Neoteric solvent-based blue biorefinery: for chemicals, functional materials and fuels from oceanic biomass
Rosy Alphons
Sequeira
 ab,
Dibyendu
Mondal†
ab,
Dibyendu
Mondal†
 *c and
Kamalesh
Prasad
*c and
Kamalesh
Prasad
 *ab
*ab
aNatural Products & Green Chemistry Division, CSIR-Central Salt and Marine Chemicals Research Institute, Gijubhai Badheka Marg, Bhavnagar- 364 002, Gujarat, India. E-mail: kamlesh@csmcri.res.in; drkamaleshp@gmail.com; Fax: +91-278-2567562; Tel: +91-278-2567760
bAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad 201002, India
cCentre for Nano & Materials Sciences, Jain University, Jain Global Campus, Kanakpura, Ramanagaram, Bangalore, 562112, Karnataka, India. E-mail: m.dibyendu@jainuniversity.ac.in; dmtapu@gmail.com
First published on 14th October 2021
Abstract
Blue biorefineries integrate the production of renewable chemicals, fuels, functional materials and marketable commodities utilising biomass of marine origin. The global climate issues, ever-increasing population and depletion of fossil resources make the development of a bioeconomy based on sustainable resources a priority. Green technologies are emerging as a potential aid for developing eco-friendly processes to treat biomass for their conversion into value-added marketable products. New types of solvent systems such as ionic liquids (ILs), deep eutectic solvents (DESs), super critical CO2 (SC-CO2) and biomass-derived solvents are gaining attention for the efficient bioprocessing of natural resources. Due to their distinguished properties, they are promising solvent systems for blue biorefineries. This review summarizes the concept of blue biorefineries, the valuable resources available from the ocean, the marketable commodities developed using marine biomass and the potential of neoteric solvent systems for blue biorefineries.
Introduction
Environmental researchers have concluded since the 1970s that the overconsumption of minerals and fossil resources will lead to the depletion of non-renewable resources, jeopardising the future.1 Recent reports suggest that 84% of the global energy is contributed by fossil fuels/resources, where coal contributes 27%, natural gas 24% and oil 3%.2 They are the main causes for greenhouse gases, environmental pollutants, global warming and oceanic acidification. If alternative resources are not found, then fossil resources will be depleted with time and further pose dangerous consequences due to the increase in atmospheric CO2. Global climate change and the extensive depletion of fossil resources due to the increasing population emphasise the need to develop a bioeconomy based on carbon neutral resources that are renewable and sustainable.Biorefineries aim to increase the value of the end product after full utilisation and processing of biomass with zero effluent discharge. They facilitate the production of biobased products using low energy, few chemicals and minimal waste generation, providing green options through decarbonization pathways. They are competitive compared to the traditional fossil-based refinery products.3,4 In the context of future industrial revolution, environmental and economical sustainability will be considered, while adopting new technologies in biorefinery concepts. This will facilitate the processing of alternative feedstock such as organic and biomass waste, employing biological and chemical unit operations to provide a plethora of commercial products (fuels, chemicals and functional materials).5–7 A shift towards biorefineries will reduce the production of CO2 emissions,8–10 thereby developing an appropriate strategy for valorising waste biomass (agriculture, aquaculture, forestry, poultry, fisheries, etc.), and lead to new socioeconomic developments, ensuring carbon neutrality.3,11,12 The classification of biorefineries is based on the source of the feedstock employed (biomass source, waste source, etc.), the biocatalyst used (fungi, algae, yeast, bacteria, etc.), the technology used (photosynthesis, thermochemical, catalysis, methanogenesis, fermentation, acidogenesis, etc.), targeted products, and generation (first, second, third and fourth).3
Several challenges exist with current biorefineries for their implementation in the prevalent scenario (fossil based) such as the availability of feedstock, demand and supply for market requirements, competitive composition, recovery and recycle of resources, and techno-economic feasibility. Furthermore, the commercialisation of biorefinery processes faces several impediments such as the collection of feedstock (from cultivation, production, harvest and storage), cost of raw materials, processing, pre-treatment, valorisation, use of land, infrastructure, logistics, food security, state of the art in research, capital expenditure, development charges, quantity, quality, market price of products and existing biorefining technologies together with other ecological, technical, economical and societal challenges.12
However, notable success has been achieved in valorisation of land-based resources such as starch, vegetable oils, and woody biomass for the production of oils, fuels, and chemicals. Woody biomass mainly consists of hemicellulose, cellulose and lignin, which are processed in a biorefinery to achieve their separation and conversion into high-value commercial products.13,14 The biorefinery concept achieved a milestone with the establishment of the first cellulosic bioethanol plant by DuPont, Nevada in November, 2015 with 30 million gallons produced per annum.15
Similar concepts could be anticipated for the valorisation of oceanic-based feedstocks for the production of value-added chemicals and products. However, research in this area is limited and a huge amount of oceanic biomass still remains unexplored. The use of marine biomass for biorefineries will be a huge paradigm shift from the conventional land-based resources. Specifically, 71% of the Earth's surface is covered by water, which accounts for inexhaustible, vast, valuable and untouched natural resources. “Blue biorefinery” refers to the production of renewable chemicals, fuels and functional materials utilising biomass from oceanic sources such as macroalgae, sea animals, seaweed, fish, molluscs, and crustaceans.16 However, the huge potential of oceans to provide renewable sources of organic carbon, nitrogen, hydrogen and other elements as starting materials for fuels, chemicals and materials is still underrated.
The benefits of blue biorefinery over the conventional land-based biorefinery is that the majority of sea plants and fishery waste are not consumed as food, and hence it does not face ethical issues such as compromised food supply due to the production of fuels, chemicals and materials from these sources.16 Further, the progress in blue biorefineries will help reduce the land area constraints, which is a prominent issue in some countries. Due to climate change and the ever-increasing population, agricultural land is already under pressure to fulfil the high demands of food supply. Accordingly, the population will not have to rely only on land-based biomass for renewable sources if ocean-based feedstocks also contribute to renewable sources. Further, the ocean-based feedstocks have certain benefits over land-based resources such as need for less infrastructure, fast growth rate of biomass, less demanding growth conditions and rich bioactive compounds that are barely explored thus far.17
Green chemical technologies are being employed for the fabrication of useful products by exploiting biomass obtained from the ocean. Efforts in this direction and the rise in the utilisation of oceanic biomass for the production of marketable products that are competitive to petro-based products can help in reducing the land space crisis. Fig. 1 shows the valuable products obtained from oceanic resources. This review highlights the achievements and opportunities for the utilisation of oceanic biomass for the production of valuable products, meeting the market demands, while maintaining environmental sustainability.
 | ||
| Fig. 1 Valuable products obtained from marine resources such as algae, seaweed, fishes, and crustaceans. | ||
Renewable resources from the ocean
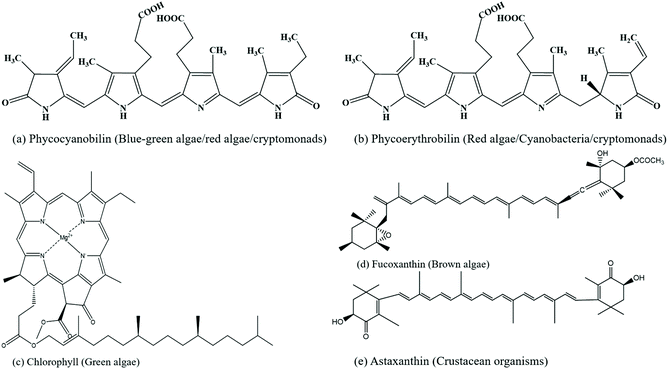
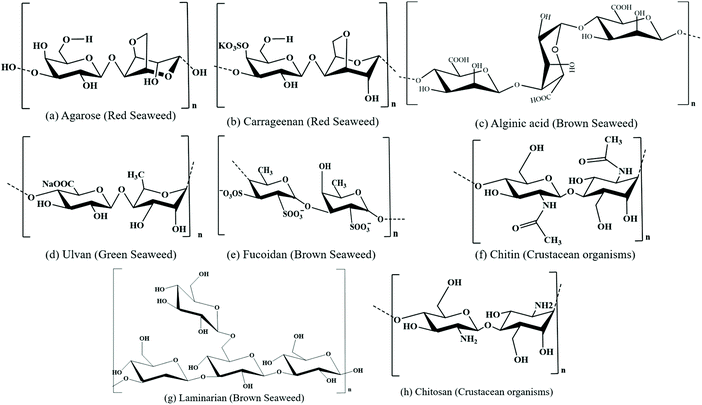
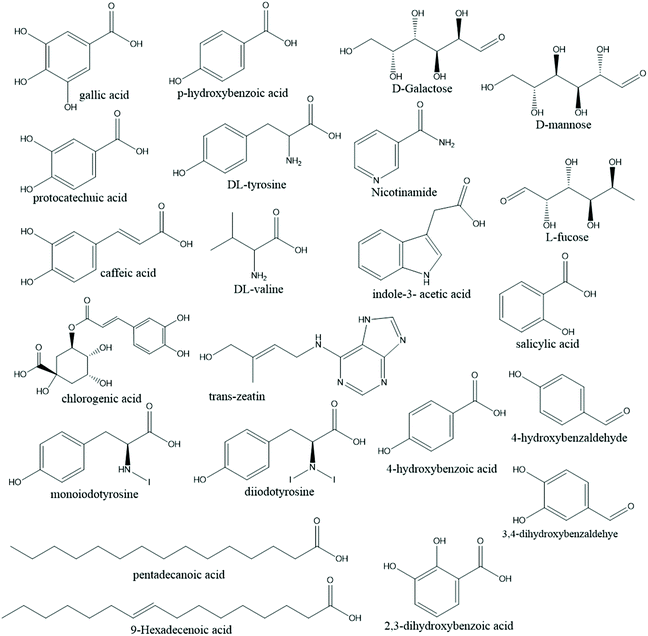
The top priorities in today's world are sustainability and renewability, where the bioeconomy is emerging as a new economic concept that outshines the traditional fossil-based models. It is automated with advanced innovations in biotechnology, ensuring resource-efficient processes, minimising waste, exploiting available resources for longer durations and adapting circular economic business models. This helps to strengthen the bridges that connect science, industry and society, further encouraging a better balance among socioeconomic, geopolitical and environmental issues. The bioeconomy utilises renewable biological resources from the terrestrial and aquatic ecosystems (crops, forests, and marine bioresources) and the term “blue bioeconomy” is generalised, where the origin, source, methods/processes and final end product are related to the aquatic ecosystem.18,19 Specifically, it is focused on the sustainable utilization of the ocean and its species to enhance and improve economic activities, while preserving them for future generations. The present and future global challenges can be addressed by the ocean as is has the potential to significantly contribute to the global economy through eco-sustainable technologies.With the commencement of biotechnology in algal research, cultivation of seaweeds and preservation of seaweed forests, eelgrass meadows are being employed, which enhance the sequestration of blue carbon from the atmosphere and oceans, further supporting biodiversity and providing key ecosystem services.20 Marine resources have plenty of valuable components such as pigments (phycoerythrin (phycoerythrobilin chromophore), phycocyanin (phycocyanobilin chromophore), allophycocyanin, chlorophyll, fucoxanthin, astaxanthin, etc.), polysaccharides (alginic acid, carrageenan, fucoidan, ulvan, chitin, cellulose, agar, etc.), and proteins. The chemical structures of some pigments and polysaccharides are shown in Fig. 2 and 3, respectively.
Several cosmeceuticals, pharmaceuticals, nutraceuticals, etc. that help in promoting the well-being and health of humans are produced using natural compounds obtained from marine sources.21 Marine-inspired biomaterials and medicines have found their place in the market (e.g., antimicrobial, antifungal, antibacterial, anticancer, and analgesic agents) and several marine molecules are getting validation in drug-discovery research.21
Seaweed farming is now prevalent in around 50 countries globally, including Japan, China, Indonesia, Philippines, and Korea.22 The major contribution of aquatic plant production comes from Indonesia, which increased from 6.7% in 2005 to 36.9% in 2014. Around 28.5 million tons of seaweeds is harvested for a number of purposes including human consumption. The global algal market was estimated to be $10–$12 billion in 2004.23 The annual global value for algal hydrocolloids such as alginic acid, carrageenan and agar is estimated to be $213 million, $240 million and $132 million, respectively. Antioxidants such as β-carotene produced from microalgae had a sale value of $392 million in 2010. The food color market in North America is expected to grow at a rate of 7.1% to reach at $441.4 million by 2020.24 Biotechnological research is being pursued in many developed and developing countries to switch to alternative energy generators such as biodiesel, bioethanol and biogas, which can be produced by marine feedstocks. These developments are expected to be commercialised by 2025.25 According to the report published in Grand View Research, Inc., the global market for algae biofuel is expected to reach 10.73 billion USD by 2025 with 8% CAGR, where the yield of algal biofuel is much higher (2–20 times) than that obtained from corn feedstock biofuel. Algal biofuel is expected to replace the existing fuels given that its demand will increase by 70% in 2025. The major players interested in this area are Reliance Life Sciences, Solazyme Inc., Algenol, Origin Oils Inc., Sapphire Energy, Proviron, Genifuels, Culture Biosystems, Solix Biofuels, Blue Marble Production, and Algae Production Systems.26 The hydrolysis of algal polysaccharides leads to the production of simple sugars, which are consumed by natural microbes and produce ethanol.27 Biofuel production mainly depends on different seaweeds such as Laminaria japonica, Ulva lactuca, Ulva pertusa, Sargassum fulvellum, and Gelidium amansii. Yeast of marine origin is also exploited for the production of bioethanol, pharmaceuticals and enzymes. Edible oils cannot be considered for the production of biofuels given that this may hinder the global food demand and supply. Hence, oils from waste or non-edible oils can make marine resources more attractive for large-scale production at cheaper rates.28,29 The enzyme market is also gaining interest with a CAGR of 5.7% from 2018 to 2024. Currently, researchers are focused on the isolation of enzymes exhibiting distinct activities from various marine microorganisms.30 Several marine enzymes such as tyrosinases, proteases, xylanases, peroxidases, agarases, amylases, and lipases are used for water treatment and in the nutraceutical/pharmaceutical sectors.31Aspergillus, Penicillium, Rhizopus, Clostridium, Vibrio fluvialis, Vibrio mimicus, Bacillus, etc. help in degrading marine polysaccharides such as chitin and chitosan.32 In addition to microbes, marine invertebrates and marine algae also have potential for the large-scale production of marine enzymes in industry. Proper identification, isolation, characterization and functionalisation of marine enzymes are essential to increase the market value of the end products.33 The functional food market will reach a CAGR of 7% by 2022 and marine organisms such as algae, fungi, bacteria, fish, sponges, molluscs, and crustaceans are a rich source of secondary metabolites and bioactive components.34 Functional foods include polysaccharides, pigments, minerals, vitamins, proteins, lipids and phenolic compounds, which have bioactivities such as antibacterial, anticoagulant, antiviral, anti-inflammatory, anti-obesity and anticancer activities. Marine algae and fish oils are good sources of poly-saturated fatty acids, which contribute in increasing the nutritional value of food products.35 Chitin and chitosan derived from crabs, shells, shrimp, cuttlefish, prawns, etc. have antibacterial and antiadipogenic activities.36 The exopolysaccharides and enzymes acquired from extremophiles present in deep oceans also have multiple applications.37 The nutraceutical market will reach up to 561.38 billion USD by 2023 at a CAGR of 6.8% from 2018 to 2023. These supplements can be given in the form of capsules, gels, powders, tablets and liquids based on their applications such as nutrition boosters (prebiotic and probiotic), herbals, phytochemicals, and dietary supplements. Several antimicrobial, antibacterial, antiviral, antiparasitic, anti-inflammatory, and analgesic drugs are obtained from marine resources, which can be further explored in the nutraceutical industry.38–40 The cosmetics market is recognized as a multibillion dollar industry, which will grow at a CAGR of 3.4% from 2019–2023. This industry is constantly working on developing beauty products derived from natural sources given that consumers demand natural, organic, environment friendly and ethical products that do not compromise their health. Several compounds extracted from marine organisms are utilized as excipients and additives in the cosmetics industry, which exhibit activities that provide photoprotection and moisturisation to the skin, prevent skin aging and enhance skin fairness.41,42 Nano-based technologies help increase the bioavailability, solubility, activity and efficiency of hydrophobic drugs. The applications are based on the surface, charge, functionality and size of the nanoformulation. Nanoparticles from marine origin are used in agricultural biotechnology for the production of fertilizers, insecticides, herbicides, pesticides, etc. Various drugs are designed using marine nanotechnology, where matter is manipulated the atomic or supramolecular level to inhibit diseases.43 The degradation of pollutants and contaminants is carried out by the action of cultured microorganisms, which is termed “bioremediation”. It is classified based on the microorganism, i.e., phycoremediation (algae), phytoremediation (plant) and mycoremediation (fungi). Recently, marine microorganisms have been studied for their tendency to remove heavy metal ions such as cadmium, chromium, lead, zinc and mercury.44–48 Sea lettuce (U. Lactuca) showed great efficiency in treating water effluents containing inorganic wastes.49 Globally, sites that are contaminated with hydrocarbons are treated by microbial remediation, which is a promising solution. The ocean is prone pollution from petroleum hydrocarbons, causing harm to the fragile and biodiversity-rich marine habitat. Due to their diverse resistance and degradation mechanisms, marine organisms have a potential for bioremediation. The tendency of nutrient assimilation by marine algae make them suitable for the production of biofuel and wastewater remediation.49
The ocean is a vast source of highly diverse and abundant species, which can provide valuable chemicals and drugs. With the successful development of efficient products, there is the possibility of the overexploitation of resources, which can harm the biodiversity.50 Hence, the judicious and sustainable use of marine resources is very important. The mass production of various valuable compounds can be achieved through various cultivation methods without compromising the marine ecosystem. Several desired metabolites can be synthesized in an industrial setup by genetic engineering based on the information on novel and potential genes present in marine microbes. Different types of cultivation methods can be employed for the rapid multiplication of marine organisms, including photobioreactors, raceway ponds, fermenters and open ponds. The blue biorefinery is an ecologically efficient and sustainable concept, which utilises complete biomass for the production of multiple metabolites.
Neoteric solvent systems: promising sustainable solvents for the biorefinery concept
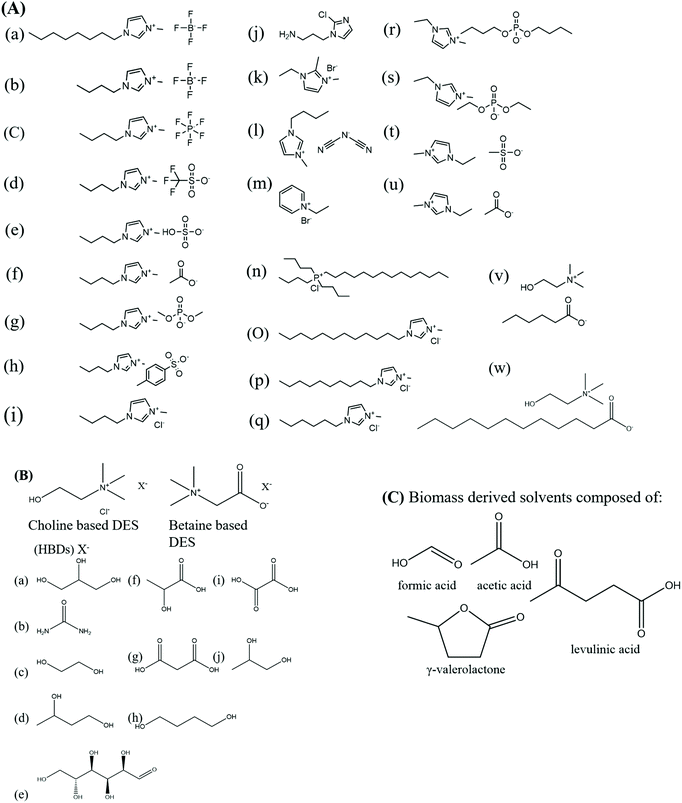
With the development of new technologies and advanced valuable products, new challenges need to be addressed. The blue biorefinery focuses on the development of products utilising natural marine resources especially sensitive biomolecules. However, care must be taken while handling these molecules given that they tend to degrade quickly and lose their structural and functional activities under harsh processing conditions. Solvents play an important role in the processing of biomolecules and must provide good solubility, while maintaining their native properties.51,52 Due to the rigid and complex structures of biomolecules, they are poorly soluble in conventional solvents, and thus proper screening of suitable solvents is necessary to provide stability. These molecules are temperature sensitive, light sensitive and pH sensitive, and thus their processing conditions need to be optimized accordingly. The organic solvents that are used exhaustively are hazardous, volatile, toxic and highly inflammable and produce huge amounts of volatile organic solvents (VOCs), questioning their environmental favourability.51 In considering the biorefinery concept, it is essential to focus on the sustainability aspect, which provides safety to workers and process/end-product safety. This demonstrates the need for mild, eco-friendly and green solvents that can conveniently process highly sensitive biomolecules, providing them with high stability and further providing solutions to pollution, energy consumption and environmental deterioration.53 Research attempts are now targeted towards the smart choice of solvents to develop advanced green technologies that can meet techno-economic demands, thus providing environmental stability. Researchers are focused on the judicious use of solvents to develop green technologies for the processing of biomolecules, which will match the techno-economic needs. To address this, solvents are ranked based on their environmental, safety, and health characteristics (ESH) by firms such as Pfizer, GSK, AstraZeneca, ACS Chemistry Institute Pharmaceutical Roundtable (GCI-PR) and Sanofi. This ranking is based on green chemistry metrics, which is a promising approach towards the development of novel sustainable technologies.54 Green solvents such as ionic liquids (ILs), deep eutectic solvents (DESs), super critical CO2 and solvents derived from natural resources (biomass derived) are finding use as “future solvents”.55 These types of new solvents were termed “neoteric solvents” by K. Seddon in 1996.56 These solvents have distinguished and unique properties such as thermal stability, low vapor pressure, low toxicity, non-volatility, air and water stability, recyclability and good solvent recovery, making them interesting for the processing industry and preparation of functional materials.51,57–64 The properties of neoteric solvents are attributed to their individual constituents, which can be tuned according to the process requirements. The solvent behavior needs to be understood at the molecular level, which can help to solve various process challenges.65–68ILs are defined as compounds composed entirely of ions and are in liquid state under 100 °C. They are regarded as designer solvents given that their physical attributes can be regulated by the combination of different sets of anions and cations, and hence they are also termed “task-specific” ILs.69,70 These systems have found extensive applications in the field of biotechnology as solvents, adjuvants, co-solvents, co-surfactants and reagents for biotransformation, biocatalysis, protein and DNA preservation and stabilization.71–74 Biopolymers show good solubility without self-aggregation, increased lifetime and stability in ILs, and their further recovery is also feasible using anti-solvents, filtration and centrifugation. The recovery rates for the products and ILs used in bioprocessing are high and these solvents also provide media for the refolding and crystallization of proteins.74 Several processes require the immobilization of biomolecules to maintain their secondary structures, and accordingly ILs, due to their high viscous nature, provide this type of medium.
Another type of neoteric solvent is deep eutectic solvents (DESs), which are defined as eutectic mixtures of Lewis or Brønsted acids and bases.75 They are composed of hydrogen bond donors (HBDs) and hydrogen bond acceptors (HBAs). The HBDs are amines, amino acids, alcohols, sugars and carboxylic acids, whereas quaternary ammonium salts act as the HBAs. The first DES was reported in 2003 by Abbot et al. in 2003, which was comprised of urea with a melting point of 133 °C and choline chloride having a melting point of 302 °C. The resulting eutectic mixture was liquid at room temperature with a melting point of 12 °C, which had a much-reduced melting point compared to the starting materials. The formation of a DES was attributed to the hydrogen bonding and van der Waals forces between the HBDs and HBAs.76 HBAs can shield the charges on certain HBDs, and hence a DES is formed, which has similar properties to that of ILs and are considered to be their analogs or fourth-generation ILs. Due to their simpler preparation methods and cheaper starting materials, DES are considered to be more cost effective compared to ILs. Nowadays, DES are prepared from starting components of natural origin, making them non-toxic, sustainable, economical and environmentally viable.77–79 These solvents exhibit huge depression in freezing points and are also recently considered for human consumption when they are composed of choline and sugars, making them potential candidates for application in the biomedical field. Most DESs have a melting point less than 150 °C, but those with a melting point less than 50 °C are desirable given that the processing conditions will be comparatively safe and cheap. The DESs with higher melting points require elevated temperatures given that they tend to solidify at room temperature, limiting their applications as green solvents.77,79
The concept of natural deep eutectic solvents (NaDESs) was introduced for the first time in 2011 by Choi et al., where DESs were synthesized using natural components such as choline, sugars, amino acids, and urea.80 These solvents, due to their natural origin, are biodegradable, biocompatible, pharmaceutically acceptable, non-toxic and also exhibit a spectrum of polarity, low melting temperatures, minimal vapor pressure and high solubility towards biopolymers such as cellulose, amino acids, DNA, and proteins.81
Several solvents can also be obtained from biomass through fermentation, esterification or enzymatic processes, and these green solvents are termed “biomass-derived solvents”. They include furfural, lactic acids, levulinic acid, fatty acid esters, hydroxymethylfurfural and their esters, glycols, terpenes alcohols of low molecular weight and glycerol derivatives.82 These solvents derived from renewable resources present a wide spectrum of applications for example levulinic acid and its derivatives are used as adjuncts for flavours, antimicrobials, gasoline, fragrance, etc.83,84 Lactates are used for the synthesis of plastic and pharmaceutical.85 Glycerol is applied in the pharmaceutical, nutraceutical, cosmetics, and food industries and source of designer solvents such as DES/ILs.86 Furfural is used in production of polymers, pharmaceuticals and fuels.87 NADESs prepared from glycol, glycerol, choline chloride, alcohols, lipids, sugars, etc. are used as extraction, reaction or chromatographic media for biomedical applications.88 They are also exploited as nontoxic cryoprotective agents.89 These biomass-derived solvents also follow the green metrics and exhibit properties expected from green solvents such as biodegradability, recyclability, high boiling point, low vapour pressure, high dissolution capacity, no toxicity to human health and the environment, and low cost given that they are derived from natural renewable sources. However, a complete evaluation of their chemical, physical, toxicological and safety properties is necessary to evaluate their green nature.90
A new type of solvent system also includes supercritical fluids such as CO2, which are sustainable for the Earth's atmosphere given that they contribute to “clean technologies”, where no secondary products are generated.91 A supercritical fluid is a single phase that occurs when held above its critical temperature and pressure. Among them, supercritical CO2 (SC-CO2) is of major interest because of its moderate critical temperature (31.1 °C) and pressure (73.8 bar). It has found applications in material processing as a solvent, solute, anti-solvent and reaction medium.92 Carbon dioxide is inexpensive, inert, non-flammable, non-toxic and easily available in large quantities as an industrial by-product. Hence, SC-CO2 has found interest as an environmental benign solvent that can avoid the use of VOCs such as benzene, chlorofluorocarbons (CFCs), and CCl4 used as conventional solvents. The CO2 used can be recycled and the process is energy efficient.93 This solvent system is generally recognized as a safe (GRAS) solvent, and hence food-grade products obtained using it are safe for human consumption.94 Furthermore, global issues such as greenhouse gas emissions and global warming can be addressed by supercritical CO2 (SC-CO2) processing plants, which can trap CO2 and create new potential applications, avoiding its release in the environment.95
Currently, neoteric solvents are extensively used in materials science, as is evident from the increasing number of publications in this field. As mentioned earlier, the extraction of biomolecules from natural resources is a difficult task and utmost consideration must be given to parameters such as temperature, light, solid liquid ratio (SLR), pH, extraction time, solvent-biomass agitation and concentration of solvent.96 Accordingly, due to the tuneable properties of neoteric solvent systems, they can maintain the required parameters from the initial steps such as cell lysis to facilitate high dissolution, maintaining pH through self-buffering ability, to the final step of enhanced product recovery.
They have been proven as potential processing media for the processing of polysaccharides, proteins, pigments, nucleic acids, lipids, antibiotics, alkaloids, amino acids, etc. without compromising their functionality and activity.51,52,57–61,63,64,68 Neoteric solvent systems act as partitioning media and provide solutions to deal with challenges such as the isolation of bioactive molecules from aqueous media. Considering industrial processes, it critical that the products are isolated with high purity and are cheap given that 40–50% of the total production expenses depend on the separation techniques. It is difficult to detect the trace amounts of hydrophilic bioactive compounds that are present in biological systems using the conventional analytical methods, and hence neoteric solvents are emerging as a new platform to deal with this problem.97 These solvent systems are now being considered as promising media for extraction, functionalization and as additives in the therapeutic industry to meet analytical demands.98–102Fig. 4 highlights the different types of neoteric solvents and their promising applications.
As mentioned earlier, the advantages of green solvents make them promising alternative solvent systems for the efficient processing of marine renewable resources to facilitate biorefineries. Innovative technologies can be incorporated with new solvent systems to enable the production of value-added bio-products, co-products and biofuels with minimum waste generation. They may further reduce unit operations, facilitating robust and safe controlled processes.
Neoteric solvents have gained recognition as topic of interest among green chemists globally due to their distinguished properties and plethora of applications. However, it must be noted that all neoteric solvents cannot be generalized as green. Besides the merits of these types of solvent systems, they have several drawbacks that also need to be addressed. Recently, Mu et al. proposed 13 different strategies to tackle the problems associated with ILs and DESs to provide a new viewpoint on the greenness of these solvents, making them feasible for industrial applications.103 Similarly, supercritical fluids have benefits such as they facilitate high diffusivity, providing faster extraction and also protection against degradation for labile molecules due to their lower operating temperatures. However, the main limitation of the expensive investment for equipment setup needs to be considered when applying this technique in bio-refineries.104
Neoteric solvents for value-added products from oceanic biomass using the biorefinery concept
Polysaccharides are interesting biomolecules present in seaweeds. Agarose present in red algae was extracted using 1-ethyl-3-methylimidazolium acetate ([C2C1im][C1CO2]) with the assistance of microwaves. Agarose with high purity was precipitated using methanol as an antisolvent.109 In 2015, Sharma et al. explored some choline-based bio-ionic liquids for the selective precipitation of agarose from a hot seaweed extract of the red seaweed Gracilaria dura under ambient reaction conditions, as shown in Fig. 5A. Among the studied ionic liquids, choline laurate exhibited the best result with 14% w/w yield of agarose with properties required for molecular biological applications and gel electrophoresis. All the ILs were also studied for their recyclability and could be recycled for three consecutive batches of agarose precipitation.110 Fucoidan and laminarian were extracted by solid-phase extraction (SPE) using packing materials modified by DES and ILs. The best results were evident for choline chloride/urea DES with high extraction efficiencies of 95.5% fucoidan and 87.6% laminarian from marine kelp. Modification of polymers by DESs and ILs showed better results for SPE and can be utilized for the sample treatment of analytes.111 These polysaccharides were also extracted from brown algae by SPE using modified magnetic graphene oxide employing an imidazolium-based IL (1-(3-aminopropyl)imidazole chloride). The extraction efficiency varied with the amount of ILs used for modification, SLR of brown algae, and agitation time. Fucoidan and laminarian yields of up to 93.3% and 87.2% were achieved, respectively.112 Similarly, κ-carrageenan found in red marine algae from the Solomon Islands was extracted using 1-butyl-3-methylimidazolium acetate ([C4C1im][C1CO2]) via subcritical water extraction of algal biomass. A remarkably high purity of polysaccharides was obtained in this novel extraction method compared to the conventional methods.113 Six cholinium-based bio-ionic liquids were employed for the selective coagulation of κ-carrageenan from Kappaphycus alvarezii seaweed extract. The bio-ionic liquid-promoted selective precipitation from the seaweed extracts is shown in Fig. 5B. Among them, choline caproate and choline laurate selectively coagulated the polysaccharide by up to 14.8%. Considering the selective binding of ILs with targeted polysaccharides, they can be a potential alternative for down-stream processing of carrageenophytes.61
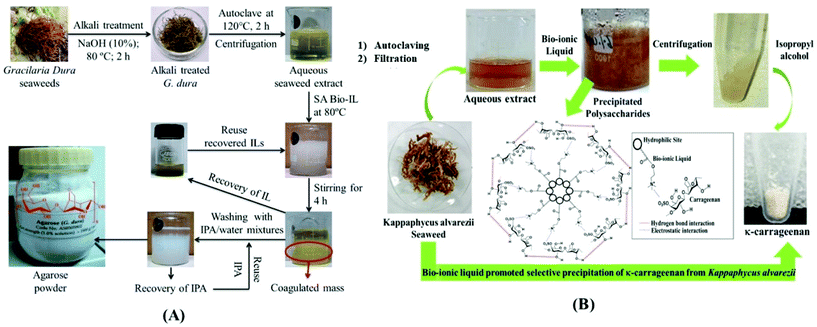 | ||
| Fig. 5 (A) Selective precipitation of agarose using bio-ionic liquid (reproduced from ref. 110 with permission from The Royal Society of Chemistry). (B) Bio-ionic liquid-promoted selective precipitation from seaweed extracts (reproduced from ref. 61 with permission from Elsevier). | ||
Pure ILs were used for the extraction of the polysaccharides in the above-mentioned cases, but this should be avoided as it may limit the process performances due to the high viscosities of ILs. Specifically, this limits the thermal transfer and agitation, thereby increasing the energy consumption and leading to expensive processing conditions. Hence, IL-based aqueous solutions are gaining interest, where even a small amount of IL enhances the extraction efficacy of a process, which can be more efficient for marine biorefineries. Furthermore, κ-carrageenan from Kappaphycus alvarezii was selectively extracted using three different types of DES comprised of cholinium cation with a combination of hydrogen bond donors such as urea, ethylene glycol and glycerol. Also, they were studied in their hydrated form for the extraction of the polysaccharide. The physicochemical and rheological properties of carrageenan obtained using DES as the solvent were the same as that obtained using conventional extraction. The hydrated DES performed better compared with the pure DES, and this can be considered as alternative solvent systems for the facile extraction of polysaccharides directly from seaweed.114
Monosaccharides (D-(þ)-galactose and L-(−)-fucose) and amino acids (DL-tyrosine and DL-valine) were extracted from kelp using air-assisted dispersive liquid–liquid microextraction (AA-DLLME) in the presence of hydrophilic–hydrophobic DESs.115Thallus laminariae (kelp) has high concentrations of minerals and vitamins and is consumed as a super food. Determination of the nicotinamide quantity in kelp was studied from the extracts obtained under ultrasound-assisted extraction in the presence of ILs, where 98.05% to 99.51% of nicotinamide was recovered in the process.116 NADES were exploited to extract biologically active compounds from the brown algae Fucus vesiculosus and Ascophyllum nodosum. Polyphenols were extracted exploiting 10 different NADES composed of components such as choline chloride, betaine, lactic acid and glucose with different mole ratios. Hydrated NADES were also studied for the extraction of phlorotannins. The results were comparable with that obtained using the conventional solvents Me2CO and ethanol.117 Several plant growth hormones such as trans-zeatin and indole-3-acetic acid were extracted using imidazolium-based ILs and their cationic and anionic interaction with the targeted molecule was studied. 1-Butyl-3-methylimidazolium hexafluorophosphate ([C4C1im][PF6]) efficiently showed higher yields such as trans-zeatin (65%) and indole-3-acetic acid (18%).118
Marine algae rich in pigments were exploited in the presence of IL-based aqueous solutions to recover highly expensive pigments, namely, phycobiliproteins from red marine algae. These are fluorescent pigments that capture light for photosynthesis in red algae. Cholinium-based ILs were successful in extracting phycobiliproteins by more than 46.5% compared to conventional techniques. Multi-products from single biomass are always desirable when considering marine biorefineries. The simultaneous extraction of hydrophobic and hydrophilic biomolecules (chlorophyll and phycobiliproteins) was achieved by Martins et al. in 2016 by tuning the properties of ILs (changing their alkyl chain length) without compromising their structural integrity.119 R-Phycoerythrin (R-PE) is a red pigment from the class of phycobiliproteins, which is abundant in Porphyra yezoensis. This protein–pigment complex has a high market value and was purified using a choline chloride-urea (ChCl-U) DES-ATPS (deep eutectic solvent aqueous two-phase system) combined with ammonium sulphate precipitation. The purity index obtained was 3.825 with a yield of 69.99% (w/w) and the results illustrated that this study provides a simple and green purification method for drug development using R-PE.120
Aqueous solutions of surface-active ILs were explored for the extraction of carotenoids especially targeting the hydrophobic pigment named fucoxanthin, which is mainly found in brown macroalgal species. Pyridinium and ammonium-based tensioactive ILs were compared with the conventional solvent ethanol.121 An integrated process was developed for the efficient separation and purification of chlorophyll a and xanthophyll found in the cyanobacteria Spirulina sp. using ILs and surfactants. Solid–liquid extraction was performed, and the results obtained were compared to that obtained using conventional organic solvents. The aqueous solutions of ammonium-based ILs could extract 25% more chlorophyll a compared with the conventional technique. Chlorophyll a was separated and purified further from xanthophyll using liquid–liquid extraction. Fig. 6 shows a schematic of the integrated process for the extraction and purification of chlorophyll a and xanthophylls. These bioactive compounds retained their ability to generate oxygen singlets and have potential applications as photosensitizers in photodynamic therapy.122
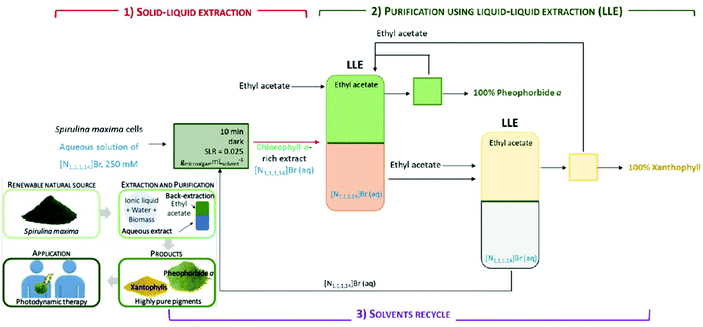 | ||
| Fig. 6 Schematic of the integrated process for the extraction and purification of chlorophyll a and xanthophylls (reproduced from ref. 122 with permission from the American Chemical Society). | ||
Chlorophyll was extracted from wild-harvested Ulva spp. using aqueous solutions of tensioactive compounds such as ILs and surfactants. The operational conditions of the extraction process were optimized and the product obtained was 5.96 mg g−1 of dry algae in the case of extraction in the presence of 250 mM of tributyltetradecylphosphonium chloride ([P4,4,4,14]Cl). This developed process was cost-effective and could maintain the stability of the final product for up to one month.123 Fucoxanthin and chlorophyll were extracted and purified from the brown macroalga Saccharina latissimi (Linnaeus) using IL, oil and water systems. The best results were obtained for the aqueous solution of tensioactive phosphonium-based IL at 350 mM and 16% sunflower oil with an SLR of 0.017 g dry biomass per mL solvent. A conceptual diagram of the process for chlorophyll and fucoxanthin recovery from S. latissima (Linnaeus) is shown in Fig. 7. The environmental and economic aspects of the procedure were evaluated and the solvents were recycled in the process.124
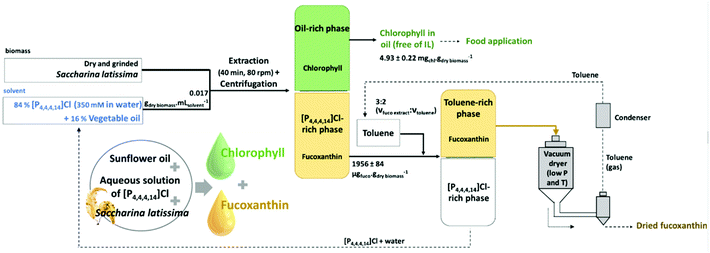 | ||
| Fig. 7 Conceptual process for the recovery of chlorophyll and fucoxanthin from S. latissima (Linnaeus) using sunflower oil and aqueous solution of IL (reproduced from ref. 124 with permission from the American Chemical Society). | ||
Ammonium-, phosphonium- and imidazolium-based ILs were used for the recovery of proteins (40% (w/w)) from the green seaweed Ulva lactuca via solid–liquid extraction. Aqueous biphasic systems and alkaline extraction assisted by mechanical agitation were also employed for efficient extraction. Consequently, 80.6% of the total proteins was recovered by 1-ethyl-3-methylimidazolium dibutyl phosphate ([C2C1im][(C4)2PO4]).125 Several organic and inorganic iodine compounds such as diiodo-tyrosine and I-monoiodo-tyrosine have been reported to be extracted from the genus Laminaria. These molecules are crucial micronutrients for human and animal health, which are usually extracted using toxic and strong alkaline solvents. In search of alternative solvents, numerous ILs have been screened and employed under ultrasonication to recover these biomolecules. A recovery yield of up to 88% was achieved using the pyridinium-based IL, namely, ethylpyridinium bromide ([C2Py]Br) at pH 6.5 in only 30 min.126
A cholinium-based DES, namely, choline chloride–oxalic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), was studied for the extraction of metals from the macroalgae Enteromorpha intestinalis. The biomass was treated under microwave digestion in the presence of the DES and compared with acid digestion (conventional process). Almost similar results were obtained in both cases, but the extraction time was reduced by 100 times in the case of the DES, thereby leading to reduced energy consumption.127 Polycyclic aromatic hydrocarbons were also extracted using the same species and the same DES by Helalat-Nezhad et al. in 2015. However, better recovery yields with lower temperatures and simpler steps compared to existing methods were achieved.128
2), was studied for the extraction of metals from the macroalgae Enteromorpha intestinalis. The biomass was treated under microwave digestion in the presence of the DES and compared with acid digestion (conventional process). Almost similar results were obtained in both cases, but the extraction time was reduced by 100 times in the case of the DES, thereby leading to reduced energy consumption.127 Polycyclic aromatic hydrocarbons were also extracted using the same species and the same DES by Helalat-Nezhad et al. in 2015. However, better recovery yields with lower temperatures and simpler steps compared to existing methods were achieved.128
Studies have revealed that the combination of different mechanical extraction techniques such as microwave,109 ultrsonication126 and subcritical water extraction107,113 with neoteric solvent extraction can lead to better extraction efficiency and also enhancement of the antioxidant activity exhibited by phenolic compounds.107 Pure solvents were employed earlier for biomass processing, but this trend has been decreasing and pure solvents are now being replaced by their respective aqueous solutions, where a small amount of solvent is sufficient for processing.107,113,119,121,126 The use of aqueous solutions of solvents can facilitate better biomass agitation with lower viscosity and provide higher product yields, while maintaining economic and environmental aspects.105,129 It is beneficial to always reduce and recover the solvents during processing, but there are still many gaps considering the reuse/recycle of solvents. Trivedi et al. and Sharma et al. reported the reuse of the solvent for up to four cycles without compromising the yield and product in the extraction of agarose.109,110Fig. 8 shows a general scheme of the extraction and purification of renewable chemicals/products from oceanic biomass. Table 1 present a summary of the literature work dealing with the extraction of value-added compounds from marine resources using neoteric solvent systems.
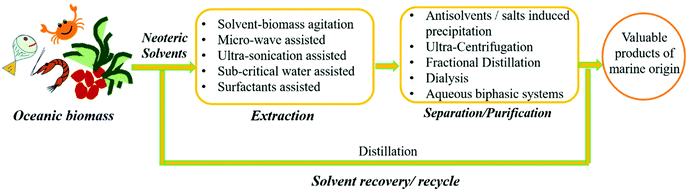 | ||
| Fig. 8 General scheme for the extraction and purification of renewable chemicals/products from oceanic biomass. | ||
| Marine resource | Compounds of interest | Solvent used | Operational parameters | Yield | Ref. |
|---|---|---|---|---|---|
| Laminaria japonica Aresch | Phenolic compounds | Imidazolium-based ILs | Ultrasonic assisted with [C4C1im][BF4], 200 W, 60 min, pH 1.25 | 88.3% recovery | 106 |
| Saccharina japonica | Antioxidants (gallic, protocatechuic, caffeic, p-hydroxybenzoic and chlorogenic acids) | [C4C1im][BF4] (0.25–1.00 M in water) | Subcritical water extraction with [C4C1im][BF4] at 0.5 M; 175 °C | Gallic, chlorogenic, protocatechuic, p-hydroxybenzoic, and caffeic acids were 7.33-, 154.9-, 572.8-, 54.8-, and 91.8-fold higher than the conventional solvent (water) | 107 |
| Saccharina japonica | Polysaccharides (fucoidan and alginate) | Choline chloride-based DES | Subcritical water assisted-DES system at 150 °C, 19.85 bar, 70% water content and S/L ratio of 36.81 mL g−1 | 28.12% alginate and 14.93% fucoidan | 108 |
| Gracilaria dura | Agarose | [Emim][OAc]; 1-ethyl-3-methylimidazolium diethyl phosphate, 1-ethyl-3-methylimidazolium acetate, [Emim][Dep] and choline acetate [Ch][OAc] | The mixture containing (algae + [Emim][OAc]) was stirred at 2 h at 80/100 °C or MW treatment at 3 s pulse for 2 min | 39 wt% | 109 |
| Gracilaria dura | Agarose | Choline-based bio-ionic liquids | Alkali-treated extracts treated with choline laurate at 80 °C | 14 wt% | 110 |
| Marine kelp | Polysaccharides (fucoidan and laminarian) | DES and ILs | Solid-phase extraction (SPE) | 95.5% fucoidan and 87.6% laminarian | 111 |
| Brown algae | Polysaccharides (fucoidan and laminarian) | Imidazolium-based IL (1-(3-aminopropyl) imidazole chloride) | SPE | 93.3% fucoidan and 87.2% laminarian | 112 |
| Solomon island red seaweed Kappaphycus alvarezii | κ-Carrageenan | 1-Butyl-3-methylimidazolium acetate ([C4C1im][C1CO2]) | 1% 1-butyl-3-methylimidazolium acetate (BMIMAc) at 150 _C/5 MPa with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 g mL−1 80 g mL−1 |
78.75 wt% | 113 |
| Kappaphycus alvarezii | κ-Carrageenan | Cholinium-based bio-ionic liquids | Choline caproate Choline laurate | 14.8 wt% | 61 |
| Kappaphycus alvarezii | κ-Carrageenan | Cholinium-based DES with hydrogen bond donors urea, ethylene glycol and glycerol | Powdered seaweed in 10 g DES at 85/95 °C for 1 h | 60.25 wt% | 114 |
| Kelp | Monosaccharides (D-(þ)-galactose, L-(−)-fucose) and amino acids (DL-tyrosine, DL-valine) | Hydrophilic and hydrophobic DESs | Air-assisted dispersive liquid–liquid microextraction (AA-DLLME) | D-(+)-Galactose, L-(−)-fucose, DL-tyrosine, and DL-valine in kelp were 16.7 ± 0.2, 8.6 ± 0.2, 2.6 ± 0.1, and 1.6 ± 0.1 mg g−1 | 115 |
| Thallus laminariae (kelp) | Nicotinamide | 1-ethyl-3-methylimidazolium mesylate, 1-ethyl-2,3-dimethylimidazolium bromide, and 1-propyl-3-methylimidazolium tetraborofluorate | Ultrasound-assisted extraction in the presence of ILs | 98.05% to 99.51% recovery | 116 |
| Brown algae Fucus vesiculosus and Ascophyllum nodosum | Polyphenols/Phlorotannins | Natural deep eutectic solvents (NaDESs) composed of choline chloride, betaine, lactic acid and glucose | Maceration for 120 min at 50 °C with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 raw-material 5 raw-material![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) extractant ratio extractant ratio |
60–72% recovery | 117 |
| Kappaphycus alvarezii | Plant hormones indole-3-acetic acid trans-zeatin | Imidazolium-based ILs; [C4C1im][PF6], [C8C1im][BF4], [C4C1im][NTf2] | 1-Butyl-3-methylimidazolium hexafluorophosphate ([C4C1im][PF6]); Agitation (450 rpm) at 25–50 °C, 5–120 min, SLR 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Recovery of trans-zeatin (65%) and indole-3-acetic (18%) acid | 118 |
| Gracilaria sp. (fresh and grinded) | Phycobiliproteins | [C2C1im]Cl, [C4C1im]Cl, [C6C1im]Cl, [C10C1im]Cl, [C12C1im]Cl, [C2C1im][C1CO2], [C4C1im][N(CN)2], [C4C1im][CF3SO3], [C4C1im][C1CO2], [C4C1im][(C1)2PO4][C4C1im][TOS], [C4C1im][CH3SO3], [C4C1im][SCN], [C4C1im][CF3CO2] and [C2C1im][C1CO2] | Ionic liquid-based extraction 1 M [N1,1,1,2OH]Cl in McIlvaine buffer (pH 5.9) for 20 min | 46.5% recovery of phycobiliproteins, higher than conventional method | 119 |
| Porphyra yezoensis | Pigment; R-phycoerythrin (R-PE) | DES; choline chloride–urea (ChCl–U) | Choline chloride–urea (ChCl–U) DES-ATPS (deep eutectic solvent aqueous two-phase system) combined with ammonium sulphate precipitation | 69.99% (w/w) recovery | 120 |
| Sargassum muticum (fresh and dried, both grinded) | Pigment; carotenoids; fucoxanthin | Surface-active ILs; Ammonium and pyridinium-based [N1,1,1,14]Br, [N1,1,1,16]Br, [C16py]Cl, [C16py]Br, AOTa, SDBSa, SDSa (1–30_ CMC in water) | Fresh algae; SDSa at 15_CMC; 90 min; Agitation (250 rpm) at room temperature, 73–107 min, SLR 0.04 | 37.4% higher than conventional | 121 |
| Spirulina sp. | Pigment; chlorophyll a and xanthophyll | Combination of ILs and surfactants | A solid–liquid ratio (SLR) of 0.025 g dry biomass per mL solvent at temperature (25 °C) and stirring (50 rpm) for 30 min in an orbital mixer. Concentration of ILs was 250 mM | 25% greater recovery of chlorophyll a compared to conventional method | 122 |
| Ulva spp. | Pigment; chlorophyll | aqueous solutions of tensioactive compounds such as ILs and surfactants | 250 mM of tributyltetradecylphosphonium chloride ([P4,4,4,14]Cl) in aqueous solution for 30 min with an SLR of 0.01 g biomass per mL solvent | 5.96 mg g−1 of dry algae | 123 |
| Saccharina latissimi (Linnaeus) | Pigment; fucoxanthin and chlorophyll | Tensioactive phosphonium-based ILs, oil and water systems | Aqueous solution of tensioactive phosphonium-based IL at 350 mM and 16% sunflower oil with SLR of 0.017 g dry biomass per mL solvent | Chlorophyll and fucoxanthin of 4.93 ± 0.22 mgchl per g drybiomass and 1956 ± 84 μgfuco per g drybiomass, respectively | 124 |
| Ulva lactuca | Proteins | Ammonium-, phosphonium- and imidazolium-based ILs N1,1,1,2OH][C1CO2], [C4C1im][C1CO2], [C2C1im][(C4)2PO4], [C4C1im][(C4)2PO4], [C4C1im]Cl, [N1,1,1,2OH]Cl, [P6,6,6,14]Cl, [P4,4,4,1][MSO4], [C4C1im][N(CN)2], and [P6,6,6,14][N(CN)2] (40% (w/w)) | Mechanical agitation and alkaline extraction with 1-ethyl-3-methylimidazolium dibutyl phosphate ([C2C1im][(C4)2PO4]). Tissue homogenizer and beads beaten for 3 cycles at 6500 rpm for 60 s, with break of 120 s between cycles | 80.6% recovery | 125 |
| Laminaria | 1-Monoiodo-tyrosine and diiodo-tyrosine | [C2C1im][L-L], [C2C1im]Br, [C4C1im][CH3SO3], [C4C1im][BF4], [C6C1im]Br, [C2C1mo]Br, [C2py]Br, [C2C1pip]Br, [C4C1pyr]Br, [C12C1im][NO3], [C12C1im][HSO4], [C12C1im]Br, and [C12C1im]Cl (100–300 mM in water) | Ethylpyridinium bromide ([C2Py]Br) at pH 6.5; 30 min. Ultrasonic-assisted (100 W, 40 kHz) at 40 °C, 15–75 min, SLR 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
88% recovery | 126 |
| Enteromorpha intestinalis | Metals Cu, Fe, Ni, and Zn | Cholinium-based DES; choline chloride–oxalic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Biomass was treated under microwave digestion in the presence of DES and compared with acid digestion | Results similar to conventional method | 127 |
| Enteromorpha intestinalis | Polycyclic aromatic hydrocarbons | Cholinium-based DES; choline chloride–oxalic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Samples were dissolved at atmospheric pressure in ChCl–Ox (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) at 55 °C for 30 min 2) at 55 °C for 30 min |
Recoveries from spiked marine fish and macroalgae samples were in the range of 71.6% to 109.6% | 128 |
Several valuable bioactive compounds have been extracted from algae using supercritical fluids.130 Volatile oil from Dictyopteris membranacea131 and Dilophus ligulatus,132 fatty acids from Sargassum hemiphyllum,133 fucosterol from Lessonia vadose,134 fucoxanthin from Sargassum muticum,135 fucoidan from Undaria pinnatifida,136 fatty acids and their esters, phenols, and sterols from Gloiopeltis tenax,137 fucoidans from Fucus evanescens, Saccharina japonica, and Sargassum oligocystum,138 lipids from Hypnea charoides,139 halogenated monoterpenes from Plocamium cartilagineum140 and separation of cis–trans geometrical isomers of β–carotene from Dunaliella bardawil.141 Curato et al. studied the antifungal activity of crude extracts utilising supercritical CO2 (SC-CO2) extraction from two brown seaweeds (Laminaria digita and Undaria pinnatifida) and three red seaweeds (Porphyra umbilicalis, Eucheuma denticulatum and Gelidium pusillum) against three postharvest pathogens (Botrytis cinerea, Monilinia laxa and Penicillium digitatum). Twenty fatty acids, three polysaccharide (laminarians, fucoidan and alginate) and phlorotannins (fucols, phlorethols, eckols, fuhalols and fucophlorethols) were quantified from the obtained extracts.142 Similarly, essential oil was extracted from the brown seaweed Undaria pinnatifida using the SC-CO2 fluid extraction technique. The optimal extraction was observed at 45 °C and 20 MPa, with the extracts showing high anti-inflammatory activity. These studies were consistent with the claims that Undaria pinnatifida can be used as a remedy for inflammation-related symptoms.143 The antioxidant activity of red seaweed Gracilaria mammillaris was examined using the seaweed extracts obtained using SC-CO2 with ethanol as a co-solvent. The results indicated that red seaweed extract can be a promising antioxidant.144 The cytotoxicity of the extract from Posidonia oceanica and Zostera marina leaves obtained by SC-CO2 extraction was studied. The extracts were rich in active compounds such as phenylpropanoids (chicoric, p-coumaric, rosmarinic, benzoic, ferulic and caffeic acids). These extracts proved to be more effective than that from the conventional Soxhlet method, and hence can be utilised as supplements for preventive purposes.145 Oil from the brown seaweeds Saccharina japonica and Sargassum horneri was extracted using SC-CO2 with ethanol as a co-solvent and the results were compared with that from the conventional process. The phenolic content and antioxidant activity of the extracts extracted by SC-CO2 were high in oil, demonstrating that it is a better method for obtaining valuable compounds from brown seaweeds.146Saccharina japonica was also exploited using SC-CO2 in the presence of sunflower oil, soyabean oil, canola oil, ethanol and water as co-solvents for the extraction of fucoxanthin, phlorotannin and carotenoids. The sunflower oil-assisted extraction method showed a higher fatty acid content with increased antioxidant activity and oil stability.147 The response surface methodology was used to understand the effect of pressure and temperature on the SC-CO2 extraction of natural dye from Sargassum sp. The process parameters were found to have significant effects on the extraction yields and the highest yield achieved was 2.7 mg-extract per g-dried sample, which contained bioactive compounds, namely, sterols, pentadecanoic acid, 14-methyl ester, 9-hexadecenoic acid, methyl ester and phytol with antimicrobial properties.148Table 2 presents a summary of the value-added compounds from marine resources using supercritical CO2 extraction.
| Marine resource | Compound of interest | Solvent used | Operational parameters | Ref. |
|---|---|---|---|---|
| Dictyopteris membranacea | Volatile oil | SC-CO2 | 40 °C; 9.1 MPa | 131 |
| Dilophus ligulatus | Volatile oil | SC-CO2 | 35–55 °C; 8.0–25.0 MPa | 132 |
| Sargassum hemiphyllum | Fatty acids | SC-CO2 | 40–50 °C; 24.1–37.9 MPa | 133 |
| Lessonia vadosa | Fucosterol | SC-CO2 | 50 °C; 18.0 MPa | 134 |
| Sargassum muticum | Fucoxanthin | SC-CO2 | 55 °C; 40.0 MPa | 135 |
| Undaria pinnatifida | Fucoidan | SC-CO2 | 40 °C; 40.0 MPa | 136 |
| Gloiopeltis tenax | Sesquiterpenes, fatty acids and their esters, phenols and sterols | SC-CO2 | 45 °C; 30.0 MPa | 137 |
| Fucus evanescens, Saccharina japonica, Sargassum oligocystum | Fucoidans | SC-CO2 | 60 °C; 55.0 MPa | 138 |
| Hypnea charoides | Lipids | SC-CO2 | 40–50 °C; 24.1–37.9 MPa | 139 |
| Plocamium cartilagineum | Halogenated monoterpenes | SC-CO2 | 40–100 °C; 25.0–40.0 MPa | 140 |
| Dunaliella bardawil | Separation of cis–trans geometrical isomers of β-SC-CO2carotene | SC-CO2 | 40 °C; 44.8 MPa | 141 |
| Brown seaweeds; Laminaria digita and Undaria pinnatifida Red Seaweeds; Porphyra umbilicalis, Eucheuma denticulatum and Gelidium pusillum | Fungicides | SC-CO2 | Pressure = 37.9 MPa, density = 0.701 g mL−1, temperature = 50 °C and flow rate = 34 kg h−1 | 142 |
| Undaria pinnatifida | Essential oil | SC-CO2 | 45 °C and 20 MPa | 143 |
| Gracilaria mammillaris | Antioxidants | SC-CO2 | 30 MPa, 60 °C | 144 |
| Posidonia oceanica and Zostera marina | Phenylpropanoids (chicoric, p-coumaric, rosmarinic, benzoic, ferulic and caffeic acids) | SC-CO2 | 250 bar, 80 °C | 145 |
| Saccharina japonica and Sargassum horneri | Antioxidant oil | SC-CO2 | 250 bar, 45 °C | 146 |
| Saccharina japonica | Fucoxanthin, phlorotannin and carotenoids | SC-CO2 and sunflower oil, soyabean oil, canola oil, ethanol and water as co-solvents | 200–300 bar, 45–55 °C | 147 |
| Sargassum sp. | Natural dye; sterols, pentadecanoic acid, 14-methyl ester, 9-hexadecenoic acid, methyl ester and phytol | SC-CO2 | 4500 psi and 65 °C | 148 |
Algal biomass extraction with SC-CO2 depends on various operating conditions and the extraction yields vary accordingly. The most influential parameter seems to be pressure, where the higher the pressure, the higher the yields with faster extraction kinetics. The influence of temperature is the opposite to that of pressure. Better results are achieved with a high CO2/algae mass ratio, and according to the above-mentioned works, it is evident that algal pre-treatment is highly efficient. Centrifugation results in concentrated algal biomass, which can be dried at low temperatures or by freeze-drying. The dried mass can then be crushed and reports show that the smaller the particle size, the faster the extraction kinetics with higher product yields.149Fig. 9 shows the chemical structures of the neoteric solvents used for the bio-processing of marine resources. Fig. 10 shows the chemical structures of the chemicals extracted from marine resources using neoteric solvents.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) was added with different contents of surfactant (hexadecyltrimethylammonium bromide) and introduced into high density polyethylene/agar bio composites through melt mixing. This showed efficient compatibilization of HDPE/agar bio composites with DES-surfactant mixtures.151 Agar films were prepared in the presence of choline-based DESs (choline chloride/urea (1
2) was added with different contents of surfactant (hexadecyltrimethylammonium bromide) and introduced into high density polyethylene/agar bio composites through melt mixing. This showed efficient compatibilization of HDPE/agar bio composites with DES-surfactant mixtures.151 Agar films were prepared in the presence of choline-based DESs (choline chloride/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and choline chloride/glycerol(1
2) and choline chloride/glycerol(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)). The biopolymer was pre-solubilised in DES followed by compression-molding and subsequent drying. The hydrophilicity of the prepared film increased with a decrease in the concentration of agar. Thus, choline chloride-based DES may be promising tools for the development of materials based on seaweed polysaccharides.152 An ionic liquid, namely, 1-butyl-3-methylimidazolium chloride [C4mim][Cl], was used for the dissolution of agarose and chitosan to form composite materials. These materials were decorated with silver oxide nanoparticles to form nanocomposites. The biopolymer acted as a reducing and stabilizing agent, and upon cooling the solution, nanocomposite ionogels with high mechanical strength were formed. The characterization showed that these materials had good thermal and conformational stability, compatibility and strong hydrogen bonding interactions. These nanocomposites showed good antimicrobial activity, and thus can be used in biotechnology and biomedical applications. Further, they can be precursors for applications such as quasi-solid dye-sensitized solar cells, actuators, sensors and electrochemical display.153 Agarose/talc composite films were fabricated using 1-n-butyl-3-methylimidazolium chloride (BmimCl) IL and urea through the gelation method. The talc particles were embedded in the agarose matrix and characterization of these composites demonstrated that BmimCl/Urea can be utilized as a coupling agent for these composites.154 Functionalisation (acetylation/carbanilation) of agarose was carried out using 1-butyl-3-methylimidazolium acetate. Acetylated agarose was hydrophobic and carbanilated agarose was hydrophilic in nature. Carbanilated agarose readily dissolved in the IL and cooling these solutions resulted in the formation of an ionogel, which was attributed their self-healing properties. It was further tested as a solid electrolyte for an activated carbon-based supercapacitor cell and showed good conductivity, which is desirable for energy storage devices and electronic skins with robustness.155 For biomedical applications, the surface properties of the materials used are very important and a simple procedure was developed to graft antibacterial polysaccharides on biomedical-grade polyurethane (PU). Seaweed polysaccharides were meticulously grafted on the surface via an isothiocyanate-alcohol reaction in 1-ethyl-3-methyl imidazolium phosphate IL. This procedure could be transposed for grafting PU surfaces bearing hydroxyl, amine or thiol groups.156 Similarly, the same ionic liquid 1-ethyl-3-methyl imidazolium phosphate was used as a solvent and catalyst to graft antibacterial seaweed polysaccharides such as ulvan, laminarian, fucan and zosterin onto poly(vinylchloride) (PVC).157
2)). The biopolymer was pre-solubilised in DES followed by compression-molding and subsequent drying. The hydrophilicity of the prepared film increased with a decrease in the concentration of agar. Thus, choline chloride-based DES may be promising tools for the development of materials based on seaweed polysaccharides.152 An ionic liquid, namely, 1-butyl-3-methylimidazolium chloride [C4mim][Cl], was used for the dissolution of agarose and chitosan to form composite materials. These materials were decorated with silver oxide nanoparticles to form nanocomposites. The biopolymer acted as a reducing and stabilizing agent, and upon cooling the solution, nanocomposite ionogels with high mechanical strength were formed. The characterization showed that these materials had good thermal and conformational stability, compatibility and strong hydrogen bonding interactions. These nanocomposites showed good antimicrobial activity, and thus can be used in biotechnology and biomedical applications. Further, they can be precursors for applications such as quasi-solid dye-sensitized solar cells, actuators, sensors and electrochemical display.153 Agarose/talc composite films were fabricated using 1-n-butyl-3-methylimidazolium chloride (BmimCl) IL and urea through the gelation method. The talc particles were embedded in the agarose matrix and characterization of these composites demonstrated that BmimCl/Urea can be utilized as a coupling agent for these composites.154 Functionalisation (acetylation/carbanilation) of agarose was carried out using 1-butyl-3-methylimidazolium acetate. Acetylated agarose was hydrophobic and carbanilated agarose was hydrophilic in nature. Carbanilated agarose readily dissolved in the IL and cooling these solutions resulted in the formation of an ionogel, which was attributed their self-healing properties. It was further tested as a solid electrolyte for an activated carbon-based supercapacitor cell and showed good conductivity, which is desirable for energy storage devices and electronic skins with robustness.155 For biomedical applications, the surface properties of the materials used are very important and a simple procedure was developed to graft antibacterial polysaccharides on biomedical-grade polyurethane (PU). Seaweed polysaccharides were meticulously grafted on the surface via an isothiocyanate-alcohol reaction in 1-ethyl-3-methyl imidazolium phosphate IL. This procedure could be transposed for grafting PU surfaces bearing hydroxyl, amine or thiol groups.156 Similarly, the same ionic liquid 1-ethyl-3-methyl imidazolium phosphate was used as a solvent and catalyst to graft antibacterial seaweed polysaccharides such as ulvan, laminarian, fucan and zosterin onto poly(vinylchloride) (PVC).157
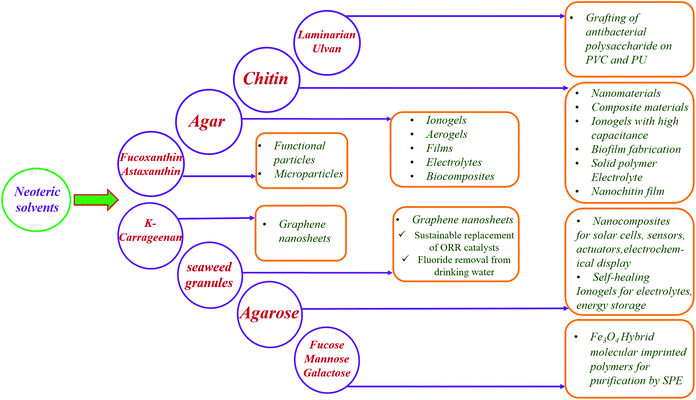
The liquid-phase exfoliation of graphite in the presence of biomass-derived solvents was carried out for the production of pristine graphene nanosheets. A bio-solvent was produced from a cultivable red seaweed Kappaphycus alvarezii for this purpose. The solvent consisted of acetic acid, levulinic acid and γ-valerolactone, which was prepared from the polysaccharide obtained through the acid hydrolysis of seaweed biomass. Fig. 11 shows a schematic of the ultra-sonication-assisted graphite exfoliation of seaweed biomass-extracted solvent mixtures. This process is cost-effective, recyclable and scalable for the large-scale production of graphene sheets.62
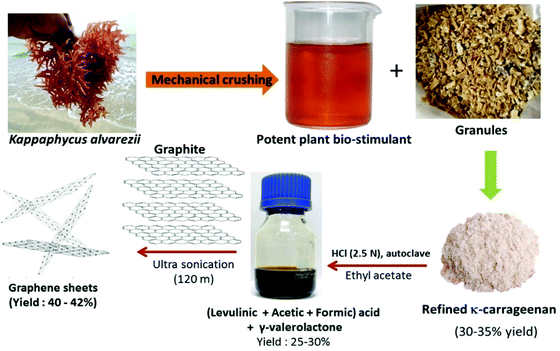 | ||
| Fig. 11 Schematic representation of ultra-sonication-assisted graphite exfoliation of seaweed biomass-extracted solvent mixture (reproduced from ref. 62 (Licensed under Creative Commons CC-BY-NC-ND). | ||
Graphene nanosheets doped with Fe3O4/Fe (Fe3O4/Fe-GN) were produced from the abundant fresh brown seaweed Sargassum tenerrimum. The DES prepared by the complexation of choline chloride and FeCl3 was utilized as a catalyst for the production of graphene nanosheets from the residual granules obtained from the juice of the seaweed. The mixture of seaweed granules and DES was pyrolyzed at 700–900 °C under a 95% N2 and 5% H2 atmosphere for the formation of Fe3O4/Fe-GN with high electrical conductivity (2384.6 mS m−1) and surface area (220 m2 g−1). The results were acceptable, showing that this can be a sustainable replacement for the existing metal-based oxygen reduction reaction (ORR) catalysts. Fig. 12 shows a schematic of the production of magnetite-functionalized graphene from Sargassum tenerrimum and scalable production method for Fe3O4/Fe-doped graphene nanosheets having electrocatalytic activity using DESs.158
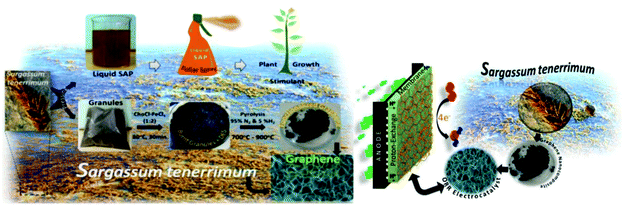 | ||
| Fig. 12 Schematic showing the production of magnetite-functionalized graphene from Sargassum tenerrimum and scalable production method for Fe3O4/Fe-doped graphene nanosheets having electrocatalytic activity using DESs (reproduced from ref. 158 with permission from The Royal Society of Chemistry). | ||
A similar type of work was done, where a choline chloride-based DES was employed for the production of metal oxide-functionalized graphene nanosheets (GNs). The functionalized GNs (Fe3O4/Fe, SnO2/SnO/Sn, or ZnO/Zn-functionalized GNs) were studied for their toxicity and were found to be non-toxic against human lung carcinoma cells. Further, they were assessed for the removal of F− from fluoride-contaminated ground water. Fig. 13 shows the deep eutectic solvent-promoted preparation of functionalized GNs from seaweed granules. These GNs can be utilized to produce safe drinking water according to the World Health Organization (WHO) standards.159
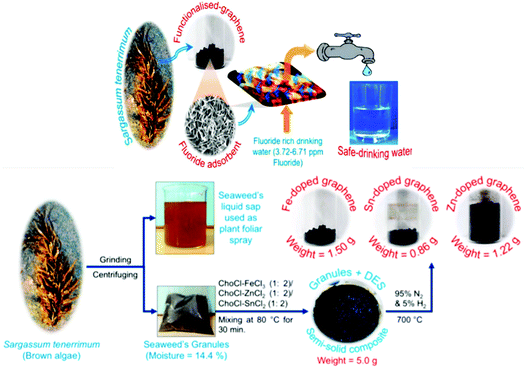 | ||
| Fig. 13 Deep eutectic solvent-promoted preparation of functionalized graphene nanosheets from seaweed granules for producing safe-drinking water (reproduced from ref. 159 with permission from the American Chemical Society). | ||
Chitin is found in abundance in crustaceans and is a biodegradable and biocompatible marine polysaccharide. However, it is poorly soluble in common solvents, which limits its applications.
It has applications in the medical, agriculture, cosmetics, and food and beverages industries and Elibol et al. summarized the recent advances in the application of DESs as extraction media and for bio-film fabrication, nanomaterial preparation, chitosan methylation, chitin dissolution and preparation of composite materials.160 Similarly, Shamshina et al. also examined the treatment (recovery, dissolution and processing) of chitin in ILs, summarizing the recent developments of ILs as catalysts, solvents and co-solvents and demonstrating the improvement in chitin processing.161 An imidazolium-based IL, namely, 1-butyl-3-metlimidazolium acetate ([BMIM]Ac), was used to dissolve a biopolymer and further explored for its gel-forming ability. The rheological properties of the formed ionogel were studied and its functionality in a supercapacitor was also examined. This gel exhibited a high capacitance and better cyclic behaviour compared to the commercial membranes.162 Recently, a carboxymethyl chitin (CMChit)-based membrane having potential as a solid polymer electrolyte (SPE) was blended with 1-butyl-3-methylimidazolium acetate (BMIM[Ac]) IL. This modified SPE showed ionic conductivity in the order of 10−4 S cm−1 and electrochemical stability of up to 2.93 V. It also showed good reversibility in symmetric cells based on Zn//SPE//Zn with potential in proton conducting batteries.163 A nanochitin film was fabricated via the aggregation of scaled-down chitin nanofibers (SD-ChNFs). The self-assembled film was prepared via regeneration from a chitin/ionic liquid iongel using methanol. The prepared film could be bent and twisted easily and exhibited excellent mechanical properties when cross-linked with ι-carrageenan.164
A simple, fast, cost-effective and clean method was developed for the fabrication of green electrolytes composed of κ-carrageenan obtained from red seaweed in the presence of 1-butyl-3-methyl-1H-imidazolium chloride ([Bmim]Cl) ionic liquid and glycerol. High conductivity was obtained for these electrolytes, which are suitable for the fabrication of environmentally friendly electrochemical devices, energy storage devices and electrochromic devices that do not require gas flow and lead to water formation.165 Polysaccharide-based ionogels were formed for the fabrication of electronic noses, which were formed by gas sensors connected to computational systems. The ionogels were prepared using three biopolymers, namely, gelatine, agar and sodium alginate, in 1-ethyl-3-methylimidazolium dicyanamide (EMIMDCA) ionic liquid and were deposited on metallic interdigitated electrodes. This nose was tested for volatile compounds such as acetone, methanol, ethanol, hexane and ethyl acetate. Hit rates of 96% were achieved.166 Ionic liquids such as 1-ethyl-3-methylimidazolium ethylsulfate, [C2mim][C2SO4], 1-ethyl-3-methylimidazolium acetate, [C2mim][OAc] and trimethyl-ethanolammonium acetate, and [Ch][Oac] were investigated for their ability to prepare electrolytes from natural polymers such as agar. The prepared electrolyte samples were thermally stable up to 190 °C. These electrolytes can be incorporated in electrochromic devices.167 A quasi-solid-state polymer electrolyte (QSPE) was prepared using agar in the presence of 1-methyl-3-propylimidazolium iodide (MPII) ionic liquid and its conductivity was recorded to be 1.48 × 10–3 S cm−1. The QSPE was sandwiched between the working and counter electrodes to fabricate a dye-sensitized solar cell (DSSC) and analyzed under a sun simulator.168
A major development in the application of SC-CO2 is the fabrication of functional particles/microparticles, which are used in the pharmaceutical, paint, cosmetics and chemical industries. Pigments such as fucoxanthin and astaxanthin from marine sources were extracted by SC-CO2 at 20 MPa and 45 °C. Microparticles with a diameter of 0.78–1.42 μm were obtained via gas-saturated solutions and the biodegradable polymer polyethylene glycol.169 Monosaccharides such as D-(+)-galactose, L-(−)-fucose, and D-(+)-mannose were modified by DESs to prepare Fe3O4 hybrid molecular imprinted polymers to purify monosaccharides from seaweed using solid-phase extraction (SPE). Higher recoveries of purified product were obtained using templates modified with DESs.170Fig. 14 shows the materials developed from marine resources using neoteric solvents (Table 3).
| Marine source | Compound of interest | Solvent used | Materials/applications | Ref. |
|---|---|---|---|---|
| Red Algae | Polysaccharide; Agar | 1-Butyl-3-methylimidazolium chloride and cosolvent dimethyl sulfoxide | Ionogels and aerogels formed with agar blended with cellulose, rice starch, zeatin protein | 150 |
| Red Algae | Polysaccharide; Agar | DES-surfactant mixture; choline chloride and glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)-hexadecyltrimethylammonium bromide 2)-hexadecyltrimethylammonium bromide |
Biocomposite with increased mechanical strength; compatibilization of HDPE/agar biocomposites | 151 |
| Red Algae | Polysaccharide; Agar | Choline-based DESs (choline chloride/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and choline chloride/glycerol(1 2) and choline chloride/glycerol(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)) 2)) |
Agar films; promising tool for development of materials based on seaweed polysaccharides | 152 |
| Red Algae; Crustacean organisms | Polysaccharides; Agarose and Chitosan | 1-Butyl-3-methylimidazolium chloride [C4mim][Cl] | Agarose-chitosan nanocomposites; potential applications in quasi-solid dye sensitized solar cells, actuators, sensors or electrochemical displays | 153 |
| Red Algae | Polysaccharides; Agarose | 1-n-Butyl-3-methylimidazolium chloride (BmimCl) IL and urea | Agarose/talc composite films | 154 |
| Red Algae | Agarose | 1-Butyl-3-methylimidazolium acetate | Ionogel with self-healing property/solid electrolyte for an activated carbon-based supercapacitor cell and showed good conductivity, which is desirable for energy storage devices and electronic skins with robustness | 155 |
| L. saccharina parmeat; U. rotundata; Fucus/Ascophyllum; Zosteraceae | Laminarin 822; Ulvan 815; Fucan 812; Zosterin 900 | 1-Ethyl-3-methyl imidazolium phosphate IL | Antibacterial seaweed polysaccharide grafting of polyurethane surfaces bearing hydroxyl, amine or thiol groups | 156 |
| L. saccharina parmeat; U. rotundata; Fucus/Ascophyllum; Zosteraceae | Laminarin 822; Ulvan 815; Fucan 812; Zosterin 900 | 1-Ethyl-3-methyl imidazolium phosphate IL | Antibacterial seaweed polysaccharide grafting of polyvinyl chloride surfaces bearing hydroxyl, amine or thiol groups | 157 |
| Kappaphycus alvarezii | Polysaccharide; κ-carrageenan | Biomass-derived solvent; solvent consisted of acetic acid, levulinic acid and γ-valerolactone | Graphene nanosheets | 62 |
| Sargassum tenerrimum | Residual granules of seaweed | DES prepared by complexation of choline chloride and FeCl3 | Graphene nanosheets doped with Fe3O4/Fe (Fe3O4/Fe-GN); sustainable replacement for the existing metal-based oxygen reduction reaction (ORR) catalysts | 158 |
| Sargassum tenerrimum | Residual granules of seaweed | Choline chloride-based DES | Functionalized graphene nanosheets GNs (Fe3O4/Fe, SnO2/SnO/Sn, or ZnO/Zn-functionalized GNs); fluoride removal from drinking water; can be utilized to produce safe drinking water according to World Health Organization (WHO) standards | 159 |
| Crustacean organisms | Chitin | DESs | DESs as extraction media, bio-film fabrication, nanomaterial preparation, chitosan methylation, chitin dissolution and composite material preparation | 160 |
| Crustacean organisms | Chitin | ILs | Recovery, dissolution and processing of chitin | 161 |
| Crustacean organisms | Chitin | 1-Butyl-3-metlimidazolium acetate ([BMIM]Ac) | Ionogels with high capacitance and better cyclic behaviour as compared to the commercial membranes | 162 |
| Crustacean organisms | Chitin | 1-Butyl-3-methylimidazolium acetate (BMIM[Ac]) IL | Carboxymethyl chitin (CMChit)-based membrane having potential as solid polymer electrolyte (SPE); conductivity in the order of 10−4 S cm−1 and electrochemical stability was up to 2.93 V | 163 |
| Crustacean organisms; Red Algae | Chitin; ι-carrageenan | ILs | Nanochitin film; bent, twisted easily and exhibited excellent mechanical properties when cross-linked with ι-carrageenan | 164 |
| Red Algae | κ-Carrageenan | 1-Butyl-3-methyl-1H-imidazolium chloride ([Bmim]Cl) ionic liquid and glycerol | Green electrolytes; potential for the fabrication of environmentally friendly electrochemical devices, energy storage devices and electrochromic devices that do not require gas flow and do not lead to water formation | 165 |
| Red algae; Brown algae | Polysaccharides; gelatine, agar and sodium alginate | 1-Ethyl-3-methylimidazolium dicyanamide (EMIMDCA) | Ionogels were formed for the fabrication of electronic noses | 166 |
| Red Algae | Agar | 1-Ethyl-3-methylimidazolium ethylsulfate, [C2mim][C2SO4], 1-ethyl-3-methylimidazolium acetate, [C2mim][Oac] and trimethyl-ethanolammonium acetate, [Ch][Oac] | Electrolytes were thermally stable up to 190 °C. These electrolytes can be incorporated in electrochromic devices | 167 |
| Red Algae | Agar | 1-Methyl-3-propylimidazolium iodide (MPII) | Quasi-solid-state polymer electrolytes (QSPEs); potential to fabricate dye-sensitized solar cells (DSSC) and analyzed under sun simulator | 168 |
| Brown Algae; Crab shells | Fucoxanthin and astaxanthin | SC-CO2 | Functional particles/microparticles, which are used in the pharmaceutical, paint, cosmetics and chemical industries | 169 |
| Seaweed | D-(+)-Galactose, L-(−)-fucose, and D-(+)-mannose | DESs | Fe3O4 hybrid molecular-imprinted polymers for the purification of monosaccharides by SPE | 170 |
Neoteric solvent-assisted process for fuels/fuel intermediates from oceanic biomass
Oceanic algae is an attractive feed stock for the production of biofuel given that it is a non-food crop, which is largely composed of readily fermented carbohydrates such as starch rather than the more recalcitrant lignocellulosic materials currently under intense development. Ionic liquid-assisted subcritical water was employed for the extraction of lipids from microalgae of Scenedesmus sp. [HNEt3] [HSO4] IL with 1% concentration at a temperature of 110 °C, resulting in a lipid yield of 35.67%. Compared with conventional methods, the triglycerol contents were higher and separation was easy as the algal cell residues assembled into microspheres.171 Three different aqueous deep eutectic solvents (aDESs), i.e., choline chloride–oxalic acid (aCh–O), choline chloride–ethylene glycol (aCh–EG) and urea–acetamide (aU–A) were explored for their efficiency in lipid extraction from microalgal biomass of Chlorella sp. The biomass was pre-treated with aDESs and the results indicated that the recovery rate of lipid increased from the untreated biomass (52.03%) to the pre-treated biomass (80.90%, 66.92% and 75.26% for (aCh–O), (aCh–EG) and (aU–A), respectively).172 DESs were also employed for the pre-treatment of algal biomass of Chlorella sp. and Chlorococcum sp. before the recovery of lipids. Among the DESs under study, choline chloride–acetic acid (Ch–Aa) showed the optimal conditions and is feasible for microalgae-based biodiesel production.173 Direct extraction and biocatalytic transformation of algal oil to biodiesel were done using binary mixtures of imidazolium-based ILs. The algal oil was extracted from microalgae Chlorella vulgaris and Chlorella protothecoides. The advantage of the biocatalytic synthesis of biodiesel of 1-hexadecyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide [C16mim][NTf2] and the ability of cellulosic biomass dissolution of 1-butyl-3-methylimidazolium chloride ([Bmim][Cl] were considered for the use of the binary mixture.174 The production of sustainable biofuel from seaweed waste biomass from the carrageenan industry (SWBC) was achieved by peracetic acid (PAA)-IL pre-treatment. The pre-treatment increased the enzymatic saccharification of SWBC. PAA + 1-hexylpyridinium chloride ([Hpy][Cl]) and PAA + 1-ethyl-3-methylimidazolium diethylphosphate ([Emim][DEP]) were used for this purpose.175 Choline amino acid-based ILs were used for the pre-treatment of biomass derived from Chlorella vulgaris and Spirulina platensis for the extraction of lipids. The lipids were dissolved in the ILs, leaving behind a carbohydrate-rich solid, which was further subjected to enzyme hydrolysis to release fermentable sugars. Herein, this opens new pathways for the production of biodiesel and bioethanol using cheap ILs.176 Protic ionic liquids (PILs) have the potential to disrupt and exudate lipids from microalgae due to their labile protons. Ten carboxylate PILs with lactam and ammonium cations were studied for the production of biodiesel from Nannochloropsis oculata and Chlorella salina. The PILs were found to inhibit lipases, which promote lipolysis in lipid samples and have negligible pigments, which favoured a reduction in the purification steps for the production of biodiesel.177 To reduce the energy consumption required for algal extraction, ILs were assisted by SC-CO2 considering that CO2 captured can compensate the energy consumption. It was evident that the lipid yield of Chlorella vulgaris increased from 68% to 75.6% upon the addition of CO2 to the IL [BMIM][BF4]. The properties of the synthesized biodiesel was on par with the European biodiesel standard, and hence this process has the potential to be commercialised.178 Mixtures of ILs and methanol were used to extract lipids from Chlorella vulgaris, where the lipid yield was comparatively higher for the [Bmim][CF3SO3] and methanol mixture than Bligh and Dyer's method (conventional technique).179 Around thirty ILs were studied with cations such as ammonium, phosphonium, pyridinium and imidazolium for the extraction of lipids from the microalgae Chlorella vulgaris. They were screened for their ability to increase the hexane extraction efficiency of lipids from freeze-dried biomass. For all the tested ILs, the chlorophyll content extracted was low. The best results were obtained for 1-ethyl-3-methylimidazolium ethylsulfate [C2mim][EtSO4] and its SLR ratio, water content and incubation time were optimised to develop an efficient extraction method.180 DESs assisted by microwaves (MWs) were investigated as pre-treatments for algal biomass to develop a new lipid extraction methodology to obtain fatty-acid-rich extract from the diatom Phaeodactylum tricornutum. Eco-friendly extractions were employed using dimethyl carbonate (DMC) and SC-CO2. Choline-based DESs with different combinations of hydrogen bond donors such as levulinic acid, ethylene glycol, urea, levulinic acid, sorbitol and urea were tested for pre-treatment. Fig. 15 shows the pre-treatment of biomass using DESs and further extraction of lipids, fatty acids, etc. The extraction efficiency of SC-CO2 improved and the total fatty acid yield also increased by 20 times, providing triglyceride of the utmost purity.181 | ||
| Fig. 15 Pre-treatment of biomass using DESs and further extraction of lipids, fatty acids, etc. (reproduced from ref. 181 with permission from the American Chemical Society). | ||
Lipid extraction from microalgae using ILs and methanol co-solvent was studied, and it was concluded that this method is highly efficient, cheap, safe and provides environmental protection. The best result was obtained for [BMIM][MeSO4] IL at 70 °C with a reaction time of 2 h.182 A switchable solvent, namely N-methylcyclohexylamine (MCHA), was employed for the extraction of a wet biomass slurry and the algal oil was recovered by IL [C4-mim][PF6] (1-butyl-3-methylimidazolium hexafluorophosphate) through the phase separation method. CO2 was used to trigger the separation of MCHA from the IL. This method can be useful to extract slurries of wet alga directly and the solvent can be recycled. The algal lipid recovery was up to 77%.183 Seaweed is a rich source of polysaccharides, and hence fresh Kappaphycus alvarezii seaweed was exploited for the production of fuel intermediates such as 5-hydroxymethyl furfural (HMF). The seaweed was crushed, and its juice was expelled, which was rich in KCl, and κ-carrageenan was extracted from the residual biomass. Further, HMF was derived from the extracted polysaccharide with Mg(HSO4)2 acid catalyst. Galactose was obtained as a by-product, which was further subjected to acid hydrolysis to yield other products such as levulinic acid (LA) and formic acid (FA).184Fig. 16 shows an integrated scheme for the total utilisation of fresh Kappaphycus alvarezii red seaweed (production of fuel intermediates, agricultural nutrients, pure water and polysaccharides). Table 4 presents a summary of biofuel extraction from marine resources using neoteric solvents. Filho et al. summarized the lipid extraction from natural feedstock using green solvents.185 Recently, Reglero et al. overviewed green technologies for the production of lipids.186
 | ||
| Fig. 16 Integrated scheme for the total utilisation of fresh Kappaphycus alvarezii red seaweed (production of fuel intermediates, agricultural nutrients, pure water and polysaccharides) (reproduced from ref. 184 with permission from The Royal Society of Chemistry). | ||
| Marine resource | Solvent used | Product/% recovery | Ref. |
|---|---|---|---|
| Scenedesmus sp. | [HNEt3] [HSO4] IL | 35.67% lipid yield | 171 |
| Chlorella sp. | Aqueous deep eutectic solvents (aDESs), i.e., choline chloride–oxalic acid (aCh–O), choline chloride–ethylene glycol (aCh–EG) and urea–acetamide (aU–A) | Lipid recovery of 80.90%, 66.92% and 75.26% for (aCh–O), (aCh–EG) and (aU–A), respectively | |
| Chlorella sp. and Chlorococcum sp. | DESs; choline chloride–acetic acid (Ch–Aa) | Yield increased by 30 times compared with conventional method | 172 |
| Chlorella vulgaris and Chlorella protothecoides | 1-Hexadecyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide [C16mim][NTf2]; 1-butyl-3-methylimidazolium chloride ([Bmim][Cl]) | 100% yield for biodiesel | 173 |
| Seaweed waste biomass of carrageenan industry (SWBC) | PAA + 1-hexylpyridinium chloride ([Hpy][Cl]) and PAA + 1-ethyl-3-methylimidazolium diethylphosphate ([Emim][DEP]) | [Hpy][Cl], [Emim][DEP] or 1-ethyl-3-methylimidazole acetate ([Emim][OAc]) produced cellulose conversions of 77%, 91%, 84% and 62%, respectively | 174 |
| Chlorella vulgaris and Spirulina platensis | Choline amino acid-based ILs | 30.6% and 51% for Chlorella vulgaris and Spirulina platensis, respectively | 175 |
| Nannochloropsis oculata and Chlorella salina | Protic ionic liquids | Lipid recovery of 134.9% and 85.4‰ for butyrolactam Hexanoate-treated N. oculata and C. salina, respectively | 176 |
| Chlorella vulgaris | [BMIM][BF4] IL-assisted with SC-CO2 | 75.6% lipid recovery | 177 |
| Chlorella vulgaris | Mixture of [Bmim][CF3SO3] IL and methanol | 12.5% and 19.0% of lipids recovered from commercial and cultivated biomass respectively | 183 |
| Chlorella vulgaris | Ammonium, phosphonium, pyridinium and imidazolium based ILs; 1-ethyl-3-methylimidazolium ethylsulfate [C2mim][EtSO4] | Better extraction yields compared to conventional method | 179 |
| Phaeodactylum tricornutum | Choline-based DESs with (levulinic acid, ethylene glycol, urea, levulinic acid, sorbitol and urea) assisted with dimethyl carbonate (DMC) and SC-CO2 | Lipids with high selectivity of 88% were extracted compared to 35% in conventional method | 180 |
| Algae | [BMIM][MeSO4] IL with methanol cosolvent | 75.6% lipid recovery | 181 |
| Algae | N-Methylcyclohexylamine (MCHA) and [C4-mim][PF6] (1-butyl-3-methylimidazolium hexafluorophosphate) | 77% lipid recovery | 182 |
Conclusion and future perspectives
Blue biorefineries utilize the surplus resources that do not interfere in the food supply chain, and hence offer a potential facility that supports the circular bioeconomy concept by facilitating the conversion of marine biomass (residues, wastes and co-products) into functional materials, fuels, chemicals and commercial commodities. Innovative technologies must be developed for the successful market execution of blue biorefineries, ensuring sustainability of the biomass-to-products chain. Marine resources are still unexplored, and researchers worldwide are actively participating in the development of smart and improved processes to address and solve problems related to the limitations of blue biorefineries. New types of solvent systems are being developed to address the sustainability issues related with biorefineries. However, limited research has been done on the use of neoteric solvents for the bioprocessing of marine resources compared to the available marine feedstocks. The strategic establishment of innovative and eco-friendly technologies employing neoteric solvents for the production of marketable products may lead to large-scale industrial applications. Although techniques are being developed for the use of neoteric solvents for blue biorefineries, considering sustainable and eco-friendly approaches, the cost of the solvents needs to be analysed for their commercial applications. However, this cannot be generalized for all neoteric solvents given that their cost depends on their starting materials. Cheaper neoteric solvents can be developed from biomass and residual feedstocks for large-scale applications. Several reports show that the addition of a small amount of these solvents to other solvents also increases the process efficiency, and thus they can be used as adjuvants. When considering these solvents for biorefinery use, processes must be developed to recover/recycle and minimize the generation of waste/by-products from these solvents to increase the economic viability of the developed techniques. The majority of neoteric solvents are generalised to be non-toxic in nature and these solvents are designer solvents that can be composed according to the process requirements. Thus, when considering the use of these solvents is biorefineries, solvents that can be synthesized with the least toxicity can have a beneficial impact on the environment (Fig. 17). In general, the next decades would be promising and exciting for the development of blue biorefineries with new types of solvent systems. | ||
| Fig. 17 Challenges, strengths and future needs to exploit neoteric solvents for the biorefinery approach. | ||
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
RS thanks DST for DST-INSPIRE Fellowship. KP thanks Council of Scientific & Industrial Research, New Delhi (MLP0027) and SERB-DST, India (CRG/2020/000416) for financial support.References
- S. Kirsch, Extr. Ind. Soc., 2020, 7, 838–840 Search PubMed.
- R. Rapier, Fossil Fuels Still Supply 84 Percent Of World Energy—And Other Eye Openers From BP's Annual Review, (https://www.forbes.com/sites/rrapier/2020/06/20/bp-review-new-highs-in-global-energy-consumption-and-carbon-emissions-in-2019/) Search PubMed.
- R. Katakojwala and S. V. Mohan, Curr. Opin. Green Sustain, 2020, 100392 Search PubMed.
- M. I. Khan, J. H. Shin and J. D. Kim, Microb. Cell Fact., 2018, 17, 1–21 CrossRef PubMed.
- F. Cherubini and S. Ulgiati, Appl. Energy, 2010, 87, 47–57 CrossRef CAS.
- S. V. Mohan, G. N. Nikhil, P. Chiranjeevi, C. N. Reddy, M. V. Rohit, A. N. Kumar and O. Sarkar, Bioresour. Technol., 2016, 215, 2–12 CrossRef PubMed.
- S. V. Mohan, S. Dahiya, K. Amulya, R. Katakojwala and T. Vanitha, Bioresour. Technol. Rep., 2019, 7, 100277 CrossRef.
- Ó. Ögmundarson, M. Herrgård, J. Forster, M. Hauschild and P. Fantke, Nat Sustain, 2020, 3, 167–174 CrossRef.
- Y. Liao, S.-F. Koelewijn, G. Van den Bossche, J. Van Aelst, S. Van den Bosch, T. Renders, K. Navare, T. Nicolaï, K. Van Aelst, M. Maesen, H. Matsushima, J. M. Thevelein, K. Van Acker, B. Lagrain, D. Verboekend and B. F. Sels, Science, 2020, 367, 1385–1390 CrossRef CAS PubMed.
- J. Moncada, J. A. Tamayo and C. A. Cardona, Chem. Eng. Sci., 2014, 118, 126–140 CrossRef CAS.
- E. D. Jong and G. Jungmeier, Biorefinery Concepts in Comparison to Petrochemical Refineries, Ind. Biorefin. White Biotechnol., 2015, 3–33, DOI:10.1016/B978-0-444-63453-5.00001-X.
- A. Azapagic, Trends Biotechnol., 2014, 32, 1–4 CrossRef CAS PubMed.
- G. Fiorentino, M. Ripa and S. Ulgiati, Biofuels, Bioprod. Biorefin., 2017, 11, 195–214 CrossRef CAS.
- E. Lipinsky, Science, 1981, 212, 1465–1471 CrossRef CAS PubMed.
- O. Rosales-Calderon and V. Arantes, Biotechnol. Biofuels, 2019, 12, 1–58 CrossRef CAS PubMed.
- F. M. Kerton, Y. Liu, K. W. Omari and K. Hawboldt, Green Chem., 2013, 15, 860–871 RSC.
- F. M. Kerton and N. Yan, Fuels, Chemicals and Materials from the Oceans and Aquatic Sources, John Wiley & Sons, 2017 Search PubMed.
- H. Vieira, M. C. Leal and R. Calado, Trends Biotechnol., 2020, 38, 940–943 CrossRef CAS PubMed.
- B. M. Cleveland, J. World Aquacult. Soc., 2018, 50, 890–893 CrossRef.
- P. I. Macreadie, A. Anton, J. A. Raven, N. Beaumont, R. M. Connolly, D. A. Friess, J. J. Kelleway, H. Kennedy, T. Kuwae and P. S. Lavery, Nat. Commun., 2019, 10, 1–13 CrossRef PubMed.
- R. Calado, M. C. Leal, H. Gaspar, S. Santos, A. Marques, M. L. Nunes and H. Vieira, in Grand challenges in marine biotechnology, Springer, 2018, pp. 317–403 Search PubMed.
- J. Hoffman, R. C. Pate, T. Drennen and J. C. Quinn, Algal Res., 2017, 23, 51–57 CrossRef.
- A. LiVecchi, A. Copping, D. Jenne, A. Gorton, R. Preus, G. Gill, R. Robichaud, R. Green, S. Geerlofs, S. Gore, D. Hume, W. McShane, C. Schmaus and H. Spence, Marine Algae, in Powering the Blue Economy; Exploring Opportunities for Marine Renewable Energy in Maritime Markets, U.S. Department of Energy, Office of Energy Efficiency and Renewable Energy, Washington, D.C, 2019, https://www.energy.gov/sites/prod/files/2019/03/f61/Chapter%205.pdf Search PubMed.
- Algae Biofuel Market, Expected with a CAGR of 2.6%, Top Companies data report covers, Market-specific challenges, Share, Growth, Trends, and Forecasts 2021–2027, RFD TV, 2021, https://www.rfdtv.com/story/44153832/algae-biofuel-market Search PubMed.
- D. Rodrigues, A. C. Freitas, L. Pereira, T. A. P. Rocha-Santos, M. W. Vasconcelos, M. Roriz, L. M. Rodríguez-Alcalá, A. M. P. Gomes and A. C. Duarte, Food Chem., 2015, 183, 197–207 CrossRef CAS PubMed.
- Algae Biofuel Market Size Worth $10.73 Billion By 2025 | CAGR: 8.8%, Grand View Research, 2017, https://www.grandviewresearch.com/press-release/global-algae-biofuel-market Search PubMed.
- N. Q. Diep, K. Sakanishi, N. Nakagoshi, S. Fujimoto, T. Minowa and X. D. Tran, International Energy Journal, 2012, 13, 113–122 Search PubMed.
- R. Jiang, K. N. Ingle and A. Golberg, Algal Res., 2016, 14, 48–57 CrossRef.
- J. J. Milledge and P. J. Harvey, J. Chem. Technol. Biotechnol., 2016, 91, 2221–2234 CrossRef CAS PubMed.
- A. Trincone, Mar. Drugs, 2017, 15, 93 CrossRef PubMed.
- C. Zhang and S.-K. Kim, Mar. Drugs, 2010, 8, 1920–1934 CrossRef CAS PubMed.
- T. E. Rao, M. Imchen and R. Kumavath, in Advances in food and nutrition research, Elsevier, 2017, vol. 80, pp. 149–163 Search PubMed.
- S. Baharum, E. Beng and M. Mokhtar, J. Biol. Sci., 2010, 10(6), 555–564 CrossRef.
- S. Lordan, R. P. Ross and C. Stanton, Mar. Drugs, 2011, 9, 1056–1100 CrossRef CAS PubMed.
- M. Kazir, Y. Abuhassira, A. Robin, O. Nahor, J. Luo, A. Israel, A. Golberg and Y. D. Livney, Food Hydrocolloids, 2019, 87, 194–203 CrossRef CAS.
- D. Rodrigues, A. C. Freitas, L. Pereira, T. A. Rocha-Santos, M. W. Vasconcelos, M. Roriz, L. M. Rodríguez-Alcalá, A. M. Gomes and A. C. Duarte, Food Chem., 2015, 183, 197–207 CrossRef CAS PubMed.
- S. P. Prabha, S. Nagappan, R. Rathna, R. Viveka and E. Nakkeeran, in Refining Biomass Residues for Sustainable Energy and Bioproducts, Elsevier, 2020, pp. 463–480 Search PubMed.
- H. A. R. Suleria, S. Osborne, P. Masci and G. Gobe, Mar. Drugs, 2015, 13, 6336–6351 CrossRef CAS PubMed.
- Y. Murti and T. Agrawal, Int. J. ChemTech Res., 2010, 2, 2198–2217 CAS.
- A. Kijjoa and P. Sawangwong, Mar. Drugs, 2004, 2, 73–82 CrossRef CAS.
- L. Alparslan, N. Şekeroğlu and A. Kijjoa, Curr. Pers. Med. Aromat. Plants (CUPMAP), 2018, 1(2), 53–66 Search PubMed.
- J.-B. Guillerme, C. Couteau and L. Coiffard, Cosmetics, 2017, 4, 35 CrossRef.
- C. R. Singh, K. Kathiresan and S. Anandhan, Afr. J. Biotechnol., 2015, 14, 1525–1532 CrossRef.
- D. Pradhan, L. B. Sukla, B. B. Mishra and N. Devi, J. Cleaner Prod., 2019, 209, 617–629 CrossRef CAS.
- F. Baltar, A. Gutiérrez-Rodríguez, M. Meyer, I. Skudelny, S. Sander, B. Thomson, S. Nodder, R. Middag and S. E. Morales, Front. Microbiol., 2018, 9, 3190 CrossRef PubMed.
- J. C. Camacho-Chab, M. D. R. Castañeda-Chávez, M. J. Chan-Bacab, R. N. Aguila-Ramírez, I. Galaviz-Villa, P. Bartolo-Pérez, F. Lango-Reynoso, C. Tabasco-Novelo, C. Gaylarde and B. O. Ortega-Morales, Int. J. Environ. Res. Public Health, 2018, 15, 314 CrossRef PubMed.
- P. Gupta and B. Diwan, Biotechnol. Rep., 2017, 13, 58–71 CrossRef PubMed.
- J. De, N. Ramaiah and L. Vardanyan, Mar. Biotechnol., 2008, 10, 471–477 CrossRef CAS PubMed.
- R. Elizondo-González, E. Quiroz-Guzmán, C. Escobedo-Fregoso, P. Magallón-Servín and A. Peña-Rodríguez, PeerJ, 2018, 6, e4459 CrossRef PubMed.
- R. C. R. Counterclockwise, Z. Out, Z. In and M. Gavrilescu, Dyn. Biochem. Process Biotechnol. Mol. Biol., 2010, 4, 1–36 Search PubMed.
- R. A. Sequeira, J. Bhatt and K. Prasad, Sustainable Chem., 2020, 1, 116–137 CrossRef.
- K. Prasad and M. Sharma, Curr. Opin. Green Sustain, 2019, 18, 72–78 CrossRef.
- C. Capello, U. Fischer and K. Hungerbühler, Green Chem., 2007, 9, 927–934 RSC.
- C. J. Clarke, W.-C. Tu, O. Levers, A. Brohl and J. P. Hallett, Chem. Rev., 2018, 118, 747–800 CrossRef CAS PubMed.
- M. C. Bubalo, S. Vidović, I. R. Redovniković and S. Jokić, J. Chem. Technol. Biotechnol., 2015, 90, 1631–1639 CrossRef.
- K. R. Seddon, J. Chem. Technol. Biotechnol., 1997, 68, 351–356 CrossRef CAS.
- R. A. Sequeira, N. Singh, M. M. Pereira, N. A. Chudasama, S. Bhattacharya, M. Sharma, D. Mondal and K. Prasad, Int. J. Biol. Macromol., 2018, 120, 378–384 CrossRef CAS PubMed.
- R. A. Sequeira, S. Dubey, M. M. Pereira, T. K. Maity, S. Singh, S. Mishra and K. Prasad, ACS Sustainable Chem. Eng., 2020, 8, 12005–12013 CrossRef CAS.
- R. A. Sequeira, M. Sharma, M. M. Pereira, N. Singh, S. Bhattacharya, N. A. Chudasama and K. Prasad, Sep. Sci. Technol., 2021, 56, 631–639 CrossRef CAS.
- G. Sharma, R. A. Sequeira, M. M. Pereira, T. K. Maity, N. A. Chudasama and K. Prasad, J. Mol. Liq., 2021, 328, 115386 CrossRef CAS.
- A. K. Das, R. A. Sequeira, T. K. Maity and K. Prasad, Food Hydrocolloids, 2021, 111, 106382 CrossRef CAS.
- N. Singh, M. Sharma, D. Mondal, D. A. Maru, M. R. Rathod, R. A. Sequeira, N. A. Chudasama and K. Prasad, Mater. Sci. Energy Technol., 2021, 4, 100–106 CAS.
- J. Bhatt, R. A. Sequeira, A. Vohra, R. V. Devkar, T. K. Maity and K. Prasad, ACS Sustainable Chem. Eng., 2020, 9, 264–272 CrossRef.
- N. A. Chudasama, R. A. Sequeira, K. Moradiya and K. Prasad, Molecules, 2021, 26, 2608 CrossRef CAS PubMed.
- J. N. Canongia Lopes and A. A. Padua, J. Phys. Chem. B, 2006, 110, 3330–3335 CrossRef CAS PubMed.
- N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123–150 RSC.
- D. R. MacFarlane, N. Tachikawa, M. Forsyth, J. M. Pringle, P. C. Howlett, G. D. Elliott, J. H. Davis, M. Watanabe, P. Simon and C. A. Angell, Energy Environ. Sci., 2014, 7, 232–250 RSC.
- A. Benedetto and P. Ballone, ACS Sustainable Chem. Eng., 2016, 4, 392–412 CrossRef CAS.
- Z. Lei, B. Chen, Y.-M. Koo and D. R. MacFarlane, Chem. Rev., 2017, 117, 6633–6635 CrossRef PubMed.
- J. H. Davis, Jr., Chem. Lett., 2004, 33(9), 1072–1077 CrossRef.
- M. Moniruzzaman and T. Ono, Biochem. Eng. J., 2012, 60, 156–160 CrossRef CAS.
- R. Financie, M. Moniruzzaman and Y. Uemura, Biochem. Eng. J., 2016, 110, 1–7 CrossRef CAS.
- H. Zhao, J. Chem. Technol. Biotechnol., 2015, 90, 19–25 CrossRef CAS PubMed.
- S. Yamaguchi, E. Yamamoto, T. Mannen, T. Nagamune and T. Nagamune, Biotechnol. J., 2013, 8, 17–31 CrossRef CAS PubMed.
- D. Yu, Z. Xue and T. Mu, Chem. Soc. Rev., 2021, 50, 8596–8638 RSC.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 70–71 RSC.
- A. Paiva, R. Craveiro, I. Aroso, M. Martins, R. L. Reis and A. R. C. Duarte, ACS Sustainable Chem. Eng., 2014, 2, 1063–1071 CrossRef CAS.
- K. Radošević, M. C. Bubalo, V. G. Srček, D. Grgas, T. L. Dragičević and I. R. Redovniković, Ecotoxicol. Environ. Saf., 2015, 112, 46–53 CrossRef PubMed.
- Q. Zhang, K. D. O. Vigier, S. Royer and F. Jerome, Chem. Soc. Rev., 2012, 41, 7108–7146 RSC.
- Y. H. Choi, J. van Spronsen, Y. Dai, M. Verberne, F. Hollmann, I. W. Arends, G.-J. Witkamp and R. Verpoorte, Plant Physiol., 2011, 156, 1701–1705 CrossRef CAS PubMed.
- Y. Dai, J. van Spronsen, G.-J. Witkamp, R. Verpoorte and Y. H. Choi, Anal. Chim. Acta, 2013, 766, 61–68 CrossRef CAS PubMed.
- S. Maiti, G. Gallastegui, G. Suresh, S. J. Sarma, S. K. Brar, P. Drogui, Y. LeBihan, G. Buelna, M. Verma and C. R. Soccol, Bioresour. Technol., 2018, 249, 673–683 CrossRef CAS PubMed.
- M. K. Ghosh, M. S. Howard, Y. Zhang, K. Djebbi, G. Capriolo, A. Farooq, H. J. Curran and S. Dooley, Combust. Flame, 2018, 193, 157–169 CrossRef CAS.
- N. A. S. Ramli, N. I. Hisham and N. A. S. Amin, Sains Malays., 2018, 47, 1131–1138 CrossRef CAS.
- A. Vivian, L. Fusaro, D. P. Debecker and C. Aprile, ACS Sustainable Chem. Eng., 2018, 6, 14095–14103 CrossRef CAS.
- A. Leal-Duaso, P. Pérez, J. A. Mayoral, E. Pires and J. I. García, Phys. Chem. Chem. Phys., 2017, 19, 28302–28312 RSC.
- L. Jaafari, H. Ibrahim, B. Jaffary and R. Idem, Renewable Energy, 2019, 130, 1176–1184 CrossRef CAS.
- Y. Liu, J. B. Friesen, J. B. McAlpine, D. C. Lankin, S.-N. Chen and G. F. Pauli, J. Nat. Prod., 2018, 81, 679–690 CrossRef CAS PubMed.
- V. I. Castro, R. Craveiro, J. M. Silva, R. L. Reis, A. Paiva and A. R. C. Duarte, Cryobiology, 2018, 83, 15–26 CrossRef CAS PubMed.
- L. Lomba, E. Zuriaga and B. Giner, Curr. Opin. Green Sustain, 2019, 18, 51–56 CrossRef.
- F. Temelli, J. Supercrit. Fluids, 2009, 47, 583–590 CrossRef CAS.
- X. Zhang, S. Heinonen and E. Levänen, RSC Adv., 2014, 4, 61137–61152 RSC.
- J. M. DeSimone and W. Tumas, Green chemistry using liquid and supercritical carbon dioxide, Oxford University Press, 2003 Search PubMed.
- J. Rutkowska and A. Stolyhwo, Food Chem., 2009, 115, 745–752 CrossRef CAS.
- C. G. Pereira and M. A. A. Meireles, Food Bioprocess Technol., 2010, 3, 340–372 CrossRef CAS.
- D. A. Esquivel-Hernández, I. P. Ibarra-Garza, J. Rodríguez-Rodríguez, S. P. Cuéllar-Bermúdez, M. d. J. Rostro-Alanis, G. S. Alemán-Nava, J. S. García-Pérez and R. Parra-Saldívar, Biofuels, Bioprod. Biorefin., 2017, 11, 215–231 CrossRef.
- I. Marrucho, L. Branco and L. Rebelo, Annu. Rev. Chem. Biomol. Eng., 2014, 5, 527–546 CrossRef CAS PubMed.
- L. Vidal, M.-L. Riekkola and A. Canals, Anal. Chim. Acta, 2012, 715, 19–41 CrossRef CAS PubMed.
- J. L. Anderson, D. W. Armstrong and G.-T. Wei, Anal. Chem., 2006, 78, 2892–2902 CrossRef PubMed.
- S. Shahriari, L. C. Tomé, J. M. Araújo, L. P. N. Rebelo, J. A. Coutinho, I. M. Marrucho and M. G. Freire, RSC Adv., 2013, 3, 1835–1843 RSC.
- B. Tang, W. Bi, M. Tian and K. H. Row, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2012, 904, 1–21 CrossRef CAS PubMed.
- D. Han and K. H. Row, Molecules, 2010, 15, 2405–2426 CrossRef CAS PubMed.
- Y. Chen and T. Mu, Green Chem. Eng., 2021, 2, 174–186 CrossRef.
- A. Pieczykolan, W. Pietrzak, E. Rój and R. Nowak, Open Chem., 2019, 17, 302–312 CAS.
- H. Passos, M. G. Freire and J. A. Coutinho, Green Chem., 2014, 16, 4786–4815 RSC.
- D. Han, T. Zhu and K.-H. Row, Bull. Korean Chem. Soc., 2011, 32, 2212–2216 CrossRef CAS.
- T. V. Dinh, P. S. Saravana, H. C. Woo and B. S. Chun, Sep. Purif. Technol., 2018, 196, 287–299 CrossRef CAS.
- P. S. Saravana, Y.-N. Cho, H.-C. Woo and B.-S. Chun, J. Cleaner Prod., 2018, 198, 1474–1484 CrossRef CAS.
- T. J. Trivedi and A. Kumar, Green Sustainable Chem., 2014, 4, 190 CrossRef.
- M. Sharma, J. P. Chaudhary, D. Mondal, R. Meena and K. Prasad, Green Chem., 2015, 17, 2867–2873 RSC.
- G. Li, Y. Dai, X. Wang and K. Row, Anal. Lett., 2019, 52, 511–525 CrossRef CAS.
- X. Wang, G. Li and K. H. Row, J. Sep. Sci., 2017, 40, 3301–3310 CrossRef CAS PubMed.
- C. R. N. Gereniu, P. S. Saravana and B.-S. Chun, Sep. Purif. Technol., 2018, 196, 309–317 CrossRef CAS.
- A. K. Das, M. Sharma, D. Mondal and K. Prasad, Carbohydr. Polym., 2016, 136, 930–935 CrossRef CAS PubMed.
- G. Li and K. H. Row, Anal. Lett., 2020, 53, 188–202 CrossRef CAS.
- F. Putri Hernat and H. Yu, Anal. Lett., 2016, 49, 1154–1162 CrossRef CAS.
- E. D. Obluchinskaya, A. V. Daurtseva, O. N. Pozharitskaya, E. V. Flisyuk and A. N. Shikov, Pharm. Chem. J., 2019, 53, 243–247 CrossRef CAS.
- A. K. Das and K. Prasad, Anal. Methods, 2015, 7, 9064–9067 RSC.
- M. Martins, F. A. Vieira, I. Correia, R. A. Ferreira, H. Abreu, J. A. Coutinho and S. P. Ventura, Green Chem., 2016, 18, 4287–4296 RSC.
- Y. Xu, Q. Wang and Y. Hou, Mar. Drugs, 2020, 18, 618 CrossRef CAS PubMed.
- F. A. Vieira, R. J. Guilherme, M. C. Neves, A. Rego, M. H. Abreu, J. A. Coutinho and S. P. Ventura, Sep. Purif. Technol., 2018, 196, 300–308 CrossRef CAS.
- M. Martins, C. M. Albuquerque, C. F. Pereira, J. A. P. Coutinho, M. G. P. M. S. Neves, D. C. G. A. Pinto, M. A. F. Faustino and S. P. M. Ventura, ACS Sustainable Chem. Eng., 2021, 9, 1772–1780 CrossRef CAS.
- M. Martins, A. P. Fernandes, M. A. Torres-Acosta, P. N. Collén, M. H. Abreu and S. P. Ventura, Sep. Purif. Technol., 2021, 254, 117589 CrossRef CAS.
- M. Martins, L. M. d. S. Mesquita, B. M. C. Vaz, A. C. R. V. Dias, M. A. Torres-Acosta, B. Quéguineur, J. A. P. Coutinho and S. P. M. Ventura, ACS Sustainable Chem. Eng., 2021, 9, 6599–6612 CrossRef CAS.
- C. Miranda, C. van den Berg and M. Smolders, Screening of ionic liquids for the extraction of proteins from the macroalga Ulva lactuca aiming at an integrated biorefinery approach, Instituto Superior Técnico of Lisbon, 2017 Search PubMed.
- L.-Q. Peng, W.-Y. Yu, J.-J. Xu and J. Cao, Food Chem., 2018, 239, 1075–1084 CrossRef CAS PubMed.
- K. Ghanemi, M.-A. Navidi, M. Fallah-Mehrjardi and A. Dadolahi-Sohrab, Anal. Methods, 2014, 6, 1774–1781 RSC.
- Z. Helalat-Nezhad, K. Ghanemi and M. Fallah-Mehrjardi, J. Chromatogr., A, 2015, 1394, 46–53 CrossRef CAS PubMed.
- A. F. M. Cláudio, M. C. Neves, K. Shimizu, J. N. C. Lopes, M. G. Freire and J. A. Coutinho, Green Chem., 2015, 17, 3948–3963 RSC.
- S. Machmudah, W. Diono, H. Kanda and M. Goto, Eng. J., 2018, 22, 13–30 CrossRef CAS.
- M. El Hattab, G. Culioli, L. Piovetti, S. E. Chitour and R. Valls, J. Chromatogr., A, 2007, 1143, 1–7 CrossRef CAS PubMed.
- P. Subra and P. Boissinot, J. Chromatogr., A, 1991, 543, 413–424 CrossRef CAS.
- P. C. Cheung, A. Y. Leung and P. O. Ang, J. Agric. Food Chem., 1998, 46, 4228–4232 CrossRef CAS.
- M. Becerra, S. Boutefnouchet, O. Córdoba, G. P. Vitorino, L. Brehu, I. Lamour, F. Laimay, A. Efstathiou, D. Smirlis and S. Michel, Phytochem. Lett., 2015, 11, 418–423 CrossRef CAS.
- P. Pérez-López, E. M. Balboa, S. González-García, H. Domínguez, G. Feijoo and M. T. Moreira, Bioresour. Technol., 2014, 161, 137–148 CrossRef PubMed.
- A. T. Quitain, T. Kai, M. Sasaki and M. Goto, Ind. Eng. Chem. Res., 2013, 52, 7940–7946 CrossRef CAS.
- J. Zheng, Y. Chen, F. Yao, W. Chen and G. Shi, Mar. Drugs, 2012, 10, 2634–2647 CrossRef PubMed.
- R. Men'shova, F. Lepeshkin, S. Ermakova, O. Pokrovskii and T. Zvyagintseva, Chem. Nat. Compd., 2013, 48, 923–926 CrossRef.
- P. C. Cheung, Food Chem., 1999, 65, 399–403 CrossRef CAS.
- D. Gao, R. Okuda and V. Lopez-Avila, J. AOAC Int., 2001, 84, 1313–1331 CrossRef CAS PubMed.
- I. Gamlieli-Bonshtein, E. Korin and S. Cohen, Biotechnol. Bioeng., 2002, 80, 169–174 CrossRef CAS PubMed.
- U. De Corato, R. Salimbeni, A. De Pretis, N. Avella and G. Patruno, Postharvest Biol. Nanotechnol., 2017, 131, 16–30 CrossRef CAS.
- J.-Y. Kang, B.-S. Chun, M.-C. Lee, J.-S. Choi, I. S. Choi and Y.-K. Hong, J. Essent. Oil-Bear. Plants, 2016, 19, 46–51 CrossRef CAS.
- M. Ospina, H. I. Castro-Vargas and F. Parada-Alfonso, J. Supercrit. Fluids, 2017, 128, 314–322 CrossRef CAS.
- C. Sevimli-Gur and O. Yesil-Celiktas, Mol. Biol. Rep., 2019, 46, 3691–3699 CrossRef CAS PubMed.
- S. P. Sivagnanam, S. Yin, J. H. Choi, Y. B. Park, H. C. Woo and B. S. Chun, Mar. Drugs, 2015, 13, 3422–3442 CrossRef CAS PubMed.
- P. S. Saravana, A. Getachew, Y.-J. Cho, J. H. Choi, Y. B. Park, H. Woo and B. Chun, J. Supercrit. Fluids, 2017, 120, 295–303 CrossRef CAS.
- N. Talib, M. R. Ahmad, M. I. A. Kadir, K. Ismail and A. F. C. Rahim, Int. J. Eng. Technol., 2018, 7, 105–108 CrossRef CAS.
- C. Crampon, O. Boutin and E. Badens, Ind. Eng. Chem. Res., 2011, 50, 8941–8953 CrossRef CAS.
- A. A. Shamsuri, D. K. Abdullah and R. Daik, Cellul. Chem. Technol., 2012, 46, 45–52 CAS.
- A. Shamsuri, R. Daik, E. Zainudin and P. Tahir, J. Reinf. Plast. Compos., 2014, 33, 440–453 CrossRef.
- A. M. Sousa, H. K. Souza, N. Latona, C. K. Liu, M. P. Gonçalves and L. Liu, Carbohydr. Polym., 2014, 111, 206–214 CrossRef CAS PubMed.
- T. J. Trivedi, K. S. Rao and A. Kumar, Green Chem., 2014, 16, 320–330 RSC.
- A. A. Shamsuri and R. Daik, Materials, 2013, 6, 682–698 CrossRef PubMed.
- T. J. Trivedi, D. Bhattacharjya, J. S. Yu and A. Kumar, ChemSusChem, 2015, 8, 3294–3303 CrossRef CAS PubMed.
- S. Bigot, G. Louarn, N. Kébir and F. Burel, Appl. Surf. Sci., 2014, 314, 301–307 CrossRef CAS.
- S. Bigot, G. Louarn, N. Kébir and F. Burel, Carbohydr. Polym., 2013, 98, 1644–1649 CrossRef CAS PubMed.
- D. Mondal, M. Sharma, C.-H. Wang, Y.-C. Lin, H.-C. Huang, A. Saha, S. K. Nataraj and K. Prasad, Green Chem., 2016, 18, 2819–2826 RSC.
- M. Sharma, D. Mondal, N. Singh, K. Upadhyay, A. Rawat, R. V. Devkar, R. A. Sequeira and K. Prasad, ACS Sustainable Chem. Eng., 2017, 5, 3488–3498 CrossRef CAS.
- N. Özel and M. Elibol, Carbohydr. Polym., 2021, 262, 117942 CrossRef PubMed.
- J. L. Shamshina and P. Berton, Front. Bioeng. Biotechnol., 2020, 8, 11 CrossRef PubMed.
- L. Deng and L.-M. Zhang, Colloids Surf., A, 2020, 586, 124220 CrossRef CAS.
- M. Latifi, A. Ahmad, N. H. Hassan, H. Ben Youcef and H. Kaddami, Carbohydr. Polym., 2021, 273, 118542 CrossRef CAS PubMed.
- T. Hashiguchi, K. Yamamoto and J.-i. Kadokawa, Carbohydr. Polym., 2021, 270, 118369 CrossRef CAS PubMed.
- S. C. Nunes, R. F. P. Pereira, N. Sousa, M. M. Silva, P. Almeida, F. M. L. Figueiredo and V. de Zea Bermudez, Adv. Sustainable Syst., 2017, 1, 1700070 CrossRef.
- M. M. O. Netto, W. B. Gonçalves, R. W. C. Li and J. Gruber, Sens. Actuators, B, 2020, 315, 128025 CrossRef CAS.
- R. Leones, F. Sentanin, L. Rodrigues, I. Marrucho, J. Esperança, A. Pawlicka and M. M. Silva, eXPRESS Polym. Lett., 2012, 6, 1007–1016 CrossRef CAS.
- S. R. Nadia, M. H. Khanmirzaei, S. Ramesh and K. Ramesh, Ionics, 2017, 23, 1585–1590 CrossRef CAS.
- K.-T. Kwon, G. Jung, J.-E. Sim and B. Chun, Korean J. Chem. Eng. 201128102044–2049 Search PubMed.
- G. Li and K. H. Row, J. Liq. Chromatogr. Relat. Technol., 2017, 40, 1037–1046 CrossRef CAS.
- X. Chen, L. Hu, R. Xing, S. Liu, H. Yu, Y. Qin, K. Li, R. Li and P. Li, Eur. J. Lipid Sci. Technol., 2015, 117, 1192–1198 CrossRef CAS.
- W. Lu, M. A. Alam, Y. Pan, J. Wu, Z. Wang and Z. Yuan, Bioresour. Technol., 2016, 218, 123–128 CrossRef CAS PubMed.
- Y. Pan, M. A. Alam, Z. Wang, D. Huang, K. Hu, H. Chen and Z. Yuan, Bioresour. Technol., 2017, 238, 157–163 CrossRef CAS PubMed.
- P. Lozano, J. M. Bernal, C. Gómez, E. Álvarez, B. Markiv, E. García-Verdugo and S. V. Luis, Catal. Today, 2020, 346, 87–92 CrossRef CAS.
- Uju, A. T. Wijayanta, M. Goto and N. Kamiya, Biomass Bioenergy, 2015, 81, 63–69 CrossRef CAS.
- T. Q. To, K. Procter, B. A. Simmons, S. Subashchandrabose and R. Atkin, Faraday Discuss., 2018, 206, 93–112 RSC.
- M. Shankar, P. K. Chhotaray, R. L. Gardas, K. Tamilarasan and M. Rajesh, Biomass Bioenergy, 2019, 123, 14–24 CrossRef CAS.
- X. Yu, J. Yang, H. Lu, S.-T. Tu and J. Yan, Appl. Energy, 2015, 160, 648–655 CrossRef CAS.
- Y.-H. Kim, Y.-K. Choi, J. Park, S. Lee, Y.-H. Yang, H. J. Kim, T.-J. Park, Y. Hwan Kim and S. H. Lee, Bioresour. Technol., 2012, 109, 312–315 CrossRef CAS PubMed.
- V. C. A. Orr, N. V. Plechkova, K. R. Seddon and L. Rehmann, ACS Sustainable Chem. Eng., 2016, 4, 591–600 CrossRef CAS.
- E. Tommasi, G. Cravotto, P. Galletti, G. Grillo, M. Mazzotti, G. Sacchetti, C. Samorì, S. Tabasso, M. Tacchini and E. Tagliavini, ACS Sustainable Chem. Eng., 2017, 5, 8316–8322 CrossRef CAS.
- W. Zhou, Z. Wang, M. A. Alam, J. Xu, S. Zhu, Z. Yuan, S. Huo, Y. Guo, L. Qin and L. Ma, Int. J. Polym. Sci., 2019, 2019, 9209210 Search PubMed.
- H.-Y. Yang, W.-J. Lu, Y.-C. Chen, K.-T. Chen, J.-C. Teng and H. Wan, Biomass Bioenergy, 2017, 100, 108–115 CrossRef CAS.
- D. Mondal, M. Sharma, P. Maiti, K. Prasad, R. Meena, A. K. Siddhanta, P. Bhatt, S. Ijardar, V. P. Mohandas, A. Ghosh, K. Eswaran, B. G. Shah and P. K. Ghosh, RSC Adv., 2013, 3, 17989–17997 RSC.
- S. S. de Jesus and R. M. Filho, Renewable Sustainable Energy Rev., 2020, 133, 110289 CrossRef CAS.
- L. Vázquez, C. Bañares, C. F. Torres and G. Reglero, Annu. Rev. Food Sci. Technol., 2020, 11, 319–337 CrossRef PubMed.
Footnote |
| † Present address: Institute of Plant Genetics of the Polish Academy of Sciences, Strzeszyńska 34, 60479, Poznan, Poland. |
| This journal is © The Royal Society of Chemistry 2021 |