MB (10 ppm); (b) schematic diagram of electron–hole transfer. (c) Influence of radical scavengers on MB. (d) Cycling runs. (e–h) The N2 adsorption/desorption isotherms of the pristine Ag2WO4, g-C3N4, and Ag2WO4@g-C3N4 composite (reproduced with permission from ref. 41 Copyright 2015, Elsevier). | ||
Chen et al.131,132 reported 2D–2D AgWO3/g-C3N4 by solvent evaporation, followed by the in situ calcination method for the degradation of dye rhodamine B (RhB) as well as the drug tetracycline (TC). The SBET was measured for g-C3N4 (70.13 m2 g−1), WO3 (11.95 m2 g−1), and AgWO3/g-C3N4 (50.62 m2 g−1). The XPS survey spectrum for the AgWO3/g-C3N4 composite gives the value of C/N/O/W/Ag. In the spectrum of C 1s, the peaks 284.7 eV and 287.9 eV are related to the coordination of N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, and N 1s show three peaks at 398.4 eV, 399.7 eV, and 400.9 eV, indicating the C
N, and N 1s show three peaks at 398.4 eV, 399.7 eV, and 400.9 eV, indicating the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C, N–(C)3, and amino (NH2) groups, respectively. For WO3, the XPS peaks appear at 35.2 eV and 37.3 eV, and the O 1s peak at 529.6 eV indicates O2− in WO3. The Ag 3d peaks (368.1 eV, 374.2 eV) are attributed to Ag0. These values indicated that AgWO3/g-C3N4 was successfully synthesized and applied for RhB and TC degradation, which were degraded to 97% and ≈90%, respectively. This data shows that the reported catalyst is most efficient for one dye (97% RhB degradation takes only 40 min), while it is not efficient for the drug (TC ≈ 90% degradation in 140 min). This catalyst is selective only for one pollutant and less efficient for others. This may be because the photocatalysts' efficiency often depends upon the adsorption of pollutants on the catalyst's surface for degradation, and the adsorption capacity depends on its surface area, surface chemistry, and pore structure. Suppose a specific photocatalyst has a high adsorption capacity for a specific pollutant. It can effectively concentrate the pollutant near active sites and enhance the degradation rate of the pollutants for this catalyst, while if the same catalyst has low adsorption capacity for another pollutant, the pollutant may not be efficiently captured by the photocatalyst and is less degraded.
N–C, N–(C)3, and amino (NH2) groups, respectively. For WO3, the XPS peaks appear at 35.2 eV and 37.3 eV, and the O 1s peak at 529.6 eV indicates O2− in WO3. The Ag 3d peaks (368.1 eV, 374.2 eV) are attributed to Ag0. These values indicated that AgWO3/g-C3N4 was successfully synthesized and applied for RhB and TC degradation, which were degraded to 97% and ≈90%, respectively. This data shows that the reported catalyst is most efficient for one dye (97% RhB degradation takes only 40 min), while it is not efficient for the drug (TC ≈ 90% degradation in 140 min). This catalyst is selective only for one pollutant and less efficient for others. This may be because the photocatalysts' efficiency often depends upon the adsorption of pollutants on the catalyst's surface for degradation, and the adsorption capacity depends on its surface area, surface chemistry, and pore structure. Suppose a specific photocatalyst has a high adsorption capacity for a specific pollutant. It can effectively concentrate the pollutant near active sites and enhance the degradation rate of the pollutants for this catalyst, while if the same catalyst has low adsorption capacity for another pollutant, the pollutant may not be efficiently captured by the photocatalyst and is less degraded.
Li et al.133 synthesized the same photocatalyst with some modifications. He prepared ultrathin Ag2WO4/g-C3N4-NS by a simple deposition–precipitation method for the degradation of methyl orange and Rhodamine B. After irradiation of 20 min in RhB, self-degradation is almost negligible, and Ag2WO4/g-C3N4-NS, NS-20 (RhB 100%) show higher photocatalytic activities than Ag2WO4 and g-C3N4-NS, NS-10, NS-30. In NS-30 (30%), further increasing Ag2WO4 reduces the photocatalytic efficacy and shows that during the photocatalytic reaction, the mass ratio of Ag2WO4 and g-C3N4-NS is crucial to the synergistic effects. For comparison, P25, g-C3N4-NS, and bulk g-C3N4-20 composite were also investigated, and less than 20% RhB could be removed under the same conditions, which has much lower photocatalytic activities than the NS-20 composite. In the absence of photocatalysts, the direct photolysis of MO is almost ignored. After 50 min in visible light, MO (6%) can be removed by Ag2WO4, g-C3N4-B, and P25 but only 15–30% by B-20 and g-C3N4-NS. Under the same conditions, about 85% MO is degraded by NS-20. Under the same experimental conditions, the corresponding kinetic constants (k) for RhB and MO by NS-20 were calculated and displayed. The reaction rate constant for RhB degradation is 0.2180 min−1, 18.9, 53.6, 10.9, and 26.5 times higher than that of Ag2WO4, g-C3N4-B, g-C3N4-NS, and B-20 samples, respectively. Similarly, the MO degradation sample is 0.0394 min−1, 28.4, 30.6, 4.8, and 9.8 times higher than that of Ag2WO4, g-C3N4-B, g-C3N4-NS, and B-20 samples, respectively. The results showed that the novel Ag2WO4/g-C3N4-NS heterostructures are excellent photocatalytic and can have great photocatalytic activity.
Huang et al.134 synthesized a novel catalyst Ag2WO4/P doped g-C3N4 (PCN) by single-step thermal polymerization for indomethacin (IDM) degradation. The experimental data shows that 30% (weight percentage) Ag2WO4 in the P-doped g-C3N4 by composites exhibits excellent photocatalyst efficiency. The BET surface of 30% Ag2WO4/PCN was determined to be 61.43 m2 g−1, and the photocatalytic system's total organic carbon (TOC) removal rate was 51.9%. The IDM degradation rate constants of 30% Ag2WO4/PCN were −0.233 per min, 4.59 times greater than that of 30% Ag2WO4/CN (−0.0508 per min). There are already a number of the Ag2WO4/CN composite used for the degradation of the drugs, but here the purpose of the doping of P into the graphitic carbon nitride may be due to the following reason: P doping in graphitic carbon nitride increases the negative surface charge and can also modify the surface chemistry and morphology of g-C3N4. It may enhance the adhesion of Ag+, which ultimately improves the stability of the phosphorus-doped Ag2WO4/CN composite, promotes efficient charge separation, and exposes more active sites. These effects contribute to the composite material's overall photocatalytic activity and performance in pollutant degradation. The overall summary is that the unique properties of Ag2WO4 (narrow bandgap, excellent photocatalytic capability) and complement characteristics of g-C3N4 offers a stable π-conjugated structure (promotes efficient charge separation), which enables the composite for the effective utilization of solar energy and enhancing the overall photocatalytic activity.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 CdWO4/g-C3N4 nanomaterials for RhB is maximum, with the highest rate constant value (k = 0.164 h−1) in the visible spectrum region. These degradation efficiency values are 1.6 and 54.6 times greater than that of pure g-C3N4 and CdWO4, respectively. SEM analysis data of CdWO4 shows that it consists of nanorods with 50–100 nm length and 20–50 nm width. The TEM analysis for pure CdWO4 shows a clear fringe with 0.301 nm interval, which forms the (1 1 1) lattice plane (CdWO4 monoclinic). Active species trapping experiments were carried out using EDTA and IPA, showing the existence of h+/·OH species, which plays a major role in the photocatalytic mechanism. The PL emissions of CdWO4 and Cd/g-C3N4 composites were observed in the 480–500 nm visible range. The PL CdWO4/g-C3N4 exhibited the lowest intensity, showing its lowest recombination. The possible reason behind this may be due to the reason that g-C3N4 and CdWO4 have different bandgap energies, and the energy levels of CdWO4 and g-C3N4 (after being combined in the composite) can align in a way that favors charge transfer and inhibits recombination. This alignment will facilitate the efficient migration of the photoexcited e−/h+ toward the respective energy levels (in CdWO4, g-C3N4) and minimize the recombination of the photoexcited e−/h+, resulting in a reduced PL signal.
10 CdWO4/g-C3N4 nanomaterials for RhB is maximum, with the highest rate constant value (k = 0.164 h−1) in the visible spectrum region. These degradation efficiency values are 1.6 and 54.6 times greater than that of pure g-C3N4 and CdWO4, respectively. SEM analysis data of CdWO4 shows that it consists of nanorods with 50–100 nm length and 20–50 nm width. The TEM analysis for pure CdWO4 shows a clear fringe with 0.301 nm interval, which forms the (1 1 1) lattice plane (CdWO4 monoclinic). Active species trapping experiments were carried out using EDTA and IPA, showing the existence of h+/·OH species, which plays a major role in the photocatalytic mechanism. The PL emissions of CdWO4 and Cd/g-C3N4 composites were observed in the 480–500 nm visible range. The PL CdWO4/g-C3N4 exhibited the lowest intensity, showing its lowest recombination. The possible reason behind this may be due to the reason that g-C3N4 and CdWO4 have different bandgap energies, and the energy levels of CdWO4 and g-C3N4 (after being combined in the composite) can align in a way that favors charge transfer and inhibits recombination. This alignment will facilitate the efficient migration of the photoexcited e−/h+ toward the respective energy levels (in CdWO4, g-C3N4) and minimize the recombination of the photoexcited e−/h+, resulting in a reduced PL signal.
Maavia et al.136 synthesized the same catalyst (g-C3N4/CdWO4) through the hydrothermal method and used it for minocycline (MC) degradation with bandgap energies calculated for CdWO4 (3.31 eV), g-C3N4 (2.67 eV), and g-C3N4/CdWO4 (2.71 eV). The FTIR spectra of CdWO4 provide information about three types of peaks. The two peaks at 522 cm−1 and 594 cm−1 are the bond stretching vibrations modes of Cd–O, and the peaks at 711 cm−1 and 820 cm−1 indicate W–O and Cd–O–W, respectively. The UV-visible spectra results show that g-C3N4 has an absorption at 460 nm and CdWO4 at about 380 nm. But the g-C3N4/CdWO4 composite shows an increase in the absorption peak intensity from 455 to 470 nm, and this g-C3N4/CdWO4 composite shows better degradation than pristine materials, may be due to the reason that g-C3N4 has good absorption capacity in the visible range, while CdWO4 absorbing UV light and by combining, g-C3N4/CdWO4 can effectively utilize both UV and visible light; this broad spectrum range allows g-C3N4/CdWO4 to degrade the pollutants efficiently. In short, combining the two materials (g-C3N4 and CdWO4) creates a unique interface, promoting the surface reactions or photocatalytic activity. These prominent factors contribute to its superior photocatalytic performance and make it a promising material for various applications in photocatalysis.
Prabavathi et al.138 reported that CoWO4/g-C3N4 nanomaterials were successfully fabricated by the hydrothermal method, followed by ultrasonication, and used for norfloxacin degradation. For the chemical bond information (Fig. 15(a)), the XPS analysis of a high-resolution spectrum of the Co 2p shows two peaks at 780.4 and 793 eV, which are related to Co 2p3/2 and Co 2p1/2, respectively, or it could be attributed to a +2 state and bandgap energy of CoWO4 (2.2 eV), g-C3N4 (2.71 eV), and CoWO4/g-C3N4 (1.85 eV), as shown in Fig. 15(b). The electron transport resistance (Rcr kΩ) values are CoWO4/g-C3N4 (9.34), CoWO4 (42.50), and g-C3N4 (47.11), which shows that CoWO4/g-C3N4 has less charge transfer resistance and good interfacial contact than the other two (Fig. 14(c and d)). The norfloxacin degradation rate/first-order rate constant k at 80 min was observed for g-C3N4 nanosheets (∼51.2%, 0.0089 S−1), CoWO4 (∼57%), and CoWO4/g-C3N4 (91%, 0.0283 S−1, 0.0105 S−1). The efficiency of CoWO4/g-C3N4 is about 3.181, 2.691 times greater than that of g-C3N4 nanosheets and CoWO4 nanorods, as shown in Fig. 14(e and f).
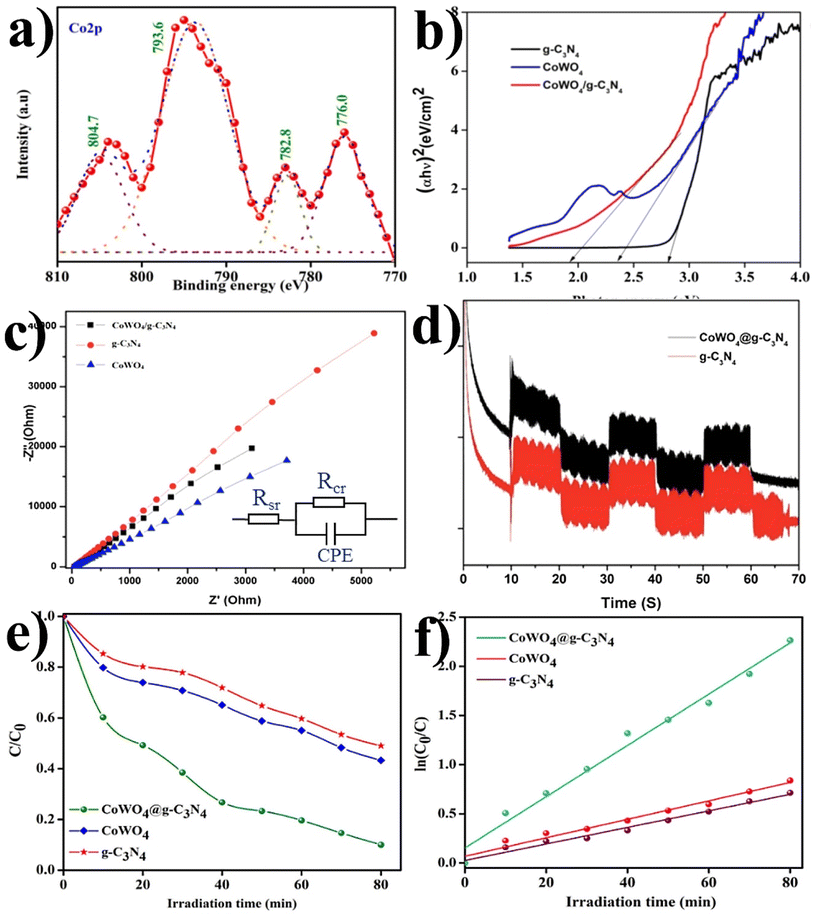 | ||
| Fig. 15 (a) XPS spectrum of Co 2p, (b) the corresponding Tauc plot. (c) Electrical impedance, (d) transient photocurrent studies, (e) photocatalytic degradation of norfloxacin, (f) corresponding kinetics curves of CoWO4/g-C3N4, CoWO4, g-C3N4, and CoWO4/g-C3N4 (reproduced with permission from ref. 93 Copyright 2019, Elsevier). | ||
Sahoo et al.139 designed CoWO4-modified g-C3N4 nanocomposite by the reflux-calculation method for ciprofloxacin (CIP) degradation. UV-Vis diffuse reflectance analysis (UV-DRS) was conducted for the optical response of CoWO4-modified g-C3N4 nanocomposite, and a characteristic absorption band was observed at 580 nm. The photoluminescence spectrum (PL) emission spectra of g-C3N4 and CoWO4/g-C3N4 at 440 nm (strong emission band) because due to the recombination of the band–band charge carrier. Similarly, the degradation rate of 57% for CIP by CoWO4 and 81% for CoWO4/g-C3N4 in 60 min was observed. During the trapping experiment, superoxide and hydroxide radicals were the main factors for the degradation of CIP. The above summary and results are in favor of the CoWO4/g-C3N4 composite, which can offer enhanced surface reactivity compared to the pristine catalyst because the combination of CoWO4 and g-C3N4 creates a unique interface between them and promotes the surface reactions and catalytic activity. Besides these, the CoWO4 nanoparticles on the surface of g-C3N4 increase the active sites available for photocatalysis, leading to improved photocatalytic efficiency and higher degradation rates of the pollutants.
Rashidizadeh et al.142 also synthesized a fabricated heterojunction g-C3N4 nanosheet/FeWO4 nanoparticle for tandem photooxidation/Knoevenagel condensation reaction under visible light and O2 as a green oxidant. Similarly, Choi et al.143 reported the heterostructure FeWO4/g-C3N4(FWO/CN) functionalized with the N-doped graphene quantum dots (NGQD) synthesized under the numerous conditions of sonication for the effectual removal of harmful vapors such as 2-butoxyethanol (2BE) and ethylbenzene (EB). C3N4/FeWO4 photocatalyst was also synthesized144via the facile sonochemical method with post-annealing treatment for the reduction of CO2. Hexangular flowerlike FeWO4 heterojunctions containing g-C3N4 photocatalyst were fabricated using the in situ growth method.145 In Fig. 16(a and b), the X-ray diffraction (XRD) patterns of each (g-C3N4 and FeWO4) of the composite exhibit the typical diffraction peaks. For pure FeWO4, the peaks at 15.60 (010), 18.81 (100), 23.72 (011), 24.50 (110), 30.50 (111), 31.41 (020), 36.42 (002), 38.30 (200), 41.11(200), 44.22 (102), 45.50 (112), 48.60 (211), 51.81(022), 53.90 (130), 61.50 (221), 65 (113), 68.70 (132), and 71.50 (041) planes of monoclinic FeWO4 (JCPDS card file no. 71-2390). g-C3N4 peaks at 12.8 and 27.5° were indexed to the (100) and (002) planes, respectively. The composite g-C3N4/FeWO4 (CNU–FWx) contains both pristine peaks in the XRD patterns. The FTIR peaks at 834 cm−1, 877 cm−1, and 566 cm−1 correspond to the characteristic peaks of the Fe–O–W and Fe–O bond. For thermostability (TGA), bulk g-C3N4 (CNU) exhibited rapid weight loss at 520 °C and at about 700 °C; its degradation was completed (Fig. 16(c)). Similarly, the rapid weight loss of g-C3N4/FeWO4's began at 430 °C and reached 500 °C. The photocurrent response of bulk and optimized materials during five periodical-off cycles is shown in Fig. 16(d) and enhanced the photocurrent response generated for optimized materials (CNU–FW90). Furthermore, a small arc radius of CNU–FWx was found in the EIS Nyquist plots, which means that there is a lesser recombination of electron–hole pairs among the interface of g-C3N4 and FeWO4, as shown in Fig. 16(e). In Fig. 16(f), FeWO4 exhibited poor activity (0.79%) for RhB and FeWO4 coated on g-C3N4. An enhancement in the photodegradation of RhB up to 97.2% was achieved in only 30 min. This is because of the arrangement of FeWO4/g-C3N4 after composite formation. The close integration between the two materials allows for effective photoexcited (e−/h+) migration, reducing the recombination and enhancing photocatalytic activity, as shown in Fig. 16(h). A schematic illustration of the photocatalytic mechanism of g-C3N4/FeWO4 is shown in Fig. 16(g). The g-C3N4/FeWO4 heterojunction demonstrates a lower valence band (VB) level compared to the pristine by 0.41 V, and a significant difference confirms the enhanced photooxidation ability of the heterojunction since the lower VB level suggests that the heterojunction has a higher capability for accepting and transferring photoexcited holes, which are crucial for driving photooxidation reactions. This unique property of the g-C3N4/FeWO4 heterojunction contributes to its stronger photooxidation ability, making it a promising material for various photocatalytic applications.
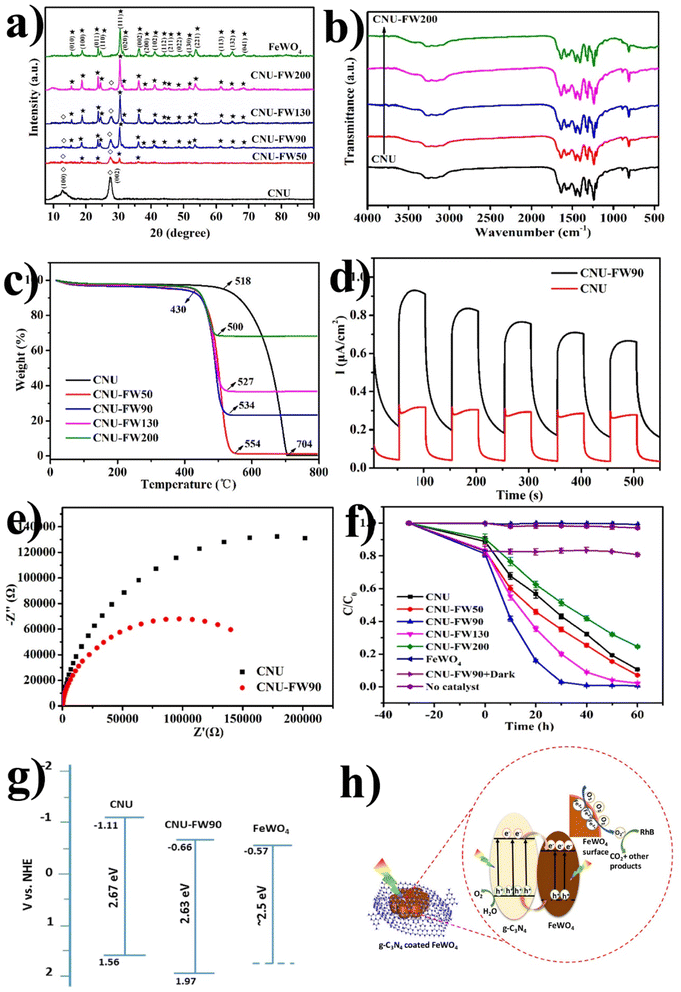 | ||
| Fig. 16 (a) XRD patterns, (b) FTIR spectrum, (c) TGA profile, (d) photocurrent response, (e) EIS Nyquist plots, (f) photocatalytic activity toward the degradation of RhB, (g) bandgap structures, (h) schematic illustration of the photocatalytic mechanism of g-C3N4/FeWO4 (reproduced with permission from ref. 145 Copyright 2018, American Chemical Society). | ||
Zhou et al.147 synthesized the same photocatalyst CuWO4/g-C3N4 by hydrothermal reaction for Rhodamine B (RhB) degradation. The 10%CuWO4/g-C3N4 composite displayed the highest degradation efficiency of 93% for RhB within 150 min, and the photodegradation efficiency remains 80% after four cycles, and the reaction mechanism of CuWO4/g-C3N4 photocatalyst was fully discussed to according to the formation of the Z-scheme system. The Z-scheme formed may be because CuWO4 and g-C3N4 have different energy band structures, and CuWO4 has a narrower bandgap than g-C3N4, resulting in the different positions of their VB and CB energy levels. The energy levels of CuWO4 and g-C3N4 align in such a way (after the combination of these two composites) that the CB of CuWO4 is higher than the CB of g-C3N4, while the VB of g-C3N4 is higher than the VB of CuWO4, and this energy level alignment forms the basis for efficient charge transfer and Z-scheme formation. Under the irradiation of light, g-C3N4 absorbs light and generates the e−/h+ pair. As discussed above, due to energy level alignment, the photoexcited electrons in the CB (g-C3N4) is readily transferred to the CB of CuWO4. Here, they are trapped and utilized for redox reactions. Similarly, the h+ generated in the VB of g-C3N4 migrated to the VB of CuWO4, creating a strong oxidation environment. This efficient charge separation and transfer between CuWO4 and g-C3N4 form the Z-scheme system.
The same material was synthesized with some modifications. 1D/2D MnWO4 nanorods were anchored on g-C3N4 nanosheets via a one-pot hydrothermal approach for the photocatalytic degradation of ofloxacin (OFX 90.4%), and the calculated bandgap for this material was 2.58 eV.149 It was experimentally observed that different combinations of the photocatalyst show different photocatalytic activity, for e.g., MnWO4@g-C3N4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 (45%), MnWO4@g-C3N4 1
0.25 (45%), MnWO4@g-C3N4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 (50.2%), MnWO4@g-C3N4 1
0.5 (50.2%), MnWO4@g-C3N4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (90.4%), MnWO4@g-C3N4 1
1 (90.4%), MnWO4@g-C3N4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 (56.8%), and MnWO4@g-C3N4 1
1.5 (56.8%), and MnWO4@g-C3N4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (45%), and when the g-C3N4 ratio was increased to 1, maximum degradation was observed due to sufficient interaction, leading to synergy toward OFX degradation. After that, a further increase in the g-C3N4 ratio results in lesser degradation efficiency due to the shielding effect among MnWO4 nanorods and OFX molecules. As we know, increasing the ratio of one material to the other material beyond a limit can cause the aggregation of particles within the composite and can also cause a mismatch in electronic and energy levels between the materials. This aggregation reduces the available surface area for catalytic reactions, hinders efficient charge transfer, limits the active sites, impedes the utilization of photoexcited electrons/holes, and hinders the accessibility of the reactants to the catalyst. Consequently, the photocatalyst may experience higher recombination rates and reduce the overall efficiency. Inorganic anions such as SO42−, CO32−, and Cl− can also affect the degradation ability of the photocatalysts because of the scavenging properties created by the formation's weak oxidative species. MnWO4/g-C3N4 (MWG) hybrid catalysts were synthesized by a facile hydrothermal approach for the degradation efficiency of RhB and 4-CP dyes.150 MWG3 (3
2 (45%), and when the g-C3N4 ratio was increased to 1, maximum degradation was observed due to sufficient interaction, leading to synergy toward OFX degradation. After that, a further increase in the g-C3N4 ratio results in lesser degradation efficiency due to the shielding effect among MnWO4 nanorods and OFX molecules. As we know, increasing the ratio of one material to the other material beyond a limit can cause the aggregation of particles within the composite and can also cause a mismatch in electronic and energy levels between the materials. This aggregation reduces the available surface area for catalytic reactions, hinders efficient charge transfer, limits the active sites, impedes the utilization of photoexcited electrons/holes, and hinders the accessibility of the reactants to the catalyst. Consequently, the photocatalyst may experience higher recombination rates and reduce the overall efficiency. Inorganic anions such as SO42−, CO32−, and Cl− can also affect the degradation ability of the photocatalysts because of the scavenging properties created by the formation's weak oxidative species. MnWO4/g-C3N4 (MWG) hybrid catalysts were synthesized by a facile hydrothermal approach for the degradation efficiency of RhB and 4-CP dyes.150 MWG3 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) displayed the high photocatalytic activity of RhB (98%), high first-order kinetics of 0.0923 min−1, long-term stability, and loss of only 2.8%. The J–V characteristics reveal that the MnWO4/g-C3N4 photoanode shows high photoconversion efficiency (PCE%) at 7.15% and pure MnWO4 at 2.34%.
1) displayed the high photocatalytic activity of RhB (98%), high first-order kinetics of 0.0923 min−1, long-term stability, and loss of only 2.8%. The J–V characteristics reveal that the MnWO4/g-C3N4 photoanode shows high photoconversion efficiency (PCE%) at 7.15% and pure MnWO4 at 2.34%.
When mixed in the proper amount and method, each composite material has optimal stoichiometry, desired synergistic effects, and photocatalytic activity. Deviating from this optimal ratio can disrupt the balance of interactions and hinder the desired functionalities. Similarly, optimizing various experimental parameters (such as catalyst loading, irradiation intensity, and reaction temperature) can enhance the photocatalytic activity.
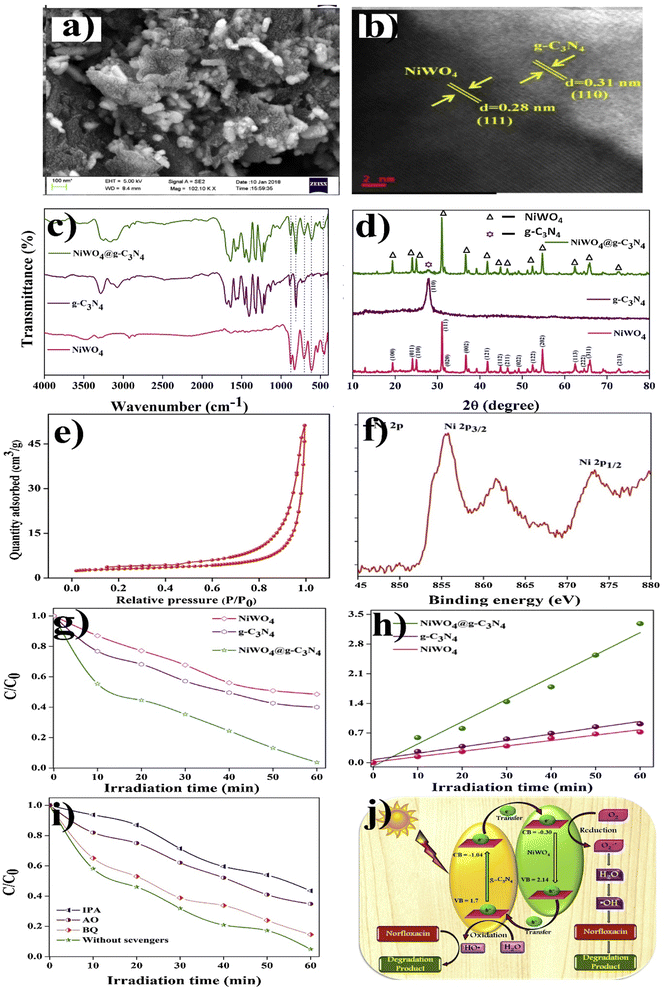 | ||
| Fig. 17 (a) SEM images, (b) TEM images, (c) FT-IR spectra, (d) XRD, (e) BET, (f) XRD of the NiWO4@g-C3N4 nanocomposite, (g) C/C0, (h) kinetic study, (i) trapping agent, and (j) mechanism of photocatalytic degradation of NRF by the NiWO4@g-C3N4 nanocomposite (reproduced with permission from ref. 152 Copyright 2019, Elsevier). | ||
g-C3N4 and NiWO4 were fabricated to establish g-C3N4/NiWO4 for the photocatalytic degradation of toluene.153 The combination of 1C/1N exhibited the highest photocatalytic degradation activity: removal efficiency (95.3%) and mineralization degree (99.1%). The g-C3N4/NiWO4 heterojunction absorbs visible light for electron excitation from the CB to VB, and due to the heterojunction, the photoexcited electrons in the CB of NiWO4 combine with the photoexcited holes in the VB of the g-C3N4, which provide the maximum amounts of accessible electrons and holes for toluene degradation under visible light. This interface-driven synergy enhances the overall performance of the composite and contributes to its superior catalytic activity in various photocatalytic applications. NiWO4@g-C3N4 may face challenges related to stability and durability because the nanocomposite's prolonged usage may undergo structural degradation, chemical leaching, or material loss, leading to reduced catalytic performance and hindering its practical applications.
Recently, ZnWO3/g-C3N4 (CZW) was reported for phenol degradation under UV irradiation.155 Their characterization is checked by different techniques such as XRD data, which shows the diffraction angles at 15.30° (0 1 0), 23.78° (0 1 1), 24.25° (0 2 1), 30.56° (1 1 1), and 36.32° (0 0 2). These peaks could be attributed to the structure of the ZnWO4. In the FT-IR spectra, two peaks at 3447 cm−1 and 1620 cm−1 indicate that the basic hydroxyl groups existed in ZnWO4, and the surface of ZnWO4 was hydroxylated. Similarly, in the XPS data, the binding energies at 1045.1 eV and 1021.7 eV are assigned to Zn2+ 2p1/2 and Zn2+ 2p3/2, respectively. The binding energy values of Zn 2p3/2 and Zn 2p1/2 were observed at 1021.7 and 1045.1 eV, respectively, which can be assigned to Zn2+ ions in ZnWO4. The BET-specific surface areas were determined for g-C3N4 (4.698 m2 g−1), ZnWO4 (4.698 m2 g−1), and 5-CZW (21.315 m2 g−1). The different combinations of photocatalysts show the different degradation efficiency of ZnWO4 (16.8%), 1-CZW (21.8%), 3-CZW (25.4%), 5-CZW (30.4%), 7-CZW (23.2%), and g-C3N4 (3.9%).
In transition metal-doped WO3/g-C3N4, the transition metal can improve the photocatalytic efficiency of the composites. Some transition metals can enhance the stability, ensure long-term performance, and extend the composite's lifespan. Moreover, transition metal dopants can modify the electronic structure and band alignment, and this modification facilitates efficient charge separation, reducing electron–hole recombination and enhancing the overall photocatalytic performance. Transition metal-doped WO3/g-C3N4 has potential in various fields such as photocatalytic water splitting to produce hydrogen, solar cell, and CO2 reduction. Furthermore, it can be employed in energy storage devices, sensors, and optoelectronic devices. However, some disadvantages of this composite are briefly described below: transition metal-doped WO3/g-C3N4 can only be degraded by a limited number of dyes, drugs, and other pollutants because different pollutants possess varying chemical structures and properties, and if a composite is well-suited to the chemical nature of a pollutant, it can form strong bonds, promote reactive species generation, and facilitate degradation. However, pollutants with complex chemical structures may be more challenging for the composite to break down effectively. Moreover, the composite possesses specific active sites that promote photodegradation and activity, and the selectivity of these active sites determines how effectively the composite can interact with pollutants. The composite with high activity can facilitate the conversion of maximum pollutants into less harmful byproducts, but the composite with selectivity can specifically target certain pollutant species. Therefore, the different composite may be designed to target specific pollutants or related pollutant classes, and the degradation depends on a combination of composite properties and pollutant characteristics.
Table 3 presents the transition metal-doped WO3/g-C3N4-based materials for the degradation and removal of various materials with their efficiency.
| Catalyst | Synthesis method | Parameters | Pollutant/application | Degradation efficiency | Time | Trapping agents | Ref. |
|---|---|---|---|---|---|---|---|
| Ag2WO4@g-C3N4 | Sono-chemical impregnation | 150 W xenon arc lamp | MB | 100% | 120 min | h+, O2˙− | 41 |
| AgWO3/g-C3N4 2D/2D heterostructure | Solvent evaporation and in situ calcination | XG500 xenon long-arc lamp | RhB | 96% (RhB), ≈90% (TC) | 40 min, 140 min | O2˙−, ·OH | 131 |
| TC | |||||||
| Ag2WO4/P doped g-C3N4 | Single-step thermal polymerization | 350 W xenon lamp | Indometacin orange (MO) | 52% | 15 min | O2˙− | 134 |
| CdWO4/g-C3N4 | Mixed-calcination method | 500 W xenon lamp | Rhodamine B (RhB) | ≈45 | 4 h | ·OH, h+ | 135 |
| g-C3N4/CdWO4 | Hydrothermal process | 250 W xenon lamp | Minocycline (MC) | 86% | 5 h | ·OH | 136 |
| CoWO4/g-C3N4 | Hydrothermal method | Tungsten–halogen | Norfloxacin (NF) | 91% | 80 min | ·OH | 138 |
| g-C3N4/FeWO4 | Solvothermal method | Sunlight | RhB, TC | 99% (RhB), 88% (TC) | 90 min | O2˙−, ·OH | 141 |
| CuWO4/g-C3N4 heterojunction | Hydrothermal method | 500 W xenon lamps | Rhodamine B (RhB) | 93% | 150 min | O2˙−, ·OH | 147 |
| MnWO3/g-C3N4 heterojunction | Facile hydrothermal method | 250 W xenon lamp | Rhodamine B (RhB) | 73% | 4 h | O2˙−, ·OH | 148 |
| 1D/2D MnWO4 nanorods on g-C3N4 | One-pot hydrothermal | 150 mW cm−2 tungsten lamp | Ofloxacin (OFX) | 90% | 70 min | h+, O2˙− | 149 |
| MnWO4/g-C3N4 hybrid | Hydrothermal method | Xenon lamp XQ-500 W | RhB, 4-CP | 98% (RhB), 91% (4-CP) | 100 min | ·OH | 150 |
| NiWO4 nanorods anchored on g-C3N4 | Hydrothermal method | Tungsten lamp (150 mW) | Norfloxacin (NRF) | 97% | 60 min | ·OH, h+ | 151 |
| g-C3N4/NiWO4 | Hydrothermal | 1000 W Xe | Toluene | 95% | 180 min | ·OH | 153 |
| g-C3N4/ZnWO4 films | Hydrothermal | UV lamp 12 W | Phenol | — | 4 h | O2˙− | 155 |
| g-C3N4/ZnWO4 | In situ hydrothermal technique | 100 W solar simulator | Rhodamine (RhB) | 99% | 180 min | ·OH, h+ | 154 |
| CoWO4/g-C3N4 | Reflux-calcinations method | Sunlight | Ciprofloxacin (CIP) | 81% | 60 min | O2˙−, ·OH | 139 |
Currently, dual Z-scheme-based various ternary composites of g-C3N4 were synthesized, stimulating the complete transformation of photoexcited electrons and giving high charge separation properties.157,158 Tang et al.159 synthesized a ternary g-C3N4/WO3/AgI photocatalytic system with a double Z-scheme for neonicotinoid degradation. The developed photocatalyst has an exclusive transmission path for photoinduced charge carriers, thus increasing the photocatalyst's activity. Guo et al.160 prepared a dual Z-scheme Bi2S3/BiVO4/MgIn2S4 nanocomposite heterojunction for the photocatalytic degradation of carbamazepine by the in situ growth method. Photoexcited charge carriers have a Z-scheme transformation path, which leads to enhanced photocatalytic activity. These ternary structures contain a better charge transformation path, thus improving the catalyst performance. Hongfei Y. et al.161 fabricated AgBr/β-Ag2WO4/g-C3N4 dual Z-scheme heterostructure by the precipitation method for tetracycline(TCH) and rhodamine B degradation. The synthesized ternary composite AgBr/β-Ag2WO4/g-C3N4 (CNAWAB7) degraded the rhodamine by almost 99.2% within just 25 min respective to CN, β-Ag2WO4, and composite of both CNAW9. The ternary composite also showed little better results for the degradation of tetracycline (60% within 25 min). The fabricated photocatalyst has better charge separation properties due to the alignment of a band, which mainly improves the photocatalytic performance of the proposed catalyst against TCH and rhodamine. However, catalytic efficiency decreases after every cycle. Mitra M. et al.161 synthesized a new ternary Z-scheme material by incorporating the nanoparticles of CoWO4 and Fe3O4 over g-C3N4 by the simple refluxing calcination method. The ternary nanocomposite with 10% g-C3N4/Fe3O4/CoWO4 degraded almost 100% Rhodamine B in 240 min. The proposed catalyst has high catalytic efficiency, but the time duration is very high to degrade Rhodamine B completely. There is a further need to improve to minimize the time factor.
Longbo J. et al.162 synthesized a new composite material (WO3/g-C3N4/Bi2O3) by single-step cocalcination strategy utilizing simple melamine, bismuth(III) nitrate pentahydrate, and tungstic acid as the starting material. The photocatalytic capability of the catalyst against TC increased by up to 80% (Fig. 10(b)). The pore volume, BET surface area, and pore diameter of the catalyst improved by preparing the composite of g-C3N4 with WO3 and Bi2O3. To effectively remove these drugs via photocatalysis, Nguyen T. et al.163 introduced another ternary Z-scheme dual Cu-NiWO4/g-C3N4 heterojunction for the photocatalytic breakdown of n-hexane under visible light. The designed catalyst gives better removal and degradation efficiency up to 5 cycles and has a better surface area (84.6 m2 g−1), which is a sign of the stability of the catalyst, but there is a need to test it for more than five cycles. Y. O. Ibrahim et al.164 synthesized a ternary nanocomposite-based TiO2/WO3/g-C3N4 photocatalyst to degrade methylene blue as a pollutant under visible light. The photocatalyst TiO2/WO3/g-C3N4 with 15 wt% g-C3N4 displayed the maximum photodegradation action with 91.5% effectiveness. The stability and reusability of the designed catalyst are not good enough, but by seeing this work, the potential photocatalyst can be formed for pollutant degradation under visible light.
M. B. Tahir et al.50 designed a photocatalyst to degrade aspirin and caffeine under visible light. They synthesized WO3-TiO2-g-C3N4 by a simple hydrothermal process. Tingting Y. et al.165 synthesized a self-sustained catalytic fuel cell system based on anodic TiO2/g-C3N4 heterojunction and cathodic WO3/W for the oxidation of triclosan or rhodamine B and reduction of NO3−-N to N2 instantaneously. When these two electrodes are combined, a microscopic current was produced without harvesting light that caused nitrate reduction into N2 at the cathode. The electrons generated by the system activate the oxygen molecules to generate reactive oxygen species, which cause the oxidation of triclosan and rhodamine B by h+ in the anode chamber. The electron produced at the anode is transferred to the cathode through an external conducting system where the reduction of nitrates occurs. The designed self-biased system removed almost ∼90% RhB and reduced ∼95% NO3–N under UV light after 4 h. Along with the generation of photo-excited electrons ∼98%, triclosan was degraded, and ∼81% NO3−-N in the cathode chamber was removed in 60 min. The catalytic mechanism of the designed photoelectrochemical and the degradation rate discussed above are shown in Fig. 18(a–c).
 | ||
| Fig. 18 (a) Degradation mechanism of the self-sustained system. (b) RhB and NO3−-N degradation rate in anode/cathode chamber and spontaneous current. (c) TCS and NO3−-N degradation rate in the anode/cathode chamber and spontaneous current (reproduced with permission from ref. 166 Copyright 2017, Elsevier). | ||
Na Lu et al.167 synthesized a Z-scheme photocatalyst for the photodegradation of antibiotic ciprofloxacin, and RGO was used as an electron mediator. They synthesized the photocatalyst g-C3N4/RGO/WO3 by simple self-assembly and photoreduction method. The light-harvesting and charge transfer properties became high with the assimilation of RGO as an electron mediator in place of conductive material within C3N4/WO3. The charge mobility properties were studied using photoluminescence (PL) and electrochemical impedance spectroscopy (EIS), and the photocatalytic degradation efficiency of the synthesized material against ciprofloxacin is still 85%. Yongchao B.168 synthesized WO3/RGO/protonated g-C3N4 (PCN) composites by the microwave-assisted hydrothermal method for the degradation of tetracycline (TC-HCL). The proposed photocatalyst by this method showed a shorter path of charge migration and a wide interface contact area. The photodegradation efficiency of the 20% WO3/RGO/PCN composite is maximum. This photocatalyst shows more than 90% catalytic efficiency relative to the previously designed one. By analyzing this work, it is possible to do more work in this field and near future synthesis of a photocatalyst with 100% efficiency, that requires minimum time for the photodegradation of multiple pollutants.
The above discussion highlights the potential of ternary-doped modified WO3/g-C3N4 for advanced photocatalysis. However, it is important to note that the specific scientific advantages may vary depending on the dopant elements, synthesis methods, and intended applications. Ternary photocatalysts typically consist of three different materials, which can increase the complexity of their synthesis, characterization, and application for optimization. Achieving precise control over the composition, morphology, and size of three components in the ternary composite can be challenging, with batch-to-batch variation potentially resulting in low reproducibility. The synthesis of ternary photocatalyst composites can be more demanding than binary or single-component photocatalysts since the longer reaction times and harsher conditions increase energy consumption and create complexity. The synthesis of ternary materials often involves more cost, leading to higher production costs; ternary photocatalysts composite often involves complex interactions, and their performance may depend on intricate mechanisms. Multiple components in the ternary composite can introduce additional chemical reactions and phase separation, leading to decreased photocatalytic activity.
Nonmetal-doped g-C3N4/WO3 materials are environment friendly and utilizing them as dopants can reduce the toxicity of heavy metals, making materials more sustainable and cost-effective. Introducing nonmetal elements into g-C3N4/WO3 can improve the material's resistance to photocorrosion, structural changes, and efficient use in photocatalytic processes. Huinan Che et al.169 synthesized nitrogen-doped graphene quantum dots (NGQDs)-modified Z-scheme g-C3N4/Bi2WO6 heterojunctions through the hydrothermal method for the photocatalytic degradation of multiple antibiotic drugs such as ciprofloxacin (CIP), tetracycline (TC), and oxytetracycline (OTC) under near-infrared and visible light. Relative to pure g-C3N4, Bi2WO6, and 60% C3N4/Bi2WO6, the visible light absorption intensity of 3% NGQDs-60% C3N4/Bi2WO6 heterojunction increased from 500 nm to 700 nm, suggesting that the incorporation of 3% NGQDs enhanced the photogenerated electron–hole pair production, which results in enhanced photocatalytic activity. The photocatalytic activity of 3% NGQDs–60% C3N4/Bi2WO6 is higher than that of other pure g-C3N4, Bi2WO6, and 60% C3N4/Bi2WO6 photocatalysts. By varying the weight of NGQDs between 1 to 7 wt%, the photocatalytic activity of the photocatalyst against TC increased. A further increase decreased the catalyst's performance, indicating that a high concentration of NGQDs reduced the light absorption capability. NGQDs are more spread over the surface of g-C3N4/Bi2WO6. The photocatalytic efficiency of 3% NGQDs-60% C3N4/Bi2WO6 within 60 min against TC was 85.2%, 89.1%, 80.8%, and 75.0%. The photocatalytic performance of the catalyst is lower against other mentioned drugs, only degrading specific pollutants. These catalysts should be designed to degrade multiple drugs under visible and UV light regions within a minimum time and with maximum reusability.
Mi G. et al.170 designed a novel photocatalyst to degrade organic solvent vapors, which are toxic. They fabricated nitrogen-doped carbon quantum dots NCQDs, g-C3N4 (CN), and Bi2WO6 (BWO) heterostructure (NCQDs/CN/BWO) by a simple sonication method. The TEM and SEM images in Fig. 18(a and b) show that the average size range of 2.5–9.4 nm and lattice distance of NCQDs was 0.320 nm; the corresponding (002) plane of graphitic carbon and the SAED pattern in the SEM image of NCQDs (inset) confirmed the diffraction ring of the (002) crystal plane. NCQDs exhibited a light blue color for suspension when subjected to UV irradiation (Fig. 18(c)). In dark, unmodified, nearly transparent CQDs solution, the color difference between unmodified CQDs and NCQDs is attributed to the absence or presence of N in the CQDs, indicating that the NCQDs sample contained N. Fig. 18(d) shows that the removal efficiency of nUD by NCQDs/CN/BWO-1 was 57.8 (average) ± 0.8%, while that observed for CN/BWO-0.5, NCQDs/BWO, NCQDs/CN, CN, and BWO was 35.6 ± 0.9%, 29.2 ± 0.4%, 26.1 ± 0.3%, 17.6 ± 0.9%, 13.4 ± 1.2%, respectively. The catalytic performance was still good after 5 cycles; however, the performance efficiency was lower. This work established a landmark for designing a more effective photocatalyst to remove these hazardous organic-based vapors from the atmosphere. Jong U. et al.143 synthesized another ternary composite NGQD/FWO/CN material to remove harmful vapors. Firstly, the FeWO4/g-C3N4 heterostructure was synthesized, and then N-doped graphene quantum dots (NGQDs) were decorated on it. The normal elimination efficiency of vaporous ethylbenzene (EBZ) and 2-butoxyethanol (2BE) over a typical NGQD/FWO/CN sample were 97.3% (±0.7%) and 55.1% (±0.6%), as shown in Fig. 19(e and f).
 | ||
| Fig. 19 (a) TEM image and size distribution and (b) HRTEM image with the SAED pattern of NCQDs. (c) UV-Vis spectra of CQDs and NCQDs suspended in deionized water. (d) Average efficiencies ± standard error for nUD and mXYL removal.171 (e) Efficiencies of the photocatalytic removal of 2BE and (f) EBZ (reproduced with permission from ref. 172 Copyright 2020, Elsevier). | ||
K. M. et al.173 synthesized a porous oxygen-doped-g-C3N4/WO3 photocatalyst for H2 production. The optimal production of hydrogen was 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 142 μmol g−1, obtained using 10 mg photocatalyst (O-g-CNW-4). However, loading more than 10 mg catalysts reduced the photocatalytic H2 evaluation. The heteroatom doping of WO3/g-C3N4 set a new site for developing a new photocatalyst for various applications. There is a further need to work in this field to synthesize photocatalysts that can be applied commercially. However, nonmetal doping in photocatalysis can have a few disadvantages, for instance, it may not have the same photocatalytic activity as the metal doping photocatalyst since they can not provide active sites for the desired chemical transformations, thus lowering the photocatalytic efficiency. Nonmetal dopants may decrease the stability of the photocatalytic material by introducing defects in the structure, reducing the material's durability over time and limiting the lifespan of the photocatalyst. Some nonmetals may have narrow absorption ranges, may not effectively utilize the visible light spectrum, and can restrict the photocatalyst's ability.
142 μmol g−1, obtained using 10 mg photocatalyst (O-g-CNW-4). However, loading more than 10 mg catalysts reduced the photocatalytic H2 evaluation. The heteroatom doping of WO3/g-C3N4 set a new site for developing a new photocatalyst for various applications. There is a further need to work in this field to synthesize photocatalysts that can be applied commercially. However, nonmetal doping in photocatalysis can have a few disadvantages, for instance, it may not have the same photocatalytic activity as the metal doping photocatalyst since they can not provide active sites for the desired chemical transformations, thus lowering the photocatalytic efficiency. Nonmetal dopants may decrease the stability of the photocatalytic material by introducing defects in the structure, reducing the material's durability over time and limiting the lifespan of the photocatalyst. Some nonmetals may have narrow absorption ranges, may not effectively utilize the visible light spectrum, and can restrict the photocatalyst's ability.
Limitations of WO3/g-C3N4-based materials
The stability of WO3/g-C3N4 limits its practical applications since some studies reported issues with WO3/g-C3N4 photocorrosion under prolonged irradiation or harsh reaction conditions. Therefore, strategies such as 0D, 1D, 2D, 3D,122,126,130,174,175 metallic and bimetallic doping, or structural modifications have been explored to enhance the stability of these photocatalysts.40,43,45,47–51The preparation method for the WO3/g-C3N4 can impact the performance efficiency and stability of the photocatalyst.100 Different synthesis methods (hydrothermal, coprecipitation, calcination, in situ synthesis, thermal condensation, acid treatment, etc.) have been employed to fabricate the WO3/g-C3N4 composite; however, the optimized synthesis parameters and achieving uniform dispersion can be challenging.122,126,130,174,175
The efficiency of WO3/g-C3N4 can vary depending on several factors (morphology, composition, and pH conditions). However, there is still room for improvement to enhance the WO3/g-C3N4 efficiency.82,176
Different studies have reported different bandgap values, even for the same doped materials of the WO3/g-C3N4-based nanocomposite.56,58,59 Advanced spectroscopic characterization can be useful to evaluate the bandgap energy of the doped WO3/g-C3N4 nanocomposite.
A deep understanding of the photoexcited electron–hole separation process is unclear on the WO3/g-C3N4-based nanocomposite. Different advanced characterization techniques should verify it for better understanding.
Efficient recycling and catalyst recovery of WO3/g-C3N4 is an important parameter in practical applications, and some techniques such as filtration, centrifugation, or magnetic separation are used to recover photocatalysts for reuse. After the separation, the catalyst shows less photocatalytic activities. The separation from the system is not fruitful; therefore, further research is needed to develop more effective and scalable methods for photocatalyst separation and recycling. It is a drawback for real applications, which could be resolved with the development of science and technology. More attention should be paid to overcoming the separation and recyclability issue of the WO3/g-C3N4-based nanocomposite.
Future perspectives
The combination of WO3 and g-C3N4 as photocatalysts has significant advances in recent research. Here are some of the most important advancements for WO3/g-C3N4 photocatalysts based on the above data.• The synergistic effect between WO3 and g-C3N4 has shown improved photocatalytic performance compared to individual components, and this advancement opens avenues for the further optimization and exploration of the WO3/g-C3N4 system.
• Researchers have made progress in understanding the mechanisms behind the improved photocatalytic activity of WO3/g-C3N4. Current research further elucidates the charge separation, transfer processes, and surface reactions contributing to enhanced performance.
• Modifying the band structure of WO3/g-C3N4 through element doping and heterojunction formation offers opportunities to tailor the photocatalytic properties. Future research can explore novel strategies to fine-tune the band structure for specific applications and improved efficiency.
• While significant progress has been made in the laboratory, scaling up the synthesis and application of WO3/g-C3N4 photocatalysts is an important area for future research. Efforts can be directed toward developing scalable synthesis methods and exploring real-world applications.
• In the future, this material can solve global energy challenges and minimize water pollution by providing green and economical cheap materials for designing different modification techniques. This material can be used in water splitting (the process that converts water into hydrogen and oxygen gases). Hydrogen gas can serve as a clean and renewable energy source soon.
• Researchers should apply these materials in environmental studies to remove volatile organic compounds for air purification. This application can improve the air quality in environments. The photocatalytic properties of the WO3/g-C3N4-based nanocomposite make them more effective in volatile organic compounds (VOCs) and nitrogen oxides (NOx) detection from air. This material can detect bimolecular, toxic gases, and environmental pollutants shortly for designing such sensing devices.
• The surface area of the WO3/g-C3N4-based nanocomposite is small. To enhance the specific surface area of the WO3/g-C3N4-based nanocomposite, further studies should be focused on, such as synthesizing mesoporous WO3/g-C3N4-based nanocomposite using different methods.
• The antimicrobial properties of the WO3/g-C3N4-based nanocomposite can be utilized for developing antibacterial coatings on surfaces, preventing the growth and spread of bacteria and infections, and medical equipment. Drug delivery in the present era is the most popular medical field. Researchers should pay attention to these materials for drug delivery. Further investigations should focus on this application in in vitro and in vivo studies and living organisms.
• Little data is available on these materials (WO3/g-C3N4) for artificial photosynthesis and nitrogen fixation. Researchers should apply this catalyst in this recent hot field.
• Transition metals are important in nanotechnology since have variable oxidation states. They adsorb on another surface and activate them. Several transition metals are not reported for doping in WO3/g-C3N4-based nanocomposite and need to be further investigated in detail. In addition, s-block and p-block elements can significantly change the properties of WO3/g-C3N4-based nanocomposites.
• Computational approaches for theoretical calculations to evaluate the degradation pathways for understanding the photocatalytic mechanism can also be helpful in WO3/g-C3N4-based materials.
• Similarly, future research work must focus on the development of superconductors. Superconductors are used mostly in levitating trains, engines, generators, highly sensitive optical sensors, detectors of magnetic fields, low-noise amplifiers, transformers, magnetic resonance imaging (MRI), and areas that apply strong magnetic fields. A limited number of publications are available on this material for a superconductor.
Conclusion
In this review, we described the history, crystalline structure, synthesis, precursor, method of fabrication, modification (element-doping and structure modification), characterization, and application of WO3/g-C3N4-based nanocomposite in photocatalysis. It is observed that the pristine g-C3N4 and WO3 show unacceptable efficiency due to their low surface area, insufficient sunlight absorption, and fast recombination of photoinduced electron–hole pairs. Strategies to improve the efficiency of WO3/g-C3N4 highlighted the various synthetic routes such as sol–gel, ultrasonic dispersion, microwave irradiation, precipitation, hydrothermal, pyrolysis method, and calcination methods are discussed. A wide range of composites, including WO3/g-C3N4, modified WO3/g-C3N4, (0D, 1D, 2D, 3D) light metal-doped WO3/g-C3N4, transition metal-doped WO3/g-C3N4, ZnO-WO3/g-C3N4, and TiO2-WO3/g-C3N4 nanomaterials, are investigated. These modifications provide fascinating properties such as a wide range of visible light responses, good redox ability, suitable bandgap, nontoxicity, environmental friendliness, high physicochemical stability, and thermal stability. A brief overview of the recent advanced publications from 2009 to 2023 is discussed for the photocatalytic degradation of organic pollutants. In future studies, WO3/g-C3N4-based nanocomposites can be applied for the betterment of human beings.Data availability
All the data is available in the manuscript.Conflicts of interest
Authors declare no competing interests.Acknowledgements
There is no funding to report for this article. Authors acknowledge the Higher Education Commission (HEC) of Pakistan for this research.References
- P. V. Nidheesh, M. Zhou and M. A. Oturan, An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes, Chemosphere, 2018, 197, 210–227 CrossRef CAS PubMed
.
- T. Shindhal,
et al., A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater, Bioengineered, 2021, 12(1), 70–87 CrossRef CAS PubMed
.
- X. Yang,
et al., Visible-near-infrared-responsive g-C3N4Hx+ reduced decatungstate with excellent performance for photocatalytic removal of petroleum hydrocarbon, J. Hazard. Mater., 2020, 381, 120994 CrossRef CAS PubMed
.
- P. G. Kougias and I. Angelidaki, Biogas and its opportunities—A review, Front. Environ. Sci. Eng., 2018, 12, 1–12 CAS
.
-
Administration, U.E.I., Annual Energy Outlook 2011: With Projections to 2035, Government Printing Office, 2011 Search PubMed
.
- P. Gao,
et al., A critical review on bismuth oxyhalide based photocatalysis for pharmaceutical active compounds degradation: Modifications, reactive sites, and challenges, J. Hazard. Mater., 2021, 412, 125186 CrossRef CAS PubMed
.
- M. M. Moura,
et al., Degradation of the mixture of the ketoprofen, meloxicam and tenoxicam drugs using TiO2/metal photocatalysers supported in polystyrene packaging waste, Water Sci. Technol., 2021, 83(4), 863–876 CrossRef PubMed
.
- H. Wang,
et al., Visible-light-driven removal of tetracycline antibiotics and reclamation of hydrogen energy from natural water matrices and wastewater by polymeric carbon nitride foam, Water Res., 2018, 144, 215–225 CrossRef CAS PubMed
.
- L. M. Bexfield,
et al., Hormones and pharmaceuticals in groundwater used as a source of drinking water across the United States, Environ. Sci. Technol., 2019, 53(6), 2950–2960 CrossRef CAS PubMed
.
- B. P. Gumbi,
et al., Detection and quantification of acidic drug residues in South African surface water using gas chromatography-mass spectrometry, Chemosphere, 2017, 168, 1042–1050 CrossRef CAS PubMed
.
- Z. Zhao,
et al., In Situ Preparation of Mn0. 2Cd0. 8S-Diethylenetriamine/Porous g-C3N4 S-Scheme Heterojunction with Enhanced Photocatalytic Hydrogen Production, Adv. Sustainable Syst., 2023, 7(1), 2100498 CrossRef CAS
.
- C. J. Ogugbue and T. J. Sawidis, Bioremediation and detoxification of synthetic wastewater containing triarylmethane dyes by Aeromonas hydrophila isolated from industrial effluent, Biotechnol. Res. Int., 2011, 2011, 967925 Search PubMed
.
- P. A. Carneiro,
et al., Homogeneous photodegradation of CI Reactive Blue 4 using a photo-Fenton process under artificial and solar irradiation, Dyes Pigm., 2007, 74(1), 127–132 CrossRef CAS
.
- A. Aziz,
et al., Chitosan-zinc sulfide nanoparticles, characterization and their photocatalytic degradation efficiency for azo dyes, Int. J. Biol. Macromol., 2020, 153, 502–512 CrossRef CAS PubMed
.
- S. Khan,
et al., Electrochemical sensors based on biomimetic magnetic molecularly imprinted polymer for selective quantification of methyl green in environmental samples, Mater. Sci. Eng., C, 2019, 103, 109825 CrossRef CAS PubMed
.
- N. H. Tran, M. Reinhard and K. Y.-H. Gin, Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review, Water Res., 2018, 133, 182–207 CrossRef CAS PubMed
.
- K. He,
et al., Advances in nanostructured silicon carbide photocatalysts, Acta Phys.-Chim. Sin., 2022, 38, 2201021 Search PubMed
.
- F. C. Moreira,
et al., Electrochemical advanced oxidation processes: a review on their application to synthetic and real wastewaters, Appl. Catal., B, 2017, 202, 217–261 CrossRef CAS
.
- N. Li,
et al., Recent Developments in Functional Nanocomposite Photocatalysts for Wastewater Treatment: A Review, Adv. Sustainable Syst., 2022, 6(7), 2200106 CrossRef CAS
.
- N. Taoufik,
et al., Comparative overview of advanced oxidation processes and biological approaches for the removal pharmaceuticals, J. Environ. Manage., 2021, 288, 112404 CrossRef CAS PubMed
.
- K. Ayoub,
et al., Application of advanced oxidation processes for TNT removal: a review, J. Hazard. Mater., 2010, 178(1–3), 10–28 CrossRef CAS PubMed
.
- P. K. Pandis,
et al., Key points of advanced oxidation processes (AOPs) for wastewater, organic pollutants and pharmaceutical waste treatment: A mini review, ChemEngineering, 2022, 6(1), 8 CrossRef CAS
.
- H. Sun,
et al., One-pot thermal polymerization route to prepare N-deficient modified g-C3N4 for the degradation of tetracycline by the synergistic effect of photocatalysis and persulfate-based advanced oxidation process, Chem. Eng. J., 2021, 406, 126844 CrossRef CAS
.
- D. Zhu and Q. Zhou, Monitoring, and Management, Action and mechanism of semiconductor photocatalysis on degradation of organic pollutants in water treatment: A review, Environ. Nanotechnol., Monit. Manage., 2019, 12, 100255 Search PubMed
.
- R. Dewil,
et al., New perspectives for advanced oxidation processes, J. Environ. Manage., 2017, 195, 93–99 CrossRef CAS PubMed
.
-
M. H. Mahdi, T. J. Mohammed, and J. A. Al-Najar, Advanced Oxidation Processes (AOPs) for treatment of antibiotics in wastewater: a review, in IOP Conference Series: Earth and Environmental Science, IOP Publishing, 2021 Search PubMed
.
- C. Comninellis,
et al., Advanced oxidation processes for water treatment: advances and trends for R&D, J. Chem. Technol. Biotechnol., 2008, 83(6), 769–776 CrossRef CAS
.
- S. A. Fast,
et al., A critical evaluation of advanced oxidation processes for emerging contaminants removal, Environ. Processes, 2017, 4, 283–302 CrossRef
.
- J. Y. Hwang,
et al., Crystal phase-dependent generation of mobile OH radicals on TiO2: Revisiting the photocatalytic oxidation mechanism of anatase and rutile, Appl. Catal., B, 2021, 286, 119905 CrossRef CAS
.
- E. S. Elmolla and M. Chaudhuri, Degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution by the UV/ZnO photocatalytic process, J. Hazard. Mater., 2010, 173(1–3), 445–449 CrossRef CAS PubMed
.
- X. Feng,
et al., High performance, recoverable Fe3O4ZnO nanoparticles for enhanced photocatalytic degradation of phenol, Chem. Eng. J., 2014, 244, 327–334 CrossRef CAS
.
- Q. Li,
et al., CdS/graphene nanocomposite photocatalysts, Adv. Energy Mater., 2015, 5(14), 1500010 CrossRef
.
- M. T. Uddin,
et al., Nanostructured SnO2–ZnO heterojunction photocatalysts showing enhanced photocatalytic activity for the degradation of organic dyes, Inorg. Chem., 2012, 51(14), 7764–7773 CrossRef CAS PubMed
.
- D. Zhu and Q. Zhou, Action and mechanism of semiconductor photocatalysis on degradation of organic pollutants in water treatment: A review, Environ. Nanotechnol., Monit. Manage., 2019, 12, 100255 Search PubMed
.
- I. Ani,
et al., Photocatalytic degradation of pollutants in petroleum refinery wastewater by TiO2-and ZnO-based photocatalysts: recent development, J. Cleaner Prod., 2018, 205, 930–954 CrossRef CAS
.
- X. Gu,
et al., Self-assembly synthesis of S-scheme g-C3N4/Bi8 (CrO4) O11 for photocatalytic degradation of norfloxacin and bisphenol A, Chin. J. Catal., 2022, 43(10), 2569–2580 CrossRef CAS
.
- J. Jia,
et al., Recent advances on g–C3N4–based Z-scheme photocatalysts: Structural design and photocatalytic applications, Int. J. Hydrogen Energy, 2023, 48(1), 196–231 CrossRef CAS
.
- H. Wang,
et al., Synthesis and applications of novel graphitic carbon nitride/metal-organic frameworks mesoporous photocatalyst for dyes removal, Appl. Catal., B, 2015, 174, 445–454 CrossRef
.
- T. Song,
et al., Preparation, structure and application of g-C3N4/BiOX composite photocatalyst, Int. J. Hydrogen Energy, 2021, 46(2), 1857–1878 CrossRef CAS
.
- Y. Gong, M. Li and Y. J. C. Wang, Carbon nitride in energy conversion and storage: recent advances and future prospects, ChemSusChem, 2015, 8(6), 931–946 CrossRef CAS PubMed
.
- K. Vignesh and M. Kang, Facile synthesis, characterization and recyclable photocatalytic activity of Ag2WO4@ g-C3N4, Mater. Sci. Eng., B, 2015, 199, 30–36 CrossRef CAS
.
- Z. Fan,
et al., Bridging effect of S–C bond for boosting electron transfer over cubic hollow CoS/g-C3N4 heterojunction toward photocatalytic hydrogen production, Langmuir, 2022, 38(10), 3244–3256 CrossRef CAS PubMed
.
- M. Yang,
et al., The influence of preparation method on the photocatalytic performance of g-C3N4/WO3 composite photocatalyst, Ceram. Int., 2014, 40(8), 11963–11969 CrossRef CAS
.
- J. Bai,
et al., Integration of 2D layered CdS/WO3 S-scheme heterojunctions and metallic Ti3C2 MXene-based Ohmic junctions for effective photocatalytic H2 generation, Chin. J. Catal., 2022, 43(2), 359–369 CrossRef CAS
.
- M. G. Peleyeju and E. Viljoen, WO3-based catalysts for photocatalytic and photoelectrocatalytic removal of organic pollutants from water A review, J. Water Process Eng., 2021, 40, 101930 CrossRef
.
- H. Wang,
et al., Structure–performance correlation guided applications of covalent organic frameworks, Mater. Today, 2022, 106–133 CrossRef
.
- V. Alman,
et al., Sunlight Assisted improved photocatalytic degradation of rhodamine B using Pd-loaded gC 3 N 4/WO 3 nanocomposite, Appl. Phys. A: Mater. Sci. Process., 2020, 126(9), 1–9 CrossRef
.
- M. Karimi-Nazarabad,
et al., Highly efficient photocatalytic and photoelectrocatalytic activity of solar light driven WO3/g-C3N4 nanocomposite, Sol. Energy Mater. Sol. Cells, 2017, 160, 484–493 CrossRef CAS
.
- C. Wang,
et al., BaWO4/g-C3N4 heterostructure with excellent bifunctional photocatalytic
performance, Chem. Eng. J., 2020, 385, 123833 CrossRef CAS
.
- M. B. Tahir, M. Sagir and K. Shahzad, Removal of acetylsalicylate and methyl-theobromine from aqueous environment using nano-photocatalyst WO3-TiO2@ g-C3N4 composite, J. Hazard. Mater., 2019, 363, 205–213 CrossRef CAS PubMed
.
- S. Li,
et al., In situ construction of a C 3 N 5 nanosheet/Bi 2 WO 6 nanodot S-scheme heterojunction with enhanced structural defects for the efficient photocatalytic removal of tetracycline and Cr (vi), Inorg. Chem. Front., 2022, 9(11), 2479–2497 RSC
.
- L. Jiang,
et al., Doping of graphitic carbon nitride for photocatalysis: A reveiw, Appl. Catal., B, 2017, 217, 388–406 CrossRef CAS
.
- C. Dong,
et al., A review on WO3 based gas sensors: Morphology control and enhanced sensing properties, J. Alloys Compd., 2020, 820, 153194 CrossRef CAS
.
- M. G. Peleyeju and E. L. Viljoen, WO3-based catalysts for photocatalytic and photoelectrocatalytic removal of organic pollutants from water–A review, J. Water Process. Eng., 2021, 40, 101930 CrossRef
.
- B. S. Kalanoor, H. Seo and S. S. Kalanur, Recent developments in photoelectrochemical water-splitting using WO3/BiVO4 heterojunction photoanode: A review, Mater. Sci. Energy Technol., 2018, 1(1), 49–62 Search PubMed
.
- J. Yao,
et al., Improved photocatalytic activity of WO3/C3N4: By constructing an anchoring morphology with a Z-scheme band structure, Solid State Sci., 2019, 95, 105926 CrossRef CAS
.
-
O. Ola, et al., Pseudocapacitive Performance of WO 3/gC 3 N 4 Nanocomposites, in 2021 IEEE 11th International Conference Nanomaterials: Applications & Properties (NAP), IEEE, 2021 Search PubMed
.
- H. Qian,
et al., One-Pot Construction of Porous WO3/g-C3N4 Nanotubes of Photocatalyst for Fast and Boosted Photodegradation of Rhodamine B and Tetracycline, J. Electron. Mater., 2023, 1–16 CAS
.
- X. Zhang and S. He, WOx/g-C3N4 layered heterostructures with controlled crystallinity towards superior photocatalytic degradation and H2 generation, Carbon, 2020, 156, 488–498 CrossRef CAS
.
- J. Liebig, Annalen, 1834, 10, 10 Search PubMed
.
- E. C. Franklin, The ammono-carbonic acids, J. Am. Chem. Soc., 1922, 44(3), 486–509 CrossRef CAS
.
- W. K. Darkwah,
et al, Mini review on the structure and properties (photocatalysis), and preparation techniques of graphitic carbon nitride nano-based particle, and its applications, Nanoscale Res. Lett., 2018, 13(1), 1–15 CrossRef CAS PubMed
.
- K. Qi, S.-y. Liu and A. Zada, Graphitic carbon nitride, a polymer photocatalyst, J. Taiwan Inst. Chem. Eng., 2020, 109, 111–123 CrossRef CAS
.
- A. O. Idris,
et al., Graphitic carbon nitride: a highly electroactive nanomaterial for environmental and clinical sensing, Sensors, 2020, 20(20), 5743 CrossRef CAS PubMed
.
- V. Battula,
et al., A true oxygen-linked heptazine based polymer for efficient hydrogen evolution, Appl. Catal., B, 2019, 244, 313–319 CrossRef CAS
.
- A. Wang,
et al., Recent advances of graphitic carbon nitride-based structures and applications in catalyst, sensing, imaging, and LEDs, Nano-Micro Lett., 2017, 9(4), 1–21 Search PubMed
.
- M. Y. Chen, D. Li, X. Lin, V. P. Dravid, Y. W. Chung, M. S. Wong and W. D. Sproul, Analytical electron microscopy and Raman spectroscopy studies of carbon nitride thin films, J. Vac. Sci. Technol., A, 1993, 11(3), 521–524 CrossRef CAS
.
- J. L. Corkill and M. L. Cohen, Calculated quasiparticle band gap of β-C3N4, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 48(23), 17622 CrossRef CAS PubMed
.
-
M. F. R. Samsudin, N. Bacho, and S. Sufian, Recent development of graphitic carbon nitride-based photocatalyst for environmental pollution remediation, in Nanocatalysts, IntechOpen. 2018 Search PubMed
.
- D. Wei,
et al., In situ construction of interconnected SnO2/nitrogen-doped Carbon@ TiO2 networks for lithium-ion half/full cells, Electrochim. Acta, 2018, 290, 312–321 CrossRef CAS
.
- Q. Hao,
et al., Graphitic carbon nitride with different dimensionalities for energy and environmental applications, Nano Res., 2020, 13(1), 18–37 CrossRef CAS
.
- B. Zhu,
et al., First-principle calculation study of tri-s-triazine-based g-C3N4: a review, Appl. Catal., B, 2018, 224, 983–999 CrossRef CAS
.
- W.-J. Ong,
et al., Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: are we a step closer to achieving sustainability?, Chem. Rev., 2016, 116(12), 7159–7329 CrossRef CAS PubMed
.
- H.-W. ZUO,
et al., Pt4 clusters supported on monolayer graphitic carbon nitride sheets for oxygen adsorption: a first-principles study, Acta Phys.-Chim. Sin., 2016, 32(5), 1183–1190 CAS
.
- B. Zhu, Review on DFT calculation of s-triazine-based carbon nitride, Carbon Energy, 2019, 1(1), 32–56 CrossRef CAS
.
- B. Zhu,
et al., First principle investigation of halogen-doped monolayer g-C3N4 photocatalyst, Appl. Catal., B, 2017, 207, 27–34 CrossRef CAS
.
- B. Zhu,
et al., First-principle calculation study of tri-s-triazine-based g-C3N4: a review, Appl. Catal., B, 2018, 224, 983–999 CrossRef CAS
.
- J. Kim,
et al., Platinized WO3 as an environmental photocatalyst that generates OH radicals under visible light, Environ. Sci. Technol., 2010, 44(17), 6849–6854 CrossRef CAS PubMed
.
- D. Migas,
et al., Tungsten oxides. I. Effects of oxygen vacancies and doping on electronic and optical properties of different phases of WO 3, J. Appl. Phys., 2010, 108(9), 093713 CrossRef
.
- S. S. Kalanur, L. T. Duy and H. Seo, Recent progress in photoelectrochemical water splitting activity of WO3 photoanodes, Top. Catal., 2018, 61(9), 1043–1076 CrossRef CAS
.
- F. Wang, C. Di Valentin and G. Pacchioni, Electronic and structural properties of WO3: a systematic hybrid DFT study, J. Phys. Chem. C, 2011, 115(16), 8345–8353 CrossRef CAS
.
- J. Fu,
et al., Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photocatalyst, Appl. Catal., B, 2019, 243, 556–565 CrossRef CAS
.
- P. Praus,
et al., Synthesis and properties of nanocomposites of WO3 and exfoliated g-C3N4, Ceram. Int., 2017, 43(16), 13581–13591 CrossRef CAS
.
- X. Han,
et al., WO3/g-C3N4 two-dimensional composites for visible-light driven photocatalytic hydrogen production, Int. J. Hydrogen Energy, 2018, 43(10), 4845–4855 CrossRef CAS
.
- H. Zhuang,
et al., In situ construction of WO 3/gC 3 N 4 composite photocatalyst with 2D–2D heterostructure for enhanced visible light photocatalytic performance, New J. Chem., 2019, 43(44), 17416–17422 RSC
.
- T. Pan,
et al., Anionic polyacrylamide-assisted construction of thin 2D-2D WO3/g-C3N4 Step-scheme heterojunction for enhanced tetracycline degradation under visible light irradiation, J. Hazard. Mater., 2020, 393, 122366 CrossRef CAS PubMed
.
- J. Chen,
et al., Fabrication of hierarchical sheet-on-sheet WO3/g-C3N4 composites with enhanced photocatalytic activity, J. Alloys Compd., 2019, 777, 325–334 CrossRef CAS
.
- Y. Li,
et al., Construction of inorganic–organic 2D/2D WO 3/gC 3 N 4 nanosheet arrays toward efficient photoelectrochemical splitting of natural seawater, Phys. Chem. Chem. Phys., 2016, 18(15), 10255–10261 RSC
.
- C. Cheng,
et al., WO3/g-C3N4 composites: one-pot preparation and enhanced photocatalytic H2 production under visible-light irradiation, Nanotechnology, 2017, 28(16), 164002 CrossRef PubMed
.
- T. Xiao,
et al., In situ construction of hierarchical WO3/g-C3N4 composite hollow microspheres as a Z-scheme photocatalyst for the degradation of antibiotics, Appl. Catal., B, 2018, 220, 417–428 CrossRef CAS
.
- M.-H. Pham,
et al., Visible light induced hydrogen generation using a hollow photocatalyst with two cocatalysts separated on two surface sides, Phys. Chem. Chem. Phys., 2014, 16(13), 5937–5941 RSC
.
- J. Yuan,
et al., TiO2/SnO2 double-shelled hollow spheres-highly efficient photocatalyst for the degradation of rhodamine B, Catal. Commun., 2015, 60, 129–133 CrossRef CAS
.
- S. L. Prabavathi,
et al., Construction of heterostructure CoWO4/g-C3N4 nanocomposite as an efficient visible-light photocatalyst for norfloxacin degradation, J. Ind. Eng. Chem., 2019, 80, 558–567 CrossRef CAS
.
- X. Xing,
et al., CoWO4 nanoparticles prepared by two methods displaying different structures and supercapacitive performances, Electrochim. Acta, 2015, 157, 15–22 CrossRef CAS
.
- K. Liu,
et al., Synthesis of direct Z-scheme MnWO4/g-C3N4 photocatalyst with enhanced visible light photocatalytic activity, Nano, 2017, 12(10), 1750129 CrossRef CAS
.
- J. Low,
et al., A review of direct Z-scheme photocatalysts, Small Methods, 2017, 1(5), 1700080 CrossRef
.
- R. Dadigala,
et al., Construction of in situ self-assembled FeWO 4/gC 3 N 4 nanosheet heterostructured Z-scheme photocatalysts for enhanced photocatalytic degradation of rhodamine B and tetracycline, Nanoscale Adv., 2019, 1(1), 322–333 RSC
.
- X. Zhang, X. Zhang and P. Yang, Transition metals decorated g-C3N4/N-doped carbon nanotube catalysts for water splitting: A review, J. Electroanal. Chem., 2021, 895, 115510 CrossRef CAS
.
- H. Jiang,
et al., Recent advances in heteroatom doped graphitic carbon nitride (g-C3N4) and g-C3N4/metal oxide composite photocatalysts, Curr. Org. Chem., 2020, 24(6), 673–693 CrossRef CAS
.
- M. W. Kadi and R. M. Mohamed, Increasing visible light water splitting efficiency through synthesis route and charge separation in measoporous g-C3N4 decorated with WO3 nanoparticles, Ceram. Int., 2019, 45(3), 3886–3893 CrossRef CAS
.
- L. Huang,
et al., Visible-light-induced WO 3/gC 3 N 4 composites with enhanced photocatalytic activity, Dalton Trans., 2013, 42(24), 8606–8616 RSC
.
- W. Yang,
et al., Hierarchical NiCo 2 O 4@ NiO core–shell hetero-structured nanowire arrays on carbon cloth for a high-performance flexible all-solid-state electrochemical capacitor, J. Mater. Chem. A, 2014, 2(5), 1448–1457 RSC
.
- L. Huang,
et al., Visible-light-induced WO 3/gC 3 N 4 composites with enhanced photocatalytic activity, Dalton Trans., 2013, 42(24), 8606–8616 RSC
.
- S. Chen,
et al., Study on the separation mechanisms of photogenerated electrons and holes for composite photocatalysts g-C3N4-WO3, Appl. Catal., B, 2014, 150, 564–573 CrossRef
.
- A. T. Doan,
et al., Graphitic gC 3 N 4-WO 3 composite: synthesis and photocatalytic properties, Bull. Korean Chem. Soc., 2014, 35(6), 1794–1798 CrossRef CAS
.
- H. Zhang,
et al., Photocatalytic selective oxidation of biomass-derived 5-hydroxymethylfurfural to 2, 5-diformylfuran on WO3/g-C3N4 composite under irradiation of visible light, J. Photochem. Photobiol., A, 2019, 371, 1–9 CrossRef CAS
.
- H. Yan,
et al., Single-source-precursor-assisted synthesis of porous WO3/g-C3N4 with enhanced photocatalytic property, Colloids Surf., A, 2019, 582, 123857 CrossRef CAS
.
- F. Chang,
et al., Binary composites WO3/g-C3N4 in porous morphology: Facile construction, characterization, and reinforced visible light photocatalytic activity, Colloids Surf., A, 2019, 563, 11–21 CrossRef CAS
.
- R. Ma, Preparation of highly dispersed WO3/few layer g-C3N4 and its enhancement of catalytic oxidative desulfurization activity, Colloids Surf., A, 2019, 572, 250–258 CrossRef CAS
.
- A. Priya,
et al., A study of photocatalytic and photoelectrochemical activity of as-synthesized WO3/g-C3N4 composite photocatalysts for AO7 degradation, Mater. Sci. Energy Technol., 2020, 3, 43–50 CAS
.
- R. Zhao,
et al., Preparation of WO3/g-C3N4 composites and their application in oxidative desulfurization, Appl. Surf. Sci., 2017, 392, 810–816 CrossRef CAS
.
- A. Navarro-Aguilar,
et al., An efficient and stable WO3/g-C3N4 photocatalyst for ciprofloxacin and orange G degradation, J. Photochem. Photobiol., A, 2019, 384, 112010 CrossRef CAS
.
- I. Aslam,
et al., The synergistic effect between WO 3 and gC 3 N 4 towards efficient visible-light-driven photocatalytic performance, New J. Chem., 2014, 38(11), 5462–5469 RSC
.
- H. Katsumata,
et al., Z-scheme photocatalytic hydrogen production over WO 3/gC 3 N 4 composite photocatalysts, RSC Adv., 2014, 4(41), 21405–21409 RSC
.
- M. Gondal,
et al., Preparation of WO 3/gC 3 N 4 composites and their enhanced photodegradation of contaminants in aqueous solution under visible light irradiation, React. Kinet., Mech. Catal., 2015, 114(1), 357–367 CrossRef CAS
.
- S. Chen,
et al., Study on the separation mechanisms of photogenerated electrons and holes for composite photocatalysts g-C3N4-WO3, Appl. Catal., B, 2014, 150, 564–573 CrossRef
.
- K.-i. Katsumata,
et al., Preparation of graphitic carbon nitride (g-C3N4)/WO3 composites and enhanced visible-light-driven photodegradation of acetaldehyde gas, J. Hazard. Mater., 2013, 260, 475–482 CrossRef CAS PubMed
.
- Y. Zang,
et al., Facile synthesis of composite g-C 3 N 4/WO 3: a nontoxic photocatalyst with excellent catalytic activity under visible light, RSC Adv., 2013, 3(33), 13646–13650 RSC
.
- J. Singh,
et al., Synthesis of coral like WO3/g-C3N4 nanocomposites for the removal of hazardous dyes under visible light, J. Alloys Compd., 2019, 808, 151734 CrossRef CAS
.
- J. Singh, A. Arora and S. Basu, Synthesis of coral like WO3/g-C3N4 nanocomposites for the removal of hazardous dyes under visible light, J. Alloys Compd., 2019, 808, 151734 CrossRef CAS
.
- Z. Lou and C. J. C. Xue, In situ growth of WO 3− x nanowires on gC 3 N 4 nanosheets: 1D/2D heterostructures with enhanced photocatalytic activity, CrystEngComm, 2016, 18(43), 8406–8410 RSC
.
- T. Pan,
et al., Anionic polyacrylamide-assisted construction of thin 2D-2D WO3/g-C3N4 Step-scheme heterojunction
for enhanced tetracycline degradation under visible light irradiation, J. Hazard. Mater., 2020, 393, 122366 CrossRef CAS PubMed
.
- F. Zhang,
et al., One-step oxygen vacancy engineering of WO3-x/2D g-C3N4 heterostructure: Triple effects for sustaining photoactivity, J. Alloys Compd., 2019, 795, 426–435 CrossRef CAS
.
- M. Antoniadou,
et al., Bifunctional g-C3N4/WO3 thin films for photocatalytic water purification, Water, 2019, 11(12), 2439 CrossRef CAS
.
- C. Xu,
et al., Facile construction of leaf-like WO 3 nanoflakes decorated on gC 3 N 4 towards efficient oxidation of alcohols under mild conditions, New J. Chem., 2018, 42(20), 16523–16532 RSC
.
- T. Xiao,
et al., In situ construction of hierarchical WO3/g-C3N4 composite hollow microspheres as a Z-scheme photocatalyst for the degradation of antibiotics, Appl. Catal., B, 2018, 220, 417–428 CrossRef CAS
.
- F. Xu,
et al., Step-by-step mechanism insights into the TiO2/Ce2S3 S-scheme photocatalyst for enhanced aniline production with water as a proton source, ACS Catal., 2021, 12(1), 164–172 CrossRef
.
- W. Wang,
et al., Efficient degradation of tetracycline via coupling of photocatalysis and photo-fenton processes over a 2D/2D α-Fe2O3/g-C3N4 S-scheme heterojunction catalyst, Acta Phys.-Chim. Sin., 2022, 38(7), 2201008 Search PubMed
.
- S. Deng,
et al., WO 3 nanosheets/gC 3 N 4 nanosheets' nanocomposite as an effective photocatalyst for degradation of rhodamine B, Appl. Phys. A: Mater. Sci. Process., 2019, 125(1), 44 CrossRef
.
- J. Chen,
et al., Fabrication of hierarchical sheet-on-sheet WO3/g-C3N4 composites with enhanced photocatalytic activity, J. Alloys Compd., 2019, 777, 325–334 CrossRef CAS
.
- J. Chen,
et al., Ag nanoparticles decorated WO3/g-C3N4 2D/2D heterostructure with enhanced photocatalytic activity for organic pollutants degradation, Appl. Surf. Sci., 2019, 467, 1000–1010 Search PubMed
.
- S. M. Hashmi,
et al., Enhancement of mechanical properties of epoxy/halloysite nanotube (HNT) nanocomposites, SN Appl. Sci., 2019, 1(4), 296 CrossRef
.
- Y. Li,
et al., In situ loading of Ag2WO4 on ultrathin g-C3N4 nanosheets with highly enhanced photocatalytic performance, J. Hazard. Mater., 2016, 313, 219–228 CrossRef CAS PubMed
.
- J. Huang,
et al., Ultrathin Ag2WO4-coated P-doped g-C3N4 nanosheets with remarkable photocatalytic performance for indomethacin degradation, J. Hazard. Mater., 2020, 392, 122355 CrossRef CAS PubMed
.
- N. Tian, H. Huang and Y. Zhang, Mixed-calcination synthesis of CdWO4/g-C3N4 heterojunction with enhanced visible-light-driven photocatalytic activity, Appl. Surf. Sci., 2015, 358, 343–349 CrossRef CAS
.
- A. Maavia,
et al., Facile synthesis of g-C3N4/CdWO4 with excellent photocatalytic performance for the degradation of Minocycline, Mater. Sci. Energy Technol., 2019, 2(2), 258–266 Search PubMed
.
- P. Xing,
et al., Preparation of CoWO4/g-C3N4 and its Ultra-Deep Desulfurization Property, Aust. J. Chem., 2016, 70(3), 271–279 CrossRef
.
- S. L. Prabavathi,
et al., Construction of heterostructure CoWO4/g-C3N4 nanocomposite as an efficient visible-light photocatalyst for norfloxacin degradation, J. Ind. Eng. Chem., 2019, 80, 558–567 CrossRef CAS
.
- S. Sahoo,
et al., Facile construction of CoWO4 modified g-C3N4 nanocomposites with enhanced photocatalytic activity under visible light irradiation, Mater. Today: Proc., 2021, 35, 193–197 CrossRef CAS
.
- D. P. Ojha,
et al., Decoration of g-C3N4 with hydrothermally synthesized FeWO4 nanorods as the high-performance supercapacitors, Chem. Phys. Lett., 2018, 712, 83–88 CrossRef CAS
.
- R. Dadigala,
et al., Construction of in situ self-assembled FeWO 4/gC 3 N 4 nanosheet heterostructured Z-scheme photocatalysts for enhanced photocatalytic degradation of rhodamine B and tetracycline, Nanoscale Adv., 2019, 1(1), 322–333 RSC
.
- A. Rashidizadeh,
et al., Graphitic Carbon Nitride Nanosheet/FeWO4 Nanoparticle Composite for Tandem Photooxidation/Knoevenagel Condensation, ACS Appl. Nano Mater., 2020, 3(7), 7057–7065 CrossRef CAS
.
- J. U. Choi and W.-K. Jo, FeWO4/g-C3N4 heterostructures decorated with N-doped graphene quantum dots prepared under various sonication conditions for efficient removal of noxious vapors, Ceram. Int., 2020, 46(8), 11346–11356 CrossRef CAS
.
- R. Bhosale,
et al., Direct Z-scheme g-C3N4/FeWO4 nanocomposite for enhanced and selective photocatalytic CO2 reduction under visible light, ACS Appl. Mater. Interfaces, 2019, 11(6), 6174–6183 CrossRef CAS PubMed
.
- L. Zhang,
et al., In situ growth of g-C3N4 on hexangular flowerlike FeWO4 microcrystals: highly efficient catalyst and the crucial roles of Fe3+/Fe2+ couple in the photoassisted oxidation and reduction reactions, J. Phys. Chem. C, 2018, 122(24), 12900–12912 CrossRef CAS
.
- R. Huang,
et al., In situ synthesis
of Cu+ self-doped CuWO4/g-C3N4 heterogeneous Fenton-like catalysts: the key role of Cu+ in enhancing catalytic performance, Sep. Purif. Technol., 2020, 250, 117174 CrossRef CAS
.
- S. Zhou,
et al., Enhanced visible light photocatalytic degradation of rhodamine B by Z-scheme CuWO 4/gC 3 N 4 heterojunction, J. Mater. Sci.: Mater. Electron., 2021, 32(3), 2731–2743 CrossRef CAS
.
- K. Liu,
et al., Synthesis of direct Z-scheme MnWO4/g-C3N4 photocatalyst with enhanced visible light photocatalytic activity, Nano, 2017, 12(10), 1750129 CrossRef CAS
.
- K. Saravanakumar,
et al., 1D/2D MnWO4 nanorods anchored on g-C3N4 nanosheets for enhanced photocatalytic degradation ofloxacin under visible light irradiation, Colloids Surf., A, 2019, 581, 123845 CrossRef
.
- V. Rathi, A. Panneerselvam and R. J. Sathiyapriya, Surface decoration of MnWO 4 nanoparticles with gC 3 N 4 nanosheets to build hetero-structure and its structural, optical and enhanced visible light photocatalytic activity, J. Mater. Sci.: Mater. Electron., 2020, 31(17), 14823–14837 CrossRef CAS
.
- V. J. C. Muthuraj,
et al, Superior visible light driven photocatalytic degradation of fluoroquinolone drug norfloxacin over novel NiWO4 nanorods anchored on g-C3N4 nanosheets, Colloids Surf., A, 2019, 567, 43–54 CrossRef
.
- V. Muthuraj, Superior visible light driven photocatalytic degradation of fluoroquinolone drug norfloxacin over novel NiWO4 nanorods anchored on g-C3N4 nanosheets, Colloids Surf., A, 2019, 567, 43–54 CrossRef
.
- V.-D. Dao,
et al., Superior visible light photocatalytic activity of g-C3N4/NiWO4 direct Z system for degradation of gaseous toluene, J. Solid State Chem., 2019, 272, 62–68 CrossRef CAS
.
- R. Koutavarapu,
et al., A novel one-pot approach of ZnWO 4 nanorods decorated onto gC 3 N 4 nanosheets: 1D/2D heterojunction for enhanced solar-light-driven photocatalytic activity, J. Mater. Sci., 2020, 55(3), 1170–1183 CrossRef CAS
.
- S. Zhan,
et al., g-C3N4/ZnWO4 films: preparation and its enhanced photocatalytic decomposition of phenol in UV, Appl. Surf. Sci., 2015, 358, 328–335 CrossRef CAS
.
- Z. Bian,
et al., Plant uptake-assisted round-the-clock photocatalysis for complete purification of aquaculture wastewater using sunlight, Environ. Sci. Technol., 2015, 49(4), 2418–2424 CrossRef CAS PubMed
.
- J. Zheng and L. J. C. E. J. Zhang, Designing 3D magnetic peony flower-like cobalt oxides/g-C3N4 dual Z-scheme photocatalyst for remarkably enhanced sunlight driven photocatalytic redox activity, Chem. Eng. J., 2019, 369, 947–956 CrossRef CAS
.
- M. Tang, Facile synthesis of dual Z-scheme g-C3N4/Ag3PO4/AgI composite photocatalysts with enhanced performance for the degradation of a typical neonicotinoid pesticide, Appl. Catal., B, 2020, 268, 118395 CrossRef CAS
.
- M. Tang,
et al., Rationally constructing of a novel dual Z-scheme composite photocatalyst with significantly enhanced performance for neonicotinoid degradation under visible light irradiation, Appl. Catal., B, 2020, 270, 118918 CrossRef CAS
.
- Y. Guo,
et al., Mediator-free direct dual-Z-scheme Bi2S3/BiVO4/MgIn2S4 composite photocatalysts with enhanced visible-light-driven performance towards carbamazepine degradation, Appl. Catal., B, 2019, 254, 479–490 CrossRef CAS
.
- H. Yin,
et al., Construction of AgBr/β-Ag2WO4/g-C3N4 ternary composites with dual Z-scheme band alignment for efficient organic pollutants removal, Sep. Purif. Technol., 2021, 272, 118251 CrossRef CAS
.
- L. Jiang,
et al., In-situ synthesis of direct solid-state dual Z-scheme WO3/g-C3N4/Bi2O3 photocatalyst for the degradation of refractory pollutant, Appl. Catal., B, 2018, 227, 376–385 CrossRef CAS
.
- N. T. T. Truc,
et al., Superior activity of Cu-NiWO4/g-C3N4 Z direct system for photocatalytic decomposition of VOCs in aerosol under visible light, J. Alloys Compd., 2019, 798, 12–18 CrossRef
.
- Y. O. Ibrahim and M. Gondal, Visible-light-driven photocatalytic performance of a Z-scheme based TiO2/WO3/g-C3N4 ternary heterojunctions, Mol. Catal., 2021, 505, 111494 CrossRef CAS
.
- T. Yu, L. Liu and F. Yang, Heterojunction between anodic TiO2/g-C3N4 and cathodic WO3/W nano-catalysts for coupled pollutant removal in a self-biased system, Chin. J. Catal., 2017, 38(2), 270–277 CrossRef CAS
.
- T. Yu, L. Liu and F. Yang, Heterojunction between anodic TiO2/g-C3N4 and cathodic WO3/W nano-catalysts for coupled pollutant removal in a self-biased system, Chin. J. Catal., 2017, 38(2), 270–277 CrossRef CAS
.
- N. Lu,
et al., Construction of Z-Scheme g-C3N4/RGO/WO3 with in situ photoreduced graphene oxide as electron mediator for efficient photocatalytic degradation of ciprofloxacin, Chemosphere, 2019, 215, 444–453 CrossRef CAS PubMed
.
- Y. Bao,
et al., Heterostructured WO3/RGO/protonated g-C3N4 three-layer nanosheets for enhanced visible-light photocatalytic activity, Appl. Surf. Sci., 2019, 496, 143639 CrossRef CAS
.
- H. Che,
et al., NGQD active sites as effective collectors of charge carriers for improving the photocatalytic performance of Z-scheme gC 3 N 4/Bi 2 WO 6 heterojunctions, Catal. Sci. Technol., 2018, 8(2), 622–631 RSC
.
- M. G. Kim and W.-K. Jo, Visible-light-activated N-doped CQDs/g-C3N4/Bi2WO6 nanocomposites with different component arrangements for the promoted degradation of hazardous vapors, J. Mater. Sci. Technol., 2020, 40, 168–175 CrossRef CAS
.
- M. G. Kim and W.-K. Jo, Visible-light-activated N-doped CQDs/g-C3N4/Bi2WO6 nanocomposites with different component arrangements for the promoted degradation of hazardous vapors, J. Mater. Sci. Technol., 2020, 40, 168–175 CrossRef CAS
.
- J. U. Choi and W.-K. Jo, FeWO4/g-C3N4 heterostructures decorated with N-doped graphene quantum dots prepared under various sonication conditions for efficient removal of noxious vapors, Ceram. Int., 2020, 46(8), 11346–11356 CrossRef CAS
.
- K. Mallikarjuna,
et al., Synthesis of oxygen-doped-g-C3N4/WO3 porous structures for visible driven photocatalytic H2 production, Phys. E, 2021, 126, 114428 CrossRef CAS
.
- C. Cheng,
et al., WO3/g-C3N4 composites: one-pot preparation and enhanced photocatalytic H2 production under visible-light irradiation, Nanotechnology, 2017, 28(16), 164002 CrossRef PubMed
.
- Y. Li,
et al., Construction of inorganic–organic 2D/2D WO 3/gC 3 N 4 nanosheet arrays toward efficient photoelectrochemical splitting of natural seawater, Phys. Chem. Chem. Phys., 2016, 18(15), 10255–10261 RSC
.
- B.-C. CHEN,
et al., Research progress on g-C3N4-based Z-scheme photocatalytic system, Acta Phys.-Chim. Sin., 2016, 32(6), 1371–1382 CAS
.
| This journal is © The Royal Society of Chemistry 2023 |