Open Access Article
Open Access ArticleSynthesis and applications of WO3 nanosheets: the importance of phase, stoichiometry, and aspect ratio
Travis G.
Novak†
a,
Jin
Kim†
b,
Paul A.
DeSario
 c and
Seokwoo
Jeon
c and
Seokwoo
Jeon
 *d
*d
aNRC Postdoctoral Associate, US Naval Research Laboratory, Washington, D.C. 20375, USA
bThin Film Materials Research Center, Korea Research Institute of Chemical Technology, Daejeon, 34114, Republic of Korea
cChemistry Division (Code 6100), U.S. Naval Research Laboratory, Washington, D.C. 20375, USA
dDepartment of Materials Science and Engineering, KAIST Institute for the Nanocentury, Advanced Battery Center, KAIST, Daejeon, 34141, Republic of Korea. E-mail: jeon39@kaist.ac.kr
First published on 5th August 2021
Abstract
Tungsten trioxide (WO3) is an abundant, versatile oxide that is widely explored for catalysis, sensing, electrochromic devices, and numerous other applications. The exploitation of WO3 in nanosheet form provides potential advantages in many of these fields because the 2D structures have high surface area and preferentially exposed facets. Relative to bulk WO3, nanosheets expose more active sites for surface-sensitive sensing/catalytic reactions, and improve reaction kinetics in cases where ionic diffusion is a limiting factor (e.g. electrochromic or charge storage). Synthesis of high aspect ratio WO3 nanosheets, however, is more challenging than other 2D materials because bulk WO3 is not an intrinsically layered material, making the widely-studied sonication-based exfoliation methods used for other 2D materials not well-suited to WO3. WO3 is also highly complex in terms of how the synthesis method affects the properties of the final material. Depending on the route used and subsequent post-synthesis treatments, a wide variety of different morphologies, phases, exposed facets, and defect structures are created, all of which must be carefully considered for the chosen application. In this review, the recent developments in WO3 nanosheet synthesis and their impact on performance in various applications are summarized and critically analyzed.
1. Introduction
Tungsten trioxide (WO3) is of interest in a great number of potential applications due to both its intrinsic properties and its wide range of options for chemical/structural modifications. WO3 is generally eco-friendly, inexpensive, and abundant.1 Pure WO3 can be stable or metastable in many phases, including monoclinic, orthorhombic, hexagonal, or cubic, with several hydrated crystalline phases of various stoichiometries (WO3·nH2O) possible as well (Table 1).2–4 In addition, WO3 is generally non-toxic,5 and its chemical stability over a wide pH range makes it useful for both liquid and gas phase applications.6| Phase | Symmetry | Notesa |
|---|---|---|
| a Temperature ranges are for bulk, stoichiometric WO3.4 | ||
| δ-WO3 | Triclinic | −43 to 17 °C |
| γ-WO3 | Monoclinic | 17 to 330 °C |
| β-WO3 | Orthorhombic | 330 to 740 °C |
| α-WO3 | Tetragonal | >720 °C |
| Cubic WO3 | Cubic | Sub-stoichiometric |
| h-WO3 | Hexagonal | Metastable at <400 °C |
| WO3·2H2O | Monoclinic | Layered WO5(OH)2 sheets |
| WO3·H2O | Orthorhombic | Corner-sharing WO5(OH) |
Bulk WO3 typically has a band gap of 2.5–3.0 ev,7 and due to considerable absorption of visible light, is one of the most widely explored photocatalysts for solar-driven applications. The WO3 band gap can be tuned for specific applications by widening through quantum confinement,8 or narrowing using oxygen vacancy formation9 or heteroatom doping.10 This versatility has led to research into WO3 photocatalysts and photoelectrocatalysts focused a wide range of reactions, including water oxidation,11–14 water reduction,15 degradation of organic pollutants,16–22 methane conversion,23 CO2 reduction,24–26 nitrate synthesis,27 and cross-coupling reactions.28 WO3 has also been extensively studied as an electrocatalyst for the hydrogen evolution reaction (HER).29
For use in sensors, WO3 is an n-type material that readily chemisorbs various gases,30 creating a charge depletion region and subsequent resistivity change. Similar to the case with photocatalysis, modification of WO3 morphology31 or chemical composition32 can alter its properties for enhancement of gas sensing performance.
WO3 is also notable for its ability to intercalate lithium ions (Li+),33 a trait that makes it applicable as both an energy storage and electrochromic material. In latter case, the mostly transparent WO3 becomes opaque upon lithiation due to the transition from W6+ to W5+ states,34 blocking the majority of visible light in films only ∼100 nm thick.35 This rapid and reversible optical switching makes WO3 a leading candidate for next-generation “smart window” technology, where the transparent-to-opaque transition could dramatically reduce heating/cooling costs in buildings.36
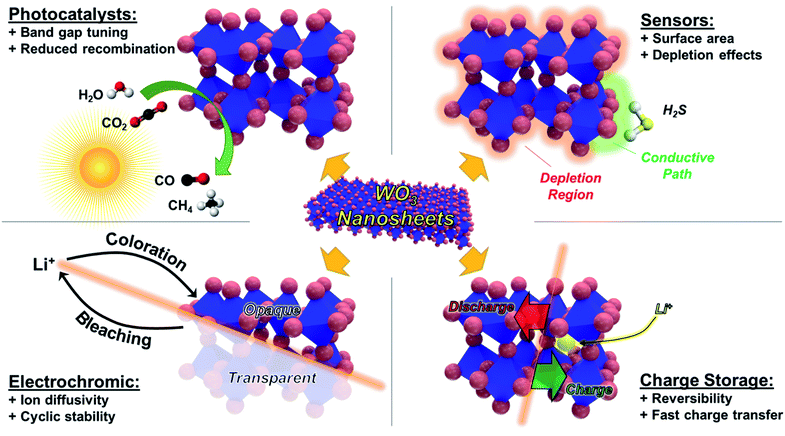
The exploitation of WO3 in nanosheet form has potential benefits for many desired applications, as illustrated in Fig. 1. Nanosheets have an abundance of exposed surface sites compared to bulk materials, beneficial for catalytic or sensing applications alike. The inherent porosity and continuous intercalation paths of nanosheets also allow for easier infiltration of ions, notably Li+, making them of an ideal morphology for electrochromic and charge storage devices. In addition, nanosheets can often be easily dispersed in aqueous or organic solvents, allowing for compatibility with techniques such as spin-casting, spray coating, or ink-jet printing.
Despite these advantages, there are still many challenges involved with applications of WO3 nanosheets, mostly related to synthesis. Unlike intrinsically layered materials such as graphite or transition metal dichalcogenides (TMDCs), WO3 is not amenable to traditional exfoliation techniques. A wide variety of alternative synthesis techniques have been reported, but these generally struggle to achieve a combination of high aspect ratio, chemical purity, and desired phase.
In this review, we summarize and critically analyse the common techniques recently reported for WO3 nanosheet synthesis, both top-down (starting from bulk) and bottom-up (starting from smaller precursor molecules). Because the synthesis technique strongly affects the morphology, stoichiometry, and phase of the resulting nanosheet, we also discuss the influence of these factors on the desired application.
2. Bottom-up synthesis of WO3 nanosheets
2.1 Hydrothermal routes
Hydrothermal synthesis typically refers to any method that involves raising the temperature and pressure of a sealed, aqueous solution to yield a desired product.37 This is often considered industrially attractive because it can be a simple, one-step route that is easily scalable, but hydrothermal routes often lack as precise control of morphology and surface chemistry as other methods.For WO3, a wide variety of different shapes can be grown through simple modification of hydrothermal synthesis conditions. Yu et al. reported synthesis of hydrated WO3 nanostructures – 0D nanoparticles, 1D nanorods, 2D nanoplates, and 3D nanoflowers – through a sodium tungstate dihydrate (Na2WO4·2H2O) route at 160 °C.38 As shown in Fig. 2, the choice of surfactant determines the morphology of the resulting WO3 structure. Addition of K2SO4 produced nanorods, while oxalic acid produced either nanoplates or nanoflowers, depending on the concentration. The orthorhombic nanoplates were reported to have an average edge length of 300 nm and thickness of 40 nm.38
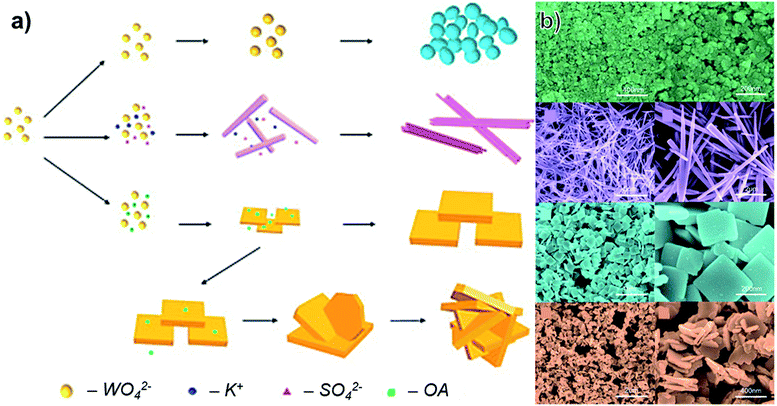 | ||
| Fig. 2 (a) Schematic of different morphologies of WO3 produced through a hydrothermal route with variation of precursors (OA: oxalic acid). (b) SEM images of the results structures at low and high magnification, adapted with permission from ref. 38, copyright 2016, Elsevier. | ||
Variations on this synthetic scheme using Na2WO4·2H2O appear frequently in WO3 literature.39–42 Sodium dodecyl sulfate (SDS) is an anionic surfactant known to functionalize and stabilize nanosheets in solution.43 Addition of SDS to WO3 hydrothermal routes is common, and it has been asserted that the SDS micelles create additional porosity in WO3 structures,39 but these often must be thermally removed post-synthesis.
At moderate temperatures, WO3 prepared through hydrothermal routes tends to be monoclinic or orthorhombic, but can be stabilized in other phases through annealing. Zhang et al. synthesized WO3 nanosheets by a typical hydrothermal route, followed by annealing in N2 at 350 °C to create hexagonal WO3 nanosheets.40 As with other annealing processes in oxides, this can affect morphology, and likely reduces aspect ratio in the case of nanosheets.
As an alternative to higher temperature routes, Xiao et al. showed that addition of Na2SO4 stabilizes hexagonal phase WO3 at a synthesis temperature of only 200 °C.44 The nanosheets produced from this route were of relatively high aspect ratio compared to other hydrothermal routes, with an average thickness of 15 nm and lateral sizes appearing to be on the order of 1 μm. Depending on the amount of Na2SO4 added, the product appeared either as discrete nanosheets or mesoporous flowers of assembled nanosheets.
Further variations on hydrothermal routes include the use of seeds or templates to create morphological variations. Shi et al. synthesized hexagonal WO3 nanosheets with dominant (100) facets using H2WO4 as a precursor and FTO as a seed layer.45 This yielded a highly porous assembly, and individual nanosheets appeared to be 100's of nm across.
For template routes, the triblock copolymer Pluronic P123 has been used to synthesize a wide variety of ultrathin oxide nanosheets, where its use promotes lateral growth and reduces agglomeration.46 It was later shown for WO3 that removal of the P123 template at 300 °C results in nanosheets that are thinner (∼10 nm) compared to the 20–30 nm of the pristine sheets, while lateral size was maintained at 100's of nm.47
Other methods using P123 have demonstrated similarly thin nanosheets. Liang et al. used P123 and WCl6 to synthesize WO3 nanosheets, finding nanosheets with an average thickness of 4.9 nm and dominant (002) facets.20 A similar method was later used to combine ultra-thin WO3 nanosheets with C3N4 to form a highly porous composite.48
2.2 Solution-phase routes at or near room temperature
As their name suggests, the hydrothermal methods described in the previous section use applied heat (most commonly 120–200 °C) to assemble of WO3 nanostructures from their starting precursors. A closely related class of synthetic technique uses similar starting molecules but relies on acid or base-catalysed reactions rather than thermal stimulus to achieve the desired products. These are often dubbed ‘self-assembly’ or ‘sol–gel’ routes depending on the precursors and methods of assembly, but there is considerable overlap as these methods could also be considered hydrothermal if done in a sealed vessel at elevated temperatures. We will therefore define methods in this section as routes where the primary reactions occur at <100 °C (post-synthesis drying/calcination steps not included).These low-temperature processes have been used to synthesize WO3 nanosheets, but typically struggle to achieve a large lateral size. Ahmed et al. synthesized WO3 nanorods and nanosheets through a sol–gel method using Na2WO4·2H2O and HCl.49 Post-synthesis calcination at 400, 450, and 500 °C was shown to generate triclinic, orthorhombic, and monoclinic WO3, respectively. Thickness was not measured, but lateral size appeared to be on the order of 100's of nm for the monoclinic nanosheets.
Similar acid-catalysed sol–gel routes can produce a wide variety of nanosheet-like structures. Wang et al. demonstrated that sol–gel synthesized tungstite (WO3·H2O) nanosheets that were preferentially [111] oriented would convert to monoclinic [200] oriented WO3 porous nanosheet arrays (PNAs) upon annealing at 400 °C.50 Average thickness was ∼20 nm post-annealing, but lateral size of individual nanosheets within the PNA was not clear.
Although not explicitly defined as a sol–gel method, Na2WO4·2H2O with nitric acid (HNO3) was found to produce polycrystalline, monoclinic nanosheets with crystallite sizes of 22–44 nm after annealing at 500 °C.51 Substitution of 1–2 at% Sn reduced wrinkles in these nanosheets. The same basic synthesis scheme was later applied to produce Cr-doped WO3 nanosheets.52
Chen et al. demonstrated a similar method for synthesis of WO3 nanosheets, using one-step method with Na2WO4 and HNO3 at 60 °C.53Fig. 3a illustrates the formation through self-assembly of the precursor molecules. The authors found that by adding different ratios of sodium oleate (NaOA) and sodium-n-octanoate (NaOct) the thickness of WO3 nanosheets could be controlled. At a NaOct/NaOA mass ratio of 0.04, nanosheets were ∼5 nm thick and 10's of nm in lateral size and of monoclinic phase (Fig. 3b–d).
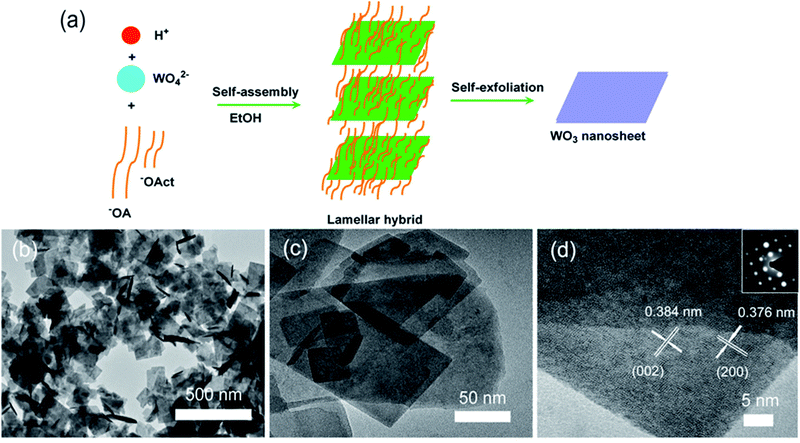 | ||
| Fig. 3 (a) Schematic of self-assembly. (b–d) SEM, TEM, and HRTEM images of WO3 nanosheets produced with a NaOct/NaOA mass ratio of 0.04. Reprinted with permission from ref. 53, copyright 2018, Elsevier. | ||
Another work regarding morphology as a function of additive concentration used oxalic acid in conjunction with Na2WO4·2H2O and HCl.54 Optimized conditions yielded monoclinic WO3 nanosheets with average thickness of ∼10 nm and lateral size appearing to be 100's of nm. The authors proposed that addition of oxalic acid promotes dissolution of H2WO4·nH2O precipitates during the growth process, effectively preventing thicker sheets from forming.
In a variation on the acid-catalysed processes, Sánchez-Martínez et al. reported ultrasonication-assisted synthesis using ammonium tungstate hydrate, HNO3, and cetyltrimethyl ammonium bromide (CTAB).55 The authors asserted that addition of CTAB promotes the formation of nanosheets; samples prepared without CTAB were irregular and of greater thickness. Optimization of CTAB concentration yielded monoclinic WO3 nanosheets that were 50 nm in average thickness and 100 s of nm in lateral size.
A unique indirect method of obtaining WO3 nanosheets has been reported through spontaneous conversion of a BaWO4-polymer nanohybrid in solution.56 Here, a WO42− solution was prepared using Na2WO4 and added to a solution of barium chloride hexahydrate (BaCl2·6H2O) and polyacrylic acid (PAA). It was asserted that this process formed a BaWO4–PAA hybrid that released Ba2− ions into the solution, leaving behind metastable, hexagonal WO3 nanosheets. Fig. 4 illustrates the interaction of PAA with BaWO4 and the conversion of WO4 tetrahedrons to WO6 octahedrons characteristic of hexagonal WO3.
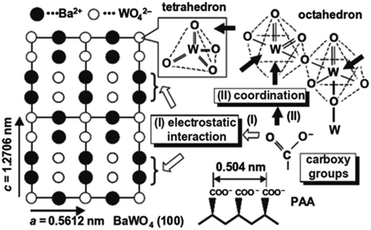 | ||
| Fig. 4 Illustration of BaWO4 (100) and the interaction with PAA molecules that leads to formation of octahedral WO6. Reprinted with permission from ref. 56, copyright 2006, John Wiley and Sons. | ||
2.3 Other bottom-up routes
While the majority of bottom-up routes utilize precursors dispersed in an aqueous or organic solvent, a few other methods exist based on other techniques. Several of these start from metallic tungsten, which can be oxidized and then assembled into nanosheets through arc-discharge or anodization.A solid–liquid phase arc discharge route was reported by Chen et al. to produce ultra-thin WO3 nanosheets.24 Here, a tungsten filament at high voltage was brought into contact with NaNO3 solution, producing WO3 nanoparticles in solution. These nanoparticles then preferentially orient and attach along the (100) and (010) axes to form nanosheets, as illustrated in Fig. 5. The rectangular nanosheets produced through this method were ultra-thin, averaging 4.5 nm in height, and 100's of nm in lateral size. A similar technique was later reported to synthesize WO3 nanoparticles that were oxygen deficient (WO3−x), which the authors attributed to electron bombardment during the arc-discharge.57
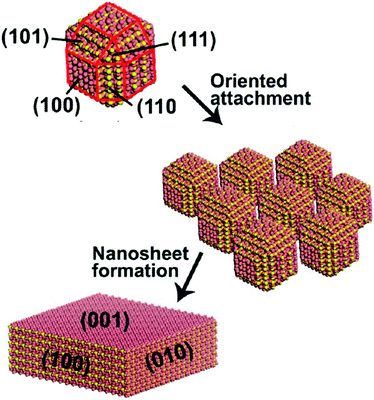 | ||
| Fig. 5 Schematic of WO3 formation from smaller nanocrystals produced through solid–liquid phase arc discharge. Reprinted with permission from ref. 24, copyright 2012, American Chemical Society. | ||
There also exist several reports of metallic tungsten structures deposited through bottom-up routes that are oxidized to form WO3 nanosheets. Wisitsora-at et al. used RF sputtering to deposit a 1 μm thick tungsten film on Al2O3, followed by anodization with HNO3 at 60 °C to create films of WO3 nanosheets.58 A similar method had previously been reported to create nanostructured WO3 films from commercial tungsten foils.59 Increasing HNO3 concentration was found to reduce thickness but also degrade crystallinity, although individual nanosheet dimensions were not specified.
Another report using RF sputtering followed by HNO3 anodization and annealing at 450 °C provided more details regarding the morphology of the nanosheet films.60 Nanosheets were found to be monoclinic and predominately (002). Average lateral size was 50–500 nm and thickness was 10–50 nm, making the nanosheets similar in both crystallinity and aspect ratio to those grown by solution-phase routes.
While these routes deposited metallic tungsten through bottom-up means and subsequently anodized, it is also possible to create WO3 nanosheets through direct deposition of the oxide. Luo et al. synthesized WO3 nanosheets through thermal evaporation starting with WO3 powder to deposit on a glass substrate.61 The amorphous nanosheets could be separated from the substrate through ultrasonication and were of large lateral size (0.5–10 μm), but thickness was not reported.
An unconventional approach was studied Fang et al. using a solid-state mechanochemical reaction induced by ball-milling between Na2WO4·2H2O and oxalate dehydrate (H2C2O4·2H2O), as illustrated in Fig. 6a.62 The resulting WO3·2H2O monoclinic nanosheets showed a preferential (100) orientation and had an average thickness of 5.67 nm (Fig. 6b). Typical lengths and widths were 50–80 nm and 8–10 nm, respectively.
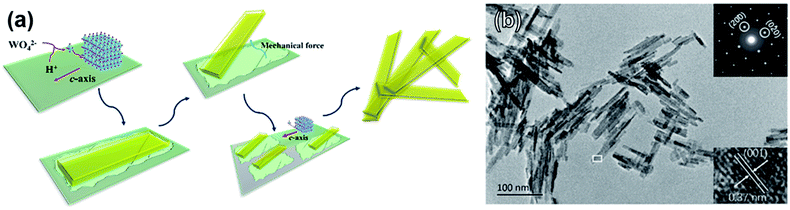 | ||
| Fig. 6 (a) Schematic of WO3·2H2O nanosheet formation through a solid-state mechanochemical reaction. (b) TEM image showing nanosheet morphology with inset selected-area diffraction pattern and lattice parameter measurement. Reprinted with permission from ref. 62, copyright 2017 American Chemical Society. | ||
Overall, there are a wide variety of bottom-up methods for WO3 synthesis that provide relatively simple and scalable synthesis and can be tuned to generate different phases of WO3. One common drawback of these routes, however, is the relatively low aspect ratio of the nanosheets produced. It is typical to see sizes of 100 s of nm or less with these techniques, potentially limiting the morphological advantages compared to WO3 produced through methods we will discuss in the following sections.
3. Top-down synthesis of WO3 nanosheets
While bottom-up methods attempt to synthesize WO3 from smaller precursor molecules, top-down methods aim to directly cleave nanosheets from the bulk source. In the case of advanced exfoliation techniques, this has been widely developed in order to enhance the aspect ratio while maintaining scalability. For WO3 this can include either exfoliation of WO3, exfoliation of another layered bulk material (WS2) followed by oxidation, or exfoliation of inherently layered hydrated WO3. Among these methods, liquid-phase exfoliation is a promising approach due to not only its scalability but also its simplicity. In this section, we will summarize the progress of top-down synthesis of WO3 nanosheets, especially in terms of liquid exfoliation, based on different source materials.3.1 Direct exfoliation of bulk WO3
Bulk materials could be exfoliated into nanosheets through external stimulation, such as ultra-sonication, and cohesive interaction between the liquid media and the bulk source. This has generated high-quality crystalline nanosheets for a wide variety of van der Waals (VDW) bonded materials, including graphite (graphene),63 TMDCs,64 and black phosphorus.65 Based on this concept, initial attempts were made by directly exfoliating raw bulk WO3 in appropriate liquid, but naturally this proved more challenging in a material that was not intrinsically layered.Perhaps the simplest top-down method reported to date, Szkoda et al. refluxed bulk WO3 powder in water over the course of 10 days at 80 °C, finding that nearly 100% exfoliation occurred and further centrifugation processes were not necessary.66 Although some atomically thin structures were observed in TEM, statistical analysis of nanosheets size/thickness was not reported, and lateral size appeared to be limited.
Another route utilizing sonication in aqueous solutions, Guan et al. sonicated bulk WO3 for 48 h in bovine serum albumin (BSA) solution to produce atomically thin WO3 nanosheets, shown in Fig. 7a.16 The exfoliation is driven by both aqueous media, which has good cohesive energy with WO3, and electrostatic forces caused by BSA molecules. Specifically, the authors observed both theoretically and experimentally that the –NH3+ groups of the BSA molecules strongly bind on the negatively charged WO3 surface in acidic media, making WO3 nanosheets separate easily from one another through electrostatic force. Because this method is based on electrostatic binding between WO3 and BSA molecules, the exfoliated monoclinic nanosheets show high crystallinity and long-term stability against restacking. After centrifugation at 2000 rpm, 49% of nanosheets were bilayer (2.1 nm thick), but average lateral size was a modest 150 nm.
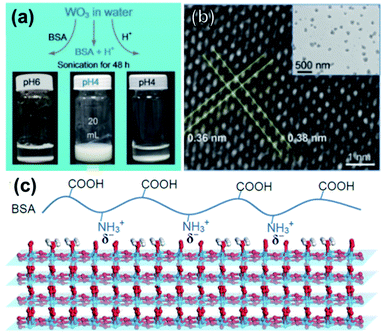 | ||
| Fig. 7 (a) Optimization of exfoliation/purification for WO3 exfoliation. (b) HRTEM of a nanosheet with lattice spacing indicated; inset shows typical lateral size of WO3 nanosheets. (c) Schematic illustration for electrostatic-driven interaction of BSA on WO3 surface. Reprinted with permission from ref. 16, copyright 2017 John Wiley and Sons. | ||
Although the above works show that it is possible to exfoliate bulk WO3 into nanostructures, there are clearly severe limitations to these approaches. Because of the strong binding in WO3, it is difficult to directionally cleave, resulting in long processing times and nanosheets of low aspect ratio. For improved exfoliation efficiency, there is clearly a need to start with an intrinsically layered material.
3.2 Exfoliation and oxidation from WS2
Unlike WO3, WS2 is a layered and highly anisotropic material, with strong covalent in-plane bonds and weaker VDW forces out-of-plane. As a result, optimized exfoliation methods can generate WS2 nanosheets that are >5 nm in thickness and with lateral sizes that range from 100's of nm to over 1 μm.67,68 Naturally, some efforts to synthesize high aspect ratio WO3 nanosheets have leveraged this approach by exfoliating WS2 and then attempting to oxidize the WS2 into WO3.By simply sonicating bulk WS2 for 4 h in DMF followed by heating at 140 °C, Pan et al. showed that oxygen-deficient (WO3−x) dots were formed.69 These dots were monoclinic and of extremely small lateral size (2.7 nm on average). Given that the lateral size of the exfoliated WS2 nanosheets were on the order of microns, it indicates that the uncontrolled oxidation process will break apart otherwise high aspect ratio flakes.
Intercalation can greatly benefit solution-phase exfoliation processes by weakening the interlayer binding forces in VDW materials, allowing for milder sonication conditions. For ionic salts, this can be easily accomplished by heating in an inert environment to allow dissociation of the salt without formation of side products. This approach has been widely reported to generate graphene of higher aspect ratio than other approaches,70–72 and is commonly adapted for other VDW materials such as WS2 and MoS2.73Fig. 8 illustrates a typical intercalation scheme using n-butyllithium (n-BuLi), which is a frequently used intercalant for TMDC nanosheet synthesis.74
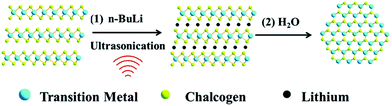 | ||
| Fig. 8 Intercalation/exfoliation scheme using n-BuLi to synthesize TMDC nanosheets. Reprinted with permission from ref. 74, copyright 2016, Royal Society of Chemistry. | ||
Yim et al. utilized this n-BuLi intercalation strategy to exfoliate WS2, followed by treatment in K2PdCl4 at 50 °C.28 This both deposited PdO nanoparticles (∼3 nm in diameter) and oxidized the WS2 nanosheets, forming a PdO@WO3 heterostructure. Only trace amounts of sulfur were detected after conversion, and the amorphous WO3 nanosheets appeared to largely maintain the high aspect ratio of WS2.
Although n-BuLi is the most widely studied, other Li-based salts can be used. Ghorai et al. intercalated different lithium salts (I, Br, Cl) in bulk WS2 to correlate intercalation efficiency to exfoliation yield.75 The authors achieved extremely high yield of 19 mg ml−1 by using LiI, which showed the best intercalation efficiency through low lattice energy compared to other salts. When exfoliating WS2 for WO3 nanosheet synthesis, there are naturally a similarly wide variety of intercalant options that have been evaluated.
While lithium-based intercalants are widely studied because they share common design principles with electrochromic and charge storage devices based on Li+, there are many other ions can readily intercalate into WS2, in some cases inducing partial oxidation in addition to interlayer expansion. As shown in Fig. 9a, Zhou et al. reported simultaneous exfoliation and oxidation of bulk WS2 into a partially oxidized WS2/WO3 hetrostructure, using supercritical CO2 (sc-CO2).76 Sc-CO2 could easily permeate into the WS2 interlayer gallery, which results not only in higher exfoliation efficiency through increased interlayer distance but also oxidation of the WS2. Although the sc-CO2 could be a simple method that could achieve exfoliation and oxidation simultaneously, the WO3 nanosheet is only partially oxidized, with some WS2 remaining, and the oxidation becomes especially ineffective for few-layer nanosheets due to limited diffusion of CO2 beyond the surface layer.
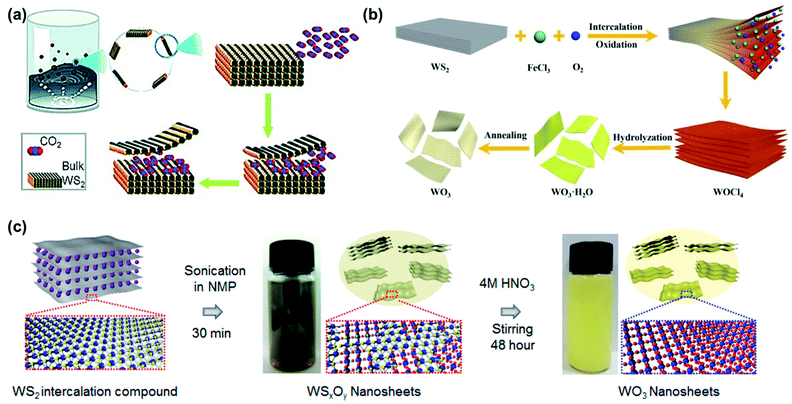 | ||
| Fig. 9 Examples of exfoliation and oxidation from WS2. (a) Supercritical CO2 used to intercalate and partially oxidize WS2, reprinted with permission from ref. 76, copyright 2015 John Wiley and Sons. (b) Use of FeCl3 to intercalate and oxidize WS2 to WOCl4, followed by hydrolysis in water to yield WO3·H2O and annealing to form WO3 nanosheets. Reprinted with permission from ref. 77, copyright 2019 John Wiley and Sons. (c) Synthesis of WO3 nanosheets through intercalation-assisted exfoliation of WS2, followed by nitric acid treatment. Reprinted with permission from ref. 78, copyright 2018 American Chemistry Society. | ||
Similarly, Tang et al. utilized FeCl3 as both an intercalant and oxidation agent of WS2, as shown in Fig. 9b.77 During the intercalation process, WS2 converts to WOCl4 which easily hydrolyses in water to hydrated WO3 (WO3·H2O). After annealing in air at 250 °C, cubic WO3 nanosheets were formed that were 3–5 nm thick on average. The authors also found that monoclinic WO3 nanosheets could be formed by increasing the annealing temperature to 400 °C.
Azam et al. used two-step exfoliation and oxidation reaction to produce WO3 nanosheet using sodium potassium tartrate tetrahydrate.78 In this system both the alkali ions and the organic chain intercalate, achieving even larger interlayer distance than intercalation with alkali ions alone and allowing for relatively mild sonication conditions.79–81 Due to the four water molecules of the hydrated salt, the WS2 nanosheet was partially oxidized into WSxOy during the intercalation process, a method known to partially oxidize structurally similar MoS2.82,83 The authors found that the nanosheets were completely oxidized into monoclinic WO3 after nitric acid treatment, as illustrated in Fig. 9c.78 After oxidation, 82% of nanosheets were <10 nm thick and lateral size was 1–20 μm, marking one of the highest average aspect ratios reported to date.
Overall, these methods generally produce the highest aspect ratio WO3 nanosheets by leveraging the inherent ease of exfoliation of WS2. The only notable drawback is process complexity: to achieve fully oxidized WO3 nanosheets generally requires separate exfoliation and oxidation steps, which tend make these routes more time consuming than single-step methods.
3.3 Exfoliation of hydrated WO3
Further research attempted to produce WO3 intercalation compounds in order to produce highly crystalline WO3 nanosheets without additional oxidation processes. Although the interlayers are strongly bonded through electrostatic interaction, the highly hydrophilic nature of the WO3 readily harbors water molecules between its interlayers. Compared to bulk WO3, layered hydrated WO3 (WO3·2H2O) can provide structural flexibility to accommodate intercalation-induced strain84 and achieve faster intercalation kinetics.85 Since intercalation of water in raw bulk WO3 is difficult, hydrated WO3 is normally prepared with tungstate precursors, mostly sodium tungstate.Similar to WS2 intercalation compounds, Wang et al. showed that WO3 also experiences expansion in interlayer spacing from 3.75 Å to 6.9 Å after water intercalation, which could weaken the binding energy (Fig. 10a).86 Kalantar-zadeh et al. demonstrated mechanical exfoliation (the scotch-tape method) of ultra-thin nanosheet from hydrated WO3, which is not possible from natural bulk WO3.87 Although this method is not scalable for industrial applications, it served as an important proof of concept that hydrated WO3 could be exfoliated in ways similar to graphite or other layered materials.
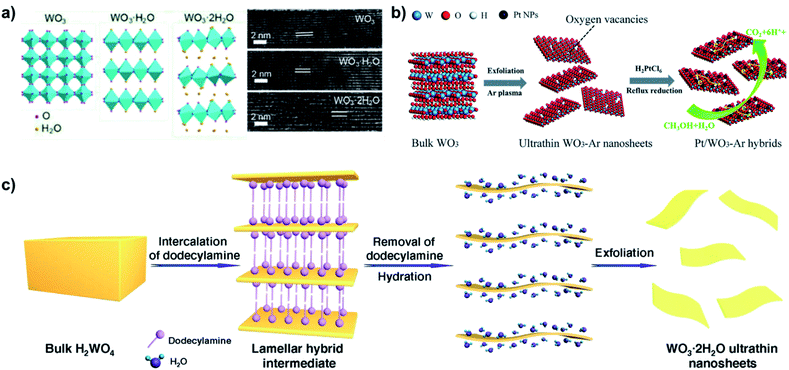 | ||
| Fig. 10 Examples of interlayer expansion and exfoliation of hydrated WO3. (a) Layer expansion of WO3 upon intercalation with water, reprinted with permission from ref. 86, copyright 2020 American Chemical Society. (b) Exfoliation of hydrated WO3 using Ar-plasma, followed by deposition of Pt nanoparticles (NPs). Reprinted with permission from ref. 88, copyright 2018 Royal Society of Chemistry. (c) Intercalation and exfoliation of hydrated WO3 using dodecylamine. Reprinted with permission from ref. 89, Springer-Nature. | ||
Inspired by this, many turned their attention to utilizing liquid-phase exfoliation for hydrated WO3. Zhang et al. exfoliated hydrated WO3, etching intercalated water molecules with Ar plasma, as shown in Fig. 10b.88 The strong etching nature of the Ar-plasma could efficiently exfoliate ultra-thin WO3 nanosheets with thickness of 1.4 nm while maintaining lateral sizes of 160–180 nm. The harsh etching process also induced numerous oxygen vacancies in the ultra-thin nanosheets.
Liang et al. further expanded the interlayer spacing from through intercalation of dodecylamine in hydrated WO3 (Fig. 10c).89 The additional expansion allows higher yield of ultra-thin nanosheets, nearly all with a thickness of 1.4 nm (corresponding to approximately twice the interlayer distance of adjacent WO3·H2O layers) and lateral size up to 500 nm.
Exfoliation of hydrated WO3 has been demonstrated to produce nanosheets with monolayer thickness in high yield. Depending on the exfoliation method or co-intercalants other than water, the chemical properties of the WO3 nanosheets can be additionally tuned as well. However, bulk hydrated WO3 is typically fabricated on the nanometer scale, making the exfoliated nanosheets also of small lateral size. Nevertheless, the methods introduced above are shown to be efficient for applications that require highly crystalline WO3 nanosheets.
4. Structure–property relationships for WO3
The diversity of WO3 synthesis techniques discussed in previous sections create vastly different materials in terms of phase, morphology, defect structures, etc. To better contextualize these synthesis methods for specific applications, it is necessary to briefly discuss how these properties imparted by the specific synthesis methods affect performance. Although we attempt to highlight primarily WO3 nanosheet based works, in this section some literature regarding other nanostructures or thin films will also be discussed as their insights are often directly applicable to the WO3 nanosheets as well.4.1 Effect of morphology and aspect ratio
The term nanosheet implies a true 2D structure, but in the case of WO3 that is very challenging to achieve. As seen in the discussion of synthesis methods, WO3 nanosheets often have aspect ratios on the order of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (i.e. 100's of nm in later size and 10's of nm in thickness). This contrasts with other layered materials such as graphene or TMDCs, where aspect ratios of 1000
1 (i.e. 100's of nm in later size and 10's of nm in thickness). This contrasts with other layered materials such as graphene or TMDCs, where aspect ratios of 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or more are possible with advanced exfoliation techniques.70–72,90 This is crucial for certain applications as higher aspect ratios can often alter charge transport properties, expose more surface sites, or promote easier ionic diffusion through the structure.
1 or more are possible with advanced exfoliation techniques.70–72,90 This is crucial for certain applications as higher aspect ratios can often alter charge transport properties, expose more surface sites, or promote easier ionic diffusion through the structure.
In the case of photocatalysts, nanostructuring WO3 in nanosheet morphologies can reduce electron–hole recombination, which is detrimental to photoefficiency. The low dimensionality of ultrathin nanosheets ensures that photogenerated charge carriers do not have to traverse large distances to reach the surface,11,16,19,20,24 and therefore have an increased probability of reacting with surface adsorbates rather than recombining with electrons.
In addition to reducing charge recombination, morphological modifications of WO3 can enhance catalysis by leveraging quantum confinement effects to widen its bandgap, shifting the edges of the conduction band (CB) or valence band (VB) for more thermodynamically favourable carrier migration and redox reactions.11,17 Although the CB potential of bulk WO3 is insufficient for O2 reduction,17,18 water reduction,15 and CO2 reduction,24 these applications have been realized by shifting the band potentials of WO3.
Chen et al. demonstrated that the combination of a widened bandgap and a CB shift to more negative potentials in ∼4–5 nm thick WO3 nanosheets provided the necessary thermodynamic power to impart high activity for photocatalytic reduction of CO2 to CH4.24 The stark contrast between photoactivity of WO3 nanosheets and commercial WO3 powder is shown in Fig. 11a. Shifting the CB to more negative potentials can also benefit photo-oxidation because as CB electrons more readily react with O2 they are less likely to recombine with holes.
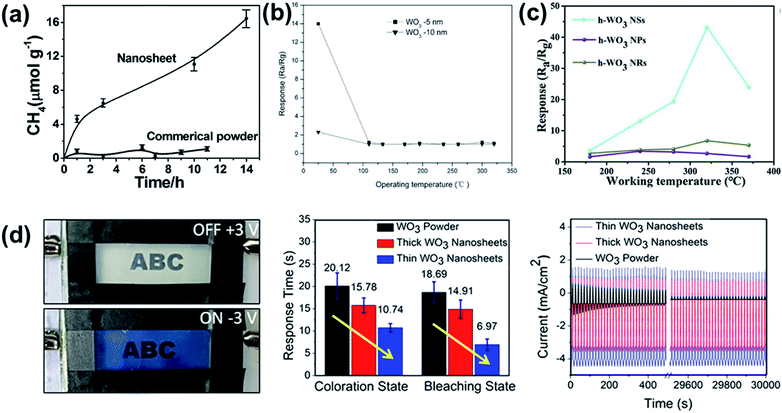 | ||
| Fig. 11 Effect of morphology for various WO3 applications. (a) Comparison of nanosheets vs. commercial powder WO3 for reduction of CO2 to CH4, reprinted with permission from ref. 24, copyright 2012 American Chemical Society. (b) Response time of WO3 nanosheets with 5 nm and 10 nm average thickness. Reprinted with permission from ref. 53, copyright 2018, Elsevier. (c) Comparison of WO3 nanosheets (NSs), nanoparticles (NPs) and nanorods (NRs) responses towards 50 ppm of ethylbenzene vapor. Reprinted with permission from ref. 40, copyright 2019, Elsevier. (d) Photographs of on and off states of WO3 nanosheet based electrochromic device and effect of WO3 thickness of electrochromic response times and stability. Reprinted with permission from ref. 78, copyright 2018, American Chemical Society. | ||
Although the VB of WO3 already provides potent oxidative potential, Liu et al. demonstrated that the additional overpotential provided by shifting the VB further positive in WO3 nanosheets improves photoelectrochemical oxygen evolution efficiency relative to bulk WO3.11 The quantum size effect is even more pronounced when pores are incorporated into WO3 nanosheets, further widening its bandgap and improving oxygen evolution activity relative to nonporous WO3 nanosheets.11
Furthermore, simply the increased surface area afforded by WO3 nanosheets can benefit photocatalytic applications. Parthibavarman et al. analyzed lateral size effect on photocatalytic properties by comparing WO3 nanosheet and WO3 nanorod based devices.19 The larger contact with methylene blue dye provided by larger surface area of WO3 nanosheet resulted in much better photocatalytic efficiency and stability than WO3 nanorod did due to more efficient electron transport.
Similarly, thinner WO3 nanosheets show generally show better performance in sensor applications. As shown in Fig. 11b, Chen et al. controlled average thickness of WO3 nanosheets from 5 nm to 10 nm by adjusting precursor ratio.53 When applied to a triethylamine sensor, the 5 nm WO3 nanosheet showed 6.4 times better response value than that of 10 nm nanosheet at room temperature.
WO3 nanosheets have also been found to be superior to other nano-morphologies in sensing applications, implying the importance of the nanosheet morphology. Zhang et al. compared volatile organic compound gas sensing properties of WO3 nanosheets, nanoparticles and nanorods, as shown in Fig. 11c.40 The much larger surface area provided from the 2D WO3 nanosheets showed 9 fold higher selectivity and superior recovery time to those of lower-dimension WO3 nanorods and nanoparticles.
Finally, there is evidence to suggest electrochromic devices benefit from the high aspect ratio of WO3 nanosheets as well. The diffusivity of Li+ is known to be higher at the surface than the interior of WO3 clusters, making high aspect ratio nanosheets an ideal morphology.91 As shown in Fig. 11d, Azam et al. compared coloration efficiencies of electrochromic devices with WO3 nanosheets of different thicknesses.78 It was found that thinner WO3 nanosheets have not only better capacity through better packing and higher exposed active surface area, but also better ion diffusivity. Overall, the device based on WO3 nanosheets with average thickness of less than 10 nm showed 3.43 times higher color modulation and ∼46.62% enhancement in response time than those of bulk WO3 devices. Other works have confirmed these principles for hydrated WO3, finding that nanosheet morphologies outperform bulk89 or nanorod92 expressions of similar chemical composition.
4.2 Effect of phase and preferentially exposed facets
Different phases of WO3 can both create different adsorption energies for surface reactions and expose different crystal facets. This is particularly important in nanosheets as the facet on the long surfaces of nanosheets have much more surface area than edge surfaces. Often it can be difficult to experimentally determine the exact effect of phase independent of other factors as it is challenging to produce structures that are morphologically identical but of different crystal structures.Although the overwhelming majority of synthesis methods discussed produce crystalline WO3 of some form, amorphous WO3 can be beneficial in certain roles. For refluxed WO3 nanoflakes, non-crystalline, hydrated WO3 was found to be superior to crystalline WO3 in supercapacitors.66
In the case of crystalline WO3, the exposed facet is particularly important for sensing applications. Song et al. compared monoclinic, triclinic, and hexagonal WO3 nanosheets and found the triclinic phase to be most effective for NO2 sensing.93 In triclinic WO3 nanosheets, the (200) surface is terminated by oxygen atoms with a coordination of one (O1C), which are most amenable to redox reactions. The (002) facets of monoclinic WO3 contain a relatively lower concentration of O1C, while hexagonal (002) facets contain no O1C at all. As seen in Fig. 12, this correlates strongly with their sensing performance for NO2.
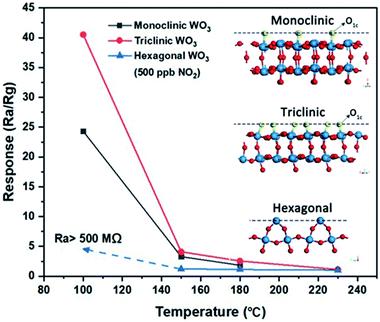 | ||
| Fig. 12 Effect of WO3 crystal structure on sensing performance of NO2. Inset shows the crystal structures with density of single coordinated oxygen atoms (O1c) on the exposed facet. Reprinted with permission from ref. 93, copyright 2020, Springer-Nature. | ||
The oxygen density of preferentially exposed facets also plays an important role in photocatalysts. Here, the high photocatalytic activity of the preferential (001) facet has been attributed to the relative ease with which this surface forms oxygen radicals26,94,95 and its low energy barrier for water oxidation.94
In electrochromic applications, there are reports of high-performance electrochromic devices from a wide variety of different WO3 phases. Earlier works regarding electrochromic WO3 asserted that while amorphous WO3 could achieve high initial values of coloration efficiency, some crystallinity was necessary for cyclic stability,96,97 as shown in Fig. 13. At least one recent report, however, has found reasonable stability from mesoporous amorphous WO3,98 which could be attributed to the ability of the highly porous structure to prevent stress accumulation upon Li+ insertion.
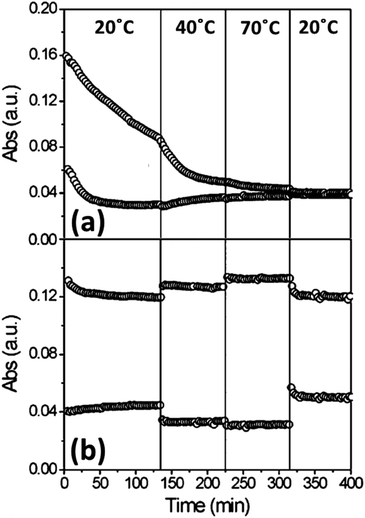 | ||
| Fig. 13 Absorption change of amorphous (a) and crystalline (b) WO3 thin films at various temperatures over time. Reprinted with permission from ref. 96, copyright 2007, American Chemical Society. | ||
Djaoued et al. used sol–gel synthesis to create porous electrochromic WO3 thin films of three different phases: hexagonal, monoclinic, and orthorhombic.99 There was minimal difference observed in optical modulation between the three films; all achieved optical modulation of at least 70%, indicating that Li+ produces similar effects in all three structures. The authors did not evaluate switching times or cyclic stability, however, so it is possible that certain phases still have electrochromic advantages in these parameters.
4.3 Effect of oxygen vacancies
Although typically labelled simply ‘WO3’, it is rare for the stoichiometry to be exactly 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; as-synthesized WO3 is typically slightly oxygen deficient. Promoting and tuning the oxygen vacancy (Ovac) concentration can enhance various properties, notably visible light absorption for photoactivity100 and adsorption energy of various molecules for catalytic or sensing applications.101
1; as-synthesized WO3 is typically slightly oxygen deficient. Promoting and tuning the oxygen vacancy (Ovac) concentration can enhance various properties, notably visible light absorption for photoactivity100 and adsorption energy of various molecules for catalytic or sensing applications.101
Incorporating oxygen vacancies or other defects into nanosheet surfaces can further improve their photocatalytic activity. Oxygen deficient WO3 can exhibit localized surface plasmon resonance (LSPR) in the IR region, improving light harvesting as shown in Fig. 14a.102 More commonly, the benefit of oxygen-vacancies on the photocatalytic activity of WO3 nanosheets has been ascribed to bandgap narrowing, thus improving visible-light utilization,12 and formation of highly active superoxide radicals at vacancy sites.21Fig. 14b shows the effect of oxygen vacancy induced band gap narrowing on visible light photocurrents in Pt/WO3 nanosheets.103
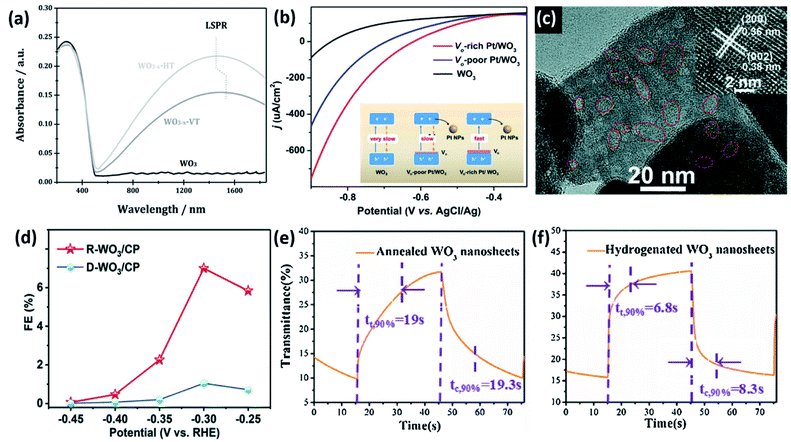 | ||
| Fig. 14 Impact of oxygen vacancies of WO3. (a) LSPR formation in oxygen vacancy right WO3 nanosheets. Reprinted with permission from ref. 102, copyright 2015, John Wiley and Sons. (b) Band gap narrowing and improved photocurrent induced by oxygen vacancy formation. Adapted from ref. 103, copyright 2020 Elsevier. (c) TEM image of oxygen vacancy rich WO3 nanosheets (R-WO3) and (d) comparison of FE for reduction of N2 to NH3. Reprinted with permission from ref. 104, copyright 2019, Royal Society of Chemistry. (e) Electrochromic response of annealed WO3 nanosheets vs. (f) hydrogenated WO3 nanosheets. Reprinted with permission from ref. 108, copyright 2017 Elsevier. | ||
Creating voids in the nanosheet surface, referred to as nanopores11 or “potholes”,27 improves carrier separation and migration and promotes adsorption and activation of chemical species. Holes traversing along the (001) surface of porous nanosheets can find pore-adsorbed reactants before they have the opportunity to recombine.11 A higher degree of band bending in porous nanosheets relative to nonporous nanosheets accounts for the improved charge transfer resistance in the former.11 Pothole voids expose more dangling surface oxygens than defect-free surfaces and thus provide electron rich sites for adsorption and activation of chemical species.27 The electron-rich pothole defects in WO3 nanosheets have been implicated in adsorbing and activating N2, thus forming a metastable N2 intermediate that is prone to conversion to NO, while no such intermediate forms in pothole-free WO3.27
For electrocatalysts, Kong et al. studied to effect on oxygen vacancies in WO3 nanosheets for N2 reduction to NH3.104 Nanosheets were prepared through a typical hydrothermal method, followed by annealing at 400 °C in an N2 atmosphere to produce oxygen-vacancy rich nanosheets (R-WO3 NSs), or the same temperature in air to produce nanosheets deficient in oxygen vacancies (D-WO3 NSs). A TEM image highlighting the lattice distortions induced by oxygen vacancies is shown in Fig. 14c. Oxygen vacancies in R-WO3 NSs were asserted to promote additional adsorption sites for N2 or reduce electron transfer resistance, resulting in Faradaic efficiency (FE) that was far greater than D-WO3 NSs (Fig. 14d).
The ability of oxygen vacancies to modify adsorption characteristics of gases also can benefit WO3 for sensing applications. In the case of oxidizers such as NO2, the electrophilic gas can be adsorbed more effectively since surface oxygen vacancies act as n-type dopants.47 Improved adsorption of NO2 in oxygen vacancy rich WO3 nanostructures had been previously predicted through DFT analysis,105 and has since been mechanistically studied over a various WO3 morphologies.106
For detection of reducing gases it could be expected that the oxygen vacancies would have the opposite effect, but this is not always the case. It was observed by Rahmani et al. that detection of H2 was improved after compensation of crystal defects like oxygen vacancies,60 but for other reducing gases such as H2S101 and NH3 (ref. 107) there is some evidence to the contrary. In the case of ammonia sensing this was attributed to band gap narrowing causing easier injections of electrons from ammonia to the CB of WO3.107
While oxygen vacancies are beneficial for certain catalytic and sensing applications, for electrochromic applications there are conflicting reports regarding their effects. For WO3 nanosheets, Zhou et al. reported that hydrogen treatment to induce oxygen vacancies produced electrochromic films that were superior in both contrast ratio and switching time to pristine WO3.108Fig. 14e and f show the difference between annealed WO3 and hydrogenated WO3, respectively. The authors posited that the oxygen vacancies could be helpful for Li+ insertion and extraction. Other oxygen-deficient WO3 nanostructures have similarly shown short coloration/bleaching times.109
A later study, however, found that oxygen vacancies are not necessarily beneficial for electrochromic films.110 Here, three different films were studied: as-deposited, annealed in O2, and annealed in Ar. The as-deposited amorphous films showed the best ΔT (87%) but poor cyclic stability, while the films annealed in Ar (which were crystalline, but with abundant oxygen vacancies) was the worst (51%). WO3 annealed in O2 showed the best cyclic stability and still retained moderate contrast (71%). This shows that while amorphous regions or other structural defects may boost electrochromic performance, oxygen vacancies specifically do not.
4.4 WO3 nanosheet based composites
The intrinsic properties of WO3 make it amenable to hybridization with various other materials to modify its electronic or optical properties. WO3 nanosheets can be particularly well-suited to these roles as their high surface area allows for easy surface doping with other chemical moieties and/or intimate contact with other nanostructures.Rapid recombination of electrons and holes in WO3 photocatalysts has been thwarted by transferring charges across heterojunctions to metal nanoparticles12,17 or other semiconductors.15,18,25 Electron transfer between WO3 nanosheets and Pt17 or Ag12 nanoparticles is demonstrated to reduce recombination of photogenerated electron–hole pairs and allow photogenerated holes to more efficiently drive oxidation of tetracycline17 or water,12 respectively. Fig. 15a shows the total organic carbon (TOC) removal curves of tetracycline with Pt/WO3.17
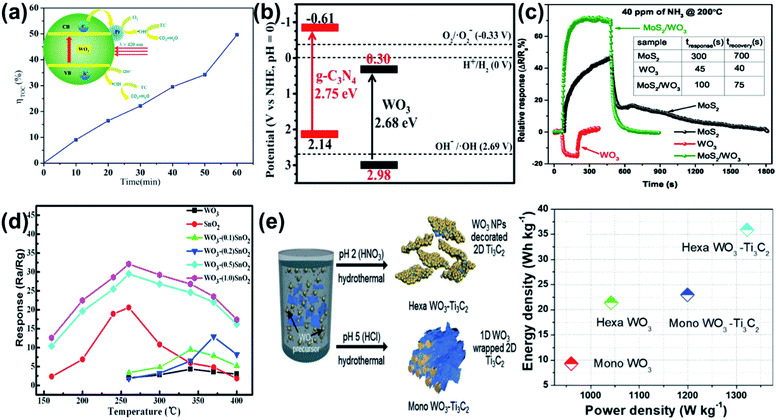 | ||
| Fig. 15 Compositing/hybridization examples for WO3. (a) Removal of tetracycline with Pt NP decorated WO3 nanosheets, with inset showing the mechanism of charge transfer. Reprinted with permission from ref. 17, copyright 2014, American Chemical Society. (b) Band diagram of g-C3N4–WO3 for S-scheme H2 photocatalysts. Reprinted from ref. 15, copyright 2019 Elsevier. (c) Response over time of MoS2, WO3, and MoS2/WO3 to ammonia. Reprinted with permission from ref. 112, copyright 2021, American Chemical Society. (d) Response of WO3, SnO2, and various weight loadings of SnO2 on WO3vs. temperature. Reprinted with permission from ref. 116, copyright 2018 Elsevier. (e) Schematic for formation of WO3–Ti3C2 hybrids and the energy density/power density plot for their use in a supercapacitor. Reprinted with permission from ref. 122, copyright 2018 John Wiley and Sons. | ||
Paired with other semiconductors, the strong oxidizing power of WO3 can be coupled with the strong reducing power of a reduction catalyst in a direct Z-scheme heterojunction composite (note: this sub-type of Z-scheme photocatalyst has recently been re-named S-scheme to avoid confusion with other types of Z-scheme photocatalysts15,111). In this configuration, when both semiconductors are excited, the VB holes of the reduction catalyst react with CB electrons in WO3, thus separating VB holes in WO3 from CB electrons in the reduction catalyst.
The strong oxidizing power of WO3 has been combined with the superior reducing power of CdS,18 gC3N4,15 or iron phthalocyanine (FePc)25 to promote efficient photocatalytic/photoelectrocatalytic ciprofloxacin degradation, water reduction, and CO2 reduction, respectively. This type of S-scheme heterojunction is particularly effective when coupling 2D materials due to the large interfacial contact area, such as is realized between ultrathin WO3 nanosheets and g-C3N4 nanosheets.15Fig. 15b shows the band structure of WO3/g-C3N4 for S-scheme H2 photocatalysts.
Similar compositing strategies for WO3 nanosheet based sensors can benefit performance by altering the electronic structure. Pairing n-type WO3 nanosheets with p-type nanosheets, such as MoS2 (ref. 112) or WS2,113 creates a strong depletion region with a built-in field across the junction which is highly sensitive to charge transfer events on the surface. Fig. 15c shows the superior sensing repose of MoS2/WO3 compared to pure MoS2 or WO3 for ammonia sensing. The same concept has been utilized in tungsten oxysulfide nanosheets to create strong depletion regions within the nanosheet surface.114
Nanosheets can also be decorated with other oxides to promote oxygen vacancies or other defect species that are sensitive to the target species.115–117Fig. 15d shows the response of pure WO3 and pure SnO2 compared to SnO2-decorated WO3 nanosheets for detection of acetone vapor.116 The authors asserted that lattice distortion at the SnO2–WO3 interface creates dangling bonds and non-uniform electron distributions that act as favourable adsorption sites.
In charge storage applications, compositing strategies for WO3 often focus on pairing the normally insulating oxide with a more conductive material to alleviate charge transport issues. WO3 paired with graphene/rGO frequently appears in literature for both supercapacitors118,119 and Li-ion battery anodes,120,121 in both cases providing faster charge transport and improved stability over pure WO3.
WO3 has also been combined with a newly explored class of 2D materials – MXene – in a similar composite concept.122 MXene sheets (Ti3C2) were mixed into a standard hydrothermal WO3 synthesis procedure. Depending on the pH of the solution, this method either generated nanorod-like monoclinic WO3 hybrids (Mono WO3–Ti3C2), or nanosheet-like hexagonal WO3 on Ti3C2 (Hexa WO3–Ti3C2). Fig. 15e shows the schematic for synthesis and the effect on supercapacitor performance metrics (power density and power density), where the Hexa WO3–Ti3C2 outperforms Mono WO3–Ti3C2 and either of the pure materials. The effect was attributed to both improved surface area of the nanosheet-like structures as well as intimate contact providing faster ionic movement between WO3 and Ti3C2.
Although by no means a comprehensive list of all WO3 nanosheet composites reported in the literature, this provides a sampling of many of the key concepts frequently utilized. Nanosheet expressions of WO3 can be easily decorated with NPs to either modify their visible light response or tune the adsorption energy for photocatalytic or sensing applications. The combination of WO3 nanosheets with other 2D materials (graphene, TMDCs, or MXene) is also widely studied to either create depletion regions at the n-type/p-type boundary or facilitate charge transfer.
5. Conclusions
The literature summarized and reviewed here shows the tremendous amount of research interest that WO3 nanosheets have garnered in recent years. For photocatalytic, sensing, electrochromic, and charge storage applications, WO3 nanosheets provide significant advantages over bulk counterparts, and in many cases advantages over other nano-morphologies as well. Because WO3 is not intrinsically layered, synthesis of high-aspect ratio nanosheets is non-trivial, and as such there is great diversity in their production. Bottom-up methods of various chemistries can successfully synthesize WO3 nanosheets in simple, one-step methods, but generally struggle to achieve high aspect ratios. Top-down methods, in particular intercalation-assisted exfoliation of WS2 followed by oxidation, provide a promising alternative for applications which benefit from nanosheets with larger lateral sizes and higher aspect ratios. Both types of synthesis offer many variations in terms of phase and stoichiometry that can be further exploited to fit the target application.Conflicts of interest
There are no conflicts to declare.Acknowledgements
T.G.N. gratefully acknowledges the National Research Council for support through a Naval Research Laboratory/National Research Council Postdoctoral Associateship.Notes and references
- C. C. Mardare and A. W. Hassel, Phys. Status Solidi A, 2019, 216, 1900047 CrossRef.
- C. Chacón, M. Rodríguez-Pérez, G. Oskam and G. Rodríguez-Gattorno, J. Mater. Sci.: Mater. Electron., 2015, 26, 5526–5531 CrossRef.
- G. N. Kustova, Y. A. Chesalov, L. M. Plyasova, I. Y. Molina and A. I. Nizovskii, Vib. Spectrosc., 2011, 55, 235–240 CrossRef CAS.
- H. Zheng, J. Z. Ou, M. S. Strano, R. B. Kaner, A. Mitchell and K. Kalantar-zadeh, Adv. Funct. Mater., 2011, 21, 2175–2196 CrossRef CAS.
- I. Angela, T. Tiina, V. Meeri, V. Heiki, K. Aleksandr, S. Mariliis, P. Suman, M. Lutz, H. Margit, K. Vambola, S. Ruth and K. Anne, Curr. Top. Med. Chem., 2015, 15, 1914–1929 CrossRef PubMed.
- L. Santos, J. P. Neto, A. Crespo, D. Nunes, N. Costa, I. M. Fonseca, P. Barquinha, L. Pereira, J. Silva, R. Martins and E. Fortunato, ACS Appl. Mater. Interfaces, 2014, 6, 12226–12234 CrossRef CAS.
- P. Dong, G. Hou, X. Xi, R. Shao and F. Dong, Environ. Sci.: Nano, 2017, 4, 539–557 RSC.
- D. Tanaka, Y. Oaki and H. Imai, Chem. Commun., 2010, 46, 5286–5288 RSC.
- M. Gillet, C. Lemire, E. Gillet and K. Aguir, Surf. Sci., 2003, 532–535, 519–525 CrossRef CAS.
- S. S. Kalanur, Catalysts, 2019, 9, 456 CrossRef CAS.
- Y. Liu, L. Liang, C. Xiao, X. Hua, Z. Li, B. Pan and Y. Xie, Adv. Energy Mater., 2016, 6, 1600437 CrossRef.
- Y. Ren, C. Li, Q. Xu, J. Yan, Y. Li, P. Yuan, H. Xia, C. Niu, X. Yang and Y. Jia, Appl. Catal., B, 2019, 245, 648–655 CrossRef CAS.
- J. J. Zhang, P. Zhang, T. Wang and J. L. Gong, Nano Energy, 2015, 11, 189–195 CrossRef CAS.
- J. Y. Zheng, G. Song, J. S. Hong, T. K. Van, A. U. Pawar, D. Y. Kim, C. W. Kim, Z. Haider and Y. S. Kang, Cryst. Growth Des., 2014, 14, 6057–6066 CrossRef CAS.
- J. W. Fu, Q. L. Xu, J. X. Low, C. J. Jiang and J. G. Yu, Appl. Catal., B, 2019, 243, 556–565 CrossRef CAS.
- G. Guan, J. Xia, S. Liu, Y. Cheng, S. Bai, S. Y. Tee, Y.-W. Zhang and M.-Y. Han, Adv. Mater., 2017, 29, 1700326 CrossRef PubMed.
- G. Zhang, W. Guan, H. Shen, X. Zhang, W. Fan, C. Lu, H. Bai, L. Xiao, W. Gu and W. Shi, Ind. Eng. Chem. Res., 2014, 53, 5443–5450 CrossRef CAS.
- X. Liu, Y. Yan, Z. Da, W. Shi, C. Ma, P. Lv, Y. Tang, G. Yao, Y. Wu, P. Huo and Y. Yan, Chem. Eng. J., 2014, 241, 243–250 CrossRef CAS.
- M. Parthibavarman, M. Karthik and S. Prabhakaran, Vacuum, 2018, 155, 224–232 CrossRef CAS.
- Y. Liang, Y. Yang, C. Zou, K. Xu, X. Luo, T. Luo, J. Li, Q. Yang, P. Shi and C. Yuan, J. Alloys Compd., 2019, 783, 848–854 CrossRef CAS.
- M. M. Zhang, C. Lai, B. S. Li, D. L. Huang, S. Y. Liu, L. Qin, H. Yi, Y. K. Fu, F. H. Xu, M. F. Li and L. Li, J. Colloid Interface Sci., 2019, 556, 557–567 CrossRef CAS PubMed.
- D. Q. Zhang, S. L. Wang, J. Zhu, H. X. Li and Y. F. Lu, Appl. Catal., B, 2012, 123, 398–404 CrossRef.
- J. Ma, K. K. Mao, J. X. Low, Z. H. Wang, D. W. Xi, W. Q. Zhang, H. X. Ju, Z. M. Qi, R. Long, X. J. Wu, L. Song and Y. J. Xiong, Angew. Chem., Int. Ed., 2021, 60, 9357–9361 CrossRef CAS PubMed.
- X. Chen, Y. Zhou, Q. Liu, Z. Li, J. Liu and Z. Zou, ACS Appl. Mater. Interfaces, 2012, 4, 3372–3377 CrossRef CAS PubMed.
- B. Li, L. Q. Sun, J. Bian, N. Sun, J. W. Sun, L. Q. Chen, Z. J. Li and L. Q. Jing, Appl. Catal., B, 2020, 270 Search PubMed.
- F. Q. Zhan, W. H. Liu, W. Z. Li, J. Li, Y. H. Yang, Q. Liu, Y. M. Li and X. D. Tang, J. Mater. Sci.: Mater. Electron., 2017, 28, 13836–13845 CrossRef CAS.
- Y. Liu, M. Cheng, Z. He, B. Gu, C. Xiao, T. Zhou, Z. Guo, J. Liu, H. He, B. Ye, B. Pan and Y. Xie, Angew. Chem., Int. Ed., 2019, 58, 731–735 CrossRef CAS PubMed.
- D. Yim, F. Raza, J. H. Park, J.-H. Lee, H.-I. Kim, J.-K. Yang, I.-J. Hwang and J.-H. Kim, ACS Appl. Mater. Interfaces, 2019, 11, 36960–36969 CrossRef CAS.
- Y. Li, X. Zhai, Y. Liu, H. Wei, J. Ma, M. Chen, X. Liu, W. Zhang, G. Wang, F. Ren and S. Wei, Frontiers in Materials, 2020, 7, 105 CrossRef.
- H. Long, W. Zeng and H. Zhang, J. Mater. Sci.: Mater. Electron., 2015, 26, 4698–4707 CrossRef CAS.
- C. Dong, R. Zhao, L. Yao, Y. Ran, X. Zhang and Y. Wang, J. Alloys Compd., 2020, 820, 153194 CrossRef CAS.
- S. Bai, Y. Ma, X. Shu, J. Sun, Y. Feng, R. Luo, D. Li and A. Chen, Ind. Eng. Chem. Res., 2017, 56, 2616–2623 CrossRef CAS.
- C. O. Avellaneda and L. O. S. Bulhões, Solid State Ionics, 2003, 165, 59–64 CrossRef CAS.
- S.-H. Lee, H. M. Cheong, J.-G. Zhang, A. Mascarenhas, D. K. Benson and S. K. Deb, Appl. Phys. Lett., 1999, 74, 242–244 CrossRef CAS.
- Y. Zhen, B. P. Jelle and T. Gao, Analytical Science Advances, 2020, 1, 124–131 CrossRef.
- B. P. Jelle, A. Hynd, A. Gustavsen, D. Arasteh, H. Goudey and R. Hart, Sol. Energy Mater. Sol. Cells, 2012, 96, 1–28 CrossRef CAS.
- S. Feng and R. Xu, Acc. Chem. Res., 2001, 34, 239–247 CrossRef CAS PubMed.
- Y. Yu, W. Zeng, M. Xu and X. Peng, Phys. E, 2016, 79, 127–132 CrossRef CAS.
- J. Shi, Z. Cheng, L. Gao, Y. Zhang, J. Xu and H. Zhao, Sens. Actuators, B, 2016, 230, 736–745 CrossRef CAS.
- D. Zhang, Y. Fan, G. Li, Z. Ma, X. Wang, Z. Cheng and J. Xu, Sens. Actuators, B, 2019, 293, 23–30 CrossRef CAS.
- O. O. Abe, Z. Qiu, J. R. Jinschek and P.-I. Gouma, Sensors, 2021, 21, 1690 CrossRef CAS.
- Y. Wicaksana, S. Liu, J. Scott and R. Amal, Molecules, 2014, 19, 17747–17762 CrossRef PubMed.
- B. Meschi Amoli, J. Trinidad, G. Rivers, S. Sy, P. Russo, A. Yu, N. Y. Zhou and B. Zhao, Carbon, 2015, 91, 188–199 CrossRef CAS.
- W. Xiao, W. Liu, X. Mao, H. Zhu and D. Wang, J. Mater. Chem. A, 2013, 1, 1261–1269 RSC.
- W. Shi, X. Guo, C. Cui, K. Jiang, Z. Li, L. Qu and J.-C. Wang, Appl. Catal., B, 2019, 243, 236–242 CrossRef CAS.
- Z. Sun, T. Liao, Y. Dou, S. M. Hwang, M.-S. Park, L. Jiang, J. H. Kim and S. X. Dou, Nat. Commun., 2014, 5, 3813 CrossRef CAS PubMed.
- Z. Wang, D. Wang and J. Sun, Sens. Actuators, B, 2017, 245, 828–834 CrossRef CAS.
- D. Wang, S. Huang, H. Li, A. Chen, P. Wang, J. Yang, X. Wang and J. Yang, Sens. Actuators, B, 2019, 282, 961–971 CrossRef CAS.
- B. Ahmed, S. Kumar, A. K. Ojha, P. Donfack and A. Materny, Spectrochim. Acta, Part A, 2017, 175, 250–261 CrossRef CAS.
- M. Wang, Y. Wang, X. Li, C. Ge, S. Hussain, G. Liu and G. Qiao, Sens. Actuators, B, 2020, 316, 128050 CrossRef CAS.
- S. B. Upadhyay, R. K. Mishra and P. P. Sahay, Sens. Actuators, B, 2014, 193, 19–27 CrossRef CAS.
- S. B. Upadhyay, R. K. Mishra and P. P. Sahay, Ceram. Int., 2016, 42, 15301–15310 CrossRef CAS.
- G. Chen, X. Chu, H. Qiao, M. Ye, J. Chen, C. Gao and C.-Y. Guo, Mater. Lett., 2018, 226, 59–62 CrossRef CAS.
- M. Yin, L. Yu and S. Liu, J. Alloys Compd., 2017, 696, 490–497 CrossRef CAS.
- D. Sánchez-Martínez, C. Gomez-Solis and L. M. Torres-Martinez, Mater. Res. Bull., 2015, 61, 165–172 CrossRef.
- Y. Oaki and H. Imai, Adv. Mater., 2006, 18, 1807–1811 CrossRef CAS.
- P. J. Boruah, R. R. Khanikar and H. Bailung, Plasma Chem. Plasma Process., 2020, 40, 1019–1036 CrossRef CAS.
- A. Wisitsora-at, D. Phokaratkul, K. Jaruwongrangsee, T. M. Daniels and W. Wlodarski, Proceedings, 2017, 1, 466 CrossRef.
- A. Z. Sadek, H. Zheng, M. Breedon, V. Bansal, S. K. Bhargava, K. Latham, J. Zhu, L. Yu, Z. Hu, P. G. Spizzirri, W. Wlodarski and K. Kalantar-zadeh, Langmuir, 2009, 25, 9545–9551 CrossRef CAS PubMed.
- M. B. Rahmani, M. H. Yaacob and Y. M. Sabri, Sens. Actuators, B, 2017, 251, 57–64 CrossRef CAS.
- J. Y. Luo, Z. Cao, F. Chen, L. Li, Y. R. Lin, B. W. Liang, Q. G. Zeng, M. Zhang, X. He and C. Li, Appl. Surf. Sci., 2013, 287, 270–275 CrossRef CAS.
- X. Fang, M. Yao, L. Guo, Y. Xu, W. Zhou, M. Zhuo, C. Shi, L. Liu, L. Wang, X. Li and W. Chen, ACS Sustainable Chem. Eng., 2017, 5, 10735–10743 CrossRef CAS.
- Y. Hernandez, V. Nicolosi, M. Lotya, F. M. Blighe, Z. Sun, S. De, I. T. McGovern, B. Holland, M. Byrne, Y. K. Gun'Ko, J. J. Boland, P. Niraj, G. Duesberg, S. Krishnamurthy, R. Goodhue, J. Hutchison, V. Scardaci, A. C. Ferrari and J. N. Coleman, Nat. Nanotechnol., 2008, 3, 563–568 CrossRef CAS.
- A. O'Neill, U. Khan and J. N. Coleman, Chem. Mater., 2012, 24, 2414–2421 CrossRef.
- T. G. Novak, H. Shin, J. Kim, K. Kim, A. Azam, C. V. Nguyen, S. H. Park, J. Y. Song and S. Jeon, ACS Appl. Mater. Interfaces, 2018, 10, 17957–17962 CrossRef CAS PubMed.
- M. Szkoda, Z. Zarach, K. Trzciński, G. Trykowski and A. P. Nowak, Materials, 2020, 13 Search PubMed.
- R. J. Smith, P. J. King, M. Lotya, C. Wirtz, U. Khan, S. De, A. O'Neill, G. S. Duesberg, J. C. Grunlan, G. Moriarty, J. Chen, J. Wang, A. I. Minett, V. Nicolosi and J. N. Coleman, Adv. Mater., 2011, 23, 3944–3948 CrossRef CAS PubMed.
- D. Xu, P. Xu, Y. Zhu, W. Peng, Y. Li, G. Zhang, F. Zhang, T. E. Mallouk and X. Fan, ACS Appl. Mater. Interfaces, 2018, 10, 2810–2818 CrossRef CAS PubMed.
- D. Pan, Z. Fang, E. Yang, Z. Ning, Q. Zhou, K. Chen, Y. Zheng, Y. Zhang and Y. Shen, Angew. Chem., Int. Ed., 2020, 59, 16747–16754 CrossRef CAS PubMed.
- T. G. Novak, J. Kim, J. Kim, H. Shin, A. P. Tiwari, J. Y. Song and S. Jeon, 2D Materials, 2019, 6, 045019 CrossRef CAS.
- T. G. Novak, J. Kim, J. Kim, A. P. Tiwari, H. Shin, J. Y. Song and S. Jeon, Adv. Funct. Mater., 2020, 30, 2001760 CrossRef CAS.
- J. Kim, N. M. Han, J. Kim, J. Lee, J.-K. Kim and S. Jeon, ACS Appl. Mater. Interfaces, 2018, 10, 37507–37516 CrossRef CAS.
- Q. Zhang, L. Mei, X. Cao, Y. Tang and Z. Zeng, J. Mater. Chem. A, 2020, 8, 15417–15444 RSC.
- L. Yuwen, H. Yu, X. Yang, J. Zhou, Q. Zhang, Y. Zhang, Z. Luo, S. Su and L. Wang, Chem. Commun., 2016, 52, 529–532 RSC.
- A. Ghorai, A. Midya, R. Maiti and S. K. Ray, Dalton Trans., 2016, 45, 14979–14987 RSC.
- P. Zhou, Q. Xu, H. Li, Y. Wang, B. Yan, Y. Zhou, J. Chen, J. Zhang and K. Wang, Angew. Chem., Int. Ed., 2015, 54, 15226–15230 CrossRef CAS.
- X.-Y. Tang, M.-F. Li, L.-F. Gao, H. Yan, S.-M. Deng, J.-B. Fan, M.-S. Zheng, S.-L. Deng, Q.-Y. Zhang, S.-Y. Xie and L.-S. Zheng, Adv. Mater. Interfaces, 2019, 6, 1901122 CrossRef CAS.
- A. Azam, J. Kim, J. Park, T. G. Novak, A. P. Tiwari, S. H. Song, B. Kim and S. Jeon, Nano Lett., 2018, 18, 5646–5651 CrossRef CAS PubMed.
- T. G. Novak, J. Kim, S. H. Song, G. H. Jun, H. Kim, M. S. Jeong and S. Jeon, Small, 2016, 12, 994–999 CrossRef CAS.
- S. H. Song, M.-H. Jang, J. Chung, S. H. Jin, B. H. Kim, S.-H. Hur, S. Yoo, Y.-H. Cho and S. Jeon, Adv. Opt. Mater., 2014, 2, 1016–1023 CrossRef CAS.
- J. Kim, S. H. Song, H.-G. Im, G. Yoon, D. Lee, C. Choi, J. Kim, B.-S. Bae, K. Kang and S. Jeon, Small, 2015, 11, 3124–3129 CrossRef CAS PubMed.
- S. H. Song, B. H. Kim, D.-H. Choe, J. Kim, D. C. Kim, D. J. Lee, J. M. Kim, K. J. Chang and S. Jeon, Adv. Mater., 2015, 27, 3152–3158 CrossRef CAS PubMed.
- T. G. Novak, J. Kim, A. P. Tiwari, J. Kim, S. Lee, J. Lee and S. Jeon, ACS Sustainable Chem. Eng., 2020, 8, 11276–11282 CrossRef CAS.
- J. B. Mitchell, N. R. Geise, A. R. Paterson, N. C. Osti, Y. Sun, S. Fleischmann, R. Zhang, L. A. Madsen, M. F. Toney, D.-e. Jiang, A. I. Kolesnikov, E. Mamontov and V. Augustyn, ACS Energy Lett., 2019, 4, 2805–2812 CrossRef CAS.
- R. Wang, C.-C. Chung, Y. Liu, J. L. Jones and V. Augustyn, Langmuir, 2017, 33, 9314–9323 CrossRef CAS PubMed.
- Z. Wang, W. Gong, X. Wang, Z. Chen, X. Chen, J. Chen, H. Sun, G. Song, S. Cong, F. Geng and Z. Zhao, ACS Appl. Mater. Interfaces, 2020, 12, 33917–33925 CrossRef CAS.
- K. Kalantar-zadeh, A. Vijayaraghavan, M.-H. Ham, H. Zheng, M. Breedon and M. S. Strano, Chem. Mater., 2010, 22, 5660–5666 CrossRef CAS.
- Y. Zhang, Y. Shi, R. Chen, L. Tao, C. Xie, D. Liu, D. Yan and S. Wang, J. Mater. Chem. A, 2018, 6, 23028–23033 RSC.
- L. Liang, J. Zhang, Y. Zhou, J. Xie, X. Zhang, M. Guan, B. Pan and Y. Xie, Sci. Rep., 2013, 3, 1936 CrossRef.
- J. Kim, G. Yoon, J. Kim, H. Yoon, J. Baek, J. H. Lee, K. Kang and S. Jeon, Carbon, 2018, 139, 309–316 CrossRef CAS.
- S.-H. Lee, H. M. Cheong, C. E. Tracy, A. Mascarenhas, J. R. Pitts, G. Jorgensen and S. K. Deb, Appl. Phys. Lett., 2000, 76, 3908–3910 CrossRef CAS.
- H. Li, J. Wang, G. Shi, H. Wang, Q. Zhang and Y. Li, RSC Adv., 2015, 5, 196–201 RSC.
- W. Song, R. Zhang, X. Bai, Q. Jia and H. Ji, J. Mater. Sci.: Mater. Electron., 2020, 31, 610–620 CrossRef CAS.
- S. C. Wang, H. J. Chen, G. P. Gao, T. Butburee, M. Q. Lyu, S. Thaweesak, J. H. Yun, A. J. Du, G. Liu and L. Z. Wang, Nano Energy, 2016, 24, 94–102 CrossRef CAS.
- H. Jin, J. Zhu, W. Chen, Z. Fang, Y. Li, Y. Zhang, X. Huang, K. Ding, L. Ning and W. Chen, J. Phys. Chem. C, 2012, 116, 5067–5075 CrossRef CAS.
- S. Sallard, T. Brezesinski and B. M. Smarsly, J. Phys. Chem. C, 2007, 111, 7200–7206 CrossRef CAS.
- S. H. Lee, R. Deshpande, P. A. Parilla, K. M. Jones, B. To, A. H. Mahan and A. C. Dillon, Adv. Mater., 2006, 18, 763–766 CrossRef CAS.
- K.-W. Kim, T. Y. Yun, S.-H. You, X. Tang, J. Lee, Y. Seo, Y.-T. Kim, S. H. Kim, H. C. Moon and J. K. Kim, NPG Asia Mater., 2020, 12, 84 CrossRef CAS.
- Y. Djaoued, S. Balaji and R. Brüning, J. Nanomater., 2012, 2012, 674168 Search PubMed.
- S. Chen, Y. Xiao, W. Xie, Y. Wang, Z. Hu, W. Zhang and H. Zhao, Nanomaterials, 2018, 8, 553 CrossRef.
- W. Yu, Z. Shen, F. Peng, Y. Lu, M. Ge, X. Fu, Y. Sun, X. Chen and N. Dai, RSC Adv., 2019, 9, 7723–7728 RSC.
- J. Yan, T. Wang, G. Wu, W. Dai, N. Guan, L. Li and J. Gong, Adv. Mater., 2015, 27, 1580–1586 CrossRef CAS PubMed.
- J.-J. Li, M. Zhang, B. Weng, X. Chen, J. Chen and H.-P. Jia, Appl. Surf. Sci., 2020, 507, 145133 CrossRef CAS.
- W. Kong, R. Zhang, X. Zhang, L. Ji, G. Yu, T. Wang, Y. Luo, X. Shi, Y. Xu and X. Sun, Nanoscale, 2019, 11, 19274–19277 RSC.
- Y. Qin and Z. Ye, Sens. Actuators, B, 2016, 222, 499–507 CrossRef CAS.
- A. Staerz, S. Somacescu, M. Epifani, T. Kida, U. Weimar and N. Barsan, ACS Sens., 2020, 5, 1624–1633 CrossRef CAS.
- C.-Y. Wang, X. Zhang, Q. Rong, N.-N. Hou and H.-Q. Yu, Chemosphere, 2018, 204, 202–209 CrossRef CAS PubMed.
- X. Zhou, X. Zheng, B. Yan, T. Xu and Q. Xu, Appl. Surf. Sci., 2017, 400, 57–63 CrossRef CAS.
- C.-C. Liao, F.-R. Chen and J.-J. Kai, Sol. Energy Mater. Sol. Cells, 2006, 90, 1147–1155 CrossRef CAS.
- H. Yu, J. Guo, C. Wang, J. Zhang, J. Liu, G. Dong, X. Zhong and X. Diao, Electrochim. Acta, 2020, 332, 135504 CrossRef CAS.
- Q. Xu, L. Zhang, B. Cheng, J. Fan and J. Yu, Chem, 2020, 6, 1543–1559 CAS.
- S. Singh, J. Deb, U. Sarkar and S. Sharma, ACS Appl. Nano Mater., 2021, 4, 2594–2605 CrossRef CAS.
- Y. Han, Y. Liu, C. Su, X. Chen, B. Li, W. Jiang, M. Zeng, N. Hu, Y. Su, Z. Zhou, Z.-g. Zhu and Z. Yang, ACS Appl. Nano Mater., 2021, 4, 1626–1634 CrossRef CAS.
- Y. Zheng, L. Sun, W. Liu, C. Wang, Z. Dai and F. Ma, J. Mater. Chem. C, 2020, 8, 4206–4214 RSC.
- C. Wang, S. Zhang, L. Qiu, S. A. Rasaki, F. Qu, T. Thomas, Y. Liu and M. Yang, J. Alloys Compd., 2020, 826, 154196 CrossRef CAS.
- M. Yin, Y. Yao, H. Fan and S. Liu, J. Alloys Compd., 2018, 736, 322–331 CrossRef CAS.
- Y. Gui, L. Yang, K. Tian, H. Zhang and S. Fang, Sens. Actuators, B, 2019, 288, 104–112 CrossRef CAS.
- R. Samal, B. Chakraborty, M. Saxena, D. J. Late and C. S. Rout, ACS Sustainable Chem. Eng., 2019, 7, 2350–2359 CrossRef CAS.
- S. Chaitoglou, R. Amade and E. Bertran, Nanoscale Res. Lett., 2017, 12, 635 CrossRef PubMed.
- S. K. Park, H. J. Lee, M. H. Lee and H. S. Park, Chem. Eng. J., 2015, 281, 724–729 CrossRef CAS.
- W. Dang, W. Wang, Y. Yang, Y. Wang, J. Huang, X. Fang, L. Wu, Z. Rong, X. Chen, X. Li, L. Huang and X. Tang, Electrochim. Acta, 2019, 313, 99–108 CrossRef CAS.
- S. B. Ambade, R. B. Ambade, W. Eom, S. H. Noh, S. H. Kim and T. H. Han, Adv. Mater. Interfaces, 2018, 5, 1801361 CrossRef.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2021 |