Open Access Article
Open Access ArticleThe role of polyplexes in developing a green sustainable approach in agriculture
Pratyush K. Das ,
Gyanendra Panda,
Kananbala Patra,
Nivedita Jena and
Mamoni Dash
,
Gyanendra Panda,
Kananbala Patra,
Nivedita Jena and
Mamoni Dash *
*
Institute of Life Sciences, DBT-ILS, Bhubaneswar, Odisha, India. E-mail: mamoni.dash@ils.res.in
First published on 30th November 2022
Abstract
Rise in global population has increased the food demands and thus the competition among farmers to produce more and more. In the race to obtain higher productivity, farmers have resorted to injudicious farming practices that include the reckless use of nitrogenous fertilizers and intensive cropping on farmlands. Such practices have paved the path for large scale infestations of crops and plants by pests thus affecting the plant productivity and crop vigour. There are several traditional techniques to control pest infestations in plants such as the use of chemical or bio-pesticides, and integrated pest management practices which face several drawbacks. Delivery of gene/nucleic acid in plants through genetic engineering approaches is a more sustainable and effective method of protection against pests. The technology of RNA interference (RNAi) provides a sustainable solution to counter pest control problems faced by other traditional techniques. The RNAi technique involves delivery of dsDNA/dsRNA or other forms of nucleic acids into target organisms thereby bringing about gene silencing. However, RNAi is also limited to its use because of their susceptibility to degradation wherein the use of cationic polymers can provide a tangible solution. Cationic polymers form stable complexes with the nucleic acids known as “polyplexes”, which may be attributed to their high positive charge densities thus protecting the exogenous nucleic acids from extracellular degradation. The current paper focuses on the utility of nucleic acids as a sustainable tool for pest control in crops and the use of cationic polymers for the efficient delivery of nucleic acids in pests thus protecting the plant from infestations.
1. Introduction
Sustainability in agriculture is brought about by effective practices and technologies that consider minimizing the environmental footprint.1 The use of nucleic acids as a sustainable means for control of pests in crops and plants has started to gain foothold. Nucleic acids which include either DNA or RNA can act as an active ingredient in pest control formulations. Upon exposure of an organism to these nucleic acids, can either act to cause mutagenesis or can cause gene silencing thereby altering the expression in a particular gene or a set of genes.2 RNA is the most common active ingredient used in topical pesticidal applications. Targeting specific gene/mRNA sequences in pathogens and pests of different plants inducing RNA silencing via specifically designed nucleic acids seems to be quite promising. Special interests of researchers have been drawn towards the use of dsRNAs, and siRNAs to induce RNAi mediated gene silencing.3 Exogenously supplied dsRNAs could induce gene silencing in an organism by means of injecting, soaking, or feeding and can cause heritable effects in certain cases.4 An RNAi study in C. elegans was a first of its kind affecting gene silencing in the organism via exposure to environmental RNAi. The resultant gene silencing as a result was evident from the systemic spread of the silencing signals to all the cells in the organism. Environmental RNAi offers to be a cost-effective and simple method to deliver dsRNA into organisms. This particular method has led to a large scale RNAi screening studies thus paving way for several innovative pest control approaches in agriculture.5–8Chemical pesticides are mostly used to control pest infestations in farmlands owing to their low cost-nature but have several negative implications on the crop9 as well as its environment.10 Moreover, aggressive uses of such chemicals have resulted in the occurrence of resistance amongst pests towards the particular pesticide. There arises an immediate need to look for alternative options that not only will help effectively control the pests but also simultaneously act as an cheap, biodegradable, and non-toxic product. Conventional gene transfer methods employing genetic engineering approaches have proved to be successful but face several drawbacks including public acceptance issues.11 As such nucleic acid based environmental RNAi approaches could be instrumental in controlling the pests of plants thus substantially improving the productivity as well as preventing large scale crop loss.
Development of nucleic acid based topical RNAi strategies for protection against pests pose several bottle necks. A major issue is the lack of amplification of silencing signal mostly due to the degradation of the active ingredient (dsRNA) post ingestion.12 The availability of RNAi effectors and their silencing inducing ability has been found to decrease with an increase in the distance from the site of its exogenous application.13 Therefore, the current research activities in relation to the development of RNAi based topical pest control products are mostly focussed on two aspects. The first is to identify specific target genes in a pest to induce high mortality rates and the other is to ensure the stability of the topically applied dsRNAs.1 Protection of the dsRNA from environmental degradation like change in pH within the pest gut is a major aspect for feeding assays. Nanotechnology offers a wide range of applications in different fields of research which includes pharmaceutics, cosmetics, electronics, food products, sensor-based devices, and environment.14,15 Similar to the drug delivery capacity, nanosystems also act as an important gene delivery agent which is now mostly being explored in the field of agriculture for disease resistance and pest control. Nanoparticles have proven to play an important role in delivery of genes or dsRNA into pests thus conferring gene silencing with high specificity and efficiency.16 Cationic polymer based nanoparticles offer an effective option for safe delivery of these dsRNAs into the pests as non-viral delivery agent. They can be easily produced, stored, and literally pose to be non-pathogenic which is an advantage of such polymers over other traditional carriers like viruses.17 In one of the approaches, nucleic acids are loaded into the nanoparticle either upon encapsulation into the matrix or chemically conjugated by appropriate surface modification. In the other approach, strong positive charges of cationic polymers efficiently bind to and condense the nucleic acids to form a polyplex. This condensation renders the polyplex to become a nanosystem, thus facilitating the entry through the cellular membranes. The polyplexes are formed upon spontaneous electrostatic condensation between the nucleic acid and the cationic polymer. These polymers are also known to enhance the endosomal escape ability. Several mechanisms of their interaction with the cell membranes have already been documented in other reviews and is beyond the scope of this review.18
The current review emphasizes on the problem of pest infestations in agricultural crops and its impact on the economy. It highlights the role of nucleic acids like dsDNA, dsRNA as an active ingredient in pest control applications over traditional pest control systems. The authors also categorically focus on the use of cationic polymers over other agents for safe and efficient delivery of nucleic acids in pests via inducing gene silencing through RNAi approach.
2. Nucleic acid delivery in plants
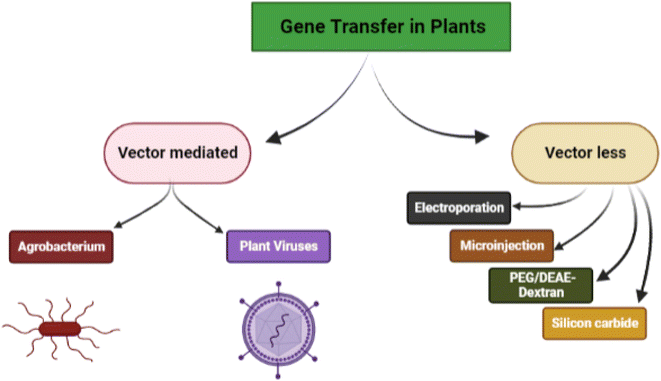
Genetic engineering in plants is an attractive and efficient way to improve crops, biosynthesize different plant products and bring about sustainability in agriculture. Several biological, chemical, and physical approaches have been successfully used in the context. The delivery of exogenous nucleic acids/genes in plants can be either through vector-mediated gene transfer (indirect method) and vector-less gene transfer (direct method) (Fig. 1).Vector-mediated gene transfer is carried out either by Agrobacterium-mediated transformation or by the use of plant viruses as vectors. This approach involves the pairing of the transgene with a vector (Ti-plasmid) that delivers it to the target cells for integration. Agrobacterium tumefaciens is a Gram-negative soil bacterium that infects dicotyledonous plants and induces crown gall tumors at affected sites. This exceptional ability of Agrobacterium tumefaciens aids in the advancement of plant transformation techniques.19 According to the researchers, the segregation of GFP signals revealed that a variety of wild Oryza species can be genetically transformed by utilising modified immature embryo technique thereby confirming the transmission of T-DNAs to the following generation.20 The bacterium because of its capability to transfer DNA into other organisms is a potential vector to produce transgenic plants that can confer resistance to some pests.
Non-Agrobacterium mediated gene transfer involves recruiting improved non-Agrobacterium strains such as Rhizobium, Ensifer, Ochrobactrum haywardense equipped with subtle mechanisms to deliver the gene into plant cells. The Transbacter technology can hasten the search for non-Agrobacterium species capable of transforming plants.21 Research findings demonstrated the propensity of trans-kingdom gene transfer with associated rhizobia; when equipped with a disarmed pEHA105 Ti plasmid and a binary plasmid (pCAMBIA1105.1R) that can be used to transfer T-DNA to a variety of plant species including Arabidopsis thaliana (model plant), Nicotiana tabacum (non-food crop), and Oryza sativa (food crop).22
Vector-less gene transfer is carried out by physical (electroporation, biolistic, microinjection, silicon carbide fibre-mediated) and chemical (polyethylene glycol-induced and DEAE-dextran mediated) gene transfer methods.
In essence, electroporation entails the employment of electrical impulses with high field strength to reversibly permeate cell membranes for DNA absorption. DNA can be delivered into intact plant cells and protoplasts using this method. The oil palm cell of the Elaeis guineensis species was successfully transformed by the electroporation technique, which led to the formation of explants with increased growth rates.23
Particle bombardment (or biolistic) commonly known as microprojectile or gene gun is a direct gene delivery technique that uses high-velocity micro-projectiles to deliver foreign DNA into plants. DNA-coated gold or tungsten microcarriers or microprojectiles are accelerated toward the target plant in order to pierce the cell wall. The transferred DNA separates from the microprojectiles after entering the cells, where it can be partially expressed or may be permanently incorporated into the host genome.24
Microinjection follows a mechanical approach to transfer the desired DNA into the target plant cells. It is employed for chromosomal modification and gene transfer. In this method, the gene is introduced into a protoplast's cytoplasm using a glass micropipette and a transgenic plant is generated by culturing the modified cell.25 In fact, this method has led to the development of transgenic tobacco and Brassica napus. Agrobacterium tumefaciens strain EHA105 containing the uidA gene was microinjected into shoot apex explants of cotton (Gossypium hirsutum L. ‘KC3’) and cultivated. In this experiment, the bacterial cell suspension was carefully microinjected 1–5 times into the pre-cultured apical meristem areas of shoot apices. Microinjections of an Agrobacterium tumefaciens cell density up to three times produced better results, but more than three injections caused severe meristematic damage and decreased the explants' survival rates.26
Sonoporation is a technique for delivering genes to targeted cells by employing ultrasound that creates small pores in the plasma membrane thereby transferring the gene of interest into the cell. In this approach, the gene–microbubble combination improves the transfer efficiency while micro bubbles lower the threshold for cavity formation.27 The expression of the hCTLA4Ig gene was suppressed using siRNA in transgenic cell cultures. The chemically synthesized siRNA duplex was coupled with polyethyleneimine and the cells were exposed to sonoporation at 40 kHz and 419 W for 90 s to enhance the delivery process. The sonoporation-delivered siRNA complexes downregulated the synthesis of hCTLA4Ig by 73%. Therefore, it can be inferred that sonoporation may improve the delivery of siRNA complexes into plant cells.28
Silicon carbide fibre-mediated transfer is a technique identical to microinjection in which the DNA is transported into the cell by using silicon carbide fibres. The silicon carbide fibres with DNA coating are vortexed with plant sample (suspension culture, calluses).29 DNA attached to the fibres penetrates the cells during mixing and is successfully integrated with the host genome.30 Silicon carbide whiskers with callus, plasmid harboring chitinase, and hygromcin genes were vortexed to deliver genes in peanut (Arachis hypogaea). In order to transform 2 g of 20 day old callus with the maximum transformation efficiency (6.88%), 200 mg of whiskers and 5 g of plasmid were employed. Hygromycin-resistant calli were grown into complete plants that produced seeds and had a far higher level of resistance to the leaf spot disease than control plants.31
In polyethylene glycol-mediated gene transfer the plasma membrane of protoplasts is destabilized by the Ca2+ ions and becomes permeable to DNA. Hence the naked DNA enters the nucleus and gets incorporated into the genome. The technique involves the protoplast isolation and suspension, addition of plasmid DNA, followed by gradual addition of 40% PEG-4000 (w/v) dissolved in calcium nitrate and mannitol solution. Protoplasts get transformed during incubation. Polyethylene glycol-mediated protoplast transfection was carried out with ribonucleoproteins comprising LbCas12a and a single guide RNA. Analysis of T1 offspring confirmed that DNA-free edits resulted at 40% frequency and the modifications are heritable.32
Delivery of exogenous biomolecules into plants is quite a difficult process due to the barrier posed by the rigid plant cell wall. The conventional nucleic acid delivery methods for crop improvement and protection pose several drawbacks which include high cost of upstream production, difficulty in plant regeneration, and propagation of elite varieties.33 Transgenic crops have issues related to non-acceptance among the public due to concerns regarding safety of human health, animals, and the environment.34
Protection of crop from infestations by pests is an uphill task and needs utmost priority. Among all the crops, the ones belonging to the Poacea family are considered economically most important which may be attributed to the large scale dependence of the human population to meet the food demands.
3. Susceptibility of plants to pests and conventional pest control measures
Nitrogen is very much essential for crops and helps in increasing the yield.35 The high leachability of several forms of nitrogen in the soil makes them unavailable for absorption by crops and thus the need for exogenous application of the element through fertilizers is a common practice.36,37 Availability of nitrogen to plants makes them more succulent and thereby more prone to be fed upon by the pests.38 Excessive use of fertilizers is also responsible for creating disturbances in the crop canopy as well as the balance between the plant and animal communities.39 Increase in crop biomass also leads to increase in the crop density thereby providing suitable breeding habitat for the pests. Pest infestations are a major concern for cultivation of crops and there has been a surge in the use of chemical pesticides in recent times which may be attributed to the large scale cultivation of crops. Increased and reckless use of these pesticides has resulted in a significant loss in the biodiversity along with pollution of the water resources.10 Pesticides affect crops and plants to a great extent.9 Pest control forms an integral part of agricultural practices and is much important as far as crop productivity is concerned.40,41 Rising populations, increased demand for food, aided with the onset of green revolution in the early 1970s led to an increase in pest infestations.42 Practices of monoculture farming along with the use of chemical fertilizers, and pesticides became more frequent. Fertilizers and pesticides tend to lose their efficiency in the environment due to alterations in their chemical composition under high temperatures, and wash off due to heavy rainfall.43 To counter the reduction in the effectiveness the farmers tend to bring about an increase in the dosage and frequency of application of the agrochemicals, thus leading to development of resistance in pests.44There are several types of pesticides used in the agricultural practices which differ from each other based upon their chemical and physical nature. Drum (1980)45 proposed the categorization of pesticides in three different ways mainly based on the origin, the target pest, and the pesticidal function as well. Natural or organic pesticides include plant phytochemicals, essential oils, and plant extracts that have been proven to be effective against several pests.46 These compounds pose negligible toxicity towards mammalian cells, short environmental persistence, and prevent the development of pest resistance due to their chemical complexity.47 Organic pesticides are although environment friendly but due to low persistence, can have varying effectiveness on targeted pests. High cost of organic pesticides is also a major issue that hinders its use on a field scale. Inorganic pesticides are generally composed of simple inorganic salts that have higher solubility in water as compared to the organic ones. Examples include sulphur, sulphates of metals like copper and iron, and lime.48 The major issue with use of inorganic pesticides is their long environmental persistence and chances of being carried further in the food chain. These pesticides being rich in metal salts tend to be more toxic to the living biota especially the soil microbiota that play a major role in crop health and vigor (Fig. 2).
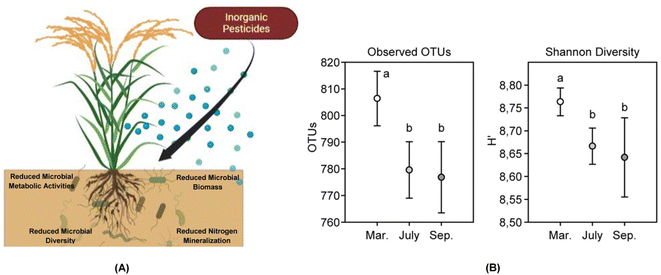 | ||
| Fig. 2 Impact of inorganic pesticides on soil microbes. (A) Inorganic pesticides are known to affect soil microbial population to a great extent. These pesticides have been found to negatively impact metabolic activities of the microbes thereby leading to death. Pesticides are known to sometimes promote the growth of a particular microbial species and retard others. This creates a disturbance in the diversity of microbial populations in the soil. Retardation of microbial growth due to application of inorganic pesticides also leads to reduced microbial biomass. The toxic nature of the pesticides not only hampers general soil microbes but also certain rhizospheric microbes colonizing the roots of the crops. The pesticidal toxicity reduces the nitrogen mineralization ability in the microbes thereby rendering the soil low on nutrient. (B) Holmsgaard et al. (2017)49 studied the responses of a bacterial community to pesticides used over an agricultural season (March to September) in a farm. The microbial diversity of the soil was significantly reduced between the month of March to July as quite evident from the reduction in the operational taxonomic units (OTUs) and the Shannon diversity index thus signifying the negative impact of the pesticides on the soil microbial community of the agricultural farm. (B has been reproduced from Holmsgaard et al. (2017)49 with permission from Elsevier, copyright 2017). | ||
Man-made or synthetic pesticides are the most commonly used pest control agents in the field of agriculture. These include the organochlorines, organophosphates, carbamates, and pyrethroids. The mode of action of these pesticides on crop pests includes alteration of nervous functions, disruption of sodium channels, paralysis, and death. These synthetic pesticides, although have a pronouncing effect on a broad range of pests, but bear a long lasting deleterious effect on the environment as well as its components (Fig. 3).
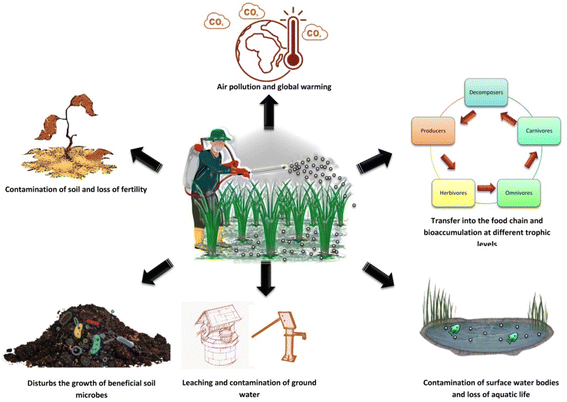 | ||
| Fig. 3 Deleterious effect of synthetic pesticides on the biotic and abiotic components of the environment. | ||
4. Greener possibilities of pest control
Since their introduction in the late 1940s, synthetic pesticides have been widely used which may be attributed to their high efficacy, ease of application, and cost friendly nature. However, the large scale uses of pesticides have led to several deleterious effects on the environment. Some of the major effect includes reduction in soil quality, contamination of ground water, accumulation of toxic chemicals in the food chain, health disorders in humans, and destruction of the biodiversity.50 The synthetic pesticides being non-specific in nature also tend to harm beneficial soil microbes, and other organisms along with the development of resistance among the pests.51 The increased resistance paves way for large scale destruction of crops thereby greatly affecting the production. Pests of the Lepidoptera family are one of the most harmful, accounting for approximately 10 million mega grams of loss in crop yield.50Green pest control technologies offer an alternative to the conventional chemical pesticides thereby preventing any sort of damage to the environment and its components. This is where the need to employ integrated pest management (IPM) comes into the limelight. IPM includes several strategies to control pests in agricultural fields. This may include either a single or combination of techniques involving genetic, mechanical, cultural, biological, and chemical tools.52
4.1. Biological based techniques
Biological based IPM strategies are most feasible and environmental friendly way to control pests of crops. The bio-based techniques are mostly dependent on the environmental conditions and utilize a broad range of bio-agents like bacteria, viruses, fungi, and other predators.50 Natural predators like spiders play a major role in keeping the pest population under control.53 Natural chemicals obtained from living organisms like plants and microbes can also be instrumental in controlling the growth and development of the pests.54 These chemicals are used as a major ingredient in the formulation of biopesticides and hold an extremely high value in a sustainable agricultural system. Plant extracts of Azadirachta indica has been successfully used as a bio-control agent for the control of brown plant hopper, thus leading to 82% of mortality.55 Bt agent is a popular bio-insecticide mostly recommended and used in China to control the outbreaks of stem borer and leaf folders in rice plants.4.2. Development of resistant varieties
Improving the resistance of crop varieties by conventional breeding approaches is also a very useful method of pest control in crops and is generally termed as the host-plant resistance mechanism. Researchers have identified 29 genes in rice plants resistance to the brown plant hopper.56 The resistance of the conventionally breed varieties however weaken after certain generations leading to further development of resistance among the pests.57Genetically modified plant varieties have been found to overcome the disadvantages posed by the conventional breed varieties. Introduction of several genes into the plants have provided breakthrough results in pest control and management.
4.3. Non-biological techniques
Traps designed to kill pests utilizing specific frequencies of light have also been used to trap pests like adult stem borers and plant hoppers.58 The major problem with these pest traps is that these lack specificity and will cause high mortality among other beneficial insects.Post-harvest conditions are a major factor involved in pest outbreaks. Rice stubbles form the main breeding grounds for pests like stem borers and their population in the subsequent season is mostly dependent on the existing stubble environment. Mechanical harvesting of the stubble helps in reducing the pest populations. Reduction in the stubble height has been found to bring about a 70–90% reduction in the surviving pests.58
Ecological engineering methods like growing nectar rich flowering plants can harbour several natural enemies of the pests.59 These enemies will help control the pest population and their outbreaks thus preventing damage or yield loss.
5. RNA interference (RNAi) as a sustainable technique for pest control
Insect pests are a major threat to plants that directly increase the pressure on global food supply which already remains affected due to the rise in population and other environmental problems. Chemical pesticides are although effective but have been found to cause collateral damage to the environment and its biotic counterparts. Moreover, they tend to kill other beneficial and non-target pests. Development of transgenic plants can counter these limitations however can lead to emergence of resistance among the pests which is also another major concern.60 In such a scenario, pest control technologies based on RNA interference (RNAi) seems more promising due to their target specific nature.61 Three different types of RNAi pathways have been identified in insects which includes the siRNA pathway (involves dsRNA/siRNA), the miRNA pathway, and the piRNA pathway.62–64 The different pathways play different roles in the insects. The siRNA pathway protects the insect from viruses and transposons65,66 while the miRNA pathway plays a major role in the regulation of genes,67 and the piRNA pathway supresses the expression of germ line transposons.68 Insect pests have been found to take dsRNA more rather than the siRNA through the process of clathrin mediated endocytosis.69,70The process of RNAi is comprised of two important steps. The first step involves the uptake of dsRNA by the cells of the pest followed by the second step which involves the processing of the same by the central RNAi machinery of the cell (Fig. 4). Hence, the cellular uptake is a major factor to be considered in RNAi based pest control strategies. dsRNA synthesized chemically can be applied directly to the leaf of a targeted plant in the form of a foliar spray. Pests ingesting upon the leaf of the plant simultaneously intake the dsRNA which is thus directed into the lumen of the pests gut. Inside the gut, uptake of the dsRNA into the cells mostly occurs via the mechanism of clathrin dependent receptor mediated endocytosis. Besides, SID1 like (SIL) proteins, extracellular vesicles (EV), and RNA binding proteins (RBPs) that are secreted post the fusion of multivesicular bodies with the plasma membrane of the insect cells may also possibly help in the uptake process. Moreover, these molecules are also thought of as a probable mechanism for facilitating the movement of the dsRNA/silencing signal from one cell to the others thereby causing systemic RNAi based silencing in certain pests. However, the actual role of these molecules in the uptake of dsRNA is yet to be clearly elucidated.71 The cells lining the gut of the insect uptake the dsRNA which is further cleaved by DICER enzyme into sRNA and then loaded into some specific members of the AGO protein family. This leads to formation of a RNA induced silencing complex (RISC) following which the guide strand of the sRNA promotes the binding of the RISC complex to the complementary target RNA. Recognition of the target results in post transcriptional gene silencing in the cytoplasm of the insect cell either by degradation of the target mRNA or by inhibiting its translation process. Sometimes transcriptional gene silencing in the nucleus of the pest cells may occur by modifications of chromatin.72
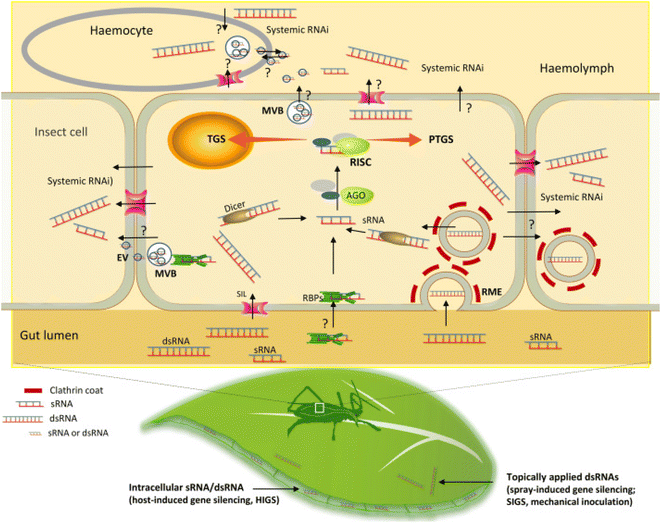 | ||
| Fig. 4 Uptake of dsRNA and the RNAi machinery in pests. This figure has been reproduced from Liu et al. (2020)72 under a creative commons license (CC BY 4.0). | ||
Besides foliar application, RNAi can also be induced directly in the host plant (host induced gene silencing) or through viruses (viral induced gene silencing).73 Host induced gene silencing involves the development of transgenic crops that have the ability to express dsRNA against a specific pest. RNAi induced gene silencing approaches can also be carried out using genetically engineered viruses that can produce the desired dsRNA in the pest it targets.74 The two later approaches involves transgenic organisms which is not widely acceptable over the globe and faces ethical issues. Moreover, development of transgenics requires high skill as well as the cost of development is too high. Table 1 elucidates some of the genes targeted in pests to induce the silencing effect, and the RNAi process involved therein.
| Plant/crop | Pest | Target gene | RNAi type | References |
|---|---|---|---|---|
| Transgenic corn crop | Diabrotica virgifera virgifera | snf7 | Host induced | 61 |
| Transgenic rice | Lepidopteran sp. | Cry (Bacillus thuringiensis) | Host induced | 75 |
| Transgenic rice | Brown plant hopper | Bph38(t), Bph37, Bph36, Bph34 | Host induced | 76 |
| Maize | Diabrotica virgifera virgifera | Troponin I | Host induced | 77 |
| Corn | O. furnacalis | CHT10 | Spray induced | 78 |
| Rice | S. exigua | Chitin synthase B | Spray induced | 79 |
| Nicotiana attenuata | Manduca sexta | MsCYPs | Virus induced | 80 |
| — | Drosophila melanogaster | Vha26, RPS13, and alpha COP | Virus induced | 81 |
Huvenne and Smagghe (2010)82 classified the RNAi process into two types based upon its silencing effect as – cell autonomous RNAi and non-cell autonomous RNAi (Fig. 5).
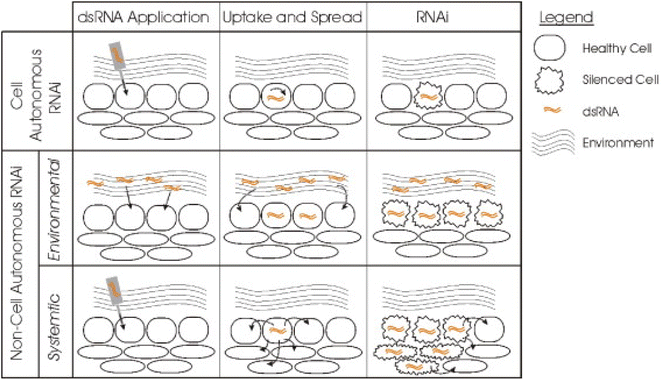 | ||
| Fig. 5 Different types of RNAi based upon their silencing effects. In the cell autonomous RNAi, the dsRNA of a gene is generally applied to or expressed in a particular cell thus limiting the silencing effect to the cell. The non-cell autonomous RNAi includes systemic and environmental RNAi. Environmental RNAi involves uptake of the dsRNA from the environment and the effect can be observed in all the cells that are able to uptake the dsRNA. This type of exposure occurs by either soaking or feeding the targeted pest. In case of systemic RNAi the silencing signal is transported from the cell where the dsRNA is applied or expressed to other different cells or tissues of the organism thus spreading the silencing effect (this figure has been reproduced from Huvenne and Smagghe (2010)82 with permission from Elsevier, copyright 2010). | ||
6. Limitations of RNAi based pest control
RNAi has become an important tool to silence targeted genes. It has several benefits when compared to other pest control agents available in the market. However, any novel inventions or products have certain levels of risk associated with them and so is the RNAi mediated pest control process (Table 2).| Limitations of RNAi | Probable reasons | Reference |
|---|---|---|
| Lethal impact on beneficial insects | Non-targeted pest sharing common key genes/similar dsRNA sequence homology with the target pest | 83 |
| Lack of uniform susceptibility between species | Enzymatic degradation of dsRNA, exposure dosage, impaired RNAi machinery, presence of virus in the target pest | 84 |
| Variation in RNAi responses within the same insect | Insufficient spread of RNAi response throughout the body, variation in pH among different organs | 85 and 87 |
| High production costs | High cost of chemicals, equipment, and maintenance conditions | 1 |
| Extracellular degradation of dsRNA | Degradation by nucleases present in the digestive system | 91 and 92 |
6.1. Impact on other beneficial insects
The RNAi process is although highly targeted and specific for a particular gene sequence, there remains a chance that it may sometimes affect a non-target insect that holds close genetic resemblance to the targeted pest. This may be a possible case if the non-targeted pest shares a common key gene with the pest and also has a close dsRNA sequence homology. The beneficial insect with respect to the pest if shares the common host and same feeding pattern, then could lead to its death.836.2. Variation of susceptibility between species
Prediction or expecting a successful gene knockdown can be quite difficult as the susceptibility of pests to dsRNA is species dependent and may vary widely. When exposed to dsRNA, insect pests belonging to coleopteran species are mostly susceptible followed by the dipterans, and hymenopterans species. The lepidopterans and hemipterans species are found to be very rarely susceptible.846.3. Variation of susceptibility among species
Populations from within the same species show differential responses to external administration of dsRNA. Study conducted by Sugahara et al. (2017)85 suggested that individuals belonging to the same laboratory strain can have different degrees of responses towards dsRNA. Similar results were also confirmed by other researchers.86,876.4. Tissue dependent variation
Several inconsistencies have been observed in RNAi response within the same insects. The susceptibility of the insect pests to dsRNA mediated gene silencing can vary in its degree from tissues to tissues or cell to cell.88 Telang et al. (2013)89 reported lower RNAi efficiency in the ovarian and head tissues of Aedes aegypti as compared to other tissues upon external application of dsRNA. Tissue dependent RNAi susceptibility has also been observed in lepidopteran species.906.5. High cost of production
The production/synthesis of target specific dsRNA for RNAi against crop pests is a costly affair and thus will increase the price of the final product available for pest control in crops like rice plants.16.6. Extracellular degradation of dsRNA
Some insects on feeding upon dsRNA have almost no effect. The dsRNA upon reaching the digestive system of the insects get degraded by the nucleases present thus inhibiting the RNAi activity.91 A study by Wynant et al. (2014)92 demonstrated the dsRNA degrading ability of digestive solution obtained from the midgut of Schistocerca gregaria. The digestive enzyme solution was found to degrade almost 150 ng of the dsRNA within 5 minutes of exposure.7. Tools to overcome RNAi instability
The problems faced due to low RNAi sensitivity in the targeted pests needs to be looked upon seriously before applying it as a potential pesticidal agent. Facilitation of uptake of dsRNA and preventing its degradation can be carried out by means of efficient delivery systems. Some of the recent delivery systems include microrganisms like bacteria, and viruses, nanoparticle based carriers, liposomes, carrier proteins, and chemical modifications.93Microorganisms specifically genetically modified bacteria that lack the RNaseIII endonuclease have been used to deliver dsRNA within the insect cells.94 The bacterial shell is thought of probably providing a protective effect to the dsRNA inside the digestive system. Pre-treatment of the bacterial cell by sonication have been found to improve the dsRNA release inside the insect most probably by weakening the bacterial cell wall.95 The selection of bacteria for the purpose is an important factor and only symbiotic bacteria or yeasts must be selected to avoid any potential pathogenicity to other organisms.96 Viruses can also be used as an effective tool for the delivery of dsRNA into the intracellular environment in pests. Viruses have been found to be specific to certain particular hosts and thus can be carefully selected as a successful delivery agent in RNAi experiments.74 Despite possessing several advantages as a delivery system for dsRNA, the application of the viral carriers in vivo has not yet been fully investigated due to many concerned safety issues. All the viruses are not host specific and may pose the chance of cross infecting several other beneficial insects which is a potential biosafety issue.
Nanoparticles can also be used as a delivery agent wherein dsRNA can be incorporated into these particles to enhance the stability and uptake efficiency. Chitosan derived nanoparticles have been found to efficiently deliver dsRNA through oral routes in A. gambiae and A. aegyptia resulting in knockdown of genes.97 Synthetically modified polymer nanoparticles have also been used to a great extent. Uptake of dsRNA complexed with a fluorescent nanoparticle led to RNAi silencing of CHT10 gene in the larvae of Asian corn borer (O. furnacalis). The fluorescent nanoparticle not only helped to visualize the dsRNA after uptake but also prevented the aggregation of the same in water.78 Several nanoparticles have been designed from cationic polymer based derivatives functionalized with guanidine side groups. The functionalization helped protect the dsRNA from degradation under high alkaline pH which is a characteristic feature of the lepidopteran gut environment (Fig. 6). The pH stable nanoparticles when fed to larvae of S. exigua resulted in the knockdown of chitin synthase B gene thus leading to increased mortality.79
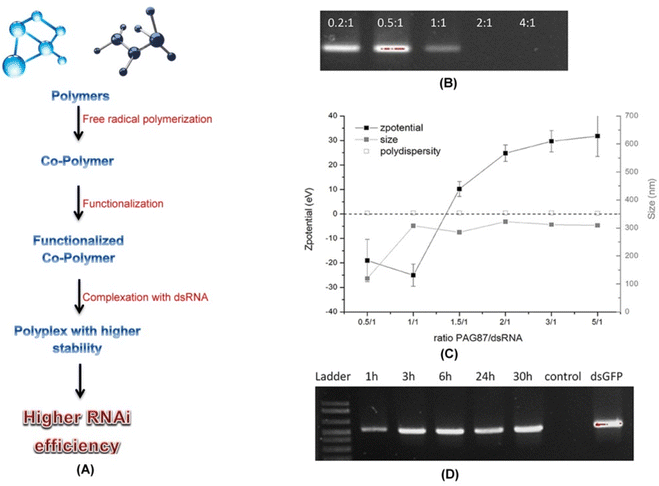 | ||
Fig. 6 Functionalized polymers and increased RNAi efficiency. (A) Schematic diagram showing steps in formation of cationic polymer based polyplexes with higher stability and RNAi efficiency. In a recent study several different combinations of polyplexes were designed by Christiaens et al. (2018)79 and were characterized. Based upon the N/P ratios, charge, size, and degradation assay the polymer PAG87 was selected as the most suitable candidate for polyplex formation with the dsRNA. (B) A 100% complexation was achieved at N/P ratios of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4 1 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as evident from the agarose gel data. (C) The zeta potential data also verified the same. (D) Results from an ex vivo assay revealed the protective nature of PAG87 due to the high content of guanidine against nucleolytic degradation of dsRNA. The polyplex when incubated in the gut juice obtained from S. exigua at pH 11 for different time periods followed by decomplexation of the dsRNA and analysis over a 1.5% agarose gel revealed protection of the dsRNA for as long as 30 hours. This supports the possible use of functionalized polymers like PAG87 in successful protection of the dsRNA at higher alkaline pH from degradation thus increasing the RNAi efficiency. (B–D) has been reproduced from Christiaens et al. (2018)79 under a creative commons license (CC BY 4.0). 1 as evident from the agarose gel data. (C) The zeta potential data also verified the same. (D) Results from an ex vivo assay revealed the protective nature of PAG87 due to the high content of guanidine against nucleolytic degradation of dsRNA. The polyplex when incubated in the gut juice obtained from S. exigua at pH 11 for different time periods followed by decomplexation of the dsRNA and analysis over a 1.5% agarose gel revealed protection of the dsRNA for as long as 30 hours. This supports the possible use of functionalized polymers like PAG87 in successful protection of the dsRNA at higher alkaline pH from degradation thus increasing the RNAi efficiency. (B–D) has been reproduced from Christiaens et al. (2018)79 under a creative commons license (CC BY 4.0). | ||
Lipid based transfection agents referred to as ‘liposomes’ are also very instrumental in increasing RNAi efficiency. Formation of liposomes occurs naturally when the transfection agents are subjected to an aqueous environment. Bilayer lipid particles are formed when positively charged lipid molecules envelope the negatively charged dsRNA.98 The liposome encapsulated dsRNA is facilitated entry into the cell through lipofection. Zhang et al. (2018)99 had successfully performed liposome mediated uptake of dsRNA in Rhipicephalus haemaphysaloides.
Carrier proteins also called as cell penetrating peptides are also an excellent prospect for delivery of dsRNA into pest cells. These peptides are cationic in nature and comprises of short chains of amino acids (10–30) with a high occurrence of basic amino acid residues like lysine and arginine.100 The cationic peptides facilitate the entry into the intracellular environment of the pest most possibly by endocytosis along with transporting the dsRNA. However, the exact cellular mechanism behind the carrier proteins mediated dsRNA uptake is still fuzzy.101 In a study by Gillet et al. (2017)115 to induce RNAi response in Anthonomus grandis, a fusion protein comprising of a peptide transduction domain and dsRNA binding domain from human protein kinase R was designed. The protein transduction domain and dsRNA binding domain along with the dsRNA forms a ribonucleic protein particle which in turn facilitates the uptake into the gut of the insect. The ribonucleic protein particle was found to increase the knockdown of chitin synthase II gene in A. grandis as compared to the naked dsRNA.
Small RNA oligonucleotides like siRNA are not effective enough to initiate a RNAi response in pests, however chemically modifying these molecules has been found to improve their activity in terms of stability and uptake.102 Modified siRNAs targeting important genes in Plutella xylostella have been found to result in increased mortality.103
8. Cationic polymers in RNAi based pest control
Cationic polymers are the positive charge bearing macromolecules. The charges may either be present in the backbone or in the side chains of the polymer. Most of these polymers contain functional amine groups that could be protonated.104 Cationic polymers have been found to be quite instrumental in several fields which involves drug delivery, gene delivery, and as antimicrobial agents.Cationic polymers act as an efficient non-viral agent for transfer of DNA material into the pests. Due to high positive charge densities, the cationic polymers interact with the negatively charged dsDNA thus forming stable complexes termed as ‘polyplexes’ or ‘nanoplexes’.105 The cationic polymers protect the dsDNA from degradation by enzymes and high alkaline pH present in the gut microenvironment of the pests. Moreover, the cationic polymers are biodegradable, less toxic, are structurally diverse, and pose high transfection efficiency.106 These polymers facilitate internalization into cellular compartments and endosomal escape through a mechanism known as proton sponge107 (Fig. 7). Cationic polymers also help in controlled release and the net positive charge of the complex facilitates binding to the anionic proteoglycans present on the cell surfaces.108 The polyplex can be used as a formulation for spraying on leaves of the crops. The complex either is ingested or enters the pest through dermal penetration. The polyplex after binding to the cell membrane of the insect enters into the cell by endocytosis. Post the uptake by the cells, the polyplex generated endocytic vesicles travel through the microtubes to fuse with early endosomes which further mature into late endosomes at pH = 5.0–6.2. These polyplex fused endosomes finally enter into degradative lysosomes. The polyplex here needs to exit the endosome to prevent degradation by the lysosome which is carried out by a mechanism known as ‘proton sponge effect’. Cationic polymers in general have a strong buffering capacity. The acidic environment inside the lysosome causes protonation of the amine groups present in the polymer, leading to influx of water and lysis of the endosome thus releasing the dsDNA into the cytosol. The next step involves unbinding of the dsDNA from the polymer nanoparticle which occurs via competitive displacement of the polymer from the dsDNA by certain intracellular polyanions. The polymeric nanoparticles can also be designed to respond to intracellular stimuli like pH and certain reducers thus inducing the disassembly process.
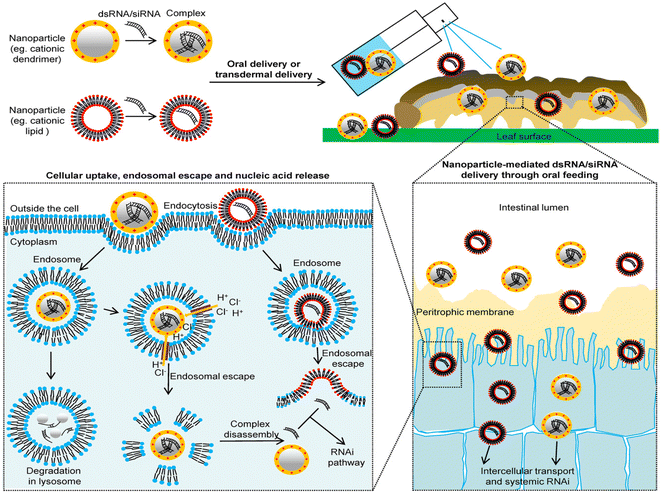 | ||
| Fig. 7 Cationic nano-polymer based delivery of dsRNA/siRNA in pests. This figure has been reproduced from Yan et al. (2021)109 under a creative commons license (CC BY-NC-ND 4.0). | ||
Several types of cationic polymers based upon their structural variations have been used for control of pests in crops. The current section summarizes some of the important cationic polymers used in pest control which includes linear homopolymers (LP), branched polymers (BP), and guanylated nanopolymers (GNP)16 (Fig. 8). LPs (linear homopolymers) are the simplest type of cationic polymers used to impart RNAi effect. Some commonly used LPs are poly[2(dimethylamino)ethyl methacrylate] (pDMAEMA), polyethyleneimine (PEI), and poly-L-lysine (PLL).110 These type of polymers are made up of a single monomer that contains an amine group inside the polymeric chain backbone. The high pKa of these cationic polymers (pDMAEMA: 7.4–7.8, PEI: 8.2–9.5, PLL: 9–11) favours the complexation with the phosphate backbone of the dsRNA through electrostatic forces. LPs till date have not been found to be efficient in the complexation and protection of the dsRNA besides the issue of non-specific cytotoxicity which may be a major concern for other beneficial organisms. The cytotoxicity effect may arise due to interaction of the polymer with cell membranes in organisms, leading to formation of pores and eventually cell death. To overcome the drawbacks posed by LPs, several architectural and functional modifications have been designed to enhance dsRNA uptake, maintain stability, and reduce the cytotoxicity. The uses of star shaped polymers for dsRNA delivery in insects have been studied by several researchers.84,111 The issues have also been tried to be overcome by simple alteration in the chemistry of the polymers which includes but not limited to variation in the molecular weight, charge density, ionic strength, and pKa.112
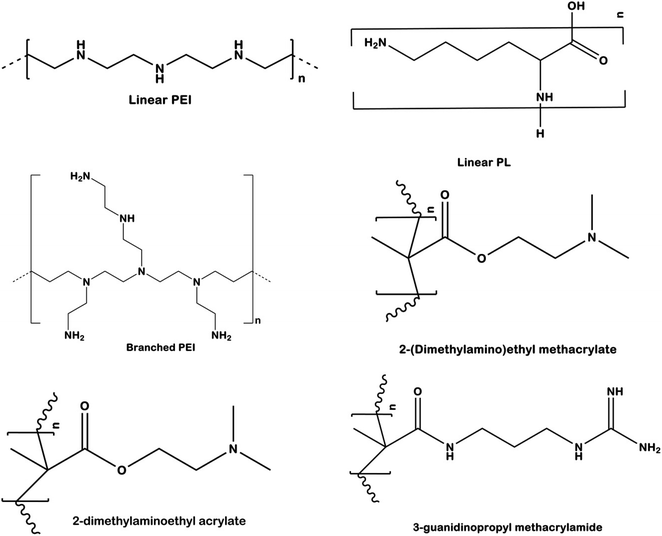 | ||
| Fig. 8 Structural variation based different cationic polymers generally used in RNAi based plant pest control. | ||
Branched polymers (BPs) were designed with the aim to improve the transfection and RNAi efficiency and reducing the levels of non-specific cytotoxicity as observed in case of LPs. Star cationic polymers with 3–5 arms shows reduced cytotoxicity as the branching increases as most of the nitrogen atoms involved in complexation remains within the dense core of the polymer. However, a higher amount of BPs is required to stabilize the DNA when compared with the LPs due to less availability of nitrogen complexing moieties.113
Several designs and developments have been made to functionalized polymers to increase their efficacy as a dsRNA vector. Certain pH responsive polymers have been prepared that releases the dsRNA within the endosome compartment of the cell by undergoing conformational changes due to a transition in pH.114 The pH transition range of the polymers however may not work for pest control strategies. Pests belonging to Lepidoptera family have a very alkaline intestinal gut pH and therefore may destabilize the dsRNA thus decreasing its efficacy. Polymers designed to protect the dsRNA under highly alkaline conditions need to seriously take into account the impact of pH on the complexation of polymers. Guanidine functionalized cationic polymers have been designed to protect the dsRNA over a high range of alkaline pH which is a characteristic feature of the lepidopteran gut microenvironment. These polymers facilitate in endocytic passage of the RNA through the cell membranes and escape from the endosomes.115 PGPMA (poly-[N-(3-guanidinopropyl)methacrylamide]) has been found to have a pKa of 12.5 thus ensuring the protonation of gunaidinium functional groups at high alkaline gut pH. Parsons et al. (2018),60 complexed PGPMA with dsRNA to form a compact polyplex at pH 10. The polyplex brought about a 92% reduction in the CDC27 mRNA in Sf9 cell lines post 48 hours of incubation. Feeding assays on 2nd and 3rd instar larvae of S. frugiperda with a diet supplemented with the polyplex (PGPMA/CDC27) for a period of 7 days resulted in approximately 30% mortality after 29 days. In another study a series of copolymers of poly-N-2aminoethylmethacrylate (PAEMA) and PDMAEMA were synthesized by free radical polymerization. The synthesized copolymers were guanidium functionalized by reacting 1H-pyrazole-1-carboxamidine hydrochloride (HPC) with part of primary amine moieties of the PAEMA. The copolymer with highest guanidine content showed higher protection efficiency towards the dsRNA when incubated with the larval midgut juice of S. exigua (pH 7.5 and 11).79 The guanylated copolymer when complexed with the dsRNA of the chitin synthase (ChSB) gene protected the dsRNA for as long as 10 hours in the gut juice of S. exigua (pH = 11). In vivo feeding assays supported the use of guanidine functionalized polyplexes in enhancing the efficiency of RNAi in S. exigua. The polymer protected dsRNA was found to exhibit 53% mortality as compared to a low mortality rate of only 16% in case of the naked dsRNA. The association of guanidine functional groups with the copolymer has been proved to likely provide enhanced dsRNA protection and thus increase the efficacy of RNAi. A study conducted by Gurusamy et al. (2020)116 evaluated the role of chitosan–dsRNA polyplex in improving RNAi in Spodoptera frugiperda. The complex showed reduced accumulation in the endosomes of the Sf9 cells and in the larval tissues thus exhibiting the protective property of chitosan. Moreover, the polyplex when fed to the S. frugiperda larvae resulted in successful knockdown of the iap gene, thus leading to retardation of growth and mortality among the larvae. In a similar fashion, Wang et al. (2020)117 studied the role of cationic polymers chitosan and lipofectamine 2000 to specifically target the glyceraldehyde-3-phosphate dehydrogenase (G3PDH) gene in the rice pest Chilo suppressalis. The chitosan–G3PDH conjugate when fed to the 2nd instar larvae brought about a 45% reduction in the expression of the gene in the pest gut. Similarly lipofectamine 2000 reduced the expression of the targeted gene to 52% in the gut tissues.
The above discussions aptly support the role of polyplexes (cationic polymer–dsRNA complex) in maintaining the stability of the dsRNA in both in vitro and in vivo. Greater stability of the complex results in higher RNAi efficiency as observed in the several studies.
Cationic polymers have been known to provide several advantages over other pest control agents. The cationic polymers act as an excellent non-viral delivery agent thereby reducing the cost. They are quite easy to produce, preserve, and exhibit no potential pathogenicity unlike the viral carriers.17 Cationic polymers are important non-viral transfection agents. Cationic polymers due to their strong positive charges can attract and condense the dsDNA thus facilitating the entry through the cellular membranes. These polymers also enhance the endosomal escape ability.18 The cationic polymers when chemically modified behave as a more target specific unit, reduce toxicity, and improve the efficiency of transfection.118
The choice of cationic polymers for pest control is a crucial aspect to be considered while working on RNAi based pest control in crops. The cationic polymers to be used should not be toxic to the environment and its components. Moreover the selected polymers should be biodegradable in nature thereby avoiding environmental persistence. The physicochemical properties of the polymers can be used as a base for deciding on its usefulness as a delivery agent in pest control. A particle size of the polymer at nanoscale, higher numbers of positive surface charges, and a suitable spatial framework are favourable parameters for selection of the polymers.17
9. Alternatives to cationic polymers
The efficacy of RNAi mechanism purely depends upon the delivery or uptake of the intact dsRNA into the cells of the pests. There are several options other than cationic polymers to reduce the degradation of the dsRNA as well as increasing the cellular uptake efficiency. These includes liposomes, bringing chemical modifications, absorption into plants via roots, direct injection into vessels, involvement of bacteria and viruses, and development of engineered or transplastomic plants (Table 3).| Delivery methods | Target species | Molecular impact | Effect | References |
|---|---|---|---|---|
| Cationic liposome | D. melanogaster, D. sechellia, D. yakuba, and D. pseudoobscura larvae | 3′ UTR of γ-tubulin gene | Mortality | 119 |
| Lipofectamine (liposome) | Drosophila suzukii | mRNA silencing | 40–50% silencing | 120 |
| Chemical modification | — | Addition of methyl group to the 2′ of ribosyl ring of 2nd base of siRNA | Increase in specificity of the dsRNA | 121 |
| Root drenching and trunk injection | Diaphorina citri | Silencing of arginine kinase (dsRNA-AK) | Increased mortality | 122 |
| Brown plant hopper | Knockdown of carboxylesterase (Ces) and cytochrome P450 (Cyp18A1) | High mortality among BPH nymphs | 123 | |
| Ostrinia furnacalis | Silencing of Kunitz-type trypsin inhibitors (dsKTI) | High mortality rate | 123 | |
| Application of bacteria and viruses | Rhodnius prolixus | Initiation of RNAi upon ingestion of recombinant bacteria | Knockdown of horizontally transmissible phenotypes | 96 |
| Bactericera cockerelli | Recombinant TMV targeting actin and V-ATPase sequences | Decrease in mRNA abundance and progeny production | 124 | |
| Engineered/transplastomic plants | Leptinotarsa decemlineata | — | 100% larval mortality | 97 |
Liposomes being non-toxic and biodegradable in nature have been found to safely deliver exogenous RNA to the target cells. Several researchers have demonstrated the effectiveness of liposomes in the RNAi process (Fig. 9). Lipofectamine, a liposome was successfully used as a transfection agent by Taning et al. (2016)120 in Drosophila suzukii. Feeding naked dsRNA did not yield any result whereas lipofectamine complexed dsRNA led to a silencing efficiency of 40–50%. A similar study by Whyard et al. (2009)119 demonstrated successful RNAi silencing effect in four different species of Drosophila when fed with γTub23C-dsRNA encapsulated with different cationic liposomes.
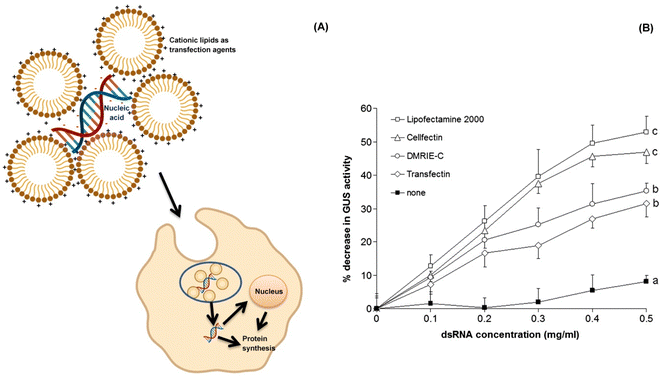 | ||
| Fig. 9 Cationic liposomes as efficient transfection agents in RNAi. (A) The cationic lipids/liposomes are amphiphilic molecules with overall positive charge. They bind to the nucleic acid as well as cell membranes with electrostatic interactions. The lipid based complex enters the target cell through endocytosis and is subsequently released into the cytoplasm. If the exogenous nucleic acid to be supplied is a DNA, then it needs to be transported into the nucleus while in case of a mRNA molecule it stays within the cytoplasm.125. (B) A study by Whyard et al. (2009)119 demonstrated the effect of different transfection agents and dsRNA concentration on the GUS gene in the gut of D. melanogaster larvae. The transfection agents when each coupled with a GUS-dsRNA concentration of 0.5 mg ml−1 brought about an improved silencing effect on the GUS gene as compared to the dsRNA alone. Among all the combinations, Lipofectamine 2000 coupled dsRNA showed maximum GUS silencing activity (more than 50%) as evident from the graph. (Fig. 9B has been reproduced from Whyard et al. (2009)119 with permission from Elsevier, copyright 2009). | ||
Chemically modifying any one or both of the strands of the dsRNA can improve the stability of the molecule. It can also help in increasing the shelf life, bio-distribution, and specificity. However, the cost of production and safety concerns needs to be assessed prior to the modifications.
RNAi based silencing could also be initiated by supply of dsRNA via absorption in roots of the plants or injecting the same into the trunk or vessels. The sucking and chewing pests thereby acquire the dsRNA naturally.126 Hunter et al. (2012)122 exposed citrus plant to dsRNA by means of root drenching and injection into the trunk. 2 g of dsRNA in 15 L of water was applied to the citrus plants and could be observed in the plant vessels until 7 weeks post the treatment. The experiment also demonstrated two hemipteran species and a leafhopper taking up the dsRNA feeding on the treated plant. Strategies like root absorption or trunk injection have some serious concerns associated. For the purpose, production of dsRNA in large mass is required thus making it a costly affair. Root application of dsRNA could be carried out on large fields through irrigation however the problem is with the short lived nature of the dsRNA in the soil. Trunk injection is more appropriate for sap sucking insects over the chewing insects. Treatment of crops like rice which are cultivated on a large scale by the method of trunk injection is almost impractical. Both trunk injection and root absorption requires repeated application at regular intervals and thus another drawback of the method.127
Bacteria mediated delivery of dsRNA pose several advantages which includes low cost and possible large scale production. Continuous and large scale production of dsRNA is made possible by the bacterial species. Viruses can also act as an efficient vector for the production of dsRNA. Several plant viruses have been studied for triggering RNAi in plants.62,124 Plants react to infections caused by viruses through the siRNA pathway. Introduction of an insect specific RNAi inducer sequence into a plant virus will produce siRNAs specific to that insect. Insect feeding on the plant can uptake the siRNA thus leading to silencing effect and mortality.
Long dsRNA are required for an effective RNAi activity in insects. However, the dsRNA expressed in plants are mostly diced into siRNAs and then taken up by insects leading to a limited RNAi effect.128 This problem can be overcome by engineering plants to express dsRNA in the organelles like chloroplasts which lack the RNAi processing ability. The chloroplasts are derived from cyanobacteria that lack RNAi pathway thus accumulating dsRNA.129
9.1. Prospects and challenges
The world is facing an expansion of population and the soaring population has put tremendous pressure on the food chain. In order to meet with the food shortage the agricultural productions have become of paramount importance. The traditional problems of lower agricultural production such as pests etc. have been taken care of by the use of hormones and chemical fertilizers. These abusive use of pesticides has caused more harm than good in de-balancing the environment. The use of pesticide has not only contributed to environmental pollution but also deteriorated the quality of agricultural products.With the growth in research tools in molecular biology, it is pertinent that scientists use such innovative tools to address the lacunae in the field. The field of RNAi offers some exciting solutions and potentials. There is tremendous scope with a lot of research to be done in the use of nucleic acid based techniques especially to improve their half-life. With a multidisciplinary approach and efforts of experts from diverse fields nucleic acids can be efficiently used for agricultural utility with solution to major problems of food, population, and pollution.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Mamoni Dash acknowledges the Ramalingaswami Fellowship 2016–17 (D.O.NO.BT/HRD/35/02/2006), Department of Biotechnology, Government of India. The funding from Biotechnology Industry Research Assistance Council (BIRAC) is highly acknowledged for the funding through the PACE grant BFD/RO/B.04/0350/2021-22, Institute of Life Sciences (ILS), Department of Biotechnology funded project BT/PR 36474/NNT/28/1701/2020, Government of India. We highly acknowledge Dr Amaresh C Panda for useful discussions regarding nucleic acids.References
- S. J. Fletcher, P. T. Reeves, B. T. Hoang and N. Mitter, A Perspective on RNAi-Based Biopesticides, Front. Plant Sci., 2020, 11, 51 CrossRef PubMed.
- J. A. Heinemann and S. Walker, Environmentally applied nucleic acids and proteins for purposes of engineering changes to genes and other genetic material, Biosafety and Health, 2019, 1(03), 113–123 CrossRef.
- A. Székács, A. S. Ammour and M. L. Mendelsohn, RNAi Based Pesticides, Frontiers Media SA, 2021, p. 714116 Search PubMed.
- J. S. Whangbo and C. P. Hunter, Environmental RNA interference, Trends Genet., 2008, 24(6), 297–305 CrossRef CAS PubMed.
- S. Ivashuta, Y. Zhang, B. E. Wiggins, P. Ramaseshadri, G. C. Segers and S. Johnson, et al., Environmental RNAi in herbivorous insects, RNA, 2015, 21(5), 840–850 CrossRef CAS PubMed.
- N. Bensoussan, S. Dixit, M. Tabara, D. Letwin, M. Milojevic and M. Antonacci, et al., Environmental RNA interference in two-spotted spider mite, Tetranychus urticae, reveals dsRNA processing requirements for efficient RNAi response, Sci. Rep., 2020, 10(1), 1–16 CrossRef PubMed.
- N. Bensoussan, M. Milojevic, K. Bruinsma, S. Dixit, S. Pham and V. Singh, et al., Localized efficacy of environmental RNAi in Tetranychus urticae, Sci. Rep., 2022, 12(1), 1–14 CrossRef PubMed.
- M. Mondal, J. Peter, O. Scarbrough and A. Flynt, Environmental RNAi pathways in the two-spotted spider mite, BMC Genomics, 2021, 22(1), 1–11 Search PubMed.
- A. Sharma, V. Kumar, R. Kumar, B. Shahzad, A. K. Thukral and R. Bhardwaj, Brassinosteroid-mediated pesticide detoxification in plants: A mini-review, Cogent Food Agric., 2018, 4(1), 1436212 CrossRef.
- K. C. Parsons, P. Mineau and R. B. Renfrew, Effects of pesticide use in rice fields on birds, Waterbirds, 2010, 33(sp1), 193–218 Search PubMed.
- J. A. Anderson, P. C. Ellsworth, J. C. Faria, G. P. Head, M. D. Owen and C. D. Pilcher, et al., Genetically engineered crops: importance of diversified integrated pest management for agricultural sustainability, Front. Bioeng. Biotechnol., 2019, 7, 24 CrossRef PubMed.
- J. Niu, C. Taning, O. Christiaens, G. Smagghe and J. Wang, Rethink RNAi in Insect Pest Control: Challenges and Perspectives, Advances in Insect Physiology, 2018, pp. 1–17 Search PubMed.
- D. Biedenkopf, T. Will, T. Knauer, L. Jelonek, A. Furch and T. Busche, et al., Phloem-mediated spreading of SIGS-derived non-coding RNAs in Hordeum vulgare, bioRxiv, 2019 DOI:10.1101/2019.12.30.891002.
- P. K. Das, C. Mohanty, G. K. Purohit, S. Mishra and S. Palo, Nanoparticle assisted environmental remediation: Applications, toxicological implications and recommendations for a sustainable environment, Environ. Nanotechnol., Monit. Manage., 2022, 18, 100679 Search PubMed.
- J. Li and K. Kataoka, Chemo-physical strategies to advance the in vivo functionality of targeted nanomedicine: the next generation, J. Am. Chem. Soc., 2020, 143(2), 538–559 CrossRef PubMed.
- C. E. Pugsley, R. E. Isaac, N. J. Warren and O. J. Cayre, Recent Advances in Engineered Nanoparticles for RNAi-Mediated Crop Protection Against Insect Pests, Front. Agron., 2021, 3, 652981 CrossRef.
- N. Bono, F. Ponti, D. Mantovani and G. Candiani, Non-Viral in Vitro Gene Delivery: It is Now Time to Set the Bar, Pharmaceutics, 2020, 12(2), 183 CrossRef CAS PubMed.
- Q. Huang, S. Li, Y.-F. Ding, H. Yin, L.-H. Wang and R. Wang, Macrocycle-wrapped polyethylenimine for gene delivery with reduced cytotoxicity, Biomater. Sci., 2018, 6(5), 1031–1039 RSC.
- A. H. A. Rashid and D. D. Lateef, Novel techniques for gene delivery into plants and its applications for disease resistance in crops, Am. J. Plant Sci., 2016, 7(1), 181–193 CrossRef CAS.
- S. Shimizu-Sato, K. Tsuda, M. Nosaka-Takahashi, T. Suzuki, S. Ono and K. N. Ta, et al., Agrobacterium-mediated genetic transformation of wild Oryza species using immature embryos, Rice, 2020, 13(1), 1–13 CrossRef PubMed.
- W. Broothaerts, H. J. Mitchell, B. Weir, S. Kaines, L. Smith and W. Yang, et al., Gene transfer to plants by diverse species of bacteria, Nature, 2005, 433(7026), 629–633 CrossRef CAS PubMed.
- D. S. Rathore and E. Mullins, Alternative Non-Agrobacterium based methods for plant transformation, Annu. Plant Rev., 2018, 891–908 Search PubMed.
- C. Darmawan, N. M. A. Wiendi, C. Utomo and T. Liwang, Electroporation-mediated genetic transformation of oil palm (Elaeis guineensis), Biodiversitas, 2020, 21(8), 3720–3726 Search PubMed.
- I. I. Ozyigit and K. Yucebilgili Kurtoglu, Particle bombardment technology and its applications in plants, Mol. Biol. Rep., 2020, 47(12), 9831–9847 CrossRef CAS PubMed.
- H. Matsuoka, T. Komazaki, Y. Mukai, M. Shibusawa, H. Akane and A. Chaki, et al., High throughput easy microinjection with a single-cell manipulation supporting robot, J. Biotechnol., 2005, 116(2), 185–194 CrossRef CAS PubMed.
- P. Gurusaravanan, S. Vinoth and N. Jayabalan, An improved Agrobacterium-mediated transformation method for cotton (Gossypium hirsutum L.‘KC3’) assisted by microinjection and sonication, In Vitro Cell. Dev. Biol.: Plant, 2020, 56(1), 111–121 CrossRef CAS.
- M. Tomizawa, F. Shinozaki, Y. Motoyoshi, T. Sugiyama, S. Yamamoto and M. Sueishi, Sonoporation: Gene transfer using ultrasound, World J. Methodol., 2013, 3(4), 39 CrossRef PubMed.
- S.-H. Cheon, K.-H. Lee, J.-Y. Kwon, S.-H. Choi, M.-N. Song and D.-I. Kim, Enhanced delivery of siRNA complexes by sonoporation in transgenic rice cell suspension cultures, J. Microbiol. Biotechnol., 2009, 19(8), 781–786 CAS.
- H. F. Kaeppler, W. Gu, D. A. Somers, H. W. Rines and A. F. Cockburn, Silicon carbide fiber-mediated DNA delivery into plant cells, Plant Cell Rep., 1990, 9(8), 415–418 CrossRef CAS PubMed.
- D. Songstad, D. Somers and R. Griesbach, Advances in alternative DNA delivery techniques, Plant Cell, Tissue Organ Cult., 1995, 40(1), 1–15 CrossRef CAS.
- Z. Akram, S. Ali, G. M. Ali, Y. Zafar, Z. H. Shah and F. Alghabari, Whisker-mediated transformation of peanut with chitinase gene enhances resistance to leaf spot disease, Crop Breed. Appl. Biotechnol., 2016, 16, 108–114 CrossRef.
- V. Sidorov, D. Wang, E. D. Nagy, C. Armstrong, S. Beach and Y. Zhang, et al., Heritable DNA-free genome editing of canola (Brassica napus L.) using PEG-mediated transfection of isolated protoplasts, In Vitro Cell. Dev. Biol.: Plant, 2022, 58(3), 447–456 CrossRef CAS.
- J. M. Dunwell, Transgenic approaches to crop improvement, J. Exp. Bot., 2000, 51(suppl_1), 487–496 CrossRef CAS PubMed.
- R. E. Goodman, S. Vieths, H. A. Sampson, D. Hill, M. Ebisawa and S. L. Taylor, et al., Allergenicity assessment of genetically modified crops—what makes sense?, Nat. Biotechnol., 2008, 26(1), 73–81 CrossRef CAS PubMed.
- J. Gu and J. Yang, Nitrogen (N) transformation in paddy rice field: Its effect on N uptake and relation to improved N management, Crop and Environment, 2022, 1(1), 7–14 CrossRef.
- K. Jantapoa, S. Pinita, L. Zhouc, W. Wangc and J. Chaiwanonb, Effects of propiconazole on rice growth and gene expression in response to nitrogen and phosphorus deficiencies, ScienceAsia, 2021, 47, 19–27 Search PubMed.
- Z. Jia and N. von Wirén, Signaling pathways underlying nitrogen-dependent changes in root system architecture: from model to crop species, J. Exp. Bot., 2020, 71(15), 4393–4404 CrossRef CAS PubMed.
- Z.-x. Lu, X.-p. Yu, K.-l. Heong and C. Hu, Effect of Nitrogen Fertilizer on Herbivores and Its Stimulation to Major Insect Pests in Rice, Rice Sci., 2007, 14(1), 56–66 CrossRef.
- G. Conway, The doubly green revolution: food for all in the twenty-first century, Cornell University Press, 2019 Search PubMed.
- E. Cardarelli and G. Bogliani, Effects of grass management intensity on ground beetle assemblages in rice field banks, Agric., Ecosyst. Environ., 2014, 195, 120–126 CrossRef.
- N. Brzezina, B. Kopainsky and E. Mathijs, Can Organic Farming Reduce Vulnerabilities and Enhance the Resilience of the European Food System? A Critical Assessment Using System Dynamics Structural Thinking Tools, Sustainability, 2016, 8(10), 971 CrossRef.
- J. Mariyono, Green revolution- and wetland-linked technological change of rice agriculture in Indonesia, Manag. Environ. Qual., 2015, 26(5), 683–700 CrossRef.
- I. K. A. Zwertvaegher, I. Van Daele, P. Verheesen, M. Peferoen and D. Nuyttens, Development and implementation of a laboratory spray device and rainfall simulator for retention research using small amounts of agroformulations, Pest Manage. Sci., 2017, 73(1), 123–129 CrossRef CAS PubMed.
- C. Thorburn, The Rise and Demise of Integrated Pest Management in Rice in Indonesia, Insects, 2015, 6(2), 381–408 CrossRef.
- C. Drum, Soil chemistry of pesticides, PPG Industries. Inc USA, 1980 Search PubMed.
- R. Pavela, History, presence and perspective of using plant extracts as commercial botanical insecticides and farm products for protection against insects–a review, Plant Prot. Sci., 2016, 52(4), 229–241 CrossRef CAS.
- D. R. George, R. D. Finn, K. M. Graham and O. A. E. Sparagano, Present and future potential of plant-derived products to control arthropods of veterinary and medical significance, Parasites Vectors, 2014, 7(1), 28 CrossRef PubMed.
- D. Gunnell, M. Eddleston, M. R. Phillips and F. Konradsen, The global distribution of fatal pesticide self-poisoning: Systematic review, BMC Public Health, 2007, 7(1), 357 CrossRef PubMed.
- P. N. Holmsgaard, S. Dealtry, V. Dunon, H. Heuer, L. H. Hansen and D. Springael, et al., Response of the bacterial community in an on-farm biopurification system, to which diverse pesticides are introduced over an agricultural season, Environ. Pollut., 2017, 229, 854–862 CrossRef CAS PubMed.
- S. Fahad, S. Saud, A. Akhter, A. A. Bajwa, S. Hassan and M. Battaglia, et al., Bio-based integrated pest management in rice: An agro-ecosystems friendly approach for agricultural sustainability, J. Saudi Soc. Agric. Sci., 2021, 20(2), 94–102 Search PubMed.
- S.-S. Liu, A. Rao and S. B. Vinson, Biological Control in China: Past, present and future—An introduction to this special issue, Biol. Control, 2014, 68(1), 5 Search PubMed.
- K. Sorby, G. Fleischer and E. Pehu, Integrated pest management in development: review of trends and implementation strategies, 2003 Search PubMed.
- J. Holt, A. Cook, T. Perfect and G. Norton, Simulation analysis of Brown Planthopper population dynamics in tropical rice: a simulation analysis, J. Appl. Ecol., 1987, 24, 87–102 CrossRef.
- K. Jabran, G. Mahajan, V. Sardana and B. S. Chauhan, Allelopathy for weed control in agricultural systems, Crop Prot., 2015, 72, 57–65 CrossRef.
- S. Senthil-Nathan, M. Y. Choi, C. H. Paik, H. Y. Seo and K. Kalaivani, Toxicity and physiological effects of neem pesticides applied to rice on the Nilaparvata lugens Stål, the brown planthopper, Ecotoxicol. Environ. Saf., 2009, 72(6), 1707–1713 CrossRef CAS PubMed.
- J. Hu, C. Xiao and Y. He, Recent progress on the genetics and molecular breeding of brown planthopper resistance in rice, Rice, 2016, 9(1), 30 CrossRef PubMed.
- J. Cheng. Rice Planthoppers in the Past Half Century in China, in Rice Planthoppers: Ecology, Management, Socio Economics and Policy, ed. Heong K. L, Cheng J. and Escalada M. M., Springer, Netherlands, Dordrecht, 2015. pp. 1–32 Search PubMed.
- H. X. Xu, X. S. Zheng and Z. X. Lu, No. 34 Striped stem borer, Chilo suppressalis, in Institute of Plant Protection, Chinese Academy of Agricultural Sciences & China Society of Plant Protection, Crop Diseases and Insect Pests in China, China Agriculture Press, Beijing, 2015, pp. 124–130 Search PubMed.
- G. M. Gurr, Z. Lu, X. Zheng, H. Xu, P. Zhu and G. Chen, et al., Multi-country evidence that crop diversification promotes ecological intensification of agriculture, Nat. Plants, 2016, 2, 16014 CrossRef PubMed.
- K. H. Parsons, M. H. Mondal, C. L. McCormick and A. S. Flynt, Guanidinium-Functionalized Interpolyelectrolyte Complexes Enabling RNAi in Resistant Insect Pests, Biomacromolecules, 2018, 19(4), 1111–1117 CrossRef CAS PubMed.
- P. M. Bachman, R. Bolognesi, W. J. Moar, G. M. Mueller, M. S. Paradise and P. Ramaseshadri, et al., Characterization of the spectrum of insecticidal activity of a double-stranded RNA with targeted activity against Western Corn Rootworm (Diabrotica virgifera virgifera LeConte), Transgenic Res., 2013, 22(6), 1207–1222 CrossRef CAS PubMed.
- R. S. Nandety, Y.-W. Kuo, S. Nouri and B. W. Falk, Emerging strategies for RNA interference (RNAi) applications in insects, Bioengineered, 2015, 6(1), 8–19 CrossRef CAS PubMed.
- B. W. Han, W. Wang, C. Li, Z. Weng and P. D. Zamore, Noncoding RNA. piRNA-guided transposon cleavage initiates Zucchini-dependent, phased piRNA production, Science, 2015, 348(6236), 817–821 CrossRef CAS PubMed.
- K. Y. Zhu and S. R. Palli, Mechanisms, Applications, and Challenges of Insect RNA Interference, Annu. Rev. Entomol., 2020, 65(1), 293–311 CrossRef CAS PubMed.
- I. Biryukova and T. Ye, Endogenous siRNAs and piRNAs derived from transposable elements and genes in the malaria vector mosquito Anopheles gambiae, BMC Genomics, 2015, 16(1), 278 CrossRef PubMed.
- L. Swevers, J. Liu and G. Smagghe, Defense Mechanisms against Viral Infection in Drosophila: RNAi and Non-RNAi, Viruses, 2018, 10(5), 230 CrossRef PubMed.
- K. J. Lucas, B. Zhao, S. Roy, A. L. Gervaise and A. S. Raikhel, Mosquito-specific microRNA-1890 targets the juvenile hormone-regulated serine protease JHA15 in the female mosquito gut, RNA Biol., 2015, 12(12), 1383–1390 CrossRef PubMed.
- M. Ninova, S. Griffiths-Jones and M. Ronshaugen, Abundant expression of somatic transposon-derived piRNAs throughout Tribolium castaneum embryogenesis, Genome Biol., 2017, 18(1), 184 CrossRef PubMed.
- K. Cappelle, C. F. R. de Oliveira, B. Van Eynde, O. Christiaens and G. Smagghe, The involvement of clathrin-mediated endocytosis and two Sid-1-like transmembrane proteins in double-stranded RNA uptake in the Colorado potato beetle midgut, Insect Mol. Biol., 2016, 25(3), 315–323 CrossRef CAS PubMed.
- N. Wynant, D. Santos, P. Van Wielendaele and J. Vanden Broeck, Scavenger receptor-mediated endocytosis facilitates RNA interference in the desert locust, Schistocerca gregaria, Insect Mol. Biol., 2014, 23(3), 320–329 CAS.
- P. Baldrich, B. D. Rutter, H. Z. Karimi, R. Podicheti, B. C. Meyers and R. W. Innes, Plant Extracellular Vesicles Contain Diverse Small RNA Species and Are Enriched in 10- to 17-Nucleotide “Tiny” RNAs, Plant Cell, 2019, 31(2), 315–324 CrossRef CAS PubMed.
- S. Liu, M. Jaouannet, D. A. Dempsey, J. Imani, C. Coustau and K. H. Kogel, RNA-based technologies for insect control in plant production, Biotechnol. Adv., 2020, 39, 107463 CrossRef CAS PubMed.
- O. Christiaens, S. Whyard, A. M. Vélez and G. Smagghe, Double-stranded RNA technology to control insect pests: Current status and challenges, Front. Plant Sci., 2020, 11, 451 CrossRef PubMed.
- A. Kolliopoulou, C. N. T. Taning, G. Smagghe and L. Swevers, Viral Delivery of dsRNA for Control of Insect Agricultural Pests and Vectors of Human Disease: Prospects and Challenges, Front. Physiol., 2017, 8, 399 CrossRef PubMed.
- M. Chen, A. Shelton and G.-y Ye, Insect-resistant genetically modified rice in China: from research to commercialization, Annu. Rev. Entomol., 2011, 56(1), 81–101 CrossRef CAS PubMed.
- Z. Li, Y. Xue, H. Zhou, Y. Li, B. Usman and X. Jiao, et al., High-resolution mapping and breeding application of a novel brown planthopper resistance gene derived from wild rice (Oryza. rufipogon Griff), Rice, 2019, 12(1), 41 CrossRef PubMed.
- E. Fishilevich, A. J. Bowling, M. L. Frey, P.-H. Wang, W. Lo and M. Rangasamy, et al., RNAi targeting of rootworm Troponin I transcripts confers root protection in maize, Insect Biochem. Mol. Biol., 2019, 104, 20–29 CrossRef CAS PubMed.
- B. He, Y. Chu, M. Yin, K. Müllen, C. An and J. Shen, Fluorescent Nanoparticle Delivered dsRNA Toward Genetic Control of Insect Pests, Adv. Mater., 2013, 25(33), 4580–4584 CrossRef CAS PubMed.
- O. Christiaens, M. G. Tardajos, Z. L. Martinez Reyna, M. Dash, P. Dubruel and G. Smagghe, Increased RNAi Efficacy in Spodoptera exigua via the Formulation of dsRNA With Guanylated Polymers, Front. Physiol., 2018, 9, 316 CrossRef PubMed.
- M. Zotti, E. A. Dos Santos, D. Cagliari, O. Christiaens, C. N. T. Taning and G. Smagghe, RNA interference technology in crop protection against arthropod pests, pathogens and nematodes, Pest Manage. Sci., 2018, 74(6), 1239–1250 CrossRef CAS PubMed.
- C. N. Taning, O. Christiaens, X. Li, L. Swevers, H. Casteels and M. Maes, et al., Engineered flock house virus for targeted gene suppression through RNAi in fruit flies (Drosophila melanogaster) in vitro and in vivo, Front. Physiol., 2018, 9, 805 CrossRef PubMed.
- H. Huvenne and G. Smagghe, Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: a review, J. Insect Physiol., 2010, 56(3), 227–235 CrossRef CAS PubMed.
- J. A. Baum, T. Bogaert, W. Clinton, G. R. Heck, P. Feldmann and O. Ilagan, et al., Control of coleopteran insect pests through RNA interference, Nat. Biotechnol., 2007, 25(11), 1322–1326 CrossRef CAS PubMed.
- O. Christiaens, T. Dzhambazova, K. Kostov, S. Arpaia, M. R. Joga and I. Urru, et al., Literature review of baseline information on RNAi to support the environmental risk assessment of RNAi-based GM plants, EFSA Supporting Publ., 2018, 15(5), 1424E Search PubMed.
- R. Sugahara, S. Tanaka, A. Jouraku and T. Shiotsuki, Geographic variation in RNAi sensitivity in the migratory locust, Gene, 2017, 605, 5–11 CrossRef CAS PubMed.
- H. M. Abd El Halim, B. M. H. Alshukri, M. S. Ahmad, E. Y. T. Nakasu, M. H. Awwad and E. M. Salama, et al., RNAi-mediated knockdown of the voltage gated sodium ion channel TcNav causes mortality in Tribolium castaneum, Sci. Rep., 2016, 6(1), 29301 CrossRef CAS PubMed.
- J. Spit, A. Philips, N. Wynant, D. Santos, G. Plaetinck and J. Vanden Broeck, Knockdown of nuclease activity in the gut enhances RNAi efficiency in the Colorado potato beetle, Leptinotarsa decemlineata, but not in the desert locust, Schistocerca gregaria, Insect Biochem. Mol. Biol., 2017, 81, 103–116 CrossRef CAS PubMed.
- C. Lenaerts, D. Cools, R. Verdonck, L. Verbakel, J. Vanden Broeck and E. Marchal, The ecdysis triggering hormone system is essential for successful moulting of a major hemimetabolous pest insect, Schistocerca gregaria, Sci. Rep., 2017, 7(1), 46502 CrossRef CAS PubMed.
- A. Telang, J. A. Rechel, J. R. Brandt and D. M. Donnell, Analysis of ovary-specific genes in relation to egg maturation and female nutritional condition in the mosquitoes Georgecraigius atropalpus and Aedes aegypti (Diptera: Culicidae), J. Insect Physiol., 2013, 59(3), 283–294 CrossRef CAS PubMed.
- O. Terenius, A. Papanicolaou, J. S. Garbutt, I. Eleftherianos, H. Huvenne and S. Kanginakudru, et al., RNA interference in Lepidoptera: an overview of successful and unsuccessful studies and implications for experimental design, J. Insect Physiol., 2011, 57(2), 231–245 CrossRef CAS PubMed.
- R. Katoch and N. Thakur, Insect gut nucleases: a challenge for RNA interference mediated insect control strategies, Int. J. Biochem. Biotechnol., 2012, 1, 198–203 Search PubMed.
- N. Wynant, D. Santos, R. Verdonck, J. Spit, P. Van Wielendaele and J. Vanden Broeck, Identification, functional characterization and phylogenetic analysis of double stranded RNA degrading enzymes present in the gut of the desert locust, Schistocerca gregaria, Insect Biochem. Mol. Biol., 2014, 46, 1–8 CrossRef CAS PubMed.
- E. Vogel, D. Santos, L. Mingels, T.-W. Verdonckt and J. V. Broeck, RNA Interference in Insects: Protecting Beneficials and Controlling Pests, Front. Physiol., 2019, 9, 1912 CrossRef PubMed.
- L. Timmons, D. L. Court and A. Fire, Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans, Gene, 2001, 263(1–2), 103–112 CrossRef CAS PubMed.
- M. Vatanparast and Y. Kim, Optimization of recombinant bacteria expressing dsRNA to enhance insecticidal activity against a lepidopteran insect, Spodoptera exigua, PLoS One, 2017, 12(8), e0183054 CrossRef PubMed.
- M. M. A. Whitten, P. D. Facey, R. Del Sol, L. T. Fernández-Martínez, M. C. Evans and J. J. Mitchell, et al., Symbiont-mediated RNA interference in insects, Proc. Biol. Sci., 2016, 283(1825), 20160042 Search PubMed.
- X. Zhang, K. Mysore, E. Flannery, K. Michel, D. W. Severson and K. Y. Zhu, et al., Chitosan/interfering RNA nanoparticle mediated gene silencing in disease vector mosquito larvae, J. Visualized Exp., 2015,(97), e52523 Search PubMed.
- B. Dalby, S. Cates, A. Harris, E. C. Ohki, M. L. Tilkins and P. J. Price, et al., Advanced transfection with Lipofectamine 2000 reagent: primary neurons, siRNA, and high-throughput applications, Methods, 2004, 33(2), 95–103 CrossRef CAS PubMed.
- Y. Zhang, J. Cui, Y. Zhou, J. Cao, H. Gong and H. Zhang, et al., Liposome mediated double-stranded RNA delivery to silence ribosomal protein P0 in the tick Rhipicephalus haemaphysaloides, Ticks Tick-borne Dis., 2018, 9(3), 638–644 CrossRef PubMed.
- J. Durzyńska, Ł. Przysiecka, R. Nawrot, J. Barylski, G. Nowicki and A. Warowicka, et al., Viral and Other Cell-Penetrating Peptides as Vectors of Therapeutic Agents in Medicine, J. Pharmacol. Exp. Ther., 2015, 354(1), 32 CrossRef PubMed.
- S. Y. Choi and E. A. David, Cell Penetrating Peptides and the Mechanisms for Intracellular Entry, Curr. Pharm. Biotechnol., 2014, 15(3), 192–199 Search PubMed.
- M. R. Joga, M. J. Zotti, G. Smagghe and O. Christiaens, RNAi Efficiency, Systemic Properties, and Novel Delivery Methods for Pest Insect Control: What We Know So Far, Front. Physiol., 2016, 7, 553 Search PubMed.
- K. He, Y. Sun, H. Xiao, C. Ge, F. Li and Z. Han, Multiple miRNAs jointly regulate the biosynthesis of ecdysteroid in the holometabolous insects, Chilo suppressalis, RNA, 2017, 23(12), 1817–1833 CrossRef CAS PubMed.
- I. Tabujew and K. Peneva, Functionalization of cationic polymers for drug delivery applications, 2014 Search PubMed.
- Y. Ho and H.-P. Too, CATIONIC POLYMER BASED GENE DELIVERY: UPTAKE AND INTRACELLULAR TRAFFICKING, Cosmos, 2014, 10(01), 17–24 CrossRef.
- Q. Liu, J. Xu, Y. Wang, C. Li, G. Han and J. Qi, et al., Synergism of CmGV and Bacillus thuringiensis against larvae of Cnaphalocrocis medinalis Güenée, J. Yangzhou Univ. Agric. Life Sci. Ed., 2013, 34(4), 89–93 CAS.
- A. Kichler, C. Leborgne, E. Coeytaux and O. Danos, Polyethylenimine-mediated gene delivery: a mechanistic study, J. Gene Med., 2001, 3(2), 135–144 CrossRef CAS PubMed.
- G. Creusat, A.-S. Rinaldi, E. Weiss, R. Elbaghdadi, J.-S. Remy and R. Mulherkar, et al., Proton Sponge Trick for pH-Sensitive Disassembly of Polyethylenimine-Based siRNA Delivery Systems, Bioconjugate Chem., 2010, 21(5), 994–1002 CrossRef CAS PubMed.
- S. Yan, B.-Y. Ren and J. Shen, Nanoparticle-mediated double-stranded RNA delivery system: A promising approach for sustainable pest management, Insect Sci., 2021, 28(1), 21–34 CrossRef CAS PubMed.
- S. Agarwal, Y. Zhang, S. Maji and A. Greiner, PDMAEMA based gene delivery materials, Mater. Today, 2012, 15(9), 388–393 CrossRef CAS.
- A. B. Cook, R. Peltier, M. Hartlieb, R. Whitfield, G. Moriceau and J. A. Burns, et al., Cationic and hydrolysable branched polymers by RAFT for complexation and controlled release of dsRNA, Polym. Chem., 2018, 9(29), 4025–4035 RSC.
- J. M. Layman, S. M. Ramirez, M. D. Green and T. E. Long, Influence of Polycation Molecular Weight on Poly(2-dimethylaminoethyl methacrylate)-Mediated DNA Delivery In Vitro, Biomacromolecules, 2009, 10(5), 1244–1252 CrossRef CAS PubMed.
- C. V. Synatschke, A. Schallon, V. Jérôme, R. Freitag and A. H. Müller, Influence of polymer architecture and molecular weight of poly(2-(dimethylamino)ethyl methacrylate) polycations on transfection efficiency and cell viability in gene delivery, Biomacromolecules, 2011, 12(12), 4247–4255 CrossRef CAS PubMed.
- Z. Cao, H. Xiao, L. Li, M. Liu, G. Lin and P. Zhai, et al., The Codelivery of siRNA and QDs by pH-Responsive Micelle for Hepatoma Cancer Cells, Front. Pharmacol., 2019, 10, 1194 CrossRef CAS PubMed.
- F. X. Gillet, R. A. Garcia, L. L. P. Macedo, E. V. S. Albuquerque, M. C. M. Silva and M. F. Grossi-de-Sa, Investigating Engineered Ribonucleoprotein Particles to Improve Oral RNAi Delivery in Crop Insect Pests, Front. Physiol., 2017, 8, 256 CrossRef PubMed.
- D. Gurusamy, K. Mogilicherla and S. R. Palli, Chitosan nanoparticles help double-stranded RNA escape from endosomes and improve RNA interference in the fall armyworm, Spodoptera frugiperda, Arch. Insect Biochem. Physiol., 2020, 104(4), e21677 CAS.
- K. Wang, Y. Peng, J. Chen, Y. Peng, X. Wang and Z. Shen, et al., Comparison of efficacy of RNAi mediated by various nanoparticles in the rice striped stem borer (Chilo suppressalis), Pestic. Biochem. Physiol., 2020, 165, 104467 CrossRef CAS PubMed.
- F. Haghiralsadat, G. Amoabediny, S. Naderinezhad, T. Forouzanfar, M. N. Helder and B. Zandieh-Doulabi, Preparation of PEGylated cationic nanoliposome-siRNA complexes for cancer therapy, Artif. Cells, Nanomed., Biotechnol., 2018, 46(sup1), 684–692 CrossRef CAS PubMed.
- S. Whyard, A. D. Singh and S. Wong, Ingested double-stranded RNAs can act as species-specific insecticides, Insect Biochem. Mol. Biol., 2009, 39(11), 824–832 CrossRef CAS PubMed.
- C. N. T. Taning, O. Christiaens, N. Berkvens, H. Casteels, M. Maes and G. Smagghe, Oral RNAi to control Drosophila suzukii: laboratory testing against larval and adult stages, J. Pest Sci., 2016, 89(3), 803–814 CrossRef.
- A. L. Jackson, S. R. Bartz, J. Schelter, S. V. Kobayashi, J. Burchard and M. Mao, et al., Expression profiling reveals off-target gene regulation by RNAi, Nat. Biotechnol., 2003, 21(6), 635–637 CrossRef CAS PubMed.
- W. B. Hunter, E. Glick, N. Paldi and B. R. Bextine, Advances in RNA interference: dsRNA treatment in trees and grapevines for insect pest suppression, Southwest. Entomol., 2012, 37(1), 85–87 CrossRef.
- H. Li, R. Guan, H. Guo and X. Miao, New insights into an RNAi approach for plant defence against piercing-sucking and stem-borer insect pests, Plant, Cell Environ., 2015, 38(11), 2277–2285 CrossRef CAS PubMed.
- A. M. Khan, M. Ashfaq, Z. Kiss, A. A. Khan, S. Mansoor and B. W. Falk, Use of recombinant tobacco mosaic virus to achieve RNA interference in plants against the citrus mealybug, Planococcus citri (Hemiptera: Pseudococcidae), PLoS One, 2013, 8(9), e73657 CrossRef CAS PubMed.
- A. Fus-Kujawa, P. Prus, K. Bajdak-Rusinek, P. Teper, K. Gawron and A. Kowalczuk, et al., An overview of methods and tools for transfection of eukaryotic cells in vitro, Front. Bioeng. Biotechnol., 2021, 634, 701031 CrossRef PubMed.
- E. C. de Andrade and W. B. Hunter, RNA interference–natural gene-based technology for highly specific pest control (HiSPeC), in, RNA Interference, I. Y. Abdurakhmonov, IntechOpen, London, 2016, DOI:10.5772/61612.
- K. A. Murphy, C. A. Tabuloc, K. R. Cervantes and J. C. Chiu, Ingestion of genetically modified yeast symbiont reduces fitness of an insect pest via RNA interference, Sci. Rep., 2016, 6(1), 22587 CrossRef CAS PubMed.
- M. Kumar, G. P. Gupta and M. V. Rajam, Silencing of acetylcholinesterase gene of Helicoverpa armigera by siRNA affects larval growth and its life cycle, J. Insect Physiol., 2009, 55(3), 273–278 CrossRef CAS PubMed.
- J. Zhang, S. A. Khan, C. Hasse, S. Ruf, D. G. Heckel and R. Bock, Full crop protection from an insect pest by expression of long double-stranded RNAs in plastids, Science, 2015, 347(6225), 991–994 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |