Open Access Article
Open Access ArticleSolid-state Suzuki–Miyaura cross-coupling reactions: olefin-accelerated C–C coupling using mechanochemistry†
Tamae
Seo
a,
Tatsuo
Ishiyama
a,
Koji
Kubota
 *ab and
Hajime
Ito
*ab and
Hajime
Ito
 *ab
*ab
aDivision of Applied Chemistry and Frontier Chemistry Center, Faculty of Engineering, Hokkaido University, Sapporo, Hokkaido, Japan. E-mail: hajito@eng.hokudai.ac.jp; kbt@eng.hokudai.ac.jp
bInstitute for Chemical Reaction Design and Discovery (WPI-ICReDD), Hokkaido University, Sapporo, Hokkaido, Japan
First published on 22nd July 2019
Abstract
The Suzuki–Miyaura cross-coupling reaction is one of the most reliable methods for the construction of carbon–carbon bonds in solution. However, examples for the corresponding solid-state cross-coupling reactions remain scarce. Herein, we report the first broadly applicable mechanochemical protocol for a solid-state palladium-catalyzed organoboron cross-coupling reaction using an olefin additive. Compared to previous studies, the newly developed protocol shows a substantially broadened substrate scope. Our mechanistic data suggest that olefin additives might act as dispersants for the palladium-based catalyst to suppress higher aggregation of the nanoparticles, and also as stabilizer for the active monomeric Pd(0) species, thus facilitating these challenging solid-state C–C bond forming cross-coupling reactions.
Introduction
Solid-state organic reactions have attracted considerable interest in a variety of research areas due to their potentially lower environmental impact and the possibility to access reaction pathways that are unavailable in solution.1,2 In addition, solid-state reactions are particularly useful for substrates that are poorly soluble in common organic solvents.2 However, the development of highly efficient solid-state organic transformations whose performance is comparable to conventional solvent-based synthetic routes remains challenging.2 This is mainly due to poor mixing efficiency of the reactants and/or catalysts in the solid state, where organic molecules are arranged tightly and regularly.2 Thus, the exploration of new strategies and concepts for solid-state organic reactions is of great interest in synthetic chemistry.1,2The Nobel-prize-winning palladium-catalyzed cross-coupling reactions arguably represent the most powerful, versatile, and well-established class of organic transformations.3 In particular, the Suzuki–Miyaura cross-coupling of aryl halides with organoboron reagents has found wide applications in both academic and industrial settings.3c–3f Conventionally, Suzuki–Miyaura cross-coupling reactions are carried out in organic solvents.3c–3f Compared to such solvent-based protocols, solid-state cross-coupling reactions have remained extremely scarce, even though this approach would present the following advantages: (1) solvent waste would be greatly reduced, especially for large-scale syntheses;1,2 (2) solid-state coupling reactions should be particularly useful for the preparation of carbon-based materials (e.g. large polyaromatic hydrocarbons) that are typically prepared from substrates that are poorly soluble due to strong intermolecular interactions (e.g., π–π interactions);4 and 3) the set-up for solid-state cross-coupling reactions should be much simpler than that of solution-based reactions. Despite these benefits, only a limited number of solid-state Suzuki–Miyaura cross-coupling reactions under mechanochemical conditions using ball milling has been reported.5–7 Moreover, the scope of these reactions is significantly restricted, i.e., only electron-deficient aryl halides are applicable, and low conversion rates are common. Thus, we have focused on re-designing palladium-based catalyst systems for the development of a broadly applicable solid-state cross-coupling protocol, which may potentially unlock versatile applications for such solid-state syntheses.
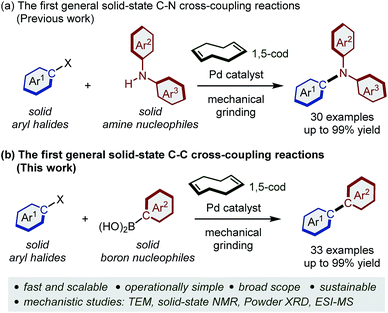
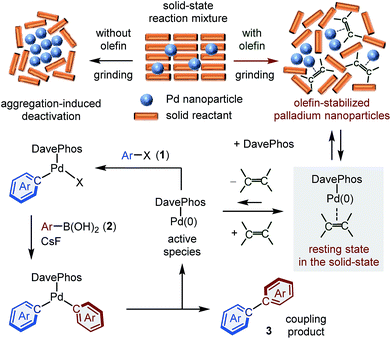
We have previously reported a rational strategy for a general and scalable solid-state Buchwald–Hartwig cross-coupling reaction using mechanochemistry (Scheme 1a).8 The key finding of this previous study was that olefin additives such as 1,5-cyclooctadiene (1,5-cod) dramatically accelerate the C–N bond-forming reaction in the solid state. Based on a transmission electron microscopy (TEM) analysis, we discovered that olefin additives can act as efficient molecular dispersants for palladium-based catalysts in the solid state, thus inhibiting the deleterious aggregation of the catalyst (Scheme 1a).8,9 Herein, we report the development of the first general and scalable solid-state Suzuki–Miyaura cross-coupling reactions using mechanochemistry (Scheme 1b). The present study is not a simple extension of our previous work,8 but represents the development of a new catalytic system for robust C–C bond-forming reaction in the solid-state. Compared to previous studies,5 the developed system shows a substantially broadened substrate scope and allows the first solid-state organoboron cross-coupling reaction of unactivated aryl chlorides.5a Furthermore, we demonstrate the solvent-free solid-state synthesis of large polyaromatic hydrocarbons and a gram-scale mechanochemical synthesis.4d,10,11 Experimental mechanistic studies to elucidate the roles for the olefin additives in these solid-state cross-coupling reactions are also described.
Results and discussion
In order to develop a potentially general and scalable solid-state organoboron cross-coupling protocol, we focused on Pd(OAc)2/DavePhos, which is a high-performance catalytic system for several cross-coupling reactions developed by Buchwald and co-workers.11,12 Initially, we compared the reactivity of liquid 1-bromobenzene (1a) and solid 4-bromo-1,1′-biphenyl (1b) in this mechanochemical palladium-catalyzed Suzuki–Miyaura cross-coupling reaction under slightly modified conditions to those originally used for the solution-based Suzuki–Miyaura coupling reaction (Scheme 2).12a Reactions were conducted in a Retsch MM400 mill in a stainless-steel milling jar (1.5 mL) at 25 Hz using one stainless-steel ball (diameter: 5 mm). In the presence of Pd(OAc)2/DavePhos, the Suzuki–Miyaura cross-coupling of liquid 1a with 4-dimethylaminophenylboronic acid (2a) proceeded readily to afford the corresponding coupling product (3a) in excellent yield (92%; Scheme 2a). In contrast, the reaction of solid 1b under the same reaction conditions resulted in a low yield of 3b (26%; Scheme 2b). These results suggest the existence of a considerable reactivity gap between liquid and solid substrates, even under mechanochemical conditions.8 This is probably due to poor mixing efficiency of the reactants and/or catalysts in the constrained solid-state reaction medium, even under ball-milling conditions.7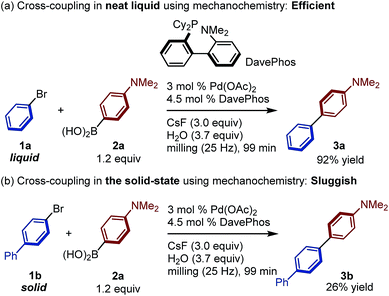 | ||
| Scheme 2 Reactivity difference between liquid and solid aryl bromides in a Suzuki–Miyaura cross-coupling reaction under solvent-free mechanochemical conditions. | ||
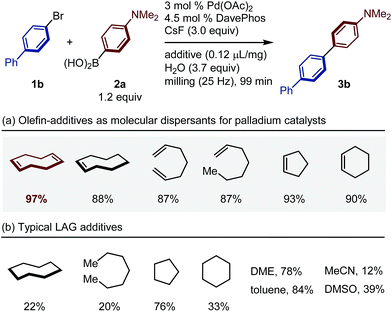
Given our recent success regarding olefin-accelerated solid-state C–N coupling reactions,8 we speculated that this strategy could also be potentially applied to solid-state organoboron cross-coupling reactions. Thus, we decided to focus on olefin additives that might act as molecular dispersants for palladium-based catalysts in solid-state media (Table 1). All liquid-assisted grinding (LAG) reactions13–16 with olefins or other liquid additives were performed at a 0.12 ratio of μL of liquid added per mg of reactant. The use of olefin additives efficiently promoted the coupling reactions of 1b with 2a (Table 1a). In particular, we found that the reaction was dramatically accelerated in the presence of 1,5-cod and the corresponding coupling product 3b was formed in quantitative yield (97%; Table 1a). In sharp contrast, the use of alkanes or typical organic solvents as LAG additives provided lower yields than those from the reaction with olefins (Table 1b), suggesting that the presence of an olefin functional group is crucial for the observed acceleration. It should be noted that the amount of olefin used in these reactions is comparable to that of the reactant (0.12 μL mg−1), which stands in sharp contrast to conventional solution-based coupling reactions, where often a 10- to 100-fold excess of solvent is used.
| a Conditions: 1b (0.3 mmol), 2a (0.36 mmol), Pd(OAc)2 (0.009 mmol), DavePhos (0.0135 mmol), CsF (0.9 mmol), H2O (20 μL), and additive (0.12 μL mg−1) in a stainless-steel ball-milling jar (1.5 mL) with a stainless-steel ball (5 mm). |
|---|
|
Subsequently, we investigated the effect of the phosphine ligand on the solid-state organoboron cross-coupling reaction (Table 2). Experiments involving catalyst systems consisting of 3 mol% Pd(OAc)2 and 4.5 mol% Buchwald-type ligands (Table 2, entries 1–6) revealed that the use of DavePhos provides the desired coupling product in the highest yield (entry 1, 97%). Simple monophosphine ligands such as tBu3P, Cy3P and Ph3P were also examined, but the product yields were moderate (50–70%, entry 7–9). In the absence of a supporting ligand, the reaction provides the product in very low yield (18%, entry 10), suggesting that the use of a phosphine ligand is crucial for this solid-state cross-coupling reaction.
| Entry | Ligand | NMR yield (%) |
|---|---|---|
| a Conditions: 1b (0.3 mmol), 2a (0.36 mmol), Pd(OAc)2 (0.009 mmol), ligand (0.0135 mmol), CsF (0.9 mmol), H2O (20 μL), and 1,5-cod (0.12 μL mg−1) in a stainless-steel ball-milling jar (1.5 mL) with a stainless-steel ball (5 mm). | ||
| 1 | DavePhos | 97 |
| 2 | XPhos | 65 |
| 3 | RuPhos | 92 |
| 4 | BrettPhos | 92 |
| 5 | tBuDavePhos | 82 |
| 6 | tBuXPhos | 70 |
| 7 | tBu3P | 50 |
| 8 | Cy3P | 60 |
| 9 | Ph3P | 43 |
| 10 | None | 18 |
| Entry | Frequency (Hz) | Number of balls | Ball size (mm) | NMR yield (%) |
|---|---|---|---|---|
| a Conditions: 1b (0.3 mmol), 2a (0.36 mmol), Pd(OAc)2 (0.009 mmol), DavePhos (0.0135 mmol), CsF (0.9 mmol), H2O (20 μL), and 1,5-cod (0.12 μL mg−1) in a stainless-steel ball-milling jar (1.5 mL). | ||||
| 1 | 10 | 1 | 5 | 70 |
| 2 | 15 | 1 | 5 | 75 |
| 3 | 20 | 1 | 5 | 70 |
| 4 | 25 | 1 | 5 | 97 |
| 5 | 30 | 1 | 5 | 99 |
| 6 | 25 | 1 | 3 | 90 |
| 7 | 25 | 2 | 5 | 97 |
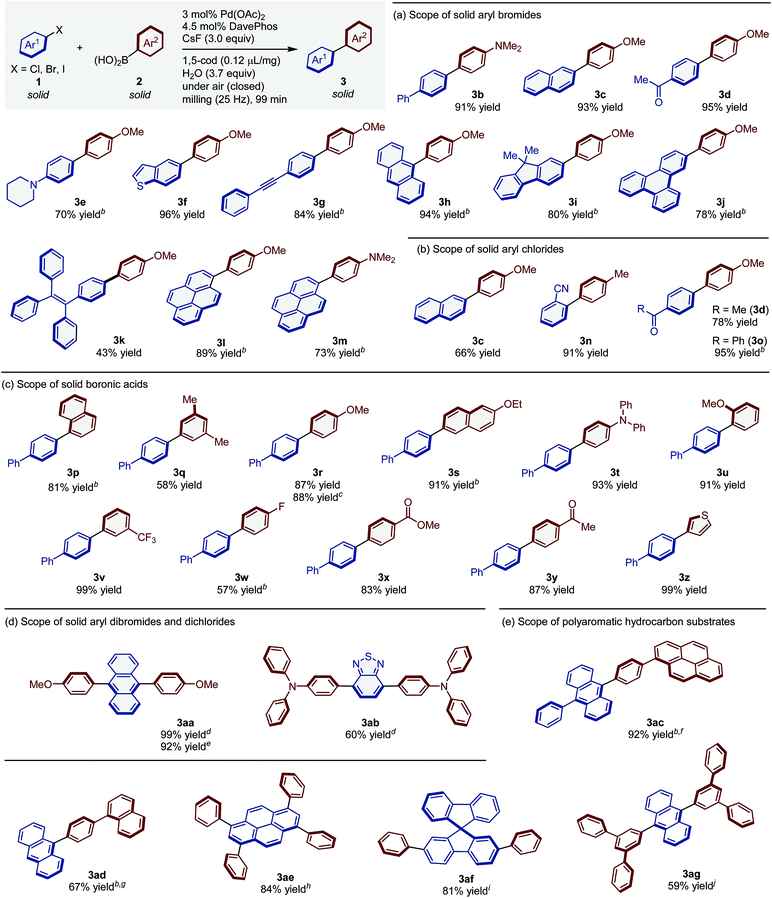
To explore the scope of the present solid-state coupling reaction, a variety of solid aryl halides was tested (Table 4a). This reaction is characterized by a broad substrate scope. The reaction of 2-bromonaphthalene (1c) and that of electron-poor aryl bromide 1d with p-methoxyboronic acid (2b) proceeded to give the desired products in excellent yield (3c: 93%; 3d: 95%). The developed conditions were also applied to the electron-rich, unactivated solid aryl bromide 1e, which afforded the desired product (3e) in good yield (70%). Aryl bromides containing a thiophene moiety (1f) and an internal alkyne (1g) also provided the corresponding products in high yield (3f: 96%; 3g: 84%). In addition, aryl bromides containing anthracene (1h), fluorene (1i), triphenylene (1j), tetraphenylethylene (1k), and pyrene (1l and 1m) moieties successfully furnished the desired coupling products (3h–3m) in moderate to excellent yield (43–94%).
| a Reaction conditions: Pd(OAc)2 (0.009 mmol), DavePhos (0.0135 mmol), 1 (0.3 mmol), 2 (0.36 mmol), CsF (0.9 mmol), H2O (20 μL), 1,5-cod (0.12μL mg−1) in a stainless-steel ball-milling jar (1.5 mL) with a stainless-steel ball (diameter: 5 mm), 25 Hz, 99 min. Isolated yields are shown. b 1,5-Cod (0.2 μL mg−1) was used. c The corresponding aryl iodide was used as the substrate. d Reaction conditions: Pd(OAc)2 (0.009 mmol), DavePhos (0.0135 mmol), 1 (0.15 mmol), 2 (0.36 mmol), CsF (0.9 mmol), H2O (20 μL), 1,5-cod (0.2μL mg−1) in a stainless-steel ball-milling jar (1.5 mL) with a stainless-steel ball (diameter: 5 mm), 25 Hz, 99 min. e The corresponding aryl chloride was used as the substrate. f Reaction time: 180 min. g 0.2 mmol scale. h Reaction conditions: Pd(OAc)2 (0.036 mmol), DavePhos (0.054 mmol), 1 (0.3 mmol), 2 (4.8 mmol), CsF (3.6 mmol), H2O (80 μL), 1,5-cod (0.2 μL mg−1) in a stainless-steel ball-milling jar (25 mL) with stainless-steel balls (diameter: 4 × 10 mm), 25 Hz, 99 min. i Reaction conditions: Pd(OAc)2 (0.018 mmol), DavePhos (0.027 mmol), 1 (0.3 mmol), 2 (2.4 mmol), CsF (1.8 mmol), H2O (40 μL), 1,5-cod (0.2 μL mg−1) in a stainless-steel ball-milling jar (5 mL) with a stainless-steel ball (diameter: 10 mm), 25 Hz, 180 min. j Reaction conditions: Pd(OAc)2 (0.03 mmol), DavePhos (0.045 mmol), 1 (0.3 mmol), 2 (0.72 mmol), CsF (1.8 mmol), H2O (40 μL), 1,5-cod (0.2 μL mg−1) in a stainless-steel ball-milling jar (5 mL) with a stainless-steel ball (diameter: 10 mm), 25 Hz, 99 min. |
|---|
|
Notably, the developed catalytic system enabled also the solid-state organoboron cross-coupling of unactivated aryl chlorides (Table 4b). The reaction of solid 2-chloronaphthalene (1c′) efficiently furnished the desired product (3c; 66%). Aryl chlorides bearing electron-withdrawing groups such as cyano and carbonyl groups also provided the desired products (3d, 3n and 3o) in high yield (78–95%).
Subsequently, we turned our attention to the scope of solid arylboronic acids (Table 4c). Simple aryl boronic acids afforded the corresponding coupling products (3p and 3q) in good yield (81% and 58%, respectively). Substrates bearing electron-donating groups were coupled efficiently to provide the products (3r–3u) in good to excellent yield (87–94%). The product 3r was also obtained using the corresponding 4-iodobiphenyl substrate (88%). Substrates bearing electron-withdrawing groups were also compatible with the applied solid-state conditions (3v–3y; 57–99%). Finally, this method was successfully applied to the solid-state synthesis of heteroaryl-containing biaryl derivatives (3z; 99%).
Under the developed conditions, cross-coupling reactions of diaryl halides in the solid state also proceeded to give the diarylated products in good yield (Table 4d). The reaction of 9,10-dibromoanthracene (1aa) with 2b afforded the double arylation product 3aa in quantitative yield (99%). Notably, 3aa could also be successfully synthesized from the corresponding aryl chloride (92%). In addition, this reaction permits the rapid construction of 2,1,3-benzothiadiazole derivatives, which are π-conjugated molecules that are frequently encountered in dye-sensitized solar cells.17 Specifically, the reaction of dibromo-substituted 2,1,3-benzothiadiazole (1ab) furnished an symmetrical D–A–π–A–D system in good yield (3ab: 60%).
We expected this solid-state organoboron cross-coupling reaction to be particularly useful for the preparation of carbon-based materials that are poorly soluble substrates (e.g. large polyaromatic hydrocarbons) due to strong intermolecular interactions.4 Therefore, we carried out a preliminary investigation on the construction of polyaromatic hydrocarbons via solid-state cross-coupling reactions using mechanochemistry (Table 4e). Structurally complex polyaromatic hydrocarbons (3ac–3ag) were successfully synthesized in good to high yield (59–92%). Conventional solvent-based coupling conditions9 for the preparation of such polyaromatic hydrocarbons often require the use of significant amounts of solvent (<0.05 M), high temperatures, and long reaction times, thus highlighting the synthetic utility of the solid-state cross-coupling method presented herein.
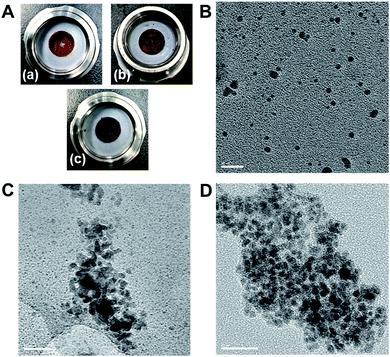
In order to further explore the synthetic utility of this protocol, we conducted a solid-state cross-coupling reaction on the gram-scale under mechanochemical conditions (Fig. 1). The solid-state cross-coupling of 9-bromoanthracene (1h) with 2b was carried out at an 8.0 mmol scale in a stainless-steel ball-milling jar (25 mL) using four stainless-steel balls (diameter: 10 mm), which afforded 3h in high yield (87%). The product could be isolated by simple re-precipitation from CH2Cl2/MeOH. For comparison, we also carried out a solution-based Suzuki–Miyaura cross-coupling reaction in dioxane12a at high reaction temperature (100 °C) using a large amount of solvent (160 mL, 0.05 M) and prolonged reaction time (24 h); however, under these conditions, 3h was obtained only in a moderate yield (52%).18 It should be noted that solution-based reactions of insoluble aromatic substrates such as 1h typically require significant amounts of dry solvent, an inert atmosphere, high-vacuum instrumentation, and large reaction flasks equipped with a reflux condenser and an oil bath on a stirring hotplate (Fig. 1).10 Compared to such complex set-ups, the present solid-state coupling reactions can be carried out in a small jar in air,19 and special operational skills are not required (for the details of the procedure, see the ESI†). This operational simplicity is likely to substantially increase the practical utility of the present solvent-free solid-state cross-coupling reactions beyond the laboratory scale.
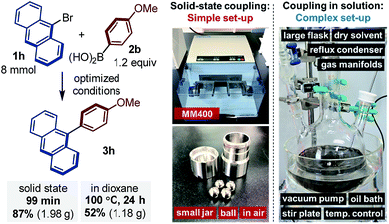 | ||
| Fig. 1 Solid-state coupling reaction on the gram scale (for detailed reaction conditions, see the ESI†). | ||
To gain mechanistic insight into the observed acceleration effect upon the addition of olefin additives, we used TEM imaging to characterize the palladium nanoparticles generated in situ in the crude reaction mixture of 1b with 2a (Fig. 2). The observed image clearly shows the formation of palladium nanoparticles (approximate size: 3–5 nm) in the reaction mixture in the presence of 1,5-cod (Fig. 2B). Notably, higher aggregation of the palladium particles was not observed. On the other hand, the image obtained for the palladium species derived from the reaction mixture in the presence of cyclooctane (Fig. 2C) and in the absence of any additive (Fig. 2D) shows that the palladium species have significantly aggregated into dense particles (Fig. 2C and 2D). These results suggest that olefin additives might act as dispersants for the palladium-based catalyst to suppress higher aggregation of the nanoparticles.8,9
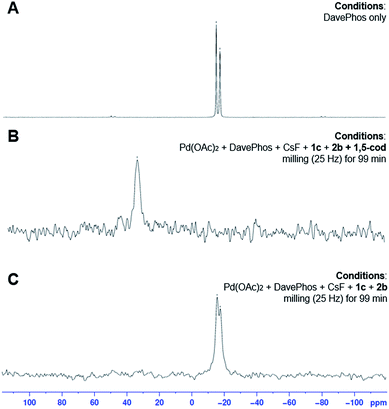
Subsequently, we used solid-state (SS) 31P NMR spectroscopy to examine if monomeric catalytic active species are formed during the cross-coupling reaction in the solid state (Fig. 3). The SS 31P NMR spectra of the reaction mixtures of 2-bromonaphthalene (1c) and p-methoxyboronic acid (2b) after grinding (25 Hz) in a ball mill in the presence of 1,5-cod showed that signals derived from DavePhos (−14.0 and −16.3 ppm) had completely disappeared, and only one signal (37.6 ppm) was observed, suggesting that a DavePhos-ligated monomeric palladium species is most likely generated (Fig. 3A and B). This is consistent with the results of Table 2, which show that the ligands significantly affect the reactivity. Notably, the SS 31P NMR spectra of the reaction mixtures of 1c and 2b after grinding (25 Hz) in the absence of 1,5-cod showed only signals derived from DavePhos (Fig. 3C). The crude reaction mixture of 1c and 2b after grinding in the presence of 1,5-cod was further characterized by ESI mass spectrometry (Scheme 3). The mass spectrum showed a peak corresponding to DavePhos-Pd(0)–(1,5-cod), which is consistent with the results of the SS 31P NMR analysis. These results suggest that the monomeric DavePhos-Pd(0)–(1,5-cod) species is most likely the resting state of the catalyst during the solid-state cross-coupling reaction in the presence of 1,5-cod.20 While such a palladium(0) complex should be unstable under ambient conditions,19 we speculate that the longevity of this palladium(0) complex might be rationalized in terms of the low diffusion efficiency of gaseous oxygen in such solid-state reaction mixtures.21
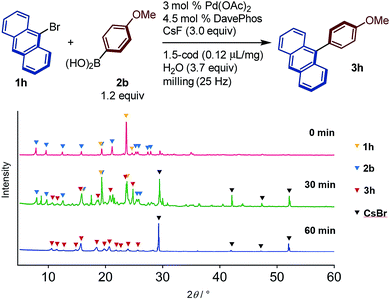
The reaction progress of the cross-coupling reaction between 1h and 2b in the presence of 1,5-cod was monitored by powder X-ray diffraction (PXRD) analysis (Fig. 4). After 30 min, new diffraction peaks derived from cross-coupling product 3h and CsBr appeared, while the peaks associated with the starting materials remained. After 60 min, the diffraction peaks derived from the starting materials had been completely disappeared, and only those of coupling product 3b and CsBr were observed, demonstrating a clean solid-to-solid conversion without melting during the reaction.8 It should also be noted that a thermographic analysis revealed a temperature of inside the milling jar after grinding for 99 min at 25 Hz was ∼35 °C, which indicates that the temperature did not significantly increase under the applied mechanochemical conditions (see the ESI† for the details).
Based on our mechanistic data, we would like to propose two possible roles for the olefin additives in these solid-state cross-coupling reactions (Fig. 5): (1) olefins might act as dispersants for the palladium-based catalyst to suppress higher aggregation of the nanoparticles, which would lead to catalyst deactivation,8,9 and (2) the active monomeric Pd(0) species could be stabilized upon coordination by the olefins; subsequently, dissociated Pd(0) species could be coordinated by DavePhos and release the olefin ligands to form [(DavePhos)Pd(0)], which could activate the C–X bond of aryl halides.8,12,22
Conclusions
In summary, we have developed the first general and scalable solid-state Suzuki–Miyaura cross-coupling reaction using mechanochemistry. While few examples of mechanochemical palladium-catalyzed coupling reactions of solid substrates have already been reported, their scope is significantly limited, and they typically exhibit low conversion rates. However, we discovered that the addition of small amounts of olefins dramatically improves the progress of these challenging solid-state C–C bond-forming cross-coupling reactions. Although the use of solvents during work up and/or purification remains a future challenge, the development of our solvent-free solid-state cross-coupling reaction constitutes a significant step in developing industrially attractive and environmentally friendly routes to a broad variety of synthetic targets.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Japan Society for the Promotion of Science (JSPS) via KAKENHI grants JP18H03907, JP17H06370 and JP19K15547 as well as by the Institute for Chemical Reaction Design and Discovery (ICReDD), which has been established by the World Premier International Research Initiative (WPI), MEXT, Japan. We would like to thank Mr Naoya Nakagawa for his help analysing the solid-state NMR. We would like to thank Ms. Nozomi Takeda for her help analysing the ICP-AES.Notes and references
-
(a) D. J. C. Constable, C. Jimenez-Gonzalez and R. K. Henderson, Org. Process Res. Dev., 2007, 11, 133 CrossRef CAS
; (b) J. H. Clark and S. J. Tavener, Org. Process Res. Dev., 2007, 11, 149 CrossRef CAS
; (c) J. M. DeSimone, Science, 2002, 297, 799 CrossRef CAS PubMed
.
-
(a)
Solvent-free organic synthesis, ed. K. Tanaka, Wiley-VCH, Weinheim, 2009 Search PubMed
; (b) Organic solid-state reactions, ed. F. Toda, Springer, 2004 Search PubMed
; (c) F. Toda, Acc. Chem. Res., 1995, 28, 480 CrossRef CAS
; (d) K. Tanaka and F. Toda, Chem. Rev., 2000, 100, 480 CrossRef
.
-
(a) C. C. C. Johansson Seehurn, M. O. Kitching, T. J. Colacot and V. Snieckus, Angew. Chem., Int. Ed., 2012, 51, 5062 CrossRef PubMed
; (b) Palladium-catalyzed coupling reactions: Practical aspects and future developments, ed. Á. Molnár, Wiley-VCH, Weinheim, 2013 Search PubMed
; (c) Metal-catalyzed cross-coupling reactions, ed. A. Meijere and F. Diederich, Wiley-VCH, Weinheim, 2nd revised edn, 2008 Search PubMed
; (d) N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457 CrossRef CAS
; (e) A. J. J. Lennox and G. C. Lloyd-Jones, Chem. Soc. Rev., 2014, 43, 412 RSC
; (f) R. Martin and S. L. Buchwald, Acc. Chem. Res., 2008, 41, 1461 CrossRef CAS PubMed
.
-
(a) L. Chen, Y. Hernandez, X. Feng and K. Müllen, Angew. Chem., Int. Ed., 2012, 51, 7640 CrossRef CAS PubMed
; (b) L. T. Scott, E. A. Jackson, Q. Zhang, B. D. Steinberg, M. Bancu and B. Li, J. Am. Chem. Soc., 2012, 134, 107 CrossRef CAS PubMed
; (c) G. Povie, Y. Segawa, T. Nishihara, Y. Miyauchi and K. Itami, Science, 2017, 356, 172 CrossRef CAS PubMed
; (d) K. Kawasumi, Q. Zhang, Y. Segawa, L. T. Scott and K. Itami, Nat. Chem., 2013, 5, 739 CrossRef CAS PubMed
; (e) Y. Koga, T. Kaneda, Y. Saito, K. Murakami and K. Itami, Science, 2018, 359, 435 CrossRef CAS PubMed
.
- For examples of solvent-free solid-state Suzuki–Miyaura cross-coupling reactions using mechanochemistry, see:
(a) G. Cravotto, D. Garella, S. Tagliapietra, A. Stolle, S. Schüßler, S. E. S. Leonhardt and B. Ondruschka, New J. Chem., 2012, 36, 1304 RSC
; (b) F. Schneider and B. Ondruschka, ChemSusChem, 2008, 1, 622 CrossRef CAS PubMed
; (c) S. F. Nielsen, D. Peters and O. Axelsson, Synth. Commun., 2000, 30, 3501 CrossRef CAS
; (d) E. N. Leadbeater and M. L. Klingensmith, Tetrahedron Lett., 2003, 44, 765 CrossRef
.
- For examples of solvent-free Suzuki–Miyaura cross-coupling reactions of liquid substrates using mechanochemistry, see:
(a) F. Schneider, A. Stolle, B. Ondruschka and H. Hopf, Org. Process Res. Dev., 2009, 13, 44 CrossRef CAS
; (b) Z.-J. Jiang, Z.-H. Li, J.-B. Yu and W.-K. Su, J. Org. Chem., 2016, 81, 10049 CrossRef CAS PubMed
.
- For recent reviews on organic syntheses using mechanochemistry, see:
(a) S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413 RSC
; (b) G.-W. Wang, Chem. Soc. Rev., 2013, 42, 7668 RSC
; (c) T. Friščić, I. Halasz, V. Štrukil, M. Eckert-Maksić and R. E. Dinnebier, ACS Cent. Sci., 2017, 3, 13 CrossRef PubMed
; (d) J. G. Hernández and C. Bolm, J. Org. Chem., 2017, 82, 4007 CrossRef PubMed
; (e) J. G. Hernández, Chem.–Eur. J., 2017, 23, 17157 CrossRef PubMed
; (f) T.-X. Métro, J. Martinez and F. Lamaty, ACS Sustainable Chem. Eng., 2017, 5, 9599 CrossRef
; (g) T. K. Achar, A. Bose and P. Mal, Beilstein J. Org. Chem., 2017, 13, 1907 CrossRef CAS PubMed
; (h) J.-L. Do and T. Friščić, Synlett, 2017, 28, 2066 CrossRef CAS
; (i) D. Tan and T. Friščić, Eur. J. Org. Chem., 2018, 18 CrossRef CAS
; (j) O. Eguaogie, J. S. Vyle, P. F. Conlon, M. A. Gîlea and Y. Liang, Beilstein J. Org. Chem., 2018, 14, 955 CrossRef CAS PubMed
; (k) J. L. Howard, Q. Cao and D. L. Browne, Chem. Sci., 2018, 9, 3080 RSC
; (l) J. Andersen and J. Mack, Green Chem., 2018, 20, 1435 RSC
; (m) N. R. Rightmire and T. P. Hanusa, Dalton Trans., 2016, 45, 2352 RSC
; (n) M. Leonardi, M. Villacampa and J. C. Menéndez, Chem. Sci., 2018, 9, 2042 RSC
; (o) C. Xu, S. De, A. M. Balu, M. Ojeda and R. Luque, Chem. Commun., 2015, 51, 6698 RSC
; (p) D. Tan and T. Friščić, Chem. Commun., 2016, 52, 7760 RSC
; (q) A. A. Gečiauskaité and F. García, Beilstein J. Org. Chem., 2017, 13, 2068 CrossRef PubMed
; (r) M. J. Muñoz-Bastista, D. Rodriguez-Padron, A. R. Puente-Santiago and R. Luque, ACS Sustainable Chem. Eng., 2018, 9, 9530 CrossRef
; (s) J. Andersen and J. Mack, Green Chem., 2018, 20, 1435 RSC
; (t) E. Colacino, M. Carta, G. Pia, A. Porcheddu, P. C. Ricci and F. Delogu, ACS Omega, 2018, 3, 9196 CrossRef CAS
; (u) D. Tan and T. Friščić, Eur. J. Org. Chem., 2018, 1, 18 CrossRef
; (v) D. Tan and F. García, Chem. Soc. Rev., 2019, 48, 2274 RSC
; (w) C. Bolm and J. D. Hernández, Angew. Chem., Int. Ed., 2019, 58, 3285 CrossRef CAS PubMed
.
- K. Kubota, T. Seo, K. Koide, Y. Hasegawa and H. Ito, Nat. Commun., 2019, 10, 111 CrossRef PubMed
.
- I. J. S. Fairlamb, Org. Biomol. Chem., 2008, 6, 3645 RSC
.
- For selected examples of Suzuki–Miyaura cross-coupling reactions for the synthesis of polyaromatic hydrocarbons under solvent-based conditions, see:
(a) J. Huang, B. Xu, J.-H. Su, H. C. Chen and H. Tian, Tetrahedron, 2010, 66, 7577 CrossRef CAS
; (b) B. Kobin, S. Behren, B. Braun-Cula and S. Hecht, J. Phys. Chem. A, 2016, 120, 5474 CrossRef CAS PubMed
; (c) S.-K. Kim, B. Yang, Y. Ma, J.-H. Lee and J.-W. Park, J. Mater. Chem., 2008, 18, 3376 RSC
; (d) S. Jeelani Basha, R. Venkatachalam, V. Babu and S. Sethuraman, Beilstein J. Org. Chem., 2013, 9, 698 CrossRef
; (e) H. Lee, B. Kim, S. Kim, J. Kim, J. Lee, H. Shin, J.-H. Lee and J. Park, J. Mater. Chem. C, 2014, 2, 4737 RSC
.
- J.-P. Corbet and G. Mignani, Chem. Rev., 2006, 106, 2651 CrossRef CAS PubMed
.
-
(a) D. W. Old, J. P. Wolfe and S. L. Buchwald, J. Am. Chem. Soc., 1998, 120, 9722 CrossRef CAS
; (b) R. Martin and S. L. Buchwald, Acc. Chem. Res., 2008, 41, 1461 CrossRef CAS PubMed
; (c) A. C. Sather and S. L. Buchwald, Acc. Chem. Res., 2016, 49, 2146 CrossRef CAS PubMed
.
- The selected example of liquid-assisted grinding, see: T. Friščić, S. L. Childs, S. A. A. Rizvi and W. Jones, CrystEngComm, 2009, 11, 418 RSC
.
- The selected example of ion- and liquid-assisted grinding, see: T. Friščić, D. G. Reid, I. Halasz, R. S. Stein, R. E. Dinnebier and M. J. Duer, Angew. Chem., Int. Ed., 2010, 49, 712 CrossRef PubMed
.
- The selected example of vapour-assisted-grinding, see: C. Jia, J. Wang, X. Feng, Q. Lin and W. Tuan, CrystEngComm, 2014, 16, 6552 RSC
.
- The selected examples of polymer-assisted-grinding, see:
(a) D. Hasa, G. S. Rauber, D. Voinovich and W. Jones, Angew. Chem., Int. Ed., 2015, 54, 7371 CrossRef CAS PubMed
; (b) D. Hasa, E. Carlino and W. Jones, Cryst. Growth Des., 2016, 16, 1772 CrossRef CAS
.
-
(a) J. Hou, H.-Y. Chen, S. Zhang, G. Li and Y. Yang, J. Am. Chem. Soc., 2008, 130, 16144 CrossRef CAS PubMed
; (b) Z. Yao, M. Zhang, H. Wu, L. Yang, R. Ki and P. Wang, J. Am. Chem. Soc., 2015, 130, 16144 Search PubMed
; (c) J. Zhang, W. Chen, A. J. Rojas, E. V. Jucov, T. V. Timofeeva, T. C. Parker, S. Barlow and S. R. Marder, J. Am. Chem. Soc., 2013, 135, 16376 CrossRef CAS
.
- When the solution-based reaction was carried out at room temperature, the product yield was significantly lower (33% NMR yield).
- The reaction under an atmosphere of Ar provided a similar result as the reaction conducted in air, suggesting that the presence/absence of oxygen is not crucial for the reaction, which is another advantage of this method.
- For structure and reactivity of [(LPd)n(1,5-cod)] complexes, see: H. G. Lee, P. J. Milner, M. T. Colvin, L. Andreas and S. L. Buchwald, Inorg. Chim. Acta, 2014, 422, 188 CrossRef CAS PubMed
.
- We have also attempted to prepare the DavePhos-Pd(0)-(1,5-cod) complex via the reaction of (1,5-cod)Pd(CH2TMS)2 and DavePhos in pentane, but the complex was probably unstable in solution and decomposed during the reaction. However, the in situ31P NMR analysis of the reaction in d8-toluene showed a signal around 35 ppm, which suggests the formation of the DavePhos-Pd(0)-(1,5-cod) complex. The chemical shift is consistent with the SS 31P NMR shown in Fig. 3B, suggesting that the observed signal (37.6 ppm) in the SS 31P NMR would be derived from the DavePhos-Pd(0)-(1,5-cod) complex.
-
(a) J. Hu and Y. Liu, Langmuir, 2005, 21, 2121 CrossRef CAS
; (b) T. Yurino, Y. Ueda, Y. Shimizu, S. Tanaka, H. Nishiyama, H. Tsurugi, K. Sato and K. Mashima, Angew. Chem., Int. Ed., 2015, 54, 14437 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9sc02185j |
| This journal is © The Royal Society of Chemistry 2019 |