Nitrogen-doped carbon nanotubes as an anode for a highly robust potassium-ion hybrid capacitor†
Xiuqi
Li‡
a,
Maoxin
Chen‡
a,
Lei
Wang
*a,
Hanjiao
Xu
a,
Jiang
Zhong
a,
Meng
Zhang
a,
Yaya
Wang
a,
Qiusheng
Zhang
a,
Lin
Mei
*a,
Tao
Wang
 *a,
Jian
Zhu
a,
Bingan
Lu
*a,
Jian
Zhu
a,
Bingan
Lu
 b and
Xidong
Duan
b and
Xidong
Duan
 *a
*a
aState Key Laboratory for Chemo/Biosensing and Chemometrics, and College of Chemistry and Chemical Engineering, Hunan Key Laboratory of Two-Dimensional Materials, Hunan University, Changsha 410082, P. R. China. E-mail: wangleihnu@hnu.edu.cn; meilinhoo@yeah.net; wangtao2014@hnu.edu.cn; xidongduan@hnu.edu.cn
bSchool of Physics and Electronics, Hunan University, Changsha 410082, P. R. China
First published on 28th September 2020
Abstract
Potassium ion hybrid capacitors (KIHCs) have drawn growing interest owing to their outstanding energy density, power density and excellent cycling stability. However, the large ionic radius of potassium triggers a huge volume change during continuous K+ insertion/extraction processes, restricting the development of KIHCs. Here, we report N-doped carbon nanotubes (NCNTs) for high-performance K+ storage. The NCNTs possess a hierarchical structure and N functional groups and not only offer sufficient space to relieve the volume expansion, but also provide highly efficient channels to transport electrons and ions. As a result, the NCNTs anode presents a high specific capacity and an excellent cycling stability with an average decay rate of 0.0238% per cycle (the lowest value among the reported carbon-based anodes for K-ions batteries) during 3600 continuous cycles. A potassium ion hybrid capacitor (KIHC) was also designed with the NCNT anode and a commercial active carbon cathode and achieved both a high energy/power density (117.1 W h kg−1/1713.4 W kg−1) and a long cycle life (2000 cycles at 1 A g−1). Moreover, the in situ Raman and ex situ element mapping characterization demonstrate the outstanding electrochemical reversibility of the NCNTs. This work provides a superior strategy to design low-cost anode materials with excellent K+ storage electrochemistry.
New conceptsPotassium ion hybrid capacitors (KIHCs), integrating the advantages of ion-full batteries and supercapacitors, possess a high energy/power density and long cycling stability, and have become a hot topic of research. In this work, N-doped carbon nanotubes (NCNTs) were synthesized as an anode for highly robust K-ions batteries (KIBs) and KIHCs. The NCNTs, possessing a hierarchical structure and a high content of N-doping can offer sufficient space to relieve volume expansion, and provide highly efficient channels to transport electrons and ions. Therefore, the NCNTs delivered a high specific reversible capacity, extraordinary rate performance and outstanding cycling stability (190.2 mA h g−1 at a high current density of 1000 mA g−1 after 3600 cycles with an average decay rate of 0.0238% per cycle). Furthermore, the KIHCs based on the NCNTs anode exhibited a high energy density (117.1 W h kg−1 at 50 mA g−1), excellent power density (1713.4 W h kg−1 at 1 A g−1), outstanding long cycle life (2000 cycles at 1 A g−1) and extraordinary capacity retention (81.6% after 2000 cycles). The working mechanism of the NCNTs electrode was presented by analyzing in situ Raman spectra and ex situ element mapping. This work highlights the effective mechanism of N doping and hierarchical porosity for carbon-based anodes on the performance of batteries and hybrid capacitors. |
1. Introduction
Hybrid capacitors (HCs), integrating the advantages of ion-full batteries and supercapacitors, possess a high energy/power density and long cycling stability, and have become a hot topic of research.1,2 In particular, lithium ion hybrid capacitors (LIHCs) have the potential to become promising novel energy storage devices in the fields of electric vehicles and portable mobile devices.3,4 However, the ever-increasing costs induced by limited lithium resources have seriously restricted their further applications.4,5 Therefore, alternative storage devices, such as sodium ion hybrid capacitors (SIHCs) and potassium ion hybrid capacitors (KIHCs), have attracted significant interest.6–11 Potassium is a chemical element with a low cost and natural abundance (2.09 wt% for K, 2.38 wt% for Na, 0.0017 wt% for Li in the earth's crust).12–14 The low negative redox potential of K/K+ (−2.93 V) compared with Na/Na+ (−2.71 V), is closer to that of Li/Li+ (−3.04 V), and affords a high voltage and energy density.15 In addition, KIHCs are safer than SIHCs and LIHCs because the potassiation potential (0.2 V vs. K/K+) is higher than the sodiation potential (0.05 V vs. Na/Na+) and the lithiation potential (0.1 V vs. Li/Li+).13,16 Moreover, compared with Li+ and Na+, K+ displays a higher transport number of smaller solvated potassium ions and a lower desolvation energy, leading to faster diffusion kinetics.17,18 However, the large ionic radius of K+, to a considerable extent, narrows the scope of suitable electrode materials.Carbon-based electrode materials are regarded as the most promising ones for large-scale applications owing to their merits of a high natural abundance and high electronic conductivity.19–22 Unfortunately, the huge volume change (61% caused by KC8vs. 10% by LiC6) during repeated K+ insertion/extraction results in a low reversible capacity, inferior cycling stability and rate capability, severely hindering the development of KIHCs.23,24 Thus, the ideal structural design of the carbon anode is essential to achieving high performance KIHCs. In addition, heteroatom doping has been confirmed to be effective in improving the electrochemical performance of the carbonaceous materials.25–27 More defects are created that produce more active sites, while the electron conductivity is improved to ameliorate kinematics, thus enhancing the charge transfer.25,28,29 The higher electronegativity of the N atom causes a stronger interaction with the cations during the cycles.28 In particular, pyridinic N can cause more mesoporous defects in the carbon material, improve the adsorption capacity of K+, and finally enhance the electrochemical energy storage capacity.30 Hence, it is of great significance to fabricate ideal carbon-based electrodes with a superb structural design and N doping for robust KIHCs.
In this work, N-doped carbon nanotubes (NCNTs) were synthesized as an anode for highly robust K-ions batteries (KIBs) and KIHCs. The NCNTs with a hierarchically structure and high-content of N doping offer fast pathways for electrons and ions, and also provide abundant reactive sites to obtain a high utilization rate of the electrode materials, as well as offer sufficient space to buffer the volume expansion. Therefore, the NCNTs deliver a high specific reversible capacity, extraordinary rate performance and outstanding cycling stability (190.2 mA h g−1 at a high current density of 1000 mA g−1 after 3600 cycles with an average decay rate of 0.0238% per cycle). Furthermore, the KIHCs based on the NCNTs anode exhibited a high energy density (117.1 W h kg−1 at 50 mA g−1), excellent power density (1713.4 W h kg−1 at 1 A g−1), outstanding long cycling life (2000 cycles at 1 A g−1) and extraordinary capacity retention (81.6% after 2000 cycles). The working mechanism of the NCNTs electrode is presented by analyzing the in situ Raman spectra and ex situ element mapping. This work highlights the effective mechanism of N doping and hierarchical porosity for carbon-based anodes for the performance of batteries and hybrid capacitors.
2. Experimental
2.1 Synthesis of the NCNTs, CCNSs and SNCNTs
The NCNTs were synthesized by a one-pot method using zinc acetate, oleic acid and urea as precursors. Firstly, 20 g zinc acetate ((CH3COO)2Zn), 22.45 ml oleic acid (C18H34O2), and 1 g urea (CH4N2O) were added to an agate mortar and ground for about 20 min until the precursor was thoroughly mixed. The ground white solid was then placed in a tube furnace and annealed at 700 °C for 2 h in an Ar atmosphere (heating rate was maintained at 3 °C min−1). Next, the as-annealed sample was immersed in concentrated hydrochloric acid (HCl, 37 wt%) and stirred for 48 h. Then, vacuum filtration was carried out until the pH = 7. Finally, the sample was dried in a vacuum to obtain the NCNTs. For comparison, the crimped carbon nanosheets (CCNSs) and sealed N-doped carbon nanotubes (SNCNTs) were also prepared by controlling the amount of urea to 0 and 3 g, respectively.2.2 Material characterization
Scanning electron microscopy (SEM) images and energy-dispersive X-ray spectroscopy (EDS) mappings of the samples were obtained on a SIGMA microscope (Zeiss, Germany) equipped with an EDS spectrometer. Transmission electron microscopy (TEM) images, X-ray diffractometry (XRD) patterns, and Raman spectra were obtained on a JEM-2100F microscope (JEOL, Japan), an XRD-6100 spectrometer with Cu-Kα radiation (Shimadzu, Japan), and an in Via-reflex confocal Raman spectrometer with a 532 nm laser as the excitation source (Renishaw, UK), respectively. Nitrogen adsorption–desorption isotherms at 77 K were measured on an ASAP 2020 absorption analyzer (Micromeritics, America). The specific surface area and the pore size distribution of the samples were deduced using the Brunauer–Emmett–Teller (BET) method and the Barrett–Joyner–Halenda method, respectively. X-ray photoelectron spectra were obtained on a K-Alpha ESCALAB 250Xi instrument (ThermoFisher-VG Scientific, USA), with Al Kα radiation as the excitation source. The binding energies were corrected for specimen charging by referencing C 1s (284.8 eV).2.3 Electrochemical measurements
The KIBs and KIHCs anodes were made by mixing superconducting carbon (SC), sodium carboxymethyl cellulose (CMC) and the as-prepared carbon samples (NCNTs, CCNSs, SNCNTs) at a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8. The NCNTs, SC and CMC were ball-milled in a mixed solvent of water and ethanol to obtain a homogeneous slurry. Then, the obtained slurry was coated onto a copper foil. After drying in a vacuum for 10 h (60 °C), the copper foil was cut into a 12 mm diameter disc using a microtome to form a working electrode with an average loading of 1–2 mg cm−2. The capacitor cathode was made by mixing SC, poly(vinylidene fluoride) (PVDF) and commercial active carbon (AC) with a mass ratio of 1
8. The NCNTs, SC and CMC were ball-milled in a mixed solvent of water and ethanol to obtain a homogeneous slurry. Then, the obtained slurry was coated onto a copper foil. After drying in a vacuum for 10 h (60 °C), the copper foil was cut into a 12 mm diameter disc using a microtome to form a working electrode with an average loading of 1–2 mg cm−2. The capacitor cathode was made by mixing SC, poly(vinylidene fluoride) (PVDF) and commercial active carbon (AC) with a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8. The AC, SC, PVDF were ball-milled in N-methyl-2-pyrrolidone (NMP) to obtain a homogeneous slurry. Then, the obtained slurry was coated onto an aluminum foil. After drying in a vacuum for 10 h (60 °C), the aluminum foil was cut into a 12 mm diameter disc using a microtome to form a capacitor cathode with an average loading of 2–5 mg cm−2. To assemble the KIBs (half-cell) with an average loading of 1–2 mg cm−2 in 2032 type coin cells, glass microfiber filters (GE Co., America), 5 M KPF6 blended with ethylene carbonate and diethyl carbonate (EC
8. The AC, SC, PVDF were ball-milled in N-methyl-2-pyrrolidone (NMP) to obtain a homogeneous slurry. Then, the obtained slurry was coated onto an aluminum foil. After drying in a vacuum for 10 h (60 °C), the aluminum foil was cut into a 12 mm diameter disc using a microtome to form a capacitor cathode with an average loading of 2–5 mg cm−2. To assemble the KIBs (half-cell) with an average loading of 1–2 mg cm−2 in 2032 type coin cells, glass microfiber filters (GE Co., America), 5 M KPF6 blended with ethylene carbonate and diethyl carbonate (EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC = 1
DMC = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Nanjing Mojiesi Energy Techlogy Co., China), and potassium metal were used as the separator, electrolyte and the counter electrode, respectively. To assemble the KIHCs (full-cell) with an average loading of 3–7 mg cm−2 in 2032 type coin cells, glass microfiber (GE Co., America), 5 M potassium bis(fluorosulfonyl)amide (KFSI) blend with ethylene carbonate and diethyl carbonate (EC
1) (Nanjing Mojiesi Energy Techlogy Co., China), and potassium metal were used as the separator, electrolyte and the counter electrode, respectively. To assemble the KIHCs (full-cell) with an average loading of 3–7 mg cm−2 in 2032 type coin cells, glass microfiber (GE Co., America), 5 M potassium bis(fluorosulfonyl)amide (KFSI) blend with ethylene carbonate and diethyl carbonate (EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC = 1
DMC = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Nanjing Mojiesi Energy Techlogy Co., China) and AC were used as the separator, electrolyte and counter electrode, respectively. The capacity of the half-cell was calculated based only on the mass of NCNTs. The capacity and capacitance of the full-cell were calculated based on the mass of both the anode and cathode. An MB-Labstar (1200/780) glove box (M. Braun Intergas Systems Co., Ltd, Germany) under an Ar atmosphere (H2O < 0.5 ppm, O2 < 0.5 ppm) was used during the entire cell assembling process. The charge–discharge and cyclic voltammetry (CV) tests were measured on a CT2001A battery test system (LANDTE Co., China) and a CHI660E electrochemical station (CHI instrument Co., China), respectively. Electrochemical impedance spectroscopy (EIS) measurements were conducted on an Autolab 302N electrochemical station (Metrohm, Switzerland) over a frequency range of 0.01 Hz to 100 kHz.
1) (Nanjing Mojiesi Energy Techlogy Co., China) and AC were used as the separator, electrolyte and counter electrode, respectively. The capacity of the half-cell was calculated based only on the mass of NCNTs. The capacity and capacitance of the full-cell were calculated based on the mass of both the anode and cathode. An MB-Labstar (1200/780) glove box (M. Braun Intergas Systems Co., Ltd, Germany) under an Ar atmosphere (H2O < 0.5 ppm, O2 < 0.5 ppm) was used during the entire cell assembling process. The charge–discharge and cyclic voltammetry (CV) tests were measured on a CT2001A battery test system (LANDTE Co., China) and a CHI660E electrochemical station (CHI instrument Co., China), respectively. Electrochemical impedance spectroscopy (EIS) measurements were conducted on an Autolab 302N electrochemical station (Metrohm, Switzerland) over a frequency range of 0.01 Hz to 100 kHz.
3. Results and discussion
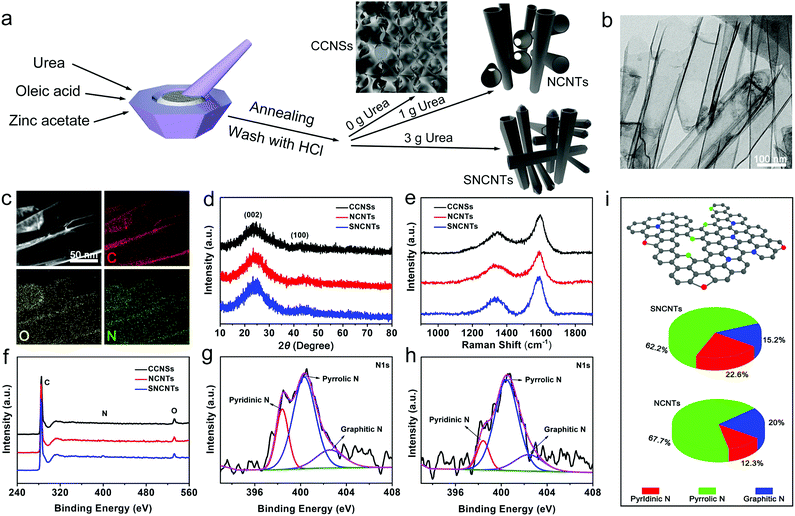
Currently, significant attention has been focused on the preparation of carbon nanotubes with hierarchically porous structures via various methods, including direct pyrolysis methods,31,32 template methods33,34 self-templating methods,35–37 and so on. As shown in Fig. 1a, oleic acid and urea could act as the carbon source and nitrogen source, respectively, and zinc oxide produced by thermal decomposition of zinc acetate acts as a self-template. Specifically, zinc acetate was decomposed to zinc oxide as a hard template, while oleic acid was carbonized to form the CCNSs, NCNTs and SNCNTs along with the walls of zinc oxides during the material synthesis processes.38–40 In these processes, urea releases ammonia gas under heating conditions, which provides an alkaline environment for the annealing process.41 As the pH increases, it is found that zinc oxide tends to form a columnar like morphology.42,43 SEM images of the CCNSs (0 g urea), NCNTs (1 g urea) and SNCNTs (3 g urea) taken before washing with HCl are shown in Fig. S1a–c (ESI†), which clearly exhibits the morphology of zinc oxide as self-templates obtained after the annealing of zinc acetate with different contents of urea. In addition, the effect of the alkaline environment and the increasing content of the urea could also enlarge the distance between the self-templates, which is beneficial to the accumulation and growth of carbonized oleic acid. Both the large space and additional carbon source from urea help nanotubes to form a thicker wall. Therefore, after the templates are washed by HCI, the shape and thickness of the CCNSs, NCNTs and SNCNTs are different with different amounts of urea. Furthermore, the morphology of the CCNSs is sheet like, that of the NCNTs is tube like and the SNCNTs are also tube like but thicker. In a typical procedure, zinc acetate, oleic acid and urea were ground until the precursor was thoroughly mixed, and then placed in a tube furnace for annealing at 700 °C. After that, the as-annealed sample was immersed in concentrated HCl and stirred for 48 h to remove the Zn+. By controlling the quality of urea during the preparation process, a different structural morphology can be obtained (Fig. 1a). The morphology and spatial structures of the NCNTs, CCNSs and SNCNTs were investigated using SEM characterization (Fig. S2, ESI†). In Fig. 1b and Fig. S3 (ESI†), the TEM images confirmed the formation mechanism of the CCNSs, NCNTs and SNCNTs, corresponding to the morphologic evolution process in the SEM images. Obviously, the diameter of the NCNTs is approximately in the range of 80–120 nm. The EDS and element mapping images shown in Fig. S4 (ESI†) and Fig. 1c show the uniform distribution of C, N, and O in the NCNTs. Similar results were obtained in the EDS pattern and element mapping images of the CCNSs and SNCNTs (Fig. S4 and S5, ESI†). The materials used in KIBs and KIHCs, and the typical internal hollow structure of the NCNTs with a large specific surface area can shorten the diffusion distance of K+ and reduce the insertion and extraction space resistance. In addition, heteroatomic (N) doping and rich potassium storage sites allow more K+ to participate in the electrochemical reaction, ensuring a high specific capacity.Fig. 1d shows the XRD patterns of the NCNTs, CCNSs and SNCNTs. The three samples show two diffraction peaks at 2θ = 23–26° and 43°, which match the (002) and (100) diffraction peaks of carbon materials, respectively.44 In Fig. 1e, the Raman spectra show that the three samples exhibit two characteristic peaks at around 1356 and 1596 cm−1, belonging to the D and G bands of the carbon materials, respectively. In general, the D and G bands represent the defective or disordered carbon and the relatively ordered carbon. The intensity ratio of the two bands ID/IG can measure the degree of disorder in the carbon material. Compared with the CCNSs and SNCNTs, the NCNTs exhibit a higher ID/IG value, which indicates the maximum degree of disorder in the NCNTs owing to its extra defect.45
Brunauer–Emmett–Teller measurements were conducted to investigate the specific surface areas, pore construction and pore size of the three samples. From the adsorption/desorption isotherms of the NCNTs, CCNSs and SNCNTs shown in Fig. S6a (ESI†), the typical IV type curves and obvious hysteresis curve (P/P0 = 0.4–1) demonstrate the presence of slit type holes within the hierarchical carbon matrix. Moreover, according to the BET analysis, the specific surface areas of NCNTs, CCNSs and SNCNTs were calculated to be 519.57, 182.92 and 393.11 m2 g−1, respectively. Owing to the hierarchically unsealed structure, the NCNTs possess the maximum specific surface area. In addition, the pore size distribution of the NCNTs, CCNSs and SNCNTs are shown in Fig. S6b (ESI†). The pore size range of the three samples is 2–5 nm, indicating the presence of mesopores in the materials. This type of porous structure can produce a large number of active sites to promote K+ diffusion, which plays an important role in enhancing the electrochemical properties of the electrodes.46
X-ray photoelectron spectroscopy (XPS) was used to detect the 1s electron binding energy of various elements in the material surface to obtain their chemical bonding state. Fig. 1f shows that the XPS results for the NCNTs and SNCNTs have obvious characteristic peaks at 285, 400 and 532 eV, corresponding to the peaks of C 1s, N 1s and O 1s, and only two apparent peaks of C 1s (285 eV) and O 1s (532 eV) appeared in the spectrum of the non-N-doped CCNSs. Table S1 (ESI†) show the proportions of C, N and O in the NCNTs are 95.57%, 1.21% and 3.22%, respectively. The proportion of elements in the CCNSs and SNCNTs are also listed in Table S1 (ESI†).
According to the high-resolution N 1s spectra of the NCNTs and SNCNTs in Fig. 1g and h, and the calculated ratio of pyridinic N, pyrrolic N and graphite N in the NCNTs and SNCNTs shown in Fig. 1i, the ratio of pyridinic N in the NCNTs is higher than that of the SNCNTs, the high ratio of pyridinic N may induce large amounts of defects and create more active sites, making NCNTs outstanding for application as energy storage devices.25,30 Based on the structural features model shown in Fig. 1i, the special six-membered ring structure introduced by N-doping can greatly enhance the adsorption capacity of the ions.47 Accordingly, as shown in Fig. S7a–c (ESI†), the high-resolution C 1s XPS spectra of all three CCNSs, NCNTs and SNCNTs are deconvoluted to three peaks (C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (284.7 eV), C
O (284.7 eV), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N/C–O (285.9 eV), and C
N/C–O (285.9 eV), and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (288.9 eV)).48,49 In addition, as shown in the high resolution O 1s XPS spectra of all three CCNSs, NCNTs and SNCNTs shown in Fig. S7d–f (ESI†), the peaks at 531.4, 532.9 and 536.6 eV in these figures can be indexed to the C
O (288.9 eV)).48,49 In addition, as shown in the high resolution O 1s XPS spectra of all three CCNSs, NCNTs and SNCNTs shown in Fig. S7d–f (ESI†), the peaks at 531.4, 532.9 and 536.6 eV in these figures can be indexed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, C–O bonds and H2O, and the N–C–O peaks at 532.1 eV can only be seen in the O 1s XPS spectra of the NCNTs and SNCNTs.50,51 Based on the evidence presented above, the characteristics of nitrogen-doped materials can be further proven by XPS.
O bonds, C–O bonds and H2O, and the N–C–O peaks at 532.1 eV can only be seen in the O 1s XPS spectra of the NCNTs and SNCNTs.50,51 Based on the evidence presented above, the characteristics of nitrogen-doped materials can be further proven by XPS.
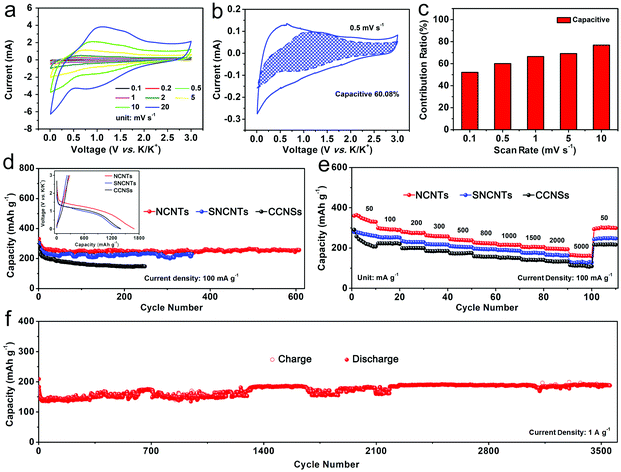
The electrochemical properties of the NCNTs, CCNSs and SNCNTs electrodes in the KIBs were investigated using CV, constant current charge–discharge and rate tests. Generally, new chemical substances that come from the anode material polarization and electrolyte decomposition will react at the solid–liquid interface, forming an irreversible passive film (solid electrolyte interface (SEI)) that can cover the surface of the electrode material during the first charge–discharge cycle. The charge–discharge curves of the NCNTs, CCNSs and SNCNTs electrodes were tested and recorded at a current density of 100 mA g−1 between 0.01–3 V (see inset in Fig. 2d and Fig. S8, ESI†). It was found that all three electrodes show a large voltage platform at approximately 1 V in the first discharge curve, we believe that the large voltage platform is derived from the irreversible reaction of the electrolyte and the formation of the SEI film,52,53 which can reduce the initial coulombic efficiency (ICE). We can increase the ICE of these three materials by optimizing the electrolytes, pre-potassiation of the anodes and engineering the defects.54–57 The next curves in the 10th, 100th, 250th and 620th cycles of the NCNTs were almost overlapped, indicating the excellent electrochemical reversibility of the NCNTs, and the charge–discharge curves at high densities of 200 mA g−1 and 1 A g−1 also support the result (Fig. S9, ESI†). As shown in the CV curves of the NCNTs anode in the first cycle (Fig. S10, ESI†), a reduction peak appeared at 0.6 V owing to the irreversible reaction of the electrolyte and the formation of the SEI film. It should be noted that after the first cycle, the CV curves of the NCNTs electrode tend to overlap, which also shows the outstanding electrochemical reversibility. Additionally, the EIS curves of the three materials are shown in Fig. S11 (ESI†). It can be seen that the NCNTs exhibit the lowest charge transfer resistance, suggesting the best electron conductivity and ion diffusion ability within these three materials. Apparently, all these results prove that NCNTs anodes have obvious advantages in the electrochemical storage of potassium ions, compared with SNCNTs and CCNSs.
The superior electrochemical properties of the NCNTs anodes are ascribed to the excellent electrochemical reaction kinetics on the electrode surface and inside. In order to analyze the kinetics of the NCNTs electrode, the CV curves at different sweep speeds (0.1–20 mV s−1) were measured, as shown in Fig. 2a. As the sweep speed gradually changes from 0.1 to 20 mV s−1, the NCNTs anode can maintain its original shape with a small movement of the peak voltage, indicating insignificant polarization of the anode.
The kinetics properties of the electrode materials can be analyzed using the following equations:58
| i = avb | (1) |
| i(V)/v1/2 = k1v1/2 + k2 | (2) |
By linearly fitting log(i) and log(v), the value of the adjustable constant b can be calculated, and it can be determined whether the electrode material has pseudocapacitance properties during charging and discharging. When the b value is equal to 0.5, the electrode material exhibits an ideal intercalation process, which is represented as a battery property; when the b value is in the range of 0.5–1, the electrode material exhibits the dual property of the battery and the pseudocapacitance; and when the b value is 1, the electrode material only exhibits a surface charge storage process and thus exhibits a pseudocapacitance property.58 After logarithmic fitting of the peak current and the scanning velocity in Fig. S12 (ESI†), the adjustable constant b is calculated to be 0.838, indicating that the electrode material exhibits the properties of both a battery and pseudocapacitance. The contribution of the capacitance charge in the overall capacity can be calculated according to eqn (2). At a specified voltage, i(V)/v1/2 and v1/2 are linearly fitted to obtain the value of the adjustable constant k1, and k1v represents the contribution of the capacitance to the current at that particular voltage. As shown in Fig. 2b, the area of the shadow portion is the result of nonlinear fitting at a scanning speed of 0.5 mV s−1, which illustrates the current response distribution contributed by the capacitance process. From the calculation, the ratio of the capacitance contribution reaches 60.08%. As the scanning speed becomes larger, the proportion of the capacitance contribution also increases, as shown in Fig. 2c. At scan speeds of 0.1, 0.5, 1, 5 and 10 mV s−1, the capacitance contribution ratio can reach 52.11%, 60.08%, 66.49%, 69.17% and 76.86%, respectively. Therefore, the NCNTs manifest a small degree of polarization and a large proportion of the capacitance contribution, representing excellent kinetics.
To study the electrochemical properties of the NCNTs anode, the cycle performances of the KIBs with the NCNTs, CCNSs, SNCNTs electrodes were measured at a current density of 100 mA g−1 between 0.01–3 V (vs. K/K+), as shown in Fig. 2d. The NCNTs electrode can deliver a discharge capacity of 257.5 mA h g−1 even after 620 cycles with a retention ratio of 77.7% (average decay rate of 0.0358% per cycle). Compared with the NCNTs, the specific capacity of the SNCNTs anode after 360 cycles is only 195.1 mA h g−1, and the capacity of the CCNSs was 147.4 mA h g−1 after 250 cycles. Obviously, at the same voltage window and current densities, the NCNTs electrodes exhibit a superior capacity and cycle stability. In addition, the cycling performance of the NCNTs electrodes at 50 and 200 mA g−1 are shown in Fig. S13 (ESI†), respectively. The NCNTs anode can maintain a capacity of 267.9 mA h g−1 at a current density of 50 mA g−1 with a decay rate of 0.105% per cycle after 280 cycles. In addition, it can still deliver a capacity of 223.2 mA h g−1 at a current density of 200 mA g−1 with a decay rate of 0.0342% per cycle after 840 cycles.
Moreover, the rate and long cycling performance at different current densities were investigated. As shown in Fig. 2e, the NCNTs anode can provide a specific capacity of 358.8, 299.2, 279.1, 259.5, 239.5, 222.6, 216.3, 205.4 and 193.3 mA h g−1 at current densities of 0.05, 0.1, 0.2, 0.3, 0.5, 0.8, 1, 1.5 and 2 A g−1, respectively. It is worth noting that the capacity can still be maintained at around 162.2 mA h g−1 even at a high current density of 5 A g−1. Moreover, the capacity can be restored to about 313.5 mA h g−1 after the current density is restored to 0.05 A g−1. Also, Fig. 2f shows the cycling performance of the NCNTs anode at a high current density of 1 A g−1. It should be noted that, even after 3600 cycles, the KIBs with the NCNTs anode can retain a capacity of 190.2 mA h g−1 with an average decay rate of 0.0238% per cycle, which is the lowest value among the as-reported carbon-based anodes so far. For the SNCNTs and CCNSs anodes, neither the capacity at various current densities nor the capacity recovery rate after returning to a small current density can be compared with that of the NCNTs electrodes.
Owing to the unique 3D structure, the NCNTs can provide an excellent diffusion channel for K+ to shuttle more freely. The outstanding rate performance, together with the excellent capacity decay rate verified above, ensure the advantages of the NCNTs in the competition for anode materials for KIBs. Table S1 (ESI†) is a statistical table showing the capacity and capacity decay rates of carbon materials. Apparently, the NCNTs exhibit an excellent energy storage performance, and the lowest capacity decay rate at a high current density (1 A g−1) among the materials reported to date.
Based on the half-cell test, capacitors with the configuration of NCNTs anode//5 M KFSI (EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC = 1
DMC = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (electrolyte)//active carbon (AC) cathode were assembled to study the electrochemical behaviors of the KIHCs. As shown in Fig. S14 (ESI†), the charging and discharging behavior of the AC cathode displays a typical capacitive process between 2.0 and 4.0 V (vs. K/K+) and provides a reversible capacity of about 37.5 mA h g−1 over 200 cycles at a current density of 100 mA g−1. In order to reasonably match the cathode and anode in the KIHCs full cell, the voltage window of the anode used for matching is 0–2 V. As shown in Fig. S15 (ESI†), both the cycling performance and the charge–discharge curves prove that the released capacities of NCNTs are almost the same under the voltage windows of 0–2 V and 0–3 V at the same current density, and the NCNTs anode used for assembling the KIHCs hybrid capacitor has undergone pre-potassiation, which means the low initial coulombic efficiency and the voltage window (0–2 V)of the anode will not affect the matching of the full cell (Fig. S16, ESI†).
1) (electrolyte)//active carbon (AC) cathode were assembled to study the electrochemical behaviors of the KIHCs. As shown in Fig. S14 (ESI†), the charging and discharging behavior of the AC cathode displays a typical capacitive process between 2.0 and 4.0 V (vs. K/K+) and provides a reversible capacity of about 37.5 mA h g−1 over 200 cycles at a current density of 100 mA g−1. In order to reasonably match the cathode and anode in the KIHCs full cell, the voltage window of the anode used for matching is 0–2 V. As shown in Fig. S15 (ESI†), both the cycling performance and the charge–discharge curves prove that the released capacities of NCNTs are almost the same under the voltage windows of 0–2 V and 0–3 V at the same current density, and the NCNTs anode used for assembling the KIHCs hybrid capacitor has undergone pre-potassiation, which means the low initial coulombic efficiency and the voltage window (0–2 V)of the anode will not affect the matching of the full cell (Fig. S16, ESI†).
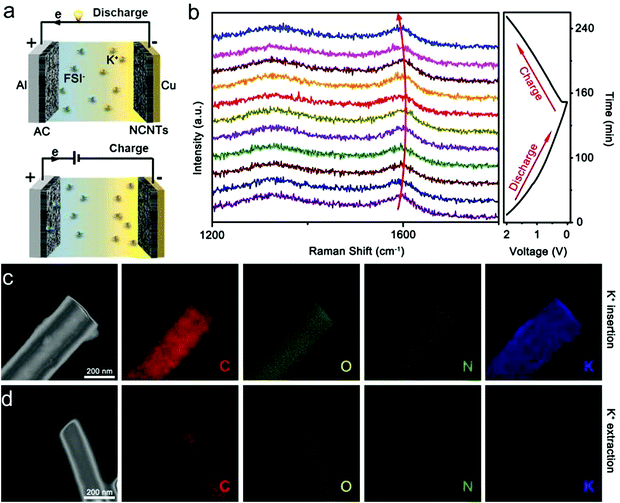
Fig. 3a displays the working schematic of the KIHCs, which reveals a capacitive cathode and a battery behavior anode. During the charge progress, K+ in the electrolyte will insert into the anode materials, while the FSI− will be adsorbed on the surface of the cathode. Conversely, the anions/cations will leave the cathode/anode and return to the electrolyte, respectively. In order to study the working mechanism of NCNTs electrode, in situ Raman spectra (Fig. 3b) were used to study the NCNTs anode during the charge–discharge process. During the discharge process, the G band of the NCNTs (1585 cm−1) blue shifts to a larger wavenumber and reaches 1606 cm−1 at a discharge voltage of 0.01 V. During the charge process, the G band (1606 cm−1) significantly red shifted to lower values with the increased voltage. When the battery is charged to 2.0 V, the G band recovers to 1583 cm−1, which indicates the potassiation/depotassiation process. TEM and element mapping were also carried out to further confirm the operation mechanism of the anode in KIHCs. When KIHC is full charged, it is clear that the amount of the K element in the anode increased significantly, indicating the successful insertion of K into the NCNTs electrode (Fig. 3c). However, the content of K in the anode remarkably reduced when the potassium ion hybrid capacitor (KIHC) was discharged to 0.01 V (Fig. 3d), implying the depotassiation occurs during the discharge process.
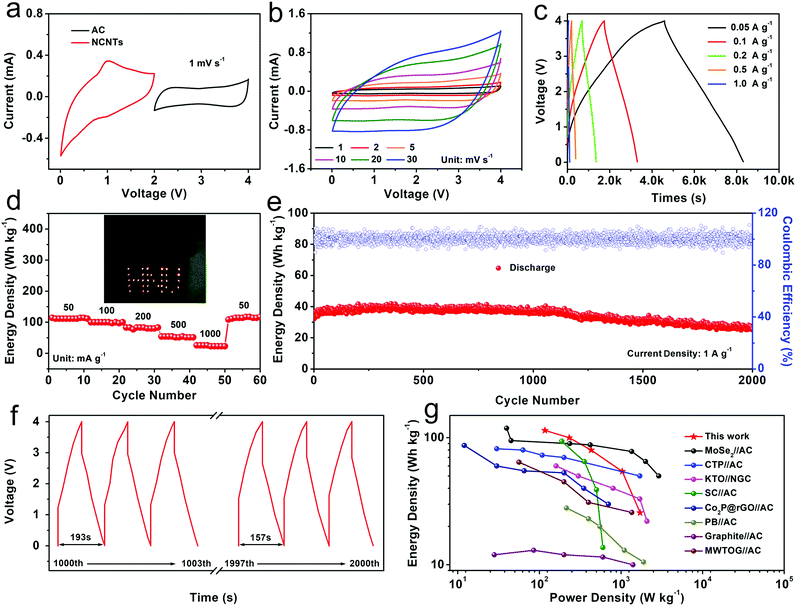
The general confirmation method of the KIHCs voltage window is to subtract the anode discharging platform from the cathode charging cut-off voltage, and then adjust the coulombic efficiency.59,60 As shown in Fig. S17 (ESI†), it is easy to determine that the cathode charging cut-off voltage is 4.0 V and there is no anode discharge platform. However, we know that the initial potassiation voltage of the graphite like carbon material is 0.01 V.45,61 Thus, we defined the voltage window for KIHC as 0.01–4 V. In view of the discrepant storage mechanisms and rate capabilities of the anode and cathode, the CV curve (Fig. 4a) is portrayed to clearly display the concordant matching with a mass ratio of about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 between the NCNTs anode and the AC cathode. Fig. 4b shows the CV curves of the KIHCs at scan speeds from 1 to 30 mV s−1 without distinct peaks and with similar rectangle shapes, exhibiting the capacitive behavior of the device. All of the CV curves can keep alike shape characteristics with a gradual increase in the scan speeds, suggesting the good reversibility of the KIHCs. In order to analyze the diffusion controlled and surface capacitive controlled process, the pseudocapacitance of the KIHCs was also measured. According to eqn (1), by linearly fitting log(i) and log(v), the values of the adjustable constant b can be calculated as 0.805, which means the electrode materials exhibit the properties of both the capacitive behavior and the diffusion controlled process.
3 between the NCNTs anode and the AC cathode. Fig. 4b shows the CV curves of the KIHCs at scan speeds from 1 to 30 mV s−1 without distinct peaks and with similar rectangle shapes, exhibiting the capacitive behavior of the device. All of the CV curves can keep alike shape characteristics with a gradual increase in the scan speeds, suggesting the good reversibility of the KIHCs. In order to analyze the diffusion controlled and surface capacitive controlled process, the pseudocapacitance of the KIHCs was also measured. According to eqn (1), by linearly fitting log(i) and log(v), the values of the adjustable constant b can be calculated as 0.805, which means the electrode materials exhibit the properties of both the capacitive behavior and the diffusion controlled process.
From the charge–discharge curves shown in Fig. 4c, the KIHCs could operate for over 8500 s with a high energy density of 115 W h kg−1 (corresponding to a single capacitance of 96.98 F g−1), and a power density of 116.8 W kg−1, which indicates its high performance in energy storage. In addition, the KIHCs exhibit an outstanding rate performance. As shown in Fig. 4d, the KIHCs can provide energy densities of 114.3, 100, 80, 54.3 and 25.7 W h kg−1 (equivalent to the single capacitances of 96.03, 88.98, 80.35, 38.5, and 19.5 F g−1), corresponding to power densities of 116.8, 231.7, 430.5, 1034.3 and 1713.4 W kg−1 at current densities of 50, 100, 200, 500 and 1000 mA g−1, respectively (all the current, power, and energy densities are calculated based on the total active material mass of the anode and cathode). Surprisingly, when the current density returned to 50 mA g−1, the energy density returned to 117.1 W h kg−1 (equivalent to the single capacitance of 107.63 F g−1), corresponding to a power density of 112.8 W kg−1, demonstrating an outstanding rate recoverability. Furthermore, the KIHCs can easily light up a “HNU” (Hunan University) logo consisting of red light-emitting diodes for a long time, as shown in the inset of Fig. 4d.
As shown in Fig. 4e, the KIHCs exhibit an outstanding cycling stability with a capacity retention of 81.6% after 2000 cycles at 1 A g−1 (energy density of 26.2 W h kg−1 (corresponding to the single capacitance of 19.75 F g−1), equivalent to a power density of 1278.1 W kg−1). The charge–discharge profiles of the 1000th–1003th and 1997th–2000th (Fig. 4f) are almost the same, which also shows the excellent cycling stability. Compared with other KIHCs, NCNTs//AC KIHCs exhibit a relatively high energy density/power density. Fig. 4g shows the statistical figure of the energy density and power density of the carbon-based KIHCs.9,62–68 Apparently, the KIHCs exhibit an excellent energy storage performance, and outstanding power density among the as-reported KIHCs devices.
4. Conclusions
In summary, the NCNTs with a hierarchical structure and high-content of N doping were designed as an anode for superior half-cell KIBs and KIHCs. The appropriate N-doping content makes this anode material form a unique, hierarchically nanotube structure, with fast conductive pathways of electrons and ions, and sufficient space to overcome the damage caused by the volume expansion, which ensures the outstanding electrochemical performance. Thus, the KIBs with the NCNTs anode maintained a high reversible specific capacity and a low average decay rate of 0.0238% among the reported carbon anodes for KIHCs. Furthermore, KIHCs designed with the NCNTs as the anode and commercial active carbon as the cathode can achieve a high energy/power density and long cycle life. Moreover, the in situ characterization confirms its high reversibility, consistent with the CV and durability electrochemical tests. Through the kinetic study, the NCNTs also displayed a high capacitance charge contribution and reduced degree of polarization. This work represents a key step towards synthesizing a high-performance anode for KIBs and KIHCs for commercial applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the China Postdoctoral Science Foundation (Grant No. 2019M652757, 2020TQ0091 and 2020M672491), and the National Natural Science Foundation of China (Grant No. 21805076 and 22005092).Notes and references
- F. Liu, Z. Chen, G. Fang, Z. Wang, Y. Cai, B. Tang, J. Zhou and S. Liang, Nano-Micro Lett., 2019, 11, 25 CrossRef CAS.
- W. Zuo, R. Li, C. Zhou, Y. Li, J. Xia and J. Liu, Adv. Sci., 2017, 4, 1600539 CrossRef.
- B. Li, J. Zheng, H. Zhang, L. Jin, D. Yang, H. Lv, C. Shen, A. Shellikeri, Y. Zheng, R. Gong, J. Zheng and C. Zhang, Adv. Mater., 2018, 30, 1705670 CrossRef.
- H. Wang, C. Zhu, D. Chao, Q. Yan and H. Fan, Adv. Mater., 2017, 29, 1702093 CrossRef.
- C. Gao, Q. Wang, S. Luo, Z. Wang, Y. Zhang, Y. Liu, A. Hao and R. Guo, J. Power Sources, 2019, 415, 165–171 CrossRef CAS.
- D. Li, X. Ren, Q. Ai, Q. Sun, L. Zhu, Y. Liu, Z. Liang, R. Peng, P. Si, J. Lou, J. Feng and L. Ci, Adv. Energy Mater., 2018, 8, 1802386 CrossRef.
- M. Zhang, X. Song, X. Ou and Y. Tang, Energy Storage Mater., 2019, 16, 65–84 CrossRef.
- H. Li, J. Lang, S. Lei, J. Chen, K. Wang, L. Liu, T. Zhang, W. Liu and X. Yan, Adv. Funct. Mater., 2018, 28, 1800757 CrossRef.
- L. Fan, K. Lin, J. Wang, R. Ma and B. Lu, Adv. Mater., 2018, 30, 1800804 CrossRef.
- H. Chen, Y. Qin, H. Cao, X. Song, C. Huang, H. Feng and X. Zhao, Energy Storage Mater., 2019, 17, 194–203 CrossRef.
- Y. Feng, S. Chen, J. Wang and B. Lu, J. Energy Chem., 2020, 43, 129–138 CrossRef.
- S. Li, Z. Zhao, C. Li, Z. Liu and D. Li, Nano-Micro Lett., 2019, 11, 14 CrossRef CAS.
- P. Xiong, P. Bai, S. Tu, M. Cheng, J. Zhang, J. Sun and Y. Xu, Small, 2018, 14, 1802140 CrossRef.
- J. Yang, Z. Ju, Y. Jiang, Z. Xing, B. Xi, J. Feng and S. Xiong, Adv. Mater., 2018, 33, 1700104 CrossRef.
- C. Liu, N. Xiao, H. Li, Q. Dong, Y. Wang, H. Li, S. Wang, X. Zhang and J. Qiu, Chem. Eng. J., 2020, 382, 121759 CrossRef CAS.
- L. Fan, R. Ma, J. Wang, H. Yang and B. Lu, Adv. Mater., 2018, 30, 1805486 CrossRef.
- Z. Jian, W. Luo and X. Ji, J. Am. Chem. Soc., 2015, 137, 11566 CrossRef CAS.
- M. Okoshi, Y. Yamada, S. Komaba, A. Yamada and H. Nakai, J. Electrochem. Soc., 2016, 164, A54–A60 CrossRef.
- L. Fan, R. Ma, Q. Zhang, X. Jia and B. Lu, Angew. Chem., Int. Ed., 2019, 58, 10500–10505 CrossRef CAS.
- G. Wang, X. Xiong, D. Xie, Z. Lin, J. Zheng, F. Zheng, Y. Li, Y. Liu, C. Yang and M. Liu, J. Mater. Chem. A, 2018, 6, 24317–24323 RSC.
- J. Ge, B. Wang, J. Wang, Q. Zhang and B. Lu, Adv. Energy Mater., 2020, 10, 1903277 CrossRef CAS.
- J. Ge, B. Wang, J. Zhou, S. Liang, A. M. Rao and B. Lu, ACS Mater. Lett., 2020, 2(7), 853–860 CrossRef CAS.
- E. Zhang, X. Jia, B. Wang, J. Wang, X. Yu and B. Lu, Adv. Sci., 2020, 2000470 CrossRef.
- K. Beltrop, S. Beuker, A. Heckmann, M. Winter and T. Placke, Energy Environ. Sci., 2017, 10, 2090–2094 RSC.
- Y. Xie, Y. Chen, L. Liu, P. Tao, M. Fan, N. Xu, X. Shen and C. Yan, Adv. Mater., 2017, 29, 1702268 CrossRef.
- Y. Yao, R. Xu, M. Chen, X. Cheng, S. Zeng, D. Li, X. Zhou, X. Wu and Y. Yu, ACS Nano, 2019, 13, 4695–4704 CrossRef CAS.
- Y. Zhong, B. Li, S. Li, S. Xu, Z. Pan, Q. Huang, L. Xing, C. Wang and W. Li, Nano-Micro Lett., 2018, 10, 56 CrossRef.
- K. Share, A. Cohn, R. Carter, B. Rogers and C. Pint, ACS Nano, 2016, 10, 9738–9744 CrossRef CAS.
- L. Fu, K. Tang, K. Song, P. Aken, Y. Yu and J. Maier, Nanoscale, 2014, 6, 1384–1389 RSC.
- J. Luo, K. Wang, X. Hua, W. Wang, J. Li, S. Zhang and S. Chen, Small, 2019, 15, 1805325 CrossRef.
- T. Liu, Z. Zhou, Y. Guo, D. Guo and G. Liu, Nat. Commun., 2019, 10, 675 CrossRef CAS.
- H. Li, J. Li, A. Thomas and Y. Liao, Adv. Funct. Mater., 2019, 29, 1904785 CrossRef.
- Z. Fan, Y. Liu, J. Yan, G. Ning, Q. Wang, T. Wei, L. Zhi and F. Wei, Adv. Energy Mater., 2012, 2, 419 CrossRef CAS.
- C. Liang, K. Hong, G. Guiochon, J. Mays and S. Dai, Angew. Chem., Int. Ed., 2004, 43, 5785 CrossRef CAS.
- W. Yang, W. Yang, F. Ding, L. Sang, Z. Ma and G. Shao, Carbon, 2017, 111, 419 CrossRef CAS.
- M. Sevilla and A. Fuertes, ACS Nano, 2014, 8, 5069 CrossRef CAS.
- L. Chen, Y. Lu, L. Yu and X. W. Lou, Energy Environ. Sci., 2017, 10, 1777 RSC.
- J. Yin, W. Zhang, N. Alhebshi, N. Salah and H. Alshareef, Small Methods, 2020, 4, 1900853 CrossRef CAS.
- X. Deng, B. Zhao, L. Zhu and Z. Shao, Carbon, 2015, 93, 48–58 CrossRef CAS.
- J. Lee, J. Kim and T. Hyeon, Adv. Mater., 2006, 18, 2073–2094 CrossRef CAS.
- S. Tischer, M. Börnhorst, J. Amsler, G. Schochb and O. Deutschmann, Phys. Chem. Chem. Phys., 2019, 21, 16785 RSC.
- C. Wu, X. Qiao, J. Chen, H. Wang, F. Tan and S. Li, Mater. Lett., 2006, 60, 1828–1832 CrossRef CAS.
- C. Wu, X. Qiao, J. Chen and H. Wang, Mater. Chem. Phys., 2007, 102, 7–12 CrossRef CAS.
- J. Ozaki, N. Kimura, T. Anahara and A. Oya, Carbon, 2007, 45, 1847–1853 CrossRef CAS.
- D. Yoon, D. Kim, K. Chung, W. Chang, S. Kim and J. Kim, Carbon, 2016, 98, 213–220 CrossRef CAS.
- H. He, Q. Gan, H. Wang, G. Xu, X. Zhang, D. Huang, F. Fu, Y. Tang, K. Amine and M. Shao, Nano Energy, 2018, 44, 217–227 CrossRef CAS.
- Z. Tan, K. Ni, G. Chen, W. Zeng, Z. Tao, M. Ikram, Q. Zhang, H. Wang, L. Sun, X. Zhu, X. Wu, H. Ji, R. Ruoff and Y. Zhu, Adv. Mater., 2017, 29, 1603414 CrossRef.
- H. Luo, M. Chen, J. Cao, M. Zhang, S. Tan, L. Wang, J. Zhong, H. Deng, J. Zhu and B. Lu, Nano-Micro Lett., 2020, 12, 113 CrossRef CAS.
- W. Luo, F. Li, W. Zhang, K. Han, J. Gaumet, H. Schaefer and L. Mai, Nano Res., 2019, 12(5), 1025–1031 CrossRef CAS.
- Q. Zhang, J. Mao, W. Pang, T. Zheng, V. Sencadas, Y. Chen, Y. Liu and Z. Guo, Adv. Energy Mater., 2018, 8, 1703288 CrossRef.
- Z. Lin, G. Waller, Y. Liu, M. Liu and C. Wong, Adv. Energy Mater., 2012, 2, 884–888 CrossRef CAS.
- J. Besenhard, M. Winter, J. Yang and W. Biberacher, J. Power Sources, 1995, 54, 228 CrossRef CAS.
- P. Verma, P. Maire and P. Novák, Electrochim. Acta, 2010, 55, 6332–6341 CrossRef CAS.
- H. He, D. Sun, Y. Tang, H. Wang and M. Shao, Energy Storage Mater., 2019, 23, 233–257 CrossRef.
- A. Ponrouch, E. Marchante, M. Courty, J. Tarascon and M. Palacin, Energy Environ. Sci., 2012, 5, 8572–8583 RSC.
- N. Liu, L. Hu, M. McDowell, A. Jackson and Y. Cui, ACS Nano, 2011, 5, 6487–6493 CrossRef CAS.
- P. Liu, Y. Li, Y. Hu, H. Li, L. Chen and X. Huang, J. Mater. Chem. A, 2016, 4, 13046–13052 RSC.
- S. Ren, R. Chen, E. Maawad, O. Dolotko, A. Guda, V. Shapovalov, D. Wang, H. Hahn and M. Fichtner, Adv. Sci., 2015, 2, 1500128 CrossRef.
- B. Deng, T. Lei, W. Zhu, L. Xiao and J. Liu, Adv. Funct. Mater., 2018, 28, 1704330 CrossRef.
- J. Ge, L. Fan, J. Wang, Q. Zhang, Z. Liu, E. Zhang, Q. Liu, X. Yu and B. Lu, Adv. Energy Mater., 2019, 8, 1903277 Search PubMed.
- J. Chen, B. Yang, H. Li, P. Ma, J. Lang and X. Yan, J. Mater. Chem. A, 2019, 7, 9247–9252 RSC.
- Y. Yi, Z. Sun, C. Li, Z. Tian, C. Lu, Y. Shao, J. Li, J. Sun and Z. Liu, Adv. Funct. Mater., 2020, 30, 1903878 CrossRef CAS.
- Z. Zhang, M. Li, Y. Gao, Z. Wei, M. Zhang, C. Wang, Y. Zeng, B. Zou, G. Chen and F. Du, Adv. Funct. Mater., 2018, 28, 1802684 CrossRef.
- S. Dong, Z. Li, Z. Xing, X. Wu, X. Ji and X. Zhang, ACS Appl. Mater. Interfaces, 2018, 10, 15542–15547 CrossRef CAS.
- Y. Wang, Z. Zhang, G. Wang, X. Yang, Y. Sui, F. Du and B. Zou, Nanoscale Horiz., 2019, 4, 1394–1401 RSC.
- Z. Le, F. Liu, P. Nie, X. Li, X. Liu, Z. Bian, G. Chen, H. Wu and Y. Lu, ACS Nano, 2017, 11, 2952–2960 CrossRef CAS.
- L. Zhou, M. Zhang, Y. Wang, Y. Zhu, L. Fu, X. Liu, Y. Wu and W. Huang, Electrochim. Acta, 2017, 232, 106–113 CrossRef CAS.
- A. Comte, Y. Reynier, C. Vincens, C. Leys and P. Azaïs, J. Power Sources, 2017, 363, 34–43 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Fig. S1–S14 and Tables S1–S4. See DOI: 10.1039/d0nh00451k |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |